UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE TO
Tender Offer Statement under Section 14(d)(1) or 13(e)(1)
of the Securities Exchange Act of 1934
BIOTIE
THERAPIES OYJ
(Name of Subject Company (Issuer))
ACORDA THERAPEUTICS, INC.
(Name of Filing Person (Offeror))
Ordinary shares, no nominal value (“Ordinary Shares”)
American Depositary Shares (“ADSs”), each representing 80 ordinary shares, no nominal value
Option rights issued under the December 6, 2011 option plan (“2011 Option Rights”)
Option rights issued under the January 2, 2014 option plan (“2014 Option Rights”)
Option rights issued under the January 4, 2016 option plan (“2016 Option Rights”)
Share units issued under the December 6, 2011 equity incentive plan (“2011 Share Rights”)
Share units under the January 2, 2014 equity incentive plan (“2014 Share Rights”)
Option rights awards under the Swiss option plan dated June 18, 2008 (“Swiss Option Rights”)
Warrants issued on May 28, 2015 (“Warrants”)
(Title of Class of Securities)
FI0009011571 (Ordinary Shares)
09074D103 (ADSs)
None
(2011 Option Rights)
None (2014 Option Rights)
None (2016 Option Rights)
None (2011 Share Rights)
None (2014 Share Rights)
None (Swiss Option Rights)
None (Warrants)
(CUSIP
Number of Class of Securities)
Jane Wasman
President, International, General Counsel and Corporate Secretary
Acorda Therapeutics, Inc.
420 Saw Mill River Road
Ardsley, NY 10502
(914)
347-4300
(Name, address and telephone number of person authorized to receive notices and communications on behalf of filing person)
Copy to:
Daniel Wolf, P.C.
Kirkland & Ellis LLP
601 Lexington Ave
New
York, NY 10022
(212) 446-4884
CALCULATION OF
FILING FEE
|
|
|
| Transaction valuation(1) |
|
Amount of filing fee(2) |
| $367,900,597.37 |
|
$37,047.59 |
| (1) |
Calculated solely for purposes of determining the filing fee. The calculation assumes the purchase of all issued and outstanding equity securities of Biotie Therapies Oyj for the following amounts: €0.2946 per
Share for 980,921,795 Shares (including Shares represented by ADSs), €0.2946 minus the applicable subscription price for each 2011 Option Right, 2014 Option Right, 2016 Option Right, 2011 Share Right and 2014 Share Right, and
€0.1664 per Warrant for 220,400,001 Warrants. The transaction valuation was calculated in euros and converted into U.S. dollars using the euro to U.S. dollar exchange rate of $1.101 per €1 as of March 4, 2016, as published by the
Federal Reserve Bank. |
| (2) |
The amount of the filing fee was calculated in accordance with Rule 0-11 of the Securities Exchange Act of 1934 (the “Exchange Act”), as amended, and Fee Rate Advisory #1 for fiscal year 2016, issued
August 27, 2015, by multiplying the transaction value by 0.0001007. |
| ¨ |
Check the box if any part of the fee is offset as provided by Rule 0-11(a)(2) and identify the filing with which the offsetting fee was previously paid. Identify the previous filing by registration statement number, or
the Form or Schedule and the date of its filing. |
|
|
|
| Amount Previously Paid: N/A |
|
Filing Party: N/A |
| Form or Registration No.: N/A |
|
Date Filed: N/A |
| ¨ |
Check the box if filing relates solely to preliminary communications made before the commencement of a tender offer. |
Check the appropriate boxes below to designate any transactions to which the statement relates:
| |
x |
third-party tender offer subject to Rule 14d-1. |
| |
¨ |
issuer tender offer subject to Rule 13e-4. |
| |
¨ |
going-private transaction subject to Rule 13e-3. |
| |
¨ |
amendment to Schedule 13D under Rule 13d-2. |
Check the following box if the filing is a final
amendment reporting the results of the tender offer: ¨
If applicable, check the appropriate
box(es) below to designate the appropriate rule provision(s) relied upon:
| |
¨ |
Rule 13e-4(i) (Cross-Border Issuer Tender Offer) |
| |
¨ |
Rule 14d-1(d) (Cross-Border Third Party Tender Offer) |
This Tender Offer Statement on Schedule TO (together with any amendments and supplements hereto,
this “Schedule TO”) is filed by (i) Acorda Therapeutics, Inc., a Delaware corporation (“Acorda” or the “Offeror”). This Schedule TO relates to the tender offer for all of the issued and
outstanding ordinary shares, no nominal value (the “Shares”), all of the outstanding American Depositary Shares, each representing 80 Shares (the “ADSs”), all of the outstanding Option Rights (as defined below), all
of the outstanding Share Rights (as defined below) and all of the outstanding warrants issued on May 28, 2015 (the “Warrants”) (the outstanding Shares, ADSs, Option Rights, Share Rights and Warrants, collectively, the
“Equity Interests”) in Biotie Therapies Oyj, a public limited liability company organized under the laws of Finland (“Biotie” or the “Company”), that are not held by the Company or its subsidiaries
(the “Tender Offer”). “Option Rights” means, collectively, option rights granted under the option plan resolved upon by the board of directors of the Company (the “Board of Directors”) on
December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Option Rights”), option rights granted under the option plan resolved upon by the Board of
Directors of the Company on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013 (the “2014 Option Rights”), option rights granted under the option plan
resolved upon by the Board of Directors of the Company on January 4, 2016 by virtue of an authorization granted by the annual general meeting of the Company held on May 26, 2015 (the “2016 Option Rights”) and option rights
granted under the Swiss option plan dated June 17, 2008 (the “Swiss Option Rights”). “Share Rights” means, collectively, share units under the equity incentive plan resolved upon by the Board of Directors of
the Company on December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Share Rights”) and share units under the equity incentive plan resolved upon
by the Board of Directors of the Company on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013 (the “2014 Share Rights”).
The Tender Offer is being made pursuant to the offer to purchase (the “Tender Offer Document”), a copy of which is attached
as Exhibit (a)(1)(A), the Letter of Transmittal for ADSs (the “Letter of Transmittal”), a copy of which is attached as Exhibit (a)(1)(B), the Acceptance Form for Shares (including any instruction letter attached thereto), a copy of
which is attached as Exhibit (a)(1)(C), the Acceptance Form for Uncertificated Equity Instruments (including any instruction letter attached thereto), a copy of which is attached as Exhibit (a)(1)(D), and the Acceptance Form for Certificated Equity
Instruments (including any instruction letter attached thereto), the form of which is attached hereto as Exhibit (a)(1)(E) (such acceptance forms and attached instructions, the “Acceptance Forms”), in each case, together with any
amendments or supplements thereto.
The information set forth in the Tender Offer Document is incorporated by reference herein to the
extent set forth in response to Items 1 through 9 and Item 11 in this Schedule TO, and is supplemented by the information specifically provided in this Schedule TO.
| Item 1. |
Summary Term Sheet. |
Regulation M-A Item 1001
The information set forth in the Section titled “Summary Term Sheet” of the Tender Offer Document is incorporated herein by
reference.
| Item 2. |
Subject Company Information. |
Regulation M-A Item 1002
(a) Name and Address. The name, address, and telephone number of the subject company’s principal executive offices are as
follows:
Biotie Therapies Oyj
Joukahaisenkatu 6, FI-20520
Turku, Finland
(+358) 2 274-8900
(b) Securities. The information set forth on the first two pages of and in
Section 5.2—“Presentation of Biotie—Share Capital” of the Tender Offer Document is incorporated herein by reference.
(c) Trading Market and Price. The information set forth in Section 2.2—“Price Range of Biotie’s Shares
(historical)”, Section 2.4—“Price Range of Biotie’s ADSs (historical)”, Section 2.5—“Grounds for Determining the Warrant Offer Price”, and Section 2.6—“Grounds for Determining the
Option Right and Share Right Offer Price” of the Tender Offer Document is incorporated herein by reference.
| Item 3. |
Identity and Background of Filing Person. |
Regulation M-A Item 1003
(a)-(c) Name and Address; Business and Background of Entities; and Business and Background of Natural Persons. The information set
forth in Section 6.1—“Offeror in Brief” and Schedule I of the Tender Offer Document is incorporated herein by reference.
| Item 4. |
Terms of the Transaction. |
Regulation M-A Item 1004
(a) Material Terms. The information set forth in the Tender Offer Document is incorporated herein by reference.
| Item 5. |
Past Contacts, Transactions, Negotiations and Agreements. |
Regulation M-A Item 1005
(a)-(b) Transactions. Significant Corporate Events. The information set forth in the Section titled “Summary Term Sheet”,
Section 1.1—“Background to the Tender Offer”, Section 3—“Summary of the Combination Agreement” and Section 6.1—“The Offeror in Brief” of the Tender Offer Document is incorporated herein by
reference.
| Item 6. |
Purposes of the Transaction and Plans or Proposals. |
Regulation M-A Item 1006
(a) Purposes. The information set forth in Section 1.3—“The Offeror’s Strategic Plans” and
Section 1.4—“The Offeror’s Future Plans with respect to Biotie Shares” of the Tender Offer Document is incorporated herein by reference.
(c) (1)-(7) Plans. The information set forth in Section 1.2—“Effect on Biotie’s Operations and Assets and
Future Position of Management and Employees”, Section 1.3—“The Offeror’s Strategic Plans”, Section 1.4—“The Offeror’s Future Plans with respect to Biotie Shares”,
Section 3—“Summary of the Combination Agreement” and Section 4.15—“Certain Effects of the Tender Offer” of the Tender Offer Document is incorporated herein by reference.
| Item 7. |
Source and Amount of Funds or Other Consideration. |
Regulation M-A Item 1007
(a) Source of Funds. The information set forth in the Section title “Summary Term Sheet” and
Section 1.5—“Financing of the Tender Offer” of the Tender Offer Document is incorporated herein by reference.
(b)
Conditions. The Tender Offer is not subject to a financing condition.
(d) Borrowed Funds. Not applicable.
| Item 8. |
Interest in Securities of the Subject Company. |
Regulation M-A Item 1008
(a) Securities Ownership. The information set forth in Section 2.1—“Grounds for Determining the Offer Price with respect
to Biotie Shares”, Section 6.1—“The Offeror in Brief” and Section 6.2—“Persons Related to the Offeror as Stipulated in Chapter 11, Section 5 of the Finnish Securities Market Act” of the Tender Offer
Document is incorporated herein by reference:
(b) Securities Transactions. None.
| Item 9. |
Persons/Assets, Retained, Employed, Compensated or Used. |
Regulation M-A Item 1009
(a) Solicitations or Recommendations. The information set forth in the Section titled “Advisors to the Offeror” and
Section 1.8—“Advisors, Fees and Expenses” of the Tender Offer Document are incorporated herein by reference.
| Item 10. |
Financial Statements. |
Not applicable because (a) the consideration offered
consists solely of cash; (b) the offer is not subject to any financing condition; and (c) the offeror is a public reporting company under Section 13(a) or 15(d) of the Exchange Act that files reports electronically on EDGAR.
| Item 11. |
Additional Information. |
Regulation M-A Item 1011
(a) Agreements, Regulatory Requirements and Legal Proceedings. The information set forth in the Section titled “Summary Term
Sheet”, Section 1.1—“Background to the Tender Offer”, Section 1.6—“Statement by Biotie’s Board of Directors”, Section 1.7—“Irrevocable Undertakings of Major Shareholders”,
Section 3—“Summary of the Combination Agreement”, Section 4.15—“Certain Effects of the Tender Offer” and Section 4.16—“Certain Legal Matters; Regulatory Approvals; Description of SEC
Relief” of the Tender Offer Document is incorporated herein by reference.
(b) Other Material Information. The
information set forth in the Tender Offer Document (other than Annexes B and C thereto), the Letter of Transmittal and the Acceptance Forms is incorporated herein by reference.
Regulation M-A Item 1016
|
|
|
Exhibit
No. |
|
|
|
|
| (a)(1)(A) |
|
Tender Offer Document. |
|
|
| (a)(1)(B) |
|
Letter of Transmittal for holders of ADSs, dated March 11, 2016 (including Internal Revenue Service Form W-9). |
|
|
| (a)(1)(C) |
|
Form of Acceptance Form and Cover Letter for Shares. |
|
|
| (a)(1)(D) |
|
Form of Acceptance Form and Cover Letter for Uncertificated Equity Instruments. |
|
|
| (a)(1)(E) |
|
Form of Acceptance Form and Cover Letter for Certificated Equity Instruments. |
|
|
|
Exhibit
No. |
|
|
|
|
| (a)(1)(F) |
|
Marketing Brochure for holders of Shares, dated March 11, 2016. |
|
|
| (a)(1)(G) |
|
Letter from the CEO of Acorda to holders of Shares, dated March 11, 2016. |
|
|
| (a)(1)(H) |
|
Instruction Letter for Account Operators, dated March 11, 2016. |
|
|
| (a)(1)(I) |
|
Letter from the Information Agent to Brokers, Dealers, Commercial Banks, Trust Companies and Nominees, dated March 11, 2016. |
|
|
| (a)(1)(J) |
|
Letter to Clients for Use by Brokers, Dealers, Commercial Banks, Trust Companies and Nominees, dated March 11, 2016. |
|
|
| (a)(1)(K) |
|
Summary Advertisement as published by the Wall Street Journal on March 11, 2016. |
|
|
| (a)(1)(L) |
|
English translation of Finnish advertisements to be as published by Finnish daily newspapers, Aamulehti, Helsingin Sanomat and Turun Sanomat, on March 14 and March 15, 2016 and displayed on television screens in customer offices of
Pohjola Bank plc. |
|
|
| (b) |
|
None. |
|
|
| (d)(1) |
|
Combination Agreement, dated as of January 19, 2016, between the Company and the Offeror (incorporated by reference to Exhibit 2.1 of the Form 8-K filed by the Offeror on January 19, 2016). |
|
|
| (d)(2) |
|
Forms of Irrevocable Undertaking. |
|
|
| (d)(3) |
|
Confidentiality Agreement, dated as of November 30, 2015, between the Company and the Offeror. |
|
|
| (g) |
|
None. |
|
|
| (h) |
|
None. |
| Item 13. |
Information Required by Schedule 13E-3. |
Not applicable.
SIGNATURE
After due inquiry and to the best of my knowledge and belief, I certify that the information set forth in this statement is true, complete and
correct.
|
|
|
| ACORDA THERAPEUTICS, INC. |
|
|
| By |
|
/s/ Michael Rogers |
| Name: |
|
Michael Rogers |
| Title: |
|
CFO |
|
|
| Date: |
|
March 11, 2016 |
EXHIBIT INDEX
|
|
|
Exhibit
No. |
|
|
|
|
| (a)(1)(A) |
|
Tender Offer Document. |
|
|
| (a)(1)(B) |
|
Letter of Transmittal for holders of ADSs, dated March 11, 2016 (including Internal Revenue Service Form W-9). |
|
|
| (a)(1)(C) |
|
Form of Acceptance Form and Cover Letter for Shares. |
|
|
| (a)(1)(D) |
|
Form of Acceptance Form and Cover Letter for Uncertificated Equity Instruments. |
|
|
| (a)(1)(E) |
|
Form of Acceptance Form and Cover Letter for Certificated Equity Instruments. |
|
|
| (a)(1)(F) |
|
Marketing Brochure for holders of Shares, dated March 11, 2016. |
|
|
| (a)(1)(G) |
|
Letter from the CEO of Acorda to holders of Shares, dated March 11, 2016. |
|
|
| (a)(1)(H) |
|
Instruction Letter for Account Operators, dated March 11, 2016. |
|
|
| (a)(1)(I) |
|
Letter from the Information Agent to Brokers, Dealers, Commercial Banks, Trust Companies and Nominees, dated March 11, 2016. |
|
|
| (a)(1)(J) |
|
Letter to Clients for Use by Brokers, Dealers, Commercial Banks, Trust Companies and Nominees, dated March 11, 2016. |
|
|
| (a)(1)(K) |
|
Summary Advertisement as published by the Wall Street Journal on March 11, 2016. |
|
|
| (a)(1)(L) |
|
English translation of Finnish advertisements to be as published by Finnish daily newspapers, Aamulehti, Helsingin Sanomat and Turun Sanomat, on March 14 and March 15, 2016 and displayed on television screens in customer offices of
Pohjola Bank plc. |
|
|
| (b) |
|
None. |
|
|
| (d)(1) |
|
Combination Agreement, dated as of January 19, 2016, between the Company and the Offeror (incorporated by reference to Exhibit 2.1 of the Form 8-K filed by the Offeror on January 19, 2016). |
|
|
| (d)(2) |
|
Forms of Irrevocable Undertaking. |
|
|
| (d)(3) |
|
Confidentiality Agreement, dated as of November 30, 2015, between the Company and the Offeror. |
|
|
| (g) |
|
None. |
|
|
| (h) |
|
None. |
Exhibit (a)(1)(A)
TENDER OFFER DOCUMENT
PUBLIC TENDER OFFER BY ACORDA THERAPEUTICS, INC.
Offer to Purchase for Cash
All outstanding ordinary shares, no nominal value,
All outstanding American Depositary Shares, each representing 80 ordinary shares, no nominal value,
All outstanding option rights issued under the December 6, 2011 option plan,
All outstanding option rights issued under the January 2, 2014 option plan,
All outstanding option rights issued under the January 4, 2016 option plan,
All outstanding share units issued under the December 6, 2011 equity incentive plan,
All outstanding share units under the January 2, 2014 equity incentive plan,
All outstanding option rights awards under the Swiss option plan dated June 18, 2008,
and
All outstanding
warrants issued on May 28, 2015
of
BIOTIE THERAPIES OYJ
THE OFFER PERIOD AND THE ASSOCIATED WITHDRAWAL RIGHTS WILL EXPIRE ON APRIL 8, 2016 AT 4:00 P.M. (FINNISH
TIME) / 9:00 A.M. (NEW YORK TIME), UNLESS THE OFFER PERIOD IS EXTENDED.
This Tender Offer (as defined below) is being made pursuant to the
Combination Agreement, dated as of January 19, 2016 (the “Combination Agreement”), by and between Acorda Therapeutics, Inc. (“Acorda” or “Offeror”) and Biotie Therapies Oyj
(“Biotie” or the “Company”).
Acorda hereby offers to acquire, in accordance with Chapter 11 of the Finnish Securities
Market Act (746/2012, as amended), the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the terms and conditions of this offer to purchase (this “Tender Offer Document”), all of the issued
and outstanding ordinary shares, no nominal value (the “Shares”), all of the outstanding American Depositary Shares, each representing 80 Shares (the “ADSs”), all of the outstanding Option Rights (as defined
below), all of the outstanding Share Rights (as defined below) and all of the outstanding warrants issued on May 28, 2015 (the “Warrants”) (the outstanding Shares, ADSs, Option Rights, Share Rights and Warrants, collectively,
the “Equity Interests”) in Biotie that are not held by the Company or its subsidiaries (the “Tender Offer”). “Option Rights” means, collectively, option rights granted under the option plan resolved
upon by the board of directors of the Company (the “Board of Directors”) on December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Option
Rights”), option rights granted under the option plan resolved upon by the Board of Directors of the Company on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4,
2013 (the “2014 Option Rights”), option rights granted under the option plan resolved upon by the Board of Directors of the Company on January 4, 2016 by virtue of an authorization granted by the annual general meeting of the
Company held on May 26, 2015 (the “2016 Option Rights”) and option rights granted under the Swiss option plan dated June 17, 2008 (the “Swiss Option Rights”). “Share Rights” means,
collectively, share units under the equity incentive plan resolved upon by the Board of Directors of the Company on December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011
(the “2011 Share Rights”) and share units under the equity incentive plan resolved upon by the Board of Directors of the Company on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the
Company held on April 4, 2013 (the “2014 Share Rights”).
Acorda is a Delaware corporation incorporated in 1995 with shares of its
common stock listed on the NASDAQ Global Market under the symbol “ACOR.”
The Company is a public limited liability company incorporated under the laws of Finland with its Shares listed
on NASDAQ Helsinki Ltd. (“Nasdaq Helsinki”) under the symbol “BTH1V” and its ADSs listed on NASDAQ Global Select Market (“Nasdaq US”) under the symbol “BITI.”
The offer price for each outstanding Share validly tendered in accordance with the terms and conditions of the Tender Offer is EUR 0.2946 in cash (the
“Share Offer Price”).
The offer price for each outstanding ADS validly tendered in accordance with the terms and conditions of the
Tender Offer is EUR 23.5680 in cash (the “ADS Offer Price”), payable in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro
exchange rate on the nearest practicable day to the applicable Closing Date (as defined below). For the avoidance of doubt, holders of ADSs who validly tender their ADSs in accordance with the terms and conditions of the Tender Offer will not be
entitled to any consideration for their ADSs (including the Shares underlying such ADSs) other than the ADS Offer Price.
The offer prices for outstanding
Option Rights validly tendered in accordance with the terms and conditions of the Tender Offer are (i) EUR 0.2846 in cash for each 2011 Option Right, (ii) EUR 0.2846 in cash for each 2014 Option Right, (iii) EUR 0.1326 in cash for
each 2016 Option Right payable, at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the
nearest practicable day to the applicable Closing Date, (iv) EUR 0.2032 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.10, (v) EUR 0.1026 in cash for each Swiss Option Right with a per Share subscription
price of CHF 0.21, (vi) EUR 0.0386 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.28, (vii) EUR 0.0112 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.31, and
(viii) EUR 0.0100 in cash for each other Swiss Option Right (collectively, the “Option Right Offer Price”).
The offer prices for
outstanding Share Rights validly tendered in accordance with the terms and conditions of the Tender Offer are (i) EUR 0.2946 in cash for each 2011 Share Right, and (ii) EUR 0.2854 in cash for each 2014 Share Right, payable, in each case,
at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the
applicable Closing Date (collectively, the “Share Right Offer Price”).
The offer price for each outstanding Warrant validly tendered in
accordance with the terms and conditions of the Tender Offer is EUR 0.1664 in cash (the “Warrant Offer Price,” and together with the Share Offer Price, the ADS Offer Price, the Option Right Offer Price and the Share Right Offer
Price, the “Offer Price”).
The Offer Price will be paid in each case without interest and less any applicable withholding taxes (in the
United States, see Section 4.13—”Material United States Federal Income Tax Consequences”).
The initial acceptance period for the
Tender Offer (the “Offer Period”) will commence on March 11, 2016 at 9:30 a.m. (Finnish time) / 2:30 a.m. (New York time) and expire on April 8, 2016 at 4:00 p.m. (Finnish time) / 9:00 a.m. (New York time) unless the Offer Period is
extended (the end of the Offer Period, as extended, the “Expiration Date”). The Offeror reserves the right to initiate a subsequent offer period in connection with the announcement of the final results with respect to the Offer
Period if the Tender Offer shall have been announced to be unconditional at that time (such subsequent offer period, the “Subsequent Offer Period”). See Section 4.1—“Terms and Conditions of the Tender
Offer” and Section 4.11—“Subsequent Offer Period”.
The completion of the Tender Offer is subject to the
satisfaction of the conditions described under Section 4.2—“Conditions to Completion of the Tender Offer” of this Tender Offer Document. The Tender Offer is not subject to a financing condition. The Offeror reserves the
right to waive any conditions to completion of the Tender Offer, subject to compliance with applicable law (including U.S. tender offer rulers that require the Tender Offer
2
remain open for at least five U.S. business days from the date of any waiver of a material condition to completion).
If the Tender Offer is consummated and all Equity Interests validly tendered and not withdrawn have been transferred to the Offeror (the date of each such
transfer, a “Closing Date”), and the Offeror has acquired more than 90 percent of all outstanding Shares (including Shares represented by ADSs), the Offeror intends, as soon as practicable after consummation of the Tender Offer, to
initiate subsequent compulsory redemption proceedings to redeem the remaining outstanding Shares (including Shares represented by ADSs) in accordance with the Finnish Companies Act (such potential proceedings, the “Subsequent Compulsory
Redemption”) and to redeem the other Equity Interests in accordance with the terms of such Equity Interests.
Holders of Equity Interests
representing approximately 65 percent of the outstanding Shares and votes on a fully diluted basis have, subject to certain customary conditions, irrevocably undertaken to accept the Tender Offer.
After careful consideration, the Board of Directors of the Company has determined that the Combination Agreement and the transactions contemplated thereby,
including the Tender Offer, are advisable, fair to and in the best interests of the Company and the holders of the Equity Interests. Accordingly, the Board of Directors has recommended that the holders of Equity Interests accept the Tender Offer and
tender their Equity Interests to Offeror in the Tender Offer.
The information on these front pages should be read in conjunction with, and is
qualified in its entirety by, the more detailed information in this Tender Offer Document, in particular Section 4—“Terms and Conditions of the Tender Offer”. You should carefully read this entire Tender Offer
Document, the Acceptance Form for Shares, and if you hold Uncertificated Equity Instruments, the Acceptance Form for Uncertificated Equity Instruments, and if you hold Certificated Equity Instruments, the Acceptance Form for Certificated Equity
Instruments, as well as any instruction letter attached to such Acceptance Forms (such acceptance forms and attached instructions, the “Acceptance Forms”), and if you hold ADSs, the related Letter of Transmittal for ADSs (the
“Letter of Transmittal”) before deciding whether to tender your Equity Interests in the Tender Offer.
THIS TENDER OFFER
DOCUMENT AND RELATED MATERIALS, INCLUDING THE LETTER OF TRANSMITTAL AND ACCEPTANCE FORMS, WILL NOT AND MAY NOT BE DISTRIBUTED, FORWARDED OR TRANSMITTED INTO OR FROM ANY JURISDICTION WHERE PROHIBITED BY APPLICABLE LAW BY ANY MEANS WHATSOEVER
INCLUDING, WITHOUT LIMITATION, MAIL, FACSIMILE TRANSMISSION, E-MAIL OR TELEPHONE. IN PARTICULAR, THE TENDER OFFER IS NOT BEING MADE, DIRECTLY OR INDIRECTLY, IN OR INTO, CANADA, JAPAN, AUSTRALIA, SOUTH AFRICA OR HONG KONG OR ANY OTHER JURISDICTION
WHERE PROHIBITED BY LAW. THE TENDER OFFER CANNOT BE ACCEPTED BY ANY SUCH USE, MEANS OR INSTRUMENTALITY OR FROM WITHIN CANADA, JAPAN, AUSTRALIA, SOUTH AFRICA OR HONG KONG OR ANY OTHER JURISDICTION WHERE PROHIBITED BY LAW.
This transaction has not been approved or disapproved by the U.S. Securities and Exchange Commission (the “SEC”) or any state securities
commission nor has the SEC or any state securities commission passed upon the fairness or merits of this transaction or upon the accuracy or adequacy of the information contained in this Tender Offer Document or related materials, including the
Letter of Transmittal and Acceptance Forms. Any representation to the contrary is unlawful.
IMPORTANT
INFORMATION
This Tender Offer Document has been prepared in accordance with Finnish and United States law, including the Finnish Securities
Market Act (746/2012, as amended, the “Finnish Securities Market Act”), Decree 1022/2012 of the Ministry of Finance and regulations and guidelines 7/2013 (FIVA 9/01.00/2013) and 9/2013 (FIVA
3
10/01.00/2013) issued by the Finnish Financial Supervisory Authority (the “FSA”), and the Exchange Act and rules and regulations promulgated thereunder.
The Offeror has undertaken to follow the Helsinki Takeover Code issued by the Securities Market Association referred to in Chapter 11 Section 28 of the
Finnish Securities Market Act. According to the statement issued by the Board of Directors of Biotie on March 4, 2016 and attached as Annex A to this Tender Offer Document, Biotie has also undertaken to follow the Helsinki Takeover Code.
The FSA has approved the Finnish language version of the Tender Offer Document but is not responsible for the accuracy of the information presented therein.
The decision number of such approval is 2/02.05.05./2016.
The Tender Offer will not be made directly or indirectly in any jurisdiction where prohibited
by applicable law or where any document, registration or other requirement would be necessary in addition to those required by the applicable law in Finland and the United States.
All information concerning the Company presented in this Tender Offer Document has been extracted from, and has been provided exclusively based upon, publicly
available information. In addition, the Company has informed the Offeror of the transaction bonuses payable to holders of 2016 Option Rights (please see Section 5.5—”Option Rights and Share Rights”). Consequently, the Offeror does not
accept any responsibility for such information except for the accurate restatement of such information herein.
This Tender Offer Document will not be
supplemented or updated with any financial information or other stock exchange releases published by the Company after the date of this Tender Offer Document nor will the Offeror otherwise separately inform holders about the publishing of such
financial information or other stock exchange releases, except to the extent required by applicable law (including if the Offeror if required to make supplemental disclosures pursuant to Rule 14d-6(c) under the Exchange Act).
This Tender Offer Document, the Letter of Transmittal and the Acceptance Forms contain important information, and you should read each carefully and in
their entirety before making a decision with respect to the Tender Offer.
FOR HOLDERS OF SHARES
If you desire to tender all or any portion of your Shares to the Offeror pursuant to the Tender Offer, you should either (a) submit a properly completed
and duly executed Acceptance Form for Shares to the account operator managing your book-entry account in accordance with its instructions and within the time limit set by such account operator, which may be prior to the expiry of the Offer Period,
or, if your account operator does not accept Acceptance Forms (e.g. Euroclear), you should contact any branch office of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd. to give your acceptance to tender your Shares;
or (b) request that your broker, dealer, commercial bank, trust company, custodian or other nominee effect the transaction for you. If you hold your Shares at a broker, dealer, commercial bank, trust company, custodian or other nominee, you
must contact that institution in order to tender such Shares to the Offeror pursuant to the Tender Offer. Please note that the Tender Offer must be accepted separately for each book-entry account. See the procedures described in
Section 4.4—“Acceptance Procedure of the Tender Offer” for further information.
Questions and requests for assistance regarding the
Tender Offer or any of the terms thereof with respect to Shares may be directed to the call service of OP Financial Group at (+358) (0) 100 0500. Requests for additional copies of this Tender Offer Document, Acceptance Forms (including the
instructions attached thereto) and other tender offer materials may be directed to the call service of OP Financial Group at (+358) (0) 100 0500. You may also contact your account operator, broker, dealer, commercial bank, trust company,
custodian or other nominee for assistance.
4
FOR HOLDERS OF ADSs
If you desire to tender all or any portion of your ADSs to the Offeror pursuant to the Tender Offer, you should either (a) send (i) an Agent’s
Message (as defined in Section 4.4—“Acceptance Procedure of the Tender Offer”) or (ii) a properly completed and duly executed Letter of Transmittal and any other required documents to Computershare Trust Company, N.A., in
its capacity as depositary for the Tender Offer (the “Depositary”) prior to the Expiration Date or end of any Subsequent Offer Period, as applicable, and follow the procedures described in Section 4.4—“Acceptance
Procedure of the Tender Offer,” or (b) request that your broker, dealer, commercial bank, trust company, custodian or other nominee effect the transaction for you. If you hold such ADSs registered in the name of a broker, dealer,
commercial bank, trust company, custodian or other nominee, you must contact that institution in order to tender such ADSs to the Offeror pursuant to the Tender Offer. There are no guaranteed delivery procedures and holders of ADSs may not accept
the Tender Offer by delivering a notice of guaranteed delivery.
Questions and requests for assistance regarding the Tender Offer or any of the terms
thereof with respect to ADSs may be directed to Innisfree M&A Incorporated, as information agent for the Tender Offer with respect to the ADSs (the “Information Agent for ADSs”), at the address and telephone number set forth for
the Information Agent for ADSs on the back cover of this Tender Offer Document. Requests for additional copies of this Tender Offer Document, the Letter of Transmittal and other tender offer materials may be directed to the Information Agent for
ADSs. You may also contact your broker, dealer, commercial bank, trust company, custodian or other nominee for assistance.
FOR
HOLDERS OF OPTION RIGHTS, SHARE RIGHTS AND WARRANTS
The acceptance procedure for Option Rights, Share Rights and Warrants depends on whether such
Equity Interests are in certificated or book-entry form. All 2011 Option Rights, the 2014 Option Rights in the 2014A tranche and all Warrants are uncertificated Equity Interests (“Uncertificated Equity Instruments”). If you desire
to tender all or any portion of such Uncertificated Equity Instruments to the Offeror pursuant to the Tender Offer, you should either (a) submit a properly completed and duly executed Acceptance Form for Uncertificated Equity Instruments to the
account operator managing your book-entry account in accordance with its instructions and within the time limit set by the account operator, which may be prior to the expiry of the Offer Period, or, if such account operator does not accept
Acceptance Forms (e.g. Euroclear), you should contact any branch office of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd. to give your acceptance to tender your Uncertificated Equity Instruments; or
(b) request that your broker, dealer, commercial bank, trust company, custodian or other nominee effect the transaction for you. If you hold such Uncertificated Equity Instruments at a broker, dealer, commercial bank, trust company, custodian
or other nominee, you must contact that institution in order to tender such Uncertificated Equity Instruments to the Offeror pursuant to the Tender Offer. Please note that the Tender Offer must be accepted separately for each type of Uncertificated
Equity Instrument and, if such Uncertificated Equity Instruments are held in more than one book-entry account, separately for each book-entry account. See the procedures described in Section 4.4—“Acceptance Procedure of the Tender
Offer” for further information.
The 2014 Option Rights in the 2014B, 2014C, 2014D and 2014M tranches, all 2016 Option Rights, all Swiss Option
Rights, all 2011 Share Rights and all 2014 Share Rights are certificated Equity Interests (“Certificated Equity Instruments”). If you desire to tender all or any portion of such Certificated Equity Instruments to the Offeror
pursuant to the Tender Offer, you should submit a properly completed and duly executed Acceptance Form for Certificated Equity Instruments to Pohjola Bank (as defined herein), in accordance with the instructions that will be sent to you together
with the Acceptance Form and within the time limit set forth in the instructions. Please note that the Tender Offer may be accepted only in respect of Certificated Equity Instruments registered in your name in the Company’s register for such
Certificated Equity Instruments on the date of acceptance of the Tender Offer. See the procedures described in Section 4.4—“Acceptance Procedure of the Tender Offer” for further information.
5
Questions and requests for assistance regarding the Tender Offer or any of the terms thereof with respect to
Option Rights, Share Rights and Warrants may be directed to the call service of OP Financial Group at (+358) (0) 100 0500. Requests for additional copies of this Tender Offer Document, Acceptance Forms (including the instructions attached
thereto) and other tender offer materials may also be directed to the call service of OP Financial Group at (+358) (0) 100 0500. You may also contact your account operator, broker, dealer, commercial bank, trust company, custodian or other
nominee for assistance.
* * * * *
This
Tender Offer Document will be available in Finnish from March 11, 2016 onwards at the branch offices of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd., at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki,
Finland, and at the offices of the Offeror at Office of the Corporate Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on the internet at www.op.fi/merkinta, http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/sijoittajat and in English from March 11, 2016 onwards on the internet at http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/investors.
FORWARD LOOKING STATEMENTS
This
Tender Offer Document includes “forward looking statements”, including statements about the expected timing and completion of the Tender Offer, and language indicating trends. Generally, words such as may, should, aim, will, expect,
intend, estimate, anticipate, believe, plan, seek, contemplate, envisage, continue or similar expressions identify forward-looking statements.
These
statements are subject to risks, uncertainties, assumptions and other important factors, many of which may be beyond the control of the Offeror, and could cause actual results to differ materially from those expressed or implied in these
forward-looking statements.
Factors that could cause actual results to differ from such statements include: the occurrence of any event, change or other
circumstances that could give rise to the termination of the Tender Offer, the failure to receive, on a timely basis or otherwise, the required approvals by government or regulatory agencies, the risk that a condition to consummating the Tender
Offer may not be satisfied, the ability of the Company to retain and hire key personnel and maintain relationships with customers, suppliers and other business partners pending the completion of the Tender Offer, and other factors.
Although the Offeror believes that the expectations reflected in such forward-looking statements are based on reasonable assumptions, no assurance can be
given that such statements will be fulfilled or prove to be correct, and no representations are made as to the future accuracy and completeness of such statements.
The Offeror undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or
otherwise, except as required by applicable laws or by any appropriate regulatory authority.
6
CERTAIN KEY DATES
The following timetable sets forth certain key dates relating to the Tender Offer, provided that the Offer Period has not been extended and the Offeror has
accepted for payment the tendered Equity Interests in accordance with the terms and conditions of the Tender Offer:
|
|
|
| January 19, 2016 |
|
Announcement of entry into the Combination Agreement |
| March 11, 2016 |
|
Offer Period commences |
| April 8, 2016 |
|
Offer Period expires |
| April 11, 2016 |
|
Announcement of the preliminary result with respect to the Offer Period |
| April 13, 2016 |
|
Announcement of the final result with respect to the Offer Period |
| April 18, 2016 |
|
Payment in cash for tendered Equity Interests* |
| * |
Estimated date. Actual time of receipt for the payment will depend on the schedules of money transactions between financial institutions. |
PERSONS RESPONSIBLE FOR THE TENDER OFFER DOCUMENT
Offeror
Acorda Therapeutics, Inc.
Address: 420 Saw Mill River Road, Ardsley, NY 10502, USA
Domicile: Delaware, U.S.A.
The Board of Directors of the
Offeror
Ron Cohen, M.D.
Barry Greene
Peder K. Jensen, M.D.
John P. Kelley
Sandra Panem, Ph.D.
Lorin J. Randall
Steven M. Rauscher
Ian F. Smith
The Executive Management of the Offeror
Rick Batycky,
Ph. D., Chief Technology Officer
Andrew R. Blight, Ph. D., Chief Scientific Officer
Ron Cohen, M. D., President and Chief Executive Officer
Andrew
Hindman, Chief Business Development Officer
David Lawrence, Chief of Business Operations
Michael Rogers, Chief Financial Officer
Lauren M. Sabella, Chief
Commercial Officer
Jane Wasman, President, International, General Counsel and Corporate Secretary
7
STATEMENT BY THE OFFEROR
This Tender Offer Document has been prepared by the Offeror pursuant to Chapter 11, Section 11 of the Finnish Securities Market Act and the Exchange Act
and rules and regulations promulgated thereunder for purposes of the Tender Offer set out herein.
The Offeror represents that to the best of its
knowledge and understanding the information contained in this Tender Offer Document is accurate and complete and no information has been omitted that is likely to affect the assessment of the merits of the Tender Offer.
All information concerning the Company presented in this Tender Offer Document has been extracted from, and has been provided exclusively based upon, publicly
available information. Consequently, the Offeror does not accept any responsibility for such information, except for the accurate restatement of such information herein.
In Ardsley, NY, March 10, 2016
Acorda Therapeutics, Inc.
ADVISORS TO THE OFFEROR
Financial advisors to the Offeror
|
|
|
| Lazard Frères & Co. LLC
30 Rockefeller Plaza New York, NY
10020 U.S.A |
|
MTS Health Partners L.P.
MTS Securities LLC 623 Fifth
Avenue, 14th Floor New York, NY 10022
U.S.A. |
Legal advisors to the Offeror in connection with the Tender Offer
|
|
|
| As to Finnish law: |
|
As to U.S. law: |
| Roschier, Attorneys Ltd.
Keskuskatu 7 A FI-00100
Helsinki Finland |
|
Kirkland & Ellis LLP
601 Lexington Avenue New York, NY
11101 U.S.A. |
Arranger of the Tender Offer
Receiving and Paying Agent for Shares, Option Rights, Share Rights and Warrants
Capital Markets Financing department of Pohjola Bank plc (“Pohjola Bank”)
Teollisuuskatu 1
FI-00510 Helsinki
Finland
Communication Agent
Hill+Knowlton Strategies, Finland
Ludviginkatu 6
FIN-00130 Helsinki
Finland
8
Depositary and Receiving and Paying Agent for ADSs
Computershare Trust Company, N.A.
By First Class, Registered
or Certified Mail:
Computershare Trust Company, N.A.
c/o Voluntary Corporate Actions
PO Box 43011
Providence, RI 02940-3011
By Express or Overnight Delivery:
Computershare Trust Company, N.A.
c/o Voluntary
Corporate Actions
250 Royall Street, Suite V
Canton, MA
02021
By Facsimile Transmission (for eligible institutions only):
Computershare Trust Company, N.A.
Facsimile: (617) 360-6810
Confirm By Telephone:
(+1) 781 575 2332
Information Agent for ADSs
Innisfree M&A
Incorporated
501 Madison Avenue, 20th Floor
New York, NY
10022
U.S.A.
(+1) 888 750 5834 (toll-free from the United
States)
(+1) 412 232 3651 (from other locations)
Banks and
Brokers may call collect at (+1) 212 750 5833
ADVISORS TO THE COMPANY
Financial advisor to the Company in connection with the Tender Offer
Guggenheim Securities, LLC
330 Madison Avenue
New York, NY 10017
U.S.A.
Legal advisors to the Company in connection with the Tender Offer
|
|
|
| As to Finnish law: |
|
As to U.S. law: |
| Hannes Snellman Attorneys Ltd.
Eteläesplanadi 20 00130
Helsinki Finland |
|
Davis Polk & Wardwell LLP
450 Lexington Avenue New York, NY
10017 U.S.A. |
9
TABLE OF CONTENTS
10
ANNEXES
|
|
|
| Annex A — Statement of Biotie’s Board of Directors |
|
|
| Annex B — Financial Statements of Biotie for the financial year ended December 31, 2014 |
|
|
| Annex C — Interim Report of Biotie for the nine months ended September 30, 2015, unaudited |
|
|
| Annex D — Stock Exchange Releases Published by Biotie on January 19, 2016 and February 17, 2016 |
|
|
| Annex E — Articles of Association of Biotie |
|
|
| Annex F — Forms of Irrevocable Undertaking |
|
|
| Annex G — Combination Agreement |
|
|
11
SUMMARY TERM SHEET
This summary term sheet highlights selected information from this offer to purchase (this “Tender Offer Document”) and may not contain all
of the information that is important to you and is qualified in its entirety by the more detailed descriptions and explanations contained in this Tender Offer Document, the related Letter of Transmittal for ADSs (the “Letter of
Transmittal”), and the Acceptance Form for Shares (including any instruction letter attached thereto), Acceptance Form for Uncertificated Equity Instruments (including any instruction letter attached thereto) and Acceptance Form for
Certificated Equity Instruments (including any instruction letter attached thereto) (such acceptance forms and attached instructions, the “Acceptance Forms”). To better understand the Tender Offer (as defined below) and for a
complete description of the legal terms of the Tender Offer, you should read this Tender Offer Document, the Letter of Transmittal and the Acceptance Forms carefully and in their entirety. Questions or requests for assistance may be directed to the
call service of OP Financial Group at (+358) (0) 100 0500 or Innisfree M&A Incorporated (the “Information Agent for ADSs”) at the address and telephone number available on the back cover of this Tender Offer Document,
as applicable. Unless otherwise indicated in this Tender Offer Document or the context otherwise requires, all references in this Tender Offer Document to “we,” “our” or “us” refer to the Offeror (as defined below).
Acorda Therapeutics, Inc. (“Acorda” or the “Offeror”) is offering to acquire, in accordance with Chapter 11 of
the Finnish Securities Market Act (746/2012, as amended the “Finnish Securities Market Act”), the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the terms and conditions of this Tender Offer
Document, the Letter of Transmittal, and the Acceptance Forms, all of the issued and outstanding ordinary shares, no nominal value (the “Shares”), all of the outstanding American Depositary Shares, each representing 80 Shares (the
“ADSs”), all of the outstanding Option Rights (as defined below), all of the outstanding Share Rights (as defined below) and all of the outstanding warrants issued on May 28, 2015 (the “Warrants”) (the
outstanding Shares, ADSs, Option Rights, Share Rights and Warrants, collectively, the “Equity Interests”) in Biotie Therapies Oyj (“Biotie” or the “Company”) that are not held by the Company or its
subsidiaries (the “Tender Offer”). “Option Rights” means, collectively, option rights granted under the option plan resolved upon by the board of directors of the Company (the “Board of Directors”)
on December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Option Rights”), option rights granted under the option plan resolved upon by the Board
of Directors of the Company on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013 (the “2014 Option Rights”), option rights granted under the option
plan resolved upon by the Board of Directors of the Company on January 4, 2016 by virtue of an authorization granted by the annual general meeting of the Company held on May 26, 2015 (the “2016 Option Rights”) and option
rights granted under the Swiss option plan dated June 17, 2008 (the “Swiss Option Rights”). “Share Rights” means, collectively, share units under the equity incentive plan resolved upon by the Board of
Directors of the Company on December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Share Rights”) and share units under the equity incentive plan
resolved upon by the Board of Directors of the Company on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013 (the “2014 Share Rights”).
The Offer Price, as defined and set forth in Section 4.1—“Terms of the Tender Offer,” for the Equity Interests is the Share Offer Price
in cash for each Share, the ADS Offer Price in cash for each ADS, the Option Right Offer Price in cash for each Option Right, the Share Right Offer Price in cash for each Share Right, and the Warrant Offer Price in cash for each Warrant, in each
case, validly tendered in the Tender Offer.
The following are some questions you, as a holder of Equity Interests of the Company, may have and
answers to those questions.
12
Who is offering to buy my Equity Interests?
Acorda is a Delaware corporation incorporated in 1995 with shares of its common stock listed on the NASDAQ Global Market under the symbol “ACOR.” See
Section 1.1—“Background to the Tender Offer” and Section 6.1—“The Offeror in Brief.”
What Equity Interests are
you offering to purchase?
We are making an offer to purchase all of the Equity Interests on the terms and subject to the conditions set forth in this
Tender Offer Document. See Section 4.1—“Terms of the Tender Offer.”
Why are you making the Tender Offer?
We are making the Tender Offer because we want to acquire control of, and ultimately the entire equity interest in, the Company. If the Tender Offer is
consummated and all Equity Interests validly tendered and not withdrawn have been transferred to the Offeror (the date of each such transfer, a “Closing Date”), and the Offeror has acquired more than 90 percent of all outstanding
Shares (including Shares represented by ADSs), the Offeror intends, as soon as practicable after consummation of the Tender Offer, to initiate subsequent compulsory redemption proceedings to redeem the remaining outstanding Shares (including Shares
represented by ADSs) in accordance with the Finnish Companies Act (such potential proceedings, the “Subsequent Compulsory Redemption”) and to redeem the other Equity Interests in accordance with the terms of such Equity Interests.
Upon acquiring all Equity Interests, the Company would be a wholly-owned direct subsidiary of the Offeror.
How much are you offering to pay and what
is the form of payment? Will I have to pay any fees or commissions?
We are offering to pay (i) the Share Offer Price of EUR 0.2946 in cash per
Share, (ii) the ADS Offer Price of EUR 23.5680 in cash per ADS, (iii) the Option Right Offer Price and Share Right Offer Price of EUR 0.2946 minus the applicable subscription price in cash for each of the Option Rights and Share Rights, as
applicable, with an applicable subscription price less than EUR 0.2946, and the Option Right Offer Price of EUR 0.01 in cash for each other Option Right and (iv) the Warrant Offer Price of EUR 0.1664 in cash per warrant. The Offer Price is paid
in each case, without interest and less any applicable withholding taxes (in the United States, see Section 4.13—“Material United States Federal Income Tax Consequences”). If you are the record owner of your Equity Interests and you
directly tender your Equity Interests to us in the Tender Offer, you will not have to pay brokerage fees, commissions or similar expenses. If you own your Equity Interests through a broker or other nominee, and your broker tenders your Equity
Interests on your behalf, your broker or nominee may charge you a fee for doing so. You should consult with your account operator, broker, custodian or nominee to determine whether any charges will apply. In connection with a Subsequent Compulsory
Redemption, if any, holders of ADSs will bear any fees and expenses charged by the depositary under the ADS deposit agreement. See Section 4.1—“Terms of the Tender Offer,” and Section 4.12—“Transfer Tax and Other
Payments.”
Is there an agreement governing the Tender Offer?
Yes. The Combination Agreement, dated as of January 19, 2016 (the “Combination Agreement”), by and between Acorda and Biotie, provides,
among other things, for the terms and conditions of the Tender Offer. See Section 3—“Summary of the Combination Agreement.”
What
are the conditions to the Tender Offer?
The completion of the Tender Offer will be subject to the Conditions of Completion as defined and set forth in
Section 4.2—“Conditions to Completion of the Tender Offer.” The Offeror reserves the right to complete the Tender Offer even if the Conditions to Completion have not been fulfilled. See Section 4.2—“Conditions to
the Completion of the Tender Offer.”
13
Do you have the financial resources to pay for all of the Equity Interests that you are offering to purchase
in the Tender Offer?
Yes. If the Conditions of Completion are satisfied, Acorda will have sufficient funds to acquire all of the Equity Interests
validly tendered in the Tender Offer and not validly withdrawn (and any Equity Interests to be acquired in a Subsequent Compulsory Redemption or otherwise). The necessary funds are available from Acorda’s cash on hand, which includes the
proceeds of the Share Issuance (as defined in Section 1.5—“Financing of the Tender Offer”). The Tender Offer is not conditioned on any financing arrangement. See Section 1.5—“Financing of the Tender Offer.”
Is your financial condition relevant to my decision to tender my Equity Interests in the Tender Offer?
We do not think that our financial condition is relevant to your decision whether to tender Equity Interests and accept the Tender Offer because:
| |
• |
|
the Tender Offer is being made for all Equity Interests solely for cash; |
| |
• |
|
the Tender Offer is not subject to any financing condition; and |
| |
• |
|
the Offeror will have sufficient funds available to purchase all Equity Interests validly tendered in the Tender Offer and not validly withdrawn, and any Equity Interests purchased in a Subsequent Compulsory Redemption
or otherwise, in light of the Offeror’s financial capacity in relation to the amount of consideration payable. |
See
Section 1.5—“Financing of the Tender Offer.”
How long do I have to decide whether to tender my Equity Interests in the Tender
Offer?
The initial acceptance period for the Tender Offer (the “Offer Period”) will commence on March 11, 2016 at 9:30 a.m. (Finnish
time) / 2:30 a.m. (New York time) and expire on April 8, 2016 at 4:00 p.m. (Finnish time) / 9:00 a.m. (New York time) unless the Offer Period is extended (the end of the Offer Period, as extended, the “Expiration Date”). You will
have until the Expiration Date, or in the event of a Subsequent Offer Period, as defined in the answer to the following question, until the end of such Subsequent Offer Period, to tender your Equity Interests in the Tender Offer, in each case
subject to the time limits set by any broker, dealer, commercial bank, trust company, custodian or other nominee that tenders your Equity Interests on your behalf, which may end prior to the Expiration Date. See Section 4.1—“Terms and
Conditions of the Tender Offer” and Section 4.11—“Subsequent Offer Period”.
Under what circumstances can or will the Tender
Offer be extended?
Subject to the following paragraph, if at the scheduled expiration time of the Offer Period any of the Conditions to Completion are
not satisfied, we will extend the Offer Period for additional periods not exceeding two (2) weeks each in accordance with this Tender Offer Document and, in each case, subject to compliance with applicable Finnish and United States legal
requirements.
The maximum duration of the Offer Period is ten (10) weeks as required by Finnish law. However, under the Finnish Securities Market
Act, if any of the Conditions to Completion has not been fulfilled due to a particular obstacle, the Tender Offer may for a special reason stay in force more than ten (10) weeks, provided that the business operations of the Company are not
hindered for longer than is reasonable, until such obstacle has been removed and until all Conditions to Completion have been satisfied. In no event are we required to extend the Tender Offer beyond June 19, 2016.
U.S. tender offer rules require that the Offeror extend the Tender Offer if the Offeror intends to materially change the Tender Offer within ten U.S. business
days of the then-scheduled Expiration Date, so that the Tender Offer will expire no less than ten U.S. business days after the publication of the material change.
14
We reserve the right to initiate a subsequent offer period in connection with the announcement of the final
results with respect to the Offer Period if the Tender Offer shall have been announced to be unconditional at that time (such subsequent offer period, the “Subsequent Offer Period”). In the event of such Subsequent Offer Period, the
Subsequent Offer Period will expire on the date and at the time determined by us in the final result announcement. The Subsequent Offer Period and any extension thereof may extend beyond ten (10) weeks from the beginning of the original Offer
Period (and, in our discretion, beyond June 19, 2016). See Section 4.11—“Subsequent Offer Period”.
How will I be notified if
the Tender Offer is extended?
If we extend the Offer Period, we will inform the Depositary and the Receiving and Paying Agent for Shares, Option
Rights, Share Rights and Warrants (each as defined in Section 1.8—“Advisors, Fees and Expenses”) of that fact and will make a public announcement of the extension by a stock exchange release at or before 4:00 p.m. (Finnish
time) / 9:00 a.m. (New York City time) on April 11, 2016. We will give notice of any further extension of the Tender Offer, at the latest on the first Finnish banking day following what would have been, but for such extension, the Expiration Date or
end of any Subsequent Offer Period, as applicable, at or before 4:00 p.m. (Finnish time) / 9:00 a.m. (New York City time). See Section 4.1—“Terms of the Tender Offer.”
How do I tender my Equity Interests?
If you hold Shares
and wish to accept the Tender Offer with respect to such Shares, then acceptances of the Tender Offer must be delivered to the account operator managing your book-entry account in accordance with its instructions and within the time limit set by the
account operator, which may be prior to the expiry of the Offer Period, or, if such account operator does not accept Acceptance Forms (e.g. Euroclear), you must contact any branch office of the cooperative banks belonging to the OP Financial Group
or Helsinki OP Bank Ltd. to tender your Shares into the Tender Offer. The Acceptance Form for Shares shall be submitted so that it is received on or prior to the Expiration Date, subject to and in accordance with the instructions of the relevant
account operator. In the event of a Subsequent Offer Period, the Acceptance Form shall be submitted so that it is received during the Subsequent Offer Period, subject to and in accordance with the instructions of the relevant account operator.
Detailed instructions are contained in the Acceptance Form for Shares and in Section 4.4—“Acceptance Procedure of the Tender Offer.” Please call the call service of OP Financial Group at (+358) (0) 100 0500 for
assistance.
If you hold ADSs and wish to accept the Tender Offer, you should complete the confirmation of a book-entry transfer of your ADSs into the
account of the Depositary at The Depository Trust Company (“DTC”) and send either (i) an Agent’s Message (as defined below) or (ii) a properly completed and duly executed Letter of Transmittal and any other required
documents to the Depositary prior to the Expiration Date or end of any Subsequent Offer Period, as applicable. These materials must reach the Depositary on or prior to the Expiration Date or end of any Subsequent Offer Period, as applicable. If you
hold such ADSs registered in the name of a broker, dealer, commercial bank, trust company, custodian or other nominee, you must contact that institution in order to tender such ADSs to the Offeror pursuant to the Tender Offer.
An “Agent’s Message” delivered in lieu of the Letter of Transmittal is a message transmitted by DTC to, and received by, the Depositary
as part of a confirmation of a book-entry transfer. The message states that DTC has received an express acknowledgment from the DTC participant tendering ADSs that such participant has received and agrees to be bound by the terms of the Letter of
Transmittal and that we may enforce such agreement against such participant.
Detailed instructions are contained in the Letter of Transmittal and in
Section 4.4—“Acceptance Procedure of the Tender Offer.” Please call Innisfree M&A Incorporated, the Information Agent for ADSs, at (+1) 888 750 5834 (toll-free from the United States) or at (+1) 412 232 3651 (from
other locations), for assistance. (Banks and Brokers may call collect at (+1) 212 750 5833).
15
If you hold Warrants or Option Rights that have been entered into the Finnish book-entry securities system and
wish to accept the Tender Offer with respect to such Equity Interests, then acceptances of the Tender Offer must be received by the account operator managing your book-entry account in accordance with its instructions and within the time limit set
by the account operator, which may be prior to the expiry of the Offer Period, or, if such account operator does not accept Acceptance Forms (e.g. Euroclear), such holder of Warrants or Option Rights shall contact any branch office of the
cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd. to give his/her acceptance to tender his/her Warrants and/or Option Rights. The Acceptance Form for Uncertificated Equity Instruments shall be submitted so that it is
received on or prior to the Expiration Date, subject to and in accordance with the instructions of the relevant account operator. In the event of a Subsequent Offer Period, the Acceptance Form shall be submitted so that it is received during the
Subsequent Offer Period, subject to and in accordance with the instructions of the relevant account operator. Detailed instructions are contained in the Acceptance Form and in Section 4.4—“Acceptance Procedure of the Tender
Offer.” Please call the call service of OP Financial Group at (+358) (0) 100 0500 for assistance.
If you hold Share Rights or Option
Rights that have not been entered into the Finnish book-entry securities system and wish to accept the Tender Offer with respect to such Equity Interests, then acceptances of the Tender Offer must be received by the Capital Markets Financing
department of Pohjola Bank plc (“Pohjola Bank”). Detailed instructions are contained in Section 4.4—“Acceptance Procedure of the Tender Offer” and in the instructions that will be sent to you together with an
Acceptance Form for Certificated Equity Instruments. Please call the call service of OP Financial Group at (+358) (0) 100 0500 for assistance. See Section 4.4—“Acceptance Procedure of the Tender Offer.”
Until what time may I withdraw previously tendered Equity Interests?
You may withdraw previously tendered Equity Interests any time prior to the Expiration Date by following the procedures for withdrawing your Equity Interests
in a timely manner. If you tendered your Equity Interests by giving instructions to a broker or other nominee, you must instruct your broker or nominee prior to the Expiration Date to arrange for the withdrawal of your Equity Interests in a timely
manner. Equity Interests tendered during a Subsequent Offer Period, if any, may not be withdrawn. See Section 4.5—“Withdrawal Rights.”
How do I withdraw previously tendered Equity Interests?
To validly withdraw any of your previously tendered Shares, Option Rights, Share Rights or Warrants, you must submit a written notice of withdrawal to the same
account operator to whom the Acceptance Form(s) for such Equity Interests was submitted. If you submitted an Acceptance Form with respect to such Equity Interests to a branch office of the cooperative banks belonging to the OP Financial Group or
Helsinki OP Bank Ltd., the notice of withdrawal must be submitted to the same branch office.
To validly withdraw any of your previously tendered ADSs,
you must deliver a written notice of withdrawal, or a facsimile of one (with original delivered via overnight courier), with the required information to the Depositary while you still have the right to withdraw such ADSs.
If you tendered your Equity Interests by giving instructions to a broker, banker or other nominee, you must instruct your broker, banker or other nominee to
arrange for the withdrawal of your Equity Interests and such broker, banker or other nominee must effectively withdraw such Equity Interests while you still have the right to withdraw Equity Interests. See Section 4.5—“Withdrawal
Rights.”
What does the Board of Directors of the Company think of the Tender Offer?
We are making the Tender Offer pursuant to the Combination Agreement, which has been approved by the Company’s Board of Directors by a unanimous decision.
The Board of Directors:
| |
• |
|
having evaluated the terms and conditions of the Tender Offer from the point of view of Biotie and the holders of its Equity Interests and other
available information, issued a statement to the effect that the |
16
| |
consideration offered by the Offeror in the Tender Offer is fair to the holders of its Equity Interests; and |
| |
• |
|
accordingly, has decided to recommend the holders of Equity Interests to accept the Tender Offer. The Board of Directors’ decision was unanimous. |
A more complete description of the Board of Directors’ reasons for authorizing and approving the Combination Agreement and the transactions contemplated
thereby, including the Tender Offer, is set forth in the Company’s Solicitation/Recommendation Statement on Schedule 14D–9 under the Exchange Act and in Annex A hereto. See Section 1.1—“Background to the Tender Offer,”
Section 1.6—“Statement of Biotie’s Board of Directors” and Annex A.
If the Tender Offer is consummated, will the Company
continue as a public company?
If following the purchase of Equity Interests in the Tender Offer we do not own all of the Equity Interests, we expect
to acquire all Equity Interests through the Subsequent Compulsory Redemption or otherwise. Once we acquire all Equity Interests, the Company will no longer be publicly-owned. Even if for some reason we do not acquire all Equity Interests and the
Subsequent Compulsory Redemption does not take place, there may be so few remaining shareholders and publicly-held ADSs that the ADSs will no longer be eligible to be traded on NASDAQ Global Select Market (“Nasdaq US”), there may
not be a public trading market for ADSs of the Company, and the Company may no longer be required to make filings with the U.S. Securities and Exchange Commission (the “SEC”) or otherwise comply with the rules of the SEC relating to
publicly-held companies. Once we own all of the Equity Interests, we plan to delist the Shares from NASDAQ Helsinki Ltd. (“Nasdaq Helsinki”). See Section 4.15—“Certain Effects of the Tender Offer.”
If I object to the price being offered, will I have appraisal rights?
Appraisal rights are not available as a result of the Tender Offer. However, if the Offeror has acquired more than 90 percent of all outstanding Shares
(including Shares represented by ADSs), the Offeror intends to initiate a Subsequent Compulsory Redemption, in which shareholders who have not tendered their Shares in the Tender Offer will be entitled to appraisal rights under Finnish law. Upon
completion of such Subsequent Compulsory Redemption, you will be entitled to payment for your Shares equal to the fair value of your Shares as determined by an arbitral tribunal. Under the general rule under Finnish law, this value is equal to the
price paid in the Tender Offer but could also be different than the price that we are offering to pay you for your Shares in the Tender Offer if there are special grounds to value the Shares differently. The Offeror intends to request that the
arbitral tribunal confirm its right to redeem the remaining Shares at a price equal to the price paid in the Tender Offer. U.S. holders of Shares subject to the Subsequent Compulsory Redemption proceedings will be treated the same as other
shareholders. Although holders of ADSs will not participate personally in the Subsequent Compulsory Redemption, subject to the terms of the ADS deposit agreement and less any fees and expenses incurred under the ADS deposit agreement, holders of
ADSs will be entitled to receive the value determined by the arbitral tribunal for each Share represented by their ADSs. See Section 1.4—“The Offeror’s Future Plans with respect to Biotie Shares.”
If you hold Equity Interests other than Shares and ADSs, you will not benefit from such appraisal rights, except to the extent your Equity Interests are
exercised or converted into Shares. As described in more detail in the answer to the following question, however, the Company may redeem certain such Equity Interests in connection with a Subsequent Compulsory Redemption in accordance with their
terms and conditions, for a price based on that paid to holders of Shares in such Subsequent Compulsory Redemption, which may be different than the price that we are offering to pay for such Equity Interests in the Tender Offer.
Any Subsequent Compulsory Redemption proceedings are expected to last between six and twelve months.
17
If I decide not to tender, how will the Tender Offer affect my Equity Interests?
If the Tender Offer is consummated and the Offeror has obtained ownership of more than 90 percent of all outstanding Shares (including Shares represented by
ADSs) and you do not tender your Shares, the Offeror intends to initiate a Subsequent Compulsory Redemption under Finnish Law. Pursuant to such Subsequent Compulsory Redemption, all of the then outstanding Shares (other than Shares held by the
Offeror or the Company) will, subject to certain rights of shareholders under Finnish law, be redeemed for cash for an amount per Share to be determined by the arbitral tribunal in the Subsequent Compulsory Redemption, with interest, but less any
applicable withholding taxes (in the United States, see Section 4.13—“Material United States Federal Income Tax Consequences”). Under the general rule under Finnish law, this value is equal to the price paid in the Tender Offer but
could also be different than the price that we are offering to pay you for your Shares in the Tender Offer if there are special grounds to value the Shares differently. The Offeror intends to request that the arbitral tribunal confirm its right to
redeem the remaining Shares at a price equal to the price paid in the Tender Offer. Therefore, if the Subsequent Compulsory Redemption takes place, the main difference to you between tendering your Shares and not tendering your Shares is that you
will be paid earlier if you tender your Shares but that no appraisal rights will be available. Even if the Subsequent Compulsory Redemption does not take place but we purchase all of the tendered Shares and ADSs, the number of shareholders and the
number of Shares and ADSs that are still in the hands of the public may be so small that there no longer will be an active public trading market (or, possibly, any public trading market) for the Shares and ADSs. Also, as described above, the Company
may no longer be required to make filings with the SEC or otherwise comply with the rules of the SEC relating to publicly-held companies. See Section 4.15—“Certain Effects of the Tender Offer.”
If the Offeror initiates a Subsequent Compulsory Redemption under Finnish Law, pursuant to terms and conditions of the Option Rights, Share Rights and
Warrants, the holders of such Equity Interests will have the following obligations and/or rights in connection with, or prior to, the Subsequent Compulsory Redemption.
2011 Option Rights, 2014 Option Rights, 2016 Option Rights and 2014 Share Rights. Should the Subsequent Compulsory Redemption be initiated during the
share subscription periods of any 2011 Option Rights, 2014 Option Rights, 2016 Option Rights or 2014 Share Rights, the holders of such Equity Interests will have an opportunity to exercise their right of share subscription within a period of time to
be determined by the Board of Directors of the Company. If such right is exercised, such holders will be entitled to participate in the Subsequent Compulsory Redemption as shareholders. If such right is not exercised, the Offeror may redeem such
Equity Interests in connection with the Subsequent Compulsory Redemption for a price based on the value determined for Shares in such Subsequent Compulsory Redemption.
Should the Subsequent Compulsory Redemption be initiated before the share subscription periods of any 2014 Option Rights, 2016 Option Rights or 2014 Share
Rights, the Board of Directors of the Company may, in its sole discretion, grant holders of such Equity Interests the right to subscribe for Shares (with the performance multiplier applicable to any senior management awards determined by the Board
of Directors of the Company in its discretion). If such right is exercised, such holders will be entitled to participate in the Subsequent Compulsory Redemption as shareholders. If such right is not exercised, the Offeror may redeem such Equity
Interests in connection with the Subsequent Compulsory Redemption for a price based on the value determined for Shares in such Subsequent Compulsory Redemption.
Notwithstanding the above, the Company has agreed with certain holders of 2016 Option Rights that, should the Tender Offer be announced before April 1,
2016, their 2016 Option Rights will be returned to the Company without consideration and the Company will instead pay such holders a transaction bonus amounting to the gross amount of the gain such holders would have received had such 2016 Option
Rights been acquired by the Offeror in the Tender Offer. See Section 5.5—“Presentation of Biotie—Option Rights and Share Rights.”
2011 Share Rights. Should the Subsequent Compulsory Redemption be initiated, each 2011 Share Right will be redeemed and the holders thereof will
receive an amount paid in cash, to be determined by the Board of Directors of Biotie in a fair and equitable manner.
18
Swiss Option Rights. Should the Subsequent Compulsory Redemption be initiated, the holders of Swiss Option
Rights will have an opportunity to exercise their right of share subscription. If such right is exercised, such holders will be entitled to participate in the Subsequent Compulsory Redemption as shareholders. All unexercised Swiss Option Rights will
be terminable by the Company.
Warrants. Should a Subsequent Compulsory Redemption be initiated, the holders of Warrants will have an
obligation to transfer their Warrants to the Offeror, for the consideration agreed in the terms and conditions of the Warrants.
What is the
market value of my Shares or ADSs as of a recent date?
On January 18, 2016, the last trading day before the Offeror and the Company announced
that they had entered into the Combination Agreement (the day of such announcement, the “Announcement Date”), the last sale price of Shares reported on Nasdaq Helsinki was EUR 0.1510 per Share. On January 15, 2016, the
last trading day before the Announcement Date, the last sale price of ADSs reported on Nasdaq US was USD 13.20 per ADS. We encourage you to obtain a recent quotation for Shares or ADSs in deciding whether to tender your Shares or ADSs. See
Section 2.2—“Price Range of Biotie’s Shares; Dividends (historical)” and Section 2.4—“Price Range of Biotie’s ADSs (historical).”
Have any holders of Equity Interests already agreed to tender their Equity Interests in the Tender Offer or to otherwise support the Tender Offer?
Yes. On January 19 and 20, 2016, in connection with the execution of the Combination Agreement, certain shareholders, ADS holders and holders of other
Equity Interests of the Company representing in total approximately 65 percent of the outstanding Shares and votes (on a fully diluted basis) in the Company have, subject to certain customary conditions, irrevocably undertaken to accept the Tender
Offer. This includes all the holders of the Warrants and members of the senior management team of the Company and one director of the Company (Mr. Don M. Bailey). Each of the Irrevocable Undertakings (as defined in
Section 1.7—“Irrevocable Undertakings of Major Shareholders”) will terminate upon certain customary termination events, including in the event the Combination Agreement is terminated. See Section 1.7—“Irrevocable
Undertakings of Major Shareholders.”
If I tender my Equity Interests, when and how will I get paid?
If the Conditions to Completion, as set forth in Section 4.2—“Conditions to Completion of the Tender Offer,” are satisfied or waived and we
accept your Equity Interests tendered during the Offer Period for payment, we will pay the applicable price for each Equity Interest so accepted in cash without interest (and less any amounts required to be deducted and withheld under any applicable
law) within six (6) Finnish banking days following expiration of the Offer Period. Actual time of receipt of the payment will depend on the schedules of money transactions between financial institutions and, if applicable, agreements between
you and your account operator or nominee in each case.
We will announce the terms of payment and settlement for the Equity Interests tendered during any
Subsequent Offer Period in connection with the announcement thereof. The sale and purchase of Equity Interests validly tendered in accordance with the terms and conditions of the Tender Offer during the Subsequent Offer Period will be executed in
intervals of approximately one (1) week.
Holders of ADSs tendering their ADSs will be paid in U.S. dollars, as determined by converting the ADS
Offer Price to the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date.
19
The Offer Price payable to holders of 2016 Option Rights, 2011 Share Rights and 2014 Share Rights will, at the
option of the holder, be payable in euros or the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to
the applicable Closing Date.
See Section 4.1—“Terms of the Tender Offer” and Section 4.4—“Acceptance Procedure of the
Tender Offer.”
What are the United States federal income tax consequences of the Tender Offer and Subsequent Compulsory Redemption, if any?
If you are a U.S. Holder (as defined in Section 4.13—“Material United States Federal Income Tax Consequences”), the receipt of
cash by you in exchange for your Equity Interests pursuant to the Tender Offer or the Subsequent Compulsory Redemption, if any, will be a taxable transaction for United States federal income tax purposes. In general, with respect to Shares, ADSs
and/or Warrants, you will recognize gain or loss in an amount equal to the difference between (i) the amount realized and (ii) your adjusted tax basis in the Shares, ADSs and/or Warrants exchanged therefor. Subject to the discussion in
Section 4.13—“Material United States Federal Income Tax Consequences,” the gain or loss that you recognize generally will be capital gain or loss and will be long-term if you have held the Shares, ADSs and/or Warrants for more
than one year. In general, with respect to Option Rights and/or Share Rights, the cash you receive will be treated as ordinary compensation income to the person who was originally granted the Option Rights or Share Rights, and the amount payable to
you in the Tender Offer or in connection with the Subsequent Compulsory Redemption, if any, may be subject to U.S. federal, and possibly state and local, withholding (including payroll taxes) owed by the original grantee. See
Section 4.13—“Material United States Federal Income Tax Consequences.”
YOU ARE URGED TO CONSULT YOUR TAX ADVISORS REGARDING THE
SPECIFIC TAX CONSEQUENCES OF TENDERING YOUR EQUITY INTERESTS IN THIS TENDER OFFER OR EXCHANGING SUCH SECURITIES FOR CASH IN CONNECTION WITH A SUBSEQUENT COMPULSORY REDEMPTION IN LIGHT OF YOUR PARTICULAR SITUATION, INCLUDING THE APPLICABILITY AND
EFFECT OF U.S. FEDERAL, STATE, LOCAL, NON-U.S. AND OTHER LAWS.
What are the Finnish income tax consequences of the Tender Offer and Subsequent
Compulsory Redemption, if any?
Disposal of securities is primarily a taxable transaction for the transferors who are generally liable to tax in
Finland. The taxation shall be carried out in accordance with applicable legislation. The capital gain accrued by Finnish tax resident individuals is primarily taxed as capital income at the 30/34 tax rate, unless the received benefit is deemed to
be received due to employment and therefore shall be taxed as earned income at the progressive rate under applicable rules. Should the transfer of Equity Interests be made by a Finnish tax resident’s business, the taxable income will be
calculated according to the applicable laws regarding each transferor and each situation. In case the transfer results in loss, the loss may be deducted during the same or subsequent tax years as provided by regulation applicable to the taxpayer in
question and the applicable statute of limitations.
When the transferor is not a Finnish tax resident, the Tender Offer and Subsequent Compulsory
Redemption should not generally result in any tax liability in Finland. It should be noted, however, that certain disposals, such as a disposal of those instruments which are taxed as employment based option rights as provided in the Finnish Income
Tax Act (1535/1992), may result in tax liability in Finland also in respect of non-tax residents of Finland.
Please see section 4.14 –
“Material Finnish Income Tax Consequences” for more information about material tax consequences in Finland.
ALL HOLDERS OF EQUITY INTERESTS
SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE SPECIFIC TAX CONSEQUENCES OF TENDERING THEIR EQUITY
20
INTERESTS IN THIS TENDER OFFER OR EXCHANGING SUCH SECURITIES FOR CASH IN CONNECTION WITH A SUBSEQUENT COMPULSORY REDEMPTION IN LIGHT OF THEIR PARTICULAR SITUATIONS, INCLUDING THE APPLICABILITY
AND EFFECT OF FINNISH TAXATION LAWS.
Who should I talk to if I have additional questions about the Tender Offer?
Questions and requests for assistance regarding the Tender Offer or any of the terms thereof with respect to Shares, Option Rights, Share Rights or Warrants
may be directed to the call service of OP Financial Group at (+358) (0) 100 0500. Requests for additional copies of this Tender Offer Document, Acceptance Forms (including the instructions attached thereto) and other tender offer materials
may be directed to the call service of OP Financial Group at (+358) (0) 100 0500. You may also contact your account operator, broker, dealer, commercial bank, trust company, custodian or other nominee for assistance.
Questions and requests for assistance regarding the Tender Offer or any of the terms thereof with respect to ADSs may be directed to Innisfree M&A
Incorporated, as Information Agent for ADSs, at the address and telephone number set forth for the Information Agent for ADSs on the back cover of this Tender Offer Document. Requests for additional copies of this Tender Offer Document, the Letter
of Transmittal and other tender offer materials may be directed to the Information Agent for ADSs. You may also contact your broker, dealer, commercial bank, trust company, custodian or other nominee for assistance.
21
| 1 |
BACKGROUND AND OBJECTIVES |
| 1.1 |
Background to the Tender Offer |
Acorda, or the Offeror, is a biotechnology company focused on
developing therapies that restore function and improve the lives of people with neurological disorders. It has an industry leading pipeline of novel neurological therapies addressing a range of disorders, including multiple sclerosis,
Parkinson’s disease, post-stroke walking deficits, epilepsy and migraine and markets three FDA-approved therapies. The Offeror is a Delaware corporation incorporated in 1995 with shares of its common stock listed on the NASDAQ Global Market
under the symbol “ACOR.”
As part of the Offeror’s strategy to continue to grow as a fully-integrated biopharmaceutical company and leading
neurology company, the Offeror is committed to expanding its pipeline through potential in-licensing and/or acquisition of neurology and/or other specialty products and technologies, focusing on late stage/near commercial or commercial products. The
Offeror also considers earlier-stage programs based on compelling science and the potential to address significant unmet medical needs. Accordingly, the Offeror continues to search for opportunities to complement its current portfolio.
The Company is a biopharmaceutical company primarily focused on developing therapeutics for central nervous system disorders. Its pipeline includes product
candidates designed to address unmet medical needs in Parkinson’s disease and related dementia, other neurodegenerative indications and primary sclerosing cholangitis, an orphan fibrotic liver disease. The Company is a public limited liability
company incorporated in Finland in 1998 with shares listed on Nasdaq Helsinki under the symbol “BTH1V” and ADSs listed on Nasdaq US under the symbol “BITI.”
In September 2015, Dr. Ron Cohen, the chief executive officer of the Offeror, expressed an interest to a member of the Board of Directors of the Company
in exploring potential opportunities for the Offeror and the Company. At an industry conference on October 12, 2015, Dr. Timo Veromaa, the chief executive officer of the Company, met with Dr. Cohen. The discussion was of an
introductory nature, but the Offeror’s interest on a high level in the Company’s development programs was discussed, although the Offeror did not make any proposal regarding a transaction during the meeting. Dr. Veromaa informed
Dr. Cohen that the Company would submit any written proposal received from the Offeror to the Board of Directors for its consideration.
On
November 12, 2015, the Company received from the Offeror a written, non-binding proposal to acquire the Company for USD 25.50 per ADS in cash. The price assumed 1.1 billion outstanding Shares on a fully-diluted basis, and the offer was
subject to a number of conditions, including due diligence. The proposed consideration represented a 90 percent premium to the prior day’s ADS price and a 50 percent premium to the 90-trading day volume-weighted average ADS price. In response
to this offer, the Company notified the Offeror on November 16, 2015 that it was engaging an advisor to help review the offer.
On November 24,
2015, on behalf of the Company, representatives from Guggenheim Securities, LLC (“Guggenheim Securities”) communicated to Lazard and MTS that the Company was evaluating the proposal. Also on November 24, 2015, the Company
agreed, contingent upon executing a satisfactory confidentiality agreement, to commence providing due diligence materials to the Offeror and schedule an in-person presentation by the Company’s senior management to representatives of the Offeror
during the following week. From November 24, 2015 through November 30, 2015, representatives of the Offeror and the Company negotiated the terms and conditions of a standard confidentiality agreement, which (i) included customary
restrictions and obligations with respect to the use of confidential information, (ii) included a customary standstill provision, and (iii) provided for the Offeror’s ability to submit private, confidential proposals to the Company
Board of Directors. On November 30, 2015, the Company and the Offeror executed and delivered the confidentiality agreement.
22
Beginning on November 30, 2015, the Company made available to the Offeror certain information through an
electronic data room. On December 3 and 4, 2015, the Company’s senior management conducted an in-person presentation to representatives of the Offeror. Representatives of Lazard and MTS and Guggenheim Securities also attended the meeting.
Thereafter, representatives of the Company conducted a series of due diligence calls with representatives of the Offeror and the Offeror’s advisors regarding intellectual property, clinical development, manufacturing, regulatory, financial and
tax matters.
On December 11, 2015, the Offeror sent the Company a letter in which it confirmed its non-binding offer of USD 25.50 per ADS,
with corresponding prices to be offered for the other Equity Interests. The letter stated that the offer assumed 1.1 billion outstanding Shares on a fully-diluted basis and was subject to reaching agreement on the terms of a definitive transaction
agreement. The letter stated that there would be no financing contingency to completing the transaction. The letter also stated that the Offeror intended to structure the transaction as a tender offer and would require a 90 percent minimum
acceptance threshold to complete the transaction.
On December 16, 2015, on behalf of the Company, the Company’s advisors communicated to the
Offeror’s advisors that the Offeror should increase the offer price to USD 31.00 per ADS in cash and that the tender offer acceptance threshold should be lowered from 90 percent to 67 percent. On December 20, 2015, Dr. Cohen and
Dr. Veromaa spoke by telephone, and Dr. Cohen conveyed that, having received the Company’s counteroffer, the Offeror’s board of directors had instructed the Offeror’s management to conduct additional due diligence to
determine whether the Offeror would be able to improve the terms of its offer. On December 21, 2015, Dr. Veromaa and Dr. Cohen again spoke by telephone, and Dr. Cohen indicated that the Offeror would contact the Company after
Christmas to discuss a potential increase in the offer price.
On December 27, 2015, Dr. Cohen and Dr. Veromaa spoke by phone, and
Dr. Cohen communicated a revised non-binding offer of USD 26.25 per ADS, subject to satisfactory completion of due diligence. Dr. Cohen also indicated that the 90 percent minimum acceptance threshold was important to the Offeror. Such
offer was subsequently confirmed in writing.
On December 29, 2015, Dr. Veromaa communicated to Dr. Cohen that the Board of Directors was
willing to continue discussions regarding a transaction on the basis of the offer price of USD 26.25 per ADS.
On December 30, 2015, the
Offeror’s legal advisors provided an initial draft of the Combination Agreement to the Company’s legal advisors, with a minimum tender condition of 90 percent. Subsequently, the Company’s legal advisors and the Offeror’s legal
advisors held several teleconferences to discuss Finnish legal requirements regarding the structuring of all cash acquisitions of Finnish-incorporated and Finnish-listed entities. During this time, the Company also responded to additional diligence
requests from the Offeror. On January 4, 2016, the Company’s legal advisors provided a revised Combination Agreement to the Offeror’s legal advisors.
On January 5, 2016, representatives from Lazard and MTS spoke to representatives from Guggenheim Securities regarding further due diligence that had been
conducted in relation to the Company’s capital structure. Lazard and MTS informed Guggenheim Securities that the preliminary offers made by the Offeror thus far had been made on the basis and the assumption that the total value of the
Company’s equity on a fully-diluted basis would be USD 363 million and that the revised offer price of USD 26.25 per ADS was based on such assumption (i.e., that all Equity Interests, including the Warrants, would be paid based on the
per Share value implied by a fully-diluted equity value of USD 363 million). Lazard and MTS further informed Guggenheim Securities that USD 363 million would be the Offeror’s final offer for the Company’s equity on a fully-diluted
basis and due to the results of further diligence, including with respect to the Company’s capital structure and the fact that the Warrant terms provided that in certain circumstances an acquirer of the Company would be required to pay an
amount determined by reference to a Black-Scholes option pricing formula in satisfaction of the Warrants, the Offeror was revising its offer price per ADS to USD 25.60, which, assuming such Warrant payments, reflected the same USD 363 million
total equity value reflected in the Offeror’s previous offer letter. Dr. Cohen discussed the same topic on a telephone call placed to Dr. Veromaa.
23
On January 6, 2016, the Offeror’s legal advisors provided a revised draft of the Combination Agreement
and a draft of irrevocable undertaking intended to be signed by certain of the Company’s shareholders and warrant holders in conjunction with the execution of the Combination Agreement. The various legal advisors engaged in discussions
regarding various terms in the revised draft, including closing certainty. On January 6, 2016, the Company’s legal advisors sent the Offeror’s legal advisors a revised draft of the Combination Agreement, and on January 7, 2016,
the Company’s legal advisors circulated to the Offeror’s legal advisors a revised draft of the irrevocable undertaking. On January 9, 2016, the Offeror’s legal advisors provided a revised draft of the Combination Agreement to the
Company’s legal advisors.
On January 10, 2016, Dr. Veromaa and Dr. Cohen met in person in San Francisco, where both had travelled to
attend an industry conference. Dr. Veromaa conveyed to Dr. Cohen that the Company’s Board of Directors had authorized the Company to proceed on the basis of the USD 25.60 per ADS offer price but noted several issues, including
closing certainty and in particular the definition of “Material Adverse Effect,” that remained outstanding and about which the Company felt strongly about resolving in a satisfactory manner.
Over the course of January 11 to 19, 2016, the parties progressed the Combination Agreement and form of irrevocable undertaking. During this period, the
Offeror contacted the Finnish Financial Supervisory Authority (the “FSA”) regarding certain aspects of the transaction structure under Finnish law. Beginning on January 14, 2016, Dr. Veromaa contacted several major
shareholders and each warrant holder and asked them to sign a confidentiality agreement. Upon receipt of a signed confidentiality agreement from each of these parties, they were provided with certain information about the transaction and a form of
irrevocable undertaking. During the course of January 15-19, 2016, certain shareholders and warrant holders expressed willingness to support the transaction and provided comments on the language of the form of irrevocable undertaking. The
various legal advisors worked to finalize the language of the irrevocable undertaking with the shareholders and warrant holders during this time period.
On January 17, 2016, the Company and the Offeror set the proposed offer price at EUR 23.5680 per ADS (and EUR 0.2946 per Share), which was
equal to the agreed offer price of USD 25.60 per ADS based on the average exchange rate from the last five trading days of USD 1.0864 to EUR 1.00.
Over the course of January 16 to 18, 2016, the various legal advisors held a series of conversations regarding the amount and structure of the
Offeror’s proposed financing and revised the Combination Agreement based on the documentation for the Offeror’s planned private placement of equity.
On January 18, 2016, the Offeror received a letter from the FSA confirming their view on certain aspects of the transaction structure under Finnish law.
On January 18, 2016, the Offeror submitted a revised letter confirming, on the basis of further due diligence of the Company’s capital
structure, an offer price per ADS of USD 25.60, with a price derived from this figure to be offered for the Shares, Option Rights and Share Rights and a price for the Warrants based on a Black-Scholes option pricing formula incorporating the price
per Share derived from the price per ADS of USD 25.60. The letter also noted that, while the transaction would not be subject to any financing contingency, the Offeror would sell to a banking institution in a private placement an estimated amount of
the Offeror’s common stock between USD 50 million and USD 100 million, the proceeds of which together with the Offeror’s existing cash resources, would be used to fund the transaction.
On January 19, 2016, the Company’s Board of Directors met via teleconference to discuss the final terms of the proposed transaction, the proposed
definitive Combination Agreement and related documents. The Board of Directors unanimously concluded that it is in the best interest of the holders of Equity Interests to approve the transaction and recommended that the holders of Equity Interests
accept the offer to be made pursuant to the terms agreed to in the Combination Agreement. The Board of Directors approved the Combination Agreement.
24
Following such meeting of the Board of Directors, on January 19, 2016, the Company and the Offeror executed
the Combination Agreement, and the Offeror simultaneously executed the agreement for the Share Issuance. At the same time, holders of approximately 60 percent of the outstanding Shares (on a fully diluted basis), representing all but two holders
approached (each of whom indicated support of the contemplated transaction but for various reasons were not able to sign an irrevocable undertaking at the time), entered into irrevocable undertakings to support the transaction. The transaction was
announced via press release shortly thereafter.
On January 20, 2016, one of the two holders of Equity Interests that had indicated support for the
transaction but had not been able to enter into an irrevocable undertaking the day before, entered into an irrevocable undertaking, which brought the percentage of outstanding Shares (on a fully diluted basis) committed via irrevocable undertakings
to approximately 65 percent.
| 1.2 |
Effect on Biotie’s Operations and Assets and Future Position of Management and Employees |
After the
completion of the Tender Offer, Biotie will become a subsidiary of Acorda. The business of Biotie will be eventually integrated into the business of Acorda based upon an integration plan currently under development by the management of Acorda but
which will not be finalized until after the completion of the Tender Offer. While the integration plan is currently not expected to have any immediate material effect on the business operations, assets or employees of Biotie in the near term, the
final and longer term impact of the integration plan can be assessed only after the completion of the Tender Offer.
Acorda plans to maintain the
operations of Biotie’s South San Francisco facility in full and currently expects that the business and operations in South San Francisco will be continued substantially as they are currently being conducted. It is currently expected that in
the near term, the completion of the Tender Offer will not have any immediate major impact on Biotie’s Turku operations and employees. Acorda will consider the longer term operating plan and scope of the Turku operations following the
completion of the Tender Offer as part of the integration planning.
To such end, Acorda has extended to certain Biotie employees in South San Francisco
and may extend to certain additional Biotie employees, the opportunity to participate in Acorda’s compensation and benefit plans in accordance with their terms. Subject to the approval of the Board of Directors of Acorda, the offers extended
prior to the printing of this Tender Offer Document include retention arrangements and equity awards consistent with Acorda’s new hire practices and equity compensation programs applicable to Acorda employees generally. Except for such
retention arrangements and equity awards, which are subject to and effective upon the completion of the Tender Offer, all such offers are subject to and effective upon the completion of the Tender Offer and any Subsequent Compulsory Redemption.
Acorda has not entered into any agreements providing for any compensation or other remuneration granted to the management or the members of the Board of
Directors of Biotie payable in return for the execution of the Combination Agreement and/or for the completion of the Tender Offer. The Company has, however, agreed with certain holders of 2016 Option Rights that, should the Tender Offer be
announced before April 1, 2016, their 2016 Option Rights will be returned to the Company without consideration and the Company will instead pay such holders a transaction bonus amounting to the gross amount of the gain such holders would have
received had such 2016 Option Rights been acquired by the Offeror in the Tender Offer. See Section 5.5—“Presentation of Biotie—Option Rights and Share Rights.”
| 1.3 |
The Offeror’s Strategic Plans |
By acquiring Biotie, Acorda expects to complement its neurology
product pipeline and portfolio. Acorda believes that the complementary nature of Biotie’s offerings with Acorda’s products and pipeline will allow the combined companies to become a leader in Parkinson’s disease therapeutic
development. Acorda also believes Biotie will benefit from Acorda’s size, capitalization and collective expertise. Acorda believes that combining Biotie’s offerings with Acorda’s existing products and brand recognition will create an
opportunity for significant revenue synergies across complementary products.
The Tender Offer and any Subsequent Compulsory Redemption will have no
material near term effect on the operations and business locations of, or on employment at Biotie. Acorda intends to keep Biotie’s South San
25
Francisco open. In the near term, the Turku office will also remain open but the Offeror will consider the longer term operating plan and scope of the Turku operations following the completion of
the Tender Offer.
Pursuant to the terms of the Combination Agreement, as soon as the Offeror has publicly confirmed that it will complete the Tender
Offer, the Board of Directors of the Company will, at the Offeror’s request, convene a general meeting of shareholders of the Company for the purpose of electing new members to the Board of Directors of the Company and addressing other agenda
items proposed by the Offeror.
The Offeror will continue to evaluate the business and operations of the Company during the pendency of the Tender Offer
and any Subsequent Compulsory Redemption and, upon consummation of the Tender Offer and any Subsequent Compulsory Redemption, will take such actions as it deems appropriate under the circumstances then existing.
Except as described above or elsewhere in this Tender Offer Document, the Offeror has no present plans or proposals that would relate to or result in
(i) any extraordinary corporate transaction involving the Company or any of its subsidiaries (such as a merger, reorganization, liquidation, relocation of any operations or sale or other transfer of a material amount of assets), (ii) any
sale or transfer of a material amount of assets of the Company or any of its subsidiaries, (iii) any material change in the Company’s capitalization or dividend policy or (iv) any other material change in the Company’s corporate
structure or business. In case the Tender Offer is consummated and the Minimum Condition (as defined in Section 4.2—“Conditions to Completion of the Tender Offer”) is not fulfilled during the Offer Period or Subsequent Offer
Period, the Offeror will consider all legally available alternatives to reach 100 percent ownership in the Company.
| 1.4 |
The Offeror’s Future Plans with respect to Biotie Shares |
Purpose of the Tender Offer. The
purpose of the Tender Offer is to acquire control of, and all of the Equity Interests in, the Company.
Redemption under the Finnish Companies
Act. Under Chapter 18 of the Finnish Companies Act (624/2006, as amended) a shareholder holding more than 90 percent of the total number of shares and voting rights in a company shall have the right to redeem the remainder of the issued and
outstanding shares in the company. Alternatively, if such greater than 90 percent shareholder has not initiated such redemption proceedings, such shareholder will have the obligation, should any minority shareholder(s) so demand, to participate in
redemption proceedings with respect to the redemption of those shares held by such demanding minority shareholder(s).
After the Tender Offer is
consummated, should the Offeror obtain more than 90 percent of the issued and outstanding Shares (including Shares represented by ADSs) and voting rights in the Company, calculated on a fully diluted basis and otherwise in accordance with Chapter 18
Section 1 of the Finnish Limited Liability Companies Act, the Offeror intends to initiate a Subsequent Compulsory Redemption as promptly as practicable under the above provisions of the Finnish Companies Act in order to acquire title to all the
outstanding Shares and ADSs in the Company. U.S. holders of Shares subject to the Subsequent Compulsory Redemption proceedings will be treated the same as other shareholders. All Equity Interests acquired by the Offeror pursuant to the Tender Offer
and the Subsequent Compulsory Redemption, if any, will be retained by the Offeror.
Appraisal Rights. As part of a Subsequent Compulsory
Redemption, whether initiated by the Offeror or by shareholders, participating shareholders who have not tendered their Shares in the Tender Offer will be entitled to appraisal rights under Finnish law.
The value of the Shares in a Subsequent Compulsory Redemption will be determined by an arbitral tribunal. Under the general rule under Finnish law, this value
will be equal to the price paid in the Tender Offer but could also be different than the Share Offer Price if there are special grounds to value the Shares differently. The Offeror intends to request that the arbitral tribunal confirm its right to
redeem the remaining Shares at a price
26
equal to the price paid in the Tender Offer. Assuming the Offeror initiates the Subsequent Compulsory Redemption, the Offeror will file an application for the appointment of arbitrators with the
Redemption Committee of the Finland Chamber of Commerce, which will thereafter appoint the arbitrator(s) for the process. The Offeror will then file a statement of claim and offer to place a security for the payment of the redemption price with the
arbitral tribunal, to which the minority shareholders and their appointed trustee will respond. Based on the Offeror’s and minority shareholders’ claims, the arbitral tribunal will determine the size of the security, and such security
would typically be placed on the date of the first oral hearing of the arbitral tribunal, which would generally occur four (4) or five (5) months after initiating the redemption process. Upon placing such security, the Offeror would obtain
advance title to the Shares. Normally, the total duration of the redemption process is approximately six (6) to twelve (12) months.
U.S.
holders of Shares subject to the Subsequent Compulsory Redemption proceedings will be treated the same as other shareholders. Although holders of ADSs will not participate personally in the Subsequent Compulsory Redemption, subject to the terms of
the ADS deposit agreement and less any fees and expenses incurred under the ADS deposit agreement, holders of ADSs will be entitled to receive the value determined by the arbitral tribunal for each Share represented by their ADSs. As holders of ADSs
will not be personally represented, their interests in the Subsequent Compulsory Redemption will be supervised by an independent trustee who represents all absent minority shareholders in the proceedings.
Holders of Equity Interests other than Shares and ADSs will not benefit from such appraisal rights, except to the extent your Equity Interests are exercised
or converted into Shares. However, the Company may redeem certain such Equity Interests in connection with a Subsequent Compulsory Redemption in accordance with their terms and conditions, for a price based on that paid to holders of Shares in such
Subsequent Compulsory Redemption. See “If I decide not to tender, how will the Tender Offer affect my Equity Interests?” in the Summary Term Sheet.
Non-Applicability of Rules Regarding “Going Private” Transactions. The SEC has adopted Rule 13e–3 under the Exchange Act, which is
applicable to certain “going private” transactions and may be applicable to a Subsequent Compulsory Redemption or another business combination following the Tender Offer unless the Offeror completes such Subsequent Compulsory Redemption or
other business combination within one year after completion of the Tender Offer and holders of Equity Interests receive consideration at least equal to that paid in the Tender Offer. The Offeror and the Company believe that Rule 13e–3 will not
be applicable to a Subsequent Compulsory Redemption because it expects that any such Subsequent Compulsory Redemption will be effected within one year following the consummation of the Tender Offer and in such Subsequent Compulsory Redemption,
holders of Equity Interests will receive the same consideration as that paid in the Tender Offer for each class of Equity Interests.
Obligation
to Make a Mandatory Offer. According to Chapter 11, Section 19 of the Finnish Securities Market Act, if a shareholder’s interest in a Finnish public company increases so as to cause such shareholder’s voting rights to exceed
either 30 percent or 50 percent of the total voting rights in such company, such shareholder is obligated to make a public tender offer (mandatory offer) for all the remaining shares and securities entitling to shares in the company. However, under
the Finnish Securities Market Act, if the relevant threshold has been exceeded by means of a voluntary public tender offer, the voluntary offer does not need to be followed by a mandatory offer provided that the initial voluntary offer has been made
for all shares and other securities entitling the holder to shares in the target company. Pursuant to the above exception, the Offeror will not have an obligation to launch a subsequent mandatory offer after the completion of the Tender Offer.
Termination of public trading, delisting and deregistration. It is the Offeror’s intention that, as soon as reasonably practicable
following the acquisition of all Equity Interests, the Company will apply for termination of public trading on and delisting of its Shares from Nasdaq Helsinki, as well as the delisting of its ADSs from Nasdaq US and the deregistration of its Shares
and ADSs under the Exchange Act.
27
| 1.5 |
Financing of the Tender Offer |
The Tender Offer will be financed through the Offeror’s internal
financing arrangements and no third party financing is required by the Offeror to complete the Tender Offer.
The Tender Offer is not conditioned upon the
Offeror’s ability to finance the purchase of Equity Interests pursuant to the Tender Offer. The Offeror estimates that the total amount of funds required to purchase all of the Equity Interests pursuant to the Tender Offer and to consummate any
Subsequent Compulsory Redemption is approximately EUR 334 million (or approximately USD 363 million based on the 5-day average of the USD to EUR exchange rate prior to the Announcement Date), excluding related transaction fees and
expenses. The Offeror has sufficient funds to consummate the Tender Offer and any Subsequent Compulsory Redemption. The funds are obtained from the Offeror’s existing cash balances, including the net proceeds from the Share Issuance (as defined
below).
On January 19, 2016, the Offeror agreed to issue USD 75 million of its common stock, par value USD 0.001 per share, to an initial
purchaser, in a private placement transaction exempt from registration under the U.S. Securities Act of 1933, as amended (the “Share Issuance”), which transaction was settled on January 26, 2016. The Offeror intends to use the
net proceeds from the Share Issuance to fund, in part, the acquisition of Equity Interests pursuant to the Tender Offer.
The Offeror does not think its
financial condition is relevant to the decision of holders of Equity Interests as to whether to tender Equity Interests and accept the Tender Offer because:
| |
• |
|
the Tender Offer is being made for all Equity Interests solely for cash; |
| |
• |
|
the Tender Offer is not subject to any financing condition; and |
| |
• |
|
the Offeror will have sufficient funds available to purchase all Equity Interests validly tendered in the Tender Offer and not validly withdrawn, and any Equity Interests purchased in a Subsequent Compulsory Redemption
or otherwise, in light of the Offeror’s financial capacity in relation to the amount of consideration payable. |
The financing
arrangements for the Tender Offer will not have any impact on the operations or obligations of the Company.
Hedging Transaction. On
January 21, 2016, the Offeror entered into a USD/EUR currency hedge arrangement to limit potential downside risk arising from the USD to EUR exchange rate in connection with the Tender Offer.
| 1.6 |
Statement by Biotie’s Board of Directors |
The Board of Directors of Biotie has recommended that
holders of Equity Interests accept the Tender Offer.
On March 4, 2016, having carefully evaluated the terms and conditions of the Tender Offer from the
point of view of Biotie and the holders of its Equity Interests and other available information, the Board of Directors of Biotie has issued a statement to the effect that the consideration offered by the Offeror in the Tender Offer is fair to the
holders of its Equity Interests. Accordingly, the Board of Directors of Biotie has decided to recommend the holders of Equity Interests to accept the Tender Offer.
In connection with such evaluation, the Board of Directors of Biotie considered numerous factors, including the opinion of Guggenheim Securities, dated
January 19, 2016, to the Board of Directors as to the fairness, from a financial point of view and as of such date, of the Share Offer Price to be received in the Tender Offer by holders of Shares and ADSs (other than Acorda and its
affiliates), which opinion was based on the matters considered, the procedures followed, the assumptions made and various limitations of and qualifications to the review undertaken as more fully described therein.
28
Biotie board member Mr. Don M. Bailey, ViVo Capital, the venture partner of which is Biotie board
member Dr. Mahendra G. Shah, and Versant Ventures, where Biotie board member Dr. Guido Magni is partner, each entered into an Irrevocable Undertaking after Biotie’s Board of Directors approved the acceptance of the Combination
Agreement on January 19, 2016 by a unanimous decision. See Section 1.7—“Irrevocable Undertakings of Major Shareholders.” Mr. Bailey, Dr. Shah and Dr. Magni did not participate in the giving of the statement of
the Biotie Board of Directors.
The statement by the Biotie Board of Directors in accordance with Chapter 11, Section 13 of the Finnish Securities
Market Act is attached to this Tender Offer Document as Annex A.
| 1.7 |
Irrevocable Undertakings of Major Shareholders |
On January 19 and January 20, 2016, in
connection with the execution of the Combination Agreement, certain of the Company’s equityholders (Invesco Asset Management Limited, Baupost Private Investments A-1, L.L.C., Baupost Private Investments B-1, L.L.C., Baupost Private Investments
C-1, L.L.C., Baupost Private Investments BVI-1, L.L.C., Baupost Private Investments BVII-1, L.L.C., Baupost Private Investments BVIII-1, L.L.C., Baupost Private Investments BVIV-1, L.L.C., Baupost Private Investments H-1, L.L.C., Baupost Private
Investments P-1, L.L.C., Baupost Private Investments Y-1, L.L.C., Armistice Capital Master Fund, Ltd., Sitra, Ilmarinen Mutual Pension Insurance Company, Vivo Capital Fund VIII, L.P., Vivo Capital Surplus Fund VIII, L.P., Versant Venture Capital
III, L.P., Versant Side Fund III, L.P., Versant Venture Capital V, L.P., Versant Affiliates Fund V, L.P., Versant Ophthalmic Affiliates Fund I, L.P., Versant Venture Capital V (Canada) LP, OrbiMed Private Investments V, LP and The Bailey 1995 Family
Trust) and holders of Option Rights and/or Share Rights (David Cook, Mehdi Paborji, Timo Veromaa, Erin Campany, Michael Samar and Stephen Bandak) (each a “Support Equityholder” and, collectively, the “Support
Equityholders”), which includes all the holders of the Warrants and members of the Company’s senior management team, entered into undertakings with the Offeror (each an “Irrevocable Undertaking” and, collectively, the
“Irrevocable Undertakings”), pursuant to which, subject to certain customary conditions, each Support Equityholder has made a binding commitment to tender their Equity Interests into the Tender Offer. Equity Interests representing
in the aggregate approximately 65 percent of the outstanding Shares and votes on a fully diluted basis in the Company are subject to the Irrevocable Undertakings.
In the Irrevocable Undertakings, each Support Equityholder irrevocably undertook, subject to certain terms and conditions, to (i) accept the Tender
Offer, and to tender or cause to be tendered and sell or cause to be sold to the Offeror, all Equity Interests owned by it, acquired by it or granted to it prior to the earlier of the Undertaking Termination Date (as defined below) and the
expiration of the Tender Offer, in the Tender Offer, (ii) deliver or cause to be delivered evidence of such acceptance pursuant to the terms of the Tender Offer to the Offeror within ten (10) business days from the beginning of the
acceptance period of the Tender Offer, and (iii) not exercise voting rights pertaining to the Shares, ADSs and any Shares subscribed for based on the other Equity Interests or any Equity Interests acquired after the date of the Irrevocable
Undertaking in favor of a transaction competing with this transaction.
Each Irrevocable Undertaking will remain in force until the first of the following
has occurred (the “Undertaking Termination Date”): (i) the failure of the Board of Directors to recommend that the holders of Shares, ADSs and Warrants accept the Tender Offer or the modification or withdrawal of such
recommendation; (ii) the completion and settlement of the Tender Offer; (iii) the making by the Offeror of a public announcement to the effect that it will not complete the Tender Offer; (iv) the termination of the Combination
Agreement; (v) any amendment being made to the Tender Offer that reduces the offer consideration or otherwise materially changes the terms and conditions of the Tender Offer in a manner adverse to such Support Equityholder; or (vi) the
Tender Offer, having been launched or published, has failed to be completed by June 19, 2016.
The foregoing summary is qualified in its entirety by
reference to each Irrevocable Undertaking, the forms of which are attached in Annex F.
29
| 1.8 |
Advisors, Fees and Expenses |
Pohjola Bank is acting as the Arranger of the Tender Offer. Pohjola is also
acting as Receiving and Paying Agent for Shares, Option Rights, Share Rights and Warrants in connection with the Tender Offer (the “Receiving and Paying Agent for Shares, Option Rights, Share Rights and Warrants”). Pohjola Bank may
request account operators brokers, bankers and other nominees to forward materials relating to the Tender Offer to beneficial owners of Shares.
Computershare Trust Company, N.A. is acting as depositary and receiving and paying agent for ADSs in connection with the Tender Offer (the
“Depositary”).
Hill+Knowlton Strategies is acting as communication agent for Shares in connection with the Tender Offer (the
“Communication Agent”).
Innisfree M&A Incorporated is acting as Information Agent for ADSs in connection with the Tender Offer. The
Information Agent for ADSs may contact holders of ADSs by mail, telephone, telecopy and personal interview and may request brokers, bankers and other nominees to forward materials relating to the Tender Offer to beneficial owners of ADSs.
Lazard Frères & Co. LLC (“Lazard”), MTS Health Partners L.P. and its affiliate, MTS Securities LLC (together,
“MTS”), and J.P. Morgan Securities LLC are serving as financial advisors, and Kirkland & Ellis LLP, Roschier, Attorneys Ltd., Covington & Burling LLP and Jones Day LLP are serving as legal advisors to Acorda in
connection with the Tender Offer.
The Receiving and Paying Agent for Shares, Option Rights, Share Rights and Warrants, the Depositary, the Communication
Agent, and the Information Agent for ADSs each will receive reasonable and customary compensation for their respective services in connection with the Tender Offer and will be reimbursed for reasonable and customary expenses and may be indemnified
against certain liabilities and expenses in connection therewith.
The Offeror will not pay any fees or commissions to any broker or dealer or to any
other person (other than to the Receiving and Paying Agent for Shares, Option Rights, Share Rights and Warrants, the Depositary, the Communication Agent, and the Information Agent for ADSs) in connection with the solicitation of tenders of Equity
Interests pursuant to the Tender Offer. Brokers, bankers and other nominees will, upon request, be reimbursed by Offeror for customary mailing and handling expenses incurred by them in forwarding offering materials to their customers.
Except as follows, the Offeror has not entered into any agreements regarding any fees or other remuneration payable to Offeror’s professional advisors as
a result of the completion of the Tender Offer. Lazard and MTS will each receive a Transaction Fee of USD 1,475,000 in connection with the consummation of the Tender Offer. Pohjola Bank, acting as Receiving and Paying Agents for Shares, Option
Rights, Share Rights and Warrants, will receive a success fee of EUR 187,500 payable by the Offeror when the Minimum Condition has been satisfied.
This Tender Offer Document has been prepared and will be applied in accordance with
Finnish and United States law, including the Finnish Securities Market Act, Decree 1022/2012 of the Ministry of Finance and regulations and guidelines 7/2013 (FIVA 9/01.00/2013) and 9/2013 (FIVA 10/01.00/2013) issued by the FSA, and the Exchange Act
and rules and regulations promulgated thereunder. Finnish courts shall have non-exclusive jurisdiction to settle disputes arising from or relating to the terms and conditions of the Tender Offer.
30
| 2 |
INFORMATION ON GROUNDS FOR PRICING OF THE TENDER OFFER |
| 2.1 |
Grounds for Determining the Offer Price with respect to Equity Interests |
The consideration offered for
the Equity Interests, or the Offer Price, is the Share Offer Price in cash for each Share, the ADS Offer Price in cash for each ADS, the Option Right Offer Price in cash for each Option Right, the Share Right Offer Price in cash for each Share
Right, and the Warrant Offer Price in cash for each Warrant, in each case, validly tendered in the Tender Offer. See Section 4.1—“Terms of the Tender Offer.”
According to Chapter 11, Section 24 of the Finnish Securities Market Act, the starting point in determining the consideration to be offered in a
voluntary tender offer for all shares and other securities entitling to shares in the target company shall be the highest price paid for the securities subject to the tender offer by the offeror or by a person related to the offeror as stipulated in
Chapter 11, Section 5 of the Finnish Securities Market Act, during a period of six (6) months preceding the Announcement Date.
Neither the
Offeror nor any other party referred to in Chapter 11, Section 5 of the Finnish Securities Market Act has during the 6-month period preceding the Announcement Date acquired any Shares or other Equity Interests in the Company in public trading
or otherwise at a price higher than the Offer Price. At the date of this Tender Offer Document, neither the Offeror nor any other party referred to in Chapter 11, Section 5 of the Finnish Securities Market Act holds any Shares or other Equity
Interests in the Company.
| 2.2 |
Price Range of Biotie’s Shares; Dividends (historical) |
The Shares are listed on Nasdaq Helsinki
under the symbol “BTH1V.” The Shares have been listed on Nasdaq Helsinki since June 3, 2004.
The chart below shows the price development
of the Shares on Nasdaq Helsinki and the trading volumes of the Shares during the last three (3) years preceding the Announcement Date, i.e., from January 22, 2013 to January 18, 2016.
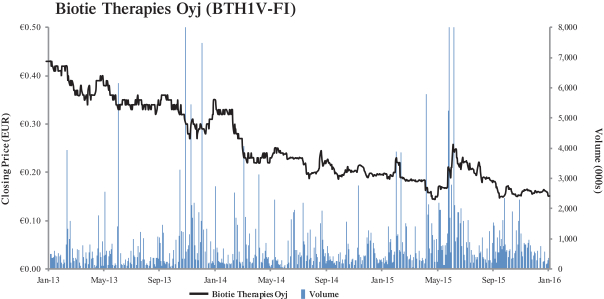
31
The following table sets forth the 3-month trading prices and trading volumes of the Shares as reported on Nasdaq
Helsinki for the last three (3) years preceding the Announcement Date.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Share prices during period |
|
|
Share turnover during period |
|
| |
|
Average(1) |
|
|
High |
|
|
Low |
|
|
Shares (mm)(2) |
|
|
EUR (mm)(3) |
|
| Trading Period: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Oct 20, 2015 — Jan 18, 2016 |
|
€ |
0.16 |
|
|
€ |
0.17 |
|
|
€ |
0.15 |
|
|
|
32.89 |
|
|
|
5.18 |
|
| Jul 21, 2015 — Oct 19, 2015 |
|
€ |
0.18 |
|
|
€ |
0.23 |
|
|
€ |
0.15 |
|
|
|
40.65 |
|
|
|
7.43 |
|
| Apr 21, 2015 — Jul 20, 2015 |
|
€ |
0.20 |
|
|
€ |
0.26 |
|
|
€ |
0.14 |
|
|
|
84.12 |
|
|
|
16.82 |
|
| Jan 20, 2015 — Apr 20, 2015 |
|
€ |
0.20 |
|
|
€ |
0.23 |
|
|
€ |
0.18 |
|
|
|
36.87 |
|
|
|
7.25 |
|
| Oct 21, 2014 — Jan 19, 2015 |
|
€ |
0.20 |
|
|
€ |
0.22 |
|
|
€ |
0.19 |
|
|
|
27.46 |
|
|
|
5.50 |
|
| Jul 22, 2014 — Oct 20, 2014 |
|
€ |
0.21 |
|
|
€ |
0.25 |
|
|
€ |
0.18 |
|
|
|
31.89 |
|
|
|
6.85 |
|
| Apr 22, 2014 — Jul 21, 2014 |
|
€ |
0.23 |
|
|
€ |
0.25 |
|
|
€ |
0.21 |
|
|
|
27.85 |
|
|
|
6.47 |
|
| Jan 21, 2014 — Apr 21, 2014 |
|
€ |
0.27 |
|
|
€ |
0.36 |
|
|
€ |
0.21 |
|
|
|
29.97 |
|
|
|
8.15 |
|
| Oct 22, 2013 — Jan 20, 2014 |
|
€ |
0.30 |
|
|
€ |
0.36 |
|
|
€ |
0.26 |
|
|
|
64.54 |
|
|
|
19.62 |
|
| Jul 23, 2013 — Oct 21, 2013 |
|
€ |
0.34 |
|
|
€ |
0.36 |
|
|
€ |
0.32 |
|
|
|
22.93 |
|
|
|
7.82 |
|
| Apr 23, 2013 — Jul 22, 2013 |
|
€ |
0.36 |
|
|
€ |
0.41 |
|
|
€ |
0.32 |
|
|
|
29.91 |
|
|
|
10.81 |
|
| Jan 22, 2013 — Apr 22, 2013 |
|
€ |
0.40 |
|
|
€ |
0.46 |
|
|
€ |
0.34 |
|
|
|
32.50 |
|
|
|
13.12 |
|
| (1) |
Represents volume weighted average Share price over period |
| (2) |
Represents cumulative trade volume over period |
| (3) |
Represents the volume weighted average Share price over the period times the cumulative trade volume over the period |
The following table sets forth the quarterly high and low sales prices per Share as reported on Nasdaq Helsinki since January 1, 2014.
|
|
|
|
|
|
|
|
|
| |
|
High |
|
|
Low |
|
| Year Ended 2014: |
|
|
|
|
|
|
|
|
| First Quarter |
|
€ |
0.36 |
|
|
€ |
0.22 |
|
| Second Quarter |
|
€ |
0.25 |
|
|
€ |
0.21 |
|
| Third Quarter |
|
€ |
0.25 |
|
|
€ |
0.18 |
|
| Fourth Quarter |
|
€ |
0.22 |
|
|
€ |
0.18 |
|
| Year Ended 2015: |
|
|
|
|
|
|
|
|
| First Quarter |
|
€ |
0.23 |
|
|
€ |
0.18 |
|
| Second Quarter |
|
€ |
0.26 |
|
|
€ |
0.14 |
|
| Third Quarter |
|
€ |
0.25 |
|
|
€ |
0.16 |
|
| Fourth Quarter |
|
€ |
0.18 |
|
|
€ |
0.15 |
|
| Year Ending 2016: |
|
|
|
|
|
|
|
|
| First Quarter (through January 18, 2016) |
|
€ |
0.17 |
|
|
€ |
0.15 |
|
On January 18, 2016, the last trading day before the Announcement Date, the last sale price of Shares reported on Nasdaq
Helsinki was EUR 0.1510 per Share; therefore, the Share Offer Price of EUR 0.2946 represents a premium of approximately 95 percent over such price. The Share Offer Price represents a premium of approximately 87 percent compared to the
volume-weighted average trading price of the Shares on Nasdaq Helsinki over the 3-month period preceding the Announcement Date, i.e. from October 20, 2015 to January 18, 2016, of EUR 0.1575. Correspondingly, the Share Offer Price
represents a premium of approximately 72 percent over the volume-weighted average trading price during the six (6) months preceding the Announcement Date, i.e. from July 21, 2015 to January 18, 2016, of EUR 0.1715.
On March 9, 2016, the last trading day prior to the approval of this Tender Offer Document, the last sale price of the Shares reported on Nasdaq Helsinki was
EUR 0.29 per Share.
32
Shareholders are urged to obtain current market quotations for Shares before making a decision with respect to
the Tender Offer.
The Company has never declared or paid any cash dividends on its capital stock. In addition, under the terms of the Combination
Agreement, the Company is not permitted to declare or pay dividends in respect of Shares unless consented to by the Offeror in writing.
| 2.3 |
Grounds for Determining the Offer Price with respect to ADSs |
As each ADS represents 80 underlying
Shares, the ADS Offer Price is EUR 23.5680 in cash, or 80 times the Share Offer Price (payable in the equivalent amount of U.S. dollars as determined based on the U.S. dollar to euro spot rate on the nearest practicable day to the applicable Closing
Date).
| 2.4 |
Price Range of Biotie’s ADSs (historical) |
The ADSs are listed on Nasdaq US under the symbol
“BITI.” The ADSs have been listed on Nasdaq US since June 11, 2015.
The chart below shows the price development of the ADSs on Nasdaq US
and the trading volumes of the ADSs from the day they were first listed through last trading day prior to the Announcement Date, i.e., from June 11, 2015 to January 15, 2016.
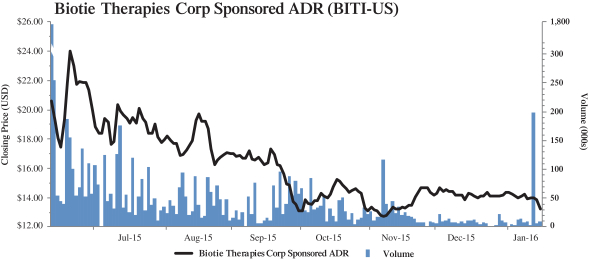
The following table sets forth the 3-month trading prices and trading volumes of the ADSs as reported on Nasdaq US from the
day they were first listed through the last trading day prior to the Announcement Date.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Share prices during period |
|
|
ADS turnover during period |
|
| |
|
Average(1) |
|
|
High |
|
|
Low |
|
|
ADSs (mm)(2) |
|
|
USD (mm)(3) |
|
| Trading Period: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Oct 17, 2015 — Jan 15, 2016 |
|
$ |
13.81 |
|
|
$ |
16.10 |
|
|
$ |
12.43 |
|
|
|
1.20 |
|
|
|
16.51 |
|
| Jul 18, 2015 — Oct 16, 2015 |
|
$ |
16.73 |
|
|
$ |
20.20 |
|
|
$ |
12.75 |
|
|
|
2.73 |
|
|
|
45.60 |
|
| Jun 11, 2015 — Jul 17, 2015 |
|
$ |
19.59 |
|
|
$ |
25.39 |
|
|
$ |
16.11 |
|
|
|
3.90 |
|
|
|
76.40 |
|
| (1) |
Represents volume weighted average ADS price over period |
| (2) |
Represents cumulative trade volume over period |
| (3) |
Represents the volume weighted average Share price over the period times the cumulative trade volume over the period |
33
The following table sets forth the quarterly high and low sales prices per ADS as reported on Nasdaq US since
June 11, 2015.
|
|
|
|
|
|
|
|
|
| |
|
High |
|
|
Low |
|
| Year Ended 2015: |
|
|
|
|
|
|
|
|
| Second Quarter (from June 11, 2015) |
|
$ |
25.39 |
|
|
$ |
16.11 |
|
| Third Quarter |
|
$ |
21.48 |
|
|
$ |
12.77 |
|
| Fourth Quarter |
|
$ |
16.10 |
|
|
$ |
12.43 |
|
| Year Ending 2016: |
|
|
|
|
|
|
|
|
| First Quarter (through January 15, 2016) |
|
$ |
14.50 |
|
|
$ |
13.03 |
|
On January 15, 2016, the last trading day before the Announcement Date, the last sale price of ADSs reported on Nasdaq US
was USD 13.20 per ADS. The volume-weighted average trading price of the ADSs on Nasdaq US over the 3-month period preceding the Announcement Date, i.e. from October 17, 2015 to January 15, 2016, was USD 13.81. Correspondingly, the
volume-weighted average trading price during the six (6) months preceding the Announcement Date, i.e. from July 18, 2015 to January 15, 2016, was USD 15.84.
Using the 5-day average of the USD to EUR exchange rate prior to the Announcement Date, the ADS Offer Price of EUR 23.5680, or USD 25.60, represented a
premium of approximately 94 percent over the last sale price of the ADSs on the last trading day prior to the Announcement Date, a premium of approximately 85 percent over the 3-month volume-weighted average trading price of the ADSs, and a premium
of approximately 62 percent over the 6-month volume-weighted average trading price of the ADSs.
On March 9, 2016, the last trading day prior to the
approval of this Tender Offer Document, the last sale price of the ADSs reported on Nasdaq US was USD 25.48 per ADS.
| 2.5 |
Grounds for Determining the Warrant Offer Price |
The Warrant Offer Price is EUR 0.1664 in cash per
Warrant validly tendered in the Tender Offer.
The Warrants are not publicly traded. The Warrant Offer Price was determined based on Section 2.11 of
the Terms and Conditions of the Warrants and the Black-Scholes value of the Warrants. Section 2.11 of the Terms and Conditions of the Warrants provides that in the event of an acquisition of the Company, the Company shall use its best efforts
to ensure that lawful and adequate provision shall be made whereby each holder of Warrants will thereafter continue to have the right to subscribe upon those same Terms and Conditions for shares in the surviving or acquiring entity with an aggregate
value equal to the Black-Scholes value of the Warrants. In lieu of receiving warrant rights in Acorda based on such Black-Scholes value, each holder of Warrants has agreed to receive such value in cash by tendering their Warrants.
On January 19, 2016, in connection with the execution of the Combination Agreement, the holders of 100 percent of the outstanding Warrants have
irrevocably undertaken to accept the Tender Offer for the Warrant Offer Price. See Section 1.7—“Irrevocable Undertakings of Major Shareholders.”
| 2.6 |
Grounds for Determining the Option Right and Share Right Offer Price |
The consideration offered for the
Option Rights and the Share Rights is the Option Right Offer Price in cash for each Option Right and the Share Right Offer Price in cash for each Share Right, in each case, validly tendered in the Tender Offer. See Section 4.1—“Terms
of the Tender Offer.”
The Option Rights and Share Rights are not publicly traded. The Option Right Offer Price and Share Right Offer Price have been
calculated by deducting the share subscription price of the respective Option Rights and Share Rights from Share Offer Price of EUR 0.2946, with a minimum Offer Price of EUR 0.01 in cash in case the
34
applicable share subscription price exceeds EUR 0.2946. The applicable subscription prices of the respective Option Rights and Share Rights pursuant to their terms and conditions are as follows:
EUR 0.01 for 2011 Option Rights, EUR 0.01 for 2014 Option Rights, EUR 0.162 for 2016 Option Rights, none for 2011 Share Rights, USD 0.01 for 2014 Share Rights, and between CHF 0.10 and CHF 0.45 for the Swiss Option Rights. For details of the
Option Rights and Share Rights, please see Section 5.5—“Option Right and Share Right Rights” below.
To the Offeror’s knowledge, no public tender offer for the Equity Interests
has been made by any third party during the twelve (12) months preceding the Announcement Date.
| 3 |
SUMMARY OF THE COMBINATION AGREEMENT |
This summary is not an exhaustive presentation of all of the terms
and conditions of the Combination Agreement. The summary aims at describing the terms and conditions of the Combination Agreement to the extent that such terms and conditions may materially affect the assessment of a holder of Equity Interests of
the terms and conditions of the Tender Offer. This summary is qualified in its entirety by reference to the full text of the Combination Agreement. A copy of the Combination Agreement has been enclosed as Annex G to this Tender Offer Document.
Capitalized terms used in this Section 3—“Summary of the Combination Agreement” but not defined in this Tender Offer Document will have the respective meanings given to them in the Combination Agreement. Holders of Equity
Interests should read the Combination Agreement for a more complete description of the provisions summarized below.
| 3.1 |
Background to the Combination Agreement |
The Offeror and Biotie entered into the Combination Agreement
on January 19, 2016 under which they agreed to effect a combination of the Offeror and Biotie pursuant to the terms and conditions of the Combination Agreement. In order to effect the combination, the Offeror will acquire, through the Tender
Offer and, if necessary, through a Subsequent Compulsory Redemption or otherwise, all of the Equity Interests.
In connection with the execution of the
Combination Agreement, certain holders of Equity Interests representing in total approximately 65 percent of the outstanding Shares and votes (on a fully diluted basis) in the Company have, subject to certain customary conditions, irrevocably
undertaken to accept the Tender Offer. See Section 1.7—“Irrevocable Undertakings of Major Shareholders.”
The background of the
transactions contemplated under the Combination Agreement has been described in more detail in Section 1.1—“Background to the Tender Offer”.
Offer Period. The Combination Agreement provides that the Offeror will commence
the Tender Offer as promptly as practicable after the date of the Combination Agreement (but no later than five (5) banking days after the Offeror received the SEC Relief, which was received on March 8, 2016). Under the Combination Agreement,
the Offer Period will initially run for twenty (20) business days and must be extended by the Offeror for one or more successive periods not to exceed two (2) weeks ending no later than the Long-Stop Date if any of the Conditions to
Completion are not satisfied or waived prior to its expiration in accordance with the terms and conditions of the Tender Offer and the Combination Agreement and subject to Applicable Law.
Offer Consideration. The Combination Agreement provides that the Offeror will offer to acquire all the Equity Interests for the applicable Offer Price
set forth in Section 4.1—“Terms of the Tender Offer”.
Conditions to Completion. The obligations of the Offeror to
accept for payment the tendered Equity Interests and to complete the Tender Offer will be subject to the fulfilment or, to the extent permitted by Applicable Law, waiver by the Offeror of the Conditions to Completion (as defined
Section 4.2—“Conditions to Completion of the Tender Offer”).
35
Payment. Subject to the terms and conditions of the Combination Agreement and to the satisfaction or
waiver of the Conditions to Completion, the Offeror will accept for payment and pay for, as promptly as practicable after the expiration of the Tender Offer, all Equity Interests validly tendered and not withdrawn. As permitted by the SEC Relief,
the Offeror expects to pay for all Equity Interests validly tendered and not withdrawn within six (6) Finnish banking days following the expiration of the Tender Offer. Actual time of receipt of the payment will depend on the schedules of money
transactions between financial institutions and, if applicable, agreements between the holder of Equity Interest and the relevant account operator or nominee in each case.
Permission by the Board of Directors of Biotie to transfer Option Rights. The Board of Directors of Biotie has under the Combination Agreement
undertaken to grant permission to the holders of the 2011, 2014 and 2016 Option Rights to transfer their 2011, 2014 and 2016 Option Rights to the Offeror by accepting the Tender Offer and tendering the 2011, 2014 and 2016 Option Rights into the
Tender Offer in accordance with the terms and conditions of the 2011, 2014 and 2016 Option Rights. Biotie has also undertaken under the Combination Agreement to cause the Board of Directors of Biotie Therapies AG to adopt such amendments to the
terms and conditions of the Swiss Option Rights as may be required for their tender into the Tender Offer.
Delisting and deregistration. Pursuant
to the Combination Agreement, subject to the Offeror acquiring more than 90 percent of the issued and outstanding Shares (including Shares represented by ADSs) and voting rights in the Company, the Offeror will cause, and the Company will use its
reasonable best efforts to assist the Offeror to cause, the Shares of the Company to be delisted from Nasdaq Helsinki, the ADSs to be delisted from Nasdaq US and the Shares and the ADSs to be deregistered under the Exchange Act as soon as permitted
and reasonably practicable under Applicable Laws (in practice it is the Offeror’s intention that, as soon as reasonably practicable following the acquisition of all Equity Interests, the Company will apply for termination of public trading on
and delisting of its Shares from Nasdaq Helsinki, as well as the delisting of its ADSs from Nasdaq US and the deregistration of its Shares and ADSs under the Exchange Act).
Withholding. The Combination Agreement provides that the Offeror and the Company are entitled to deduct and withhold from any amount payable pursuant
to the Combination Agreement such amounts as are required to be deducted and withheld under any applicable provision of Applicable Law to the extent such withholdings or deductions are required to be made at the time such payments are made. The
transfer tax, if any, levied on the sale and purchase of the Equity Interests will, subject to certain conditions, be borne by the Offeror.
Recommendation. The Company has represented in the Combination Agreement that the Company’s Board of Directors, by a unanimous board decision,
decided to recommend that the holders of the Equity Interests accept the Tender Offer and tender their Equity Interests in the Tender Offer and to issue a formal statement to this effect. Biotie board member Mr. Don M. Bailey, ViVo
Capital, the venture partner of which is Biotie board member Dr. Mahendra G. Shah, and Versant Ventures, where Biotie board member Dr. Guido Magni is partner, each entered into an Irrevocable Undertaking after Biotie’s Board of
Directors approved the acceptance of the Combination Agreement on January 19, 2016 by a unanimous decision. See Section 1.7—“Irrevocable Undertakings of Major Shareholders.” Mr. Bailey, Dr. Shah and Dr. Magni
did not participate in the giving of the statement of the Biotie Board of Directors.
Subject to certain provisions therein, including those described in
the following paragraph, under the terms of the Combination Agreement, the Board of Directors of the Company may, at any time prior to the Closing Date, cancel or change the recommendation if, and only if, the Board of Directors of Biotie determines
in good faith that failure to cancel or change the recommendation would be inconsistent with its fiduciary duties.
Pursuant to the Combination Agreement,
the Board of Directors of Biotie has, in the event of a possible competing public tender offer or other written competing proposal, undertaken not to withdraw or change its recommendation for the Tender Offer unless the Board of Directors determines
in good faith, after taking advice from its external legal counsel and financial advisor, that the competing public tender offer or other competing proposal, judged as a whole, is superior from a financial point of view to the Tender Offer, and that
therefore it would no longer be in the best interest of the holders of the Equity Interests to accept the Tender Offer and that
36
failure to cancel or change the recommendation would be inconsistent with the Board of Director’s fiduciary duties toward the holders of the Equity Interests under applicable Finnish laws
and guidance on board duties in the Helsinki Takeover Code; provided that simultaneously with cancelling or changing the recommendation the Company must enter into a definitive written agreement with respect to the competing proposal (but not with
respect to a competing public tender offer). Should the Offeror enhance the Tender Offer so as to be at least as favorable to the holders of Biotie’s Equity Interests as the competing public tender offer or competing proposal, the Board of
Directors has undertaken to confirm and uphold the recommendation for the Tender Offer, as enhanced.
| 3.3 |
Representations and Warranties |
The Combination Agreement contains representations and warranties made
by the Company to the Offeror and representations and warranties made by the Offeror to the Company. The purpose of this summary of the Combination Agreement is to provide the holders of Equity Interests with information regarding the terms of the
Combination Agreement and is not intended to modify or supplement any factual disclosures about the Company or the Offeror in the Company or the Offeror’s public reports filed with the SEC. In particular, the assertions contained in these
representations and warranties are qualified by information in confidential disclosure schedules provided by the Company to the Offeror in connection with the signing of the Combination Agreement. These disclosure schedules contain information that
modifies, qualifies and creates exceptions to the representations and warranties set forth in the Combination Agreement. Moreover, certain representations and warranties in the Combination Agreement were used for the purpose of allocating risk among
the Company and the Offeror, rather than establishing matters of fact. Accordingly, the representations and warranties in the Combination Agreement may not constitute the actual state of facts about the Company or the Offeror. Holders of Equity
Interests are not third-party beneficiaries of the Combination Agreement and should not rely on the representations, warranties and covenants or any descriptions thereof as characterizations of the actual state of facts or conditions of the Company,
the Offeror or any of their respective subsidiaries or affiliates.
In the Combination Agreement, the Company has made customary representations and
warranties to the Offeror with respect to, among other things:
| |
• |
|
Biotie and its subsidiaries being validly incorporated and in good standing (where applicable) and having the requisite power and authority to carry on their business; |
| |
• |
|
Biotie having the power and authority to enter into, and the enforceability of, the Combination Agreement and the Biotie’s obligations thereunder; |
| |
• |
|
the absence of any competing proposal; |
| |
• |
|
the latest consolidated financial statements and interim report of Biotie having been prepared in accordance with relevant laws and accounting standards; |
| |
• |
|
Biotie having disclosed all information required to be disclosed under applicable Finnish and US laws and regulations and the rules and regulations of Nasdaq Helsinki and Nasdaq US, and no information being misleading
or untrue in any material respect; |
| |
• |
|
the number of Equity Interests, and there being no further option rights or other securities entitling to shares in Biotie; |
| |
• |
|
Biotie and its subsidiaries being in compliance with all applicable laws, Health Laws and laws relating to the employment of labor, any benefit plans or collective agreements, and all material contracts of the Company;
|
| |
• |
|
there being no material claims, litigation or other legal proceedings pending or threatened against Biotie or its subsidiaries; |
| |
• |
|
Biotie and its subsidiaries being the owner or having a valid license to use all intellectual property necessary for the Company or its subsidiaries
to conduct their respective businesses and there being no |
37
| |
infringement or other violation of any intellectual property rights, nor any pending or threatened claims with respect to any intellectual property; |
| |
• |
|
Biotie and its subsidiaries having filed all tax returns, having paid all material taxes due and there being no tax-related actions or disputes pending or threatened with respect to Biotie or its material subsidiaries;
|
| |
• |
|
Biotie and its subsidiaries having sufficient internal accounting controls in place and not having knowingly made or accepted any unlawful payments; and |
| |
• |
|
being in good standing and in compliance with regulatory requirements relating to any Covered Product. |
Some
of the representations and warranties in the Combination Agreement made by the Company are qualified as to “materiality” or “Material Adverse Effect.” Material Adverse Effect has the meaning given to it in
Section 4.2—“Conditions to Completion of the Tender Offer.”
In the Combination Agreement, the Offeror has made customary
representations and warranties to the Company with respect to, among other things:
| |
• |
|
valid organization and the requisite power and authority to carry on its business; |
| |
• |
|
the power and authority to enter into, and the enforceability of, the Combination Agreement and the Offeror’s obligations thereunder; |
| |
• |
|
sufficient financing being available to finance the Tender Offer and there being no need for third party financing to complete the Tender Offer; |
| |
• |
|
the concurrent entry into, and enforceability of the Share Issuance; |
| |
• |
|
the compliance of this Tender Offer Document with applicable laws, rules and regulations, and no information herein being misleading or untrue in any material respect; and |
| |
• |
|
the absence of certain agreements with holders of Equity Interests and the Company’s management and Board of Directors (except for the Irrevocable Undertakings). |
The representations and warranties will automatically terminate upon consummation of the Tender Offer, thereby having no further effect after such date.
Reasonable Best Efforts. Under the Combination Agreement, each of the Parties has
undertaken to use its reasonable best efforts to do, or cause to be done, and to assist and cooperate with the other Party in making all necessary registrations and filings, obtaining all requisite consents, approvals or waivers from and providing
all necessary notices to third parties, informing their employees of the Tender Offer, and executing and delivering any additional resolutions or instruments necessary. The Parties have also undertaken to co-operate and consult with each other in
communications with regulatory authorities, to comply with the Helsinki Takeover Code and to use their reasonable best efforts to facilitate the fulfilment of the Minimum Condition.
To satisfy the obligations described in the preceding paragraph, the Offeror has undertaken to divest, hold separate, or enter into any license or restriction
on the ownership or operation of the Offeror or the Company, provided that in no event will the Offeror be obligated to take such action that would have a material adverse effect on the business of the Company and its subsidiaries, taken a whole, or
the business of the Offeror and its subsidiaries, taken as a whole (with the business of the Offeror and its subsidiaries, taken as a whole, deemed for such purposes to be equivalent in size to the business of the Company and its subsidiaries, taken
as a whole).
38
SEC Relief. The Combination Agreement provides that the parties would cooperate with one another to obtain
exemptive relief from the SEC with respect to permitting the Tender Offer to be conducted in accordance with the terms and conditions set forth in this Tender Offer Document. Such relief was obtained on March 8, 2016. See
Section 4.16—“Certain Legal Matters; Regulatory Approvals; Description of SEC relief.”
No Solicitation. Biotie has
undertaken, and has undertaken to cause its subsidiaries and representatives to undertake, in each case subject to the Fiduciary Duties of the Board of Directors and the rights of the Company set forth in the Combination Agreement:
| |
• |
|
not to solicit, knowingly encourage, facilitate or promote or discuss with or provide confidential information with respect to the Company to any Person in connection with any competing offer or any proposal for such
offer or for any other transaction involving the transfer of any material portion of Biotie’s assets or businesses, whether through a public tender offer or by sale or transfer of assets, license, option, warrant, sale of shares, reorganization
or merger, transfer of employees in a hiring action by a third party or otherwise, or any other similar corporate transaction that would constitute or result in any competing transaction that would harm or hinder the completion of the Tender Offer
(“Competing Proposal”); |
| |
• |
|
to cease and cause to be terminated any discussions, negotiations or other activities related to any Competing Proposal conducted prior to January 19, 2016; |
| |
• |
|
not to, upon receipt of a Competing Proposal or a competing offer, directly or indirectly, knowingly facilitate, promote, or encourage or discuss with or provide confidential information with respect to the Company to
the Persons making such Competing Proposal (“Promoting Measures”); |
| |
• |
|
to promptly provide the Offeror any information regarding the Company provided to any third party to the extent such information has not been previously provided to the Offeror; |
| |
• |
|
to promptly inform the Offeror in writing about any Competing Proposal, including, to the extent available to the Company, the identity of the offeror and the price and other main terms and conditions of such Competing
Proposal, and of any material amendments to such price and terms and conditions by the third-party offeror, and will keep the Offeror informed, on a current basis, of the status and material terms of any such Competing Proposal, including any
material amendments or proposed amendments as to price and other material terms thereof; and |
| |
• |
|
to provide the Offeror with an opportunity to negotiate with the Board of Directors of the Company about matters arising from the Competing Proposal. |
The Combination Agreement does not prohibit the Board of Directors of Biotie from (i) complying with Rule 14e-2(a) under the Exchange Act with
regard to a Competing Proposal so long as any such action taken or statement made to so comply also complies with the Combination Agreement; or (ii) issuing a “stop, look and listen” communication or similar communication of the type
contemplated by Rule 14d-9(f) under the Exchange Act.
Conduct of Business of the Company. The Combination Agreement provides that, except as
contemplated or permitted by the Combination Agreement, expressly set forth in the disclosure schedules to the Combination Agreement, required by law or consented to in writing by the Offeror, during the period from the date of the Combination
Agreement to the Closing Date, the Company has undertaken to, and to cause each of its subsidiaries to:
| |
• |
|
conduct their respective businesses only in the ordinary course of business consistent with past practice and in all cases based on prudent business judgment; |
| |
• |
|
subject to specified thresholds and exceptions, to refrain from making or implementing: |
| |
• |
|
any material changes in its business; |
39
| |
• |
|
any hiring of new employees; |
| |
• |
|
any material corporate transactions; |
| |
• |
|
any agreements not at arms’ length or otherwise outside the ordinary course of business; |
| |
• |
|
any material amendments, waivers under, or termination with respect to Material Contracts or the entry into any new agreement containing change of control provisions, restrictions on business, licenses of material
intellectual property, contingent payment provisions, or that otherwise relate to the exploitation of material intellectual property; |
| |
• |
|
any changes to its organizational documents or accounting principles or practices; |
| |
• |
|
any commencement, settlement or compromise of material legal proceedings or claims; |
| |
• |
|
any act or omission that would reasonably be expected to result in the abandonment, encumbrance or grant of material intellectual property rights; |
| |
• |
|
any dividends, changes in capitalization, transfer or encumbrance of treasury shares or grant, transfer or disposal of any option rights for shares in the Company; |
| |
• |
|
any changes in agreements with or compensation or benefits of material employees; |
| |
• |
|
any action that would increase liability for taxes; and/or |
| |
• |
|
any agreement or commitment to do any of the foregoing. |
In addition, the Company has undertaken to provide
the Offeror with advance notice of, and the opportunity to observe, scheduled meetings and discussions with regulatory authorities concerning regulatory matters relating to the Covered Products. The Company has also undertaken to provide the Offeror
with a reasonable opportunity to review and provide comments with respect to any material filing and any material correspondence or other material communication proposed to be submitted or otherwise transmitted to a regulatory authority relating to
a Covered Product, and to keep the Offeror promptly informed of any material communication with or from any regulatory authority.
Access to
Information. Biotie has undertaken to use its reasonable best efforts to provide the Offeror with access to information regarding Biotie and its subsidiaries that the Offeror may reasonably need in preparation of regulatory filings or that the
Offeror may otherwise reasonably require for purposes of the Tender Offer. Biotie has further undertaken to afford to the representatives of the Offeror reasonable access to its employees, properties, books and records and to furnish to the Offeror
all information concerning its business, properties and personnel as may be reasonably requested.
Biotie has also undertaken to use reasonable
best efforts to provide the Offeror with a list of issuance, renewal, maintenance and other payments and of any other actions required to be taken to maintain its applied for and registered intellectual property, and to use its reasonable best
efforts to cause or request such payments to be made and such actions to be taken.
Notice of Certain Events and Public Announcements. Each Party
has undertaken to notify the other Party of certain events and to consult with each other before issuing any public announcements relating to the Tender Offer.
General Meeting of Shareholders. The Board of Directors of Biotie has undertaken to convene, at request of the Offeror, an extraordinary general
meeting of shareholders of the Company after the completion of the Tender Offer for the purpose of electing new members to the Board of Directors of the Company.
Indemnification and Insurance. For a period of six (6) years after the Closing Date, the Offeror has agreed, with certain exceptions, to indemnify
and hold harmless, and to advance fees, costs and expenses related to any
40
litigation, claim or proceeding involving, the present and former directors and officers of Biotie and its subsidiaries in respect of acts or omissions occurring at or prior to the Closing
Date. The Parties have agreed to obtain and fully pay the premium for the extension of the Company’s existing directors’ and officers’ insurance policies and fiduciary liability insurance policies for a claims period of at least six
(6) years from and after the Closing Date with terms, conditions and levels of coverage at least as favorable as the Company’s existing policies; provided that in no event will the annual premiums be in excess of 300 percent of the amount
per annum the Company paid in its fiscal year ended December 31, 2015.
Employee Matters. The Combination Agreement provides that for a
period of one year following the Closing Date, the Offeror will provide the employees of the Company and its subsidiaries that are employed in the United States and who remain employed with (i) an annual rate of salary or wages that is no less
favorable than that provided by the Company as of immediately prior to the Closing Date and (ii) bonus opportunities, employee benefits and severance benefits that are substantially comparable to those provided by the Offeror to its
similarly situated employees. Further, should the Offeror elect to transition any such employee from a Biotie benefit plan to a welfare plan of the Offeror, the Offeror will use its reasonable best efforts to waive applicable limitations in its
welfare plans with respect to participation and coverage requirements and to credit such employees for copayments, deductibles or similar payments made under a Biotie plan during the relevant plan year as well as credit for service with the
Company, any of its subsidiaries or any predecessor employer for purposes of satisfying any applicable requirements or determining benefit levels or eligibility. Under the Combination Agreement, and contingent upon the consummation of the Tender
Offer, the Company undertakes to terminate any employee benefit plan(s) intended to qualify as qualified cash or deferred arrangements under Section 401(k) of the Code (as defined below) and the Offeror undertakes to allow any of the
Company’s employees that were participants under such plan to participate in a similar plan of the Offeror and to distribute or roll over the balances and up to one outstanding loan under such Company plan in accordance with its terms. The
Combination Agreement provides that the rights described in this “Employee Matters” paragraph are solely for the benefit of the Parties and that no employee, consultant, contractor or director of the Company or any of its subsidiaries will
be regarded as a third-party beneficiary thereof.
Financing. The Offeror agreed to use reasonable best efforts to receive the proceeds
from the Share Issuance, the proceeds of which were received on January 26, 2016. See Section 1.5—“Financing of the Tender Offer.”
| 3.5 |
Termination of the Combination Agreement |
The Combination Agreement may be terminated with immediate
effect at any time prior to the Closing Date as follows:
| |
• |
|
by mutual written consent of the Parties; |
| |
• |
|
by either Party, if there is a final, nonappealable legal restraint or prohibition preventing the consummation of the Tender Offer; |
| |
• |
|
by either Party if the Closing Date has not occurred by June 19, 2016; |
| |
• |
|
by the Company if the Board of Directors of Biotie has in compliance with the provisions set forth in the Combination Agreement cancelled or changed its recommendation to the holders of Biotie securities to accept the
Tender Offer; |
| |
• |
|
by the Offeror if the Board of Directors of Biotie has for any reason cancelled or changed its recommendation to the holders of Biotie securities to accept the Tender Offer; |
| |
• |
|
by the Company if (i) the Offeror has failed to perform in any material respect any covenant or agreement set forth in the Combination Agreement or (ii) any of the representations and warranties of the Offeror
fail to be true and correct in such a manner so as to reasonably be expected to prevent the consummation of the Tender Offer, and in each case, such failure is incapable of being cured by the Long-Stop Date, or if capable of being cured prior to the
Long-Stop Date, has not been cured within thirty (30) days of the Company providing written notice thereof; or |
41
| |
• |
|
by the Offeror if (i) the Company has failed to perform in any material respect any covenant or agreement set forth in the Combination Agreement, (ii) any of the representations and warranties of the Company
fail to be true and correct (the materiality threshold for such determination being set forth in the Combination Agreement), and in each case, such failure is incapable of being cured by the Long-Stop Date, or if capable of being cured prior to the
Long-Stop Date, has not been cured within thirty (30) days of the Offeror providing written notice thereof. |
In case of any termination
of the Combination Agreement, the Offeror will withdraw the Tender Offer. If the Combination Agreement is is terminated as a result of the Board of Directors of Biotie cancelling or changing its recommendation for the Tender Offer due to a competing
offer or Competing Proposal in accordance with the Combination Agreement, the Company will pay to the Offeror a termination fee of USD 4.5 million for the Offeror’s reasonable costs for the evaluation, negotiation and preparation of the
Tender Offer.
| 3.6 |
Governing Law, Jurisdiction and Specific Performance |
The Combination Agreement is governed by the laws
of Finland. All disputes arising out of or relating to the Combination Agreement will be settled by final, binding arbitration in English in London, United Kingdom and will be conducted under the Rules of the International Chamber of Commerce and in
accordance with the provisions of the Combination Agreement. Pending the commencement or completion of any such arbitral proceeding, each Party is entitled to apply to a court of competent jurisdiction for a precautionary measure, temporary remedy
or order or preliminary injunction where necessary to protect its interests.
The Combination Agreement may not be amended except by written instrument signed by both
Parties.
| 4 |
TERMS AND CONDITIONS OF THE TENDER OFFER |
| 4.1 |
Terms of the Tender Offer |
Object of the Tender Offer. Through the Tender Offer, the Offeror
offers to acquire all of the Equity Interests in the Company that are not held by the Company or any of its subsidiaries, on the terms and subject to the conditions set forth below.
According to the terms and conditions of the 2011 Option Rights, 2014 Option Rights and 2016 Option Rights as well as the 2011 Share Rights and 2014 Share
Rights, such Option Rights and Share Rights are not freely transferable. The Board of Directors of the Company may, however, permit the transfer of the Option Rights and Share Rights before such date, and, under the Combination Agreement, the Board
of Directors of the Company has undertaken to grant such permission to the holders of Option Rights and Share Rights to transfer their Option Rights and Share Rights to the Offeror by accepting the Tender Offer and tendering the Option Rights into
the Tender Offer in accordance with the terms and conditions of the Tender Offer. The Board of Directors of the Company has granted the permission to transfer the Option Rights and Share Rights in connection with the Tender Offer.
According to the terms and conditions of the Swiss Option Rights, such Option Rights are not freely transferable without the consent of the board of directors
of Biotie Therapies AG, a wholly-owned subsidiary of Biotie. The Board of Directors of the Company has undertaken to cause Biotie Therapies AG to grant such consent to holders of the Swiss Option Rights who accept the Tender Offer and tender their
Swiss Option Rights in accordance with the terms and conditions of the Tender Offer. The Board of Directors of Biotie Therapies AG has granted the consent in question.
Offer Price. The offer price for each outstanding Share validly tendered in accordance with the terms and conditions of the Tender Offer is EUR 0.2946
in cash (the “Share Offer Price”).
42
The offer price for each outstanding ADS validly tendered in accordance with the terms and conditions of the
Tender Offer is EUR 23.5680 in cash (the “ADS Offer Price”), payable in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro
exchange rate on the nearest practicable day to the applicable Closing Date. For the avoidance of doubt, holders of ADSs who validly tender their ADSs in accordance with the terms and conditions of the Tender Offer will not be entitled to any
consideration for their ADSs (including the Shares underlying such ADSs) other than the ADS Offer Price.
The offer prices for outstanding Option Rights
validly tendered in accordance with the terms and conditions of the Tender Offer are as follows:
| |
(i) |
EUR 0.2846 in cash for each 2011 Option Right; |
| |
(ii) |
EUR 0.2846 in cash for each 2014 Option Right; |
| |
(iii) |
EUR 0.1326 in cash for each 2016 Option Right; |
| |
(iv) |
EUR 0.2032 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.10; |
| |
(v) |
EUR 0.1026 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.21; |
| |
(vi) |
EUR 0.0386 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.28; |
| |
(vii) |
EUR 0.0112 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.31; and |
| |
(viii) |
EUR 0.0100 in cash for each other Swiss option right. |
(The Option Right offer prices set forth in items
(i) – (viii) above jointly referred to as the “Option Right Offer Price”.)
The offer prices for outstanding Share Rights
validly tendered in accordance with the terms and conditions of the Tender Offer are as follows:
| |
(i) |
EUR 0.2946 in cash for each 2011 Share Right; and |
| |
(ii) |
EUR 0.2854 in cash for each 2014 Share Right. |
(The Share Right offer prices set forth in items
(i) – (ii) above jointly referred to as the “Share Right Offer Price”.)
The offer price for each outstanding Warrant
validly tendered in accordance with the terms and conditions of the Tender Offer is EUR 0.1664 in cash (the “Warrant Offer Price”).
The
offer price payable to holders of 2016 Option Rights, 2011 Share Rights and 2014 Share Rights will, at the option of the holder, be payable in euros or the equivalent amount of U.S. dollars determined as near to the payment date as reasonably
practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date.
The Share
Offer Price, ADS Offer Price, Option Right Offer Price, Share Right Offer Price and Warrant Offer Price are collectively referred to as the “Offer Price”.
The Offer Price will be paid in each case without interest and less any applicable withholding taxes (in the United States, see Section
4.13—“Material United States Federal Income Tax Consequences”).
Offer Period. The Offer Period commences on March 11, 2016 at 9:30
a.m. (Finnish time) / 2:30 a.m. (New York City time) and expires on April 8, 2016 at 4:00 p.m. (Finnish time) / 9:00 a.m. (New York City time), unless the Offer Period is extended as set forth below.
43
Subject to the following paragraph, if at the scheduled expiration time of the Offer Period any of the Conditions
to Completion are not satisfied, the Offeror will extend the Offer Period for additional periods not exceeding two (2) weeks each in accordance with these terms and conditions and, in each case, subject to compliance with applicable Finnish and
United States legal requirements.
The maximum duration of the Offer Period is ten (10) weeks as required by Finnish law. However, if any of the
Conditions to Completion has not been fulfilled due to a particular obstacle, the Offeror may extend the Offer Period beyond ten (10) weeks until such obstacle has been removed and until all Conditions to Completion have been satisfied. In no
event is the Offeror required to extend the Tender Offer beyond June 19, 2016.
U.S. tender offer rules require that the Offeror extend the Tender
Offer if the Offeror intends to materially change the Tender Offer within ten U.S. business days of the then-scheduled Expiration Date, so that the Tender Offer will expire no less than ten U.S. business days after the publication of the material
change.
The Offeror reserves the right to initiate a Subsequent Offer Period in connection with the announcement of the final results with respect to the
Offer Period if the Tender Offer shall have been announced to be unconditional at that time. See Section 4.11—“Subsequent Offer Period.”
If the Offer Period is extended the Offeror will make a public announcement of the extension by a stock exchange release at or before 4:00 p.m. (Finnish time)
/ 9:00 a.m. (New York City time) on April 11, 2016. The Offeror will give notice of any further extension of the Tender Offer, at the latest on the first Finnish banking day following what would have been, but for such extension, the Expiration Date
or end of any Subsequent Offer Period, as applicable, at or before 4:00 p.m. (Finnish time) / 9:00 a.m. (New York City time).
| 4.2 |
Conditions to Completion of the Tender Offer |
The obligation of the Offeror to accept for payment the
Equity Interests validly tendered and not withdrawn during the Offer Period will be subject to the fulfilment or, to the extent permitted by applicable law, waiver by the Offeror of the following conditions on or prior to the date of the
Offeror’s announcement of the preliminary result with respect to the Offer Period (“Conditions to Completion”):
| |
(a) |
the valid tender of outstanding Shares (including outstanding Shares represented by validly tendered ADSs and validly tendered Warrants) representing, together with any outstanding Shares (including outstanding Shares
represented by ADSs and Warrants) otherwise acquired by the Offeror, more than 90 percent of the issued and outstanding Shares and voting rights of the Company, calculated on a fully diluted basis and otherwise in accordance with Chapter 18
Section 1 of the Finnish Limited Liability Companies Act (the “Minimum Condition”) (as used in this paragraph, “fully diluted basis” means an equation in which the numerator represents the aggregate number of
outstanding Shares (including outstanding Shares represented by ADSs) and Warrants that have been validly tendered or otherwise acquired by the Offeror and the denominator represents the aggregate number of all outstanding Shares (including
outstanding Shares represented by ADSs) and Warrants, as well as Shares issuable upon the vesting and exercise of those Option Rights and Share Rights that have not been validly tendered into the Tender Offer or otherwise acquired by the Offeror);
|
| |
(b) |
the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Act (as discussed in Section 4.16—“Certain Legal Matters; Regulatory Approvals; Description of SEC Relief,”
such waiting period expired on February 16, 2016); |
| |
(c) |
no Material Adverse Effect (as defined below) having occurred in the Company after January 19, 2016; |
| |
(d) |
the Offeror not, after January 19, 2016, having received information previously undisclosed to it that describes a Material Adverse Effect to the Company that occurred prior to January 19, 2016;
|
| |
(e) |
no information made public by the Company or disclosed by the Company to the Offeror being materially inaccurate, incomplete, or misleading, and the
Company not having failed to make public |
44
| |
any information that should have been made public by it under applicable laws, including without limitation the rules of Nasdaq Helsinki and Nasdaq US, provided that, in each case, the
information made public, disclosed or not disclosed or the failure to disclose information constitutes a Material Adverse Effect to the Company; |
| |
(f) |
no court or regulatory authority of competent jurisdiction (including without limitation the FSA or the SEC) having given an order or issued any regulatory action preventing or enjoining the completion of the Tender
Offer; |
| |
(g) |
the Board of Directors of the Company having issued its recommendation for the Tender Offer and the recommendation remaining in force and not being modified or changed in a manner detrimental to the Offeror; and
|
| |
(h) |
the Combination Agreement not having been terminated and remaining in force and no event having occurred that, with the passage of time, would give the Offeror the right to terminate the Combination Agreement under
specified sections of the Combination Agreement that give the Offeror the right to terminate the Combination Agreement in response to a breach of the agreement by the Company. |
Fulfillment of the Conditions to Completion, including fulfillment of the Minimum Condition, will be determined on the next Finnish banking day after the
Expiration Date, based on the preliminary results with respect to the Offer Period then available. Such results may be subject to change based on a finalization count, which will be available on the third (3rd) Finnish banking day after the
Expiration Date. However, no such change will impact fulfillment of the Conditions to Completion.
“Material Adverse Effect” means
| |
(a) |
any divestment or reorganization of any material part or asset of the Company or its subsidiaries or any recapitalization thereof; or |
| |
(b) |
any event, condition, circumstance, development, occurrence, change, effect or fact (any such item an “Effect”) that individually or in the
aggregate, has, results in or would reasonably be expected to have or result in a material adverse effect on the (i) business, assets, condition (financial or otherwise) or results of operations, of the Company and its subsidiaries, taken as a
whole, excluding any Effect resulting from (A) changes in the financial or securities markets, or economic, political or regulatory conditions generally, except to the extent such change has a disproportionate effect on the Company relative to
other industry participants, (B) changes in IFRS or changes in the regulatory accounting requirements applicable to any industry in which the Company and its subsidiaries operate, except to the extent such change has a disproportionate effect
on the Company relative to other industry participants, (C) changes (including changes of applicable law) or conditions generally affecting the industry in which the Company and its subsidiaries operate, except to the extent such change has a
disproportionate effect on the Company relative to other industry participants, (D) acts of war, sabotage or terrorism or natural disasters, except to the extent such change has a disproportionate effect on the Company relative to other
industry participants, (E) the announcement or pendency of the transactions contemplated by, or the performance of obligations under, the Combination Agreement, including but not limited to any loss of or change in relationships between the
Company or any of its subsidiaries and any customer, supplier, distributor, business partner, employee, similar party, governmental authority or any other persons and any shareholder or derivative litigation relating to the execution and performance
of the Combination Agreement or the announcement or the anticipated consummation of the transactions contemplated thereby (it being understood that this clause (E) shall not apply with respect to a representation or warranty contained in the
Combination Agreement to the extent that the purpose of such representation or warranty is to address the consequences resulting from the execution and delivery of the Combination Agreement or the consummation, announcement or pendency of the
transactions contemplated by, or the performance of obligations under, the Combination Agreement), (F) any failure by the Company and its subsidiaries to meet any internal, published or third-party budgets, projections, forecasts or predictions
of financial performance for any period (provided that the |
45
| |
exception in this clause (F) shall not prevent or otherwise affect a determination that any Effect underlying such failure has resulted in or contributed to a Material Adverse Effect),
(G) any action taken (or omitted to be taken) at the express request of the Offeror, (H) any action taken by the Company or any of its subsidiaries that is required or expressly contemplated by the Combination Agreement, (I) the
results of any pre-clinical or clinical testing sponsored by the Company, any of its competitors or any of their respective collaboration partners or the increased incidence or severity of any previously identified side effects, adverse events or
safety observations, or reports of new side effects, adverse events or safety observations, with respect to any products or product candidates of the Company or any of its competitors (but not, in each case, the underlying cause of such results,
side effects, adverse events or safety observations to the extent such cause related to any Specified Event (as defined below)) (and, it being understood that this clause (I) shall not apply with respect to a representation or warranty
contained in the Combination Agreement to the extent that both (A) the purpose of such representation or warranty is to address the conduct of pre-clinical or clinical testing sponsored by the Company or any of its collaboration partners or any
products or product candidates of the Company and (B) as January 19, 2016, to the knowledge of the Company, the Effect that would otherwise be excluded by this clause (I) has occurred), (J) the effect of the continued incurrence
by the Company of net losses (provided that the exception in this clause (J) shall not prevent or otherwise affect a determination that any Effect underlying such change has resulted in or contributed to a Material Adverse Effect), or
(K) a change in the price and/or trading volume of the Shares or ADSs on Nasdaq Helsinki, Nasdaq US or any other exchange or market on which they trade or are quoted for purchase and sale (provided that the exception in this clause
(K) shall not prevent or otherwise affect a determination that any Effect underlying such change has resulted in or contributed to a Material Adverse Effect), or (ii) ability of the Company to consummate the transactions contemplated
hereby. |
“Specified Event” means an event where the Company, U.S. Food and Drug Administration, European Medicines Agency
or any Institutional Review Board or Data Safety Monitoring Board terminates or suspends, or recommends that the sponsor terminates or suspends, a tozadenant clinical trial for safety reasons.
The Offeror reserves the right to waive any of the Conditions to Completion that have not been satisfied, subject to compliance with applicable law (including
U.S. tender offer rulers that require the Tender Offer remain open for at least five U.S. business days from the date of any waiver of a material Condition to Completion).
The Offeror can only invoke a condition set forth in the Conditions to Completion to cause the withdrawal of the Tender Offer if the nonfulfillment of the
condition has a material impact on the Offeror from the perspective of the anticipated acquisition as referred to in the Finnish Financial Supervisory Authority’s recommendations and guidelines 9/2013 (Takeover bid and the obligation to launch
a bid).
The Offeror will announce the fulfilment of the Conditions to Completion or the decision to waive any of the Conditions to Completion that have
not been satisfied by a stock exchange release on the first business day following the Expiration Date at or before 4:00 p.m. (Finnish time) / 9:00 a.m. (New York time).
| 4.3 |
Obligation to Increase the Tender Offer or to Pay Compensation |
If the Offeror or any party referred to
in Chapter 11, Section 5 of the Finnish Securities Market Act acquires, before the expiry of the Offer Period, any Equity Interests at a higher price than the applicable Offer Price or otherwise on terms that are more favorable than those of
the Tender Offer, the Offeror must according to Chapter 11, Section 25 of the Finnish Securities Market Act amend the terms and conditions of the Tender Offer to correspond to such acquisition on more favorable terms (obligation to increase the
offer). The Offeror shall then, without delay, make public the triggering of such obligation to increase the offer and pay to all holders of Equity Interests accepted for payment pursuant to the Tender Offer, whether or not such Equity Interests
were tendered prior to such increase, the difference between the higher price and the price initially offered in the Tender Offer.
46
If the Offeror or any party referred to in Chapter 11, Section 5 of the Finnish Securities Market Act
acquires, during the nine (9) months following the expiry of the Offer Period, any Equity Interests at a higher price than the applicable Offer Price or otherwise on terms that are more favorable than those of the Tender Offer, the Offeror must
according to Chapter 11, Section 25 of the Finnish Securities Market Act compensate those holders of securities who have accepted the Tender Offer for the amount equal to the difference between such acquisition on more favorable terms and the
consideration offered in the Tender Offer (obligation to compensate). The Offeror shall then, without delay, make public the triggering of such obligation to compensate and pay the difference between the acquisition on more favorable terms and the
consideration offered in the Tender Offer within one month after the triggering of the obligation to compensate to the holders of securities who have accepted the Tender Offer. According to Chapter 11, Section 25, Subsection 5 of the Finnish
Securities Market Act, such obligation to compensate will not, however, be triggered in case the payment of a higher price than the applicable Offer Price is based on an arbitral award pursuant to the Finnish Companies Act, including an award issued
in a Subsequent Compulsory Redemption, provided that the Offeror or any party referred to in Chapter 11, Section 5 of the Finnish Securities Market Act has not offered to acquire Equity Interests on terms that are more favorable than those of
the Tender Offer before or during the arbitral proceedings.
Prior to the expiration of the Tender Offer, the Offeror, the managers of the Offeror and
their respective affiliates are prohibited by Rule 14e-5 under the Exchange Act from directly or indirectly purchasing or arranging to purchase any of the Equity Interests outside of the Tender Offer, except pursuant to certain limited exceptions
provided therein. Following the expiration of the Tender Offer, and subject to applicable law, the Offeror expressly reserves the absolute right, in its sole discretion from time to time in the future, to purchase any Equity Interests, whether or
not any Equity Interests are purchased pursuant to the Tender Offer, through open market purchases, privately negotiated transactions, tender offers, exchange offers or otherwise, upon such terms and at such prices as it may determine, which may be
more or less than the price to be paid pursuant to the Tender Offer and could be for cash or other consideration taking into account, however, the obligation to compensate described in the previous paragraph.
| 4.4 |
Acceptance Procedure of the Tender Offer |
Holders of Equity Interests may only accept the Tender Offer
unconditionally (subject to the withdrawal rights set forth in Section 4.5—“Withdrawal Rights”). Acceptance given during the Offer Period is effective through the Expiration Date.
Shares
The Tender Offer must be accepted
separately for each book-entry account. A shareholder of the Company must have a cash account in a financial institution operating in Finland or abroad. A shareholder may only accept the Tender Offer for every Share on the book-entry account
specified in the Acceptance Form for Shares on the date of the execution of the sale and purchase of the Shares and partial acceptances of the Tender Offer will require separate book-entry accounts.
Most Finnish book-entry account operators will send a notification of the Tender Offer, including instructions and the Acceptance Form for Shares to their
customers who are registered as shareholders in the shareholders’ register of the Company maintained by Euroclear Finland Ltd. (“Euroclear”). Shareholders who do not receive such notification from their account operator or
asset manager can contact any branch office of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd. where such shareholders will receive necessary information and instructions on how to give their acceptance.
A shareholder in the Company whose Shares are registered in the name of a nominee and who wishes to accept the Tender Offer shall effect such acceptance in
accordance with the nominee’s instructions.
47
Pledged Shares may only be tendered with the consent of the relevant pledgee. The obtaining of such consent shall
be the responsibility of the relevant shareholder in the Company. The consent by the pledgee shall be delivered in writing to the account operator.
A
shareholder in the Company who is registered as a shareholder in the shareholders’ register of the Company and who wishes to accept the Tender Offer shall submit a properly completed and duly executed Acceptance Form to the account operator
managing the shareholder’s book-entry account in accordance with its instructions and within the time limit set by the account operator, which may be prior to the expiry of the Offer Period, or, if such account operator does not accept
Acceptance Forms (e.g. Euroclear), such shareholder shall contact any branch office of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd. to give his/her acceptance to tender the Shares. The Acceptance Form shall be
submitted so that it is received on or before the Expiration Date, subject to and in accordance with the instructions of the relevant account operator. In the event of a Subsequent Offer Period, the Acceptance Form shall be submitted so that it is
received during the Subsequent Offer Period, subject to and in accordance with the instructions of the relevant account operator.
The method of delivery
of the Acceptance Form for Shares is at the shareholder’s option and risk, and the delivery will be deemed made only when actually received by such account operator or cooperative bank belonging to the OP Financial Group or Helsinki OP Bank
Ltd. The Offeror may also reject any partial tender of the Shares. The Offeror reserves the right to reject any acceptance given in an incorrect or incomplete manner.
By accepting the Tender Offer, the shareholders of the Company authorize Pohjola Bank or a party authorized by Pohjola Bank or the account operator managing
the shareholder’s book-entry account to enter a transfer restriction or a sales reservation on the shareholder’s book-entry account after the shareholder has delivered their acceptance of the Tender Offer. In addition, the shareholders who
have accepted the Tender Offer authorize Pohjola Bank or a party authorized by Pohjola Bank or the account operator managing the shareholder’s book-entry account to perform the necessary entries and to take all other actions required to
technically execute the Tender Offer and to sell all the Shares owned by such shareholder at the time of the execution trades under the Tender Offer to the Offeror in accordance with the terms and conditions of the Tender Offer.
A shareholder that has validly accepted the Tender Offer and that has not properly withdrawn its acceptance in accordance with the terms and conditions of the
Tender Offer may not sell or otherwise dispose of its tendered Shares. A transfer restriction in respect of the Shares will be registered in the relevant book-entry account after a shareholder has submitted the Acceptance Form for the Tender Offer.
If the Tender Offer is not completed or if the tender is properly withdrawn by the shareholder in accordance with the terms and conditions of the Tender Offer, the transfer restriction registered on the tendered Shares in the relevant book-entry
account will be removed as soon as possible and within approximately three (3) Finnish banking days following the announcement that the Tender Offer will not be completed or the receipt of a notice of withdrawal in accordance with the terms and
conditions of the Tender Offer.
ADSs
Holders
of ADSs may tender their ADSs by taking, or causing to be taken, the following actions on or prior to the Expiration Date or end of any Subsequent Offer Period, as applicable:
| |
• |
|
a book-entry transfer of their ADSs into the account of the Depositary at DTC, pursuant to the procedures described below; |
| |
• |
|
the delivery to the Depositary at one of its addresses set forth on the back cover of this Tender Offer Document of either: |
| |
(i) |
an Agent’s Message (as defined below); or |
| |
(ii) |
a properly completed and duly executed Letter of Transmittal, or a facsimile copy with an original manual signature, with any required signature guarantees; and |
48
| |
• |
|
delivery to the Depositary at one of its addresses set forth on the back cover of this Tender Offer Document of any other documents required by the Letter of Transmittal. |
There are no guaranteed delivery procedures and holders of ADSs may not accept the Tender Offer by delivering a notice of guaranteed delivery.
An “Agent’s Message” delivered in lieu of the Letter of Transmittal is a message transmitted by DTC to, and received by, the Depositary
as part of a confirmation of a book-entry transfer. The message states that DTC has received an express acknowledgment from the DTC participant tendering ADSs that such participant has received and agrees to be bound by the terms of the Letter of
Transmittal and that we may enforce such agreement against such participant.
Within two (2) business days after the date of this Tender Offer
Document, the Depositary will establish and maintain an account at DTC with respect to ADSs for purposes of this Tender Offer. Any financial institution that is a participant in DTC’s systems may make book-entry delivery of ADSs by causing DTC
to transfer such ADSs into the account of the Depositary in accordance with DTC’s procedure for the transfer.
ADSs held in “street
name.” If the beneficial owner of ADSs is not the registered holder of such ADSs but holds such ADSs in “street name” through a broker, bank or custodian, such beneficial owner should contact the broker, bank or custodian through
which such ADSs are held to discuss the appropriate procedures for tendering.
Signature Guarantees. In general, signatures on the Letter of
Transmittal must be guaranteed by a firm that is a member of the Medallion Signature Guarantee Program, or by any other “eligible guarantor institution,” as such term is defined in Rule 17Ad-15 under the Exchange Act (each an
“Eligible Institution”). However, signature guarantees are not required in cases where ADSs are tendered:
| |
• |
|
by a registered holder of ADSs who has not completed either the box entitled “Special Payment Instructions” or the box entitled “Special Delivery Instructions” on the Letter of Transmittal; or
|
| |
• |
|
for the account of an Eligible Institution. |
Delivery of Tendered ADSs. The method of delivery of ADSs,
the Letter of Transmittal and all other required documents is at the election and risk of the tendering holder. Delivery of all such documents will be deemed made only when actually received by the Depositary (including, in the case of a book-entry
transfer, by confirmation of such transfer). If such delivery is by mail, it is recommended that all such documents be sent by properly insured registered mail with return receipt requested. In all cases, sufficient time should be allowed to ensure
timely delivery.
Payment for Tendered ADSs. The cash consideration for ADSs will be payable in U.S. dollars calculated by using the open
market spot exchange rate for the U.S. dollar to euro on the nearest practicable day to the applicable Closing Date.
Option Rights, Share
Rights and Warrants
The acceptance procedure for Option Rights, Share Rights and Warrants depends on whether such Equity Interests are in
book-entry form or certificated. All 2011 Option Rights, the 2014 Option Rights in the 2014A tranche and all Warrants (“Uncertificated Equity Instruments”) are registered in the Finnish book-entry securities system. The 2014 Option
Rights in the 2014B, 2014C, 2014D and 2014M tranches, all 2016 Option Rights, all Swiss Option Rights, all 2011 Share Rights and all 2014 Share Rights (“Certificated Equity Instruments”) are certificated and have not been registered
in the Finnish book-entry securities system.
Uncertificated Equity Instruments. Uncertificated Equity Instruments comprise all 2011 Option Rights,
the 2014 Option Rights in the 2014A tranche and all Warrants. The Tender Offer must be accepted separately for each
49
type of Uncertificated Equity Instrument and, if such Uncertificated Equity Instruments are held in more than one book-entry account, separately for each book-entry account. A holder of
Uncertificated Equity Instruments may only accept the Tender Offer for every Uncertificated Equity Instrument on the book-entry account specified in the Acceptance Form for Uncertificated Equity Instruments on the date of the execution of the sale
and purchase of the Uncertificated Equity Instruments. A holder of Uncertificated Equity Instruments must have a cash account in a financial institution operating in Finland or abroad.
Most of the Finnish book-entry account operators will send a notification of the Tender Offer, including instructions and an Acceptance Form for
Uncertificated Equity Instruments to their customers who are holders of Uncertificated Equity Instruments. Holders of Uncertificated Equity Instruments who do not receive such notification from their account operator or asset manager can contact the
call service of OP Financial Group at (+358) (0) 100 0500 for assistance where such holders of Uncertificated Equity Instruments will receive necessary information and instructions on how to give their acceptance.
Holders of Uncertificated Equity Instruments whose holdings are registered in the name of a nominee and who wish to accept the Tender Offer shall effect such
acceptance in accordance with the nominee’s instructions.
Pledged Uncertificated Equity Instruments may only be tendered with the consent of the
relevant pledgee. The obtaining of such consent shall be the responsibility of the relevant holder of Uncertificated Equity Instruments. The consent by the pledgee shall be delivered in writing to the account operator.
A holder of Uncertificated Equity Instruments who wishes to accept the Tender Offer shall submit a properly completed and duly executed Acceptance Form for
Uncertificated Equity Instruments to the account operator managing the holder’s book-entry account in accordance with its instructions and within the time limit set by the account operator, which may be prior to the expiry of the Offer Period,
or, if such account operator does not accept Acceptance Forms (e.g. Euroclear), such holder of Uncertificated Equity Instruments shall contact any branch office of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd. to
give his/her acceptance to tender the Uncertificated Equity Instruments. The Acceptance Form shall be submitted so that it is received during the Offer Period or, if the Offer Period has been extended, during such extended Offer Period, subject to
and in accordance with the instructions of the relevant account operator. In the event of a Subsequent Offer Period, the Acceptance Form shall be submitted so that it is received during the Subsequent Offer Period, subject to and in accordance with
the instructions of the relevant account operator.
The method of delivery of Acceptance Forms is at the option and risk of the holder of Uncertificated
Equity Instruments, and the delivery will be deemed made only when actually received by such account operator or cooperative bank belonging to the OP Financial Group or Helsinki OP Bank Ltd. The Offeror reserves the right to reasonably reject any
acceptance given in an incorrect or incomplete manner.
By accepting the Tender Offer, the holder of Uncertificated Equity Instruments authorizes Pohjola
Bank or a party authorized by Pohjola Bank or the account operator managing the holder’s book-entry account to enter a transfer restriction or a sales reservation after the holder of Uncertificated Equity Instruments has delivered its
acceptance of the Tender Offer. In addition, the holder of Uncertificated Equity Instruments who has accepted the Tender Offer authorizes Pohjola Bank or a party authorized by Pohjola Bank or the account operator managing the holder’s
book-entry account to perform the necessary entries and to all other actions required to technically execute the Tender Offer and to sell all the Uncertificated Equity Instruments owned by such holder of Uncertificated Equity Instruments at the time
of the execution trades under the Tender Offer to the Offeror in accordance with the terms and conditions of the Tender Offer.
A holder of Uncertificated
Equity Instruments that has validly accepted the Tender Offer and that has not properly withdrawn its acceptance in accordance with the terms and conditions of the Tender Offer may not sell or otherwise dispose of its tendered Uncertificated Equity
Instruments. A transfer restriction in respect of the Uncertificated Equity Instruments will be registered in the relevant book-entry account after a holder of
50
Uncertificated Equity Instruments has submitted the Acceptance Form for the Tender Offer. If the Tender Offer is not completed or if the tender is properly withdrawn by the holder of
Uncertificated Equity Instruments in accordance with the terms and conditions of the Tender Offer, the transfer restriction registered on the tendered Uncertificated Equity Instruments in the relevant book-entry account will be removed as soon as
possible and within approximately three (3) Finnish banking days following the announcement that the Tender Offer will not be completed or the receipt of a notice of withdrawal in accordance with the terms and conditions of the Tender Offer.
Certificated Equity Instruments. Certificated Equity Instruments comprise the 2014 Option Rights in the 2014B, 2014C, 2014D and 2014M tranches,
all 2016 Option Rights, all Swiss Option Rights, all 2011 Share Rights and all 2014 Share Rights. A holder of Certificated Equity Instruments may only accept the Tender Offer in respect of Certificated Equity Instruments registered in his or her
name in the Company’s register for such Certificated Equity Instruments on the date of acceptance of the Tender Offer. A holder of Certificated Equity Instruments must have a cash account in a financial institution operating in Finland or
abroad.
Pohjola Bank will send a notification of the Tender Offer, including instructions and an Acceptance Form for Certificated Equity
Instruments to all holders of Certificated Equity Instruments who are registered during the Offer Period in the registry of holders of Certificated Equity Instruments held by the Company. If the holders of Certificated Equity Instrument do not
receive such notification and Acceptance Form from Pohjola Bank, or if the instructions and Acceptance Form cannot be sent because the address of the holder of the Certificated Equity Instrument is unknown, the holders of Certificated Equity
Instruments can contact the call service of OP Financial Group at (+358) (0) 100 0500 for assistance.
Pledged Certificated Equity Instruments
may only be tendered with the consent of the relevant pledgee. The obtaining of such consent shall be the responsibility of the relevant holder of Certificated Equity Instruments. The consent by the pledgee shall be delivered in writing together
with the Acceptance Form.
A holder of Certificated Equity Instruments who wishes to accept the Tender Offer shall submit a properly completed and duly
executed Acceptance Form for Certificated Equity Instruments to Pohjola Bank in accordance with the instructions that will be sent to such a holder of Certificated Equity Instruments together with the Acceptance Form and within the time limit set in
the instructions, which may be prior to the expiry of the Offer Period.
The Acceptance Form shall be submitted so that it is received on or prior to the
Expiration Date, subject to and in accordance with the instructions of Pohjola Bank. In the event of a Subsequent Offer Period, the acceptance form shall be submitted so that it is received during the Subsequent Offer Period, subject to and in
accordance with the instructions of Pohjola Bank. Pohjola Bank may set a separate time limit for delivering the Acceptance Form that ends already before the expiry of the Offer Period.
The method of delivery of Acceptance Forms is at the option and risk of the holder of Certificated Equity Instruments, and the delivery will be deemed made
only when actually received by Pohjola Bank. The Offeror reasonably reserves the right to reject any acceptance given in an incorrect or incomplete manner.
A holder of Certificated Equity Instruments that has validly accepted the Tender Offer may not sell or otherwise dispose of its tendered Certificated Equity
Instruments. By accepting the Tender Offer the holder of Certificated Equity Instruments authorizes Pohjola Bank to sell their Certificated Equity Instruments to the Offeror in accordance with the terms and conditions of the Tender Offer.
In accordance with Chapter 11, Section 16, Subsection 1 of the Finnish
Securities Market Act and Section 14(d)(5) of the Securities Exchange Act of 1934, as amended, and Rule 14d-7 thereunder, the Equity
51
Interests validly tendered in accordance with the terms and conditions of the Tender Offer may be withdrawn at any time on or before the Expiration Date and such withdrawal is permitted in
respect of any or all Equity Interests so tendered. If this Tender Offer Document is supplemented in accordance with Chapter 11, Section 11 of the Finnish Securities Market Act and the Offer Period is continued as a result of such supplement, an
acceptance of the Tender Offer may be withdrawn in accordance with this section until the end of the new Expiration Date of the continued Offer Period. After the Expiration Date, the Equity Interests already tendered may no longer be withdrawn.
Shares and Uncertificated Equity Instruments. The proper withdrawal of Shares and Uncertificated Equity Instruments requires that a written notice of
withdrawal is submitted to the same account operator to whom the Acceptance Form for such Equity Interests was submitted. If the Acceptance Form with respect to such Equity Interests was submitted to a branch office of a cooperative bank
belonging to the OP Financial Group or Helsinki OP Bank Ltd., the notice of withdrawal must be submitted to the same branch office. If such Equity Interests are registered in the name of a nominee, the holder of such Equity Interests shall instruct
the nominee to submit the notice of withdrawal.
If a holder of Shares or Uncertificated Equity Instruments withdraws its acceptance of the Tender
Offer in accordance with the terms and conditions of the Tender Offer, the transfer restriction registered on the tendered Shares and Uncertificated Equity Instruments in the relevant book-entry account will be removed as soon as possible and within
approximately three (3) Finnish banking days following the receipt of a notice of withdrawal in accordance with the terms and conditions of the Tender Offer.
ADSs. The proper withdrawal of ADSs requires that a written, or facsimile transmission of, notice of withdrawal be received by the Depositary in a
timely manner. The notice must specify the name of the person who tendered the ADS being withdrawn, the number of ADSs being withdrawn, the name and number of the account at DTC to be credited with the withdrawal of ADSs and the name of the
registered holder, if different from that of the person who tendered the ADS.
Certificated Equity Instruments. The proper withdrawal of
Certificated Equity Instruments requires that a written notice of withdrawal is submitted to Pohjola Bank plc in accordance with the instructions sent to holders of Certificated Equity Instruments together with the Acceptance Form for the
Certificated Equity Instruments. The notice of withdrawal must be received on or before the Expiration Date.
Re-tendering. Withdrawn Equity
Interests may be re-tendered by following the acceptance procedures described in Section 4.4—“Acceptance Procedure of the Tender Offer” above prior to the expiry of the Offer Period or, if the Offer Period has been extended,
prior to the expiry of such extended Offer Period.
The account operator managing the relevant book-entry account or the nominee may charge a fee
for withdrawals in accordance with its price lists.
In the event of a Subsequent Offer Period, the acceptance of the Tender Offer will be binding and
cannot be withdrawn, unless otherwise provided under mandatory Finnish and/or United States law.
| 4.6 |
Announcement of the Results of the Tender Offer |
The Offeror will announce the preliminary result with
respect to the Offer Period on the first (1st) Finnish banking day following the Expiration Date. The preliminary result announcement will confirm (i) the initial percentage of the Equity Interests that have been validly tendered and not
properly withdrawn and (ii) whether the Offeror will complete the Tender Offer and accept the Equity Interests tendered into the Tender Offer. As set forth in Section 4.2—“Conditions to Completion of the Tender Offer,” the
Offeror’s obligation to consummate the Tender Offer is subject to the fulfilment or waiver by the Offeror of the Conditions to Completion, including the Minimum Condition, on or prior to the date of such preliminary result announcement. For the
avoidance of doubt, the satisfaction of the Minimum Condition shall be determined based on the preliminary results with respect to the Offer Period.
52
The Offeror will announce the final result with respect to the Offer Period on or about the third
(3rd) Finnish banking day following the Expiration Date. The final result announcement will confirm the final percentage of the Equity Interests that have been validly tendered into the Tender Offer and not properly withdrawn.
The Offeror will announce the initial percentage of the Equity Interests validly tendered during any Subsequent Offer Period on the first (1st) Finnish
banking day following the expiry of the Subsequent Offer Period and the final percentage on or about the second (2nd) Finnish banking day following the expiry of the Subsequent Offer Period.
| 4.7 |
Terms of Payment and Settlement of Shares |
If the Tender Offer is consummated, the sale and purchase of
the Shares validly tendered and not properly withdrawn in accordance with the terms and conditions of the Tender Offer during the Offer Period will be executed on the fourth (4th) Finnish banking day following the Expiration Date. The sale and
purchase of the Shares will take place on Nasdaq Helsinki unless prohibited by the rules applicable to securities trading on Nasdaq Helsinki. Otherwise the sale and purchase of the Shares will take place outside of Nasdaq Helsinki.
Settlement will be effected against payment, and the Closing Date of the Shares with respect to the Offer Period will occur, on or about the second
(2nd) Finnish banking day following the execution of the sale and purchase of the Shares, or on or about the sixth (6th) Finnish banking day following the Expiration Date. Therefore the payment of the Share Offer Price will be made on such
Closing Date into the bank account connected to the shareholder’s book-entry account or, in the case of shareholders whose holdings are registered in the name of a custodian or a nominee, into the bank account specified by the custodian or
nominee. Actual time of receipt for the payment will depend on the schedules of money transactions between financial institutions and agreements between the shareholder and account operator or nominee in each case.
The Offeror will announce the terms of payment and settlement for the Shares tendered during any Subsequent Offer Period in connection with the announcement
thereof. The sale and purchase of Shares validly tendered in accordance with the terms and conditions of the Tender Offer during the Subsequent Offer Period will be executed in intervals of approximately one (1) week.
The Offeror reserves the right to postpone the payment of the Share Offer Price if payment is prevented or suspended due to a force majeure
event, but will immediately effect such payment once the force majeure event preventing or suspending payment is resolved.
| 4.8 |
Terms of Payment and Settlement of ADSs |
The sale and purchase of the ADSs validly tendered and not
withdrawn during the Offer Period in accordance with the terms and conditions of the Tender Offer will be executed no later than on the sixth (6th) Finnish banking day following the Expiration Date.
The Offeror will announce the terms of payment and settlement for the ADSs tendered during any Subsequent Offer Period in connection with the announcement
thereof. The sale and purchase of ADSs validly tendered in accordance with the terms and conditions of the Tender Offer during the Subsequent Offer Period will be executed in intervals of approximately one (1) week.
The ADS Offer Price will be payable in U.S. dollars calculated by using the open market spot exchange rate for the U.S. dollar to euro on the nearest
practicable day to the applicable Closing Date.
The Offeror reserves the right to postpone the payment of the ADS Offer Price if payment is prevented or
suspended due to a force majeure event, but will immediately effect such payment once the force majeure event preventing or suspending payment is resolved.
53
| 4.9 |
Terms of Payment and Settlement of Option Rights, Share Rights and Warrants |
Uncertificated Equity
Instruments. The sale and purchase of the Uncertificated Equity Instruments, which comprise all 2011 Option Rights, the 2014 Option Rights in the 2014A tranche and all Warrants, validly tendered and not properly withdrawn in accordance with the
terms and conditions of the Tender Offer during the Offer Period will be executed on the fourth (4th) Finnish banking day following the Expiration Date. The sale and purchase of the Uncertificated Equity Instruments will take place outside of
Nasdaq Helsinki.
Settlement will be effected against payment, and the Closing Date of the Uncertificated Equity Instruments with respect to the Offer
Period will occur, on or about the second (2nd) Finnish banking day following the execution of the sale and purchase of the Uncertificated Equity Instruments, or on or about the sixth (6th) Finnish banking day following the Expiration
Date. Therefore the payment of the applicable Offer Price will be made on such Closing Date into the bank account connected to the book-entry account of the holder of the Uncertificated Equity Instruments or, in the case of holders whose holdings
are registered in the name of a custodian or a nominee, into the bank account specified by the custodian or nominee. Actual time of receipt for the payment will depend on the schedules of money transactions between financial institutions and
agreements between the holder and account operator, custodian or nominee in each case.
The Offeror will announce the terms of payment and settlement for
the Uncertificated Equity Instruments tendered during any Subsequent Offer Period in connection with the announcement thereof. The sale and purchase of Uncertificated Equity Instruments validly tendered in accordance with the terms and conditions of
the Tender Offer during the Subsequent Offer Period will be executed in intervals of approximately one (1) week.
The Offeror reserves the right to
postpone the payment of the applicable Offer Price if payment is prevented or suspended due to a force majeure event, but will immediately effect such payment once the force majeure event preventing or suspending payment is resolved.
Certificated Equity Instruments. The sale and purchase of the Certificated Equity Instruments, which comprise the 2014 Option Rights in the 2014B,
2014C, 2014D and 2014M tranches, all 2016 Option Rights, all Swiss Option Rights, all 2011 Share Rights and all 2014 Share Rights, validly tendered and not properly withdrawn in accordance with the terms and conditions of the Tender Offer during the
Offer Period will be executed, and the Closing Date for the Certificated Equity Instruments with respect to the Offer Period will occur, on or about the sixth (6th) Finnish banking day following the Expiration Date.
The Offeror will make the payment of the applicable Offer Price for the relevant Certificated Equity Instruments on such Closing Date. Actual time of receipt
for the payment depends on the schedules of money transactions between financial institutions and agreements between the shareholder and account operator or nominee in each case. Holders of Certificated Equity Instruments will be required to provide
Pohjola Bank their bank account information and certain additional information specified in the instructions that will be sent to the holders of Certificated Equity Instruments together with their Acceptance Form.
The Offeror will announce the terms of payment and settlement for the Certificated Equity Instruments tendered during any Subsequent Offer Period in
connection with the announcement thereof. The sale and purchase of Certificated Equity Instruments validly tendered in accordance with the terms and conditions of the Tender Offer during the Subsequent Offer Period will be executed in intervals of
approximately one (1) week.
The Offeror reserves the right to postpone the payment of the applicable Offer Price if payment is prevented or
suspended due to a force majeure event, but will immediately effect such payment once the force majeure event preventing or suspending payment is resolved.
54
| 4.10 |
Transfer of Ownership |
Shares. Title to the Shares validly tendered in the Tender
Offer will pass to the Offeror on the applicable Closing Date against the payment of the Share Offer Price by the Offeror to the tendering shareholder. In the event of a Subsequent Offer Period, title to the Shares validly tendered in the Tender
Offer during the Subsequent Offer Period will pass to the Offeror against the payment of the Share Offer Price by the Offeror to the tendering shareholder as promptly as reasonably practicable following their tender.
ADSs. Title to the ADSs validly tendered in the Tender Offer will pass to the Offeror on the applicable Closing Date against the payment of the ADS
Offer Price by the Offeror to the tendering ADS holder. In the event of a Subsequent Offer Period, title to the outstanding ADSs validly tendered in the Tender Offer during the Subsequent Offer Period will pass to the Offeror against the
payment of the ADS Offer Price by the Offeror to the tendering ADS holder as promptly as reasonably practicable following their tender.
Option
Rights, Share Rights and Warrants. Title to Option Rights, Share Rights and Warrants that are validly tendered in the Tender Offer will pass to the Offeror on the applicable Closing Date against the payment of the applicable Offer Price for the
relevant Equity Interests by the Offeror to the tendering holder of such Equity Interests. In the event of a Subsequent Offer Period, title to Option Rights, Share Rights and Warrants validly tendered in the Tender Offer during the Subsequent Offer
Period will pass to the Offeror against the payment of the applicable Offer Price for the relevant Equity Interests by the Offeror to the tendering holder of such Equity Interests as promptly as reasonably practicable following their
tender.
| 4.11 |
Subsequent Offer Period |
The Offeror reserves the right to commence a Subsequent Offer
Period in connection with the announcement of the final result with respect to the Offer Period if the Tender Offer shall have been announced to be unconditional at that time. In the event of such Subsequent Offer Period, the Subsequent Offer Period
will expire on the date and at the time determined by the Offeror in the final result announcement. The Subsequent Offer Period may extend beyond ten (10) weeks from the beginning of the original Offer Period and, in the Offeror’s
discretion, beyond June 19, 2016. Acceptance of Equity Interests tendered during the Subsequent Offer Period follows the procedures outlined in Section 4.4–“Acceptance Procedure of the Tender Offer”. Except in the event of a
supplement to the Tender Offer Document in accordance with Chapter 11, Section 11 of the Finnish Securities Market Act, no withdrawal rights are available during the Subsequent Offer Period, and payment for and acceptance of Equity Interests
validly tendered during the Subsequent Offer Period will take place on a periodic basis in intervals of approximately one (1) week and payment will be effected by the Offeror no later than five (5) Finnish banking days after the end of the
relevant one week interval. The Offeror will announce the terms of payment and settlement for the Equity Interests tendered during any Subsequent Offer Period in connection with the announcement thereof.
| 4.12 |
Transfer Tax and Other Payments |
The Offeror will pay the Finnish transfer tax, if any,
payable on the sale and purchase of Equity Interests. The Offeror will not, however, pay any Finnish transfer tax that is levied in addition to the aforementioned transfer tax solely because the Offer Price has to be paid to a person other than the
person in whose name such Equity Interests have been registered. If the Offer Price has to be paid to a person other than the person in whose name the Equity Interests have been registered, the Offeror has the right to subtract from the Offer Price
the amount of this additional transfer tax levied on the Offer Price, unless the person requesting the payment pays in advance to the Offeror the said transfer tax that is levied as a result of a payment to a person other than a registered holder of
Equity Interests, or establishes to the satisfaction of the Offeror that such tax has been paid or is not payable.
The Offeror will not, however, be
responsible for payment of such transfer tax, where the tax liability is based on the Finnish Tax Administration’s position on taxation of employment options stated in its guidance (record
55
no. A186/200/2015). As further discussed in Section 4.14—“Material Finnish Income Tax Consequences,” such transfer tax liability with respect to employment based option
arrangements arises at the moment when a subscription right is granted, but the amount of payable transfer tax can only be determined upon exercising the right (e.g., in connection with the Tender Offer).
Fees charged by account operators, asset managers, nominees or any other person for registering the release of any pledges or other possible restrictions
preventing a sale of the relevant Equity Interests, as well as fees relating to a withdrawal of the tender by a holder of Equity Interests in accordance with Section 4.5—“Withdrawal Rights” above, will be borne by the holders of
Equity Interests incurring such fees. The Offeror will be responsible for other customary fees relating to book-entry registrations required for the purposes of the Tender Offer, the sale and purchase of the Equity Interests tendered under the
Tender Offer or the payment of the Offer Price.
In connection with a Subsequent Compulsory Redemption, if any, holders of ADSs will bear any fees and
expenses charged by the depositary under the ADS deposit agreement.
| 4.13 |
Material United States Federal Income Tax Consequences |
The following discussion is a
summary of U.S. federal income tax consequences generally applicable to you if you are a U.S. Holder (as defined below) upon the tender of Equity Interests in the Tender Offer or an exchange of such securities for cash in connection with a
Subsequent Compulsory Redemption, if any. For purposes of the discussion in this Section 4.13, except as otherwise noted, references to Shares include ownership interests in Shares through ADSs. This discussion is based on the U.S. Internal
Revenue Code of 1986, as amended (the “Code”), final, proposed and temporary U.S. Treasury Regulations promulgated thereunder, administrative pronouncements, and judicial decisions as of the date hereof, all of which are subject to
change, possibly on a retroactive basis, and to differing interpretation, which may result in tax consequences different from those described below. This discussion is not binding on the U.S. Internal Revenue Service (the “IRS”),
and the IRS or a court in the event of an IRS dispute may challenge any of the conclusions set forth below.
This discussion does not address any U.S.
federal estate, gift, or other non-income tax consequences, consequences of the Medicare tax on net investment income or any state, local, or non-U.S. tax consequences of the Tender Offer or Subsequent Compulsory Redemption, if any. This discussion
is a summary for general information purposes only and does not consider all aspects of U.S. federal income taxation that may be relevant to you in light of your individual investment circumstances or if you are a certain type of holder subject to
special tax rules, including: (i) holders that are banks, financial institutions, or insurance companies; regulated investment companies, mutual funds, or real estate investment trusts; brokers or dealers in securities or currencies or traders
in securities that elect to apply a mark-to-market accounting method; or tax-exempt organizations, (ii) holders that own Equity Interests as part of a straddle, hedge, constructive sale, conversion transaction, or other integrated investment,
(iii) holders that acquired Shares or Warrants in connection with the exercise of employee share options or otherwise as compensation for services, (iv) holders that have a “functional currency” other than the U.S. dollar,
(v) retirement plans, individual retirement accounts, or other tax-deferred accounts, (vi) U.S. expatriates, (vii) holders that are subject to alternative minimum tax, (viii) holders that actually or constructively own 10 percent
or more of our voting stock, (ix) entities subject to the anti-inversion rules of Section 7874 of the Code or (x) partnerships or other entities classified as partnerships for U.S. federal income tax purposes. This discussion assumes
that all Option Rights and Share Rights held by a U.S. Holder were granted to the holder as compensation in exchange for services.
As used herein, a
“U.S. Holder” is any beneficial owner of Equity Interests who or that is (i) an individual citizen or resident of the United States for U.S. federal income tax purposes, (ii) a corporation (or other entity treated as a
corporation for U.S. federal income tax purposes) created or organized under the laws of the United States, any state thereof, or the District of Columbia, (iii) an estate the income of which is subject to U.S. federal income taxation
regardless of its source or (iv) a trust which (a) is subject to the primary jurisdiction of a court within the
56
United States and for which one or more U.S. persons have authority to control all substantial decisions, or (b) has a valid election in effect under applicable U.S. Treasury Regulations to
be treated as a U.S. person for U.S. federal income tax purposes.
If a partnership (including any entity classified as a partnership for U.S. federal
income tax purposes) is a beneficial owner of Equity Interests, the U.S. federal income tax treatment of a partner in the partnership generally will depend on the status of the partner and the activities of the partners and the partnership. Any
partner of a partnership holding Equity Interests is urged to consult its own tax advisor.
ALL HOLDERS OF EQUITY INTERESTS SHOULD CONSULT THEIR TAX
ADVISORS REGARDING THE SPECIFIC TAX CONSEQUENCES OF TENDERING THEIR EQUITY INTERESTS IN THIS TENDER OFFER OR EXCHANGING SUCH SECURITIES FOR CASH IN CONNECTION WITH A SUBSEQUENT COMPULSORY REDEMPTION IN LIGHT OF THEIR PARTICULAR SITUATIONS, INCLUDING
THE APPLICABILITY AND EFFECT OF U.S. FEDERAL, STATE, LOCAL, NON-U.S. AND OTHER LAWS.
Consequences to U.S. Holders of Shares or Warrants
Assuming that each U.S. Holder holds its Shares and/or Warrants as capital assets within the meaning of Section 1221 of the Code, and subject
to the rules described under “Passive Foreign Investment Company Considerations” and “Exchange of Foreign Currencies” below, the receipt of cash, either as consideration upon consummation of the Tender Offer or in connection with
a Subsequent Compulsory Redemption, if any, will be a taxable transaction for U.S. federal income tax purposes. A U.S. Holder who so exchanges Shares or Warrants for cash generally will recognize gain or loss in an amount equal to the difference
between (i) the amount realized and (ii) such U.S. Holder’s adjusted tax basis in the Shares or Warrants exchanged therefor. Subject to the discussion below under “Passive Foreign Investment Company Considerations,” and
“Exchange of Foreign Currencies” such gain or loss will be capital gain or loss and will be long-term if such U.S. Holder has held such Shares or Warrants for more than one year.
Passive Foreign Investment Company Considerations
There can be no assurance that the Company was not a “passive foreign investment company” within the meaning of Section 1297 of the Code
(“PFIC”) for its taxable year ended December 31, 2015 or in previous taxable years. In addition, the Company’s PFIC status is tested each year and is dependent on the composition of its assets and income and the value of
its assets from time to time. Because the Company currently holds, and has stated that it expects to continue to hold, a substantial amount of cash and other passive assets, and because the value of the Company’s assets is uncertain and likely
to fluctuate over time, there can be no assurance that the Company will not be a PFIC for 2016.
In general, the Company will be classified as a PFIC in
any taxable year if either (a) the average quarterly value of its gross assets that produce passive income or are held for the production of passive income is at least 50 percent of the average quarterly value of our total gross assets
(the “asset test”) or (b) at least 75 percent of its gross income for the taxable year is passive income (such as certain dividends, interest or royalties). For this purpose, the Company will be treated as owning its proportionate
share of the assets and earning its proportionate share of the income in any other corporation in which it owns, directly or indirectly, at least 25 percent (by value) of the stock. For purposes of the asset test: (a) any cash and cash invested
in short-term, interest bearing debt instruments or bank deposits that are readily convertible into cash will generally count as producing passive income or held for the production of passive income and (b) the relevant legislative history
indicates that it is generally permissible to determine the total value of the Company’s assets based on the Company’s market capitalization.
57
If the Company is a PFIC for the current taxable year or has been a PFIC during any prior year in which a
U.S. Holder held Shares and you have not made a valid mark-to-market election or qualified electing fund election (a “QEF Election”) (discussed below), any gain recognized by you on the disposition of a Share generally
(a) would be allocated ratably to each day in your holding period for the Share, (b) the amount allocated to the current year and any tax year prior to the first taxable year in which we were a PFIC would be taxed as ordinary income in the
current year, (c) the amount allocated to each other taxable year would be subject to tax at the highest applicable marginal rate in effect for that year and (d) an interest charge at the rate for underpayments of taxes would be imposed on
the resulting tax allocated to such period. A U.S. Holder of Warrants is likely taxed in a manner similar to a U.S. Holder of Shares if the holder realizes gain on the sale of the Warrants. If you exercise Warrants to purchase Shares and the rules
described above apply to such Warrant, the holding period over which any income realized is allocated would include the holding period of the Warrants. Holders of Warrants are urged to consult with their own tax advisors regarding the application of
the PFIC rules with respect to the disposition of their Warrants.
If the Company was a PFIC in any year in which a U.S. Holder held Shares and certain
conditions relating to the regular trading of Shares have been met in the past, a U.S. Holder of Shares may have been able to make a so- called “mark-to-market” election with respect to their Shares. If a U.S. Holder made this election in
a timely fashion, then instead of the tax treatment described in the preceding paragraph, any gain recognized by the U.S. Holder as a result of the disposition of Shares in this Tender Offer (or a Subsequent Compulsory Redemption) would generally be
treated as ordinary income or ordinary loss (limited to the extent of the net amount of previously included income as a result of the mark-to-market election, if any). It is not clear under current law whether the mark-to-market election is
available with respect to the Warrants. Holders of Warrants should consult their own tax advisors regarding the availability of such an election.
If the
Company is or was a PFIC for any year and you have made a QEF Election with respect to the Company and any lower-tier PFIC in the first taxable year that the Company and each lower-tier PFIC are treated as PFICs with respect to you and maintained
the election for each following year, you will be currently taxable on your pro rata share of the relevant PFIC’s ordinary earnings and net capital gain (at ordinary income and capital gain rates, respectively) for each taxable year that the
entity is classified as a PFIC. Your tax basis in your Shares is increased by an amount equal to any income included under the QEF Election and decreased by any amount distributed on the Shares that is not included in your income. On the disposition
of the Shares in the Tender Offer, you will recognize capital gain or loss on the disposition of Shares in an amount equal to the difference between the amount realized and your adjusted tax basis in the Shares, as determined in U.S. dollars. You
should consult your tax advisor regarding the ability to make a QEF Election in your particular circumstances.
You may not make a QEF Election with
respect to your Warrants. As a result, if the Company was a PFIC at any time during the period that you held Warrants, any gain recognized by you upon the sale or other disposition of a Warrant (other than upon exercise of a Warrant) will likely be
subject to the ordinary income allocation and interest charge rules described above.
If the Company is a PFIC for the current taxable year or has been a
PFIC during any prior year in which a U.S. Holder held Shares, a U.S. Holder generally would be required to file IRS Form 8621 with respect to the disposition of Shares. The PFIC rules are complex, and you should consult your own tax advisors
regarding the applicable consequences of tendering your Shares or Warrants in the Tender Offer if we are a PFIC or have been a PFIC during any prior year in which you held Shares or Warrants.
Exchange of Foreign Currencies
A U.S. Holder that
receives foreign currency on a sale of Equity Interests generally will realize an amount of gain or loss (or ordinary income in the case of Option Rights or Share Rights, as described below) based on the U.S. dollar value of the foreign currency on
the date of the sale. Any gain or loss recognized on the subsequent sale, conversion or disposition of such foreign currency will be U.S. source ordinary income or loss. However, if such
58
foreign currency is converted into U.S. dollars on the date received by the U.S. Holder, a cash basis or electing accrual basis U.S. Holder should not recognize any gain or loss on such
conversion. An accrual basis U.S. Holder that holds Shares will realize an amount equal to the U.S. dollar value of Shares on the date the tender is accepted. Such U.S. Holder will then recognize a gain or loss, if any, measured by the difference
between the U.S. dollar value of the applicable Offer Price on the date the tender is accepted, and the U.S. dollar amount actually received on the date of payment. The recognized gain or loss, if any, will be ordinary income or loss, and will
generally be income or loss from sources within the U.S. for foreign tax credit limitation purposes.
In general, any foreign currency loss will be
treated as a “reportable transaction” for U.S. federal income tax purposes to the extent that the amount of the loss equals or exceeds certain threshold amounts (USD 50,000 in the case of individuals or trusts, whether or not the loss
flows through from an S corporation or partnership, and USD 10 million in the case of corporate taxpayers). You should consult your own tax advisor concerning the application of the reportable transaction regulations to the Tender Offer,
including any requirement to file IRS Form 8886.
Consequences of the Tender Offer to U.S. Holders of Option Rights or Share Rights
The receipt of cash in exchange for your Option Rights and Share Rights pursuant to the Tender Offer will be a taxable transaction for U.S.
federal income tax purposes. The cash you receive for your tendered Option Rights and Share Rights pursuant to the Tender Offer will be treated as ordinary compensation income to the person who was originally granted the Option Rights or Share
Rights, and the amount payable to you in the Tender Offer or in connection with Subsequent Compulsory Redemption, if any, may be subject to U.S. federal, and possibly also state and local, withholding (including payroll taxes) owed by the original
grantee. You should consult your tax advisor regarding the consequences of tendering Option Rights or Stock Rights in the Tender Offer given your own circumstances including under the rules described above.
Information Reporting and Backup Withholding
You
may be subject, under certain circumstances, to information reporting and backup withholding with respect to the amount of cash received in the Tender Offer (or in connection with a Subsequent Compulsory Redemption, if any).
Backup Withholding
Under the backup withholding
rules, a holder of Equity Interests that is a U.S. Person may be subject to backup withholding unless such holder is an exempt recipient and, when required, demonstrates this fact or provides a taxpayer identification number, makes certain
certifications on IRS Form W-9, and otherwise complies with the applicable requirements. If such holder does not provide a correct taxpayer identification number, such holder may also be subject to penalties imposed by the IRS. Holders should
consult their tax advisors regarding qualification for exemption from backup withholding and the procedure for obtaining such an exemption.
A tendering
holder that is a U.S. Person generally is required to provide a correct Taxpayer Identification Number (“TIN”) on IRS Form W-9, which is available on the IRS’s website at https://www.irs.gov/, and to certify, under penalties of
perjury, that such number is correct and that such holder is not subject to backup withholding of U.S. federal income tax. If a tendering holder that is a U.S. Person is subject to backup withholding, such holder must cross out Item (2) of Part II
of IRS Form W-9. If the correct TIN is not provided, then the holder may be subject to a penalty imposed by the IRS and backup withholding of payments to the holder pursuant to the Tender Offer. See the instructions to IRS Form W-9 for additional
information regarding qualifying for an exemption from backup withholding. Holders are advised to consult their respective tax advisors as to their qualifications for obtaining an exemption from backup withholding and the procedure for obtaining
such an exemption.
59
Certain U.S. Persons (including, among others, certain corporations) are not subject to backup withholding.
Exempt holders should furnish their TIN, check the “Exempt payee” box on the IRS Form W-9 and sign, date and return the IRS Form W-9 in order to avoid erroneous backup withholding. See the instructions to the IRS Form W-9, which are
available on the IRS’s website at https://www.irs.gov/.
The TIN of a holder that is a U.S. Person will generally be the Social Security
number or employer identification number of such holder. If such holder holds the Equity Interests in more than one name or if the Equity Interests are not in the name of the actual owner, please consult the instructions to the IRS Form W-9 for
additional guidance on which number to report. If the Offeror has reason to know that the tendering holder is a U.S. Person and the tendering holder does not provide a TIN by the time of payment, a portion of all payments of the purchase price may
be subject to backup withholding as described below.
If backup withholding applies with respect to a holder, a portion (currently, 28%) of any payment
made to such holder is required to be withheld and paid over to the IRS. Backup withholding is not an additional tax. Rather, the United States federal income tax liability of persons subject to backup withholding will be reduced by the amount of
tax withheld. If backup withholding results in an overpayment of taxes, a refund may be obtained from the IRS if required information is timely furnished to the IRS.
IF YOU ARE A U.S. PERSON, FAILURE TO COMPLETE AND RETURN THE IRS FORM W-9 MAY RESULT IN BACKUP WITHHOLDING OF A PORTION OF ANY PAYMENTS MADE TO YOU
PURSUANT TO THE TENDER OFFER. PLEASE REVIEW THE INSTRUCTIONS TO THE IRS FORM W-9 FOR ADDITIONAL DETAILS.
Other Information Reporting
Certain U.S. Holders may be required to report information with respect to their investment in Equity Interests not held through a custodial
account with a financial institution to the IRS. The Code also imposes penalties if a U.S. Holder is required to report such information to the IRS and fails to do so. U.S. Holders should consult their tax advisors regarding their reporting
obligation with respect to the disposition of their Equity Interests.
| 4.14 |
Material Finnish Income Tax Consequences |
The following summary is based on the tax laws in
force and taxation practice in Finland as of the date hereof. Changes in Finnish tax laws and taxation practice may affect, also retroactively, the application of the tax rules discussed in this summary. The following summary is not exhaustive and
it concentrates only on certain general aspects of the taxation of Biotie shareholders and holders of Option Rights, Share Rights and Warrants under the Finnish law. The summary does not explain or take into account the legislation of other
jurisdictions than Finland.
The following summary does not cover tax consequences related to Biotie shareholders and holders of Option Rights, Share
Rights and Warrants under special provisions, including, for instance, income tax-exempt entities, sole proprietorships, partnerships and limited partnerships. Neither does the summary cover (i) the tax consequences of shareholders or holders
of Option Rights, Share Rights and Warrants subject to tax in Finland based on the applicability of Finnish CFC regulation or (ii) the Finnish inheritance or gift taxation implications.
ALL HOLDERS OF EQUITY INTERESTS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE SPECIFIC TAX CONSEQUENCES OF TENDERING THEIR EQUITY INTERESTS IN THIS
TENDER OFFER OR EXCHANGING SUCH SECURITIES FOR CASH IN CONNECTION WITH A SUBSEQUENT COMPULSORY REDEMPTION IN LIGHT OF THEIR PARTICULAR SITUATIONS, INCLUDING THE APPLICABILITY AND EFFECT OF FINNISH TAXATION LAWS.
60
The following summary is based on: (i) The Income Tax Act (1535/1992 as amended); (ii) The Business
Income Tax Act (360/1968 as amended); and (iii) The Transfer Tax Act (931/1996 as amended).
In addition, relevant Finnish case law as well as
decisions and statements made by the Finnish tax authorities in effect and available as of the date hereof have been taken into account.
General
Residents and non-residents persons in Finland are treated differently in taxation. Tax residents in Finland are obliged to pay tax for their
worldwide income. Non-residents are obligated to pay tax only for their income from Finland. Additionally, non-residents in Finland are obligated to pay tax on the income from a permanent establishment located in Finland. The double tax treaties
entered into by Finland may prevent the application of Finnish domestic legislation and prevent taxation of income received from Finland by a non-resident.
An individual is deemed resident in Finland for tax purposes when such an individual stays in Finland for a continuous period exceeding six (6) months
with only short and temporary absences or has his main abode and home in Finland. A Finnish citizen who has moved abroad is deemed resident until three (3) years have elapsed from the end of the year during which he or she left the country,
unless he or she proves that no more substantial ties to Finland exist on his part during the tax year concerned. The earned income, including salary, of a Finnish tax resident individual is taxed at a progressive rate. Capital income is currently
taxed at 30 percent rate. However, if the total amount of capital income accrued during a calendar year exceeds EUR 30,000, the excess is taxed at a rate of 34 percent. Corporate entities established under the Finnish legislation are generally
liable to tax in Finland and therefore liable to tax in Finland on their worldwide income. The corporate income tax rate is currently 20 percent.
Taxation of the profits accrued on the sale of the Shares, Option Rights, Share Rights and Warrants
Taxation of Finnish Resident Individuals
The capital gain
accrued on the sale of Shares, Option Rights, Share Rights and Warrants is taxed as capital income of Finnish tax resident individuals. However, please note the rules regarding employment based option arrangements provided in the section 66.3 of the
Income Tax Act explained below, which affect the taxation of the received benefit to be generally taxed as earned income. According to the rules in force since the beginning of 2016, capital loss generated from the sale of the Shares, Option Rights,
Share Rights and Warrants is tax deductible from all capital income. This applies to capital losses arising during or after the year 2016. Those capital losses which are accrued prior to 2016 are deductible only from capital gains and not from all
capital income. Capital losses may be deducted during the same tax year and during the five (5) following tax years. Capital losses are not taken into account when confirming the amount of tax credit for capital loss.
Notwithstanding the above, capital gains arising from the sale of assets are exempt from tax provided that the proceeds of all assets sold by the resident
individual during the calendar year do not, in the aggregate, exceed EUR 1,000 (not including proceeds from a sale of assets that is tax-exempt pursuant to Finnish tax laws). Correspondingly, capital losses are not tax deductible if (i) the
acquisition cost of all assets sold during the calendar year does not, in the aggregate, exceed EUR 1,000 and (ii) the proceeds of all assets sold by the resident individual during the calendar year do not, in the aggregate, exceed EUR 1,000.
If the sale of the Shares, Option Rights, Share Rights and Warrants relates to the seller’s business, the income received from the sale shall be divided so as to be taxed partially as earned income at the progressive rate and partially as
capital income at 30/34 rate. Losses accrued in business are deducted as described below in connection with “Taxation of Finnish Corporations”.
Any capital gain or loss is calculated by deducting the original acquisition cost and sales related expenses from the sales price. Alternatively, individuals
may, instead of deducting the actual acquisition costs, choose to apply a so-called presumptive acquisition cost, which is equal to 20 percent of the sales price or, if the shares have been
61
held for at least ten years, 40 percent of the sales price. If the presumptive acquisition cost is used instead of the actual acquisition cost, any sales related expenses are deemed to be
included therein and, therefore, may not be separately deducted from the sales price.
The abovementioned rules regarding the taxation of capital gains
and losses are not applicable to instruments which are regarded as employment based option rights under the section 66.3 of the Income Tax Act. For instance, such instruments can be option rights received on the basis of an incentive program where
the option right has not yet been used to subscribe for shares. According to the rules on taxation of employment based option rights, the disposal of the option right is considered as an exercise of the option and the benefit received from the
disposal is taxed as earned income of the employee at the progressive rate. If the employee has already subscribed for shares according to the terms and conditions of the incentive program, the disposal of said shares is taxed as capital gain or
loss as described above.
Taxation of Finnish Corporations
Finnish corporations are liable to tax on their worldwide income. The taxable income of a Finnish corporation is defined separately for business activities,
agriculture and other activities. The sales price of Shares, Option Rights, Share Rights and Warrants is usually regarded as the taxable income of the business activities or other activities of a corporation. The income from both sources is taxable
at the flat tax rate of 20 percent. The capital gain and loss are calculated by deducting original acquisition cost and sales related expenses from the sales price. The undepreciated acquisition cost of the disposed instruments is deductible from
the income of the source that the disposed Shares, Option Rights, Share Rights and Warrants belonged to for the corporation. The possible loss accrued from the disposal of Shares, Option Rights, Share Rights and Warrants belonging to the business
income source may be deducted from other income of the business income source. The tax loss carry forwards of business may be deducted from business income during the following ten (10) tax years. Capital losses belonging to the other income
source are tax deductible during the same year or the following five (5) years.
As an exception to the above, the capital gains received by a
corporation from the disposal of the shares that belong to its business activities, which the corporation has continuously owned for a minimum period of one year and which entitle it to at least 10 percent of the share capital of the company owned,
may be tax exempt under certain conditions. Capital losses arising from the disposal of the said shares are not deductible in taxation. The losses incurred from the disposal of shares belonging to the business activities and which may not be
disposed tax free, are deductible in taxation only from profits accrued from the disposal of shares belonging to the business activities during the tax year and following five (5) years.
Taxation of Non-residents
Non-residents who are not
generally liable for tax in Finland usually will not be subject to Finnish taxes on capital gains realized on the sale of shares or subscription rights of a Finnish company, unless the non-resident taxpayer is deemed to have a permanent
establishment for income tax purposes in Finland and the shares are considered as assets of that permanent establishment. In addition, non-resident holders of employment based option rights, as described in the section 66.3 of the Income Tax Act,
are liable to tax in those situations and only to the extent that the benefit received from the disposal of the option right is accrued from the time the employee worked mainly in Finland on behalf of a Finnish employer. The benefit received shall
be taxed as earned income. However, a double taxation treaty applicable to the situation may limit the right to the tax in Finland.
Transfer Tax
Transfer tax is generally not payable on the transfer of securities subject to public trading against fixed cash consideration. The transaction is
not subject to transfer tax provided that an investment service company or a foreign investment service company or another investment service provider, as defined in the Finnish Act on Investment Services (747/2012, as amended), is brokering or
serving as a party to the transaction or that the
62
transferee has been approved as a trading party in the market where the transfer is executed. If the broker or other party to the transfer is not a Finnish investment firm, Finnish credit
institution or Finnish branch or office of a foreign investment firm or credit institution, the transfer will be tax exempt provided that the transferee liable for tax notifies the Finnish tax authorities of the transfer within two months thereof or
the broker submits an annual declaration concerning the transfer to the Finnish Tax Administration as set forth in the Act on Assessment Procedure. Tax exemption does not apply to transfers executed as equity investments or distribution of funds or
to transfers in which consideration comprises in full or in part of work performed, or to certain other transfers set out in the Transfer Tax Act.
The
buyer is liable to pay transfer tax amounting to 1.6 percent of the transaction price in transfers of Shares, Option Rights, Share Rights and Warrants that do not fulfil the above criteria but which are regarded as securities in the light of
Transfer Tax Act, such as option rights and other special rights under the Finnish Companies Act entitling to subscription of shares. If the buyer is not generally liable for tax in Finland or if the buyer is not a foreign credit institution’s
or investment firm’s or management company’s Finnish branch, the seller must charge the tax from the buyer. If the broker is a Finnish stockbroker or a credit institution or a foreign stockbroker’s or credit institution’s Finnish
branch, it is liable to charge the transfer tax from the buyer and give an account thereof to the buyer. If neither party to the transaction is generally liable for tax in Finland or if neither party to the transaction is a foreign credit
institution’s, investment firm’s or management company’s Finnish branch or office, transfer tax will not be payable on the transfer (excluding transfers of qualified real estate company shares). No transfer tax is levied if the amount
of the tax is less than EUR 10.
In addition, the employees taking part in the incentive programs of the Company shall take into account the current view
of the Finnish Tax Administration regarding transfer tax liability of employment based option rights and its realization in connection with the disposal of rights. The received option rights and other special rights under the Finnish Companies Act
entitling to subscription of shares, such as the share units granted in connection with the share award plan, are primarily considered as subscription rights under the Transfer Tax Act, i.e. for the purposes of transfer taxation they are viewed as
securities. The transfer tax liability with respect to employment based option arrangements arises at the moment when the subscription right is granted, but the amount of payable transfer tax can only be determined upon exercising the right.
| 4.15 |
Certain Effects of the Tender Offer |
Market for the Shares and ADSs. The purchase of
Shares and ADSs by the Offeror pursuant to the Tender Offer will reduce the number of holders of Shares and ADSs and the number of Shares and ADSs that might otherwise trade publicly, which could adversely affect the liquidity and market value of
the remaining Shares and ADSs held by persons other than the Offeror. The Offeror cannot predict whether the reduction in the number of Shares or ADSs that might otherwise trade publicly would have an adverse or beneficial effect on the market price
for, or marketability of, the Shares or ADSs or whether such reduction would cause future market prices to be greater or less than the prices offered in this Tender Offer.
Stock Quotation. The Shares are quoted on Nasdaq Helsinki and the ADSs are quoted on Nasdaq US. Upon consummation of the Tender Offer, depending upon
the aggregate market value and the number of Equity Interests not purchased pursuant to the Tender Offer or any subsequent open market or privately negotiated purchases, as well as the number of public securityholders, the ADSs may no longer meet
the quantitative requirements for continued listing on Nasdaq US and the Shares and ADSs may become eligible for deregistration under the Exchange Act. We intend to apply for such deregistration and delist the ADSs from Nasdaq US.
In addition, once we own all of the Equity Interests, it is our intention that the Company will apply for termination of public trading of Shares on the
Nasdaq Helsinki and delist the Shares from Nasdaq Helsinki.
According to the published guidelines of the Financial Industry Regulatory Authority, the
ADSs might no longer be eligible for continued inclusion in Nasdaq US if, among other things, the number of publicly-held ADSs falls
63
below 500,000, the aggregate market value of the publicly-held ADSs is less than USD 1 million, or there are fewer than three market makers for the ADSs. ADSs held by officers or directors
of the Company or their immediate families, or by any beneficial owner of 10 percent or more of the ADSs, ordinarily will not be considered to be publicly-held for this purpose.
If the Shares cease to be listed on the Nasdaq Helsinki or the ADSs cease to be listed on the Nasdaq US, the market for the Shares and ADSs could be adversely
affected. It is possible that the Shares or ADSs would be traded on other securities exchanges (with trades published by such exchanges), The Nasdaq Capital Market, the OTC Bulletin Board or in a local or regional over–the–counter market.
The extent of the public market for the Shares and ADSs and the availability of such quotations would, however, depend upon the number of holders of Shares and ADSs and the aggregate market value of the Shares and ADSs remaining at such time, the
interest in maintaining a market in the Shares and ADSs on the part of securities firms, the possible termination of registration of the ADSs under the Exchange Act and other factors.
Exchange Act Registration. The Shares and the ADSs currently are registered under the Exchange Act. The purchase of the Shares and the ADSs pursuant to
the Tender Offer may result in the Shares and the ADSs becoming eligible for deregistration under the Exchange Act. Registration of the Shares and the ADSs may be terminated by the Company upon application to the SEC if the outstanding ADSs are not
listed on a “national securities exchange” and if there are fewer than 300 holders of record of the Shares and the ADSs that are U.S. residents.
We intend to seek to cause the Company to apply for termination of registration of the Shares and the ADSs as soon as possible after consummation of the
Tender Offer if the requirements for termination of registration are met. Termination of registration of the Shares and the ADSs under the Exchange Act would reduce the information required to be furnished by the Company to the holders of Equity
Interests and to the SEC and would make certain provisions of the Exchange Act no longer applicable with respect to the Shares and the ADSs. In addition, if the Shares and the ADSs are no longer registered under the Exchange Act, the requirements of
Rule 13e–3 with respect to “going private” transactions would no longer be applicable to the Company. Furthermore, the ability of “affiliates” of the Company and persons holding “restricted securities” of the
Company to dispose of such securities pursuant to Rule 144 under the U.S. Securities Act of 1933, as amended, may be impaired or eliminated.
If
registration of the Shares and the ADSs is not terminated prior to any Subsequent Compulsory Redemption, then the registration of the Shares and the ADSs under the Exchange Act will be terminated following completion of the Subsequent Compulsory
Redemption.
| 4.16 |
Certain Legal Matters; Regulatory Approvals; Description of SEC Relief |
General.
Except as described in this Section 4.16, the Offeror is not aware of any pending legal proceeding relating to the Tender Offer. Except as described in this Section 4.16, based on its examination of publicly available information
filed by the Company with the SEC and other publicly available information concerning the Company, the Offeror is not aware of any governmental license or regulatory permit that appears to be material to the Company’s business that might be
adversely affected by the Offeror’s acquisition of Equity Interests as contemplated herein or of any approval or other action by any governmental, administrative or regulatory authority or agency, domestic or foreign, that would be required for
the acquisition or ownership of Equity Interests by the Offeror as contemplated herein. Should any such approval or other action be required, the Offeror currently contemplates that, except as described below under “Takeover Statutes,”
such approval or other action will be sought. While the Offeror does not currently intend to delay acceptance for payment of Equity Interests tendered pursuant to the Tender Offer, pending the outcome of any such matter, there can be no
assurance that any such approval or other action, if needed, would be obtained or would be obtained without substantial conditions or that if such approvals were not obtained or such other actions were not taken, adverse consequences might
not result to the Company’s business, or certain parts of the Company’s business might not
64
have to be disposed of, any of which could cause the Offeror to elect to terminate the Tender Offer without the purchase of Equity Interests thereunder under certain conditions. See
Section 4.2—“Conditions to Completion of the Tender Offer.”
U.S. State Takeover Statutes. A number of U.S. states
(including Delaware) have adopted takeover laws and regulations which purport, to varying degrees, to be applicable to attempts to acquire securities of corporations which are incorporated in such states or which have substantial assets,
stockholders, principal executive offices or principal places of business therein (“State Takeover Laws”).
The Offeror is not
aware of any State Takeover Laws that are applicable to the Tender Offer or potential Subsequent Compulsory Redemption and has not attempted to comply with any such State Takeover Laws. If any government official or third party should seek to apply
any such State Takeover Law to the Tender Offer or potential Subsequent Compulsory Redemption or other business combination between the Offeror or any of its affiliates and the Company, the Offeror will take such action as then appears desirable,
which action may include challenging the applicability or validity of such State Takeover Law in appropriate court proceedings. In the event it is asserted that one or more State Takeover Laws is applicable to the Tender Offer or potential
Subsequent Compulsory Redemption and an appropriate court does not determine that it is inapplicable or invalid as applied to the Tender Offer or potential Subsequent Compulsory Redemption, as applicable, the Offeror might be required to file
certain information with, or to receive approvals from, the relevant state authorities or holders of Equity Interests, and the Offeror might be unable to accept for payment or pay for Equity Interests tendered pursuant to the Tender Offer, or be
delayed in continuing or consummating the Tender Offer or potential Subsequent Compulsory Redemption. In such case, the Offeror may not be obligated to accept for payment or pay for any tendered Equity Interests. See
Section 4.2—“Conditions to Completion of the Tender Offer.”
U.S. Antitrust Compliance. Under the Hart-Scott-Rodino Antitrust
Improvements Act of 1976 (the “HSR Act”), and the related rules and regulations that have been issued by the Federal Trade Commission (the “FTC”), certain transactions may not be consummated until certain
information and documentary material have been furnished for review by the FTC and the Antitrust Division of the U.S. Department of Justice (the “Antitrust Division”) and certain waiting period requirements have been satisfied.
These requirements apply to Offeror’s acquisition of the Equity Interests in the Tender Offer.
Under the HSR Act, the purchase of Equity
Interests in the Tender Offer may not be completed until the expiration of a 15–calendar–day waiting period which began when the Offeror filed a Premerger Notification and Report Form under the HSR Act with the FTC and the Antitrust
Division on January 29, 2016. The required waiting period with respect to the Tender Offer expired at 11:59 p.m., New York City Time, on February 16, 2016.
We believe that the only material regulatory filing required to consummate the Tender Offer is the filing of the Premerger Notification and Report Form
pursuant to the HSR Act.
SEC No Action Relief. We have been granted no action and/or exemptive relief by the SEC from certain of its otherwise
applicable rules to allow the Tender Offer to proceed in the manner described in this Tender Offer Document. In particular, the SEC has granted the following:
| |
• |
|
relief from the provisions of Rule 14e-1(d) under the Exchange Act to permit the Offeror to announce the preliminary results and any extension of the Offer Period on the next Finnish banking day after the Expiration
Date; |
| |
• |
|
relief from the provisions of Rule 14e-1(c) and Rule 14d-11(c) under the Exchange Act to permit the Offeror to tender payment for the tendered Equity Interests within six (6) Finnish banking days after the
Expiration Date (and within nine (9) Finnish banking days for Option Rights and Share Rights that are held in certificated form) and to commence a Subsequent Offer Period while settling the initial offering in this manner; |
65
| |
• |
|
relief from the provisions of Rule 14d-11(d) under the Exchange Act to permit the Offeror to commence a Subsequent Offer Period on the next Finnish banking day following the announcement of the final results with
respect to the Offer Period; and |
| |
• |
|
relief from the provisions of Rule 14d-11(e) under the Exchange Act to permit the Offeror to accept the Equity Interests tendered during a Subsequent Offer Period period on a periodic basis (approximately every week)
and to pay for such Equity Interests within five (5) Finnish banking days after the end of each weekly settlement, in accordance with Finnish law and customary Finnish market practice. |
| 4.17 |
Dividends and Distributions |
As discussed in Section 3.4—“Summary of the
Combination Agreement—Undertakings,” the Combination Agreement provides that from the date of the Combination Agreement to the Closing Date, without the prior written approval of the Offeror, the Company will not, and will not allow its
subsidiaries to make or implement any dividends, changes in capitalization, transfer or encumbrance of treasury shares or grant, transfer or disposal of any option rights for shares in the Company.
The Offeror reserves the right to amend the terms and conditions of the Tender
Offer in accordance with Chapter 11, Section 15, Subsection 2 of the Finnish Securities Market Act and other applicable law, including U.S. tender offer rules, and subject to the terms and conditions of the Combination Agreement and this
Tender Offer Document.
Subject to the Combination Agreement, the Offeror reserves the right to extend the Offer Period and to amend the terms and
conditions of the Tender Offer (including a potential withdrawal of the Tender Offer) in accordance with Chapter 11, Section 17 of the Finnish Securities Market Act if, during the Offer Period or any Subsequent Offer Period, a third party
announces a competing public tender offer for the Equity Interests.
The Offeror will have sole discretion to determine all other issues relating to the
Tender Offer, subject to the requirements of applicable Finnish and United States law and subject to the Combination Agreement.
The Tender Offer will not
be made directly or indirectly in any jurisdiction where prohibited by applicable law or where any document, registration or other requirement would be necessary in addition to those required by the applicable law in Finland and the United States.
This Tender Offer Document and related materials, including the Letter of Transmittal and Acceptance Forms, will not and may not be distributed,
forwarded or transmitted into or from any jurisdiction where prohibited by applicable law by any means whatsoever including, without limitation, mail, facsimile transmission, e-mail or telephone. In particular, the Tender Offer is not being made,
directly or indirectly, in or into, Canada, Japan, Australia, South Africa or Hong Kong or any other jurisdiction where prohibited by law. The Tender Offer cannot be accepted by any such use, means or instrumentality or from within Canada, Japan,
Australia, South Africa or Hong Kong or any other jurisdiction where prohibited by law.
No person has been authorized to give any information or to make
any representation on behalf of the Offeror not contained herein, in the Letter of Transmittal or in the Acceptance Forms and, if given or made, such information or representation must not be relied upon as having been authorized.
The Offeror has filed with the SEC a Tender Offer Statement on Schedule TO pursuant to Rule 14d–3 of the General Rules and Regulations under the Exchange
Act, together with exhibits furnishing certain additional information with respect to the Tender Offer and potential Subsequent Compulsory Redemption, and may file amendments thereto. A copy of such documents, and any amendments thereto, may be
examined at, and copies may be obtained from, the SEC in the manner set forth under Section 5.1—“Presentation of Biotie—General Overview.”
66
The following description of the Company and its business has been provided
exclusively based upon the Company’s Registration Statement on Form F-1 (No. 333-204147), as amended (including the financial statements published by the Company for the financial year ended December 31, 2014) and the interim report for
the nine-month period ended September 30, 2015 included by the Company on the Form 6-K dated November 12, 2015, and is qualified in its entirety by reference to such reports.
The Company’s full name is Biotie Therapies Oyj, or Biotie Therapies Corp. in
English. The Company is a public limited liability company incorporated in Finland in 1998 with Shares listed on Nasdaq Helsinki under the symbol “BTH1V” and ADSs listed on Nasdaq US under the symbol “BITI.” The Company is
registered in the Finnish Trade Register under the business identity code 1475830-6. The Company is domiciled in Turku, Finland, and its registered address is Joukahaisenkatu 6, FI-20520 Turku, Finland. The Company’s head offices are located at
the same address. The Company’s primary operating subsidiary, Biotie Therapies, Inc., is located in South San Francisco, California.
The Company is
a biopharmaceutical company primarily focused on developing therapeutics for central nervous system disorders. Its pipeline includes product candidates designed to address unmet medical needs in Parkinson’s disease and related dementia, other
neurodegenerative indications and primary sclerosing cholangitis, an orphan fibrotic liver disease. In addition, the Company has successfully developed a product for alcohol dependence that is being commercialized by H. Lundbeck A/S. The
Company’s lead product candidate, tozadenant, is an orally administered, potent and selective inhibitor of the adenosine A2a receptor that the Company is developing for the treatment of Parkinson’s.
According to the articles of association of the Company, the field of activity of the Company is the development, manufacture and sale of products and
equipment used in the biotechnology, pharmaceutical industry and laboratory environment, diagnostics, production of medical and psychotherapy services and consultancy related thereto, research and educational services as well as publishing of books,
magazines and other publications in the field. The Company may purchase and sell as well as lease and rent real estate for its operations as well as own, sell and possess shares and other securities provided that it does not engage in professional
trading in securities or investment.
The Company is a “foreign private issuer” as defined in Rule 3b-4(c) under the Exchange Act and is subject
to the information and reporting requirements of the Exchange Act. In accordance therewith the Company is obligated to file reports and other information with the SEC relating to its business, financial condition and other matters. Certain
information, as of particular dates, concerning the Company’s business, principal physical properties, capital structure, material pending litigation, operating results, financial condition, directors and officers, the principal holders of the
Company’s securities, any material interests of such persons in transactions with the Company, and other matters are required to be disclosed in reports filed with the SEC. Such reports and other information should be available for inspection
at the public reference facilities maintained by the SEC at 100 F Street N.E., Room 1580, Washington, D.C. 20549. Copies of such materials may also be obtained by mail, upon payment of the SEC’s customary fees, by writing to its principal
office at 100 F Street N.E., Washington, D.C. 20549. The SEC also maintains electronic reading rooms on the Internet at http://www.sec.gov that contains reports and other information regarding issuers that file electronically with the SEC. The
Company also maintains a website at http://www.biotie.com. The information contained in, accessible from or connected to the Company’s website is not incorporated into, or otherwise a part of, this Tender Offer Document or any of the
Company’s filings with the SEC. The website addresses referred to in this paragraph are inactive text references and are not intended to be actual links to the websites.
Although the Offeror has no knowledge that any such information is untrue, the Offeror takes no responsibility for the accuracy or completeness of information
contained in this Tender Offer Document with respect to the Company or any of its subsidiaries or affiliates or for any failure by the Company to disclose any events which may have occurred or may affect the significance or accuracy of any such
information.
67
On the date of this Tender Offer Document, the registered share capital of the Company is
EUR 279,218,058.00 and the Company has issued (i) a total of 1,089,608,083 Shares which consist of 108,686,288 treasury Shares held by the Company and 980,921,795 Shares issued and outstanding (of which, as of the close of business on March 7,
2016, 308,692,080 Shares are represented by 3,858,651 ADSs), (ii) 435,000 2011 Option Rights, (iii) 7,160,125 2014 Option Rights, (iv) 34,778,560 2016 Option Rights, (v) 25,000 2011 Share Rights, (vi) 5,652,188 2014 Share
Rights, (vii) 1,949,116 Swiss Option Rights and (viii) 220,400,001 Warrants.
The Company has a single share series and each Share entitles its
holder to one (1) vote at the General Meetings of Shareholders. The Shares carry equal rights to dividends.
The articles of association of the
Company do not include any provisions or restrictions on voting rights that deviate from the provisions of the Finnish Companies Act.
Each ADS represents 80 Shares. The ADSs have been registered and delivered by the Bank of New York
Mellon, as depositary under the deposit agreement, by and between the Company, the Bank of New York Mellon, ADS holders and all other persons indirectly or beneficially holding ADSs. The rights of the ADS holders are governed by such deposit
agreement. The ADSs are registered with the SEC on Form F-6 (File no. 333-204707). For holders of ADSs, the deposit agreement may contain important information, and the holders of ADSs are encouraged to read the form of deposit agreement on file
with the SEC.
The Company has 220,400,001 outstanding Warrants entitling their holders to subscribe for
Shares in the Company during the subscription period from November 1, 2015 until November 1, 2020. The Warrants were issued on May 28, 2015. Each Warrant entitles its holder to subscribe for one (1) new Share or one
(1) treasury Share in Biotie for the subscription price of EUR 0.17. In aggregate, the Warrants entitle holders of Warrants to subscribe to 220,400,001 Shares in Biotie. The terms and conditions of the Warrants are governed by Finnish law.
| 5.5 |
Option Rights and Share Rights |
Biotie has six equity incentive plans as follows: the Swiss Option Plan,
the Stock Option Plan 2011, the Equity Incentive Plan 2011, the Stock Option Plan 2014, the Equity Incentive Plan 2014 and the Stock Option Plan 2016.
The Swiss company Synosia, which the Company acquired in February 2011 and which is now operating under the name Biotie Therapies AG, has a stock option plan,
which the Company refers to as the Swiss Option Plan, under which the Swiss Option Rights have been granted to its employees, directors and consultants. In connection with the completion of the acquisition of Synosia, the Swiss Option Plan was
amended so that the holders of Swiss Option Rights would receive Biotie Shares instead of Synosia shares upon exercise of the Swiss Option Rights. An aggregate maximum of 14,912,155 of Biotie Shares were made available for issuance under the amended
plan. The subscription price for the Swiss Option Rights varies between CHF 0.10 and CHF 0.45 and each Swiss Option Right entitles the holder to receive one (1) Share. 1,949,116 Shares remain issuable under the Swiss Option Plan.
The Board of Directors of the Company established the Stock Option Plan 2011 on December 6, 2011. This plan was established to grant 2011 Option Rights,
mainly to the Company’s European employees, and 7,401,000 Shares were authorized for issuance pursuant to 2011 Option Rights under the plan. The 2011 Option Rights are issued in three tranches, 2011A, 2011B and 2011C with each option entitling
the holder to one (1) Share for the subscription price of EUR 0.01. 435,000 Shares remain issuable under the Stock Option Plan 2011.
68
The Board of Directors of the Company established the Equity Incentive Plan 2011 on December 6, 2011. This
plan was established to grant 2011 Stock Rights, mainly to the Company’s employees in the United States. 2011 Stock Rights representing an aggregate of a maximum of 4,599,000 Shares may be delivered under the Equity Incentive Plan 2011. Each
2011 Stock Right entitles the holder for one (1) Share. 25,000 Shares remain issuable under the Equity Incentive Plan 2011.
The Board of Directors
of the Company established the Stock Option Plan 2014 on January 2, 2014. This plan was established to grant 2014 Option Rights to the Company’s European employees. The maximum total number of 2014 Option Rights that may be issued is
10,337,500, entitling the recipients to an aggregate of up to 10,337,500 Shares. The 2014 Option Rights are issued in five tranches, 2014A, 2014B, 2014C, 2014D and 2014M with options in tranches 2014A-D entitling the holder to one (1) Share for
the subscription price of EUR 0.01 and the options in tranche 2014M entitling the holder to up to three (3) Shares for each option for the subscription price of EUR 0.01. As a result of the implementation of the Stock Option Plan 2016,
there will be no further awards made under the Stock Option Plan 2014, and hence, there will be no awards made under tranches 2014E or 2014F. 7,160,125 Shares remain issuable under the Stock Option Plan 2014.
The Board of Directors of the Company established the Equity Incentive Plan 2014 on January 2, 2014. This plan was established to grant 2014 Stock
Rights, mainly to the Company’s employees in the United States. A maximum of 14,002,500 Shares may be delivered under the Equity Incentive Plan 2014. The 2014 Stock Rights are issued in five tranches, 2014A, 2014B, 2014C, 2014D and 2014M with
rights in tranches 2014A-D entitling the holder to one (1) Share for the subscription price of USD 0.01 and rights in tranche 2014M entitling the holder to up to three (3) Shares for the subscription price of USD 0.01. 5,652,188 Shares
remain issuable under the Equity Incentive Plan 2014.
On January 4, 2016, the Board of Directors of the Company approved a new share-based incentive
plan, the Stock Option Plan 2016, for the Company’s employees pursuant to which 2016 Option Rights may be granted in the 2016 to 2017 period. The maximum number of Shares that may be issued pursuant to the Stock Option Plan 2016 is 80,000,000
Shares. The 2016 Option Rights are to be divided into several tranches so that 2016 Option Rights that have equal exercise price and exercise period will form one tranche. The subscription price for each tranche of 2016 Option Rights is the fair
market value of the Shares, as determined based on the closing price of the Shares on Nasdaq Helsinki on the date on which such tranche was issued. There are 34,934,440 2016 Option Rights issued and outstanding. The Company has agreed with certain
holders of 2016 Option Rights, holding a total of 19,744,800 2016 Option Rights, that should the Tender Offer be announced before April 1, 2016, such 2016 Option Rights will be returned to the Company without consideration and the Company will
instead pay such holders a transaction bonus amounting to the gross amount of the gain such holders would have received had such 2016 Option Rights been acquired by the Offeror in the Tender Offer. Such payment will be paid net of tax and other
deductions required by law.
To the knowledge of the Offeror, 108,686,288 Shares are held by the Company as treasury
Shares representing approximately 9.97 percent of all the Shares (including such treasury Shares) and votes in the Company. To the knowledge of the Offeror, the Board of Directors of the Company has no authorizations to acquire additional Shares of
the Company.
| 5.7 |
Authorization regarding the Issuance of Equity Interests |
At the annual general meeting of shareholders
of the Company held on May 26, 2015, the Board of Directors of the Company received authorization to issue Shares, options and other special rights, in one or several issues, representing no more than 95,000,000 new Shares in the aggregate.
Based on such authorization, the Board of Directors may issue Shares (i) in accordance with a shareholder’s pre-emptive rights and (ii) in a directed issuance of Shares, options or other special rights in deviation from the
pre-emptive rights of shareholders
69
provided that the deviation is justified by a weighty financial reason for the Company, such as financing of an acquisition, other arrangement concerning the business of the Company or
development of its capital structure, or incentivizing to the Company’s personnel. The Board of Directors is authorized to decide on other terms and conditions related to such issues of Shares, option and other special rights. The authorization
is in force until June 30, 2016.
| 5.8 |
Shareholders’ Agreements |
The Offeror is not aware of any shareholders’ agreements or other
agreements relating to the use of voting rights in the Company.
| 5.9 |
Board of Directors, Chief Executive Officer and Auditors |
In accordance with the provisions of the
Finnish Companies Act and the articles of association of the Company (see Annex E), the supervision and administration of the Company are divided between the shareholders represented at the Company’s general meeting of shareholders, the Board
of Directors of the Company and the Chief Executive Officer of the Company.
Under the articles of association of the Company, the Board of Directors of
the Company shall consist of at least three (3) and not more than ten (10) members. On the date of this Tender Offer Document, the Board of Directors of the Company consists of the following persons: Mr. William M. Burns,
Mr. Bernd Kastler, Mr. Don M. Bailey, Ms. Merja Karhapää, Dr. Ismail Kola, Dr. Guido Magni, and Dr. Mahendra G. Shah.
The Chief Executive Officer of the Company at the date of this Tender Offer Document is Dr. Timo Veromaa. The auditor of the Company is
PricewaterhouseCoopers Oy (the responsible auditor being Mr. Samuli Perälä, Authorized Public Accountant).
| 5.10 |
Financial Information |
The audited consolidated financial statements of the Company for the financial
year ended December 31, 2014 are included in this Tender Offer Document (see Annex B) in the form published by the Company. On the date of this Tender Offer Document, said financial statements have been presented to and adopted by the annual
general meeting of shareholders of the Company.
The unaudited interim report of the Company for the nine (9) months ended September 30, 2015 is
included in this Tender Offer Document in the form published by the Company (see Annex C).
| 5.11 |
Future Prospects Published by the Company |
The future prospects of the Company have been described in
the financial statements of the Company for the financial year ended December 31, 2014 and in the interim report of the Company for the nine (9) months ended September 30, 2015 (see Annex B and Annex C). Other stock exchange releases
recently published by the Company that may have a material effect on the value of the Equity Interests have been attached to this Tender Offer Document as Annex D.
| 5.12 |
Articles of Association |
The articles of association of the Company are included in this Tender Offer
Document (see Annex E).
| 6 |
PRESENTATION OF THE OFFEROR |
The Offeror’s full name is Acorda Therapeutics, Inc. The Offeror is a
Delaware corporation incorporated in 1995 with shares of its common stock listed on the NASDAQ Global Market under the symbol “ACOR.” Its Company
70
Identification Number is Delaware Secretary of State File No. 2481090. The registered address of the Offeror is in Wilmington, Delaware, USA, and its registered address is at 1209 Orange
Street, Wilmington, (New Castle County), Delaware 19801, USA. The address of the Offeror’s principal executive offices is at 420 Saw Mill River Road, Ardsley, New York 10502. The telephone number of the Offeror is (+1) 914 347 4300. The
Offeror is a biotechnology company focused on developing therapies that restore function and improve the lives of people with neurological disorders. It has an industry leading pipeline of novel neurological therapies addressing a range of
disorders, including multiple sclerosis, Parkinson’s disease, post-stroke walking deficits, epilepsy and migraine and markets three FDA-approved therapies, including Ampyra (dalfampridine) Extended Release Tablets, Zanaflex Capsules and tablets
and Qutenza.
The name, citizenship, business address, business phone number, present principal occupation or employment and past material occupation,
positions, offices or employment for at least the last five (5) years for each director of the Offeror and the name, citizenship, business address, business phone number, present principal occupation or employment and past material occupation,
positions, offices or employment for at least the past five (5) years of each of the executive officers of the Offeror and certain other information are set forth in Schedule I hereto.
Certain Relationships Between the Offeror and the Company. Except as described in this Tender Offer Document, (i) neither the Offeror nor, to the
best knowledge of the Offeror, any of the persons listed in Schedule I to this Tender Offer Document or any associate or majority–owned subsidiary of the Offeror or any of the persons so listed beneficially owns or has any right to
acquire, directly or indirectly, any Shares or ADSs and (ii) neither the Offeror nor, to the best knowledge of the Offeror, any of the persons or entities referred to above nor any director, executive officer or subsidiary of any of the
foregoing has effected any transaction in the Shares or ADSs during the past sixty (60) days.
Except as provided in the Combination Agreement
or as otherwise described in this Tender Offer Document, none of the Offeror, or its subsidiaries, nor, to the best knowledge of the Offeror, any of the persons listed in Schedule I to this Tender Offer Document, has any present or proposed material
agreement, arrangement, understanding or relationship with the Company or any of its executive officers, directors, controlling persons or subsidiaries. Except as provided in the Combination Agreement or as otherwise described in this Tender Offer
Document, neither the Offeror nor, to the best knowledge of the Offeror, any of the persons listed in Schedule I to this Tender Offer Document, has any agreement, arrangement, or understanding with any other person with respect to any securities of
the Company, including, but not limited to, any contract, arrangement, understanding or relationship concerning the transfer or voting of such securities, finders’ fees, joint ventures, loan or option arrangements, puts or calls, guarantees of
loans, guarantees against loss, guarantees of profits, division of profits or loss or the giving or withholding of proxies.
Except as set forth in this
Tender Offer Document, none of the Offeror nor, to the best knowledge of the Offeror, any of the persons listed on Schedule I hereto, has had any business relationship or transaction with the Company or any of its executive officers, directors or
affiliates that is required to be reported under the rules and regulations of the SEC applicable to the Tender Offer.
Except as set forth in this Tender
Offer Document, there have been no material contacts, negotiations or transactions between the Offeror or any of its subsidiaries or, to the best knowledge of the Offeror, any of the persons listed in Schedule I to this Tender Offer Document, on the
one hand, and the Company or its affiliates, on the other hand, concerning a merger, consolidation or acquisition, tender offer or other acquisition of the Company’s securities, an election of the Company’s directors or a sale or other
transfer of a material amount of the Company’s assets during the past two (2) years.
None of the persons listed in Schedule I has, during the
past five (5) years, been convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors). None of the persons listed in Schedule I to this Tender Offer Document has, during the past five (5) years, been a party
to any judicial or administrative
71
proceeding (except for matters that were dismissed without sanction or settlement) that resulted in a judgment, decree or final order enjoining the person from future violations of, or
prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
Available
Information. Pursuant to Rule 14d–3 under the Exchange Act, the Offeror filed with the SEC a Tender Offer Statement on Schedule TO, of which this Tender Offer Document forms a part, and exhibits to the Schedule TO. Additionally, the Offeror
is subject to the information reporting requirements of the Exchange Act and, in accordance therewith, is required to file periodic reports, proxy statements and other information with the SEC relating to its business, financial condition and other
matters. The Schedule TO and the exhibits thereto, and such reports, proxy statements and other information, can be inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C.
20549–0213. Information regarding the public reference facilities may be obtained from the SEC by telephoning 1–800–SEC–0330. The Offeror’s filings are also available to the public on the SEC’s internet site
(http://www.sec.gov). Copies of such materials may also be obtained by mail from the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549–0213 at prescribed rates.
| 6.2 |
Persons Related to the Offeror as Stipulated in Chapter 11, Section 5 of the Finnish Securities Market Act |
The Acorda group of companies consists of Acorda and numerous direct or indirect subsidiaries. Neither the Offeror, any Acorda group company nor any natural or
legal person cooperating with the Offeror on the basis of an agreement or arrangement to exercise or acquire significant control of Biotie (as referred to in Chapter 11, Section 5 of the Finnish Securities Market Act) owns at the date of this
Tender Offer Document any Shares or other Equity Interests in the Company or has during the 6-month period preceding the Announcement Date acquired any Shares or other Equity Interests in the Company in public trading or otherwise.
| 6.3 |
Biotie’s Ownership in the Offeror |
To the best knowledge of the Offeror, the Company does not at
the date of this Tender Offer Document own any shares or securities entitling to shares in the Offeror or in the entities referred to in Chapter 11, Section 5 of the Finnish Securities Market Act.
72
APPENDICES
Schedule I — Directors and Officers of the Offeror
Annex A — Statement of Biotie’s Board of Directors
Annex B — Financial Statements of Biotie for the financial year ended December 31, 2014
Annex C — Interim Report of Biotie for the nine months ended September 30, 2015, unaudited
Annex D — Stock Exchange Releases Published by Biotie on January 19, 2016 and February 17, 2016
Annex E — Articles of Association of Biotie
Annex F
— Forms of Irrevocable Undertaking
Annex G — Combination Agreement
73
SCHEDULE I
DIRECTORS AND EXECUTIVE OFFICERS OF THE OFFEROR
The following table sets forth the name, present principal occupation or employment and past material occupations, positions, offices or employment for at
least the past five years for each director and executive officer of the Offeror. The current business address of each person is 420 Saw Mill River Road, Ardsley, NY 10502, USA and the current phone number is (+1) 914 347 4300. Unless otherwise
indicated, each such person is a citizen of the United States of America.
|
|
|
| Name |
|
Present Principal Occupation or Employment;
Material Positions Held During the Past Five Years |
| Rick Batycky, Ph.D. |
|
Rick Batycky, Ph.D. has been Chief Technology Officer and Site Head at Acorda since October 2014. He joined Acorda as part of the Company’s acquisition of Civitas Therapeutics, a Chelsea, MA-based biopharmaceutical company that
Dr. Batycky co-founded in 2009, serving as Chief Scientific Officer. Dr. Batycky has close to two decades of experience in drug development, with a focus on inhaled therapies. Prior to founding Civitas, Dr. Batycky was the Chief Scientific
Officer and Sr. Vice President of Research & Development at Pulmatrix. He was previously the Vice President of Research and Development at Alkermes, overseeing many facets of product development across pulmonary, injectable and oral platforms.
Dr. Batycky was an original member of Advanced Inhalation Research (AIR®), where he oversaw product development utilizing the
ARCUS® technology for pulmonary delivery. Acorda has global development rights to the ARCUS technology as part of the Civitas acquisition. Dr. Batycky held several academic posts prior to
joining AIR. Dr. Batycky received his B.Sc. in Chemical Engineering from University of Calgary and his S.M. and Ph.D. in Chemical Engineering from the Massachusetts Institute of Technology (MIT). |
|
|
| Andrew R. Blight, Ph.D. |
|
Andrew R. Blight, Ph.D., has been our Chief Scientific Officer since January 2004 and previously served as our Executive Vice President, Research and Development from 2000 to 2004, and Vice President, Research and Development, from
1998 to 2000. Prior to joining the Company, Dr. Blight spent approximately six years as Professor and Director of the Neurosurgery Research Laboratory at the University of North Carolina at Chapel Hill. Dr. Blight held prior academic
positions at Purdue University and New York University. Dr. Blight is a leader in SCI pathophysiology research and has made several important contributions to the field, particularly on the role of demyelination in SCI. He also pioneered the
therapeutic application of 4-AP in SCI animal models and in human clinical trials. Dr. Blight is a member of the editorial board of the Journal of Neurotrauma and has served as a member of the
Neurological Sciences and Disorders-A (NSDA) review committee at the National Institutes of Health (NIH). He was previously Secretary, Treasurer and Vice President of the National Neurotrauma Society. Dr. Blight received his B.S. in Zoology and
his Ph.D. in Zoology/Neurobiology from the University of Bristol, U.K. |
74
|
|
|
| Name |
|
Present Principal Occupation or Employment;
Material Positions Held During the Past Five Years |
| Ron Cohen, M.D. |
|
Ron Cohen, M.D., has served as our President and Chief Executive Officer since he founded the Company in 1995. Dr. Cohen previously was a principal in the startup of Advanced Tissue Sciences, Inc., a biotechnology company
engaged in the growth of human organ tissues for transplantation. Dr. Cohen received his B.A. with honors in Psychology from Princeton University, and his M.D. from the Columbia College of Physicians & Surgeons. He completed his
residency in Internal Medicine at the University of Virginia Medical Center, and is Board Certified in Internal Medicine. Dr. Cohen currently serves on the board of directors of VBL Therapeutics. In addition, within the last five years, he
previously served on the board of directors of Dyax Corp. Dr. Cohen previously was a Director and Chairman of the New York Biotechnology Association. He currently serves as Chair of the board of the Biotechnology Industry Organization (BIO). He
also serves as a member of the Columbia-Presbyterian Health Sciences Advisory Council and was awarded Columbia University’s Alumni Medal for Distinguished Service. In 2010, Dr. Cohen was named NeuroInvestment’s (now called
NeuroPerspective) CEO of the Year and in 2009 he was recognized by PharmaVoice Magazine as one of the 100 Most Inspirational People in the Biopharmaceutical Industry. Dr. Cohen is a recipient of the Ernst & Young Entrepreneur of the
Year Award for the New York Metropolitan Region, and an inductee into the National Spinal Cord Injury Association’s “Spinal Cord Injury Hall of Fame.” In 2010, Dr. Cohen was recognized by the New York Biotechnology Association as the
NYBA “The Cure Starts Here” Business Leader of the Year. Dr. Cohen is the principal strategist in the Company’s commitment to being a fully-integrated biopharmaceutical company that is a leading innovator in neurology. His
extensive knowledge of the Company and its history provides our Board with valuable perspectives to advance our business and the interests of our stockholders. |
|
|
| Barry Greene |
|
Barry Greene has been a member of our Board since January 2007. Mr. Greene currently serves as President and Chief Operating Officer of Alnylam Pharmaceuticals, Inc., a biopharmaceutical company based in Cambridge, MA.
Mr. Greene joined Alnylam in September 2003, bringing over 15 years of experience in the healthcare industry and in consulting. Prior to Alnylam, he was General Manager of Oncology at Millennium Pharmaceuticals, Inc., where he led the
company’s global strategy and execution for its oncology business, including strategic business direction and execution, culminating in the successful approval and launch of VELCADE (bortezomib) in mid-2003. Prior to joining Millennium in
February 2001, Mr. Greene served as Executive Vice President and Chief Business Officer for Mediconsult.com. Prior to Mediconsult.com, Mr. Greene’s past experiences included being Vice President of Marketing and Customer Services
for AstraZeneca (formerly AstraMerck); Vice President Strategic Integration with responsibility for the AstraZeneca North American post-merger integration; and partner of Andersen Consulting, responsible for the pharmaceutical/biotechnology
marketing and sales practice. He currently serves as member of the board of directors of Karyopharm Therapeutics Inc. Mr. Greene previously was a member of the boards of directors of Regulus Therapeutics, LLC and Intercept Pharmaceuticals, Inc.
Mr. Greene received his B.S. in Industrial Engineering from the University of Pittsburgh and serves as a Senior Scholar at Duke University, Fuqua School of Business. Mr. Greene brings to our Board extensive experience in the healthcare industry
as well as practical experience guiding new drugs through the commercialization process. |
75
|
|
|
| Name |
|
Present Principal Occupation or Employment;
Material Positions Held During the Past Five Years |
| Andrew Hindman |
|
Andrew Hindman has been our Chief Business Development Officer since May 2014. Prior to joining the Company, Mr. Hindman held several senior executive level positions in the biopharmaceutical industry, most recently from 2011
to 2014 as President, Chief Executive Officer and member of the Board of Tobira Therapeutics, a privately-held biotechnology company located in South San Francisco, CA. At Tobira, Mr. Hindman was responsible for developing a new corporate strategy,
building new leadership and operational teams, and raising operating capital. Prior thereto, Mr. Hindman held senior corporate development and commercial operating positions, including from 2010 to 2011 at Nodality, Inc., from 2008 to 2010 at Onyx
Pharmaceuticals, Inc., and from 1998 to 2008 at Gilead Sciences, Inc. Mr. Hindman holds a B.A. in biochemistry and economics, graduating Phi Beta Kappa, from Wesleyan University and an executive MBA from Columbia University and the University of
California Berkeley, Haas School of Business. |
|
|
| Peder K. Jensen, M.D. |
|
Peder K. Jensen, M.D., has been a member of our Board of Directors since April 2011. Dr. Jensen is currently president of Bay Way Consultants, LLC, a consulting firm based in San Rafael, CA founded by Dr. Jensen in 2010 that advises
pharmaceutical and biotechnology companies. Dr. Jensen’s experience includes over 20 years with Schering-Plough Corporation, a global pharmaceutical company, and then Merck & Co., Inc. after the merger of Schering-Plough with Merck in 2009.
During his tenure at Schering-Plough/Merck, Dr. Jensen held a number of global senior research and development positions, including Vice President Clinical Research, SPRI, Executive Vice President Worldwide Drug Development, SPRI, and most recently
Corporate Senior Vice President, and General Manager, R&D for Japan and Asia/Pacific from 2006 to 2010. Dr. Jensen has more than 24 years of global drug development experience across a variety of therapeutic areas, including neurology,
cardiovascular, anti-infective, oncology and immunology. Over the course of his career, Dr. Jensen has been responsible for more than 40 new drug approvals worldwide, including in the U.S., Europe and Japan. Dr. Jensen also currently serves on the
board of directors of FivePrime Therapeutics, Inc. Dr. Jensen previously was a member of the board of directors of BioCryst Pharmaceuticals, Inc. Dr. Jensen received his M.D. from the University of Copenhagen. Dr. Jensen’s extensive global
pharmaceutical experience, combined with his specific knowledge in developing new and innovative medical treatments in many different therapeutic areas, including neurology, makes him well positioned to provide advice and guidance to the Company on
its research and development programs. |
|
|
| John P. Kelley |
|
John P. Kelley has been a member of our Board of Directors since December 2008. Mr. Kelley is currently Chief Executive Officer of Tenax Therapeutics, Inc. (formerly named Oxygen Biotherapeutics, Inc.), a Morrisville, NC-based
company that focuses on developing products for the critical care market, where he also serves as a member of the board of directors. Mr. Kelley has held this position since November 2013. From 2011 to 2013, Mr. Kelley was President, Chief Executive
Officer, and a director of Phyxius Pharma, Inc., a privately-held development stage pharmaceutical company co-founded by Mr. Kelley in 2011 focused on developing products for use in acute care settings. Mr. Kelley became Chief Executive Officer of
Tenax Therapeutics when it acquired Phyxius Pharma in 2013. Previously, Mr. Kelley was the President and Chief Operating Officer of The Medicines Company, a pharmaceutical company providing acute care hospital products worldwide, from 2004 to
2009. He also served |
76
|
|
|
| Name |
|
Present Principal Occupation or Employment;
Material Positions Held During the Past Five Years |
|
|
|
|
on The Medicines Company’s board of directors from 2005 to 2009. From 2000 to 2004, Mr. Kelley held a series of positions at Aventis, a global pharmaceutical company, including Senior Vice President, Global Marketing and
Medical, where he was accountable for worldwide brand management. Prior to the formation of Aventis, he held a series of positions at Hoechst Marion Roussel, Inc., a life sciences firm focused on pharmaceuticals, including, from 1998 to 1999,
Vice President, Commercial Director, U.S. and, from 1995 to 1998, Vice President of Marketing. Mr. Kelley received a B.A. from Wilkes University and an M.B.A. from Rockhurst University. Mr. Kelley’s extensive knowledge of the
pharmaceutical industry as well as his operations and marketing experience make him well positioned to provide advice and guidance to the Company at this stage of its development. The Board has determined that Mr. Kelley qualifies as an audit
committee financial expert. Based on his public company and broad corporate experience, Mr. Kelley serves as Chair of our Compensation Committee. |
|
|
| David Lawrence |
|
David Lawrence has been our Chief of Business Operations since October 2013. Prior to that, from January 2005 to October 2013, he served as our Chief Financial Officer. He previously served as our Vice President, Finance from
January 2001 through 2004, and Director, Finance from 1999 to 2001. From 1991 to 1999, Mr. Lawrence held several positions for Tel-Air Communications, Inc., including Vice President and Controller. Prior to Tel-Air, he held the financial
management positions of Controller and Finance Manager for Southwestern Bell and Metromedia Telecommunications, respectively. Mr. Lawrence received his undergraduate degree in Accounting from Roger Williams College, and an M.B.A in Finance from
Iona College. Mr. Lawrence is a founding member and currently serves on the board of directors and as Treasurer of The Brian Ahearn Children’s Fund. |
|
|
| Sandra Panem, Ph.D. |
|
Sandra Panem, Ph.D., has been a member of our Board since 1998. She is currently a partner at New York, NY-based Cross Atlantic Partners, which she joined in 2000. She is also currently President of NeuroNetworks Fund, a New York,
NY-based not-for-profit venture capital fund focusing on neurodisorders which she co-founded in December 2014. From 1994 to 1999, Dr. Panem was President of Vector Fund Management, the then asset management affiliate of Vector Securities
International. Prior thereto, Dr. Panem served as Vice President and Portfolio Manager for the Oppenheimer Global BioTech Fund, a mutual fund that invested in public and private biotechnology companies. Previously, she was Vice President at
Salomon Brothers Venture Capital, a fund focused on early and later-stage life sciences and technology investments. Dr. Panem was also a Science and Public Policy Fellow in economic studies at the Brookings Institution, and an Assistant
Professor of Pathology at the University of Chicago. She received a B.S. in biochemistry and a Ph.D. in microbiology from the University of Chicago. Dr. Panem currently serves on the boards of directors of Labcyte, Inc.,
GenomeQuest, Inc., and BioLineRx Ltd. Dr. Panem’s experience investing in life sciences companies, and her long-standing relationship with the Company as a Board representative of one of its earliest investors, provides historical
perspective on the Company and the life sciences industry. Based on her broad industry and corporate experience, Dr. Panem serves as Chair of our Nominations and Governance Committee. |
77
|
|
|
| Name |
|
Present Principal Occupation or Employment;
Material Positions Held During the Past Five Years |
| Lorin J. Randall |
|
Lorin J. Randall has been a member of our Board since January 2006. Mr. Randall, a financial consultant, was Senior Vice President and Chief Financial Officer of Eximias Pharmaceutical Corporation, a development-stage drug
development company, from 2004 to 2006. From 2002 to 2004, Mr. Randall served as Senior Vice President and Chief Financial Officer of i-STAT Corporation, a publicly-traded manufacturer of medical diagnostic devices that was acquired by Abbott
Laboratories in 2004. From 1995 to 2001, Mr. Randall was Vice President and Chief Financial Officer of CFM Technologies, Inc., a publicly-traded manufacturer of semiconductor manufacturing equipment. Mr. Randall previously served on
the board of Quad Systems Corporation, a publicly-traded manufacturer of electronics manufacturing equipment where he served as Chairman of the Audit Committee. He currently serves on the boards of directors of Athersys, Inc., and
Nanosphere, Inc. In addition, within the last five years, he previously served on the boards of directors of Tengion, Inc. and MotoLogic, Inc. Mr. Randall received a B.S. in accounting from The Pennsylvania State University and an M.B.A.
from Northeastern University. As a former Chief Financial Officer of a number of publicly-traded companies, Mr. Randall possesses financial acumen acquired through working experience, including an understanding of financial matters and the
preparation and analysis of financial statements. The Board has determined that Mr. Randall qualifies as an audit committee financial expert. |
|
|
| Steven M. Rauscher |
|
Steven M. Rauscher has served on our Board since March 2005. He is Founder & Principal of BioPharm Physicians, LLC, a Lexington, MA-based company formed in 2010 to provide consulting services to physicians and companies in the
biopharmaceutical industry. Previously, he was Client Partner with Euromedica, a life sciences executive search firm, from 2012 to 2014. Prior thereto, he was President and Chief Executive Officer of Oscient Pharmaceuticals Corporation, a commercial
stage biopharmaceutical company, from 2000 to 2009. He joined Oscient in 2000 having served as a member of the Board of Directors since 1993. Previously, Mr. Rauscher was Chief Executive Officer of AmericasDoctor, a company providing clinical
research services to the pharmaceutical industry. Prior to AmericasDoctor, he held a number of leadership positions at Abbott Laboratories, including Vice President of Corporate Licensing, Vice President of Business Development, International
Division and Vice President of Sales, U.S. Pharmaceuticals. Mr. Rauscher received a B.S. from Indiana University and an M.B.A. from the University of Chicago. Having served as a Chief Executive Officer of a commercial stage biopharmaceutical
company as well as in other executive roles in a variety of companies in our industry, Mr. Rauscher brings to our Board leadership skills and expertise in managing the challenges of a biopharmaceutical company. Based on his management and
operational experience and expertise in the pharmaceutical industry, Mr. Rauscher serves as the Chair of our Compliance Committee and oversees the non-financial governance and risk management processes of the
Company. |
78
|
|
|
| Name |
|
Present Principal Occupation or Employment;
Material Positions Held During the Past Five Years |
| Michael Rogers |
|
Michael Rogers has been our Chief Financial Officer since October 2013. Prior to joining the Company, Mr. Rogers was the Executive Vice President and Chief Financial Officer of BG Medicine, Inc., a publicly-traded life sciences
company based in Waltham, MA, from June 2009 to October 2012. Prior to that, Mr. Rogers was the Executive Vice President, Chief Financial Officer and Treasurer of Indevus Pharmaceuticals, Inc. from 1999 until the company’s sale to Endo
Pharmaceuticals in 2009. He also served as Chief Financial Officer at Advanced Health Corporation and AutoImmune, Inc. Mr. Rogers has more than 23 years of experience in the biopharmaceutical industry, including as an investment banker at Lehman
Brothers and PaineWebber, where he focused on life sciences companies. Mr. Rogers received his B.A. from Union College, and an M.B.A. from the Darden School of Business at the University of Virginia. He currently serves on the Board of Directors for
pSivida Corp. and previously served on the Board of Coronado Biosciences, Inc. |
|
|
| Lauren M. Sabella |
|
Lauren M. Sabella has been our Chief Commercial Officer since February 2015. Prior to that, from January 2010 to February 2015, she was our Executive Vice President, Commercial Development. Ms. Sabella was the Founder and Principal
of Tugboat Consulting Group, an independent consulting practice assisting companies in the commercialization process. Ms. Sabella also served as Corporate Officer and VP of Commercial Development at Altus Pharmaceuticals from May 2006 to September
2008, with responsibility for all aspects of commercialization. Prior to joining Altus, Ms. Sabella was employed by Boehringer Ingelheim Pharmaceuticals for 18 years in positions of increasing responsibility. In her last role, she served as VP of
Sales, Eastern Zone, where she led the successful sales launch of Spiriva and ran both Primary Care and Specialty Divisions, including Neurology, Urology and Cardio/Pulmonary. Prior to this role, she had over ten years of marketing experience where
she led several brand launches including Mobic, an NSAID which became a $1 billion brand. Ms. Sabella holds a B.B.A. from Hofstra University. |
|
|
| Ian Smith |
|
Ian Smith has been a member of our Board since February 2007. Mr. Smith currently serves as Executive Vice President and Chief Financial Officer of Boston, MA-based global biotechnology company Vertex
Pharmaceuticals, Inc., a position he has held since February 2006. From November 2003 to February 2006, he was Senior Vice President and Chief Financial Officer, and from October 2001 to November 2003, he served as Vice President and Chief
Financial Officer, at Vertex. From 1999 to 2001, Mr. Smith served as a partner in the Life Science and Technology Practice Group of Ernst & Young LLP. Mr. Smith initially joined Ernst & Young’s U.K. firm in
1987, and then joined its Boston office in 1995. Mr. Smith currently is a member of the board of directors of Infinity Pharmaceuticals, Inc. In addition, he previously served on the board of directors of ToleRx, Inc. Mr. Smith holds a B.A.
in accounting and finance from Manchester Metropolitan University, U.K., is a member of the American Institute of Certified Public Accountants and is a Chartered Accountant of England and Wales. Mr. Smith has extensive global financial and
accounting experience and possesses an understanding of financial matters and the preparation and analysis of financial statements. The Board has determined that Mr. Smith qualifies as an audit committee financial expert. Based on his extensive
financial experience, Mr. Smith serves as Chair of our Audit Committee. Mr. Smith is a citizen of the United Kingdom. |
79
|
|
|
| Name |
|
Present Principal Occupation or Employment;
Material Positions Held During the Past Five Years |
| Jane Wasman |
|
Jane Wasman has been our President, International, General Counsel and Corporate Secretary since October 2012. Prior to that, from January 2012 until October 2012, she was our Chief, Strategic Development, General Counsel and
Corporate Secretary; and from May 2004 until January 2012, she was our Executive Vice President, General Counsel and Corporate Secretary. Prior to joining the Company, from 1995 to 2004, Ms. Wasman held various leadership positions at Schering-Plough Corporation, including Staff Vice President and Associate General Counsel responsible for legal support for U.S. Pharmaceuticals operations, including sales, marketing and compliance; FDA regulatory
matters; licensing and mergers and acquisitions; and global research and development. She served as Staff Vice President, International in 2001 and as Staff Vice President, European Operations-Legal from 1998 to 2000. Previously, Ms. Wasman
specialized in litigation at Fried, Frank, Harris, Shriver & Jacobson. She also served as Associate Counsel to the U.S. Senate Committee on Veteran’s Affairs. Ms. Wasman graduated Magna Cum Laude and Phi Beta Kappa from Princeton
University and earned her J.D. from Harvard Law School. Ms. Wasman is a member of the board of directors and the executive committee of the board of the New York Biotechnology Association (NYBA). |
80
Annex A
STATEMENT OF THE BOARD OF DIRECTORS OF BIOTIE THERAPIES CORP. REGARDING THE VOLUNTARY PUBLIC TENDER OFFER BY ACORDA THERAPEUTICS, INC.
Acorda Therapeutics, Inc. (hereinafter the “Offeror” or “Acorda”) and Biotie Therapies Corp. (hereinafter
“Biotie” or the “Company”) have announced a voluntary public tender offer (the “Offer”) by Acorda for the issued and outstanding ordinary shares, no nominal value, of the Company (the
“Shares”), the outstanding American Depositary Shares, each representing 80 Shares (the “ADSs”), and the outstanding Equity Instruments (as defined below) in Biotie by stock exchange release dated January 19,
2016.
The Board of Directors of Biotie (the “Board” or the “Biotie Board”) hereby issues the following statement
regarding the Offer as required by Finnish securities laws (Chapter 11, Section 13 of the Finnish Securities Market Act 746/2012, as amended).
The Tender Offer in Brief
Biotie and Acorda have on
January 19, 2016 entered into a combination agreement (the “Combination Agreement”) setting out, among other matters, the terms and conditions pursuant to which the Offer shall be made by Acorda.
Subject to the Combination Agreement, Acorda has resolved to make the Offer for the outstanding (i) Shares; (ii) ADSs; (iii) option rights
under the option plan resolved upon by the Board on December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Option Rights”), options rights under
the option plan resolved upon by the Board on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013, (the “2014 Option Rights”) and option rights under the
option plan resolved upon by the Board on January 4, 2016 by virtue of an authorization granted by the annual general meeting of the Company held on May 26, 2015 (the “2016 Option Rights”); (iv) share units under the
equity incentive plan resolved upon by the Board on December 6, 2011 by virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Share Rights”) and share units under
the equity incentive plan resolved upon by the Board on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013 (the “2014 Share Rights” and, together with
the 2011 Share Rights, the “Share Rights”); (v) option rights under the Swiss option plan dated June 18, 2008 (the “Swiss Option Rights” and together with the 2011 Option Rights, the 2014 Option Rights and
the 2016 Option Rights, the “Option Rights”); and (vi) warrants issued on May 28, 2015 by virtue of an authorization granted by the annual general meeting of the Company held on May 26, 2015 (the
“Warrants”).
The outstanding Option Rights, the Share Rights and the Warrants that have been granted to holders are hereinafter jointly
referred to as the “Equity Instruments.” The Shares that are not held by the Company or any of its subsidiaries including all the Shares represented by ADSs are hereinafter referred to as the “Shares.” The
outstanding Shares, the ADSs and the Equity Instruments are hereinafter jointly referred to as the “Equity Interests.”
The Offer will be
made in accordance with the terms and conditions reflected in the tender offer document (hereinafter referred to as the “Offer to Purchase”) to be published by Acorda before the acceptance period of the Offer commences. The
acceptance period of the Offer is expected to commence by mid-March 2016 and will remain open for an initial period of at least 20 U.S. business days (the “Offer Period”).
Acorda has offered to purchase all Equity Interests of the Company that are not held by the Company or any of its subsidiaries for a consideration of:
| |
(i) |
EUR 0.2946 in cash for each outstanding Share; |
| |
(ii) |
EUR 23.5680 in cash for each outstanding ADS, payable in the equivalent amount of U.S. dollars for each outstanding ADS determined as near to the
payment date as reasonably practicable based on the |
| |
U.S. dollar spot rate against the euro exchange rate on the nearest practicable date to the Closing Date (as defined below); |
| |
(iii) |
EUR 0.2846 in cash for each outstanding 2011 Option Right; |
| |
(iv) |
EUR 0.2846 in cash for each outstanding 2014 Option Right; |
| |
(v) |
EUR 0.1326 in cash for each outstanding 2016 Option Right, payable, at the option of the holder, in in euros or the equivalent amount of U.S. dollars for each outstanding 2016 Option Right determined as near to the
payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable date to the Closing Date; |
| |
(vi) |
EUR 0.2946 in cash for each outstanding 2011 Share Right, payable, at the option of the holder, in in euros or the equivalent amount of U.S. dollars for each outstanding 2011 Share Right determined as near to the
payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable date to the Closing Date; |
| |
(vii) |
EUR 0.2854 in cash for each outstanding 2014 Share Right, payable, at the option of the holder, in in euros or the equivalent amount of U.S. dollars for each outstanding 2014 Share Right determined as near to the
payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable date to the Closing Date; |
| |
(viii) |
EUR 0,2032 in cash for each outstanding Swiss Option Right with a per share subscription price of CHF 0.10; |
| |
(ix) |
EUR 0.1026 in cash for each Swiss Option Right with a per share subscription price of CHF 0.21; |
| |
(x) |
EUR 0.0386 in cash for each Swiss Option Right with a per share subscription price of CHF 0.28; |
| |
(xi) |
EUR 0.0112 in cash for each Swiss Option Right with a per share subscription price of CHF 0.31; |
| |
(xii) |
EUR 0.0100 in cash for each other Swiss Option Right; and |
| |
(xiii) |
EUR 0.1664 in cash for each outstanding Warrant. |
Pursuant to the terms of the Combination Agreement, the
completion of the Offer is subject to the following conditions (the “Conditions”):
| |
(i) |
the valid tender of outstanding Shares (including outstanding Shares represented by validly tendered ADSs and validly tendered Warrants) representing, together with any outstanding Shares (including outstanding Shares
represented by ADSs and Warrants) otherwise acquired by Acorda, more than ninety percent (90%) of the issued and outstanding Shares and voting rights of the Company, calculated on a fully diluted basis and otherwise in accordance with Chapter
18 Section 1 of the Finnish Limited Liability Companies Act (21.7.2006/624) (the “Minimum Acceptance Condition”); as used in this paragraph “fully diluted basis” means an equation in which the numerator
represents the aggregate number of outstanding Shares (including outstanding Shares represented by ADSs) and Warrants that have been validly tendered or otherwise acquired by Acorda and the denominator represents the aggregate number of all
outstanding Shares (including outstanding Shares represented by ADSs) and Warrants, as well as Shares issuable upon the vesting and exercise of those outstanding Equity Instruments (other than Warrants) that have not been validly tendered into the
Offer or otherwise acquired by Acorda; |
| |
(ii) |
the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended and the rules and regulations thereunder (the “HSR Act”), which
waiting period has expired on February 16, 2016; |
| |
(iii) |
no material adverse effect (as defined in the Combination Agreement) having occurred on the Company after January 19, 2016; |
| |
(iv) |
Acorda not, after January 19, 2016, having received information previously undisclosed to it that describes a material adverse effect on the Company that occurred prior to January 19, 2016; |
| |
(v) |
no information made public by the Company or disclosed by the Company to Acorda being materially inaccurate, incomplete, or misleading, and the Company not having failed to make public any information that should have
been made public by it under applicable laws, including without limitation the rules of NASDAQ Helsinki Ltd. (“Nasdaq Helsinki”) and NASDAQ Stock Market LLC (“Nasdaq US”), provided that, in each case, the
information made public, disclosed or not disclosed or the failure to disclose information constitutes a material adverse effect on the Company; |
| |
(vi) |
no court or regulatory authority of competent jurisdiction (including without limitation the Finnish Financial Supervisory Authority (the “FIN-FSA”) or the US Securities and Exchange Commission (the
“SEC”)) having given an order or issued any regulatory action preventing or enjoining the completion of the Offer; |
| |
(vii) |
the Board having issued its recommendation for the Offer and the recommendation remaining in force and not being modified or changed in a manner detrimental to Acorda; and |
| |
(viii) |
the Combination Agreement not having been terminated and remaining in force and no event having occurred that, with the passage of time, would give Acorda the right to terminate the Combination Agreement under specified
sections of the Combination Agreement that give Acorda the right to terminate the Combination Agreement in response to a breach of the agreement by the Company. |
Fulfillment of the Minimum Acceptance Condition and other Conditions will be determined as of the expiration of the Offer Period on the next Finnish banking
day after the expiration of the Offer Period, when the preliminary results of the Offer will be available. Acorda has reserved the right to complete the Offer even if the conditions have not been fulfilled.
If any Condition is neither satisfied nor waived at the conclusion of the Offer Period, Acorda will extend the acceptance period for additional periods not
exceeding two weeks each. The maximum duration of the Offer Period (including any extensions) is ten weeks as required under Finnish law. However, if any of the Conditions has not been fulfilled due to a particular obstacle, Acorda may, subject to
the consent of the FIN-FSA, extend the Offer Period beyond ten weeks until such obstacle has been removed and until all Conditions have been satisfied all in accordance with the terms and conditions of the Offer as agreed to and set forth in the
Combination Agreement. In no event is the Offeror required to extend the Tender Offer beyond June 19, 2016.
Acorda’s intent is to acquire 100%
of the Equity Interests of the Company. If at the completion of the Offer Period (including any and all extensions), the Minimum Acceptance Condition is satisfied or waived but Acorda does not own 100% of the Equity Interests, Acorda may seek to
acquire the remaining Equity Interests that were not acquired pursuant to the Offer by commencing a subsequent offer period (the “Subsequent Offer Period”) in accordance with the guidelines issued by the FIN-FSA and U.S. federal
securities laws and, if at completion of any Subsequent Offer Period or the Initial Offer Period Acorda does not own 100% of the Equity Interests, by entering into subsequent compulsory redemption proceedings (“Subsequent Compulsory
Redemption”) to redeem the remaining Shares (including Shares represented by ADSs) in accordance with the Finnish Companies Act or, in the case of the outstanding Equity Instruments, pursuant to the terms and conditions of such Equity
Instruments, or by using any other legally available alternative to reach 100% ownership of the Equity Interests.
Acorda has entered into irrevocable
undertakings (“Irrevocable Undertakings”) with certain of the Company’s equityholders including certain entities affiliated with each of Versant Ventures, The Baupost Group, Vivo Capital, OrbiMed, Invesco, Ilmarinen, Sitra and
Armistice, members of the Company’s senior management, one director of the Company (Mr. Bailey) and certain employees of the Company (collectively, the “Support Equityholders”) pursuant to which the Support Equityholders agreed
to tender and not withdraw all Equity Interests owned by such Support Equityholders into the Offer, subject to certain terms and conditions. The Support Equityholders collectively hold approximately 65% (on a fully diluted basis) of the outstanding
Shares and votes in Biotie.
Acorda’s intention is to cause the ADSs and the Shares to be delisted from Nasdaq US and Nasdaq Helsinki, respectively,
and deregistered under the United States Exchange Act of 1934 as soon as permitted and practicable under applicable laws and regulations following completion of the Offer.
Acorda did not hold any Shares or other Equity Interests in Biotie at the time of the announcement of the Offer
on January 19, 2016. The full terms and conditions of the Offer as well as further information about the Offer will be included in more detail in the Offer to Purchase.
Background for the statement of the Board of Directors
Pursuant to the Finnish Securities Market Act (Chapter 11, Section 13), the Board of Directors of Biotie has an obligation to prepare a public statement
regarding the Offer. The statement must include a well-founded assessment on the Offer from the perspective of Biotie and its shareholders as well as on the strategic plans and their likely effects on the operations of and employment in Biotie as
presented by Acorda in the Offer to Purchase.
For the purposes of issuing this statement, Acorda has submitted to the Board of Directors of Biotie a
draft version of the Offer to Purchase which Acorda has also filed with the Finnish Financial Supervisory Authority for approval on March 1, 2016.
Assessment of the Board of Directors from the perspective of Biotie and its shareholders
In evaluating the Combination Agreement and the Offer, the Board consulted with senior management of the Company, as well as Guggenheim Securities, Davis Polk
and Hannes Snellman. In the course of making the determination that the Offer is in the best interests of Biotie’s security holders and recommending that the security holders of the Company accept the Offer and tender their outstanding Shares,
ADSs and Equity Instruments, as applicable, pursuant to the Offer, the Board considered numerous factors, including the factors listed below, which are listed in no particular order of importance, each of which, in the view of the Board, supported
such determinations:
| |
• |
|
Financial Terms/Premium to Market Price. The Board considered the relationship of the consideration offered pursuant to the Offer to the historical market price of the Shares, including that the consideration
represents a premium of approximately 95% over the trading price at which the Shares closed on the Nasdaq Helsinki on January 18, 2016, the last trading day prior to the announcement of the entry into the Combination Agreement.
|
| |
• |
|
Cash Consideration. The Board considered the fact that the entire consideration will be payable in cash, which provides Company security holders with immediate liquidity and a high degree of certainty of value.
|
| |
• |
|
Product Development and Regulatory Risks. The Board considered the fact its product candidates tozadenant, SYN120 and BTT 1023 are in various phases of clinical development. The Company does not expect to have
top line efficacy data for its Phase 3 double-blind clinical trial (and extension) of tozadenant until the second half of 2017 and, as disclosed, will need to generate additional clinical safety data after that to be able to submit a new drug
application, or NDA, to the FDA. With respect to the Phase 2a trial of SYN120 in Parkinson’s dementia the Company expects to announce top-line data by the end of 2016. Enrolment for the Phase 2 clinical trial of BTT 1023 in PSC opened at the
end of the first quarter of 2015, and the requisite number of patients to enable interim data is expected by the end of 2016. Clinical trials are expensive and can take many years to complete, and the outcomes are inherently uncertain. The Board
considered the risks inherent in the development and eventual commercialization of its product candidates, the risks related to seeking approval for marketing from the FDA and EMA (including any potential conditions or contingencies of such
approvals) and the risks related to market acceptance of Company product candidates, if approved, and other factors affecting the revenues and profitability of biopharmaceutical products generally. |
| |
• |
|
Product Launch and Commercialization Risks. The Board also considered the risks and considerable costs as well as additional financing needs
associated with a successful launch and commercialization by the Company of its product candidates. The Board recognizes that Acorda has successfully commercialized products in the neurology field in the past and has a broad pipeline of future
product candidates. As such, Acorda will be able to launch any potential future products including tozadenant |
| |
and SYN120 without the need to build up a commercial infrastructure, which would be a significant cost for the Company if it were to commercialize the products on its own. |
| |
• |
|
The Company’s Operating and Financial Condition and Prospects. The Board is familiar with the current and historical financial condition and results of operations of the Company, as well as the prospects and
strategic objectives of the Company. Having considered different valuation methods, the Board believes, on the basis of this familiarity, that the consideration to be received by the Company’s shareholders, ADS holders and the holders of the
Equity Instruments in the Offer fairly reflects the Company’s intrinsic value. |
| |
• |
|
Strategic Alternatives. The Board has investigated and considered the trends in the markets and the industry and certain strategic alternatives available to the Company. Such alternatives include, but are not
limited to, remaining an independent public company (with resulting long-term capital needs for the already disclosed additional clinical safety study for tozadenant and the commercialization phase of tozadenant or for potential additional
investment in the Phase 2 products, such as SYN120, which could result in significant dilution to the shareholders of the Company), and partnering with others. The Board has also considered the risks and uncertainties associated with such
alternatives and the challenges associated with the industry’s current and expected competitive environment. The Board determined not to pursue those alternatives in light of its belief that the Offer maximized risk-adjusted shareholder value
and represented the best alternative reasonably available to shareholders. |
| |
• |
|
Market Check. The Board considered the fact that the Company and its advisors had conducted a strategic review conducted in 2014, during which forty companies were approached regarding a potential strategic
transaction or acquisition of the Company and that the process resulted in only one company submitting a non-binding offer, prior to the completion of significant due diligence, to acquire the Company for all-stock consideration with a value
implying no premium over the Company’s then-share price. Following the submission of the non-binding offer, that party did not engage with the Company to conduct due diligence or entertain further discussions surrounding terms of a potential
transaction and ultimately withdrew its offer in October 2014. The Board also considered the fact that, following Acorda’s indication of interest in acquiring the Company, the Company and its advisors had contacted six parties that the Company
believed to be most likely to be interested in a transaction with the Company in order to determine such parties’ interests in a potential transaction with the Company and that each of those parties ultimately declined to pursue a transaction
with the Company that would be superior to Acorda’s offer. |
| |
• |
|
Likelihood of Consummation. The Board considered that the Offer would reasonably likely be consummated in light of the facts that (i) Acorda has the financial ability and willingness to consummate the Offer,
(ii) the Offer is not subject to any financing condition and (iii) the other conditions to the Offer are reasonable and customary. |
| |
• |
|
Speed of Completion. The Board considered the anticipated timing of the consummation of the Offer, and the structure of the transaction as a tender offer for the outstanding Shares, ADSs and Equity Instruments,
which, subject to the satisfaction or waiver of the applicable conditions set forth in the Combination Agreement, should allow Company security holders to receive the consideration for their Shares, ADSs and Equity Instruments in a relatively short
timeframe. The Board considered that the potential for closing the Offer in a relatively short timeframe could also reduce the amount of time in which the Company’s business would be subject to the potential disruption and uncertainty pending
closing. |
| |
• |
|
Ability to Respond to Third-Party Takeover Proposals. The Board considered the terms and conditions of the Combination Agreement related to the Company’s ability to respond to third parties making takeover
proposals under certain circumstances, including: |
| |
• |
|
the right of the Company, under certain circumstances and subject to certain conditions, to furnish non-public information to, and to participate in discussions with, third parties in response to certain written
proposals relating to alternative acquisition transactions; and |
| |
• |
|
the right of the Board, under certain circumstances and subject to certain conditions, to withdraw or change its recommendation in favor of the Offer if the failure to do so would be inconsistent with its fiduciary
duties and to terminate the Combination Agreement in order to enter into a written definitive agreement providing for an alternative acquisition transaction. |
| |
• |
|
Terms of the Offer. The Board considered the terms and conditions of the Offer and the Combination Agreement. In addition, the Board viewed as desirable provisions in the Combination Agreement that prohibit
Acorda from changing the terms of the Offer, without the consent of the Company, in a manner that (i) decreases the Offer consideration, (ii) changes the form of consideration to be paid in the Offer, (iii) decreases the number of
Shares, ADSs or outstanding Equity Instruments sought in the Offer, (iv) extends or otherwise changes the expiration date of the Offer (except for the Subsequent Offer Period or as otherwise provided in the Combination Agreement),
(v) imposes additional conditions to the Offer or (vi) otherwise amends, modifies or supplements the conditions to the Offer or terms and conditions of the Offer to the detriment in any material respect to the holders of Equity Interests.
|
| |
• |
|
Guggenheim Securities’ Opinion. The Board considered the opinion of Guggenheim Securities, dated January 19, 2016, to the Board as to the fairness, from a financial point of view and as of such date, of
the EUR 0.2946 per Share consideration to be received in the Offer by holders of Shares and ADSs (other than Acorda and its affiliates). The full text of Guggenheim Securities’ written opinion, which is attached as Appendix 1 to this
statement and which you should read carefully and in its entirety, is subject to the assumptions, limitations, qualifications and other conditions contained in such opinion and is necessarily based on economic, capital markets and other conditions,
and the information made available to Guggenheim Securities, as of the date of such opinion. |
Guggenheim Securities’
opinion was provided to the Board (in its capacity as such) for its information and assistance in connection with its evaluation of the Share consideration from a financial point of view, did not constitute a recommendation to the Board with respect
to the Offer and does not constitute advice or a recommendation to any security holder of the Company as to whether to tender any securities pursuant to the Offer or how to act with respect to the Offer or any other matter. Guggenheim
Securities’ opinion did not address the Company’s underlying business or financial decision to pursue the Offer, the relative merits of the Offer as compared to any alternative business or financial strategies that might exist for the
Company or the effects of any other transaction in which the Company might engage. Guggenheim Securities’ opinion addressed only the fairness, from a financial point of view, of the Share consideration to holders of Shares and ADSs (other than
Acorda and its affiliates) to the extent expressly specified therein and did not address any other term, aspect or implication of the Offer or the Combination Agreement, including, without limitation, the form or structure of the Offer, any
consideration payable in respect, or any tender, exercise, redemption, conversion, rollover or assumption, of other securities (including warrants, options and other equity-based grants) of the Company or any term, aspect or implication of any
tender agreement or any other agreement, transaction document or instrument contemplated by the Combination Agreement or otherwise or to be entered into or amended in connection with the Offer.
| |
• |
|
Irrevocable Undertakings. The Board considered that certain shareholders of the Company, solely in their capacities as shareholders, are supportive of the transaction and have agreed, pursuant to and subject to
the conditions of the Irrevocable Undertakings, to tender their Equity Interests, representing approximately 65% (on a fully diluted basis) of the Shares and votes in Biotie as of January 14, 2016, the second to last trading day prior to
announcement of the transaction, into the Offer, subject to the terms and conditions of the Irrevocable Undertakings. |
The Board also
considered a number of uncertainties and risks in its deliberations concerning the transactions contemplated by the Combination Agreement, including the Offer, including:
| |
• |
|
No Ongoing Equity Interest in the Company. The Board considered the fact that the shareholders of the Company will have no ongoing equity interest in the Company going forward, meaning that the shareholders will
cease to participate in the Company’s future potential growth or to benefit from potential increases in the value of the Shares. |
| |
• |
|
Inability to Solicit Other Takeover Proposals. The Board considered the covenant in the Combination Agreement prohibiting the Company from further soliciting other potential acquisition proposals, and restricting
its ability to entertain other potential acquisition proposals unless certain conditions are satisfied. |
| |
• |
|
Termination Fee and Expenses. The Board considered the fact that that the Company would be obligated to pay a termination fee of $4,500,000 as compensation for Acorda’s reasonable transaction costs if the
Combination Agreement is terminated under certain circumstances, including to accept a superior proposal, and that the amount of the termination fee was reasonable, would not likely deter competing bids and would not likely be required to be paid
unless the Company entered into a more favorable transaction. |
| |
• |
|
Failure to Close. The Board considered that the conditions to Acorda’s obligation to accept for payment and pay for the Equity Interests tendered pursuant to the Offer were subject to conditions, and the
possibility that such conditions may not be satisfied, including as a result of events outside of the Company’s control. The Board considered the fact that, if the Offer is not consummated, the Company’s directors and other employees will
have expended extensive time and effort and will have experienced significant distractions from their work during the pendency of the transaction, and the Company will have incurred significant transaction costs attempting to consummate the
transaction. The Board also considered the fact that, if the Offer is not completed, the market’s perception of the Company’s continuing business could potentially result in a loss of vendors, business partners, collaboration partners and
employees and that the trading price of the Shares and ADSs could be adversely affected. |
| |
• |
|
Interim Operating Covenants. The Board considered that, under the terms of the Combination Agreement, the Company has agreed that it will carry on its business in the ordinary course consistent with past practice
and, subject to specified exceptions, that the Company will not take a number of actions related to the conduct of its business without the prior written consent of Acorda. The Board further considered that these terms may limit the ability of the
Company to pursue business opportunities that it would otherwise pursue. |
| |
• |
|
Effect of Announcement. The Board considered the effect of the public announcement of the transaction on the Company’s operations, Share and ADS price and employees, as well as its ability to attract and
retain key personnel while the transaction is pending. |
| |
• |
|
No Reverse Termination Fee. The Board considered the fact that Acorda will be able to terminate the Combination Agreement under certain circumstances that may be outside of the Company’s control, without the
payment of any reverse termination fee to the Company. |
| |
• |
|
Interests of the Board. The Board considered the potential conflict of interest created by the fact that the Company’s directors have financial interests in the transactions contemplated by the Combination
Agreement, including the Offer, that may be different from or in addition to those of other security holders. |
| |
• |
|
Transaction Costs. The Board considered the fact that the Company has and will continue to incur significant transaction costs and expenses in connection with the proposed transaction, regardless of whether or
not such transaction is consummated. |
The discussion of factors considered by the Board described above is not intended to be exhaustive;
rather it summarizes the material factors considered. Due to the variety of factors and the quality and amount of information considered, the Board did not find it practicable to, and did not make specific assessments to, quantify or assign relative
weights to the specific factors considered in (i) making the determination that the Combination Agreement and the transactions contemplated thereby were fair to and in the best interests of the Company’s shareholders, (ii) approving,
adopting and declaring advisable the Combination Agreement and the transactions contemplated thereby and (iii) recommending that the holders of Equity Interests accept the Offer and tender their Equity Interests pursuant to the Offer. Instead,
the Board made its determination after consideration of all factors taken together. In addition, individual members of the Board may have given different weight to different factors.
Strategic Plans of the Offeror and Their Likely Effects on Operations and Employment
According to Acorda, the business of Biotie will eventually be integrated into the business of Acorda. Acorda has stated that the final and longer-term impact
of the integration can be assessed only after the completion of the Tender Offer. Acorda expects that the acquisition of Biotie will complement its portfolio of finished or development products in the field of neurology.
Acorda states in the Offer to Purchase that the Offer will have no immediate material near term effect on the operations, and business locations of, or
employment at Biotie. Acorda intends to keep Biotie’s South San Francisco open and to maintain its operations in full. In the near term, Acorda expects that the completion of the Tender Offer will not have any immediate major impact on
Biotie’s Turku operations and employees. Acorda will consider the longer-term operating plan and scope of the Turku operations following the completion of the Tender Offer.
Based on the information provided by the Offeror, the Biotie Board believes that the strategic plans of the Offeror pursuant to the Offer would not generally
have a significant effect on the business or operations of Biotie. The Biotie Board notes that the Offer may have an effect on employment in the Company with regard to duplicative functions. Based on the statements of Acorda, the possible impact of
the planned arrangements on the status of the management and employees of Biotie will be assessed in connection with the integration that the Offeror plans to effect after the completion of the Offer.
In preparing its statement, the Board of Directors of Biotie has relied on information provided in the draft Offer to Purchase by Acorda and has not
independently verified this information.
Financing of the Offer
According to information provided by the Offeror, the Offeror intends to finance the Offer through cash on its balance sheet, which includes the proceeds of a
private placement to a banking institution of USD 75.0 million of the Offeror’s shares that was executed concurrently with the execution of the Combination Agreement and settled on January 26, 2016. The Offeror has stated that the
Offer is not conditional upon obtaining any external financing for the Offer.
The Recommendation of the Board of Directors of Biotie
The Board of Directors of Biotie has conducted a number of meetings and carefully evaluated the Offer and its terms and conditions based on the draft Offer to
Purchase and other available information.
The Board of Directors of Biotie believes that the consideration offered by Acorda for the ADSs, the Shares and
the Equity Instruments is fair to the holders of such securities.
Based on the above factors, the Board of Directors of Biotie has decided, by unanimous
decision, to recommend that the holders of the ADSs, the Shares and the Equity Instruments accept the Offer and tender their Equity Interests pursuant to the Offer.
This statement is based on an assessment of the issues and factors which the Board of Directors has concluded to be material in evaluating the Offer,
including, but not limited to, the information and assumptions on the business operations and finances of Biotie at the date of this statement and their expected future development.
The Board of Directors of Biotie notes that the holders of the ADSs, the Shares and the Equity Instruments should also take into account the risks related to
non-acceptance of the Offer. The completion of the Offer reduces the number of Biotie shareholders and the number of ADSs and Shares which would otherwise be publicly traded. Depending on the number of ADSs and Shares validly tendered in the Offer,
this could have an adverse effect on the liquidity and value of the ADSs and Shares.
Other Matters
Board member Mr. Don M. Bailey, ViVo Capital, the venture partner of which is Board member Dr. Mahendra G. Shah, and Versant Ventures,
where Board member Dr. Guido Magni is partner, representing in total approximately 27% of the outstanding shares and votes in Biotie (on a fully diluted basis), have entered into Irrevocable Undertakings to accept Acorda’s Offer. Mr
Bailey, ViVo Capital and Versant Euro Ventures entered into the Irrevocable Undertakings after Biotie’s Board of Directors approved the execution of of the Combination Agreement on January 19, 2016. Board members Mr. Bailey,
Dr. Shah and Dr. Magni have not participated in the giving of this statement of the Biotie Board. In matters related to the Offer, Biotie has committed itself to complying with the Helsinki Takeover Code (Finnish: Ostotarjouskoodi)
referred to in Chapter 11, Section 28 of the Finnish Securities Markets Act.
This statement of the Biotie Board does not constitute investment or
tax advice, and the Biotie Board does not specifically evaluate herein the general price development or the risks relating to the Shares in general. Shareholders must independently decide whether to accept the Offer, and they should take into
account all relevant information available to them, including information presented in the Offer to Purchase and this statement.
Pursuant to Chapter 18
of the Finnish Companies Act (624/2006, as amended), a shareholder with more than 90% of all shares and votes in a company shall have the right to acquire, and subject to a demand by the other shareholders also be obligated to redeem, the shares
owned by the other shareholders. Provided Acorda acquires such amount of Shares, the Shares held by Biotie’s security holders who have not accepted the Offer and the Shares underlying ADSs held by holders of ADSs who have not accepted the Offer
may be redeemed through compulsory redemption proceedings under the Finnish Companies Act under the conditions set out therein.
Guggenheim Securities,
LLC, has acted as the financial adviser to Biotie with respect to the Offer, while Davis Polk & Wardwell LLP has acted as legal counsel with respect to U.S. law and Hannes Snellman Attorneys Ltd has acted as legal counsel with respect to
Finnish law.
Helsinki, 4 March 2016
The Board of
Directors of Biotie
For more information, please contact:
William M. Burns, Chairman of the Board of Directors
Contact
requests:
Virve Nurmi, Biotie Therapies Corp.
tel. +358 2
274 8900, e-mail: virve.nurmi@biotie.com
DISTRIBUTION
www.biotie.com
Nasdaq Helsinki Ltd
NASDAQ Stock Exchange
Major media
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
SOME OF THE STATEMENTS CONTAINED IN THIS RELEASE ARE FORWARD-LOOKING STATEMENTS, INCLUDING STATEMENTS REGARDING THE EXPECTED CONSUMMATION OF THE ACQUISITION,
WHICH INVOLVES A NUMBER OF RISKS AND UNCERTAINTIES, INCLUDING THE SATISFACTION OF CLOSING CONDITIONS FOR THE ACQUISITION, SUCH AS THE TENDER OF AT LEAST 90% OF THE OUTSTANDING SHARES AND VOTING RIGHTS OF THE COMPANY, THE POSSIBILITY THAT THE
TRANSACTION WILL NOT BE COMPLETED AND OTHER RISKS AND UNCERTAINTIES DISCUSSED IN THE COMPANY’S PUBLIC FILINGS WITH THE SEC, INCLUDING
THE “RISK FACTORS” SECTION OF THE COMPANY’S REGISTRATION STATEMENT ON FORM F-1 (NO. 333-204147), AS WELL AS THE TENDER OFFER DOCUMENTS TO BE FILED BY ACORDA AND THE
SOLICITATION/RECOMMENDATION STATEMENT TO BE FILED BY THE COMPANY. THESE STATEMENTS ARE BASED ON CURRENT EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS, AND INVOLVE KNOWN AND UNKNOWN RISKS, UNCERTAINTIES AND OTHER FACTORS THAT MAY CAUSE
RESULTS, LEVELS OF ACTIVITY, PERFORMANCE OR ACHIEVEMENTS TO BE MATERIALLY DIFFERENT FROM ANY FUTURE STATEMENTS. THESE STATEMENTS ARE GENERALLY IDENTIFIED BY WORDS OR PHRASES SUCH AS “BELIEVE”, “ANTICIPATE”, “EXPECT”,
“INTEND”, “PLAN”, “WILL”, “MAY”, “SHOULD”, “ESTIMATE”, “PREDICT”, “POTENTIAL”, “CONTINUE” OR THE NEGATIVE OF SUCH TERMS OR OTHER SIMILAR EXPRESSIONS. IF
UNDERLYING ASSUMPTIONS PROVE INACCURATE OR UNKNOWN RISKS OR UNCERTAINTIES MATERIALIZE, ACTUAL RESULTS AND THE TIMING OF EVENTS MAY DIFFER MATERIALLY FROM THE EXPECTED RESULTS AND/OR TIMING DISCUSSED IN THE FORWARD-LOOKING STATEMENTS, AND YOU SHOULD
NOT PLACE UNDUE RELIANCE ON THESE STATEMENTS. THE COMPANY DISCLAIMS ANY INTENT OR OBLIGATION TO UPDATE ANY FORWARD-LOOKING STATEMENTS AS A RESULT OF DEVELOPMENTS OCCURRING AFTER THE PERIOD COVERED BY THIS RELEASE OR OTHERWISE.
ADDITIONAL INFORMATION AND WHERE TO FIND IT
THE OFFER
HAS NOT BEEN COMMENCED. THIS RELEASE IS FOR INFORMATIONAL PURPOSES ONLY AND DOES NOT CONSTITUTE AN OFFER TO PURCHASE OR A SOLICITATION OF AN OFFER TO SELL COMPANY SECURITIES. THE SOLICITATION AND OFFER TO BUY COMPANY SECURITIES WILL ONLY BE MADE
PURSUANT TO AN OFFER TO PURCHASE AND RELATED MATERIALS. AT THE TIME THE OFFER IS COMMENCED, ACORDA WILL FILE A TENDER OFFER STATEMENT ON SCHEDULE TO WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION (THE “SEC”) AND THEREAFTER, THE COMPANY
WILL FILE A SOLICITATION/RECOMMENDATION STATEMENT ON SCHEDULE 14D-9 WITH RESPECT TO THE OFFER. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THESE MATERIALS CAREFULLY WHEN THEY BECOME AVAILABLE SINCE THEY WILL CONTAIN IMPORTANT INFORMATION,
INCLUDING THE TERMS AND CONDITIONS OF THE OFFER. THE OFFER TO PURCHASE, SOLICITATION/RECOMMENDATION STATEMENT AND RELATED MATERIALS WILL BE FILED BY ACORDA AND THE COMPANY WITH THE SEC, AND INVESTORS AND SECURITY HOLDERS MAY OBTAIN A FREE COPY OF
THESE MATERIALS (WHEN AVAILABLE) AND OTHER DOCUMENTS FILED BY ACORDA AND THE COMPANY WITH THE SEC AT THE WEBSITE MAINTAINED BY THE SEC AT WWW.SEC.GOV. INVESTORS AND SECURITY HOLDERS MAY ALSO OBTAIN FREE COPIES OF THE SOLICITATION/RECOMMENDATION
STATEMENT AND OTHER DOCUMENTS FILED WITH THE SEC BY THE COMPANY AT WWW.BIOTIE.COM.
Attachment: Guggenheim Securities’ Opinion
January 19, 2016
The Board of Directors Biotie Therapies
Corp.
Joukahaisenkatu 6, FI-20520
Turku, Finland
Members of the Board:
We understand that Biotie Therapies
Corp. (“Biotie”) and Acorda Therapeutics, Inc. (“Acorda”) intend to enter into a Combination Agreement (the “Agreement”), pursuant to which Acorda will commence a tender offer (the “Tender Offer”) to purchase
all outstanding ordinary shares, no nominal value, of Biotie (“Biotie Ordinary Shares”) and all outstanding American Depositary Shares, each representing 80 Biotie Ordinary Shares (“Biotie ADSs” and, together with Biotie Ordinary
Shares, “Biotie Shares”), at a purchase price of €0.2946 per Biotie Ordinary Share in cash (the “Offer Price”). The terms and conditions of the Tender Offer are more fully set forth in the Agreement.
You have asked us to render our opinion as to whether the Offer Price to be received in the Tender Offer by holders of Biotie Shares (other than Acorda and
its affiliates) pursuant to the Agreement is fair, from a financial point of view, to such holders.
In the course of performing our reviews and analyses
for rendering our opinion, we have:
| |
• |
|
Reviewed a draft of the Agreement dated as of January 18, 2016; |
| |
• |
|
Reviewed certain publicly available business and financial information regarding Biotie; |
| |
• |
|
Reviewed certain non-public business and financial information regarding Biotie’s businesses and prospects (including certain unadjusted and probability-adjusted financial projections relating to Biotie and
estimates as to potentially realizable existing net operating loss carryforwards expected to be utilized by Biotie), all as prepared and provided to us by Biotie’s senior management; |
| |
• |
|
Discussed with Biotie’s senior management their views of Biotie’s businesses, operations, historical and projected financial results and future prospects; |
| |
• |
|
Reviewed the historical prices of Biotie Shares; |
| |
• |
|
Performed discounted cash flow analyses based on the probability-adjusted financial projections for Biotie and the estimates as to potentially realizable existing net operating loss carryforwards expected to be utilized
by Biotie, in each case as furnished to us by Biotie; |
| |
• |
|
Reviewed implied transaction values and financial metrics of certain mergers and acquisitions that we deemed relevant in evaluating the Tender Offer; |
| |
• |
|
Reviewed implied enterprise values of certain publicly traded companies that we deemed relevant in evaluating Biotie; and |
| |
• |
|
Conducted such other studies, analyses, inquiries and investigations as we deemed appropriate. |
With respect to the information used in arriving at our opinion:
| |
• |
|
We have relied upon and assumed the accuracy, completeness and reasonableness of all industry, business, financial, legal, regulatory, tax, accounting, actuarial and other information (including, without limitation, any
financial projections, estimates as to potentially realizable existing net operating loss carryforwards expected to be utilized by Biotie, other estimates and other forward-looking information) furnished by or discussed with Biotie or obtained from
public sources, data suppliers and other third parties. |
| |
• |
|
We (i) do not assume any responsibility, obligation or liability for the accuracy, completeness, reasonableness, achievability or independent verification of, and we have not independently verified, any such
information (including, without limitation, any financial projections, estimates as to potentially realizable existing net operating loss carryforwards expected to be utilized by Biotie, other estimates and other forward-looking information),
(ii) express no view, opinion, representation or warranty (in each case, express or implied) regarding the (a) reasonableness of operating loss carryforwards expected to be utilized by Biotie, other estimates and other forward-looking
information or the assumptions upon which they are based or (b) probability adjustments included in such financial projections and (iii) have relied upon the assurances of Biotie’s senior management that they are unaware of any facts
or circumstances that would make such information (including, without limitation, any financial projections, estimates as to potentially realizable existing net operating loss carryforwards expected to be utilized by Biotie, other estimates and
other forward-looking information) incomplete, inaccurate or misleading. |
| |
• |
|
Specifically, with respect to any (i) financial projections, estimates as to potentially realizable existing net operating loss carryforwards expected to be utilized by Biotie, other estimates and other
forward-looking information furnished by or discussed with Biotie, (a) we have been advised by Biotie’s senior management, and we have assumed, that such financial projections, estimates as to potentially realizable existing net operating
loss carryforwards expected to be utilized by Biotie, other estimates and other forward-looking information utilized in our analyses have been reasonably prepared on bases reflecting the best currently available estimates and judgments of
Biotie’s senior management as to the expected future performance of Biotie and (b) we have assumed that such financial projections, estimates as to potentially realizable existing net operating loss carryforwards expected to be utilized by
Biotie, other estimates and other forward-looking information have been reviewed by Biotie’s Board of Directors with the understanding that such information will be used and relied upon by us in connection with rendering our opinion and
(ii) financial projections, other estimates and/or other forward-looking information obtained by us from public sources, data suppliers and other third parties, we have assumed that such information is reasonable and reliable. We have been
advised by Biotie’s Board of Directors and senior management, based on their assessments as to the relative likelihood of achieving the future financial results for Biotie as reflected in the unadjusted and probability-adjusted financial
projections relating to Biotie, to rely for purposes of our analyses and opinion on such probability-adjusted financial projections, as applicable. |
| |
• |
|
We have relied upon, without independent verification, the assessments of Biotie’s senior management as to, among other things, (i) the potential impact on Biotie of market and other trends in and prospects
for, and governmental, regulatory and legislative matters relating to or affecting, the biotechnology, life sciences and pharmaceutical sectors in which Biotie operates, (ii) Biotie’s existing and future products and product candidates,
including the validity of, and risks associated with, such products, product candidates and intellectual property, and (iii) the probabilities of successful commercialization of, and peak worldwide sales attributable to, such products and
product candidates (including, without limitation, the timing and probabilities of successful development, testing, manufacturing and marketing thereof; approval thereof by relevant governmental authorities; prospective product-related sales prices,
annual sales price increases and volumes with respect thereto; the validity and life of patents with respect thereto; and the potential impact of competition thereon). We have assumed that there will not be any developments with respect to any such
matters that would be meaningful in any respect to our analyses or opinion. |
| |
• |
|
We have utilized a publicly available Euro to United States Dollar exchange rate, and we have assumed that such exchange rate is reasonable to utilize for purposes of our analyses and that any currency or exchange rate
fluctuations will not be meaningful in any respect to our analyses or opinion. We have not assessed or considered, for purposes of our analyses and opinion, foreign currency exchange risks associated with the Tender Offer. |
During the course of our engagement, we were asked by Biotie’s Board of Directors to solicit indications of interest from various third parties regarding
a potential transaction with Biotie, and we have considered the results of such solicitation process in rendering our opinion.
In arriving at our
opinion, we have not performed or obtained any independent appraisal of the assets or liabilities (including any contingent, derivative or off-balance sheet assets and liabilities) of Biotie or any other entity or the solvency or fair value of
Biotie or any other entity, nor have we been furnished with any such appraisals. We are not expressing any view or rendering any opinion regarding the tax consequences to Biotie or holders of Biotie Shares or any other securities of the Tender
Offer. We are not legal, regulatory, tax, consulting, accounting, appraisal or actuarial experts and nothing in our opinion should be construed as constituting advice with respect to such matters; accordingly, we have relied on the assessments of
Biotie and its other advisors with respect to such matters.
In rendering our opinion, we have assumed that, in all respects material to our analyses,
(i) the final executed form of the Agreement will not differ from the draft that we have reviewed, (ii) Biotie and Acorda will comply with all terms of the Agreement and (iii) the representations and warranties of Biotie and Acorda
contained in the Agreement are true and correct and all conditions to the obligations of each party to the Agreement to consummate the Tender Offer will be satisfied without any waiver thereof. We also have assumed that the Tender Offer will be
consummated in a timely manner, in accordance with the terms of the Agreement and in compliance with all applicable laws, documents and other requirements, without any limitations, restrictions, conditions, waivers, amendments or modifications
(regulatory, tax-related or otherwise) that would be meaningful in any respect to our analyses or opinion.
In rendering our opinion, we do not express
any view or opinion as to the price or range of prices at which Biotie Shares or other securities of Biotie may trade or otherwise be transferable at any time, including subsequent to the announcement or consummation of the Tender Offer. We have
acted as a financial advisor to Biotie in connection with the Tender Offer and will receive a customary fee for such services, a substantial portion of which is contingent on successful consummation of the Tender Offer and a portion of which is
payable upon delivery of our opinion and will be credited against the fee payable upon consummation of the Tender Offer. In addition, Biotie has agreed to reimburse us for certain expenses and to indemnify us against certain liabilities arising out
of our engagement.
Guggenheim Securities, LLC (“Guggenheim Securities”) has been previously engaged during the past three years and is
currently engaged by Biotie to provide certain financial advisory or investment banking services in connection with matters unrelated to the Tender Offer, for which we have received (or expect to receive) customary fees. Specifically during the past
three years, as Biotie’s Board of Directors is aware, Guggenheim Securities has acted as financial advisor to Biotie in connection with various acquisition and disposition activities, partnership arrangements and other general advisory matters.
Guggenheim Securities has not provided financial advisory or investment banking services during the past three years to Acorda for which Guggenheim Securities has received fees. Guggenheim Securities may seek to provide Biotie, Acorda and their
respective affiliates with certain financial advisory and investment banking services unrelated to the Tender Offer in the future.
Guggenheim Securities
and its affiliates and related entities engage in a wide range of financial services activities for our and their own accounts and the accounts of our and their customers, including: asset, investment and wealth management; investment banking,
corporate finance, mergers and acquisitions and restructuring; merchant banking; fixed income and equity sales, trading and research; and derivatives, foreign exchange and futures. In the ordinary course of these activities, Guggenheim Securities or
its affiliates and related entities may (i) provide such financial services to Biotie, Acorda, other participants in the Tender Offer or their respective affiliates, subsidiaries, investment funds and portfolio companies, for which services
Guggenheim Securities or
its affiliates and related entities has received, and may receive, compensation and (ii) directly or indirectly, hold long or short positions, trade and otherwise conduct such activities in
or with respect to certain bank debt, debt or equity securities and derivative products of or relating to Biotie, Acorda, other participants in the Tender Offer or their respective affiliates, subsidiaries, investment funds and portfolio companies.
Furthermore, Guggenheim Securities or its affiliates and related entities and our or their directors, officers, employees, consultants and agents may have investments in Biotie, Acorda, other participants in the Tender Offer or their respective
affiliates, subsidiaries, investment funds and portfolio companies.
Consistent with applicable legal and regulatory guidelines, Guggenheim Securities has
adopted certain policies and procedures to establish and maintain the independence of its research departments and personnel. As a result, Guggenheim Securities’ research analysts may hold views, make statements or investment recommendations
and publish research reports with respect to Biotie, Acorda, other participants in the Tender Offer or their respective affiliates, subsidiaries, investment funds and portfolio companies and the Tender Offer that differ from the views of Guggenheim
Securities’ investment banking personnel.
Our opinion has been provided to Biotie’s Board of Directors (in its capacity as such) for its
information and assistance in connection with its evaluation of the Offer Price. Our opinion may not be disclosed publicly, made available to third parties or reproduced, disseminated, quoted from or referred to at any time, in whole or in part,
without our prior written consent; provided, however, that this letter may be included in its entirety in any Tender Offer Solicitation/Recommendation Statement on Schedule 14D-9 required to be distributed to the holders of Biotie Shares in
connection with the Tender Offer pursuant to the United States Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder, and in any Tender Offer statement required to be distributed to the holders of Biotie
Shares in connection with the Tender Offer pursuant to the Finnish Securities Market Act.
Our opinion and any materials provided in connection therewith
do not constitute a recommendation to Biotie’s Board of Directors with respect to the Tender Offer, nor does our opinion constitute advice or a recommendation to any security holder of Biotie as to whether to tender any securities pursuant to
the Tender Offer or how any such security holder should act with respect to the Tender Offer or any other matter. Our opinion does not address Biotie’s underlying business or financial decision to pursue the Tender Offer, the relative merits of
the Tender Offer as compared to any alternative business or financial strategies that might exist for Biotie or the effects of any other transaction in which Biotie might engage. Our opinion addresses only the fairness, from a financial point of
view, of the Offer Price to holders of Biotie Shares (other than Acorda and its affiliates) to the extent expressly specified herein. We do not express any view or opinion as to any other term, aspect or implication of the Tender Offer or the
Agreement, including, without limitation, the form or structure of the Tender Offer, any consideration payable in respect, or any tender, exercise, redemption, conversion, rollover or assumption, of other securities (including warrants, options and
other equity-based grants) of Biotie or any term, aspect or implication of any tender agreement or any other agreement, transaction document or instrument contemplated by the Agreement or otherwise or to be entered into or amended in connection with
the Tender Offer, or the fairness, financial or otherwise, of the Tender Offer to, or of any consideration to be paid to or received by, the holders of any class of securities (other than the fairness, from a financial point of view, of the Offer
Price to holders of Biotie Shares (other than Acorda and its affiliates) to the extent expressly specified herein), creditors or other constituencies of Biotie. Furthermore, we do not express any view or opinion as to the fairness, financial or
otherwise, of the amount or nature of any compensation payable to or to be received by any of Biotie’s or Acorda’s directors, officers or employees, or any class of such persons, in connection with the Tender Offer relative to the Offer
Price or otherwise. Our opinion does not address the individual circumstances of specific holders with respect to rights or aspects which may distinguish such holders or the securities of Biotie held by such holders and our opinion does not address,
take into consideration or give effect to any rights, preferences, restrictions or limitations or other attributes of any such securities nor does our opinion in any way address proportionate allocation or relative fairness. We have assumed that
each outstanding Biotie ADS has a value equivalent to 80 Biotie Ordinary Shares.
Our opinion has been authorized for issuance by the Fairness Opinion and
Valuation Committee of Guggenheim Securities. Our opinion is subject to the assumptions, limitations, qualifications and other conditions contained herein and is necessarily based on economic, capital markets and other conditions, and the
information made
available to us, as of the date hereof. As Biotie is aware, the global capital markets have been experiencing and remain subject to volatility, and Guggenheim Securities expresses no view or
opinion as to any potential effects of such volatility on Biotie or the Tender Offer. We assume no responsibility for updating or revising our opinion based on facts, circumstances or events occurring after the date hereof.
Based on and subject to the foregoing, it is our opinion that, as of the date hereof, the Offer Price to be received in the Tender Offer by holders of Biotie
Shares (other than Acorda and its affiliates) pursuant to the Agreement is fair, from a financial point of view, to such holders.
Very truly yours,
GUGGENHEIM SECURITIES, LLC
Annex B
INDEX TO FINANCIAL STATEMENTS
B-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Biotie Therapies Oyj
In our opinion, the accompanying consolidated statements of financial position and the related consolidated statements of comprehensive
income, of changes in shareholders’ equity and of cash flows present fairly, in all material respects, the financial position of Biotie Therapies Oyj and its subsidiaries at December 31, 2014 and 2013, and the results of their operations
and their cash flows for each of the two years in the period ended December 31, 2014 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board. These financial statements are the
responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test
basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that
our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers Oy
Helsinki, Finland
March 17, 2015
B-2
Biotie Therapies Oyj
Consolidated Statements of Comprehensive Income
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
For the year ended
December 31, |
|
| (€ in thousands, except per share data) |
|
Note |
|
|
2014 |
|
|
2013 |
|
| Revenue |
|
|
3 |
|
|
|
14,901 |
|
|
|
27,712 |
|
| Research and development expenses |
|
|
4, 5, 6 |
|
|
|
(17,192 |
) |
|
|
(17,807 |
) |
| Impairment of in-process research and development assets |
|
|
11 |
|
|
|
(27,605 |
) |
|
|
— |
|
| General and administrative expenses |
|
|
5, 6 |
|
|
|
(7,326 |
) |
|
|
(8,971 |
) |
| Other operating income |
|
|
7 |
|
|
|
1,132 |
|
|
|
565 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating (loss) income |
|
|
|
|
|
|
(36,090 |
) |
|
|
1,499 |
|
| Interest income |
|
|
8 |
|
|
|
— |
|
|
|
37 |
|
| Interest expenses |
|
|
8 |
|
|
|
(687 |
) |
|
|
(726 |
) |
| Other net financial income (expenses) |
|
|
8 |
|
|
|
1,612 |
|
|
|
2,841 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (Loss) income before taxes |
|
|
|
|
|
|
(35,165 |
) |
|
|
3,651 |
|
| Income tax benefit |
|
|
9, 24 |
|
|
|
— |
|
|
|
2,195 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net (loss) income |
|
|
|
|
|
|
(35,165 |
) |
|
|
5,846 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
|
|
|
| Items that will not be reclassified to profit and loss: |
|
|
|
|
|
|
|
|
|
|
|
|
| Remeasurements of post-employment benefit obligations |
|
|
18, 21 |
|
|
|
(81 |
) |
|
|
— |
|
| Items that may be subsequently reclassified to profit and loss: |
|
|
|
|
|
|
|
|
|
|
|
|
| Currency translation differences |
|
|
18 |
|
|
|
6,593 |
|
|
|
(2,629 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total other comprehensive income (loss) |
|
|
|
|
|
|
6,512 |
|
|
|
(2,629 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total comprehensive (loss) income |
|
|
|
|
|
|
(28,653 |
) |
|
|
3,217 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net (loss) income attributable to equity holders of the parent |
|
|
|
|
|
|
(35,165 |
) |
|
|
5,846 |
|
| Total comprehensive (loss) income attributable to equity holders of the parent |
|
|
|
|
|
|
(28,653 |
) |
|
|
3,217 |
|
| (Loss) earnings per share (EPS) basic & diluted, €
|
|
|
10 |
|
|
|
(0.08 |
) |
|
|
0.01 |
|
All activities relate to continuing operations.
The accompanying notes form an integral part of the consolidated financial statements.
B-3
Biotie Therapies Oyj
Consolidated Statements of Financial Position
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
As at December 31, |
|
| (€ in thousands) |
|
Note |
|
|
2014 |
|
|
2013 |
|
| ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-current assets |
|
|
|
|
|
|
|
|
|
|
|
|
| Intangible assets |
|
|
11 |
|
|
|
47,356 |
|
|
|
68,744 |
|
| Goodwill |
|
|
11 |
|
|
|
5,799 |
|
|
|
5,315 |
|
| Property, plant and equipment |
|
|
12 |
|
|
|
653 |
|
|
|
627 |
|
| Investment property |
|
|
13 |
|
|
|
— |
|
|
|
817 |
|
| Other financial assets |
|
|
14 |
|
|
|
324 |
|
|
|
242 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total non-current assets |
|
|
|
|
|
|
54,132 |
|
|
|
75,745 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current assets |
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts receivables and other receivables |
|
|
15 |
|
|
|
1,806 |
|
|
|
575 |
|
| Financial assets at fair value through profit or loss |
|
|
16 |
|
|
|
24,941 |
|
|
|
33,457 |
|
| Cash and cash equivalents |
|
|
17 |
|
|
|
7,452 |
|
|
|
10,221 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
|
|
|
|
34,199 |
|
|
|
44,253 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
|
|
|
|
|
88,331 |
|
|
|
119,998 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| EQUITY AND LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
| Shareholders’ equity |
|
|
|
|
|
|
|
|
|
|
|
|
| Share capital |
|
|
18 |
|
|
|
193,285 |
|
|
|
193,285 |
|
| Reserve for invested unrestricted equity |
|
|
18 |
|
|
|
5,378 |
|
|
|
5,252 |
|
| Other reserves |
|
|
18 |
|
|
|
9,029 |
|
|
|
2,517 |
|
| Retained earnings. |
|
|
|
|
|
|
(155,069 |
) |
|
|
(120,688 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total equity |
|
|
|
|
|
|
52,623 |
|
|
|
80,366 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-current financial liabilities |
|
|
20 |
|
|
|
20,690 |
|
|
|
20,690 |
|
| Pension benefit obligation |
|
|
21 |
|
|
|
670 |
|
|
|
569 |
|
| Other non-current liabilities |
|
|
22 |
|
|
|
9,671 |
|
|
|
8,918 |
|
| Non-current deferred revenues |
|
|
23 |
|
|
|
2,000 |
|
|
|
2,972 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total non-current liabilities |
|
|
|
|
|
|
33,031 |
|
|
|
33,149 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
| Current deferred revenues |
|
|
23 |
|
|
|
— |
|
|
|
743 |
|
| Accounts payable and other current liabilities |
|
|
25 |
|
|
|
2,677 |
|
|
|
5,740 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
|
|
|
|
2,677 |
|
|
|
6,483 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
|
|
|
|
35,708 |
|
|
|
39,632 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total shareholders’ equity and liabilities |
|
|
|
|
|
|
88,331 |
|
|
|
119,998 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the consolidated financial statements.
B-4
Biotie Therapies Oyj
Consolidated Statements of Changes in Shareholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
ATTRIBUTABLE TO EQUITY HOLDERS OF THE PARENT |
|
| (€ in thousands) |
|
Note |
|
|
Share capital |
|
|
Reserve for
invested
unrestricted
equity |
|
|
Other
reserves |
|
|
Retained
earnings |
|
|
Shareholders’
equity total |
|
| Balance at January 1, 2013 |
|
|
|
|
|
|
193,285 |
|
|
|
4,882 |
|
|
|
5,146 |
|
|
|
(128,282 |
) |
|
|
75,031 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income for the period |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,846 |
|
|
|
5,846 |
|
| Other comprehensive loss |
|
|
18 |
|
|
|
— |
|
|
|
— |
|
|
|
(2,629 |
) |
|
|
— |
|
|
|
(2,629 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total comprehensive income (loss) |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
(2,629 |
) |
|
|
5,846 |
|
|
|
3,217 |
|
| Share based compensation |
|
|
19 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,748 |
|
|
|
1,748 |
|
| Options and RSU exercised |
|
|
19 |
|
|
|
— |
|
|
|
370 |
|
|
|
— |
|
|
|
— |
|
|
|
370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
370 |
|
|
|
(2,629 |
) |
|
|
7,594 |
|
|
|
5,335 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2013 |
|
|
|
|
|
|
193,285 |
|
|
|
5,252 |
|
|
|
2,517 |
|
|
|
(120,688 |
) |
|
|
80,366 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss for the period |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(35,165 |
) |
|
|
(35,165 |
) |
| Other comprehensive income |
|
|
18 |
|
|
|
— |
|
|
|
— |
|
|
|
6,512 |
|
|
|
— |
|
|
|
6,512 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total comprehensive income (loss) |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
6,512 |
|
|
|
(35,165 |
) |
|
|
(28,653 |
) |
| Share based compensation |
|
|
19 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
784 |
|
|
|
784 |
|
| Options and RSU exercised |
|
|
19 |
|
|
|
— |
|
|
|
126 |
|
|
|
— |
|
|
|
— |
|
|
|
126 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
126 |
|
|
|
6,512 |
|
|
|
(34,381 |
) |
|
|
(27,743 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2014 |
|
|
|
|
|
|
193,285 |
|
|
|
5,378 |
|
|
|
9,029 |
|
|
|
(155,069 |
) |
|
|
52,623 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the consolidated financial statements.
B-5
Biotie Therapies Oyj
Consolidated Statements of Cash Flows
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
Note |
|
|
2014 |
|
|
2013 |
|
| Cash flow from operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Net (loss) income |
|
|
|
|
|
|
(35,165 |
) |
|
|
5,846 |
|
| Adjustments |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-cash impairment of in-process R&D assets |
|
|
11 |
|
|
|
27,605 |
|
|
|
— |
|
| Other non-cash transactions |
|
|
26 |
|
|
|
777 |
|
|
|
1,814 |
|
| Interest expenses |
|
|
8 |
|
|
|
687 |
|
|
|
726 |
|
| Other financial income/expenses, net |
|
|
8 |
|
|
|
(1,612 |
) |
|
|
(2,841 |
) |
| Interest income |
|
|
8 |
|
|
|
— |
|
|
|
(37 |
) |
| Income taxes |
|
|
9 |
|
|
|
— |
|
|
|
(2,195 |
) |
| Change in working capital |
|
|
|
|
|
|
|
|
|
|
|
|
| Change in accounts receivables and other receivables |
|
|
|
|
|
|
(1,108 |
) |
|
|
2,241 |
|
| Change in accounts payable and other liabilities |
|
|
|
|
|
|
(3,479 |
) |
|
|
3,305 |
|
| Change in deferred revenue |
|
|
|
|
|
|
(1,770 |
) |
|
|
1,731 |
|
| Interest paid |
|
|
|
|
|
|
(27 |
) |
|
|
(44 |
) |
| Interest received |
|
|
|
|
|
|
— |
|
|
|
31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flow from (used in) operating activities |
|
|
|
|
|
|
(14,092 |
) |
|
|
10,577 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flow from investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Investments in financial assets at fair value through profit and loss |
|
|
16 |
|
|
|
— |
|
|
|
(15,492 |
) |
| Proceeds from sale of financial assets at fair value through profit and loss |
|
|
16 |
|
|
|
9,773 |
|
|
|
2,000 |
|
| Proceeds from sale of investment property |
|
|
13 |
|
|
|
1,350 |
|
|
|
— |
|
| Change in other financial assets |
|
|
14 |
|
|
|
(53 |
) |
|
|
(192 |
) |
| Investments in property, plant and equipment |
|
|
12 |
|
|
|
(146 |
) |
|
|
(329 |
) |
| Investments in intangible assets |
|
|
11 |
|
|
|
(50 |
) |
|
|
(52 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash from (used in) investing activities |
|
|
|
|
|
|
10,874 |
|
|
|
(14,065 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flow from financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from share issue and option exercise |
|
|
|
|
|
|
126 |
|
|
|
370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash from financing activities |
|
|
|
|
|
|
126 |
|
|
|
370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net decrease in cash and cash equivalents |
|
|
|
|
|
|
(3,092 |
) |
|
|
(3,118 |
) |
| Effect on changes in exchange rates on cash and cash equivalents |
|
|
|
|
|
|
323 |
|
|
|
(214 |
) |
| Cash and cash equivalents at the beginning of the period |
|
|
|
|
|
|
10,221 |
|
|
|
13,553 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents at the end of the period |
|
|
17 |
|
|
|
7,452 |
|
|
|
10,221 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the consolidated financial statements.
B-6
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements
Biotie Therapies Oyj (“Biotie” or the
“Company”) is a specialized drug development company incorporated and domiciled in Finland, with its headquarters at Joukahaisenkatu 6, Turku, Finland, focused on products for neurodegenerative and psychiatric disorders. Biotie operates
primarily in Finland and in the United States. Biotie has developed Selincro (nalmefene) for alcohol dependence, which received European marketing authorization in 2013 and is currently being rolled out across Europe by partner Lundbeck. The current
development products include tozadenant for Parkinson’s disease, which is transitioning into Phase 3 development, and two additional compounds which are in Phase 2 development for cognitive disorders including Parkinson’s disease dementia
and primary sclerosing cholangitis, a rare fibrotic disease of the liver. Biotie’s shares are listed on Nasdaq Helsinki. As used in these consolidated financial statements, unless the context indicates otherwise, all references to
“Biotie” or the “Company” or the “Group” refer to Biotie Therapies Oyj and all its consolidated subsidiaries.
The financial statements were approved for issue by the Board of Directors on March 13, 2015.
| 2. |
Summary of Significant Accounting Policies |
The consolidated financial statements of Biotie have been prepared
in accordance with International Financial Reporting Standards (“IFRS”) and IFRIC Interpretations, as issued by the International Accounting Standards Board (“IASB”).
The principal accounting policies applied in the preparation of these consolidated financial statements are set out below. These policies have
been consistently applied to all the years presented, unless otherwise stated.
The consolidated financial statements have been prepared
assuming that the Company will continue as a going concern and under the historical cost convention, except for financial assets at fair value through profit and loss. The Directors have considered the financial position of the Company, its cash
position and forecast cash flows for the twelve month period from the date of signing these financial statements in its going concern consideration. They have also considered the Company’s business activities, the key policies for managing
financial risks and the key factors affecting the likely development of the business in 2015. In the light of this review, the Directors have a reasonable expectation that the Company has adequate resources to continue in operational existence for
the foreseeable future. Accordingly, they continue to adopt the going concern basis in preparing these financial statements.
The
preparation of financial statements under IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the end of the
reporting period, as well as the reported amounts of income and expenses during the reporting period. Although these estimates are based on management’s best knowledge of current events and actions, actual results may ultimately differ from
them. The areas involving a higher degree of judgment or complexity, or areas where assumptions and estimates are significant to the consolidated financial statements are disclosed in note 2.24.
The Notes to the consolidated financial statements are presented in thousands of euros, rounded to the nearest thousand, unless otherwise
stated. As a result, rounding differences may exist.
B-7
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| 2.2 |
Changes in accounting policy and disclosures |
(a) The following amendment to IFRS
standards became effective for annual periods beginning on January 1, 2014 and has been adopted by the Company in the preparation of the consolidated financial statements
| |
• |
|
Amendments to IAS 32 Financial statements – presentation |
The adoption of this amendment
did not impact the Company’s financial position or results of operations. Other standards, amendments to standards and interpretations which are effective for the financial year beginning on January 1, 2014 are not material to the Group.
(b) New and amended IFRS standards and IFRIC interpretations effective after December 31, 2014
The following standards have been issued, but are not effective until after December 31, 2014, and are considered relevant for the
Company. The Company is currently assessing their potential impact on the accounting policies, financial position and performance of the Company.
i. IFRS 9, Financial Instruments
IFRS 9, ‘Financial instruments’, addresses the classification, measurement and recognition of financial assets and financial
liabilities. The complete version of IFRS 9 was issued in July 2014. It replaces the guidance in IAS 39 that relates to the classification and measurement of financial instruments. IFRS 9 retains, but simplifies, the mixed measurement model and
establishes three primary measurement categories for financial assets: amortized cost, fair value through other comprehensive income (loss) and fair value through profit and loss. The basis of classification depends on the entity’s business
model and the contractual cash flow characteristics of the financial asset. Investments in equity instruments are required to be measured at fair value through profit or loss with the irrevocable option to present changes in fair value in other
comprehensive income (loss) not recycling. There is a new expected credit losses model that replaces the incurred loss impairment model used in IAS 39. For financial liabilities there were no changes to classification and measurement except for the
recognition of changes in own credit risk in other comprehensive income (loss) and for liabilities designated at fair value through profit or loss. IFRS 9 relaxes the requirements for hedge effectiveness by replacing the bright line hedge
effectiveness tests. It requires an economic relationship between the hedged item and hedging instrument and for the ‘hedged ratio’ to be the same as the one management actually uses for risk management purposes. Contemporaneous
documentation is still required, but is different to that currently prepared under IAS 39. The standard is effective for accounting periods beginning on or after January 1, 2018. Early adoption is permitted. The Company is assessing the impact
of IFRS 9.
ii. IFRS 15, Revenue from Contracts with Customers
IFRS 15, ‘Revenue from contracts with customers’ deals with revenue recognition and establishes principles for reporting useful
information to users of financial statements about the nature, amount, timing and uncertainty of revenue and cash flows arising from an entity’s contracts with customers. Revenue is recognized when a customer obtains control of a good or
service and thus has the ability to direct the use of, and obtain the benefits from, the good or service. The standard replaces IAS 18 ‘Revenue’ and IAS 11 ‘Construction contracts’ and related interpretations. The standard is
effective for annual periods beginning on or after January 1, 2017 and earlier application is permitted. The Company is assessing the impact of IFRS 15.
B-8
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
(a) Subsidiaries
Subsidiaries are all entities over which the Company has control. The Company controls an entity when it is exposed to, or has rights to,
variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are consolidated from the date on which control is transferred to the Company and are de-consolidated
from the date that control ceases. The acquisition method of accounting is used to account for subsidiaries acquired through a business combination.
Intra-group transactions, balances and unrealized gains and losses on transactions between group companies are eliminated. Unrealized losses
are also eliminated, unless the transaction provides evidence of an impairment of the asset transferred. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Company.
(b) Business combinations
Business combinations are accounted for using the acquisition method. The acquisition cost is measured as the aggregate of the fair value of
consideration transferred, measured at acquisition date, and the amount of any non-controlling interest in the acquiree. Acquisition costs incurred are expensed and included in general and administrative expenses.
| 2.4 |
Foreign currency translation |
(a) Functional and presentation currency
Items included in the financial statements of each of the Group entities are measured using the currency of the primary economic environment in
which the entity operates (the functional currency). The consolidated financial statements are presented in euros, which is the parent company’s functional and the Group’s presentation currency.
(b) Transactions and balances
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of the transactions.
Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in the statement of
comprehensive income.
Foreign exchange gains and losses that relate to borrowings and cash and cash equivalents are presented in the
statement of comprehensive income within other net financial income (expenses), except when deferred in the cumulative translation account included in other comprehensive income as part of qualifying net investments. All other foreign exchange gains
or losses are presented in the consolidated statement of comprehensive income within operating income.
(c) Group companies
The results and financial position of all group entities that have a functional currency different from the presentation currency are
translated into the presentation currency as follows:
| |
• |
|
Assets and liabilities for each balance sheet presented are translated at the closing rate at the date of that balance sheet. |
B-9
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| |
• |
|
Income and expenses for each income statement are translated at average exchange rate (unless this average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in
which case income and expenses are translated at the rate on the dates of the transactions). |
| |
• |
|
All resulting exchange differences are recognized in other comprehensive income (loss). |
Goodwill and fair value adjustments arising on the acquisition of a foreign entity are treated as assets and liabilities of the foreign entity
and translated at the closing rate. Exchange differences arising are recognized in other comprehensive income (loss).
| 2.5 |
Notes to the statement of cash flows |
The statement of cash flows has been prepared
using the indirect method. The cash disclosed in the statement of cash flows comprise cash and cash equivalents, excluding restricted cash balances. Cash comprises cash on hand and demand deposits. Cash equivalents are short-term, highly liquid
investments with original maturities of three months or less that are readily convertible to known amounts of cash and are subject to an insignificant risk of changes in value. Cash flows denominated in foreign currencies have been translated at the
average exchange rates. Exchange differences, if any, affecting cash items are shown separately in the statement of cash flows. Interest paid and received, dividends received and income tax are included in the cash flow from operating activities.
Biotie operates in one reportable segment, which comprises the
development of pharmaceutical products. The Chief Executive Officer is identified as the chief operating decision maker. The Chief Executive Officer reviews the consolidated operating results regularly to make decisions about resources and to assess
overall performance.
In 2014, €7,978 thousand (2013: €23,557 thousand) of the total revenue was received from Belgium and
€6,923 thousand (2013: €4,155 thousand) of the total revenue was received from Denmark.
Non-current assets (other than
financial instruments and deferred income tax assets) by country were €2,652 thousand (2013: €1,305 thousand) in Finland, €19,142 thousand (2013: €26,360 thousand) in Switzerland and €28,054 thousand (2013:
€42,136 thousand) in the United States.
The Company’s revenue arises from collaboration and licensing
agreements with pharmaceutical companies that may include a) licenses, b) development and approval milestone payments, c) development funding income, d) commercial milestone payments and e) royalties. For these agreements, the Company applies
revenue recognition criteria to the separately identifiable components of a single transaction. The total arrangement consideration is allocated to separately identifiable components by reference to their fair values. Revenue is recognized when the
amount can be measured reliably, when it is probable that future economic benefits will flow to the Company, when costs incurred in respect of the sale can be measured reliably, and, if applicable, when specific criteria have been met for each of
the Company’s activities, in accordance with the principles described below. Costs under these types of arrangements are expensed as incurred and therefore the pattern of cost recognition may be different than revenue recognition.
B-10
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
(a) Licenses
Revenues in connection with out-licensing of the Company’s patents and other intellectual property are recognized when the following
criteria have been met:
| |
• |
|
The Company has transferred to the buyer the significant risks and rewards of ownership of the patents and intellectual property; and |
| |
• |
|
The Company does not retain either the continuing managerial involvement to the degree usually associated with ownership or the effective control over the patent and intellectual property. |
Where the above criteria are not met, up-front and option payments received in connection with product out-licensing activities are deferred
and recognized over the period of the license term or the option period on a straight-line basis, even where such fees are non-refundable.
(b) Development and approval milestone payments
Revenue related to non-refundable fixed-fee development and approval milestone payments are recognized when a milestone has been achieved and
the Company has no further performance obligations in respect of the milestone.
In certain agreements, where the non-refundable milestone
payments are primarily received to reimburse development costs for specific development activities, revenue is recognized as the lower of the non-refundable cash received under the agreement and that based on the percentage of completion method
until the contingency is resolved, which is measured on the efforts and costs incurred to date in relation to the total estimated costs to complete the contract.
Any milestone payments that have been received but for which the earnings process has not been completed are accounted for as deferred revenue
(a liability) on the statement of financial position.
(c) Development funding income
Development funding income is recognized once the development activities have been incurred and the Company has no further performance
obligations.
(d) Commercial milestone payments
Commercial milestone payments are non-refundable and are due when sales by license partners have reached certain pre-agreed levels. The
milestone payments are recognized in full when the Company can verify that the milestone has been reached, which is normally evidenced through partner acceptance in accordance with the license agreement terms.
(e) Royalties
Royalty
revenue is recognized on an accrual basis in accordance with the relevant agreement.
The Company has received grants, from time to time, from government and certain
charitable organizations, such as the Michael J. Fox Foundation, to support certain research projects. These grants are recognized as
B-11
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
income and presented in other operating income when management has reasonable assurance that the Company will comply with the conditions attached to those grants and that such grants will be
received. Income is recognized when costs under each grant are incurred in accordance with the terms and conditions of the grant and the collectability of the receivable is probable. Further, grants relating to costs are deferred and recognized in
the consolidated statement of comprehensive income over the period necessary to match them with the costs they are intended to compensate and only to the extent that the cumulative accrued eligible costs at the time of recognition are less than the
cumulative received grant to reflect any obligation to return any portion of the grant for which the related costs have not been incurred at the end of the grant period.
| 2.9 |
Property, plant and equipment |
Property, plant and equipment comprises mainly machinery
and technical equipment used in research and development and leasehold improvements. They are stated at historical cost less depreciation and any impairment loss. Historical cost includes expenditure that is directly attributable to the acquisition
of the items. Depreciation is calculated using the straight-line method to allocate each item’s acquisition cost or impaired amount to its residual value during its estimated useful life, as follows:
|
|
|
|
|
| Asset group |
|
Useful life |
|
| Machinery and technical equipment |
|
|
3–12 years |
|
| Other equipment |
|
|
3–8 years |
|
| Leasehold improvements |
|
|
5 years |
|
The residual value and the useful life of an asset are reviewed, and adjusted if appropriate, at each balance
sheet date.
Gains and losses on the disposals are determined by comparing proceeds with the carrying amount and are recognized in the
consolidated statement of comprehensive income within other operating income.
Investment properties are land and buildings which are held to earn
rental income or for capital appreciation or for both.
Investment properties are initially recorded at cost, including transaction costs,
and after initial recognition stated at historical cost less depreciation (at straight-line) and any impairment losses. The fair values for the investment properties are disclosed in Note 13. The fair values are generally assessed using
internationally accepted valuation methods, such as taking comparable properties as a guide to current market prices or by applying the discounted cash flow method, unless a property specific valuation is available.
As at December 31, 2014, the Company’s investment property had been disposed of and, accordingly, the Company no longer holds assets
in this category.
(a) Goodwill
Goodwill arising in a business combination is recognized as an asset at the date that control is acquired. Goodwill is measured as the excess
of the sum of consideration transferred, the amount of any non-controlling interest in the acquiree and the fair value of the acquirer’s previously held equity interest (if any) in the entity over the fair value of the identifiable assets
acquired and liabilities assumed.
B-12
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
Goodwill is not amortized but is tested for impairment annually, or more frequently when
there is an indication that goodwill may be impaired. For the purpose of impairment testing, goodwill is allocated to a cash-generating unit (CGU) or groups of CGUs expected to benefit from the synergies of the business combination giving rise to
the goodwill. The company has determined that it only has one CGU as its material assets are used in the development of all the products and management regularly reviews all activities of the group as a single component and, as a result, goodwill is
monitored at the operating segment level. If the recoverable amount, which is the higher of value in use and the fair value less cost of disposal, of the CGU is less than its carrying amount, the impairment loss is allocated first to reduce the
carrying amount of any goodwill to nil and then to the other assets of the unit pro-rata on the basis of the carrying amount of each asset in the CGU. An impairment loss recognized for goodwill is not reversed in a subsequent period.
On disposal of a CGU, the attributable amount of goodwill is included in the determination of the profit or loss on disposal.
(b) Research and development expenses
Research expenditures are recognized as expenses as incurred. Costs include salaries and support costs, such as rent and leasing, which are
directly attributable to the Company’s research and development programs. Costs incurred on development projects are recognized as intangible assets as of the date that it meets all the criteria for recognition, one of which is to establish
that it is probable that future economic benefits that are attributable to the asset will flow to the Company. Given the current stage of the development of the Company’s products and product candidates, no internal development expenditures
have yet been capitalized as management considers that the uncertainties inherent in developing pharmaceutical products prohibits the capitalization of internal development expense as an intangible asset until marketing approval has been received
from the relevant regulatory agencies.
(c) Other intangible assets
Intangible assets include in-process research and development, production licenses, computer software and other intangible assets.
In-process research and development projects acquired in a business combination are capitalized at their fair values at the date of
acquisition. They will be amortized from the date when marketing approval has been received from the relevant regulatory agencies. Prior to that, acquired in-process research and development projects are not amortized, but are subject to annual
impairment testing, or more frequently when there is an indicator of impairment.
Production licenses, computer software and other
intangible assets are capitalized on the basis of the costs incurred and amortized using straight-line depreciation over their estimated useful lives. The amortization periods are as follows:
|
|
|
|
|
| Asset group |
|
Useful life |
|
| Production licenses |
|
|
17–20 years |
|
| Computer software |
|
|
3–4 years |
|
| 2.12 |
Impairment of non-financial assets |
Non-financial assets that are subject to
amortization are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. Intangible assets that have an
B-13
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
indefinite useful life, such as goodwill, or intangible assets not ready to use, such as in-process R&D assets, are not subject to amortization and are tested annually for impairment, or
whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
An impairment loss is recognized
for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less cost of disposal and value in use. The Company’s impairment review methodology
applied is based on the fair value less cost of disposal. The fair value of the asset is determined from the discounted future net cash flows expected to be derived from the asset or, in the case of goodwill, the CGU. The discounted future net cash
flows of development assets are adjusted for the probability of future development success; the discount rate used reflects the time value of money and appropriate risk premiums for the asset. The fair value less cost of disposal is then compared to
the carrying amount of the asset. An impairment charge is recognized in the consolidated statement of comprehensive income for the amount by which the asset’s carrying amount exceeds its fair value less cost of disposal. Intangible assets,
other than goodwill, that have been previously impaired, are reviewed for possible reversal of the impairment at each subsequent reporting date; any such reversal would be recognized in the consolidated statement of comprehensive income on the
period in which it was identified.
The Company classifies its financial assets in the following
categories:
| |
• |
|
at fair value through profit or loss |
The classification depends on the purpose for which the financial
assets were acquired and in which they were classified at initial recognition. The Company applies a consistent policy in recognizing an asset based on the trade date, which is the date that the Company commits to buy or sell the asset. Financial
assets are initially recognized at fair value, transaction costs are included in the fair value unless the asset is recognized at fair value through profit or loss.
(a) Financial assets at fair value through profit and loss
Financial assets are classified as at fair value through profit and loss when they are either acquired for trading purposes or when the
management designates them initially as at fair value through profit or loss. The Company’s financial assets in this category comprise investments in money market and investment funds that have been designated at fair value through profit and
loss at initial recognition. Financial assets at fair value through profit and loss are measured and their performance is evaluated by management on a fair value basis.
Realized and unrealized gains and losses arising from changes in their fair value are recognized in the consolidated statement of
comprehensive income within other financial income (expense) in the period in which they arise.
(b) Loans and receivables
Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market nor held
by the Company for trading. Accounts receivable and other receivables are included in this category. These are initially measured at fair value plus transaction costs. Subsequent to initial recognition assets are carried at amortized cost using the
effective interest method less any impairment. Interest income is recognized in the consolidated statement of comprehensive income within interest income.
B-14
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
(c) Impairment of loans and receivables
Accounts and other receivables are assessed for indicators of impairment at each reporting date. Receivables are impaired where there is
objective evidence that, as a result of one or more events that occurred after initial recognition, the estimated future cash flows of the receivables have been affected. Evidence of impairment may include indicators such as a debtor’s
significant financial difficulty, default or delinquency in interest or principal payments, or the probability that it will enter bankruptcy.
If in a subsequent period, the amount of the impairment loss decreases and the decrease can be related objectively to an event occurring after
the impairment was recognized, the reversal of the previously recognized impairment loss is recognized in the consolidated statement of comprehensive income.
(a) Group companies as the lessee
Leases of tangible assets, where the Company has substantially all the risks and rewards of ownership, are classified as finance leases.
Finance leases are capitalized at the inception of the lease at the lower of the fair value of the leased property or the present value of the minimum lease payments. Each lease payment is allocated between the finance charge and the reduction of
the outstanding liability so as to achieve a constant rate on the finance balance outstanding. Lease obligations are included in current and non-current financial liabilities net of finance charges. The interest element of the payments is expensed.
An asset recognized under a finance lease is depreciated over its useful life.
Leases where a significant portion of the risks and
rewards of ownership are retained by the lessor are classified as other operating leases. Payments made under operating leases are charged to the consolidated statement of comprehensive income on a straight-line basis over the period of the lease.
(b) Group companies as the lessor
Leases in which the Company has not transferred substantially all the risks and rewards of ownership are classified as operating leases. Rental
payments received are recorded in other operating income on a straight-line basis over the lease term.
| 2.15 |
Cash and cash equivalents |
Cash and cash equivalents comprise cash on hand, demand
deposits and other short-term, highly liquid investments with original maturities of less than three months.
Trade payables are obligations to pay for goods or services that have
been acquired in the ordinary course of business from suppliers. Trade payables are classified as current liabilities if payment is due within one year or less (or in the normal operating cycle of the business if longer). If not, they are presented
as non-current liabilities.
Shares are classified as equity. Incremental costs directly attributable
to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds of the share issue.
B-15
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
When a Group company purchases Parent Company’s shares (treasury shares), the
consideration paid, including any directly attributable incremental costs (net of income taxes) is deducted from equity attributable to the Company’s equity holders until the shares are cancelled, reissued or disposed of. Where such shares are
subsequently sold or reissued, any consideration received, net of any directly attributable incremental transaction costs and the related income tax effect, is included in the equity attributable to the Company’s equity holders.
Reserve for invested unrestricted equity includes, under the Finnish Companies Act, the exercise value of shareholders’ investment
comprising the share subscription prices and exercise prices of share options.
Borrowings are recognized initially at fair value net of transaction costs
incurred. Financial liabilities are included in current and non-current liabilities based on their maturities. Borrowings are subsequently recognized at amortized cost, any difference between the proceeds, net of transaction costs, and the
redemption value is recognized in the consolidated statement of comprehensive income over the period of the borrowings using the effective interest method.
Compound financial instruments issued by the Group comprise of a convertible note that can be converted to share capital at the option of the
holder and the number of shares to be issued is fixed and does not vary with changes in their fair value. The liability component of a compound financial instrument is recognized initially at the fair value of a similar liability that does not have
an equity conversion option. The equity component is recognized initially at the difference between the fair value of the compound financial instrument as a whole and the fair value of the liability component. Any directly attributable transaction
costs are allocated to the liability and equity components in proportion to their initial carrying amounts. Subsequent to initial recognition, the liability component of a compound financial instrument is measured at amortized cost using the
effective interest method. The equity component of a compound financial instrument is not re-measured subsequent to initial recognition except on conversion or expiry.
The fair value of the liability portion of the convertible loan was determined at inception using a market interest rate for the equivalent
non-convertible loan. Based on the fair value calculation at the date of inception in 1999, there was no significant separate conversion option component in the convertible capital loan and as such the entire convertible loan has been classified as
a financial liability.
For loans from a government agency (such as Finnish Funding Agency for Technology and Innovation, Tekes) issued at
below market rates and awarded post IAS 20 amendment (effective January 1, 2009), the Company separately accounts for the grant and liability components and records the benefit of the below market rate loan as grant income. The remaining
liability is measured at amortized cost. Following the forgiveness of certain of the Tekes loans in 2013, the grant components related to such loans have been fully utilized.
A financial liability, or a portion of a financial liability, is derecognized when it is extinguished i.e. when the obligation specified in
the contract is discharged, cancelled or expires. For government loans that have been forgiven as a result of a separate event not part of the original terms and conditions, the forgiveness of the loan is treated as a gain on extinguishment and the
resulting gain has been recorded through the consolidated statement of comprehensive income within other financial income (expense). Interest costs on interest-bearing liabilities are expensed as incurred using the effective interest method.
Income tax expense consists of current and deferred taxes. The income tax
effects of items recognized in other comprehensive income or directly in equity are similarly recognized in other comprehensive income or
B-16
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
equity, respectively. The current income tax charge is calculated on the basis of the tax laws enacted in the countries where the Group operates and generates taxable income.
Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is subject to
interpretation. It establishes provisions where appropriate on the basis of amounts expected to be paid to the tax authorities.
Deferred
income tax is recognized on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. Temporary differences arise primarily from in-process R&D intangible assets,
R&D credits and deferrals, depreciation on property, plant and equipment and net operating tax loss carryforwards.
Deferred income
tax assets are recognized only to the extent that it is probable that future taxable profit will be available against which temporary differences can be utilized.
Deferred taxes are determined using a tax rate enacted, or substantially enacted, as of the date of the balance sheet date in the respective
countries. However, deferred taxes are not recognized if they arise from the initial recognition of goodwill, or in the initial recognition of an asset or liability in a transaction other than a business combination that at the time of the
transaction affects neither accounting nor taxable profit nor loss.
| 2.20 |
Earnings (loss) per share |
Basic earnings (loss) per share is calculated by dividing the
net income (loss) attributable to shareholders by the weighted average number of shares in issue during the year, excluding shares purchased by the Company and held as treasury shares.
Diluted earnings (loss) per share is calculated by adjusting the weighted average number of shares outstanding assuming conversion of all
dilutive potential shares.
The Company has both defined contribution and defined benefit plans.
(a) Defined contribution plans
A defined contribution plan is a pension plan under which the Company pays fixed contributions into a separate entity, which is not part of the
Group. The Company has no legal or constructive obligations to pay further contributions if the fund does not hold sufficient assets to pay all employees the benefits relating to employee service in the current and prior periods. The contributions
are recognized as employee benefit expense when they are due. Prepaid contributions are recognized as an asset to the extent that a cash refund or a reduction in the future payments is available.
(b) Defined benefit plans
The Company has two closed schemes classified as defined benefit plans related to former German employees based on a defined amount of pension
benefit that these former employees will receive at retirement. The liability recognized in the consolidated statement of financial position in respect of these defined benefit pension plans is the present value of the defined benefit obligation at
the reporting date less the fair value of plan assets. The defined benefit obligation is calculated annually by independent actuaries using the projected unit
B-17
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
credit method. The present value of the defined benefit obligation is determined by discounting the estimated future cash outflows using interest rates of high quality corporate bonds that are
denominated in the currency in which the benefits will be paid, and that have terms to maturity approximating to the terms of the related pension liability. In countries where there is no deep market in such bonds, the market rates on government
bonds are used.
Actuarial gains and losses arising from experience adjustments and changes in actuarial assumptions are charged or
credited to equity in other comprehensive income in the period in which they arise.
Past-service costs are recognized immediately as an
expense. As the Company’s remaining defined benefit obligations are closed schemes, the Company does not incur current service costs and the charge in the statement of comprehensive income comprises the interest cost that is presented within
personnel expenses.
| 2.22 |
Share-based compensation |
The Company operates a number of equity-settled share-based
compensation plans (share option rights and restricted share units “RSU”) plans under which it receives services from employees as consideration for equity instruments of the Company. Option rights and RSU have been measured at their fair
values at the grant dates and the fair values are expensed on a straight-line basis over the vesting period, based on the Company’s estimate of shares that will eventually vest. The fair value is determined using the Black–Scholes option
pricing model with market-based inputs.
At each reporting date, the Company revises its estimate of the number of equity instruments that
are expected to vest. The impact of the revision of the original estimates, if any, is recognized in consolidated statement of comprehensive income with a corresponding adjustment to equity. When options are exercised and RSU subscribed for,
generally the Company issues new shares from treasury shares. Option rights that are exercised and RSU subscribed for with an exercise price are recognized in the reserve for invested unrestricted equity.
| 2.23 |
Provisions and contingent liabilities |
Provisions are recognized when the Company has a
present legal or constructive obligation as a result of past events, it is probable that an outflow of resources will be required to settle the obligation, and a reliable estimate of the amount can be made. Provisions are measured at the present
value of the expenditures expected to be required to settle the obligation using a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the obligation. The increase in a provision due to passage
of time is recognized in interest expenses.
| 2.24 |
Critical accounting estimates and judgments in applying the Company’s accounting policies |
In the application of the Company’s accounting policies, which are described above, management is required to make judgments, estimates
and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant.
Actual results may differ from these estimates.
The estimates and assumptions are reviewed on an on-going basis. Revisions to accounting
estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revisions and future periods if the revision affects both current and future periods.
B-18
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
The following are the critical judgments, apart from those involving estimations (which are
dealt with separately below), that management has made in the process of applying the Company’s accounting policies and that have the most significant effect on the amounts recognized in the financial statements.
(a) Revenue recognition
The Company’s revenue arises from collaboration and licensing agreements with pharmaceutical companies that may include licenses,
development and approval milestone payments, development funding income, commercial milestone payments and royalties. These agreements which often require significant analysis and judgment by management in order to determine the appropriate method
of revenue recognition.
Where such arrangements can be divided into separate units of accounting (each unit constituting a separate
earnings process), the arrangement consideration is allocated to the different units based on their relative fair values and recognized over the respective performance period. This analysis requires considerable estimates and judgments, including
estimates of the relative fair values of the various elements included in such agreements and the estimated length of the respective performance periods. Depending upon how such judgment is exercised, the timing and amount of revenue recognized
could differ significantly. Revenue in the various accounting units containing elements is recognized when the criteria for revenue recognition regarding the elements of that accounting unit have been met according to their type and only to the
extent of the consideration that is not contingent upon completion or performance of the remaining elements in the contract.
Revenues on
licenses are recognized when, in the judgment of management, significant risks and rewards of ownership have been transferred to the buyer and where the Company does not retain either the continuing managerial involvement to the degree usually
associated with ownership or effective control.
Non-refundable development, approval and commercial milestone payments are recognized
when a milestone has been achieved and the Company has no further performance obligations. This is normally when the Company is informed by the partner that the milestone has been achieved.
Any milestone payments that have been received but for which earnings process has not been completed are reported as deferred revenue
(liability) in the statement of financial position. Any change in the estimated development period may lead to an adjustment of the recognition amount and time. In case the estimated development schedule was to be delayed, the annual income would
lessen since the amount of the total revenue would be allocated over a longer period of time.
In certain agreements, where development
milestones are primarily received to reimburse development costs for specific development activities, revenue is recognized as the lower of the non-refundable cash received under the agreement and that based on the percentage of completion method.
This is based on the efforts and costs incurred to date in relation to the total estimated costs to complete the contract. Any change in the estimated costs to complete could cause a change in the amount of revenue that should have been recognized
to date. Refer to note 3 for details in revenue.
(b) Share-based payments
Option rights and share units have been measured at their fair value at the grant date and are recognized as an expense in the consolidated
statement of comprehensive income over the vesting period. A Black-Scholes pricing model is used to value the option rights and share units that have been granted and critical judgments need to be exercised in determining the appropriate assumptions
to include in the model, as well as to determine the most appropriate way of recognizing the compensation expense. The Company’s shares are listed on the
B-19
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
Nasdaq OMX Helsinki Ltd., or the Finnish Stock Exchange and therefore, the market based inputs used to fair value the option rights and share units include company-specific historical share price
and volatility information. The key assumptions in the model are detailed in note 19. The Group reviews and updates, as appropriate, each of the underlying assumptions at the date of the financial statements. The impact of any changes in the
estimates or assumptions is recorded in the statement of comprehensive income.
(c) Impairment of intangible assets and goodwill
The Group has significant investments in intangible assets and goodwill, which are tested for impairment in accordance with the
accounting policies above. The recoverable amounts of the assets have been determined based on fair value less cost of disposal. The determination of the fair values requires management to make a number of estimates and assumptions related to future
expectations of success and to use discount rates and other inputs that are relevant to the specific assets. Should it be required to recognize impairments in the consolidated statement of comprehensive income as a result of the impairment testing,
this could have a material effect on the Group’s results and financial position, although this would not have any impact on the Company’s cash or liquid resources balances. Key assumptions regarding intangible assets and goodwill
impairment testing, including the impairments recognized in 2014, are discussed in note 11.
(d) Research and development expenses
The stage of a particular development asset generally forms the basis for the decision whether costs incurred on research and
development projects can be capitalized or not. In general, the Company’s view is that research and development expenses may not be capitalized until marketing approval has been received from the relevant regulatory agencies, as this is
considered to be the first point at which it may be appropriate to conclude the future revenues can be generated. Expenses before that point are recognized as they are incurred in the consolidated statement of comprehensive income and when a project
reaches that point, it is reviewed at each reporting period to assess whether in management’s judgment it meets the capitalization criteria.
As of each balance sheet date, the Company estimates the level of service performed by its vendors or other counterparties and the associated
costs incurred for the services performed. As part of the process of preparing the Company’s financial statements, the Company is required to estimate its accrued expenses, which are predominantly in respect of research and development
activities. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf, estimating the level of service performed and the associated cost incurred for the service when it has
not yet been invoiced or notified of the actual cost; in most cases, this is done by discussion with the vendors. The majority of the Company’s service providers invoice the Company monthly in arrears for services performed. The Company makes
estimates of its accrued expenses at each balance sheet date in its financial statements based on the facts and circumstances known to it at that date. Although the Company does not expect its estimates to be materially different from the amounts
actually incurred, its understanding of the status and timing of services performed relative to actual status and timing may vary and could result in it reporting amounts that are too high or too low in a particular period. When the actual amounts
are known, any difference is recognized in the consolidated statement of comprehensive income. Refer to note 4 for detail.
(e) Income
taxes
The Company is subject to income taxes in Finland, the United States, Switzerland and Germany. Significant judgment is required
in determining the use of net operating loss carry forwards and the taxation of up-front and milestone payments for income tax purposes. There are many transactions and calculations for which the ultimate tax determination is uncertain. Where the
final tax outcome of these matters is different from
B-20
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
the amounts that were initially recorded, such differences may impact the current and deferred income tax assets and liabilities in the period in which such determination is made.
The companies in the Biotie group generally have a history of recent tax losses and the Group recognizes deferred tax assets arising from
unused tax losses or tax credits only to the extent the relevant fiscal entity has sufficient taxable temporary differences or there is convincing other evidence that sufficient taxable profits will be available against which the unused tax losses
or unused tax credits can by utilized by the fiscal entity. Management’s judgment is currently that sufficient convincing other evidence is not available and a deferred tax asset is, therefore, not recognized. Refer to notes 9 and 24 for detail
on income taxes.
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Commercial milestone payments from Lundbeck license agreement |
|
|
6,000 |
|
|
|
4,000 |
|
| Royalties from Lundbeck license agreement |
|
|
923 |
|
|
|
155 |
|
| Phase 2 development milestones from UCB collaboration agreement |
|
|
— |
|
|
|
15,286 |
|
| Phase 3 development milestones from UCB collaboration agreement |
|
|
5,047 |
|
|
|
8,271 |
|
| Phase 3 development funding income from UCB |
|
|
2,931 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
14,901 |
|
|
|
27,712 |
|
|
|
|
|
|
|
|
|
|
Lundbeck License Agreement
In 2007, the Company entered into a license agreement with Lundbeck to develop, manufacture and commercialize Selincro (nalmefene) for any
purpose. Lundbeck is responsible for the manufacturing and commercialization of Selincro (nalmefene), as well as registration, maintenance and defense of all trademarks related to such products. Pursuant to the license agreement, Lundbeck agreed to
make milestone payments to the Company upon the achievement of specified regulatory and commercial milestones as well as royalties on sales of Selincro (nalmefene).
UCB License and Collaboration Agreement and UCB Termination and Transition Agreement
As part of an acquisition in February 2011, the Company assumed a license and collaboration agreement with UCB to develop and commercialize
tozadenant. Following a review of the tozadenant Phase 2 data in February 2013, UCB exercised an option and paid the Company a nonrefundable Phase 2 development milestone. Until the end of March 2014, UCB paid Phase 3 development milestones. In
March 2014, UCB terminated the collaboration agreement and the Company and UCB entered into a termination and transition agreement pursuant to which UCB agreed to fund certain transitional activities.
B-21
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| 4. |
Research and development expenses |
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Outsourced services |
|
|
(10,088 |
) |
|
|
(10,816 |
) |
| Internal research and development expenses |
|
|
(1,478 |
) |
|
|
(1,373 |
) |
| Personnel costs |
|
|
(5,456 |
) |
|
|
(5,511 |
) |
| Depreciation and amortization |
|
|
(170 |
) |
|
|
(107 |
) |
|
|
|
|
|
|
|
|
|
| Total |
|
|
(17,192 |
) |
|
|
(17,807 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Salaries |
|
|
(6,488 |
) |
|
|
(5,817 |
) |
| Obligatory personnel expenses |
|
|
(509 |
) |
|
|
(347 |
) |
| Voluntary personnel expenses (including fringe benefits) |
|
|
(914 |
) |
|
|
(895 |
) |
| Pension expenses—contribution-based pension plans |
|
|
(343 |
) |
|
|
(281 |
) |
| Pension expenses—benefit-based pension plans |
|
|
(20 |
) |
|
|
(21 |
) |
| Share based compensation |
|
|
(784 |
) |
|
|
(1,748 |
) |
|
|
|
|
|
|
|
|
|
| Total |
|
|
(9,058 |
) |
|
|
(9,109 |
) |
|
|
|
|
|
|
|
|
|
| Personnel costs by operation |
|
|
|
|
|
|
|
|
| Research and development personnel costs |
|
|
(5,456 |
) |
|
|
(5,511 |
) |
| General and administrative personnel costs |
|
|
(3,602 |
) |
|
|
(3,598 |
) |
|
|
|
|
|
|
|
|
|
| Total |
|
|
(9,058 |
) |
|
|
(9,109 |
) |
|
|
|
|
|
|
|
|
|
The average number of personnel in 2014 was 36 (2013: 35). Share-based compensation disclosures are included
in Note 19 and management benefits in Note 29.
| 6. |
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Depreciation and amortization by type of asset |
|
|
|
|
|
|
|
|
| Intangible assets |
|
|
(74 |
) |
|
|
(53 |
) |
| Machinery and equipment |
|
|
(183 |
) |
|
|
(75 |
) |
| Investment property |
|
|
(24 |
) |
|
|
(39 |
) |
|
|
|
|
|
|
|
|
|
| Total |
|
|
(281 |
) |
|
|
(167 |
) |
|
|
|
|
|
|
|
|
|
| Depreciation and amortization by operation |
|
|
|
|
|
|
|
|
| Research and development |
|
|
(170 |
) |
|
|
(107 |
) |
| Administration |
|
|
(111 |
) |
|
|
(60 |
) |
|
|
|
|
|
|
|
|
|
| Total |
|
|
(281 |
) |
|
|
(167 |
) |
|
|
|
|
|
|
|
|
|
B-22
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| 7. |
Other operating income |
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Rent from investment property |
|
|
366 |
|
|
|
565 |
|
| Net gain on sale of investment property, see note 13 |
|
|
433 |
|
|
|
— |
|
| Grant income |
|
|
333 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
1,132 |
|
|
|
565 |
|
|
|
|
|
|
|
|
|
|
Grant income has been recognized in respect of the grant from the Michael J. Fox Foundation in relation to the
SYN120 Phase 2a study.
| 8. |
Financial income and expenses |
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Interest income |
|
|
|
|
|
|
|
|
| Interest income |
|
|
— |
|
|
|
37 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
— |
|
|
|
37 |
|
|
|
|
|
|
|
|
|
|
| Interest expenses |
|
|
|
|
|
|
|
|
| Interest on Tekes loans |
|
|
(519 |
) |
|
|
(558 |
) |
| Interest on convertible capital loan |
|
|
(168 |
) |
|
|
(168 |
) |
|
|
|
|
|
|
|
|
|
| Total |
|
|
(687 |
) |
|
|
(726 |
) |
|
|
|
|
|
|
|
|
|
| Other net financial income (expenses) |
|
|
|
|
|
|
|
|
| Unrealized and realized gains from assets recorded at fair value in profit and loss |
|
|
264 |
|
|
|
242 |
|
| Extinguishment of debt (Tekes loan forgiveness) |
|
|
— |
|
|
|
3,175 |
|
| Net gains (losses) from foreign exchange |
|
|
1,348 |
|
|
|
(576 |
) |
|
|
|
|
|
|
|
|
|
| Total |
|
|
1,612 |
|
|
|
2,841 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Current income tax |
|
|
— |
|
|
|
— |
|
| Deferred income tax |
|
|
— |
|
|
|
2,195 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
— |
|
|
|
2,195 |
|
|
|
|
|
|
|
|
|
|
| (Loss) income before tax |
|
|
(35,165 |
) |
|
|
3,651 |
|
| Tax benefit (charge) calculated at domestic tax rates applicable to (loss) income in the respective countries |
|
|
11,881 |
|
|
|
(745 |
) |
| Tax effects of: |
|
|
|
|
|
|
|
|
| Expenses not deductible for tax purposes |
|
|
— |
|
|
|
(106 |
) |
| Utilization of previously unrecognized tax losses |
|
|
93 |
|
|
|
3,128 |
|
| Tax losses for which no tax asset was recognized |
|
|
(11,974 |
) |
|
|
(82 |
) |
|
|
|
|
|
|
|
|
|
| Income tax benefit |
|
|
— |
|
|
|
2,195 |
|
|
|
|
|
|
|
|
|
|
B-23
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| 10. |
(Loss) earnings per share |
(a) Basic (loss) earnings per share
Basic (loss) earnings per share is calculated by dividing the net (loss) income attributable to equity holders of the parent by the weighted
average number of shares in issue during the year, excluding shares purchased by the Company and held as treasury shares.
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Net (loss) income attributable to equity holders of the parent (€ in thousands) |
|
|
(35,165 |
) |
|
|
5,846 |
|
| Weighted average number of outstanding shares (thousands) |
|
|
450,686 |
|
|
|
446,214 |
|
|
|
|
|
|
|
|
|
|
| Basic (loss) earnings per share (€ per share) |
|
|
(0.08 |
) |
|
|
0.01 |
|
|
|
|
|
|
|
|
|
|
(b) Diluted (loss) earnings per share
Diluted (loss) earnings per share is calculated by adjusting the weighted average number of shares outstanding assuming conversion of all
dilutive potential shares. The Company has three kinds of potentially dilutive instruments comprising stock options, restricted share units (RSU) and the convertible capital loan. For the year ended December 31, 2014, because there was a loss
for the year the potential dilutive shares had an anti-dilutive effect (i.e. decrease loss per share) and are, therefore, excluded from the calculation of diluted earnings (loss) per share.
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Net (loss) income attributable to equity holders of the parent (€ in thousands) |
|
|
(35,165 |
) |
|
|
5,846 |
|
| Interest expense on convertible debt (net of tax) (€ in thousands) |
|
|
— |
|
|
|
127 |
|
|
|
|
|
|
|
|
|
|
| Net (loss) income used to determine diluted earnings (loss) per share (€ in thousands) |
|
|
(35,165 |
) |
|
|
5,973 |
|
|
|
|
|
|
|
|
|
|
| Weighted average number of outstanding shares (thousands) |
|
|
450,686 |
|
|
|
446,214 |
|
| Adjustments for: |
|
|
|
|
|
|
|
|
| Share options and RSU (thousands) |
|
|
— |
|
|
|
5,296 |
|
| Assumed conversion of convertible debt (thousands) |
|
|
— |
|
|
|
828 |
|
| Weighted average number of outstanding shares for diluted (loss) earnings per share (thousands) |
|
|
450,686 |
|
|
|
452,338 |
|
|
|
|
|
|
|
|
|
|
| Diluted (loss) earnings per share (€ per share) |
|
|
(0.08 |
) |
|
|
0.01 |
|
|
|
|
|
|
|
|
|
|
B-24
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| 11. |
Intangible assets and goodwill |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
In-process
R&D |
|
|
Production
licenses |
|
|
Software |
|
|
Other
intangible
assets |
|
|
Intangible
assets
total |
|
|
Goodwill |
|
|
Total |
|
| Book value January 1, 2013 |
|
|
70,534 |
|
|
|
530 |
|
|
|
21 |
|
|
|
— |
|
|
|
71,085 |
|
|
|
5,497 |
|
|
|
76,582 |
|
| Additions |
|
|
— |
|
|
|
— |
|
|
|
42 |
|
|
|
10 |
|
|
|
52 |
|
|
|
— |
|
|
|
52 |
|
| Amortization |
|
|
— |
|
|
|
(38 |
) |
|
|
(15 |
) |
|
|
— |
|
|
|
(53 |
) |
|
|
— |
|
|
|
(53 |
) |
| Translation differences |
|
|
(2,340 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,340 |
) |
|
|
(182 |
) |
|
|
(2,522 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Book value December 31, 2013 |
|
|
68,194 |
|
|
|
492 |
|
|
|
48 |
|
|
|
10 |
|
|
|
68,744 |
|
|
|
5,315 |
|
|
|
74,059 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| At December 31, 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Acquisition cost |
|
|
98,297 |
|
|
|
762 |
|
|
|
267 |
|
|
|
10 |
|
|
|
99,336 |
|
|
|
5,549 |
|
|
|
104,885 |
|
| Accumulated amortization and impairment |
|
|
(27,763 |
) |
|
|
(270 |
) |
|
|
(219 |
) |
|
|
— |
|
|
|
(28,252 |
) |
|
|
— |
|
|
|
(28,252 |
) |
| Translation differences |
|
|
(2,340 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,340 |
) |
|
|
(234 |
) |
|
|
(2,574 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Book value December 31, 2013 |
|
|
68,194 |
|
|
|
492 |
|
|
|
48 |
|
|
|
10 |
|
|
|
68,744 |
|
|
|
5,315 |
|
|
|
74,059 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Book value January 1, 2014 |
|
|
68,194 |
|
|
|
492 |
|
|
|
48 |
|
|
|
10 |
|
|
|
68,744 |
|
|
|
5,315 |
|
|
|
74,059 |
|
| Additions |
|
|
— |
|
|
|
— |
|
|
|
50 |
|
|
|
— |
|
|
|
50 |
|
|
|
— |
|
|
|
50 |
|
| Amortization |
|
|
— |
|
|
|
(38 |
) |
|
|
(36 |
) |
|
|
— |
|
|
|
(74 |
) |
|
|
— |
|
|
|
(74 |
) |
| Impairment |
|
|
(27,605 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(27,605 |
) |
|
|
— |
|
|
|
(27,605 |
) |
| Translation differences |
|
|
6,241 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,241 |
|
|
|
484 |
|
|
|
6,725 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Book value December 31, 2014 |
|
|
46,830 |
|
|
|
454 |
|
|
|
62 |
|
|
|
10 |
|
|
|
47,356 |
|
|
|
5,799 |
|
|
|
53,155 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| At December 31, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Acquisition cost |
|
|
98,297 |
|
|
|
762 |
|
|
|
317 |
|
|
|
10 |
|
|
|
99,386 |
|
|
|
5,549 |
|
|
|
104,935 |
|
| Accumulated amortization and impairment |
|
|
(55,368 |
) |
|
|
(308 |
) |
|
|
(255 |
) |
|
|
— |
|
|
|
(55,931 |
) |
|
|
— |
|
|
|
(55,931 |
) |
| Translation differences |
|
|
3,901 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,901 |
|
|
|
250 |
|
|
|
4,151 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Book value December 31, 2014 |
|
|
46,830 |
|
|
|
454 |
|
|
|
62 |
|
|
|
10 |
|
|
|
47,356 |
|
|
|
5,799 |
|
|
|
53,155 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In-process R&D assets represent the fair value assigned to development projects that the Company acquired
through business combinations, which at the time of the acquisition have not led to marketing approvals that are required for commercialization. Until December 31, 2014, in-process R&D (IPR&D) assets comprised the SYN115, SYN120 and
SYN117 programs, which were acquired in the Synosia 2011 acquisition. Amounts capitalized as IPR&D are not amortized until marketing approval has been received from the relevant regulatory agencies. These assets are tested for impairment
annually and whenever there is an indication that the asset may be impaired.
IPR&D and goodwill have been tested for impairment at
December 31, 2014 and December 31, 2013, our annual testing date, by assessing their respective recoverable amounts to their carrying values. For the purpose of this analysis, fair value less costs of disposal was deemed to be the
recoverable amount. The fair value less costs of disposal is determined from the discounted future net cash flows expected to be derived from the asset or, in the case of goodwill, the CGU utilizing market participant assumptions. The fair values
represent a Level 3 fair value measurement category in the fair value hierarchy.
For IPR&D, the factors and assumptions used in the
determination of the discounted future net cash flows expected to be derived from the asset are highly sensitive, as market prices for the individual assets are not available and depend on assumptions specific to the nature of the Group’s
activities, including: the selected
B-25
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
discount rates; the expected development costs; the probability of success of the clinical trials; the time to commercialization; the expected revenue that will be generated from the expected
market size and market penetration; the expected treatment costs; the expected sales and marketing resources that will be required; the expected patent life; and the tax rates that will be applicable in any year. Management projects cash flows for
each development asset for three years beyond the end of the expected patent life and, therefore, does not include any terminal value. Due to the above factors, actual cash flows and values could vary significantly from forecasted cash flows and
related recoverable amount derived using discounting techniques.
The analyses concluded at December 31, 2014 and December 31,
2013, in respect of the in-process R&D assets, that:
| |
• |
|
Tozadenant (SYN115) was not subject to impairment at either balance sheet date and has increased its fair value during 2014 as a result of UCB’s decision to terminate the license agreement and return its rights to
the Company. This resulted in the Company having global rights to the asset as opposed to shared economics through the in-license agreement in place in 2013. |
| |
• |
|
The development pathway for SYN120 in 2013 was a Phase 2a study in Alzheimer’s disease for which the recoverable amount supported the carrying value as of December 31, 2013. Following the receipt of funding in
July 2014 for a study in Parkinson’s disease dementia and the termination of the UCB license agreement with respect to tozadenant, management concluded as of December 31, 2014 that the Company could not immediately fund the
Alzheimer’s disease study. Accordingly, the Company’s development efforts are currently targeted to the development pathway in Parkinson’s disease dementia and the December 31, 2014 impairment review has been conducted in
relation to the development for Parkinson’s disease dementia. This has resulted in SYN120 being subject to an impairment charge of €16,458 thousand as at December 31, 2014, which has been recorded through the statement of
comprehensive income as impairment of in-process R&D assets. As the asset has been written-down to its recoverable amount as at December 31, 2014, any changes in the assumptions could result in either a further impairment being recognized
or, alternatively, in a reversal of the impairment charge. This could be the case if the Company’s development plans change, for example if the planned Alzheimer’s disease study could be funded, which could increase the fair value of the
asset. |
| |
• |
|
Nepicastat (SYN117) was concluded to have a recoverable amount of nil as of December 31, 2014, as a result of the receipt of negative top-line data in respect of the Phase 2a study in January 2015 following the
completion of the clinical treatment phase in August 2014. As a result, the IP&D for nepicastat (SYN117) asset was fully impaired as at the reporting date and a non-cash impairment charge of €11,147 thousand has been recorded through
the statement of comprehensive income as impairment of in-process R&D assets”. The 2013 carrying value was not impaired as the study was still in progress and the recoverable amount fully supported its carrying value. |
For goodwill, the Company assesses the aggregate fair value of the business as a whole, as there is only one CGU. The fair value of the
business as a whole is determined by projecting cash flows for the business through to three years beyond the expected longest patent life of its current products and, consequently, does not include a terminal value; these projections include a fair
value for Selincro license agreement, which has no carrying value in the statements of financial position because it was an internally generated asset. The analyses of the aggregate fair value of the business concluded that there was no impairment
of goodwill at either December 31, 2014 or December 31, 2013.
The sensitivity analysis conducted for each asset and for
goodwill in 2014 and 2013 indicated, as stated above, that only with respect to SYN120, any reasonably possible negative change in any of the key assumptions would cause further impairment as the asset has been written down to its fair value in
2014. In 2013, the only
B-26
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
asset where any reasonable possible negative change in any of the key assumptions would cause impairment was SYN120, for which an increase in the discount rate of approximately 1.5 percentage
points or a reduction in revenues of approximately 3 percentage points might have resulted in an impairment loss being recognized.
The
discount rates used in the determination of the recoverable amount for IPR&D asset was 14.5% as at December 31, 2013 and December 31, 2014 and for goodwill 14.0-14.5% as at December 31, 2014 and December 31, 2013,
respectively.
All of the IPR&D assets that remain in development are subject to inherent risks and uncertainties in drug development
and it is possible that additional non-cash impairment charges are recognized in the future.
| 12. |
Property, plant and equipment |
|
|
|
|
|
| (€ in thousands) |
|
Machinery and
equipment |
|
| Book value on January 1, 2013 |
|
|
256 |
|
| Additions |
|
|
446 |
|
| Depreciation |
|
|
(75 |
) |
|
|
|
|
|
| Book value December 31, 2013 |
|
|
627 |
|
|
|
|
|
|
| At December 31, 2013 |
|
|
|
|
| Acquisition cost |
|
|
4,667 |
|
| Accumulated depreciation |
|
|
(4,040 |
) |
|
|
|
|
|
| Book value December 31, 2013 |
|
|
627 |
|
|
|
|
|
|
| Book value on January 1, 2014 |
|
|
627 |
|
| Additions |
|
|
209 |
|
| Depreciation |
|
|
(183 |
) |
|
|
|
|
|
| Book value December 31, 2014 |
|
|
653 |
|
|
|
|
|
|
| At December 31, 2014 |
|
|
|
|
| Acquisition cost |
|
|
4,876 |
|
| Accumulated depreciation |
|
|
(4,223 |
) |
|
|
|
|
|
| Book value December 31, 2014 |
|
|
653 |
|
|
|
|
|
|
The table includes assets the Company has leased through finance leases, comprising of equipment used in
research and development which is as follows:
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Acquisition cost—capitalized on the basis of finance lease |
|
|
1,907 |
|
|
|
1,907 |
|
| Accumulated depreciation |
|
|
(1,649 |
) |
|
|
(1,592 |
) |
|
|
|
|
|
|
|
|
|
| Book value December 31 |
|
|
258 |
|
|
|
315 |
|
|
|
|
|
|
|
|
|
|
Finance lease agreements are made for 2 to 3 years. Monthly lease payments are fixed, and include bargain
purchase options which correspond approximately to one month’s lease payment. In 2014, no new finance leases were made.
B-27
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Book value January 1 |
|
|
817 |
|
|
|
846 |
|
| Additions |
|
|
3 |
|
|
|
10 |
|
| Depreciation |
|
|
(24 |
) |
|
|
(39 |
) |
| Disposals |
|
|
(796 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| At December 31 |
|
|
— |
|
|
|
817 |
|
|
|
|
|
|
|
|
|
|
As at December 31, 2013, the fair value of the investment property was estimated to be
€1,100 thousand based on a purchase offer for the property received from an external party contemplating the purchase of the property (a level 2 disclosure) representing a price that the Company would have received to sell the asset in an
orderly transaction between market participants.
During 2014, the investment property was sold realizing a net gain after related costs of
€433 thousand.
| 14. |
Other financial assets |
Other financial assets comprise primarily restricted cash
balances in escrow securing long-term operating lease commitments. Other financial assets amounted to €324 thousand as at December 31, 2014 compared to €242 thousand as at December 31, 2013.
| 15. |
Accounts receivables and other receivables |
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Accounts receivable |
|
|
1,270 |
|
|
|
102 |
|
| VAT receivables |
|
|
41 |
|
|
|
65 |
|
| Income tax receivable |
|
|
— |
|
|
|
48 |
|
| Other receivables |
|
|
91 |
|
|
|
213 |
|
| Prepaid expenses and accrued income |
|
|
404 |
|
|
|
147 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
1,806 |
|
|
|
575 |
|
|
|
|
|
|
|
|
|
|
Accounts receivables are classified as loans and receivables and, therefore, measured at amortized cost. The
provision for impairment was nil at December 31, 2014 and December 31, 2013, respectively. The fair values of current accounts receivable and other receivables correspond to their carrying values due to their short maturities. Further, the
carrying value represents the maximum credit risk exposure for the Company. As at December 31, 2014 and December 31, 2013 there were no past due or impaired account receivables.
| 16. |
Financial assets at fair value through profit or loss |
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Short term |
|
|
24,941 |
|
|
|
33,457 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
24,941 |
|
|
|
33,457 |
|
|
|
|
|
|
|
|
|
|
B-28
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
Financial assets at fair value through profit and loss, consisting mainly of investments to
money market funds, are measured at their fair value based on quoted bid prices at the reporting date. The fair values are based on fund manager reports and are classified either within Level 1 or Level 2 in the fair value hierarchy. For Level 1,
the fair value measurement is directly obtained from an active market. For Level 2, the fair value measurement is based on observable quoted market information, although it is not directly obtained from an active market (Level 1). According to the
Company’s investment policy, money market funds held in Europe must have a Morning Star rating of three stars or higher. Money market funds in the U.S. must be rated AAA by Moody’s or AAA by Standard & Poor´s.
| 17. |
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Bank accounts |
|
|
7,452 |
|
|
|
10,221 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
7,452 |
|
|
|
10,221 |
|
|
|
|
|
|
|
|
|
|
There are no significant concentrations of credit risk as the cash balance is diversified with cash deposits
in the respective operating countries of the Company.
(a) Share Capital
Movements in our shares outstanding, treasury shares and our total registered shares are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
| (Number of shares) |
|
Outstanding
shares |
|
|
Treasury
shares |
|
|
Total registered
shares |
|
| As at January 1, 2013 |
|
|
444,098,961 |
|
|
|
8,611,777 |
|
|
|
452,710,738 |
|
| Share options and RSU exercised |
|
|
2,114,987 |
|
|
|
(2,114,987 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As at December 31, 2013 |
|
|
446,213,948 |
|
|
|
6,496,790 |
|
|
|
452,710,738 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As at January 1, 2014 |
|
|
446,213,948 |
|
|
|
6,496,790 |
|
|
|
452,710,738 |
|
| New shares issued at no consideration (treasury shares) |
|
|
— |
|
|
|
5,769,035 |
|
|
|
5,769,035 |
|
| Cancellations of treasury shares |
|
|
— |
|
|
|
(2,511,599 |
) |
|
|
(2,511,599 |
) |
| Share options and RSU exercised |
|
|
4,482,067 |
|
|
|
(4,482,067 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As at December 31, 2014 |
|
|
450,696,015 |
|
|
|
5,272,159 |
|
|
|
455,968,174 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company’s total authorized number of shares is 455,968,174. All issued shares are fully paid. The
shares have no par value. On 31 December 2014 the total number of shares held in treasury represented approximately 1.2% (2013: 1.4%) of the total registered shares. Treasury shares have been issued without consideration for the purpose of the
Company’s share-based compensation plans.
The total number of stock options and restricted stock units outstanding as at
December 31, 2014 was 2,824,772, for which the Company holds an equivalent amount of treasury shares which it will use to settle these if they are exercised.
B-29
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
(b) Reserve for invested unrestricted equity
Reserve of invested unrestricted equity includes, under the Finnish Companies Act, the exercise value of shareholders’ investment
comprising share subscription prices and exercise prices of share options.
(c) Other reserves
Other reserves include the foreign currency translation reserve and a reserve for the remeasurements of the post-employment pension obligations
recorded through other comprehensive income.
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Balance at January 1, |
|
|
2,517 |
|
|
|
5,146 |
|
| Change in foreign currency translation reserve |
|
|
6,593 |
|
|
|
(2,629 |
) |
| Re-measurement of post-employment benefit obligations |
|
|
(81 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, |
|
|
9,029 |
|
|
|
2,517 |
|
|
|
|
|
|
|
|
|
|
(a) Stock Option Plan 2011 and Equity Incentive Plan 2011
The Stock Option Plan 2011, primarily for European employees, and the Equity Incentive Plan 2011, primarily for US employees, were
approved at the Company’s 2011 general shareholders’ meeting as part of the Company’s incentive scheme determined by the Board of Directors. These plans contain both a service requirement condition at vesting and individual specified
non-market performance targets during the year of grant.
i. Stock Option Plan 2011
The options are forfeited in case the employee leaves the Company before the options vest, unless the Board of Directors approves otherwise.
After the beginning of the share subscription period, the vested options may be freely transferred or exercised. The fair value of the options was determined at the grant date by using the Black & Scholes option valuation model and expensed
over the vesting period. Grant dates for the option plan may vary depending on the date when the company and employees agree to the key terms and conditions of the share-based payment arrangement. The maximum number of stock options that could be
awarded under the plan was 7,401,000, in three equal tranches designated as 2011A, 2011B and 2011C.
Key characteristics and terms of the
option plan are listed in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Stock Option Plan 2011 |
|
| Option rights |
|
2011A |
|
|
2011B |
|
|
2011C |
|
| Maximum number of stock options |
|
|
2,467,000 |
|
|
|
2,467,000 |
|
|
|
2,467,000 |
|
| The number of shares subscribed by one option |
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
| Exercise price, € |
|
|
0.01 |
|
|
|
0.01 |
|
|
|
0.01 |
|
| Dividend adjustment |
|
|
No |
|
|
|
No |
|
|
|
No |
|
| Beginning of subscription period, date |
|
|
January 1, 2014 |
|
|
|
January 1, 2015 |
|
|
|
January 1, 2016 |
|
| End of subscription period, date (expiration) |
|
|
February 28, 2015 |
|
|
|
February 29, 2016 |
|
|
|
February 28, 2017 |
|
| Vesting conditions |
|
|
Service until beginning of the subscription period |
|
B-30
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
The changes in the number of options in the plan during the years ended December 31,
2014 and 2013 is shown in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2014 |
|
|
|
|
2013 |
|
| Number of options |
|
2011A |
|
|
2011B |
|
|
2011C |
|
|
|
|
2011A |
|
|
2011B |
|
|
2011C |
|
| Outstanding at 1 January |
|
|
1,844,250 |
|
|
|
1,830,500 |
|
|
|
2,267,500 |
|
|
|
|
|
2,119,250 |
|
|
|
2,178,000 |
|
|
|
— |
|
| Granted |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
200,000 |
|
|
|
2,267,500 |
|
| Forfeited |
|
|
— |
|
|
|
(37,500 |
) |
|
|
(37,500 |
) |
|
|
|
|
(275,000 |
) |
|
|
(547,500 |
) |
|
|
— |
|
| Exercised |
|
|
(1,844,250 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Outstanding at 31 December |
|
|
— |
|
|
|
1,793,000 |
|
|
|
2,230,000 |
|
|
|
|
|
1,844,250 |
|
|
|
1,830,500 |
|
|
|
2,267,500 |
|
All options were fair valued at grant date and recognized as an expense to personnel expenses included in
research and development costs and general and administrative costs based on the employee’s function over the vesting period. The effect of stock option plans 2011A, 2011B and 2011C on the Company’s earnings 2014 (2013) was €472
(916) thousand. The fair values of the stock options have been determined by using the Black–Scholes option valuation model. The most significant inputs used to estimate the fair value of the stock options granted during the year ended
December 31, 2013 are as follows:
|
|
|
|
|
|
|
|
|
| Determination of fair value |
|
Granted 2013 |
|
| Option plan |
|
|
2011B |
|
|
|
2011C |
|
| Share price at grant date |
|
|
€0.42 |
|
|
|
€0.34 |
|
| Subscription price |
|
|
€0.01 |
|
|
|
€0.01 |
|
| Volatility * |
|
|
45.0 |
% |
|
|
45.0 |
% |
| Maturity, years |
|
|
3.01 |
|
|
|
3.87 |
|
| Interest rate |
|
|
0.37 |
% |
|
|
0.30 |
% |
| Expected dividends |
|
|
— |
|
|
|
— |
|
|
|
|
| Valuation model |
|
|
Black-Scholes |
|
|
|
Black-Scholes |
|
| Option fair value, € |
|
|
0.41 |
|
|
|
0.33 |
|
| Effect on earnings 2013, € in thousands |
|
|
38 |
|
|
|
192 |
|
| Effect on earnings 2014, € in thousands |
|
|
44 |
|
|
|
298 |
|
| * |
Expected volatility was determined by calculating the historical volatility of the Company’s share using monthly observations over corresponding maturity. |
ii. Equity Incentive Plan 2011
The Equity Incentive Plan 2011 includes three consecutive discretionary periods, calendar years 2011 (2011A), 2012 (2011B) and 2013
(2011C) in which the restricted share units may be granted. Each discretionary period is followed by an approximately two year vesting period, ending on January 5, 2014, January 5, 2015 and January 5, 2016, respectively
after which the Company’s shares will be delivered to employees on the basis of the granted share units. Should an employee’s employment or service in the Company end before the end of a vesting period, the corresponding share units will
be forfeited, unless the Board of Directors approves otherwise. Grant dates for the equity incentive plan may vary depending on the date when the company and employees agree to the key terms and conditions of the share-based payment arrangement. A
maximum of 4,599,000 of our shares may be delivered under the plan, but there is no maximum that can be issued in any one year.
B-31
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
Key characteristics and terms of the plan are listed in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2011A |
|
|
2011B |
|
|
2011C |
|
| The number of shares delivered for one share unit |
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
| Exercise price |
|
|
€0 |
|
|
|
€0 |
|
|
|
€0 |
|
| Dividend adjustment |
|
|
No |
|
|
|
No |
|
|
|
No |
|
| Vesting date |
|
|
January 5, 2014 |
|
|
|
January 5, 2015 |
|
|
|
January 5, 2016 |
|
| Beginning of delivery of shares |
|
|
January 6, 2014 |
|
|
|
January 6, 2015 |
|
|
|
January 6, 2016 |
|
| End of delivery of shares* |
|
|
February 28, 2014 |
|
|
|
February 28, 2015 |
|
|
|
February 29, 2016 |
|
| Vesting conditions |
|
|
Service until the vesting date |
|
| * |
The end delivery of shares may be extended up to March 15 in the following year, should the employee holding the share units be in a period in which they are not able to trade shares. |
The changes in the number of share units in the plan during the years ended December 31, 2014 and 2013 is shown in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2014 |
|
|
|
|
2013 |
|
| Number of share units |
|
2011A |
|
|
2011B |
|
|
2011C |
|
|
|
|
2011A |
|
|
2011B |
|
|
2011C |
|
| Number of share units at January 1 |
|
|
1,477,410 |
|
|
|
1,389,000 |
|
|
|
1,482,500 |
|
|
|
|
|
1,514,910 |
|
|
|
1,614,000 |
|
|
|
— |
|
| Granted |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
150,000 |
|
|
|
1,482,500 |
|
| Forfeited |
|
|
— |
|
|
|
(734,625 |
) |
|
|
(687,500 |
) |
|
|
|
|
(37,500 |
) |
|
|
(375,000 |
) |
|
|
— |
|
| Delivered |
|
|
(1,477,410 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Number of share units at December 31 |
|
|
— |
|
|
|
654,375 |
|
|
|
795,000 |
|
|
|
|
|
1,477,410 |
|
|
|
1,389,000 |
|
|
|
1,482,500 |
|
The total effect of the Equity Incentive Plan 2011 on the Company’s 2014 (2013) earnings was credit
of €114 thousand (an expense of €708 thousand). The fair value of the restricted share units was determined as the closing share price for Biotie share on the grant date. The fair value of the 2011A and 2011B grants was
€0.47 per share. The fair value for the 2011C grants was €0.41 per share.
(b) Swiss option plan
The Company’s Swiss subsidiary, Biotie Therapies AG, also has a stock option plan approved in 2008. Vesting of the options is related to
continued service to the Company. The maximum contractual term of each option is ten years. The plan has been closed to new grants from February 1, 2011. An aggregate maximum of 14,912,155 shares in Biotie Therapies Corp. has been subscribed to
under the plan and such shares have been issued to Biotie Therapies AG to be further conveyed to the option holders when they potentially exercise their option rights in accordance with the terms and conditions of the option rights. Grant dates for
the option plan may vary depending on the date when the company and employees agree to the key terms and conditions of the share-based payment arrangement. The last day for the share subscriptions based on the option rights in the Swiss option plan
is December 7, 2020.
B-32
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
The key characteristics and terms of the option plan are listed in the table below:
|
|
|
|
|
| Swiss Option Plan |
|
|
|
| Maximum number of stock options |
|
|
14,912,155 |
|
| The number of shares subscribed by one option |
|
|
1 |
|
| Exercise price range |
|
|
€0.08–0.37 |
|
| Dividend adjustment |
|
|
No |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2014 |
|
|
2013 |
|
| Transactions during the period — Swiss
Option Plan |
|
Number of
options |
|
|
Weighted
average exercise
price, € |
|
|
Number of
options |
|
|
Weighted
average exercise
price, € |
|
| Outstanding at January 1, 2014 |
|
|
5,295,754 |
|
|
|
0.28 |
|
|
|
8,256,813 |
|
|
|
0.24 |
|
| Forfeited |
|
|
(1,310,575 |
) |
|
|
0.35 |
|
|
|
(846,072 |
) |
|
|
0.33 |
|
| Exercised |
|
|
(1,160,407 |
) |
|
|
0.08 |
|
|
|
(2,114,987 |
) |
|
|
0.18 |
|
| Outstanding at December 31, 2014 |
|
|
2,824,772 |
|
|
|
0.26 |
|
|
|
5,295,754 |
|
|
|
0.24 |
|
At December 31, 2014 there were no unvested options; at December 31, 2013 there were 483,866
unvested options. Share options outstanding at December 31, 2014 have the following expiry dates and exercise prices:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Grant date |
|
|
|
Vesting date |
|
Expiry date |
|
Exercise price, € |
|
|
|
|
Number of option rights |
|
| June 18, 2008 |
|
|
|
June 18, 2012 |
|
June 18, 2018 |
|
|
0.17 |
|
|
|
|
|
684,670 |
|
| June 18, 2008 |
|
|
|
June 18, 2012 |
|
June 18, 2018 |
|
|
0.29 |
|
|
|
|
|
155,588 |
|
| June 18, 2008 |
|
|
|
June 18, 2012 |
|
June 18, 2018 |
|
|
0.23 |
|
|
|
|
|
67,296 |
|
| June 18, 2008 |
|
|
|
June 18, 2012 |
|
June 18, 2018 |
|
|
0.22 |
|
|
|
|
|
29,094 |
|
| September 15, 2008 |
|
|
|
December 10, 2011 |
|
September 15, 2018 |
|
|
0.30 |
|
|
|
|
|
134,592 |
|
| January 23, 2009 |
|
|
|
December 10, 2012 |
|
January 23, 2019 |
|
|
0.32 |
|
|
|
|
|
422,843 |
|
| March 11, 2010 |
|
|
|
March 11, 2014 |
|
March 11, 2020 |
|
|
0.08 |
|
|
|
|
|
351,151 |
|
| December 7, 2010 |
|
|
|
December 7, 2014 |
|
December 7, 2020 |
|
|
0.37 |
|
|
|
|
|
979,538 |
|
The total effect of the Swiss Option Plan on the Company’s 2014 (2013) earnings was a credit of
€50 thousand (expense of €124 thousand).
(c) Stock Option Plan 2014 and Equity Incentive Plan 2014
The Stock Option Plan 2014, primarily for European employees, and the Equity Incentive Plan 2014, primarily for US employees, were approved at
the Company’s 2014 general shareholders’ meeting as part of the Company’s incentive scheme determined by the Board of Directors. These plans contain both a service requirement condition at vesting for all awards and for the management
awards, designated 2014M awards, there is an additional specified market performance requirement that determines the number of awards earned.
i. Stock Option Plan 2014
The options are forfeited in case the employee leaves the Company before the options vest, unless the Board of Directors approves otherwise.
After the beginning of the share subscription period, the vested options may freely be transferred or exercised. The fair value of the options was determined at the grant date by using the Black & Scholes option valuation model and expensed
over the vesting period. Grant dates for the option plan may vary depending on the date when the company and employees agree to the key terms and conditions of the
B-33
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
share-based payment arrangement. The maximum number of options that could be awarded under the plan is 10,337,500, of which 4,320,000 are 2014M awards that are subject to an additional specified
market performance requirement at vesting. The 2014M awards include an additional incentive (a market condition) for the senior management team to have a portion of their potential awards over the three year period ending December 31, 2016
based solely on the increase in the share price of the Company for the vesting period. The 2014M awards will not vest unless the Company’s share price growth during that three year period is greater than 35%; however, if the share price growth
is greater than 35%, there will be an increasing return up to a maximum of three times the initial awards for a share price growth of at least 100% over the three year vesting period. The 2014M market condition has been incorporated into the
Black-Scholes model, by determining the probability of the share price growth increase over the three year period based on historical share price movements.
Key characteristics and terms of the option plan are listed in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Option Plan 2014 |
|
| Option rights |
|
2014A |
|
|
2014B |
|
|
2014C |
|
|
2014D |
|
|
2014E |
|
|
2014F |
|
|
2014M |
|
| Maximum number of stock options |
|
|
468,125 |
|
|
|
1,404,375 |
|
|
|
518,125 |
|
|
|
1,554,375 |
|
|
|
518,125 |
|
|
|
1,554,375 |
|
|
|
4,320,000 |
|
| The number of shares subscribed by one option |
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
| Exercise price, € |
|
|
0.01 |
|
|
|
0.01 |
|
|
|
0.01 |
|
|
|
0.01 |
|
|
|
0.01 |
|
|
|
0.01 |
|
|
|
0.01 |
|
| Dividend adjustment |
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
| Beginning of subscription period |
|
|
January 1,
2016 |
|
|
|
January 1,
2017 |
|
|
|
January 1,
2017 |
|
|
|
January 1,
2018 |
|
|
|
January 1,
2018 |
|
|
|
January 1,
2019 |
|
|
|
January 1,
2017 |
|
| End of subscription period |
|
|
February 28,
2017 |
|
|
|
February 28,
2018 |
|
|
|
February 28,
2018 |
|
|
|
February 28,
2019 |
|
|
|
February 28,
2019 |
|
|
|
February 29,
2020 |
|
|
|
February 28,
2018 |
|
| Vesting conditions |
|
|
Service condition until beginning of the subscription period. |
|
|
|
Market
performance
condition
and a
service
condition
until
beginning of
the
subscription
period |
|
The change in the number of options, or senior management option units in the case of the 2014M tranche, in
the plan during the year ended December, 31 2014 are shown in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2014 |
|
| Number of options |
|
2014A |
|
|
2014B |
|
|
2014M |
|
| Outstanding at 1 January |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Granted |
|
|
468,125 |
|
|
|
1,404,375 |
|
|
|
1,440,000 |
|
| Forfeited |
|
|
(9,375 |
) |
|
|
(28,125 |
) |
|
|
— |
|
| Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Outstanding at 31 December |
|
|
458,750 |
|
|
|
1,376,250 |
|
|
|
1,440,000 |
|
All options were fair valued at grant date and will be recognized as an expense to personnel expenses included
in research and development costs and general and administrative costs based on the employee’s function over the vesting period. Stock options 2014A, 2014B and 2014M were still unvested at December 31,
B-34
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
2014 and their effect on the Company’s earnings 2014 was €279 thousand (no impact to 2013 as the plan had not started). The fair values of the stock options have been determined by
using the Black–Scholes option valuation model. The most significant inputs used to estimate the fair value of the stock options granted during the year ended December 31, 2014 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
| Determination of fair value |
|
Granted 2014 |
|
| Option plan |
|
|
2014A |
|
|
|
2014B |
|
|
|
2014M |
|
| Share price at grant date |
|
|
€0.31 |
|
|
|
€0.31 |
|
|
|
€0.31 |
|
| Subscription price |
|
|
€0.01 |
|
|
|
€0.01 |
|
|
|
€0.01 |
|
| Volatility * |
|
|
50.0 |
% |
|
|
50.0 |
% |
|
|
50.0 |
% |
| Maturity, years |
|
|
3.14 |
|
|
|
4.14 |
|
|
|
4.14 |
|
| Interest rate |
|
|
0.29 |
% |
|
|
0.43 |
% |
|
|
0.43 |
% |
| Expected dividends |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Valuation model |
|
|
Black-Scholes |
|
|
|
Black-Scholes |
|
|
|
Black-Scholes |
|
| Option fair value, € |
|
|
0.30 |
|
|
|
0.30 |
|
|
|
0.15 |
|
| Effect on earnings 2014, € in thousands |
|
|
69 |
|
|
|
138 |
|
|
|
73 |
|
| * |
Expected volatility was determined by calculating the historical volatility of the Company’s share using monthly observations over corresponding maturity. |
ii. Equity Incentive Plan 2014
The Equity Incentive Plan 2014 includes three consecutive discretionary periods, calendar years 2014, 2015 and 2016, in which the restricted
share units, or senior management units, may be granted. Each discretionary period is followed by a subscription period of approximately two years (for 2014A, 2014C and 2014E awards) or approximately three years (for 2014B, 2014D, 2014F and 2014M
awards), ending on January 5, 2016, January 5, 2017, January 5, 2018 or January 5, 2019, after which the Company’s shares will be delivered to employees on the basis of the granted share units. Should an
employee’s employment or service with the Company end before the end of a subscription period, the corresponding share units will be forfeited, unless the Board of Directors agree otherwise. A maximum of 14,002,500 of our shares may be
delivered under the plan, of which 2,520,000 are 2014M awards that are subject to an additional specified market performance requirement at vesting, which is the same as that described in the Stock Option Plan 2014 above. Grant dates for the equity
incentive plan may vary depending on the date when the company and employees agree to the key terms and conditions of the share-based payment arrangement. There is no maximum number of share units that can be awarded in any one year, but all the
2014M awards must have been awarded in 2014.
B-35
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
Key characteristics and terms of the Equity Incentive Plan 2014 are listed in the table
below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2014A |
|
|
2014B |
|
|
2011C |
|
|
2014D |
|
|
2014E |
|
|
2014F |
|
|
2014M |
|
| The number of shares delivered for one restricted share unit |
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
| Exercise price, USD equivalent of |
|
|
€0.01 |
|
|
|
€0.01 |
|
|
|
€0.01 |
|
|
|
€0.01 |
|
|
|
€0.01 |
|
|
|
€0.01 |
|
|
|
€0.01 |
|
| Dividend adjustment |
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
|
|
No |
|
| Vesting date |
|
|
January 5,
2016 |
|
|
|
January 5,
2017 |
|
|
|
January 5,
2017 |
|
|
|
January 5,
2018 |
|
|
|
January 5,
2018 |
|
|
|
January 5,
2019 |
|
|
|
January 5,
2017 |
|
| Beginning of delivery of shares |
|
|
January 6,
2016 |
|
|
|
January 6,
2017 |
|
|
|
January 6,
2017 |
|
|
|
January 6,
2018 |
|
|
|
January 6,
2018 |
|
|
|
January 6,
2019 |
|
|
|
January 6,
2017 |
|
| End of delivery of shares |
|
|
February 29,
2016 |
|
|
|
February 28,
2017 |
|
|
|
February 28,
2017 |
|
|
|
February 28,
2018 |
|
|
|
February 28,
2018 |
|
|
|
February 28,
2019 |
|
|
|
February
28, 2017 |
|
| Vesting conditions |
|
|
Service condition until the vesting date |
|
|
|
Market
performance
condition
and a
service
condition
until
beginning
of the
subscription
period |
|
The change in the number of share units, or senior management share units in the case of the 2014M tranche, in
the plan during the year ended December, 31 2014 are shown in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2014A |
|
|
2014B |
|
|
2014M |
|
| Number of share units at January 1 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Granted |
|
|
622,812 |
|
|
|
1,868,438 |
|
|
|
840,000 |
|
| Forfeited |
|
|
(213,125 |
) |
|
|
(639,375 |
) |
|
|
— |
|
| Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Number of share units at December 31 |
|
|
409,687 |
|
|
|
1,229,063 |
|
|
|
840,000 |
|
The total effect of the Equity Incentive Plan 2014 on the Company’s 2014 earnings was an expense of
€197 thousand (no impact to 2013 as the plan had not started). The fair value of the restricted share units was determined as the closing share price of the Company’s shares on the grant date. The fair value of the 2014A, 2014B was
€0.30 per share and for the 2014M grants the fair value was €0.15 per share.
| 20. |
Non-current financial liabilities |
|
|
|
|
|
|
|
|
|
| |
|
Carrying amount as at
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Non-convertible capital loans from Tekes |
|
|
16,318 |
|
|
|
16,318 |
|
| Long-term R&D loans from Tekes |
|
|
2,690 |
|
|
|
2,690 |
|
| Convertible capital loan |
|
|
1,682 |
|
|
|
1,682 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
20,690 |
|
|
|
20,690 |
|
|
|
|
|
|
|
|
|
|
Fair values of the loans are determined by discounting estimated future cash flows of the loans using
appropriate spot interest rates at the reporting date. The discount rate considers the specific features of each loan (as described below), and estimated margin for the Company’s own credit risk. Future cash flows are based on
B-36
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
the Company’s best estimate on the timing of the repayment of the loan principal and payment of related capitalized interests. Given that the inputs to the valuation technique rely on
unobservable market data, fair value measures of the loans are classified in Level 3 in the fair value hierarchy.
After considering the
relevant inputs as described above, the Company has determined that it would not be reasonable to present fair values for the loans, as the Group only has access to Tekes loans and a convertible loan, i.e. similar government grant loans the Group
already has. As the financing cost and other terms of such loans would be largely identical to the Company’s current loans, there would be no difference in the nominal amount of the loans the Group would receive, if it refinanced its existing
loan package.
Capital loans and R&D loans (excluding the capitalized interest) are due as follows:
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Under 1 year |
|
|
— |
|
|
|
— |
|
| 1–5 years |
|
|
1,614 |
|
|
|
2,152 |
|
| Over 5 years |
|
|
19,076 |
|
|
|
18,538 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
20,690 |
|
|
|
20,690 |
|
|
|
|
|
|
|
|
|
|
€18,000 thousand of the capital loans are due for repayment in less than one year. Nonetheless, the
repayment of capital loans and accrued interest is governed by a restrictive condition, according to which the capital plus the accrued interest must only be returned if the restricted equity of the parent company, including the equity of the
consolidated subsidiaries, for the last financial period is fully covered. Interest on the non-convertible capital loans shall be paid only if the parent company, including the equity of the consolidated subsidiaries, has sufficient funds for profit
distribution as per the adopted balance sheet for the most recently ended fiscal year. The loans shall also accrue interest in fiscal years in which retained earnings are not sufficient for distributions to equity holders. All capital loans are
therefore classified as long-term debt.
(a) Non-convertible capital loans from Tekes
As at December 31, 2014, non-convertible capital loans granted by Tekes comprised a total of 14 non-convertible capital loans, comprising
an aggregate amount of €16,318 thousand following the forgiveness of two loans (carrying value €1,088 thousand) in 2013. The proceeds from the loans have been fully used to fund a number of research & development projects. The
majority of the remaining loans were drawn prior to 2009 and the maturities range from 8 to 10 years from draw down. The interest rate per annum for these loans is the base rate set by the Ministry of Finance minus one (1) percentage point,
subject to a minimum rate of 3%. As the base rate has been lower than the minimum of 3%, the interest rate for these loans has been 3% for both periods presented. Further, these loans and accumulated accrued interest are not repayable until the
Company’s restricted equity on a consolidated basis is fully covered. Restricted equity of the Company represents share capital and as of December 31, 2014 (the last financial period) totaled €193,285 thousand. Distributable funds
totalled a debit of €140,662 thousand as of December 31, 2014 (for the last financial period) and are not greater than zero. Therefore, restricted equity is not considered to be fully covered as defined under the Finnish Companies Act. Since
the Company has not had distributable funds since the drawdown of these loans, interest recorded through the financial expenses is accrued and presented under other non-current liabilities in the statement of financial position as the Company does
not expect it will have distributable funds in the foreseeable future. The accumulated interest on non-convertible capital loans amounts to €6,034 thousand as at December 31, 2014 (€5,545 thousand as at December 31, 2013).
B-37
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
(b) R&D loans from Tekes
As at December 31, 2014, the Company had €2,690 thousand of R&D loans granted by Tekes. R&D loans amounting to
€1,714 thousand were extinguished in 2013 following the Tekes’ decision to forgive such loans. R&D loans are granted to a defined product development project and cover a contractually defined portion of the projects’ R&D
expenses. The interest rate for these loans is the base rate set by the Ministry of Finance minus three (3) percentage points, subject to a minimum rate of 1%. Repayment of these loans shall be initiated after 5 years, thereafter loan
principals shall be paid back in equal installments over a 5 year period. More information on repayment schedule is provided in the Note 27. The accumulated interest on R&D loans amounts to €108 thousand as at December 31, 2014
(€81 thousand as at December 31, 2013).
(c) Convertible capital loan
As at December 31, 2014, the Company had a convertible capital loan that was issued originally in 1999 to certain shareholders and venture
capital organizations in the aggregate amount of €1,682 thousand. The original subscription period began on June 1, 2000, and ended on December 31, 2005. As of December 31, 2014, the convertible capital loan can be converted, at
any time at the option of the holder, into 828,000 of our shares, the interest rate is 10% per annum. The repayment of the capital loan and its interest is governed by a restrictive condition, according to which the capital may only be returned
if the restricted equity of the parent company, including the consolidated subsidiaries, for the last financial period is fully covered. Interest on the convertible capital loan shall be paid only if the parent company, including the consolidated
subsidiaries, has sufficient funds for profit distribution as of the most recently ended fiscal year. The loan shall also yield interest from the fiscal years in which the financial statements do not present sufficient funds available for profit
distribution. As at December 31, 2014, accumulated interest on convertible capital loan amounted to €3,379 thousand and is recorded in other non-current liabilities in the statement of financial position. The convertible capital loan
can also be converted into shares of the Company under the terms of the agreement. The accumulated interest on the convertible capital loan amounts to €3,379 thousand as at December 31, 2014 (€3,211 thousand as at
December 31, 2013).
| 21. |
Pension benefit obligations |
Pension benefit obligations are recognized for certain
former employees in Biotie Therapies GmbH under two separate closed defined pension benefit schemes. The calculations are based on the Heubeck Mortality Charts RT 2005G. As the schemes are closed schemes in nature, there is no current service cost
and accordingly, the income statement charge comprises interest expenses and past service costs, if applicable, and is presented under research and development or general and administrative expenses, as applicable.
(a) Principal actuarial assumptions for calculation of pension benefit obligations:
|
|
|
|
|
|
|
|
|
| |
|
2014 |
|
|
2013 |
|
| Discount rate |
|
|
2.2 |
% |
|
|
3.5 |
% |
| Future pension increases |
|
|
1.8 |
% |
|
|
2.0 |
% |
| Rate of fluctuation of employees |
|
|
2.0 |
% |
|
|
2.0 |
% |
(b) Liabilities in the statement of financial position:
|
|
|
|
|
|
|
|
|
| |
|
As at
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Present value of unfunded pension obligations; equal to net liability in the statement of financial position |
|
|
670 |
|
|
|
569 |
|
B-38
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
(c) Personnel expenses recognized it the consolidated statement of comprehensive income
from defined benefit obligations:
|
|
|
|
|
|
|
|
|
| |
|
For the year ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Interest expenses |
|
|
20 |
|
|
|
21 |
|
|
|
|
|
|
|
|
|
|
| Total defined benefit pension expenses |
|
|
20 |
|
|
|
21 |
|
|
|
|
|
|
|
|
|
|
| Remeasurements recognized in other comprehensive income |
|
|
(81 |
) |
|
|
— |
|
(d) Changes in the present value of the defined pension obligation:
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Opening defined pension obligation January 1 |
|
|
569 |
|
|
|
573 |
|
| Interest expenses |
|
|
20 |
|
|
|
21 |
|
| Actuarial losses (gains) |
|
|
81 |
|
|
|
(25 |
) |
|
|
|
|
|
|
|
|
|
| Defined pension obligation December 31 |
|
|
670 |
|
|
|
569 |
|
|
|
|
|
|
|
|
|
|
The sensitivity of the defined obligation to changes in the weighted principal assumptions is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Impact on defined benefit obligation |
|
| |
|
Change in
assumption |
|
|
Increase in
assumption |
|
|
Decrease in
assumption |
|
| Discount rate |
|
|
0.25 |
% |
|
|
Decrease by 3.7 |
% |
|
|
Increase by 3.9 |
% |
| Future pension increase |
|
|
0.25 |
% |
|
|
Increase by 3.1 |
% |
|
|
Decrease by 3.0 |
% |
The two closed schemes have a total of 7 participants and the expected duration of the arrangements is 15.4
years.
| 22. |
Other non-current liabilities |
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Accrued accumulated interest |
|
|
9,438 |
|
|
|
8,780 |
|
| Deferred rent |
|
|
140 |
|
|
|
18 |
|
| Finance lease |
|
|
93 |
|
|
|
120 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
9,671 |
|
|
|
8,918 |
|
|
|
|
|
|
|
|
|
|
Accumulated accrued interest comprises accrued interest from Tekes loans and convertible capital loan.
Interest on the Tekes loans and convertible capital loan shall not be paid until there are sufficient cumulative distributable funds in the
consolidated financial statements for the most recently ended fiscal year. The carrying values of other non-current liabilities are reasonable approximations of their fair values.
B-39
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
The non-current deferred revenues comprises of up-front license fees
of €2,000 thousand as at December 31, 2014 and 2013, under the Lundbeck license agreement, which will be recorded as revenue when the product receives the related marketing authorization. Management cannot estimate the exact timing
for the recognition of the Lundbeck fee as revenues due to uncertainties in the marketing approval process, which is outside the Company’s control.
The remaining deferred revenue balance as at December 31, 2013 related to the developmental milestone payments from the UCB license
agreement, which have been recorded as revenue when the associated costs were incurred during 2014.
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Deferred revenues, non-current |
|
|
2,000 |
|
|
|
2,972 |
|
| Deferred revenues, current |
|
|
— |
|
|
|
743 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
2,000 |
|
|
|
3,715 |
|
|
|
|
|
|
|
|
|
|
Deferred income tax assets and liabilities are offset when there is a
legally enforceable right to offset current tax assets against current tax liabilities and when the deferred income tax assets and liabilities relate to income taxes levied by the same taxation authority on either the taxable entity or different
taxable entities where there is an intention to settle the balances on a net basis. The deferred tax assets have been recognized to the extent of existing deferred tax liabilities (i.e. a net zero deferred tax position). Temporary differences
comprise primarily in process R&D intangible assets, R&D credits and deferrals, depreciation on property, plant and equipment and net operating loss carryforwards.
The movement in deferred tax assets and liabilities (prior to offsetting of balances within the same tax jurisdiction) during the period is as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
At
January 1, |
|
|
Credited
(charged) to the
statement of
comprehensive
income |
|
|
Currency
translation
differences |
|
|
At
December 31, |
|
| Deferred tax assets 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Temporary differences |
|
|
4,355 |
|
|
|
(4,221 |
) |
|
|
(134 |
) |
|
|
— |
|
| Tax loss carry-forwards |
|
|
16,188 |
|
|
|
6,416 |
|
|
|
(501 |
) |
|
|
22,103 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total deferred tax assets |
|
|
20,543 |
|
|
|
2,195 |
|
|
|
(635 |
) |
|
|
22,103 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Deferred tax liabilities 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| In-process R&D |
|
|
22,781 |
|
|
|
— |
|
|
|
(678 |
) |
|
|
22,103 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total deferred tax liabilities |
|
|
22,781 |
|
|
|
— |
|
|
|
(678 |
) |
|
|
22,103 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net deferred tax assets (liabilities) |
|
|
(2,238 |
) |
|
|
2,195 |
|
|
|
43 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B-40
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
At
January 1, |
|
|
Credited
(charged) to the
statement of
comprehensive
income |
|
|
Currency
translation
differences |
|
|
At
December 31, |
|
| Deferred tax assets 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Temporary differences |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Tax loss carry-forwards |
|
|
22,103 |
|
|
|
(9,650 |
) |
|
|
2,054 |
|
|
|
14,507 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total deferred tax assets |
|
|
22,103 |
|
|
|
(9,650 |
) |
|
|
2,054 |
|
|
|
14,507 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Deferred tax liabilities 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| In-process R&D |
|
|
22,103 |
|
|
|
(9,650 |
) |
|
|
2,054 |
|
|
|
14,507 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total deferred tax liabilities |
|
|
22,103 |
|
|
|
(9,650 |
) |
|
|
2,054 |
|
|
|
14,507 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net deferred tax assets (liabilities) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred income tax assets are recognized to the extent that the realization of the related tax benefit is
probable. The Company did not recognize deferred income tax assets of €42,743 thousand (2013: €39,448 thousand) in respect of losses amounting to €120,583 thousand (2013: €114,709 thousand) and temporary differences of
€73,632 thousand (2013: €69,911 thousand) that can be carried forward against future taxable income, as it was not certain that they would be realized due to the history of operating losses.
Biotie’s tax loss carryforwards at December 31, 2014 expire as follows as:
|
|
|
|
|
| Years of expiration |
|
Tax loss
carryforwards
EUR
thousand |
|
| 2015–16 |
|
|
22,754 |
|
| 2017–18 |
|
|
10,482 |
|
| 2019–20 |
|
|
12,601 |
|
| 2021–22 |
|
|
10,956 |
|
| 2023–24 |
|
|
— |
|
| After 2025 |
|
|
54,317 |
|
| Tax loss carryforwards without expiration date* |
|
|
9,473 |
|
|
|
|
|
|
| Total |
|
|
120,583 |
|
|
|
|
|
|
| * |
Consists of the tax loss carryforwards of the German subsidiary. |
| 25. |
Accounts payable and other current liabilities |
|
|
|
|
|
|
|
|
|
| |
|
As at
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Accounts payable |
|
|
1,048 |
|
|
|
399 |
|
| Payroll related accruals |
|
|
441 |
|
|
|
663 |
|
| Accrued expenses |
|
|
1,188 |
|
|
|
4,678 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
2,677 |
|
|
|
5,740 |
|
|
|
|
|
|
|
|
|
|
B-41
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| 26. |
Other non-cash transactions adjustments to cash flow from operating activities |
|
|
|
|
|
|
|
|
|
| |
|
For the year
ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Depreciation and amortization |
|
|
281 |
|
|
|
167 |
|
| Share-based compensation |
|
|
784 |
|
|
|
1,748 |
|
| (Gain) on disposal of investment property |
|
|
(554 |
) |
|
|
— |
|
| Other adjustments |
|
|
266 |
|
|
|
(101 |
) |
|
|
|
|
|
|
|
|
|
| Non-cash adjustments to cash flow from operating activities |
|
|
777 |
|
|
|
1,814 |
|
|
|
|
|
|
|
|
|
|
| 27. |
Financial risk management |
The operations of the Company and its subsidiaries expose
them to financial risks. The main risk that the Group is exposed to is liquidity risk, with capital management being another important area given the Group’s financing structure. The Company’s risk management principles focus on the
unpredictability of the financial markets and aims at minimizing any undesired impacts on the Group’s financial result. The Board of Directors defines the general risk management principles and approves operational guidelines concerning
specific areas including but not limited to liquidity risk, foreign exchange risk, interest rate risk, credit risk, the use of derivatives and investment of the Company’s liquid assets. During the reporting periods, the Company or its
subsidiaries have not entered into any derivative contracts.
(a) Capital management and liquidity risks
The Company’s objective when managing capital is to safeguard the Group’s ability to continue as a going concern. Capital is the
equity and the capital loans and R&D loans, as reported in the Company’s consolidated statement of financial position (refer to notes 18 and 20).
Significant financial resources are required to advance the drug development programs into commercialized pharmaceutical products. The Company
relies on its ability to fund the operations of the Company through three major sources of financing – collaboration and licensing agreements, research and development grants and loans and equity or debt financing.
Entering into commercialization, collaboration and licensing agreements with larger pharmaceutical companies entitles the Company and its
subsidiaries to receive up-front and follow-on milestones related to agreed regulatory or commercial points, as well as royalty payments from these partners. Activities in the area of business development are targeted at securing such agreements.
Consideration of these activities is part of the management’s duties and is monitored by the Board of Directors, which ultimately decides on entering into such agreements.
The Company relies on different sources of research and development grants and loans. These funds, which are provided through regional,
national or EU level institutions or industry or therapeutic area related bodies have been historically available to the Company. The Group strictly complies with all rules and legal obligations pertaining to these funding programs and is in regular
contact with the funding agencies providing these. Availability of such funds in the future cannot be guaranteed and thus this poses a potential risk to the income situation of the Company in the future.
B-42
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
Funding of the Group’s operations is possible based on equity or debt financing. While
such equity financing has been available in the past (the last such financing was a €30 million share issue in September 2012), there can be no assurance that sufficient funds can be secured in order to permit the Company to carry out its
planned activities. Current capital market conditions are very volatile. The current financial market situation and the repercussions to the overall investor’s sentiment pose a severe risk of not being able to secure additional financing in the
future. To partly manage this risk, the Company has secured an option to raise up to €20 million through an equity facility with a reputable US investor group until November 2015. In addition, management is in constant dialogue with
financial investors, investment banks, debt providers and other market participants.
There can be no assurance that sufficient financing
can be secured in order to permit the Company to carry out its planned activities. To protect the continuity of the Company’s operations, sufficient liquidity and capital has to be maintained. The Company aims to have funds to finance at least
one year’s operations at all times. The Company can influence the amount of capital by adapting its cost basis according to the financing available. Management monitors liquidity on the basis of the amount of funds. These are reported to the
Board on a monthly basis.
The Company’s Board of Directors approves the operational plans and budget. The Board follows up the
implementation of these plans and the financial status of the Company on a monthly basis.
The Company has low risk securities (money
market funds) and bank accounts which are as follows:
|
|
|
|
|
|
|
|
|
| |
|
As at December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Money market funds |
|
|
24,941 |
|
|
|
33,457 |
|
| Bank accounts |
|
|
7,452 |
|
|
|
10,221 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
32,393 |
|
|
|
43,678 |
|
|
|
|
|
|
|
|
|
|
As at December 31, 2014, the contractual maturity of loans and interests was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
2015 |
|
|
2016 |
|
|
2017 |
|
|
2018-
thereafter |
|
|
Total |
|
| Capital loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment of loans |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
18,000 |
|
|
|
18,000 |
|
| Interest expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
9,438 |
|
|
|
9,438 |
|
| R&D loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment of loans |
|
|
— |
|
|
|
— |
|
|
|
538 |
|
|
|
2,152 |
|
|
|
2,690 |
|
| Interest expenses |
|
|
27 |
|
|
|
27 |
|
|
|
22 |
|
|
|
32 |
|
|
|
108 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
27 |
|
|
|
27 |
|
|
|
560 |
|
|
|
29,622 |
|
|
|
30,236 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As at December 31, 2014, the Company also had accounts payables €1,048 thousand and other
current liabilities €1,629 thousand due within one year (see note 25).
B-43
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
As at December 31, 2013, the contractual maturity of loans and interest was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
2014 |
|
|
2015 |
|
|
2016 |
|
|
2017-
thereafter |
|
|
Total |
|
| Capital loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment of loans |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
18,000 |
|
|
|
18,000 |
|
| Interest expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
8,780 |
|
|
|
8,780 |
|
| R&D loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment of loans |
|
|
— |
|
|
|
490 |
|
|
|
538 |
|
|
|
1,662 |
|
|
|
2,690 |
|
| Interest expenses |
|
|
27 |
|
|
|
22 |
|
|
|
16 |
|
|
|
16 |
|
|
|
81 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
27 |
|
|
|
512 |
|
|
|
554 |
|
|
|
28,458 |
|
|
|
29,551 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As at December 31, 2013, the Company also had accounts payables €399 thousand and other current
liabilities €5,341 thousand due within one year (see note 25).
(b) Market risk
i. Foreign exchange risk
The Company operates internationally but is mainly exposed to translation risk in respect of US dollar and Swiss Franc from its investments in
foreign operations. Further, the Company has granted an inter-company borrowing (amount of €36,044 thousand as at December 31, 2014) to its US subsidiary that qualifies for part of the net investment and exchange differences that are
deferred to the cumulative translation account which is a component of other comprehensive income (loss). The Company may also need to consider transfer of balances between currencies as appropriate to manage the currency in which income is received
and expenses are incurred. The Company’s policy is not to hedge translation risk, which is not considered a significant risk.
The
Company and its subsidiaries are not exposed to significant transaction risk, as the group companies mainly operate in their functional currencies. As of December 31, 2014, the Company had cash and cash equivalents of €1,334 thousand
in US dollar, €222 thousand in Swiss Franc, €49 thousand in Pound Sterling and money market funds of €5,345 thousand in US dollar.
ii. Interest rate risk
The Company’s interest rate risk arises from borrowings from Tekes and private investors. Borrowings carry fixed interest rates and hence
do not expose the Company to variable interest rate risk. The Company’s loans from Tekes are mainly tied to the base rate defined by the Finnish Ministry of Finance, which is reset rarely, with a floor at 3%. During the periods presented, the
interest rate level has been below the floor, so the Company has accrued for the floor interest of 3% on the loans. Hence an increase in base rates would not have any material impact on the Group’s profit or loss. Further, accumulated accrued
interest is not payable until the Company is profitable, and its restricted equity is fully covered. Surplus cash is invested in short term interest funds and they also expose the Company mainly to fair value interest rate risk. Due to the current
low interest rate level, the low risk profile of the funds and the interest not being immediately payable, the interest rate exposure is considered insignificant.
(c) Credit and counterparty risk
Deposit and security receivables from the banks expose the Company to credit risk. The Company prefers to work with partners with good credit
ratings. Management monitors the sufficiency of the liquid assets and
B-44
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
exposure to credit risk regularly. The Company currently derives a significant proportion of its collaborative income from a small group of partners. This risk of concentration of creditors is
partly mitigated by the fact that the Company’s collaboration partners are typically large and internationally reputable pharmaceutical companies which are financially solid. These collaborations are governed by contractual relationships that
typically address and describe remedies for situations in which interests of The Company and the partner are no longer in line. In addition, the Company aims to collaborate on different development programs with as many partners as possible in order
to spread the risk of creditor concentration.
Banks used by the Company for its deposits are among Europe’s most reputable financial
institutions. The Company invests liquid assets in low risk securities with high ratings and interest bearing bank accounts.
| 28. |
Commitments and contingent liabilities |
Operating lease commitments
|
|
|
|
|
|
|
|
|
| |
|
As at
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Due within a year |
|
|
843 |
|
|
|
675 |
|
| Due in 1–5 years |
|
|
1,937 |
|
|
|
2,287 |
|
| Due later than 5 years |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Total operating lease commitments |
|
|
2,780 |
|
|
|
2,962 |
|
|
|
|
|
|
|
|
|
|
Operating lease commitments comprise rent commitments for leasehold properties and lease commitments for motor
vehicles, machines and equipment with leases of 3 to 5 years. The Group’s operating leases are non-cancellable and they do not include redemption or extension options.
On December 31, 2014, Biotie had outstanding contractual payment obligations (contractual commitments), primarily for contract research
work services related to ongoing clinical development programs, totaling €232 thousand (December 31, 2013: €2,713 thousand).
The Company has entered into various license agreements that contingently trigger one-off payments upon achievement of certain milestones, the
payment of royalties and certain other payments. Because the achievement and timing of these payments are uncertain, our commitments under these agreements have not yet been recognized.
B-45
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
| 29. |
Transactions with related parties |
(a) Key management compensation
The Group’s 2014 management team consists of Timo Veromaa (President and CEO), David Cook (Chief Financial Officer), Stephen Bandak (Chief
Medical Officer) and Mehdi Paborji (Chief Operating Officer). The compensation paid or payable for key management for employee services is shown below.
|
|
|
|
|
|
|
|
|
| |
|
For the year
ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Salaries and other short-term employee benefits |
|
|
1,995 |
|
|
|
1,388 |
|
| Post-employment benefits (payments to defined contribution plans) |
|
|
79 |
|
|
|
37 |
|
| Share-based payments |
|
|
540 |
|
|
|
582 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
2,614 |
|
|
|
2,007 |
|
|
|
|
|
|
|
|
|
|
(b) Stock options awarded to management
Management was awarded 720,000 share options, 1,440,000 senior management option units, 420,000 share units and 840,000 senior management share
units during 2014 under the 2014M plan (1,400,000 share options, nil senior management option units, 350,000 share units and nil senior management share units during 2013). At the end of the fiscal year, the number of outstanding options and share
units granted to management amounted to 4,099,568 options, 1,440,000 senior management option units, 1,070,000 share units and 840,000 senior management share units (at the end of year 2013: 4,356,020 options and 940,000 share units). The senior
management option units and senior management share units under the 2014M plan are subject to a multiplier that is dependent on the growth in the Company’s share price over the three year period ending December 31, 2016 and may result in a
minimum of nil options and nil share units up to a maximum of 4,320,000 options and 2,520,000 share units being awarded to senior management at the end of the period.
(c) Compensation of the President and CEO
|
|
|
|
|
|
|
|
|
| |
|
For the year
ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Salary and other short-term employee benefits |
|
|
527 |
|
|
|
570 |
|
| Post-employment benefits (payments to defined contribution plans) |
|
|
42 |
|
|
|
— |
|
| Share-based payments |
|
|
271 |
|
|
|
314 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
840 |
|
|
|
884 |
|
|
|
|
|
|
|
|
|
|
The President and CEO’s (Timo Veromaa) contract may be terminated by the Company with a six month notice
period and by the President and CEO with a three month notice period. If the Company terminates the contract with the President and CEO, the President and CEO is, in addition to his salary during the notice period, entitled to a severance pay
corresponding to 12 months of salary.
B-46
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
(d) Compensation of the members of the Board of Directors
|
|
|
|
|
|
|
|
|
| |
|
For the year
ended
December 31, |
|
| (€ in thousands) |
|
2014 |
|
|
2013 |
|
| Peter Fellner* |
|
|
12 |
|
|
|
48 |
|
| William Burns |
|
|
54 |
|
|
|
36 |
|
| Merja Karhapää |
|
|
42 |
|
|
|
36 |
|
| Bernd Kastler |
|
|
44 |
|
|
|
36 |
|
| Guido Magni |
|
|
42 |
|
|
|
36 |
|
| Ismail Kola |
|
|
39 |
|
|
|
36 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
233 |
|
|
|
228 |
|
|
|
|
|
|
|
|
|
|
| * |
Board member until April 3, 2014 |
| 30. |
Investments in subsidiaries |
The Group had the following subsidiaries as at
December 31, 2014, which have been included in the consolidation.
|
|
|
|
|
|
|
|
|
| Subsidiaries |
|
Domicile |
|
Nature of business |
|
Share of
ownership % |
|
| Biotie Therapies AG |
|
Switzerland |
|
Operative
(Drug development) |
|
|
100 |
|
| Biotie Therapies Inc. |
|
USA |
|
Operative
(Drug development) |
|
|
100 |
|
| Biotie Therapies GmbH |
|
Germany |
|
Non-operative |
|
|
100 |
|
| Biotie Therapies International Ltd |
|
Finland |
|
Non-operative |
|
|
100 |
|
| 31. |
Events after the reporting date |
After the reporting period on January 20, 2015,
the Company announced that it had transferred shares of the Company held as treasury shares, that were issued on December 17, 2014, pursuant to the Stock Option Plan 2011 (942,500 shares conveyed) and the Equity Incentive Plan 2011 (66,875
shares conveyed). As a result of the transfer, the total number of voting rights attached to the Company´s shares increased to 451,705,390 votes, and the total number of the Company´s shares held by the Company or its fully owned
subsidiary was 4,262,784. The conveyance does not affect the number of registered shares (total of 455,968,174 shares).
In January 2015,
following the completion of the clinical treatment phase in August 2014, the Company announced top-line results from a Phase 2 study investigating nepicastat for cocaine dependence. When compared to placebo, nepicastat did not meet the primary
efficacy endpoint of an increased proportion of subjects remaining abstinent from cocaine during the last two weeks of the treatment period. Nepicastat was generally well tolerated in the study. The 11-week, 179-patient study was conducted at 10 US
clinics under a Collaborative Research and Development Agreement (CRADA) with the National Institute on Drug Abuse (NIDA) at the US National Institutes of Health. See note 11 for further details related to the impairment of the related in-process
R&D asset.
B-47
Biotie Therapies Oyj
Notes to the Consolidated Financial Statements — (Continued)
After the reporting period on February 17, 2015, the Company announced that The
Committee for Orphan Medicinal Products (COMP) of the European Medicines Agency (EMA) had in its February 2015 meeting issued a positive opinion recommending orphan drug designation for BTT1023 for the treatment of primary sclerosing cholangitis
(PSC).
After the reporting period on February 20, 2015, the Company announced further detail on its Phase 3 clinical development
plan for tozadenant.
After the reporting period on February 27, 2015, the Company announced that it had transferred shares of the
Company held as treasury shares, that were issued on December 17, 2014, pursuant to the Stock Option Plan 2011 (130,000 shares conveyed) and the Equity Incentive Plan 2011 (137,500 shares conveyed). As a result of the transfer, the total number
of voting rights attached to the Company´s shares increased to 451,972,890 votes and the total number of the Company´s shares held by the Company or its fully owned subsidiary was 3,995,284. The conveyance does not affect the number of
registered shares (total of 455,968,174 shares).
B-48
Annex C

