0001501796false00015017962024-11-122024-11-12
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): November 12, 2024 |
Aura Biosciences, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-40971 |
32-0271970 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
80 Guest Street |
|
Boston, Massachusetts |
|
02135 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 617 500-8864 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.00001 par value per share |
|
AURA |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On November 12, 2024, Aura Biosciences, Inc. (the “Company”) issued a press release announcing its financial results for the quarter ended September 30, 2024. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 2.02, including Exhibit 99.1 hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01 Other Events.
On November 12, 2024, the Company updated its corporate presentation for use in meetings with investors, analysts, and others. A copy of the corporate presentation is filed as Exhibit 99.2 for purposes of Section 18 of the Exchange Act.
Forward Looking Statements
Statements contained under this Item 8.01 regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements about the initiation, timing, progress, results, and cost of the Company’s research and development programs and the Company’s current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and the Company’s research and development programs; statements regarding the Company’s expectations for an improved quality of life of patients after treatment with bel-sar and changes to the treatment paradigm for patients; the Company’s ability to successfully manufacture its drug substances and product candidates for preclinical use, for clinical trials and on a larger scale for commercial use, if approved; the ability and willingness of the Company’s third-party strategic collaborators to continue research and development activities relating to the Company’s development candidates and product candidates; the Company’s ability to commercialize its products, if approved; the Company’s ability to obtain funding for its operations necessary to complete further development and commercialization of its product candidates; the Company’s ability to obtain and maintain regulatory approval of its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to serve those markets; the Company’s financial performance; the Company’s expected cash runway into the second half of 2026; and the implementation of the Company’s business model, including strategic plans for its business and product candidates.
Any forward-looking statements are neither promises nor guarantees, and investors should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond the Company’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements, including, without limitation, uncertainties inherent in clinical trials and in the availability and timing of data from ongoing clinical trials; the expected timing for submissions for regulatory approval or review by governmental authorities; the risk that the results of the Company’s preclinical and clinical trials may not be predictive of future results in connection with future clinical trials; the risk that interim data from ongoing clinical trials may not be predictive of final data from completed clinical trials; the risk that governmental authorities may disagree with the Company’s clinical trial designs even where the Company has obtained agreement with governmental authorities on the design of such trials, such as the Phase 3 Special Protocol agreement with the United States Food and Drug Administration; whether the Company will receive regulatory approvals to conduct trials or to market products; whether the Company’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; the Company’s ongoing and planned preclinical activities; and the Company’s ability to initiate, enroll, conduct or complete ongoing and planned clinical trials. These risks, uncertainties, and other factors include those risks and uncertainties described under the heading “Risk Factors” in the Company’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the United States Securities and Exchange Commission (“SEC”) and in subsequent filings made by the Company with the SEC, which are available on the SEC’s website at www.sec.gov. Except as required by law, the Company disclaims any intention or responsibility for updating or revising any forward-looking statements contained under this Item 8.01 in the event of new information, future developments or otherwise. These forward-looking statements are based on the Company’s current expectations and speak only as of the date hereof and no representations or warranties (express or implied) are made about the accuracy of any such forward-looking statements.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Aura Biosciences, Inc. |
|
|
|
|
Date: |
November 12, 2024 |
By: |
/s/ Amy Elazzouzi |
|
|
|
Amy Elazzouzi
Vice President, Finance (Interim Principal Financial Officer and Interim Principal Accounting Officer) |
Exhibit 99.1

Aura Biosciences Reports Third Quarter 2024 Financial Results and Business Highlights
Positive Phase 2 End of Study Data with Bel-sar in Early-Stage Choroidal Melanoma; Ongoing Phase 3 CoMpass Trial Recently Received Authorization to Start Enrolling Patients in Europe
Multiple Clinical Complete Responses Observed with Single Low Dose of Bel-sar in Ongoing Phase 1 Trial in Non-Muscle Invasive Bladder Cancer (NMIBC); Phase 1 Expansion Preparation in Progress
Strong Cash Position Expected to Support Operations into 2H 2026
BOSTON, MA – November 12, 2024 – Aura Biosciences, Inc. (NASDAQ: AURA), a clinical-stage biotechnology company developing precision therapies for solid tumors designed to preserve organ function, today reported financial results for the third quarter ended September 30, 2024, and provided recent business highlights.
“This is a transformative time for our Company, as we presented the first positive data in NMIBC, which we believe provides clinical evidence of the potential of bel-sar in solid tumors beyond the eye,” said Elisabet de los Pinos, PhD, Chief Executive Officer of Aura Biosciences. “We believe that bel-sar’s innovative mechanism of action may provide the first immune-ablative treatment in bladder cancer, with the goal to potentially offer safe and durable responses with a focal approach that is easily delivered by urologists in the office.”
In addition to positive early data from an ongoing Phase 1 trial of bel-sar in patients with NMIBC, the Company also recently presented positive Phase 2 end of study data in early-stage choroidal melanoma and continues to progress the ongoing Phase 3 CoMpass trial.
“I am excited for bel-sar’s potential for patients who are diagnosed with indeterminate lesions or small choroidal melanoma where we currently have no good treatment options. We either wait for the disease to progress or treat with radiation, which leads to irreversible vision loss,” said Carol L. Shields, MD, Chief of the Ocular Oncology Service at Wills Eye Hospital and Professor of Ophthalmology at Thomas Jefferson University in Philadelphia. “If approved, bel-sar may represent the opportunity to treat choroidal melanoma at an earlier stage of medical intervention and set a new standard of care in a disease that has had no new therapies approved for decades.”
Recent Pipeline Developments
Early-Stage Choroidal Melanoma
Early-stage choroidal melanoma represents an area of high unmet need with no drugs approved. The Company previously received Orphan Drug Designation from the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) and Fast Track designation from the FDA for the treatment of early-stage choroidal melanoma.
Update on Ongoing Phase 3 CoMpass Trial: CoMpass is the first registration-enabling study in early-stage choroidal melanoma. The study is a global, Phase 3, randomized trial evaluating bel-sar treatment against a sham control arm and includes an enrichment strategy to enroll 100 patients with documented growth, an approach agreed upon under a Special Protocol Assessment (SPA) agreement with the FDA.
oThe Company recently received authorization from the EMA to commence the trial under the European Union (EU) Clinical Trial Regulation (CTR) process. This approval was later than anticipated due to a requirement for additional testing to support drug substance characterization that has been successfully met. This authorization permits the Company to start enrolling patients in the study in the EU. The study started enrolling in the United States in December 2023 and currently has sites activated in the United Kingdom, Australia and Canada.
oTo identify appropriate patients to meet the enrichment strategy of documented growth, the Company has enabled a pre-screening ‘run in’ period. Globally, since June 2024, investigators have registered over 100 patients in pre-screening as having met initial enrollment criteria for the study. The Company continues to monitor the overall timeline for the study, with European sites in the process of being activated.
The Company announced positive Phase 2 end of study results evaluating bel-sar as a first-line treatment for early-stage choroidal melanoma.
Clinical Data: The Phase 2 results demonstrated that bel-sar achieved an 80% tumor control rate (n=8/10) and durability of response at 12 months among Phase 3-eligible patients who received the therapeutic regimen. Visual acuity preservation was achieved in 90% of these patients. We believe the Phase 2 results are a significant achievement that support the design of the ongoing Phase 3 trial.
Safety Data: The safety profile of bel-sar was highly favorable in all participants. There were no treatment-related serious adverse events reported. Ocular treatment-related adverse events were mild (Grade 1).
Additional Ocular Oncology Indications:
In addition to early-stage choroidal melanoma, bel-sar is being explored for metastases to the choroid and cancers of the ocular surface. These three ocular oncology indications have a collective incidence of greater than 60,000 patients annually in the United States and Europe.
Metastases to the Choroid
The Company is initiating clinical development for bel-sar as a potential treatment for metastases to the choroid, an indication with high unmet medical need and no approved therapies. The Company aims to enroll the first patients in a Phase 2 trial in 2024. Metastases to the choroid represents the second potential ocular oncology indication for bel-sar, affecting approximately 20,000 patients annually in the United States and Europe. The Company previously received FDA Fast Track designation for bel-sar as a treatment in this indication.
Cancers of the Ocular Surface
The Company’s third potential ocular oncology indication is cancers of the ocular surface, which affect approximately 35,000 patients in the United States and Europe annually. The Company continues to advance its preclinical work designed to be IND-enabling in cancers of the ocular surface.
Bladder Cancer
The Company announced positive early data from an ongoing Phase 1 clinical trial of bel-sar in patients with NMIBC.
Clinical Data: In these early data from the first 8 patients treated with a single low dose of bel-sar with light activation, a clinical complete response was observed in 4 out of 5 patients with low grade disease; visual tumor shrinkage was observed on cystoscopy in 2 out of 3 patients with high grade disease where tumor cells were still present on histopathological evaluation. For this analysis, clinical complete response was defined as the absence of tumor cells on histopathologic evaluation. In addition, immune activation was noted in all patients with available immune staining in both treated target and untreated non-target bladder tumors with rapid infiltration of effector CD8+ and CD4+ T-cells within days after treatment. This data provides evidence of a bladder urothelial field effect, potentially indicating a broader immune response in the bladder beyond the target tumor in these patients.
Safety Data: In the safety analysis as of the September 9, 2024 data cut-off date (n=12), bel-sar was well-tolerated, with less than 10% Grade 1 and no Grade 2 or higher drug-related adverse events reported. No serious adverse events have been reported.
Future Development: The Company plans to continue development of bel-sar in bladder cancer with an initial focus on low-grade, intermediate risk NMIBC patients, through a planned trial expansion to test additional doses and treatment regimens with the opportunity to assess early durability of response at 3 months. In parallel, the Company is planning regulatory discussions on the design of the next trial with the goal of expediting clinical development in this patient population.
Recent Corporate Events
•The Company announced the appointment of Sabine Doris Brookman-May, MD, FEBU as the Company’s Senior Vice President, Therapeutic Area Head Urologic Oncology.
•The Company hosted a virtual ocular oncology investor event featuring Ivana Kim, MD (Mass Eye and Ear) and Prithvi Mruthyunjaya, MD, MHS (Stanford University Byers Eye Institute) to discuss the Phase 2 end of study data on Thursday, September 12, 2024. A replay of the webcast is available on the “Investors & Media” page under the “Events & Presentations” section of Aura’s website at https://ir.aurabiosciences.com/events-and-presentations.
•The Company hosted a virtual urologic oncology investor event featuring Max Kates, MD (Johns Hopkins), Joe Jacob, MD (Syracuse University), Neal Shore, MD (Carolina Urologic Research Center) and Gary Steinberg, MD (RUSH University) to discuss the early Phase 1 data on Thursday, October 17, 2024. A replay of the webcast is available on the “Investors & Media” page under the “Events & Presentations” section of Aura’s website at https://ir.aurabiosciences.com/events-and-presentations.
Third Quarter 2024 Financial Results
•As of September 30, 2024, the Company had cash and cash equivalents and marketable securities totaling $174.4 million. The Company believes its current cash and cash equivalents and marketable securities are sufficient to fund its operations into the second half of 2026.
•Research and development expenses increased to $17.0 million for the three months ended September 30, 2024 from $15.4 million for the three months ended September 30, 2023 primarily due to manufacturing and development costs for bel-sar and higher personnel expenses related to growth of the Company.
•General and administrative expenses increased to $6.2 million for the three months ended September 30, 2024 from $5.1 million for the three months ended September 30, 2023. General and administrative expenses include $1.6 million and $1.2 million of stock-based compensation for the three months ended September 30, 2024 and 2023, respectively. The increase was primarily driven by personnel expenses, as well as increases in general corporate expenses related to the growth of the Company.
•Net loss for the three months ended September 30, 2024 was $21.0 million compared to $18.5 million for the three months ended September 30, 2023.
About Aura Biosciences
Aura Biosciences is a clinical-stage biotechnology company focused on developing precision therapies for solid tumors that aim to preserve organ function. Our lead candidate, bel-sar (AU-011), is currently in late-stage development for primary choroidal melanoma and in early-stage development in other ocular oncology indications and bladder cancer. Aura Biosciences is headquartered in Boston, MA. Our mission is to grow as an innovative global oncology company that positively transforms the lives of patients.
For more information, visit aurabiosciences.com. Follow us on X (formerly Twitter) @AuraBiosciences and visit us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, and other federal securities laws. Any statements that are not statements of historical fact may be deemed to be forward-looking statements. Words such as “may,” “will,” “could,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “seeks,” “endeavor,” “potential,” “continue” or the negative of such words or other similar expressions can be used to identify forward-looking statements. These forward-looking statements include express or implied statements regarding Aura’s future expectations, plans and prospects, including, without limitation, statements regarding the therapeutic potential of bel-sar for the treatment of various cancers; statements regarding Aura’s plans and expectations for its ongoing and future clinical trials of bel-sar in various oncology indications and the preclinical development of bel-sar in cancers of the ocular surface; statements regarding Aura’s beliefs and expectations for bel-sar’s ability to provide durable responses in bladder cancer patients and choroidal melanoma patients; statements regarding Aura’s expectations for an improved quality of life of patients after treatment with bel-sar and changes to the treatment paradigm for patients; statements regarding bel-sar’s potentially immune ablative effects; statements regarding bel-sar’s safety profile; statements regarding Aura’s beliefs and expectations for the high unmet medical need for an effective local treatment in ocular and urologic oncology; statements regarding Aura’s expectations for the estimated patient populations and related market opportunities for bel-sar; statements regarding the potential for regulatory approval of bel-sar; and statements regarding the Company’s expected cash runway.
The forward-looking statements in this press release are neither promises nor guarantees, and investors should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties and other factors, many of which are beyond Aura’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements, including, without limitation, uncertainties inherent in clinical trials and in the availability and timing of data from ongoing clinical trials; the expected timing for submissions for regulatory approval or review by governmental authorities; the risk that the results of Aura’s preclinical and clinical trials may not be predictive of future results in connection with future clinical trials; the risk that early or interim data from ongoing clinical trials may not be predictive of final data from completed clinical trials; the risk that governmental authorities may disagree with Aura’s clinical trial designs even where Aura has obtained agreement with governmental authorities on the design of such trials, such as the Phase 3 SPA agreement with the FDA; whether Aura will receive regulatory approvals to conduct trials or to market products; whether Aura’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; Aura’s ongoing and planned preclinical activities; and Aura’s ability to initiate, enroll, conduct or complete ongoing and planned clinical trials. These risks, uncertainties and other factors include those risks and uncertainties described under the heading “Risk Factors” in Aura’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the United States Securities and Exchange Commission (SEC) and in subsequent filings made by Aura with the SEC, which are available on the SEC’s website at www.sec.gov. Except as required by law, Aura disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Aura’s current expectations and speak only as of the date hereof and no representations or warranties (express or implied) are made about the accuracy of any such forward-looking statements.
Investor Contact:
Alex Dasalla
Head of Investor Relations and Corporate Communications
IR@aurabiosciences.com
Media Contact:
Kimberly Ha
KKH Advisors
kimberly.ha@kkhadvisors.com
917-291-5744
Aura Biosciences, Inc.
Condensed Consolidated Statement of Operations and Comprehensive Loss
(Unaudited)
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
|
|
2024 |
|
|
2023 |
|
2024 |
|
|
2023 |
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
17,036 |
|
|
$ |
15,428 |
|
$ |
50,968 |
|
|
$ |
44,952 |
|
General and administrative |
|
|
6,196 |
|
|
|
5,060 |
|
|
17,341 |
|
|
|
15,256 |
|
Total operating expenses |
|
|
23,232 |
|
|
|
20,488 |
|
|
68,309 |
|
|
|
60,208 |
|
Total operating loss |
|
|
(23,232 |
) |
|
|
(20,488 |
) |
|
(68,309 |
) |
|
|
(60,208 |
) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
Interest income, including amortization and accretion income |
|
|
2,258 |
|
|
|
1,981 |
|
|
7,395 |
|
|
|
5,981 |
|
Other expense |
|
|
(25 |
) |
|
|
(5 |
) |
|
(83 |
) |
|
|
(50 |
) |
Total other income |
|
|
2,233 |
|
|
|
1,976 |
|
|
7,312 |
|
|
|
5,931 |
|
Loss before income taxes |
|
|
(20,999 |
) |
|
|
(18,512 |
) |
|
(60,997 |
) |
|
|
(54,277 |
) |
Income tax provision, net |
|
|
(43 |
) |
|
|
— |
|
|
(88 |
) |
|
|
— |
|
Net loss |
|
$ |
(21,042 |
) |
|
$ |
(18,512 |
) |
$ |
(61,085 |
) |
|
$ |
(54,277 |
) |
Net loss per common share—basic and diluted |
|
$ |
(0.42 |
) |
|
$ |
(0.48 |
) |
$ |
(1.23 |
) |
|
$ |
(1.43 |
) |
Weighted average common stock outstanding—basic and diluted |
|
|
49,663,532 |
|
|
|
38,185,197 |
|
|
49,554,930 |
|
|
|
37,943,139 |
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(21,042 |
) |
|
$ |
(18,512 |
) |
$ |
(61,085 |
) |
|
$ |
(54,277 |
) |
Other comprehensive items: |
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain (loss) on marketable securities |
|
|
790 |
|
|
|
89 |
|
|
68 |
|
|
|
(62 |
) |
Total other comprehensive income (loss) |
|
|
790 |
|
|
|
89 |
|
|
68 |
|
|
|
(62 |
) |
Total comprehensive loss |
|
$ |
(20,252 |
) |
|
$ |
(18,423 |
) |
$ |
(61,017 |
) |
|
$ |
(54,339 |
) |
Aura Biosciences, Inc.
Condensed Consolidated Balance Sheets
(Unaudited)
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
September 30, 2024 |
|
|
December 31, 2023 |
|
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
25,407 |
|
|
$ |
41,063 |
|
Marketable securities |
|
|
148,970 |
|
|
|
185,087 |
|
Restricted cash and deposits |
|
|
— |
|
|
|
19 |
|
Prepaid expenses and other current assets |
|
|
9,104 |
|
|
|
5,625 |
|
Total current assets |
|
|
183,481 |
|
|
|
231,794 |
|
Restricted cash and deposits, net of current portion |
|
|
768 |
|
|
|
768 |
|
Right of use assets - operating lease |
|
|
17,744 |
|
|
|
18,854 |
|
Other long-term assets |
|
|
22 |
|
|
|
509 |
|
Property and equipment, net |
|
|
3,325 |
|
|
|
3,150 |
|
Total Assets |
|
$ |
205,340 |
|
|
$ |
255,075 |
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
|
1,991 |
|
|
|
1,787 |
|
Short-term operating lease liability |
|
|
3,126 |
|
|
|
2,687 |
|
Accrued expenses and other current liabilities |
|
|
9,597 |
|
|
|
7,883 |
|
Total current liabilities |
|
|
14,714 |
|
|
|
12,357 |
|
Long-term operating lease liability |
|
|
15,958 |
|
|
|
16,870 |
|
Total Liabilities |
|
|
30,672 |
|
|
|
29,227 |
|
Commitments and Contingencies |
|
|
|
|
|
|
Stockholders’ Equity: |
|
|
|
|
|
|
Common stock, $0.00001 par value, 150,000,000 authorized at September 30, 2024 and December 31, 2023, and 49,778,861 and 49,350,788 shares issued and outstanding at September 30, 2024 and December 31, 2023, respectively |
|
|
— |
|
|
|
— |
|
Additional paid-in capital |
|
|
522,454 |
|
|
|
512,617 |
|
Accumulated deficit |
|
|
(348,393 |
) |
|
|
(287,308 |
) |
Accumulated other comprehensive income |
|
|
607 |
|
|
|
539 |
|
Total Stockholders’ Equity |
|
|
174,668 |
|
|
|
225,848 |
|
Total Liabilities and Stockholders’ Equity |
|
$ |
205,340 |
|
|
$ |
255,075 |
|

