FDA Approves Pfizer's Fragmin for Use as a Pediatric Anticoagulant
16 Mai 2019 - 7:21PM
Dow Jones News
By Stephen Nakrosis
The U.S. Food and Drug Administration on Thursday said it
approved for the first time an anticoagulant for pediatric patients
to treat potentially life-threatening blood clots.
The FDA said it approved Fragmin, or dalteparin sodium, for
pediatric patients one month of age and older. Pfizer holds the
application for Fragmin, the agency said.
Fragmin is used to reduce the recurrence of symptomatic venous
thromboembolism, or VTE, which can include blood clots in the deep
veins of the leg and blood clots in the lungs.
VTE usually develops as a secondary complication from conditions
including cancer, congenital heart disease and trauma or surgery,
the agency said.
--Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
May 16, 2019 13:06 ET (17:06 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
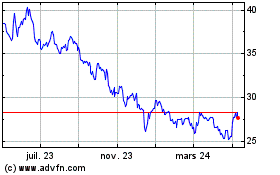
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
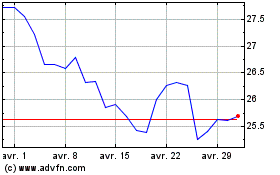
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024