Press release Biocartis Group NV: Biocartis and APIS Assay
Technologies Sign Collaboration to Develop and Commercialise a
Breast Cancer Subtyping Test on the Idylla™ platform
PRESS RELEASE: 4 April 2023, 07:00 CEST
Biocartis and APIS Assay Technologies
Sign Collaboration to Develop and
Commercialise
a Breast Cancer Subtyping Test on the
Idylla™ platform
Mechelen, Belgium, 4
April 2023 - Biocartis
Group NV (the ‘Company’ or ‘Biocartis’), an innovative molecular
diagnostics company (Euronext Brussels: BCART), and APIS Assay
Technologies Ltd., a private UK based company specializing in
molecular diagnostics, today announce that they have entered into a
new partnership agreement which targets the development of APIS’
Breast Cancer Subtyping assay on Biocartis’ rapid and easy-to-use
molecular diagnostics platform Idylla™.
Under the terms of the partnership agreement,
APIS will lead the development of the Breast Cancer Subtyping test
on Idylla™, while Biocartis will lead the commercialisation through
its growing Idylla™ network.
Breast cancer is the most commonly diagnosed
cancer among women, accounting for 11.7% of all cancer cases
globally1. In 2020, it was estimated that there were over 2.2
million new cases of breast cancer worldwide1. Invasive breast
cancer is classified into distinct categories with differing tumour
behaviour and prognosis2. Based on the expression of hormone
receptors that are present in breast cancer cells (HER2, ER, PR)3
and a proliferation marker (Ki67)4, the main molecular subtypes of
invasive breast cancer can be distinguished.5 The presence or
absence of these markers can guide the selection of appropriate
treatment options. ER and PR are important indicators of how well a
breast cancer tumour will respond to hormone therapy. HER2, on the
other hand, is an important predictor of response to targeted
therapy, such as trastuzumab6. The detection of these markers is
routinely performed with IHC7.
APIS’ current Breast Cancer Subtyping Kit is an
RNA-based diagnostic assay for detecting mRNA expression of
standard biomarkers (HER2, ER, PR, Ki67) and novel proliferative
biomarkers from pre-operative core-needle biopsy (CNB) or resected
formalin-fixed paraffin-embedded (FFPE) breast tumour tissue. The
test aims to address a number of unmet needs in current practice,
including improving reproducibility and accuracy in the Ki67
proliferation measurement and assessment of low HER2 expression
status. The latter is especially important as recent studies have
shown HER2-low patients to be responsive to a new category of HER2
targeting therapies.8,9
Currently, the APIS Breast Cancer Subtyping Kit
is available as a manual kit for in vitro diagnostic use10, mainly
addressing centralized expert laboratories. The kit is currently
offered by APIS in the UK and will be broadly commercialised11 by
Biocartis ahead of the Idylla™ version of the assay becoming
available. While the manual kit already offers a reduced time for
results interpretation (as compared to current IHC based
workflows), the Idylla™ version of the Breast Cancer Subtyping
assay will further benefit from the workflow and decentralization
advantages of the Idylla™ platform. This is expected to allow for
the fastest time to results and for improved access to the most
accurate biomarker results for patients worldwide.
Herman Verrelst, Chief Executive Officer
of Biocartis, commented: “We are excited about partnering
with APIS Assay Technologies to improve molecular classification
for breast cancer patients. The APIS team is highly experienced in
IVD development and will lead the porting of the assay to our
Idylla™ platform. Our sales team has become highly proficient in
distribution of manual kits across its extensive laboratory
network, while developing Idylla™ version of partner assays. The
combination of the APIS Breast Cancer Subtyping test with the
Idylla™ PIK3CA-AKT1 Mutation Assay that is under development will
allow us to offer a complementary set of assays in the breast
cancer domain.”
Joachim Schorr, Chief Executive Officer
of APIS Assay Technologies, added: “We are very much
looking forward to our collaboration with the Biocartis team. The
Idylla™ platform and its all-in-one cartridge-based tests provide
an optimised, fully automated solution for fast and effective
treatment selection for breast cancer patients. Furthermore,
Biocartis’ global presence will further allow our innovative
solution to benefit breast cancer patients worldwide. The
collaboration with Biocartis will provide the ability to perform
Breast Cancer Subtyping analysis also directly in pathology labs
utilising the Idylla™ platform’s ease of use and integrated FFPE
sample-to-result performance.”
