Filed Pursuant to Rule 424(b)(5)
Registration No. 333-271648
The information in
this preliminary prospectus supplement is not complete and may be changed. This preliminary prospectus supplement and the accompanying
prospectus are not an offer to sell these securities and are not soliciting an offer to buy these securities in any jurisdiction where
the offer or sale is not permitted.
SUBJECT TO COMPLETION,
DATED SEPTEMBER 8, 2023
PRELIMINARY PROSPECTUS SUPPLEMENT
(To Prospectus dated May 17, 2023)
Up to [*] Shares of Common Stock

We are offering on a “best efforts”
basis up to [*] shares of our common stock, par value $0.001per share, at a public offering price
of $ per share, to certain investors pursuant
to this prospectus supplement and the accompanying prospectus.
Our common stock is listed on The Nasdaq Capital
Market under the symbol “TNON.” On September 8, 2023, the last reported sale price of our common stock on The Nasdaq Capital
Market was $[*].
The final public offering price will be determined
through negotiation between us, the placement agent and the investors based upon a number of factors, including our history and our prospects,
the industry in which we operate, our past and present operating results, the previous experience of our executive officers and the general
condition of the securities markets at the time of this offering.
The aggregate market value of our outstanding
shares of common stock held by non-affiliates, or public float, was approximately $4,938,875 based on 22,612,856 outstanding shares of
common stock as of September 8, 2023 (a date that will be within 60 days of the date of this prospectus supplement), of which approximately
15,196,538 shares are held by non-affiliates, and a per share price of $0.325, based on the last sale price of our common stock on July
13, 2023, a date that will be within 60 days of the date of this prospectus supplement. One-third of our public float, calculated in accordance
with General Instruction I.B.6 of Form S-3, is equal to approximately $1,646,291. During the 12 calendar months prior to and including
the date of this prospectus supplement, we have sold securities with an aggregate market value of $0 pursuant to General Instruction I.B.6
of Form S-3. In no event will we sell securities registered on this registration statement in a public primary offering with a value exceeding
more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million pursuant to
General Instruction I.B.6 of Form S-3.
The securities will be offered at a fixed price
and are expected to be issued in a single closing. We expect this offering to be completed not later than two business days following
the commencement of sales in this offering and we will deliver all securities to be issued in connection with this offering delivery versus
payment/receipt versus payment upon receipt of investor funds received by us. Accordingly, neither we nor the placement agent have made
any arrangements to place investor funds in an escrow account or trust account since the placement agent will not receive investor funds
in connection with the sale of the securities offered hereunder.
We engaged Maxim Group LLC as our exclusive placement
agent (“Maxim” or the “placement agent”) to use its reasonable best efforts to solicit offers to purchase our
securities in this offering. The placement agent has no obligation to purchase any of the securities from us or to arrange for the purchase
or sale of any specific number or dollar amount of the securities. There is no required minimum number of securities that must be sold
as a condition to completion of this offering. We have agreed to pay the placement agent the placement agent fees set forth in the table
below. See “Plan of Distribution” in this prospectus supplement for more information.
Investing in our securities involves a high
degree of risk. See “Risk Factors” beginning on page S-4 of this prospectus supplement for a discussion of information
that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission
(“SEC”) nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
supplement is truthful or complete. Any representation to the contrary is a criminal offense.
We are an “emerging growth company”
as that term is used in the Jumpstart Our Business Startups Act of 2012, and we have elected to comply with certain reduced public company
reporting requirements.
| | |
Per Share | | |
Total | |
| Public offering price | |
$ | | | |
$ | | |
| Placement agent fees(1) | |
$ | | | |
$ | | |
| Proceeds, before expenses, to us | |
$ | | | |
$ | | |
| (1) |
Represents a cash fee equal to 7% of the aggregate purchase price paid by investors in this offering. In addition, we have agreed to reimburse certain expenses of the placement agent in connection with this offering. See “Plan of Distribution” beginning on page S-7 of this prospectus supplement for a description of the compensation to be received by the placement agent. |
We anticipate that delivery of the shares of common
stock against payment therefor will be made on or before September [*], 2023.
Maxim Group LLC
The date of this prospectus supplement is ,
2023.
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
ABOUT
THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying
prospectus form part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC. This document
is in two parts. The first part is this prospectus supplement, which describes the specific terms of this offering and also adds to and
updates information contained in the accompanying prospectus and the documents incorporated by reference herein or therein. The second
part, the accompanying prospectus, provides more general information. Generally, when we refer to this prospectus in this prospectus supplement,
we are referring to both parts of this document combined. If the description of this offering varies between this prospectus supplement
and the accompanying prospectus, you should rely on the information in this prospectus supplement, which supersedes the information in
the accompanying prospectus. This prospectus supplement contains information about the shares offered in this offering and may add, update
or change information in the accompanying prospectus. Before you invest in any of the shares offered under this prospectus supplement,
you should carefully read both this prospectus supplement and the accompanying prospectus together with the additional information described
under the headings “Where You Can Find More Information” and “Information We Incorporate By Reference.”
We are offering to sell, and seeking offers to
buy, securities only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying
prospectus and the offering of the shares of common in certain jurisdictions may be restricted by law. Persons outside the United States
who come into possession of this prospectus supplement and the accompanying prospectus must inform themselves about, and observe any restrictions
relating to, the offering of the shares of common stock and the distribution of this prospectus supplement and the accompanying prospectus
outside the United States. This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection
with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying
prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
You should rely only on the information contained
in, or incorporated by reference into, this prospectus supplement, the accompanying prospectus, the documents incorporated by reference
into this prospectus supplement or the accompanying prospectus, and in any free writing prospectus that we may authorize for use in connection
with this offering. We have not, and the Placement Agent has not, authorized any other person to provide you with different information.
If anyone provides you with different or inconsistent information, you should not rely on it.
Throughout this prospectus supplement, unless
otherwise designated or the context suggests otherwise,
| |
● |
generally, when we refer to this prospectus in this prospectus supplement, we are referring to both the prospectus supplement and the prospectus combined..; |
| |
● |
all references to the “Tenon,” the “Company,” the “registrant,” “we,” “our” or “us” in this prospectus mean Tenon Medical, Inc.; |
| |
● |
“year” or “fiscal year” means the year ending December 31st; and |
| |
● |
all dollar or $ references, when used in this prospectus, refer to United States dollars. |
PROSPECTUS
Supplement SUMMARY
This summary provides a brief overview of the
key aspects of our business and our securities. The reader should read the entire prospectus carefully, especially the risks of investing
in our common stock discussed under “Risk Factors.” Some of the statements contained in this prospectus, including statements
under “Prospectus Supplement Summary” and “Risk Factors” as well as those noted in the documents incorporated
herein by reference, are forward-looking statements and may involve a number of risks and uncertainties. Our actual results and future
events may differ significantly based upon a number of factors. The reader should not put undue reliance on the forward-looking statements
in this document, which speak only as of the date on the cover of this prospectus.
The Company
We are a medical device company that offers a
novel, less invasive approach to the sacroiliac joint using a single, robust, titanium implant for treatment of the most common types
of sacroiliac joint (the “SI-Joint”) disorders that cause lower back pain. The system features the CATAMARAN™ Fixation
Device which passes through both the axial and sagittal planes of the ilium and sacrum, stabilizing and transfixing the SI joint along
its longitudinal axis. The angle and trajectory of the Catamaran surgical approach is also designed to provide a pathway away from critical
neural and vascular structures and into the strongest cortical bone. We received U.S. Food and Drug Administration (“FDA”)
clearance in 2018 for The CATAMARANTM SI-Joint Fusion System (“The CATAMARAN System”). We commercially launched
The CATAMARAN System nationally in October 2022 at the North American Spine Society (“NASS”) meeting held in Chicago. Currently,
our only commercial focus is the U.S. market.
Recent Developments
Recent issuances under the 2022 Plan.
Between May 2022 and September 2023, we granted 23 restricted stock units (“RSU”) to
20 individuals under the 2022 Plan, which in aggregate convert into 1,393,530 shares of our common stock. Nine of these RSUs vest one
third on the first anniversary with the balance vesting semi-annually over the next two years. Eight of these RSUs vest one sixth semi-annually
over 3 years and four of these RSUs vest one third annually on their anniversary with one sixth of the balance vesting semi-annually over
the next 2 years. One of these RSUs vests in 25% increments over seven months and one vested 100% upon grant. During this same time period
we granted under the 2022 Plan (i) non-statutory options to 4 individuals to purchase in aggregate 98,950 shares of our common stock and
(ii) incentive stock options to 15 individuals to purchase in aggregate 193,000 shares of our common stock at exercise prices between
$0.29 and $2.75 per share. Fifteen of these options vest 33% on the first anniversary with the balance of the shares vesting monthly over
the next two years, three of these options vest monthly over two years and the remaining option is subject to vesting 50% on the first
anniversary with the balance of the shares vesting monthly over the next year. All RSUs and options expire 10 years from the date of grant.
Nasdaq Notice of Failure to Comply
with Continued Listing Standards. On July 20, 2023, we received a letter from the Nasdaq Listing Qualifications Department notifying
us that, for the 30 consecutive business day period between June 6, 2023 through July 19, 2023, our common stock had not maintained a
minimum closing bid price of $1.00 per share required for continued listing on The Nasdaq Capital Market pursuant to Nasdaq Listing Rule
5550(a)(2) (the “Bid Price Rule”). Pursuant to Nasdaq Listing Rule 5810(c)(3)(A), the Company was provided an initial period
of 180 calendar days, or until January 16, 2024 (the “Compliance Period”), to regain compliance with the Bid Price Rule.
In order to regain compliance with the
Bid Price Rule, our Common Stock would be required to maintain a minimum closing bid price of $1.00 for a minimum of ten consecutive business
days during the Compliance Period, unless extended by Nasdaq under Nasdaq Rule 5810(c)(3)(H), prior to January 16, 2024.
If we do not regain compliance with
the Bid Price Rule by January 16, 2024, we may be eligible for an additional 180-day period to regain compliance if we meet all of the
other Nasdaq listing criteria and if Nasdaq does not believe we will not be able to regain compliance within such 180-day period. If we
cannot regain compliance during the Compliance Period or any subsequently granted compliance period, our common stock will be subject
to delisting. Our Common Stock continues to be listed on the Nasdaq Capital Market under the symbol “TNON”. We are currently
evaluating our options for regaining compliance.
The Notice has no immediate effect on the listing
or trading of our common stock on The Nasdaq Capital Market and does not affect our business, operations, or reporting requirements with
the SEC.
Corporate Information
Our principal executive offices are located at
104 Cooper Court, Los Gatos, CA 95032. Our website address is www.tenonmed.com. The information included on our website is not part of
this prospectus.
Implications of Being an Emerging Growth Company
We are an “emerging growth company,”
as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). We will remain an emerging growth company
until the earlier of (i) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock
pursuant to an effective registration statement under the Securities Act; (ii) the last day of the fiscal year in which we have total
annual gross revenues of $1.235 billion or more; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during
the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under applicable SEC rules. We expect
that we will remain an emerging growth company for the foreseeable future, but cannot retain our emerging growth company status indefinitely
and will no longer qualify as an emerging growth company on or before the last day of the fiscal year following the fifth anniversary
of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act. For so long
as we remain an emerging growth company, we are permitted and intend to rely on exemptions from specified disclosure requirements that
are applicable to other public companies that are not emerging growth companies.
These exemptions include:
| ● | being permitted to provide
only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly
reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| ● | not being required to comply
with the requirement of auditor attestation of our internal controls over financial reporting; |
| ● | not being required to comply
with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or
a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
| ● | reduced disclosure obligations
regarding executive compensation; and |
| ● | not being required to hold
a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We have taken advantage of certain reduced reporting
requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from
other public companies in which you hold stock.
An emerging growth company can take advantage
of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards.
This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply
to private companies. We have irrevocably elected to avail ourselves of this extended transition period and, as a result, we will not
be required to adopt new or revised accounting standards on the dates on which adoption of such standards is required for other public
reporting companies.
We are also a “smaller reporting company”
as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and have elected to take
advantage of certain of the scaled disclosure available for smaller reporting companies.
SUMMARY
OF THE OFFERING
| Common stock offered by us |
|
Up to [*] shares of common stock. |
| |
|
|
| Common stock to be outstanding after the offering(1) |
|
[*] shares of common stock assuming the sale of all of the shares of common stock being offered in connection with this offering |
| Use of Proceeds |
|
We estimate the net proceeds to us from this offering will be approximately $[*], after deducting the placement agent fee and estimated offering expenses payable by us. We intend to use the net proceeds from this offering for working capital and general corporate purposes. See the section of this prospectus supplement titled “Use of Proceeds” beginning on page S-6 |
| |
|
|
| Listing |
|
Our common stock and a class of our warrants trade on The Nasdaq Capital Market under the symbol “TNON” and “TNONW,” respectively. |
| |
|
|
| Risk Factors |
|
You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page S-4 of this prospectus supplement before deciding whether or not to invest in shares of our common stock. |
| |
|
|
| Transfer Agent and registrar |
|
Vstock Transfer, LLC. |
| |
|
|
| Reasonable best efforts |
|
We have agreed to offer and sell the securities offered hereby to the purchasers through the placement agent. The placement agent is not required to buy or sell any specific number or dollar amount of the securities offered hereby, but it will use its reasonable best efforts to solicit offers to purchase the securities offered by this prospectus. See “Plan of Distribution” on page S-7 of this prospectus supplement. |
| (1) | The number of shares of common
stock to be outstanding after this offering is based on 22,612,856 shares of common stock outstanding as of September 8, 2023, and excludes: |
| ● | 1,905,906 shares of our common
stock issuable pursuant to options and restricted stock units granted pursuant to our equity incentive plan; |
| ● | 96,000 shares of our common
stock issuable upon the exercise of warrants issued to the underwriters in our initial public offering that closed on April 29, 2022;
and |
| ● | 20,000,000 shares issuable
upon the exercise of warrants issued to investors in the closing of our public offering of 10,000,000 units on June 16, 2023. |
Unless otherwise indicated, all information
in this prospectus supplement reflects or assumes the following:
| ● | The sale and issuance of all
of the shares of common stock being offered hereunder; and |
| ● | No exercise or forfeiture of
the outstanding stock options or remaining warrants after September 8, 2023 |
RISK
FACTORS
Investing in our securities involves a high
degree of risk. You should carefully consider the risks listed below and other information included and incorporated by reference
in this prospectus supplement and accompanying prospectus. There may also be risks of which we are currently unaware, or that we
currently regard as immaterial based on the information available to us that later prove to be material. If any of these risks occur,
our business, operating results and financial condition could be seriously harmed, the trading price of our common stock could decline,
and you could lose some or all of your investment.
Risks Related to this Offering
This is a best-efforts offering, no minimum amount of securities
is required to be sold and we may not raise the amount of capital we believe is required for our business plans.