Innovating the future of cancer care to cure patients and preserve organ function November 2024 Exhibit 99.2

Legal disclosure This presentation contains forward-looking statements, all of which are qualified in their entirety by this cautionary statement. Many of the forward-looking statements contained herein can be identified by the use of forward-looking words such as "may", "anticipate", "believe", "could', "expect", "should", "plan", "intend", "estimate", "will", "potential" and "ongoing", among others, although not all forward-looking statements contain these identifying words. These forward-looking statements include statements about the initiation, timing, progress, results and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available and our research and development programs; statements regarding our expectations for an improved quality of life of patients after treatment with bel-sar and changes to the treatment paradigm for patients; our ability to successfully manufacture our drug substances and product candidates for preclinical use, for clinical trials and on a larger scale for commercial use, if approved; the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and product candidates; our ability to commercialize our products, if approved; our ability to obtain funding for our operations necessary to complete further development and commercialization of our product candidates; our ability to obtain and maintain regulatory approval of our product candidates; the size and growth potential of the markets for our product candidates and our ability to serve those markets; our financial performance; our expected cash runway into the second half of 2026; and the implementation of our business model, including strategic plans for our business and product candidates. Except as otherwise noted, these forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to update or revise any of such statements to reflect events or circumstances occurring after this presentation. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC), as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the SEC, which are available on the SEC's website at www.sec.gov. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. We caution you not to place undue reliance on the forward-looking statements contained in this presentation. This presentation discusses product candidates that are under preclinical or clinical evaluation and that have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA) or any other regulatory authority. Until finalized in a clinical study report, clinical trial data presented herein remain subject to adjustment as a result of clinical site audits and other review processes. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction.
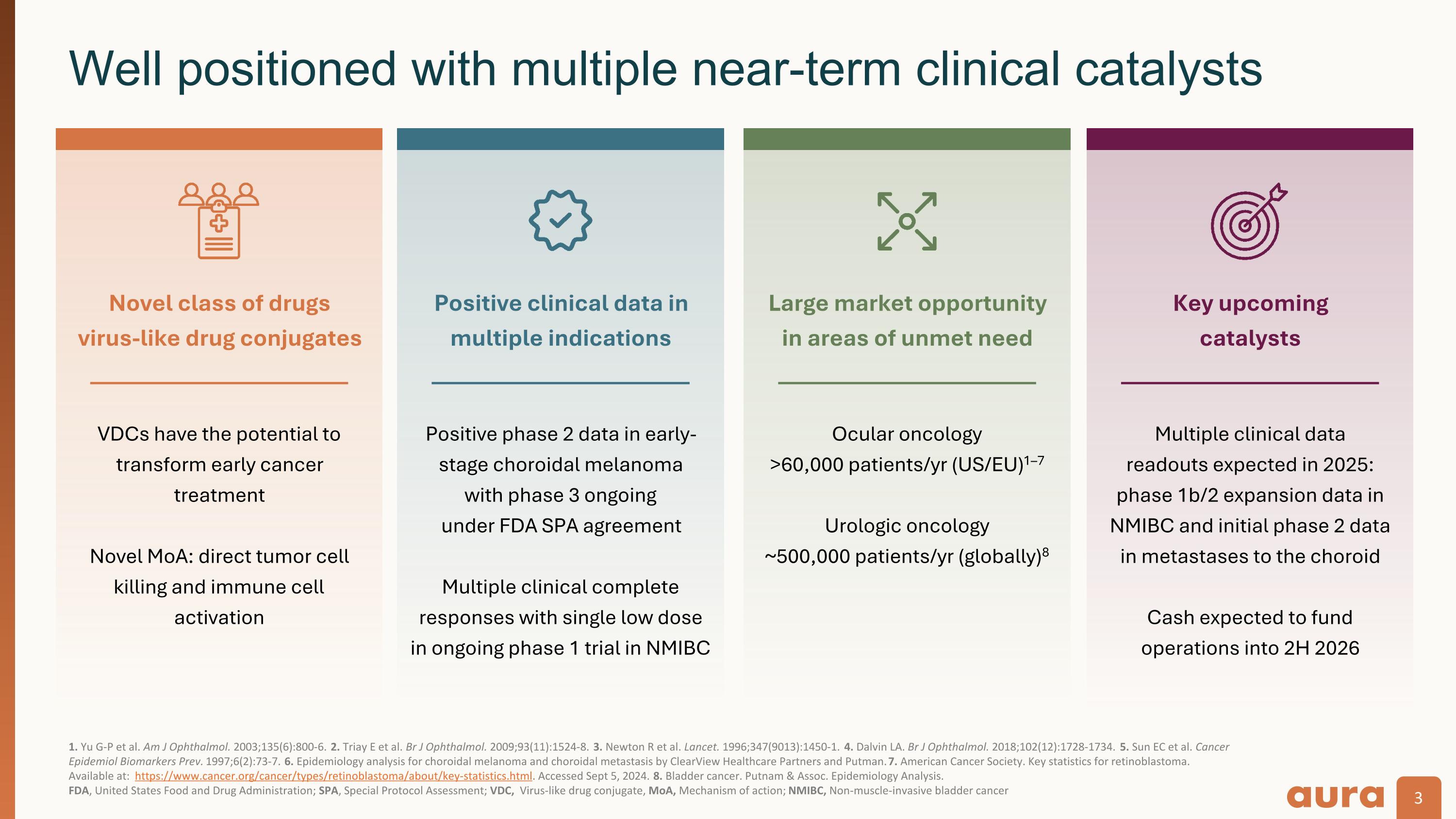
1. Yu G-P et al. Am J Ophthalmol. 2003;135(6):800-6. 2. Triay E et al. Br J Ophthalmol. 2009;93(11):1524-8. 3. Newton R et al. Lancet. 1996;347(9013):1450-1. 4. Dalvin LA. Br J Ophthalmol. 2018;102(12):1728-1734. 5. Sun EC et al. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73-7. 6. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putman. 7. American Cancer Society. Key statistics for retinoblastoma. Available at: https://www.cancer.org/cancer/types/retinoblastoma/about/key-statistics.html. Accessed Sept 5, 2024. 8. Bladder cancer. Putnam & Assoc. Epidemiology Analysis.�FDA, United States Food and Drug Administration; SPA, Special Protocol Assessment; VDC, Virus-like drug conjugate, MoA, Mechanism of action; NMIBC, Non-muscle-invasive bladder cancer Well positioned with multiple near-term clinical catalysts VDCs have the potential to transform early cancer treatment Novel MoA: direct tumor cell killing and immune cell activation Novel class of drugs virus-like drug conjugates Positive phase 2 data in early-stage choroidal melanoma with phase 3 ongoing under FDA SPA agreement Multiple clinical complete responses with single low dose in ongoing phase 1 trial in NMIBC Positive clinical data in multiple indications Ocular oncology >60,000 patients/yr (US/EU)1–7 Urologic oncology ~500,000 patients/yr (globally)8 Large market opportunity in areas of unmet need Multiple clinical data�readouts expected in 2025: phase 1b/2 expansion data in NMIBC and initial phase 2 data in metastases to the choroid Cash expected to fund�operations into 2H 2026 Key upcoming catalysts
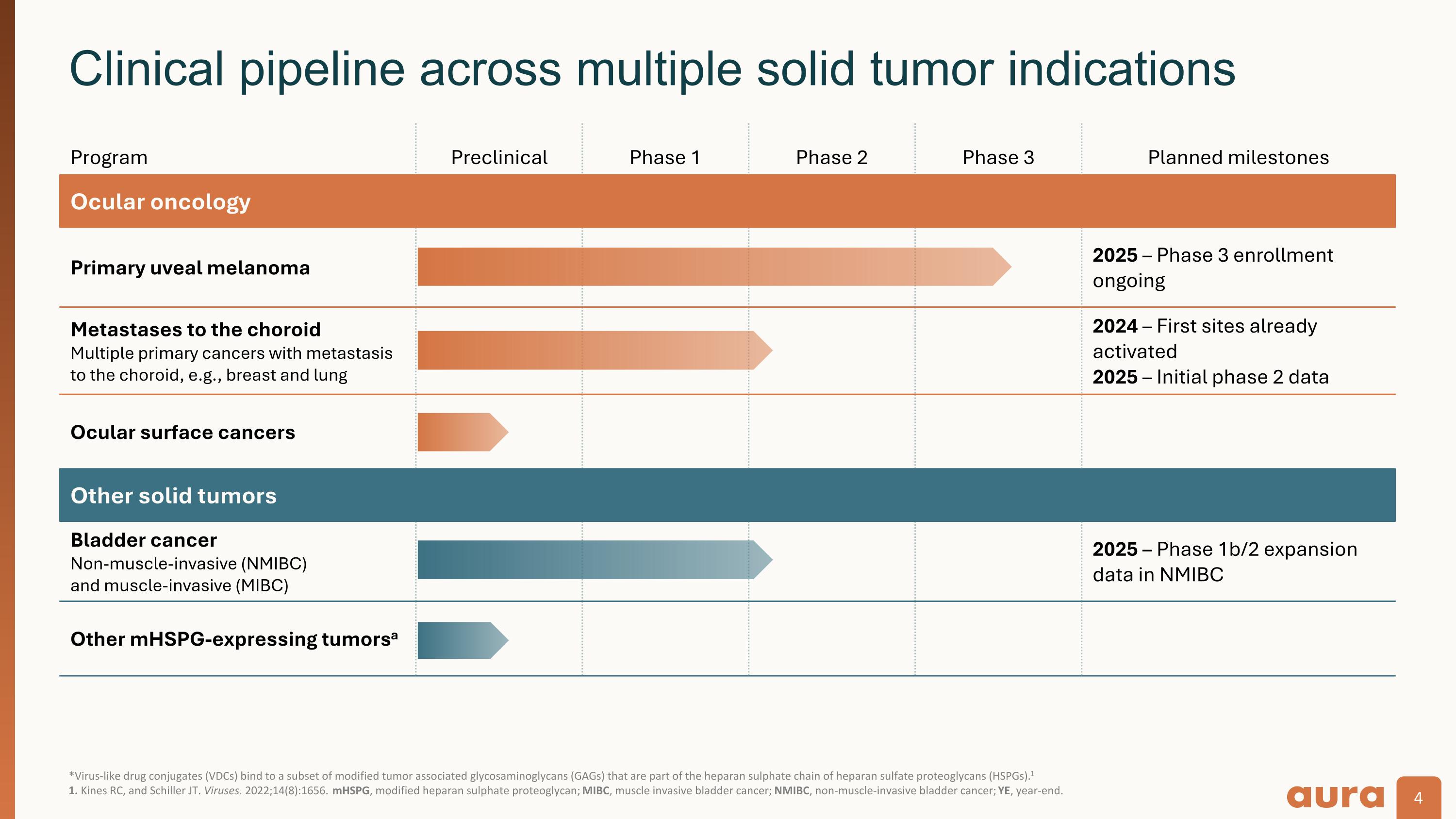
*Virus-like drug conjugates (VDCs) bind to a subset of modified tumor associated glycosaminoglycans (GAGs) that are part of the heparan sulphate chain of heparan sulfate proteoglycans (HSPGs).1�1. Kines RC, and Schiller JT. Viruses. 2022;14(8):1656. mHSPG, modified heparan sulphate proteoglycan; MIBC, muscle invasive bladder cancer; NMIBC, non-muscle-invasive bladder cancer; YE, year-end. Clinical pipeline across multiple solid tumor indications Program Preclinical Phase 1 Phase 2 Phase 3 Planned milestones Ocular oncology Primary uveal melanoma 2025 – Phase 3 enrollment ongoing Metastases to the choroid Multiple primary cancers with metastasis to the choroid, e.g., breast and lung 2024 – First sites already activated�2025 – Initial phase 2 data Ocular surface cancers Other solid tumors Bladder cancer Non-muscle-invasive (NMIBC)�and muscle-invasive (MIBC) 2025 – Phase 1b/2 expansion data in NMIBC Other mHSPG-expressing tumorsa

