Innate Pharma Shares Rise 8% After Cancer Treatment Gets Fast Track Designation
08 Juin 2023 - 4:53PM
Dow Jones News
By Chris Wack
Innate Pharma shares were up 8% to $3.25 after Sanofi said that
the U.S. Food and Drug Administration has granted Fast Track
Designation for SAR'579/IPH6101 for the treatment of hematological
malignancies.
Fast Track Designation is an FDA process designed to facilitate
the development, and expedite the review of, medicines to treat
serious conditions and fill unmet medical need.
SAR'579 is a trifunctional anti-CD123 cell engager from a joint
research collaboration between Innate Pharma and Sanofi, now under
development by partner Sanofi.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
June 08, 2023 10:38 ET (14:38 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
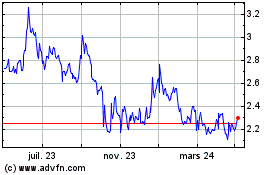
Innate Pharma (EU:IPH)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
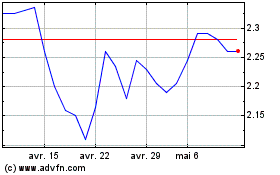
Innate Pharma (EU:IPH)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024