The placement agent has agreed to use its reasonable
best efforts to solicit offers to purchase the securities being offered in this offering. The placement agent has no obligation to buy
any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There
is no required minimum number of securities or amount of proceeds that must be sold as a condition to completion of this offering. Because
there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement agent
fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above. We may
sell fewer than all of the securities offered hereby, which may significantly reduce the amount of net proceeds received by us as described
in the “Use of Proceeds” section herein. Thus, we may not raise the amount of capital we believe is required for our
operations in the short-term and even if we raise the maximum offering amount in this public offering, we will need to raise additional
funds in the future, which may not be available or available on terms acceptable to us. For more on the risks related to our funding requirements,
see “Risk Factors--Risks Related to Our Business and Operations--We have incurred losses in the past, our financial statements
have been prepared on a going concern basis and we may be unable to achieve or sustain profitability in the future” contained
in our most recently filed Annual Report on Form 10-K.
Regardless of the amount of cash that is
raised in this public offering, the Company will require additional financing in the future to continue as a going concern.
The Company will not generate sufficient revenues
in the foreseeable future to fund its operations. Accordingly, regardless of the amount of net proceeds that are raised in this offering,
we will require additional financing in the future to continue as a going concern. If we are unable to raise additional capital or generate
sufficient cash from operations to adequately fund our operations, we will, at a minimum, need to curtail planned business activities
to reduce costs, which we expect will harm our ability to execute on our business plan and continue operations.
We may not be able to maintain the listing
of our common stock on Nasdaq, which could adversely affect our liquidity and the trading volume and market price of our common stock
and decrease or eliminate your investment.
On July 20, 2023, we received a letter from Nasdaq
notifying us that we were no longer in compliance with the $1.00 minimum bid price requirement for continued listing on Nasdaq under Nasdaq
Listing Rule 5550(a)(2). Although Nasdaq has granted us 180 calendar days, or until January 16, 2024, to regain compliance with the Bid
Price Rule, there can be no assurance that we will regain such compliance and Nasdaq could make a determination to delist our Common Stock.
Any delisting determination by Nasdaq could seriously
decrease or eliminate the value of an investment in our Common Stock and other securities linked to our common stock. While a listing
on an over-the-counter exchange could maintain some degree of a market in our common stock, we could face substantial material adverse
consequences, including, but not limited to, the following: limited availability for market quotations for our common stock; reduced liquidity
with respect to and decreased trading prices of our common stock; a determination that shares of our common stock are “penny stock”
under the Securities and Exchange Commission rules, subjecting brokers trading our common stock to more stringent rules on disclosure
and the class of investors to which the broker may sell the common stock; limited news and analyst coverage for our Company, in part due
to the “penny stock” rules; decreased ability to issue additional securities or obtain additional financing in the future;
and potential breaches under or terminations of our agreements with current or prospective large stockholders, strategic investors and
banks. The perception among investors that we are at heightened risk of delisting could also negatively affect the market price of our
securities and trading volume of our common stock.
The price of our common stock may be adversely
affected by the future issuance and sale of shares of our common stock or other equity securities.
We cannot predict the size of future issuances
or sales of our common stock or other equity securities, future acquisitions or capital raising activities, or the effect, if any, that
such issuances or sales may have on the market price of our common stock. The issuance and sale of substantial amounts of common
stock or other equity securities or announcement that such issuances and sales may occur, could adversely affect the market price of our
common stock.
Future sales by stockholders, or the perception
that such sales may occur, may depress the price of our common stock.
The sale or availability for sale of substantial
amounts of our shares in the public market or exercise of common stock warrants and options or settlement of restricted stock units, or
the perception that such sales could occur, could adversely affect the market price of our common stock and also could impair our ability
to raise capital through future offerings of our shares. As of September 8, 2023 we had 22,612,856 outstanding shares of common
stock. Any decline in the price of our common stock may encourage short sales, which could place further downward pressure on the
price of our common stock and may impair our ability to raise additional capital through the sale of equity securities.
The issuance of shares upon exercise of
derivative securities may cause immediate and substantial dilution to our existing stockholders.
The issuance of shares upon exercise of options
and settlement of outstanding restricted stock units may result in substantial dilution to the interests of other stockholders since these
selling stockholders may ultimately convert or exercise and sell all or a portion of the full amount issuable upon exercise. If
all derivative securities outstanding as of September 8, 2023 were converted or exercised into shares of common stock, there would be
approximately an additional 22,001,906 million shares of common stock outstanding as a result. The issuance of these shares
will have the effect of further diluting the proportionate equity interest and voting power of holders of our common stock.
Since we have broad discretion in how we
use the proceeds from this offering, we may use the proceeds in ways in which you disagree.
Our management will have significant flexibility
in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these
net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used
appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for
our company. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial
condition, operating results and cash flow.
Purchasers of our common stock will incur
immediate dilution.
Purchasers of shares of common stock in this offering
will experience immediate and substantial dilution because the purchase price of the common stock will be higher than the net tangible
book value per share of the outstanding common stock immediately after this offering. In addition, purchasers will experience dilution,
which may be substantial, when we issue additional shares of common stock that we are permitted or required to issue under options, our
stock equity incentive plans or other employee or director compensation plans. Because the sales of the shares offered hereby will
be made directly into the market, the prices at which we sell these shares will vary and these variations may be significant. Purchasers
of the shares we sell, as well as our existing stockholders, will experience significant dilution if we sell shares at prices significantly
below the price at which they invested. See “Dilution” for a more detailed discussion of the dilution you will incur
if you purchase common stock in this offering.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, the documents incorporated by
reference herein and therein, and other written and oral statements we make from time to time contain certain “forward-looking”
statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended
(the “Exchange Act”). You can identify these forward-looking statements by the fact they use words such as “could,”
“expect,” “anticipate,” “estimate,” “target,” “may,” “project,”
“guidance,” “intend,” “plan,” “believe,” “will,” “potential,”
“opportunity,” “future,” and other words and terms of similar meaning and expression in connection with any discussion
of future operating or financial performance. You can also identify forward-looking statements by the fact that they do not relate strictly
to historical or current facts. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties,
including factors that could delay, divert, or change any of them, and could cause actual outcomes to differ materially from current expectations.
These statements are likely to relate to, among other things, our business strategy, our research and development, our product development
efforts, our ability to commercialize our product candidates, the activities of our licensees, our prospects for initiating partnerships
or collaborations, the timing of the introduction of products, the effect of new accounting pronouncements, uncertainty regarding our
future operating results and our profitability, anticipated sources of funds as well as our plans, objectives, expectations, and intentions.
We have included more detailed descriptions of
these risks and uncertainties and other risks and uncertainties applicable to our business that we believe could cause actual results
to differ materially from any forward-looking statement in the “Risk Factors” sections of this prospectus and the documents
incorporated by reference herein including, but not limited to, the risk factors incorporated by reference from our filings with the SEC.
We encourage you to read those descriptions carefully. Although we believe we have been prudent in our plans and assumptions, no assurance
can be given that any goal or plan set forth in forward-looking statements can be achieved. We caution investors not to place significant
reliance on forward-looking statements; such statements need to be evaluated in light of all the information contained and incorporated
by reference in this prospectus. Furthermore, the statements speak only as of the date of each document, and we undertake no obligation
to update or revise these statements.
USE
OF PROCEEDS
We estimate the net proceeds to us from
this offering will be approximately $[*], after deducting the placement agent fee and estimated offering expenses payable by us. We intend
to use the net proceeds from this offering for working capital and general corporate purposes.
The amount, timing and nature of specific expenditures
of net proceeds from this offering will depend on a number of factors, including the timing, scope, progress and results of our development
efforts and the timing and progress of any collaboration efforts. As of the date of this prospectus supplement, we cannot specify with
certainty all of the particular uses of the proceeds from this offering. Accordingly, we will retain broad discretion over the use of
such proceeds.
DIVIDEND POLICY
We have not declared any cash dividends since
inception and we do not anticipate paying any dividends in the foreseeable future. Instead, we anticipate that all of our earnings will
be used to provide working capital, to support our operations, and to finance the growth and development of our business, including potentially
the acquisition of, or investment in, businesses, technologies or products that complement our existing business. The payment of dividends
is within the discretion of the Board and will depend on our earnings, capital requirements, financial condition, prospects, applicable
Delaware law, which provides that dividends are only payable out of surplus or current net profits, and other factors our Board might
deem relevant. There are no restrictions that currently limit our ability to pay dividends on our common stock other than those generally
imposed by applicable state law.
DILUTION
Purchasers of our securities in this offering
will experience an immediate and substantial dilution in the as adjusted net tangible book value of their shares of our common stock.
Dilution in as adjusted net tangible book value represents the difference between the public offering price per share of common stock
and the as adjusted net tangible book value per share of our common stock immediately after the offering.
The historical net tangible book value of our
common stock as of June 30, 2023 was $903,000 or $0.04 per share. Historical net tangible book value per share of our common stock represents
our total tangible assets (total assets less intangible assets) less total liabilities divided by the number of shares of our common stock
outstanding as of that date. After giving effect to the sale of up to [*] shares of common stock in this offering at a public offering
price of $[*] per share of common stock for net proceeds of approximately $[*] as if such offering and such share issuances had occurred
on June 30, 2023, our as adjusted net tangible book value as of June 30, 2023, would have been $[*] or approximately $[*] per share of
our common stock. This represents an immediate increase in net tangible book value per share of $[*] to the existing stockholders and
an immediate dilution in net tangible book value per share of $[*] to new investors. We determine dilution by subtracting the as adjusted
net tangible book value per share after this offering from the amount of cash that a new investor paid for a share of common stock in
this offering. The following table illustrates this per share dilution to new investors:
| Public offering price per share |
|
|
|
|
$ |
[* |
] |
| Historical net tangible book value per share as of June 30, 2023 |
$ |
0.04 |
|
|
|
|
|
| Increase in net tangible book value per share after giving effect to the offering |
$ |
[* |
] |
|
|
|
|
| As adjusted net tangible book value per share as of June 30, 2023 |
|
|
|
|
$ |
[* |
] |
| Dilution in net tangible book value per share to new investors |
|
|
|
|
$ |
[* |
] |
The above
discussion and table are based on 21,623,769 shares of our common stock outstanding as of
June 30, 2023, and excludes as of such date: (i) 1,905,906 shares of our common stock issuable pursuant to options and RSUs granted
pursuant to our equity incentive plan; (ii) 96,000 shares our common stock issuable upon the exercise of the warrants issued to our underwriters
in our initial public offering and (iii) 20,000,000 shares issuable upon the exercise of warrants issued to investors in the closing of
our public offering of 10,000,000 units on June 16, 2023.
To the extent that outstanding options or warrants
are exercised, you will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or
strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional
capital is raised through the sale of equity or convertible debt securities, the issuance of these securities may result in further dilution
to our stockholders.
DESCRIPTION
OF SECURITIES WE ARE OFFERING
Description of the Common Stock
We are offering shares of our common stock in
this offering. A description of the common stock we are offering pursuant to this prospectus supplement is set forth under the heading
“Description of Common Stock,” starting on page 9 of the accompanying prospectus. As of September 8, 2023, we
had 22,612,856 shares of common stock outstanding.
PLAN OF DISTRIBUTION
We are offering
up to [*] shares of common stock at a public offering price of $[*] per share, for gross proceeds of $[*] before deduction of placement
agent commissions and offering expenses, in a best-efforts offering.
Pursuant to a placement agency agreement, dated
as of September 8, 2023, we have engaged Maxim Group LLC to act as our exclusive placement
agent (“Maxim” or the “placement agent”) to solicit offers to purchase the securities offered by this prospectus.
The placement agent is not purchasing or selling any securities, nor is it required to arrange for the purchase and sale of any specific
number or dollar amount of securities, other than to use its “reasonable best efforts” to arrange for the sale of the securities
by us. Therefore, we may not sell the entire amount of securities being offered. There is no minimum amount of proceeds that is a condition
to closing of this offering. We have entered into securities purchase agreement directly with certain investors, which was at the investor’s
option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely
on this prospectus in connection with the purchase of our securities in this offering. The placement agent may engage one or more subagents
or selected dealers in connection with this offering.
The placement agency agreement provides that the
placement agent’s obligations are subject to conditions contained in the placement agency agreement.
We will deliver the securities being issued to the
investors upon receipt of investor funds for the purchase of the securities offered pursuant to this prospectus. We expect to deliver
the securities being offered pursuant to this prospectus on or about September [*], 2023.
Placement Agent Fees, Commissions and Expenses
Upon the closing of this offering, we will pay the
placement agent a cash transaction fee equal to 7% of the aggregate gross cash proceeds to us from the sale of the securities in the offering.
Pursuant to the placement agency agreement, we will agree to reimburse the placement agent for certain out-of-pocket expenses of the placement
agent payable by us, in an aggregate amount not to exceed $50,000. The placement agency agreement, however, will provide that in the event
this offering is terminated, the placement agent will only be entitled to the reimbursement of out-of-pocket accountable expenses actually
incurred in accordance with Financial Industry Regulatory Authority, Inc. (“FINRA”) Rule 5110(f)(2)(C).
The following table shows the public offering
price, placement agent fees and proceeds, before expenses, to us.
| | |
Per Share | | |
Total | |
| Public offering price | |
$ | | | |
| | |
| Placement agent fees (7%) | |
$ | | | |
| | |
| Proceeds, before expenses, to us | |
$ | | | |
| | |
We estimate that the total
expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding
the placement agent commission, will be approximately $[*], all of which are payable by us. This figure does not include, among other
things, the placement agent’s fees and expenses (including the legal fees, costs and expenses for the placement agent’s legal
counsel) up to $50,000.
Lock-Up Agreements
Subject
to certain limited exceptions, we have agreed for a period of 30 days after the closing date not to (i) issue, enter into any agreement
to issue or announce the issuance or proposed issuance of any shares of our common stock or other securities convertible into or exercisable
or exchangeable for shares of our common stock or (ii) file any registration statement or amendment or supplement thereto, other
than this prospectus or filing a registration statement on Form S-8 in connection with any employee benefit plan, in each case
without prior written consent of the placement agent. Each of our officers and directors have agreed, for a period of 30 days after the
closing of this offering, subject to certain exceptions, not to offer, sell, contract to sell, encumber, grant any option for the sale
of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares
of our common stock without the prior written consent of the placement agent.
The placement agent may in
its sole discretion and at any time without notice release some or all of the shares of common stock subject to lock-up agreements prior
to the expiration of the lock-up period. When determining whether or not to release shares from the lock-up agreements, the placement
agent will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares of common
stock for which the release is being requested and market conditions at the time.
Indemnification
We have agreed to indemnify the placement agent
against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the placement agent may
be required to make for these liabilities.