Bel-sar is a potential first-in-class therapy for multiple solid tumors
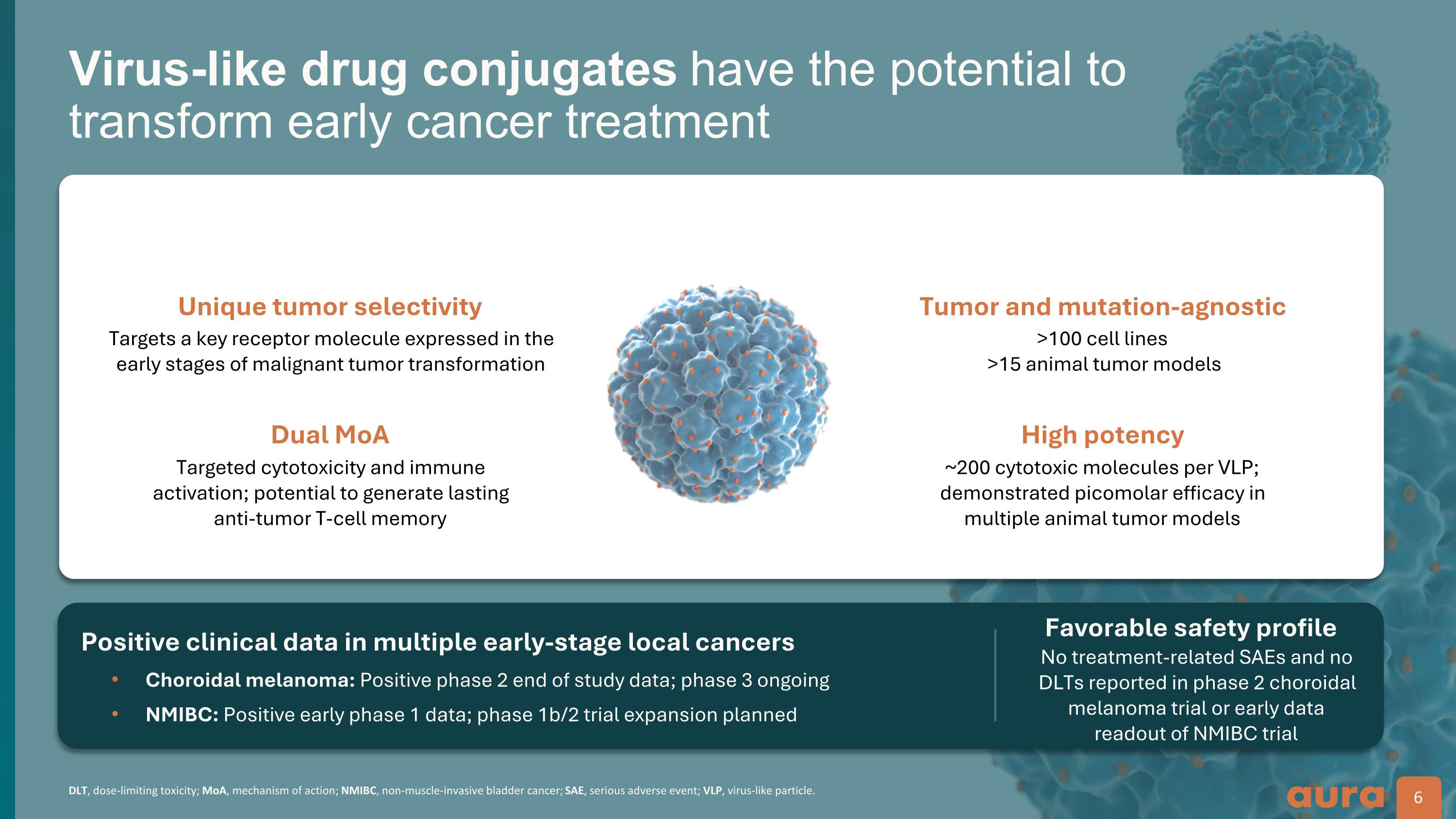
DLT, dose-limiting toxicity; MoA, mechanism of action; NMIBC, non-muscle-invasive bladder cancer; SAE, serious adverse event; VLP, virus-like particle. Virus-like drug conjugates have the potential to transform early cancer treatment 6 Positive clinical data in multiple early-stage local cancers Choroidal melanoma: Positive phase 2 end of study data; phase 3 ongoing NMIBC: Positive early phase 1 data; phase 1b/2 trial expansion planned Favorable safety profile Unique tumor selectivity Dual MoA Targets a key receptor molecule expressed in the early stages of malignant tumor transformation Targeted cytotoxicity and immune activation; potential to generate lasting anti-tumor T-cell memory Tumor and mutation-agnostic High potency >100 cell lines� >15 animal tumor models ~200 cytotoxic molecules per VLP; demonstrated picomolar efficacy in multiple animal tumor models No treatment-related SAEs and no DLTs reported in phase 2 choroidal melanoma trial or early data readout of NMIBC trial
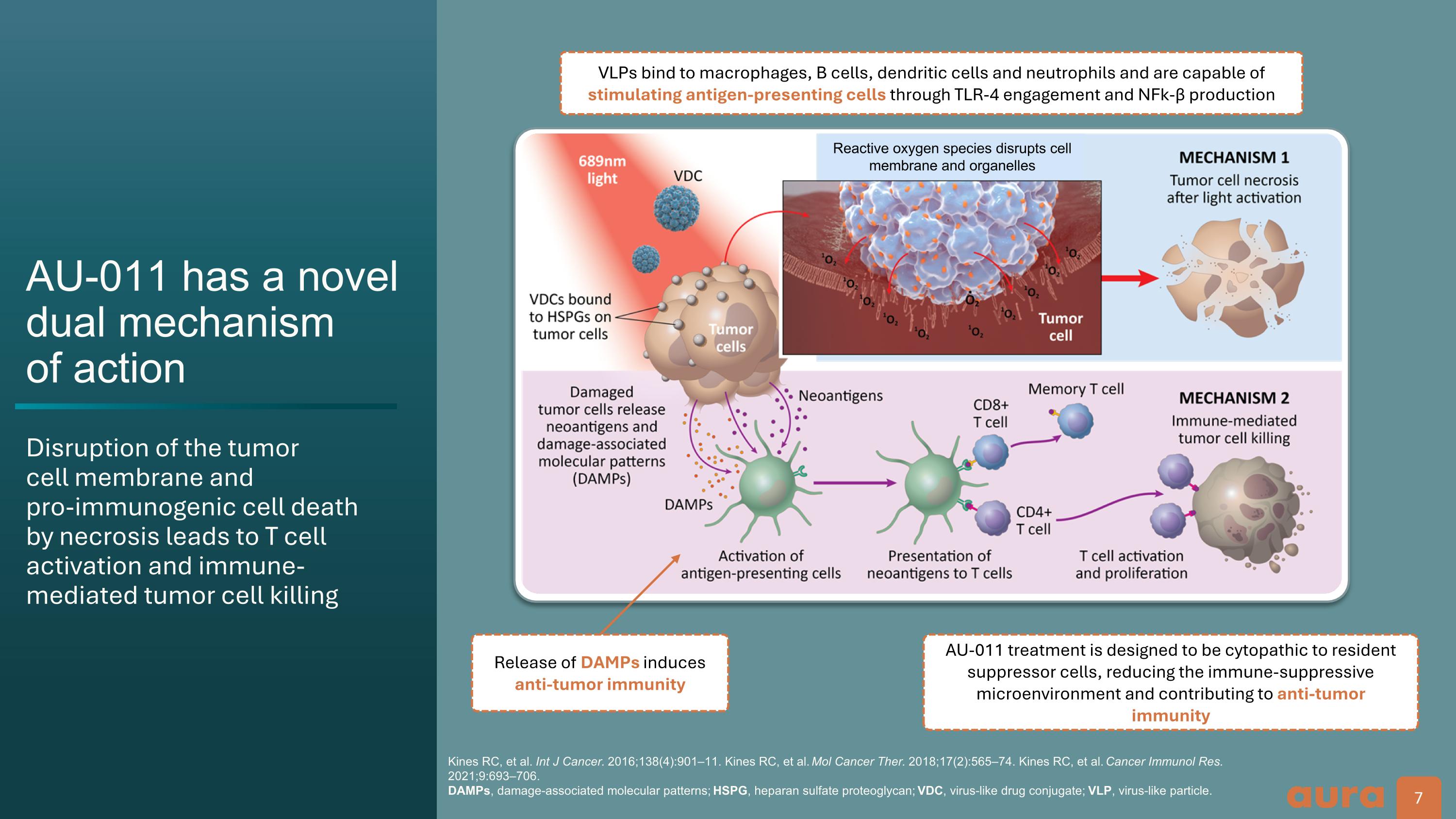
AU-011 has a novel dual mechanism�of action Disruption of the tumor�cell membrane and�pro-immunogenic cell death�by necrosis leads to T cell activation and immune-mediated tumor cell killing Kines RC, et al. Int J Cancer. 2016;138(4):901–11. Kines RC, et al. Mol Cancer Ther. 2018;17(2):565–74. Kines RC, et al. Cancer Immunol Res. 2021;9:693–706.�DAMPs, damage-associated molecular patterns; HSPG, heparan sulfate proteoglycan; VDC, virus-like drug conjugate; VLP, virus-like particle. Release of DAMPs induces anti-tumor immunity AU-011 treatment is designed to be cytopathic to resident suppressor cells, reducing the immune-suppressive microenvironment and contributing to anti-tumor immunity VLPs bind to macrophages, B cells, dendritic cells and neutrophils and are capable of stimulating antigen-presenting cells through TLR-4 engagement and NFk-β production Reactive oxygen species disrupts cell �membrane and organelles

Ocular Oncology Bel-sar target indications: Primary uveal melanoma | Metastases to the choroid | Ocular surface cancers
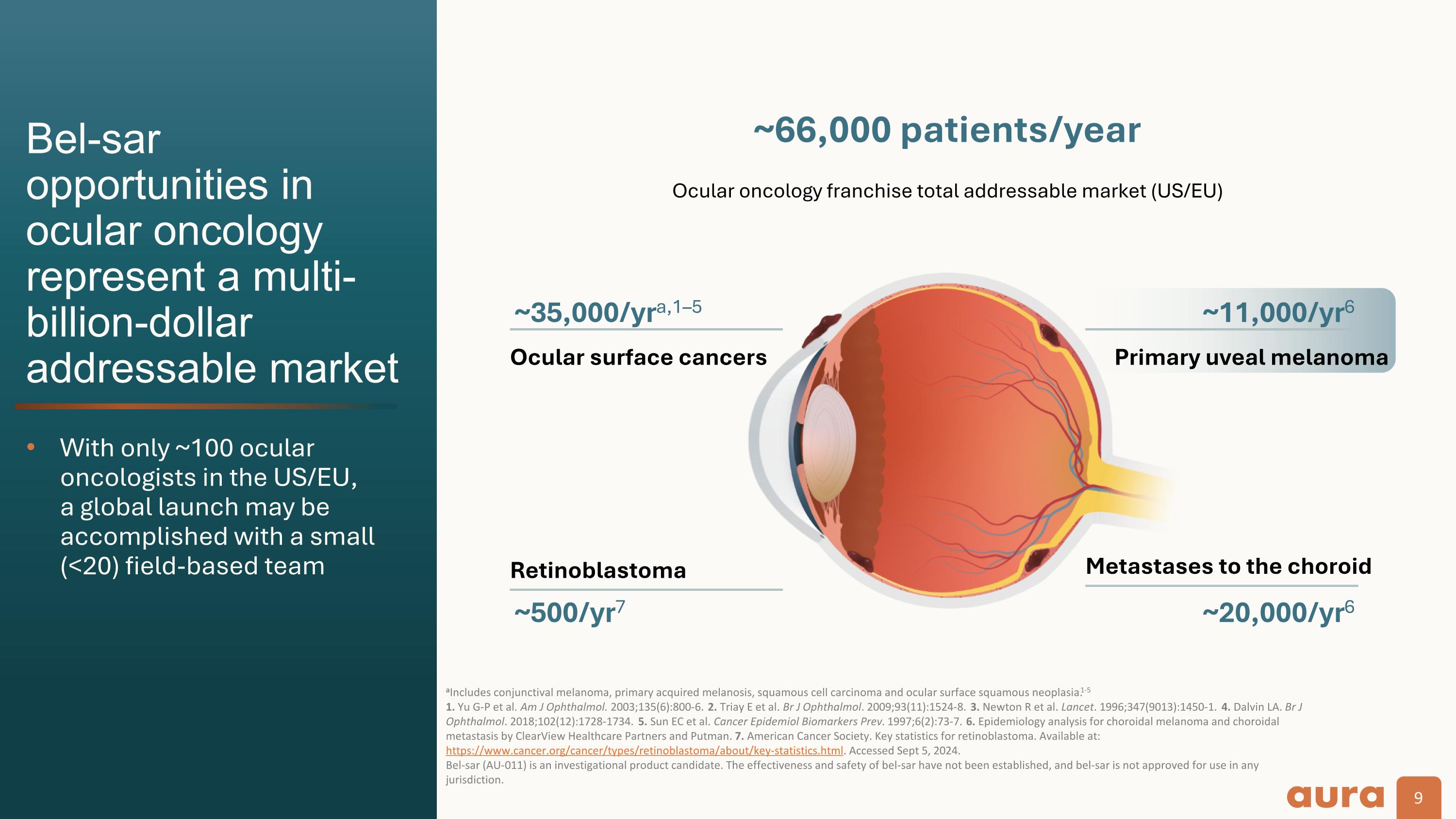
Bel-sar opportunities in ocular oncology represent a multi-billion-dollar addressable market With only ~100 ocular oncologists in the US/EU,�a global launch may be accomplished with a small�(<20) field-based team aIncludes conjunctival melanoma, primary acquired melanosis, squamous cell carcinoma and ocular surface squamous neoplasia.1-5 1. Yu G-P et al. Am J Ophthalmol. 2003;135(6):800-6. 2. Triay E et al. Br J Ophthalmol. 2009;93(11):1524-8. 3. Newton R et al. Lancet. 1996;347(9013):1450-1. 4. Dalvin LA. Br J Ophthalmol. 2018;102(12):1728-1734. 5. Sun EC et al. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73-7. 6. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putman. 7. American Cancer Society. Key statistics for retinoblastoma. Available at: https://www.cancer.org/cancer/types/retinoblastoma/about/key-statistics.html. Accessed Sept 5, 2024. Bel-sar (AU-011) is an investigational product candidate. The effectiveness and safety of bel-sar have not been established, and bel-sar is not approved for use in any jurisdiction. Ocular surface cancers ~66,000 patients/year ~35,000/yra,1–5 Primary uveal melanoma ~11,000/yr6 Metastases to the choroid ~20,000/yr6 Retinoblastoma ~500/yr7 Ocular oncology franchise total addressable market (US/EU)

Bel-sar is in phase 3 for primary uveal melanoma, the most common primary intraocular cancer�in adults Primary uveal melanoma is a high unmet medical need With no approved vision-preserving therapies, the current standard-of-care is radiotherapy – treatment that leads to legal blindness4,5 1. Heiting, G. Iris/uvea of the eye. Available at: https://www.allaboutvision.com/en-gb/resources/uvea-iris-choroid/. Accessed Oct. 3, 2023. 2. Kaliki S and Shields CL. Eye (Lond). 2017;31(2):241-257. 3. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putman. 4. Jarczak J, Karska-Basta I, Romanowska-Dixon B. Deterioration of visual acuity after brachytherapy and proton therapy of uveal melanoma, and methods of counteracting this complication based on recent publications. Medicina (Kaunas). 2023;59(6):1131. 5.. Tsui I, Beardsley RM, McCannel TA, Oliver SC, et al. Visual acuity, contrast sensitivity and color vision three years after iodine-125 brachytherapy for choroidal and ciliary body melanoma. Open Ophthalmol J. 2015;9:131-5. Choroid is 90% of the uvea1 Uvea: Choroid, ciliary body and iris Ciliary body Iris Most common primary intraocular cancer in adults2,3 50% of patients develop metastasis within 15 years (metastatic uveal melanoma)2 ~80% of patients diagnosed with early-stage disease3 Choroidal melanoma ~11,000/yr3 Bel-sar has the potential to provide a treatment option that preserves vision

aEach figure represents ~250 persons. Shields CL et al. Choroidal and ciliary body melanoma. Available at: https://eyewiki.aao.org/Choroidal_and_Ciliary_Body_Melanoma Accessed September 9, 2024. Singh AD, et al. Ophthalmology. 2005;112(10):1784–89. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putman. CM, choroidal melanoma; Enuc., enucleation. Current treatment paradigm for primary uveal melanoma Indeterminate lesions Small melanomas Risk Factors Growth Small CM Observation Incidence: Patients� US/EUa Local – Early (~8,000) Local – Late (~2,300) Metastatic (~2,000) SIZE �(mm): Small Medium Large Metastatic Radiotherapy Radiotherapy 1 2.5 – 3 >10 Enuc. Systemic chemotherapy (KIMMTRAK®)
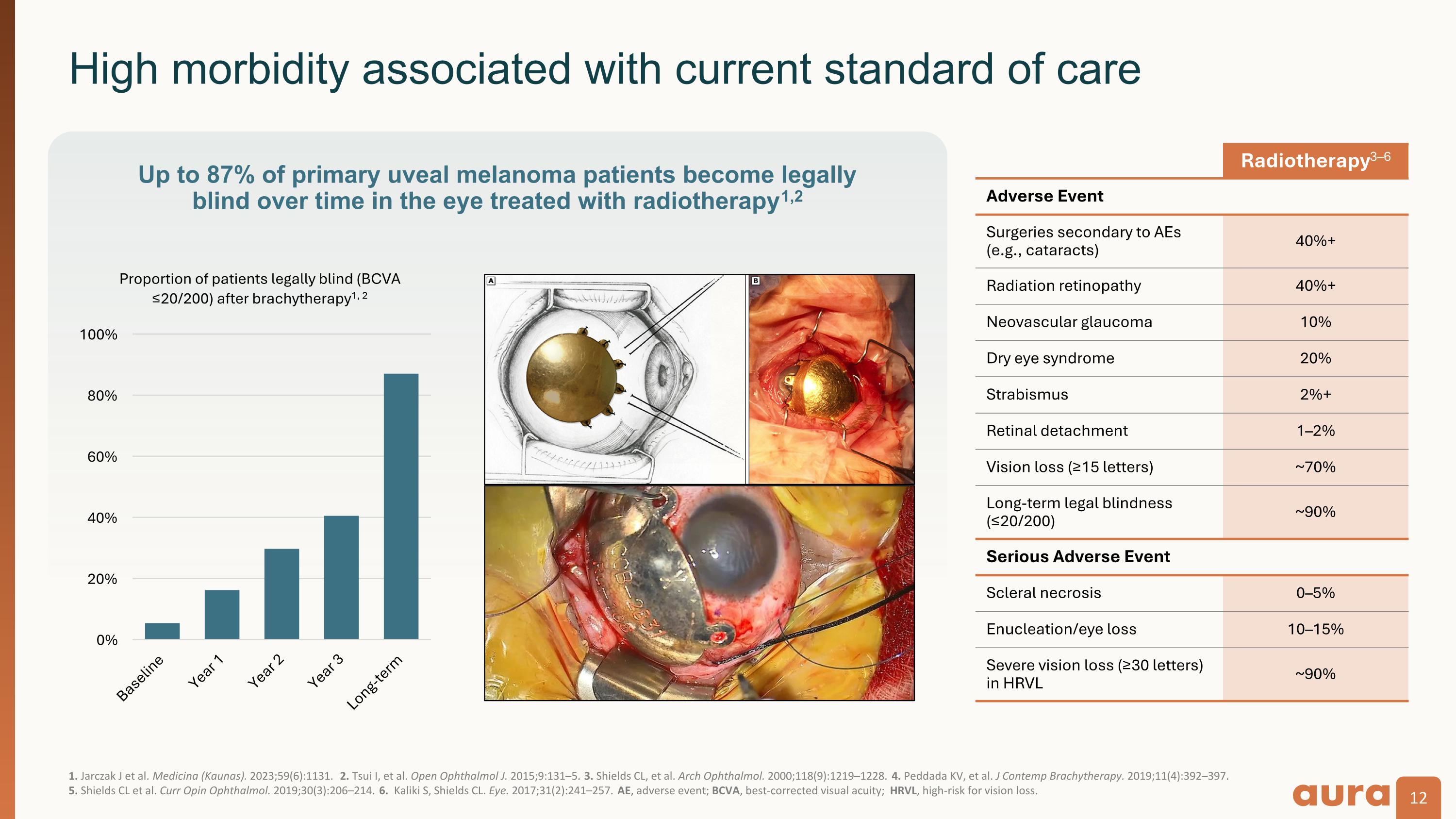
1. Jarczak J et al. Medicina (Kaunas). 2023;59(6):1131. 2. Tsui I, et al. Open Ophthalmol J. 2015;9:131–5. 3. Shields CL, et al. Arch Ophthalmol. 2000;118(9):1219–1228. 4. Peddada KV, et al. J Contemp Brachytherapy. 2019;11(4):392–397.�5. Shields CL et al. Curr Opin Ophthalmol. 2019;30(3):206–214. 6. Kaliki S, Shields CL. Eye. 2017;31(2):241–257. AE, adverse event; BCVA, best-corrected visual acuity; HRVL, high-risk for vision loss. High morbidity associated with current standard of care Up to 87% of primary uveal melanoma patients become legally blind over time in the eye treated with radiotherapy1,2 Radiotherapy3–6 Adverse Event Surgeries secondary to AEs (e.g., cataracts) 40%+ Radiation retinopathy 40%+ Neovascular glaucoma 10% Dry eye syndrome 20% Strabismus 2%+ Retinal detachment 1–2% Vision loss (≥15 letters) ~70% Long-term legal blindness (≤20/200) ~90% Serious Adverse Event Scleral necrosis 0–5% Enucleation/eye loss 10–15% Severe vision loss (≥30 letters) in HRVL ~90%

