ABIONYX Announces First Patient Enrolled in Phase 2a Clinical Study With CER-001, the Bio-HDL for the Treatment of Septic Pat...
08 Juin 2021 - 7:30AM
Business Wire
- Evaluation of the clinical activity by dosage level of
CER-001 in the prevention of Acute Kidney Injury in ICU patients
with septicemia
- A potentially modifying effect on the progression of the
inflammatory cascade in sepsis
Regulatory News:
ABIONYX Pharma (FR0012616852 - ABNX - PEA PME eligible),
a new generation biotech company dedicated to the discovery and
development of innovative therapies for patients, today announces
that the first patient has been enrolled in a Phase 2a clinical
study evaluating CER-001, the Bio-HDL, as a potential treatment for
septic patients at high risk of developing acute kidney injury.
"After positive clinical results in an ultra-rare kidney
disease, we believe that CER-001 could have a scavenger role in
reducing circulating endotoxin, as well as inflammation and
endothelial damage. Several other AKI/sepsis models showed that HDL
is a critical factor in modifying the disease," said Professor
Loreto Gesualdo, full Professor, Head of the Nephrology, Dialysis
and Transplantation unit, University of Bari Aldo Moro, Italy.
"Following the promising results obtained in our preclinical
LPS-induced sepsis model, we are now starting patient enrollment in
RACERS (a RAndomized study comparing short-term
CER-001 infusions at different doses to prevent
Sepsis-induced acute kidney injury). We are strongly
committed to improve therapeutic options and mortality in sepsis
patients."
RACERS is a randomized Phase 2a, open labelled,
placebo-controlled, parallel-group study evaluating the safety and
efficacy of intravenously administered CER-001 in ICU patients with
sepsis at high risk for AKI based on their Sequential Organ Failure
Assessment (SOFA score). A total of 20 patients will be randomized
to receive 8 doses of CER-001 or placebo over 6 days. The primary
endpoint of the study will be the onset and severity of AKI
according to KDIGO criteria as well as safety and tolerability of
the dosage regimens in order to select the optimal dose of
CER-001.
The clinical study is partnered with the University of Bari and
the Consorzio per Valutazioni Biologiche e Farmacologiche (CBVF)
and is already fully funded.
About ABIONYX Pharma
ABIONYX Pharma is a new generation biotech company dedicated to
the discovery and development of innovative therapies for patients.
The biotech develops a Bio-HDL, a bioproduct that mimics the
natural HDL for the treatment of kidney diseases and the delivery
of targeted drugs.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20210607005696/en/
NewCap Investor relations Louis-Victor Delouvrier
abionyx@newcap.eu +33 (0)1 44 71 98 53
NewCap Media relations Nicolas Merigeau abionyx@newcap.eu
+33 (0)1 44 71 94 98
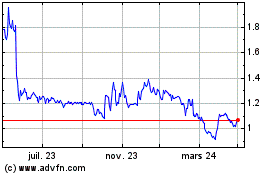
Abionyx Pharma (EU:ABNX)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
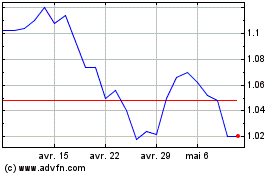
Abionyx Pharma (EU:ABNX)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024