|
|
|
|
|
| BIOTIE THERAPIES CORP. |
|
INTERIM REPORT |
|
November 12 2015 at 9.00 a.m. |
Biotie interim report 1 January – 30 September 2015
Biotie (Nasdaq Helsinki BTH1V; NASDAQ: BITI) announces its interim report for the three and nine month periods ended September 30, 2015.
Company Highlights
July – September 2015
| |
• |
|
Tozadenant, Biotie’s lead pipeline program, advanced into Phase 3 development in Parkinson’s disease, as patient recruitment commenced into the TOZ-PD study. TOZ-PD is a 450-patient double-blind, placebo-controlled Phase 3 study with an open-label extension and is being conducted under a Special Protocol Assessment (SPA) with the U.S. Food and Drug Administration (FDA). |
| |
• |
|
Phase 2 studies with SYN120 in Parkinson’s disease dementia and BTT1023 in primary sclerosing cholangitis, which are being conducted by third parties, continued to recruit patients. |
| |
• |
|
Biotie’s revenue for three months ended September 30, 2015 (three months ended September 30, 2014) was €0.8 million (€7.2 million) and the financial result was a net loss of
€7.9 million (net income of €2.9 million). |
| |
• |
|
At September 30, 2015 Biotie had cash and cash equivalents and short term investments (reported as financial assets held at fair value through profit and loss), which together are referred to as liquid assets, of
€84.0 million (€94.2 million, June 30, 2015; €32.4 million, December 31, 2014). Operating cash flow for the nine months ended September 30, 2015 was €23.0 million outflow (€10.3 million outflow
for the nine months ended September 30, 2014). |
Key figures (unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
3 months to
September 30,
2015 |
|
|
3 months to
September 30,
2014 |
|
|
9 months to
September 30,
2015 |
|
|
9 months to
September 30,
2014 |
|
| Revenues |
|
|
786 |
|
|
|
7,192 |
|
|
|
2,987 |
|
|
|
13,051 |
|
| Research and development costs |
|
|
(7,252 |
) |
|
|
(4,221 |
) |
|
|
(19,611 |
) |
|
|
(11,931 |
) |
| Net (loss)/profit |
|
|
(7,920 |
) |
|
|
2,927 |
|
|
|
(22,818 |
) |
|
|
(2,645 |
) |
| (Loss)/earnings per share (€) |
|
|
(0.01 |
) |
|
|
0.01 |
|
|
|
(0.03 |
) |
|
|
(0.01 |
) |
| Cash flow used in operating activities |
|
|
|
|
|
|
|
|
|
|
(23,049 |
) |
|
|
(10,263 |
) |
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
September 30,
2015 |
|
|
December 31,
2014 |
|
| Liquid assets |
|
|
84,020 |
|
|
|
32,393 |
|
| Equity |
|
|
109,667 |
|
|
|
52,623 |
|
| Equity ratio (%) |
|
|
74.9 |
|
|
|
61.0 |
|
Timo Veromaa, Biotie’s President and CEO commented, “The third quarter was marked by the start of the Phase 3
trial with tozadenant, our novel investigational product for patients with Parkinson’s disease. Tozadenant has a new mechanism of action and is designed to improve control of Parkinson’s symptoms in patients
C-1