Regulation M
The placement agent may be deemed to be an underwriter
within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale
of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities
Act. As an underwriter, the placement agent would be required to comply with the requirements of the Securities Act and the Exchange Act,
including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of
purchases and sales of our securities by the placement agent acting as principal. Under these rules and regulations, the placement agent
(i) may not engage in any stabilization activity in connection with our securities and (ii) may not bid for or purchase any of our securities
or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed
its participation in the distribution.
Determination
of Offering Price
The actual
offering price of the securities we are offering, was negotiated between us, the placement agent and the investors in the
offering based on the trading of our shares of common stock prior to the offering, among other
things. Other factors considered in determining the public offering price of the securities we are offering include our history and prospects,
the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment
of our management, the general conditions of the securities markets at the time of the offering and such other factors as were deemed
relevant.
Electronic Distribution
A prospectus in electronic format may be made
available on a website maintained by the placement agent. In connection with the offering, the placement agent or selected dealers may
distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF
will be used in connection with this offering.
Other than the prospectus in electronic format,
the information on the placement agent’s website and any information contained in any other website maintained by the placement
agent is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or
endorsed by us or the placement agent in its capacity as placement agent and should not be relied upon by investors.
Certain Relationships
The placement agent and its affiliates have and
may in the future provide, from time to time, investment banking and financial advisory services to us in the ordinary course of business,
for which they may receive customary fees and commissions.
On May 4,
2023, we entered into an equity distribution agreement with the placement agent (the “Equity Distribution Agreement”), pursuant
to which we may sell shares of our Common Stock having an aggregate offering price of up to $5,523,274 from time to time through the placement
agent. The placement agent will be entitled to a transaction fee at a fixed rate of 3.0% of the gross sales price of shares of common
stock sold under the Equity Distribution Agreement. As of the date hereof, no shares of our common stock have been sold under the Equity
Distribution Agreement.
On June
14, 2023, the Company entered into a placement agency agreement with the placement agent related to a public offering by the Company,
which closed on June 16, 2023, pursuant to which the placement agent received a cash fee equal of approximately $392,000.
Transfer Agent and Registrar
The transfer agent and registrar for our common
stock is Vstock Transfer, LLC, whose address is 18 Lafayette Place, Woodmere, NY 11598
and telephone number is (212) 828-8436.
Listing
Our common stock is listed on The Nasdaq Capital
Market under the symbol “TNON.” A class of our warrants are listed on The Nasdaq Capital Market under the symbol “TNONW.”
Selling Restrictions
Canada. The securities may be sold
in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National
Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients,
as defined in National Instrument 31 103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale
of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of
applicable securities laws.
Securities legislation in certain provinces or
territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment
thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the
time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any
applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or
consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument
33 105 Underwriting Conflicts (NI 33 105), the underwriters are not required to comply with the disclosure requirements
of NI 33-105 regarding underwriters conflicts of interest in connection with this offering.
European Economic Area. In relation
to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”)
an offer to the public of any securities may not be made in that Relevant Member State, except that an offer to the public in that Relevant
Member State of any securities may be made at any time under the following exemptions under the Prospectus Directive, if they have been
implemented in that Relevant Member State:
| |
● |
to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| |
● |
to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives for any such offer; or |
| |
● |
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by us or any underwriters of a prospectus pursuant to Article 3 of the Prospectus Directive. |
For the purposes of this provision, the expression
an “offer to the public” in relation to any securities in any Relevant Member State means the communication in any form and
by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide
to purchase any securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that
Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010
PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the
Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
United Kingdom. Each underwriter
has represented and agreed that:
| |
● |
it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (the FSMA) received by it in connection with the issue or sale of the securities in circumstances in which Section 21(1) of the FSMA does not apply to us; and |
| |
● |
it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the securities in, from or otherwise involving the United Kingdom. |
Switzerland. The securities may
not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (the SIX) or on any other stock
exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for
issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses
under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland.
Neither this document nor any other offering or marketing material relating to the securities or the offering may be publicly distributed
or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or
marketing material relating to the offering, or the securities have been or will be filed with or approved by any Swiss regulatory authority.
In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market
Supervisory Authority FINMA, and the offer of securities has not been and will not be authorized under the Swiss Federal Act on Collective
Investment Schemes (CISA). Accordingly, no public distribution, offering or advertising, as defined in CISA, its implementing ordinances
and notices, and no distribution to any non-qualified investor, as defined in CISA, its implementing ordinances and notices, shall
be undertaken in or from Switzerland, and the investor protection afforded to acquirers of interests in collective investment schemes
under CISA does not extend to acquirers of securities.
Australia. No placement document,
prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission
(ASIC), in relation to the offering.
This prospectus does not constitute a prospectus,
product disclosure statement or other disclosure document under the Corporations Act 2001 (the Corporations Act) and does
not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the
Corporations Act.
Any offer in Australia of the securities may only
be made to persons (the Exempt Investors) who are “sophisticated investors” (within the meaning of section 708(8)
of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise
pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the securities without
disclosure to investors under Chapter 6D of the Corporations Act.
The securities applied for by Exempt Investors
in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering,
except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption
under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D
of the Corporations Act. Any person acquiring securities must observe such Australian on-sale restrictions.
This prospectus contains general information only
and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not
contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether
the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice
on those matters.
Notice to Prospective Investors in the Cayman
Islands. No invitation, whether directly or indirectly, may be made to the public in the Cayman Islands to subscribe for our securities.
Taiwan. The securities have not
been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations
and may not be sold, issued or offered within Taiwan through a public offering or in circumstances which constitutes an offer within the
meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory Commission
of Taiwan. No person or entity in Taiwan has been authorized to offer, sell, give advice regarding or otherwise intermediate the offering
and sale of the securities in Taiwan.
Notice to Prospective Investors in Hong
Kong. The contents of this prospectus have not been reviewed by any regulatory authority in Hong Kong. You are advised to exercise
caution in relation to the offer. If you are in any doubt about any of the contents of this prospectus, you should obtain independent
professional advice. Please note that (i) our shares may not be offered or sold in Hong Kong, by means of this prospectus or any document
other than to “professional investors” within the meaning of Part I of Schedule 1 of the Securities and Futures Ordinance
(Cap.571, Laws of Hong Kong) (SFO) and any rules made thereunder, or in other circumstances which do not result in the document being
a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong) (CO) or which do not constitute an
offer or invitation to the public for the purpose of the CO or the SFO, and (ii) no advertisement, invitation or document relating to
our shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere)
which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do
so under the securities laws of Hong Kong) other than with respect to the shares which are or are intended to be disposed of only to persons
outside Hong Kong or only to “professional investors” within the meaning of the SFO and any rules made thereunder.
Notice to Prospective Investors in the People’s
Republic of China. This prospectus may not be circulated or distributed in the PRC and the shares may not be offered or sold,
and will not offer or sell to any person for re-offering or resale directly or indirectly to any resident of the PRC except pursuant to
applicable laws, rules and regulations of the PRC. For the purpose of this paragraph only, the PRC does not include Taiwan and the special
administrative regions of Hong Kong and Macau.
Israel. This document does not constitute
a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed with or approved by the Israel
Securities Authority. In the State of Israel, this document is being distributed only to, and is directed only at, and any offer of the
shares is directed only at, investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily
of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the
Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals”,
each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case
purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in
the Addendum). Qualified investors will be required to submit written confirmation that they fall within the scope of the Addendum, are
aware of the meaning of same and agree to it.
EXPERTS
The consolidated financial statements of Tenon
Medical, Inc. included in Tenon Medical Inc.’s Annual Report on Form 10-K for the year ended December 31, 2022, have been audited
by Armanino LLP, an independent registered public accounting firm, as set forth in their report thereon which is incorporated herein by
reference. Such financial statements have been incorporated by reference in reliance upon the report pertaining to such financial
statements of such firm given upon their authority as experts in accounting and auditing.
LEGAL
MATTERS
Certain legal matters with respect to the validity
of the securities being offered by this prospectus will be passed upon by Carmel, Milazzo & Feil LLP, New York, New York. Ellenoff,
Grossman & Schole LLP, New York, New York is acting as counsel for the representative of the placement agent with respect to the offering.
WHERE
YOU CAN FIND MORE INFORMATION
We have filed a registration
statement pon Form S-3 (including the exhibits, schedules and amendments thereto) with the Securities and Exchange Commission under the
Securities Act with respect to the shares of our common stock offered by this prospectus. This prospectus is part of that registration
statement and does not contain all the information included in the registration statement.
For further information
with respect to our common stock and us, you should refer to the registration statement, its exhibits and the material incorporated by
reference therein. Portions of the exhibits have been omitted as permitted by the rules and regulations of the Securities and Exchange
Commission. Statements made in this prospectus as to the contents of any contract, agreement or other document referred to are not
necessarily complete. In each instance, we refer you to the copy of the contracts or other documents filed as an exhibit to the
registration statement, and these statements are hereby qualified in their entirety by reference to the contract or document. The
registration statement may be obtained from the web site that the Securities and Exchange Commission maintains at http://www.sec.gov.
We file annual, quarterly and current reports and other information with the Securities and Exchange Commission.
INFORMATION WE INCORPORATE
BY REFERENCE
The SEC allows us to “incorporate
by reference” into this prospectus supplement and the accompanying prospectus the information in documents we file with it, which
means that we can disclose important information to you by referring you to those documents. The information incorporated by reference
is considered to be a part of this prospectus supplement and the accompanying prospectus, and information that we file later with the
SEC will automatically update and supersede this information. Any statement contained in any document incorporated or deemed to be incorporated
by reference herein shall be deemed to be modified or superseded for purposes of this prospectus supplement and the accompanying prospectus
to the extent that a statement contained in or omitted from this prospectus supplement or the accompanying prospectus, or in any other
subsequently filed document which also is or is deemed to be incorporated by reference herein or therein, modifies or supersedes such
statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part
of this prospectus supplement or the accompanying prospectus.
We incorporate by reference
the documents listed below and any future documents that we file with the SEC (excluding any portion of such documents that are furnished
and not filed with the SEC) under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus supplement
until the offering of the securities is terminated:
| ● | our Annual Report on Form 10-K
for the year ended December 31, 2022; |
| ● | our Current Reports on Form
8-K filed on May 19, 2023, June 20, 2023 (including the Form 8-K/A filed on July 18, 2023), July 11, 2023, July 21, 2023, July 28, 2023;
September 8, 2023 and |
| ● | the description of our Common
Stock set forth in the registration statement on Form 8-A filed on April 26, 2022 and the description of our warrants set forth in the
registration statement on Form 8-A filed on June 14, 2023. |
We will not, however, incorporate
by reference in this prospectus supplement or the accompanying prospectus any documents or portions thereof that are not deemed “filed”
with the SEC, including any information furnished pursuant to Item 2.02 or Item 7.01 of our current reports on Form 8-K unless, and except
to the extent, specified in such current reports.
You can obtain any of the filings incorporated
by reference into this prospectus through us or from the SEC through the SEC’s website at http://www.sec.gov. We will provide, at
no charge, to each person, including any beneficial owner, to whom a copy of this prospectus is delivered, upon written or oral request
of such person, a copy of any or all of the reports and documents referred to above which have been or may be incorporated by reference
into this prospectus. Written or telephone requests should be directed to: Tenon Medical, Inc., 104 Cooper Court, Los Gatos, CA 95032,
telephone number 408) 649-5760, Attention: Chief Financial Officer.
You should rely only on the information contained
or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized anyone else to provide you with different
or additional information. We will not make an offer of these securities in any state where the offer is not permitted. You should not
assume that the information in this prospectus or any supplement is accurate as of any date other than the date of those documents.
PROSPECTUS
$50,000,000

Tenon Medical, Inc.
Common Stock
Preferred Stock
Warrants
Debt Securities
Rights
Units
This prospectus will allow us to issue, from time
to time at prices and on terms to be determined at or prior to the time of the offering, up to $50,000,000 of any combination of the securities
described in this prospectus, either individually or in units. We may also offer common stock or preferred stock upon conversion of or
exchange for the debt securities; common stock upon conversion of or exchange for the preferred stock; common stock, preferred stock or
debt securities upon the exercise of warrants, rights or performance of purchase contracts; or any combination of these securities upon
the performance of purchase contracts.
This prospectus describes the general terms of
these securities and the general manner in which these securities will be offered. We will provide you with the specific terms of any
offering in one or more supplements to this prospectus. The prospectus supplements will also describe the specific manner in which these
securities will be offered and may also supplement, update or amend information contained in this document. You should read this prospectus
and any prospectus supplement, as well as any documents incorporated by reference into this prospectus or any prospectus supplement, carefully
before you invest.
Our securities may be sold directly by us to you,
through agents designated from time to time or to or through underwriters or dealers. For additional information on the methods of sale,
you should refer to the section entitled “Plan of Distribution” in this prospectus and in the applicable prospectus supplement.
If any underwriters or agents are involved in the sale of our securities with respect to which this prospectus is being delivered, the
names of such underwriters or agents and any applicable fees, commissions or discounts and over-allotment options will be set forth in
a prospectus supplement. The price to the public of such securities and the net proceeds that we expect to receive from such sale will
also be set forth in a prospectus supplement.
Pursuant to General Instruction I.B.6 of Form
S-3, in no event will we sell our securities in public primary offerings with a value exceeding more than one-third of our public float
in any 12-month period so long as our public float remains below $75.0 million. As of May 16, 2023, the aggregate market value of our
outstanding common stock held by non- affiliates, or public float, was approximately $16,569,823, based on 7,742,908 shares of our outstanding
common stock that were held by non-affiliates on such date and a price of $2.14 per share, which was the price at which our common stock
was last sold on the Nasdaq Capital Market on March 17, 2023, calculated in accordance with General Instruction I.B.6 of Form S-3. We
have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 during the twelve-month period that ends on and includes
the date hereof. Our common stock is listed on The Nasdaq Capital Market under the symbol “TNON.”
On May 15, 2023, the last reported sale price
of our common stock was $1.80 per share. The applicable prospectus supplement will contain information, where applicable, as to any other
listing, if any, on The Nasdaq Capital Market or any securities market or other securities exchange of the securities covered by the prospectus
supplement. Prospective purchasers of our securities are urged to obtain current information as to the market prices of our securities,
where applicable.
Investing in our securities involves a high
degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks that we have described on
page 7 of this prospectus under the caption “Risk Factors.” We may include specific risk factors in supplements to this prospectus
under the caption “Risk Factors.” This prospectus may not be used to sell our securities unless accompanied by a prospectus
supplement.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete.
Any representation to the contrary is a criminal offense.
Currently, we are an “emerging growth
company” as defined in Section 2(a) of the Securities Act of 1933, as amended, and are subject to reduced public company reporting
requirements. Please read “Implications of Being an Emerging Growth Company.”
You should read carefully and consider the
“Risk Factors” referenced on page 7 of this prospectus, as well as those contained in the applicable prospectus supplement
and in the documents that are incorporated by reference herein or the applicable prospectus supplement.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus.
