First Patients Treated With Theraclion's SONOVEIN® in the US
11 Mai 2022 - 6:45PM
Business Wire
Regulatory News:
THERACLION (ISIN: FR0010120402; Mnemo: ALTHE), an innovative
company developing a scalable robotic platform for non-invasive
echotherapy, announces today the treatment of varicose veins
patients with SONOVEIN for the first time in the United States
(US).
The study has been approved by the FDA (Food and Drug
Administration) a few months ago. Since then, Theraclion has
installed its state-of-the-art SONOVEIN platform and trained
physicians. The first patients have been treated by Dr Steven
Elias, Dr Nicos Labropoulos and Dr Antonios Gasparis, all three
internationally recognized vein specialists with more than 30 years
of experience.
“After this clinical trial, a full pivotal study will be
conducted for FDA review in view of the market authorisation. Our
qualitative pre-clinical studies, the CE marking since 2019 and our
clinical data from our European centers should support a fast
approval for our pivotal study” said Michel Nuta, MD, Chief Medical
Officer, Vice President Veins at Theraclion SA.
This trial is in line with Theraclion’s strategy to focus on key
markets. The US is the largest market for varicose veins with an
estimated 2.3 million procedures representing a $5 Billion
healthcare spending.
SONOVEIN is the only non-invasive option for varicose veins. It
does not require incisions, leaves no scars and allows patients an
immediate return to daily activities. This advanced technological
solution unleashes new treatment opportunities and improves both
patient’s and physician’s experiences.
About Theraclion
At Theraclion we believe that surgery, as we know it, is
outdated. It converts optimistic patients into anxious individuals,
brilliant doctors into exhausted system executors and stretches
healthcare systems to the limit. We have disrupted this convention
by creating extracorporeal treatment platforms. We replace surgery
with a robotic treatment from outside the body using High Intensity
Focussed Ultrasound (HIFU). Our leading edge echotherapy platforms
are currently CE marked in non-invasive treatment of varicose veins
with SONOVEIN® and of breast fibroadenomas and thyroid nodules with
Echopulse®.
Located in Malakoff, near Paris, our employees live and breathe
innovation by extensive clinical research and harness artificial
intelligence. The market of varicose veins treatment alone requires
around 5 million procedures annually. It is a dynamic market in
which we change paradigms by making non-invasive echotherapy the
new standard.
For more information Please visit www.theraclion.com and
our patient website www.echotherapy.com
Theraclion is listed on Euronext Growth Paris Eligible for the
PEA-PME scheme Mnemonic: ALTHE - ISIN code: FR0010120402 LEI:
9695007X7HA7A1GCYD29
View source
version on businesswire.com: https://www.businesswire.com/news/home/20220511005913/en/
Theraclion
David Auregan Chief Operating Officer
david.auregan@theraclion.com
Anja Kleber VP Marketing, Market Access & Sales Francophonia
anja.kleber@theraclion.com
Theraclion (EU:ALTHE)
Graphique Historique de l'Action
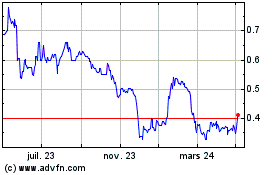
De Mar 2024 à Avr 2024
Theraclion (EU:ALTHE)
Graphique Historique de l'Action
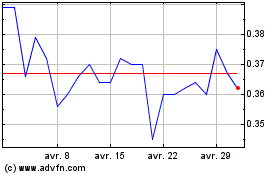
De Avr 2023 à Avr 2024