SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
PURSUANT
TO RULE 13a-16 OR 15d-163
UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For
the month of November 2024
Alterity
Therapeutics Limited
(Name
of Registrant)
Level 14, 350 Collins Street,
Melbourne, Victoria 3000 Australia
(Address
of Principal Executive Office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
This
Form 6-K is being incorporated by reference into our Registration Statement on Form S-8 (Files No. 333-251073, 333-248980
and 333-228671) and our
Registration Statements on Form F-3 (Files No. 333-274816, 333-251647, 333-231417
and 333-250076)
ALTERITY
THERAPEUTICS LIMITED
(a
development stage enterprise)
The
following exhibits are submitted:
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
Alterity Therapeutics Limited |
| |
|
|
| |
By: |
/s/ Geoffrey P. Kempler |
| |
|
Geoffrey P. Kempler |
| |
|
Chairman |
Date:
November 12, 2024
2
Exhibit 99.1

Alterity Therapeutics Announces
Presentation on Tracking the Progression of Multiple System Atrophy at International Symposium
MELBOURNE,
AUSTRALIA AND SAN FRANCISCO, USA – 12 November 2024: Alterity Therapeutics (ASX: ATH,
NASDAQ: ATHE) (“Alterity” or “the Company”), a biotechnology company dedicated to developing disease
modifying treatments for neurodegenerative diseases, today announced the presentation of data from Alterity’s Biomarkers of
progression in Multiple System Atrophy (bioMUSE) natural history study at the 35th International Symposium on the
Autonomic Nervous System.
“The data presented highlights our
work to better understand not only how multiple system atrophy (MSA) initially presents, but also how it progresses over time,”
said David Stamler, M.D., Chief Executive Officer of Alterity. “The presentation describes the use of state-of-the-art technology
that goes beyond traditional MRI methods to track the change in volume in specific regions of the brain affected in patients with MSA.
Importantly, we observed that significant reductions in brain volume over 12 months correlated with clinical worsening of the disease.
The results underscore the importance of utilizing advanced neuroimaging and analytical methods in evaluating MSA which we have implemented
in our Phase 2 clinical trials.”
The platform presentation entitled,
“The MSA Atrophy Index: A Marker of Clinical Progression in Multiple System Atrophy”, was presented by Paula Trujillo Diaz,
PhD, Research Assistant Professor, Department of Neurology, Vanderbilt University Medical Center.
While previous MRI studies have reported
brain volume reductions in regions implicated in MSA, tracking these changes reliably has been challenging. In this study, machine learning
tools were used to precisely define the neuroanatomy and a specific brain atrophy measure was designed to track disease progression in
MSA patients over one year. The results were then correlated with clinical measures of disease progression. The poster introduces the
MSA Atrophy Index (MSA-AI), a composite atrophy marker derived from the lentiform nucleus (LN, putamen and globus pallidus) and olivopontocerebellar
(OPC, cerebellum and brainstem) regions, as a potential biomarker for assessing MSA progression. These methods can enhance the understanding
of MSA progression and provide support for using brain atrophy markers for the evaluation of disease-modifying therapies.
About bioMUSE
Biomarkers of progression in Multiple
System Atrophy (bioMUSE) is a natural history study that aims to track the progression of individuals with MSA, a parkinsonian disorder
without approved therapy. The study is being conducted in collaboration with Vanderbilt University Medical Center in the U.S. under the
direction of Daniel Claassen, M.D., M.S., Professor of Neurology and Principal Investigator. Natural history studies are important for
characterizing disease progression in selected patient populations. The study has provided rich data for optimizing the design of Alterity’s
randomized ATH434-201 Phase 2 clinical trial and enrolled approximately 20 individuals with clinically probable or clinically established
MSA. BioMUSE continues to provide vital information on early stage MSA patients, informs the selection of biomarkers suitable to evaluate
target engagement and preliminary efficacy, and delivers clinical data to characterize disease progression in a patient population that
mirrors those currently enrolling in the Phase 2 clinical trial.