----- END ----
More information: Renate
DegraveHead of Corporate Communications & Investor Relations
Biocartise-mail rdegrave@biocartis.com tel
+32 15 631 729
mobile +32 471 53 60 64
About Biocartis
Biocartis (Euronext Brussels: BCART) is an
innovative molecular diagnostics (MDx) company providing next
generation diagnostic solutions aimed at improving clinical
practice for the benefit of patients, clinicians, payers and
industry. Biocartis' proprietary MDx Idylla™ platform is a fully
automated sample-to-result, real-time PCR (Polymerase Chain
Reaction) system that offers accurate, highly reliable molecular
information from virtually any biological sample in virtually any
setting. Biocartis is developing and marketing a continuously
expanding test menu addressing key unmet clinical needs, with a
focus in oncology, which represents the fastest growing segment of
the MDx market worldwide. Today, Biocartis offers tests supporting
melanoma, colorectal and lung cancer, as well as for COVID-19, flu,
RSV and sepsis. More information: www.biocartis.com. Follow us
on Twitter: @Biocartis_,Facebook or LinkedIn
About APIS
APIS is leveraging systems biology,
interrogating multi-OMICs biodata, and deploying innovative
‘Clickmer’ ligand binding technology, for the validation and
translation of biomarker and therapeutic assets into clinical
utility. APIS has deep expertise and capabilities in IVD
development of molecular & immune assays for ultimate product
realization as diagnostic tests. In addition, APIS' expertise in
bioinformatics and software development is offered as an agile
service to our clients, to develop bespoke, end-to-end multi-OMICs
solutions and platform development. The new APIS Breast Cancer
Subtyping Kit is a highly reproducible, RT-qPCR based IVD product
(in certain territories10) and RUO product for detecting standard
(HER2, ER, PR, Ki67) and novel proliferative biomarkers. The
results of this assay can help direct clinicians to the right
treatment, more quickly, with higher accuracy. For more
information, visit https://www.apisassay.com/, or follow APIS on
Twitter @ApisAssay or LinkedIn.
Biocartis and Idylla™ are registered trademarks
in Europe, the United States and other countries. The Biocartis and
Idylla™ trademark and logo are used trademarks owned by Biocartis.
Please refer to the product labeling for applicable intended uses
for each individual Biocartis product.
This press release is not for distribution,
directly or indirectly, in any jurisdiction where to do so would be
unlawful. Any persons reading this press release should inform
themselves of and observe any such restrictions. Biocartis takes no
responsibility for any violation of any such restrictions by any
person. This press release does not constitute an offer or
invitation for the sale or purchase of securities in any
jurisdiction. No securities of Biocartis may be offered or sold in
the United States of America absent registration with the United
States Securities and Exchange Commission or an exemption from
registration under the U.S. Securities Act of 1933, as amended.
Forward-looking statements
Certain statements, beliefs and opinions in this
press release are forward-looking, which reflect the Company's or,
as appropriate, the Company directors' or managements' current
expectations and projections concerning future events such as the
Company's results of operations, financial condition, liquidity,
performance, prospects, growth, strategies and the industry in
which the Company operates. By their nature, forward-looking
statements involve a number of risks, uncertainties, assumptions
and other factors that could cause actual results or events to
differ materially from those expressed or implied by the
forward-looking statements. These risks, uncertainties, assumptions
and factors could adversely affect the outcome and financial
effects of the plans and events described herein. A multitude of
factors including, but not limited to, changes in demand,
competition and technology, can cause actual events, performance or
results to differ significantly from any anticipated development.
Forward-looking statements contained in this press release
regarding past trends or activities are not guarantees of future
performance and should not be taken as a representation that such
trends or activities will continue in the future. In addition, even
if actual results or developments are consistent with the
forward-looking statements contained in this press release, those
results or developments may not be indicative of results or
developments in future periods. No representations and warranties
are made as to the accuracy or fairness of such forward-looking
statements. As a result, the Company expressly disclaims any
obligation or undertaking to release any updates or revisions to
any forward-looking statements in this press release as a result of
any change in expectations or any change in events, conditions,
assumptions or circumstances on which these forward-looking
statements are based, except if specifically required to do so by
law or regulation. Neither the Company nor its advisers or
representatives nor any of its subsidiary undertakings or any such
person's officers or employees guarantees that the assumptions
underlying such forward-looking statements are free from errors nor
does either accept any responsibility for the future accuracy of
the forward-looking statements contained in this press release or
the actual occurrence of the forecasted developments. You should
not place undue reliance on forward-looking statements, which speak
only as of the date of this press release.
1 WHO Globocan;
https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf2
13th St. Gallen International Breast Cancer Conference 2013, Expert
panel consensus opinion3 ER: estrogen receptor, PR: progesterone
receptor, HER2: human epidermal growth factor receptor 24 Ki67:
marker of proliferation Ki-675 Four main molecular subtypes:
Luminal A, Luminal B, HER2, and Basal-like (triple negative)6
Trastuzumab, sold among others under the brand name Herceptin®, is
a monoclonal antibody used to treat HER2-positive breast cancer7
IHC or Immunohistochemistry is a laboratory method that uses
antibodies to check for certain antigens (markers) in a sample of
tissue. Source: https://www.cancer.gov/ 8 Trastuzumab:deruxtecan
(Enhertu®), an antibody-drug conjugate, is the first HER2-directed
therapy approved for patients with HER2-low metastatic breast
cancer9 Nicolò et al. The HER2-low revolution in breast oncology:
steps forward and emerging challenges Ther Adv Med Oncol (2023) 15:
1758835923115284210 Registered as IVD in the UK, awaiting CE
marking under EU IVD Regulation11 In the European Union and
selected export markets
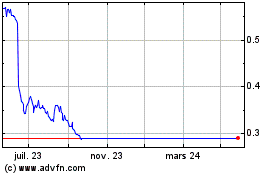
Biocartis Group NV (EU:BCART)
Graphique Historique de l'Action
De Déc 2024 à Jan 2025
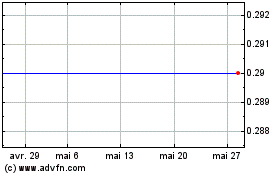
Biocartis Group NV (EU:BCART)
Graphique Historique de l'Action
De Jan 2024 à Jan 2025