experiencing levodopa end-of-dose “wearing off” episodes. We have already shown a significant clinical benefit of tozadenant in our published Phase 2b study which based on our
discussions with the FDA we believe will be accepted as the first pivotal study. The ongoing Phase 3 study has identical design in terms of enrollment criteria and endpoints, but we have increased patient numbers per dose arm to further improve
statistical power and have focused on doses that have demonstrated to have been optimal in the Phase 2b trial. While tozadenant is clearly the focus for Biotie, we continue to make progress with our mid-stage pipeline – SYN120, a 5-HT6/5-HT2a
antagonist for Parkinson’s disease dementia and other cognitive disorders, and BTT1023, which addresses a novel target in fibrotic liver disease. Both of these products are in Phase 2 trials and data expected by the end of next year.”
Product Portfolio Review:
Selincro® (nalmefene) is a dual-acting opioid system modulator and the first therapy approved in Europe for the reduction of alcohol consumption in alcohol dependent individuals.
Biotie has licensed global rights to Selincro to Lundbeck. Under the terms of the agreement with Lundbeck, Biotie is eligible for up to €94 million
in upfront and milestone payments, of which €22.5 million had been received at September 30, 2015, plus royalties on sales of Selincro. Biotie is eligible to receive further potential milestone payments on launches in certain ex-EU
markets and if the product reaches certain pre-determined sales. Biotie will continue to receive royalties on sales and will make a contribution to Lundbeck towards post approval commitment studies.
Lundbeck received European marketing authorization for Selincro in February 2013 and the product has since been introduced in Europe. Favorable reimbursement
decisions were made in the second half of 2014 in a number of key markets, including France, Spain and the United Kingdom.
Lundbeck and Otsuka
Pharmaceutical Co. Ltd. are collaborating, as part of their existing alliance, to develop and commercialize nalmefene in Japan, and a 660-patient Phase 3 study in Japan was commenced in Q1 2015.
Tozadenant (SYN115) is an orally administered, potent and selective adenosine A2a receptor antagonist being developed for the treatment of
Parkinson’s disease.
In a 420-patient Phase 2b trial, tozadenant displayed clinically important and statistically significant effects across
pre-specified primary and multiple secondary endpoints at a number of doses. In addition, tozadenant has been found to be generally safe and well tolerated in the ten clinical trials that have been conducted to date. Full data from the Phase 2b
study were published in Lancet Neurology in July 2014.
In July 2015, Biotie announced the start of the tozadenant Phase 3 study in Parkinson’s
disease (study TOZ-PD). The Company has agreed on a Special Protocol Assessment for TOZ-PD with the FDA. Based on discussions with the FDA at the End of Phase 2 meeting, Biotie believes that the planned Phase 3 clinical program, together with
existing data, could form the basis for approval of tozadenant as an adjunctive treatment to levodopa in Parkinson’s patients experiencing end-of-dose wearing off
episodes. The TOZ-PD study will use the primary and secondary endpoints and enrollment criteria used in the Phase 2b clinical trial. The study is expected to enroll 450 patients experiencing levodopa related end-of-dose wearing off, who will be
randomized to receive twice daily doses of 60mg or 120mg of tozadenant or placebo in addition to their standard anti-Parkinson’s disease medications for 24 weeks. The primary endpoint will be the reduction in the number of hours spent in the
“off” state in patients taking tozadenant as compared to placebo between baseline and week 24, as assessed by patient-completed diaries and averaged over three consecutive days. The double-blind placebo controlled period is
C-2