Any representation to the contrary is a criminal offense.
The date of this prospectus is May 17, 2023.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement
that we filed with the Securities and Exchange Commission (the “SEC”) under the Securities Act of 1933, as amended (the “Securities
Act”), using a “shelf” registration process for the delayed offering and sale of securities pursuant to Rule 415 under
the Securities Act. Under the shelf process, we may, from time to time, sell any of the securities described in this prospectus in one
or more offerings and selling security holders may offer such securities owned by them from time to time.
This prospectus provides you with a general description
of the securities we may offer. Each time we or selling security holders sell securities, we will provide one or more prospectus supplements
that will contain specific information about the terms of the offering. The prospectus supplement may also add, update, or change information
contained in this prospectus. You should read both this prospectus and the accompanying prospectus supplement together with the additional
information described under the heading “Where You Can Find More Information.”
We have not authorized anyone to provide you with
any additional information. This prospectus and any accompanying prospectus supplement do not constitute an offer to sell or the solicitation
of an offer to buy any securities other than the securities described in the accompanying prospectus supplement or an offer to sell or
the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You should assume
that the information appearing in this prospectus, any prospectus supplement, the documents incorporated by reference, and any related
free writing prospectus is accurate only as of their respective dates. Our business, financial condition, results of operations, and prospects
may have changed materially since those dates.
As used in this prospectus, unless the context
otherwise requires, the terms “we,” “us,” “our,” and “our company” mean, collectively,
Tenon Medical, Inc. and its subsidiaries.
CAUTIONARY NOTE ABOUT FORWARD-LOOKING STATEMENTS
This prospectus, the documents incorporated by
reference herein and therein, and other written and oral statements we make from time to time contain certain “forward-looking”
statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended
(the “Exchange Act”). You can identify these forward-looking statements by the fact they use words such as “could,”
“expect,” “anticipate,” “estimate,” “target,” “may,” “project,”
“guidance,” “intend,” “plan,” “believe,” “will,” “potential,”
“opportunity,” “future,” and other words and terms of similar meaning and expression in connection with any discussion
of future operating or financial performance. You can also identify forward-looking statements by the fact that they do not relate strictly
to historical or current facts. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties,
including factors that could delay, divert, or change any of them, and could cause actual outcomes to differ materially from current expectations.
These statements are likely to relate to, among other things, our business strategy, our research and development, our product development
efforts, our ability to commercialize our product candidates, the activities of our licensees, our prospects for initiating partnerships
or collaborations, the timing of the introduction of products, the effect of new accounting pronouncements, uncertainty regarding our
future operating results and our profitability, anticipated sources of funds as well as our plans, objectives, expectations, and intentions.
We have included more detailed descriptions of
these risks and uncertainties and other risks and uncertainties applicable to our business that we believe could cause actual results
to differ materially from any forward-looking statement in the “Risk Factors” sections of this prospectus and the documents
incorporated by reference herein including, but not limited to, the risk factors incorporated by reference from our filings with the SEC.
We encourage you to read those descriptions carefully. Although we believe we have been prudent in our plans and assumptions, no assurance
can be given that any goal or plan set forth in forward-looking statements can be achieved. We caution investors not to place significant
reliance on forward-looking statements; such statements need to be evaluated in light of all the information contained and incorporated
by reference in this prospectus. Furthermore, the statements speak only as of the date of each document, and we undertake no obligation
to update or revise these statements.
This summary highlights selected information
that is presented in greater detail elsewhere, or incorporated by reference, in this prospectus. It does not contain all of the information
that may be important to you and your investment decision. Before investing in our securities, you should carefully read this entire prospectus,
including the matters set forth in the section titled “Risk Factors” and the financial statements and related notes and other
information that we incorporate by reference herein, including our Annual Report on Form 10-K
Introduction
Tenon Medical, Inc. (the “Company”),
was incorporated in the State of Delaware on June 19, 2012 and was headquartered in San Ramon, California until June 2021 when it relocated
to Los Gatos, California. The Company is a medical device company that offers a novel, less invasive approach to the sacroiliac joint
using a single, robust, titanium implant for treatment of the most common types of sacroiliac joint (the “SI-Joint”) disorders
that cause lower back pain. The system features the CATAMARAN™ Fixation Device which passes through both the axial and sagittal
planes of the ilium and sacrum, stabilizing and transfixing the SI joint along its longitudinal axis. The angle and trajectory of the
Catamaran surgical approach is also designed to provide a pathway away from critical neural and vascular structures and into the strongest
cortical bone. The Company received U.S. Food and Drug Administration (“FDA”) clearance in 2018 for The CATAMARANTM
SI-Joint Fusion System (“The CATAMARAN System”). The Company commercially launched The CATAMARAN System nationally in October
2022 at the North American Spine Society meeting held in Chicago. Currently, the Company’s only commercial focus is the US market.
The Opportunity
We estimate that over 30 million American
adults have chronic lower back pain.
Published clinical studies have shown that 15%
to 30% of all chronic lower back pain is associated with the SI-Joint. For patients whose chronic lower back pain stems from the Sacroiliac
Joint (“SI-Joint”), our experience in both clinical trials and commercial settings indicates The CATAMARAN System could be
beneficial for patients who are properly diagnosed and screened for surgery by trained healthcare providers.
In 2019, approximately 475,000 patients in the
United States were estimated to have received an aesthetic injection to temporarily alleviate pain emanating from the SI-Joint and/or
to diagnose SI-Joint pain. Additionally, several non-surgical technologies have been introduced in the past 10 years to address patients
who do not respond to injection therapy, including systemic oral medications and opioids.
To date, the penetration of a surgical solution
for this market has been relatively low (5-7%). We believe this is due to complex surgical approaches and suboptimal implant design of
existing options. The penetration of this market with an optimized surgical solution is Tenon’s focus.
We believe the SI-Joint is the last major joint
to be successfully addressed by the orthopedic implant industry. Studies have shown that disability resulting from disease of the SI-Joint
is comparable to the disability associated with a number of other serious orthopedic conditions, such as knee and hip arthritis and degenerative
disc disease, each of which has surgical solutions where an implant is used, and a multi-billion-dollar market exists.
The SI-Joint
The SI-Joint is a strong weight bearing synovial
joint situated between the lumbar spine and the pelvis and is aligned along the longitudinal load bearing axis of the human spine when
in an upright posture. It functions as a force transfer conduit where it transfers axial loads bi-directionally from the spine to the
pelvis and lower extremities and allows forces to be transmitted from the extremities to the spine. It also provides load sharing between
the hip and spine to contribute towards attenuation of impact shock and stress from activities of daily living.
The SI-Joint is a relatively immobile joint that
connects the sacrum (the spinal segment that is attached to the base of the lumbar spine at the L5 vertebra) and the ilium of the pelvis.
Each SI-Joint is approximately 2mm wide and irregularly shaped.
Motion of the SI-Joint features vertical shear
and rotation. Although the rotational forces about the SI-Joint are relatively low, repetitive motions created by daily activities such
as walking, jogging, twisting at the hips, and jumping can increase the stresses on the SI-Joint. If the SI-Joint is compromised through
injury or degeneration, the load bearing and motion restraints from the surrounding anatomical structures of the SI-Joint will be compromised
resulting in abnormal stress transfers across the joint to these structures, thereby further augmenting the degenerative cascade of the
SI-Joint. Eventual pain and cessation of an individual’s normal activities due to a painful and unstable SI-Joint have led to an
increase in the recent development of SI-Joint stabilization devices.
Non-Surgical Treatment of Sacroiliac Joint
Disease
Several non-surgical treatments exist for suspected sacroiliac
joint pain. These conservative steps often provide desired relief for the patient. Non-surgical treatments include:
:
including opiates and non-steroidal anti-inflammatory medications.
:
which can involve exercises as well as massage.
| ● | Intra-Articular
Injections of Steroid Medications |
:
which are typically performed by physicians who specialize in pain treatment or anesthesia.
:
or the cauterizing of the lateral branches of the sacral nerve roots.
When conservative steps fail to deliver sustained
pain relief and return to quality of life, specific diagnostic protocols are utilized to explore if a surgical option should be considered.
Diagnosis
Historically, diagnosing pain from the SI-Joint
was not routinely a focus of orthopedic or neurosurgery training during medical school or residency programs. Due to its invasiveness,
post-operative pain, and muscle disruption along with a difficult procedure overall, the open SI-Joint fusion procedure was rarely taught
in these settings.
The emergence of various SI-Joint surgical technologies
has generated a renewed discussion of SI-Joint issues. Of particular focus is the diagnostic protocol utilized to properly select patients
for S-I Joint surgery. Patients with low back pain typically start with primary care physicians who often refer to pain specialists. Here,
the patient will go through traditional physical therapy combined with oral medications (anti-inflammatory, narcotic, etc.). If the patient
fails to respond to these steps the pain specialist may move to therapeutic injections of the SI-Joint. These injections may serve to
lessen inflammation to the point that the patient is satisfied. However, the impact from these injections is often transient. In this
case the patient is often referred to a trained physician to determine if the patient may be a candidate for surgical intervention. A
series of provocative tests in clinic, combined with a specific injection protocol to isolate the SI-Joint as the pain generator is then
utilized to confirm the need for surgical intervention. Published literature has shown this technique to be a very effective step to determine
the best treatment to alleviate pain.
Limitations of Existing Treatment Options
Surgical fixation and fusion of the SI-Joint with
an open surgical technique was first reported in 1908, with further reports in the 1920s. The open procedure uses plates and screws, requires
a 6 to 12-inch incision and is extremely invasive. Due to the invasiveness and associated morbidity, the use of this
procedure is limited to cases involving significant trauma, tumor, etc.
Less invasive surgical options along with implant
design began to emerge over the past 15 years. These options feature a variety of approaches and implant designs and have been met with
varying degrees of adoption. Lack of a standard and accepted diagnostic approach, complexity of approach, high morbidity of approach,
abnormally high complication rates and inability to radiographically confirm fusion have all been cited as reasons for low adoption of
these technologies.
The CATAMARAN™ SI-Joint Fusion System
Solution
Until October 2022 Tenon sold The CATAMARAN™
SI-Joint Fusion System (“The CATAMARAN System”) to a limited number of clinician advisors to refine the product for a full
commercial launch. In October 2022 Tenon initiated a full commercial launch at the NASS meeting in Chicago. The CATAMARAN System includes
instruments and implants designed to prepare and fixate the SI-Joint for fusion. We believe The CATAMARAN System will address a large
market opportunity with a superior product and is distinct from other competitive offerings in the following ways:
| |
● |
Transfixes the SI joint |
| |
● |
Inferior Posterior Sacroiliac Fusion Approach |
| |
● |
Reduced Approach Morbidity |
| |
● |
Direct And Visualized Approach to the SI-Joint |
| |
● |
Single Implant Technique |
| |
● |
Insertion Trajectory Away from the Neural Foramen |
| ● | Insertion
Trajectory Away from Major Vascular Structures |
| ● | Autologous
Bone Grafting in the Ilium, Sacrum and Bridge |
| ● | Radiographic
Confirmation of Bridging Bone Fusion of the SI-Joint |
The
fixation device and its key features are shown below:
 |
Key Features
“Pontoon” in the ilium
“Pontoon” in the sacrum
“Pontoons and Bridge” filled with autologous bone from
drilling process
Leading edge osteotome creates defect and facilitates ease of insertion |
The CATAMARAN System is a singular implant designed
with several proprietary components which allow for it to be explicitly formatted to transfix the SI-Joint with a single approach and
implant. This contrasts with several competitive implant systems that require multiple approach pathways and implants to achieve fixation.
In addition, the Inferior Posterior approach is designed to be direct to the joint and through limited anatomical structures which may
minimize the morbidity of the approach. The implant features a patented dual pontoon open cell design which enables the clinician to pack
the pontoons with the patient’s own autologous bone designed to promote bone fusion across the joint. The CATAMARAN System is designed
specially to resist vertical shear and rotation of the joint in which it was implanted, helping stabilize the joint in preparation for
eventual fusion.
The instruments we have developed are proprietary
to The CATAMARAN System and specifically designed to facilitate an Inferior Posterior approach that is unique to the system.
Tenon also has developed a proprietary 2D placement
protocol as well as a protocol for 3D navigation utilizing the latest techniques in spine surgery. These Tenon advancements are intended
to further enhance the safety of the procedure and encourage more physicians to adopt the procedure.
The CATAMARAN System, as mentioned previously,
is placed in the densest aspect of the SI-Joint as confirmed by the pre-op planning images below:
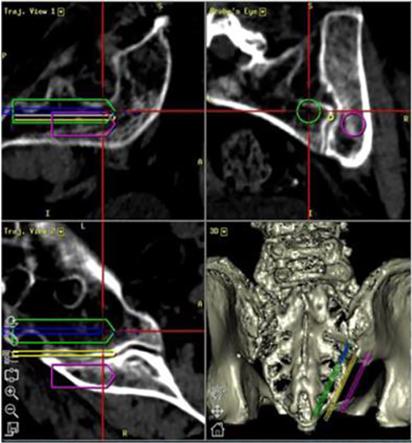 |
|
Surgical Plan Key:
Yellow: Guidewire
Purple: Lateral Pontoon (Ilium)
Green: Medial Pontoon (Sacrum)
|
| |
|
| |
Notes:
Upper Right Quadrant: The green and purple pontoons represent the placement
in the dense bone inferior – contrasted with the dorsal gap superiorly where competitive systems are most often placed.
Lower Right Quadrant: The yellow and purple outlines represent The
CATAMARAN System pontoons, illustrating the angle of insertion is away from the sacral neuro foramen providing for a much
safter trajectory for device implantation. |
The Procedure
We believe The CATAMARAN System and its differentiated
characteristics allow for an efficient and effective procedure designed to deliver short-term stabilization and long-term fusion that
can be confirmed radiographically. Shown below is an illustration demonstrating the unique placement of The CATAMARAN System inserted
Inferior Posterior and coming directly down to and transfixing the joint
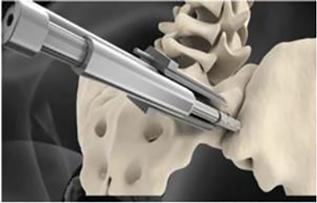 |
|
 |
The CATAMARAN System procedure is typically performed
under general anesthesia using a specially designed instrument set we provide to prepare for the Inferior Posterior access to the SI-Joint.
Specially designed imaging and navigation protocols are designed to ensure the clinician has the proper Entry Point, Trajectory, Angle
and Depth (ETAD™) so that the pontoons of The CATAMARAN System are placed for maximum fixation. The CATAMARAN System incorporates
two pontoons and is designed so that when the system is impacted into the bone one pontoon is on the Illum side and the other is in the
Sacrum side with the bridge spanning the joint, preventing shear and rotation of the joint. The device also features an open cell design
where the patient’s own (autologous) bone is packed into the pontoons and the bridge to facilitate fusion across the joint. The
leading edge of the bridge is designed to act as an osteotome, providing a self-created deficit upon insertion. These features are designed
to create an ideal environment for bone ingrowth and fusion. Below is a fluoroscopic image of an implanted CATAMARAN Fixation Device spanning
the SI-Joint.
