Pfizer Sees Positive Top-Line Results in Abrocitinib Study
15 Mai 2019 - 1:30PM
Dow Jones News
By Chris Wack
Pfizer Inc. (PFE) said Wednesday that it saw positive top-line
results from a Phase 3 pivotal study for its investigational oral
Janus kinase 1 inhibitor, abrocitinib, in patients aged 12 and
older with moderate to severe atopic dermatitis.
The pharmaceutical company said in a release that top-line
results showed that by week 12, the percentage of patients
achieving each co-primary efficacy endpoint and each key secondary
endpoint with either dose of abrocitinib was statistically
significantly higher than placebo.
Pfizer said the results demonstrate response to treatment for a
statistically significant number of patients during the first two
to four weeks following the first dose.
The company said that safety results showed that both doses of
abrocitinib were well-tolerated and there were no unexpected safety
events. The discontinuation rates due to an adverse event were low
in each treatment arm compared to placebo.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
May 15, 2019 07:15 ET (11:15 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
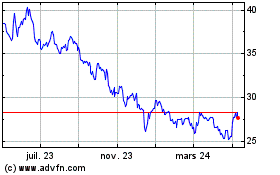
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
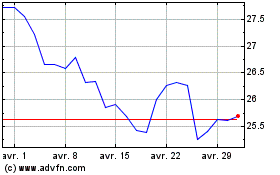
Pfizer (NYSE:PFE)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024