expected to be followed by a 52 week open label treatment period to collect additional clinical safety data. The study is currently planned to be conducted in the United States, Canada and
selected European countries. Based on current estimates top-line data from the double-blind portion is expected to be available by the end of 2017.
Providing the double-blind portion of TOZ-PD meets its primary efficacy endpoint, another open label trial is expected to be initiated in a separate
population of 450 patients to establish the requisite number of unique exposures required for approval.
Biotie has exclusive worldwide rights to develop
and commercialize tozadenant for all uses to treat or prevent human diseases and disorders under a license agreement with F. Hoffmann-La Roche Ltd (Roche).
SYN120 is an oral, dual antagonist of the 5-HT6 and 5-HT2A receptors. These two distinct properties could result in a unique therapeutic profile for
SYN120 combining pro-cognitive and antipsychotic activities in neuro-degenerative diseases, such as Parkinson’s and Alzheimer’s. SYN120 has completed single and multiple ascending dose Phase 1 clinical studies and a Phase 1 positron
emission tomography imaging study to determine therapeutic dose for subsequent Phase 2 studies. In these trials, doses well above the anticipated therapeutic dose were well tolerated.
In July 2014, Biotie was awarded a grant of up to $2.0 million from the Michael J. Fox Foundation (MJFF) to investigate SYN120 in Parkinson’s disease
patients with dementia, and patient enrollment into a Phase 2a study primarily funded under the grant was commenced in December 2014. The SYNAPSE study is an 80 patient, Phase 2a, randomized, double-blind, multi-center, placebo-controlled trial in
patients with Parkinson’s disease dementia. Patients are randomized 1:1 to placebo or SYN120 dosed once daily over a 16 week treatment period. In addition to assessing safety and tolerability, the main focus of the study is to establish
efficacy of SYN120 on cognition using the Cognitive Drug Research (CDR) Computerized Cognition Battery as the primary efficacy endpoint. The study is being conducted by the Parkinson Study Group (PSG) at approximately 12 specialist sites in the
United States. Biotie and the PSG share responsibility for the design and execution of the study, and top-line results of the study are expected by the end of 2016.
Biotie has exclusive worldwide rights to develop and commercialize SYN120 under a license agreement with Roche and will be able to use data from the
MJFF-funded study for any future regulatory submission for SYN120, including Alzheimer’s disease, although further clinical development plans in such indications will depend on the availability of funding.
BTT1023 is a fully human monoclonal antibody that specifically binds to vascular adhesion protein 1 (VAP-1), an endothelial cell adhesion receptor
expressed on blood vessels. Recent investigation has shown that VAP-1, in addition to its previously demonstrated role in inflammation, is also involved in the process of fibrosis, which can occur in several organs and is poorly treated with current
drugs.
In July 2014, Biotie partnered with the University of Birmingham, UK, who were awarded grant funding to conduct an investigator-sponsored, Phase
2, proof of concept study with BTT1023 in primary sclerosing cholangitis (PSC), a chronic and progressive orphan fibrotic disease for which there are currently no FDA-approved treatments. The grant was awarded by the UK’s National Institute for
Health Research (NIHR) Efficacy and Mechanism Evaluation Programme, funded and managed by NIHR on behalf of the Medical Research Council - NIHR partnership. The grant holder and Co-Investigator for the study is Professor David Adams, Director
of the NIHR Biomedical Research Unit in Liver Disease and Centre for Liver Research at the University of Birmingham.
The BUTEO study being funded under
the grant opened for patient recruitment in March 2015. It is an open label, single arm, multi-center study that will evaluate efficacy, safety and pharmacokinetic properties of BTT1023 in 41 patients with PSC. Patients will receive BTT1023 via
intravenous infusion every two weeks over
C-3

an 11 week treatment period. The primary efficacy endpoint is a reduction of elevated levels of alkaline phosphatase, a blood biomarker of bile duct inflammation; secondary endpoints include
various measures of liver injury and fibrosis.
The two-stage study design includes a pre-planned interim analysis. Based on current estimates, it is
expected that the requisite number of patients will have been treated by the end of 2016 to enable the interim analysis to be completed.
The European
Commission has granted BTT1023 Orphan Drug Designation in the EU for the treatment of PSC, and Biotie also intends to pursue orphan drug designation for BTT1023 in the United States. Biotie retains full rights to BTT1023.
Management Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with the condensed consolidated financial information contained herein, which has been
prepared in accordance with International Accounting Standard 34, Interim Financial Reporting. The Company presents its consolidated financial information in euros.
Overview
In the periods presented the Company has
earned revenue from Lundbeck, in the form of royalties and commercial milestones for Selincro, and from UCB in the form of Phase 3 development milestones and Phase 3 development funding for tozadenant. The accounting policies that the Company
applies in recognizing these revenues are set out in detail in note 2 to the consolidated financial statements for the year ended December 31, 2014.
The Company’s research and development activities are central to its business model and expenditure on research and development is recognized as an
expense in the period in which it is incurred. The Company’s current research and development activities mainly relate to the following key programs: Phase 3 clinical trial of tozadenant in Parkinson’s disease which started recruiting
patients in July 2015; Phase 2a clinical trial of SYN120 in Parkinson’s disease dementia which is currently recruiting patients; and Phase 2 clinical trial of BTT1023 in primary sclerosing cholangitis, which is currently recruiting patients.
General and administrative expenses consist of salary-related and external costs related to the Company’s executive, finance and other support
functions, including the costs associated of compliance with the ongoing requirements of being a listed company on Nasdaq in the United States and on the Nasdaq OMX market in Helsinki, including insurance, general administration overhead, investor
relations, legal and professional fees and audit fees.
Other operating income consists primarily of grant income and rent received on a sub-lease; prior
to September 2014 it also included rent from an investment property.
Our policy is to invest funds in low-risk investments, which primarily consists
of money market funds and interest-bearing saving and investment accounts. Savings and deposit accounts generate a small amount of interest income. Interest expenses consist primarily of non-cash interest in respect of the Tekes loans and the
convertible capital loan.
Other net financial income (expense) primarily relates to all non-interest related items and comprises net foreign exchange
gains (losses) that arise from our intercompany borrowings, and unrealized and realized gains from money market funds, that are reflected as financial assets held at fair value through profit and loss.
The Company does not generally pay any corporate income taxes, as there are currently cumulative operating losses in each subsidiary company.
C-4

Results of Operations: comparison of the nine months ended September 30, 2015 and
September 30, 2014
Revenue
Revenue
decreased 77% by €10.1 million to €3.0 million for the nine months ended September 30, 2015 compared to €13.1 million for the nine months ended September 30, 2014. The decrease was primarily due to the payment
of the Phase 3 development milestones from UCB for tozadenant of €5.0 million in the first three months of 2014, which did not recur thereafter due to the termination of the related agreement and €6.0 million of commercial
milestones from Lundbeck for Selincro that were received in the third quarter of 2014. This was partially offset by an increase in royalties from Lundbeck for Selincro of €1.9 million as a result of increased sales and the first commercial
milestone for Selincro received in 2015 of €0.5 million in the three months ended June 30, 2015. The Company also recognized revenue related to Phase 3 development funding from UCB in the periods that ended June 30,
2014, September 30, 2014 and March 31, 2015, but not in the three months ended June 30, 2015 and September 30, 2015.
Research
and development expenses
Research and development expenses increased by €7.7 million for the nine months ended September 30, 2015 to
€19.6 million, compared to €11.9 million for the nine months ended September 30, 2014. The majority of the expenditure in each period was in relation to tozadenant, with the increase mainly being due to the stage of the
development activities. During the three months ended September 30, 2015, the company paid a total €1.3 million of regulatory milestones in respect of tozadenant and BTT1023.
General and administrative expenses
General and
administrative expenses increased by €0.4 million to €5.7 million for the nine months ended September 30, 2015, as compared to €5.3 million for the nine months ended September 30, 2014.
Other operating income
Other operating income for the
nine months ended September 30, 2015 amounted to €0.2 million, comprising sub-lease rental income and grant income from MJFF. This is €0.6 million lower than the €0.8 million for the nine months ended
September 30, 2014, comprising rental income from an investment property in Germany that was sold in September 2014.
Interest income
Interest income was minimal for both of the nine months ended September 30, 2015 and 2014.
Interest expenses
Interest expenses consist of non-cash
interest expenses accrued on the Tekes loans and the convertible capital loans, which remained broadly stable. As a result, interest expenses were €0.5 million for both of the nine month periods ended September 30, 2015 and 2014.
Other net financial income (expenses)
Other net
financial income (expenses) mainly comprises net foreign exchange differences and was a net loss of €0.3 million for the nine months ended September 30, 2015, compared to a €1.2 million gain for the nine months ended
September 30, 2014
C-5

Other comprehensive income (loss)
Other comprehensive income (loss) comprises currency translation differences, which mainly arise from the translation of in-process R&D assets and goodwill
in our foreign subsidiaries. It was a gain of €5.1 million for the nine months ended September 30, 2015, an increase of €0.2 million as compared to the gain of €4.9 million for the nine months ended
September 30, 2014. The movement for the nine month period ended September 30, 2015 is due to the significant devaluation in the Euro against the United States Dollar and Swiss Franc mainly during the three month period ended
March 31, 2015.
Liquidity and Capital resources
Cash flows
Net cash outflow from operating activities for
the nine months ended September 30, 2015 was €23.0 million, an increase of €12.7 million as compared to the net cash outflow of €10.3 million during the same period in 2014, due to a higher net loss.
Net cash outflow from investing activities was €1.2 million for the nine months ended September 30, 2015, a decrease of €6.8 million
as compared to the net cash inflow of €5.6 million in the same period in 2014, due to investment in and proceeds from sale of financial assets at fair value through profit or loss.
Net cash inflow from financing activities was €74.3 million for the nine months ended September 30, 2015, an increase of
€74.3 million compared to the inflow of €0.0 million for the same period in 2014. The reason for the increase was the net proceeds received from the issue of the convertible notes on May 28, 2015 of €30.2 million and
the issue of share capital associated with the US public offering on June 16, 2015 of €44.1 million. The remaining inflows relate solely to the proceeds from share issues in respect of employee equity plans and are minimal in both periods.
Liquid assets, comprising cash and cash equivalents and financial assets at fair value through profit and loss, totaled €84.0 million at
September 30, 2015 as compared to €32.4 million at December 31, 2014. The increase of €51.6 million was mainly due to the net proceeds received from the issue of the convertible notes and US public offering of
€74.1 million, which was partially offset by utilization of cash flow for financing the operating activities, principally research and development expenses.
Cash and funding sources
Our main sources of revenue
during the periods presented were from UCB in relation to tozadenant and milestones and royalties from Lundbeck in relation to Selincro sales.
On
May 29, 2015, the Company announced that it had completed the issuance of in total 220,400,001 convertible notes and 220,400,001 warrants, which may be exercised at an exercise price of €0.17 within a period of five years starting six
months after their date of issue, to certain US investors and certain existing shareholders pursuant to the authorization granted by the Annual General Meeting of shareholders on May 26, 2015. The total principal amount raised from the issuance
of the convertible notes was €33.1 million. The warrants were issued free of charge to the subscribers of the convertible notes.
On June 16,
2015, the Company announced that it had closed its US public offering. It was confirmed that the Company had offered 3,806,047 American Depositary Shares (ADS) in its US public offering at a price to the public of $14.888 per ADS for gross proceeds
of $56.7 million (€50.2 million at the fixed ECB exchange rate of $1.1279 per euro as at June 10, 2015, the date of pricing). The share to ADS ratio is 80 to one, and the ADS
C-6

represent 304,483,760 newly issued shares in the Company with a subscription price of €0.165 (rounded figure) per new share (at the above mentioned fixed exchange rate). This includes the
full exercise of the underwriters’ over-allotment option. The issuance of new shares by the Company for the purpose of the completion of the US public offering was based on the authorization granted by the Annual General Meeting of
shareholders on May 26, 2015. Following the completion of the US public offering the automatic conversion of the convertible notes issued by the Company to certain US investors and existing shareholders and the issue of 220,400,001 new shares
to such noteholders at the pre-determined conversion price of €0.15 per new share has also been effected.
We have no ongoing material financial
commitments, such as lines of credit or guarantees, which are expected to affect our liquidity over the next five years, other than research and development loans, some of which are due for repayment as described in note 13 to the unaudited
condensed consolidated financial statements for the nine months ended September 30, 2015.
Personnel
During the reporting period January – September 2015 (2014), the average number of employees amounted to 38 (35) and at the end of the reporting
period, Biotie employed 39 people (35 people).
Equity rights
Swiss Option Plan
The Swiss company Biotie Therapies AG
has a stock option plan under which stock options have been granted to employees, directors and consultants. In connection with the completion of the acquisition of Synosia, the option plan was amended so that instead of shares in Synosia an
aggregate maximum of 14,912,155 shares in Biotie may be subscribed for based on the plan.
The Swiss subsidiary holds and has held Biotie’s shares
and such shares have been conveyed to satisfy the terms and conditions of the Swiss option plan. The conveyed shares previously held by the Company’s subsidiary have been treated as treasury shares and such shares have not carried any voting
rights. As of September 30, 2015 a total of 9,794,865 shares have already been delivered on the basis of the Swiss option plan. As a result of certain of the stock options being cancelled, a total of 2,053,134 stock options remain outstanding
and as a result, the outstanding shares and votes of Biotie may be further increased.
As at September 30, 2015, Biotie Therapies AG holds 2,605,691
shares in the Company as treasury shares to settle the remaining options.
2011 Plans
In December 2011, the Board of Directors of Biotie approved two share-based incentive plans for the Group employees; a stock option plan for mainly its
European employees and an equity incentive plan for mainly its US employees (together the 2011 plans).
On December 17, 2014, pursuant to the
authorization of the Annual General Meeting of Shareholders held on April 3, 2014, the Board of Directors resolved to issue 2,447,375 new shares to the company itself without consideration in accordance with Chapter 9 Section 20 of the
Finnish Companies Act (624/2006, as amended). The shares were issued for the purposes of conveying them to employees entitled to the shares pursuant to the terms and conditions of the 2011 plans. The treasury shares are of the same class as the
existing shares in the Company. The shares were registered in the Finnish Trade Register on December 23, 2014. At September 30, 2015 none of these shares were still held by the Company.
C-7

Stock Option Plan 2011: The maximum total number of stock options issued is 7,401,000, and they
entitle their owners to subscribe for a maximum total of 7,401,000 new shares in the company or existing shares held by the company. After giving effect to shares already issued, forfeitures and some of the instruments based on the plan having been
left unallocated, a maximum of 1,957,500 shares on September 30, 2015 may still be issued pursuant to the plan.
A total of 1,793,000 shares were
subscribed for during the period January - September 2015 under the plan and 1,793,000 of the treasury shares issued on December 17, 2014 were used for these share subscriptions.
Equity Incentive Plan 2011: The maximum number of share units to be granted and the number of corresponding shares to be delivered on the basis of the
plan will be total of 4,599,000 shares. However, due to share issues already made pursuant to the plan, forfeitures and some of the instruments based on the plan having been left unallocated, a maximum of 660,000 shares on September 30, 2015
may still be issued pursuant to the plan.
A total of 654,375 shares have been conveyed to employees without consideration during the period January -
September 2015 pursuant to the authorization of the Annual General Meeting of the Shareholders held on April 3, 2014 under the plan and 654,375 of the treasury shares issued on December 17, 2014 have been used for these share conveyances.
2014 Plans
On January 2, 2014 the Board of
Directors of Biotie approved three year incentive plans for employees. A stock option plan mainly for its European employees and an equity incentive plan mainly for its US employees.
Stock Option Plan 2014: The maximum total number of stock options to be awarded is 10,337,500, of which 4,320,000 relate to the Senior Management team
only. Stock options entitle their owners to subscribe for a maximum total of 10,337,500 new shares in the company or existing shares held by the Company. The Board of Directors shall decide on the distribution of the stock options.
Equity Incentive Plan 2014: The maximum number of share units to be granted and the number of corresponding shares to be delivered under the plan will
be a total of 14,002,500 shares, of which 2,520,000 relate to the Senior Management team only.
Available Facilities
Biotie has a standby equity distribution agreement (SEDA) in place with US fund Yorkville. Yorkville is under certain pre-agreed terms and conditions obliged
to subscribe and pay for Biotie shares in multiple tranches up to a total value of €20 million during the period until November 12, 2015 at Biotie’s discretion. The purpose of this arrangement is to have an option to secure the
financing of Biotie’s working capital in the short and medium term. Biotie last made use of this arrangement in 2010, raising a total amount of €1.1 million, but since then has not conveyed any shares under this agreement.
Share capital and shares
After the US public offering,
which closed on June 16, 2015, Biotie has shares quoted on NASDAQ OMX (Small Cap) in Helsinki (ticker: BTH1V) and American Depositary Shares (ADS) quoted on NASDAQ (Global Select Market) in the United States (ticker: BITI), where each ADS
represents 80 of the Company’s shares. The Company’s shares all have equal rights and each share entitles the holder to one vote at the general meeting of shareholders.
C-8

On September 30, 2015 the registered number of shares in Biotie Therapies Corp. was 980,851,935. Of
these shares 2,605,691 were held by the Company or its group companies. The registered share capital of Biotie was €279,218,058.55 (FAS).
Market
capitalization and trading
The key data for each of the shares listed in Helsinki and the ADS listed in the United States during the nine month period
ended September 30 2015 is shown below.
|
|
|
|
|
|
|
|
|
| |
|
Shares listed
in Helsinki |
|
|
ADS listed
in the United
States* |
|
| Price at end of period |
|
€ |
0.16 |
|
|
$ |
13.09 |
|
| Highest price during period |
|
€ |
0.26 |
|
|
$ |
25.39 |
|
| Lowest price during period |
|
€ |
0.14 |
|
|
$ |
12.77 |
|
| Average price during period |
|
€ |
0.20 |
|
|
$ |
18.17 |
|
| Market capitalization at end of period |
|
€ |
154 million |
|
|
$ |
159.7 million |
|
| Trading volume during period |
|
|
157,665,690 shares |
|
|
|
6,085,578 ADS |
|
| Turnover during period |
|
€ |
31.3 thousand |
|
|
$ |
114.0 thousand |
|
| * |
All trading information in relation to ADS listed on the NASDAQ market in the United States relates to the period since June 11, 2015, which was the first day of trading on that market. |
Annual General Meeting
The Annual General Meeting of
Biotie Therapies Corp. was held on May 26, 2015 and the resolutions of the meeting were published in a stock exchange release on the same day.
Risks and uncertainties
A detailed analysis of the risks
that Biotie faces are set out in the Company’s Registration Statement on Form F-1 as filed with the U.S. Securities and Exchange Commission on June 10, 2015 and the following summary of the key risks should be read in conjunction with that
document.
| |
• |
|
We have incurred net losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future. As of September 30, 2015, our retained earnings were an
accumulated deficit of €177.6 million. We may never achieve or sustain profitability. |
| |
• |
|
Impairment charges or write-downs on our assets could have a significant impact on our results of operations and financial results. |
| |
• |
|
We depend significantly on the success of tozadenant and our other product candidates. Tozadenant and our other product candidates are still in clinical development. If our clinical trials are not successful, we do not
obtain regulatory approval or we are unable, or unable to find a partner, to commercialize tozadenant or our other product candidates, or we experience significant delays in doing so, our business, financial condition and results of operations will
be materially adversely affected. |
| |
• |
|
Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes. |
| |
• |
|
The results of previous clinical trials may not be predictive of future results and clinical trials of product candidates may not be successful. |
C-9

| |
• |
|
Clinical development, regulatory review and approval by the FDA, the EMA and comparable foreign regulatory authorities are lengthy, time consuming, expensive and inherently unpredictable activities. If we are ultimately
unable to obtain regulatory approval for our product candidates, our business will be substantially harmed. |
| |
• |
|
The FDA’s agreement to our SPA for our Phase 3 trial of tozadenant does not guarantee any particular outcome from regulatory review, including ultimate approval and may not lead to a faster development or
regulatory review or approval process. |
| |
• |
|
Collaborations on products and product candidates are important to our business, and future collaborations may also be important to us. If we are unable to maintain any of these collaborations, if these collaborations
are not successful, or if we fail to enter into new strategic relationships, our business could be adversely affected. |
| |
• |
|
We rely on third parties to conduct our nonclinical and clinical trials and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or comply
with regulatory requirements, we may not be able to obtain regulatory approval for, or commercialize, our product candidates and our business could be substantially harmed. |
| |
• |
|
We currently rely on third-party suppliers and other third parties for production of our product candidates and our dependence on these third parties may impair the advancement of our research and development programs
and the development of our product candidates. |
| |
• |
|
If we are unable to obtain and maintain sufficient intellectual property protection for our product or product candidates, or if the scope of our intellectual property protection is not sufficiently broad, our ability
to commercialize our product and product candidates successfully and to compete effectively may be adversely affected. |
| |
• |
|
Our relationships with healthcare professionals, institutional providers, principal investigators, consultants, customers (actual and potential) and third party payors are, and will continue to be, subject, directly and
indirectly, to health care fraud and abuse, false claims, marketing expenditure tracking and disclosure, government report pricing, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such
laws, we could face penalties, including, without limitation, civil, criminal and administrative penalties, damages, fines, exclusion from government-funded health care programs and the curtailment or restructuring of our operations.
|
| |
• |
|
We cannot assure of the adequacy of our capital resources to successfully complete the development and commercialization of our product candidates, and a failure to obtain additional capital, if needed, could force us
to delay, limit, reduce or terminate our product development or commercialization efforts. The adequacy of our capital resources is particularly dependent on cash generation from milestones and royalties in connection with sales of Selincro and
other sources of non-dilutive funding. |
| |
• |
|
As a foreign private issuer in the United States, we are permitted to adopt certain Finnish practices in relation to corporate governance matters that differ significantly from NASDAQ corporate governance listing
standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards in the United States. |
Biotie continues to face a number of potential risks and uncertainties which could have a material effect on the Group’s performance over the remaining
three months of the financial year and thereafter and could cause actual results to differ from expected and historical results.
C-10

Outlook for 2015 and key upcoming milestones
Selincro® (nalmefene): Biotie anticipates that Lundbeck will continue to make sales of Selincro
in European markets during 2015 following the positive pricing and reimbursement decisions received in the second half of 2014. In addition to royalties, Biotie may also receive further milestone payments if the product reaches certain
pre-determined sales.
Tozadenant (SYN115): The Phase 3 clinical study, which is expected to be the second pivotal study required for registration,
commenced patient recruitment in July 2015. Top-line data from the double-blind part of the study is expected by the end of 2017, followed by the open-label portion of the study and a separate open-label study. Additional studies required for a
regulatory filing package will continue to be completed prior to regulatory submissions.
SYN120: Patient enrollment into an 80-patient Phase 2
study with SYN120 in Parkinson’s disease dementia (the SYNAPSE study) started in December 2014. The study, funded by MJFF, is being conducted by the Parkinson Study Group at approximately 12 specialist sites in the United States. Top-line
results of the study are expected by the end of 2016.
BTT1023: Patient enrollment into an investigator-sponsored Phase 2 study in primary
sclerosing cholangitis (the BUTEO study) started in March 2015. The 41-patient study is being conducted in the UK and is supported by grant funding from the UK’s National Institute for Health Research. It is expected that the requisite number
of patients will have been treated by the end of 2016 to enable a pre-planned interim analysis in this two-stage study.
Financial: During the
remainder of 2015, the Company expects to continue receiving Selincro royalties from Lundbeck. Research and development expenses will continue on all development products, with the tozadenant Phase 3 study now recruiting patients. Following the
financing received from the convertible notes and the US public offering in Q2 2015, the Company has a strong level of liquid resources that are expected to be sufficient for all the Company’s currently planned development activities; these
liquid resources will decrease over time, as they are invested in the Company’s product development programs.
Strategic: The
Company’s primary focus is to ensure that the Phase 3 clinical study for tozadenant is efficiently and effectively executed, with the top-line data expected by the end of 2017. SYN120 and BTT1023, funded largely by non-dilutive financing, are
both expected to reach significant potential inflection points by the end of 2016.
Key events after the reporting period
Biotie announced on October 7, 2015 that, pursuant to the authorization of the Annual General Meeting of Shareholders held on May 26, 2015, the Board
of Directors of Biotie has resolved to issue 106,088,336 shares to the Company itself without consideration in accordance with Chapter 9 Section 20 of the Finnish Companies Act (624/2006, as amended). The Treasury Shares are issued to
facilitate the timely delivery by the Company of such Treasury Shares underlying the warrants issued in May 2015 to certain US investors and certain existing shareholders based on the authorization granted by the Annual General Meeting of the
Company on May 26, 2015, if and when such above-mentioned warrants are exercised.
The Treasury Shares were registered with the Finnish Trade
Register on October 8, 2015, and admitted trading on NASDAQ OMX Helsinki Ltd on October 9, 2015. The Treasury Shares are of the same class as the existing shares in the Company.
As a result of this Biotie has 1,086,940,271 shares in total of which 978,246,244 will be outstanding shares.
C-11

Biotie announced on November 2, 2015 the change in the number of votes relating to the Swiss subsidiary
of the Company, Biotie Therapies AG (previously Synosia Therapeutics Holding AG and Biotie Therapies Holding AG) conveyed Biotie shares against consideration pursuant to the option programs in October in total 7,739.
After the conveyances the total amount of voting rights is 978,253,983 and the number of the Company’s share held by the Biotie Group is 108,686,288
(9.99 per cent). The conveyance does not affect the number of registered shares (total 1,086,940,271).
Conference call
An analyst and media conference call will take place on 12 November 2015 3:00 pm Finnish time (8:00 pm Eastern time). The conference call will be
held in English.
Lines are to be reserved ten minutes before the start of conference call. The event can also be viewed as a live webcast at
www.biotie.com. An on demand version of the conference will be published on Biotie’s website later during the day
US callers: +1212 444
0412
UK callers: +44(0)20 3427 1900
Finnish callers:
+358(0)9 6937 9543
Access code: 1072486
In case you need
additional information or assistance, please contact: Virve Nurmi, IR Manager, Tel: +358 2 2748 911
About Biotie
Biotie is a specialized drug development company focused on products for neurodegenerative and psychiatric disorders. Biotie’s development has delivered
Selincro (nalmefene) for alcohol dependence, which received European marketing authorization in 2013 and is currently being rolled out across Europe by partner Lundbeck. The current development products include tozadenant for Parkinson’s
disease, which is in Phase 3 development, and two additional compounds which are in Phase 2 development for cognitive disorders including Parkinson’s disease dementia, and primary sclerosing cholangitis (PSC), a rare fibrotic disease of the
liver.
Biotie’s shares are listed on NASDAQ Helsinki (BTH1V) and ADS on Nasdaq Stock Market LLC (BITI).
Group structure: The parent company of the group is Biotie Therapies Corp. The domicile of the company is Turku, Finland. The Company has two operative
subsidiaries, Biotie Therapies Inc, located in South San Francisco, United States of America and Biotie Therapies AG, located in Zurich, Switzerland.
The
Group also has two non-operational subsidiaries, Biotie Therapies GmbH located in Radebeul, Germany and Biotie Therapies International Ltd located in Finland.
Forward looking statements: This interim report may contain statements that constitute “forward-looking statements” within the meaning of
Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are statements other than historical fact and may include statements that address future operating, financial or
business performance or Biotie’s strategies or expectations. In some cases, you can identify these statements by forward-looking words such as “may,”
C-12

“might,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,”
“projects,” “potential,” “outlook” or “continue,” and other comparable terminology. Forward-looking statements are based on management’s current expectations and beliefs and involve significant risks and
uncertainties that could cause actual results, developments and business decisions to differ materially from those contemplated by these statements. These risks and uncertainties include, but are not limited to, the timing and conduct of clinical
trials of Biotie’s product candidates, plans to pursue research and development of product candidates, the clinical utility of Biotie’s product candidates, the timing or likelihood of regulatory filings and approvals, Biotie’s
intellectual property position, expectations regarding payments under Biotie’s collaborations and Biotie’s competitive position. These risks and uncertainties also include those described under the captions “Risk Factors” and
“Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Biotie’s Registration Statement on Form F-1 and future filings with the Securities and Exchange Commission. Forward-looking statements
speak only as of the date they are made, and Biotie does not undertake any obligation to update them in light of new information, future developments or otherwise, except as may be required under applicable law. All forward-looking statements are
qualified in their entirety by this cautionary statement.
Turku, 12 November 2015
Biotie Therapies Corp.
Board of Directors
C-13

CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
For the three month
period ended
September 30, |
|
|
For the nine month
period ended
September 30, |
|
| (€ in thousands, except per share data) |
|
Note |
|
|
2015 |
|
|
2014 |
|
|
2015 |
|
|
2014 |
|
| Revenue |
|
|
3 |
|
|
|
786 |
|
|
|
7,192 |
|
|
|
2,987 |
|
|
|
13,051 |
|
| Research and development expenses |
|
|
|
|
|
|
(7,252 |
) |
|
|
(4,221 |
) |
|
|
(19,611 |
) |
|
|
(11,931 |
) |
| General and administrative expenses |
|
|
|
|
|
|
(2,189 |
) |
|
|
(1,587 |
) |
|
|
(5,694 |
) |
|
|
(5,257 |
) |
| Other operating income |
|
|
|
|
|
|
169 |
|
|
|
507 |
|
|
|
235 |
|
|
|
776 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating (loss)/profit |
|
|
|
|
|
|
(8,486 |
) |
|
|
1,891 |
|
|
|
(22,083 |
) |
|
|
(3,361 |
) |
| Interest income |
|
|
|
|
|
|
2 |
|
|
|
1 |
|
|
|
3 |
|
|
|
4 |
|
| Interest expenses |
|
|
|
|
|
|
(171 |
) |
|
|
(175 |
) |
|
|
(478 |
) |
|
|
(486 |
) |
| Other net financial income (expenses) |
|
|
|
|
|
|
735 |
|
|
|
1,210 |
|
|
|
(260 |
) |
|
|
1,198 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (Loss)/profit before taxes |
|
|
|
|
|
|
(7,920 |
) |
|
|
2,927 |
|
|
|
(22,818 |
) |
|
|
(2,645 |
) |
| Income tax |
|
|
4 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net (loss)/profit |
|
|
|
|
|
|
(7,920 |
) |
|
|
2,927 |
|
|
|
(22,818 |
) |
|
|
(2,645 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Items that may be subsequently reclassified to profit or loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Currency translation differences* |
|
|
|
|
|
|
(1,978 |
) |
|
|
3,913 |
|
|
|
5,060 |
|
|
|
4,866 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total other comprehensive (loss)/income |
|
|
|
|
|
|
(1,978 |
) |
|
|
3,913 |
|
|
|
5,060 |
|
|
|
4,866 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total comprehensive (loss)/income |
|
|
|
|
|
|
(9,898 |
) |
|
|
6,840 |
|
|
|
(17,758 |
) |
|
|
2,220 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net (loss)/income attributable to equity holders of the parent |
|
|
|
|
|
|
(7,920 |
) |
|
|
2,927 |
|
|
|
(22,818 |
) |
|
|
(2,645 |
) |
| Total comprehensive (loss)/income attributable to equity holders of the parent |
|
|
|
|
|
|
(9,898 |
) |
|
|
6,840 |
|
|
|
(17,758 |
) |
|
|
2,221 |
|
| Loss/(earnings) per share (EPS) basic & diluted, € |
|
|
5 |
|
|
|
(0.01 |
) |
|
|
0.01 |
|
|
|
(0.03 |
) |
|
|
(0.01 |
) |
| * |
The translation differences mainly arise in relation to in-process R&D assets and goodwill. The movement for the nine month period ended September 30, 2015 is due to the significant devaluation in the Euro
against the United States Dollar and Swiss Franc mainly during the three month period ended March 31, 2015. |
All activities relate to
continuing operations.
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
C-14

CONDENSED CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
As at
September 30,
2015 |
|
|
As at
December 31,
2014 |
|
| (€ in thousands) |
|
Note |
|
|
(unaudited) |
|
|
|
|
| ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-current assets |
|
|
|
|
|
|
|
|
|
|
|
|
| Intangible assets |
|
|
6 |
|
|
|
51,555 |
|
|
|
47,356 |
|
| Goodwill |
|
|
6 |
|
|
|
6,328 |
|
|
|
5,799 |
|
| Property, plant and equipment |
|
|
7 |
|
|
|
609 |
|
|
|
653 |
|
| Non-current pre-payments |
|
|
8 |
|
|
|
3,590 |
|
|
|
— |
|
| Other financial assets |
|
|
|
|
|
|
337 |
|
|
|
324 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total non-current assets |
|
|
|
|
|
|
62,419 |
|
|
|
54,132 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current assets |
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts receivable and other receivables |
|
|
|
|
|
|
1,965 |
|
|
|
1,806 |
|
| Financial assets at fair value through profit or loss |
|
|
9 |
|
|
|
26,471 |
|
|
|
24,941 |
|
| Cash and cash equivalents |
|
|
|
|
|
|
57,549 |
|
|
|
7,452 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
|
|
|
|
85,985 |
|
|
|
34,199 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
|
|
|
|
|
148,404 |
|
|
|
88,331 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| EQUITY AND LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
| Shareholders’ equity |
|
|
|
|
|
|
|
|
|
|
|
|
| Share capital |
|
|
11 |
|
|
|
267,418 |
|
|
|
193,285 |
|
| Reserve for invested unrestricted equity |
|
|
|
|
|
|
5,417 |
|
|
|
5,378 |
|
| Other reserves |
|
|
|
|
|
|
14,089 |
|
|
|
9,029 |
|
| Retained earnings |
|
|
|
|
|
|
(177,257 |
) |
|
|
(155,069 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total equity |
|
|
|
|
|
|
109,667 |
|
|
|
52,623 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-current financial liabilities |
|
|
9 |
|
|
|
20,690 |
|
|
|
20,690 |
|
| Pension benefit obligation |
|
|
|
|
|
|
670 |
|
|
|
670 |
|
| Other non-current liabilities |
|
|
|
|
|
|
10,143 |
|
|
|
9,671 |
|
| Non-current deferred revenues |
|
|
|
|
|
|
2,000 |
|
|
|
2,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total non-current liabilities |
|
|
|
|
|
|
33,503 |
|
|
|
33,031 |
|
| Current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and other current liabilities |
|
|
|
|
|
|
5,234 |
|
|
|
2,677 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
|
|
|
|
5,234 |
|
|
|
2,677 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
|
|
|
|
38,737 |
|
|
|
35,708 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total shareholders’ equity and liabilities |
|
|
|
|
|
|
148,404 |
|
|
|
88,331 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
C-15

CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY (UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Attributable to equity holders of the parent company |
|
| (€ in thousands) |
|
Note |
|
|
Share
capital |
|
|
Reserve
for invested
unrestricted
equity |
|
|
Other
reserves |
|
|
Retained
earnings |
|
|
Share-
holders’
equity
total |
|
| Balance at January 1, 2014 |
|
|
|
|
|
|
193,285 |
|
|
|
5,252 |
|
|
|
2,517 |
|
|
|
(120,688 |
) |
|
|
80,366 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss for the period |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,645 |
) |
|
|
(2,645 |
) |
| Other comprehensive income |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
4,866 |
|
|
|
— |
|
|
|
4,866 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total comprehensive income (loss) |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
4,866 |
|
|
|
(2,645 |
) |
|
|
2,221 |
|
| Share based compensation |
|
|
12 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
596 |
|
|
|
596 |
|
| Options and RSU exercised |
|
|
12 |
|
|
|
— |
|
|
|
86 |
|
|
|
— |
|
|
|
— |
|
|
|
86 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
86 |
|
|
|
4,866 |
|
|
|
(2,049 |
) |
|
|
2,903 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at September 30, 2014 |
|
|
|
|
|
|
193,285 |
|
|
|
5,338 |
|
|
|
7,383 |
|
|
|
(122,737 |
) |
|
|
83,269 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at January 1, 2015 |
|
|
|
|
|
|
193,285 |
|
|
|
5,378 |
|
|
|
9,029 |
|
|
|
(155,069 |
) |
|
|
52,623 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss for the period |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(22,818 |
) |
|
|
(22,818 |
) |
| Other comprehensive income |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
5,060 |
|
|
|
— |
|
|
|
5,060 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total comprehensive income (loss) |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
5,060 |
|
|
|
(22,818 |
) |
|
|
(17,758 |
) |
| Share based compensation |
|
|
12 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
630 |
|
|
|
630 |
|
| Options and RSU exercised |
|
|
12 |
|
|
|
— |
|
|
|
39 |
|
|
|
— |
|
|
|
— |
|
|
|
39 |
|
| Issue of convertible notes and warrants |
|
|
11 |
|
|
|
33,060 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
33,060 |
|
| Transaction costs related to convertible note issue |
|
|
|
|
|
|
(2,844 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,844 |
) |
| Issue of share capital |
|
|
11 |
|
|
|
50,239 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
50,239 |
|
| Transaction costs related to share issue |
|
|
|
|
|
|
(6,322 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6,322 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
74,133 |
|
|
|
39 |
|
|
|
5,060 |
|
|
|
(22,188 |
) |
|
|
57,044 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at September 30, 2015 |
|
|
|
|
|
|
267,418 |
|
|
|
5,417 |
|
|
|
14,089 |
|
|
|
(177,257 |
) |
|
|
109,667 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
C-16

CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
For the nine month
period ended
September 30, |
|
| (€ in thousands) |
|
Note |
|
|
2015 |
|
|
2014 |
|
| Cash flow from operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
|
|
|
|
|
(22,818 |
) |
|
|
(2,645 |
) |
| Adjustments for: |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-cash transactions |
|
|
13 |
|
|
|
412 |
|
|
|
466 |
|
| Interest income |
|
|
|
|
|
|
(3 |
) |
|
|
(4 |
) |
| Interest expenses |
|
|
|
|
|
|
478 |
|
|
|
486 |
|
| Other net financial income (expenses) |
|
|
|
|
|
|
260 |
|
|
|
(1,198 |
) |
| Change in working capital: |
|
|
|
|
|
|
|
|
|
|
|
|
| Change in accounts receivables and other receivables |
|
|
|
|
|
|
(3,638 |
) |
|
|
(3,601 |
) |
| Change in accounts payable and other liabilities |
|
|
|
|
|
|
2,287 |
|
|
|
(2,325 |
) |
| Change in deferred revenue |
|
|
|
|
|
|
— |
|
|
|
(1,415 |
) |
| Interest paid |
|
|
|
|
|
|
(27 |
) |
|
|
(27 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash used in operating activities |
|
|
|
|
|
|
(23,049 |
) |
|
|
(10,263 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flow from investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Investments in financial assets at fair value through profit and loss |
|
|
|
|
|
|
(30,588 |
) |
|
|
— |
|
| Proceeds from sale of financial assets at fair value through profit and loss |
|
|
|
|
|
|
29,488 |
|
|
|
4,440 |
|
| Proceeds from sale of investment property |
|
|
|
|
|
|
— |
|
|
|
1,350 |
|
| Change in other financial assets |
|
|
|
|
|
|
— |
|
|
|
(51 |
) |
| Investments in property, plant and equipment |
|
|
|
|
|
|
(87 |
) |
|
|
(133 |
) |
| Investments in intangible assets |
|
|
|
|
|
|
(14 |
) |
|
|
(30 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash (used in)/from investing activities |
|
|
|
|
|
|
(1,201 |
) |
|
|
5,576 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flow from financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from option exercise and RSU delivery |
|
|
|
|
|
|
39 |
|
|
|
86 |
|
| Net proceeds from convertible note and warrants issue |
|
|
|
|
|
|
30,216 |
|
|
|
— |
|
| Net proceeds from share issue |
|
|
|
|
|
|
44,085 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash from financing activities |
|
|
|
|
|
|
74,340 |
|
|
|
86 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net increase in cash and cash equivalents |
|
|
|
|
|
|
50,089 |
|
|
|
(4,601 |
) |
| Effect of changes in exchange rates on cash and cash equivalents |
|
|
|
|
|
|
8 |
|
|
|
(27 |
) |
| Cash and cash equivalents at the beginning of the period |
|
|
|
|
|
|
7,452 |
|
|
|
10,221 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents at the end of the period |
|
|
|
|
|
|
57,549 |
|
|
|
5,593 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
C-17

NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
1. General Information
Biotie Therapies Oyj
(“Biotie” or the “Company”) is a specialized drug development company incorporated and domiciled in Finland, with its headquarters at Joukahaisenkatu 6, Turku, Finland, focused on products for neurodegenerative and psychiatric
disorders. Biotie operates primarily in Finland and in the United States. Biotie’s development has delivered Selincro (nalmefene) for alcohol dependence, which received European marketing authorization in 2013 and is currently being rolled out
across Europe by partner Lundbeck. The current development products include tozadenant for Parkinson’s disease, which is in Phase 3 development, and two additional compounds which are in Phase 2 development for cognitive disorders including
Parkinson’s disease dementia and primary sclerosing cholangitis, a rare fibrotic disease of the liver. Biotie’s shares are listed on NASDAQ Helsinki (BTH1V) and on Nasdaq Stock Market LLC (BITI). As used in these condensed consolidated
financial statements, unless the context indicates otherwise, all references to “Biotie” or the “Company” or the “Group” refer to Biotie Therapies Oyj and all its consolidated subsidiaries.
The condensed consolidated financial statements were approved for issue by the Board of Directors on November 12, 2015.
2. Summary of Significant Accounting Policies
2.1
Basis of Preparation
These unaudited condensed consolidated financial statements for the nine months ended September 30, 2015 of the Company have
been prepared in accordance with International Accounting Standard IAS 34, “Interim Financial Reporting”. Certain information and disclosures normally included in consolidated financial statements prepared in accordance with International
Financial Reporting Standards (“IFRS”) have been condensed or omitted. However, in the opinion of management, these financial statements contain all adjustments necessary to present a fair statement of results. All adjustments are deemed
to be of a normal, recurring nature. As explained in note 1 to the annual consolidated financial statements to the year ended December 31, 2014, where necessary, comparative figures have been reclassified to conform to changes in presentation
in the current year. The results of operations for the interim periods are not necessarily indicative of the results to be expected for the full year. Accordingly, these condensed consolidated financial statements should be read in conjunction with
the annual consolidated financial statements for the year ended December 31, 2014.
The preparation of financial statements in conformity with IFRS
requires management to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the end of the reporting period, as well as the reported amounts
of income and expenses during the reporting period. Although these estimates are based on management’s best knowledge of current events and actions, actual results may ultimately differ from them. The areas involving a higher degree of judgment
or complexity, or areas where assumptions and estimates are significant to the unaudited condensed consolidated financial statements are disclosed in note 2.10.
The notes to the condensed consolidated financial statements have been rounded to thousand Euros, unless otherwise stated.
2.2 Changes in Accounting Policies and Disclosures
The
Company adopted new IFRS standards, amendments or interpretations during the nine months ended September 30, 2015 that had no material impact to the condensed consolidated financial statements. The accounting policies applied are consistent
with those discussed in the Company’s annual consolidated financial statements.
C-18

(a) New and amended IFRS standards and IFRIC interpretations not yet adopted by the Company
The Company has decided not to implement early IFRS 9 “Financial Instruments”, which is effective for accounting periods ending on or after
January 1, 2018 with early adoption permitted, or IFRS 15 “Revenue from Contracts with Customers”, which is effective for accounting periods ending on or after January 1, 2018 with retrospective effect. The Company is currently
assessing the impact of both new standards. There are no other standards which are currently available for early adoption which are expected to have a significant effect on the condensed consolidated financial statements of the Company.
2.3 Consolidation
Subsidiaries are all entities over
which the Company has control. The Company controls an entity when it is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries
are consolidated from the date at which control is transferred to the Company and are de-consolidated from the date that control ceases. The acquisition method of accounting is used to account for subsidiaries acquired through a business
combination.
Intra-group transactions, balances and unrealized gains and losses on transactions between group companies are eliminated. Unrealized losses
are also eliminated, unless the transaction provides evidence of an impairment of the asset transferred. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Company.
2.4 Segment Reporting
Biotie continues to operate in one
reportable segment, which comprises the development of pharmaceutical products. The Chief Executive Officer is identified as the chief operating decision maker. The Chief Executive Officer reviews the consolidated operating results regularly to make
decisions about the resources and to assess overall performance.
2.5 Seasonality of Operations
The Company’s results have varied substantially, and are expected to continue to vary, from quarter to quarter depending on the royalty streams and level
of development activities within the quarter. The Company, therefore, believes that period to period comparisons should not be relied upon as indicative of future financial results. The Company believes that its ordinary activities are not linked to
any particular seasonal factors.
2.6 Cash and Cash Equivalents
Cash and cash equivalents comprise cash on hand, demand deposits and other short-term highly liquid investments with original maturities of less than three
months.
2.7 Share capital
Shares are classified as
equity. Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds of the share issue.
C-19

When a Group company purchases Parent Company’s shares (treasury shares), the consideration paid,
including any directly attributable incremental costs (net of income taxes) is deducted from equity attributable to the Company’s equity holders until the shares are cancelled, reissued or disposed of, Where such shares are subsequently sold or
reissued, any consideration received net of any directly attributable incremental transaction costs and the related income tax effect is included in the equity attributable to the Company’s equity holders.
In April and May 2015, the Company issued convertible notes and warrants in exchange for cash in an arms’ length transaction that had been approved by
the Company’s shareholders. The convertible notes and warrants issued by the Company have a fixed-to-fixed ratio and do not contain an obligation for a cash redemption by the Company. Accordingly, both instruments met the equity classification
criteria at inception and the proceeds received, net of directly attributable incremental costs, were recorded as share capital. In accordance with the terms and conditions of the note agreements, the convertible notes automatically converted into
the Company’s shares at the date of the US Offering on June 16, 2015 and as of September 30, 2015 there are no outstanding convertible notes. The warrants continue to be outstanding and at upon exercise of a warrant, the subscription
price to be paid in cash for each warrant exercised will be recorded as share capital.
Under the Finnish Companies Act reserve for unrestricted equity
includes the part of a subscription price of a share that is not credited to share capital as well as other equity inputs that are not to be credited to some other reserve. Exercise prices of the share options are included in the reserve for
unrestricted equity.
2.8 Income taxes
Income tax
expense consists of current and deferred taxes. The income tax effects of items recognized in other comprehensive income or directly in equity are similarly recognized in other comprehensive income or equity, respectively. The current income tax
charge is calculated on the basis of the tax laws enacted in the countries where the Company operates and generates taxable income. Taxes on income in interim periods are accrued using tax rates that would be expected to be applicable to total
annual profit or loss.
Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is
subject to interpretation. It establishes provisions where appropriate on the basis of amounts expected to be paid to the tax authorities.
Deferred
income tax is recognized on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. Temporary differences arise primarily from in-process R&D intangible assets,
R&D credits and deferrals, depreciation on property, plant and equipment and net operating loss tax carryforwards.
Deferred income tax assets are
recognized only to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilized.
Deferred taxes are determined using a tax rate enacted, or substantially enacted, as of the date of the balance sheet date in the respective countries.
However, deferred taxes are not recognized if they arise from the initial recognition of goodwill, or in the initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects
neither accounting nor taxable profit nor loss.
2.9 Earnings (loss) per share
Basic earnings (loss) per share is calculated by dividing the net income (loss) attributable to shareholders by the weighted average number of ordinary shares
in issue during the period, excluding ordinary shares purchased by the Company and held as treasury shares.
C-20

Diluted earnings (loss) per share is calculated by adjusting the weighted average number of ordinary shares
outstanding assuming the conversion of all dilutive potential ordinary shares.
2.10 Provisions and Contingent Liabilities
Provisions are recognized when the Company has a present legal or constructive obligation as a result of past events, it is probable that an outflow of
resources will be required to settle the obligation, and a reliable estimate of the amount can be made. Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax rate that
reflects the current market assessments of the time value of money and the risks specific to the obligation. The increase in a provision due to passage of time is recognized in interest expenses.
2.11 Critical Accounting Estimates and Judgments
The
preparation of condensed consolidated financial statements requires management to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and
associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
In preparing these condensed consolidated financial statements, the significant judgments made by management in applying the Company’s accounting
policies and the key sources of estimation uncertainty were the same as those that applied to the Company’s annual consolidated financial statements. The condensed consolidated financial statements do not include all disclosures for critical
accounting estimates and judgment that are required for the annual consolidated financial statements and should be read in conjunction with the Company’s annual consolidated financial statements for the year ended December 31, 2014.
3. Revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the three month
period ended
September 30, |
|
|
For the nine month
period ended
September 30, |
|
| (€ in thousands) |
|
2015 |
|
|
2014 |
|
|
2015 |
|
|
2014 |
|
| Royalties from Lundbeck license agreement |
|
|
786 |
|
|
|
242 |
|
|
|
2,274 |
|
|
|
378 |
|
| Commercial milestone payments from Lundbeck license agreement |
|
|
— |
|
|
|
6,000 |
|
|
|
500 |
|
|
|
6,000 |
|
| Phase 3 development milestones from UCB collaboration agreement |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,047 |
|
| Phase 3 development funding from UCB |
|
|
— |
|
|
|
950 |
|
|
|
213 |
|
|
|
1,626 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
786 |
|
|
|
7,192 |
|
|
|
2,987 |
|
|
|
13,051 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. Income Tax
No income
tax charge or benefit has been recognized in the nine month period ended September 30, 2015, or the corresponding period in 2014. Management’s judgment is that sufficient evidence is not currently available that future taxable profits will
be available against which the unused tax losses or unused tax credits can be utilized by the fiscal entities and, therefore, a deferred tax asset has not been recognized.
C-21

5. (Loss)/Earnings Per Share
(a) Basic (loss)/earnings per share
Basic (loss)/earnings
per share is calculated by dividing the net (loss)/income attributable to shareholders of the parent by the weighted average number of ordinary shares in issue during the period, excluding ordinary shares purchased by the Company and held as
treasury shares.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the three month
period ended
September 30, |
|
|
For the nine month
period ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
|
2015 |
|
|
2014 |
|
| Net (loss)/income attributable to equity holders of the parent (€ in thousands) |
|
|
(7,920 |
) |
|
|
2,927 |
|
|
|
(22,818 |
) |
|
|
(2,645 |
) |
| Weighted average number of outstanding shares (in thousands) |
|
|
978,246 |
|
|
|
456,032 |
|
|
|
659,086 |
|
|
|
456,032 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic (loss)/earnings per share (€ per share) |
|
|
(0.01 |
) |
|
|
0.01 |
|
|
|
(0.03 |
) |
|
|
(0.01 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b) Diluted (loss)/earnings per share
Diluted (loss)/earnings per share is calculated by adjusting the weighted average number of ordinary shares outstanding assuming conversion of all dilutive
potential ordinary shares. The Company has four kinds of potentially dilutive instruments comprising stock options, restricted share units (RSU), a convertible capital loan and warrants over its shares. For the three and nine month periods ended
September 30, 2015 and the nine month period ended September 30, 2014, because there was a loss for the period the potential dilutive shares have an anti-dilutive effect (i.e. decrease the loss per share) and are, therefore, excluded from
the calculation of diluted loss per share. Consequently, the dilutive loss per share is the same as the basic loss per share shown above. For the three month period ended September 30, 2014 there was no difference between the basic earnings per
share and the dilutive earnings per share.
6. Intangible Assets and Goodwill
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
In-process
R&D |
|
|
Production
licenses |
|
|
Software |
|
|
Other
intangible
assets |
|
|
Intangible
assets
total |
|
|
Goodwill |
|
| Book value January 1, 2015 |
|
|
46,830 |
|
|
|
454 |
|
|
|
62 |
|
|
|
10 |
|
|
|
47,356 |
|
|
|
5,799 |
|
| Additions |
|
|
— |
|
|
|
— |
|
|
|
14 |
|
|
|
— |
|
|
|
14 |
|
|
|
— |
|
| Amortization |
|
|
— |
|
|
|
(29 |
) |
|
|
(39 |
) |
|
|
(10 |
) |
|
|
(77 |
) |
|
|
— |
|
| Translation differences |
|
|
4,263 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,263 |
|
|
|
530 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Book value September 30, 2015 |
|
|
51,093 |
|
|
|
425 |
|
|
|
37 |
|
|
|
— |
|
|
|
51,555 |
|
|
|
6,329 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| At September 30, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Acquisition cost |
|
|
98,297 |
|
|
|
762 |
|
|
|
331 |
|
|
|
10 |
|
|
|
99,400 |
|
|
|
5,549 |
|
| Accumulated amortization and impairment |
|
|
(55,368 |
) |
|
|
(337 |
) |
|
|
(294 |
) |
|
|
(10 |
) |
|
|
(56,009 |
) |
|
|
|
|
| Translation differences |
|
|
8,164 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
8,164 |
|
|
|
780 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Book value September 30, 2015 |
|
|
51,093 |
|
|
|
425 |
|
|
|
37 |
|
|
|
— |
|
|
|
51,555 |
|
|
|
6,329 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The amortization charge was €77 thousand for the nine month period ended September 30, 2015 (€77 thousand
for the nine month period ended September 30, 2014) and €14 thousand for the three month period ended September 30, 2015 (€20 thousand for the three month period ended September 30, 2014).
C-22

In-process R&D assets represents the fair value assigned to development projects that the Company
acquired through business combinations, which at the time of the acquisition had not led to marketing approvals that are required for commercialization. Until December 31, 2014 in-process R&D assets comprised the tozadenant (SYN115), SYN120
and nepicastat (SYN117) programs, which were acquired in the Synosia 2011 acquisition; however, at December 31, 2014 the nepicastat (SYN117) in-process R&D asset was written off in full and, therefore, from March 31, 2015 in-process
R&D assets only comprised the tozadenant (SYN115) and SYN120 in-process R&D assets. Amounts capitalized as in-process R&D assets are not amortized until marketing approval has been received for the relevant regulatory authorities.
In-process R&D assets are tested for impairment annually, at December 31, and whenever there is an indication that the asset may be impaired; there have been no such indications during the nine months ended September 30, 2015.
For goodwill, the Company assesses the aggregate fair value of the business as a whole, as there is only one cash generating unit, on an annual basis at
December 31 and whenever there is an indication that goodwill may be impaired; there have been no such indications during the nine months ended September 30, 2015.
7. Property Plant & Equipment
|
|
|
|
|
| (€ in thousands) |
|
Machinery
and
equipment |
|
| Book value January 1, 2015 |
|
|
653 |
|
| Additions |
|
|
87 |
|
| Depreciation |
|
|
(145 |
) |
| Translation differences |
|
|
13 |
|
|
|
|
|
|
| Book value September 30, 2015 |
|
|
609 |
|
|
|
|
|
|
| At September 30, 2015 |
|
|
|
|
| Acquisition cost |
|
|
4,928 |
|
| Accumulated depreciation |
|
|
(4,333 |
) |
| Translation differences |
|
|
13 |
|
|
|
|
|
|
| Book value September 30, 2015 |
|
|
609 |
|
|
|
|
|
|
The depreciation charge was €145 thousand for the nine month period ended September 30, 2015 (€118
thousand for the nine month period ended September 30, 2014) and €77 thousand for the three month period ended September 30, 2015 (€34 thousand for the three month period ended September 30, 2014).
8. Non-current pre-payments
The Company has made
advances to the CRO (Contract Research Organization) in connection with the tozadenant Phase 3 trial in Parkinson’s disease. These advances cover various activities that are expected to take place near the completion of the project. The CRO
will hold such advances in escrow until the activities are performed. The Company classifies these deposits as non-current assets as they are not expected to be utilized within the next 12 month period.
C-23

9. Financial Assets Held at Fair Value through Profit and Loss and Non-Current Financial Liabilities
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
As at |
|
| |
|
September 30, |
|
|
December 31, |
|
| |
|
2015 |
|
|
2014 |
|
| (€ in thousands) |
|
(unaudited) |
|
|
|
|
| Assets |
|
|
|
|
|
|
|
|
| Financial assets held at fair value through profit or loss |
|
|
26,471 |
|
|
|
24,491 |
|
| Liabilities |
|
|
|
|
|
|
|
|
| Non-current financial liabilities |
|
|
20,690 |
|
|
|
20,690 |
|
Financial assets held at fair value through profit or loss, consisting mainly of investments to money market funds, are
measured at their fair value based on quoted bid prices at the reporting date. The fair values are based on fund manager reports and are classified within Level 1or Level 2 in the fair value hierarchy. For Level 1, the fair value measurement is
directly obtained from an active market. For Level 2, the fair value measurement is based on observable quoted market information, although it is not directly obtained from an active market (Level 1). According to the Company’s investment
policy, money market funds held in Europe must have a Morning Star rating of three stars or higher. Money market funds in the U.S. must be rated AAA by Moody’s or AAA by Standard and Poor’s.
Non-current financial liabilities consist of non-convertible capital loans from Tekes, long-term R&D loans from Tekes and a convertible capital loan which
are carried at cost. For fair value disclosure purposes only, the valuation technique that would be used to measure the non-current financial liabilities would rely on unobservable market data and therefore the fair value measures of the loans would
be classified as Level 3 in the fair value hierarchy. The Company has determined that it would not be reasonable to present fair values for the loans, as the Group only has access to Tekes loans and a convertible loan, i.e. similar government grant
loans the Company already has with largely identical terms to the current loans.
10. Financial Risk Management and Financial Instruments
The operations of the Company expose it to financial risks. The main risk that the Company is exposed to is liquidity risk, with capital management being
another important area given the Company’s financing structure. The Company’s risk management principles focus on the unpredictability of the financial markets and aims at minimizing any undesired impacts on the Group’s financial
result. The Board of Directors defines the general risk management principles and approves operational guidelines concerning specific areas including but not limited to liquidity risk, foreign exchange risk, interest rate risk, credit risk, the use
of derivatives and investment of the Company’s liquid assets. During the periods presented, the Company or its subsidiaries have not entered into any derivative contracts.
The condensed consolidated financial statements do not include all financial risk management information and disclosures required in the annual consolidated
financial statements and should be read in conjunction with the Company’s annual consolidated financial statements as at December 31, 2014. There have been no changes in the financial management team that is responsible for financial risk
management or in the Company’s financial risk management policies since December 31, 2014.
C-24

The Company has low risk securities (money market funds) and bank accounts which are as follows:
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
As at |
|
| |
|
September 30, |
|
|
December 31, |
|
| (€ in thousands) |
|
2015 |
|
|
2014 |
|
| Money market funds |
|
|
26,471 |
|
|
|
24,941 |
|
| Bank accounts |
|
|
57,549 |
|
|
|
7,452 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
84,020 |
|
|
|
32,393 |
|
|
|
|
|
|
|
|
|
|
As at September 30, 2015, the contractual maturities of loans and interest are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
2015 |
|
|
2016 |
|
|
2017 |
|
|
2018 -
thereafter |
|
|
Total |
|
| Capital loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment of loans |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
18,000 |
|
|
|
18,000 |
|
| Interest expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
9,767 |
|
|
|
9,767 |
|
| R&D loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment of loans |
|
|
— |
|
|
|
— |
|
|
|
538 |
|
|
|
2,152 |
|
|
|
2,690 |
|
| Interest expenses |
|
|
— |
|
|
|
27 |
|
|
|
22 |
|
|
|
32 |
|
|
|
81 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
— |
|
|
|
27 |
|
|
|
560 |
|
|
|
29,951 |
|
|
|
30,538 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As at September 30, 2015, the Company also had accounts payables of €1,354 thousand and other current
liabilities of €4,347 thousand due within one year.
11. Share Capital
Movements in the Company’s shares outstanding, treasury shares and total registered shares during the nine months ended September 30, 2015 are shown
in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares |
|
Outstanding
shares |
|
|
Treasury
shares |
|
|
Total
registered
shares |
|
| As at January 1, 2015 |
|
|
450,696,015 |
|
|
|
5,272,159 |
|
|
|
455,968,174 |
|
| Share options and RSU exercised |
|
|
2,666,468 |
|
|
|
(2,666,468 |
) |
|
|
— |
|
| Issue of convertible notes |
|
|
220,400,001 |
|
|
|
— |
|
|
|
220,400,001 |
|
| Issue of share capital |
|
|
304,483,760 |
|
|
|
— |
|
|
|
304,483,760 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As at September 30, 2015 |
|
|
978,246,244 |
|
|
|
2,605,691 |
|
|
|
980,851,935 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company’s total authorized number of shares is 980,851,935. All issued shares are fully paid. The shares have no par
value. On September 30, 2015 the total number of shares held in treasury represented approximately 0.3% (December 31, 2014: 1.2%) of the total registered shares. Treasury shares have been issued without consideration for the purpose of the
Company’s share-based compensation plans.
On May 29, 2015, the Company announced that it had completed the issuance of in total 220,400,001
convertible notes and 220,400,001 warrants to certain US investors and certain existing shareholders pursuant to the authorization granted by the Annual General Meeting of shareholders on May 26, 2015. The total principal amount raised from the
issuance of the convertible notes was €33.1 million. The warrants were issued free of charge to the subscribers of the convertible notes. Each convertible noted entitled the holder to convert such
C-25

convertible note into one new share in the Company at a conversion price of €0.15 per share and there would be an automatic conversion into new shares in the Company upon completion of
the US public offering. The subscribers of the convertible notes for each convertible note also received one warrant entitling the holder to subscribe for one new treasury share in the Company at a subscription price of €0.17.
On June 16, 2015, the Company announced that it had closed its US public offering. It was confirmed that the Company had offered 3,806,047 American
Depositary Shares (ADS) in its US public offering at a price to the public of $14.888 per ADS for gross proceeds of $56.7 million (€50.2 million at the fixed ECB exchange rate of $1.1279 per euro as at June 10, 2015, the date of pricing).
The share to ADS ratio is 80 to one, and the ADSs represent 304,483,760 newly issued shares in the Company with a subscription price of €0.165 (rounded figure) per new share (at the above mentioned fixed exchange rate). This includes the full
exercise of the underwriters’ over-allotment option. The issuance of new shares by the Company for the purpose of the completion of the US public offering was based on the authorization granted by the Annual General Meeting of shareholders
on May 26, 2015. Following the completion of the US public offering the automatic conversion of the convertible notes issued by the Company to certain US investors and existing shareholders and the issue of 220,400,001 new shares to such
noteholders at the pre-determined conversion price of €0.15 per new share has also been effected.
The total number of stock options and
restricted stock units outstanding as at September 30, 2015 was 2,053,134, for which the Company holds an equivalent amount of treasury shares which it will use to settle these if they are exercised.
At September 30, 2015, the Company also had 220,400,001 warrants that were outstanding, following their issuance on May 28, 2015. The warrants
entitle the holders to one share for each warrant at a subscription price of €0.17 per share and they may only be subscribed during a five year period beginning on the date five months after their issuance. The Company has authorization
from the Annual General Meeting of the shareholders on May 26, 2015 to issue 220,400,001 shares to settle the warrants should they be exercised and on October 7, 2015, after the reporting date, issued 106,088,336 shares to itself using
this authorization and will continue to hold them as treasury shares until such time as the warrants are exercised.
12. Share Based Payments
The condensed consolidated financial statements do not include all disclosures for share based payments that are required in the annual consolidated financial
statements and should be read in conjunction with the Company’s annual consolidated financial statements for the year ended December 31, 2014.
(a) Stock Option Plan 2011 and Equity Incentive Plan 2011
The Stock Option Plan 2011, primarily for European employees, and the Equity Incentive Plan 2011, primarily for US employees, were approved at the
Company’s 2011 general shareholders’ meeting as part of the Company’s incentive scheme determined by the Board of Directors. These plans contain both a service requirement condition at vesting and individual specified non-market
performance targets during the year of grant.
i. Stock Option Plan 2011
The fair value of the options was determined at the grant date by using the Black-Scholes option valuation model and expensed over the vesting period. The
maximum number of stock options that could be awarded under the plan was 7,401,000, in three equal tranches designated as 2011A, 2011B and 2011C.
C-26

There were no options outstanding for the 2011A tranche as at December 31, 2014. The changes in the
number of options in the plan during the nine months ended September 30, 2015 is shown in the table below.
|
|
|
|
|
|
|
|
|
| Number of options |
|
2011B |
|
|
2011C |
|
| Outstanding at January 1, 2015 |
|
|
1,793,000 |
|
|
|
2,230,000 |
|
| Forfeitures |
|
|
— |
|
|
|
(272,500 |
) |
| Exercised |
|
|
(1,793,000 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Outstanding at September 30, 2015 |
|
|
— |
|
|
|
1,957,500 |
|
|
|
|
|
|
|
|
|
|
All options were fair valued at grant date and recognized as an expense, over the vesting period, to personnel expenses
included in research and development costs and general and administrative costs based on the employee’s function over the vesting period. The expense recognized during the nine months ended September 30, 2015 was €101 thousand
(the expense for nine months ended September 30, 2014 was €363 thousand). The subscription price for all options is €0.01.
ii.
Equity Incentive Plan 2011
The Equity Incentive Plan 2011 includes three consecutive discretionary periods, calendar years 2011 (2011A), 2012
(2011B) and 2013 (2011C) in which the restricted share units may be granted. Each discretionary period is followed by an approximately two year vesting period, ending on January 5, 2014, January 5, 2015 and January 5,
2016, respectively after which the Company’s shares will be delivered to employees on the basis of the granted share units. A maximum of 4,599,000 shares may be delivered under the plan, but there is no maximum that can be issued in any one
year. As at December 31, 2014, all shares had been delivered under the 2011A tranche.
The changes in the number of share units in the plan during
the nine months ended September 30, 2015 is shown in the table below.
|
|
|
|
|
|
|
|
|
| Number of share units |
|
2011B |
|
|
2011C |
|
| Outstanding at January 1, 2015 |
|
|
654,375 |
|
|
|
795,000 |
|
| Forfeitures |
|
|
— |
|
|
|
(135,000 |
) |
| Exercised |
|
|
(654,375 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Outstanding at September 30, 2015 |
|
|
— |
|
|
|
660,000 |
|
|
|
|
|
|
|
|
|
|
The fair value of the restricted share units was determined as the closing share price for Biotie share on the grant date. The
expense recognized during the nine months ended September 30, 2015 was €34 thousand (the net reversal of the expense for the nine months ended September 30, 2014 was €(87) thousand). The exercise price for all share
units is €0.
(b) Swiss option plan
The
Company’s Swiss subsidiary, Biotie Therapies AG, also has a stock option plan approved in 2008. Vesting of the options is related to continued service to the Company. The maximum contractual term of each option is ten years. The plan has been
closed to new grants from February 1, 2011. An aggregate maximum of 14,912,155 shares in Biotie Therapies Corp. has been subscribed to under the plan and such shares have been issued to Biotie Therapies AG to be further conveyed to the
option holders when they potentially exercise their option rights in accordance with the terms and conditions of the option rights. The last day for the share subscriptions based on the option rights in the Swiss option plan is December 7,
2020.
C-27

The changes in the number of options in the plan during the nine months ended September 30, 2015 is
shown in the table below.
|
|
|
|
|
|
|
|
|
| Number of options |
|
Options |
|
|
Weighted
average
exercise
price |
|
| Outstanding at January 1, 2015 |
|
|
2,824,772 |
|
|
€ |
0.24 |
|
| Forfeitures |
|
|
(529,328 |
) |
|
|
|
|
| Exercised |
|
|
(242,310 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding at September 30, 2015 |
|
|
2,053,134 |
|
|
€ |
0.29 |
|
|
|
|
|
|
|
|
|
|
The expense recognized during the nine months ended September 30, 2015 was nil thousand (the net reversal of the expense
for the nine months ended September 30, 2014 was €(29) thousand).
(c) Stock Option Plan 2014 and Equity Incentive Plan 2014
The Stock Option Plan 2014, primarily for European employees, and the Equity Incentive Plan 2014, primarily for US employees, were approved at the
Company’s 2014 general shareholders’ meeting as part of the Company’s incentive scheme determined by the Board of Directors. These plans contain both a service requirement condition at vesting for all awards and for the management
awards, designated 2014M awards, there is an additional specified market performance requirement that determines the number of awards earned.
i. Stock
Option Plan 2014
The fair value of the options was determined at the grant date by using the Black-Scholes option valuation model and expensed over
the vesting period. The maximum number of options that could be awarded under the plan is 10,337,500, of which 4,320,000 are 2014M awards that are subject to an additional specified market performance requirement at vesting. The 2014M awards include
an additional incentive (a market condition) for the senior management team to have a portion of their potential awards over the three years ending December 31, 2016 to be based solely on an increase in the share price of the Company for the
vesting period. The 2014M awards will not vest unless the Company’s share price growth during that three year period is greater than 35%; however, if the share price growth is greater than 35%, there will be an increasing return up to a maximum
of three times the initial awards for a share price growth of at least 100% over the three year vesting period. The 2014M market condition has been incorporated into the Black-Scholes model, by determining the probability of the share price growth
increase over the three year period based on historical share price movements.
The changes in the number of options, or senior management option units in
the case of the 2014M tranche, in the plan during the nine months ended September 30, 2015 is shown in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of options |
|
2014A |
|
|
2014B |
|
|
2014C |
|
|
2014D |
|
|
2014M |
|
| Outstanding at January 1, 2015 |
|
|
458,750 |
|
|
|
1,376,250 |
|
|
|
— |
|
|
|
— |
|
|
|
1,440,000 |
|
| Forfeitures |
|
|
(75,000 |
) |
|
|
(225,000 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Granted |
|
|
— |
|
|
|
— |
|
|
|
389,250 |
|
|
|
1,167,750 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding at September 30, 2015 |
|
|
383,750 |
|
|
|
1,151,250 |
|
|
|
389,250 |
|
|
|
1,167,750 |
|
|
|
1,440,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All options were fair valued at grant date and will be recognized to personnel expenses, as research and development expenses
or general and administrative expenses, over the vesting period. The most significant
C-28

inputs used to estimate the fair value of the stock options granted during the nine months ended September 30, 2015 are as follows:
|
|
|
|
|
|
|
|
|
| Option plan |
|
2014C |
|
|
2014D |
|
| Share price at grant date |
|
€ |
0.20 |
|
|
€ |
0.20 |
|
| Subscription price |
|
€ |
0.01 |
|
|
€ |
0.01 |
|
| Volatility* |
|
|
50 |
% |
|
|
50 |
% |
| Maturity, years |
|
|
3 |
|
|
|
4 |
|
| Interest rate |
|
|
0.00 |
% |
|
|
0.00 |
% |
| Expected dividends |
|
|
— |
|
|
|
— |
|
| Valuation model |
|
|
Black-Scholes |
|
|
|
Black-Scholes |
|
| Option fair value, € |
|
|
0.19 |
|
|
|
0.19 |
|
| Effect on earnings, € in thousands |
|
|
27 |
|
|
|
53 |
|
| * |
Expected volatility was determined by calculating the historical volatility of the Company’s share using monthly observations over corresponding maturity. |
The expense recognized during the nine months ended September 30, 2015 was €242 thousand (for the nine months ended September 30,
2014: €209 thousand).
ii. Equity Incentive Plan 2014
The Equity Incentive Plan 2014 includes three consecutive discretionary periods, calendar years 2014, 2015 and 2016 in which the restricted share units, or
senior management units, may be granted. Each discretionary period is followed by a subscription period of approximately two years (for 2014A, 2014C and 2014E awards) or approximately three years (for 2014B, 2014D, 2014F and 2014M awards), ending on
January 5, 2016, January 5, 2017, January 5, 2018 or January 5, 2019, after which the Company’s shares will be delivered to employees on the basis of the granted share units. A maximum of 14,002,500 shares may be
delivered under the plan, of which 2,520,000 are 2014M awards that are subject to an additional specified market performance requirement at vesting, which is the same as that described in the Stock Option Plan 2014 above. There is no maximum number
of share units that can be awarded in any one year, but all the 2014M awards must be awarded in 2014.
The changes in the number of share units, or senior
management share units in the case of the 2014M tranche, in the plan during the nine months ended September 30, 2015 is shown in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of units |
|
2014A |
|
|
2014B |
|
|
2014C |
|
|
2014D |
|
|
2014M |
|
| Outstanding at January 1, 2015 |
|
|
409,687 |
|
|
|
1,229,063 |
|
|
|
— |
|
|
|
— |
|
|
|
840,000 |
|
| Forfeitures |
|
|
(34,375 |
) |
|
|
(114,375 |
) |
|
|
(46,875 |
) |
|
|
(140,625 |
) |
|
|
— |
|
| Granted |
|
|
— |
|
|
|
— |
|
|
|
550,938 |
|
|
|
1,652,812 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding at September 30, 2015 |
|
|
375,312 |
|
|
|
1,114,688 |
|
|
|
504,063 |
|
|
|
1,512,187 |
|
|
|
840,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The effect on the Company’s earnings for the nine months ended September 30, 2015 was €252 thousand (for
the nine months ended September 30, 2014: €141 thousand). The fair value of the restricted share units was determined by using the closing share price of the Company’s shares on the grant date. The fair value of the share units
granted in the nine months ended September 30, 2015 was €0.19 per share for the 2014C and 2014D. The exercise price for all units is the USD equivalent of €0.01.
C-29

13. Non-cash Transactions to Cash Flow from Operating Activities
|
|
|
|
|
|
|
|
|
| |
|
For the nine month
period ended
September 30, |
|
| (€ in thousands) |
|
2015 |
|
|
2014 |
|
| Depreciation and amortization |
|
|
212 |
|
|
|
237 |
|
| Share-based compensation |
|
|
629 |
|
|
|
788 |
|
| Other adjustments |
|
|
(429 |
) |
|
|
(549 |
) |
|
|
|
|
|
|
|
|
|
| Non-cash adjustments to cash flow from operating activities |
|
|
412 |
|
|
|
476 |
|
|
|
|
|
|
|
|
|
|
14. Commitments and Contingencies
Operating lease commitments
|
|
|
|
|
|
|
|
|
| (€ in thousands) |
|
September 30,
2015 |
|
|
As at
December 31,
2014 |
|
| Due within a year |
|
|
907 |
|
|
|
843 |
|
| Due in 1-5 years |
|
|
1,485 |
|
|
|
1,937 |
|
| Due later than 5 years |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
2,392 |
|
|
|
2,780 |
|
|
|
|
|
|
|
|
|
|
Operating lease commitments comprise rent commitments for leasehold properties and lease commitments for motor vehicles,
machines and equipment with leases of 3 to 5 years. The Company’s operating leases are non-cancellable and they do not include redemption or extension options.
On September 30, 2015, Biotie had outstanding contractual payment obligations (contractual commitments), primarily for contract research work services
related to ongoing clinical development programs, totaling €571 thousand (December 31, 2014: €232 thousand).
15. Transactions with
Related Parties
During the periods ended September 30, 2015 and 2014, the Company’s management team was paid regular salaries and
contributions to post-employment benefit schemes. Additionally, the members of the Board of Directors were paid regular Board and committee fees. No loans, advances or guarantees were made to the management team or Board of Directors as of
September 30, 2015 or 2014.
The condensed consolidated financial statements do not include all disclosures for related party transactions that are
required in the annual consolidated financial statements and should be read in conjunction with the Company’s annual consolidated financial statements for the year ended December 31, 2014.
16. Events After the Reporting Date
Biotie announced on
October 7, 2015 that, pursuant to the authorization of the Annual General Meeting of Shareholders held on May 26, 2015, the Board of Directors of Biotie has resolved to issue 106,088,336 shares to the Company itself without consideration
in accordance with Chapter 9 Section 20 of the Finnish Companies Act (624/2006, as amended). The Treasury Shares are issued to facilitate the timely delivery by the Company of such Treasury Shares underlying the warrants issued in May 2015 to
certain US investors and certain existing
C-30

shareholders based on the authorization granted by the Annual General Meeting of the Company on May 26, 2015, if and when such above-mentioned warrants are exercised.
The Treasury Shares were registered with the Finnish Trade Register on October 8, 2015, and admitted trading on NASDAQ OMX Helsinki Ltd on
October 9, 2015. The Treasury Shares are of the same class as the existing shares in the Company.
As a result of this Biotie has 1,086,940,271
shares in total of which 978,246,244 will be outstanding shares.
Biotie announced on November 2, 2015 the change in the number of votes relating to
the Swiss subsidiary of the Company, Biotie Therapies AG (previously Synosia Therapeutics Holding AG and Biotie Therapies Holding AG) conveyed Biotie shares against consideration pursuant to the option programs in October in total 7,739.
After the conveyances the total amount of voting rights is 978,253,983 and the number of the Company’s share held by the Biotie Group is 108,686,288
(9.99 per cent). The conveyance does not affect the number of registered shares (total 1,086,940,271).
C-31

KEY FIGURES
The formulas for the calculation of the key figures are presented in the notes of the consolidated financial statements for the year ended December 31,
2014
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the nine months |
|
|
For the year |
|
| |
|
ended
September 30, |
|
|
ended
December 31, |
|
| (€ in thousands, unless stated) |
|
2015 |
|
|
2014 |
|
|
2014 |
|
| Business development |
|
|
|
|
|
|
|
|
| Revenues |
|
|
2,987 |
|
|
|
13,051 |
|
|
|
14,901 |
|
| Personnel on average |
|
|
38 |
|
|
|
35 |
|
|
|
36 |
|
| Personnel at end of period |
|
|
39 |
|
|
|
35 |
|
|
|
38 |
|
| Research and development costs |
|
|
(19,611 |
) |
|
|
(11,931 |
) |
|
|
(17,192 |
) |
| Capital expenditure |
|
|
101 |
|
|
|
163 |
|
|
|
196 |
|
| Profitability |
|
|
|
|
|
|
|
|
| Operating (loss) |
|
|
(22,083 |
) |
|
|
(3,361 |
) |
|
|
(36,090 |
) |
| as percentage of revenues, % |
|
|
(739.3 |
) |
|
|
(25.8 |
) |
|
|
(242.2 |
) |
| (Loss) before taxes |
|
|
(22,818 |
) |
|
|
(2,645 |
) |
|
|
(35,165 |
) |
| as percentage of revenues, % |
|
|
(763.9 |
) |
|
|
(20.3 |
) |
|
|
(236.0 |
) |
| Financial positon |
|
|
|
|
|
|
|
|
| Liquid assets |
|
|
84,020 |
|
|
|
35,867 |
|
|
|
32,393 |
|
| Shareholders’ equity |
|
|
109,667 |
|
|
|
83,269 |
|
|
|
52,623 |
|
| Balance sheet total |
|
|
148,404 |
|
|
|
119,345 |
|
|
|
88,331 |
|
| Financial ratios |
|
|
|
|
|
|
|
|
| Return on equity, % |
|
|
(36.9 |
) |
|
|
(4.3 |
) |
|
|
(52.9 |
) |
| Return on capital employed, % |
|
|
(30.6 |
) |
|
|
(4.7 |
) |
|
|
(39.5 |
) |
| Equity ratio, % |
|
|
74.9 |
|
|
|
71.0 |
|
|
|
61.0 |
|
| Gearing, % |
|
|
(57.7 |
) |
|
|
(18.2 |
) |
|
|
(22.2 |
) |
| Per share data |
|
|
|
|
|
|
|
|
| (Loss) per share (EPS) basic, € |
|
|
(0.03 |
) |
|
|
(0.01 |
) |
|
|
(0.08 |
) |
| (Loss) per share (EPS) diluted, € |
|
|
(0.03 |
) |
|
|
(0.01 |
) |
|
|
(0.08 |
) |
| Shareholders’ equity per share, € |
|
|
0.15 |
|
|
|
0.18 |
|
|
|
0.12 |
|
| Dividend per share, € |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Pay-out ratio, % |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Effective dividend yield, % |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| P/E-ratio |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Share price |
|
|
|
|
|
|
|
|
| On NASDAQ-OMX market in Helsinki |
|
|
|
|
|
|
|
|
| Lowest share price, € |
|
|
0.14 |
|
|
|
0.18 |
|
|
|
0.18 |
|
| Highest share price, € |
|
|
0.26 |
|
|
|
0.36 |
|
|
|
0.36 |
|
| Average share price, € |
|
|
0.20 |
|
|
|
0.25 |
|
|
|
0.24 |
|
| End of period share price, € |
|
|
0.16 |
|
|
|
0.22 |
|
|
|
0.19 |
|
| Market capitalization, € million |
|
|
154.0 |
|
|
|
98.5 |
|
|
|
87.5 |
|
| On NASDAQ market in the United States* |
|
|
|
|
| Lowest ADS price, $ |
|
|
12.77 |
|
|
|
n/a |
|
|
|
n/a |
|
| Highest ADS price, $ |
|
|
25.39 |
|
|
|
n/a |
|
|
|
n/a |
|
| Average ADS price, $ |
|
|
18.17 |
|
|
|
n/a |
|
|
|
n/a |
|
| End of period ADS price, $ |
|
|
13.09 |
|
|
|
n/a |
|
|
|
n/a |
|
| Market capitalization, $ million |
|
|
159.7 |
|
|
|
n/a |
|
|
|
n/a |
|
C-32