Tenon believes the surgical approach and implant
design it has developed, along with the 2D and 3D protocols for proper implantation will be received well by the clinician community who
have been looking for a next generation device. Our initial clinical results indicate that The CATAMARAN System is promoting fusion across
the joint as evidenced by post-op CT scans (the recognized gold standard widely accepted by the Clinical community).
Post-Op fluoroscopic image of
implant spanning the SI-Joint |
|
6-Month CT-Scan showing clear
bridging bone fusion |
 |
|
 |
A preliminary 18 case series (Michael Joseph Chaparro,
MD, F.A.A.N.S., F.A.C.S.) has documented that The CATAMARAN System does in fact promote fusion across the SI-Joint, which many of our
competitors have not been able to demonstrate. While products from some of our competitors use screws and triangular wedges to treat the
SI-Joint, most do not effectively resist the vertical shear and twisting within the joint. This 18 patient series was presented at the
North American Spine Society Annual Meeting in Chicago, IL in October 2022.
An independent biomechanical study (Lisa Ferrara,
Ph.D. OrthoKinetic Technologies, LLC now part of Element) demonstrated that a single CATAMARAN SIJ Fixation Device was superior to predicate
device in the areas of Fixation Strength, Shear Stiffness, Dynamic Endurance and Pullout Strength. We hold issued patents on The CATAMARAN
System and its unique features including the dual pontoons and the open cell structure for bone graft packing. We also hold an issued
patent for the method of placing The CATAMARAN System into the SI-Joint where one pontoon is in the ilium and the other in the sacrum.
The CATAMARAN System’s unique design has
already demonstrated radiographically confirmed fusion in initial patients. We believe that this beneficial advantage along with a simpler,
safer, and less painful procedure will make this the procedure of choice for most physicians. Tenon has initiated post market, IRB controlled
clinical trials to demonstrate this technology delivers on these advantages.
Corporate Information
Our principal executive offices are located at
104 Cooper Court, Los Gatos, California 95032. Our website address is www.tenonmed.com. The information included on our website or in
any social media associated with the Company is not part of this prospectus.
Implications of Being an Emerging Growth Company
We are an “emerging growth company,”
as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). We will remain an emerging growth company
until the earlier of (i) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock
pursuant to an effective registration statement under the Securities Act; (ii) the last day of the fiscal year in which we have total
annual gross revenues of $1.235 billion or more; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during
the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under applicable SEC rules. We expect
that we will remain an emerging growth company for the foreseeable future, but cannot retain our emerging growth company status indefinitely
and will no longer qualify as an emerging growth company on or before the last day of the fiscal year following the fifth anniversary
of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act. For so long
as we remain an emerging growth company, we are permitted and intend to rely on exemptions from specified disclosure requirements that
are applicable to other public companies that are not emerging growth companies.
These exemptions include:
| ● | being
permitted to provide only two years of audited financial statements, in addition to any required
unaudited interim financial statements, with correspondingly reduced “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| ● | not
being required to comply with the requirement of auditor attestation of our internal controls
over financial reporting; |
| ● | not
being required to comply with any requirement that may be adopted by the Public Company Accounting
Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s
report providing additional information about the audit and the financial statements; |
| ● | reduced
disclosure obligations regarding executive compensation; and |
| ● | not
being required to hold a nonbinding advisory vote on executive compensation and shareholder
approval of any golden parachute payments not previously approved. |
We have taken advantage of certain reduced reporting
requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from
other public companies in which you hold stock.
An emerging growth company can take advantage
of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards.
This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply
to private companies. We have irrevocably elected to avail ourselves of this extended transition period and, as a result, we will not
be required to adopt new or revised accounting standards on the dates on which adoption of such standards is required for other public
reporting companies.
We are also a “smaller reporting company”
as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and have elected
to take advantage of certain of the scaled disclosure available for smaller reporting companies.
RISK FACTORS
Investing in our securities involves a high
degree of risk. You should carefully consider the risks described in the documents incorporated by reference in this prospectus and any
prospectus supplement, as well as other information we include or incorporate by reference into this prospectus and any applicable prospectus
supplement, before making an investment decision. Our business, financial condition or results of operations could be materially adversely
affected by the materialization of any of these risks. The trading price of our securities could decline due to the materialization of
any of these risks, and you may lose all or part of your investment. This prospectus and the documents incorporated herein by reference
also contain forward-looking statements that involve risks and uncertainties. Actual results could differ materially from those anticipated
in these forward-looking statements as a result of certain factors, including the risks described in the documents incorporated herein
by reference, including the risks described in Part I, Item 1A, Risk Factors in our most recent Annual Report on Form 10-K, together with
the other information set forth in this prospectus, and in the other documents that we include or incorporate by reference into this prospectus,
as updated by our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings we make with the SEC, the risk factors
described under the caption “Risk Factors” in any applicable prospectus supplement and any risk factors set forth in our other
filings with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, before making a decision about investing in our
common stock. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently
known to us or that we currently deem immaterial may also affect our operations. If any risks actually occur, our business, financial
condition and results of operations may be materially and adversely affected. In such an event, the trading price of our common stock
could decline and you could lose part or all of your investment.
For more information about our SEC filings, please
see “Where You Can Find More Information” and “Incorporation by Reference.”
Additional risks not presently known or that we
presently consider to be immaterial could subsequently materially and adversely affect our financial condition, results of operations,
business, and prospects.
USE OF PROCEEDS
The principal purposes of this offering are to
increase our capitalization and financial flexibility, increase our visibility in the marketplace and create a public market for our common
stock. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from
this offering. However, we currently intend to use the net proceeds from this offering to hire additional employees, continue the commercial
launch of our product including training clinicians on The CATAMARAN System procedure, continue clinical marketing studies that are focused
on capturing post-market safety and efficacy data, gathering system feedback and initiating product refinements, other sales and marketing
activities and for working capital and general corporate purposes. See “Business—Research & Development.”
We will retain broad discretion in the allocation
of the net proceeds from this offering and could utilize the proceeds in ways that do not necessarily improve our results of operations
or enhance the value of our common stock.
SELLING SECURITY HOLDERS
Selling security holders are persons or entities
that, directly or indirectly, have acquired or will from time to time acquire from us, our securities in various private transactions.
Such selling security holders may be parties to registration rights agreements with us, or we otherwise may have agreed or will agree
to register their securities for sale. Certain holders of our securities, as well as their transferees, pledgees, donees, or successors,
all of whom we refer to as “selling security holders,” may from time to time offer and sell the securities pursuant to this
prospectus and any applicable prospectus supplement.
The applicable prospectus supplement will set
forth the name of each selling security holder and the number of and type of securities beneficially owned by such selling security holder
that are covered by such prospectus supplement. The applicable prospectus supplement will also disclose whether any of the selling security
holders have held any position or office with, have been employed by, or otherwise have had a material relationship with us during the
three years prior to the date of the prospectus supplement.
DESCRIPTION OF SECURITIES
The descriptions of the securities contained in
this prospectus, together with the applicable prospectus supplements, summarize the material terms and provisions of the various types
of securities that we may offer. We will describe in the applicable prospectus supplement relating to any securities the particular terms
of the securities offered by that prospectus supplement. If we so indicate in the applicable prospectus supplement, the terms of the securities
may differ from the terms we have summarized below. We will also include in the prospectus supplement information, where applicable, about
material U.S. federal income tax considerations relating to the securities, and the securities exchange, if any, on which the securities
will be listed.
We may sell from time to time common stock, preferred
stock, debt securities, warrants to purchase any such securities, or any combination of the foregoing.
In this prospectus, we refer to the common stock,
preferred stock, debt securities, and warrants to be sold by us collectively as “securities.”
If we issue debt securities at a discount from
their original stated principal amount, then we will use the issue price, and not the principal amount, of such debt securities for purposes
of calculating the total dollar amount of all securities issued under this prospectus.
This prospectus may not be used to consummate
a sale of securities unless it is accompanied by a prospectus supplement.
DESCRIPTION OF COMMON STOCK
General
We are authorized to issue up to 130,000,000 shares
of common stock, par value $0.001 per share, with 11,251,299 shares issued and outstanding as of May 4, 2023.
Each share of our common stock has the same relative
rights and is identical in all respects with each other share of common stock.
The holders of our common stock are entitled to
the following rights:
Voting Rights
Each share of our common stock entitles its holder
to one vote per share on all matters to be voted or consented upon by the stockholders. Holders of our common stock are not entitled to
cumulative voting rights with respect to the election of directors.
Election of Directors
The holders of our common stock, voting as a separate
class, shall be entitled to elect one member of our Board of Directors.
Dividend Rights
Subject to limitations under Delaware law and
preferences that may apply to any shares of preferred stock that we may decide to issue in the future, holders of our common stock are
entitled to receive ratably such dividends or other distributions, if any, as may be declared by our Board out of funds legally available
therefor.
Liquidation Rights
In the event of the liquidation, dissolution or
winding up of our business, the holders of our common stock are entitled to share ratably in the assets available for distribution after
the payment of all of our debts and other liabilities, subject to the prior rights of the holders of our preferred stock.
Other Rights
The holders of our common stock have no subscription,
redemption or conversion privileges. Our common stock does not entitle its holders to preemptive rights. All of the outstanding shares
of our common stock are fully paid and non-assessable. The rights, preferences and privileges of the holders of our common stock are subject
to the rights of the holders of shares of any series of preferred stock which we may issue in the future.
Exclusive Forum
Our Certificate of Incorporation provides that,
unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole
and exclusive forum for (a) any derivative action or proceeding brought on behalf of the Company, (b) any action asserting a claim of
breach of a fiduciary duty owed by any director, officer, employee or agent of the Company to the Company or the Company’s stockholders,
(c) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our Certificate of Incorporation
or Bylaws, or (d) any action asserting a claim governed by the internal affairs doctrine, in each case subject to said Court of Chancery
having personal jurisdiction over the indispensable parties named as defendants therein. This exclusive forum provision may limit the
ability of our stockholders to bring a claim in a judicial forum that such stockholders find favorable for disputes with us or our directors
or officers, which may discourage lawsuits against us or our directors or officers. Our Certificate of Incorporation also provides that
this choice of forum provision does not apply to claims arising under federal securities laws.
Section 203 of the Delaware General Corporation
Law
We are subject to the provisions of Section 203
of the DGCL regulating corporate takeovers. This statute prevents certain Delaware corporations, under certain circumstances, from engaging
in a “business combination” with:
| ● | a stockholder who owns 15%
or more of our outstanding voting stock (otherwise known as an “interested stockholder”); |
| ● | an affiliate of an interested
stockholder; or |
| ● | an associate of an interested
stockholder, for three years following the date that the stockholder became an interested stockholder. |
A “business combination” includes
a merger or sale of more than 10% of our assets. However, the above provisions of Section 203 do not apply if:
| ● | our board of directors approves
the transaction that made the stockholder an “interested stockholder,” prior to the date of the transaction; or |
| ● | after the completion of the
transaction that resulted in the stockholder becoming an interested stockholder, that stockholder owned at least 85% of our voting stock
outstanding at the time the transaction commenced, other than statutorily excluded shares of common stock. |
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be VStock
Transfer LLC.
Listing
Our common stock is listed on the Nasdaq Capital
Market under the symbol “TNON”.
DESCRIPTION OF PREFERRED STOCK
This section describes the general terms and provisions
of the preferred stock that we may offer by this prospectus. The prospectus supplement will describe the specific terms of the series
of the preferred stock offered through that prospectus supplement. Those terms may differ from the terms discussed below. Any series of
preferred stock that we issue will be governed by our certificate of incorporation, as amended, including the certificate of designations
relating to such series of preferred stock, and our by-laws.
As of May 4, 2023 we had 20,000,000 authorized
shares of preferred stock. We currently do not have any shares of preferred stock outstanding.
Our Board of Directors without the approval of
the stockholders may issue up to 20,000,000 shares of preferred stock in one or more series and with respect to each series of preferred
stock, the Board of Directors will fix the rights, preferences, privileges, and restrictions of the preferred stock of each series in
the certificate of designations relating to that series. We will incorporate by reference as an exhibit to the registration statement
that includes this prospectus the form of any certificate of designations that describes the terms of the series of preferred stock we
are offering before the issuance of the related series of preferred stock. This description will include the following, to the extent
applicable:
| ● | the title and stated value; |
| ● | the number of shares we are
offering; |
| ● | the liquidation preference
per share; |
| ● | the dividend rate, period and
payment date, and method of calculation for dividends, if any; |
| ● | whether any dividends will
be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; |
| ● | the provisions for a sinking
fund, if any; |
| ● | the provisions for redemption
or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; |
| ● | any listing of the preferred
stock on any securities exchange or market; |
| ● | whether the preferred stock
will be convertible into our common stock and, if applicable, the conversion price, or how it will be calculated, and the conversion
period; |
| ● | whether the preferred stock
will be exchangeable into debt securities and, if applicable, the exchange price, or how it will be calculated, and the exchange period; |
| ● | voting rights, if any, of the
preferred stock; |
| ● | preemptive rights, if any; |
| ● | restrictions on transfer, sale,
or other assignment, if any; |
| ● | whether interests in the preferred
stock will be represented by depositary shares; |
| ● | a discussion of any material
or special U.S. federal income tax considerations applicable to the preferred stock; |
| ● | the relative ranking and preferences
of the preferred stock as to dividend rights and rights if we liquidate, dissolve, or wind up our affairs; any limitations on issuance
of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock as to dividend rights and
rights if we liquidate, dissolve, or wind up our affairs; and |
| ● | any other specific terms, preferences,
rights, or limitations of, or restrictions on, the preferred stock. |
When we issue shares of preferred
stock under this prospectus, the shares, when issued in accordance with the terms of the applicable agreement, will be validity issued,
fully paid, and non-assessable and will not have, or be subject to, any preemptive or similar rights.
Section 242 of DGCL provides
that the holders of each class or series of stock will have the right to vote separately as a class on certain amendments to our certificate
of incorporation, as amended, that would affect the class or series of preferred stock, as applicable. This right is in addition to any
voting rights that may be provided for in the applicable certificate of designation.
DESCRIPTION OF WARRANTS
General
As of May 4, 2023, warrants to purchase approximately
96,000 shares of our common stock with a weighted average exercise price per share of $5.00 were outstanding.
We may offer by means of this prospectus warrants
for the purchase of our common stock or preferred stock. We may issue warrants separately or together with any other securities offered
by means of this prospectus, and the warrants may be attached to or separate from such securities. Each series of warrants will be issued
under a separate warrant agreement to be entered into between us and a warrant agent specified therein. The warrant agent will act solely
as our agent in connection with the warrants of such series and will not assume any obligation or relationship of agency or trust for
or with any holders or beneficial owners of warrants.