About Multiple System Atrophy
Multiple System Atrophy (MSA) is a
rare, neurodegenerative disease characterized by failure of the autonomic nervous system and impaired movement. The symptoms reflect the
progressive loss of function and death of different types of nerve cells in the brain and spinal cord. It is a rapidly progressive disease
and causes profound disability. MSA is a Parkinsonian disorder characterized by a variable combination of slowed movement and/or rigidity,
autonomic instability that affects involuntary functions such as blood pressure maintenance and bladder control, and impaired balance
and/or coordination that predisposes to falls. A pathological hallmark of MSA is the accumulation of the protein α-synuclein within
glia, the support cells of the central nervous system, and neuron loss in multiple brain regions. MSA affects at least 15,000 individuals
in the U.S., and while some of the symptoms of MSA can be treated with medications, currently there are no drugs that are able to slow
disease progression and there is no cure.1
| 1 | Multiple System Atrophy | National Institute of Neurological
Disorders and Stroke (nih.gov) |
About Parkinson’s Disease
Parkinson’s
disease (PD) is the second most common neurodegenerative disorder and causes unintended or uncontrollable movements of the body
along with neuropsychiatric and other nonmotor features. The precise cause of PD is unknown, but some cases are hereditary while
others are thought to occur from a combination of genetics and environmental factors that trigger the disease. In PD, brain cells
become damaged or die in the substantia nigra, the part of the brain that produces dopamine--a chemical needed to produce smooth,
purposeful movement. The cardinal symptoms of PD are tremors, rigidity, slowing of movements, and later in disease, impaired
balance. Other symptoms may include difficulty swallowing, chewing, or speaking; emotional changes; urinary problems or
constipation; dementia or other cognitive problems; fatigue; and problems sleeping.3 Nearly one million people in the
U.S. and more than 10 million people worldwide are living with PD. Approximately 60,000 Americans are diagnosed with PD each
year.4
| 1 | Beauchamp et al, “ATH434 Rescues Pre-motor
Hyposmia in a Mouse Model of Parkinsonism, Neurotherapeutics, DOI:10.1007/s13311-022-01300-0 |
| 2 | Xiao, et al, “Hyposmia: a possible biomarker of Parkinson’s
disease” Neurosci Bull. 2014 Feb; 30(1): 134–140. |
| 3 | National Institute of Health: Neurological Disorders and Stroke,
Parkinson’s Disease Information Page; |
About Alzheimer’s Disease
Alzheimer’s disease is a progressive neurologic
disorder that causes the brain to shrink (atrophy) and brain cells to die. Alzheimer’s disease is the most common cause of dementia —
a continuous decline in thinking, behavioral, and social skills that affects a person’s ability to function independently. Approximately
5.8 million people in the United States age 65 and older live with Alzheimer’s disease. Of those, 80% are 75 years old and older. Out
of the approximately 50 million people worldwide with dementia, between 60% and 70% are estimated to have Alzheimer’s disease. Medications
may temporarily improve or slow progression of symptoms, but there is no treatment that cures Alzheimer’s disease or alters the disease
process in the brain. In advanced stages of the disease, complications from severe loss of brain function, such as dehydration, malnutrition
or infection, result in death.1
About Alterity Therapeutics Limited
Alterity Therapeutics is a clinical
stage biotechnology company dedicated to creating an alternate future for people living with neurodegenerative diseases. The Company’s
lead asset, ATH434, has the potential to treat various Parkinsonian disorders and is currently being evaluated in two Phase 2 clinical
trials in Multiple System Atrophy. Alterity also has a broad drug discovery platform generating patentable chemical compounds to treat
the underlying pathology of neurological diseases. The Company is based in Melbourne, Australia, and San Francisco, California, USA. For
further information please visit the Company’s web site at www.alteritytherapeutics.com.
Authorisation & Additional information
This announcement was authorized by David Stamler, CEO of Alterity
Therapeutics Limited.
Investor
and Media Contacts:
Australia
Ana Luiza Harrop
we-aualteritytherapeutics@we-worldwide.com
+61 452 510 255
U.S.
Remy Bernarda
remy.bernarda@iradvisory.com
+1 (415) 203-6386
Forward Looking Statements
This press release contains “forward-looking
statements” within the meaning of section 27A of the Securities Act of 1933 and section 21E of the Securities Exchange Act of 1934.
The Company has tried to identify such forward-looking statements by use of such words as “expects,” “intends,” “hopes,”
“anticipates,” “believes,” “could,” “may,” “evidences” and “estimates,” and
other similar expressions, but these words are not the exclusive means of identifying such statements.
Important factors that could cause
actual results to differ materially from those indicated by such forward-looking statements are described in the sections titled “Risk
Factors” in the Company’s filings with the SEC, including its most recent Annual Report on Form 20-F as well as reports on
Form 6-K, including, but not limited to the following: statements relating to the Company’s drug development program, including, but not
limited to the initiation, progress and outcomes of clinical trials of the Company’s drug development program, including, but not limited
to, ATH434, and any other statements that are not historical facts. Such statements involve risks and uncertainties, including, but not
limited to, those risks and uncertainties relating to the difficulties or delays in financing, development, testing, regulatory approval,
production and marketing of the Company’s drug components, including, but not limited to, ATH434, the ability of the Company to
procure additional future sources of financing, unexpected adverse side effects or inadequate therapeutic efficacy of the Company’s drug
compounds, including, but not limited to, ATH434, that could slow or prevent products coming to market, the uncertainty of obtaining patent
protection for the Company’s intellectual property or trade secrets, the uncertainty of successfully enforcing the Company’s patent
rights and the uncertainty of the Company freedom to operate.
Any forward-looking statement made
by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made.
We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time,
whether as a result of new information, future developments or otherwise.
3
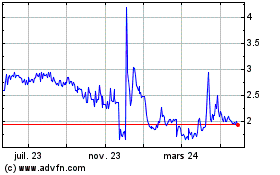
Alterity Therapeutics (NASDAQ:ATHE)
Graphique Historique de l'Action
De Oct 2024 à Nov 2024
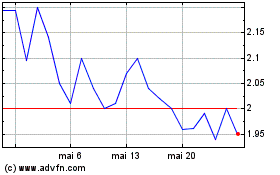
Alterity Therapeutics (NASDAQ:ATHE)
Graphique Historique de l'Action
De Nov 2023 à Nov 2024