|
|
|
|
|
|
|
|
|
|
|
|
|
| Trade of shares |
|
|
|
|
|
|
|
|
|
|
|
|
| On NASDAQ-OMX market in Helsinki |
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares traded |
|
|
157,665,690 |
|
|
|
94,167,267 |
|
|
|
124,604,223 |
|
| as percentage of all shares, % |
|
|
16.1 |
|
|
|
20.6 |
|
|
|
27.3 |
|
| On NASDAQ market in the United States* |
|
|
|
|
|
|
|
|
|
|
|
|
| Number of ADS traded |
|
|
6,085,578 |
|
|
|
n/a |
|
|
|
n/a |
|
| as percentage of all shares (after conversion factor), % |
|
|
49.6 |
|
|
|
n/a |
|
|
|
n/a |
|
| Number of shares during the period |
|
|
661,691,846 |
|
|
|
456,032,398 |
|
|
|
455,958,187 |
|
| Number of shares at end of the period |
|
|
980,851,935 |
|
|
|
456,032,398 |
|
|
|
455,968,174 |
|
| Number of shares during the period, fully diluted |
|
|
748,720,366 |
|
|
|
456,032,398 |
|
|
|
455,958,187 |
|
| Number of shares at end of the period fully diluted |
|
|
1,202,896,665 |
|
|
|
456,032,398 |
|
|
|
455,968,174 |
|
| * |
All trading information in relation to shares listed on the NASDAQ market in the United States relates to the period since June 11, 2015, which was the first day of trading on that market |
Biotie Therapies Corp.
Joukahaisenkatu 6
FI-20520 Turku
Finland
Tel. +358 2 274 89 00
Fax +358 2 274 89 10
www.biotie.com
For further information please contact:
David Cook
Chief Financial Officer
email: david.cook@biotie.com
Tel: +358 2 2748 900
Virve Nurmi
Senior Manager, Investor Relations
email: virve.nurmi@biotie.com
Tel: +358 2 2748 911
The Trout Group LLC
Lauren Williams
Managing Director
email: lwilliams@troutgroup.com
Tel: +44 203 780 4972
Jennifer Porcelli
Vice President
email: jporcelli@troutgroup.com
Tel: +1 646 378 2962
C-33
Annex D
BIOTIE THERAPIES CORP. STOCK EXCHANGE RELEASE 17 February 2016, 09:00 a.m.
U.S. antitrust regulation waiting period relating to tender offer has expired
Biotie Therapies Corp. (“Biotie” or the “Company”) has announced on 19 January 2016 that the Company and Acorda
Therapeutics, Inc. (“Acorda”) have entered into a combination agreement whereby Acorda will make a public tender offer to purchase all of the issued and outstanding shares, American Depositary Shares, stock options, share units and
warrants in Biotie that are not owned by Biotie or any of its subsidiaries (the “Tender Offer”).
The completion of the Tender Offer will
be subject to the fulfilment of certain conditions; including, among others, that the expiration or termination of any applicable waiting period under the U.S. Hart-Scott-Rodino Act. The Company has received information that the waiting period has
expired. The Acorda announcement in full has been annexed to this release.
The launch of the Tender Offer will be announced separately.
Turku, February 17, 2016
Biotie Therapies Corp.
Timo Veromaa
President and CEO
For further information, please contact:
Virve Nurmi, Biotie
Therapies Corp.
tel. +358 2 274 8900, e-mail: virve.nurmi@biotie.com
DISTRIBUTION:
www.biotie.com
Nasdaq Helsinki Ltd
Main Media
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
SOME OF THE STATEMENTS CONTAINED IN THIS ANNOUNCEMENT ARE FORWARD-LOOKING STATEMENTS, INCLUDING STATEMENTS REGARDING THE EXPECTED CONSUMMATION OF THE
ACQUISITION, WHICH INVOLVES A NUMBER OF RISKS AND UNCERTAINTIES, INCLUDING THE SATISFACTION OF CLOSING CONDITIONS FOR THE ACQUISITION, SUCH AS REGULATORY APPROVAL FOR THE TRANSACTION AND THE TENDER OF AT LEAST 90% OF THE OUTSTANDING SHARES AND
VOTING RIGHTS OF THE COMPANY, THE POSSIBILITY THAT THE TRANSACTION WILL NOT BE COMPLETED AND OTHER RISKS AND UNCERTAINTIES DISCUSSED IN THE COMPANY’S PUBLIC FILINGS WITH THE SEC, INCLUDING THE “RISK FACTORS” SECTION OF THE
COMPANY’S REGISTRATION STATEMENT ON FORM F-1 (NO. 333-204147), AS WELL AS THE TENDER OFFER DOCUMENTS TO BE FILED BY ACORDA AND THE SOLICITATION/RECOMMENDATION STATEMENT TO BE FILED BY THE COMPANY. THESE STATEMENTS ARE BASED ON CURRENT
EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS, AND INVOLVE KNOWN AND UNKNOWN RISKS, UNCERTAINTIES AND OTHER FACTORS THAT MAY CAUSE RESULTS, LEVELS OF ACTIVITY, PERFORMANCE OR ACHIEVEMENTS TO BE MATERIALLY DIFFERENT FROM ANY FUTURE STATEMENTS.
THESE STATEMENTS ARE GENERALLY IDENTIFIED BY WORDS OR PHRASES SUCH AS “BELIEVE”, “ANTICIPATE”, “EXPECT”, “INTEND”, “PLAN”, “WILL”, “MAY”, “SHOULD”, “ESTIMATE”,
“PREDICT”, “POTENTIAL”, “CONTINUE” OR THE NEGATIVE OF SUCH TERMS OR OTHER SIMILAR EXPRESSIONS. IF UNDERLYING ASSUMPTIONS PROVE INACCURATE OR UNKNOWN RISKS OR
UNCERTAINTIES MATERIALIZE, ACTUAL RESULTS AND THE TIMING OF EVENTS MAY DIFFER MATERIALLY FROM THE EXPECTED RESULTS AND/OR TIMING DISCUSSED IN THE FORWARD-LOOKING STATEMENTS, AND YOU SHOULD NOT
PLACE UNDUE RELIANCE ON THESE STATEMENTS. ACORDA AND THE COMPANY DISCLAIM ANY INTENT OR OBLIGATION TO UPDATE ANY FORWARD-LOOKING STATEMENTS AS A RESULT OF DEVELOPMENTS OCCURRING AFTER THE PERIOD COVERED BY THIS ANNOUNCEMENT OR OTHERWISE.
ADDITIONAL INFORMATION AND WHERE TO FIND IT
THE TENDER
OFFER FOR THE OUTSTANDING SHARES, AMERICAN DEPOSITARY SHARES, WARRANTS AND OTHER OUTSTANDING EQUITY INSTRUMENTS IN THE COMPANY (THE “OFFER”) HAS NOT BEEN COMMENCED. THIS ANNOUNCEMENT IS FOR INFORMATIONAL PURPOSES ONLY AND DOES NOT
CONSTITUTE AN OFFER TO PURCHASE OR A SOLICITATION OF AN OFFER TO SELL COMPANY SECURITIES. THE SOLICITATION AND OFFER TO BUY COMPANY SECURITIES WILL ONLY BE MADE PURSUANT TO AN OFFER TO PURCHASE AND RELATED MATERIALS. AT THE TIME THE OFFER IS
COMMENCED, ACORDA WILL FILE A TENDER OFFER STATEMENT ON SCHEDULE TO WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION (THE “SEC”) AND THEREAFTER, THE COMPANY WILL FILE A SOLICITATION/RECOMMENDATION STATEMENT ON SCHEDULE 14D-9 WITH RESPECT
TO THE OFFER. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THESE MATERIALS CAREFULLY WHEN THEY BECOME AVAILABLE SINCE THEY WILL CONTAIN IMPORTANT INFORMATION, INCLUDING THE TERMS AND CONDITIONS OF THE OFFER. THE OFFER TO PURCHASE,
SOLICITATION/RECOMMENDATION STATEMENT AND RELATED MATERIALS WILL BE FILED BY ACORDA AND THE COMPANY WITH THE SEC, AND INVESTORS AND SECURITY HOLDERS MAY OBTAIN A FREE COPY OF THESE MATERIALS (WHEN AVAILABLE) AND OTHER DOCUMENTS FILED BY ACORDA AND
THE COMPANY WITH THE SEC AT THE WEBSITE MAINTAINED BY THE SEC AT WWW.SEC.GOV. INVESTORS AND SECURITY HOLDERS MAY ALSO OBTAIN FREE COPIES OF THE SOLICITATION/RECOMMENDATION STATEMENT AND OTHER DOCUMENTS FILED WITH THE SEC BY THE COMPANY AT
WWW.BIOTIE.COM.
Attachment: The Acorda announcement Expiration of U.S. anti-trust regulation waiting period
ACORDA THERAPIES INC. PRESS RELEASE 17 February 2016 at 8:30 a.m. (EET)
Acorda Announces Expiration of the Hart-Scott-Rodino Waiting Period for its Tender Offer for Biotie Therapies
ARDSLEY, N.Y. – February 17, 2016 – Acorda Therapeutics, Inc. (Nasdaq: ACOR) today announced that the waiting period under the Hart-Scott-Rodino
Antitrust Improvements Act of 1976, as amended (HSR), for its tender offer for Biotie Therapies Corp. (Nasdaq Helsinki: BTH1V; Nasdaq: BITI) has expired.
Acorda announced on January 19, 2016 that it entered into an agreement to acquire Biotie for €0.2946 per share and €23.5680 per
ADS in cash. Pursuant to the terms of the agreement, Acorda will offer to acquire all outstanding shares, American Depositary Shares, and other equity securities of Biotie through a public tender offer.
The expiration of the HSR waiting period satisfies one of the conditions to the tender offer, which has not yet been commenced. The closing of the tender
offer will be subject to customary terms and conditions, unless waived by Acorda, including the valid tender to (or other acquisition by) Acorda of at least 90 percent of the issued and outstanding shares and voting rights of Biotie on a fully
diluted basis as described in more detail in the agreement between Acorda and Biotie.
Lazard, MTS Health Partners and J.P. Morgan Securities LLC are
serving as financial advisors, and Kirkland & Ellis LLP, Roschier Attorneys Ltd., Covington & Burling LLP and Jones Day LLP are serving as legal advisors to Acorda in connection with the tender offer. Guggenheim Securities is
serving as Biotie Therapies’ financial advisor, and Davis Polk & Wardwell LLP and Hannes Snellman Attorneys Ltd. are serving as Biotie’s legal advisors.
ACORDA THERAPEUTICS, INC.
FURTHER INFORMATION
For further information, please contact:
Felicia Vonella,
Investor relations
Tel. + 1 914 326 5146, e-mail: fvonella@acorda.com
About Acorda Therapeutics
Founded in 1995, Acorda
Therapeutics is a biotechnology company focused on developing therapies that restore function and improve the lives of people with neurological disorders.
Acorda has an industry leading pipeline of novel neurological therapies addressing a range of disorders, including multiple sclerosis, Parkinson’s
disease, post-stroke walking deficits, epilepsy and migraine. Acorda markets three FDA-approved therapies, including AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg. For further
information, please visit www.acorda.com.
About Biotie Therapies
Biotie is a specialized drug development company focused on products for neurodegenerative and psychiatric disorders. Biotie’s development has delivered
Selincro (nalmefene) for alcohol dependence, which received European marketing authorization in 2013 and is currently being rolled out across Europe by partner H. Lundbeck A/S. The current development products include tozadenant for
Parkinson’s disease, which is in Phase 3 development, and two additional compounds which are in Phase 2 development for cognitive disorders including Parkinson’s disease dementia, and primary sclerosing cholangitis (PSC), a rare fibrotic
disease of the liver. For further information, please visit www.biotie.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements, other than statements of historical facts, regarding management’s expectations,
beliefs, goals, plans or prospects should be considered forward-looking. These statements are subject to risks and uncertainties that could cause actual results to differ materially, including the ability to complete the Biotie transaction on a
timely basis or at all; the ability to realize the benefits anticipated to be realized by the Biotie transaction and the Civitas transaction; the ability to successfully integrate Biotie’s operations and Civitas’ operations, respectively,
into our operations; we may need to raise additional funds to finance our expanded operations and may not be able to do so on acceptable terms; our ability to successfully market and sell Ampyra in the U.S.; third party payers (including
governmental agencies) may not reimburse for the use of Ampyra or our other products at acceptable rates or at all and may impose restrictive prior authorization requirements that limit or block prescriptions; the risk of unfavorable results from
future studies of Ampyra or from our other research and development programs, including CVT-301, Plumiaz, or any other acquired or in-licensed programs; we may not be able to complete development of, obtain regulatory approval for, or successfully
market CVT-301, Plumiaz, or any other products under development; the occurrence of adverse safety events with our products; delays in obtaining or failure to obtain regulatory approval of or to successfully market Fampyra outside of the U.S. and
our dependence on our collaboration partner Biogen in connection therewith; competition; failure to protect our intellectual property, to defend against the intellectual property claims of others or to obtain third party intellectual property
licenses needed for the commercialization of our products; and failure to comply with regulatory requirements could result in adverse action by regulatory agencies. In addition, the compounds being acquired from Biotie are subject to all the risks
inherent in the drug development process, and there can be no assurance that these compounds will receive regulatory approval or be commercially successful. These and other risks are described in greater detail in our filings with the Securities and
Exchange Commission. We may not actually achieve the goals or plans described in our forward-looking statements, and investors should not place undue reliance on these statements. Forward-looking statements made in this release are made only as of
the date hereof, and we disclaim any intent or obligation to update any forward-looking statements as a result of developments occurring after the date of this release.
Additional Information
The tender offer described in
this release has not yet commenced, and this release is neither an offer to purchase nor a solicitation of an offer to sell securities. At the time the tender offer is commenced, we will file, or will cause a new wholly owned subsidiary to file,
with the SEC a tender offer statement on Schedule TO. Investors and holders of Biotie equity securities are strongly advised to read the tender offer statement (including an offer to purchase, letter of transmittal and related tender offer
documents) and the related solicitation/recommendation statement on Schedule 14D-9 that will be filed by Biotie with the SEC, because they will contain important information. These documents will be available at no charge on the SEC’s website
at www.sec.gov upon the commencement of the tender offer. In addition, a copy of the offer to purchase, letter of transmittal and other related tender offer documents (once they become available) may be obtained free of charge by directing a request
to us at www.acorda.com or Office of the Corporate Secretary, 420 Saw Mill River Road, Ardsley, New York 10502.
In addition to the offer to
purchase, the related letter of transmittal and certain other offer documents, as well as the solicitation/recommendation statement, we file annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and
copy any reports, statements or other information filed by us at the SEC public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at
1-800-SEC-0330 for further information on the public reference room. Our filings with the SEC are also available to the public from commercial document-retrieval
services and at the website maintained by the SEC at www.sec.gov.
THE OFFER WILL NOT BE MADE DIRECTLY OR INDIRECTLY IN ANY JURISDICTION WHERE EITHER
AN OFFER OR PARTICIPATION THEREIN IS PROHIBITED BY APPLICABLE LAW OR WHERE ANY TENDER OFFER DOCUMENT OR REGISTRATION OR OTHER REQUIREMENTS WOULD APPLY IN ADDITION TO THOSE UNDERTAKEN IN FINLAND AND THE UNITED STATES.
IN ADDITION, THE TENDER OFFER DOCUMENTS, THIS RELEASE AND RELATED MATERIALS AND ACCEPTANCE FORMS WILL NOT AND
MAY NOT BE DISTRIBUTED, FORWARDED OR TRANSMITTED INTO OR FROM ANY JURISDICTION WHERE PROHIBITED BY APPLICABLE LAW. IN PARTICULAR, THE TENDER OFFER IS NOT BEING MADE, DIRECTLY OR INDIRECTLY, IN OR INTO, CANADA, JAPAN, AUSTRALIA, SOUTH AFRICA OR HONG
KONG. THE TENDER OFFER CANNOT BE ACCEPTED BY ANY SUCH USE, MEANS OR INSTRUMENTALITY OR FROM WITHIN CANADA, JAPAN, AUSTRALIA, SOUTH AFRICA OR HONG KONG.
This release is for information only and does not constitute a tender offer document or an offer, solicitation of an offer or an invitation to a sales offer.
Potential investors in Finland shall accept the tender offer only on the basis of the information provided in a tender offer document approved by the Finnish Financial Supervisory Authority and related materials.
BIOTIE STOCK EXCHANGE RELEASE 19 January 2016 at 9:45 a.m. (EET)
ACORDA ANNOUNCES A RECOMMENDED CASH TENDER OFFER FOR ALL SHARES, ADSs AND OTHER EQUITY INSTRUMENTS IN BIOTIE
Acorda Therapeutics, Inc. (“Acorda”) and Biotie Therapies Corp. (“Biotie” or the “Company”) have today
entered into a combination agreement (“Combination Agreement”) whereby Acorda, either directly or through a wholly-owned subsidiary (jointly the “Offeror”), will make a public tender offer in Finland
and in the United States to purchase all of the issued and outstanding shares, American Depositary Shares (“ADSs”), stock options, share units and warrants in Biotie that are not owned by Biotie or any of its subsidiaries (the
“Tender Offer”).
The price offered for each share validly tendered into the Tender Offer will be EUR 0.2946 in cash, representing a
premium of approximately 95 per cent compared to the closing price of the Biotie shares on Nasdaq Helsinki Ltd. (“Nasdaq Helsinki”) on 18 January 2016, the last trading day on Nasdaq Helsinki preceding this announcement.
This represents a premium of approximately 84 per cent compared to the 90 trading day volume-weighted average on Nasdaq Helsinki.
The price offered
for each ADS will be EUR 23.5680 in cash, payable in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable
day to the closing date of the Tender Offer. As of January 18, 2016, this would be equivalent to USD 25.60 per ADS in cash, based on an exchange rate of 1.0864 USD to EUR 1.00, representing a premium of approximately 94 per cent
compared to the closing price of the Biotie ADSs on the Nasdaq Stock Market LLC (“Nasdaq US”) on 15 January 2016, the last trading day on the Nasdaq US preceding this announcement. Acorda will also offer to acquire all of the
outstanding option rights, share units and warrants issued by Biotie.
The Board of Directors of Biotie recommends that the holders of Biotie shares,
ADSs, option rights, share units and warrants accept the Tender Offer. The Board’s decision has been unanimous. The Board of Directors of Biotie will issue its complete statement regarding the Tender Offer in accordance with the Finnish
Securities Market Act before the commencement of the Tender Offer. In connection with the Tender Offer, the Board of Directors of Biotie has received an opinion from Biotie’s financial advisor.
Certain factors considered by the Board of Directors of Biotie when giving its recommendation include (i) the costs required to gain approval and to
subsequently launch the products, which could require an additional dilutive financing (ii) the various strategic alternatives available to the Company, taking into account discussions with other possible counterparties; (iii) the risks of
a successful launch of the products for the Company to be able to realize the full economic value of the products and (iv) the fact that the offer is a cash offer and not subject to a financing condition.
Certain Biotie shareholders and ADS holders representing in total approximately 59 per cent (on the fully diluted basis) of the outstanding shares and
votes in Biotie have subject to certain customary conditions irrevocably undertaken to accept the Tender Offer. This includes all holders of Biotie warrants and members of the management team of Biotie who have subject to certain customary
conditions irrevocably undertaken to tender their equity instruments into the Tender Offer.
Board Member Mr. Don M Bailey, ViVo Capital, whose
venture partner is Board Member Mr. Mahendra G. Shah, and Versant Euro Ventures, whose managing director is Board Member Mr. Guido Magni, representing in total approximately 27 per cent (on the fully diluted basis) of the
outstanding shares and votes in Biotie (which is included in the 59 per cent figure mentioned in the paragraph above), have subject to certain customary conditions irrevocably undertaken to accept the Tender Offer. Mr. Bailey, ViVo Capital
and Versant Euro Ventures have made the commitment in question after Biotie’s Board of Directors approved the entry into the Combination Agreement. Board Members Mr. Bailey, Mr. Shah and Mr. Magni shall not participate in the
giving of the Board of Directors’ statement regarding the Tender Offer.
Dr. Ron Cohen, Acorda’s President and CEO stated that “Our acquisition of Biotie positions Acorda
as a leader in Parkinson’s disease therapeutic development, with three clinical-stage compounds that have the potential to improve the lives of people with Parkinson’s. Tozadenant, Biotie’s most advanced clinical program, is a
promising therapy being developed to reduce daily OFF time. Adenosine A2a receptor antagonists may be the first new class of drugs approved for the treatment of Parkinson’s in the U.S. in over 20 years. Approximately 350,000 people with
Parkinson’s in the U.S. experience OFF periods, and if approved, tozadenant could provide a much needed treatment option.”
Dr. Cohen
added, “Tozadenant is a compelling opportunity with potential market exclusivity to 2030. The Phase 2 data were highly statistically significant and clinically meaningful. We are targeting an NDA filing by the end of 2018.”
Mr. William M. Burns, Chairman of the Board of Biotie commented “We have carefully assessed the terms and conditions of the Offer and believe
that it is an attractive offer to shareholders that recognizes the strategic value of Biotie.”
Mr. Burns continued, “With the shared
mission to improve the lives of patients with neurological diseases, this transaction will allow Acorda and Biotie to bring together their expertise and resources in order to fully maximize the potential of tozadenant, an A2a receptor antagonist in
Phase 3 for Parkinson’s disease, and SYN120, a dual 5-HT6/5-HT2A receptor antagonist in Phase 2 for cognitive and psychotic disorders, and to bring new medicines to patients. We are excited about this offer for our shareholders, the Biotie team
and for patients.”
BACKGROUND AND REASONS FOR THE TENDER OFFER
Acorda is a biotechnology company focused on developing therapies that improve the lives of people with neurological disorders, with its common stock listed on
Nasdaq US.
Biotie is a specialized drug development company focused on products for neurodegenerative and psychiatric disorders. Through the acquisition,
Acorda will obtain worldwide rights to tozadenant, an oral adenosine A2a receptor antagonist currently in Phase 3 development in Parkinson’s disease (PD). In clinical trials, tozadenant reduced average daily OFF time as an adjunct to treatment
regimens including levodopa.
Further expanding its Parkinson’s pipeline, Acorda will also obtain global rights to SYN-120, an oral, 5-HT6/5-HT2A dual receptor antagonist in Phase 2 development for Parkinson’s-related dementia, with support from the Michael J. Fox Foundation.
The acquisition also includes two other assets: BTT1023, a fully human monoclonal antibody in Phase 2 development for primary sclerosing cholangitis (PSC), a
chronic liver disease; and Selincro, a European Medicines Agency (EMA)-approved therapy for reduction of alcohol consumption marketed by H. Lundbeck A/S in multiple European countries and for which the Company receives royalties.
THE TERMS AND CONDITIONS OF THE TENDER OFFER
The price
offered for each share validly tendered into the Tender Offer will be EUR 0.2946 per share in cash.
The price offered for each ADS will be EUR
23.5680 in cash, payable in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the closing date
of the Tender Offer.
The price offered for each stock option or share unit issued by Biotie pursuant to its option and equity incentive plans and
convertible into Biotie shares will be the greater of (i) EUR 0.2946 minus the applicable subscription price and (ii) EUR 0.01 in cash. The price offered for each warrant will be EUR 0.1664 in cash.
The specific prices for each of the stock options, share units and warrants have been set out in Annex A of this
release.
The completion of the Tender Offer will be subject to the following conditions:
| |
(a) |
the valid tender of outstanding shares (including outstanding shares represented by validly tendered ADSs and validly tendered warrants) representing, together with any outstanding shares (including outstanding shares
represented by ADSs and warrants) otherwise acquired by the offeror, more than ninety percent (90%) of the issued and outstanding shares and voting rights of the Company, calculated on a Fully Diluted Basis and otherwise in accordance with
Chapter 18 Section 1 of the Finnish Limited Liability Companies Act (21.7.2006/624); as used in this paragraph “Fully Diluted Basis” means an equation in which the numerator represents the aggregate number of Outstanding Shares
(including Outstanding Shares represented by ADSs) and Warrants that have been validly tendered or otherwise acquired by the Offeror and the denominator represents the aggregate number of all Outstanding Shares (including Outstanding Shares
represented by ADSs) and Warrants, as well as shares issuable upon the vesting and exercise of those Outstanding Equity Instruments (other than Warrants) that have not been validly tendered into the Tender Offer or otherwise acquired by the Offeror;
|
| |
(b) |
the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Act; |
| |
(c) |
no material adverse effect (as defined in the Combination Agreement) having occurred in Biotie after 19 January 2016; |
| |
(d) |
the Offeror not, after 19 January 2016, having received information previously undisclosed to it that describes a material adverse effect to the Company that occurred prior to 19 January 2016;
|
| |
(e) |
no information made public by the Company or disclosed by the Company to the Offeror being materially inaccurate, incomplete, or misleading, and the Company not having failed to make public any information that should
have been made public by it under applicable laws, including without limitation the rules of Nasdaq Helsinki and Nasdaq US, provided that, in each case, the information made public, disclosed or not disclosed or the failure to disclose information
constitutes a material adverse effect to the Company; |
| |
(f) |
no court or regulatory authority of competent jurisdiction (including without limitation the Finnish Financial Supervisory Authority or the SEC) having given an order or issued any regulatory action preventing or
enjoining the completion of the Tender Offer; |
| |
(g) |
the Board of Directors of the Company having issued its recommendation for the Tender Offer and the recommendation remaining in force and not being modified or changed in a manner detrimental to the Offeror; and
|
| |
(h) |
the Combination Agreement not having been terminated and remaining in force and no event having occurred that, with the passage of time, would give the Offeror the right to terminate the Combination Agreement under
specified sections of the Combination Agreement that give the Offeror the right to terminate the Combination Agreement in response to a breach of the agreement by the Company. |
The Offeror reserves the right to complete the Tender Offer even if the conditions to completion of the Tender Offer have not been fulfilled.
The Tender Offer will commence after the Offeror has obtained certain regulatory relief from the US Securities and Exchange, which is expected to be obtained
no later than the end of February 2016, and initially run for twenty (20) banking days. The Offeror reserves the right to extend and is obligated to extend the acceptance period from time to time in accordance with the terms and conditions of
the Tender Offer.
The Offeror will make the filings required under the Hart-Scott-Rodino Act which requires the Offeror to delay the completion of the
Tender Offer until the applicable waiting period pursuant to the Hart-Scott-Rodino Act has expired or been terminated. The initial waiting period under the Hart-Scott-Rodino Act is thirty days, unless it is earlier terminated or extended by a
request for additional information. The Offeror currently does not believe that the completion of the Tender Offer would require regulatory approvals from competition authorities outside the United States.
The Tender Offer will be financed through cash on Acorda’s balance sheet and the gross proceeds of a private
placement to a banking institution] of approximately USD 75 million of Acorda’s common stock that was executed concurrently with the execution of the Combination Agreement and that is expected to close by 26 January, 2016. The Tender
Offer is not conditional upon obtaining any external financing for the Tender Offer.
The detailed terms and conditions of the Tender Offer and
information on how to accept the Tender Offer will be included in the tender offer documents that will be published by the Offeror before the commencement of the acceptance period in Finland and in the United States.
Acorda and Biotie have undertaken to follow the Helsinki Takeover Code issued by the Finnish Securities Market Association as referred to in the Finnish
Securities Market Act and will comply with the US Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder, subject to any relief from the US Securities and Exchange Commission.
On the date of this release, Biotie’s share capital amounts to EUR 279,218,058.55 and the number of shares issued to 1,089,608,083. The Offeror does not
currently hold any shares, ADSs, option rights, share units or warrants in Biotie.
COMBINATION AGREEMENT
The Combination Agreement sets forth the principal terms under which the Offeror will make the Tender Offer.
Under the Combination Agreement, the Board of Directors of Biotie has undertaken, in the event of a competing offer, not to cancel or change its
recommendation for the Tender Offer, unless
| |
i. |
it determines in good faith, after taking advice from external legal counsel and financial advisor that the competing offer is superior to the Offeror’s offer and that therefore it would no longer be in the best
interest of the shareholders, ADS holders and holders of stock options, share units and warrants of Biotie to accept the Tender Offer and that failure to cancel or change the Board of Directors’ recommendation for the Tender Offer would be
inconsistent with the Board of Directors’ fiduciary duties towards Biotie’s shareholders and holders of other equity instruments under Finnish laws; and |
| |
ii. |
prior to and as a precondition for cancelling or changing its recommendation, the Board of Directors has complied with certain agreed procedures allowing Acorda to assess the competing offer and to enhance the Tender
Offer. |
Should Acorda enhance the Tender Offer so as to be at least equally favourable to Biotie’s shareholders as the competing offer,
the Board of Directors has undertaken to confirm and uphold the recommendation for the Tender Offer, as enhanced.
Biotie has also agreed not to, directly
or through its representatives, solicit or encourage any competing offers or proposals for such offers or other transactions competing with the Tender Offer, nor to facilitate or promote any such competing proposals, unless the Board of Directors
has determined that, with respect to an unsolicited competing proposal, failure to take such measures would be inconsistent with the Board of Directors’ fiduciary duties. Biotie has agreed to inform Acorda of any competing proposals and to
provide Acorda an opportunity to negotiate with the Board of Directors of Biotie on matters arising from such competing proposals.
The Combination
Agreement further includes certain representations, warranties and undertakings by both parties customary in transactions of a similar nature, such as conduct of business by Biotie in the ordinary course of business before the completion of the
Tender Offer and cooperation by the parties in necessary regulatory filings and in completing the Tender Offer in the most expeditious manner practicable.
Biotie has further undertaken to compensate Acorda for its reasonable transaction costs in the amount of USD 4,500,000 in the event the Combination
Agreement is terminated in connection with the Board of Directors of Biotie withdrawing or changing its recommendation for the Tender Offer.
Acorda’s intention is to cause the shares of Biotie to be delisted from Nasdaq Helsinki and the ADSs to be
delisted from Nasdaq US as soon as permitted and reasonably practicable under applicable laws and regulations.
The foregoing summary is not complete and
is qualified by reference to the full text of the Combination Agreement.
EFFECT ON BIOTIE’S CURRENT ORGANIZATION
Biotie is headquartered in Turku, Finland, with a subsidiary based in South San Francisco, California. Following the close of the acquisition, Acorda plans to
maintain the operations of South San Francisco in full, including the staff at that site. The future of Biotie’s location in Turku will be considered separately later on. With this addition, Acorda will have operations in three major U.S.
biotechnology centers: New York, Boston and San Francisco.
ADVISORS
Guggenheim Securities, served as Biotie Therapies’ financial advisor, Davis Polk & Wardwell LLP and Hannes Snellman Attorneys Ltd. served as
Biotie’s legal advisors.
Lazard, MTS Health Partners and J.P. Morgan Securities LLC served as financial advisors and Kirkland & Ellis,
Roschier Attorneys Ltd., Covington & Burling LLP and Jones Day LLP served as legal advisor to Acorda in connection with the Tender Offer.
BIOTIE THERAPIES CORP.
Board of Directors
FURTHER INFORMATION
For further information, please
contact:
Biotie
Timo Veromaa, President and Chief Executive
Officer
Tel. +358 2 274 8900, e-mail: timo.veromaa@biotie.com
David Cook, Chief Financial Officer
Tel. +358 2 274 8900,
e-mail: david.cook@biotie.com
Virve Nurmi, Investor Relations Manager
Tel. +358 2 274 8900, e-mail: virve.nurmi@biotie.com
The Trout
Group LLC
Lauren Williams
Managing Director
Tel: +44 203 780 4972, e-mail: lwilliams@troutgroup.com
Jennifer Porcelli
Vice President
Tel: +1 646 378 2962, e-mail: jporcelli@troutgroup.com
Acorda
Jeff Macdonald (+ 1 914 326 5232, jmacdonald@acorda.com); or
Sijoittajasuhteet: Felicia Vonella, (+ 1 914 326 5146, fvonella@acorda.com)
INFORMATION REGARDING BIOTIE
Biotie is a specialized drug development company focused on products for neurodegenerative and psychiatric disorders. Biotie’s development has delivered
Selincro (nalmefene) for alcohol dependence, which received European marketing authorization in 2013 and is currently being marketed across Europe by partner Lundbeck. The current development products include tozadenant for Parkinson’s disease,
which is in Phase 3 development, and two additional compounds which are in Phase 2 development for cognitive disorders including Parkinson’s disease dementia, and primary sclerosing cholangitis (PSC), a rare fibrotic disease of the liver.
INFORMATION REGARDING ACORDA
Founded in 1995, Acorda is
a biotechnology company focused on developing therapies that improve the lives of people with neurological disorders, with its common stock listed on Nasdaq US.
Acorda has an industry leading pipeline of novel neurological therapies addressing a range of disorders, including multiple sclerosis, Parkinson’s
disease, post-stroke walking deficits, epilepsy and migraine. Acorda markets three FDA-approved therapies, including AMPYRA (dalfampridine) Extended Release Tablets, 10 mg.
ANNEX A
The specific prices for each of the stock
options, share units and warrants are as follows:
|
|
|
| Outstanding Equity Instrument |
|
Price offered in the Tender Offer (EUR in
cash) |
| 2011 Option Rights |
|
|
| 2011C (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014 Option Rights |
|
|
| 2014A (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014B (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014C (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014D (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014M (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2016 Option Rights |
|
|
| Stock option 2016 (subscription price EUR 0.162 per share) |
|
0.1326 for each stock option |
| 2011 Share Rights |
|
|
| 2011 Share right |
|
0.2946 for each share right |
| 2014 Share Rights |
|
|
| 2014A (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014B (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014C (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014D (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014M (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| Swiss Option Rights |
|
|
| Swiss option (subscription price CHF 0.10 per share) |
|
0.2032 for each stock option |
| Swiss option (subscription price CHF 0.21 per share) |
|
0.1026 for each stock option |
| Swiss option (subscription price CHF 0.28 per share) |
|
0.0386 for each stock option |
| Swiss option (subscription price CHF 0.31 per share] |
|
0.0112 for each stock option |
| Swiss option (subscription price CHF 0.35 per share) |
|
0.0100 for each stock option |
| Swiss option (subscription price CHF 0.38 per share) |
|
0.0100 for each stock option |
| Swiss option (subscription price CHF 0.45 per share) |
|
0.0100 for each stock option |
| Warrants |
|
|
| Warrants (subscription price EUR 0.17 per share) |
|
0.1664 for each warrant |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
SOME OF THE STATEMENTS CONTAINED IN THIS ANNOUNCEMENT ARE FORWARD-LOOKING STATEMENTS, INCLUDING STATEMENTS REGARDING THE EXPECTED CONSUMMATION OF THE
ACQUISITION, WHICH INVOLVES A NUMBER OF RISKS AND UNCERTAINTIES, INCLUDING THE SATISFACTION OF CLOSING CONDITIONS FOR THE ACQUISITION, SUCH AS REGULATORY APPROVAL FOR THE TRANSACTION AND THE TENDER OF AT LEAST 90% OF THE OUTSTANDING SHARES AND
VOTING RIGHTS OF THE COMPANY, THE POSSIBILITY THAT THE TRANSACTION WILL NOT BE COMPLETED AND OTHER RISKS AND UNCERTAINTIES DISCUSSED IN THE COMPANY’S PUBLIC FILINGS WITH THE SEC, INCLUDING THE “RISK FACTORS” SECTION OF THE
COMPANY’S REGISTRATION STATEMENT ON FORM F-1 (NO. 333-204147), AS WELL AS THE TENDER OFFER DOCUMENTS TO BE FILED BY ACORDA AND THE SOLICITATION/RECOMMENDATION STATEMENT TO BE FILED BY THE COMPANY. THESE STATEMENTS ARE BASED ON CURRENT
EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS, AND INVOLVE KNOWN AND UNKNOWN RISKS, UNCERTAINTIES AND OTHER FACTORS THAT MAY CAUSE RESULTS, LEVELS OF ACTIVITY, PERFORMANCE OR ACHIEVEMENTS TO BE MATERIALLY DIFFERENT FROM ANY FUTURE STATEMENTS.
THESE STATEMENTS ARE GENERALLY IDENTIFIED BY WORDS OR PHRASES SUCH AS “BELIEVE”, “ANTICIPATE”, “EXPECT”, “INTEND”, “PLAN”, “WILL”, “MAY”, “SHOULD”, “ESTIMATE”,
“PREDICT”, “POTENTIAL”, “CONTINUE” OR THE NEGATIVE OF SUCH TERMS OR OTHER SIMILAR EXPRESSIONS. IF UNDERLYING ASSUMPTIONS PROVE INACCURATE OR UNKNOWN RISKS OR UNCERTAINTIES MATERIALIZE, ACTUAL RESULTS AND THE TIMING
OF EVENTS MAY DIFFER MATERIALLY FROM THE EXPECTED RESULTS AND/OR TIMING DISCUSSED IN THE FORWARD-LOOKING STATEMENTS, AND YOU SHOULD NOT PLACE UNDUE RELIANCE ON THESE STATEMENTS. ACORDA AND THE COMPANY DISCLAIM ANY INTENT OR OBLIGATION TO UPDATE ANY
FORWARD-LOOKING STATEMENTS AS A RESULT OF DEVELOPMENTS OCCURRING AFTER THE PERIOD COVERED BY THIS ANNOUNCEMENT OR OTHERWISE.
ADDITIONAL INFORMATION
AND WHERE TO FIND IT
THE TENDER OFFER FOR THE OUTSTANDING SHARES, AMERICAN DEPOSITARY SHARES, WARRANTS AND OTHER OUTSTANDING EQUITY INSTRUMENTS IN THE
COMPANY (THE “OFFER”) HAS NOT BEEN COMMENCED. THIS ANNOUNCEMENT IS FOR INFORMATIONAL PURPOSES ONLY AND DOES NOT CONSTITUTE AN OFFER TO PURCHASE OR A SOLICITATION OF AN OFFER TO SELL COMPANY SECURITIES. THE SOLICITATION AND OFFER TO BUY
COMPANY SECURITIES WILL ONLY BE MADE PURSUANT TO AN OFFER TO PURCHASE AND RELATED MATERIALS. AT THE TIME THE OFFER IS COMMENCED, ACORDA WILL FILE A TENDER OFFER STATEMENT ON SCHEDULE TO WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION (THE
“SEC”) AND THEREAFTER, THE COMPANY WILL FILE A SOLICITATION/RECOMMENDATION STATEMENT ON SCHEDULE 14D-9 WITH RESPECT TO THE OFFER. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THESE MATERIALS CAREFULLY WHEN THEY BECOME AVAILABLE SINCE
THEY WILL CONTAIN IMPORTANT INFORMATION, INCLUDING THE TERMS AND CONDITIONS OF THE OFFER. THE OFFER TO PURCHASE, SOLICITATION/RECOMMENDATION STATEMENT AND RELATED MATERIALS WILL BE FILED BY ACORDA AND THE COMPANY WITH THE SEC, AND INVESTORS AND
SECURITY HOLDERS MAY OBTAIN A FREE COPY OF THESE MATERIALS (WHEN AVAILABLE) AND OTHER DOCUMENTS FILED BY ACORDA AND THE COMPANY WITH THE SEC AT THE WEBSITE MAINTAINED BY THE SEC AT WWW.SEC.GOV. INVESTORS AND SECURITY HOLDERS MAY ALSO OBTAIN FREE
COPIES OF THE SOLICITATION/RECOMMENDATION STATEMENT AND OTHER DOCUMENTS FILED WITH THE SEC BY THE COMPANY AT WWW.BIOTIE.COM.
Annex E
Articles of association
1 § Trade name of the
company
The trade name of the company is Biotie Therapies Oyj, in English Biotie Therapies Corp.
2 § Domicile of the company
The domicile of the
company is Turku.
3 § Field of activity of the company
The field of activity of the company is the development, manufacture and sale of products and equipment used in the biotechnology, pharmaceutical industry and
laboratory environment, diagnostics, production of medical and psychotherapy services and consultancy related thereto, research and educational services as well as publishing of books, magazines and other publications in the field. The company may
purchase and sell as well as lease and rent real estate for its operations as well as own, sell and possess shares and other securities provided that it does not engage in professional trading in securities or investment.
4 § Minimum and maximum share capital and shares
The shares in the company do not have a nominal value. The share capital of the company may be increased or reduced without amending the Articles of
Association.
5 § Board of directors
The Board
of Directors consists of at least three (3) and at most ten (10) members.
The term of office of the member of the Board of Directors shall
expire at the end of the annual general meeting of shareholders following their election.
The Board of Directors elects one of its members as the
Chairman. The Managing Director of the company cannot be elected as the Chairman of the Board of Directors.
The Board of Directors constitutes a quorum
when more than half of the members of the Board of Directors are present.
6 § Managing director
The company has a Managing Director elected by the Board of Directors.
7 § Representation and procuration
The Company is
represented by the Managing Director and the Chairman of the Board of Directors, each alone, and two members of the Board of Directors together.
The
board of directors decides on procuration. Power of procuration may only be given so that the holders of procuration shall sign the trade name two together or each alone with a member of the Board of Directors
8 § Accounting period
The accounting period of the
company is a calendar year.
9 § Auditors
The
company has at least one (1) and at most two (2) auditors. At least one of the auditors shall be a firm of auditors approved by the Central Chamber of Commerce.
The term of office of the auditor shall expire at the end of the annual general meeting of shareholders following
the election.
10 § Summons to the meetings
The
shareholders of the company are summoned to the shareholders’ meeting by publishing the summons on the company’s website. The summons shall be published not earlier than two (2) months before the last registration date mentioned in
the summons and not later than three (3) weeks prior to the date of the meeting. In addition, the Board of Directors shall publish a summary notice of the shareholders’ meeting in one or more national daily newspaper, or by sending the
notice of the shareholders’ meeting as a registered letter or other verifiable way to the shareholders’ address, which is registered in the share register.
11 § Advance registration
A shareholder, who wishes
to participate in the shareholders’ meeting, shall register himself in advance not later than on the date mentioned in the summons, which may not be later than ten (10) days prior to the date of the meeting.
12 § Annual general meeting
Annual general meeting
of shareholders shall be held annually within six (6) months from the end of the accounting period. The Board of Directors of the company shall decide on the date of the annual general meeting of shareholders.
In the meeting
1. the shareholders shall be presented with the
financial statements including the consolidated financial statements and annual report;
2. the auditors’ report
and
3. the adoption of the financial statements and the
consolidated financial statements;
4. the measures prompted by the profit or loss shown in the adopted balance sheet;
5. the granting of discharge from liability of the members of the Board of Directors and the Managing Director;
6. the remuneration of the members of the Board of Directors and the auditors; and
7. the number of the members of the Board of Directors and auditors;
shall be resolved on,
as well as
8. the members of the Board of Directors; and
9. the auditors
shall be appointed.
13 § Book-entry system
The shares of the company are incorporated in the book-entry system after the registration date set by the board of directors. The right to obtain funds from
the company and the right of subscription in a raise of the share capital shall, after the registration date, belong only to a person:
1. who is entered
in the shareholder register as a shareholder on a record date;
2. whose right to performance is, on the record date, registered in the book-entry account
of a shareholder registered in the shareholder register as well as entered in the shareholder register; or
3. if the share is registered in the name of a
nominee, whose book-entry account the share is entered into on the record date, and the custodian of whose shares is, on the record date, entered into the shareholder register as custodian of the shares.
Annex F
EXECUTION VERSION
January 19, 2016
| TO: |
Acorda Therapeutics, Inc. |
420 Saw Mill River Road, Ardsley, NY 10502
Dear Sirs,
We the undersigned (“we”, “us”, “our” or “ourselves”) refer to the Combination Agreement, dated as of the date hereof
(the “Combination Agreement”), which provides for, among other things, your proposed acquisition of all of the outstanding shares, American Depositary Shares and other applicable equity instruments in Biotie Therapies Oyj (the
“Target”) through an all cash tender offer (or, with respect to Warrants (as defined below), if so requested by the Bidder (as defined below), a purchase on terms consistent with this undertaking and the Combination Agreement) to be
made by Acorda Therapeutics, Inc. or its designated subsidiary (the “Bidder”) in Finland and in the United States with the Offer Consideration (as defined below) specified herein and otherwise on terms and conditions consistent with
the Combination Agreement and to be specified in a tender offer document (such cash tender offer by the Bidder, the “Tender Offer”), subject to the Board of Directors of the Target undertaking to recommend the Tender Offer. We
understand that as part of or in connection with the Tender Offer, the Bidder would offer to the
| |
i. |
shareholders of the Target € 0.2946 in cash for every share in the Target (“Shares”) held by each such shareholder (the “Share Consideration”); |
| |
ii. |
holders of the American Depository Shares of the Target (“ADSs”) € 23.5680 in cash for each outstanding ADS payable in the equivalent amount of U.S. dollars for each outstanding ADS determined as
near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the completion date of the Tender Offer (the “ADS Consideration”); and
|
| |
iii. |
holders of warrants of the Target issued on May 28, 2015 (“Warrants”, with, for the avoidance of doubt, each Warrant representing the right to subscribe for a single Share) € 0.1664 in
cash for each Warrant (the “Warrant Consideration”). |
The Share Consideration, the ADS Consideration and the Warrant
Consideration, as adjusted pursuant to the terms hereof, are hereinafter referred jointly to as the “Offer Consideration”. In each case, the Offer Consideration shall represent the full and final consideration payable for the
relevant instruments in the Tender Offer and, in the case of the Warrants, shall be in full and final satisfaction of the Target’s obligations to us under the governing documents for the Warrants.
We are the beneficial owner, as at the date hereof, of the number of Shares, ADSs and Warrants in the Target set forth on our signature page hereto, and such
Shares, ADSs and/or Warrants constitute, as of the date hereof, all of the Shares, ADSs and Warrants held of record, beneficially owned or for which voting power or disposition power is otherwise held by us. We have good and marketable title, free
and clear of any liens, other than liens pursuant to this undertaking and any transfer restrictions of general applicability as may be provided under applicable securities laws or in the terms and conditions of the Warrants, to all Shares, ADSs
and/or Warrants, as applicable, described in the immediately preceding sentence.
Subject to the terms and conditions hereof and of the Tender Offer, we
hereby irrevocably undertake to (i) accept the Tender Offer, and to tender or cause to tendered and sell or cause to be sold to the Bidder the Shares, ADSs and Warrants, as applicable, that we currently own, and any additional Shares, ADSs and
Warrants, as applicable, that we may hereafter acquire prior to the earlier of the Termination Date (as defined below) and the expiration of the Tender Offer, in the Tender Offer for the Offer Consideration applicable thereto, (ii) deliver or
cause to be delivered evidence of such acceptance pursuant to the terms of the Tender Offer to the Bidder within ten (10) business days from the beginning of the acceptance period of the
Tender Offer, and (iii) not exercise voting rights pertaining to the Shares, ADSs and any Shares subscribed based on the Warrants in favor of a Competitive Transaction (as defined below), in each case subject to the following paragraph.
Subject to the terms and conditions hereof, with respect to the Warrants and if so requested by the Bidder prior to the launch of the Tender Offer, in lieu of
accepting the Tender Offer and tendering the Warrants, we hereby irrevocably undertake to, subject to and upon the consummation of the Tender Offer and subject to this undertaking having not been previously terminated in accordance with the terms
hereof, sell to the Bidder (and the Bidder hereby irrevocably undertakes to purchase from us) the Warrants that we currently own, and any Warrants that we may hereafter acquire prior to the expiration of the Tender Offer, for the Warrant
Consideration and on terms consistent with the Combination Agreement; provided that such purchase and sale may not require us to make any representations and warranties, or to provide any covenants or other agreements, other than those that would
have been required to be made or provided by us in connection with tender of the Warrants in the Tender Offer.
Subject to the terms and conditions
hereof, we further undertake not to, except, in each case, in accordance with the terms of this undertaking: (i) sell, transfer, grant any option with respect to, pledge or otherwise dispose of (collectively, “Transfer”) any of
the Shares, ADSs and Warrants that we own or control prior to the earlier of the Termination Date and the expiration of the Tender Offer; provided, that nothing in this undertaking will limit our ability to convert any Warrants into Shares, indirect
Transfers or Transfers of any Shares, ADSs or Warrants to one or more of our affiliates that agree in writing to be bound hereby and assume our obligations hereunder, (ii) directly or indirectly, solicit any inquiries with respect to or solicit
or accept any public or private proposal or offer (including, without limitation, any proposal or offer to all holders of Shares, ADSs and Warrants), other than the Tender Offer, for the Shares, ADSs and Warrants we own or control prior to the
earlier of the Termination Date and the expiration of the Tender Offer (a “Competitive Transaction”) (it being understood that this undertaking is entered into solely in our capacity as a holder of Shares, ADSs and
Warrants, as applicable, and nothing in this undertaking shall (or shall require us to attempt to) limit or restrict us or any designee of us who is a director or officer of the Target from acting in such capacity or voting in such person’s
sole discretion in such capacity on any matter, however always in accordance with, where applicable, the fiduciary duties of the Board of Directors of the Target under Finnish laws); or (iii) withdraw our acceptance of the Tender Offer in
respect of any of such Shares, ADSs and Warrants regardless of any right of withdrawal contained in the terms and conditions of the Tender Offer or any legal right to withdraw, except in accordance with the terms of this undertaking, including the
withdrawal rights provided hereunder. Notwithstanding the foregoing, in the event that (A) the Target enters into discussions with a competing bidder in accordance with the Combination Agreement and (B) the Board of Directors of the Target
requests that we engage in discussions with the competing bidder, then nothing in this undertaking shall limit or restrict us from engaging in such discussions, provided that we will not, prior to the earlier of the Termination Date and the
expiration of the Tender Offer, (i) discuss, offer or otherwise negotiate with any competing bidder regarding the acquisition or other transfer to such competing bidder of any of our Shares. ADSs or Warrants at a price lower than the Share
Consideration, ADS Consideration or Warrant Consideration, as applicable, and (ii) will not enter into any undertaking with respect to any such competing transaction unless and until this undertaking has terminated in accordance with its terms.
The Bidder undertakes and agrees that should the terms and conditions of the Tender Offer, including the Offer Consideration, be revised or amended by
the Bidder for the benefit of the holders of Shares, ADSs and Warrants, such revision or amendment shall also be for our benefit. The Bidder further undertakes and agrees that, if the Bidder enters into an undertaking or similar agreement with any
other holder of Shares, ADSs or Warrants that provides such other holder with rights and benefits that are more favorable in any material respect to such other holder than the rights and benefits established in favor of us under this undertaking,
then (i) the Bidder shall promptly provide us with a copy of the other undertaking or agreement (and, in any case, within one business day of the execution thereof) and (ii) we shall have the right to elect to receive all such rights and
benefits under the
other undertaking or agreement, and this undertaking shall be amended accordingly. In addition, for the avoidance of doubt, we expressly do not waive any rights under Chapter 11, Section 25
of the Finnish Securities Market Act applicable from time to time.
This undertaking shall remain valid and in force until the first of the
following has taken place, at which date and time this undertaking shall immediately and automatically terminate in full (the “Termination Date”): (i) the Board of Directors of the Target has failed to recommend that the
holders of Shares, ADSs and Warrants accept the Tender Offer or has modified or withdrawn such recommendation; (ii) the completion and settlement of the Tender Offer; (iii) the Bidder has made a public announcement to the effect that it
will not complete the Tender Offer; (iv) the Combination Agreement has been terminated; (v) any amendment has been made to the Tender Offer that reduces the Offer Consideration or otherwise materially changes the terms and conditions of
the Tender Offer in a manner adverse to us; or (vi) the Tender Offer, having been launched or published, has failed to be completed by June 19, 2016.
We agree not to, without the prior written consent of the Bidder (such consent not to be unreasonably withheld, delayed or conditioned), make or cause to be
made any announcement or disclosure to any third party in respect of any unpublished information concerning the contemplated Tender Offer or this undertaking except (i) for disclosure to the Target, (ii) for disclosure to our directors,
officers, employees, partners, managers, members, agents and representatives who are charged with an obligation of confidentiality, (iii) for disclosure required by any applicable law, regulation or order of court, governmental authority,
self-regulatory organization or stock exchange or (iv) to enforce our rights under, or defend ourselves in any action brought in connection with, this undertaking.
This undertaking shall be governed by, and construed in accordance with, the laws of Finland. All disputes arising out of or relating to the present contract
shall be finally settled by final, binding arbitration conducted under the Rules of Arbitration of the International Chamber of Commerce (the “ICC Rules”). The arbitral panel shall be comprised of three arbitrators to be appointed
as follows: within 14 days after the commencement of arbitration, each party shall select one person to act as arbitrator and the two selected shall select a third arbitrator within 10 days of their appointment. If the arbitrators selected by the
parties are unable or fail to agree upon the third arbitrator, the third arbitrator shall be selected by the ICC. Either party may apply to the arbitrator seeking injunctive relief until the arbitration award is rendered or the controversy is
otherwise resolved. Either party also may, without waiving any remedy under this undertaking, seek from any court having jurisdiction any interim or provisional relief that is necessary to protect the rights or property of that party, pending the
arbitration panel’s determination of the merits of the controversy. The arbitration shall be conducted in English in London, United Kingdom, and judgment on the award rendered by the arbitrators may be entered in any court having jurisdiction
thereof. In the event of a conflict between this paragraph and the ICC Rules, this paragraph shall govern.
We agree and give our consent that a copy of
this undertaking may be provided to the Board of Directors of the Target and that this undertaking may be referred to and the main terms and conditions hereof may be disclosed in any public announcement that the Bidder or the Target may make in
connection with the Tender Offer or in the offer document related thereto; provided, that the Bidder or the Target, as applicable, shall (i) provide us with an advance copy of any such announcement or offer document that refers to us or our
affiliates by name and a reasonable opportunity to review and comment on any such disclosures or public announcements to the extent of such references and (ii) shall not include in any such announcement or offer document any reference to us or
our affiliates by name or, in connection therewith, the existence or terms and conditions of this undertaking if we reasonably object to the form or content thereof; provided that, nothing in the foregoing shall restrict the Bidder or the Company
from making any such disclosures or public announcements necessary to comply with applicable law.
This undertaking is given on the understanding
that nothing in this undertaking shall cause us to be deemed to be acting in concert with the Bidder or any other holder of Shares, ADSs or Warrants of the Company.
|
|
|
| Yours faithfully, |
|
| [INSERT NAME OF SHAREHOLDER] |
|
|
| By: |
|
|
| Name: |
|
|
|
| Number of Shares (not including Shares represented by ADSs or Warrants): |
|
|
| Number of ADSs: |
|
|
| Number of Warrants: |
|
|
|
|
|
| Acknowledged and accepted. |
|
| Acorda Therapeutics, Inc. |
|
|
| By: |
|
|
| Name: |
EXECUTION VERSION
January 19, 2016
| TO: |
Acorda Therapeutics, Inc. |
420 Saw Mill River Road, Ardsley, NY 10502
Dear Sirs,
We the undersigned (“we”, “us”, “our” or “ourselves”) refer to the Combination Agreement, dated as of the date hereof
(the “Combination Agreement”), which provides for, among other things, your proposed acquisition of all of the outstanding shares, American Depositary Shares and other applicable equity instruments in Biotie Therapies Oyj (the
“Target”) through an all cash tender offer (or, with respect to Warrants (as defined below), if so requested by the Bidder (as defined below), a purchase on terms consistent with this undertaking and the Combination Agreement) to be
made by Acorda Therapeutics, Inc. or its designated subsidiary (the “Bidder”) in Finland and in the United States with the Offer Consideration (as defined below) specified herein and otherwise on terms and conditions consistent with
the Combination Agreement and to be specified in a tender offer document (such cash tender offer by the Bidder, the “Tender Offer”), subject to the Board of Directors of the Target undertaking to recommend the Tender Offer. We
understand that as part of or in connection with the Tender Offer, the Bidder would offer to the
| |
i. |
shareholders of the Target € 0.2946 in cash for every share in the Target (“Shares”) held by each such shareholder (the “Share Consideration”); |
| |
ii. |
holders of the American Depository Shares of the Target (“ADSs”) € 23.5680 in cash for each outstanding ADS payable in the equivalent amount of U.S. dollars for each outstanding ADS determined as
near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the completion date of the Tender Offer (the “ADS Consideration”); |
| |
iii. |
holders of warrants of the Target issued on May 28, 2015 (“Warrants”, with, for the avoidance of doubt, each Warrant representing the right to subscribe for a single Share) € 0.1664 in
cash for each Warrant (the “Warrant Consideration”); |
| |
iv. |
holders of the following option and share rights: |
| |
a. |
the 2011 option rights (“2011 Option Rights”); |
| |
b. |
the 2014 option rights (“2014 Option Rights”); |
| |
c. |
the 2016 option rights (“2016 Option Rights”); |
| |
d. |
the option rights issued by Biotie Therapies AG (“Swiss Option Rights”); |
| |
e. |
the 2011 share rights (“2011 Share Rights”); and |
| |
f. |
the 2014 share rights (“2014 Share Rights” and, the option and share rights listed in (a) through (f), together, the “Equity Instruments”); |
in each case, the Share Consideration minus the applicable subscription price of such Equity Instrument (the “Equity Instrument
Consideration”).
The offer price payable to of the 2016 Option Rights and the 2011 and 2014 Share Rights shall be payable at the option of the
holder in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the Closing Date.
The Share Consideration, the ADS Consideration, the Warrant Consideration and the Equity Instrument
Consideration, as adjusted pursuant to the terms hereof, are hereinafter referred jointly to as the “Offer Consideration”. In each case, the Offer Consideration shall represent the full and final consideration payable for the
relevant instruments in the Tender Offer and, in the case of the Warrants, shall be in full and final satisfaction of the Target’s obligations to us under the governing documents for the Warrants.
We are the beneficial owner, as at the date hereof, of the number of Shares, ADSs, Warrants and Equity Instruments in the Target set forth on our signature
page hereto, and such Shares, ADSs, Warrants and/or Equity Instruments constitute, as of the date hereof, all of the Shares, ADSs, Warrants and Equity Instruments held of record, beneficially owned or for which voting power or disposition power is
otherwise held by us. We have good and marketable title, free and clear of any liens, other than liens pursuant to this undertaking and any transfer restrictions of general applicability as may be provided under applicable securities laws or in the
terms and conditions of the Warrants and/or Equity Instruments, to all Shares, ADSs, Warrants and/or Equity Instruments, as applicable, described in the immediately preceding sentence.
Subject to the terms and conditions hereof and of the Tender Offer, we hereby irrevocably undertake to (i) accept the Tender Offer, and to tender or
cause to be tendered and sell or cause to be sold to the Bidder the Shares, ADSs, Warrants and Equity Instruments, as applicable, that we currently own, and any additional Shares, ADSs, Warrants and Equity Instruments, as applicable, that we may
hereafter acquire or be granted prior to the earlier of the Termination Date (as defined below) and the expiration of the Tender Offer (“Additional Instruments”), in the Tender Offer for the Offer Consideration applicable thereto,
(ii) deliver or cause to be delivered evidence of such acceptance pursuant to the terms of the Tender Offer to the Bidder within ten (10) business days from the beginning of the acceptance period of the Tender Offer, and (iii) not
exercise voting rights pertaining to the Shares, ADSs and any Shares subscribed based on the Warrants, Equity Instruments or any Additional Instruments in favor of a Competitive Transaction (as defined below), in each case subject to the following
paragraph.
Subject to the terms and conditions hereof, with respect to the Warrants and if so requested by the Bidder prior to the launch of the Tender
Offer, in lieu of accepting the Tender Offer and tendering the Warrants, we hereby irrevocably undertake to, subject to and upon the consummation of the Tender Offer and subject to this undertaking having not been previously terminated in accordance
with the terms hereof, sell to the Bidder (and the Bidder hereby irrevocably undertakes to purchase from us) the Warrants that we currently own, and any Warrants that we may hereafter acquire prior to the expiration of the Tender Offer, for the
Warrant Consideration and on terms consistent with the Combination Agreement; provided that such purchase and sale may not require us to make any representations and warranties, or to provide any covenants or other agreements, other than those that
would have been required to be made or provided by us in connection with tender of the Warrants in the Tender Offer.
Subject to the terms and conditions
hereof, we further undertake not to, except, in each case, in accordance with the terms of this undertaking: (i) sell, transfer, grant any option with respect to, pledge or otherwise dispose of (collectively, “Transfer”) any of
the Shares, ADSs, Warrants, Equity Instruments or Additional Instruments that we own or control prior to the earlier of the Termination Date and the expiration of the Tender Offer; provided, that nothing in this undertaking will limit our ability to
convert any Warrants, Equity Instruments or Additional Instruments, as applicable, into Shares, indirect Transfers or Transfers of any Shares, ADSs or Warrants to one or more of our affiliates that agree in writing to be bound hereby and assume our
obligations hereunder, (ii) directly or indirectly, solicit any inquiries with respect to or solicit or accept any public or private proposal or offer (including, without limitation, any proposal or offer to all holders of Shares, ADSs,
Warrants and Equity Instruments), other than the Tender Offer, for the Shares, ADSs, Warrants, Equity Instruments and Additional Instruments we own or control prior to the earlier of the Termination Date and the expiration of the Tender Offer (a
“Competitive Transaction”) (it being understood that this undertaking is entered into solely in our capacity as a holder of Shares, ADSs, Warrants, Equity Instruments and Additional Instruments, as applicable, and
nothing in this undertaking shall (or shall require us to attempt to) limit or restrict us or any designee of us who is a director or officer of the Target from acting in such capacity or voting in such person’s sole discretion in such capacity
on any matter, however always in accordance with, where applicable, the fiduciary duties of the Board
of Directors of the Target under Finnish laws); or (iii) withdraw our acceptance of the Tender Offer in respect of any of such Shares, ADSs, Warrants, Equity Instruments or Additional
Instruments regardless of any right of withdrawal contained in the terms and conditions of the Tender Offer or any legal right to withdraw, except in accordance with the terms of this undertaking, including the withdrawal rights provided hereunder.
Notwithstanding the foregoing, in the event that (A) the Target enters into discussions with a competing bidder in accordance with the Combination Agreement and (B) the Board of Directors of the Target requests that we engage in
discussions with the competing bidder, then nothing in this undertaking shall limit or restrict us from engaging in such discussions, provided that we will not, prior to the earlier of the Termination Date and the expiration of the Tender Offer,
(i) discuss, offer or otherwise negotiate with any competing bidder regarding the acquisition or other transfer to such competing bidder of any of our Shares. ADSs, Warrants, Equity Instruments or Additional Instruments at a price lower than
the Share Consideration, ADS Consideration, Warrant Consideration or Equity Instrument Consideration, as applicable, and (ii) will not enter into any undertaking with respect to any such competing transaction unless and until this undertaking
has terminated in accordance with its terms.
The Bidder undertakes and agrees that should the terms and conditions of the Tender Offer, including the
Offer Consideration, be revised or amended by the Bidder for the benefit of the holders of Shares, ADSs, Warrants and Equity Instruments, such revision or amendment shall also be for our benefit. The Bidder further undertakes and agrees that, if the
Bidder enters into an undertaking or similar agreement with any other holder of Shares, ADSs, Warrants or Equity Instruments that provides such other holder with rights and benefits that are more favorable in any material respect to such other
holder than the rights and benefits established in favor of us under this undertaking, then (i) the Bidder shall promptly provide us with a copy of the other undertaking or agreement (and, in any case, within one business day of the execution
thereof) and (ii) we shall have the right to elect to receive all such rights and benefits under the other undertaking or agreement, and this undertaking shall be amended accordingly. In addition, for the avoidance of doubt, we expressly do not
waive any rights under Chapter 11, Section 25 of the Finnish Securities Market Act applicable from time to time.
This undertaking shall remain valid
and in force until the first of the following has taken place, at which date and time this undertaking shall immediately and automatically terminate in full (the “Termination Date”): (i) the Board of Directors of the
Target has failed to recommend that the holders of Shares, ADSs and Warrants accept the Tender Offer or has modified or withdrawn such recommendation; (ii) the completion and settlement of the Tender Offer; (iii) the Bidder has made a
public announcement to the effect that it will not complete the Tender Offer; (iv) the Combination Agreement has been terminated; (v) any amendment has been made to the Tender Offer that reduces the Offer Consideration or otherwise
materially changes the terms and conditions of the Tender Offer in a manner adverse to us; or (vi) the Tender Offer, having been launched or published, has failed to be completed by June 19, 2016.
We agree not to, without the prior written consent of the Bidder (such consent not to be unreasonably withheld, delayed or conditioned), make or cause to be
made any announcement or disclosure to any third party in respect of any unpublished information concerning the contemplated Tender Offer or this undertaking except (i) for disclosure to the Target, (ii) for disclosure required by any
applicable law, regulation or order of court, governmental authority, self-regulatory organization or stock exchange or (iii) to enforce our rights under, or defend ourselves in any action brought in connection with, this undertaking.
This undertaking shall be governed by, and construed in accordance with, the laws of Finland. All disputes arising out of or relating to the present contract
shall be finally settled by final, binding arbitration conducted under the Rules of Arbitration of the International Chamber of Commerce (the “ICC Rules”). The arbitral panel shall be comprised of three arbitrators to be appointed
as follows: within 14 days after the commencement of arbitration, each party shall select one person to act as arbitrator and the two selected shall select a third arbitrator within 10 days of their appointment. If the arbitrators selected by the
parties are unable or fail to agree upon the third arbitrator, the third arbitrator shall be selected by the ICC. Either party may apply to the arbitrator seeking
injunctive relief until the arbitration award is rendered or the controversy is otherwise resolved. Either party
also may, without waiving any remedy under this undertaking, seek from any court having jurisdiction any interim or provisional relief that is necessary to protect the rights or property of that party, pending the arbitration panel’s
determination of the merits of the controversy. The arbitration shall be conducted in English in London, United Kingdom, and judgment on the award rendered by the arbitrators may be entered in any court having jurisdiction thereof. In the event
of a conflict between this paragraph and the ICC Rules, this paragraph shall govern.
We agree and give our consent that a copy of this undertaking may be
provided to the Board of Directors of the Target and that this undertaking may be referred to and the main terms and conditions hereof may be disclosed in any public announcement that the Bidder or the Target may make in connection with the Tender
Offer or in the offer document related thereto.
This undertaking is given on the understanding that nothing in this undertaking shall cause us to be
deemed to be acting in concert with the Bidder or any other holder of Shares, ADSs, Warrants or Equity Instruments of the Company.
|
|
|
| Yours faithfully, |
|
| [INSERT NAME OF HOLDER OF EQUITY INSTRUMENTS] |
|
| By:
|
| Name: |
|
|
|
|
|
| Number of Shares (not including Shares represented by ADSs or Warrants): |
|
|
| Number of ADSs: |
|
|
| Number of Warrants: |
|
|
| Number of 2011 Option Rights: |
|
|
| Number of 2014 Option Rights: |
|
|
| Number of 2016 Option Rights |
|
|
| Number of Swiss Option Rights: |
|
|
| Number of 2011 Share Rights: |
|
|
| Number of 2014 Share Rights: |
|
|
|
|
|
| Acknowledged and accepted. |
|
| Acorda Therapeutics, Inc. |
|
|
| By: |
|
|
| Name: |
Annex G
EXECUTION VERSION
Combination Agreement
between
Acorda
Therapeutics, Inc.
and
Biotie Therapies Corp.
19 January 2016
Table of Contents
Contents
|
|
|
|
|
|
|
|
|
| 1. |
|
Definitions |
|
G-3 |
| 2. |
|
Tender Offer |
|
G-10 |
|
|
2.1 |
|
Launch of the Tender Offer |
|
G-10 |
|
|
2.2 |
|
Consideration |
|
G-11 |
|
|
2.3 |
|
Conditions to completion |
|
G-12 |
|
|
2.4 |
|
Permission by the Board of Directors of the Company to transfer Outstanding Equity Instruments |
|
G-13 |
|
|
2.5 |
|
Delisting and Deregistration |
|
G-13 |
|
|
2.6 |
|
Withholding |
|
G-14 |
|
|
|
| 3. |
|
Recommendation by the Company’s Board of Directors |
|
G-14 |
|
|
3.1 |
|
The Recommendation |
|
G-14 |
|
|
3.2 |
|
Cancellation of the Recommendation |
|
G-14 |
|
|
|
|
3.2.1 |
|
Fiduciary Duties |
|
G-14 |
|
|
|
|
3.2.2 |
|
Competing Offer |
|
G-15 |
|
|
|
| 4. |
|
Representations and warranties |
|
G-16 |
|
|
|
|
|
|
4.1 |
|
General |
|
G-16 |
|
|
4.2 |
|
Representations and warranties by the Company |
|
G-16 |
|
|
|
|
4.2.1 |
|
Organization and qualification, subsidiaries |
|
G-16 |
|
|
|
|
4.2.2 |
|
Authority relative to this Agreement, no conflicts |
|
G-16 |
|
|
|
|
4.2.3 |
|
No competing transaction |
|
G-17 |
|
|
|
|
4.2.4 |
|
Accounts |
|
G-17 |
|
|
|
|
4.2.5 |
|
Disclosed information |
|
G-17 |
|
|
|
|
4.2.6 |
|
Shares and securities entitling to shares |
|
G-18 |
|
|
|
|
4.2.7 |
|
Compliance with applicable laws and regulations |
|
G-18 |
|
|
|
|
4.2.8 |
|
Employee matters |
|
G-19 |
|
|
|
|
4.2.9 |
|
Compliance with material contracts |
|
G-20 |
|
|
|
|
4.2.10 |
|
Litigation and proceedings |
|
G-20 |
|
|
|
|
4.2.11 |
|
Intellectual property |
|
G-20 |
|
|
|
|
4.2.12 |
|
Taxes |
|
G-24 |
|
|
|
|
4.2.13 |
|
Certain Business Practices |
|
G-24 |
|
|
|
|
4.2.14 |
|
Health Regulatory Compliance |
|
G-25 |
|
|
4.3 |
|
Representations and warranties by the Offeror |
|
G-25 |
|
|
|
|
4.3.1 |
|
Organization and qualification |
|
G-25 |
|
|
|
|
4.3.2 |
|
Authority relative to this Agreement |
|
G-25 |
|
|
|
|
4.3.3 |
|
Sufficient financing |
|
G-25 |
|
|
|
|
4.3.4 |
|
Disclosure Documents |
|
G-26 |
|
|
|
|
4.3.5 |
|
Absence of Certain Agreements |
|
G-26 |
|
|
4.4 |
|
Effect and Breach of Warranties |
|
G-27 |
|
|
|
| 5. |
|
Undertakings |
|
G-27 |
|
|
5.1 |
|
Reasonable best efforts |
|
G-27 |
|
|
5.2 |
|
No solicitation of transactions |
|
G-28 |
|
|
5.3 |
|
Conduct of business pending the closing |
|
G-29 |
|
|
5.4 |
|
Access to information |
|
G-31 |
|
|
5.5 |
|
Notice of certain events |
|
G-32 |
|
|
5.6 |
|
Public announcements |
|
G-32 |
|
|
5.7 |
|
General meeting of shareholders |
|
G-32 |
|
|
5.8 |
|
Director and Officer Liability |
|
G-33 |
|
|
5.9 |
|
Employee Matters |
|
G-34 |
|
|
5.10 |
|
Financing |
|
G-35 |
G-i
|
|
|
|
|
|
|
|
|
| 6. |
|
Termination |
|
G-35 |
|
|
6.1 |
|
Termination by either Party |
|
G-36 |
|
|
6.2 |
|
Termination by the Company |
|
G-36 |
|
|
6.3 |
|
Termination by the Offeror |
|
G-36 |
|
|
6.4 |
|
Consequences of termination or expiration |
|
G-37 |
|
|
|
| 7. |
|
Miscellaneous |
|
G-37 |
|
|
7.1 |
|
Notices |
|
G-37 |
|
|
7.2 |
|
Expenses |
|
G-38 |
|
|
7.3 |
|
Schedules incorporated |
|
G-38 |
|
|
7.4 |
|
Headings |
|
G-39 |
|
|
7.5 |
|
Assignment |
|
G-39 |
|
|
7.6 |
|
No waiver |
|
G-39 |
|
|
7.7 |
|
Governing law and jurisdiction |
|
G-39 |
|
|
7.8 |
|
Specific Performance |
|
G-40 |
|
|
7.9 |
|
Amendments |
|
G-40 |
|
|
7.10 |
|
Entire agreement |
|
G-40 |
|
|
7.11 |
|
Severability |
|
G-40 |
|
|
7.12 |
|
Confidentiality |
|
G-40 |
|
|
7.13 |
|
Counterparts |
|
G-40 |
List of Schedules
|
|
|
|
|
| Schedule 2.1 |
|
Main terms and conditions of the Tender Offer |
|
G-42 |
| Schedule 5.6 |
|
Form of stock exchange releases announcing the Tender Offer |
|
G-47 |
G-ii
This Combination Agreement (this “Agreement”) is entered into on 19 January 2016
between
| (a) |
Acorda Therapeutics, Inc., a corporation organized and existing under the laws of Delaware, having its principal executive office in 420 Saw Mill River Road, Ardsley, NY 10502, United States of America
(the “Offeror”); and |
| (b) |
Biotie Therapies Corp., a corporation organized and existing under the laws of Finland, having its registered office in Joukahaisenkatu 6, FI-20520 Turku, Finland with the Business Identity Code 1475830-6 (the
“Company”). |
The Offeror and the Company are hereinafter jointly referred to as the “Parties” and each of them a
“Party”.
Background
| A. |
The Company is a Finnish public limited liability company engaged in the development of biopharmaceutical therapeutics for central nervous system disorders, the shares of which are listed on NASDAQ Helsinki Ltd.
(“Nasdaq Helsinki”) and the American Depositary Shares of which (each representing 80 shares of the Company) (the “ADSs”) are listed on NASDAQ Stock Market LLC (“Nasdaq US”). As of the date of this
Agreement, the Company has |
| |
i. |
issued a total of 1,089,608,083 shares of which 980,921,795 are outstanding and 108,686,288 are held as treasury shares by the Company (the “Shares”). Of the outstanding shares, as of the close of
business on 14 January 2016, 368,367,520 are represented by 4,604,594 ADSs; |
| |
ii. |
granted and outstanding a total of 435,000 option rights under the option plan resolved upon by the Board of Directors of the Company on 6 December 2011 by virtue of an authorization granted by the annual general
meeting of the Company held on 6 May 2011 (the “2011 Option Rights”), a total of 7,160,125 option rights under the option plan resolved upon by the Board of Directors of the Company on 2 January 2014 by virtue of an
authorization granted by the annual general meeting of the Company held on 4 April 2013, (the “2014 Option Rights”) and a total of 34,934,440 option rights under the option plan resolved upon by the Board of Directors of
the Company on 4 January 2016 by virtue of an authorization granted by the annual general meeting of the Company held on 26 May 2015 (the “2016 Option Rights”); |
| |
iii. |
granted and outstanding a total of 25,000 share units under the equity incentive plan resolved upon by the Board of Directors of the Company on 6 December 2011 (the “2011 Share Rights”) and a total
of 5,715,313 share units under the equity incentive plan resolved upon by the Board of Directors of the Company on 2 January 2014 by virtue of an authorization granted by the annual general meeting of the Company held on 4 April 2013 (the
“2014 Share Rights”); |
| |
iv. |
granted and outstanding a total of 2,027,628 option rights awards under the Swiss option plan dated 18 June 2008 (the “Swiss Option Rights”); and |
| |
v. |
issued and outstanding 220,400,001 warrants on 28 May 2015 by virtue of an authorization granted by the annual general meeting of the Company held on 26 May 2015 (the “Warrants”).
|
The 2011 Option Rights, the 2014 Option Rights, the 2016 Option Rights, the 2011 Share Rights, the 2014 Share Rights, the
Swiss Options Rights and the Warrants that have been granted to holders (other than the Company or any of its subsidiaries) are hereinafter jointly referred to as the “Outstanding Equity Instruments”.
| B. |
The Offeror is a Delaware corporation engaged in the biopharmaceutical business. As of the date of this Agreement the Offeror does not directly or indirectly hold any Shares, ADSs or Outstanding Equity Instruments
|
G-1
| C. |
The Offeror has conducted a due diligence review on the Company and its business and affairs based on information provided by the Company pursuant to a confidentiality agreement between the Parties dated
30 November 2015 (the “Confidentiality Agreement”). |
| D. |
The Offeror has proposed by letters dated November 12, 2015, December 11, 2015, December 28, 2015 and January 18, 2016 to make an offer to acquire all Shares and Outstanding Equity
Instruments in the Company, and based on the terms and conditions set forth herein the Board of Directors of each of the Parties has determined that it is in the best interest of their respective companies and their shareholders to effect the
combination as set forth in this Agreement, and each Board has approved the terms hereof. |
| E. |
The Parties intend, upon the terms and subject to the conditions of this Agreement, that in order to effect the combination, the Offeror will acquire all the Shares that are not held by the Company or any of its
subsidiaries including all the Shares represented by ADSs (the “Outstanding Shares”) and all the Outstanding Equity Instruments through a voluntary public tender offer in Finland and the United States in accordance with the Finnish
Securities Market Act (the “Finnish SMA”), the United States Securities Exchange Act of 1934, as amended (the “US Exchange Act”), and the rules and regulations promulgated thereunder and other Applicable Laws,
including the Helsinki Takeover Code that entered into force on 1 January 2014 (the “Tender Offer”) and, if necessary, through subsequent compulsory redemption proceedings in accordance with the Finnish Companies Act and/or the
terms and conditions of the Outstanding Equity Instruments, as more fully described below. |
| F. |
The Parties acknowledge that certain shareholders who, as of the date of this Agreement, represent approximately 49.4 percent of the Shares, approximately 61.4 percent of the Outstanding Equity Instruments (excluding
the Warrants) and 100 percent of the outstanding Warrants, have through undertakings dated 19 January 2016 irrevocably and unconditionally undertaken (i) to accept the Tender Offer and to tender their Shares and/or ADSs, as applicable,
into the Tender Offer and, if such shareholders hold Warrants, to either accept the Tender Offer and tender their Warrants into the Tender Offer or, on the same economic terms, to sell their Warrants to the Offeror, and (ii) in the case of
certain other holders of Outstanding Equity Instruments, to accept the Tender Offer and tender their Outstanding Equity Instruments into the Tender Offer, in the case of both clause (i) and (ii), pursuant to the terms of the applicable
irrevocable undertakings. |
| G. |
The Parties acknowledge that it is the intention of the Offeror to cause the Shares to be delisted from Nasdaq Helsinki and Nasdaq US and deregistered under the US Exchange Act as soon as permitted and reasonably
practicable under Applicable Laws. |
G-2
As used in this Agreement, unless expressly otherwise stated or evident in
the context, the following terms shall have the following meanings, the singular (where appropriate) shall include the plural and vice versa and references to Schedules and Sections shall mean Schedules and Sections of this Agreement:
|
|
|
|
|
| 1.1 |
|
2011 Option Rights |
|
has the meaning set out in the background. |
|
|
|
| 1.2 |
|
2014 Option Rights |
|
has the meaning set out in the background. |
|
|
|
| 1.3 |
|
2016 Option Rights |
|
has the meaning set out in the background. |
|
|
|
| 1.4 |
|
2011 Share Rights |
|
has the meaning set out in the background. |
|
|
|
| 1.5 |
|
2014 Share Rights |
|
has the meaning set out in the background. |
|
|
|
| 1.6 |
|
Actual Knowledge of the Company |
|
means (i) the actual knowledge of any Key Employee of the Company or (ii) the actual knowledge of any member of the Board of Directors of the Company, in each case as of the Signing Date and without any duty of
inquiry. |
|
|
|
| 1.7 |
|
ADSs |
|
has the meaning set out in the background. |
|
|
|
| 1.8 |
|
Agreement |
|
means this Combination Agreement. |
|
|
|
| 1.9 |
|
Applicable Anti-Corruption Laws |
|
has the meaning set out in Section 4.2.13. |
|
|
|
| 1.10 |
|
Applicable Law |
|
means with respect to any Person, any federal, state or local law (statutory, common or otherwise), constitution, treaty, convention, ordinance, code, rule, regulation, order, injunction, judgment, decree, ruling or other similar
requirement enacted, adopted, promulgated or applied by a Governmental Authority that is binding upon or applicable to such Person, as amended unless expressly specified otherwise. |
|
|
|
| 1.11 |
|
Applicable Subscription Price |
|
means, with respect to any Outstanding Equity Instrument, the subscription price applicable to such Outstanding Equity Instrument as set out for each Outstanding Equity Instrument in Schedule 5.6. In the case of any such
subscription price denominated in a currency other than EUR, the Applicable Subscription Price shall be equal to the equivalent amount of EUR determined as near to the payment date as reasonably practicable based on U.S. dollar or Swiss franc, as
applicable, spot rate against the euro exchange rate on the nearest practicable day to the Closing Date. For the avoidance of doubt, for the 2011 Share Rights that have no subscription price the Applicable Subscription Price shall be equal to
zero. |
G-3
|
|
|
|
|
| 1.12 |
|
banking day |
|
means any day other than any day on which the banks in Finland are authorized to be closed, provided that, for purposes of Section 3.2, banking days shall not be deemed to include any day in which the banks in New York are
authorized by law or executive order to be closed. |
|
|
|
| 1.13 |
|
BidCo |
|
has the meaning set out in Section 7.5. |
|
|
|
| 1.14 |
|
C.F.R. |
|
means the U.S. Code of Federal Regulations. |
|
|
|
| 1.15 |
|
Closing Date |
|
means the date when the title to the Outstanding Shares and the Outstanding Equity Instruments validly tendered in the Tender Offer is transferred to Offeror. |
|
|
|
| 1.16 |
|
Collective Agreement |
|
has the meaning set out in Section 4.2.8. |
|
|
|
| 1.17 |
|
Company |
|
has the meaning set out in the introductory section. |
|
|
|
| 1.18 |
|
Company Disclosure Schedule |
|
means the disclosure schedule dated the date hereof regarding this Agreement that has been provided by the Company to the Offeror. |
|
|
|
| 1.19 |
|
Company Intellectual Property |
|
has the meaning set out in Section 4.2.11. |
|
|
|
| 1.20 |
|
Company Licensee |
|
has the meaning set out in Section 4.2.11. |
|
|
|
| 1.21 |
|
Company Licensor |
|
has the meaning set out in Section 4.2.11. |
|
|
|
| 1.22 |
|
Company Partner |
|
means any partner or other third party which pursuant to a contract with the Company or any of its subsidiaries co-develops, co-promotes, co-markets or otherwise has a license or other right
to research, develop, manufacture, supply, test, distribute, market, promote, offer for sale, sell or import or to otherwise exploit any Covered Product. |
|
|
|
| 1.23 |
|
Company SEC Document |
|
means the publicly available reports, schedules, forms, statements, prospectuses, registration statements and other documents filed or furnished by the Company with the SEC since March 17, 2015. |
|
|
|
| 1.24 |
|
Competing Offer |
|
has the meaning set out in Section 3.2.2. |
|
|
|
| 1.25 |
|
Competing Proposal |
|
has the meaning set out in Section 5.2. |
|
|
|
| 1.26 |
|
Confidentiality Agreement |
|
has the meaning set out in the background |
|
|
|
| 1.27 |
|
Covered Product(s) |
|
means tozadenant, Selincro, BTT1023 and SYN 120, each individually (as well as, for purposes of Sections 4.2.7 and 4.2.14 only, Nepicastat). |
G-4
|
|
|
|
|
| 1.28 |
|
Data Room |
|
has the meaning set out in Section 4.2.5. |
|
|
|
| 1.29 |
|
Due Diligence Information |
|
has the meaning set out in Section 4.2.5. |
|
|
|
| 1.30 |
|
Effect |
|
has the meaning set out in Section 1.42. |
|
|
|
| 1.31 |
|
Equity Purchase Agreement |
|
has the meaning set out in Section 4.3.3. |
|
|
|
| 1.32 |
|
Equity Sale |
|
has the meaning set out in Section 4.3.3. |
|
|
|
| 1.33 |
|
EMA |
|
means the European Medicines Agency. |
|
|
|
| 1.34 |
|
FDA |
|
means the U.S. Food and Drug Administration. |
|
|
|
| 1.35 |
|
Fiduciary Duties |
|
has the meaning set out in Section 3.2.1. |
|
|
|
| 1.36 |
|
Financial Statements |
|
has the meaning set out in Section 4.2.4. |
|
|
|
| 1.37 |
|
Governmental Authority |
|
means any transnational, domestic or foreign federal, state or local governmental, regulatory or administrative authority, arbitrator, department, court, agency or official, including any political subdivision thereof. |
|
|
|
| 1.38 |
|
Health Authorities |
|
means the Governmental Authorities that administer Health Laws, including the FDA, the EMA, and comparable regulatory authorities. |
|
|
|
| 1.39 |
|
Health Laws |
|
means any law or regulation of any national or supranational governmental authority the purpose of which is to ensure the safety, efficacy and quality of medicines or pharmaceuticals by regulating the research, development,
manufacturing and distribution of these products, including laws and regulations relating to good laboratory practices, good clinical practices, clinical trial registration and reporting, investigational use, registration and listing, product
marketing authorization, manufacturing facilities compliance and approval, good manufacturing practices, import/export, labeling, advertising, promotional practices, safety surveillance, record keeping and filing of required reports such as the U.S.
Food, Drug and Cosmetic Act of 1938, as amended, and the Public Health Service Act, as amended, in each case including the associated rules and regulations promulgated thereunder |
|
|
|
| 1.40 |
|
IFRS |
|
International Financial Reporting Standards, as in effect from time to time. |
|
|
|
| 1.41 |
|
Intellectual Property |
|
means all intellectual property, including all applications, registrations and renewals thereof, all common law, statutory and other rights associated therewith in any jurisdiction, and, without limiting the generality of the
foregoing, including all (i) issued patents, pending patent |
G-5
|
|
|
|
|
|
|
|
|
|
|
|
applications and provisional applications and all continuations, continuations-in-part, divisionals, extensions, reissues and reexaminations and
counterparts thereof; (ii) trademarks, trade dress, Internet domain names, business names and all other indicia of origin and goodwill associated therewith; (iii) works of authorship, copyrights, moral rights and database rights; (iv) software,
including code, databases and related documentation; (v) know-how, trade secrets and other technology, including inventions, improvements, manufacturing, analytical and other methods or processes, specifications, formulations, protocols,
assays, pre-clinical, clinical and other data and business, technical and other information, batch, patent, trademark and other records; and (vi) tangible embodiments of the foregoing in any form or medium. |
|
|
|
| 1.42 |
|
Interim Report |
|
has the meaning set out in Section 4.2.4. |
|
|
|
| 1.43 |
|
Internal Revenue Code |
|
means the United States Internal Revenue Code of 1986, as amended. |
|
|
|
| 1.44 |
|
Key Employees |
|
means (i) Timo Veromaa, the Chief Executive Officer of the Company, (ii) David Cook, the Chief Financial Officer of the Company, (iii) Stephen Bandak, the Chief Medical Officer of the Company and (iv) Mehdi Paborji, the Chief
Operating Officer of the Company. |
|
|
|
| 1.45 |
|
Knowledge of the Company |
|
means (i) the actual knowledge of any Key Employee of the Company, after due inquiry, or (ii) the actual knowledge of any member of the Board of Directors of the Company. |
|
|
|
| 1.46 |
|
Launch Announcement |
|
has the meaning set out in Section 2.1. |
|
|
|
| 1.47 |
|
Launch Date |
|
means the first day of the Offer Period. |
|
|
|
| 1.48 |
|
Material Adverse Effect |
|
(A) means any divestment or reorganization of any material part or asset of the Company or its subsidiaries or any recapitalization thereof;
or (B) any event, condition, circumstance, development, occurrence, change, effect or
fact (any such item an “Effect”) that individually or in the aggregate, has, results in or would reasonably be expected to have or result in a material adverse effect on the (i) business, assets, condition (financial or otherwise)
or results of operations, of the Company and its subsidiaries, taken as a whole, excluding any Effect resulting from (A) changes in the financial or securities markets, or economic, political or regulatory conditions generally, except to the extent
such change has a disproportionate effect on the Company relative to other industry participants, (B) changes in IFRS or changes in the regulatory accounting requirements applicable to any industry in which the Company and its subsidiaries operate,
except to the extent such change has a disproportionate |
G-6
|
|
|
|
|
|
|
|
|
|
|
|
effect on the Company relative to other industry participants, (C) changes (including changes of Applicable Law) or conditions generally affecting the industry in which the Company and its subsidiaries operate, |
|
|
|
|
except to the extent such change has a disproportionate effect on the Company relative to other industry participants, (D) acts of war, sabotage or terrorism or natural disasters, except to the extent such change has a
disproportionate effect on the Company relative to other industry participants, (E) the announcement or pendency of the transactions contemplated by, or the performance of obligations under, this Agreement, including but not limited to any loss of
or change in relationships between the Company or any of its subsidiaries and any customer, supplier, distributor, business partner, employee, similar party, Governmental Authority or any other Persons and any shareholder or derivative litigation
relating to the execution and performance of this Agreement or the announcement or the anticipated consummation of the transactions contemplated thereby (it being understood that this clause (E) shall not apply with respect to a representation or
warranty contained in this Agreement to the extent that the purpose of such representation or warranty is to address the consequences resulting from the execution and delivery of this Agreement or the consummation, announcement or pendency of the
transactions contemplated by, or the performance of obligations under, this Agreement), (F) any failure by the Company and its subsidiaries to meet any internal, published or third-party budgets, projections, forecasts or predictions of financial
performance for any period (provided that the exception in this clause (F) shall not prevent or otherwise affect a determination that any Effect underlying such failure has resulted in or contributed to a Material Adverse Effect), (G) any action
taken (or omitted to be taken) at the express request of the Offeror, (H) any action taken by the Company or any of its subsidiaries that is required or expressly contemplated by this Agreement, (I) the results of any pre-clinical or clinical
testing sponsored by the Company, any of its competitors or any of their respective collaboration partners or the increased incidence or severity of any previously identified side effects, adverse events or safety observations, or reports of new
side effects, adverse events or safety observations, with respect to any products or product candidates of the Company or any of its competitors (but not, in each case, the underlying cause of such results, side effects, adverse events or safety
observations to the extent such cause related to any Specified Event) (and, it being understood that this clause (I) shall not apply with respect to a representation or warranty contained in this Agreement to the extent that both (A) the
purpose of such representation |
G-7
|
|
|
|
|
|
|
|
|
or warranty is to address the conduct of pre-clinical or clinical testing sponsored by the Company or any of its collaboration partners or any products or product candidates of the Company and (B) as of the Signing Date, to the
Knowledge of the Company, the Effect that would otherwise be excluded by this clause (I) has occurred), (J) the effect of the continued incurrence by the Company of net losses (provided that the exception in this clause (J) shall not
prevent or otherwise affect a determination that any Effect underlying such change has resulted in or contributed to a Material Adverse Effect), or (K) a change in the price and/or trading volume of the Shares or ADSs on the Nasdaq Helsinki, the
Nasdaq US or any other exchange or market on which they trade or are quoted for purchase and sale (provided that the exception in this clause (K) shall not prevent or otherwise affect a determination that any Effect underlying such change has
resulted in or contributed to a Material Adverse Effect), or (ii) ability of the Company to consummate the transactions contemplated hereby. |
|
|
|
| 1.49 |
|
Material Contracts |
|
has the meaning set out in Section 4.2.9 |
|
|
|
| 1.50 |
|
Nasdaq Helsinki |
|
has the meaning set out in the background. |
|
|
|
| 1.51 |
|
Nasdaq US |
|
has the meaning set out in the background. |
|
|
|
| 1.52 |
|
Offer Period |
|
has the meaning set out in Section 2.1. |
|
|
|
| 1.53 |
|
Offeror |
|
has the meaning set out in the introductory section. |
|
|
|
| 1.54 |
|
Outstanding Equity Instruments |
|
has the meaning set out in the background. |
|
|
|
| 1.55 |
|
Outstanding Shares |
|
has the meaning set out in the background. |
|
|
|
| 1.56 |
|
Party and Parties |
|
has the meaning set out in the introductory section. |
|
|
|
| 1.57 |
|
Person |
|
means any person or entity as the context requires. |
|
|
|
| 1.58 |
|
Promoting Measures |
|
has the meaning set out in Section 5.2(c) |
|
|
|
| 1.59 |
|
Purchaser |
|
has the meaning set out in Section 4.3.3. |
|
|
|
| 1.60 |
|
Recommendation |
|
has the meaning set out in Section 3.1. |
|
|
|
| 1.61 |
|
Replacement Financing |
|
has the meaning set out in Section 5.10. |
|
|
|
| 1.62 |
|
Report of the Board of Directors |
|
has the meaning set out in Section 4.2.4. |
|
|
|
| 1.63 |
|
Required Actions |
|
has the meaning set out in Section 5.4. |
G-8
|
|
|
|
|
| 1.64 |
|
Rules |
|
has the meaning set out in Section 7.7. |
|
|
|
| 1.65 |
|
Sarbanes -Oxley Act |
|
means the United States Sarbanes-Oxley Act of 2002, as amended. |
|
|
|
| 1.66 |
|
SEC |
|
means the United States Securities and Exchange Commission. |
|
|
|
| 1.67 |
|
SEC Relief |
|
has the meaning set out in Section 2.1. |
|
|
|
| 1.68 |
|
Shares |
|
has the meaning set out in the background. |
|
|
|
| 1.69 |
|
Signing Date |
|
means the date of this Agreement. |
|
|
|
| 1.70 |
|
Specified Event |
|
means the Company, FDA, EMA or any IRB or DSMB terminates or suspends, or recommends that the sponsor terminates or suspends, a tozadenant clinical trial for safety reasons. |
|
|
|
| 1.71 |
|
Swiss Option Rights |
|
has the meaning set out in the background. |
|
|
|
| 1.72 |
|
Tax |
|
means (i) all direct and indirect statutory, governmental, federal, state, local, municipal and foreign net income, gross income, gross receipts, value added, sales, use, ad valorem, transfer, franchise, profits, service, service
use, withholding, payroll, employment, social security, pension contributions, excise, severance, stamp, occupation, premium, property, unclaimed property, escheat, windfall profits, customs duties and any other taxes and similar charges, levies,
assessments, duties or withholdings of any kind whatsoever, whether disputed or not, imposed by any authority, including all penalties, additions and interest with respect thereto, (ii) any liability for payment of amounts described in clause (i)
whether as a result of transferee liability, of being a member of an affiliated, consolidated, combined, unitary or similar group for any period, or otherwise through operation of Applicable Law, and (iii) any liability for the payment of amounts
described in clauses (i) or (ii) as a result of any tax sharing, tax indemnity or tax allocation agreement or any other express or implied agreement to indemnify any other Person, and Taxes shall be construed accordingly. |
|
|
|
| 1.73 |
|
Tax Return |
|
means any written or electronic return, certificate, declaration, report, statement and document filed or required to be filed with respect to Taxes, amendments thereof, and schedules and attachments thereto. |
|
|
|
| 1.74 |
|
Tekes |
|
has the meaning set out in Section 5.1(b). |
|
|
|
| 1.75 |
|
Tender Offer |
|
has the meaning set out in the background. |
G-9
|
|
|
|
|
| 1.76 |
|
US Exchange Act |
|
has the meaning set out in the background. |
|
|
|
| 1.77 |
|
US Securities Act |
|
means the United States Securities Act of 1933, as amended. |
|
|
|
| 1.78 |
|
Warrants |
|
has the meaning set out in the background. |
|
|
|
| 1.79 |
|
Warranties |
|
has the meaning set out in Section 4.1. |
In addition, whenever the context may require, any pronoun shall include the corresponding masculine, feminine and neuter
forms. The words “include”, “includes” and “including” shall be deemed to be followed by the phrase “without limitation”. The word “will” shall be construed to have the same meaning and effect as the
word “shall” and the word “or” when used in this Agreement is not exclusive.
| 2.1 |
Launch of the Tender Offer |
Subject to the terms and conditions of this Agreement, the
Offeror shall launch the Tender Offer by means of a stock exchange release to be published in accordance with the rules of Nasdaq Helsinki and Nasdaq US including the terms and conditions of the Tender Offer (the “Launch
Announcement”) as promptly as practicable after the date of this Agreement and commence the Tender Offer as promptly as practicable after the date of the Launch Announcement, which commencement shall be no later than five (5) banking
days after the Offeror has received the SEC Relief (as defined below). The main terms and conditions of the Tender Offer shall be substantially in the form attached as Schedule 2.1 hereto, subject to direction from the SEC and/or the Finnish
Financial Supervisory Authority. Unless extended in accordance with this Agreement and the terms and conditions of the Tender Offer, the acceptance period under the Tender Offer shall expire on the date that is twenty (20) business days (for
this purpose calculated in accordance with Rule 14d-1(g)(3) of the US Exchange Act) (the “Offer Period”) after the Tender Offer is commenced in the United States. If any of the conditions to completion set forth in Section 2.3
is not satisfied or waived on any scheduled expiration date of the Tender Offer, and provided that this Agreement has not been terminated pursuant to Section 6, the Offer Period shall be extended by the Offeror from time to time in accordance
with the terms and conditions of the Tender Offer in compliance with applicable Finnish and United States legal requirements; provided that (a) the Offeror shall not be required to extend the Tender Offer beyond the Long-stop Date, (b) the
Offeror shall not be required to extend the Tender Offer if prohibited from doing so based on Applicable Law, having applied to applicable Governmental Authorities for such extensions up to and including the Long-stop Date, and (c) no such
individual extension shall be for a period of more than two weeks, unless the Parties otherwise agree. Notwithstanding the above, should the non-satisfaction of the conditions to completion relate to condition 2.3(a) only, and should the number of
Outstanding Shares (calculated in accordance with condition 2.3(a)) validly tendered be below two-thirds (2/3) of the issued and outstanding Shares and voting rights in the Company after the expiry of the ten (10) week maximum acceptance
period under Finnish law, the Offeror shall have no obligation to further extend the Tender Offer, unless the Offeror otherwise agrees.
The Parties shall cooperate with one another and use their respective reasonable best efforts to obtain from the SEC, as promptly as
practicable after the date hereof, exemptive relief with respect to permitting the Tender Offer to be conducted in accordance with the main terms and conditions of the Tender Offer set forth in Schedule 2.1 (the “SEC Relief”),
provided that, if the SEC Relief is not obtained by 23 February 2016, the Parties shall cooperate to restructure the Tender Offer (including, as applicable, by launching one or more separate tender offers in the United States, Finland or other
jurisdictions and one
G-10
or more separate tender offers for the Outstanding Shares, the ADSs or any of the Outstanding Equity Instruments) so as to permit the Tender Offer to be made without receipt of the SEC Relief.
Notwithstanding anything to the contrary set forth in the preceding paragraph, the Offeror shall be required to modify the terms and conditions of the Tender Offer as necessary to comply with any relief, direction or instruction set forth in any
“no action” letter or comment letter from the SEC or any written communication from the Finnish Financial Supervisory Authority (including, as applicable, any relief, direction or instruction to launch one or more separate tender offers in
the United States, Finland or other jurisdictions and one or more separate tender offers for the Outstanding Shares, the ADSs or any of the Outstanding Equity Instruments); provided, however, that (i) the Offeror will not be required to
increase the price offered for any Outstanding Shares or Outstanding Equity Instruments, offer alternative consideration for any Outstanding Shares or Outstanding Equity Instruments, waive any condition to completion set forth in Section 2.3,
or waive the right to terminate this Agreement pursuant to Section 6, and (ii) the Company must provide its prior written consent to any modification for which its consent would be required pursuant to the immediately following paragraph
(assuming for this purpose that the modification were being made pursuant to such paragraph).
For the avoidance of doubt, the Offeror may
otherwise amend the terms and conditions of the Tender Offer as provided for in this Agreement, in the terms and conditions of the Tender Offer, in the Finnish SMA and other relevant Finnish laws and in the US Exchange Act and the rules and
regulations promulgated thereunder, provided that, without the prior written consent of the Company, Offeror shall not:
| |
(i) |
decrease the consideration set forth in Section 2.2; |
| |
(ii) |
change the form of consideration to be paid; |
| |
(iii) |
decrease the number of Shares or Outstanding Equity Instruments sought in the Tender Offer; |
| |
(iv) |
extend or otherwise change the expiration date of the Tender Offer, except for a potential subsequent offer period provided for in the terms and conditions of the Tender Offer or as required by the first paragraph of
this Section 2.1; |
| |
(v) |
impose additional conditions to completion; or |
| |
(vi) |
otherwise amend, modify or supplement any of the conditions to completion or terms and conditions of the Tender Offer to the detriment in any material respect of the holders of the Shares, ADSs or Outstanding Equity
Instruments. |
In the Tender Offer, the Offeror shall offer to acquire all the ADSs, the
Outstanding Shares and all the Outstanding Equity Instruments validly tendered and not withdrawn for a consideration of
| |
(i) |
EUR 0.2946 in cash for each Outstanding Share; |
| |
(ii) |
EUR 23.5680 in cash for each outstanding ADS, payable in the equivalent amount of U.S. dollars for each outstanding ADS determined as near to the payment date as reasonably practicable based on U.S. dollar spot rate
against the euro exchange rate on the nearest practicable day to the Closing Date; |
| |
(iii) |
EUR 0.2946 minus the Applicable Subscription Price in cash for each 2011 Option Right; |
| |
(iv) |
EUR 0.2946 minus the Applicable Subscription Price in cash for each 2014 Option Right; |
| |
(v) |
EUR 0.2946 minus the Applicable Subscription Price in cash for each 2016 Option Right, payable, at the option of the holder, in the equivalent amount of U.S. dollars for each outstanding 2016 Option Right
determined as near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the Closing Date; |
G-11
| |
(vi) |
EUR 0.2946 minus the Applicable Subscription Price in cash for each 2011 Share Right, payable, at the option of the holder, in the equivalent amount of U.S. dollars for each outstanding 2011 Share Right
determined as near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the Closing Date; |
| |
(vii) |
EUR 0.2946 minus the Applicable Subscription Price in cash for each 2014 Share Right, payable, at the option of the holder, in the equivalent amount of U.S. dollars for each outstanding 2014 Share Right
determined as near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the Closing Date; |
| |
(viii) |
EUR 0.2946 minus the Applicable Subscription Price in cash for each Swiss Option Right with an Applicable Subscription Price less than EUR 0.2946; |
| |
(ix) |
EUR 0.01 in cash for each Swiss Option Right with an Applicable Subscription Price equal to or greater than EUR 0.2946; and |
| |
(x) |
EUR 0.1664 in cash for each Warrant. |
| 2.3 |
Conditions to completion |
The obligation of the Offeror to accept for payment the
tendered Outstanding Shares and Outstanding Equity Instruments and to complete the Tender Offer shall be subject to the fulfillment or, to the extent permitted by Applicable Law, waiver by Offeror of the following conditions on or prior to the date
of the Offeror’s announcement of the final result of the Tender Offer:
| |
(a) |
the valid tender of Outstanding Shares (including Outstanding Shares represented by validly tendered ADSs and validly tendered Warrants) representing, together with any Outstanding Shares (including Outstanding Shares
represented by ADSs and Warrants) otherwise acquired by the Offeror, more than ninety percent (90%) of the issued and outstanding Shares and voting rights of the Company, calculated on a Fully Diluted Basis and otherwise in accordance with
Chapter 18 Section 1 of the Finnish Limited Liability Companies Act (21.7.2006/624); as used in this paragraph “Fully Diluted Basis” means an equation in which the numerator represents the aggregate number of Outstanding Shares
(including Outstanding Shares represented by ADSs) and Warrants that have been validly tendered or otherwise acquired by the Offeror and the denominator represents the aggregate number of all Outstanding Shares (including Outstanding Shares
represented by ADSs) and Warrants, as well as shares issuable upon the vesting and exercise of those Outstanding Equity Instruments (other than Warrants) that have not been validly tendered into the Tender Offer or otherwise acquired by the Offeror.
|
| |
(b) |
the expiration or termination of any applicable waiting period under the HSR Act; |
| |
(c) |
no Material Adverse Effect having occurred after the Signing Date; |
| |
(d) |
the Offeror not, after the Signing Date, having received information previously undisclosed to it that describes a Material Adverse Effect that occurred prior to the Signing Date; |
| |
(e) |
no information made public by the Company or disclosed by the Company to the Offeror being materially inaccurate, incomplete, or misleading, and the Company not having failed to make public any information that should
have been made public by it under Applicable Laws, including without limitation the rules of Nasdaq Helsinki and Nasdaq US, provided that, in each case, the information made public, disclosed or not disclosed or the failure to disclose information
constitutes a Material Adverse Effect; |
| |
(f) |
no court or regulatory authority of competent jurisdiction (including without limitation the Finnish Financial Supervisory Authority or the SEC) having given an order or issued any regulatory action preventing or
enjoining the completion of the Tender Offer; |
G-12
| |
(g) |
the Board of Directors of the Company having issued the Recommendation and the Recommendation remaining in force and not being modified or changed in a manner detrimental to the Offeror; and |
| |
(h) |
this Agreement not having been terminated and remaining in force and no event having occurred that, with the passage of time, would give the Offeror the right to terminate this Agreement under Section 6.3(b)-(e).
|
Subject to the terms and conditions set forth in this Agreement and to the satisfaction or waiver of the conditions to
completion set forth above, the Offeror shall accept for payment and pay for, as promptly as practicable after the expiration of the Tender Offer in accordance with Applicable Law, all Shares, ADSs and Outstanding Equity Instruments validly tendered
and not withdrawn pursuant to the Tender Offer.
| 2.4 |
Permission by the Board of Directors of the Company to transfer Outstanding Equity Instruments |
The Board of Directors of the Company shall within two (2) banking days after the publication of the Launch Announcement, in accordance
with section I.6.1 of the terms and conditions of the 2011 Option Rights, section I.7.1 of the terms and conditions of the 2014 Option Rights and section I.6.1 of the terms and conditions of the 2016 Option Rights, grant a permission to the holders
of the 2011, 2014 and 2016 Option Rights to transfer their outstanding 2011, 2014 and 2016 Option Rights to the Offeror by accepting the Tender Offer and tendering the outstanding 2011, 2014 and 2016 Option Rights into the Tender Offer in accordance
with the terms and conditions thereof, and waive any other applicable lock-up or holding requirements relating to the Outstanding Equity Instruments and/or any net return of such instruments to allow the transfer of such Outstanding Equity
Instruments and/or Shares received as net return of such instruments to the Offeror by tendering such Outstanding Equity Instruments and/or Shares into the Tender Offer, or alternatively in respect of the 2016 Option Rights held by individuals who
have executed separate agreements concerning their obligation to return their 2016 Option Rights to the Company should a transaction for change of control of the Company be announced during Q1/2016, cause such 2016 Option Rights to be returned to
the Company in accordance with the terms and conditions of those agreements.
The Board of Directors (and, in respect of the Swiss Option
Rights, the Board of Directors of Biotie Therapies AG) shall within two (2) banking days after the publication of the Launch Announcement grant or procure the granting of a corresponding permission to the holders of the other Outstanding Equity
Instruments to transfer their Outstanding Equity Instruments to the Offeror by accepting the Tender Offer and tendering the Outstanding Equity Instruments into the Tender Offer in accordance with the terms and conditions thereof.
The Board of Directors of Biotie Therapies AG shall no later than two (2) banking days after the publication of the Launch Announcement
adopt such amendments to the terms and conditions of the Swiss Option Rights as may be required for Section 8.3. of such terms and conditions to apply to the Tender Offer.
| 2.5 |
Delisting and Deregistration |
Subject to the Offeror acquiring more than ninety percent
(90%) of the issued and outstanding Shares and voting rights in the Company, the Offeror shall cause the Shares of the Company to be delisted from Nasdaq Helsinki and the ADSs to be delisted from Nasdaq US and deregistered under the US Exchange
Act as soon as permitted and reasonably practicable under Applicable Laws.
If the Offeror resolves to cause said delisting and
deregistration, the Company shall use its reasonable best efforts to assist the Offeror in such procedure, including without limitation promptly taking any required corporate decisions and making any necessary filings to effect such delisting and
deregistration, in each case, subject to compliance with Applicable Law.
G-13
Notwithstanding any other provision in this Agreement to the contrary, the
Offeror, the Company, and their respective affiliates shall be entitled to deduct and withhold from any amount payable pursuant to this Agreement such amounts as are required to be deducted and withheld under any applicable provision of Applicable
Law (and, for the avoidance of doubt, the Company or the Offeror, as applicable, shall deduct and withhold all Taxes as are required by applicable Tax law in respect of payments made in accordance with Sections 2.2(iii) through 2.2(viii) of this
Agreement) to the extent such withholdings or deductions are required to be made at the time such payments are made. Amounts withheld pursuant to this Section 2.6 and duly and timely remitted to the proper governmental authority shall be
treated for all purposes of this Agreement as having been paid to the Person in respect of which such deduction and withholding was made.
The consummation of the sale and purchase of the Outstanding Shares (but not ADSs) shall take place over the Helsinki Stock Exchange to the
extent possible. The transfer tax, if any, levied on the sale and purchase of the Outstanding Shares and Outstanding Equity Instruments shall be borne by the Offeror; provided that if any amount is to be paid to a Person other than the Person
in whose name the Outstanding Shares and Outstanding Equity Instruments are registered, it shall be a condition to such payment, subject to Applicable Law in relation to the conduct of the Tender Offer, that the Person requesting such payment shall
pay in advance to the Offeror any transfer or other taxes required as a result of such payment to a Person other than the registered holder of such Outstanding Shares or Outstanding Equity Instruments or establish to the satisfaction of the Offeror
that such tax has been paid or is not payable.
| 3. |
Recommendation by the Company’s Board of Directors |
The Board of Directors of the Company has, by a unanimous board
decision, decided to recommend that the holders of the Outstanding Shares and the Outstanding Equity Instruments accept the Tender Offer and tender their Outstanding Shares and Outstanding Equity Instruments in the Tender Offer and to issue a formal
statement to this effect substantially in the form set out in Section 3.1 of the Company Disclosure Schedule, it being understood that the tender offer document, or a draft thereof, has not been available for the Board of Directors (the
“Recommendation”), all as required pursuant to the Finnish Securities Markets Act and the US Exchange Act and the rules and regulations promulgated thereunder. The Board of Directors of the Company undertakes to issue and publish
the Recommendation no later than three (3) banking days after having received the draft of the tender offer document submitted by the Offeror to the Finnish Financial Supervisory Authority for approval and the Company shall file a duly
completed Schedule 14D-9 with the SEC on the same day that the Offeror files its tender offer document with the SEC. The fact that the Board of Directors of the Company recommends the acceptance of the Tender Offer will also be disclosed in the
stock exchange releases announcing the Tender Offer in accordance with Section 5.6 below.
| 3.2 |
Cancellation of the Recommendation |
Subject to Sections 3.2.2 and 5.2, the Board of Directors of the
Company may, at any time prior to the Closing Date, cancel or change the Recommendation if, and only if, the Board of Directors of the Company determines in good faith due to an Effect occurring after the date hereof or an Effect occurring prior to
the date hereof of which the Board of Directors of the Company was not aware as of the date hereof, after taking advice from its external legal counsel and financial advisor, that failure to cancel or change the Recommendation would be inconsistent
with the Board of Directors of the Company’s fiduciary duties towards the holders of Outstanding Shares and the Outstanding Equity Instruments under Finnish laws and guidance on board duties in the Helsinki Takeover Code (as defined below)
(such duties referred to as the “Fiduciary Duties”).
G-14
Prior to and as a precondition for cancelling or changing its Recommendation, as provided above,
the Board of Directors of the Company shall (i) promptly notify the Offeror of its intention to cancel or change the Recommendation, (ii) in good faith provide the Offeror with an opportunity to negotiate with the Board of Directors of the
Company about matters arising from the changed circumstances in view of the Tender Offer, and (iii) give the Offeror at least four (4) banking days from the date of informing the Offeror of the intention to cancel or change the
Recommendation, to enhance the terms and conditions of its offer. If the Offeror enhances the terms and conditions of its offer such that the enhanced offer, in the reasonable opinion of the Board of Directors of the Company rendered in good faith,
after taking advice from external legal counsel and financial advisor, is in the interest of the holders of the Outstanding Shares and the Outstanding Equity Instruments, the Board of Directors of the Company shall confirm and uphold the
Recommendation for the Tender Offer, as enhanced.
In the event a third party publishes its decision to offer to purchase
any or all Outstanding Shares and Outstanding Equity Instruments through a public tender offer (“Competing Offer”) or the Company receives a written Competing Proposal, the Board of Directors of the Company may, subject to the provisions
of this Section 3.2.2 (but not subject to or in accordance with the provisions of Section 3.2.1 above), cancel or change the Recommendation if and only if it determines in good faith, after taking advice from external legal counsel and
financial advisor that the Competing Offer or Competing Proposal, judged as a whole, is superior from a financial point of view to the Offeror’s offer (including to the extent enhanced, as described below), and that therefore it would no longer
be in the best interest of the holders of the Outstanding Shares and the Outstanding Equity Instruments to accept the Tender Offer and that failure to cancel or change the Recommendation would be inconsistent with the Board of Directors’
Fiduciary Duties; provided that simultaneously with cancelling or changing the Recommendation in accordance with this Section 3.2.2 with respect to a Competing Proposal that is not a Competing Offer, the Company shall enter into a definitive
written agreement with respect to such Competing Proposal.
Prior to and as a precondition for cancelling or changing its Recommendation in
response to a Competing Offer or a Competing Proposal, the Board of Directors of the Company shall (i) comply with its obligations under Section 5.2, (ii) promptly notify the Offeror with reasonably detailed information regarding the
Competing Offer or the Competing Proposal (including the identity of the offeror, pricing and material terms and conditions), (iii) in good faith provide the Offeror with an opportunity to negotiate with the Board of Directors of the Company
about matters arising from the Competing Offer or the Competing Proposal, and (iv) give the Offeror at least four (4) banking days from the earlier of (a) the date of publishing the Competing Offer or (b) the date of informing
the Offeror in writing of the other Competing Proposal, to enhance its offer pursuant to this Agreement. The Board of Directors of the Company shall give due consideration to any enhanced offer received from the Offeror pursuant hereto. In the event
of any material revisions to the Competing Offer or the Competing Proposal for which notice was previously given by the Company pursuant to the first sentence of this paragraph, the Company shall be required to deliver a new written notice and to
comply again with the requirements set forth in the first two sentences of this paragraph, except that the Company shall give the Offeror at least two (2) banking days from the date of publishing the Competing Offer, or from the date of
informing the Offeror in writing of the Competing Proposal, to enhance its offer pursuant to this Agreement (rather than the four (4) banking days otherwise contemplated by the first sentence of this paragraph).
If a Competing Offer is published or if the Offeror is informed in writing of a Competing Proposal in accordance with the above and
Section 5.2 but the Offeror enhances its offer as described above such that the enhanced offer, in the opinion of the Board of Directors of the Company rendered in good faith, after taking advice from reputable external legal counsel and
financial advisor, is at least equally favorable to the holders of the Outstanding Shares and the Outstanding Equity Instruments as the Competing Offer or the Competing Proposal, the Board of Directors of the Company shall confirm and uphold the
Recommendation for the Tender Offer, as enhanced.
G-15
| 4. |
Representations and warranties |
On the Signing Date and the Closing Date, the Company gives to the Offeror the
representations and warranties set forth in Section 4.2 except as disclosed in any Company SEC Document (excluding all cautionary and forward-looking disclosures, including without limitation, those contained under the captions “Risk
Factors” or “Forward Looking Statements” or similarly titled captions) filed before the Signing Date or as set forth in the Company Disclosure Schedule (it being agreed that the disclosure of any item in any section or subsection of
the Company Disclosure Schedule shall be deemed disclosure with respect to any other section or subsection to which the relevance of such item is reasonably apparent), and the Offeror gives to the Company the representations and warranties set forth
in Section 4.3 (collectively the “Warranties”).
| 4.2 |
Representations and warranties by the Company |
The Parties acknowledge that the Offeror
has conducted a financial, business and legal due diligence on the Company and its business and affairs based on (i) publicly available information and (ii) information provided by the Company, including responses to questions that the
Offeror or its advisers have presented in relation thereto.
The Offeror acknowledges that except for representations and warranties
contained in Section 4.2 the Company makes no other representations or warranties, and the Offeror has not relied upon or otherwise been induced by any other express or implied representation or warranty with respect to the Company or with
respect to the Due Diligence Information or any other information made available to the Offeror.
| 4.2.1 |
Organization and qualification, subsidiaries |
The Company and each of its subsidiaries
has been validly incorporated and exists in accordance with the laws of the jurisdiction of its incorporation and has the requisite power and authority and all governmental approvals, licenses and permits necessary to own, use and operate its
property and to carry on its business as presently conducted in each jurisdiction where it owns assets or operates its business, except for any failure to be so incorporated and existing and those powers, authorities, approvals, licenses and permits
the absence of which would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole.
The Company and each of its subsidiaries is duly qualified to do business and, where applicable, is in good standing under the laws of each
jurisdiction in which either the ownership or use of the assets and properties owned by it, or the nature of the activities conducted by it, requires such qualification, except where failure to be so qualified or in good standing would not
reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect.
| 4.2.2 |
Authority relative to this Agreement, no conflicts |
This Agreement has been duly
authorised and validly executed and delivered by the Company and constitutes a legal, valid and binding obligation of the Company enforceable against it in accordance with its terms, subject, in each case, to the effect of bankruptcy, moratorium,
reorganization, administration, insolvency, recovery, attachment and other mandatory laws affecting creditors’ rights generally. The Company has all necessary corporate power and authority, and has taken all necessary corporate action, to duly
authorise and execute this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated herein to be consummated by it.
The execution, delivery and performance by the Company of this Agreement and any related documents to which the Company is or will be a party,
and the consummation of the transactions contemplated
G-16
hereby and thereby, do not and will not: (i) conflict with or violate the organizational documents of the Company or any of its subsidiaries; (ii) assuming compliance with the matters
referred to in Section 5.1, conflict with or violate any Applicable Law; (iii) assuming compliance with the matters referred to in Section 5.1, except as set forth on Section 4.2.2 of the Company Disclosure Schedule, result in
any breach of, or constitute a default (or an event that, with notice or lapse of time or both, would become a default or breach) under, require any consent of or notice to any Person pursuant to, or give to others any right of termination,
amendment, acceleration or cancellation of, or the right to exercise any option or other contingent or similar right, or result in the creation of an encumbrance on any property or asset of the Company or any of its subsidiaries pursuant to, or
otherwise adversely affect the rights of the Company or any of its subsidiaries under, or result in the loss of any benefit under, any contract or permit, license or approval of the Company or any of its subsidiaries, with only such exceptions, in
the case of each of clauses (ii) through (iii), as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole.
| 4.2.3 |
No competing transaction |
The Company is not as of the date hereof in a process of
discussing, or aware of, any proposal, offer or indication of interest that would reasonably be expected to lead to or constitute a Competing Proposal.
The audited consolidated financial statements of the Company as of and for the
year ended December 31, 2014 (the “Financial Statements”) have been prepared by the Company in all material respects in accordance with IFRS. The Financial Statements give a true and fair view of the financial position,
financial performance, and cash flows of the Company and its consolidated subsidiaries in all material respects in accordance with IFRS.
The unaudited consolidated interim report of the Company for the nine (9) months ended September 30, 2015 (the “Interim
Report”) has been prepared by the Company in all material respects in accordance with applicable Finnish laws and the International Accounting Standards (IAS) 34, Interim Financial Reporting. The Interim Report gives a true and fair view of
the consolidated financial position, financial performance, and cash flows of the Company and its consolidated subsidiaries in all material respects in accordance with the International Accounting Standards (IAS) 34, Interim Financial Reporting.
| 4.2.5 |
Disclosed information |
The Company has filed, furnished, disclosed and submitted to
Nasdaq Helsinki, the Finnish Financial Supervisory Authority, Nasdaq US, and the SEC all information and documents required to be so disclosed and submitted by and pursuant to applicable Finnish and U.S. laws and regulations and the rules and
requirements of Nasdaq Helsinki, the Finnish Financial Supervisory Authority, Nasdaq US, and the SEC, and all such filings, furnished information and documents, disclosures and submissions, as of their respective filing, furnishing, disclosure and
submission dates, complied in all material respects with all applicable requirements of such laws, regulations, rules and requirements. The information contained in the Financial Statements and in the Interim Report, as supplemented by any
subsequently published information, including stock exchange releases and press releases, taken as a whole, contains all material facts concerning the Company and its operations that are required to have been published pursuant to the Finnish SMA,
the US Exchange Act, the US Securities Act, the Sarbanes-Oxley Act, and the rules and regulations promulgated thereunder or the stock exchange rules of Nasdaq Helsinki or Nasdaq US, and does not, as of the Signing Date, contain any untrue statement
of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading.
G-17
Except as would not reasonably be expected to have, individually or in the aggregate, a material
effect on the Company and its subsidiaries, taken as a whole, (i) none of the information that the Company has provided to the Offeror in the virtual data room (the “Data Room”) made available by the Company to the Offeror (the
“Due Diligence Information”) and none of the information otherwise provided by the Company in the course of the Offeror’s due diligence review, pursuant to the Confidentiality Agreement or pursuant to Section 5.4 of this
Agreement, is misleading in any material respect and (ii) none of such information contains any untrue statement of a material fact or omits to state any material fact that renders the information provided misleading in any material respect.
Furthermore, neither the Due Diligence Information nor any other information provided by the Company to the Offeror in the course of the Offeror’s due diligence review, to the Knowledge of the Company, contains any insider information as
defined in the Finnish SMA or any material non-public information within the meaning of the United States federal securities laws, except such information that will be published prior to or in connection with the announcement of the Tender Offer in
accordance with Section 5.6 below.
For the avoidance of doubt, except in the case of public disclosures the adequacy of which will be
determined in accordance with Applicable Laws, including regulations, rules and requirements, matters will be treated as “disclosed” or “provided” to the Offeror pursuant to this Agreement only if and to the extent that the
nature and scope of the matter disclosed is reasonably apparent from the face of the disclosure and it is fairly disclosed, included and uploaded in such form as part of the Due Diligence Information prior to the Signing Date.
| 4.2.6 |
Shares and securities entitling to shares |
As of the date of this Agreement, the Company
has issued and outstanding a total of (i) 980,921,795 Shares (of which, as of the close of business on 14 January 2016, 368,367,520 Shares are represented by 4,604,594 ADSs), (ii) 435,000 2011 Option Rights, (iii) 7,160,125 2014
Option Rights, (iv) 34,934,440 2016 Option Rights, (v) 25,000 2011 Share Rights, (vi) 5,715,313 2014 Share Rights, (vii) 2,027,628 Swiss Option Rights and (viii) 220,400,001 Warrants. Except for the Shares and the
Outstanding Equity Instruments, (x) as of the date of this Agreement there are no option rights (including synthetic options), warrants, call rights, depository receipts, subscription, pre-emptive or other rights of any kind or other securities
entitling to, exchangeable for, convertible into, or linked to the value of the Shares, and (y) the Board of Directors of the Company has not used and will not use any authorizations granted by a general meeting of shareholders of the Company
(i) to grant any such rights or issue or transfer any shares in the Company or to acquire its own shares, (ii) to grant or allocate any further option rights nor (iii) to issue any other securities in the Company, except as provided
in Section 4.2.6 of the Company Disclosure Schedule. All of the Shares are validly issued, fully paid and nonassessable.
| 4.2.7 |
Compliance with applicable laws and regulations |
Neither the Company nor any of its
subsidiaries is or has been since January 1, 2013, in any material respect, in conflict or in material breach or default with respect to or in violation of any applicable laws, regulations, orders or judgments and all other Applicable Laws,
including, without limitation, all applicable transfer pricing, anti-bribery, anti-fraud, export control, data protection and privacy regulations and policies, except to the extent any conflict, breach or default would not reasonably be expected to
have, individually or in the aggregate, a material effect on the Company and its Subsidiaries, taken as a whole. No material claims, investigations or proceedings concerning breach of any laws or permits have been since January 1, 2013 or are
currently pending or, to the knowledge of the Company, threatened against the Company or any of its subsidiaries, and the Company is not as of the date hereof aware of any circumstances giving rise to or that would reasonably be expected to give
rise to any such breach, claims, investigations or proceedings, except to the extent any claim, investigation or proceeding would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company or its
subsidiaries, taken as whole.
G-18
The conduct of the business of the Company and its subsidiaries is, and at all times since
January 1, 2013 has been, in compliance with all applicable Health Laws, except for such non-compliance as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken
as a whole. The Covered Products are being, and at all times have been, researched, developed, tested, studied, manufactured, stored, supplied, licensed, offered for sale, sold, imported or otherwise exploited, as applicable, by or on behalf of the
Company or any of its subsidiaries, including to the Knowledge of the Company by any Company Partner, in compliance with all applicable Health Laws, except for such non-compliance as would not reasonably be expected to have, individually or in the
aggregate, a material effect on the Company and its subsidiaries, taken as a whole. Neither the Company nor any of its subsidiaries, nor, to the Knowledge of the Company, any director, officer, employee or agent of the Company or any of its
subsidiaries, has been convicted of any crime or engaged in any conduct for which debarment is mandated by 21 U.S.C. § 335a(a) or any similar Applicable Law or authorized by 21 U.S.C. § 335a(b) or any similar Applicable Law, or mandate any
exclusion from participation in any U.S. federal healthcare programs.
The Company and each of its subsidiaries are in compliance with all
Applicable Laws relating to the employment of labor, including but not limited to those related to wages, hours, collective bargaining, pension, discrimination in employment and employment practices, occupational health and safety and the payment
and withholding of Taxes and other sums as required by the appropriate governmental authority, and there is no claim, charge or proceeding asserted, pending or, to the Knowledge of the Company, threatened against the Company or any of its
subsidiaries with respect to such laws, except for any such non-compliance, claim, charge or proceeding that would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as
a whole. All employees of the Company and its subsidiaries are legally authorized for employment in each jurisdiction in which they provide services.
Neither the Company nor any of its subsidiaries is in breach of any applicable collective agreement and there are no strikes or other
industrial labor action pending or, to the Knowledge of the Company, threatened against the Company or any of its subsidiaries, nor are there any circumstances giving rise to or that could reasonably be expected to give rise to any such strikes or
action, except for any such breach, strike or action that would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole. Section 4.2.8 of the Company
Disclosure Schedule includes a complete list of all material share incentive, share option, bonus, deferred compensation, pension, profit sharing, severance pay, termination pay or retirement plans, benefits or similar arrangements in force or
otherwise proposed or agreed with respect to any current or former directors, officers or employees or independent contractors of the Company or any of its subsidiaries and there are no other material similar plans, benefits or arrangements in force
or otherwise proposed or agreed with respect to any current or former directors, officers or employees or independent contractors of the Company or any of its subsidiaries. All compensation and benefit plans, policies or arrangements of the Company
and its subsidiaries have been operated in compliance with the governing rules of such plans, policies or arrangements and Applicable Law, except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the
Company and its subsidiaries, taken as a whole.
Neither the Company nor any of its subsidiaries is a party to any contract or arrangement
(written or otherwise), other than disclosed in Section 4.2.8 of the Company Disclosure Schedule, that would require material or enhanced payments by or benefits from the Company or any of its subsidiaries in excess of the applicable statutory
termination payments to any Person, or that would accelerate the time of payment, funding or vesting of any payments by or benefits from the Company or any of its subsidiaries, (i) upon termination of such Person’s employment or service
with the Company or any of its subsidiaries or (ii) in connection with the transactions contemplated by this Agreement, including but not limited to the Tender Offer.
G-19
Neither the Company nor any of its subsidiaries has any indemnity or gross-up obligation with
respect to any Taxes imposed under Section 4999 or Section 409A of the Internal Revenue Code.
| 4.2.9 |
Compliance with material contracts |
Section 4.2.9 of the Company Disclosure
Schedule lists each agreement that is material to the respective businesses or operations of the Company and its subsidiaries, including any license or other agreement relating to any Covered Product (collectively, the Material
Contracts”). Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, neither the Company nor any of its subsidiaries is or has been in
default under or in violation of the performance of any of its obligations under any Material Contract, and no event has occurred which (whether with or without notice, lapse of time, or both) would constitute a default thereunder by the Company or
any of its subsidiaries. Neither the Company nor any of its subsidiaries has received or given any notice of breach or termination of any agreement that is material to their respective businesses or operations, nor are there to the Knowledge of the
Company as of the date hereof any circumstances that give rise to or that would reasonably be expected to give rise to any such breach or termination.
| 4.2.10 |
Litigation and proceedings |
Except as would not reasonably be expected to have,
individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, within the past five (5) years, no material claims, lawsuits, actions, or investigations or legal, administrative, arbitration or other
proceedings (including but not limited to liquidation, receivership, or other similar proceedings) have been or are pending or, to the Knowledge of the Company, threatened against, or involve the Company or any of its subsidiaries (including any of
its or their licensees) or any of their assets or any director, officer or Key Employee of the Company or any of its subsidiaries, nor are there any circumstances to the Knowledge of the Company as of the date hereof giving rise to or that would
reasonably be expected to give rise to any such claims, lawsuits, actions, investigations or proceedings.
| 4.2.11 |
Intellectual property |
| |
(a) |
Section 4.2.11(a) of the Company Disclosure Schedule sets forth a complete and correct list of all of the patents, patent applications, provisional applications, registered copyrights, registered and applications
to register trademarks, and domain names owned solely or jointly by, or exclusively licensed to, the Company or any of its subsidiaries (collectively, the “Scheduled IP”) (specifying for each, the owner(s), including any joint
owners, and if any Person other than the Company or any of its subsidiaries is an owner, the corresponding license or other agreement). Each item of Scheduled IP (i) is subsisting and in full force and effect and, to the Knowledge of the
Company, is valid and enforceable, (ii) each item of Scheduled IP that is owned by (x) the Company, or (y) to the Actual Knowledge of the Company, any Company Licensor, has been filed, prosecuted and maintained in accordance with all
Applicable Laws. Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, each of the Company and its subsidiaries (and, to the Actual Knowledge of
the Company, each Company Licensor and Company Licensee) has, as applicable, complied in all material respects with all of its obligations and duties to the respective patent and trademark offices with respect to the filing, prosecution and
maintenance of the Scheduled IP. All issuance, renewal, maintenance and other payments that have been due and payable with respect to, or actions (including the making of any filings) required to be taken in order to file, protect or maintain, any
issued patent or pending patent application or any other registration or application for registration of any Scheduled IP owned by the Company or its subsidiaries or, to the Actual Knowledge of the Company, any Scheduled IP owned or controlled by
any Company Licensor, in each case as of the date hereof, have been paid or taken by or on behalf of the Company or the applicable third party as of the earlier of (x) the deadline, and (y) the date hereof. |
G-20
| |
(b) |
The Company or its subsidiary (i) owns (whether solely or jointly), with such ownership free and clear of all liens, licenses and other encumbrances (including any options and other contingent rights to obtain any
license or other right, title or interest in or to any such Intellectual Property (except solely for the following licenses: (1) (A) to the extent the right to use confidential information under a non-disclosure agreement would be deemed
to be an implied non-exclusive license, any such implied non-exclusive license under any such non-disclosure agreement (but only during the term thereof), or (B) to the extent the right to use data, know-how or other similar Intellectual
Property solely to conduct services under a clinical trial agreement for the Company or its subsidiary would be deemed to be an implied non-exclusive license, any such implied non-exclusive license under any such clinical trial agreement (but only
during the term thereof), provided that each such agreement (clauses (A) and (B)), was entered into in the ordinary course of business consistent with past practice, and (2) the license agreements disclosed in Section 4.2.11(b)(i) of
the Company Disclosure Schedule) all Intellectual Property owned or purported to be owned by the Company or any of its subsidiaries, and (ii) has a valid and enforceable written license to use all Intellectual Property that is licensed or
purported to be licensed to the Company of any of its subsidiaries in the conduct of the business of the Company and its subsidiaries (collectively, all Intellectual Property covered by clauses (i) and/or (ii), including all Scheduled IP, the
“Company Intellectual Property”). Any Company Intellectual Property jointly owned by the Company is disclosed in Section 4.2.11(b)(ii) of the Company Disclosure Schedule and specifies the other joint owner(s) and any
corresponding agreement to which the Company or its subsidiary is a party. |
| |
(c) |
To the Actual Knowledge of the Company and except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, no Person (other
than the Company and its subsidiaries, and any Company Licensor that is a party to a valid and enforceable written license agreement, which agreement, if material, is set out in Section 4.2.9 of the Company Disclosure Schedule) owns any
Intellectual Property that is necessary for exploitation of any Covered Product (it being understood, for clarity, that the foregoing representation and warranty in this Section 4.2.11(c) is not intended to be, and shall not be construed as,
any representation or warranty with respect to infringement, misappropriation or other violation of any Intellectual Property). |
| |
(d) |
To the Knowledge of the Company and except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, during the past five
(5) years, the conduct of the business of the Company and its subsidiaries, including the making, use, licensing, sale, offer for sale, import and provision of the products or services made, licensed, sold, offered for sale, imported or
provided by or on behalf of the Company or any of its subsidiaries has not been and is not misappropriating, misusing, infringing or otherwise violating any Intellectual Property owned or controlled by another Person, or breaching or causing a
default under any applicable terms or conditions of any Material Contract, in each case, in any material respect, and, to the Knowledge of the Company, there are no circumstances giving rise to or that could reasonably be expected to give rise to
any such misappropriation, misuse, infringement, breach, default or other violation. |
| |
(e) |
Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as
a whole, (i) the Company Intellectual Property, including all Scheduled IP, constitutes and includes all of the Intellectual Property, used or held for use in, and that is necessary and sufficient for the Company and its subsidiaries to
conduct, their respective businesses and, to the Actual Knowledge of the Company, will allow the Company and its subsidiaries after the Closing Date to continue to operate those businesses in the same manner in which such businesses are currently
being and have been conducted prior to the Closing Date and as such businesses are currently proposed to be conducted, including as necessary and sufficient to develop, manufacture and otherwise exploit the products of the Company and its
subsidiaries that are currently being developed, manufactured or otherwise exploited by or on behalf of the Company or any of its subsidiaries, including, for clarity, the Covered Products, (ii) the Company and its subsidiaries have the sole
and exclusive right to develop, manufacture and otherwise exploit the Covered Products, and (iii) no Scheduled IP owned by the Company or, to the Actual Knowledge of the Company, other Company Intellectual Property, is subject
|
G-21
| |
to any outstanding consent, settlement, decree, order, injunction, judgment or ruling, including any that limits the disposition, use, enforcement or other exploitation thereof (it being
understood, for clarity, that the representations and warranties in clauses (i) – (iii) of this Section 4.2.11(e) are not intended to be, and shall not be construed as, any representation or warranty with respect to infringement,
misappropriation or other violation of any Intellectual Property). |
| |
(f) |
Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, there are, and during the past five (5) years, there
have been no, pending or, to the Knowledge of the Company, threatened, applications, proceedings, litigation or other claims or actions against the Company or any of its subsidiaries or, to the Actual Knowledge of the Company, but only to the extent
relating to any Company Intellectual Property or Covered Product, any of its or their respective current or former licensees (including any sublicensees) (each, a “Company Licensee”) or current or former licensors (including any
sublicensors) (each, a “Company Licensor”) affecting any part of the Company Intellectual Property (including any claim or action pursuant to which any Person has challenged the ownership, inventorship, patentability,
registerability, validity or enforceability of any Company Intellectual Property, and to the Actual Knowledge of the Company there are no circumstances giving rise to or that could reasonably be expected to give rise to any such applications,
proceedings, litigation, claims or actions. |
| |
(g) |
To the Knowledge of the Company, (i) no Person or entity has been or is (A) misappropriating any Company Intellectual Property owned by, or exclusively licensed to, the Company or any of its Subsidiaries or
(B) infringing or otherwise violating any of the Scheduled IP, or (ii) in the case of any Company Intellectual Property that has been licensed (including sublicensed) to another Person by the Company or any of its subsidiaries, no such
Company Licensee has been or is in material breach of any related license agreement, including any applicable Material Contract. |
| |
(h) |
Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, (i) there is, and during the past five (5) years,
there has been, no pending or, to the Knowledge of the Company, threatened, application, proceeding, litigation, claim, or action asserted against, or in any material respect by, the Company or any of its subsidiaries or, to the Actual Knowledge of
the Company, any Company Licensee or Company Licensor, nor is the Company aware of any circumstances giving rise to or that could reasonably be expected to give rise to, any such application, proceeding, litigation, claim, or action, alleging
misappropriation, infringement, misuse, or other violation of any Intellectual Property owned or controlled by, as applicable, the Company or any of its subsidiaries or any other Person, in each case, with respect to, or otherwise to the extent
relating to, the business of the Company or any of its subsidiaries or any of their respective products or services, and (ii) during the past five (5) years none of the Company or any of its subsidiaries (or, to the Actual Knowledge of the
Company, any Company Licensee or Company Licensor) has received or sent (A) any written notice of any alleged misappropriation, infringement, misuse, or other violation of any Intellectual Property owned or controlled by, as applicable, any
other Person (if such notice was received by the Company or its subsidiary) or the Company or its subsidiary (if such notice was sent by the Company or its subsidiary), or (B) any written invitation to take a license under, as applicable any
Intellectual Property of any other Person (if such invitation was received by the Company or its subsidiary), or any Company Intellectual Property (if such invitation was sent by the Company or its subsidiary), in each case, with respect to, or
otherwise to the extent relating to, the business of the Company or its subsidiaries or any of their respective products or services. |
| |
(i) |
Except, in each case, as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its
subsidiaries, taken as a whole, (i) the Company and its subsidiaries have taken all necessary or appropriate steps in accordance with all Applicable Laws to maintain, protect and enforce the Company’s and its subsidiaries’ rights in
and to the Intellectual Property owned or purported to be owned by or that is licensed to, or is otherwise used by or on behalf of, the Company or any of its subsidiaries in any material respect, including to maintain the Company’s and its
subsidiaries’ rights in the Scheduled IP and to protect the secrecy and value of any trade secrets and other |
G-22
| |
confidential information and the Company’s and its subsidiaries’ ownership or other rights with respect to any Company Intellectual Property, (ii) without limiting the generality
of the foregoing, each current or former employee, consultant and contractor of the Company or any of its subsidiaries who develops or otherwise creates or has developed or otherwise created any material Intellectual Property for the Company or any
of its subsidiaries, including any material Intellectual Property that relates to the Company’s or such subsidiary’s products or services, has executed proprietary information, confidentiality and assignment agreements substantially in the
Company’s standard forms, which have been disclosed in the Data Room, that duly and validly assign, pursuant to a present assignment, to the Company or such subsidiary all rights to, and sole ownership of, any such material Intellectual
Property that is or was created, developed, written, invented, conceived or discovered by the employee, consultant, or contractor, as applicable, and including all associated rights that otherwise appropriately protect such Intellectual Property,
all in accordance with Applicable Laws, (iii) each such current and former employee, consultant and contractor of the Company and its subsidiaries has been paid all compensation for his or her development and assignment of such Intellectual
Property, including all associated rights, on behalf of the Company and its subsidiaries to which he or she is entitled under, as applicable, the Company’ and its subsidiaries’ policies or any Applicable Laws, (iv) all current and
former employees, consultants and contractors of the Company and its subsidiaries have signed agreements in the Company’s standard form, which has been disclosed in the Data Room, in which they agree not to use or disclose any confidential or
proprietary information of the Company or the applicable subsidiary except as explicitly authorized by the Company or such subsidiary, and (v) each Company Licensor and Company Licensee, has, to the Actual Knowledge of the Company, caused its
current and former employees, consultants and contractors to execute agreements comparable to, or more stringent than, the assignment and confidentiality agreements contemplated in the foregoing clauses (ii) and (iv) with respect to any
Intellectual Property licensed by such Person to the Company or its subsidiary and as required under any agreement between the Company and/or its subsidiary and such Person and/or its affiliates. |
| |
(j) |
Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company and its subsidiaries, taken as a whole, the execution and delivery of this Agreement, the compliance
with the provisions hereof and the consummation of the transactions contemplated by this Agreement, assuming compliance with the matters referred to in Section 5.1, do not and will not conflict with, or result in any violation or default (with
or without notice or lapse of time, or both) under, or give rise to a right of termination, cancellation or acceleration of, or the right to exercise any option or other contingent or similar right, or result in the creation of an encumbrance on, or
result in the loss of rights or otherwise adversely affect the rights of the Company or any of its subsidiaries with respect to, any Company Intellectual Property (including any Intellectual Property owned or purported to be owned by, licensed to,
or otherwise used or held for use by or on behalf of, any of the Company or its subsidiaries), or any term of any agreement pursuant to which any Intellectual Property is transferred or licensed to the Company or any of its subsidiaries, including
any applicable Material Contract. |
| |
(k) |
Except as set forth on Section 4.2.11(k) of the Company Disclosure Schedule, to the Knowledge of the Company, neither the Company nor any of its subsidiaries, nor, to the Actual Knowledge of the Company, any
Company Licensor or Company Licensee, has used, or is a party to any agreement to use, any funding, facilities, personnel or Intellectual Property of any academic institution, research center or governmental authority (or any Person working for or
on behalf of any of the foregoing Persons) (collectively, “Governmental/Academic Persons”) in connection with any research, development or other activities, and no Governmental/Academic Person has, or will be entitled to
have, any right, title or interest (including any “march in”, ownership or license rights) in or to any Company Intellectual Property or any improvement, method or process relating to any of the foregoing (including any claim or option or
any other right, title or interest in or to any of the foregoing). |
G-23
The Company and each of its subsidiaries have timely filed all Tax Returns
required to be filed under Applicable Law in any jurisdiction in which the Company or such subsidiary is or has been subject to Tax with the relevant authorities, and such Tax Returns are true, correct and complete in all material aspects and have
been prepared in material compliance with Applicable Law. The Company and each of its subsidiaries have timely paid, withheld or collected all material Taxes due (regardless of whether Taxes are shown on such Tax Returns and regardless of whether a
notice of assessment or collection has been issued by the relevant authorities). The Company and each of its subsidiaries have formed adequate reserves in their books and relevant accounts for all unpaid Taxes in conformity with applicable
accounting principles concerning any relevant financial period.
No audits, actions or disputes are pending or, to the Knowledge of the
Company, threatened with respect to material Taxes of the Company or any of its subsidiaries. No claim for assessment or collection of material Taxes has been or, to the Knowledge of the Company, is presently being asserted against the Company or
any of its subsidiaries. No liens or encumbrances for material Taxes exist upon any of the assets of the Company or any of its subsidiaries (other than Taxes not yet due and payable).
Neither the Company nor any of its subsidiaries has been a “controlled corporation” or a “distributing corporation” in any
transaction occurring during the two-year period ending on the date hereof that was purported or intended to be governed in whole or in part by Sections 355 or 361 of the Internal Revenue Code (or any similar provision of state, local or non-U.S.
Tax law). Neither the Company nor any of its subsidiaries has been a party to a “listed transaction” within the meaning of United States Treasury Regulations Section 1.6011-4(b)(2).
| 4.2.13 |
Certain Business Practices |
Except as would not reasonably be expected to have,
individually or in the aggregate, a material effect on the Company or its subsidiaries, taken as whole, to the Knowledge of the Company, none of the Company, any of its subsidiaries, or any representatives of the Company or any of its subsidiaries
have, directly or indirectly, whether in cash, property or services (i) used any corporate or other funds for unlawful contributions, gifts, entertainment or other unlawful payments related to political activity, (ii) made any bribes,
kickbacks, influence payments, or other unlawful payment to U.S. or non-U.S. government officials or employees or to U.S. or non-U.S. political parties or campaigns, or otherwise taken any action that would cause them to be in violation of
(a) the Foreign Corrupt Practices Act of 1977, as amended, or any rules or regulations thereunder; (b) the UK Bribery Act 2010; (c) the Finnish Penal Code (rikoslaki, 39/1889, as amended); and (d) any other applicable
anti-corruption and/or anti-bribery laws, statutes, rules, regulations, ordinances, judgments, orders, decrees, injunctions, and writs of any governmental authority of any jurisdiction applicable to the Company or its subsidiaries (whether by virtue
of jurisdiction or organization or conduct of business) (collectively, the “Applicable Anti-Corruption Laws”) or (iii) made or accepted any other unlawful payment.
The books, records and accounts of the Company and its subsidiaries have at all times been maintained in all material respects in accordance
with applicable accounting standards.
The Company and its subsidiaries have developed and maintained a system of internal accounting
controls sufficient to provide reasonable assurance that: (i) transactions are executed and access to assets is permitted only in accordance with the Company’s or its subsidiaries’ applicable policies and procedures and
management’s general or specific authorization, and (ii) transactions have been recorded as necessary to permit preparation of periodic financial statements and to maintain accountability for assets and has otherwise established reasonable
and adequate internal controls and procedures intended to ensure compliance with Applicable Anti-Corruption Laws.
G-24
| 4.2.14 |
Health Regulatory Compliance |
Except as would not reasonably be expected to have,
individually or in the aggregate, a material effect on the Company or its subsidiaries, taken as whole, none of the Company, any of its subsidiaries or, to the Knowledge of the Company, any Company Partner has received any written notice or other
communication from any Health Authority or any institutional review board (or ethics committee) (i) withdrawing or placing on “clinical hold” any Covered Product or otherwise suspending or terminating clinical research involving any
Covered Product (ii) alleging any violation of any Health Law by the Company or any of its subsidiaries in connection with any Covered Product or (iii) threatening any regulatory enforcement action against the Company, any of its
subsidiaries, or any Company Partner. Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company or its subsidiaries, taken as whole, the preclinical studies and clinical trials conducted
by or at the direction of the Company and its subsidiaries with respect to any Covered Product were, and if still pending, are, being conducted in all respects in accordance with approved clinical protocols, informed consents, applicable
requirements of the relevant Health Authorities and applicable requirements of good laboratory practices and good clinical practices, and to the Knowledge of the Company, there has not been any failure by any Company Partner to such conduct such
preclinical studies and clinical trials in compliance with such applicable requirements, regulations and guidances. Except as would not reasonably be expected to have, individually or in the aggregate, a material effect on the Company or its
subsidiaries, taken as whole, material regulatory authorizations related to any Covered Product are, in all respects, (i) in good standing and full force and effect and (ii) in compliance with all formal filing and maintenance
requirements.
| 4.3 |
Representations and warranties by the Offeror |
| 4.3.1 |
Organization and qualification |
The Offeror has been validly incorporated and exists in
accordance with the laws of Delaware, United States and has the requisite power and authority and all necessary governmental approvals, licenses and permits necessary to own, use and operate its property and to carry on its business as presently
conducted in all material respects.
| 4.3.2 |
Authority relative to this Agreement |
This Agreement has been duly authorised and
validly executed and delivered by the Offeror and constitutes a legal, valid and binding obligation of the Offeror enforceable against it in accordance with its terms, subject, in each case, to the effect of bankruptcy, moratorium, reorganization,
administration, insolvency, recovery, attachment and other mandatory laws affecting creditors’ rights generally. The Offeror has all necessary corporate power and authority, and has taken all necessary corporate action, to duly authorise and
execute this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated herein to be consummated by it in accordance with the terms and conditions of this Agreement.
| 4.3.3 |
Sufficient financing |
The Offeror, following the closing of the Equity Sale, will have
and will maintain through the Closing sufficient finances available (including finances available under any Replacement Financing) to complete the Tender Offer, and will finance the Tender Offer through cash on its balance sheet and proceeds of the
Equity Sale and any Replacement Financing, and no other third party financing is required by the Offeror to complete the Tender Offer and subsequent acquisition (including compulsory redemption) of any remaining Shares and Outstanding Equity
Instruments.
Concurrently with the Offeror’s entry into this Agreement, the Offeror has entered into a purchase agreement (the
“Equity Purchase Agreement”) with J.P. Morgan Securities LLC (the “Purchaser”)
G-25
pursuant to which the Purchaser has committed to purchase $75,000,000 of shares of common stock, par value $0.001, of the Offeror (the “Equity Sale”). The Offeror has delivered
to the Company a true and complete copy of the form of Equity Purchase Agreement and will promptly deliver to the Company a true and complete copy of the executed Equity Purchase Agreement. There are no side letters (other than engagement or related
letters (none of which contain terms that would reasonably be expected to adversely affect the amount or closing of the Equity Sale)) related to the Equity Sale.
As of its signing on the date hereof, (i) the Equity Purchase Agreement is a valid and binding obligation of the Offeror and, to the
knowledge of the Offeror, the Purchaser, and (ii) no event has occurred which would constitute a default (or an event which with notice or lapse of time or both would constitute a default), or the failure of any condition, on the part of the
Offeror or, to the knowledge of the Offeror, the Purchaser under the Equity Purchase Agreement. There are no conditions precedent to the closing of the Equity Sale and receipt by the Offeror of the net proceeds of the Equity Sale other than the
conditions precedent set forth in the Equity Purchase Agreement, and the Offeror has no reason to believe that it will not be able to satisfy any closing condition in the Equity Purchase Agreement or that the full amount of net proceeds of the
Equity Sale will not be paid to the Offeror on or before January 26, 2016.
The Offeror acknowledges and agrees that notwithstanding
anything to the contrary in this Agreement, the closing of the Equity Sale or any Replacement Financing shall not be a condition to the obligation of the Offeror to consummate the Tender Offer and the other transactions contemplated hereby.
| 4.3.4 |
Disclosure Documents |
The Tender Offer documents, when filed and distributed or
disseminated, will comply as to form in all material respects with applicable rules, regulations, all other Applicable Laws and, at the time of such filing or the filing of any amendment or supplement thereto, at the time of such distribution or
dissemination and at the time of consummation of the Tender Offer, will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of the
circumstances under which they were made, not misleading. The representations and warranties in this Section will not apply to statements or omissions included or incorporated by reference in the Tender Offer documents based upon information
supplied to the Offeror by the Company or any of its representatives or advisors specifically for use or incorporation by reference therein.
| 4.3.5 |
Absence of Certain Agreements |
As of the date hereof, neither the Offeror nor any of its
affiliates has entered into any contract, arrangement or understanding (in each case, whether oral or written), or authorized, committed or agreed to enter into any contract, arrangement or understanding (in each case, whether oral or written),
pursuant to which: (a) any holder of Shares, ADSs or Outstanding Equity Instruments would be entitled to receive consideration of a different amount or nature than the consideration specified in Section 2.2 or, except for the irrevocable
undertakings referred to in clause F in the background, pursuant to which any holder of Shares, ADSs or Outstanding Equity Instruments agrees to tender such instruments into the Tender Offer or otherwise sell such instruments to the Offeror or
(b) any third party has agreed to provide, directly or indirectly, equity capital to the Offeror or its affiliates to finance in whole or in part the Tender Offer or the other transactions contemplated by this Agreement.
Other than this Agreement, as of the date hereof, there are no contracts, undertakings, commitments, agreements or obligations or
understandings, except for the irrevocable undertakings referred to in clause F in the background, between the Offeror or any of its affiliates, on the one hand, and any member of the Company’s management or the Board of Directors of the
Company, on the other hand, relating in any way to the transactions contemplated by this Agreement or the operations of the Company after the Closing.
G-26
| 4.4 |
Effect and Breach of Warranties |
The Warranties shall automatically terminate upon
consummation of closing of the Tender Offer thereby having no further effect after the Closing Date. Nothing herein shall relieve any Party from liability for fraud or willful breach of any Warranty hereunder.
| 5.1 |
Reasonable best efforts |
Each of the Parties agrees to use its reasonable best efforts
to do, or cause to be done, and to assist and cooperate with the other Party in:
| |
(a) |
the making of all necessary registrations and filings with the Finnish Financial Supervisory Authority, the SEC, Nasdaq Helsinki, Nasdaq US and the Antitrust Division of the U.S. Department of Justice and U.S. Federal
Trade Commission (such filings to be made as promptly as practicable), and with any other governmental entities or regulatory authorities, as may be required, and the obtaining of all necessary clearances, approvals and waivers from such
authorities, including, without limitation, the responses to any relief, direction or instruction received from the SEC or Finnish Financial Supervisory Authority and the documents to be submitted and filed pursuant to Section 3.1;
|
| |
(b) |
obtaining any requisite consent from, or providing any requisite notice to, the Finnish Funding Agency for Technology and Innovation (“Tekes”) in relation to the Tender Offer as a result of any Company
projects having received funding from Tekes; |
| |
(c) |
the obtaining of all necessary consents, approvals or waivers from and the provision of all necessary notices to third parties, provided that neither the Company nor the Offeror shall be required to incur any material
expenses or take or commit to take any actions that would materially adversely affect the Company or the Offeror, as applicable, in order to obtain any such consents, approvals or waivers from or provide any such notices to third parties;
|
| |
(d) |
informing their employees of the Tender Offer, as required by Applicable Law; and |
| |
(e) |
the execution and/or delivery of any additional corporate resolutions and/or instruments necessary to consummate the transactions contemplated herein, and to fully carry out the purposes of this Agreement.
|
The Parties shall co-operate and consult with each other, provide each other the opportunity to review and comment, and give
reasonable and good faith consideration to each other’s comments in connection with the making of all such filings, including all correspondence with the Finnish Financial Supervisory Authority, the SEC, Nasdaq Helsinki and Nasdaq US, and
thereafter shall keep each other timely apprised of all material developments and proposed responses and further communications in connection therewith (and shall co-operate and consult with each other in responding to comments on any filings from
any such authorities or exchanges).
The Offeror’s reasonable best efforts under clause (a) of this Section 5.1 shall
include the obligation to divest, hold separate, or enter into any license or restriction on the ownership or operation of the Offeror or the Company, provided that in no event shall the Offeror be obligated to divest, hold separate, or enter into
any license or restriction on the ownership or operation of the Offeror or the Company unless such divestment, holding separate or entry into license or restriction on the ownership or operation would not have a material adverse effect on the
business of the Company and its subsidiaries, taken as a whole, or the business of the Offeror and its Subsidiaries, taken as a whole (with the business of the Offeror and its subsidiaries, taken as a whole, deemed for purposes of this proviso to be
equivalent in size to the business of the Company and its subsidiaries, taken as a whole) (a “Burdensome Effect”). In the event of any claim asserted by any Governmental Authority under Applicable Law, and prior to the Long-stop
Date,
G-27
the Offeror shall use its reasonable best efforts to oppose (which reasonable best efforts shall include, in the event of any such claim asserted in court, by litigating in opposition to) any
claim that would prevent the completion of the Tender Offer from occurring, including if such claim would have a Burdensome Effect.
Subject to the terms of this Agreement, each of the Parties undertake to comply with the Helsinki Takeover Code issued by the Finnish
Securities Market Association, in force as of 1 January 2014 (the “Helsinki Takeover Code”), and shall not deviate from any of the recommendations of the code, except as may be required due to any irrevocable undertakings, as
referred to herein, that may be given by certain members of the Board of Directors of the Company.
The Parties shall use their respective
reasonable best efforts to facilitate the fulfilment of the conditions to completion in Section 2.3(a), including without limitation, in the case of the Offeror, at the reasonable request of the Company, by sending a marketing letter to all
shareholders of the Company the addresses of which are provided to the Offeror by the Company, by publishing an advertisement in one Finnish national and one Finnish business newspaper of its choice regarding the Tender Offer, and by engaging a
reputable financial adviser experienced in communicating with retail investors in the Finnish market in connection with the technical implementation of the Tender Offer, provided that neither Party shall be required to pay any consideration
(monetary or otherwise) to the shareholders of the Company (other than the consideration to be paid by the Offeror in the Tender Offer).
| 5.2 |
No solicitation of transactions |
The Company undertakes and shall cause its subsidiaries
and direct its officers, directors, employees and representatives (including, for the avoidance of doubt, any investment bankers, legal counsel and other external advisors) to undertake, as between the Signing Date and the Closing Date:
| |
(a) |
except as provided in clause (c) below, not to solicit, knowingly encourage, facilitate or promote or discuss with or provide confidential information with respect to the Company to any Person in connection with
any Competing Offer or any proposal for a Competing Offer or for any other transaction involving the transfer of all or any material portion of the Company’s assets or businesses, whether through a public tender offer or by sale or transfer of
assets, license, option, warrant, sale of shares, reorganization or merger, transfer of employees in a hiring action by a third party (other than the Offeror or its representatives) or otherwise, or any other similar corporate transaction that would
constitute or result in any competing transaction that would harm or hinder the completion of the Tender Offer (“Competing Proposal”); |
| |
(b) |
to cease and cause to be terminated any discussions, negotiations or other activities related to any Competing Proposal conducted prior to the Signing Date; |
| |
(c) |
not to, upon receipt of a Competing Proposal or a Competing Offer, directly or indirectly, knowingly facilitate, promote, or encourage or discuss with or provide confidential information with respect to the Company to
the Persons making such Competing Proposal (“Promoting Measures”), unless the Board of Directors of the Company determines in good faith, after having received advice from external legal counsel and financial advisor, that the
failure to take such Promoting Measures would be inconsistent with the Fiduciary Duties of the Board of Directors of the Company. The Company shall promptly provide the Offeror any information regarding the Company provided to any third party to the
extent such information has not been previously provided to the Offeror; |
| |
(d) |
to promptly inform the Offeror in writing about any Competing Proposal, including, to the extent available to the Company, the identity of the offeror and the price and other main terms and conditions of such Competing
Proposal, and of any material amendments to such price and terms and conditions by the third-party offeror. The Company shall keep the Offeror informed, on a current basis, of the status and material terms of any such Competing Proposal, including
any material amendments or proposed amendments as to price and other material terms thereof; and |
G-28
| |
(e) |
to provide the Offeror with an opportunity to negotiate with the Board of Directors of the Company about matters arising from the Competing Proposal. |
For the avoidance of doubt, nothing in this Section 5.2 shall be deemed to limit in any way whatsoever the rights and/or obligations of
the Company (or the Board of Directors of the Company) under Section 3.2.
In addition, nothing contained herein shall prevent the
Board of Directors of the Company from (i) complying with Rule 14e-2(a) under the Exchange Act with regard to a Competing Proposal or a Competing Offer so long as any such action taken or statement made to so comply also complies with
Section 3.2 and the other terms of this Section 5.2; or (ii) issuing a “stop, look and listen” communication or similar communication of the type contemplated by Rule 14d-9(f) under the Exchange Act.
| 5.3 |
Conduct of business pending the closing |
Except with the prior written consent of the
Offeror (which shall not be unreasonably withheld, conditioned or delayed) or as expressly contemplated by this Agreement, or as set forth in Section 5.4 of the Company Disclosure Schedule, or as mandated by Applicable Law, as between the
Signing Date and the Closing Date:
| |
(a) |
the Company undertakes to, and shall cause each of its subsidiaries to, conduct their respective businesses only in the ordinary course of business consistent with past practice and in all cases based on prudent
business judgment; |
| |
(b) |
the Company shall provide the Offeror with advance notice of all scheduled meetings, conferences, and discussions by the Company with regulatory authorities concerning any regulatory matters relating to the Covered
Products not later than ten (10) days after the Company receives notice of the scheduling of such meeting, conference, or discussion, unless circumstances necessitate a shorter period. Upon the Offeror’s written request, the Company and
the Offeror will coordinate to permit one (1) representative of the Offeror to accompany the Company in an observational role to any such scheduled meetings, subject to Applicable Law and only to the extent permitted by the applicable
regulatory authority. The Company and the Offeror shall use reasonable efforts to agree in advance on the scheduling of such meetings, conferences and discussions with any regulatory authority and on the agenda and objectives to be accomplished at
any such meetings, conferences and discussions. Each party shall provide all such assistance as is reasonably requested by the other in the preparation and conduct of any such scheduled meetings, conferences and discussions, including any
inspections by regulatory authorities with respect to any Covered Product. Upon the Offeror’s request, the Company shall (a) provide the Offeror with a reasonable opportunity to review and provide comments with respect to (and the Company
will consider in good faith any such comments), any material filing proposed to be made by or on behalf of any of the Company, and any material correspondence or other material communication proposed to be submitted or otherwise transmitted to a
regulatory authority by or on behalf of any of the Company relating to a Covered Product, and (b) keep the Offeror promptly informed of any material communication (written or oral) with or from any regulatory authority; provided, however, that
the Company shall make all decisions regarding these issues prior to the Closing Date. It being understood, however, that nothing contained in this Section 5.3(b) is intended to give the Offeror, directly or indirectly, the right to control or
direct the operations of the Company or any of its subsidiaries prior to the Closing Date; and |
| |
(c) |
without limiting the generality of the foregoing, unless previously agreed by the Offeror in writing, the Company undertakes to, and shall cause each of its subsidiaries to undertake to, refrain from making or
implementing: |
| |
(i) |
any material changes in its business (including without limitation termination by it of Key Employees, suppliers, resellers or other material business relationships) or corporate structure; and/or |
G-29
| |
(ii) |
any hiring of new employees other than with respect to employees having initial annual base compensation not exceeding $100,000 USD (or its equivalent); and/or |
| |
(iii) |
any material corporate transactions, investments, loans, incurrence of indebtedness for borrowed money, incurrence of liens or other encumbrances on material assets, acquisitions or divestments outside the ordinary
course of business; and/or |
| |
(iv) |
any agreements or commitments that are not entered into on arms’ length terms or in the ordinary course of business; and/or |
| |
(v) |
any material amendments to, waivers under, or termination of any Material Contracts other than in the ordinary course of business, or any new agreement that contains (A) a change in control provision that would be
unfavorably implicated by the transactions contemplated hereunder, (B) any provision that would restrict the Company or its subsidiaries or, following the Closing Date, its affiliates from competing in any line of business, developing any
products or clinical candidates or hiring the employees of a competitor, (C) any license of material intellectual property, (D) any obligation to make contingent payments based, directly or indirectly, on future product sales, regulatory
approvals or any material transaction or (E) otherwise relates to the exploitation of any material Intellectual Property owned by the Company or its subsidiaries; and/or |
| |
(vi) |
any change of their articles of association, by-laws or other constituting documents or any material change to their accounting principles or practices other than such arising out of or relating to any recent or
proposed changes of EU, Finnish or U.S. laws and regulations; and/or |
| |
(vii) |
any commencement, settlement or compromise of any material legal proceedings or of claims against third parties; and/or |
| |
(viii) |
any act or omission that would reasonably be expected to result in the abandonment, encumbrance (including any grant or exercise of any option or other contingent right), assignment or other conveyance of title (in
whole or in part) of, or an exclusive license or any other grant of any right or other license (including any covenant not to sue) to, any material Company Intellectual Property, other than non-exclusive licenses granted in the ordinary course of
business consistent with past practice for research and development purposes; and/or |
| |
(ix) |
(1) any distribution of dividends or other funds from the Company or its subsidiaries (other than distributions of dividends or other funds from wholly-owned subsidiaries of the Company to the Company or to other
wholly-owned subsidiaries of the Company), (2) a change in the number of shares issued or outstanding, share capital or rights to acquire share capital of the Company or its subsidiaries, including without limitation by reclassification,
recapitalisation, stock split, combination, repurchase, redemption or issuance of any shares or securities exercisable for, convertible into or exchangeable for shares in the Company or in its subsidiaries (other than upon the exercise or conversion
of Outstanding Equity Instruments outstanding on the date hereof or issued after the date hereof in compliance with this Agreement), (3) any sale, pledge, transfer or other disposal or encumbrance of any shares in the Company or in any of its
subsidiaries that are held or obtained by the Company or any of its subsidiaries (other than upon the exercise or conversion of Outstanding Equity Instruments outstanding on the date hereof or issued after the date hereof in compliance with this
Agreement), and/or (4) any grant, allocation, sale, transfer or disposal of any option rights held by the Company or any of its subsidiaries or of any other shares or securities exercisable for, convertible into or exchangeable for shares in
the Company or in any of its subsidiaries; and/or |
| |
(x) |
any change in the current or future agreements with, compensation of or other benefits (including without limitation by way of synthetic options, bonus, insurance, retention, severance or pension arrangements) of the
Board of Directors of the Company and/or each of the Persons employed by or serving the Company or its subsidiaries with annual base compensation exceeding $100,000 USD (or its equivalent); and/or |
G-30
| |
(xi) |
any action that would have the effect of increasing the liability for Taxes of the Company or any of its subsidiaries for any period ending after the Closing Date; and/or |
| |
(xii) |
any agreement or commitment to do any of the foregoing. |
| 5.4 |
Access to information |
Upon the Offeror’s request, and subject to Applicable Law
and appropriate restrictions for confidential and/or competitively sensitive information, the Company shall use its reasonable best efforts to give any information regarding the Company or its subsidiaries to the Offeror that:
| |
(a) |
the Offeror may reasonably need in preparation of the competition filings or any other regulatory filings referred to in Section 5.1 and of the tender offer documentation related to the Tender Offer; and/or
|
| |
(b) |
the Offeror may otherwise reasonably require for the purposes of the Tender Offer and other transactions contemplated under this Agreement, including without limitation, for preparing the Schedule TO to be filed
with the SEC or obtaining the SEC Relief (including, without limitation, providing information to the Offeror regarding the number of Outstanding Shares, ADSs and Outstanding Equity Instruments held by U.S. holders (within the meaning of, and as
determined under, Rule 14d-1 under the US Exchange Act)) and the offer document to be filed with the Finnish Financial Supervisory Authority, provided that the Offeror shall always have access to all information needed to evaluate the existence of
an actual or potential breach of Warranty or a Material Adverse Effect. |
Subject to Applicable Law, upon reasonable notice,
the Company shall also (and shall cause its representatives and subsidiaries to) afford to the officers and other authorized representatives of the Offeror, reasonable access, during normal business hours throughout the period prior to the Closing
Date, to its employees, properties, books, contracts, personnel files and records and, during such period, the Company shall (and shall cause its subsidiaries to) furnish to the Offeror all information concerning its business, properties and
personnel as may be reasonably requested by the Offeror, provided that no investigation or provision of information pursuant to this Section 5.4 shall affect or be deemed to modify any representation or warranty made by the Company herein,
provided, further, that notwithstanding the investigation or provision of information by any party, no party shall be deemed to make any representation or warranty except as expressly set forth in this Agreement. Any investigation pursuant to this
Section shall be conducted in such manner as not to interfere unreasonably with the conduct of the business of the Company and its subsidiaries.
Promptly, but no later than thirty (30) days, after the date hereof, the Company shall provide to the Offeror a list of all issuance,
renewal, maintenance and other payments and of any other actions (including filings and responses to office actions) required to be taken in order to maintain any Scheduled IP owned by the Company or any of its subsidiaries, and the Company shall
use its reasonable best efforts to provide to the Offeror such information and list with respect to all Scheduled IP not owned by the Company or any of its subsidiaries (including, for the avoidance of doubt, by promptly requesting the applicable
information from any Company Licensor) which, in each case, to the Knowledge of the Company, are required to be, as applicable, made or taken with respect to the Scheduled IP within one hundred eighty (180) days of the date of the Signing Date.
With respect to any and all Required Actions (as defined in this sentence below) required to be taken to maintain any Scheduled IP that have deadlines which fall within the period prior to and as of the Closing Date and/or within the one hundred
twenty (120) days of the date hereof, (i) the Company shall, with respect to Scheduled IP owned by the Company or any of its subsidiaries (or with respect to which the Company or its subsidiary has primary prosecution and/or maintenance
rights), pay all issuance, renewal, maintenance and other payments, and take any other actions (including making filings and responding to office actions) in order to maintain the Scheduled IP (collectively, “Required Actions”), in
each case, by the earlier of (x) the deadline and
G-31
(y) the Closing Date, and (ii) the Company shall, with respect to Scheduled IP not owned by the Company or any of its subsidiaries, use reasonable best efforts to promptly and timely
request the applicable Company Licensor to, in a timely manner, take any Required Actions (including to pay any applicable maintenance or renewal payments), in each case, by the earlier of (x) the deadline and (y) the Closing Date, and the
Company shall follow up as reasonably needed with such Company Licensor and keep the Offeror reasonably updated. The Company shall use reasonable best efforts to provide the Offeror with a list of the Company’s and its subsidiaries’ key
contacts for these matters for each Company Licensor, including such persons name and contact details, and a list of any prosecution counsel that handles any of these matters on behalf of the Company or any of its subsidiaries and such other similar
information as may be reasonably requested by the Offeror from time, solely for use post-Closing.
In case any insider information as
defined in the Finnish SMA or any material non-public information within the meaning of the United States federal securities laws is disclosed to the Offeror, the Company undertakes to publish such information prior to or in connection with the
announcement of the Tender Offer in accordance with Section 5.6 below in order to enable the Offeror to launch and complete the Tender Offer without breaching any applicable insider laws or regulations.
| 5.5 |
Notice of certain events |
Each Party shall, without delay, notify the other Party in
writing if it becomes aware of an event, change or circumstance that could or can reasonably be expected to result in or constitute a breach of Warranty made or to be made by that Party or constitute a Material Adverse Effect or that would delay or
impede such Party’s ability to consummate the transactions contemplated by this Agreement or to fulfil its obligations set forth in this Agreement.
Notwithstanding Section 3 of the Confidentiality Agreement,
the Parties agree that the stock exchange releases to be published by the Parties in connection with the announcement of the Tender Offer and this Agreement shall be in the form of Schedule 5.6. The Parties shall otherwise consult with each other
before issuing any releases or otherwise making any public statements with respect to this Agreement or any transactions contemplated hereunder, and shall not issue any such release or announcement without the other Party’s prior written
approval, unless required to do so by Applicable Law or relevant stock exchange regulations and only provided that it is not practically possible to consult and obtain the approval of the other Party prior to such release or announcement.
| 5.7 |
General meeting of shareholders |
The Board of Directors of the Company shall not convene
a general meeting of shareholders of the Company to address the Tender Offer or any matter which would have a material effect on the Tender Offer except as provided in this Agreement or except as required by mandatory law or, subject to the Company
having complied with its obligations under Sections 3.2 and 5.2 above, if the Board of Directors of the Company determines in good faith, after taking advice from external legal counsel and financial advisor, that failure to convene a general
meeting of shareholders would be inconsistent with the Fiduciary Duties of the Board of Directors.
It is understood and agreed that as
soon as the Offeror has publicly confirmed that it will complete the Tender Offer, the Board of Directors of the Company will, at the Offeror’s request, convene an extraordinary general meeting of shareholders of the Company for the purpose of
electing new members to the Board of Directors and addressing other agenda items proposed by the Offeror, if any.
The Offeror shall
subsequently grant the resigning members of the Board of Directors of the Company discharge from liability at the next Annual General Meeting of Shareholders of the Company following the Closing Date and, with respect to the financial year started
January 1, 2016, at the Annual General
G-32
Meeting of Shareholders of the Company where the financial statements for such financial year are considered, provided that no material findings to the contrary are made in the audit of the
Company’s financial statements for the respective accounting periods or otherwise.
| 5.8 |
Director and Officer Liability |
For six (6) years after the Closing Date, the
Offeror and the Company shall indemnify and hold harmless the present and former directors and officers of the Company and its subsidiaries (each, an “Indemnified Person”) in respect of acts or omissions occurring at or prior to the
Closing Date to the extent permitted by Applicable Law, provided, however, that no such obligation shall arise with respect to acts or omissions finally determined by a court of competent jurisdiction to amount to fraud, willful misconduct, criminal
activity or a breach of the duty of loyalty and that nothing herein shall be construed as a requirement on the Offeror or the Company to effect any change to the Articles of Association of the Company. In the event that any Indemnified Person is
made party to any action, litigation, claim, suit, investigation or proceeding (an “Action”) arising out of or relating to matters that would be indemnifiable pursuant to the immediately preceding sentence, the Offeror and the
Company shall advance fees, costs and expenses (including reasonable attorney’s fees and disbursements) as incurred by such Indemnified Person in connection with and prior to the final disposition of such Action, subject to the execution by
such Indemnified Person of appropriate undertakings to repay such advanced fees, costs and expenses if it is ultimately determine that such Indemnified Person is not entitled to indemnification. For the avoidance of doubt, nothing in this Agreement
shall limit the right of the Company to claim damages from its directors under the Finnish Companies Act, provided that this shall not affect the undertakings and obligations of the Offeror in this Section 5.8.
Prior to the Closing Date, the Company shall or, if the Company is unable to, Offeror shall cause Company after the Closing Date to, obtain and
fully pay the premium for the extension of the directors’ and officers’ liability coverage of the Company’s existing directors’ and officers’ insurance policies and the Company’s existing fiduciary liability insurance
policies (collectively, “D&O Insurance”), in each case for a claims reporting or discovery period of at least six years from and after the Closing Date with respect to any claim related to any period at or prior to the Closing
Date from an insurance carrier with the same or better credit rating as the Company’s current insurance carrier with respect to D&O insurance with terms, conditions, retentions and limits of liability that are no less favorable than the
coverage provided under the Company’s existing policies with respect to any actual or alleged error, misstatement, misleading statement, act, omission, neglect, breach of duty or any matter claimed against a director or officer of the Company
or any of its subsidiaries by reason of him or her serving in such capacity that existed or occurred at or prior to the Closing Date (including in connection with this Agreement or the transactions or actions contemplated hereby). If the Company for
any reason fails to obtain such “tail” insurance policies as of the Closing Date, the Offeror or the Company shall continue to maintain in effect, for a period of at least six years from and after the Closing Date, the D&O Insurance in
place as of the date hereof with terms, conditions, retentions and limits of liability that are no less favorable than the coverage provided under the Company’s existing policies as of the date hereof, or the Offeror shall cause the Company to
purchase comparable D&O Insurance for such six-year period with terms, conditions, retentions and limits of liability that are no less favorable than as provided in the Company’s existing policies as of the date hereof. In no event shall
the Offeror be required to, and the Company shall not, expend for such policies pursuant to the previous sentence an annual premium amount in excess of 300% of the amount per annum the Company paid in its last full fiscal year (it being agreed that
if the aggregate premiums of such insurance coverage exceed such amount, the Company shall be obligated to obtain a policy with the greatest coverage available, with respect to matters occurring prior to the Closing Date, for a cost not exceeding
such amount).
In the event that the Offeror, the Company or any of their respective successors or assigns (i) consolidates with or
merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or (ii) transfers all or substantially all of its properties and assets to any
G-33
Person, then, and in each such case, proper provision shall be made so that the successors and assigns of such Person shall assume the obligations set forth in this Section 5.8.
The rights of each Indemnified Person under this Section 5.8 shall be in addition to any rights such Person may have under Applicable Law.
These rights shall survive consummation of the transactions contemplated hereby and are intended to benefit, and shall be enforceable by, each Indemnified Person.
(a) For a period commencing on the Closing Date and ending on the
first anniversary of the Closing Date (or, such shorter period of employment, as the case may be), Offeror shall provide the employees of the Company and its subsidiaries who remain employed by the Offeror or any of its subsidiaries (the
“Continuing Employees”) shall receive from Offeror (i) an annual rate of salary or wages that is no less favorable than the annual rate of salary or wages provided to such Continuing Employee by the Company and its subsidiaries
as of immediately prior to the Closing Date, (ii) bonus opportunities and employee benefits that are substantially comparable in the aggregate to the bonus opportunities or employee benefits, as applicable, provided by the Offeror to its
similarly situated employees and (iii) severance benefits that are substantially comparable in the aggregate to the severance benefits provided by the Offeror to its similarly situated employees.
(b) As of the Closing Date and solely with respect to Continuing Employees, should the Offeror elect to transition a Continuing Employee from a
Company employee benefit plan to a welfare plan of the Offeror, the Offeror shall, or shall cause its subsidiaries to, use reasonable best efforts to waive all limitations as to any pre-existing condition, actively-at-work requirements or waiting
periods in its applicable welfare plans with respect to participation and coverage requirements applicable to each Continuing Employee (and his or her eligible dependents) under any welfare plans that such Continuing Employee (and his or her
eligible dependents) may be eligible to participate in after the Closing and to credit each Continuing Employee (and his or her eligible dependents) for any copayments, deductibles, offsets or similar payments made under a company plan during the
plan year that includes the Closing Date for purposes of satisfying any applicable copayment, deductible, offset or similar requirements under the comparable plans of the Offeror or any of its subsidiaries. In addition, as of the Closing Date, the
Offeror shall and shall cause any applicable subsidiary to, give Continuing Employees full credit for purposes of eligibility, vesting, participation in, benefit plan accruals and determination of level of benefits under any employee benefit and
compensation plans or arrangements (other than with respect to defined benefit plans or where such service credit would result in duplication of benefits) maintained by the Offeror or an applicable subsidiary that such Continuing Employees may be
eligible to participate in after the Closing Date for such Continuing Employee’s service with the Company or any of its subsidiaries (as well as service with any predecessor employer) to the same extent that such service was credited for
purposes of any comparable Company employee benefit plan immediately prior to the Closing.
(c) Notwithstanding the foregoing provisions of this Section 5.9, the provisions of Sections 5.9(a) and (b) shall apply only with
respect to Continuing Employees who are employed in the United States. With respect to Continuing Employees not described in the preceding sentence, Offeror shall, and shall cause its subsidiaries to, comply with all Applicable Laws, directives and
regulations relating to employees and employee benefits matters applicable to such employees.
(d) Effective no later than the day
immediately prior to the Closing Date and contingent upon the consummation of the transaction contemplated by this Agreement, the Company shall take or cause to be taken all actions necessary to terminate the employee benefit plan(s) (including the
adoption of resolutions by the board of directors of the Company sponsoring such employee benefit plan(s) in a form reasonably acceptable to Offeror) sponsored by the Company or its subsidiaries intended to qualify as qualified cash or deferred
arrangements under Section 401(k) of the Internal Revenue Code (the “Company 401(k) Plan”). Effective on or as soon as administratively practicable following the Closing
G-34
Date, each employee of any Company who is a participant in a Company 401(k) Plan (each, an “401(k) Participant”) shall be allowed to participate in a qualified cash or deferred
arrangement under Section 401(k) of the Internal Revenue Code that is sponsored and maintained by Offeror or one of its Affiliates (the “Offeror 401(k) Plan”). As soon as practicable following the Closing Date, Offeror shall take, or
cause to be taken, any and all actions as may be required, including amendments to the Company 401(k) Plan(s) and/or the Offeror 401(k) Plan to permit, as applicable, (i) the account balances of each 401(k) Participant to be distributed in
accordance with the terms of such plan and Applicable Law and (ii) the 401(k) Participants who are participants in such plan to roll over any such distributions (including a rollover of up to one (1) outstanding participant loan per 401(k)
Participant) into the Offeror 401(k) Plan.
(e) Prior to the Closing Date, Offeror and the Company shall cooperate in good faith to
complete an analysis of any potential Tax liability or loss of Tax deduction under Sections 280G or 4999 of the Internal Revenue Code that may result from the transactions contemplated by this Agreement.
(f) The provisions of this Section 5.9 are solely for the benefit of the Parties to this Agreement and their respective successors and
assigns, and no current or former employee, individual consultant, individual independent contractor, individual self-employed contractor leased employee or director of the Company or any of its subsidiaries (each, a “Company Service
Provider”) or any other individual associated therewith shall be regarded for any purpose as a third-party beneficiary of this Section 5.9. In no event shall the terms of this Agreement be deemed to confer upon any Company Service
Provider any right to continued employment with the Offeror or any of its affiliates (including, following the Closing Date, the Company). Nothing herein shall be deemed to establish, amend, modify or cause to be adopted any Company employee benefit
plan or any other benefit plan, program, agreement or arrangement maintained or sponsored by the Offeror or the Company or any of their respective affiliates.
The Offeror shall use its reasonable best efforts to take, or cause to be
taken, all actions and to do, or cause to be done, all things necessary to obtain the proceeds from the Equity Sale. Without limiting the foregoing, from and after the date hereof, the Offeror shall not agree to any amendment or modification to be
made to, or to any waiver of any provision or remedy under, the Equity Purchase Agreement without the prior written consent of the Company if such amendments, modifications or waivers would reasonably be expected to (x) reduce the aggregate
amount of funds or commitments payable to the Offeror under the Equity Purchase Agreement or (y) impose new or additional conditions precedent to the closing of the Equity Sale.
If any portion of the Equity Sale becomes unavailable in the manner or from the sources contemplated in the Equity Purchase Agreement, the
Offeror shall notify the Company and shall use reasonable best efforts to take, or cause to be taken, all actions and do, or cause to be done, all things reasonably necessary or desirable to arrange to obtain any such portion from alternative
sources as promptly as practicable, to the extent such financing is necessary to complete the Tender Offer and subsequent acquisition (including compulsory redemption) of any remaining Shares and Outstanding Equity Instruments.
If and to the extent that the Equity Sale is replaced, supplemented or superseded by any alternative financing (“Replacement
Financing”) in accordance with the terms hereof, the terms “Equity Sale”, “Equity Purchase Agreement” and “Purchaser” shall each be deemed to be modified, mutatis mutandis, to refer to such alternative
financing and any financing sources and commitments with respect thereto.
This Agreement enters into force on the Signing Date and shall
automatically terminate upon consummation of the Tender Offer on the Closing Date. Furthermore, this Agreement may be terminated by either Party as set forth in this Section 6.
G-35
| 6.1 |
Termination by either Party |
Either Party may terminate this Agreement with immediate
effect at any time prior to the Closing Date by giving to the other Party a written notice thereof, if at least one of the following events occurs:
| |
(a) |
by mutual consent of the Parties duly authorized by their respective Board of Directors; |
| |
(b) |
a final, nonappealable injunction or other order issued by any court of competent jurisdiction or other final, nonappealable legal restraint or prohibition preventing the consummation of the Tender Offer shall have
taken effect after the date hereof and shall still be in effect; or |
| |
(c) |
the Closing Date has not occurred by 19 June 2016 (the “Long-stop Date”), provided that the right to terminate this Agreement pursuant to this Section 6.1(c) shall not be available to any
Party whose breach of any representation, warranty, undertaking or obligation set forth in this Agreement has been the cause of, or resulted in, the failure of the Closing Date to occur by such date. |
| 6.2 |
Termination by the Company |
The Company may terminate this Agreement with immediate
effect at any time prior to the Closing Date by giving to the Offeror a written notice thereof if (a) the Board of Directors of the Company has, having complied with the provisions set forth in this Agreement (including without limitation
Sections 3.2 and 5.2), cancelled or changed the Recommendation or (b) (i) the Offeror shall have failed to perform in any material respect any covenant or agreement of the Offeror set forth in this Agreement and such failure is
incapable of being cured prior to the Long-stop Date or, if capable of being cured prior to the Long-stop Date, has not been cured within thirty (30) days of the Company providing written notice thereof, or (ii) any of the representations
and warranties of the Offeror are not true and correct in all respects, where such failure to be true and correct in all respects (A) would, individually or in the aggregate, reasonably be expected to prevent the consummation of the Tender
Offer prior to the Long-stop Date and (B) is incapable of being cured prior to the Long-stop Date or, if capable of being cured prior to the Long-stop Date, has not been cured within thirty (30) days of the Company providing written notice
thereof; provided that, at the time of the delivery of such notice of termination, the Company shall not be in material breach of its obligations under this Agreement.
| 6.3 |
Termination by the Offeror |
The Offeror may terminate this Agreement with immediate
effect at any time prior to the Closing Date by giving the Company a written notice thereof if at least one of the following events occurs:
| |
(a) |
the Board of Directors of the Company has, for any reason whatsoever, cancelled or changed the Recommendation in a manner detrimental to the Offeror; |
| |
(b) |
the Company shall have failed to perform in any material respect any covenant or agreement of the Company set forth in this Agreement; |
| |
(c) |
any of the Warranties set forth in Section 4.2.6 are not true and correct in all respects, except where the failure of such Warranties to be so true and correct is de minimis; |
| |
(d) |
any of the Warranties set forth in (i) the first paragraph of Section 4.2.1 or (ii) the first paragraph of Section 4.2.2 (in the case of each of sub-clauses (i) and (ii), without giving effect
to any qualification as to “materiality” or “Material Adverse Effect” qualifiers set forth therein), are not true and correct in all material respects; or |
| |
(e) |
any of the other Warranties set forth in this Agreement (without giving effect to any qualification as to “materiality” or “Material Adverse Effect” qualifiers set forth therein) are not true and
correct in all respects, except where the failure of such Warranties to be so true and correct would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, |
G-36
provided that, (1) in the case of each of clauses (a) through (e), at the time
of the delivery of such notice of termination, the Offeror shall not be in material breach of its obligations under this Agreement and (2) in the case of each of clauses (b) through (e), such failure to perform or of such Warranties to be
so true and correct is incapable of being cured prior to the Long-stop Date or, if capable of being cured prior to the Long-stop Date, has not been cured within thirty (30) days of the Offeror providing written notice thereof.
| 6.4 |
Consequences of termination or expiration |
In case of any termination or expiration of
this Agreement, the Offeror shall promptly (and in any event within 24 hours following such termination or expiration) withdraw the Tender Offer and shall not acquire any Outstanding Shares or Outstanding Equity Instruments pursuant thereto.
Any termination or expiration shall be without prejudice to any remedies available to the Parties under this Agreement or under law for breach
of a contractual obligation, it being understood, however, that the non-breaching Party shall be entitled to claim solely direct damages of the non-breaching Party pursuant to this Agreement. Nothing in this Agreement shall, however, limit a
Party’s liability for fraud or willful misconduct.
If this Agreement is, as a result of a Competing Offer or a Competing Proposal,
terminated by the Company on the basis of Section 6.2(a) or by the Offeror on the basis of Section 6.3(a), the Company shall pay to the Offeror a termination fee of USD $4,500,000 as compensation for the Offeror’s reasonable costs for
the evaluation, negotiation and preparation of the Tender Offer. Such termination fee shall be paid by the Company upon termination of this Agreement in immediately available funds to a bank account identified by the Offeror.
If this Agreement is terminated by the Company on the basis of Section 6.2 as a result of a Competing Offer, and the Offeror nevertheless
confirms its intention to enhance the Tender Offer in writing, the Company shall allow the Offeror the possibility to conduct further due diligence on terms equal to those applied with respect to the third party offeror making said Competing Offer,
provided that the Company shall not be required to allow the Offeror to conduct further diligence if the Company has entered into a definitive agreement providing for a Competing Offer, provided, further that the Offeror shall be treated (i) on
the same terms as any other third party in connection with making unsolicited offers under such other definitive agreement (including with respect to diligence and information access) and (ii) in accordance with the principles set forth in the
Helsinki Takeover Code.
Notwithstanding the termination of this Agreement, the following Sections shall survive: 5.6, 6.4 and 7 and, in
the event the Tender Offer is completed, also the following Sections shall survive: 2.5, 5.7, 5.8 and 5.9.
All notices, requests, claims, demands and other communications hereunder shall
be in writing and shall be given and shall be deemed to have been duly given if delivered personally (notice deemed given upon receipt), sent by e-mail (notice deemed given upon receipt), sent by an internationally recognized courier service (notice
deemed given upon receipt of proof of delivery) or mailed by registered or certified mail,
G-37
return receipt requested (notice deemed given upon receipt) to the respective parties at the following addresses:
|
|
|
| If to the Company: |
|
Biotie Therapies Corp. |
| attention: |
|
David Cook, Chief Financial Officer |
| address: |
|
Joukahaisenkatu 6, FI-20520d, Turku, Finland |
| e-mail: |
|
David.Cook@biotie.com |
|
|
| With a copy to: |
|
Davis Polk & Wardwell LLP |
| attention: |
|
Michael Davis |
|
|
Sophia Hudson |
| address: |
|
450 Lexington Avenue, New York, NY 10017 |
| e-mail: |
|
michael.davis@davispolk.com |
|
|
sophia.hudson@davispolk.com |
|
|
| With a copy to: |
|
Hannes Snellman Attorneys Ltd. |
| attention: |
|
Mikko Heinonen |
|
|
Klaus Ilmonen |
| address: |
|
Eteläesplanadi 20, 00130 Helsinki, Finland |
| e-mail: |
|
mikko.heinonen@hannessnellman.com |
|
|
klaus.ilmonen@hannessnellman.com |
|
|
| If to the Offeror: |
|
Acorda Therapeutics, Inc. |
| attention: |
|
Jane Wasman |
| address: |
|
420 Saw Mill River Road, Ardsley, NY 10502 |
| e-mail: |
|
jwasman@acorda.com |
|
|
| With a copy to: |
|
Kirkland & Ellis LLP |
| attention: |
|
Daniel Wolf, P.C. |
|
|
Joshua Zachariah |
| address: |
|
601 Lexington Avenue, New York, NY 10022 |
| e-mail: |
|
daniel.wolf@kirkland.com |
|
|
joshua.zachariah@kirkland.com |
|
|
| And a copy to: |
|
Roschier |
| attention: |
|
Paula Linna |
|
|
Antti Ihamuotila |
| address: |
|
Keskuskatu 7 A, FI-00100 Helsinki, Finland |
| e-mail: |
|
Paula.Linna@roschier.com |
|
|
Antti.Ihamuotila@roschier.com |
or at such other address as the respective Party may hereafter specify in writing to the other Party.
Subject to Section 6.4, all expenses incurred in connection with this
Agreement and the transactions contemplated hereunder (including without limitation the due diligence review) shall be paid by the Party incurring such expenses whether or not the Tender Offer or any other transactions contemplated herein are
consummated.
The Company confirms that its expenses for external advisors in connection with the transactions contemplated under this
Agreement are not expected to exceed $8,000,000.
| 7.3 |
Schedules incorporated |
Each schedule to which reference is made herein and which is
attached hereto shall be incorporated in this Agreement by such reference.
G-38
The headings of this Agreement are for convenience of reference only and shall
not in any way limit or affect the meaning or interpretation of the provisions of this Agreement.
Neither this Agreement nor any of the rights, interests or obligations
hereunder shall be assigned by any of the Parties hereto (whether by operation of law or otherwise) without the prior written consent of the other Party. Subject to the preceding sentence, this Agreement shall be binding upon and shall inure to the
benefit of the Parties and their respective successors and assignees.
Notwithstanding anything contained in this Agreement to the
contrary, nothing in this Agreement, expressed or implied, is intended to confer on any Person other than the Parties or their respective successors and assigns and the Indemnified Persons (with respect to Section 5.8) any rights, remedies,
obligations or liabilities under or by reason of this Agreement.
Notwithstanding the foregoing, the Offeror may transfer all its rights
and obligations under this Agreement to a wholly-owned direct or indirect subsidiary to be used as an acquisition vehicle in the Tender Offer (the “BidCo”), provided that the Offeror shall upon such transfer automatically be deemed
to guarantee, as for its own debt (fi. omavelkainen takaus), the performance of BidCo’s obligations in relation to the Tender Offer and to the Company under this Agreement. In case of such a transfer, the BidCo shall replace the Offeror
as a Party to this Agreement and be entitled to enforce the terms of this Agreement.
Failure by any Party at any time or times to require performance of any
provisions of this Agreement shall in no manner affect its right to enforce the same, and the waiver by any Party of any breach of any provision of this Agreement must be in writing and shall not be construed to be a waiver by such Party of any
succeeding breach of such provision or waiver by such Party of any breach of any other provision hereof.
| 7.7 |
Governing law and jurisdiction |
This Agreement shall be governed by and construed in
accordance with the substantive laws of Finland, excluding its conflicts of laws provisions.
Subject to Section 7.8, all disputes
arising out of or relating to the present contract shall be finally settled by final, binding arbitration conducted under the Rules of Arbitration of the International Chamber of Commerce (the “ICC Rules”).
The arbitral tribunal shall be comprised of three arbitrators to be appointed as follows: each party shall nominate one person to act as
arbitrator in accordance with Article 12(4) of the ICC Rules and the two selected shall nominate a third arbitrator within 10 days of the confirmation of their appointment. If the arbitrators nominated by the parties are unable or fail to agree upon
the third arbitrator, the third arbitrator shall be selected by the International Court of Arbitration of the ICC.
Either party may apply
to the arbitral tribunal for injunctive relief until the arbitral award is rendered or the controversy is otherwise resolved. Either party also may, without waiving any remedy under this agreement, seek from any competent judicial authority any
interim or provisional relief that is necessary to protect the rights or property of that party, at any stage pending final determination of the dispute by the arbitral tribunal.
Consistent with the expedited nature of arbitration, each party will, upon the written request of the other party, promptly provide the other
with copies of documents relevant to the issues raised by any claim or
G-39
counterclaim. The party to whom the request is directed must respond in writing within 20 days after receipt of the requests. Objections, if any, must state with specificity the grounds for
objecting to the request. Any dispute regarding document production, or the relevance or scope thereof, shall be determined by the arbitral tribunal, which determination shall be conclusive. All document production shall be completed within 90 days
following the appointment of the arbitral tribunal.
Each party shall be entitled to examine the other party’s witnesses by way of
deposition. Depositions shall be limited to a maximum of five per party and shall be held within 30 days of the making of a written request. Additional depositions may be scheduled only with the permission of the arbitral tribunal and for good cause
shown. Each deposition shall be limited to a maximum of one day’s duration. All objections are reserved for determination by the arbitral tribunal.
The arbitration shall be conducted in English in London, United Kingdom, and judgment on the award rendered by the arbitrators may be entered
in any court having jurisdiction thereof.
The Parties agree that irreparable damage would occur and that the
Parties would not have any adequate remedy at law in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that, notwithstanding
anything in this Agreement to the contrary, each Party may apply to a court of competent jurisdiction for a precautionary measure, temporary procedural remedy, temporary restraining order or preliminary injunction where necessary to protect its
interests and shall be entitled to such relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement pending the commencement or completion of any arbitration proceedings pursuant to
Section 7.7.
This Agreement may not be amended except by an instrument in writing signed
by both Parties hereto.
Without prejudice to the Confidentiality Agreement, this Agreement
constitutes the entire agreement among the Parties with respect to the subject matter hereof and supersedes all prior agreements and understandings among the Parties with respect thereto.
If any term of this Agreement is invalid or unenforceable, such term shall
be reformed or deleted, as the case may be, but only to the extent necessary. The remaining terms shall remain in full force and effect.
Except as required by applicable law regulations or other Applicable
Law, including applicable stock exchange regulations, the Parties undertake, during the term of this Agreement and after the termination thereof for any reason whatsoever, not to disclose the content, in any manner whatsoever, of this Agreement to
any third parties (other than their advisors) without the prior written consent of the other Party. Each Party acknowledges, however, that this Agreement shall be made public in connection with the filing of the Schedule TO with the SEC and in the
Finnish tender offer document.
This Agreement has been executed in two (2) identical counterparts,
one (1) for each Party.
G-40
In witness whereof, the Parties hereto have duly executed this Agreement as of the day and
year first above written.
|
|
|
|
|
|
|
| Acorda Therapeutics, Inc. |
|
|
|
Biotie Therapies Corp. |
|
|
|
|
|
|
| /s/ Ron Cohen |
|
|
|
/s/ Timo Veromaa |
|
|
| Name: Ron Cohen |
|
|
|
Name: Timo Veromaa |
|
|
| Title: Chief Executive Officer |
|
|
|
Title: Chief Executive Officer |
|
|
G-41
SCHEDULE REFERENCE
Schedule 2.1
MAIN TERMS AND CONDITIONS
OF THE TENDER OFFER
Object of the Tender Offer
Through the Tender Offer, the Offeror offers to acquire all of the ADSs, Outstanding Shares and Outstanding Equity Instruments in the Company on the terms and
subject to the conditions set forth below.
Offer Price
The offer price for each Outstanding Share validly tendered in accordance with the terms and conditions of the Tender Offer is EUR 0.2946 in cash (the
“Share Offer Price”).
The offer price for each outstanding ADS validly tendered in accordance with the terms and conditions of the Tender Offer
is EUR 23.5680 in cash (the “ADS Offer Price”), payable in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest
practicable day to the Closing Date.
The offer prices for Outstanding Equity Instruments validly tendered in accordance with the terms and conditions of
the Tender Offer are as follows:
The specific prices for each of the stock options, share units and warrants are as follows:
|
|
|
| Outstanding Equity Instrument |
|
Price offered in the Tender Offer
(EUR in cash) |
| 2011 Option Rights |
|
|
| 2011C (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014 Option Rights |
|
|
| 2014A (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014B (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014C (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014D (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014M (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2016 Option Rights |
|
|
| Stock option 2016 (subscription price EUR 0.162 per share) |
|
0.1326 for each stock option |
| 2011 Share Rights |
|
|
| 2011 Share right |
|
0.2946 for each share right |
| 2014 Share Rights |
|
|
| 2014A (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014B (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014C (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014D (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014M (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
G-42
|
|
|
| Outstanding Equity Instrument |
|
Price offered in the Tender Offer
(EUR in
cash) |
| Swiss Option Rights |
|
|
| Swiss option (subscription price CHF 0.10 per share) |
|
0.2032 for each stock option |
| Swiss option (subscription price CHF 0.21 per share) |
|
0.1026 for each stock option |
| Swiss option (subscription price CHF 0.28 per share) |
|
0.0386 for each stock option |
| Swiss option (subscription price CHF 0.31 per share) |
|
0.0112 for each stock option |
| Swiss option (subscription price CHF 0.35 per share) |
|
0.0100 for each stock option |
| Swiss option (subscription price CHF 0.38 per share) |
|
0.0100 for each stock option |
| Swiss option (subscription price CHF 0.45 per share) |
|
0.0100 for each stock option |
| Warrants |
|
|
| Warrants (subscription price EUR 0.17 per share) |
|
0.1664 for each warrant |
The offer price payable to holders of the 2016 Option Rights and the 2011 and 2014 Share Rights shall, at the option of the
holder, be payable in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the Closing Date.
Offer Period
The Offer Period1 commences on [ ] [ ] 2016 at 9:30 a.m. (Finnish time) / 2:30 a.m. (New York City time) and expires on [ ] [ ] 2016 at 4:00 p.m. (Finnish time) / 9.00 a.m. (New York City time), unless the Offer
Period is extended as set forth below.
Subject to the following paragraph, if at the scheduled expiration time of the Tender Offer any of the Conditions
to Completion (as defined below) are not satisfied, the Offeror will extend the Offer Period for additional periods not exceeding two (2) weeks each in accordance with these terms and conditions and, in each case, subject to compliance with
applicable Finnish and United States legal requirements. The Offeror will give notice of the possible extension of the Offer Period by a stock exchange release at or before 4:00 p.m. (Finnish time) / 9.00 a.m. (New York City time) on [ ] [ ]
2016.2 The Offeror will give notice of any further extension of the Offer Period, in each case, at the latest on the first Finnish banking day following the expiry of the extended Offer Period at
or before 4:00 p.m. (Finnish time) / 9.00 a.m. (New York City time).
If the Offeror extends the Offer Period, the Offer Period will expire on the date
and at the time to which the Offeror extends the Offer Period. The maximum duration of the Offer Period (including any extended Offer Period) is ten (10) weeks as required under Finnish law. However, if any of the Conditions to Completion (as
defined below) has not been fulfilled due to a particular obstacle, the Offeror may extend the Offer Period beyond ten (10) weeks until such obstacle has been removed and until all Conditions to Completion have been satisfied. In no event shall
the Offeror extend the Tender Offer beyond 19 June 2016.
The Offeror also reserves the right to extend the Offer Period for an additional two
(2) week period in connection with the announcement of the final result of the Tender Offer if the Tender Offer shall have been announced to be unconditional at that time (such extended Offer Period shall be referred to as the “Subsequent
Offer Period”). In the event of such Subsequent Offer Period, the Subsequent Offer Period will expire on the date and at the time determined by the Offeror in the final result announcement. Any Subsequent Offer Period may extend beyond ten
(10) weeks (and, in the Offeror’s discretion, beyond 19 June 2016).
| 1 |
Note to draft: Offer Period of at least 20 US business days in accordance with Rule 14d-1(g)(3). |
| 2 |
Note to draft: This date will be the first Finnish banking date following the expiry of the initial Offer Period, release to be issued before 9 am New York time. |
G-43
Conditions to Completion of the Tender Offer
The obligation of the Offeror to accept for payment the validly tendered and not withdrawn Outstanding Shares, ADSs and Outstanding Equity Instruments and to
complete the Tender Offer shall be subject to the fulfillment or, to the extent permitted by applicable law, waiver by the Offeror of the following conditions on or prior to the date of the Offeror’s announcement of the final result of the
Tender Offer (“Conditions to Completion”):
| (a) |
the valid tender of outstanding shares (including outstanding shares represented by validly tendered ADSs and validly tendered warrants) representing, together with any outstanding shares (including outstanding shares
represented by ADSs and warrants) otherwise acquired by the Offeror, more than ninety percent (90%) of the issued and outstanding shares and voting rights of the Company, calculated on a fully diluted basis and otherwise in accordance with
Chapter 18 Section 1 of the Finnish Limited Liability Companies Act (as used in this paragraph, “fully diluted basis” means an equation in which the numerator represents the aggregate number of outstanding shares (including
outstanding shares represented by ADSs) and warrants that have been validly tendered or otherwise acquired by the Offeror and the denominator represents the aggregate number of all outstanding shares (including outstanding shares represented by
ADSs) and warrants, as well as shares issuable upon the vesting and exercise of those outstanding equity instruments (other than warrants) that have not been validly tendered into the Tender Offer or otherwise acquired by the Offeror);
|
| (b) |
the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Act; |
| (c) |
no material adverse effect (as defined below) having occurred in the Company after 19 January 2016; |
| (d) |
the Offeror not, after 19 January 2016, having received information previously undisclosed to it that describes a material adverse effect to the Company that occurred prior to 19 January 2016;
|
| (e) |
no information made public by the Company or disclosed by the Company to the Offeror being materially inaccurate, incomplete, or misleading, and the Company not having failed to make public any information that should
have been made public by it under applicable laws, including without limitation the rules of Nasdaq Helsinki and Nasdaq US, provided that, in each case, the information made public, disclosed or not disclosed or the failure to disclose information
constitutes a material adverse effect to the Company; |
| (f) |
no court or regulatory authority of competent jurisdiction (including without limitation the Finnish Financial Supervisory Authority or the SEC) having given an order or issued any regulatory action preventing or
enjoining the completion of the Tender Offer; |
| (g) |
the Board of Directors of the Company having issued its recommendation for the Tender Offer and the recommendation remaining in force and not being modified or changed in a manner detrimental to the Offeror; and
|
| (h) |
the Combination Agreement not having been terminated and remaining in force and no event having occurred that, with the passage of time, would give the Offeror the right to terminate the Combination Agreement under
specified sections of the Combination Agreement that give the Offeror the right to terminate the Combination Agreement in response to a breach of the agreement by Birch. |
| “Material |
Adverse Effect” means |
| (A) |
any divestment or reorganization of any material part or asset of the Company or its subsidiaries or any recapitalization thereof; or |
| (B) |
any event, condition, circumstance, development, occurrence, change, effect or fact (any such item an “Effect”) that individually or in the
aggregate, has, results in or would reasonably be expected to have or result in a material adverse effect on the (i) business, assets, condition (financial or otherwise) or results of operations, of the Company and its subsidiaries, taken as a
whole, excluding any Effect resulting from (A) changes in the financial or securities markets, or economic, political or regulatory conditions generally, except to the extent such change has a disproportionate effect on the Company relative to
other industry participants, (B) changes in IFRS or changes in the regulatory accounting requirements applicable to any industry in which the Company and its subsidiaries operate, except to the extent such change has a disproportionate effect
on the Company relative to other industry participants, (C) changes (including |
G-44
| |
changes of Applicable Law) or conditions generally affecting the industry in which the Company and its subsidiaries operate, except to the extent such change has a disproportionate effect on the
Company relative to other industry participants, (D) acts of war, sabotage or terrorism or natural disasters, except to the extent such change has a disproportionate effect on the Company relative to other industry participants, (E) the
announcement or pendency of the transactions contemplated by, or the performance of obligations under, the Combination Agreement, including but not limited to any loss of or change in relationships between the Company or any of its subsidiaries and
any customer, supplier, distributor, business partner, employee, similar party, Governmental Authority or any other Persons and any shareholder or derivative litigation relating to the execution and performance of the Combination Agreement or the
announcement or the anticipated consummation of the transactions contemplated thereby (it being understood that this clause (E) shall not apply with respect to a representation or warranty contained in the Combination Agreement to the extent
that the purpose of such representation or warranty is to address the consequences resulting from the execution and delivery of the Combination Agreement or the consummation, announcement or pendency of the transactions contemplated by, or the
performance of obligations under, the Combination Agreement), (F) any failure by the Company and its subsidiaries to meet any internal, published or third-party budgets, projections, forecasts or predictions of financial performance for any
period (provided that the exception in this clause (F) shall not prevent or otherwise affect a determination that any Effect underlying such failure has resulted in or contributed to a Material Adverse Effect), (G) any action taken (or
omitted to be taken) at the express request of the Offeror, (H) any action taken by the Company or any of its subsidiaries that is required or expressly contemplated by the Combination Agreement, (I) the results of any pre-clinical or
clinical testing sponsored by the Company, any of its competitors or any of their respective collaboration partners or the increased incidence or severity of any previously identified side effects, adverse events or safety observations, or reports
of new side effects, adverse events or safety observations, with respect to any products or product candidates of the Company or any of its competitors (but not, in each case, the underlying cause of such results, side effects, adverse events or
safety observations to the extent such cause related to any Specified Event) (and, it being understood that this clause (I) shall not apply with respect to a representation or warranty contained in the Combination Agreement to the extent that
both (A) the purpose of such representation or warranty is to address the conduct of pre-clinical or clinical testing sponsored by the Company or any of its collaboration partners or any products or product candidates of the Company and
(B) as of the Signing Date, to the Knowledge of the Company, the Effect that would otherwise be excluded by this clause (I) has occurred), (J) the effect of the continued incurrence by the Company of net losses (provided that the
exception in this clause (J) shall not prevent or otherwise affect a determination that any Effect underlying such change has resulted in or contributed to a Material Adverse Effect), or (K) a change in the price and/or trading volume of
the Shares or ADSs on the Nasdaq Helsinki, the Nasdaq US or any other exchange or market on which they trade or are quoted for purchase and sale (provided that the exception in this clause (K) shall not prevent or otherwise affect a
determination that any Effect underlying such change has resulted in or contributed to a Material Adverse Effect), or (ii) ability of the Company to consummate the transactions contemplated hereby. |
“Specified Event” means the Company, FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency) or any IRB or DSMB terminates or
suspends, or recommends that the sponsor terminates or suspends, a tozadenant clinical trial for safety reasons.
The Offeror reserves the right to waive,
to the extent permitted by applicable law, any of the Conditions to Completion that have not been satisfied.
The Offeror can only invoke a condition set
forth in the Conditions to Completion to cause the withdrawal of the Tender Offer if the nonfulfillment of the condition has a material impact on the Offeror from the perspective of the anticipated acquisition as referred to in the FIN-FSA’s
recommendations and guidelines 9/2013 (Takeover bid and the obligation to launch a bid).
G-45
The Offeror will announce the fulfilment of the Conditions to Completion or the decision to waive any of the
Conditions to Completion that have not been satisfied by a stock exchange release.
Withdrawal Rights
In accordance with Chapter 11, Section 16, Subsection 1 of the Finnish Securities Market Act and Section 14(d)(5) of the Securities Exchange Act of
1934, as amended, and Rule 14d-7 thereunder (except to the extent of any SEC relief granted), the Outstanding Shares, ADSs and Outstanding Equity Instruments validly tendered in accordance with the terms and conditions of the Tender Offer may be
withdrawn at any time during the Offer Period or, if the Offer Period has been extended, during such extended Offer Period, until the Offeror has announced that all the Conditions to Completion have been satisfied or waived by the Offeror, thereby
declaring the Tender Offer unconditional, provided, however, the Offeror shall grant withdrawal rights after the Tender Offer has been declared unconditional to the extent permitted, or required, by Applicable Law. After the Offer Period (as it may
be extended), the Outstanding Shares, ADSs and Outstanding Equity Instruments already tendered may no longer be withdrawn except as required by Applicable Law.
In the event of a Subsequent Offer Period, the acceptance of the Tender Offer shall be binding and cannot be withdrawn, unless otherwise provided under
mandatory Finnish and/or United States law.
Announcement of the Result of the Tender Offer
The Offeror will announce the preliminary result of the Tender Offer on or about the first
(1st) Finnish banking day following the expiry of the Offer Period or, if applicable, the extended Offer Period. The Offeror will announce the final result of the Tender Offer on or about the
[third (3rd)] Finnish banking day following the expiry of the Offer Period or, if applicable, the extended Offer Period. The final result announcement will confirm (i) the percentage of the
Outstanding Shares, ADSs and Outstanding Equity Instruments that have been validly tendered and not properly withdrawn and (ii) whether the Tender Offer will be completed.
The Offeror will announce the initial percentage of the Outstanding Shares, ADSs and Outstanding Equity Instruments validly tendered during a possible
Subsequent Offer Period on or about the first (1st) Finnish banking day following the expiry of the Subsequent Offer Period and the final percentage on or about the [third (3rd)] Finnish
banking day following the expiry of the Subsequent Offer Period.
Transfer of Ownership
Title to the Outstanding Shares validly tendered in the Tender Offer will pass to the Offeror on the Closing Date against the payment of the Share Offer Price
by the Offeror to the tendering shareholder. In the event of a Subsequent Offer Period, title to the Outstanding Shares validly tendered in the Tender Offer during the Subsequent Offer Period will pass to the Offeror against the payment of the Share
Offer Price by the Offeror to the tendering shareholder as promptly as reasonably practicable following their tender.
Title to the outstanding ADSs
validly tendered in the Tender Offer will pass to the Offeror on the Closing Date against the payment of the ADS Offer Price by the Offeror to the tendering ADS holder. In the event of a Subsequent Offer Period, title to the outstanding ADSs validly
tendered in the Tender Offer during the Subsequent Offer Period will pass to the Offeror against the payment of the ADS Offer Price by the Offeror to the tendering ADS holder as promptly as reasonably practicable following their tender.
Title to Outstanding Equity Instruments validly tendered in the Tender Offer will pass to the Offeror on the Closing Date against the payment of the offer
price for the relevant Outstanding Equity Instrument by the Offeror to the tendering holder such Outstanding Equity Instrument. In the event of a Subsequent Offer Period, title to Outstanding Equity Instruments validly tendered in the Tender Offer
during the Subsequent Offer Period will pass to the Offeror against the payment of the offer price for the relevant Outstanding Equity Instrument by the Offeror to the tendering holder of such Outstanding Equity Instrument as promptly as reasonably
practicable following their tender.
G-46
Schedule 5.6
ACORDA PRESS RELEASE 19 January 2016 at 9:45 a.m. (EET)
ACORDA ANNOUNCES A RECOMMENDED CASH TENDER OFFER FOR ALL SHARES, ADSs AND OTHER EQUITY INSTRUMENTS IN BIOTIE
Acorda Therapeutics, Inc. (“Acorda”) and Biotie Therapies Corp. (“Biotie” or the “Company”) have today
entered into a combination agreement (“Combination Agreement”) whereby Acorda, either directly or through a wholly-owned subsidiary (jointly the “Offeror”), will make a public tender offer in Finland and in the
United States to purchase all of the issued and outstanding shares, American Depositary Shares (“ADSs”), stock options, share units and warrants in Biotie that are not owned by Biotie or any of its subsidiaries (the “Tender
Offer”).
The price offered for each share validly tendered into the Tender Offer will be EUR 0.2946 in cash, representing a premium of
approximately 95 per cent compared to the closing price of the Biotie shares on Nasdaq Helsinki Ltd. (“Nasdaq Helsinki”) on 18 January 2016, the last trading day on Nasdaq Helsinki preceding this announcement. This
represents a premium of approximately 84 per cent compared to the 3 month volume-weighted average trading price on Nasdaq Helsinki and approximately 56 per cent compared to the 6 month volume-weighted average trading price on Nasdaq
Helsinki.
The price offered for each ADS will be EUR 23.5680 in cash, payable in the equivalent amount of U.S. dollars determined as near to the payment
date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the closing date of the Tender Offer. As of January 18, 2016, this would be equivalent to 25.60 USD per ADS in
cash, based on the average exchange rate from the last 5 trading days to January 15 of 1.0864 USD to 1.00 EUR, representing a premium of approximately 94 per cent compared to the closing price of the Biotie ADSs on the Nasdaq Stock Market
LLC (“Nasdaq US”) on 15 January 2016, the last trading day on the Nasdaq US preceding this announcement. Acorda will also offer to acquire all of the outstanding option rights, share units and warrants issued by Biotie.
The Board of Directors of Biotie recommends that the holders of Biotie shares, ADSs, option rights, share units and warrants accept the Tender Offer. The
Board’s decision has been unanimous. The Board of Directors of Biotie will issue its complete statement regarding the Tender Offer in accordance with the Finnish Securities Market Act before the commencement of the Tender Offer. In connection
with the Tender Offer, the Board of Directors of Biotie has received an opinion from Biotie’s financial advisor.
Certain factors considered by the
Board of Directors of Biotie when giving its recommendation include (i) the costs required to gain approval and to subsequently launch the products, which could require an additional dilutive financing, (ii) the various strategic
alternatives available to the Company, taking into account discussions with other possible counterparties; (iii) the risks of a successful launch of the products for the Company to be able to realize the full economic value of the products and
(iv) the fact that the offer is a cash offer and not subject to a financing condition.
Certain Biotie shareholders and ADS holders representing in
total approximately 59 per cent of the outstanding shares and votes in Biotie on a fully diluted basis have subject to certain customary conditions irrevocably undertaken to accept the Tender Offer, including all the holders of Biotie warrants
and members of the management team of Biotie.
Board Member Mr. Don M Bailey, Vivo Capital, whose venture partner is Board Member
Mr. Mahendra G. Shah, and Versant Euro Ventures, whose partner is Board Member Mr. Guido Magni, representing in total approximately 27 per cent of the outstanding shares and votes in Biotie on a fully diluted basis (which is
included in the 59 per cent mentioned in the paragraph above), have subject to certain customary conditions irrevocably undertaken to accept the Tender Offer. Mr. Bailey, Vivo Capital and Versant Euro Ventures have made the commitment in
question after Biotie’s Board of Directors approved the entry into the Combination Agreement.
G-47
Board Members Mr. Bailey, Mr. Shah and Mr. Magni shall not participate in the giving of the Board of Directors’ statement regarding the Tender Offer.
“Our acquisition of Biotie positions Acorda as a leader in Parkinson’s disease therapeutic development, with three clinical-stage compounds that
have the potential to improve the lives of people with Parkinson’s. Tozadenant, Biotie’s most advanced clinical program, is a promising therapy being developed to reduce daily OFF time,” said Ron Cohen, M.D., Acorda’s President
and CEO. “Adenosine A2a receptor antagonists may be the first new class of drugs approved for the treatment of Parkinson’s in the U.S. in over 20 years. Approximately 350,000 people with Parkinson’s in the U.S. experience OFF periods,
and if approved, tozadenant could provide a much needed treatment option.”
Dr. Cohen added, “Tozadenant is a compelling opportunity with
potential market exclusivity to 2030. The Phase 2 data were highly statistically significant and clinically meaningful. We are targeting an NDA filing by the end of 2018.”
Mr. William M. Burns, Chairman of the Board of Biotie commented “We have carefully assessed the terms and conditions of the Offer and believe
that it is an attractive offer to shareholders that recognizes the strategic value of Biotie.”
Mr. Burns continued, “With the shared
mission to improve the lives of patients with neurological diseases, this transaction will allow Acorda and Biotie to bring together their expertise and resources in order to fully maximize the potential of tozadenant, an A2a receptor antagonist in
Phase 3 for Parkinson’s disease, and SYN120, a dual 5-HT6/5-HT2A receptor antagonist in Phase 2 for cognitive and psychotic
disorders, and to bring new medicines to patients. We are excited about this offer for our shareholders, the Biotie team and for patients.”
BACKGROUND AND REASONS FOR THE TENDER OFFER
Acorda is a
biotechnology company focused on developing therapies that improve the lives of people with neurological disorders, with its common stock listed on Nasdaq US.
Biotie is a specialized drug development company focused on products for neurodegenerative and psychiatric disorders. Through the acquisition, Acorda will
obtain worldwide rights to tozadenant, an oral adenosine A2a receptor antagonist currently in Phase 3 development in Parkinson’s disease (PD). In clinical trials, tozadenant reduced average daily OFF time as an adjunct to treatment regimens
including levodopa.
Further expanding its Parkinson’s pipeline, Acorda will also obtain global rights to SYN-120, an oral, 5-HT6/5-HT2A dual
receptor antagonist in Phase 2 development for Parkinson’s-related dementia, with support from the Michael J. Fox Foundation.
The acquisition also
includes two other assets: BTT1023, a fully human monoclonal antibody in Phase 2 development for primary sclerosing cholangitis (PSC), a chronic liver disease; and Selincro, a European Medicines Agency (EMA)-approved therapy for reduction of alcohol
consumption marketed by H. Lundbeck A/S in multiple European countries and for which the Company receives royalties.
THE TERMS AND CONDITIONS OF THE
TENDER OFFER
The price offered for each share validly tendered into the Tender Offer will be EUR 0.2946 per share in cash.
The price offered for each ADS will be EUR 23.5680 in cash, payable in the equivalent amount of U.S. dollars determined as near to the payment date as
reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the closing date of the Tender Offer.
G-48
The price offered for each stock option or share unit issued by Biotie pursuant to its option and equity
incentive plans and convertible into Biotie shares will be the greater of (i) EUR 0.2946 minus the applicable subscription price and (ii) EUR 0.01 in cash. The price offered for each warrant will be EUR 0.1664 in cash.
The specific prices for each of the stock options, share units and warrants have been set out in Annex A of this release.
The completion of the Tender Offer will be subject to the following conditions:
| |
(a) |
the valid tender of outstanding shares (including outstanding shares represented by validly tendered ADSs and validly tendered warrants) representing, together with any outstanding shares (including outstanding shares
represented by ADSs and warrants) otherwise acquired by the Offeror, more than ninety percent (90%) of the issued and outstanding shares and voting rights of the Company, calculated on a Fully Diluted Basis and otherwise in accordance with
Chapter 18 Section 1 of the Finnish Limited Liability Companies Act (21.7.2006/624); as used in this paragraph “Fully Diluted Basis” means an equation in which the numerator represents the aggregate number of outstanding shares
(including outstanding shares represented by ADSs) and warrants that have been validly tendered or otherwise acquired by the Offeror and the denominator represents the aggregate number of all outstanding shares (including outstanding shares
represented by ADSs) and warrants, as well as shares issuable upon the vesting and exercise of those outstanding equity instruments (other than warrants) that have not been validly tendered into the Tender Offer or otherwise acquired by the Offeror;
|
| |
(b) |
the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Act; |
| |
(c) |
no material adverse effect (as defined in the Combination Agreement) having occurred in Biotie after 19 January 2016; |
| |
(d) |
the Offeror not, after 19 January 2016, having received information previously undisclosed to it that describes a material adverse effect to the Company that occurred prior to 19 January 2016;
|
| |
(e) |
no information made public by the Company or disclosed by the Company to the Offeror being materially inaccurate, incomplete, or misleading, and the Company not having failed to make public any information that should
have been made public by it under applicable laws, including without limitation the rules of Nasdaq Helsinki and Nasdaq US, provided that, in each case, the information made public, disclosed or not disclosed or the failure to disclose information
constitutes a material adverse effect to the Company; |
| |
(f) |
no court or regulatory authority of competent jurisdiction (including without limitation the Finnish Financial Supervisory Authority or the SEC) having given an order or issued any regulatory action preventing or
enjoining the completion of the Tender Offer; |
| |
(g) |
the Board of Directors of the Company having issued its recommendation for the Tender Offer and the recommendation remaining in force and not being modified or changed in a manner detrimental to the Offeror; and
|
| |
(h) |
the Combination Agreement not having been terminated and remaining in force and no event having occurred that, with the passage of time, would give the Offeror the right to terminate the Combination Agreement under
specified sections of the Combination Agreement that give the Offeror the right to terminate the Combination Agreement in response to a breach of the agreement by the Company. |
The Offeror reserves the right to complete the Tender Offer even if the conditions to completion of the Tender Offer have not been fulfilled.
The Tender Offer will commence after the Offeror has obtained certain regulatory relief from the US Securities and Exchange Commission, which is expected to
be obtained no later than the end of February 2016, and initially run for twenty (20) banking days. The Offeror reserves the right to extend and is obligated to extend the acceptance period from time to time in accordance with the terms and
conditions of the Tender Offer.
G-49
The Offeror will make the filings required under the Hart-Scott-Rodino Act, which requires the Offeror to delay
the completion of the Tender Offer until the applicable waiting period pursuant to the Hart-Scott-Rodino Act has expired or been terminated. The initial waiting period under the Hart-Scott-Rodino Act is thirty days, unless it is earlier terminated
or extended by a request for additional information. The Offeror currently does not believe that the completion of the Tender Offer would require regulatory approvals from competition authorities outside the United States.
The Tender Offer will be financed through cash on Acorda’s balance sheet and the gross proceeds of a private placement to a banking institution of
approximately USD 75 million of Acorda’s common stock that was executed concurrently with the execution of the Combination Agreement and that is expected to close by 26 January 2016. The Tender Offer is not conditional upon obtaining
any external financing for the Tender Offer.
The detailed terms and conditions of the Tender Offer and information on how to accept the Tender Offer will
be included in the tender offer documents that will be published by the Offeror before the commencement of the acceptance period in Finland and in the United States.
Acorda and Biotie have undertaken to follow the Helsinki Takeover Code issued by the Finnish Securities Market Association as referred to in the Finnish
Securities Market Act and will comply with the US Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder, subject to any relief from the US Securities and Exchange Commission.
On the date of this release, Biotie’s share capital amounts to EUR 279,218,058.55 and the number of shares issued to 1,089,608,083. The Offeror does not
currently hold any shares, ADSs, option rights, share units or warrants in Biotie.
COMBINATION AGREEMENT
The Combination Agreement sets forth the principal terms under which the Offeror will make the Tender Offer.
Under the Combination Agreement, the Board of Directors of Biotie has undertaken, in the event of a competing offer, not to cancel or change its
recommendation for the Tender Offer, unless
| |
i. |
it determines in good faith, after taking advice from external legal counsel and financial advisor that the competing offer is superior to the Offeror’s offer and that therefore it would no longer be in the best
interest of the shareholders, ADS holders and holders of stock options, share units and warrants of Biotie to accept the Tender Offer and that failure to cancel or change the Board of Directors’ recommendation for the Tender Offer would be
inconsistent with the Board of Directors’ fiduciary duties towards Biotie’s shareholders and holders of other equity instruments under Finnish laws; and |
| |
ii. |
prior to and as a precondition for cancelling or changing its recommendation, the Board of Directors has complied with certain agreed procedures allowing Acorda to assess the competing offer and to enhance the Tender
Offer. |
Should Acorda enhance the Tender Offer so as to be at least equally favourable to Biotie’s shareholders as the competing offer,
the Board of Directors has undertaken to confirm and uphold the recommendation for the Tender Offer, as enhanced.
Biotie has also agreed not to, directly
or through its representatives, solicit or encourage any competing offers or proposals for such offers or other transactions competing with the Tender Offer, nor to facilitate or promote any such competing proposals, unless the Board of Directors
has determined that, with respect to an unsolicited competing proposal, failure to take such measures would be inconsistent with the Board of Directors’ fiduciary duties. Biotie has agreed to inform Acorda of any competing proposals and to
provide Acorda an opportunity to negotiate with the Board of Directors of Biotie on matters arising from such competing proposals.
G-50
The Combination Agreement further includes certain representations, warranties and undertakings by both parties
customary in transactions of a similar nature, such as conduct of business by Biotie in the ordinary course of business before the completion of the Tender Offer and cooperation by the parties in necessary regulatory filings and in completing the
Tender Offer in the most expeditious manner practicable.
Biotie has further undertaken to compensate Acorda for its reasonable transaction costs in the
amount of USD 4,500,000 in the event the Combination Agreement is terminated in connection with the Board of Directors of Biotie withdrawing or changing its recommendation for the Tender Offer.
Acorda’s intention is to cause the shares of Biotie to be delisted from Nasdaq Helsinki and the ADSs to be delisted from Nasdaq US as soon as permitted
and reasonably practicable under applicable laws and regulations.
The foregoing summary is not complete and is qualified by reference to the full text of
the Combination Agreement.
EFFECT ON BIOTIE’S CURRENT ORGANIZATION
Biotie is headquartered in Turku, Finland, with a subsidiary based in South San Francisco, California. Following the close of the acquisition, Acorda plans to
maintain the operations of the South San Francisco in full, including the staff at that site. The future of Biotie’s location in Turku will be considered separately later on. With this addition, Acorda will have operations in three major U.S.
biotechnology centers: New York, Boston and San Francisco.
ADVISORS
Lazard, MTS Health Partners, L.P. and J.P. Morgan Securities LLC served as financial advisors, and Kirkland & Ellis, Roschier Attorneys Ltd.,
Covington & Burling LLP and Jones Day LLP served as legal advisors to Acorda in connection with the Tender Offer.
Guggenheim Securities served
as Biotie Therapies’ financial advisors, and Davis Polk & Wardwell LLP and Hannes Snellman Attorneys Ltd. served as Biotie’s legal advisors.
ACORDA THERAPEUTICS, INC.
Board of Directors
FURTHER INFORMATION
For further information, please
contact:
Acorda
Jeff Macdonald, Senior Director,
Corporate Communications
Tel. + 1 914 326 5232, e-mail: jmacdonald@acorda.com
Felicia Vonella, Investor relations
Tel. + 1 914 326 5146,
e-mail: fvonella@acorda.com
Biotie
Timo Veromaa,
President and Chief Executive Officer
G-51
Tel. +358 2 274 8900, e-mail: timo.veromaa@biotie.com
David Cook, Chief Financial Officer
Tel. +358 2 274 8900,
e-mail: david.cook@biotie.com
Virve Nurmi, Investor Relations Manager
Tel. +358 2 274 8900, e-mail: virve.nurmi@biotie.com
The Trout
Group LLC
Lauren Williams, Managing Director
Tel: +44 203
780 4972, e-mail: lwilliams@troutgroup.com
Jennifer Porcelli, Vice President
Tel: +1 646 378 2962, e-mail: jporcelli@troutgroup.com
INFORMATION REGARDING ACORDA
Founded in 1995, Acorda is
a biotechnology company focused on developing therapies that improve the lives of people with neurological disorders, with its common stock listed on Nasdaq US.
Acorda has an industry leading pipeline of novel neurological therapies addressing a range of disorders, including multiple sclerosis, Parkinson’s
disease, post-stroke walking deficits, epilepsy and migraine. Acorda markets three FDA-approved therapies, including AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg.
INFORMATION REGARDING BIOTIE
Biotie is a specialized
drug development company focused on products for neurodegenerative and psychiatric disorders. Biotie’s development has delivered Selincro (nalmefene) for alcohol dependence, which received European marketing authorization in 2013 and is
currently being marketed across Europe by partner Lundbeck. The current development products include tozadenant for Parkinson’s disease, which is in Phase 3 development, and two additional compounds which are in Phase 2 development for
cognitive disorders including Parkinson’s disease dementia, and primary sclerosing cholangitis (PSC), a rare fibrotic disease of the liver.
ANNEX
A
The specific prices for each of the stock options, share units and warrants are as follows:
|
|
|
| Outstanding Equity Instrument |
|
Price offered in the Tender Offer
(EUR in cash) |
| 2011 Option Rights |
|
|
| 2011C (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014 Option Rights |
|
|
| 2014A (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014B (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014C (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014D (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
| 2014M (subscription price EUR 0.01 per share) |
|
0.2846 for each stock option |
G-52
|
|
|
| Outstanding Equity Instrument |
|
Price offered in the Tender Offer
(EUR in cash) |
| 2016 Option Rights |
|
|
| Stock option 2016 (subscription price EUR 0.162 per share) |
|
0.1326 for each stock option |
| 2011 Share Rights |
|
|
| 2011 Share right |
|
0.2946 for each share right |
| 2014 Share Rights |
|
|
| 2014A (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014B (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014C (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014D (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| 2014M (subscription price USD 0.01 per share) |
|
0.2854 for each share right |
| Swiss Option Rights |
|
|
| Swiss option (subscription price CHF 0.10 per share) |
|
0.2032 for each stock option |
| Swiss option (subscription price CHF 0.21 per share) |
|
0.1026 for each stock option |
| Swiss option (subscription price CHF 0.28 per share) |
|
0.0386 for each stock option |
| Swiss option (subscription price CHF 0.31 per share) |
|
0.0112 for each stock option |
| Swiss option (subscription price CHF 0.35 per share) |
|
0.0100 for each stock option |
| Swiss option (subscription price CHF 0.38 per share) |
|
0.0100 for each stock option |
| Swiss option (subscription price CHF 0.45 per share) |
|
0.0100 for each stock option |
| Warrants |
|
|
| Warrants (subscription price EUR 0.17 per share) |
|
0.1664 for each warrant |
DISCLAIMER
This press
release includes forward-looking statements. All statements, other than statements of historical facts, regarding management’s expectations, beliefs, goals, plans or prospects should be considered forward-looking. These statements are subject
to risks and uncertainties that could cause actual results to differ materially, including the ability to complete the Biotie transaction on a timely basis or at all; the ability to realize the benefits anticipated to be realized by the Biotie
transaction and the Civitas transaction; the ability to successfully integrate Biotie’s operations and Civitas’ operations, respectively, into our operations; we may need to raise additional funds to finance our expanded operations and may
not be able to do so on acceptable terms; our ability to successfully market and sell Ampyra in the U.S.; third party payers (including governmental agencies) may not reimburse for the use of Ampyra or our other products at acceptable rates or at
all and may impose restrictive prior authorization requirements that limit or block prescriptions; the risk of unfavorable results from future studies of Ampyra or from our other research and development programs, including CVT-301, Plumiaz, or any
other acquired or in-licensed programs; we may not be able to complete development of, obtain regulatory approval for, or successfully market CVT-301, Plumiaz, or any other products under development; the occurrence of adverse safety events with our
products; delays in obtaining or failure to obtain regulatory approval of or to successfully market Fampyra outside of the U.S. and our dependence on our collaboration partner Biogen in connection therewith; competition; failure to protect our
intellectual property, to defend against the intellectual property claims of others or to obtain third party intellectual property licenses needed for the commercialization of our products; and failure to comply with regulatory requirements could
result in adverse action by regulatory agencies. In addition, the compounds being acquired from Biotie are subject to all the risks inherent in the drug development process, and there can be no assurance that these compounds will receive regulatory
approval or be commercially successful. These and other risks are described in greater detail in our filings with the Securities and Exchange Commission. We may not actually achieve the goals or plans described in our forward-looking statements, and
investors should not place undue reliance on these statements. Forward-looking statements made in this release are made only as of the date hereof, and we disclaim any intent or obligation to update any forward-looking statements as a result of
developments occurring after the date of this release.
G-53
Additional Information
The tender offer described in this release has not yet commenced, and this release is neither an offer to purchase nor a solicitation of an offer to sell
securities. At the time the tender offer is commenced, we will file, or will cause a new wholly owned subsidiary to file, with the SEC a tender offer statement on Schedule TO. Investors and holders of Biotie Equity Interests are strongly advised to
read the tender offer statement (including an offer to purchase, letter of transmittal and related tender offer documents) and the related solicitation/recommendation statement on Schedule 14D-9 that will be filed by Biotie with the SEC, because
they will contain important information. These documents will be available at no charge on the SEC’s website at www.sec.gov upon the commencement of the tender offer. In addition, a copy of the offer to purchase, letter of transmittal and other
related tender offer documents (once they become available) may be obtained free of charge by directing a request to us at www.acorda.com or Office of the Corporate Secretary, 420 Saw Mill River Road, Ardsley, New York 10502.
In addition to the offer to purchase, the related letter of transmittal and certain other offer documents, as well as the solicitation/recommendation
statement, we file annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy any reports, statements or other information filed by us at the SEC public reference room at 100 F Street, N.E.,
Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. Our filings with the SEC are also available to the public from commercial document-retrieval services and at the website maintained
by the SEC at www.sec.gov.
THE OFFER WILL NOT BE MADE DIRECTLY OR INDIRECTLY IN ANY JURISDICTION WHERE EITHER AN OFFER OR PARTICIPATION THEREIN IS
PROHIBITED BY APPLICABLE LAW OR WHERE ANY TENDER OFFER DOCUMENT OR REGISTRATION OR OTHER REQUIREMENTS WOULD APPLY IN ADDITION TO THOSE UNDERTAKEN IN FINLAND AND THE UNITED STATES.
IN ADDITION, THE TENDER OFFER DOCUMENTS, THIS RELEASE AND RELATED MATERIALS AND ACCEPTANCE FORMS WILL NOT AND MAY NOT BE DISTRIBUTED, FORWARDED OR
TRANSMITTED INTO OR FROM ANY JURISDICTION WHERE PROHIBITED BY APPLICABLE LAW. IN PARTICULAR, THE TENDER OFFER IS NOT BEING MADE, DIRECTLY OR INDIRECTLY, IN OR INTO, CANADA, JAPAN, AUSTRALIA, SOUTH AFRICA OR HONG KONG. THE TENDER OFFER CANNOT BE
ACCEPTED BY ANY SUCH USE, MEANS OR INSTRUMENTALITY OR FROM WITHIN CANADA, JAPAN, AUSTRALIA, SOUTH AFRICA OR HONG KONG.
This announcement is for
information only and does not constitute a tender offer document or an offer, solicitation of an offer or an invitation to a sales offer. Potential investors in Finland shall accept the tender offer only on the basis of the information provided in a
tender offer document approved by the Finnish Financial Supervisory Authority and related materials.
G-54
Exhibit (a)(1)(B)
LETTER OF TRANSMITTAL
TO TENDER AMERICAN DEPOSITARY SHARES (“ADSs”) EACH REPRESENTING 80 ORDINARY SHARES, NO NOMINAL VALUE
OF
BIOTIE THERAPIES
OYJ
PURSUANT TO THE TENDER OFFER DOCUMENT
BY
ACORDA
THERAPEUTICS, INC.
THE OFFER PERIOD AND THE ASSOCIATED WITHDRAWAL RIGHTS WILL EXPIRE ON APRIL 8, 2016 AT 4:00 P.M.
(FINNISH TIME) / 9:00 A.M. (NEW YORK TIME), UNLESS THE OFFER PERIOD IS EXTENDED.
This Letter of Transmittal relates to the
offer to purchase filed with the United States Securities and Exchange Commission on March 11, 2016 (the “Tender Offer Document”) pursuant to which Acorda Therapeutics, Inc., a Delaware corporation (“Acorda” or
the “Offeror”), is offering to purchase (the “Tender Offer”) all outstanding ordinary shares, no nominal value (“Shares”), all outstanding American Depositary Shares (“ADSs”), each
representing 80 Shares, all of the outstanding Option Rights (as defined in the Tender Offer Document), all of the outstanding Share Rights (as defined in the Tender Offer Document) and all of the outstanding warrants issued on May 28, 2015
(the “Warrants”) (the outstanding Shares, ADSs, Option Rights, Share Rights and Warrants, collectively, the “Equity Interests”) in Biotie Therapies Oyj (“Biotie” or the “Company”),
a Finnish limited liability Company, upon and subject to the terms and conditions set forth in the Tender Offer Document, this Letter of Transmittal and the related acceptance forms for Option Rights, Share Rights and Warrants.
Please deliver this properly completed and duly executed Letter of Transmittal and accompanying documents to Computershare Trust Company,
N.A., in its capacity as Depositary for the Tender Offer, at one of the addresses or the facsimile set forth below.
The Depositary
for the Tender Offer is:

