FDA Approves Two-Drug Combination HIV Treatment
08 Avril 2019 - 11:00PM
Dow Jones News
By Maria Armental
A two-drug, single-tablet regimen to treat the most prevalent
type of the AIDS virus has been approved for sale in the U.S., the
Food and Drug Administration said Monday.
Dovato (dolutegravir and lamivudine)--developed by
GlaxoSmithKline PLC and Pfizer Inc.'s HIV venture ViiV
Healthcare--is the first FDA two-drug, fixed-dose, complete regimen
for adults infected with the human immunodeficiency virus type 1
who have never received treatment for HIV.
The current standard of care for such patients is a three-drug
treatment, noted Dr. Debra Birnkrant, director of the FDA's
division of antiviral products.
Dovato will carry a label warning patients with HIV and
hepatitis B to have additional treatment for hepatitis B or use a
different drug regimen.
Patients with HIV and hepatitis B who have taken products with
lamivudine, one of the ingredients in Dovato, have developed
hepatitis B variants associated with resistance to lamivudine and
may have severe liver problems, including liver failure, when they
stop taking drugs containing lamivudine, the FDA said.
The approval is part of the U.S. push to effectively end the HIV
epidemic, reducing new infections by 75% in the next five years and
by 90% in the next 10 years.
Write to Maria Armental at maria.armental@wsj.com
(END) Dow Jones Newswires
April 08, 2019 16:45 ET (20:45 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
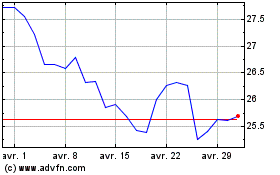
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
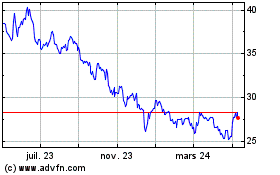
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024