Bel-sar has the potential to be the first approved vision-preserving therapy in primary uveal melanoma No radiation-�related morbidity Vision�preservation Local tumor control Reduce metastasis�risk with early treatment Improve safety�and quality of life Treatment Goals In-office procedure Two injections (2 min. each) 30 min. apart 10-30 min. procedure Delivery via�suprachoroidal injection Light activation with standard ophthalmic laser Suprachoroidal Bel-sar (AU-011) is an investigational product candidate. The effectiveness and safety of bel-sar have not been established, and bel-sar is not approved for use in any jurisdiction.
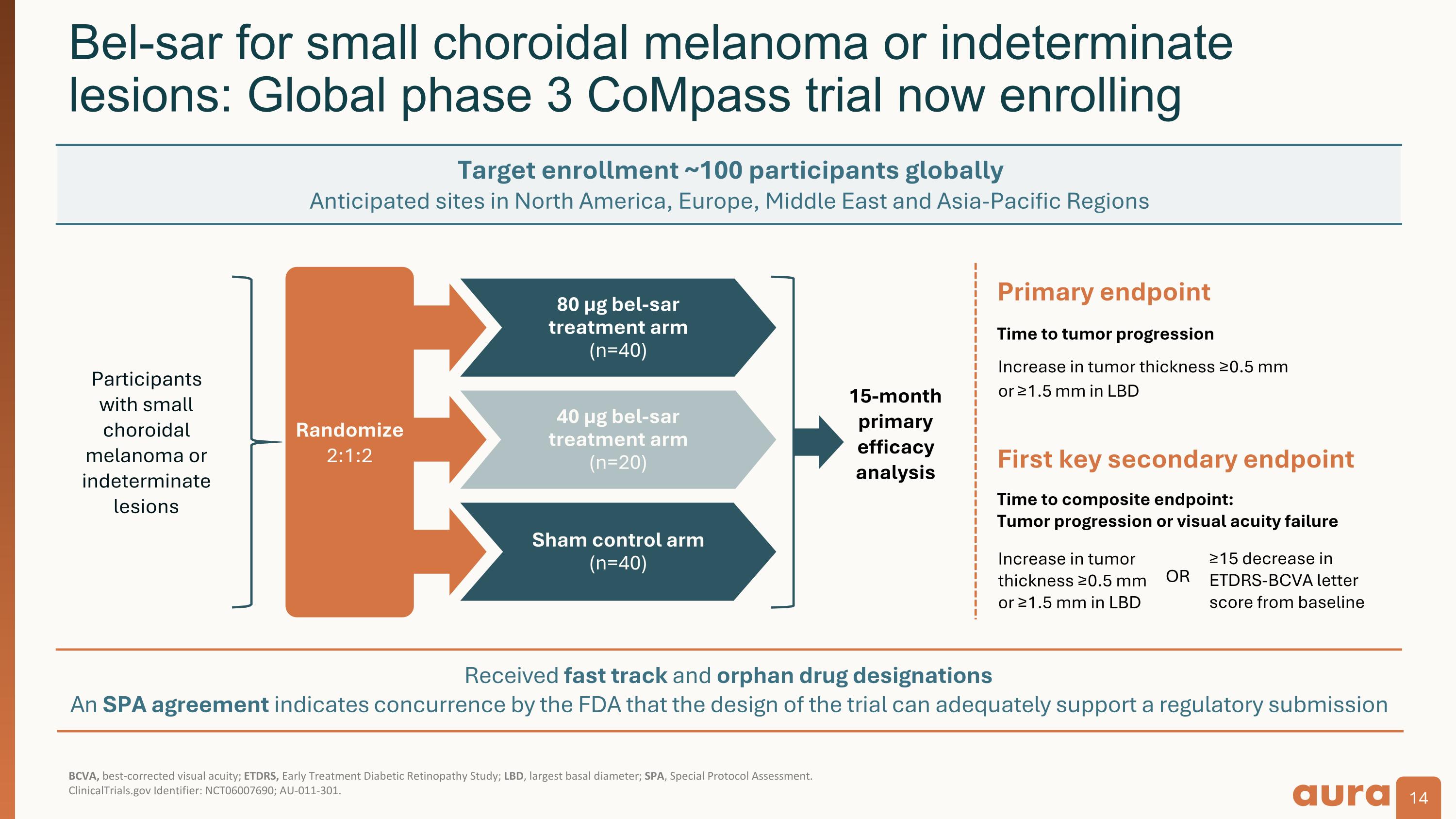
Received fast track and orphan drug designations An SPA agreement indicates concurrence by the FDA that the design of the trial can adequately support a regulatory submission BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; LBD, largest basal diameter; SPA, Special Protocol Assessment.�ClinicalTrials.gov Identifier: NCT06007690; AU-011-301. Bel-sar for small choroidal melanoma or indeterminate lesions: Global phase 3 CoMpass trial now enrolling 15-month primary efficacy analysis 80 µg bel-sar treatment arm�(n=40) 40 µg bel-sar treatment arm�(n=20) Sham control arm (n=40) Participants�with small choroidal melanoma or indeterminate lesions Randomize 2:1:2 First key secondary endpoint Primary endpoint Time to tumor progression Increase in tumor thickness ≥0.5 mm or ≥1.5 mm in LBD Time to composite endpoint: Tumor progression or visual acuity failure ≥15 decrease in ETDRS-BCVA letter score from baseline Increase in tumor thickness ≥0.5 mm or ≥1.5 mm in LBD OR Target enrollment ~100 participants globally Anticipated sites in North America, Europe, Middle East and Asia-Pacific Regions
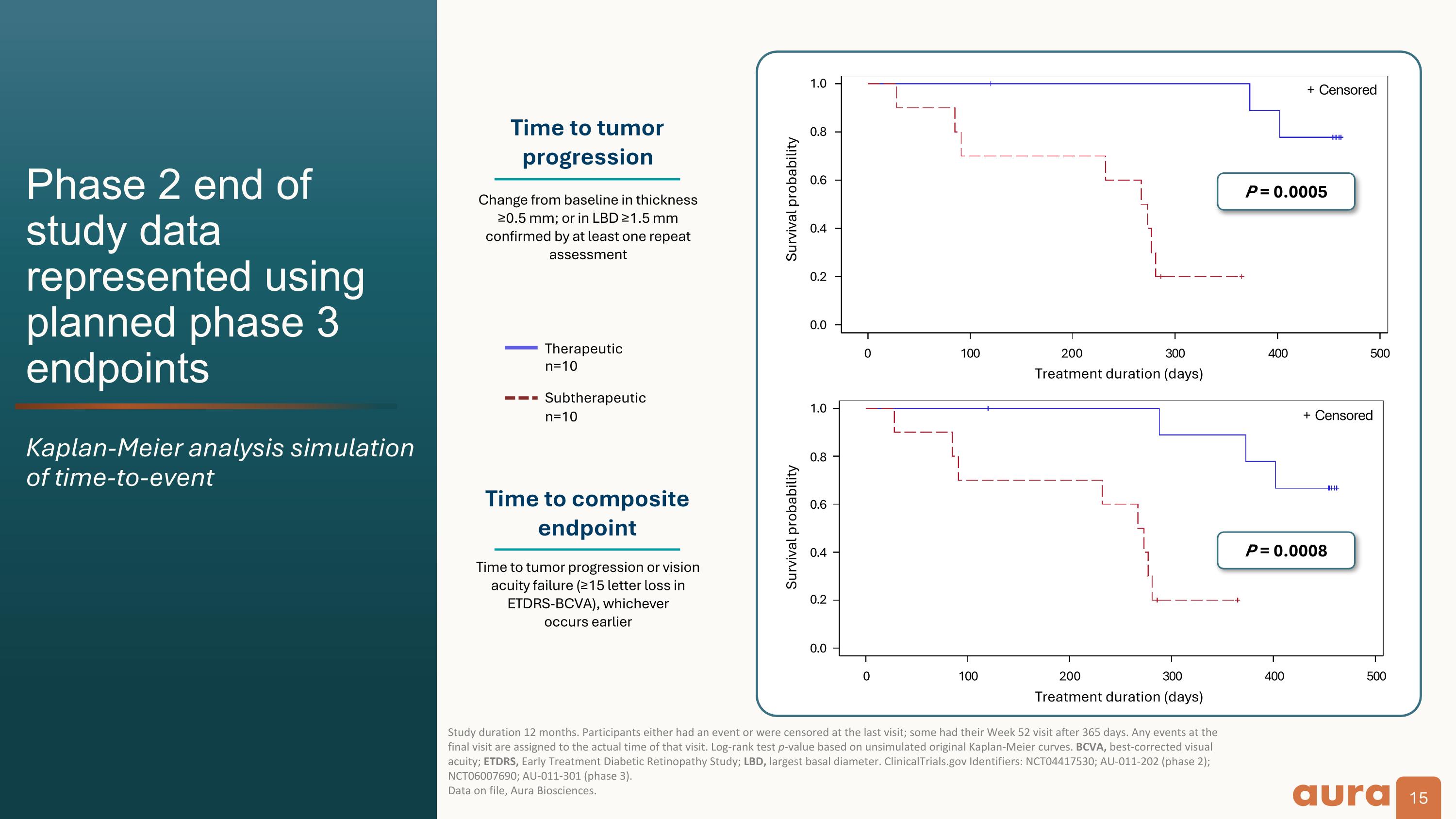
Phase 2 end of study data represented using planned phase 3 endpoints Kaplan-Meier analysis simulation of time-to-event Study duration 12 months. Participants either had an event or were censored at the last visit; some had their Week 52 visit after 365 days. Any events at the final visit are assigned to the actual time of that visit. Log-rank test p-value based on unsimulated original Kaplan-Meier curves. BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; LBD, largest basal diameter. ClinicalTrials.gov Identifiers: NCT04417530; AU-011-202 (phase 2); NCT06007690; AU-011-301 (phase 3).�Data on file, Aura Biosciences. Survival probability P = 0.0005 Time to tumor progression Time to composite endpoint Change from baseline in thickness ≥0.5 mm; or in LBD ≥1.5 mm confirmed by at least one repeat assessment Therapeutic�n=10 Subtherapeutic n=10 Time to tumor progression or vision acuity failure (≥15 letter loss in ETDRS-BCVA), whichever�occurs earlier 0.0 0.2 0.4 0.6 0.8 1.0 + Censored 0.0 0.2 0.4 0.6 0.8 1.0 0 100 200 300 400 500 P = 0.0008 Survival probability 0 100 200 300 400 500 + Censored Treatment duration (days) Treatment duration (days)

Bel-sar opportunities in ocular oncology represent a multi-billion-dollar addressable market With only ~100 ocular oncologists in the US/EU,�a global launch may be accomplished with a small�(<20) field-based team aIncludes conjunctival melanoma, primary acquired melanosis, squamous cell carcinoma and ocular surface squamous neoplasia.1-5 1. Yu G-P et al. Am J Ophthalmol. 2003;135(6):800-6. 2. Triay E et al. Br J Ophthalmol. 2009;93(11):1524-8. 3. Newton R et al. Lancet. 1996;347(9013):1450-1. 4. Dalvin LA. Br J Ophthalmol. 2018;102(12):1728-1734. 5. Sun EC et al. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73-7. 6. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putman. 7. American Cancer Society. Key statistics for retinoblastoma. Available at: https://www.cancer.org/cancer/types/retinoblastoma/about/key-statistics.html. Accessed Sept 5, 2024. Bel-sar (AU-011) is an investigational product candidate. The effectiveness and safety of bel-sar have not been established, and bel-sar is not approved for use in any jurisdiction. Ocular surface cancers ~66,000 patients/year ~35,000/yra,1–5 Primary uveal melanoma ~11,000/yr6 Metastases to the choroid ~20,000/yr6 Retinoblastoma ~500/yr7 Ocular oncology franchise total addressable market (US/EU)

Metastases to the choroid is a high unmet medical need and potentially doubles the ocular oncology market opportunity Metastases to the choroid decrease vision and quality of life in patients fighting metastatic cancer 1. Mathis T et al. Prog Ret Eye Res. 2019;68:144-176. 2. Shields CL et al. Ophthalmology. 1997;104(8):1265-76. 3. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putman. 4. Cohen VML. Eye (Lond). 2013;27(2):137-41. GI, gastrointestinal. Metastases to the choroid originate from multiple primary cancers1 ~1/4 of patients have tumors bilaterally2 Choroidal metastases ~20,000/yr (US/EU)3 Skin 2% Kidney 2% Breast 40–53% Lung 20–29% GI 4% Prostate 2% Standard of care is daily radiotherapy for up to 4 weeks,4 with a high burden to patients and radiation-associated complications
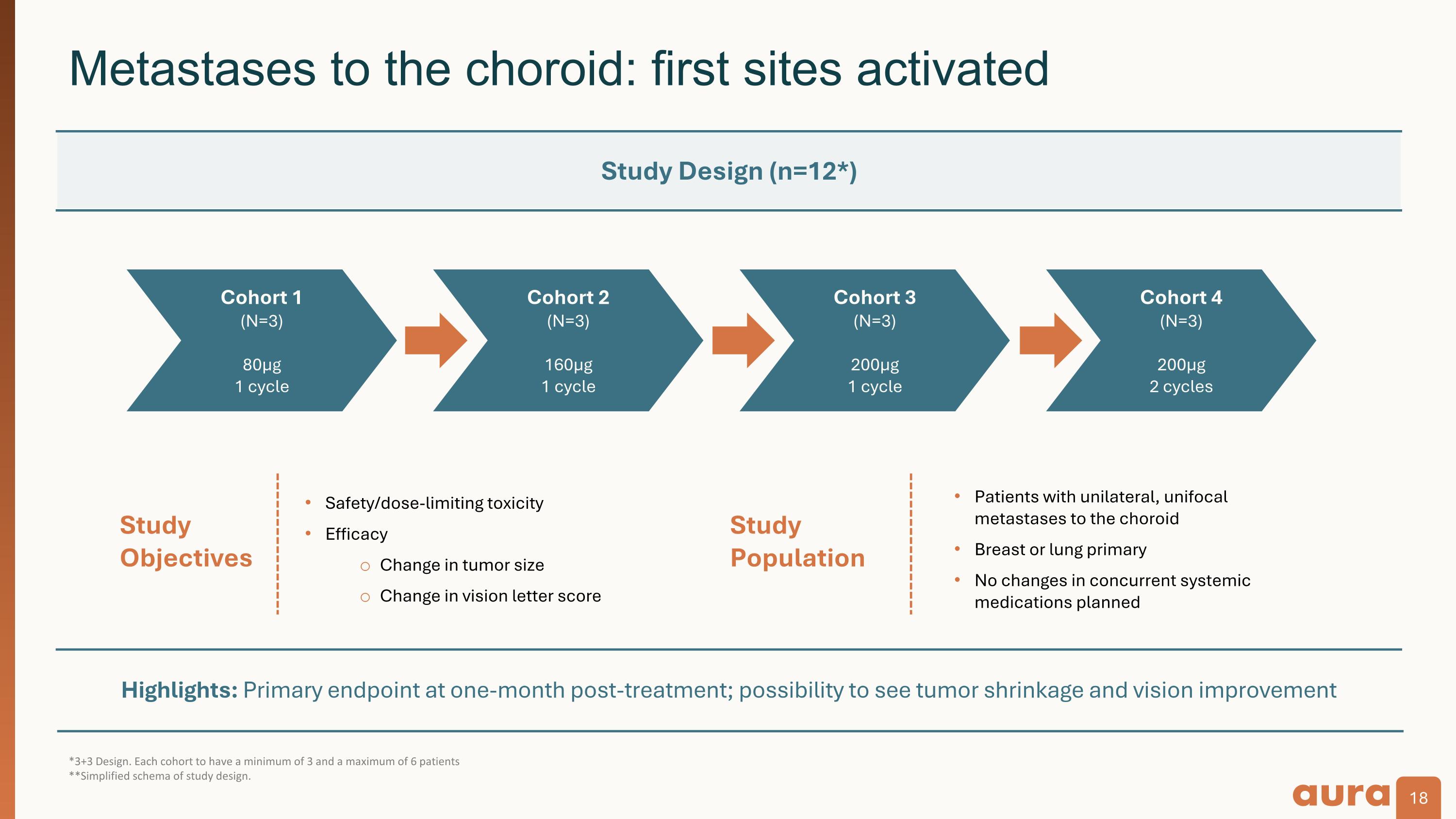
Highlights: Primary endpoint at one-month post-treatment; possibility to see tumor shrinkage and vision improvement *3+3 Design. Each cohort to have a minimum of 3 and a maximum of 6 patients **Simplified schema of study design. Metastases to the choroid: first sites activated Study�Population Study�Objectives Safety/dose-limiting toxicity Efficacy Change in tumor size Change in vision letter score Patients with unilateral, unifocal metastases to the choroid Breast or lung primary No changes in concurrent systemic medications planned Study Design (n=12*) Cohort 1 (N=3) 80µg 1 cycle Cohort 2 (N=3) 160µg 1 cycle Cohort 3 (N=3) 200µg 1 cycle Cohort 4 (N=3) 200µg 2 cycles

Urologic Oncology Bel-sar target indications: Non-muscle-invasive bladder cancer | Muscle-invasive bladder cancer