|
|
|
|
|
| By First Class, Registered or Certified Mail: |
|
By Express or Overnight Delivery: |
|
*By Facsimile Transmission (for eligible institutions only): |
| Computershare Trust Company, N.A.
c/o Voluntary Corporate Actions PO Box 43011
Providence, RI 02940-3011 |
|
Computershare Trust Company, N.A.
c/o Voluntary Corporate Actions 250 Royall Street, Suite V
Canton, MA 02021 |
|
Computershare Trust Company, N.A.
Facsimile: (617) 360-6810 Confirm
By Telephone: (781) 575-2332 |
DELIVERY OF THIS LETTER OF TRANSMITTAL TO AN ADDRESS OTHER THAN AS SET FORTH ABOVE, OR TRANSMISSION OF
INSTRUCTIONS VIA FACSIMILE TO A NUMBER OTHER THAN AS SET FORTH ABOVE, WILL NOT CONSTITUTE A VALID DELIVERY TO THE DEPOSITARY. YOU MUST SIGN THIS LETTER OF TRANSMITTAL IN THE APPROPRIATE SPACE PROVIDED BELOW, WITH SIGNATURE GUARANTEE IF REQUIRED, AND
COMPLETE THE IRS FORM W-9 INCLUDED HEREIN, OR THE APPROPRIATE IRS FORM W-8, AS APPLICABLE.
Voluntary Corporate Action: COY BTHB
THE INSTRUCTIONS CONTAINED WITHIN THIS LETTER OF TRANSMITTAL SHOULD BE READ CAREFULLY BEFORE
THIS LETTER OF TRANSMITTAL IS COMPLETED. DELIVERY OF THIS LETTER OF TRANSMITTAL MAY BE USED ONLY TO TENDER AMERICAN DEPOSITARY SHARES. IT MAY NOT BE USED TO TENDER ORDINARY SHARES OR OTHER EQUITY INTERESTS OF BIOTIE THERAPIES OYJ.
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
2 |
|
|
DESCRIPTION OF ADSs TENDERED
|
|
|
| Name(s) and Address(es) of Registered Holder(s) |
|
Number of ADS(s) Tendered
(Please attach additional signed list, if necessary) |
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| Total ADSs Tendered |
|
|
Unless an Agent’s Message (as
defined below) is utilized, this Letter of Transmittal is to be used by holders of ADSs to facilitate the delivery of ADSs by book-entry transfer to an account maintained by the Depositary at The Depositary Trust Company (“DTC”) and
pursuant to the procedures set forth in Section 4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer Document. DELIVERY OF DOCUMENTS TO DTC WILL NOT CONSTITUTE DELIVERY TO THE DEPOSITARY. See Instruction 2.
The term “Agent’s Message” means a message transmitted by DTC to, and received by, the Depositary as part of a confirmation of
a book-entry transfer. The message states that DTC has received an express acknowledgment from the DTC participant tendering ADSs that such participant has received and agrees to be bound by the terms of the Letter of Transmittal and that the
Offeror may enforce such agreement against such participant.
Acceptance of the Tender Offer in respect of Shares (except
insofar as they are represented by ADSs), Share Rights, Option Rights or Warrants cannot be made by means of this Letter of Transmittal. If you wish to tender your Shares, Share Rights, Option Rights or Warrants, you must follow the procedures
set forth In Section 4.4––“Acceptance Procedure of the Tender Offer” of the Tender Offer Document. See Instruction 10.
There will be no guaranteed delivery tender procedures in connection with the Tender Offer.
Your bank or broker can assist you in completing this Letter of Transmittal. The instructions included with this Letter of Transmittal must
be followed. Questions and requests for assistance or for additional copies of the Tender Offer Document and this Letter of Transmittal may be directed to the Information Agent for ADSs, at the address and telephone numbers indicated below.
TENDER OF ADSs
| ¨ |
CHECK HERE TO CONFIRM THAT TENDERED ADSs ARE BEING DELIVERED BY BOOK-ENTRY TRANSFER TO THE DEPOSITARY’S ACCOUNT AT DTC AND COMPLETE THE FOLLOWING (ONLY PARTICIPANTS IN DTC MAY DELIVER ADSs BY BOOK-ENTRY TRANSFER):
|
|
|
|
| Name of Tendering Institution: |
|
|
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
3 |
|
|
NOTE: ALL SIGNATURES MUST BE PROVIDED BELOW
PLEASE READ THE INSTRUCTIONS SET FORTH IN THIS LETTER OF TRANSMITTAL CAREFULLY
Ladies and Gentlemen:
The undersigned hereby
tenders to Acorda Therapeutics, Inc. (“Acorda” or the “Offeror”), the above-described American Depositary Shares (the “ADSs”), each of which represents 80 ordinary shares, no nominal value (the
“Shares”) of Biotie Therapies Oyj, a public limited liability company incorporated in Finland (“Biotie” or the “Company”), pursuant to the Offeror’s offer to purchase (the “Tender
Offer”) for cash all outstanding Shares, ADSs and certain other securities of the Company, upon and subject to the terms and conditions set forth in the offer to purchase filed with the United States Securities and Exchange Commission on
March 11, 2016 (the “Tender Offer Document”), receipt of which is hereby acknowledged, this Letter of Transmittal and the related acceptance forms for Option Rights, Share Rights and Warrants.
Upon the terms and subject to the conditions of the Tender Offer (and if the Tender Offer is extended or amended, the terms of any such
extension or amendment), and effective upon acceptance for payment of the ADSs tendered herewith in accordance with the terms of the Tender Offer, the undersigned hereby sells, assigns and transfers to or upon the order of the Offeror all right,
title and interest in and to all of the ADSs that are being tendered hereby, and any and all dividends, distributions, Shares or other securities or rights issued or issuable in respect thereof on or after the date hereof (collectively,
“Distributions”) and irrevocably constitutes and appoints Computershare Trust Company, N.A. (the “Depositary”) the true and lawful agent and attorney-in-fact of the undersigned with respect to such ADSs (and any and
all Distributions), with full power of substitution (such power of attorney being deemed to be an irrevocable power coupled with an interest), to (i) transfer ownership of such ADSs (and any and all Distributions) on the account books
maintained by DTC, together, in any such case, with all accompanying evidences of transfer and authenticity, to or upon the order of the Offeror, (ii) present such ADSs (and any and all Distributions) for transfer on the books of Biotie and
(iii) receive all benefits and otherwise exercise all rights of beneficial ownership of such ADSs (and any and all Distributions), all in accordance with the terms of the Tender Offer.
By executing this Letter of Transmittal, the undersigned hereby irrevocably appoints the Offeror’s officers and designees, and each of
them, the attorneys-in-fact and proxies of the undersigned, each with full power of substitution, to vote at any annual or special meeting of Biotie’s shareholders or any adjournment or postponement thereof or otherwise in such manner as each
such attorney-in-fact and proxy or his or her substitute may in his or her sole discretion deem proper with respect to, to execute any written consent concerning any matter as each such attorney-in-fact and proxy or his or her substitute may in his
or her sole discretion deem proper with respect to, and to otherwise act as each such attorney-in-fact and proxy or his or her substitute may in his or her sole discretion deem proper with respect to, all of the ADSs (and any and all Distributions)
tendered hereby and accepted for payment by the Offeror. This appointment will be effective if and when, and only to the extent that, the Offeror accepts such ADSs for payment pursuant to the Tender Offer. This power of attorney and proxy are
irrevocable and are granted in consideration of the acceptance for payment of such ADSs in accordance with the terms of the Tender Offer. Such acceptance for payment will, without further action, revoke any prior powers of attorney and proxies
granted by the undersigned at any time with respect to such ADSs (and any and all Distributions), and no subsequent powers of attorney, proxies, consents or revocations may be given by the undersigned with respect thereto (and, if given, will not be
deemed effective). The Offeror reserves the right to require that, in order for the ADSs to be deemed validly tendered, immediately upon the Offeror’s acceptance for payment of such ADSs, the Offeror must be able to exercise full voting,
consent and other rights with respect to such ADSs (and any and all Distributions), including voting at any meeting of Biotie’s shareholders.
The undersigned hereby represents and warrants that the undersigned has full power and authority to tender, sell, assign and transfer the ADSs
tendered hereby (and any and all Distributions) and that, when the same are accepted for payment by the Offeror, the Offeror will acquire good, marketable and unencumbered title
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
4 |
|
|
thereto (and to any and all Distributions), free and clear of all liens, restrictions, charges and encumbrances and the same will not be subject to any adverse claims. The undersigned hereby
represents and warrants that the undersigned is a participant in DTC whose name appears on a security position listing as the owner of the ADSs. The undersigned will, upon request, execute and deliver any additional documents deemed by the
Depositary or the Offeror to be necessary or desirable to complete the sale, assignment and transfer of the ADSs tendered hereby (and any and all Distributions). In addition, effective upon acceptance for payment, the undersigned will remit and
transfer promptly to the Depositary for the account of the Offeror all Distributions in respect of the ADSs tendered hereby, accompanied by appropriate documentation of transfer, and, pending such remittance and transfer or appropriate assurance
thereof, the Offeror will be entitled to all rights and privileges as owner of each such Distribution and may withhold the entire purchase price of the ADSs tendered hereby or deduct from such purchase price the amount or value of such Distribution
as determined by the Offeror in its sole discretion.
All authority herein conferred or agreed to be conferred will survive the death or
incapacity of the undersigned, and any obligation of the undersigned hereunder will be binding upon the heirs, executors, administrators, personal representatives, trustees in bankruptcy, successors and assigns of the undersigned. Except as stated
in the Tender Offer Document, this tender is irrevocable.
The undersigned understands that the valid tender of the ADSs pursuant to any
one of the procedures described in Section 4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer Document and in the Instructions hereto will constitute the undersigned’s acceptance of the terms and conditions
of the Tender Offer (and if the Tender Offer is extended or amended, the terms and conditions of any such extension or amendment). The Offeror’s acceptance of the undersigned’s ADS, for payment will constitute a binding agreement between
the undersigned and the Offeror upon the terms and subject to the conditions of the Tender Offer. Without limiting the foregoing, if the price to be paid in the Tender Offer is amended, the price to be paid to the undersigned will be the amended
price notwithstanding the fact that a different price is stated in this Letter of Transmittal. The undersigned recognizes that, under certain circumstances set forth in the Tender Offer Document, the Offeror may not be required to accept for payment
any of the ADSs tendered hereby.
Unless otherwise indicated herein in the box entitled “Special Payment Instructions,” the undersigned hereby
instructs the Depositary to credit any ADSs tendered hereby by book-entry transfer that are not accepted for payment by crediting the account at DTC designated above. The undersigned recognizes that the Offeror has no obligation, pursuant to the
“Special Payment Instructions,” to transfer any ADSs from the name of the registered holder thereof if the Offeror does not accept for payment any of the ADSs so tendered.
The undersigned understands that the Offeror reserves the right to transfer or assign, in whole at any time, or in part from time to time, to one or more of
its affiliates, the right to purchase all or any portion of the ADSs tendered pursuant to the Tender Offer, but any such transfer or assignment will not relieve the Offeror of its obligations under the Tender Offer and will in no way prejudice the
rights of holders to receive payment for ADSs validly tendered and accepted for payment pursuant to the Tender Offer. Any reference to the Offeror herein shall also be deemed to be a reference to any such permitted assignee.
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
5 |
|
|
SPECIAL PAYMENT INSTRUCTIONS (See Instructions 1, 4, 5 and 6)
To be completed ONLY if the check for the purchase price of ADSs accepted for payment is to be issued in the name of someone other than the
undersigned.
|
|
|
| Issue: |
|
¨ Check |
|
|
| Name: |
|
|
|
|
(Please Print) |
|
|
| Address: |
|
|
|
|
(Include Zip Code) |
(Taxpayer Identification or Social Security Number)
(Also complete IRS Form W-9 included herein, or the appropriate IRS Form W-8, as applicable)
(Account Number)
SPECIAL
DELIVERY INSTRUCTIONS
(See Instructions 1, 4, 5 and 6)
To be completed ONLY if the check for the purchase price of ADSs accepted for payment is to be mailed to someone other than the undersigned or
to the undersigned at an address other than that shown above under “Description of ADSs Tendered.”
|
|
|
| Mail: |
|
¨ Check |
|
|
| Name: |
|
|
|
|
(Please Print) |
|
|
| Address: |
|
|
|
|
(Include Zip Code) |
(Taxpayer Identification or Social Security Number)
(Also complete IRS Form W-9 included herein, or the appropriate IRS Form W-8, as applicable)
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
|
|
|
IMPORTANT
ADS HOLDER: SIGN HERE
(Please complete IRS Form W-9 included herein, or the appropriate IRS Form W-8, as applicable)
(Signature(s) of Holder(s))
|
|
|
|
|
| Capacity (Full Title): |
|
|
|
|
(See Instructions) |
|
|
|
|
|
| Address: |
|
|
|
|
(Include Zip Code) |
|
|
|
|
|
| Area Code and Telephone Number: |
|
|
|
|
|
|
|
| Taxpayer Identification or Social Security Number: |
|
|
|
|
(See IRS Form W-9 included herein, or the
appropriate IRS Form W-8, as applicable) |
Dated:
, 2016
(Must be signed by the registered holder(s) exactly as name(s) appear(s) on a security position listing and documents transmitted herewith. If
signature is by a trustee, executor, administrator, guardian, attorney-in-fact, officer of a corporation or other person acting in a fiduciary or representative capacity, please set forth full title and see Instruction 4.)
GUARANTEE OF SIGNATURES
(If required—See Instructions 1 and 5. For use by Eligible Institutions only.)
|
|
|
|
|
| Address: |
|
|
|
|
(Include Zip Code) |
|
|
|
|
|
| Area Code and Telephone Number: |
|
|
Dated:
, 2016
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
|
|
|
INSTRUCTIONS
FORMING PART OF THE TERMS AND CONDITIONS OF THE TENDER OFFER
1. Guarantee of Signatures. No signature guarantee is required on this Letter of Transmittal if (a) this Letter of Transmittal is signed
by the registered holder(s) (which term, for purposes of this section, includes any participant in DTC’s systems whose name appears on a security position listing as the owner of the ADSs) of ADSs tendered hereby, unless such registered
holder(s) has (have) completed either the box entitled “Special Payment Instructions” or the box entitled “Special Delivery Instructions” on the Letter of Transmittal, or (b) such ADSs are tendered for the account of a
financial institution (including most commercial banks, savings and loan associations and brokerage houses) that is a participant in the Securities Transfer Agents Medallion Program, the New York Stock Exchange Medallion Signature Program or the
Stock Exchange Medallion Program or by any other “eligible guarantor institution,” as such term is defined in Rule I7Ad-15 under the Securities Exchange Act of 1934, as amended (each, an “Eligible Institution”). In all
other cases, all signatures on this Letter of Transmittal must be guaranteed by an Eligible Institution. See Instruction 4.
2.
Requirements of Tender. Unless an Agent’s Message is utilized, this Letter of Transmittal is to be completed by ADS holders if tenders are to be made pursuant to the procedure for tender by book-entry transfer set forth in
Section 4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer Document. Timely confirmation (a “Book-Entry Confirmation”) of a book-entry transfer of ADS holders into the Depositary’s account at
DTC, as well as this Letter of Transmittal (or a facsimile hereof), properly completed and duly executed, with any required signature guarantees, or an Agent’s Message in connection with a book-entry transfer, and any other documents required
by this Letter of Transmittal, must be received by the Depositary at one of its addresses set forth herein on or prior to the Expiration Date (as defined in “Summary Term Sheet” of the Tender Offer Document).
The term “Agent’s Message” means a message transmitted by DTC to, and received by, the Depositary as part of a confirmation of
a book-entry transfer. The message states that DTC has received an express acknowledgment from the DTC participant tendering ADSs that such participant has received and agrees to be bound by the terms of the Letter of Transmittal and that the
Offeror may enforce such agreement against such participant.
The method of delivery of this Letter of Transmittal and all other
required documents, including delivery through DTC, is at the option and the risk of the tendering holder, and the delivery will be deemed made only when actually received by the Depositary (including, in the case of book-entry transfer, receipt of
a book-entry confirmation). If delivery is by mail, registered mail with return receipt requested, properly insured, is recommended. In all cases, sufficient time should be allowed to ensure timely delivery. There will be no guaranteed delivery
tender procedures in connection with the Tender Offer.
No alternative, conditional or contingent tenders will be accepted and no
fractional ADSs will be purchased. All tendering ADS holders, by execution of this Letter of Transmittal (or a facsimile hereof), waive any right to receive any notice of the acceptance of their ADSs for payment.
3. Inadequate Space. If the space provided herein is inadequate, the number of ADSs and any other required information should be listed on a
separate signed schedule attached hereto.
4. Signatures on Letter of Transmittal. If any of the ADSs tendered hereby are held of record
by two or more joint owners, all such owners must sign this Letter of Transmittal. If any of the tendered ADSs are registered in the names of different holders, it will be necessary to complete, sign and submit as many separate Letters of
Transmittal as there are different registrations of such ADSs.
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
8 |
|
|
If this Letter of Transmittal is signed by a trustee, executor, administrator, guardian,
attorney-in-fact, officer of a corporation or other person acting in a fiduciary or representative capacity, such person should so indicate when signing, and proper evidence satisfactory to the Offeror of the authority of such person so to act must
be submitted.
5. Transfer Taxes. Except as otherwise provided in this Instruction 5, the Offeror will pay all transfer taxes with respect
to the transfer and sale of any ADSs to it or its order pursuant to the Offer (for the avoidance of doubt, transfer taxes do not include United States federal income tax or backup withholding taxes). If, however, payment of the purchase price is to
be made to any person other than the registered holder(s), the amount of any transfer taxes (whether imposed on the registered holder(s) or such other person) payable on account of the transfer to such other person will be deducted from the purchase
price of such ADSs purchased unless evidence satisfactory to the Offeror of the payment of such taxes, or exemption therefrom, is submitted.
6. Special Payment and Delivery Instructions. If a check is to be issued in the name of a person other than the signatory of this Letter of
Transmittal or if a check is to be returned to a person other than the person(s) signing this Letter of Transmittal or to an address other than that shown in this Letter of Transmittal, the appropriate boxes on this Letter of Transmittal must be
completed.
7. IRS Form W-9 or IRS Form W-8. A tendering ADS holder that is a U.S. Person (as defined below) is required to provide the
Depositary with a correct Taxpayer Identification Number (“TIN”) on IRS Form W-9, which is provided herein, and to certify, under penalties of perjury, that such number is correct and that such holder is not subject to backup withholding
of U.S. federal income tax. If a tendering ADS holder that is a U.S. Person is subject to backup withholding, such holder must cross out Item (2) of Part II of IRS Form W-9. Failure to provide the information on the IRS Form W-9 may subject a
tendering ADS holder that is a U.S. Person to a penalty imposed by the IRS and U.S. federal backup income tax withholding (current at a rate of 28%) of any payments made to such holder or other payee pursuant to the Tender Offer.
Certain ADS holders (including, among others, certain corporations and certain foreign individuals and entities) are not subject to backup
withholding and should indicate their exempt status on IRS Form W-9. ADS holders that are not U.S. Persons should submit an appropriate and properly completed IRS Form W-8, a copy of which may be obtained from the Depositary, in order to avoid
backup withholding.
For this purpose, a “U.S. Person” is an individual who is a U.S. citizen or U.S. resident alien, a
partnership, corporation, company, or association created or organized in the United States or under the laws of the United States or an estate the income of which is subject to tax in the United States regardless of its source or a trust, which
(a) is subject to the primary jurisdiction of a court within the United States and for which one or more U.S. persons have authority to control all substantial decisions, or (b) has a valid election in effect under applicable U.S. Treasury
Regulations to be treated as a U.S. person for U.S. federal income tax purposes. See the instructions to IRS Form W-9 or the appropriate Form W-8, as applicable, for additional information regarding qualifying for an exemption from backup
withholding. ADS holders are advised to consult their respective tax advisors as to their qualifications for obtaining an exemption from backup withholding and the procedure for obtaining such an exemption.
8. Requests for Assistance or Additional Copies. Questions and requests for assistance or additional copies of the Tender Offer Document, this
Letter of Transmittal and IRS Form W-8 may be directed to the Information Agent for ADSs or Depositary at the addresses and phone numbers set forth herein, or to your broker, dealer, commercial bank or trust company.
9. Irregularities and Waiver of Conditions. All questions as to purchase price, the form of documents and the validity, eligibility
(including, without limitation, time of receipt) and acceptance for payment of any tender of ADSs will be determined by the Offeror in its sole discretion, subject to applicable law, which determinations shall be final and binding on all parties.
The Offeror reserves the absolute right to reject any or all tenders of
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
9 |
|
|
ADSs it determines not to be in proper form or the acceptance of which or payment for which may, in the opinion of the Offeror, be unlawful. The Offeror also reserves the absolute right to waive
any defect or irregularity in the tender of any particular ADSs, and, subject to applicable law, the Offeror’s interpretation of the terms of the Tender Offer (including, without limitation, these instructions), will be final and binding on all
parties. No tender of ADSs will be deemed to be properly made until all defects and irregularities have been cured or waived. Unless waived, any defects or irregularities in connection with tenders must be cured within such time as the Offeror shall
determine. None of the Offeror, the Depositary, the Information Agent for ADSs or any other person is or will be obligated to give notice of any defects or irregularities in tenders, and none of them will incur any liability for failure to give any
such notice.
Subject to the terms and conditions set forth in Section 4.2—“Conditions to Completion of the Tender
Offer” of the Tender Offer Document and the rules and regulations of the Securities and Exchange Commission, the Offeror reserves the right, in its sole discretion, to waive, at any time and from time to time, any of the specified conditions of
the Tender Offer (if waivable), in whole or in part.
10. Holders of Shares Not Represented by ADSs, Share Rights, Option Rights or
Warrants. Tenders of Equity Interests other than ADSs cannot be made by means of this Letter of Transmittal. If you wish to tender such Equity Interests you must follow the procedures set forth in Section 4.4––“Acceptance
Procedure of the Tender Offer” of the Tender Offer Document.
11. No Interest; Foreign Exchange Currency. Under no circumstances will
interest be paid on the purchase price of the ADSs, regardless of any delay in payment for such securities. The cash consideration paid to tendering ADS holders will be payable in U.S. dollars calculated by using the spot exchange rate for the U.S.
dollar against the euro exchange rate on the nearest practicable day to the Closing Date (as defined in the Tender Offer Document).
12.
Expiration Date. The initial acceptance period for the Tender Offer (the “Offer Period”) will commence on March 11 at 9:30 a.m. (Finnish time) / 2:30 a.m. (New York time) and expire on April 8 at 4:00 p.m. (Finnish time) / 9:00 a.m.
(New York time) unless the Offer Period is extended (the end of the Offer Period, as extended, the “Expiration Date”), subject to the time limits set by any broker, dealer, commercial bank, trust company, custodian or other nominee
that tenders ADSs on behalf of a holder, which may end prior to the Expiration Date.
If the Offeror extends the Offer Period, the Offeror will inform the
Depositary of that fact and will make a public announcement of the extension by a stock exchange release at or before 4:00 p.m. (Finnish time) / 9:00 a.m. (New York time) on April 11, 2016. The Offeror will give notice of any further extension of
the Tender Offer, at the latest on the first Finnish banking day following what would have been, but for such extension, the Expiration Date or end of any Subsequent Offer Period (as defined in the Tender Offer Document), as applicable, at or before
4:00 p.m. (Finnish time) / 9:00 a.m. (New York City time).
IMPORTANT: THIS LETTER OF TRANSMITTAL (OR A MANUALLY SIGNED FACSIMILE
HEREOF) TOGETHER WITH ANY REQUIRED SIGNATURE GUARANTEES, OR AN AGENT’S MESSAGE, AND ANY OTHER REQUIRED DOCUMENTS, MUST BE RECEIVED BY THE DEPOSITARY ON OR PRIOR TO THE EXPIRATION DATE AND ADSs MUST BE DELIVERED PURSUANT TO THE PROCEDURES FOR
BOOK-ENTRY TRANSFER ON OR PRIOR TO THE EXPIRATION DATE.
IMPORTANT TAX INFORMATION
Under United States federal income tax law, a stockholder who is a U.S. Person (as defined below) surrendering ADSs must, unless an exemption
applies, provide the Depositary (as payer) with the stockholder’s
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
10 |
|
|
correct TIN on IRS Form W-9, a copy of which is included in this Letter of Transmittal. If the stockholder is an individual, then the stockholder’s TIN is such stockholder’s Social
Security number. If the correct TIN is not provided, then the stockholder may be subject to a penalty imposed by the IRS and payments of cash to the stockholder (or other payee) pursuant to the Tender Offer may be subject to backup withholding of a
portion of all payments of the purchase price.
Certain stockholders (including, among others, corporations and certain foreign
individuals and entities) may not be subject to backup withholding and reporting requirements. In order for an exempt foreign stockholder to avoid backup withholding, such person should complete, sign and submit an appropriate IRS Form W-8, signed
under penalties of perjury, attesting to his, her or its exempt status. An appropriate IRS Form W-8 can be obtained from the Depositary. Such stockholders should consult a tax advisor to determine which IRS Form W-8 is appropriate. Exempt
stockholders, other than foreign stockholders, should furnish their TIN, check the “Exempt payee” box on the IRS Form W-9 and sign, date and return the IRS Form W-9 to the Depositary in order to avoid erroneous backup withholding. See the
instructions enclosed with the IRS Form W-9 included in this Letter of Transmittal for additional instructions.
If backup withholding
applies, the Depositary is required to withhold and pay over to the IRS a portion (currently, 28%) of any payment made to a stockholder. Backup withholding is not an additional tax. Rather, the United States federal income tax liability of persons
subject to backup withholding will be reduced by the amount of tax withheld. If backup withholding results in an overpayment of taxes, a refund may be obtained from the IRS if required information is timely furnished to the IRS.
For this purpose, a “U.S. Person” is an individual who is a U.S. citizen or U.S. resident alien, a partnership, corporation,
company, or association created or organized in the United States or under the laws of the United States or an estate the income of which is subject to tax in the United States regardless of its source or a trust, which (a) is subject to the
primary jurisdiction of a court within the United States and for which one or more U.S. persons have authority to control all substantial decisions, or (b) has a valid election in effect under applicable U.S. Treasury Regulations to be treated
as a U.S. person for U.S. federal income tax purposes. See the instructions to IRS Form W-9 or the appropriate Form W-8, as applicable, for additional information regarding qualifying for an exemption from backup withholding. ADS holders are advised
to consult their respective tax advisors as to their qualifications for obtaining an exemption from backup withholding and the procedure for obtaining such an exemption.
Purpose of IRS Form W-9
To prevent backup withholding on payments that are made to a stockholder that is a U.S. Person (as defined above) with respect to ADSs
purchased pursuant to the Tender Offer, the stockholder is required to notify the Depositary of the stockholder’s correct TIN by completing the IRS Form W-9 included in this Letter of Transmittal certifying that (1) the TIN provided on the
IRS Form W-9 is correct (or that such stockholder is awaiting a TIN), (2) the stockholder is not subject to backup withholding because (i) the stockholder is exempt from backup withholding, (ii) the stockholder has not been notified
by the IRS that the stockholder is subject to backup withholding as a result of a failure to report all interest and dividends or (iii) the IRS has notified the stockholder that the stockholder is no longer subject to backup withholding, and
(3) the stockholder is a U.S. Person (as defined above).
The following section, entitled “What Number to Give the
Depositary,” is applicable only to stockholders that are U.S. Persons.
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
11 |
|
|
What Number to Give the Depositary
The tendering holder is required to give the Depositary the TIN, generally the Social Security number or employer identification number, of
the record holder of all ADSs tendered hereby. If such ADSs are in more than one name or are not in the name of the actual owner, consult the instructions enclosed with the IRS Form W-9 included in this Letter of Transmittal for additional guidance
on which number to report. If the tendering stockholder has not been issued a TIN and has applied for a number or intends to apply for a number in the near future, such stockholder should write “Applied For” in the space for the TIN on the
IRS Form W-9, sign and date the IRS Form W-9 and sign and date the Certificate of Awaiting Taxpayer Identification Number below. If the tendering stockholder writes “Applied For” in the space for the TIN and the Depositary is not provided
with a TIN by the time of payment, the Depositary will withhold a portion of all payments of the purchase price, which will be refunded if a TIN is provided to the Depositary within sixty (60) days of the Depositary’s receipt of the
Certificate of Awaiting Taxpayer Identification Number. If the Depositary is provided with an incorrect TIN in connection with such payments, then the stockholder may be subject to a penalty imposed by the IRS and backup withholding on any payments
made to such stockholder or other payee pursuant to the Tender Offer.
| NOTE: |
FAILURE TO COMPLETE AND RETURN THE IRS FORM W-9 INCLUDED IN THIS LETTER OF TRANSMITTAL MAY RESULT IN BACKUP WITHHOLDING OF A PORTION OF ANY PAYMENTS MADE TO YOU PURSUANT TO THE OFFER. PLEASE REVIEW THE ENCLOSED
INSTRUCTIONS ENCLOSED WITH THE IRS FORM W-9 INCLUDED IN THIS LETTER OF TRANSMITTAL FOR ADDITIONAL DETAILS. YOU MUST COMPLETE THE FOLLOWING CERTIFICATE IF YOU WROTE “APPLIED FOR” IN THE SPACE FOR THE TIN ON THE IRS FORM W-9.
|
CERTIFICATE OF AWAITING TAXPAYER IDENTIFICATION NUMBER
I certify under penalties of perjury that a taxpayer identification number has not been issued to me, and
either (1) I have mailed or delivered an application to receive a taxpayer identification number to the appropriate IRS Center or Social Security Administration Office, or (2) I intend to mail or deliver an application in the near future.
I understand that if I do not provide a taxpayer identification number by the time of payment, a portion of all reportable payments made to me will be withheld, but that such amounts will be refunded to me if I then provide a Taxpayer Identification
Number within sixty (60) days.
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
12 |
|
|
|
|
|
|
|
|
|
|
| Form W-9
(Rev. December 2014) Department of the Treasury
Internal Revenue Service |
|
Request for Taxpayer
Identification Number and Certification |
|
Give Form to the
requester. Do not send to
the IRS. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Print or type
See Specific Instructions
on page 2. |
|
1 Name (as shown on your income tax return). Name is required on this line; do not leave
this line blank. |
|
|
|
|
|
|
|
|
| |
2 Business name/disregarded entity name, if different from above
|
|
|
|
|
|
|
|
|
|
|
| |
3 Check appropriate box for federal tax classification; check only one of the following seven boxes: |
|
|
|
|
|
4 Exemptions (codes apply only to
certain entities, not individuals; see
instructions on
page 3): Exempt payee code (if
any)
Exemption from FATCA reporting
code (if
any)
(Applies to accounts maintained outside the U.S.) |
| |
¨ |
|
Individual/sole proprietor or
single-member LLC |
|
¨ |
|
C Corporation |
|
¨ |
|
S Corporation |
|
¨ |
|
Partnership |
|
¨ |
|
Trust/estate |
|
|
|
| |
¨ Limited liability company. Enter the tax classification (C=C corporation, S=S corporation, P=partnership) u
Note. For a single-member LLC that is disregarded, do not check LLC; check the appropriate
box in the line above for
the tax classification of the single-member owner. ¨ Other (see instructions) u |
|
|
|
| |
5 Address (number, street, and apt. or suite no.) |
|
Requester’s name and address (optional) |
|
|
|
|
|
|
|
|
| |
6 City, state, and
ZIP code |
|
|
|
|
|
|
| |
|
7 List account
number(s) here (optional) |
|
|
|
|
|
| Part I |
|
Taxpayer Identification Number (TIN) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Enter your TIN in the appropriate box. The TIN provided must match the name given on line 1 to avoid backup withholding. For individuals,
this is generally your social security number (SSN). However, for a resident alien, sole proprietor, or disregarded entity, see the Part I instructions on page 3. For other entities, it is your employer identification number (EIN). If you do not
have a number, see How to get a TIN on page 3. Note. If the account is in more than
one name, see the instructions for line 1 and the chart on page 4 for guidelines on whose number to enter. |
|
Social security number |
| |
|
| |
|
|
|
|
|
|
– |
|
|
|
|
|
– |
|
|
|
|
|
|
|
|
| |
or |
| |
Employer identification number |
|
|
| |
|
|
|
| |
|
|
|
|
– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Under penalties of perjury, I certify that:
| 1. |
|
The number shown on this form is my correct taxpayer identification number (or I am waiting for a number to be issued to me); and |
| 2. |
|
I am not subject to backup withholding because: (a) I am exempt from backup withholding, or (b) I have not been notified by the Internal Revenue Service (IRS) that I am subject to backup withholding as a result of a
failure to report all interest or dividends, or (c) the IRS has notified me that I am no longer subject to backup withholding; and |
| 3. |
|
I am a U.S. citizen or other U.S. person (defined below); and |
| 4. |
|
The FATCA code(s) entered on this form (if any) indicating that I am exempt from FATCA reporting is correct. |
Certification instructions. You must cross out item 2 above if you have been notified by the IRS that you are currently subject to backup withholding because you
have failed to report all interest and dividends on your tax return. For real estate transactions, item 2 does not apply. For mortgage interest paid, acquisition or abandonment of secured property, cancellation of debt, contributions to an
individual retirement arrangement (IRA), and generally, payments other than interest and dividends, you are not required to sign the certification, but you must provide your correct TIN. See the instructions on page 3.
|
|
|
|
|
Sign
Here |
|
Signature of
U.S. person u |
|
Date
u |
General Instructions
Section
references are to the Internal Revenue Code unless otherwise noted.
Future developments. Information about developments affecting Form W-9 (such as legislation enacted after we release it) is at www.irs.gov/fw9.
Purpose of Form
An individual or entity (Form W-9 requester) who is required
to file an information return with the IRS must obtain your correct taxpayer identification number (TIN) which may be your social security number (SSN), individual taxpayer identification number (ITIN), adoption taxpayer identification number
(ATIN), or employer identification number (EIN), to report on an information return the amount paid to you, or other amount reportable on an information return. Examples of information returns include, but are not limited to, the following:
• Form 1099-INT (interest earned or paid)
• Form 1099-DIV (dividends,
including those from stocks or mutual funds)
• Form 1099-MISC (various types of income, prizes, awards, or gross proceeds)
• Form 1099-B (stock or mutual fund sales and certain other transactions by brokers)
• Form 1099-S (proceeds from real estate transactions)
• Form 1099-K (merchant card and third party network transactions)
• Form 1098 (home mortgage interest), 1098-E (student loan interest), 1098-T (tuition)
• Form 1099-C (canceled debt)
• Form 1099-A (acquisition or abandonment of
secured property)
Use Form W-9 only if you are a U.S. person (including a resident alien), to provide your correct TIN.
If you do not return
Form W-9 to the requester with a TIN,
you might be subject to backup withholding. See What
is backup withholding? on page 2.
By signing the filled-out form, you:
1. Certify that the TIN you are giving is correct (or you are waiting for a number to be issued),
2. Certify that you are not subject to backup withholding, or
3. Claim exemption from backup withholding if you are a U.S. exempt payee. If applicable, you are also certifying that as a U.S. person, your allocable
share of any partnership income from a U.S. trade or business is not subject to the withholding tax on foreign partners’ share of effectively connected income, and
4. Certify that FATCA code(s) entered on this form (if any) indicating that you are exempt from the FATCA reporting, is correct. See What is FATCA
reporting? on page 2 for further information.
|
|
|
|
|
| |
|
Cat. No. 10231X |
|
Form W-9 (Rev.
12-2014) |
| Form W-9 (Rev. 12-2014) |
Page 2 |
Note. If you are a U.S. person and a requester gives you a form other than Form W-9 to request your TIN,
you must use the requester’s form if it is substantially similar to this Form W-9.
Definition of a U.S. person. For
federal tax purposes, you are considered a U.S. person if you are:
• An individual who is a U.S. citizen or U.S. resident alien;
• A partnership, corporation, company, or association created or organized in the United States or under the laws of the United States;
• An estate (other than a foreign estate); or
• A domestic trust (as
defined in Regulations section 301.7701-7).
Special rules for partnerships. Partnerships that conduct a trade or business
in the United States are generally required to pay a withholding tax under section 1446 on any foreign partners’ share of effectively connected taxable income from such business. Further, in certain cases where a Form W-9 has not been received,
the rules under section 1446 require a partnership to presume that a partner is a foreign person, and pay the section 1446 withholding tax. Therefore, if you are a U.S. person that is a partner in a partnership conducting a trade or business in the
United States, provide Form W-9 to the partnership to establish your U.S. status and avoid section 1446 withholding on your share of partnership income.
In the cases below, the following person must give Form W-9 to the partnership for purposes of establishing its U.S. status and avoiding withholding on
its allocable share of net income from the partnership conducting a trade or business in the United States:
• In the case of a disregarded entity with a U.S.
owner, the U.S. owner of the disregarded entity and not the entity;
• In the case of a grantor trust with a U.S. grantor or other U.S. owner, generally, the
U.S. grantor or other U.S. owner of the grantor trust and not the trust; and
• In the case of a U.S. trust (other than a grantor trust), the U.S. trust (other
than a grantor trust) and not the beneficiaries of the trust.
Foreign person. If you are a foreign person or the U.S. branch of a
foreign bank that has elected to be treated as a U.S. person, do not use Form W-9. Instead, use the appropriate Form W-8 or Form 8233 (see Publication 515, Withholding of Tax on Nonresident Aliens and Foreign Entities).
Nonresident alien who becomes a resident alien. Generally, only a nonresident alien
individual may use the terms of a tax treaty to reduce or eliminate U.S. tax on certain types of income. However, most tax treaties contain a provision known as a “saving clause.” Exceptions specified in the saving clause may permit an
exemption from tax to continue for certain types of income even after the payee has otherwise become a U.S. resident alien for tax purposes.
If you
are a U.S. resident alien who is relying on an exception contained in the saving clause of a tax treaty to claim an exemption from U.S. tax on certain types of income, you must attach a statement to Form W-9 that specifies the following five items:
1. The treaty country. Generally, this must be the same treaty under which you claimed exemption from tax as a nonresident alien.
2. The treaty article addressing the income.
3.
The article number (or location) in the tax treaty that contains the saving clause and its exceptions.
4. The type and amount of income that
qualifies for the exemption from tax.
5. Sufficient facts to justify the exemption from tax under the terms of the treaty article.
Example. Article 20 of the U.S.-China income tax treaty allows an exemption from tax for scholarship income received by a
Chinese student temporarily present in the United States. Under U.S. law, this student will become a resident alien for tax purposes if his or her stay in the United States exceeds 5 calendar years. However, paragraph 2 of the first Protocol to the
U.S.-China treaty (dated April 30,1984) allows the provisions of Article 20 to continue to apply even after the Chinese student becomes a resident alien of the United States. A Chinese student who qualifies for this exception (under paragraph 2 of
the first protocol) and is relying on this exception to claim an exemption from tax on his or her scholarship or fellowship income would attach to Form W-9 a statement that includes the information described above to support that exemption.
If you are a nonresident alien or a foreign entity, give the requester the appropriate completed Form W-8 or Form 8233.
Backup Withholding
What is backup
withholding? Persons making certain payments to you must under certain conditions withhold and pay to the IRS 28% of such payments. This is called “backup withholding.” Payments that may be subject to backup withholding
include interest, tax-exempt interest, dividends, broker and barter exchange transactions, rents, royalties, nonemployee pay, payments made in settlement of payment card and third party network transactions, and certain payments from fishing boat
operators. Real estate transactions are not subject to backup withholding.
You will not be subject to backup withholding on payments you receive if
you give the requester your correct TIN, make the proper certifications, and report all your taxable interest and dividends on your tax return.
Payments you receive will be subject to backup withholding if:
1. You do not furnish your TIN to the requester,
2. You do not certify your TIN when required (see the Part II instructions on page 3 for details),
3. The IRS tells the requester that you furnished an incorrect TIN,
4. The IRS tells you that you are subject to backup withholding because you did not report all your interest and dividends on your tax return (for
reportable interest and dividends only), or
5. You do not certify to the requester that you are not subject to backup withholding under 4 above
(for reportable interest and dividend accounts opened after 1983 only).
Certain payees and payments are exempt from backup withholding. See
Exempt payee code on page 3 and the separate Instructions for the Requester of Form W-9 for more information.
Also see
Special rules for partnerships above.
What is FATCA reporting?
The Foreign Account Tax Compliance Act (FATCA) requires a participating foreign financial institution to report all United States account holders that are specified
United States persons. Certain payees are exempt from FATCA reporting. See Exemption from FATCA reporting code on page 3 and the Instructions for the Requester of Form W-9 for more information.
Updating Your Information
You must provide updated information to any
person to whom you claimed to be an exempt payee if you are no longer an exempt payee and anticipate receiving reportable payments in the future from this person. For example, you may need to provide updated information if you are a C corporation
that elects to be an S corporation, or if you no longer are tax exempt. In addition, you must furnish a new Form W-9 if the name or TIN changes for the account; for example, if the grantor of a grantor trust dies.
Penalties
Failure to furnish
TIN. If you fail to furnish your correct TIN to a requester, you are subject to a penalty of $50 for each such failure unless your failure is due to reasonable cause and not to willful neglect.
Civil penalty for false information with respect to withholding. If
you make a false statement with no reasonable basis that results in no backup withholding, you are subject to a $500 penalty.
Criminal
penalty for falsifying information. Willfully falsifying certifications or affirmations may subject you to criminal penalties including fines and/or imprisonment.
Misuse of TINs. If the requester discloses or uses TINs in violation of federal law, the requester may be subject to civil and criminal penalties.
Specific Instructions
Line 1
You must enter one of the following on this line; do not leave this line blank. The name should match the name on your tax return.
If this Form W-9 is for a joint account, list first, and then circle, the name of the person or entity whose number you entered in Part I of Form W-9.
a. Individual. Generally, enter the name shown on your tax return. If you have changed your last name without informing the Social
Security Administration (SSA) of the name change, enter your first name, the last name as shown on your social security card, and your new last name.
Note. ITIN applicant: Enter your individual name as it was entered on your Form W-7 application, line 1a. This should also be
the same as the name you entered on the Form 1040/1040A/1040EZ you filed with your application.
b. Sole proprietor or
single-member LLC. Enter your individual name as shown on your 1040/1040A/1040EZ on line 1. You may enter your business, trade, or “doing business as” (DBA) name on line 2.
c. Partnership, LLC that is not a single-member LLC,
C Corporation, or S Corporation. Enter the entity’s name as shown on the entity’s tax return on line 1 and any business, trade, or DBA name on line 2.
d. Other entities. Enter your name as shown on required U.S. federal tax documents on line 1. This name should match the name shown on the
charter or other legal document creating the entity. You may enter any business, trade, or DBA name on line 2.
e. Disregarded entity.
For U.S. federal tax purposes, an entity that is disregarded as an entity separate from its owner is treated as a “disregarded entity.” See Regulations section 301.7701-2(c)(2)(iii). Enter the owner’s name on line 1. The name of
the entity entered on line 1 should never be a disregarded entity. The name on line 1 should be the name shown on the income tax return on which the income should be reported. For example, if a foreign LLC that is treated as a disregarded entity for
U.S. federal tax purposes has a single owner that is a U.S. person, the U.S. owner’s name is required to be provided on line 1. If the direct owner of the entity is also a disregarded entity, enter the first owner that is not disregarded for
federal tax purposes. Enter the disregarded entity’s name on line 2, “Business name/disregarded entity name.” If the owner of the disregarded entity is a foreign person, the owner must complete an appropriate Form W-8 instead of a
Form W-9. This is the case even if the foreign person has a U.S. TIN.
| Form W-9 (Rev. 12-2014) |
Page 3 |
Line 2
If you have a business
name, trade name, DBA name, or disregarded entity name, you may enter it on line 2.
Line 3
Check the appropriate box in line 3 for the U.S. federal tax classification of the person whose name is entered on line 1. Check only one box in line 3.
Limited Liability Company (LLC). If the name on line 1 is an LLC treated as a partnership for U.S. federal tax
purposes, check the “Limited Liability Company” box and enter “P” in the space provided. If the LLC has filed Form 8832 or 2553 to be taxed as a corporation, check the “Limited Liability Company” box and in the space
provided enter “C” for C corporation or “S” for S corporation. If it is a single-member LLC that is a disregarded entity, do not check the “Limited Liability Company” box; instead check the first box in line 3
“Individual/sole proprietor or single-member LLC.”
Line 4, Exemptions
If you are exempt from backup withholding and/or FATCA reporting, enter in the appropriate space in line 4 any code(s) that may apply to you.
Exempt payee code.
• Generally, individuals (including sole
proprietors) are not exempt from backup withholding.
• Except as provided below, corporations are exempt from backup withholding for certain payments,
including interest and dividends.
• Corporations are not exempt from backup withholding for payments made in settlement of payment card or third party network
transactions.
• Corporations are not exempt from backup withholding with respect to attorneys’ fees or gross proceeds paid to attorneys, and corporations
that provide medical or health care services are not exempt with respect to payments reportable on Form 1099-MISC.
The following codes identify
payees that are exempt from backup withholding. Enter the appropriate code in the space in line 4.
1—An organization exempt from tax under
section 501(a), any IRA, or a custodial account under section 403(b)(7) if the account satisfies the requirements of section 401(f)(2)
2—The
United States or any of its agencies or instrumentalities
3—A state, the District of Columbia, a U.S. commonwealth or possession, or any of
their political subdivisions or instrumentalities
4—A foreign government or any of its political subdivisions, agencies, or instrumentalities
5—A corporation
6—A dealer in
securities or commodities required to register in the United States, the District of Columbia, or a U.S. commonwealth or possession
7—A
futures commission merchant registered with the Commodity Futures Trading Commission
8—A real estate investment trust
9—An entity registered at all times during the tax year under the Investment Company Act of 1940
10—A common trust fund operated by a bank under section 584(a)
11—A financial institution
12—A
middleman known in the investment community as a nominee or custodian
13—A trust exempt from tax under section 664 or described in section
4947
The following chart shows types of payments that may be exempt from backup withholding. The chart applies to the exempt payees listed above, 1
through 13.
|
|
|
| IF the payment is for . . . |
|
THEN the payment is exempt for . . . |
| Interest and dividend payments |
|
All exempt payees except for 7 |
| Broker transactions |
|
Exempt payees 1 through 4 and 6 through 11 and all C corporations. S corporations must not enter an exempt payee code because they are exempt only for sales of noncovered securities
acquired prior to 2012. |
| Barter exchange transactions and patronage dividends |
|
Exempt payees 1 through 4 |
| Payments over $600 required to be reported and direct sales over $5,0001 |
|
Generally, exempt payees
1 through 52 |
| Payments made in settlement of payment card or third party network transactions |
|
Exempt payees 1 through 4 |
| 1 |
See Form 1099-MISC, Miscellaneous Income, and its instructions. |
| 2 |
However, the following payments made to a corporation and reportable on Form 1099-MISC are not exempt from backup withholding: medical and health care payments, attorneys’ fees, gross proceeds paid to an attorney
reportable under section 6045(f), and payments for services paid by a federal executive agency.
|
Exemption from FATCA reporting code. The following codes identify payees that are exempt from reporting under
FATCA. These codes apply to persons submitting this form for accounts maintained outside of the United States by certain foreign financial institutions. Therefore, if you are only submitting this form for an account you hold in the United States,
you may leave this field blank. Consult with the person requesting this form if you are uncertain if the financial institution is subject to these requirements. A requester may indicate that a code is not required by providing you with a Form W-9
with “Not Applicable” (or any similar indication) written or printed on the line for a FATCA exemption code.
A—An organization
exempt from tax under section 501(a) or any individual retirement plan as defined in section 7701(a)(37)
B—The United States or any of its
agencies or instrumentalities
C—A state, the District of Columbia, a U.S. commonwealth or possession, or any of their political subdivisions
or instrumentalities
D—A corporation the stock of which is regularly traded on one or more established securities markets, as described in
Regulations section 1.1472-1(c)(1)(i)
E—A corporation that is a member of the same expanded affiliated group as a corporation described in
Regulations section 1.1472-1(c)(1)(i)
F—A dealer in securities, commodities, or derivative financial instruments (including notional principal
contracts, futures, forwards, and options) that is registered as such under the laws of the United States or any state
G—A real estate
investment trust
H—A regulated investment company as defined in section 851 or an entity registered at all times during the tax year under the
Investment Company Act of 1940
I—A common trust fund as defined in section 584(a)
J—A bank as defined in section 581
K—A
broker
L—A trust exempt from tax under section 664 or described in section 4947(a)(1)
M—A tax exempt trust under a section 403(b) plan or section 457(g) plan
Note. You may wish to consult with the financial institution requesting this form to determine whether the FATCA code and/or exempt payee code should be
completed.
Line 5
Enter your address (number, street, and apartment or
suite number). This is where the requester of this Form W-9 will mail your information returns.
Line 6
Enter your city, state, and ZIP code.
Part I. Taxpayer Identification Number
(TIN)
Enter your TIN in the appropriate box. If you are a resident alien and you do not have and are not eligible to get an SSN, your TIN is your IRS
Individual taxpayer identification number (ITIN). Enter it in the social security number box. If you do not have an ITIN, see How to get a TIN below.
If you are a sole proprietor and you have an EIN, you may enter either your SSN or EIN. However, the IRS prefers that you use your SSN.
If you are a single-member LLC that is disregarded as an entity separate from its owner (see Limited Liability Company (LLC) on this page), enter
the owner’s SSN (or EIN, if the owner has one). Do not enter the disregarded entity’s EIN. If the LLC is classified as a corporation or partnership, enter the entity’s EIN.
Note. See the chart on page 4 for further clarification of name and TIN combinations.
How to get a TIN. If you do not have a TIN, apply for one immediately. To apply for an SSN, get Form SS-5, Application for a Social Security Card, from your
local SSA office or get this form online at www.ssa.gov. You may also get this form by calling 1-800-772-1213. Use Form W-7, Application for IRS Individual Taxpayer Identification Number, to apply for an ITIN, or Form SS- 4, Application for
Employer Identification Number, to apply for an EIN. You can apply for an EIN online by accessing the IRS website at www.irs.gov/businesses and clicking on Employer Identification Number (EIN) under Starting a Business. You can get Forms W-7
and SS-4 from the IRS by visiting IRS. gov or by calling 1-800-TAX-FORM (1-800-829-3676).
If you are asked to complete Form W-9 but do not have a
TIN, apply for a TIN and write “Applied For” in the space for the TIN, sign and date the form, and give it to the requester. For interest and dividend payments, and certain payments made with respect to readily tradable instruments,
generally you will have 60 days to get a TIN and give it to the requester before you are subject to backup withholding on payments. The 60-day rule does not apply to other types of payments. You will be subject to backup withholding on all such
payments until you provide your TIN to the requester.
Note. Entering “Applied For” means that you have already applied for a TIN or that you
intend to apply for one soon.
Caution: A disregarded U.S. entity that has a foreign owner must use the
appropriate Form W-8.
| Form W-9 (Rev. 12-2014) |
Page 4 |
Part II. Certification
To
establish to the withholding agent that you are a U.S. person, or resident alien, sign Form W-9. You may be requested to sign by the withholding agent even if items 1, 4, or 5 below indicate otherwise.
For a joint account, only the person whose TIN is shown in Part I should sign (when required). In the case of a disregarded entity, the person identified
on line 1 must sign. Exempt payees, see Exempt payee code earlier.
Signature requirements. Complete the certification as indicated in items 1 through 5
below.
1. Interest, dividend, and barter exchange accounts opened before 1984 and broker accounts considered active during 1983.
You must give your correct TIN, but you do not have to sign the certification.
2. Interest, dividend, broker, and barter exchange accounts
opened after 1983 and broker accounts considered inactive during 1983. You must sign the certification or backup withholding will apply. If you are subject to backup withholding and you are merely providing your correct TIN to the
requester, you must cross out item 2 in the certification before signing the form.
3. Real estate transactions. You must sign
the certification. You may cross out item 2 of the certification.
4. Other payments. You must give your correct TIN, but you do
not have to sign the certification unless you have been notified that you have previously given an incorrect TIN. “Other payments” include payments made in the course of the requester’s trade or business for rents, royalties, goods
(other than bills for merchandise), medical and health care services (including payments to corporations), payments to a nonemployee for services, payments made in settlement of payment card and third party network transactions, payments to certain
fishing boat crew members and fishermen, and gross proceeds paid to attorneys (including payments to corporations).
5. Mortgage interest
paid by you, acquisition or abandonment of secured property, cancellation of debt, qualified tuition program payments (under section 529), IRA, Coverdell ESA, Archer MSA or HSA contributions or distributions, and pension distributions.
You must give your correct TIN, but you do not have to sign the certification.
What Name and Number To Give the Requester
|
|
|
|
|
|
|
| |
For this type of account: |
|
Give name and SSN of: |
| |
1. |
|
|
Individual |
|
The individual |
| |
2. |
|
|
Two or more individuals (joint account) |
|
The actual owner of the account or, if combined funds, the first individual on the account1 |
| |
3. |
|
|
Custodian account of a minor (Uniform Gift to Minors Act) |
|
The minor2 |
| |
4. |
|
|
a. The usual revocable savings trust (grantor is also trustee) |
|
The grantor-trustee1 |
|
|
|
|
b. So-called trust account that is not a legal or valid trust under state law |
|
The actual owner1 |
| |
5. |
|
|
Sole proprietorship or disregarded entity owned by an individual |
|
The owner3 |
| |
6. |
|
|
Grantor trust filing under Optional Form 1099 Filing Method 1 (see Regulations section 1.671-4(b)(2)(i)(A)) |
|
The grantor* |
| For this type of account: |
|
Give name and EIN of: |
| |
7. |
|
|
Disregarded entity not owned by an individual |
|
The owner |
| |
8. |
|
|
A valid trust, estate, or pension trust |
|
Legal entity4 |
| |
9. |
|
|
Corporation or LLC electing corporate status on Form 8832 or Form 2553 |
|
The corporation |
| |
10. |
|
|
Association, club, religious, charitable, educational, or other tax-exempt organization |
|
The organization |
| |
11. |
|
|
Partnership or multi-member LLC |
|
The partnership |
| |
12. |
|
|
A broker or registered nominee |
|
The broker or nominee |
| |
13. |
|
|
Account with the Department of Agriculture in the name of a public entity (such as a state or local government, school district, or prison) that receives agricultural program payments |
|
The public entity |
| |
14. |
|
|
Grantor trust filing under the Form 1041 Filing Method or the Optional Form 1099 Filing Method 2 (see Regulations section 1.671-4(b)(2)(i)(B)) |
|
The trust |
| 1 |
List first and circle the name of the person whose number you furnish. If only one person on a joint account has an SSN, that person’s number must be furnished. |
| 2 |
Circle the minor’s name and furnish the minor’s SSN. |
| 3 |
You must show your individual name and you may also enter your business or DBA name on the “Business name/disregarded entity” name line. You may use either your SSN or EIN (if you have one), but the IRS
encourages you to use your SSN. |
| 4 |
List first and circle the name of the trust, estate, or pension trust. (Do not furnish the TIN of the personal representative or trustee unless the legal entity itself is not designated in the account title.)
Also see Special rules for partnerships on page 2. |
| * |
Note. Grantor also must provide a Form W-9 to trustee of trust. |
Note. If no name is circled when more than
one name is listed, the number will be considered to be that of the first name listed.
Secure Your Tax Records from Identity Theft
Identity theft occurs when someone uses your personal information such as your name, SSN, or other identifying information, without your permission, to commit fraud or
other crimes. An identity thief may use your SSN to get a job or may file a tax return using your SSN to receive a refund.
To reduce your risk:
• Protect your SSN,
• Ensure your employer is protecting your SSN, and
• Be careful when choosing a tax preparer.
If your tax records are affected by identity theft and you receive a notice from the IRS, respond right away to the name and phone number printed on the
IRS notice or letter.
If your tax records are not currently affected by identity theft but you think you are at risk due to a lost or stolen purse or
wallet, questionable credit card activity or credit report, contact the IRS Identity Theft Hotline at 1-800-908-4490 or submit Form 14039.
For more
information, see Publication 4535, Identity Theft Prevention and Victim Assistance.
Victims of identity theft who are experiencing economic harm or a
system problem, or are seeking help in resolving tax problems that have not been resolved through normal channels, may be eligible for Taxpayer Advocate Service (TAS) assistance. You can reach TAS by calling the TAS toll-free case intake line at
1-877-777-4778 or TTY/TDD 1-800-829-4059.
Protect yourself from suspicious emails or phishing schemes. Phishing is the creation and use of email and websites
designed to mimic legitimate business emails and websites. The most common act is sending an email to a user falsely claiming to be an established legitimate enterprise in an attempt to scam the user into surrendering private information that will
be used for identity theft.
The IRS does not initiate contacts with taxpayers via emails. Also, the IRS does not request personal detailed
information through email or ask taxpayers for the PIN numbers, passwords, or similar secret access information for their credit card, bank, or other financial accounts.
If you receive an unsolicited email claiming to be from the IRS, forward this message to
phishing@irs.gov. You may also report misuse of the IRS name, logo, or other IRS property to the Treasury Inspector General for
Tax Administration (TIGTA) at 1-800-366-4484. You can forward suspicious emails to the Federal Trade Commission at:spam@uce.gov or contact them at
www.ftc.gov/idtheft or 1-877-IDTHEFT (1-877-438-4338).
Visit IRS.gov to learn more about identity theft and how to reduce your risk.
Privacy Act Notice
Section 6109 of the Internal Revenue Code requires you to provide your correct TIN to persons (including federal agencies) who are required to file information returns
with the IRS to report interest, dividends, or certain other income paid to you; mortgage interest you paid; the acquisition or abandonment of secured property; the cancellation of debt; or contributions you made to an IRA, Archer MSA, or HSA. The
person collecting this form uses the information on the form to file information returns with the IRS, reporting the above information. Routine uses of this information include giving it to the Department of Justice for civil and criminal litigation
and to cities, states, the District of Columbia, and U.S. commonwealths and possessions for use in administering their laws. The information also may be disclosed to other countries under a treaty, to federal and state agencies to enforce civil and
criminal laws, or to federal law enforcement and intelligence agencies to combat terrorism. You must provide your TIN whether or not you are required to file a tax return. Under section 3406, payers must generally withhold a percentage of taxable
interest, dividend, and certain other payments to a payee who does not give a TIN to the payer. Certain penalties may also apply for providing false or fraudulent information.
Questions and requests for assistance or for additional copies of the Tender Offer Document, the
Letter of Transmittal and other tender offer materials should be directed to the Information Agent for ADSs or the Depositary at their respective telephone numbers and locations listed herein. Additional copies of the foregoing materials will be
furnished promptly at the Offeror’s expense. You may also contact your broker, dealer, commercial bank, trust company or other nominee for assistance concerning the Tender Offer.
The Information Agent for ADSs for the Tender Offer is:

