J&J's Janssen Gets CHMP Positive Option for Spravato
18 Octobre 2019 - 2:10PM
Dow Jones News
By Colin Kellaher
Johnson & Johnson's (JNJ) Janssen Pharmaceutical Cos. unit
on Friday said the European Medicines Agency's Committee for
Medicinal Products for Human Use recommended approval of its
Spravato nasal spray for adults with treatment-resistant major
depressive disorder.
The drug maker said the recommendation covers Spravato in
combination with a selective serotonin reuptake inhibitor or
serotonin and norepinephrine reuptake inhibitor for adults with
treatment-resistant depression, meaning they haven't responded to
at least two different treatments.
The European Commission, which generally follows the CHMP's
recommendations, is expected to make a final decision by the end of
the year, Janssen said.
The U.S. Food and Drug Administration in March approved Spravato
for patients with treatment-resistant depression. The nasal spray
is a close chemical relation to ketamine, an anesthetic that is
often abused as a party drug but has been shown to have a
fast-acting impact on depression symptoms.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
October 18, 2019 07:55 ET (11:55 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
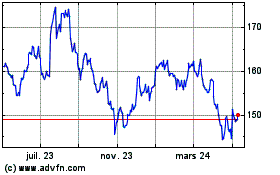
Johnson and Johnson (NYSE:JNJ)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
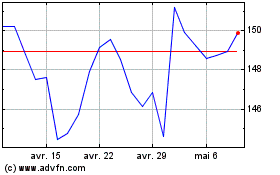
Johnson and Johnson (NYSE:JNJ)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024