1. GLOBOCAN 2022. Bladder. Available at: https://gco.iarc.who.int/media/globocan/factsheets/cancers/30-bladder-fact-sheet.pdf. [Accessed October 1, 2024]. 2. Sung H, et al. CA Cancer J Clin. 2021;71(3):209–49. 3. Burger M, et al. Eur Urol. 2013;63(2):234–41. 4. Flaig TW, et al. J Natl Compr Canc Netw. 2018;16(9):1041–53. 5. Clark O, et al. Pharmacoecon Open. 2024 Aug 18. doi: 10.1007/s41669-024-00512-8. [Online ahead of print]. 6. Lamm DL, et al. J Urol. 2000;163(4):1124-9. 7. Shore ND, et al. Urol Oncol. 39(10):642–63. 8. Shalata AT, et al. Cancers (Basel). 2022;14(20):5019. BCG, Bacillus Calmette-Guerin; MIBC, muscle-invasive bladder cancer. QoL, quality of life; TURBT, transurethral resection of bladder tumor. Bladder cancer: High unmet medical need for function-preserving organ-sparing therapies 9th most common�cancer worldwide1 >$6 billion Annual cost�of treatment in US5 One of the highest lifetime treatment costs of all cancers Conventional bladder cancer treatments are suboptimal Short- and long-term side effects Considerable impact on QoL Inadequate efficacy Multiple TURBT surgeries Disease progression/metastasis Loss of bladder/cystectomy MIBC�25% NMIBC 75% Ranked 13th for mortality1 20 20 The majority of bladder cancer patients present with NMIBC3 ~70-80% of patients with NMIBC develop� recurrence after treatment8 NMIBC MIBC >600,000 614,298 diagnosed in 20221�(>7% increase from 2020)1,2 cases/year globally1 84% Patients are receiving fewer courses of BCG due to global shortage7 of patients do not complete a full course of BCG treatment6
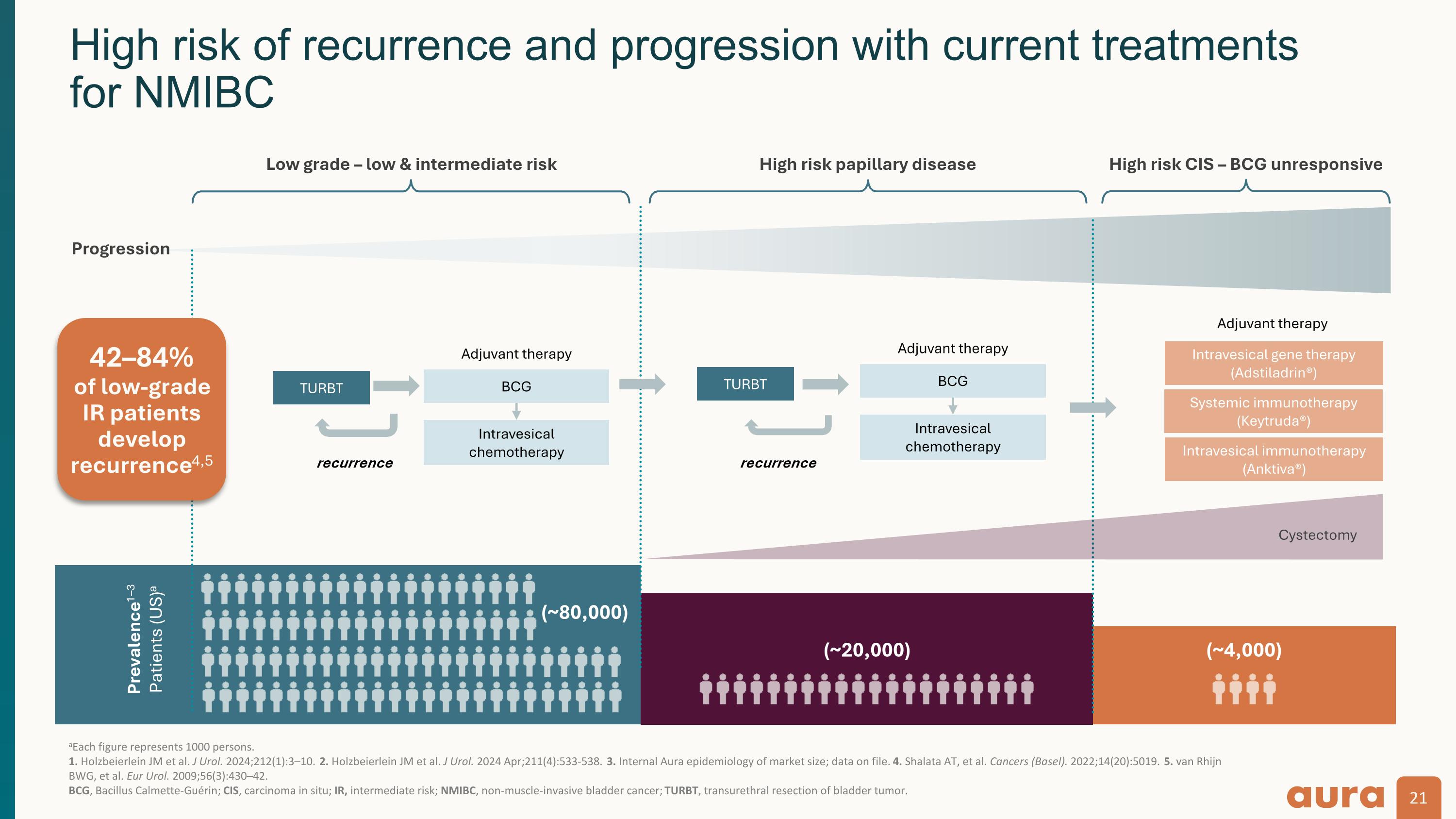
aEach figure represents 1000 persons. 1. Holzbeierlein JM et al. J Urol. 2024;212(1):3–10. 2. Holzbeierlein JM et al. J Urol. 2024 Apr;211(4):533-538. 3. Internal Aura epidemiology of market size; data on file. 4. Shalata AT, et al. Cancers (Basel). 2022;14(20):5019. 5. van Rhijn BWG, et al. Eur Urol. 2009;56(3):430–42. �BCG, Bacillus Calmette-Guérin; CIS, carcinoma in situ; IR, intermediate risk; NMIBC, non-muscle-invasive bladder cancer; TURBT, transurethral resection of bladder tumor. Prevalence1–3 Patients (US)a (~80,000) (~20,000) Progression Low grade – low & intermediate risk High risk papillary disease High risk CIS – BCG unresponsive BCG Intravesical chemotherapy (~4,000) TURBT recurrence Intravesical gene therapy (Adstiladrin®) Systemic immunotherapy (Keytruda®) Cystectomy TURBT recurrence Adjuvant therapy Adjuvant therapy Intravesical immunotherapy (Anktiva®) BCG Intravesical chemotherapy Adjuvant therapy High risk of recurrence and progression with current treatments�for NMIBC 42–84%�of low-grade IR patients develop recurrence4,5

AU-011 as a potential front-line immune ablative therapy�in NMIBC AU-011 has a dual mechanism of action and can potentially reduce the treatment burden NMIBC, non–muscle-invasive bladder cancer; TURBT, transurethral resection of bladder tumor. Treatment goals Focal treatment with direct tumor cell killing Reduce risk of recurrence and progression Avoid TURBT/operating room Stimulate broad anti-tumor T cell response Front-line early intervention for local disease Decreased treatment burden with favorable safety profile
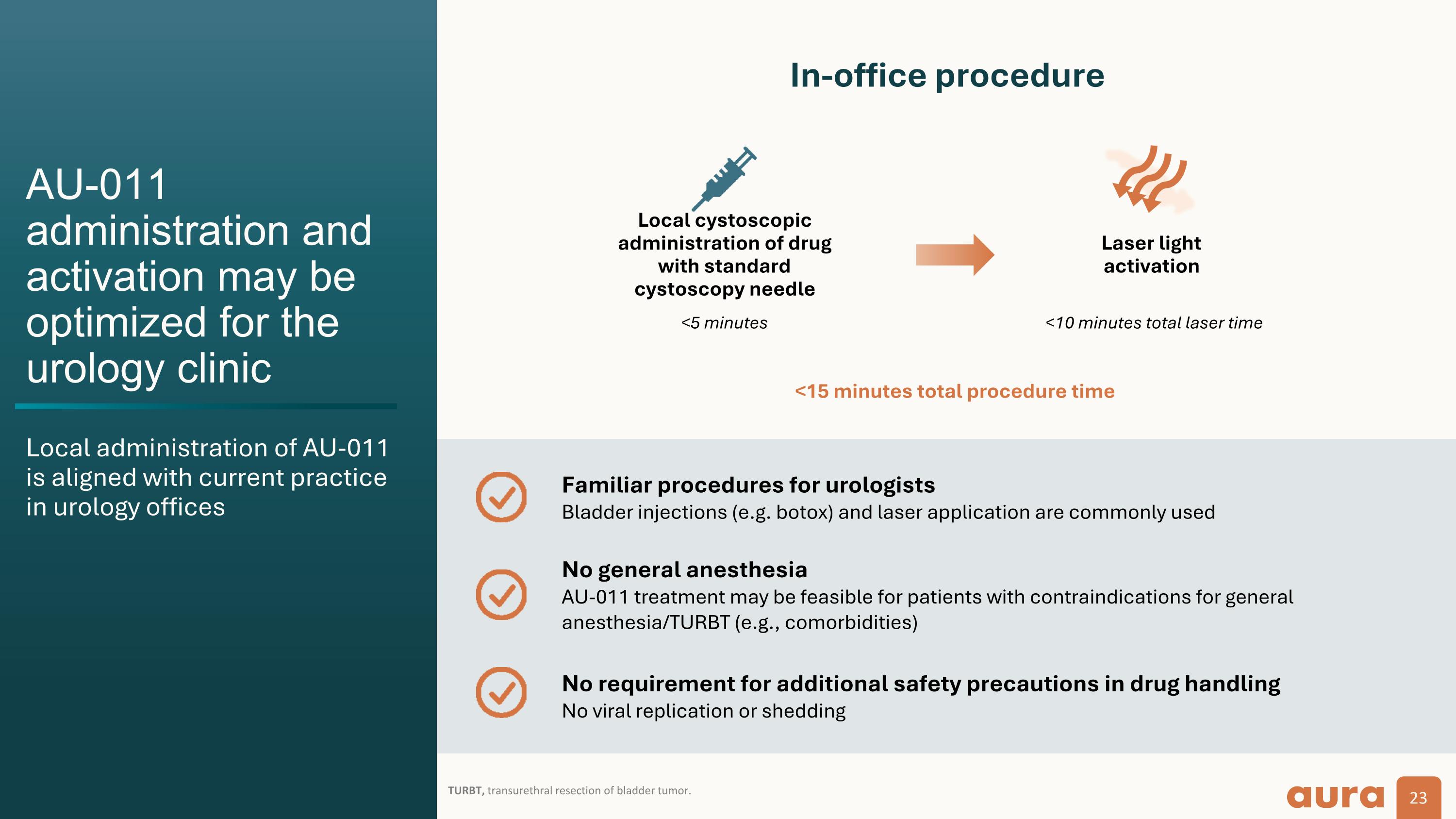
AU-011 administration and activation may be optimized for the urology clinic Local administration of AU-011�is aligned with current practice in urology offices TURBT, transurethral resection of bladder tumor. In-office procedure Local cystoscopic administration of drug with standard cystoscopy needle Laser light activation <10 minutes total laser time <15 minutes total procedure time <5 minutes Familiar procedures for urologists�Bladder injections (e.g. botox) and laser application are commonly used No general anesthesia�AU-011 treatment may be feasible for patients with contraindications for general anesthesia/TURBT (e.g., comorbidities) No requirement for additional safety precautions in drug handling�No viral replication or shedding
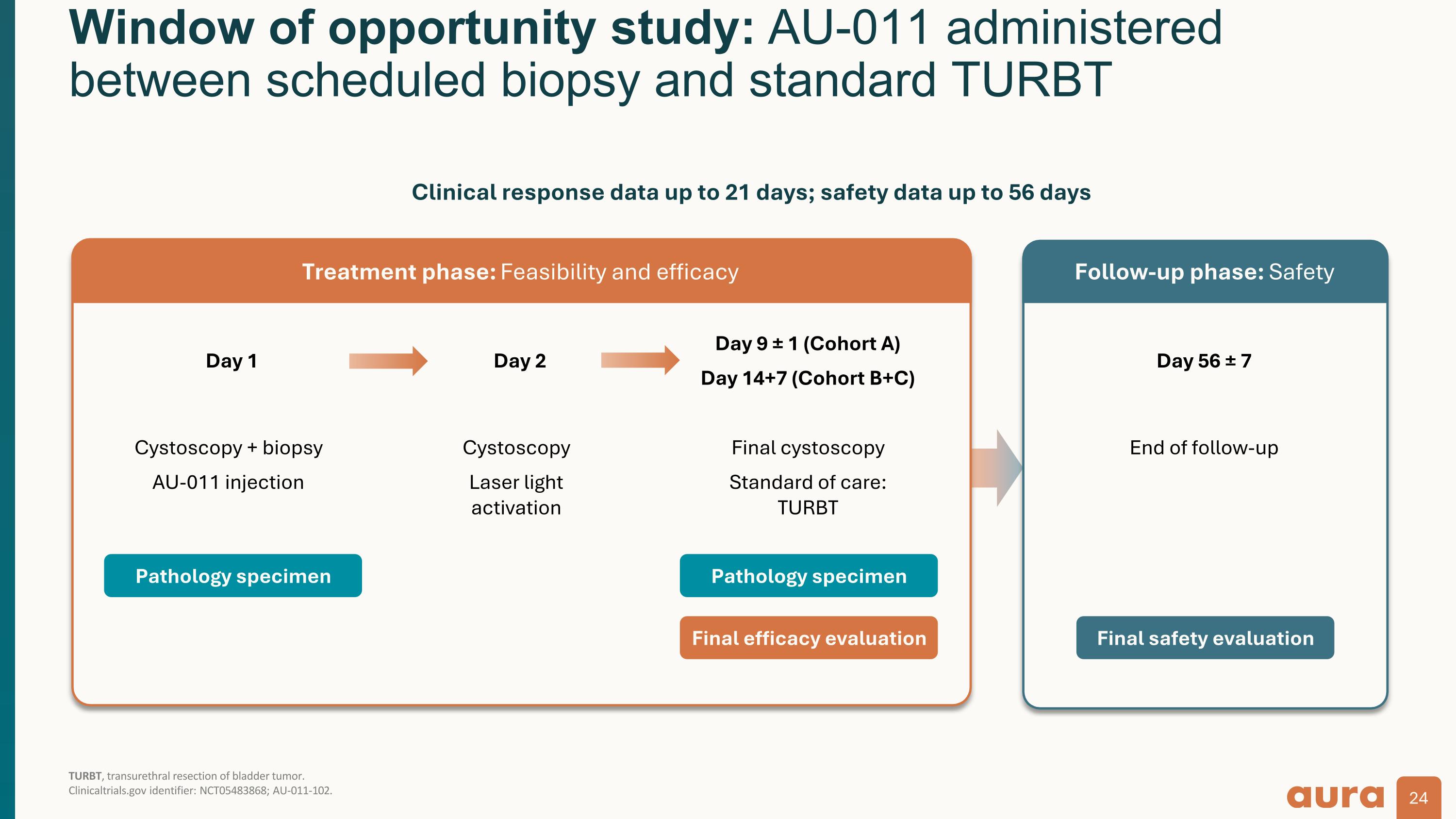
Window of opportunity study: AU-011 administered between scheduled biopsy and standard TURBT TURBT, transurethral resection of bladder tumor.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Day 1 Cystoscopy + biopsy AU-011 injection Day 2 Cystoscopy Laser light activation Day 9 ± 1 (Cohort A) Day 14+7 (Cohort B+C) Final cystoscopy Standard of care: TURBT Day 56 ± 7 End of follow-up Pathology specimen Pathology specimen Final efficacy evaluation Final safety evaluation Treatment phase: Feasibility and efficacy Follow-up phase: Safety Clinical response data up to 21 days; safety data up to 56 days

