Pfizer Says EC Approves Vizimpro for Non-Small Cell Lung Cancer
03 Avril 2019 - 4:33PM
Dow Jones News
By Michael Dabaie
Pfizer Inc. (PFE) said Wednesday the European Commission
approved Vizimpro as monotherapy for the first-line treatment of
lung cancer.
The EC approved Vizimpro for adult patients with locally
advanced or metastatic non-small cell lung cancer with epidermal
growth factor receptor-activating mutations. The EC's approval was
supported by data from the Archer 1050 Phase 3 study. The primary
endpoint was progression-free survival as determined by blinded
Independent Radiologic Central review. A statistically significant
improvement in PFS as determined by the IRC was demonstrated, the
company said.
In 2012, Pfizer and SFJ Pharmaceuticals agreed to conduct the
Archer 1050 study across multiple sites. SFJ Pharmaceuticals
provided the funding and conducted the trial to generate the
clinical data used to support this application. Pfizer retains all
rights to commercialize Vizimpro globally.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
April 03, 2019 10:18 ET (14:18 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
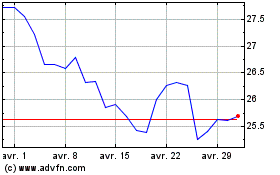
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
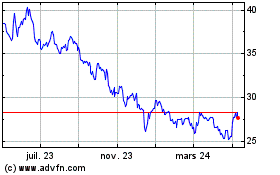
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024