Amylyx Pharmaceuticals to Host Virtual Webcast to Discuss Interim Data from Phase 2 HELIOS Study of AMX0035 in Wolfram Syndrome on April 10, 2024
08 Avril 2024 - 3:00PM
Business Wire
Amylyx Pharmaceuticals, Inc. (Nasdaq: AMLX) (“Amylyx” or the
“Company”) today announced the Company will host a virtual webcast
with management and Fumihiko Urano, MD, PhD, Principal Investigator
of the HELIOS clinical trial and the Samuel E. Schechter Professor
of Medicine in the Division of Endocrinology, Metabolism &
Lipid Research at Washington University School of Medicine in St.
Louis to discuss interim data from HELIOS, a Phase 2 trial of
AMX0035 for the treatment of Wolfram syndrome on April 10, 2024 at
1:30pm ET.
A live webcast of the presentation can be accessed under “Events
and Presentations” in the Investor section of the Company’s
website, https://investors.amylyx.com/news-events/events, and will
be available for replay for 90 days following the event.
Dr. Fumihiko Urano is a physician and medical researcher
specializing in Wolfram syndrome. Dr. Urano serves as a Professor
of Medicine and of Pathology & Immunology and is the Samuel E.
Schechter Professor of Medicine at Washington University School of
Medicine in St. Louis. Dr. Urano is a driving force in the study of
Wolfram syndrome and related disorders, including WFS1-related
disorders/Wolfram-like disorders. As director of the Wolfram
Syndrome Clinic and the Wolfram Syndrome International Registry
& Clinical Study at Washington University, Dr. Urano treats
patients with Wolfram syndrome and related disorders, and leads
basic science, clinical, translational, and interventional studies
of Wolfram syndrome and related disorders.
About the HELIOS Trial
The HELIOS trial (NCT05676034) is a 12-participant, open-label,
proof of biology, Phase 2 trial designed to study the effect of
AMX0035 on safety and tolerability, and various measures of
endocrinological, neurological, and ophthalmologic function in
adult participants living with Wolfram syndrome.
About Wolfram Syndrome
Wolfram syndrome is an autosomal recessive neurodegenerative
disease characterized by childhood-onset diabetes, optic nerve
atrophy, and neurodegeneration. Common manifestations of Wolfram
syndrome include diabetes mellitus, optic nerve atrophy, central
diabetes insipidus, sensorineural deafness, neurogenic bladder, and
progressive neurologic difficulties. Genetic and experimental
evidence suggests that endoplasmic reticulum (ER) dysfunction is a
critical pathogenic component of Wolfram syndrome. The prognosis of
Wolfram syndrome is poor, and many people with the disease die
prematurely with severe neurological disabilities.
About AMX0035
AMX0035 is an oral, fixed-dose combination of sodium
phenylbutyrate (PB) and taurursodiol (TURSO; also known as
ursodoxicoltaurine outside of the U.S.). AMX0035 was designed to
slow or mitigate neurodegeneration by targeting endoplasmic
reticulum (ER) stress and mitochondrial dysfunction, two connected
central pathways that lead to cell death and neurodegeneration. We
believe that our proprietary combination of PB and TURSO and their
complementary mechanisms of action will allow us to synergistically
target abnormal cell death to better prevent neurodegeneration than
treatment targeted at either mechanism of action alone. AMX0035 is
being studied as a potential treatment for Wolfram syndrome and
progressive supranuclear palsy, two neurodegenerative diseases.
About Amylyx Pharmaceuticals
Amylyx Pharmaceuticals, Inc. is committed to supporting and
creating more moments for the neurodegenerative disease community
through the discovery and development of innovative new treatments.
Amylyx is headquartered in Cambridge, Massachusetts. For more
information, visit amylyx.com and follow us on LinkedIn and X,
(formerly Twitter). For investors, please visit
investors.amylyx.com.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240408195225/en/
Media Amylyx Media Team +1 (857) 799-7274
amylyxmediateam@amylyx.com
Investors Lindsey Allen Amylyx Pharmaceuticals, Inc. +1 (857)
320-6244 Investors@amylyx.com
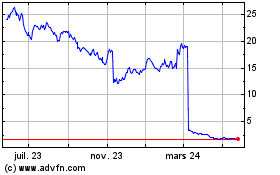
Amylyx Pharmaceuticals (NASDAQ:AMLX)
Graphique Historique de l'Action
De Nov 2024 à Déc 2024
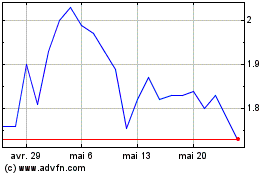
Amylyx Pharmaceuticals (NASDAQ:AMLX)
Graphique Historique de l'Action
De Déc 2023 à Déc 2024