Phase 1 trial of AU-011 for bladder cancer designed to evaluate safety, feasibility, and mechanism of action Safety Review Board completed after each cohort. Patients followed for safety after TURBT to 56 days. NMIBC, non-muscle-invasive bladder cancer; MoA, mechanism of action; TURBT, transurethral resection of bladder tumor.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Total 100 µg NMIBC (N=5) 50 µg at tumor base 50 µg within lamina propria Drug only (No light) Total 100 µg NMIBC (N=4) 50 µg at tumor base 50 µg within lamina propria Cohort A: Drug + light Total 100 µg NMIBC (N=3) 100 µg at tumor base Cohort B: Drug + light Study objectives Safety & dose-limiting toxicity Focal distribution�of AU-011 Feasibility of technique Focal�necrosis Markers of�immune activation Histopathological assessment completed at time of standard of care TURBT Part 1 (n=5) AU-011 + focal light activation Single dose window of opportunity study in NMIBC all-comers AU-011 alone Part 2 (n=~10) Cohort C: Drug + light Total 200 µg NMIBC (N~3) 200 µg at tumor base
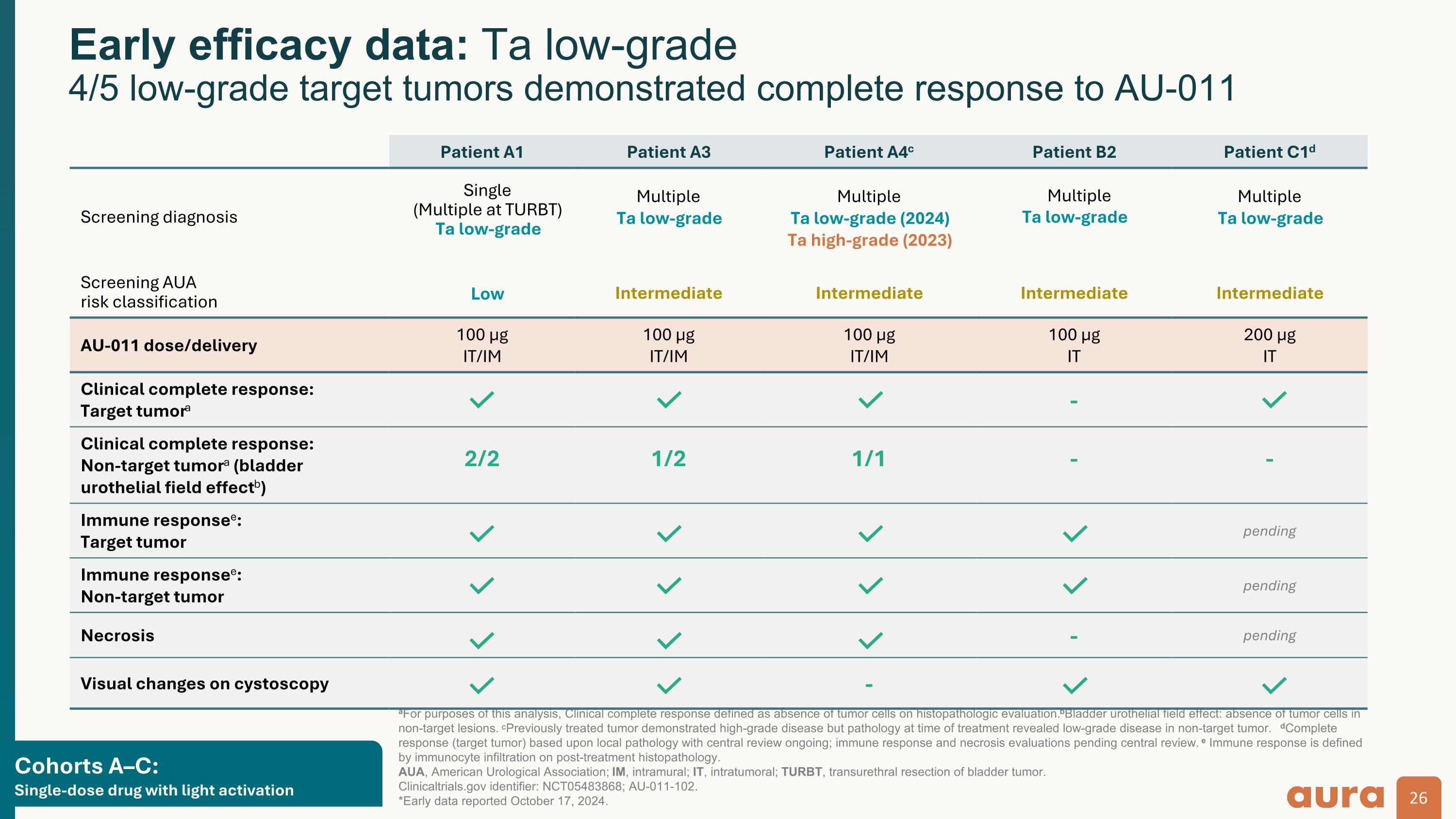
Cohorts A–C:�Single-dose drug with light activation aFor purposes of this analysis, Clinical complete response defined as absence of tumor cells on histopathologic evaluation. bBladder urothelial field effect: absence of tumor cells in non-target lesions. CPreviously treated tumor demonstrated high-grade disease but pathology at time of treatment revealed low-grade disease in non-target tumor. dComplete response (target tumor) based upon local pathology with central review ongoing; immune response and necrosis evaluations pending central review. e Immune response is defined by immunocyte infiltration on post-treatment histopathology.�AUA, American Urological Association; IM, intramural; IT, intratumoral; TURBT, transurethral resection of bladder tumor.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. *Early data reported October 17, 2024. Early efficacy data: Ta low-grade�4/5 low-grade target tumors demonstrated complete response to AU-011 Patient A1 Patient A3 Patient A4c Patient B2 Patient C1d Screening diagnosis Single (Multiple at TURBT) Ta low-grade Multiple Ta low-grade Multiple Ta low-grade (2024) Ta high-grade (2023) Multiple Ta low-grade Multiple Ta low-grade Screening AUA�risk classification Low Intermediate Intermediate Intermediate Intermediate AU-011 dose/delivery 100 µg�IT/IM 100 µg�IT/IM 100 µg�IT/IM 100 µg�IT 200 µg�IT Clinical complete response:�Target tumora - Clinical complete response: Non-target tumora (bladder urothelial field effectb) 2/2 1/2 1/1 - - Immune responsee:�Target tumor pending Immune responsee: Non-target tumor pending Necrosis - pending Visual changes on cystoscopy -

Cohorts A + B:�Single-dose drug with light activation aClinical complete response defined as absence of tumor cells on histopathologic evaluation. bBladder urothelial field effect: absence of tumor cells in non-target lesions. cImmune response is defined by immunocyte infiltration on post-treatment histopathology�AUA, American Urological Association; BCG, Bacillus Calmette-Guerin; CIS, carcinoma in situ; IM, intramural; IT, intratumoral; TURBT, transurethral resection of bladder tumor.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. *Early data reported October 17, 2024. Early efficacy data: Ta high-grade�3/3 high-grade tumors demonstrated immune response to AU-011 Patient A2 Patient B1 Patient B3 Screening diagnosis Single Ta high-grade Multiple Ta high-grade Single Ta high-grade Screening AUA�risk classification High High Intermediate AU-011 dose/�delivery 100 µg�IT/IM 100 µg�IT 100 µg�IT Clinical complete response: Target tumora - - - Clinical complete response: Non-target tumora (bladder urothelial field effectb) NA - NA Immune responsec: Target tumor Immune responsec: Non-target tumor NA NA Necrosis - - - Visual changes on cystoscopy Tumor Visually Smaller Tumor Visually Smaller -
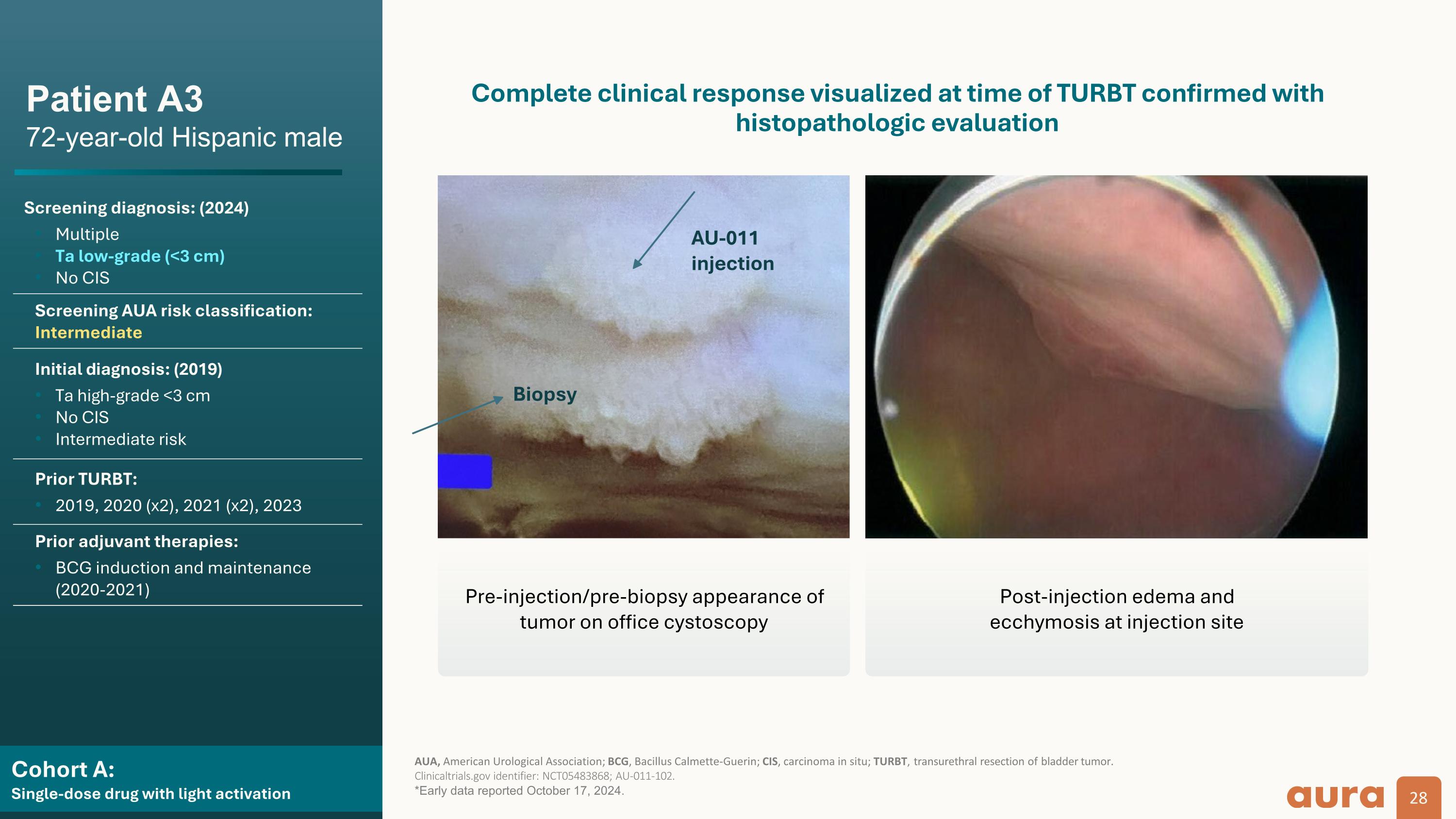
Cohort A:�Single-dose drug with light activation Patient A3�72-year-old Hispanic male Screening diagnosis: (2024) Multiple Ta low-grade (<3 cm) No CIS Screening AUA risk classification: Intermediate Initial diagnosis: (2019) Ta high-grade <3 cm No CIS Intermediate risk Prior TURBT: 2019, 2020 (x2), 2021 (x2), 2023 Prior adjuvant therapies: BCG induction and maintenance (2020-2021) AUA, American Urological Association; BCG, Bacillus Calmette-Guerin; CIS, carcinoma in situ; TURBT, transurethral resection of bladder tumor.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. *Early data reported October 17, 2024. Complete clinical response visualized at time of TURBT confirmed with histopathologic evaluation Biopsy AU-011 injection Pre-injection/pre-biopsy appearance of tumor on office cystoscopy Post-injection edema and�ecchymosis at injection site

Patient A3: AU-011 focal distribution, necrosis, and positive immune staining (target lesion) Cohort A:�Single-dose drug with light activation H&E CD3 CD4 CD8 Pre-treatment Post-treatment H&E, hematoxylin and eosin.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. *Early data reported October 17, 2024.

Light-activated cohorts (A + B):��Strong evidence of immune-mediated mechanism of action aPatients for which biopsies were available. bOrganized aggregates of immune cells.�MOA, mechanism of action�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. *Early data reported October 17, 2024. 100% (7/7) of target tumors showed infiltration of effector CD8+ T and CD4+ cells, as early as 7 days after laser activation 100% (7/7) of non-target tumorsa (in the five patients with available immune staining) showed T cell infiltration, supportive of a bladder urothelial field effect Focal eosinophilic infiltration was observed in 57% (4/7) target tumors and in 14% (1/7) non-target tumors, supportive of a local innate immune response to tumor necrosis Generation of lymphoid folliclesb was observed in 71% (5/7) target tumors, supportive of a local adaptive immune response AU-011 showed evidence of producing pro-immunogenic�changes in situ that have the potential to bridge, activate, and enhance adaptive immunity, consistent with its expected MOA
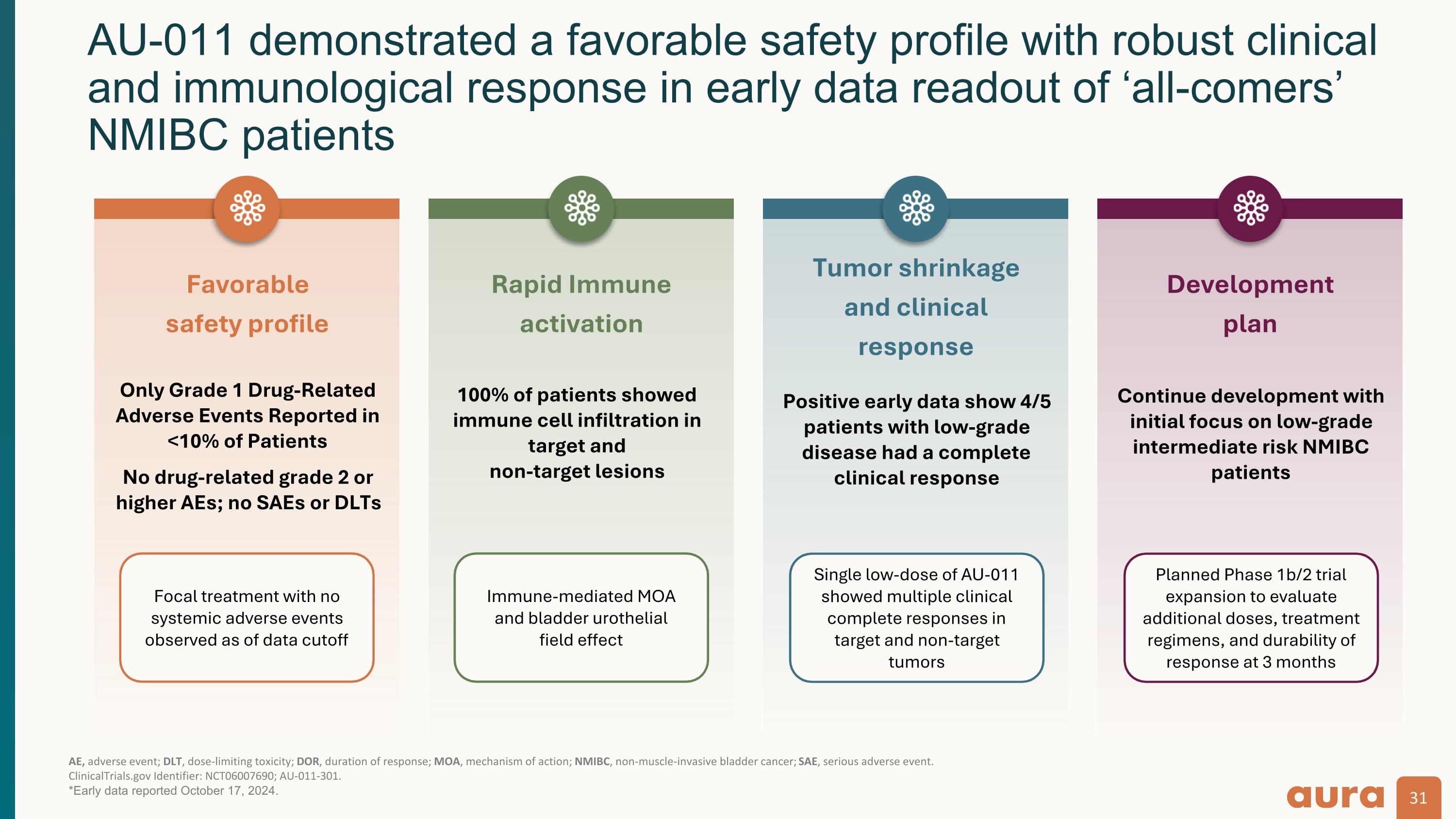
AU-011 demonstrated a favorable safety profile with robust clinical and immunological response in early data readout of ‘all-comers’ NMIBC patients AE, adverse event; DLT, dose-limiting toxicity; DOR, duration of response; MOA, mechanism of action; NMIBC, non-muscle-invasive bladder cancer; SAE, serious adverse event.�ClinicalTrials.gov Identifier: NCT06007690; AU-011-301. *Early data reported October 17, 2024. Rapid Immune activation 100% of patients showed immune cell infiltration in target and�non-target lesions Tumor shrinkage and clinical response Positive early data show 4/5 patients with low-grade disease had a complete clinical response Development plan Continue development with initial focus on low-grade intermediate risk NMIBC patients Favorable safety profile Only Grade 1 Drug-Related Adverse Events Reported in <10% of Patients No drug-related grade 2 or higher AEs; no SAEs or DLTs Focal treatment with no systemic adverse events observed as of data cutoff Immune-mediated MOA and bladder urothelial�field effect Single low-dose of AU-011 showed multiple clinical complete responses in target and non-target tumors Planned Phase 1b/2 trial expansion to evaluate additional doses, treatment regimens, and durability of response at 3 months
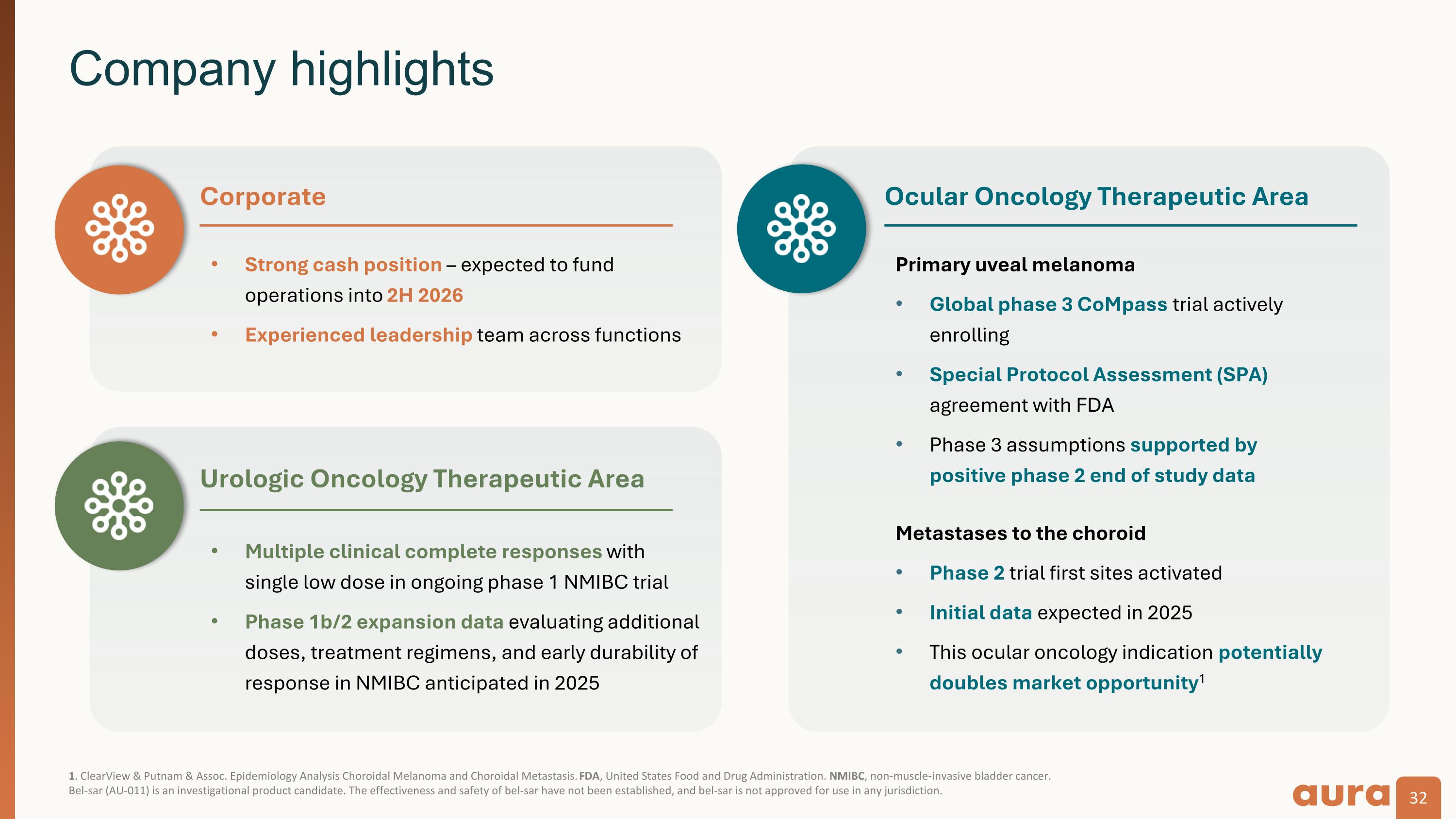
1. ClearView & Putnam & Assoc. Epidemiology Analysis Choroidal Melanoma and Choroidal Metastasis. FDA, United States Food and Drug Administration. NMIBC, non-muscle-invasive bladder cancer. Bel-sar (AU-011) is an investigational product candidate. The effectiveness and safety of bel-sar have not been established, and bel-sar is not approved for use in any jurisdiction. Company highlights Strong cash position – expected to fund operations into 2H 2026 Experienced leadership team across functions Corporate Ocular Oncology Therapeutic Area Primary uveal melanoma Global phase 3 CoMpass trial actively enrolling Special Protocol Assessment (SPA) agreement with FDA Phase 3 assumptions supported by�positive phase 2 end of study data Metastases to the choroid Phase 2 trial first sites activated Initial data expected in 2025 This ocular oncology indication potentially�doubles market opportunity1 Urologic Oncology Therapeutic Area Multiple clinical complete responses with single low dose in ongoing phase 1 NMIBC trial Phase 1b/2 expansion data evaluating additional doses, treatment regimens, and early durability of response in NMIBC anticipated in 2025