When we refer to a series of securities in this
section, we mean all securities issued as part of the same series under any applicable indenture, agreement, or other instrument. When
we refer to the prospectus supplement, we mean the applicable prospectus supplement describing the specific terms of the security you
purchase. The terms used in the prospectus supplement will have the meanings described in this prospectus, unless otherwise specified.
The following description of warrants does not
purport to be complete and is qualified in its entirety by reference to the description of a particular series of warrants contained in
an applicable prospectus supplement. For information relating to our capital stock, see “Description of Common Stock,” and
“Description of Preferred Stock.”
Agreements
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. Each series of warrants may be evidenced
by certificates and may be issued under a separate indenture, agreement, or other instrument to be entered into between us and a bank
that we select as agent with respect to such series. The agent, if any, will have its principal office in the United States and have a
combined capital and surplus of at least $50,000,000. Warrants in book-entry form will be represented by a global security registered
in the name of a depositary, which will be the holder of all the securities represented by the global security. Those who own beneficial
interests in a global security will do so through participants in the depositary’s system, and the rights of these indirect owners
will be governed solely by the applicable procedures of the depositary and its participants.
General Terms of Warrants
The prospectus supplement relating to a series
of warrants will identify the name and address of the warrant agent, if any. The prospectus supplement will describe the following terms,
where applicable, of the warrants in respect of which this prospectus is being delivered:
| ● | the
title and issuer of the warrants; |
| ● | the
aggregate number of warrants; |
| ● | the
price or prices at which the warrants will be issued; |
| ● | the
currencies in which the price or prices of the warrants may be payable; |
| ● | the
designation, amount, and terms of the securities purchasable upon exercise of the warrants; |
| ● | the
designation and terms of the other securities with which the warrants are issued and the number of warrants issued with each such security
or each principal amount of security; |
| ● | if
applicable, the date on and after which the warrants and any related securities will be separately transferable; |
| ● | any
securities exchange or quotation system on which the warrants or any securities deliverable upon exercise of such securities may be listed; |
| ● | the
price or prices at which and currency or currencies in which the securities purchasable upon exercise of the warrants may be purchased; |
| ● | the
date on which the right to exercise the warrants shall commence and the date on which such right shall expire; |
| ● | the
minimum or maximum amount of warrants that may be exercised at any one time; |
| ● | whether
the warrants will be issued in fully registered for or bearer form, in global or non-global form, or in any combination of these forms; |
| ● | information
with respect to book-entry procedures, if any; |
| ● | a
discussion of certain U.S. federal income tax considerations; and |
| ● | any
other material terms of the warrants, including terms, procedures, and limitations relating to the exchange and exercise of the
warrants. |
Exercise of Warrants
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. If any warrant is exercisable for
other securities or other property, the following provisions will apply. Each such warrant may be exercised at any time up to any expiration
date and time mentioned in the prospectus supplement relating to those warrants. After the close of business on any applicable expiration
date, unexercised warrants will become void.
Warrants may be exercised by delivery of the certificate
representing the securities to be exercised, or in the case of global securities by delivery of an exercise notice for those warrants,
together with certain information, and payment to any agent in immediately available funds, as provided in the prospectus supplement,
of the required purchase amount, if any. Upon receipt of payment and the certificate or exercise notice properly executed at the office
indicated in the prospectus supplement, we will, in the time period the relevant agreement provides, issue and deliver the securities
or other property purchasable upon such exercise. If fewer than all of the warrants represented by such certificates are exercised, a
new certificate will be issued for the remaining amount of warrants.
If mentioned in the prospectus supplement, securities
may be surrendered as all or part of the exercise price for warrants.
Antidilution Provisions
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. In the case of warrants to purchase
common stock, the exercise price payable and the number of shares of common stock purchasable upon warrant exercise may be adjusted in
certain events, including:
| ● | the
issuance of a stock dividend to common stockholders or a combination, subdivision, or reclassification of common stock; |
| ● | the
issuance of rights, warrants, or options to all common and preferred stockholders entitling them to purchase common stock for an aggregate
consideration per share less than the current market price per share of common stock; |
| ● | any
distribution to our common stockholders of evidences of our indebtedness of assets, excluding cash dividends or distributions referred
to above; and |
| ● | any
other events mentioned in the prospectus supplement. |
The
prospectus supplement will describe which, if any, of these provisions shall apply to a particular series of warrants.
Unless otherwise specified in the applicable prospectus
supplement, no adjustment in the number of shares purchasable upon warrant exercise will be required until cumulative adjustments require
an adjustment of at least 1% of such number and no fractional shares will be issued upon warrant exercise, but we will pay the cash value
of any fractional shares otherwise issuable.
Modification
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. We and any agent for any series
of warrants may amend any warrant or rights agreement and the terms of the related warrants by executing a supplemental agreement, without
any such warrant holders’ consent, for the purpose of:
| ● | curing
any ambiguity, any defective or inconsistent provision contained in the agreement, or making any other corrections to the agreement that
are not inconsistent with the provisions of the warrant certificates; |
| |
● |
evidencing
the succession of another corporation to us and its assumption of our covenants contained in the agreement and the securities; |
| |
● |
appointing
a successor depository, if the securities are issued in the form of global securities; |
| |
● |
evidencing
a successor agent’s acceptance of appointment with respect to any securities; |
| |
● |
adding
to our covenants for the benefit of securityholders or surrendering any right or power we have under the agreement; |
| |
● |
issuing
warrants in definitive form, if such securities are initially issued in the form of global securities; or |
| |
● |
amending
the agreement and the warrants as we deem necessary or desirable and that will not adversely affect the interests of
the applicable warrant holders in any material respect. |
We and any agent for any series of warrants may
also amend any agreement and the related warrants by a supplemental agreement with the consent of the holders of a majority of the warrants
of any series affected by such amendment, for the purpose of adding, modifying, or eliminating any of the agreement’s provisions
or of modifying the rights of the holders of warrants. However, no such amendment that:
| ● | reduces
the number or amount of securities receivable upon any exercise of any such security; |
| ● | shortens
the time period during which any such security may be exercised; |
| ● | otherwise
adversely affects the exercise rights of warrant holders in any material respect; or |
| ● | reduces
the number of securities the consent of holders of which is required for amending the agreement or the related warrants; |
may be made without the consent of each holder
affected by that amendment.
Consolidation, Merger, and Sale of Assets
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. Any agreement with respect to warrants
will provide that we are generally permitted to merge or consolidate with another corporation or other entity. Any such agreement will
also provide that we are permitted to sell our assets substantially as an entirety to another corporation or other entity or to have another
entity sell its assets substantially as an entirety to us. With regard to any series of warrants, however, we may not take any of these
actions unless all of the following conditions are met:
| ● | if
we are not the successor entity, the person formed by the consolidation or into or with which we merge or the person to which our properties
and assets are conveyed, transferred, or leased must be an entity organized and existing under the laws of the United States, any state,
or the District of Columbia and must expressly assume the performance of our covenants under any relevant indenture, agreement, or other
instrument; and |
| |
● |
we or
that successor corporation must not immediately be in default under that agreement. |
Enforcement by Holders of Warrants
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. Any agent for any series of warrants
will act solely as our agent under the relevant agreement and will not assume any obligation or relationship of agency or trust for any
securityholder. A single bank or trust company may act as agent for more than one issue of securities. Any such agent will have no duty
or responsibility in case we default in performing our obligations under the relevant agreement or warrant, including any duty or responsibility
to initiate any legal proceedings or to make any demand upon us. Any securityholder may, without the agent’s consent or consent
of any other securityholder, enforce by appropriate legal action its right to exercise any warrant exercisable for any property.
Replacement of Certificates
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. We will replace any destroyed, lost,
stolen, or mutilated warrant or rights certificate upon delivery to us and any applicable agent of satisfactory evidence of the ownership
of that certificate and of its destruction, loss, theft or mutilation, and (in the case of mutilation) surrender of that certificate to
us or any applicable agent, unless we have, or the agent has, received notice that the certificate has been acquired by a bona fide purchaser.
That securityholder will also be required to provide indemnity satisfactory to us and the relevant agent before a replacement certificate
will be issued.
Title
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any warrants we issue pursuant to this prospectus. We, any agents for any series of
warrants, and any of their agents may treat the registered holder of any certificate as the absolute owner of the securities evidenced
by that certificate for any purpose and as the person entitled to exercise the rights attaching to the warrants so requested, despite
any notice to the contrary.
DESCRIPTION OF DEBT SECURITIES
Any debt securities we may issue, offered by this
prospectus and any accompanying prospectus supplement, will be issued under an indenture to be entered into between our company and the
trustee identified in the applicable prospectus supplement. The terms of the debt securities will include those stated in the indenture
and those made part of the indenture by reference to the Trust Indenture Act of 1939, as in effect on the date of the indenture. We have
filed a copy of the form of indenture as an exhibit to the registration statement in which this prospectus is included. The indenture
will be subject to and governed by the terms of the Trust Indenture Act of 1939.
Unless otherwise specified in the applicable prospectus
supplement, the debt securities will represent direct, unsecured obligations of our company and will rank equally with all of our other
unsecured indebtedness.
The following statements relating to the debt
securities and the indenture are summaries, qualified in their entirety to the detailed provisions of the indenture.
General
We may issue the debt securities in one or more
series with the same or various maturities, at par, at a premium, or at a discount. We will describe the particular terms of each series
of debt securities in a prospectus supplement relating to that series, which we will file with the SEC.
The prospectus supplement will set forth, to the
extent required, the following terms of the debt securities in respect of which the prospectus supplement is delivered:
| ● | the
title of the series; |
| |
● |
the aggregate
principal amount; |
| |
● |
the issue
price or prices, expressed as a percentage of the aggregate principal amount of the debt securities; |
| |
● |
any limit
on the aggregate principal amount; |
| |
● |
the date
or dates on which principal is payable; |
| |
● |
the interest
rate or rates (which may be fixed or variable) or, if applicable, the method used to determine such rate or rates; |
| |
● |
the date
or dates from which interest, if any, will be payable and any regular record date for the interest payable; |
| |
● |
the place
or places where principal and, if applicable, premium and interest, is payable; |
| |
● |
the terms
and conditions upon which we may, or the holders may require us to, redeem or repurchase the debt securities; |
| |
● |
the denominations
in which such debt securities may be issuable, if other than denominations of $1,000, or any integral multiple of that number; |
| |
● |
whether
the debt securities are to be issuable in the form of certificated debt securities (as described below) or global debt securities
(as described below); |
| |
● |
the portion of principal amount that will be payable upon declaration of acceleration of the maturity date if other than the principal amount of the debt securities; |
| |
● |
the currency of denomination; |
| |
● |
the designation of the currency, currencies, or currency units in which payment of principal and, if applicable, premium and interest, will be made; |
| |
● |
if payments of principal and, if applicable, premium or interest, on the debt securities are to be made in one or more currencies or currency units other than the currency of denomination, the manner in which the exchange rate with respect to such payments will be determined; |
| |
● |
if amounts of principal and, if applicable, premium and interest may be determined by reference to an index based on a currency or currencies, or by reference to a commodity, commodity index, stock exchange index, or financial index, then the manner in which such amounts will be determined; |
| |
● |
the provisions, if any, relating to any collateral provided for such debt securities; |
| |
● |
any addition to or change in the covenants and/or the acceleration provisions described in this prospectus or in the indenture; |
| |
● |
any events of default, if not otherwise described below under “Events of Default”; |
| |
● |
the terms and conditions, if any, for conversion into or exchange for shares of common stock or preferred stock; |
| |
● |
any depositaries, interest rate calculation agents, exchange rate calculation agents, or other agents; and |
| |
● |
the terms and conditions, if any, upon which the debt securities shall be subordinated in right of payment to other indebtedness of our company. |
We may issue discount debt securities that provide
for an amount less than the stated principal amount to be due and payable upon acceleration of the maturity of such debt securities in
accordance with the terms of the indenture. We may also issue debt securities in bearer form, with or without coupons. If we issue discount
debt securities or debt securities in bearer form, we will describe material U.S. federal income tax considerations and other material
special considerations which apply to these debt securities in the applicable prospectus supplement.
We may issue debt securities denominated in or
payable in a foreign currency or currencies or a foreign currency unit or units. If we do, we will describe the restrictions, elections,
and general tax considerations relating to the debt securities and the foreign currency or currencies or foreign currency unit or units
in the applicable prospectus supplement.
Exchange and/or Conversion Rights
We may issue debt securities that can be exchanged
for or converted into shares of common stock or preferred stock. If we do, we will describe the terms of exchange or conversion in the
prospectus supplement relating to these debt securities.
Transfer and Exchange
We may issue debt securities that will be represented
by either:
| |
● |
“book-entry securities,” which means that there will be one or more global securities registered in the name of a depositary or a nominee of a depositary; or |
| |
● |
“certificated securities,” which means that they will be represented by a certificate issued in definitive registered form. |
We will specify in the prospectus supplement applicable
to a particular offering whether the debt securities offered will be book-entry or certificated securities.
Certificated Debt Securities
Those who hold certificated debt securities may
transfer or exchange such debt securities at the trustee’s office or at the paying agent’s office or agency in accordance
with the terms of the indenture. There will be no service charge for any transfer or exchange of certificated debt securities, but there
may be a requirement to pay an amount sufficient to cover any tax or other governmental charge payable in connection with such transfer
or exchange.
Those who hold certificated debt securities may
effect the transfer of certificated debt securities and of the right to receive the principal of, premium, and/or interest, if any, on
the certificated debt securities only by surrendering the certificate representing the certificated debt securities and having us or the
trustee issue a new certificate to the new holder.
Global Securities
If we decide to issue debt securities in the form
of one or more global securities, then we will register the global securities in the name of the depositary for the global securities
or the nominee of the depositary, and the global securities will be delivered by the trustee to the depositary for credit to the accounts
of the holders of beneficial interests in the debt securities.
The prospectus supplement will describe the specific
terms of the depositary arrangement for debt securities of a series that are issued in global form. None of us, the trustee, any payment
agent, or the security registrar will have any responsibility or liability for any aspect of the records relating to or payments made
on account of beneficial ownership interests in a global debt security or for maintaining, supervising, or reviewing any records relating
to these beneficial ownership interests.
No Protection in the Event of Change of Control
The indenture does not have any covenants or other
provisions providing for a put or increased interest or otherwise that would afford holders of debt securities additional protection in
the event of a recapitalization transaction, a change of control of our company, or a highly leveraged transaction. If we offer any covenants
or provisions of this type with respect to any debt securities covered by this prospectus, we will describe them in the applicable prospectus
supplement.
Covenants
Unless otherwise indicated in this prospectus
or a prospectus supplement, the debt securities will not have the benefit of any covenants that limit or restrict our business or operations,
the pledging of our assets, or the incurrence by us of indebtedness. We will describe in the applicable prospectus supplement any material
covenants in respect of a series of debt securities.
