Merck Gets FDA OK For Expanded Pifeltro, Delstrigo Indications
20 Septembre 2019 - 1:52PM
Dow Jones News
By Colin Kellaher
Merck & Co. (MRK) on Friday said the U.S. Food and Drug
Administration approved supplemental new-drug applications
expanding the use of the HIV-1 treatments Pifeltro and
Delstrigo.
The Kenilworth, N.J., drug maker said the approval expands the
indications for Pifeltro, in combination with other antiretroviral
agents, and Delstrigo as a complete regimen to include adults with
HIV-1 infection who are virologically suppressed on a stable
antiretroviral regimen with no history of treatment failure and no
known substitutions associated with resistance to Pifeltro or the
individual components of Delstrigo.
The FDA last year approved Pifeltro and Delstrigo for the
treatment of HIV-1 infection in adults with no prior antiretroviral
treatment history.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
September 20, 2019 07:37 ET (11:37 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
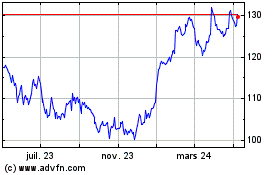
Merck (NYSE:MRK)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
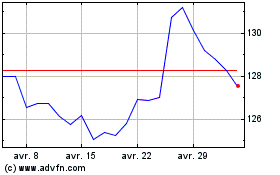
Merck (NYSE:MRK)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024