Appendix Ocular Oncology

Goal: To determine safety, optimal dose and therapeutic regimen with suprachoroidal administration One cycle = Doses on days 1, 8, and 15. a12 patients enrolled, 1 patient who discontinued after 1 cycle due to unrelated SAEs is not included in data analysis (n=11). bCohort 2: 2 participants were planned; third participant was additionally enrolled due to dose error in 1 participant.�LBD, largest basal diameter; QW, every week; SAE, serious adverse event. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Phase 2 trial of bel-sar for choroidal melanoma: �Open-label, dose-escalation with suprachoroidal administration Trial design – 22 participants enrolled Patient population representative of early-stage disease: Small choroidal melanoma and indeterminate lesions Endpoints Tumor progression Growth in tumor height ≥0.5 mm or ≥1.5 mm in LBD relative to baseline Visual acuity loss ≥15 letters decrease from baseline Tumor thickness growth rate Change in rate of growth of tumor thickness 1 dose:�20 μg x 1 laser 1 dose:�40 μg x 1 laser 1 dose:�40 μg x 2 lasers 2 doses:�40 μg x 2 lasers QW x 2 9 doses:�80 μg x 2 lasers QW x 3,�3 cycles Subtherapeutic regimens�(N=10) 1–2 doses (n=9); 2 cycles (6 doses; n=1) Therapeutic regimen�(N=11)a 3 cycles (9 doses) Cohort 1 (n=1) Cohort 2 (n=3b) Cohort 3 (n=2) Cohort 4 (n=3) Cohort 5 (n=3) Cohort 6 (n=10) 6–9 doses:�40 μg x 2 lasers QW x 3,�up to 3 cycles (20 µg) (40 µg) (40 µg) (80 µg) (240–360 µg) (720 µg) Total intended dose

Baseline characteristics All study participants aHigh risk for vision loss defined as tumor edge within either 3 mm of foveal center or 3 mm of optic disc edge. BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; LBD, largest basal diameter. Data on file, Aura Biosciences. All patients (n=22) Female (%) 54.5 White, not Hispanic or Latino (%) 100 Subretinal fluid at screening (%) 100 Orange pigment at screening (%) 86.4 Documented growth prior to screening (%) 86.4�(100% of therapeutic group) Mean age at screening (years, ± SD) 59.2 (±16.5) Mean baseline BCVA in study eye (ETDRS letters, ± SD) 83.2 (±7.2) Mean baseline LBD (mm, ± SD) 8.5 (±1.4) Mean baseline tumor thickness (mm, ± SD) 2.0 (±0.5) Mean tumor distance to closest vision-critical structure at screening (mm, ± SD) 2.0 (±2.3) Tumors at high risk for vision loss (%)a 73%�(80% [8/10] of therapeutic group)
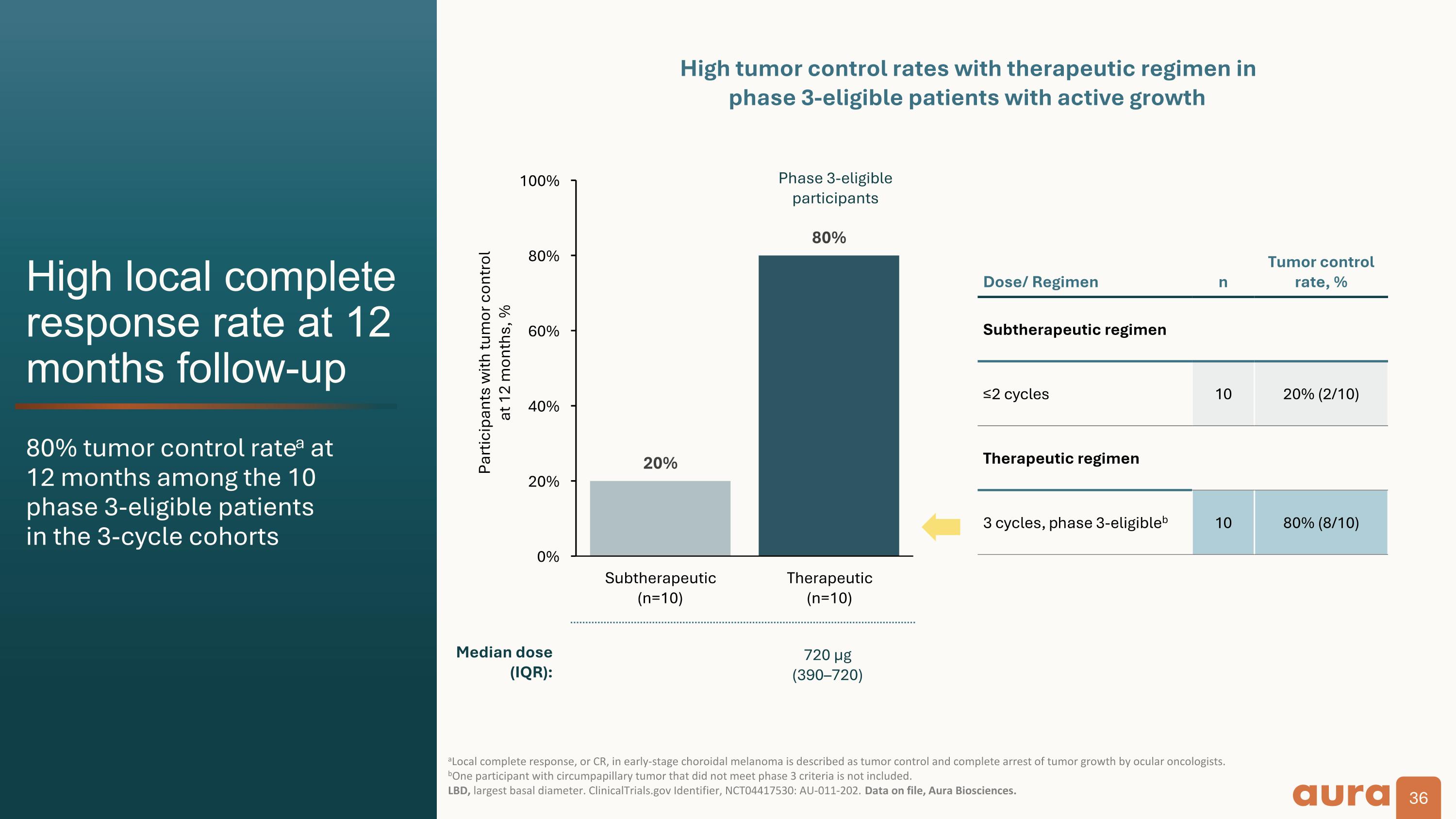
High local complete response rate at 12 months follow-up 80% tumor control ratea at�12 months among the 10�phase 3-eligible patients�in the 3-cycle cohorts aLocal complete response, or CR, in early-stage choroidal melanoma is described as tumor control and complete arrest of tumor growth by ocular oncologists.�bOne participant with circumpapillary tumor that did not meet phase 3 criteria is not included.�LBD, largest basal diameter. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Participants with tumor control at 12 months, % Dose/ Regimen n Tumor control rate, % Subtherapeutic regimen ≤2 cycles 10 20% (2/10) Therapeutic regimen 3 cycles, phase 3-eligibleb 10 80% (8/10) Phase 3-eligible participants High tumor control rates with therapeutic regimen in�phase 3-eligible patients with active growth Median dose (IQR): 140 µg�(80160) 720 µg�(390–720)

