Pfizer Says FDA Approves Lorbrena for Certain Lung Cancer Patients
02 Novembre 2018 - 10:37PM
Dow Jones News
By Stephen Nakrosis
Pfizer Inc. (PFE) said Friday the U.S. Food and Drug
Administration approved lorbrena for certain lung cancer
patients.
The approval was based on a multicenter Phase 1/2 study,
B7461001, which evaluated lorbrena for the treatment of patients
with certain types of lung cancer whose disease had progressed
while receiving other treatments.
Continued approval for this indication may be contingent upon
verification and description of clinical benefit in a confirmatory
trial, the company said.
Pfizer said the latest approval is the company's third for an
oncology treatment, including two lung cancer medicines, within two
months.
--Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
November 02, 2018 17:22 ET (21:22 GMT)
Copyright (c) 2018 Dow Jones & Company, Inc.
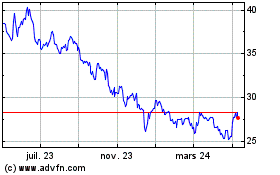
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
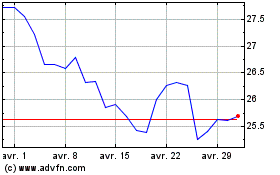
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024