Consolidation, Merger, and Sale of Assets
We will agree in the indenture that we will not
consolidate with or merge into any other person, or convey, transfer, sell, or lease our properties and assets substantially as an entirety
to any person, unless:
| ● | the person formed by the consolidation
or into or with which we are merged or the person to which our properties and assets are conveyed, transferred, sold, or leased, is a
corporation organized and existing under the laws of the United States, any state, or the District of Columbia, or a corporation or comparable
legal entity organized under the laws of a foreign jurisdiction and, if we are not the surviving person, the surviving person has expressly
assumed all of our obligations, including the payment of the principal of, and premium, if any, and interest on the debt securities and
the performance of the other covenants under the indenture; and |
| ● | immediately after giving effect
to the transaction, no event of default, and no event which, after notice or lapse of time or both, would become an event of default,
has occurred and is continuing under the indenture. |
Events of Default
Unless otherwise specified in the applicable prospectus
supplement, the following events will be events of default under the indenture with respect to debt securities of any series:
| |
● |
we fail to pay any principal or premium, if any, when it becomes due and such default is not cured within 5 business days; |
| |
● |
we fail to pay any interest within 30 days after it becomes due; |
| |
● |
we fail to comply with any other covenant in the debt securities or the indenture for 60 days after written notice specifying the failure from the trustee or the holders of not less than 25% in aggregate principal amount of the outstanding debt securities of that series; and |
| |
● |
certain events involving bankruptcy, insolvency, or reorganization of our company or any of our significant subsidiaries. |
The trustee may withhold notice to the holders
of the debt securities of any series of any default, except in payment of principal of, or premium, if any, or interest on the debt securities
of a series, if the trustee considers it to be in the best interest of the holders of the debt securities of that series to do so.
If an event of default (other than an event of
default resulting from certain events of bankruptcy, insolvency, or reorganization) occurs, and is continuing, then the trustee or the
holders of not less than 25% in aggregate principal amount of the outstanding debt securities of any series may accelerate the maturity
of the debt securities. If this happens, the entire principal amount, plus the premium, if any, of all the outstanding debt securities
of the affected series plus accrued interest to the date of acceleration will be immediately due and payable. At any time after the acceleration,
but before a judgment or decree based on such acceleration is obtained by the trustee, the holders of a majority in aggregate principal
amount of outstanding debt securities of such series may rescind and annul such acceleration if:
| |
● |
all events of default (other than nonpayment of accelerated principal, premium, or interest) have been cured or waived; |
| |
● |
all lawful interest on overdue interest and overdue principal has been paid; and |
| |
● |
the rescission would not conflict with any judgment or decree. |
In addition, if the acceleration occurs at any
time when we have outstanding indebtedness which is senior to the debt securities, the payment of the principal amount of outstanding
debt securities may be subordinated in right of payment to the prior payment of any amounts due under the senior indebtedness, in which
case the holders of debt securities will be entitled to payment under the terms prescribed in the instruments evidencing the senior indebtedness
and the indenture.
If an event of default resulting from certain
events of bankruptcy, insolvency, or reorganization occurs, the principal, premium, and interest amount with respect to all of the debt
securities of any series will be due and payable immediately without any declaration or other act on the part of the trustee or the holders
of the debt securities of that series.
The holders of a majority in principal amount
of the outstanding debt securities of a series will have the right to waive any existing default or compliance with any provision of the
indenture or the debt securities of that series and to direct the time, method, and place of conducting any proceeding for any remedy
available to the trustee, subject to certain limitations specified in the indenture.
No holder of any debt security of a series will
have any right to institute any proceeding with respect to the indenture or for any remedy under the indenture, unless:
| |
● |
the holder gives to the trustee written notice of a continuing event of default; |
| |
● |
the holders of at least 25% in aggregate principal amount of the outstanding debt securities of the affected series make a written request and offer reasonable indemnity to the trustee to institute a proceeding as trustee; |
| |
● |
the trustee fails to institute a proceeding within 60 days after such request; and |
| |
● |
the holders of a majority in aggregate principal amount of the outstanding debt securities of the affected series do not give the trustee a direction inconsistent with such request during such 60-day period. |
These limitations do not, however, apply to a
suit instituted for payment on debt securities of any series on or after the due dates expressed in the debt securities.
Modification and Waiver
From time to time, we and the trustee may, without
the consent of holders of the debt securities of one or more series, amend the indenture or the debt securities of one or more series,
or supplement the indenture, for certain specified purposes, including:
| |
● |
to provide that the surviving entity following a change of control of our company permitted under the indenture will assume all of our obligations under the indenture and debt securities; |
| |
● |
to provide for certificated debt securities in addition to uncertificated debt securities; |
| |
● |
to comply with any requirements of the SEC under the Trust Indenture Act of 1939; |
| |
● |
to cure any ambiguity, defect, or inconsistency, or make any other change that does not materially and adversely affect the rights of any holder; and |
| |
● |
to appoint a successor trustee under the indenture with respect to one or more series. |
From time to time, we and the trustee may, with
the consent of holders of at least a majority in principal amount of the outstanding debt securities, amend or supplement the indenture
or the debt securities, or waive compliance in a particular instance by us with any provision of the indenture or the debt securities.
We may not, however, without the consent of each holder affected by such action, modify or supplement the indenture or the debt securities,
or waive compliance with any provision of the indenture or the debt securities in order to:
| |
● |
reduce the amount of debt securities whose holders must consent to an amendment, supplement, or waiver to the indenture or such debt security; |
| |
● |
reduce the rate of or change the time for payment of interest; |
| |
● |
reduce the principal of or change the stated maturity of the debt securities; |
| |
● |
make any debt security payable in money other than that stated in the debt security; |
| |
● |
change the amount or time of any payment required, or reduce the premium payable upon any redemption, or change the time before which no such redemption may be made; |
| |
● |
waive a default in the payment of the principal of, premium, if any, or interest on the debt securities or a redemption payment; or |
| |
● |
take any other action otherwise prohibited by the indenture to be taken without the consent of each holder affected by the action. |
Defeasance of Debt Securities and Certain Covenants in Certain Circumstances
The indenture will permit us, at any time, to
elect to discharge our obligations with respect to one or more series of debt securities by following certain procedures described in
the indenture. These procedures will allow us either:
| |
● |
to defease and be discharged from any and all of our obligations with respect to any debt securities except for the following obligations (which discharge is referred to as “legal defeasance”): |
| |
(1) |
to register the transfer or exchange of such debt securities; |
| |
|
|
| |
(2) |
to replace temporary or mutilated, destroyed, lost, or stolen debt securities; |
| |
|
|
| |
(3) |
to compensate and indemnify the trustee; or |
| |
|
|
| |
(4) |
to maintain an office or agency in respect of the debt securities and to hold monies for payment in trust; or |
| |
● |
to be released from our obligations with respect to the debt securities under certain covenants contained in the indenture, as well as any additional covenants which may be contained in the applicable supplemental indenture (which release is referred to as “covenant defeasance”). |
In order to exercise either defeasance option,
we must deposit with the trustee or other qualifying trustee, in trust for that purpose:
| |
● |
U.S. Government Obligations (as described below) or Foreign Government Obligations (as described below), which through the scheduled payment of principal and interest in accordance with their terms will provide money; or |
| |
● |
a combination of money and/or U.S. Government Obligations and/or Foreign Government Obligations sufficient in the written opinion of a nationally-recognized firm of independent accountants to provide money; |
which in each case specified above, provides a
sufficient amount to pay the principal of, premium, if any, and interest, if any, on the debt securities of the series, on the scheduled
due dates, or on a selected date of redemption in accordance with the terms of the indenture.
In addition, defeasance may be effected only if,
among other things:
| |
● |
in the case of either legal or covenant defeasance, we deliver to the trustee an opinion of counsel, as specified in the indenture, stating that as a result of the defeasance neither the trust nor the trustee will be required to register as an investment company under the Investment Company Act of 1940; |
| |
● |
in the case of legal defeasance, we deliver to the trustee an opinion of counsel stating that we have received from, or there has been published by, the Internal Revenue Service a ruling to the effect that, or there has been a change in any applicable federal income tax law with the effect that (and the opinion shall confirm that), the holders of outstanding debt securities will not recognize income, gain, or loss for U.S. federal income tax purposes solely as a result of such legal defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner, including as a result of prepayment, and at the same times as would have been the case if legal defeasance had not occurred; |
| |
● |
in the case of covenant defeasance, we deliver to the trustee an opinion of counsel to the effect that the holders of the outstanding debt securities will not recognize income, gain, or loss for U.S. federal income tax purposes as a result of covenant defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner, and at the same times as would have been the case if covenant defeasance had not occurred; and |
| |
● |
certain other conditions described in the indenture are satisfied. |
If we fail to comply with our remaining obligations
under the indenture and applicable supplemental indenture after a covenant defeasance of the indenture and applicable supplemental indenture,
and the debt securities are declared due and payable because of the occurrence of any undefeased event of default, the amount of money
and/or U.S. Government Obligations and/or Foreign Government Obligations on deposit with the trustee could be insufficient to pay amounts
due under the debt securities of the affected series at the time of acceleration. We will, however, remain liable in respect of these
payments.
The term “U.S. Government Obligations”
as used in the above discussion means securities that are direct obligations of or non-callable obligations guaranteed by the United States
of America for the payment of which obligation or guarantee the full faith and credit of the United States of America is pledged.
The term “Foreign Government Obligations”
as used in the above discussion means, with respect to debt securities of any series that are denominated in a currency other than U.S.
dollars (1) direct obligations of the government that issued or caused to be issued such currency for the payment of which obligations
its full faith and credit is pledged or (2) obligations of a person controlled or supervised by or acting as an agent or instrumentality
of such government the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by that government,
which in either case under clauses (1) or (2), are not callable or redeemable at the option of the issuer.
Regarding the Trustee
We will identify the trustee with respect to any
series of debt securities in the prospectus supplement relating to the applicable debt securities. You should note that if the trustee
becomes a creditor of our company, the indenture and the Trust Indenture Act of 1939 limit the rights of the trustee to obtain payment
of claims in certain cases, or to realize on certain property received in respect of any such claim, as security or otherwise. The trustee
and its affiliates may engage in, and will be permitted to continue to engage in, other transactions with us and our affiliates. If, however,
the trustee acquires any “conflicting interest” within the meaning of the Trust Indenture Act of 1939, it must eliminate such
conflict or resign.
The holders of a majority in principal amount
of the then outstanding debt securities of any series may direct the time, method, and place of conducting any proceeding for exercising
any remedy available to the trustee. If an event of default occurs and is continuing, the trustee, in the exercise of its rights and powers,
must use the degree of care and skill of a prudent person in the conduct of his or her own affairs. Subject to that provision, the trustee
will be under no obligation to exercise any of its rights or powers under the indenture at the request of any of the holders of the debt
securities, unless they have offered to the trustee reasonable indemnity or security.
DESCRIPTION OF RIGHTS
General
We may issue rights to our stockholders to purchase
shares of our common stock, preferred stock or the other securities described in this prospectus. We may offer rights separately or together
with one or more additional rights, debt securities, preferred stock, common stock or warrants, or any combination of those securities
in the form of units, as described in the applicable prospectus supplement. Each series of rights will be issued under a separate rights
agreement to be entered into between us and a bank or trust company, as rights agent. The rights agent will act solely as our agent in
connection with the certificates relating to the rights of the series of certificates and will not assume any obligation or relationship
of agency or trust for or with any holders of rights certificates or beneficial owners of rights. The following description sets forth
certain general terms and provisions of the rights to which any prospectus supplement may relate. The particular terms of the rights to
which any prospectus supplement may relate and the extent, if any, to which the general provisions may apply to the rights so offered
will be described in the applicable prospectus supplement. To the extent that any particular terms of the rights, rights agreement or
rights certificates described in a prospectus supplement differ from any of the terms described below, then the terms described below
will be deemed to have been superseded by that prospectus supplement. We encourage you to read the applicable rights agreement and rights
certificate for additional information before you decide whether to purchase any of our rights. We will provide in a prospectus supplement
the following terms of the rights being issued:
| |
● |
the date of determining the stockholders entitled to the rights distribution; |
| |
● |
the aggregate number of shares of common stock, preferred stock or other securities purchasable upon exercise of the rights; |
| |
● |
the aggregate number of rights issued; |
| |
● |
whether the rights are transferrable and the date, if any, on and after which the rights may be separately transferred; |
| |
● |
the date on which the right to exercise the rights will commence, and the date on which the right to exercise the rights will expire; |
| |
● |
the method by which holders of rights will be entitled to exercise; |
| |
● |
the conditions to the completion of the offering, if any; |
| |
● |
the withdrawal, termination and cancellation rights, if any; |
| |
● |
whether there are any backstop or standby purchaser or purchasers and the terms of their commitment, if any; |
| |
● |
whether stockholders are entitled to oversubscription rights, if any; |
| |
● |
any applicable material United States federal income tax considerations; and |
| |
● |
any other terms of the rights, including terms, procedures and limitations relating to the distribution, exchange and exercise of the rights, as applicable. |
Each right will entitle the holder of rights to
purchase for cash the principal amount of shares of common stock, preferred stock or other securities at the exercise price provided in
the applicable prospectus supplement. Rights may be exercised at any time up to the close of business on the expiration date for the rights
provided in the applicable prospectus supplement.
Holders may exercise rights as described in the
applicable prospectus supplement. Upon receipt of payment and the rights certificate properly completed and duly executed at the corporate
trust office of the rights agent or any other office indicated in the prospectus supplement, we will, as soon as practicable, forward
the shares of common stock, preferred stock or other securities, as applicable, purchasable upon exercise of the rights. If less than
all of the rights issued in any rights offering are exercised, we may offer any unsubscribed securities directly to persons other than
stockholders, to or through agents, underwriters or dealers or through a combination of such methods, including pursuant to standby arrangements,
as described in the applicable prospectus supplement.
Rights Agent
The rights agent for any rights we offer will
be set forth in the applicable prospectus supplement.
DESCRIPTION OF UNITS
This section outlines some of the provisions of
the units and the unit agreements that we may enter into. This information may not be complete in all respects and is qualified entirely
by reference to the unit agreement with respect to the units of any particular series. The specific terms of any series of units will
be described in the applicable prospectus supplement. If so described in a particular supplement, the specific terms of any series of
units may differ from the general description of terms presented below.
We may issue units comprised of one or more debt
securities, shares of common stock, shares of preferred stock, and warrants in any combination. Each unit will be issued so that the holder
of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations
of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the
unit may not be held or transferred separately, at any time or at any time before a specified date.
The applicable prospectus supplement may describe:
| |
● |
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| |
● |
any provisions of the governing unit agreement that differ from those described below; and |
| |
● |
any provisions for the issuance, payment, settlement, transfer, or exchange of the units or of the securities comprising the units. |
The provisions described in this section, as well
as those described under “Description of Common Stock,” “Description of Preferred Stock,” “Description of
Warrants,” and “Description of Debt Securities” will apply to the securities included in each unit, to the extent relevant.
