Avadel Pharmaceuticals to Intervene in Jazz Pharmaceuticals Lawsuit Over FDA Ruling
23 Juin 2023 - 9:05PM
Dow Jones News
By Denny Jacob
Avadel Pharmaceuticals intends to intervene in a lawsuit filed
by Jazz Pharmaceuticals against the U.S. Food and Drug
Administration, Avadel Chief Executive Greg Divis said.
Ireland-based biopharmaceutical company Jazz Pharmaceuticals
said Thursday in a regulatory filing that its lawsuit alleges that
the FDA acted outside its authority under the Orphan Drug Act when
it approved Avadel's new drug application for Lumryz and granted it
orphan drug exclusivity.
The lawsuit alleges the FDA acted without lawful basis when the
agency determined that Lumryz boosted patient care and was
purportedly clinically superior to two similar drugs that Jazz
Pharmaceutical produces, Xywav and Xyrem.
Divis said that Avadel would "vigorously defend FDA's decisions
to approve Lumryz and grant it orphan drug exclusivity, which were
entirely proper under the law and rooted in the facts." He added
that Lumryz launched earlier this month and is on track with
expectations.
Jazz Pharmaceuticals said Xywav already had orphan drug
exclusivity and described Lumryz as an extended-release
reformulation of Xyrem. The drugs treat cataplexy, or excessive
daytime sleepiness, in adults with narcolepsy.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
June 23, 2023 14:50 ET (18:50 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
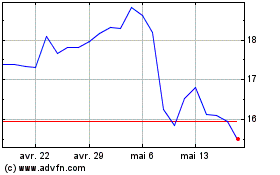
Avadel Pharmaceuticals (NASDAQ:AVDL)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
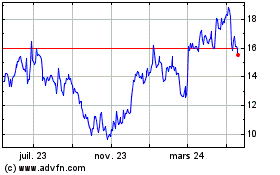
Avadel Pharmaceuticals (NASDAQ:AVDL)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024