Rate of tumor growth ± SE, mm/yr P < 0.0001 Rate of tumor growth with bel-sar treatment In phase 3-eligible patients, the 3-cycle regimen resulted in cessation of growth among responders (N=8) Tumor thickness growth rates/slopes estimated using Mixed Models for Repeat Measures (MMRM); random intercept and slope model for Historical and Study periods. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Post-treatment actual growth rate Untreated projected growth rate Pre-treatment actual growth rate
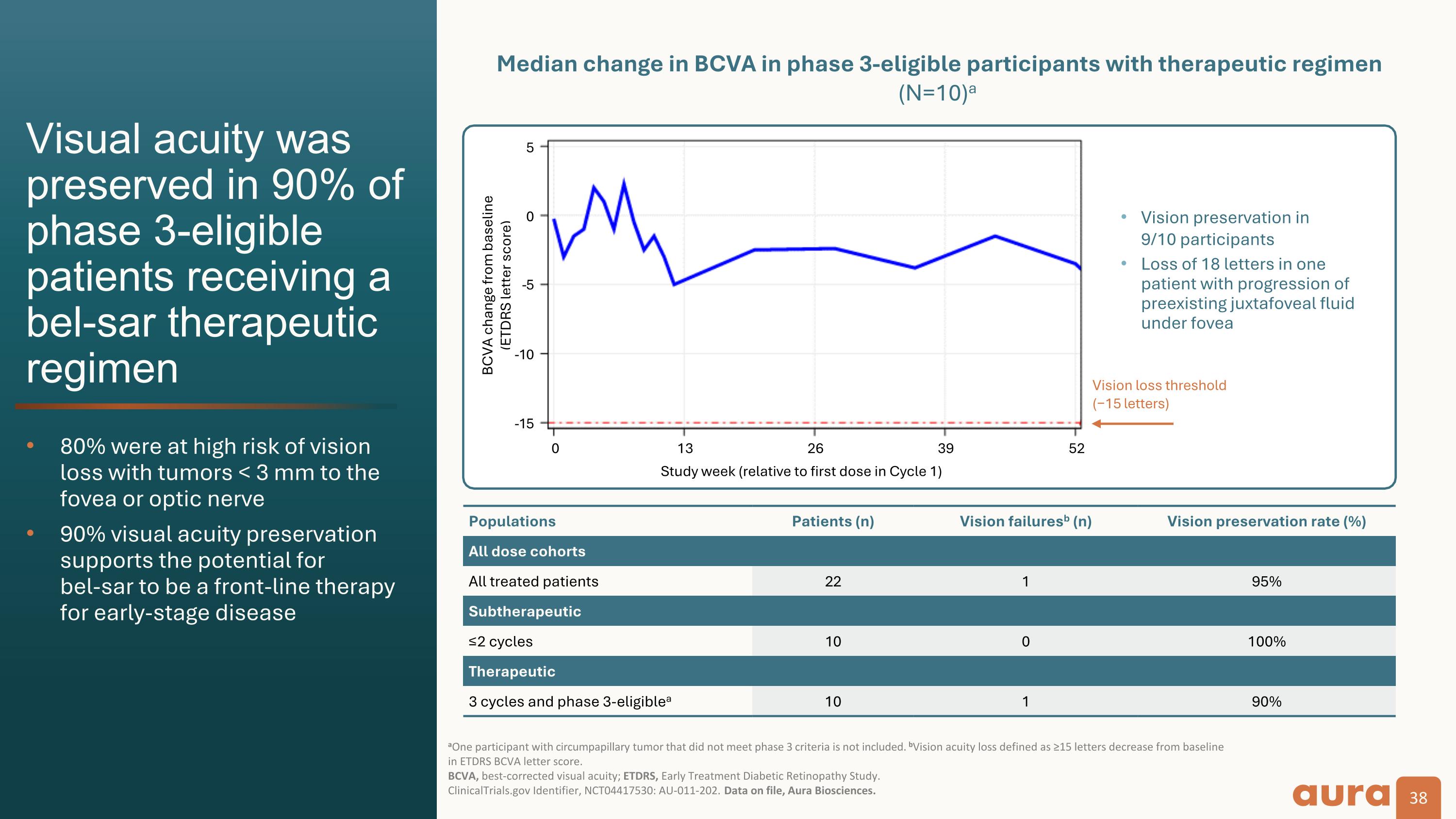
Vision loss threshold�(−15 letters) Populations Patients (n) Vision failuresb (n) Vision preservation rate (%) All dose cohorts All treated patients 22 1 95% Subtherapeutic ≤2 cycles 10 0 100% Therapeutic 3 cycles and phase 3-eligiblea 10 1 90% BCVA change from baseline�(ETDRS letter score) Median change in BCVA in phase 3-eligible participants with therapeutic regimen (N=10)a Visual acuity was preserved in 90% of phase 3-eligible patients receiving a bel-sar therapeutic regimen 80% were at high risk of vision loss with tumors < 3 mm to the fovea or optic nerve 90% visual acuity preservation supports the potential for�bel-sar to be a front-line therapy for early-stage disease aOne participant with circumpapillary tumor that did not meet phase 3 criteria is not included. bVision acuity loss defined as ≥15 letters decrease from baseline in ETDRS BCVA letter score. �BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Study week (relative to first dose in Cycle 1) Vision preservation in�9/10 participants Loss of 18 letters in one patient with progression of preexisting juxtafoveal fluid under fovea -5 0 5 -5 -10 -15 0 13 26 39 52
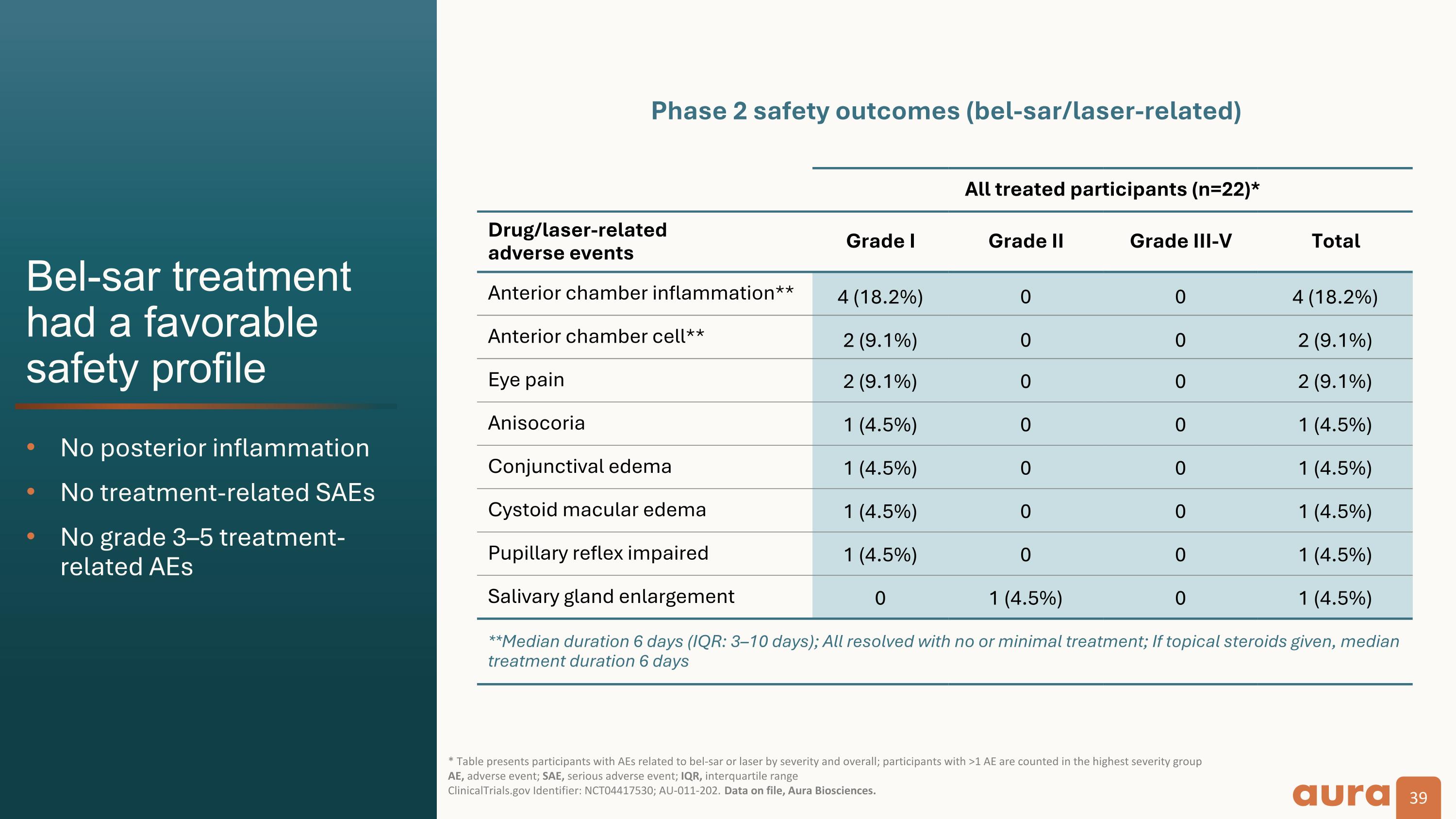
Bel-sar treatment�had a favorable�safety profile No posterior inflammation No treatment-related SAEs No grade 3–5 treatment-related AEs * Table presents participants with AEs related to bel-sar or laser by severity and overall; participants with >1 AE are counted in the highest severity group AE, adverse event; SAE, serious adverse event; IQR, interquartile range�ClinicalTrials.gov Identifier: NCT04417530; AU-011-202. Data on file, Aura Biosciences. All treated participants (n=22)* Drug/laser-related�adverse events Grade I Grade II Grade III-V Total Anterior chamber inflammation** 4 (18.2%) 0 0 4 (18.2%) Anterior chamber cell** 2 (9.1%) 0 0 2 (9.1%) Eye pain 2 (9.1%) 0 0 2 (9.1%) Anisocoria 1 (4.5%) 0 0 1 (4.5%) Conjunctival edema 1 (4.5%) 0 0 1 (4.5%) Cystoid macular edema 1 (4.5%) 0 0 1 (4.5%) Pupillary reflex impaired 1 (4.5%) 0 0 1 (4.5%) Salivary gland enlargement 0 1 (4.5%) 0 1 (4.5%) **Median duration 6 days (IQR: 3–10 days); All resolved with no or minimal treatment; If topical steroids given, median treatment duration 6 days Phase 2 safety outcomes (bel-sar/laser-related)
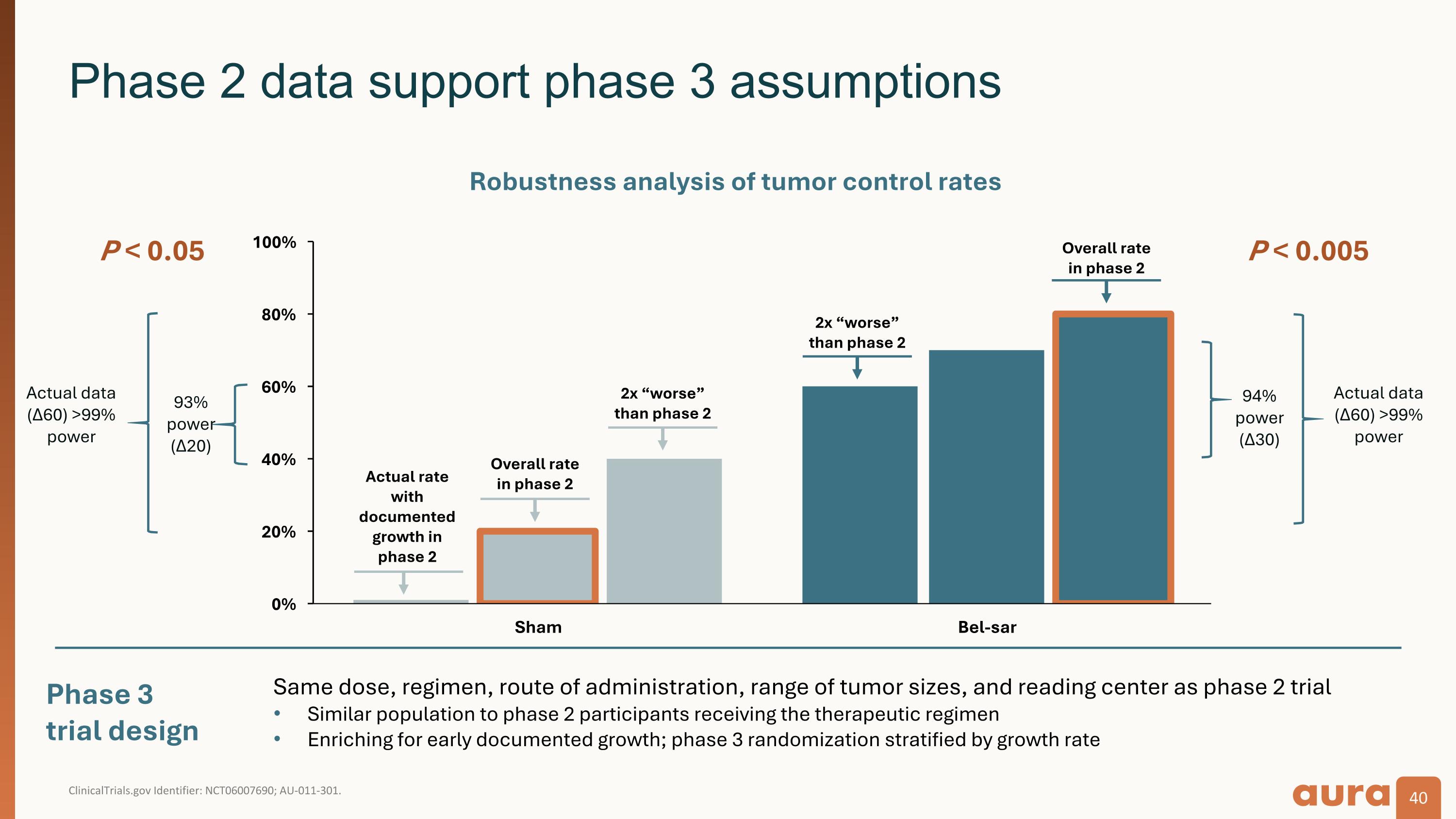
ClinicalTrials.gov Identifier: NCT06007690; AU-011-301. Phase 2 data support phase 3 assumptions Phase 3 trial design P < 0.005 93% power (Δ20) Actual data (Δ60) >99% power P < 0.05 Robustness analysis of tumor control rates Overall rate�in phase 2 2x “worse” than phase 2 2x “worse” than phase 2 Actual rate�with documented growth in�phase 2 Overall rate�in phase 2 94% power (Δ30) Actual data (Δ60) >99% power Same dose, regimen, route of administration, range of tumor sizes, and reading center as phase 2 trial Similar population to phase 2 participants receiving the therapeutic regimen Enriching for early documented growth; phase 3 randomization stratified by growth rate

Appendix Urologic Oncology
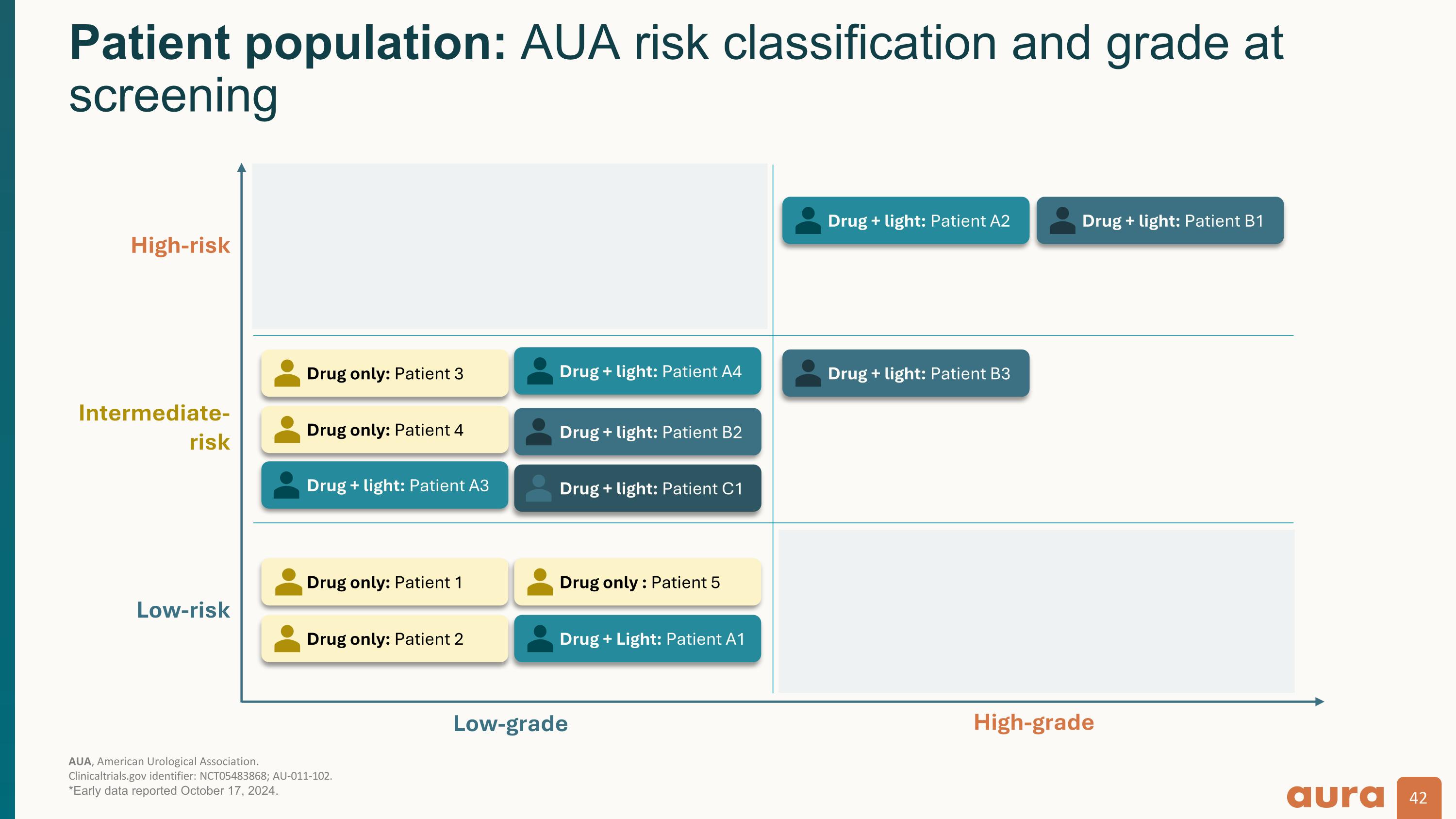
AUA, American Urological Association.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. *Early data reported October 17, 2024. Patient population: AUA risk classification and grade at screening Low-grade High-grade Low-risk Intermediate-�risk High-risk Drug only: Patient 3 Drug only: Patient 4 Drug + light: Patient A3 Drug + light: Patient B2 Drug + light: Patient B3 Drug + light: Patient A2 Drug + light: Patient A4 Drug + light: Patient B1 Drug only: Patient 1 Drug only: Patient 2 Drug only : Patient 5 Drug + Light: Patient A1 Drug + light: Patient C1
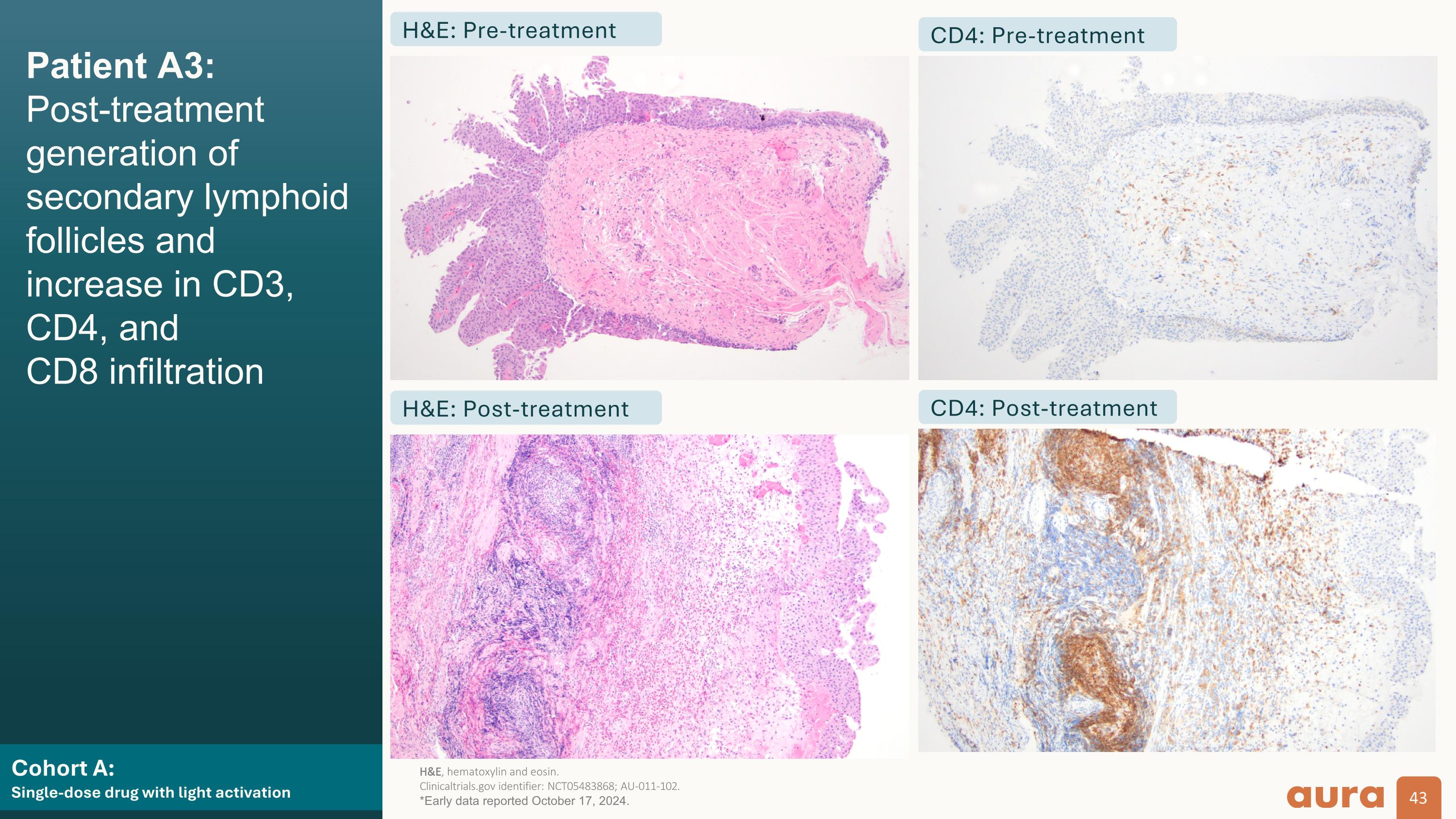
Cohort A:�Single-dose drug with light activation Patient A3:�Post-treatment generation of secondary lymphoid follicles and increase in CD3, CD4, and�CD8 infiltration H&E: Pre-treatment H&E: Post-treatment CD4: Pre-treatment CD4: Post-treatment H&E, hematoxylin and eosin.�Clinicaltrials.gov identifier: NCT05483868; AU-011-102. *Early data reported October 17, 2024.
v3.24.3
Document And Entity Information
|
Nov. 12, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Nov. 12, 2024
|
| Entity Registrant Name |
Aura Biosciences, Inc.
|
| Entity Central Index Key |
0001501796
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-40971
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
32-0271970
|
| Entity Address, Address Line One |
80 Guest Street
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02135
|
| City Area Code |
617
|
| Local Phone Number |
500-8864
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Title of 12(b) Security |
Common Stock, $0.00001 par value per share
|
| Trading Symbol |
AURA
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Aura Biosciences (NASDAQ:AURA)
Graphique Historique de l'Action
De Jan 2025 à Fév 2025
Aura Biosciences (NASDAQ:AURA)
Graphique Historique de l'Action
De Fév 2024 à Fév 2025