Issuance in Series
We may issue units in such amounts and in as many
distinct series as we wish. This section summarizes terms of the units that apply generally to all series. Most of the financial and other
specific terms of your series will be described in the applicable prospectus supplement.
Unit Agreements
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any units we issue pursuant to this prospectus. We will issue the units under one or
more unit agreements to be entered into between us and a bank or other financial institution, as unit agent. We may add, replace, or terminate
unit agents from time to time. We will identify the unit agreement under which each series of units will be issued and the unit agent
under that agreement in the applicable prospectus supplement.
The following provisions will generally apply
to all unit agreements unless otherwise stated in the applicable prospectus supplement.
Enforcement of Rights
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any units we issue pursuant to this prospectus. The unit agent under a unit agreement
will act solely as our agent in connection with the units issued under that agreement. The unit agent will not assume any obligation or
relationship of agency or trust for or with any holders of those units or of the securities comprising those units. The unit agent will
not be obligated to take any action on behalf of those holders to enforce or protect their rights under the units or the included securities.
Except as indicated in the next paragraph, a holder
of a unit may, without the consent of the unit agent or any other holder, enforce its rights as holder under any security included in
the unit, in accordance with the terms of that security and the indenture, warrant agreement, rights agreement or other instrument under
which that security is issued. Those terms are described elsewhere in this prospectus under the sections relating to debt securities,
preferred stock, common stock, or warrants, as relevant.
Notwithstanding the foregoing, a unit agreement
may limit or otherwise affect the ability of a holder of units issued under that agreement to enforce its rights, including any right
to bring a legal action, with respect to those units or any securities, other than debt securities, that are included in those units.
Limitations of this kind will be described in the applicable prospectus supplement.
Modification without Consent of Holders.
Unless otherwise provided in the applicable prospectus supplement, the following provisions will apply to any units we issue pursuant
to this prospectus. We and the applicable unit agent may amend any unit or unit agreement without the consent of any holder:
| |
● |
to correct or supplement any defective or inconsistent provision; or |
| |
● |
to make any other change that we believe is necessary or desirable and will not adversely affect the interests of the affected holders in any material respect. |
We do not need any approval to make changes that
affect only units to be issued after the changes take effect. We may also make changes that do not adversely affect a particular unit
in any material respect, even if they adversely affect other units in a material respect. In those cases, we do not need to obtain the
approval of the holder of the unaffected unit; we need only obtain any required approvals from the holders of the affected units.
Modification with Consent of Holders.
Unless otherwise provided in the applicable prospectus supplement, the following provisions will apply to any units we issue pursuant
to this prospectus. We may not amend any particular unit or a unit agreement with respect to any particular unit unless we obtain the
consent of the holder of that unit, if the amendment would:
| |
● |
impair any right of the holder to exercise or enforce any right under a security included in the unit if the terms of that security require the consent of the holder to any changes that would impair the exercise or enforcement of that right; or |
| |
● |
reduce the percentage of outstanding units or any series or class the consent of whose holders is required to amend that series or class, or the applicable unit agreement with respect to that series or class, as described below. |
Any other change to a particular unit agreement and the units issued
under that agreement would require the following approval:
| |
● |
if the change affects only the units of a particular series issued under that agreement, the change must be approved by the holders of a majority of the outstanding units of that series; or |
| |
● |
if the change affects the units of more than one series issued under that agreement, it must be approved by the holders of a majority of all outstanding units of all series affected by the change, with the units of all the affected series voting together as one class for this purpose. |
These provisions regarding changes with majority approval also apply
to changes affecting any securities issued under a unit agreement, as the governing document.
In each case, the required approval must be given
by written consent.
Unit Agreements Will Not Be Qualified Under
Trust Indenture Act. No unit agreement will be qualified as an indenture, and no unit agent will be required to qualify as a trustee,
under the Trust Indenture Act. Therefore, holders of units issued under unit agreements will not have the protections of the Trust Indenture
Act with respect to their units.
Mergers and Similar Transactions Permitted;
No Restrictive Covenants or Events of Default
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any units we issue pursuant to this prospectus. The unit agreements will not restrict
our ability to merge or consolidate with, or sell our assets to, another corporation or other entity or to engage in any other transactions.
If at any time we merge or consolidate with, or sell our assets substantially as an entirety to, another corporation or other entity,
the successor entity will succeed to and assume our obligations under the unit agreements. We will then be relieved of any further obligation
under these agreements.
The unit agreements will not include any restrictions
on our ability to put liens on our assets, including our interests in our subsidiaries, nor will they restrict our ability to sell our
assets. The unit agreements also will not provide for any events of default or remedies upon the occurrence of any events of default.
Governing Law
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any units we issue pursuant to this prospectus. The unit agreements and the units will
be governed by Delaware or New York law as decided by the Company at the time of issuance.
Form, Exchange, and Transfer
Unless otherwise provided in the applicable prospectus
supplement, the following provisions will apply to any units we issue pursuant to this prospectus. We will issue each unit in global—that
is, book-entry—form only. Units in book-entry form will be represented by a global security registered in the name of a depositary,
which will be the holder of all the units represented by the global security. Those who own beneficial interests in a unit will do so
through participants in the depositary’s system, and the rights of these indirect owners will be governed solely by the applicable
procedures of the depositary and its participants.
In addition, we will issue each unit in registered
form, unless we say otherwise in the applicable prospectus supplement. Bearer securities would be subject to special provisions, as we
describe below under “Securities Issued in Bearer Form.”
Each unit and all securities comprising the unit
will be issued in the same form.
If we issue any units in registered, non-global
form, the following will apply to them.
The units will be issued in the denominations
stated in the applicable prospectus supplement. Holders may exchange their units for units of smaller denominations or combined into fewer
units of larger denominations, as long as the total amount is not changed.
| |
● |
Holders may exchange or transfer their units at the office of the unit agent. Holders may also replace lost, stolen, destroyed, or mutilated units at that office. We may appoint another entity to perform these functions or perform them ourselves. |
| |
● |
Holders will not be required to pay a service charge to transfer or exchange their units, but they may be required to pay for any tax or other governmental charge associated with the transfer or exchange. The transfer or exchange, and any replacement, will be made only if our transfer agent is satisfied with the holder’s proof of legal ownership. The transfer agent may also require an indemnity before replacing any units. |
| |
● |
If we have the right to redeem, accelerate, or settle any units before their maturity, and we exercise our right as to less than all those units or other securities, we may block the exchange or transfer of those units during the period beginning 15 days before the day we mail the notice of exercise and ending on the day of that mailing, in order to freeze the list of holders to prepare the mailing. We may also refuse to register transfers of or exchange any unit selected for early settlement, except that we will continue to permit transfers and exchanges of the unsettled portion of any unit being partially settled. We may also block the transfer or exchange of any unit in this manner if the unit includes securities that are or may be selected for early settlement. |
Only the depositary will be entitled to transfer or exchange a unit
in global form, since it will be the sole holder of the unit.
Payments and Notices
In making payments and giving notices with respect
to our units, we will follow the procedures we plan to use with respect to our debt securities, where applicable.
PLAN OF DISTRIBUTION
We and any selling security holders may sell the
securities covered by this prospectus directly to purchasers or through underwriters, broker-dealers, or agents, who may receive compensation
in the form of discounts, concessions, or commissions from us. These discounts, concessions, or commissions as to any particular underwriter,
broker-dealer, or agent may be in excess of those customary in the types of transactions involved. In addition, we may issue the securities
as a dividend or distribution or in a subscription rights offering to our existing security holders.
The securities may be sold in one or more transactions
at fixed prices, at prevailing market prices at the time of sale, at varying prices determined at the time of sale, or at negotiated prices.
These sales may be effected in transactions which may involve crosses or block transactions.
If underwriters are used in an offering of securities,
such offered securities may be resold in one or more transactions:
| |
● |
on any national securities exchange or quotation service on which the common stock or the preferred stock may be listed or quoted at the time of sale, including, as of the date of this prospectus, the Nasdaq Capital Market in the case of the common stock; |
| |
● |
in the over-the-counter market; |
| |
● |
in transactions otherwise than on these exchanges or services or in the over-the-counter market; or |
| |
● |
through the writing of options, whether the options are listed on an options exchange or otherwise. |
Each prospectus supplement will state the terms of the offering, including,
but not limited to:
| |
● |
the names of any underwriters, dealers, or agents; |
| |
● |
the public offering or purchase price of the securities and the net proceeds that we will receive from the sale; |
| |
● |
any underwriting discounts and commissions or other items constituting underwriters’ compensation; |
| |
● |
any discounts, commissions, or fees allowed or paid to dealers or agents; and |
| |
● |
any securities exchange on which the offered securities may be listed. |
If we sell securities to underwriters, we will
execute an underwriting agreement with them at the time of the sale and will name them in the applicable prospectus supplement. In connection
with these sales, the underwriters may be deemed to have received compensation in the form of underwriting discounts and commissions.
The underwriters also may receive commissions from purchasers of securities for whom they may act as agent. Unless we specify otherwise
in the applicable prospectus supplement, the underwriters will not be obligated to purchase the securities unless the conditions set forth
in the underwriting agreement are satisfied, and if the underwriters purchase any of the securities offered by such prospectus supplement,
they will be required to purchase all of such offered securities. The underwriters may acquire the securities for their own account and
may resell the securities from time to time in one or more transactions, including negotiated transactions, at a fixed public offering
price or varying prices determined at the time of sale. The underwriters may sell the securities to or through dealers, and those dealers
may receive discounts, concessions, or commissions from the underwriters as well as from the purchasers for whom they may act as agent.
We may designate agents who agree to use their
reasonable efforts to solicit purchasers for the period of their appointment or to sell securities on a continuing basis. We may also
sell securities directly to one or more purchasers without using underwriters or agents.
Under agreements entered into with us, underwriters
and agents may be entitled to indemnification by us against certain civil liabilities, including liabilities under the Securities Act,
or to contribution for payments the underwriters or agents may be required to make. The underwriters, agents, and their affiliates may
engage in financial or other business transactions with us and our subsidiaries in the ordinary course of business.
The aggregate proceeds to us from the sale of the securities will be
the purchase price of the securities less discounts and commissions, if any.
In order to comply with the securities laws of
certain states, if applicable, any securities covered by this prospectus must be sold in such jurisdictions only through registered or
licensed brokers or dealers. In addition, in certain states securities may not be sold unless they have been registered or qualified for
sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
In order to facilitate the offering of the securities,
any underwriters may engage in transactions that stabilize, maintain, or otherwise affect the price of the securities or any other securities
the prices of which may be used to determine payments on such securities. Specifically, any underwriters may overallot in connection with
the offering, creating a short position for their own accounts. In addition, to cover overallotments or to stabilize the price of the
securities or of any such other securities, the underwriters may bid for, and purchase, the securities or any such other securities in
the open market. Finally, in any offering of the securities through a syndicate of underwriters, the underwriting syndicate may reclaim
selling concessions allowed to an underwriter or a dealer for distributing the securities in the offering if the syndicate repurchases
previously distributed securities in transactions to cover syndicate short positions, in stabilization transactions, or otherwise. Any
of these activities may stabilize or maintain the market price of the securities above independent market levels. Any such underwriters
are not required to engage in these activities and may end any of these activities at any time.
The applicable prospectus supplement may provide
that the original issue date for your securities may be more than three scheduled business days after the trade date for your securities.
Accordingly, in such a case, if you wish to trade securities on any date prior to the third business day before the original issue date
for your securities, you will be required, by virtue of the fact that your securities initially are expected to settle in more than three
scheduled business days after the trade date for your securities, to make alternative settlement arrangements to prevent a failed settlement.
The securities may be new issues of securities
and may have no established trading market. The securities may or may not be listed on a national securities exchange. We can make no
assurance as to the liquidity of or the existence of trading markets for any of the securities.
In order to comply with the securities laws of
some states, if applicable, the shares of common stock offered by this prospectus must be sold in such jurisdictions only through registered
or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied
with.
To the extent required, this prospectus may be
amended or supplemented from time to time to describe a specific plan of distribution.
Transfer Agent and Registrar
The transfer agent and registrar for our common
stock is VStock Transfer, LLC.
LEGAL MATTERS
The validity of the securities that may be offered
hereby will be passed upon for us by Carmel, Milazzo & Feil LLP, New York, New York. Additional legal matters may be passed upon for
us or any underwriters, dealers, or agents by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements of Tenon
Medical, Inc. as of and for the years ended December 31, 2022 and 2021 have been incorporated by reference herein and in the registration
statement in reliance on the report of Armanino LLP, an independent registered public accounting firm, given on the authority of said
firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the information requirements
of the Exchange Act and file annual, quarterly, and special reports, proxy statements, and other information with the SEC. Our SEC filings
are available to you on the SEC’s website at www.sec.gov. You may also obtain information about us by visiting our website
at https://www.tenonmed.com. The information contained on or accessible through our website is not incorporated by reference and
is not part of this prospectus.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to
incorporate by reference much of the information that we file with the SEC, which means that we can disclose important information to
you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus is considered
to be part of this prospectus. This prospectus incorporates by reference the documents listed below (other than any portions of such documents
that are not deemed “filed” under the Exchange Act in accordance with the Exchange Act and applicable SEC rules):
| |
● |
our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 10, 2023; |
| |
● |
our Quarterly Report on Form 10-Q for the period ended March 31, 2023, filed with the SEC on May 9, 2023; |
| |
● |
the description of our common stock contained in the Registration Statement on Form 8A12B (File No. 001-41364) relating thereto, filed on April 26, 2022, including any amendment or report filed for the purpose of updating such description; and |
| |
● |
any future filings made with the SEC under Section 13(a), 13(c) or 15(d) of the Exchange Act. |
Certain statements in
and portions of this prospectus update and replace information in the above listed documents incorporated by reference. Likewise, statements
in or portions of a future document incorporated by reference in this prospectus may update and replace statements in and portions of
this prospectus or the above listed documents.
We will provide you without
charge, upon your written or oral request, a copy of any of the documents incorporated by reference in this prospectus, other than exhibits
to such documents which are not specifically incorporated by reference into such documents. Please direct your written or telephone requests
to:
Tenon Medical, Inc.
Attn: Chief Financial
Officer
104 Cooper Court
Los Gatos, California
95032
Telephone: (408) 649-5760
.

TENON MEDICAL, INC.
Up to [*] Shares of
Common Stock
PRELIMINARY PROSPECTUS
SUPPLEMENT
Maxim Group LLC
September ,
2023
Tenon Medical (NASDAQ:TNON)
Graphique Historique de l'Action
De Oct 2024 à Nov 2024

Tenon Medical (NASDAQ:TNON)
Graphique Historique de l'Action
De Nov 2023 à Nov 2024