501 Madison Avenue, 20th Floor
New York, New York 10022
ADS
holders May Call Toll-Free: (888) 750-5834
Banks & Brokers May Call Collect: (212) 750-5833
|
|
|
|
|
| Voluntary Corporate Action: COY BTHB |
|
|
|
|
Exhibit (a)(1)(C)
|
|
|
|
|

|
|
COVER LETTER |
|
|
Dear customer
TENDER OFFER BY ACORDA THERAPEUTICS, INC. FOR ALL SHARES, AMERICAN DEPOSITARY SHARES AND OTHER EQUITY INTERESTS IN BIOTIE THERAPIES CORP.
Acorda Therapeutics, Inc. (the “Offeror”) is making a public tender offer in Finland and in the United States to purchase all of the issued
and outstanding shares (“Shares”), American Depositary Shares (“ADSs”), stock options (“Option Rights”), share units (“Share Rights”) and warrants (“Warrants”) in Biotie Therapies Corporation
(“Biotie” or the “Company”) that are not owned by Biotie or any of its subsidiaries (the “Tender Offer”).
The price
offered for each Share validly tendered in the Tender Offer will be EUR 0.2946 in cash.
The price offered for all 2011 Option Rights, the 2014
Option Rights in the 2014A tranche and all Warrants of the Company (the “Uncertificated Equity Instruments”), which are registered in the Finnish book-entry securities systems, is EUR 0.2846 in cash for each 2011 Option Right, EUR 0.2846
in cash for each 2014 option right and EUR 0.1664 in cash for each warrant.
The offer period will commence on March 11, 2016 at 9:30 a.m.
(Finnish time) and expire on April 8, 2016 at 4:00 p.m. (Finnish time) unless the offer period is extended under the terms and conditions of the Tender Offer (the end of the offer period, as extended, the “Expiration Date”).
The Offeror may extend or discontinue the extended offer period at any time in accordance with the terms and conditions of the Tender Offer. The Offeror also retains the right to initiate a subsequent offer period in accordance with the terms and
conditions of the Tender Offer in connection with the announcement of the final result of the Tender Offer.
The completion of the Tender Offer is
subject to the satisfaction of the conditions described in the tender offer document relating to the Tender Offer (the “Tender Offer Document”). The Offeror reserves the right to waive any conditions to completion of the Tender Offer.
Tender Offer acceptance procedure
If you
wish to accept the Tender Offer, please complete and sign the enclosed acceptance form duly and return it to the account operator managing your book-entry account so that it is received on or prior to the Expiration Date, subject to and in
accordance with the instructions of the relevant account operator.
The Tender Offer may be accepted unconditionally only and for each
book-entry account and for all Shares, Option Rights and Warrants included in the book-entry account at the time of execution of the Tender Offer. The amount of tendered Shares, Options Rights and Warrants shall be notified in the acceptance form
for each book-entry account separately. With respect to pledged Shares and Option Rights, the pledgee’s consent is required for the acceptance of the Tender Offer.
You will not incur any costs as a result of accepting the Tender Offer under its terms and conditions. The Offeror is liable for any asset transfer tax
on the transaction that may be collected in Finland. The possible asset transfer tax related to the tendering of the Option Rights and Warrants is described in more detail in the Tender Offer Document. Possible fees charged by account operators,
asset managers, nominees or any other person for registering the release of any pledges or other possible restrictions preventing a sale of the relevant Shares, Option Rights or Warrants will be borne by the holders of such equity interests
incurring such fees.
In case you accept the Tender Offer, a restriction on the right of disposal will be imposed on the Shares, Option Rights and
Warrants in your book-entry account. Following the end of the offer period or the extended offer period, this restriction will be removed and, if the Offeror has accepted the tendered securities for payment, the sale and purchase of the Shares,
Option Rights and Warrants offered for sale is expected to be executed on the fourth (4th) Finnish banking day following the end of the offer period, which will occur on or about April 14, 2016 unless the offer period is extended. The
price offered for the Shares and Option Rights is expected to be paid into your bank account on the second (2nd) Finnish banking day following the execution date, which will occur on or about April 18, 2016 unless the offer period is
extended, subject to the timetable for money transactions between financial institutions.
In the event of a subsequent offer period, the Offeror will announce the terms and conditions for the
settlement and payment for the validly tendered Shares, Option Rights and Warrants in connection with the announcement of such subsequent offer period. The payment for and acceptance of Shares, Option Rights and Warrants validly tendered during the
subsequent offer period will, however, take place on a periodic basis in intervals of approximately one (1) week and payment will be effected by the Offeror no later than five (5) banking days after the end of the relevant one week
interval.
If you buy additional Shares, Option Rights or Warrants after you have accepted the Tender Offer and after the registration of the
restriction on the right of disposal, you must separately accept the Tender Offer for such additional Shares, Option Rights and Warrants by sending a new completed acceptance form related to such additional Shares. In case you have accepted the
Tender Offer for the Shares and/or Options Rights and/or Warrants in your book-entry account, but you do not accept the Tender Offer separately for the Shares, Option Rights or Warrants subsequently purchased, your acceptance will only relate to the
Shares, Option Rights and Warrants that you had in your book-entry account when you accepted the Tender Offer for your Shares, Options Rights and Warrants for the first time.
Withdrawal right
You may withdraw your
acceptance at any time before the Expiration Date. The transfer restriction registered on the tendered Shares and Uncertificated Equity Instruments in the relevant book-entry account will be removed as soon as possible and within approximately three
(3) Finnish banking days following the receipt of a notice of withdrawal in accordance with the terms and conditions of the Tender Offer. Your account operator may charge a separate fee for such withdrawal. In the event of a subsequent offer
period, the acceptance of the Tender Offer will be binding and cannot be withdrawn, unless otherwise provided under mandatory Finnish and/or United States law.
Tender Offer Document and additional information on the Tender Offer
This Tender Offer Document will be available in Finnish from March 11, 2016 onwards at the branch offices of the cooperative banks belonging to the
OP Financial Group or Helsinki OP Bank Plc, at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki, Finland, and at the offices of the Offeror at Office of the Corporate Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on the internet at www.op.fi/merkinta,
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/sijoittajat and in English from March 11, 2016 onwards on the internet at
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/investors.
Yours sincerely
[Name of the Bank]
|
|
|
| <BANK NAME> |
|
ACCEPTANCE FORM (SHARES) |
|
|
| Name and postal address |
|
Address for return |
|
|
|
| «CO»
«NIMI»
«OSOITE»
«POSTINUMERO» «POSTITOIMIPAIKKA» |
|
BANK NAME DEPARTMENT
ADDRESS POST CODE |
| |
To reach the bank no later than [●] |
| Owner |
|
Bank account number
|
| Personal identity number or business identity code
|
|
Number of book-entry account |
PUBLIC TENDER OFFER BY ACORDA THERAPEUTICS, INC. FOR ALL SHARES, AMERICAN DEPOSITARY SHARES AND OTHER EQUITY INTERESTS
IN BIOTIE THERAPIES CORP.
Acorda Therapeutics, Inc. (the “Offeror”) is making a public tender offer in Finland and in the United
States to purchase all of the issued and outstanding shares (“Shares”), American Depositary Shares (“ADSs”), stock options (“Option Rights”), share units (“Share Rights”) and warrants (“Warrants”) in
Biotie Therapies Corp. (“Biotie” or the “Company”) that are not owned by Biotie or any of its subsidiaries (the “Tender Offer”). Capitalized terms used but not defined herein have the meanings given to them in the
Tender Offer document.
The price offered is EUR 0,2946 in cash for each Share of the Company.
The offer period will begin on March 11, 2016 at 9.30 a.m. (Finnish time) and end on April 8, 2016 at 4.00 p.m. (Finnish time), unless the
offer period is extended in accordance with the terms and conditions of the Tender Offer. The Tender Offer is conditional.
A shareholder may
only accept the Tender Offer unconditionally, and for all of the shares of the Company in the book-entry account specified above. The Offeror intends to execute the sale and purchase of the shares that are properly tendered in the Tender Offer by no
later than the fourth (4th) banking day after the offer period ends. The offer price for the shares will be paid into the bank account specified above on or about the second (2nd) banking day following the execution of the sale and
purchase.
Acceptance of the Tender Offer in respect of ADSs, Option Rights, Share Rights or Warrants in Biotie cannot be made by means of this
acceptance form. If you wish to tender your ADSs, Option Rights, Share Rights or Warrants, you must follow the procedures set forth in Section 4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer document.
This Tender Offer Document will be available in Finnish from March 11, 2016 onwards at the branch offices of the cooperative banks belonging to the
OP Financial Group or Helsinki OP Bank Plc, at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki, Finland, and at the offices of the Offeror at Office of the Corporate Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on the internet at
www.op.fi/merkinta, http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/sijoittajat and in English from
March 11, 2016 onwards on the internet at http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/investors.
The following section is
applicable only to U.S. Persons (as defined in the Tender Offer document):
APPLICABLE ONLY TO U.S. PERSONS
If you are a U.S. Person (as defined in the Tender Offer document), please read Section 4.13 of the
Tender Offer document carefully and return a completed IRS Form W-9, which can obtained at the IRS’s website at https://www.irs.gov/ to
Ernst & Young LLP electronically via email to melanie.mueller4@ey.com. IF YOU ARE A U.S. PERSON, FAILURE TO COMPLETE AND RETURN THE IRS
FORM W-9 MAY RESULT IN BACKUP WITHHOLDING OF A PORTION OF ANY PAYMENTS OTHERWISE PAYABLE TO YOU PURSUANT TO THE TENDER OFFER. Please see the instructions in Section 4.13 of the Tender Offer document and to the IRS Form W-9 for further
information.
THE FOLLOWING SHARES OF THE COMPANY WERE HELD IN BOOK-ENTRY ACCOUNT FOR THE OWNER AS OF [●], FOR WHICH THE OWNER ACCEPTS THE TENDER OFFER:
SHARES
2 (2)
AUTHORISATION
I have examined the terms and
conditions of the Tender Offer and the Tender Offer document, and I hereby approve said terms and conditions. I warrant that I have sufficient investment experience in order to understand the risks involved in the Tender Offer. I also warrant that I
am not resident or located in Australia, Canada, Hong Kong, Japan or South Africa or any other state or territory with statutory limitations on the right to make the Tender Offer, nor has this acceptance form been sent from such a state or
territory. I further warrant that my domicile or registered address is not in such a state or territory. The bank is not obliged to assess the suitability of the services or the securities for the client if the execution or brokerage takes place at
the client’s request, and the services are connected to simplified products set out in the Finnish Securities Market Act, including listed shares and related option rights.
I hereby authorise <Bank name> or its appointee to register a restriction on transfer of shares of the Company in my book-entry account referred to
above and to make all other necessary entries in the book-entry account on my behalf to complete and clear the sale, to sell all of the shares of the Company in the said book-entry account, and to credit the offer price to the bank account shown
above in accordance with the terms and conditions of the Tender Offer.
|
|
|
| Place and date |
|
Signature(s) 1) |
| Telephone number during banking
hours |
|
|
| Appendices (e.g. extract from
trade register, authorisation) 2) |
|
Clarification of names of signatories |
1) Signature(s) of parent(s), guardian(s) or trustee(s) of any
shareholder under 18 years of age to be included
2) An extract from the trade register issued no
earlier than three months before signing must be enclosed to the authorisation when the Tender Offer is accepted by a corporation.
The consent of all co-owners is
required in cases of joint ownership.
Shares that have been pledged may be tendered only with the written consent of the pledgee.
When accepting the Tender Offer please return this acceptance form, completed and signed, to <bank name> referred to above so that it reaches the said bank no
later than by [●].
Exhibit (a)(1)(D)
|
|
|
|
|

|
|
COVER LETTER |
|
|
Dear customer
TENDER OFFER BY
ACORDA THERAPEUTICS, INC. FOR ALL SHARES, AMERICAN DEPOSITARY SHARES AND OTHER EQUITY INTERESTS IN BIOTIE THERAPIES CORP.
Acorda Therapeutics,
Inc. (the “Offeror”) is making a public tender offer in Finland and in the United States to purchase all of the issued and outstanding shares (“Shares”), American Depositary Shares (“ADSs”), stock options (“Option
Rights”), share units (“Share Rights”) and warrants (“Warrants”) in Biotie Therapies Corporation (“Biotie” or the “Company”) that are not owned by Biotie or any of its subsidiaries (the “Tender
Offer”).
The price offered for each Share validly tendered in the Tender Offer will be EUR 0.2946 in cash.
The price offered for all 2011 Option Rights, the 2014 Option Rights in the 2014A tranche and all Warrants of the Company (the “Uncertificated
Equity Instruments”), which are registered in the Finnish book-entry securities systems, is EUR 0.2846 in cash for each 2011 Option Right, EUR 0.2846 in cash for each 2014 option right and EUR 0.1664 in cash for each warrant.
The offer period will commence on March 11, 2016 at 9:30 a.m. (Finnish time) and expire on April 8, 2016 at 4:00 p.m. (Finnish time) unless
the offer period is extended under the terms and conditions of the Tender Offer (the end of the offer period, as extended, the “Expiration Date”). The Offeror may extend or discontinue the extended offer period at any time in
accordance with the terms and conditions of the Tender Offer. The Offeror also retains the right to initiate a subsequent offer period in accordance with the terms and conditions of the Tender Offer in connection with the announcement of the final
result of the Tender Offer.
The completion of the Tender Offer is subject to the satisfaction of the conditions described in the tender offer
document relating to the Tender Offer (the “Tender Offer Document”). The Offeror reserves the right to waive any conditions to completion of the Tender Offer.
Tender Offer acceptance procedure
If you
wish to accept the Tender Offer, please complete and sign the enclosed acceptance form duly and return it to the account operator managing your book-entry account so that it is received on or prior to the Expiration Date, subject to and in
accordance with the instructions of the relevant account operator.
The Tender Offer may be accepted unconditionally only and for each
book-entry account and for all Shares, Option Rights and Warrants included in the book-entry account at the time of execution of the Tender Offer. The amount of tendered Shares, Options Rights and Warrants shall be notified in the acceptance form
for each book-entry account separately. With respect to pledged Shares and Option Rights, the pledgee’s consent is required for the acceptance of the Tender Offer.
You will not incur any costs as a result of accepting the Tender Offer under its terms and conditions. The Offeror is liable for any asset transfer tax
on the transaction that may be collected in Finland. The possible asset transfer tax related to the tendering of the Option Rights and Warrants is described in more detail in the Tender Offer Document. Possible fees charged by account operators,
asset managers, nominees or any other person for registering the release of any pledges or other possible restrictions preventing a sale of the relevant Shares, Option Rights or Warrants will be borne by the holders of such equity interests
incurring such fees.
In case you accept the Tender Offer, a restriction on the right of disposal will be imposed on the Shares, Option Rights and
Warrants in your book-entry account. Following the end of the offer period or the extended offer period, this restriction will be removed and, if the Offeror has accepted the tendered securities for payment, the sale and purchase of the Shares,
Option Rights and Warrants offered for sale is expected to be executed on the fourth (4th) Finnish banking day following the end of the offer period, which will occur on or about April 14, 2016 unless the offer period is extended. The
price offered for the Shares and Option Rights is expected to be paid into your bank account on the second (2nd) Finnish banking day following the execution date, which will occur on or about April 18, 2016 unless the offer period is
extended, subject to the timetable for money transactions between financial institutions.
In the event of a subsequent offer period, the Offeror will announce the terms and conditions for the
settlement and payment for the validly tendered Shares, Option Rights and Warrants in connection with the announcement of such subsequent offer period. The payment for and acceptance of Shares, Option Rights and Warrants validly tendered during the
subsequent offer period will, however, take place on a periodic basis in intervals of approximately one (1) week and payment will be effected by the Offeror no later than five (5) banking days after the end of the relevant one week
interval.
If you buy additional Shares, Option Rights or Warrants after you have accepted the Tender Offer and after the registration of the
restriction on the right of disposal, you must separately accept the Tender Offer for such additional Shares, Option Rights and Warrants by sending a new completed acceptance form related to such additional Shares. In case you have accepted the
Tender Offer for the Shares and/or Options Rights and/or Warrants in your book-entry account, but you do not accept the Tender Offer separately for the Shares, Option Rights or Warrants subsequently purchased, your acceptance will only relate to the
Shares, Option Rights and Warrants that you had in your book-entry account when you accepted the Tender Offer for your Shares, Options Rights and Warrants for the first time.
Withdrawal right
You may withdraw your acceptance at any
time before the Expiration Date. The transfer restriction registered on the tendered Shares and Uncertificated Equity Instruments in the relevant book-entry account will be removed as soon as possible and within approximately three (3) Finnish
banking days following the receipt of a notice of withdrawal in accordance with the terms and conditions of the Tender Offer. Your account operator may charge a separate fee for such withdrawal. In the event of a subsequent offer period, the
acceptance of the Tender Offer will be binding and cannot be withdrawn, unless otherwise provided under mandatory Finnish and/or United States law.
Tender Offer
Document and additional information on the Tender Offer
This Tender Offer Document will be available in Finnish from March 11, 2016
onwards at the branch offices of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Plc, at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki, Finland, and at the offices of the Offeror at Office of the Corporate
Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on the internet at www.op.fi/merkinta,
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/sijoittajat and in English from March 11, 2016 onwards on the internet at
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/investors.
Yours sincerely
[Name of the Bank]
|
|
|
| <BANK NAME> |
|
ACCEPTANCE FORM (UNCERTIFICATED EQUITY
INSTRUMENTS) |
|
|
|
| Name and postal address |
|
Address for return |
|
|
| «CO» «NIMI»
«OSOITE» «POSTINUMERO»
«POSTITOIMIPAIKKA» |
|
BANK NAME DEPARTMENT
ADDRESS POST CODE |
|
|
|
|
To reach the bank no later than [●] |
|
|
|
| Owner
|
|
Bank account number
|
| Personal identity number or business identity code
|
|
Number of book-entry
account |
PUBLIC TENDER OFFER BY ACORDA THERAPEUTICS, INC FOR ALL SHARES, AMERICAN DEPOSITARY SHARES AND OTHER EQUITY INTERESTS
IN BIOTIE THERAPIES CORP.
Acorda Therapeutics, Inc. (the “Offeror”) is making a public tender offer in Finland and in
the United States to purchase all of the issued and outstanding shares (“Shares”), American Depositary Shares (“ADSs”), stock options (“Option Rights”), share units (“Share Rights”) and warrants
(“Warrants”) in Biotie Therapies Corporation (“Biotie” or the “Company”) that are not owned by Biotie or any of its subsidiaries (the “Tender Offer”). Capitalized terms used but not defined herein have the
meanings given to them in the Tender Offer document.
The price offered for Uncertificated Equity Instruments (as defined in the
Tender Offer document) of the Company, which are registered in the Finnish book-entry securities systems, is EUR 0.2846 in cash for each 2011 Option Right and EUR 0.2846 in cash for each 2014 Option Right and EUR 0.1664 in cash for each
Warrant.
The offer period will begin on March 11 at 9.30 a.m. (Finnish time) and end on April 8 at 4.00 p.m.
(Finnish time), unless the offer period is extended in accordance with the terms and conditions of the Tender Offer. The Tender Offer is conditional.
A holder of Uncertificated Equity Instruments may only accept the Tender Offer unconditionally, and for all of the Uncertificated Equity
Instruments of the Company in the book-entry account specified above. The Offeror intends to execute the sale and purchase of the Uncertificated Equity Instruments that are properly tendered in the Tender Offer by no later than the fourth
(4th) business day after the offer period ends. The offer price for the Uncertificated Equity Instruments will be paid into the bank account specified above on or about the second (2nd) banking day following execution of the sale and
purchase.
Acceptance of the Tender Offer in respect of Shares, ADSs and Certificated Equity Instruments (as defined in the Tender
Offer Document) in Biotie cannot be made by means of this acceptance form. If you wish to tender your Shares, ADSs or Certificated Equity Instruments, you must follow the procedures set forth in Section 4.4—“Acceptance Procedure
of the Tender Offer” of the Tender Offer document.
This Tender Offer Document will be available in Finnish from March 11,
2016 onwards at the branch offices of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Plc, at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki, Finland, and at the offices of the Offeror at Office of the Corporate
Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on the internet at www.op.fi/merkinta, http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/sijoittajat and in English from March 11, 2016 onwards on the internet at
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/investors.
The following section is applicable only to U.S. Persons (as defined in the Tender Offer document):
|
| APPLICABLE ONLY TO U.S. PERSONS
If you are a U.S. Person (as defined in the Tender Offer document), please read
Section 4.13 of the Tender Offer document carefully and return a completed IRS Form W-9, which can obtained at the IRS’s website at https://www.irs.gov/ to Ernst & Young LLP electronically via
email to melanie.mueller4@ey.com. IF YOU ARE A U.S. PERSON, FAILURE TO COMPLETE AND RETURN THE IRS FORM W-9 MAY RESULT IN BACKUP WITHHOLDING OF A PORTION OF ANY PAYMENTS OTHERWISE PAYABLE TO YOU PURSUANT TO THE
TENDER OFFER. Please see the instructions in Section 4.13 of the Tender Offer document and to the IRS Form W-9 for further information. |
|
| THE FOLLOWING (SERIES) UNCERTIFICATED EQUITY INSTRUMENTS OF THE COMPANY WERE
HELD IN BOOK-ENTRY ACCOUNT FOR THE OWNER AS OF [●], FOR WHICH THE OWNER ACCEPTS THE TENDER OFFER:
|
2 (2)
AUTHORISATION
I have examined the terms and conditions of the Tender Offer and the Tender Offer Document, and I hereby approve said terms and conditions. I warrant that
I have sufficient investment experience in order to understand the risks involved in the Tender Offer. I also warrant that I am not resident or located in Australia, Canada, Hong Kong, Japan or South Africa or any other state or territory with
statutory limitations on the right to make the Tender Offer, nor has this acceptance form been sent from such a state or territory. I further warrant that my domicile or registered address is not in such a state or territory. The bank is not obliged
to assess the suitability of the services or the securities for the client if the execution or brokerage takes place at the client’s request, and the services are connected to simplified products set out in the Finnish Securities Market Act,
including listed shares and related option rights.
I hereby authorise <Bank name> or its appointee to register a restriction on transfer of
option rights of the Company in my book-entry account referred to above and to make all other necessary entries in the book-entry account on my behalf to complete and clear the sale, to sell all of the Uncertificated Equity Instruments of the
Company in the said book-entry account, and to credit the offer price to the bank account shown above in accordance with the terms and conditions of the Tender Offer.
|
|
|
| Place and date
|
|
Signature(s) 1) |
| Telephone number during banking hours
|
|
|
|
Appendices (e.g. extract from trade register, authorisation) 2)
|
|
Clarification of names of signatories
|
1) Signature(s) of parent(s), guardian(s) or trustee(s) of any holder of option
rights under 18 years of age to be included
2) An extract from the trade register issued no earlier than three
months before signing must be enclosed to the authorisation when the Tender Offer is accepted by a corporation.
The consent of all co-owners is required in cases of
joint ownership.
Uncertificated Equity Instruments that have been pledged may be tendered only with the written consent of the pledgee.
When accepting the Tender Offer please return this acceptance form, completed and signed, to <bank name> referred to above so that it reaches the said bank no
later than by [●].
Exhibit (a)(1)(E)
|
|
|
|

|
|
COVER LETTER |
Dear Holder of Certificated Equity Instruments
TENDER OFFER BY ACORDA THERAPEUTICS, INC. FOR ALL SHARES, AMERICAN DEPOSITARY SHARES AND OTHER EQUITY INTERESTS IN BIOTIE THERAPIES CORP.
Acorda Therapeutics, Inc. (the “Offeror”) is making a public tender offer in Finland and in the United States to purchase all of the issued
and outstanding shares (“Shares”), American Depositary Shares (“ADSs”), stock options (“Option Rights”), share units (“Share Rights”) and warrants (“Warrants”) in Biotie Therapies Corporation
(“Biotie” or the “Company”) that are not owned by Biotie or any of its subsidiaries (the “Tender Offer”). Capitalized terms used but not defined herein have the meanings given to them in the Tender Offer document.
The prices offered for the Certificated Equity Instruments (as defined in the Tender Offer document) of the Company, which are not registered in the
Finnish book-entry securities systems and which comprise the 2014 Option Rights in the 2014B, 2014C, 2014D and 2014M tranches, all 2016 Option Rights, all Swiss Option Rights, all 2011 Share Rights and all 2014 Share Rights and, are as follows:
|
|
|
| i. |
|
EUR 0.2846 in cash for each 2014 Option Right, |
| ii. |
|
EUR 0.1326 in cash for each 2016 Option Right payable, at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment
date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date, |
| iii. |
|
EUR 0.2032 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.10, |
| iv. |
|
EUR 0.1026 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.21, |
| v. |
|
EUR 0.0386 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.28, |
| vi. |
|
EUR 0.0112 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.31, |
| vii. |
|
EUR 0.0100 in cash for each other Swiss Option Right, |
| viii. |
|
EUR 0.2946 in cash for each 2011 Share Right, payable, at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment
date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date, and |
| ix. |
|
EUR 0.2854 in cash for each 2014 Share Right, payable, at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment
date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date. |
The offer period will commence on March 11, 2016 at 9:30 a.m. (Finnish time) and expire on April 8, 2016 at 4:00
p.m. (Finnish time) unless the offer period is extended under the terms and conditions of the Tender Offer (the end of the offer period, as extended, the “Expiration Date”). The Offeror may extend or discontinue the extended offer period
at any time in accordance with the terms and conditions of the Tender Offer. The Offeror also retains the right to initiate a subsequent offer period in accordance with the terms and conditions of the Tender Offer in connection with the announcement
of the final result of the Tender Offer.
The completion of the Tender Offer is subject to the satisfaction of the conditions described in the
tender offer document relating to the Tender Offer (the “Tender Offer Document”). The Offeror reserves the right to waive any conditions to completion of the Tender Offer.
Tender Offer acceptance procedure
If you
wish to accept the Tender Offer, please return the acceptance form completed and signed together with a copy of your passport, which is used for identifying purposes, to Pohjola Bank plc either:
| |
1. |
In the response envelope attached to the delivery package of the acceptance form so that it is received on or prior to the Expiration Date; or
|
| |
2. |
Via email to Pohjola Bank plc to the following email address: juho.hallenberg@pohjola.fi so that it is received on or
prior to the Expiration Date. |
If you choose to send the acceptance form and the copy of your passport via email, please attach the
duly completed and signed acceptance form and the copy of your passport in a commonly used electronic form (.pdf, .jpg or similar))
If you
are a U.S. Person (as defined in the Tender Offer document), please complete and return an IRS Form W-9 by following the instructions contained in the acceptance form.
TO ENSURE YOUR ACCEPTANCE IS TIMELY RECEIVED, WE ENCOURAGE YOU TO SUBMIT THE ACCEPTANCE USING THE ELECTRONIC ACCEPTANCE PROCEDURES.
A holder of Certificated Equity Instruments may only accept the Tender Offer unconditionally, and for all of the Certificated Equity Instruments of the
Company. The amount of tendered Certificated Equity Instruments shall be notified in the acceptance form for each Certificated Equity Instrument separately. If a holder’s Certificated Equity Instruments are subject to a pledge or other transfer
restriction that would prohibit the transfer of such Certificated Equity Instruments to the Offeror, such holder must obtain consent to transfer the Certificated Equity Instruments in the Tender Offer prior to delivering their acceptance of the
Tender Offer.
You will not incur any costs as a result of accepting the Tender Offer under its terms and conditions. The Offeror is liable for any
asset transfer tax on the transaction that may be collected in Finland. The possible asset transfer tax related to the tendering of the Certificated Equity Instruments is described in more detail in the Tender Offer Document. Possible fees charged
by account operators, asset managers, nominees or any other person for registering the release of any pledges or other possible restrictions preventing a sale of the relevant Certificated Equity Interests will be borne by the holders of such equity
interests incurring such fees.
In case you accept the Tender Offer and, if the Offeror has accepted the tendered securities for payment, the sale
and purchase of the Certificated Equity Instruments offered for sale is expected to be executed on or about the sixth (6th) Finnish banking day (the “Closing Date”) following the end of the offer period, which will occur on or about
April 18, 2016 unless the offer period is extended. The Offeror will make the payment of the applicable Offer Price for the relevant Certificated Equity Instruments on such Closing Date. Actual time of receipt for the payment depends on the
schedules of money transactions between financial institutions and agreements between the shareholder and account operator or nominee in each case.
In the event of a subsequent offer period, the Offeror will announce the terms and conditions for the settlement and payment for the validly tendered
Certificated Equity Interests in connection with the announcement of such subsequent offer period. The payment for and acceptance of Certificated Equity Interests validly tendered during the subsequent offer period will, however, take place on
a periodic basis in intervals of approximately one (1) week and payment will be effected by the Offeror no later than five (5) banking days after the end of the relevant one week interval.
Withdrawal right
You may withdraw your
acceptance at any time on or prior to the Expiration Date by sending a notification of the withdrawal in writing to Pohjola Bank plc to the following address:
Pohjola Bank plc
Attn. Juho Hallenberg
Teollisuuskatu 1
FI-00510 Helsinki
Finland
To be effective, such
notification must be received by Pohjola Bank plc on or prior to the Expiration Date.
In the event of a subsequent offer period, the acceptance of
the Tender Offer will be binding and cannot be withdrawn, unless otherwise provided under mandatory Finnish and/or United States law.
Tender
Offer Document and additional information on the Tender Offer
Please read the terms and conditions of the Tender Offer set forth in the Tender
Offer Document. This Tender Offer Document will be available in Finnish from March 11, 2016 onwards at the branch offices of the cooperative
banks belonging to the OP Financial Group or Helsinki OP Bank Plc, at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki, Finland, and at the offices of the Offeror at Office of the Corporate
Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on the internet at www.op.fi/merkinta,
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/sijoittajat and in English from March 11, 2016 onwards on the internet at
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/investors.
Yours sincerely
Pohjola Bank
|
|
|
| POHJOLA BANK PLC |
|
ACCEPTANCE FORM (CERTIFICATED EQUITY INSTRUMENTS)
|
| Name and postal address |
|
Address for return |
|
|
|
| «CO» «NIMI»
«OSOITE» «POSTINUMERO»
«POSTITOIMIPAIKKA» |
|
BANK NAME DEPARTMENT
ADDRESS POST CODE
|
| |
To reach the bank no later than April 8, 2016 |
|
|
|
| Beneficiary’s full name
|
|
Beneficiary’s full address |
| Bank account number (in IBAN form if residence in Europe)
|
|
Bank identifier code (“BIC code”), or clearing code |
| Beneficiary’s bank
|
|
Address of beneficiary’s bank |
| Personal identity number (Finnish citizen), or date of birth (for a
non-Finnish citizen) |
PUBLIC TENDER OFFER BY ACORDA THERAPEUTICS, INC. FOR ALL SHARES, AMERICAN DEPOSITARY SHARES
AND OTHER EQUITY INTERESTS IN BIOTIE THERAPIES CORP.
Acorda Therapeutics, Inc. (the “Offeror”) is making a public tender offer in Finland and in the United States to purchase all of the issued and
outstanding shares (“Shares”), American Depositary Shares (“ADSs”), stock options (“Option Rights”), share units (“Share Rights”) and warrants (“Warrants”) in Biotie Therapies Corp.
(“Biotie” or the “Company”) that are not owned by Biotie or any of its subsidiaries (the “Tender Offer”). Capitalized terms used but not defined herein have the meanings given to them in the Tender Offer document.
The prices offered for Certificated Equity Instruments of the Company, which are not registered in the Finnish book-entry securities systems and which
comprise the 2014 Option Rights in the 2014B, 2014C, 2014D and 2014M tranches, all 2016 Option Rights, all Swiss Option Rights, all 2011 Share Rights and all 2014 Share Rights and, are as follows:
|
|
|
| i. |
|
EUR 0.2846 in cash for each 2014 Option Right, |
| ii. |
|
EUR 0.1326 in cash for each 2016 Option Right payable, at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment
date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date, |
| iii. |
|
EUR 0.2032 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.10, |
| iv. |
|
EUR 0.1026 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.21, |
| v. |
|
EUR 0.0386 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.28, |
| vi. |
|
EUR 0.0112 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.31, |
| vii. |
|
EUR 0.0100 in cash for each other Swiss Option Right, |
| viii. |
|
EUR 0.2946 in cash for each 2011 Share Right payable, at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment
date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date, and |
| ix. |
|
EUR 0.2854 in cash for each 2014 Share Right payable at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment
date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date. |
The offer period will begin on March 11 at 9.30 a.m. (Finnish time) and end on April 8 at 4.00 p.m. (Finnish time),
unless the offer period is extended in accordance with the terms and conditions of the Tender Offer. The Tender Offer is conditional.
A holder
of Certificated Equity Instruments may only accept the Tender Offer unconditionally, and for all of the Certificated Equity Instruments of the Company specified above. The Offeror intends to execute the sale and purchase of the Certificated Equity
Instruments, and the Closing Date for the Certificated Equity Instruments with respect to the Offer Period will occur, on or about the sixth (6th) Finnish banking day following the Expiration Date. The Offeror will make the payment of the applicable
Offer Price for the relevant Certificated Equity Instruments on such Closing Date. Actual time of receipt of payment depends on the schedules of money transactions between financial institutions and agreements between the shareholder and account
operator or nominee in each case.
Acceptance of the Tender Offer in respect of shares, ADSs and Uncertificated Equity Instruments in Biotie
cannot be made by means of this acceptance form. If you wish to tender your Shares, ADSs or Uncertificated Equity Instruments, you must follow the procedures set forth in Section 4.4—“Acceptance Procedure of the Tender Offer” of
the Tender Offer document.
This Tender Offer Document will be available in Finnish from March 11, 2016 onwards at the branch offices of the
cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Plc, at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki, Finland, and at the offices of the Offeror at Office of the Corporate
Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on the internet at www.op.fi/merkinta, http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/sijoittajat and in English from March 11, 2016 onwards on the internet at
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and
www.biotie.com/investors.
2 (3)
The following section is applicable only to U.S. Persons (as defined in the Tender Offer document):
APPLICABLE ONLY TO U.S. PERSONS
If you are a U.S. Person (as defined in the Tender Offer document), please read Section 4.13 of the Tender Offer document carefully
and return a completed IRS Form W-9, which can obtained at the IRS’s website at https://www.irs.gov/ to Ernst & Young LLP electronically via email
to melanie.mueller4@ey.com. IF YOU ARE A U.S. PERSON, FAILURE TO COMPLETE AND RETURN THE IRS FORM W-9 MAY RESULT IN BACKUP WITHHOLDING OF A
PORTION OF ANY PAYMENTS OTHERWISE PAYABLE TO YOU PURSUANT TO THE TENDER OFFER. Please see the instructions in Section 4.13 of the Tender Offer document and to the IRS Form W-9 for further information.
THE FOLLOWING SERIES OF CERTIFICATED EQUITY INSTRUMENTS OF THE COMPANY WERE REGISTERED UNDER YOUR NAME AS THE HOLDER OF SUCH
CERTIFICATED EQUITY INSTRUMENTS IN THE REGISTER OF THE COMPANY AS OF MARCH 10, 2016, FOR WHICH YOU AS THE HOLDER OF SUCH CERTIFICATED EQUITY INSTRUMENTS HEREBY ACCEPT THE TENDER OFFER:
|
|
|
|
|
| CERTIFICATED EQUITY INSTRUMENT |
|
AMOUNT OF CERTIFICATED EQUITY INSTRUMENTS IN THE COMPANY’S REGISTER |
|
ACCEPTANCE (X =
“YES”, BLANK = “NO”) |
| [OPTION RIGHT SCHEDULE] |
|
[AMOUNT] |
|
[X] |
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
IDENTIFICATION OF A HOLDER OF CERTIFICATED
EQUITY INSTRUMENTS
In order to identify you as a legitimate holder of Certificated Equity Instruments specified above, you must provide Pohjola Bank plc with a
copy of your passport.
Please send a copy of your passport together with this acceptance form in the response envelope or, alternatively, via email to Pohjola Bank
plc to the following email address: juho.hallenberg@pohjola.fi. If you choose to send the acceptance form and the copy of your passport via email, please
enclose the completed and signed acceptance form and the copy of your passport as attachments in a commonly used electronic form (.pdf, .jpg or similar).
An
acceptance form received without a copy of your passport does not qualify you as having accepted the Tender Offer and tendered your Certificated Equity Instruments.
OPTION TO RECEIVE PAYMENT IN U.S. DOLLARS
Pursuant to Section 4.1 of the Tender
Offer document, the offer price payable to holders of 2016 Option Rights, 2011 Share Rights and 2014 Share Rights will, at the option of the holder, be payable in euros or the equivalent amount of U.S. dollars determined based on the U.S. dollar
spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date.
If you hold 2016 Option Rights, 2011 Share Rights or 2014
Share Rights, check the box below to indicate your election to receive the offer price in U.S. dollars, determined as described in the previous paragraph:
¨ YES, I would like to receive payment for my 2016 Option Rights, 2011 Share Rights and 2014 Share Rights in U.S. dollars as described above.
Unless you choose to receive the offer price in U.S. dollars, the offer price will be paid to you in euros.
AUTHORISATION
I have examined the terms and
conditions of the Tender Offer and the Tender Offer document, and I hereby approve said terms and conditions. I warrant that I have sufficient investment experience in order to understand the risks involved in the Tender Offer. I also warrant that I
am not resident or located in Australia, Canada, Hong Kong, Japan or South Africa or any other state or territory with statutory limitations on the right to make the Tender Offer, nor has this acceptance form been sent from such a state or
territory. I further warrant that my domicile or registered address is not in such a state or territory. The bank is not obliged to assess the suitability of the services or the securities for the client if the execution or brokerage takes place at
the client’s request, and the services are connected to simplified products set out in the Finnish Securities Market Act, including listed shares and related option rights.
3 (3)
I hereby authorise Pohjola Bank plc or its appointee to take all necessary actions on my behalf to
complete and clear the sale, to sell all of the Certificated Equity Instruments registered under my name in the register of the Company, and to credit the offer price to the bank account shown above in accordance with the terms and conditions of the
Tender Offer.
|
|
|
| Place and date
|
|
Signature(s)
1) |
| Telephone number during banking
hours |
|
|
| Appendices (e.g. extract from trade register, authorisation) 2) |
|
Clarification of names of signatories
|
1) Signature(s) of parent(s), guardian(s) or trustee(s) of any holder of option
rights under 18 years of age to be included
2) An extract from the trade register issued no earlier than three
months before signing must be enclosed to the authorisation when the Tender Offer is accepted by a corporation.
The consent of all co-owners is required in cases of
joint ownership.
Certificated Equity Instruments that have been pledged may be tendered only with the written consent of the pledgee.
When accepting the Tender Offer please return this acceptance form completed and signed together with a copy of your passport, which is used for
identifying purposes, to Pohjola Bank plc either:
i) In the response envelope attached to the delivery package of this acceptance form, or
ii) Via email to Pohjola Bank plc to the following email address:
juho.hallenberg@pohjola.fi (if you choose to send the acceptance form and the copy of your passport via email, please attach the duly completed and signed
acceptance form and the copy of your passport in a commonly used electronic form (.pdf, .jpg or similar))
PLEASE MAKE SURE THAT YOUR DULY COMPLETED AND
SIGNED ACCEPTANCE FORM AND THE COPY OF YOUR PASSPORT REACH POHJOLA BANK PLC NO LATER THAN BY MARCH 8, 2016.
TO ENSURE YOUR ACCEPTANCE IS TIMELY RECEIVED, WE
ENCOURAGE YOU TO SUBMIT THE ACCEPTANCE USING THE ELECTRONIC ACCEPTANCE PROCEDURES
Exhibit (a)(1)(F)

ACORDA’S CASH TENDER OFFER FOR ALL SHARES, ADSs AND OTHER EQUITY INSTRUMENTS IN BIOTIE
Marketing brochure. This is not a tender offer document.1 The Tender Offer Document is available at
op.fi/merkinta, ir.acorda.com/investors/Biotie-Therapies-Tender-Offer and biotie.com/investors.
RATIONALE OF THE TENDER OFFER

ACORDA (THE OFFEROR) AND BIOTIE have entered into the Combination Agreement on
January 19, 2016, whereby Acorda has made a Tender Offer on March 11, 2016 for all the Equity Interests in Biotie. The Offer Period has started on March 11, 2016.
Acorda’s strategy is to build a leading neurology company. Accordingly, Acorda is searching for opportunities to complement its
current neurology product pipeline and portfolio via acquisitions and in-licensing opportunities. Acorda and Biotie are both focused on improving the lives of people with neurological disorders, Acorda with an industry leading pipeline of novel
therapies addressing a range of neurological disorders including Parkinson’s disease, and Biotie, developing therapeutics for central nervous system disorders also including Parkinson’s disease. The products of Biotie complement
Acorda’s pipeline and its expertise in developing and commercializing neurology therapies.
Acorda believes that the
business combination will benefit from Acorda’s capitalization and expertise in neurology development and commercialization. The combined entity:
| ¢ |
|
has the potential to become a leader in Parkinson’s disease therapeutic development based on the complementary nature of the product portfolios of both companies, and |
| ¢ |
|
will create an opportunity for significant revenue synergies across complementary products via combination of Biotie’s offerings with Acorda’s existing products and pipeline
|
We believe that the combination of Biotie’s offerings with Acorda’s existing products, pipeline,
infrastructure and capitalization will create an opportunity for significant value creation. Through this acquisition, Acorda is positioned to become a leader in developing novel therapies for Parkinson’s disease.
The Tender Offer and any Subsequent Compulsory Redemption will have no material near term effect on the operations and business locations
of, or on employment at Biotie. Acorda intends to keep Biotie’s South San Francisco open. In the near term, the Turku office will also remain open but the Offeror will consider the longer term operating plan and scope of the Turku operations
following the completion of the Tender Offer. ¢
1 Capitalized terms used herein, or the
letter from Acorda’s CEO to shareholders that were enclosed herewith,
but not defined herein, or therein, have the meanings
given to them in the Tender Offer Document.
2

ACCEPTING THE
TENDER OFFER
/ HOW TO ACT
|
|
|
| THE OFFER PERIOD OF THE TENDER OFFER FOR ALL SHARES, ADSS AND OTHER EQUITY INTERESTS IN BIOTIE STARTED ON MARCH 11, 2016. THE ACCEPTANCE FORM AND INSTRUCTIONS WILL BE
PROVIDED BY MOST FINNISH ACCOUNT OPERATORS AND CUSTODIAN BANKS SEPARATELY FROM THIS MARKETING BROCHURE TO THEIR CUSTOMERS WHO ARE REGISTERED AS BIOTIE SHAREHOLDERS. IF YOU DO NOT RECEIVE THE ACCEPTANCE FORM FROM YOUR ACCOUNT OPERATOR OR CUSTODIAN
BANK YOU CAN CONTACT ANY BRANCH OFFICE OF THE COOPERATIVE BANKS BELONGING TO THE OP FINANCIAL GROUP OR HELSINKI OP BANK LTD WHERE YOU WILL RECEIVE NECESSARY INFORMATION AND CAN GIVE YOUR ACCEPTANCE. |
|

|
INSTRUCTIONS TO ACCEPT THE TENDER OFFER
A Biotie shareholder who wishes to accept the Tender Offer must submit an Acceptance Form
| ¢ |
|
to the account operator managing the shareholder’s book-entry account, or |
| ¢ |
|
any branch office of the cooperative bank belonging to the OP Financial Group or Helsinki OP Bank Ltd |
at the latest on the expiration date of the tender offer or, if the offer period is extended, during the tender offer, but always in accordance with the
instructions and within the time limit set by the account operator. Holders of equity interests may only accept the tender offer unconditionally.
A shareholder that has validly accepted the Tender Offer and that has not properly withdrawn its acceptance in accordance with the terms and conditions of the Tender Offer may not sell or otherwise dispose of its tendered Shares. A
transfer restriction in respect of the Shares will be registered in the relevant book-entry account after a shareholder has submitted the acceptance for the Tender Offer. If the Tender Offer is not completed or if the tender is properly withdrawn by
the shareholder in accordance with the terms and conditions of the Tender Offer, the transfer restriction registered on the tendered Shares in the relevant book-entry account will be removed.
WITHDRAWAL RIGHTS
The Shares may be withdrawn at any time on or before the expiration date of the Offer Period, as the same may be extended.
The proper withdrawal of Shares requires that a written notice of withdrawal is submitted to the same account operator to whom the
Acceptance Form for such Shares was submitted. If the Acceptance Form with respect to such Shares was submitted to a branch office of a cooperative bank belonging to the OP Financial Group or Helsinki OP Bank Ltd, the notice of withdrawal must be
submitted to the same branch office. ¢

 3
3
ABOUT ACORDA
ACORDA IS A BIOTECHNOLOGY COMPANY focused on developing therapies that restore
function and improve the lives of people with neurological disorders. It has an industry leading pipeline of novel neurological therapies addressing a range of disorders including Parkinson’s disease, epilepsy, post-stroke walking deficits,
migraine, and multiple sclerosis. Acorda markets three FDA approved therapies, including Ampyra Extended Release Tablets.
Acorda
was incorporated in 1995 and has its common stock listed on the NASDAQ Global Market (NASDAQ: ACOR). ¢

|
|
How much will I receive for my Shares?
|
The offer price will be EUR 0.2946 per Share in cash.
|
| Is the
price offered for Biotie Shares good? |
The offer price represents a premium of approximately 95 percent compared to the closing price of the Biotie
Shares on Nasdaq Helsinki on January 18, 2016, the last trading day on Nasdaq Helsinki preceding the announcement of the Tender Offer and a premium of approximately 87 percent compared to the 3 month volume-weighted average trading price on
Nasdaq Helsinki prior to such announcement.
On March 4, 2016, having evaluated the terms and conditions of the Tender Offer
from the point of view of Biotie and the holders of its Equity Interests and other available information, the Board of Directors of has decided to recommend the holders of Equity Interests to accept the Tender Offer.
In connection with such evaluation, the Board of Directors of Biotie considered numerous factors, including the opinion of Guggenheim
Securities, LLC, dated January 19, 2016, to the Board of Directors as to the fairness, from a financial point of view and as of such date, of the Share Offer Price to be received in the Tender Offer by the holders of Shares and ADSs (other than
Acorda and its affiliates), which opinion was based on the matters considered, the procedures followed, the assumptions made and various limitations of and qualifications to the review undertaken as more fully described therein.
|
| How do
I accept the offer? |
You shall submit a properly completed and duly executed Acceptance Form to the account operator managing
your book-entry account in accordance with its instructions and within the time limit set by your account operator. If your account operator does not accept Acceptance Forms (e.g. Euroclear), you shall contact any branch office of the cooperative
banks belonging to the OP Financial Group or Helsinki OP Bank Ltd to give your acceptance to tender the Shares.
4


Q&A
Have any of the shareholders committed to accept
the Tender Offer? Shareholders representing in total approximately 65 percent (on a fully diluted basis) of the outstanding Shares and votes in Biotie have irrevocably undertaken to accept the Tender Offer. This includes all holders of Biotie
warrants and members of the senior management team of Biotie, who have, subject to certain customary conditions, irrevocably undertaken to tender their Equity Interests into the Tender Offer.
What happens if I don’t accept the offer?
If the Offeror receives more than 90 percent of
Biotie’s Shares and votes, the Offeror can initiate compulsory redemption proceeding in order to gain full ownership of all of Biotie’s Shares. If a compulsory redemption proceeding is initiated and you have not accepted the Tender Offer,
you may have to wait for several months before you receive the cash payment from your Shares.
When do I get paid from my Shares?
If you accept the Tender Offer during the acceptance period, the cash payment for your Shares will be made to your account approximately on April 18, 2016 assuming that the Offer
Period is not extended and the Offeror has resolved to complete the Tender Offer.
I didn’t receive the Acceptance Form.
How should I act?
You can request the Acceptance Form from your account operator, custodian bank or
any branch office of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Ltd.
How do I accept the Tender Offer if I don’t have an
account operator or custodian bank?
You can visit any branch office of the cooperative banks belonging to the OP Financial Group or Helsinki OP Bank Plc, from whom
you will receive all the necessary information and guidance on how to tender your Shares.Can I cancel my acceptance and if so how?
Tendered Shares may be withdrawn at
any time on or before the expiration date of the Offer Period, as the same may be extended.
The proper withdrawal of Shares requires that a written notice of
withdrawal is submitted to the same account operator to whom the Acceptance Form for such Shares was submitted. If the Acceptance Form with respect to such Shares was submitted to a branch office of a cooperative bank belonging to the OP Financial
Group or Helsinki OP Bank Ltd, the notice of withdrawal must be submitted to the same branch office.
Does accepting the Tender Offer cost me anything?
There is no cost to the holders of Shares associated with accepting the Tender Offer according to the terms and conditions.
5
CERTAIN CONDITIONS OF THE
TENDER
OFFER
This marketing brochure presents a summary of Acorda’s Tender Offer for Biotie Shares and other Equity Interests and certain main
terms and conditions of the Tender Offer. The terms and conditions of the Tender Offer are presented thoroughly in the Tender Offer Document (“Tender Offer Document”). Please read the Tender Offer Document carefully when deciding whether
to accept the offer.
OFFER PRICE
The offer price for each outstanding Share is EUR 0.2946 in cash. The offer price represents a premium of
| ¢ |
|
approximately 95 per cent compared to the closing price of the Biotie shares
|
| |
|
on Nasdaq Helsinki Ltd. (“Nasdaq Helsinki”) on 18 January 2016, the last trading day on Nasdaq Helsinki preceding the announcement of the Combination Agreement.
|
| ¢ |
|
approximately 87 per cent compared to the 3 month volume-weighted average trading price on Nasdaq Helsinki prior to such announcement. |
The offer price for each outstanding ADS is EUR 23.5680 in cash
| ¢ |
|
The offer price represents a premium of approximately 94 percent compared to the closing price of the Biotie ADSs on the Nasdaq Stock Market LLC on January 15, 2016, the last trading day on
the Nasdaq Global Select Market preceding the announcement of the Tender Offer. |
Acorda will also offer to acquire
all of the outstanding Option Rights, Share Units and Warrants issued by Biotie. The more detailed offer prices related to the outstanding Option Rights, Share Units and Warrants can be found in the Tender Offer Document under the section “4.1
Terms of the tender offer”.
OFFER PERIOD
The Offer Period commences on March 11, 2016 at 9:30 a.m. (Finnish time) / 2:30 a.m. (New York Time) and expires on April 8, 2016 at
4:00 p.m. (Finnish time) / 9:00 a.m. (New York Time). The Offeror reserves the right to continue the Offer Period according to terms and conditions in the Tender Offer Document.
CONDITIONS TO COMPLETION OF THE TENDER OFFER
The obligation of the Offeror to accept for payment the Equity Interests validly tendered and not withdrawn during the Offer Period will be
subject to the fulfilment or, to the extent permitted by applicable law, waiver by the Offeror of the following conditions on or prior to the date of the Offeror’s announcement of the preliminary result with respect to the Offer Period
| (a) |
the valid tender of outstanding Shares (including outstanding Shares represented by validly tendered ADSs and validly tendered Warrants) representing, together with any outstanding Shares
(including outstanding Shares represented by ADSs and Warrants) otherwise |

6

| |
acquired by the Offeror, more than 90 percent of the issued and outstanding Shares and voting rights of the Company, calculated on a fully diluted basis as set forth in the Tender Offer Document;
|
| (b) |
the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Act (as discussed in the Tender Offer Document, such waiting period expired on February 16, 2016);
|
| (c) |
no Material Adverse Effect (as defined in the Tender Offer Document) having occurred in the Company after January 19, 2016; |
| (d) |
the Offeror not, after January 19, 2016, having received information previously undisclosed to it that describes a Material Adverse Effect to the Company that occurred prior to
January 19, 2016; |
| (e) |
no information made public by the Company or disclosed by the Company to the Offeror being materially inaccurate, incomplete, or misleading, and the Company not having failed to make public any
information that should have been made public by it under applicable
|
| |
laws, including without limitation the rules of Nasdaq Helsinki and Nasdaq US, provided that, in each case, the information made public, disclosed or not disclosed or the failure to disclose
information constitutes a Material Adverse Effect to the Company; |
| (f) |
no court or regulatory authority of competent jurisdiction (including without limitation the FSA or the SEC) having given an order or issued any regulatory action preventing or enjoining the
completion of the Tender Offer; |
| (g) |
the Board of Directors of the Company having issued its recommendation for the Tender Offer and the recommendation remaining in force and not being modified or changed in a manner detrimental to
the Offeror; and |
| (h) |
the Combination Agreement not having been terminated and remaining in force and no event having occurred that, with the passage of time, would give the Offeror the right to terminate the
Combination Agreement under specified sections of the Combination Agreement that give the Offeror the right to terminate
|
| |
the Combination Agreement in response to a breach of the agreement by the Company. |
Fulfillment of the Conditions to Completion, including fulfillment of the Minimum Condition, will be determined on the next Finnish banking day after the
Expiration Date, based on the preliminary results with respect to the Offer Period then available. Such results may be subject to change based on a finalization count, which will be available on the third (3rd) Finnish banking day after the
Expiration Date. However, no such change will impact fulfillment of the Conditions to Completion.
In addition, the Tender Offer is not subject to any
financing condition. The Offeror has sufficient funds available to purchase all Equity Interests validly tendered in the Tender Offer and not validly withdrawn, and any Equity Interests purchased in a Subsequent Compulsory Redemption or otherwise,
in light of the Offeror’s financial capacity in relation to the amount of consideration payable.

 7
7

|
|
|
|
|
|
|
|
|
|
TENDER OFFFER DOCUMENT |
|
|
|
|
|
|
|
The Tender Offer Document is available starting from 11.3.2016 on the internet at op.fi/merkinta, ir.acorda.com/investors/ Biotie-Therapies-Tender-Offer and
biotie.com/investors as well as approximately on March 11, 2016 from Biotie group management and in person at OP Financial Group branch offices. |
|
|
|
|
|
|
|
ADDITIONAL QUESTIONS? |
|
|
|
|
|
|
|
If you wish to request additional information on the Tender Offer you can contact any OP Financial Group branch office or account operator. You may also obtain additional
information by calling the OP Call service at OP 0100 0500. |
|
|
|
|
|
|
|
FORWARD LOOKING STATEMENTS |
|
|
|
|
|
|
|
This marketing brochure includes “forward looking statements”, including statements about the expected timing and completion of the Tender Offer. All statements,
other than statements of historical facts, regarding management’s expectations, beliefs, goals, plans or prospects should be considered forward-looking. These statements are subject to risks and uncertainties that could cause actual results to
differ materially, including the occurrence of an event, change or other circumstance that could give rise to the termination of the Tender Offer and the risk that a condition to consummating the Tender Offer may not be satisfied. No assurance can
be given that such statements will be fulfilled or prove to be correct, and no representations are made as to the future accuracy and completeness of such statements. Forward-looking statements made in this document are made only as of the date
hereof, and Acorda undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information or otherwise, except as required by applicable laws. |
|
|
|
|
|
|
|
ADDITIONAL INFORMATION |
|
|
|
|
|
|
|
This communication is for informational purposes only and does not constitute a tender offer document nor an offer to purchase or a solicitation of an offer to sell
securities. Acorda has filed a tender offer document on Schedule TO with the SEC on March 11, 2016 (as from time to time amended and supplemented, the “Schedule TO”) and the Finnish Financial Supervisory Authority has approved the
tender offer document on March 10, 2016. The offer to purchase all equity interests of Biotie held by U.S. holders (within the meaning of Rule 14d-1(d) under the Securities Exchange Act of 1934, as amended, which defines a U.S. holder as
“any security holder resident in the United States”) is only being made pursuant to the offer to purchase/tender offer document, the related letter of transmittal and acceptance forms filed with the SEC by Acorda as a part of its Schedule
TO. Investors and security holders are urged to read the Schedule TO (including the offer to purchase/tender offer document, the related letter of transmittal and acceptance forms and other related materials), as it may be amended from time to time,
because it contains important information about the Tender Offer, including its terms and conditions, and should be read carefully before any decision is made with respect to the Tender Offer. Investors and security holders may obtain free copies of
these statements and other materials filed with the SEC at the website maintained by the SEC at www.sec.gov, or by directing requests for such materials to the call service of OP Financial Group, at (+358) (0) 100 0500, Innisfree M&A
Incorporated, at (+1) 888 750 5834, or to us, at www. acorda.com or Office of the Corporate Secretary, 420 Saw Mill River Road, Ardsley, New York 10502. The tender offer document is available in Finnish also at www.op.fi/merkinta. |
|
|
|
|
|
|
|
THE TENDER OFFER WILL NOT BE MADE DIRECTLY OR INDIRECTLY IN ANY JURISDICTION WHERE EITHER AN OFFER OR PARTICIPATION THEREIN IS PROHIBITED BY APPLICABLE LAW OR WHERE ANY
TENDER OFFER DOCUMENT OR REGISTRATION OR OTHER REQUIREMENTS WOULD APPLY IN ADDITION TO THOSE UNDERTAKEN IN FINLAND AND THE UNITED STATES. |
|
|
Exhibit (a)(1)(G)

Dear Shareholder of Biotie Therapies,
On January 19, 2016, Acorda Therapeutics, Inc. and Biotie Therapies Corp. entered into a combination agreement pursuant to which Acorda is
making a public Tender Offer in Finland and in the United States to purchase all Shares, American Depositary Shares and other Equity Interests in Biotie.
|
|
|
|
|
| “We have carefully assessed the terms and conditions of
the Offer and believe that it is an attractive offer to shareholders that recognizes the strategic value of Biotie.” |
|
|
|
The price offered for each Share will be EUR 0.2946 in cash, representing a premium of approximately 95 per cent compared to the
closing price of the Biotie Shares on Nasdaq Helsinki on the last trading day on Nasdaq Helsinki preceding the announcement of the Combination Agreement and a premium of approximately 87 percent compared to the 3 month volume-weighted average
trading price on Nasdaq Helsinki prior to such announcement. |
| – William M. Burns,
Chairman of the Board of
Directors |
|
|
|
Acorda and Biotie are both focused on improving the lives of people with neurological disorders, Acorda with an industry leading pipeline of novel therapies addressing a range of neurological
disorders including Parkinson’s disease, and Biotie, developing therapeutics for central nervous system disorders also including Parkinson’s disease. The products of Biotie complement Acorda’s pipeline and its expertise in developing
and commercializing neurology therapies. We believe that the combination of Biotie’s offerings with Acorda’s existing products, pipeline, infrastructure and capitalization will create an opportunity for significant value creation. Through
this acquisition, Acorda is positioned to become a leader in developing novel therapies for neurological diseases, particularly Parkinson’s disease. |
The Board of Directors of Biotie by a unanimous decision recommends that the holders of
Biotie Shares accept the Tender Offer. To date, Biotie shareholders and holders of American Depository Shares and other Equity Interests representing in total approximately 65% of the outstanding Shares in Biotie (on a fully diluted basis) have
already irrevocably undertaken to accept the Tender Offer, subject to certain customary conditions.
The initial acceptance period for the
Tender Offer commenced on March 11, 2016 and it will expire on April 8, 2016, unless extended in accordance with the terms and conditions of the Tender Offer.
Please direct any questions regarding this transaction to Pohjola Bank, arranger of the Tender Offer.
Kind regards,

Ron Cohen, M.D.
President and CEO
Acorda
Therapeutics, Inc.
About Acorda Therapeutics
Founded in 1995, Acorda is a biotechnology company focused on developing therapies that restore function and improve the lives of people with
neurological disorders. Acorda has an industry leading pipeline of novel neurological therapies addressing a range of disorders, including Parkinson’s disease, epilepsy, post-stroke walking deficits, migraine, and multiple sclerosis. Acorda
markets three FDA-approved therapies, including AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg (marketed outside the United States as FAMPYRA). Acorda is listed on NASDAQ US under the symbol “ACOR.”
Exhibit (a)(1)(H)
|
|
|

|
|
INSTRUCTIONS TO ACCOUNT
OPERATORS |
| Translation only for information purposes |
|
|
TENDER OFFER BY ACORDA THERAPEUTICS, INC. FOR ALL SHARES, AMERICAN DEPOSITARY SHARES AND OTHER EQUITY INTERESTS IN BIOTIE
THERAPIES CORP.
Acorda Therapeutics, Inc. (the “Offeror”) is making a public tender
offer in Finland and in the United States to purchase all of the issued and outstanding shares, American Depositary Shares (“ADSs”), stock options, share units and warrants in Biotie Therapies Corp. (“Biotie”) that are not owned
by Biotie or any of its subsidiaries (the “Tender Offer”).
Pohjola Bank plc acts as the Arranger of the Tender Offer.
The following timetable sets forth certain key dates relating to the
Tender Offer, provided that the offer period has not been extended and the Offeror has accepted for payment the tendered Equity Interests in accordance with the terms and conditions of the Tender Offer:
|
|
|
|
|
| • |
|
Announcement of entry into the Combination Agreement |
|
January 19, 2016 |
| • |
|
Tender Offer Document published (online and in print) |
|
March 11, 2016 |
| • |
|
Offer period commences |
|
March 11, 2016 |
| • |
|
Offer period ends* |
|
April 8, 2016 |
| • |
|
Announcement of the preliminary result of the Tender Offer |
|
April 11, 2016 |
| • |
|
Announcement of the final result of the Tender Offer |
|
April 13, 2016 |
| • |
|
Sale and purchase of tendered equity interests |
|
April 14, 2016 |
| • |
|
Payment in cash for tendered equity interests** |
|
April 18, 2016 |
| * |
The Offeror reserves the right to extend the offer period. In such occasion the extension is published by press release. |
| ** |
Estimated date. Actual time of receipt for the payment will depend on the schedules of money transactions between financial institutions. |
| 3. |
Terms and conditions of the Tender Offer and the Offer Document |
The Tender Offer
Document, of which the FSA has approved on March 10, 2016 the Finnish language version but is not responsible for the accuracy of the information presented therein, sets forth the terms and conditions of the Tender Offer.
This Tender Offer Document will be available in Finnish from March 11, 2016 onwards at the branch offices of the cooperative banks
belonging to the OP Financial Group or Helsinki OP Bank Plc, at Nasdaq Helsinki, Fabianinkatu 14, FI-00100 Helsinki, Finland, and at the offices of the Offeror at Office of the Corporate Secretary, 420 Saw Mill River Road, Ardsley, NY, 10502 and on
the internet at www.op.fi/merkinta, http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/sijoittajat and in English from March 11, 2016 onwards on the internet at
http://ir.acorda.com/investors/Biotie-Therapies-Tender-Offer/default.aspx and www.biotie.com/investors.
| 4. |
Instructions for acceptance of Tender Offer |
| |
• |
|
The holder of shares or other equity interests may accept the Tender Offer only in full. |
| |
• |
|
Acceptances are made separately per each book-entry account |
| |
• |
|
Separate acceptance forms for shares and for other equity instruments |
| |
• |
|
All fields shall be completed |
| |
• |
|
The Tender Offer cannot be accepted from any jurisdiction where prohibited by law or the terms of the Tender Offer |
| |
• |
|
A transfer restriction in respect of the shares and equity instruments will be registered in the relevant book-entry account after acceptance has been submitted |
| |
• |
|
Withdrawal of acceptance is possible in accordance with the terms and conditions of the Tender Offer |
| |
• |
|
Please see the Tender Offer Document for further information |
| 5. |
Foreign shareholders and holders of other equity interests |
Acceptance forms with
instructions may be sent, and acceptances may be received from countries belonging to European Economic Area.
The Tender Offer Document
and related materials, including the letter of transmittal and acceptance forms, will not and may not be distributed, forwarded or transmitted into or from any jurisdiction where prohibited by applicable law by any means whatsoever including,
without limitation, mail, facsimile transmission, e-mail or telephone. In particular, the Tender Offer is not being made, directly or indirectly, in or into, Canada, Japan, Australia, South Africa or Hong Kong or any other jurisdiction where
prohibited by law. The Tender Offer cannot be accepted by any such use, means or instrumentality or from within Canada, Japan, Australia, South Africa or Hong Kong or any other jurisdiction where prohibited by law.
With respect to any other jurisdictions, please contact the Arranger in order to be able to distribute any materials or receive the
acceptances.
Contact information:
Juho Hallenberg / Director ECM, Pohjola Bank plc, Capital Markets Financing / juho.hallenberg@pohjola.com / Tel. +358 50 545 6826 or +358 10
252 4064.
Exhibit (a)(1)(l)
Offer to Purchase for Cash
All outstanding ordinary shares, no nominal value,
All outstanding American Depositary Shares, each representing 80 ordinary shares,
All outstanding option rights issued under the December 6, 2011 option plan,
All outstanding option rights issued under the January 2, 2014 option plan,
All outstanding option rights issued under the January 4, 2016 option plan,
All outstanding share units issued under the December 6, 2011 equity incentive plan,
All outstanding share units under the January 2, 2014 equity incentive plan,
All outstanding option rights awards under the Swiss option plan dated June 18, 2008,
and
All outstanding
warrants issued on May 28, 2015
of
BIOTIE THERAPIES OYJ, a Finnish limited liability company
by
ACORDA
THERAPEUTICS, INC.
March 11, 2016
THE OFFER PERIOD AND THE ASSOCIATED WITHDRAWAL RIGHTS WILL EXPIRE ON APRIL 8, 2016 AT 4:00 P.M.
(FINNISH TIME) / 9:00 A.M. (NEW YORK TIME), UNLESS THE OFFER PERIOD IS EXTENDED.
1
March 11, 2016
To
Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees:
We have been appointed by Acorda Therapeutics, Inc., a Delaware
corporation (“Acorda” or the “Offeror”), to act as Information Agent for ADSs in connection with the Offeror’s offer to purchase (the “Tender Offer”) for cash all outstanding ordinary shares,
no nominal value (“Shares”), all outstanding American Depositary Shares (“ADSs”), each representing 80 Shares, all of the outstanding Option Rights (as defined in the Tender Offer Document (as defined below)), all
of the outstanding Share Rights (as defined in the Tender Offer Document) and all of the outstanding warrants issued on May 28, 2015 (the “Warrants”) (the outstanding Shares, ADSs, Option Rights, Share Rights and Warrants,
collectively, the “Equity Interests”) in Biotie Therapies Oyj (“Biotie” or the “Company”), a Finnish limited liability Company, upon and subject to the terms and conditions set forth in the offer to
purchase filed with the United States Securities and Exchange Commission on March 11, 2016 (the “Tender Offer Document”), the related Letter of Transmittal enclosed herewith (the “Letter of Transmittal”), and the
related acceptance forms for Option Rights, Share Rights and Warrants.
The Tender Offer is subject to certain conditions set forth in
the Tender Offer Document. See Section 4.2—“Conditions to Completion of the Tender Offer” of the Tender Offer Document.
Please note that there will be no guaranteed delivery tender procedures in connection with the Tender Offer.
Please furnish copies of the enclosed materials to those of your clients for whose accounts you hold ADSs registered in your name or in the
name of your nominee.
1. The Tender Offer Document.
2. The Letter of Transmittal to be used by holders of ADSs to accept the Tender Offer and tender ADSs and for the information of your clients
(manually signed facsimile copies of the Letter of Transmittal may be used to tender ADSs).
3. The Company’s
Solicitation/Recommendation Statement on Schedule 14D-9.
4. A printed form of letter that may be sent to your clients for whose accounts
you hold ADSs registered in your name or in the name of your nominee, with space provided for obtaining such clients’ instructions with regard to the Tender Offer.
In order to tender ADSs in the Tender Offer, (i) a duly executed and properly completed Letter of Transmittal and any required signature
guarantees and other documents, or (ii) an Agent’s Message (as defined in Section 4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer Document) in connection with a book-entry delivery of ADSs and other
required documents should be sent to Computershare Trust Company, N.A., in its capacity as depositary for the Tender Offer (the “Depositary”), and such ADSs should be tendered by book-entry transfer into the Depositary’s
account maintained at The Depositary Trust Company (“DTC”), all in accordance with the instructions set forth in the Letter of Transmittal and the Tender Offer Document. The Offeror will not pay any fees or commissions to any broker
or dealer or to any other person (other than to the Receiving and Paying Agent for Shares, Option Rights, Share Rights and Warrants, the Depositary, the Communication Agent, and the Information Agent for ADSs (each as defined in the Tender Offer
Document)) in connection with the solicitation of tenders of Equity Interests pursuant to the Tender Offer. The Offeror will, however, upon request, reimburse you for customary mailing and handling costs incurred by you in forwarding the enclosed
materials to your customers.
The Offeror will pay or cause to be paid all transfer taxes applicable to its purchase of ADSs pursuant to
the Tender Offer, except as otherwise provided in Instruction 5 of the Letter of Transmittal.
2
PLEASE CONTACT YOUR CLIENTS AS PROMPTLY AS POSSIBLE. PLEASE NOTE THAT THE OFFER PERIOD AND THE
ASSOCIATED WITHDRAWAL RIGHTS WILL EXPIRE ON APRIL 8, 2016 AT 4:00 P.M. (FINNISH TIME) / 9:00 A.M. (NEW YORK TIME), UNLESS THE OFFER PERIOD IS EXTENDED.
THE TENDER OFFER CANNOT BE ACCEPTED IN RESPECT OF SHARES OR ANY EQUITY INTEREST OTHER THAN THE ADSs BY MEANS OF A LETTER OF TRANSMITTAL. A
FORM OF ACCEPTANCE FOR ACCEPTING THE OFFER IN RESPECT OF THE SHARES AND OTHER EQUITY INTERESTS OTHER THAN ADSs CAN BE OBTAINED BY CALLING THE CALL SERVICE OF OP FINANCIAL GROUP AT (+358) (0) 100 0500.
Any inquiries you may have with respect to the Tender Offer should be addressed to, and additional copies of the enclosed materials may be
obtained from the undersigned, the Information Agent for ADSs, at the address and telephone number set forth on the back cover of the Tender Offer Document.
|
| Very truly yours, |
|
| |
| Innisfree M&A Incorporated |
NOTHING CONTAINED HEREIN OR IN THE ENCLOSED DOCUMENTS SHALL CONSTITUTE YOU OR ANY OTHER PERSON AS AN
AGENT OF THE OFFEROR, BIOTIE, THE RECEIVING AND PAYING AGENT FOR SHARES, OPTION RIGHTS, SHARE RIGHTS AND WARRANTS, THE DEPOSITARY, THE COMMUNICATION AGENT, OR THE INFORMATION AGENT FOR ADSs (EACH AS DEFINED IN THE TENDER OFFER DOCUMENT) OR ANY
AFFILIATE OF ANY OF THE FOREGOING OR AUTHORIZE YOU OR ANY OTHER PERSON TO USE ANY DOCUMENT OR MAKE ANY STATEMENT ON BEHALF OF ANY OF THEM IN CONNECTION WITH THE TENDER OFFER OTHER THAN THE DOCUMENTS ENCLOSED HEREWITH AND THE STATEMENTS CONTAINED
THEREIN.
3
Exhibit (a)(1)(J)
Offer to Purchase for Cash
All outstanding ordinary shares, no nominal value,
All outstanding American Depositary Shares, each representing 80 ordinary shares,
All outstanding option rights issued under the December 6, 2011 option plan,
All outstanding option rights issued under the January 2, 2014 option plan,
All outstanding option rights issued under the January 4, 2016 option plan,
All outstanding share units issued under the December 6, 2011 equity incentive plan,
All outstanding share units under the January 2, 2014 equity incentive plan,
All outstanding option rights awards under the Swiss option plan dated June 18, 2008,
and
All outstanding
warrants issued on May 28, 2015
of
BIOTIE THERAPIES OYJ, a Finnish limited liability company
by
ACORDA
THERAPEUTICS, INC.
March 11, 2016
THE OFFER PERIOD AND THE ASSOCIATED WITHDRAWAL RIGHTS WILL EXPIRE ON APRIL 8, 2016 AT 4:00 P.M.
(FINNISH TIME) / 9:00 A.M. (NEW YORK TIME), UNLESS THE OFFER PERIOD IS EXTENDED.
March 11, 2016
To Our Clients:
Enclosed for your consideration are the offer
to purchase filed with the United States Securities and Exchange Commission on March 11, 2016 (as it may be amended or supplemented from time to time, the “Tender Offer Document”), and the related Letter of Transmittal (as it may be
amended or supplemented from time to time, the “Letter of Transmittal”) in connection with the offer (the “Tender Offer”) by Acorda Therapeutics, Inc., a Delaware corporation (“Acorda” or the
“Offeror”), to purchase all outstanding ordinary shares, no nominal value (“Shares”), all outstanding American Depositary Shares (“ADSs”), each representing 80 Shares, all of the outstanding Option
Rights (as defined in the Tender Offer Document), all of the outstanding Share Rights (as defined in the Tender Offer Document) and all of the outstanding warrants issued on May 28, 2015 (the “Warrants”) (the outstanding Shares,
ADSs, Option Rights, Share Rights and Warrants, collectively, the “Equity Interests”) in Biotie Therapies Oyj (“Biotie” or the “Company”), a Finnish limited liability Company, upon and subject to
the terms and conditions set forth in the Tender Offer Document, the Letter of Transmittal, and the related acceptance forms for Shares, Option Rights, Share Rights and Warrants.
Also enclosed is the Company’s Solicitation/Recommendation Statement on Schedule 14D-9.
We or our nominees are the holder of record of ADSs held for your account. A tender of such ADSs can be made only by us as the holder of record and pursuant
to your instructions.
We request instructions as to whether you wish us to tender any or all of the ADSs held by us for your account, upon the terms
and subject to the conditions set forth in the enclosed Tender Offer Document and the Letter of Transmittal.
Please note carefully the following:
| |
1. |
The offer price for ADSs in the Tender Offer is EUR 23.5680 in cash per ADS, less any applicable withholding taxes. |
| |
2. |
The Tender Offer is being made for all Equity Interests. |
| |
3. |
The Tender Offer is being made pursuant to the Combination Agreement, dated as of January 19, 2016 (the “Combination Agreement”), by and between the Offeror and the Company. |
| |
4. |
The board of directors of the Company has determined that the Combination Agreement and the transactions contemplated thereby, including the Tender Offer, are advisable, fair to and in the best interests of the Company
and the holders of the Equity Interests. Accordingly, the board of directors of the Company has recommended that the holders of Equity Interests accept the Tender Offer and tender their Equity Interests to the Offeror in the Tender Offer.
|
| |
5. |
The offer period and the associated withdrawal rights will expire on April 8, 2016 at 4:00 p.m. (Finnish time) / 9 a.m. (New York time), unless the offer period is extended by the Offeror. Previously tendered
Equity Interests may be withdrawn at any time until the offer period has expired. |
| |
6. |
There will be no guaranteed delivery tender procedures in connection with the Tender Offer. |
| |
7. |
The Tender Offer is conditioned, among other things, upon the valid tender to (or other acquisition by) the Offeror of at least 90% of the issued and outstanding Shares (including Shares represented by ADSs) and voting
rights on a fully diluted basis. In addition, the Tender Offer is subject to certain conditions described in Section 4.2—“Conditions to Completion of the Tender Offer” of the Tender Offer Document. |
| |
8. |
Any transfer taxes applicable to the sale of ADSs to the Offeror pursuant to the Tender Offer will be paid by the Offeror, except as otherwise provided in the Letter of Transmittal. |
If you wish to have us tender any or all of your ADSs, then please so instruct us by completing, executing, detaching and returning to us the Instruction Form
on the detachable part hereof. An envelope to return your instructions to us is enclosed. If you authorize tender of your ADSs, then all such ADSs will be tendered unless otherwise specified on the Instruction Form.
Your prompt action is requested. Your Instruction Form should be forwarded to us in ample time to permit us to submit the tender on your behalf before the
expiration of the offer period.
The Tender Offer is not being made to, nor will tenders be accepted from or on behalf of, holders of Equity Interests
in any jurisdiction in which the making of the Tender Offer or acceptance thereof would not be in compliance with the laws of such jurisdiction.
2
INSTRUCTION FORM
With Respect to the Offer to Purchase for Cash
All outstanding ordinary shares, no nominal value,
All outstanding American Depositary Shares, each representing 80 ordinary shares,
All outstanding option rights issued under the December 6, 2011 option plan,
All outstanding option rights issued under the January 2, 2014 option plan,
All outstanding option rights issued under the January 4, 2016 option plan,
All outstanding share units issued under the December 6, 2011 equity incentive plan,
All outstanding share units under the January 2, 2014 equity incentive plan,
All outstanding option rights awards under the Swiss option plan dated June 18, 2008,
and
All outstanding
warrants issued on May 28, 2015
of
BIOTIE THERAPIES OYJ, a Finnish limited liability company
by
ACORDA
THERAPEUTICS, INC.
The undersigned acknowledge(s) receipt of your letter and the enclosed Tender Offer Document filed with the United States
Securities and Exchange Commission on March 11, 2016 (as it may be amended or supplemented from time to time, the “Tender Offer Document”), and the related Letter of Transmittal (as it may be amended or supplemented from time
to time, the “Letter of Transmittal”) in connection with the offer (the “Tender Offer”) by Acorda Therapeutics, Inc., a Delaware corporation (“Acorda” or the “Offeror”), to purchase
all outstanding ordinary shares, no nominal value (“Shares”), all outstanding American Depositary Shares (“ADSs”), each representing 80 Shares, all of the outstanding Option Rights (as defined in the Tender Offer
Document), all of the outstanding Share Rights (as defined in the Tender Offer Document) and all of the outstanding warrants issued on May 28, 2015 (the “Warrants”) (the outstanding Shares, ADSs, Option Rights, Share Rights and
Warrants, collectively, the “Equity Interests”) in Biotie Therapies Oyj (“Biotie” or the “Company”), a Finnish limited liability Company, upon and subject to the terms and conditions set forth in
the Tender Offer Document and Letter of Transmittal, and the related acceptance forms for Shares, Option Rights, Share Rights and Warrants.
3
The undersigned hereby instruct(s) you to tender to the Offeror the number of ADSs indicated below or, if no
number is indicated, all ADSs held by you for the account of the undersigned, upon the terms and subject to the conditions set forth in the Tender Offer.
ACCOUNT
NUMBER:
NUMBER OF ADSs BEING
TENDERED HEREBY: ADSs*
The method of delivery
of this document is at the election and risk of the tendering holder of ADSs. If delivery is by mail, then registered mail with return receipt requested, properly insured, is recommended. In all cases, sufficient time should be allowed to ensure
timely delivery.
Unless otherwise indicated, it will be assumed that all ADSs held by us for your account are to be tendered.
Dated:
, 2016
(Signature(s))
(Please Print Name(s))
Address
(Include Zip Code)
Area
Code and Telephone No.
Taxpayer Identification or Social Security No.
4
Exhibit (a)(1)(K)
This announcement is neither an offer to purchase nor a solicitation of an offer to sell Equity Interests (as defined below). The Tender Offer (as defined
below) is made solely by the Tender Offer Document (as defined below) and the related Letter of Transmittal (as defined below) and Acceptance Forms (as defined below) and any amendments or supplements thereto. The Tender Offer is not being made to
(nor will tenders be accepted from or on behalf of) holders of Equity Interests in any jurisdiction in which the making of the Tender Offer or the acceptance thereof would not be in compliance with the securities, blue sky or other laws of such
jurisdiction or any administrative or judicial action pursuant thereto. The Offeror (as defined below) may, in its discretion, take such action as it deems necessary to make the Tender Offer to holders of Equity Interests in such jurisdiction. In
those jurisdictions where applicable laws require that the Tender Offer be made by a licensed broker or dealer, the Tender Offer will be deemed to be made on behalf of the Offeror by one or more registered brokers or dealers licensed under the laws
of such jurisdiction to be designated by the Offeror.
Notice of Offer to Purchase for Cash
All outstanding ordinary shares, no nominal value,
All outstanding American Depositary Shares, each representing 80 ordinary shares, no nominal value,
All outstanding option rights issued under the December 6, 2011 option plan,
All outstanding option rights issued under the January 2, 2014 option plan,
All outstanding option rights issued under the January 4, 2016 option plan,
All outstanding share units issued under the December 6, 2011 equity incentive plan,
All outstanding share units under the January 2, 2014 equity incentive plan,
All outstanding option rights awards under the Swiss option plan dated June 18, 2008,
and
All outstanding
warrants issued on May 28, 2015
of
BIOTIE THERAPIES OYJ
by
ACORDA THERAPEUTICS, INC.
Acorda
Therapeutics Inc. (“Acorda” or the “Offeror”), a Delaware corporation, is offering to acquire, in accordance with Chapter 11 of the Finnish Securities Market Act (746/2012, as amended), the U.S. Securities Exchange
Act of 1934, as amended (the “Exchange Act”), and the terms and conditions of the offer to purchase dated March 11, 2016 (the “Tender Offer Document”), all of the issued and outstanding ordinary shares, no nominal
value (the “Shares”), all of the outstanding American Depositary Shares, each representing 80 Shares (the “ADSs”), all of the outstanding Option Rights (as defined below), all of the outstanding Share Rights (as
defined below) and all of the outstanding warrants issued on May 28, 2015 (the “Warrants”) (the outstanding Shares, ADSs, Option Rights, Share Rights and Warrants, collectively, the “Equity Interests”) in
Biotie Therapies OYJ (“Biotie” or the “Company”), a public limited liability company organized under the laws of Finland, that are not held by the Company or its subsidiaries (the “Tender Offer”).
“Option Rights” means, collectively, option rights granted under the option plan resolved upon by the board of directors of the Company (the “Board of Directors”) on December 6, 2011 by virtue of an authorization
granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Option Rights”), option rights granted under the option plan resolved upon by the Board of Directors of the Company on January 2, 2014 by
virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013 (the “2014 Option Rights”), option rights granted under the option plan resolved upon by the Board of Directors of the
Company on January 4, 2016 by virtue of an authorization granted by the annual general meeting of the Company held on May 26, 2015 (the “2016 Option Rights”) and option rights granted under the Swiss option plan dated
June 17, 2008 (the “Swiss Option Rights”). “Share Rights” means, collectively, share units under the equity incentive plan resolved upon by the Board of Directors of the Company on December 6, 2011 by
virtue of an authorization granted by the annual general meeting of the Company held on May 6, 2011 (the “2011 Share Rights”) and share units under the equity incentive plan resolved upon by the Board of Directors of the
Company on January 2, 2014 by virtue of an authorization granted by the annual general meeting of the Company held on April 4, 2013 (the “2014 Share Rights”).
The offer price for each outstanding Share validly tendered in accordance with the terms and conditions of the
Tender Offer is EUR 0.2946 in cash (the “Share Offer Price”).
The offer price for each outstanding ADS validly tendered in accordance
with the terms and conditions of the Tender Offer is EUR 23.5680 in cash (the “ADS Offer Price”), payable in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S.
dollar spot rate against the euro exchange rate on the nearest practicable day to the applicable Closing Date (as defined below).
The offer prices for
outstanding Option Rights validly tendered in accordance with the terms and conditions of the Tender Offer are (i) EUR 0.2846 in cash for each 2011 Option Right, (ii) EUR 0.2846 in cash for each 2014 Option Right, (iii) EUR 0.1326 in cash for each
2016 Option Right, payable, at the option of the holder, in euros or the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the
nearest practicable date to the applicable Closing Date, (iv) EUR 0.2032 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.10, (v) EUR 0.1026 in cash for each Swiss Option Right with a per Share subscription price of
CHF 0.21, (vi) EUR 0.0386 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.28, (vii) EUR 0.0112 in cash for each Swiss Option Right with a per Share subscription price of CHF 0.31, and (viii) EUR 0.0100 in cash for
each other Swiss Option Right (collectively, the “Option Right Offer Price”).
The offer prices for outstanding Share Rights validly
tendered in accordance with the terms and conditions of the Tender Offer are (i) EUR 0.2946 in cash for each 2011 Share Right, and (ii) EUR 0.2854 in cash for each 2014 Share Right, payable, in each case, at the option of the holder, in euros or the
equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable date to the applicable Closing Date (collectively, the
“Share Right Offer Price”).
The offer price for each outstanding Warrant validly tendered in accordance with the terms and conditions of
the Tender Offer is EUR 0.1664 in cash (the “Warrant Offer Price,” and together with the Share Offer Price, the ADS Offer Price, the Option Right Offer Price and the Share Right Offer Price, the “Offer Price”). The
Offer Price will be paid in each case without interest and less any applicable withholding taxes.
The initial acceptance period for the Tender Offer (the
“Offer Period”) will commence on March 11 at 9:30 a.m. (Finnish time) / 2:30 a.m. (New York time) and expire on April 8 at 4:00 p.m. (Finnish time) / 9:00 a.m. (New York time) unless the Offer Period is extended (the end of the
Offer Period, as extended, the “Expiration Date”). The Offeror reserves the right to initiate a Subsequent Offer Period (as defined below) in connection with the announcement of the final results with respect to the Offer Period if
the Tender Offer shall have been announced to be unconditional at that time.
THE OFFER PERIOD AND THE ASSOCIATED WITHDRAWAL RIGHTS WILL EXPIRE ON
APRIL 8, 2016 AT 4:00 P.M. (FINNISH TIME) / 9:00 A.M. (NEW YORK TIME), UNLESS THE OFFER PERIOD IS EXTENDED.
Tendering equityholders who have Equity
Interests registered in their names and who tender ADSs directly to Computershare Trust Company, N.A., in its capacity as depositary for the Tender Offer (the “Depositary”), or Shares, Option Rights, Share Rights or Warrants
directly to the account operator managing the applicable book-entry account or otherwise to a branch office of the cooperative bank belonging to the OP Financial Group or Helsinki OP Bank Plc, will not be obligated to pay brokerage fees or
commissions or, except as set forth in the Letter of Transmittal for ADSs (the “Letter of Transmittal”) or the Acceptance Form for Shares (including any instruction letter attached thereto) or Acceptance Form for Option Rights,
Share Rights and Warrants (including any instruction letter attached thereto) (such acceptance forms and attached instructions, the “Acceptance Forms”), as applicable, transfer taxes on the purchase of Equity Interests by the
Offeror pursuant to the Tender Offer. Equityholders who hold their Equity Interests through a broker or bank should consult with such institution as to whether it charges any service fees or commissions.
The Tender Offer is being made pursuant to the Combination Agreement, dated as of January 19, 2016 (the “Combination Agreement”), by and
between Acorda and Biotie. The Combination Agreement is more fully described in the Tender Offer Document.
2
The completion of the Tender Offer is subject to the satisfaction or waiver of certain limited conditions. The
Tender Offer is not subject to a financing condition. The Tender Offer is conditioned upon, among other things, the valid tender of outstanding Shares (including outstanding Shares represented by validly tendered ADSs and validly tendered Warrants)
representing, together with any outstanding Shares (including outstanding Shares represented by ADSs and Warrants) otherwise acquired by the Offeror, more than 90 percent of the issued and outstanding Shares and voting rights of the Company,
calculated on a fully diluted basis and otherwise in accordance with Chapter 18 Section 1 of the Finnish Limited Liability Companies Act (the “Minimum Condition”).
Subject to the provisions of, and in accordance with, the Combination Agreement, the terms and conditions of the Tender Offer described in the Tender Offer
Document, the Finnish Securities Market Act and other relevant Finnish laws and the Exchange Act and the rules and regulations promulgated thereunder, the Offeror reserves the right to waive or otherwise modify or amend any of the terms and
conditions of the Tender Offer.
The purpose of the Tender Offer is to acquire control of, and all of the outstanding Equity Interests in, the Company. If
the Tender Offer is consummated and all Equity Interests validly tendered and not withdrawn have been transferred to the Offeror (the date of each such transfer, a “Closing Date”), and the Offeror has acquired more than 90 percent
of all the Shares (including the Shares represented by ADSs), the Offeror intends, as soon as practicable after consummation of the Tender Offer, to initiate subsequent compulsory redemption proceedings to redeem the remaining Shares (including the
Shares represented by ADSs) in accordance with the Finnish Companies Act (such potential proceedings, the “Subsequent Compulsory Redemption”) and to redeem the other Equity Interests in accordance with the terms of such Equity
Interests. All of the then-outstanding Shares (other than Shares held by the Offeror or the Company) will, subject to certain rights of shareholders under Finnish law, be redeemed for cash for an amount per Share to be determined by the arbitrator
in the Subsequent Compulsory Redemption, with interest but less any applicable withholding taxes. Under the general rule under Finnish law, this value is equal to the price paid in the Tender Offer but could also be different than such price if
there are special grounds to value the Shares differently. If the Subsequent Compulsory Redemption takes place, no appraisal rights will be available to those who have tendered their Shares in the Tender Offer.
After careful consideration, the Board of Directors of the Company has determined that the Combination Agreement and the transactions contemplated thereby,
including the Tender Offer, are advisable, fair to and in the best interests of the Company and the holders of the Equity Interests. Accordingly, the Board of Directors has recommended that the holders of Equity Interests accept the Tender Offer and
tender their Equity Interests to the Offeror in the Tender Offer.
In general, if at the scheduled expiration time of the Offer Period any of the
Conditions to Completion are not satisfied, the Offeror will extend the Offer Period for additional periods not exceeding two (2) weeks each in accordance with the Tender Offer Document and, in each case, subject to compliance with applicable
Finnish and United States legal requirements. The maximum duration of the Offer Period is ten (10) weeks as required by Finnish law. However, if any of the Conditions to Completion has not been fulfilled due to a particular obstacle, the Offeror
may, subject to the decision of the Finnish Financial Supervisory Authority, extend the Offer Period beyond ten (10) weeks until such obstacle has been removed and until all Conditions to Completion have been satisfied. In no event is the Offeror
required to extend the Tender Offer beyond June 19, 2016. The Offeror reserves the right to commence a subsequent offer period (a “Subsequent Offer Period”) in connection with the announcement of the final result with respect
to the Offer Period if the Tender Offer shall have been announced to be unconditional at that time. In the event of such Subsequent Offer Period, the Subsequent Offer Period will expire on the date and at the time determined by the Offeror in the
final result announcement. The Subsequent Offer Period may extend beyond ten (10) weeks (and, in the Offeror’s discretion, beyond June 19, 2016).
If the Offer Period is extended the Offeror will make a public announcement of the extension by a stock exchange release at or before 4:00 p.m. (Finnish time)
/ 9:00 a.m. (New York time) on April 11, 2016. The Offeror will give notice of any further extension of the Tender Offer, at the latest on the first Finnish banking day following what would have been, but for such extension, the Expiration Date or
end of any Subsequent Offer Period, as applicable, at or before 4:00 p.m. (Finnish time) / 9:00 a.m. (New York time).
Holders of Shares who wish to
accept the Tender Offer with respect to such Shares must deliver acceptances of the Tender Offer to the account operator managing their book-entry account in accordance with such account operator’s
3
instructions and within the time limit set by the account operator or, if such account operator does not accept Acceptance Forms (e.g., Euroclear), holders must contact any branch office of the
cooperative bank belonging to the OP Financial Group or Helsinki OP Bank Plc to tender their Shares into the Tender Offer. The Acceptance Form for Shares shall be submitted so that it is received on or prior to the Expiration Date, subject to and in
accordance with the instructions of the relevant account operator. In the event of a Subsequent Offer Period, the Acceptance Form shall be submitted so that it is received during the Subsequent Offer Period, subject to and in accordance with the
instructions of the relevant account operator. Detailed instructions are contained in the Acceptance Form for Shares and in Section 4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer Document.
Holders of ADSs who wish to accept the Tender Offer should complete the confirmation of a book-entry transfer of their ADSs into the account of the Depositary
at The Depository Trust Company (“DTC”) and send either (i) an Agent’s Message (as defined below) or (ii) a properly completed and duly executed Letter of Transmittal and any other required documents to the Depositary prior to
the Expiration Date or end of any Subsequent Offer Period, as applicable. These materials must reach the Depositary on or prior to the Expiration Date or end of any Subsequent Offer Period, as applicable. Holders of ADSs who hold such ADSs
registered in the name of a broker, dealer, commercial bank, trust company, custodian or other nominee must contact that institution in order to tender such ADSs to the Offeror pursuant to the Tender Offer.
An “Agent’s Message” delivered in lieu of the Letter of Transmittal is a message transmitted by DTC to, and received by, the Depositary
as part of a confirmation of a book-entry transfer. The message states that DTC has received an express acknowledgment from the DTC participant tendering ADSs that such participant has received and agrees to be bound by the terms of the Letter of
Transmittal and that we may enforce such agreement against such participant.
Detailed instructions are contained in the Letter of Transmittal and in
Section 4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer Document.
Holders of Warrants or Option Rights that have been
entered into the Finnish book-entry securities system who wish to accept the Tender Offer with respect to such Equity Interests must deliver acceptances of the Tender Offer to the account operator managing their book-entry account in accordance with
such account operator’s instructions and within the time limit set by the account operator or, if such account operator does not accept Acceptance Forms (e.g. Euroclear), such holder of Warrants or Option Rights shall contact any branch office
of the cooperative bank belonging to the OP Financial Group or Helsinki OP Bank Plc to give his/her acceptance to tender his/her Warrants and/or Option Rights. The Acceptance Form for Option Rights, Share Rights and Warrants shall be submitted so
that it is received on or prior to the Expiration Date, subject to and in accordance with the instructions of the relevant account operator. In the event of a Subsequent Offer Period, the Acceptance Form shall be submitted so that it is received
during the Subsequent Offer Period, subject to and in accordance with the instructions of the relevant account operator. Detailed instructions are contained in the Acceptance Form and in Section 4.4—“Acceptance Procedure of the Tender
Offer” of the Tender Offer Document.
Holders of Share Rights or Option Rights that have not been entered into the Finnish book-entry securities
system who wish to accept the Tender Offer with respect to such Equity Interests must deliver acceptances of the Tender Offer to the Capital Markets Financing department of Pohjola Bank plc. Detailed instructions are contained in Section
4.4—“Acceptance Procedure of the Tender Offer” of the Tender Offer Document and in the instructions that will be attached to the Acceptance Form for Option Rights, Share Rights and Warrants.
Holders of Equity Interests may withdraw previously tendered Equity Interests any time prior to the Expiration Date by following the procedures for
withdrawing their Equity Interests in a timely manner. Equity Interests tendered during a Subsequent Offer Period, if any, may not be withdrawn.
To
validly withdraw any of previously tendered Shares, Option Rights, Share Rights or Warrants, holders must submit a written notice of withdrawal to the same account operator to whom the Acceptance Form(s) for such Equity Interests was submitted. If
an Acceptance Form was submitted with respect to such Equity Interests to a branch office of the cooperative bank belonging to the OP Financial Group or Helsinki OP Bank Plc, the notice of withdrawal must be submitted to the same branch office.
4
To validly withdraw any previously tendered ADSs, holders must deliver a written notice of withdrawal, or a
facsimile of one (with original delivered via overnight courier), with the required information to the Depositary.
If such Equity Interests were tendered
by giving instructions to a broker or other nominee, holders must instruct their broker or nominee prior to the Expiration Date to arrange for the withdrawal of such Equity Interests in a timely manner.
The sale and purchase of the Shares validly tendered and not properly withdrawn in accordance with the terms and conditions of the Tender Offer will be
executed on the fourth Finnish banking day following the Expiration Date. The sale and purchase of the Shares will take place on NASDAQ Helsinki Ltd. (“Nasdaq Helsinki”) unless prohibited by the rules applicable to securities
trading on Nasdaq Helsinki. The sale and purchase of the ADSs validly tendered and not withdrawn during the Offer Period in accordance with the terms and conditions of the Tender Offer will be executed no later than on the sixth Finnish banking day
following the Expiration Date. The sale and purchase of the Option Rights, Share Rights and Warrants, validly tendered and not properly withdrawn in accordance with the terms and conditions of the Tender Offer, will be executed no later than the
sixth Finnish banking day following the Expiration Date.
The receipt of cash as payment for the Equity Interests pursuant to the Tender Offer or pursuant
to a Subsequent Compulsory Redemption, if any, will be a taxable transaction for United States federal income tax purposes. For a summary of the material United States federal income tax consequences of the Tender Offer and Subsequent Compulsory
Redemption, if any, see the Tender Offer Document. ALL HOLDERS OF EQUITY INTERESTS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE SPECIFIC TAX CONSEQUENCES OF TENDERING THEIR EQUITY INTERESTS IN THE TENDER OFFER OR EXCHANGING SUCH SECURITIES FOR
CASH IN CONNECTION WITH A SUBSEQUENT COMPULSORY REDEMPTION IN LIGHT OF THEIR PARTICULAR SITUATIONS, INCLUDING THE APPLICABILITY AND EFFECT OF U.S. FEDERAL, STATE, LOCAL, NON-U.S. AND OTHER LAWS.
The information required to be disclosed by paragraph (d)(1) of Rule 14d-6 of the General Rules and Regulations under the Exchange Act is contained in the
Tender Offer Document and is incorporated herein by reference.
The Tender Offer Document and the related Letter of Transmittal and Acceptance Forms
contain important information and each document should be read carefully and in its entirety before any decision is made with respect to the Tender Offer.
Questions and requests for assistance regarding the Tender Offer or any of the terms thereof with respect to Shares, Option Rights, Share Rights or Warrants
may be directed to the call service of OP Financial Group at (+358) (0) 100 0500. Questions and requests for assistance regarding the Tender Offer or any of the terms thereof with respect to ADSs may be directed to Innisfree M&A Incorporated
(the “Information Agent for ADSs”), at the address and telephone number set forth below. Requests for copies of the Tender Offer Document, the Letter of Transmittal, the Acceptance Forms and other tender offer materials may be
directed to the call service of OP financial Group at (+358) (0) 100 0500 or the Information Agent for ADSs, as applicable. Such copies will be furnished promptly at the Offeror’s expense. Holders of Equity Interests may also contact their
broker, dealer, commercial bank, trust company, custodian or other nominee for assistance.
The Offeror will not pay any fees or commissions to any broker
or dealer or to any other person (other than to the Receiving and Paying Agent for Shares, Option Rights, Share Rights and Warrants, the Depositary, the Communication Agent, and the Information Agent for ADSs) in connection with the solicitation of
tenders of Equity Interests pursuant to the Tender Offer. Brokers, bankers and other nominees will, upon request, be reimbursed by the Offeror for customary mailing and handling expenses incurred by them in forwarding offering materials to their
customers.
5
|
|
|
| The Receiving and Paying Agent for Shares, Option
Rights, Share Rights and Warrants is: |
|
The Information Agent for ADSs is: |
|

Capital Markets Financing
department of Pohjola Bank plc
Teollisuuskatu 1 FI-00510
Helsinki Finland
For assistance, please contact the
call service of OP Financial Group at
(+358) (0) 100 0500 |
|

Innisfree M&A
Incorporated 501 Madison Avenue, 20th Floor
New York, NY 10022 U.S.A.
ADS holders within the United States may call
1 888 750-5834
ADS holders from other locations may call
(+1) 412 232-3651
Banks and Brokers may call collect at
(+1) 212 750-5833 |
March 11, 2016
6
Exhibit (a)(1)(L)
ACORDA
THERAPEUTICS’ TENDER OFFER FOR ALL
THE EQUITY INTERESTS IN BIOTIE
Offer Period 11.3.–8.4.2016
A separate marketing brochure has been sent to all shareholders. Please check your mail!
ACORDA CREATES LEADING SOLUTIONS IN THE FIGHT AGAINST PARKINSON’S DISEASE.
The Tender Offer Document is available in branch offices of OP Pohjola Group and at op.fi/ merkinta,
ir.acorda.com/investors/Biotie-Therapies-Tender-Offer and biotie.com/investors
Additional information also
available in OP Call Service 0100 0500.
ACORDA THERAPEUTICS’ TENDER OFFER FOR ALL THE EQUITY INTERESTS IN BIOTIE
Offer Period 11.3.–8.4.2016
ACORDA THERAPEUTICS’ TENDER OFFER FOR ALL THE EQUITY INTERESTS IN BIOTIE
A separate marketing brochure has been sent to all shareholders. Please check your mail!
ACORDA THERAPEUTICS’ TENDER OFFER FOR ALL THE EQUITY INTERESTS IN BIOTIE
The Tender Offer Document is available in branch offices of OP Pohjola Group and at op.fi/merkinta, ir.acorda.com/investors/Biotie-Therapies-Tender-Offer and biotie.com/sijoittajat
ACORDA THERAPEUTICS’ TENDER OFFER FOR ALL THE EQUITY INTERESTS IN BIOTIE
Additional information also available in OP Call Service 0100 0500
Arranger of the Tender Offer Pohjola Pankki Oyj
Exhibit (d)(2)
EXECUTION VERSION
January 19, 2016
| TO: |
Acorda Therapeutics, Inc. |
| |
420 Saw Mill River Road, Ardsley, NY 10502 |
Dear Sirs,
We the undersigned (“we”, “us”, “our” or “ourselves”) refer to the Combination Agreement, dated as of the date hereof
(the “Combination Agreement”), which provides for, among other things, your proposed acquisition of all of the outstanding shares, American Depositary Shares and other applicable equity instruments in Biotie Therapies Oyj (the
“Target”) through an all cash tender offer (or, with respect to Warrants (as defined below), if so requested by the Bidder (as defined below), a purchase on terms consistent with this undertaking and the Combination Agreement) to be
made by Acorda Therapeutics, Inc. or its designated subsidiary (the “Bidder”) in Finland and in the United States with the Offer Consideration (as defined below) specified herein and otherwise on terms and conditions consistent with
the Combination Agreement and to be specified in a tender offer document (such cash tender offer by the Bidder, the “Tender Offer”), subject to the Board of Directors of the Target undertaking to recommend the Tender Offer. We
understand that as part of or in connection with the Tender Offer, the Bidder would offer to the
| |
i. |
shareholders of the Target € 0.2946 in cash for every share in the Target (“Shares”) held by each such shareholder (the “Share Consideration”); |
| |
ii. |
holders of the American Depository Shares of the Target (“ADSs”) € 23.5680 in cash for each outstanding ADS payable in the equivalent amount of U.S. dollars for each outstanding ADS determined as
near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the completion date of the Tender Offer (the “ADS Consideration”); and
|
| |
iii. |
holders of warrants of the Target issued on May 28, 2015 (“Warrants”, with, for the avoidance of doubt, each Warrant representing the right to subscribe for a single Share) € 0.1664 in
cash for each Warrant (the “Warrant Consideration”). |
The Share Consideration, the ADS Consideration and the Warrant
Consideration, as adjusted pursuant to the terms hereof, are hereinafter referred jointly to as the “Offer Consideration”. In each case, the Offer Consideration shall represent the full and final consideration payable for the
relevant instruments in the Tender Offer and, in the case of the Warrants, shall be in full and final satisfaction of the Target’s obligations to us under the governing documents for the Warrants.
We are the beneficial owner, as at the date hereof, of the number of Shares, ADSs and Warrants in the Target set forth on our signature page hereto, and such
Shares, ADSs and/or Warrants constitute, as of the date hereof, all of the Shares, ADSs and Warrants held of record, beneficially owned or for which voting power or disposition power is otherwise held by us. We have good and marketable title, free
and clear of any liens, other than liens pursuant to this undertaking and any transfer restrictions of general applicability as may be provided under applicable securities laws or in the terms and conditions of the Warrants, to all Shares, ADSs
and/or Warrants, as applicable, described in the immediately preceding sentence.
Subject to the terms and conditions hereof and of the Tender Offer, we hereby irrevocably undertake to
(i) accept the Tender Offer, and to tender or cause to tendered and sell or cause to be sold to the Bidder the Shares, ADSs and Warrants, as applicable, that we currently own, and any additional Shares, ADSs and Warrants, as applicable, that we
may hereafter acquire prior to the earlier of the Termination Date (as defined below) and the expiration of the Tender Offer, in the Tender Offer for the Offer Consideration applicable thereto, (ii) deliver or cause to be delivered evidence of
such acceptance pursuant to the terms of the Tender Offer to the Bidder within ten (10) business days from the beginning of the acceptance period of the Tender Offer, and (iii) not exercise voting rights pertaining to the Shares, ADSs and
any Shares subscribed based on the Warrants in favor of a Competitive Transaction (as defined below), in each case subject to the following paragraph.
Subject to the terms and conditions hereof, with respect to the Warrants and if so requested by the Bidder prior to the launch of the Tender Offer, in lieu of
accepting the Tender Offer and tendering the Warrants, we hereby irrevocably undertake to, subject to and upon the consummation of the Tender Offer and subject to this undertaking having not been previously terminated in accordance with the terms
hereof, sell to the Bidder (and the Bidder hereby irrevocably undertakes to purchase from us) the Warrants that we currently own, and any Warrants that we may hereafter acquire prior to the expiration of the Tender Offer, for the Warrant
Consideration and on terms consistent with the Combination Agreement; provided that such purchase and sale may not require us to make any representations and warranties, or to provide any covenants or other agreements, other than those that would
have been required to be made or provided by us in connection with tender of the Warrants in the Tender Offer.
Subject to the terms and conditions
hereof, we further undertake not to, except, in each case, in accordance with the terms of this undertaking: (i) sell, transfer, grant any option with respect to, pledge or otherwise dispose of (collectively, “Transfer”) any of
the Shares, ADSs and Warrants that we own or control prior to the earlier of the Termination Date and the expiration of the Tender Offer; provided, that nothing in this undertaking will limit our ability to convert any Warrants into Shares, indirect
Transfers or Transfers of any Shares, ADSs or Warrants to one or more of our affiliates that agree in writing to be bound hereby and assume our obligations hereunder, (ii) directly or indirectly, solicit any inquiries with respect to or solicit
or accept any public or private proposal or offer (including, without limitation, any proposal or offer to all holders of Shares, ADSs and Warrants), other than the Tender Offer, for the Shares, ADSs and Warrants we own or control prior to the
earlier of the Termination Date and the expiration of the Tender Offer (a “Competitive Transaction”) (it being understood that this undertaking is entered into solely in our capacity as a holder of Shares, ADSs and
Warrants, as applicable, and nothing in this undertaking shall (or shall require us to attempt to) limit or restrict us or any designee of us who is a director or officer of the Target from acting in such capacity or voting in such person’s
sole discretion in such capacity on any matter, however always in accordance with, where applicable, the fiduciary duties of the Board of Directors of the Target under Finnish laws); or (iii) withdraw our acceptance of the Tender Offer in
respect of any of such Shares, ADSs and Warrants regardless of any right of withdrawal contained in the terms and conditions of the Tender Offer or any legal right to withdraw, except in accordance with the terms of this undertaking, including the
withdrawal rights provided hereunder. Notwithstanding the foregoing, in the event that (A) the Target enters into discussions with a competing bidder in accordance with the Combination Agreement and (B) the Board of Directors of the Target
requests that we engage in discussions with the competing bidder, then nothing in this undertaking shall limit or restrict us from engaging in such discussions, provided that we will not, prior to the earlier of the Termination Date and the
expiration of the Tender Offer, (i) discuss, offer or otherwise negotiate with any competing bidder regarding the acquisition or other transfer to such competing bidder of any of our Shares. ADSs or Warrants at a price lower than the Share
Consideration, ADS Consideration or Warrant Consideration, as applicable, and (ii) will not enter into any undertaking with respect to any such competing transaction unless and until this undertaking has terminated in accordance with its terms.
The Bidder undertakes and agrees that should the terms and conditions of the Tender Offer, including the Offer
Consideration, be revised or amended by the Bidder for the benefit of the holders of Shares, ADSs and Warrants, such revision or amendment shall also be for our benefit. The Bidder further undertakes and agrees that, if the Bidder enters into an
undertaking or similar agreement with any other holder of Shares, ADSs or Warrants that provides such other holder with rights and benefits that are more favorable in any material respect to such other holder than the rights and benefits established
in favor of us under this undertaking, then (i) the Bidder shall promptly provide us with a copy of the other undertaking or agreement (and, in any case, within one business day of the execution thereof) and (ii) we shall have the right to
elect to receive all such rights and benefits under the other undertaking or agreement, and this undertaking shall be amended accordingly. In addition, for the avoidance of doubt, we expressly do not waive any rights under Chapter 11,
Section 25 of the Finnish Securities Market Act applicable from time to time.
This undertaking shall remain valid and in force until the
first of the following has taken place, at which date and time this undertaking shall immediately and automatically terminate in full (the “Termination Date”): (i) the Board of Directors of the Target has failed to
recommend that the holders of Shares, ADSs and Warrants accept the Tender Offer or has modified or withdrawn such recommendation; (ii) the completion and settlement of the Tender Offer; (iii) the Bidder has made a public announcement to
the effect that it will not complete the Tender Offer; (iv) the Combination Agreement has been terminated; (v) any amendment has been made to the Tender Offer that reduces the Offer Consideration or otherwise materially changes the terms
and conditions of the Tender Offer in a manner adverse to us; or (vi) the Tender Offer, having been launched or published, has failed to be completed by June 19, 2016.
We agree not to, without the prior written consent of the Bidder (such consent not to be unreasonably withheld, delayed or conditioned), make or cause to be
made any announcement or disclosure to any third party in respect of any unpublished information concerning the contemplated Tender Offer or this undertaking except (i) for disclosure to the Target, (ii) for disclosure to our directors,
officers, employees, partners, managers, members, agents and representatives who are charged with an obligation of confidentiality, (iii) for disclosure required by any applicable law, regulation or order of court, governmental authority,
self-regulatory organization or stock exchange or (iv) to enforce our rights under, or defend ourselves in any action brought in connection with, this undertaking.
This undertaking shall be governed by, and construed in accordance with, the laws of Finland. All disputes arising out of or relating to the present contract
shall be finally settled by final, binding arbitration conducted under the Rules of Arbitration of the International Chamber of Commerce (the “ICC Rules”). The arbitral panel shall be comprised of three arbitrators to be appointed
as follows: within 14 days after the commencement of arbitration, each party shall select one person to act as arbitrator and the two selected shall select a third arbitrator within 10 days of their appointment. If the arbitrators selected by the
parties are unable or fail to agree upon the third arbitrator, the third arbitrator shall be selected by the ICC. Either party may apply to the arbitrator seeking injunctive relief until the arbitration award is rendered or the controversy is
otherwise resolved. Either party also may, without waiving any remedy under this undertaking, seek from any court having jurisdiction any interim or provisional relief that is necessary to protect the rights or property of that party, pending the
arbitration panel’s determination of the merits of the controversy. The arbitration shall be conducted in English in London, United Kingdom, and judgment on the award rendered by the arbitrators may be entered in any court having jurisdiction
thereof. In the event of a conflict between this paragraph and the ICC Rules, this paragraph shall govern.
We agree and give our consent that a copy of this undertaking may be provided to the Board of Directors of the
Target and that this undertaking may be referred to and the main terms and conditions hereof may be disclosed in any public announcement that the Bidder or the Target may make in connection with the Tender Offer or in the offer document related
thereto; provided, that the Bidder or the Target, as applicable, shall (i) provide us with an advance copy of any such announcement or offer document that refers to us or our affiliates by name and a reasonable opportunity to review and comment
on any such disclosures or public announcements to the extent of such references and (ii) shall not include in any such announcement or offer document any reference to us or our affiliates by name or, in connection therewith, the existence or
terms and conditions of this undertaking if we reasonably object to the form or content thereof; provided that, nothing in the foregoing shall restrict the Bidder or the Company from making any such disclosures or public announcements necessary to
comply with applicable law.
This undertaking is given on the understanding that nothing in this undertaking shall cause us to be deemed to be acting in
concert with the Bidder or any other holder of Shares, ADSs or Warrants of the Company.
|
| Yours faithfully, |
|
| [INSERT NAME OF SHAREHOLDER] |
|
| By:
|
| Name: |
|
|
|
| Number of Shares (not including Shares represented by ADSs or Warrants): |
|
|
|
|
|
| Number of ADSs: |
|
|
|
|
|
| Number of Warrants: |
|
|
|
|
|
|
| Acknowledged and accepted. |
|
| Acorda Therapeutics, Inc. |
|
| By:
|
| Name: |
EXECUTION VERSION
January 19, 2016
| TO: |
Acorda Therapeutics, Inc. |
| |
420 Saw Mill River Road, Ardsley, NY 10502 |
Dear Sirs,
We the undersigned (“we”, “us”, “our” or “ourselves”) refer to the Combination Agreement, dated as of the date hereof
(the “Combination Agreement”), which provides for, among other things, your proposed acquisition of all of the outstanding shares, American Depositary Shares and other applicable equity instruments in Biotie Therapies Oyj (the
“Target”) through an all cash tender offer (or, with respect to Warrants (as defined below), if so requested by the Bidder (as defined below), a purchase on terms consistent with this undertaking and the Combination Agreement) to be
made by Acorda Therapeutics, Inc. or its designated subsidiary (the “Bidder”) in Finland and in the United States with the Offer Consideration (as defined below) specified herein and otherwise on terms and conditions consistent with
the Combination Agreement and to be specified in a tender offer document (such cash tender offer by the Bidder, the “Tender Offer”), subject to the Board of Directors of the Target undertaking to recommend the Tender Offer. We
understand that as part of or in connection with the Tender Offer, the Bidder would offer to the
| |
i. |
shareholders of the Target € 0.2946 in cash for every share in the Target (“Shares”) held by each such shareholder (the “Share Consideration”); |
| |
ii. |
holders of the American Depository Shares of the Target (“ADSs”) € 23.5680 in cash for each outstanding ADS payable in the equivalent amount of U.S. dollars for each outstanding ADS determined as
near to the payment date as reasonably practicable based on U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the completion date of the Tender Offer (the “ADS Consideration”); |
| |
iii. |
holders of warrants of the Target issued on May 28, 2015 (“Warrants”, with, for the avoidance of doubt, each Warrant representing the right to subscribe for a single Share) € 0.1664 in
cash for each Warrant (the “Warrant Consideration”); |
| |
iv. |
holders of the following option and share rights: |
| |
a. |
the 2011 option rights (“2011 Option Rights”); |
| |
b. |
the 2014 option rights (“2014 Option Rights”); |
| |
c. |
the 2016 option rights (“2016 Option Rights”); |
| |
d. |
the option rights issued by Biotie Therapies AG (“Swiss Option Rights”); |
| |
e. |
the 2011 share rights (“2011 Share Rights”); and |
| |
f. |
the 2014 share rights (“2014 Share Rights” and, the option and share rights listed in (a) through (f), together, the “Equity Instruments”); |
in each case, the Share Consideration minus the applicable subscription price of such Equity Instrument (the “Equity Instrument
Consideration”).
The offer price payable to of the 2016 Option Rights and the 2011 and 2014 Share Rights shall be payable at the
option of the holder in the equivalent amount of U.S. dollars determined as near to the payment date as reasonably practicable based on the U.S. dollar spot rate against the euro exchange rate on the nearest practicable day to the Closing Date.
The Share Consideration, the ADS Consideration, the Warrant Consideration and the Equity Instrument Consideration, as adjusted pursuant to the terms hereof,
are hereinafter referred jointly to as the “Offer Consideration”. In each case, the Offer Consideration shall represent the full and final consideration payable for the relevant instruments in the Tender Offer and, in the case of
the Warrants, shall be in full and final satisfaction of the Target’s obligations to us under the governing documents for the Warrants.
We are the
beneficial owner, as at the date hereof, of the number of Shares, ADSs, Warrants and Equity Instruments in the Target set forth on our signature page hereto, and such Shares, ADSs, Warrants and/or Equity Instruments constitute, as of the date
hereof, all of the Shares, ADSs, Warrants and Equity Instruments held of record, beneficially owned or for which voting power or disposition power is otherwise held by us. We have good and marketable title, free and clear of any liens, other than
liens pursuant to this undertaking and any transfer restrictions of general applicability as may be provided under applicable securities laws or in the terms and conditions of the Warrants and/or Equity Instruments, to all Shares, ADSs, Warrants
and/or Equity Instruments, as applicable, described in the immediately preceding sentence.
Subject to the terms and conditions hereof and of the Tender
Offer, we hereby irrevocably undertake to (i) accept the Tender Offer, and to tender or cause to be tendered and sell or cause to be sold to the Bidder the Shares, ADSs, Warrants and Equity Instruments, as applicable, that we currently own, and
any additional Shares, ADSs, Warrants and Equity Instruments, as applicable, that we may hereafter acquire or be granted prior to the earlier of the Termination Date (as defined below) and the expiration of the Tender Offer (“Additional
Instruments”), in the Tender Offer for the Offer Consideration applicable thereto, (ii) deliver or cause to be delivered evidence of such acceptance pursuant to the terms of the Tender Offer to the Bidder within ten (10) business
days from the beginning of the acceptance period of the Tender Offer, and (iii) not exercise voting rights pertaining to the Shares, ADSs and any Shares subscribed based on the Warrants, Equity Instruments or any Additional Instruments in favor
of a Competitive Transaction (as defined below), in each case subject to the following paragraph.
Subject to the terms and conditions hereof, with
respect to the Warrants and if so requested by the Bidder prior to the launch of the Tender Offer, in lieu of accepting the Tender Offer and tendering the Warrants, we hereby irrevocably undertake to, subject to and upon the consummation of the
Tender Offer and subject to this undertaking having not been previously terminated in accordance with the terms hereof, sell to the Bidder (and the Bidder hereby irrevocably undertakes to purchase from us) the Warrants that we currently own, and any
Warrants that we may hereafter acquire prior to the expiration of the Tender Offer, for the Warrant Consideration and on terms consistent with the Combination Agreement; provided that such purchase and sale may not require us to make any
representations and warranties, or to provide any covenants or other agreements, other than those that would have been required to be made or provided by us in connection with tender of the Warrants in the Tender Offer.
Subject to the terms and conditions hereof, we further undertake not to, except, in each case, in accordance with the terms of this undertaking:
(i) sell, transfer, grant any option with respect to, pledge or otherwise dispose of (collectively, “Transfer”) any of the Shares, ADSs, Warrants, Equity Instruments or Additional Instruments that we own or control prior to the
earlier of the Termination Date and the expiration of the Tender Offer; provided, that nothing in this undertaking will limit our
ability to convert any Warrants, Equity Instruments or Additional Instruments, as applicable, into Shares, indirect Transfers or Transfers of any Shares, ADSs or Warrants to one or more of our
affiliates that agree in writing to be bound hereby and assume our obligations hereunder, (ii) directly or indirectly, solicit any inquiries with respect to or solicit or accept any public or private proposal or offer (including, without
limitation, any proposal or offer to all holders of Shares, ADSs, Warrants and Equity Instruments), other than the Tender Offer, for the Shares, ADSs, Warrants, Equity Instruments and Additional Instruments we own or control prior to the earlier of
the Termination Date and the expiration of the Tender Offer (a “Competitive Transaction”) (it being understood that this undertaking is entered into solely in our capacity as a holder of Shares, ADSs, Warrants, Equity
Instruments and Additional Instruments, as applicable, and nothing in this undertaking shall (or shall require us to attempt to) limit or restrict us or any designee of us who is a director or officer of the Target from acting in such capacity or
voting in such person’s sole discretion in such capacity on any matter, however always in accordance with, where applicable, the fiduciary duties of the Board of Directors of the Target under Finnish laws); or (iii) withdraw our acceptance
of the Tender Offer in respect of any of such Shares, ADSs, Warrants, Equity Instruments or Additional Instruments regardless of any right of withdrawal contained in the terms and conditions of the Tender Offer or any legal right to withdraw, except
in accordance with the terms of this undertaking, including the withdrawal rights provided hereunder. Notwithstanding the foregoing, in the event that (A) the Target enters into discussions with a competing bidder in accordance with the
Combination Agreement and (B) the Board of Directors of the Target requests that we engage in discussions with the competing bidder, then nothing in this undertaking shall limit or restrict us from engaging in such discussions, provided that we
will not, prior to the earlier of the Termination Date and the expiration of the Tender Offer, (i) discuss, offer or otherwise negotiate with any competing bidder regarding the acquisition or other transfer to such competing bidder of any of
our Shares. ADSs, Warrants, Equity Instruments or Additional Instruments at a price lower than the Share Consideration, ADS Consideration, Warrant Consideration or Equity Instrument Consideration, as applicable, and (ii) will not enter into any
undertaking with respect to any such competing transaction unless and until this undertaking has terminated in accordance with its terms.
The Bidder
undertakes and agrees that should the terms and conditions of the Tender Offer, including the Offer Consideration, be revised or amended by the Bidder for the benefit of the holders of Shares, ADSs, Warrants and Equity Instruments, such revision or
amendment shall also be for our benefit. The Bidder further undertakes and agrees that, if the Bidder enters into an undertaking or similar agreement with any other holder of Shares, ADSs, Warrants or Equity Instruments that provides such other
holder with rights and benefits that are more favorable in any material respect to such other holder than the rights and benefits established in favor of us under this undertaking, then (i) the Bidder shall promptly provide us with a copy of
the other undertaking or agreement (and, in any case, within one business day of the execution thereof) and (ii) we shall have the right to elect to receive all such rights and benefits under the other undertaking or agreement, and this
undertaking shall be amended accordingly. In addition, for the avoidance of doubt, we expressly do not waive any rights under Chapter 11, Section 25 of the Finnish Securities Market Act applicable from time to time.
This undertaking shall remain valid and in force until the first of the following has taken place, at which date and time this undertaking shall immediately
and automatically terminate in full (the “Termination Date”): (i) the Board of Directors of the Target has failed to recommend that the holders of Shares, ADSs and Warrants accept the Tender Offer or has modified or
withdrawn such recommendation; (ii) the completion and settlement of the Tender Offer; (iii) the Bidder has made a public announcement to the effect that it will not complete the Tender Offer; (iv) the Combination Agreement has been
terminated; (v) any amendment has been made to the Tender Offer that reduces the Offer Consideration or otherwise materially changes the terms and conditions of the Tender Offer in a manner adverse to us; or (vi) the Tender Offer, having
been launched or published, has failed to be completed by June 19, 2016.
We agree not to, without the prior written consent of the Bidder (such consent not to be unreasonably withheld,
delayed or conditioned), make or cause to be made any announcement or disclosure to any third party in respect of any unpublished information concerning the contemplated Tender Offer or this undertaking except (i) for disclosure to the Target,
(ii) for disclosure required by any applicable law, regulation or order of court, governmental authority, self-regulatory organization or stock exchange or (iii) to enforce our rights under, or defend ourselves in any action brought in
connection with, this undertaking.
This undertaking shall be governed by, and construed in accordance with, the laws of Finland. All disputes arising out
of or relating to the present contract shall be finally settled by final, binding arbitration conducted under the Rules of Arbitration of the International Chamber of Commerce (the “ICC Rules”). The arbitral panel shall be comprised
of three arbitrators to be appointed as follows: within 14 days after the commencement of arbitration, each party shall select one person to act as arbitrator and the two selected shall select a third arbitrator within 10 days of their appointment.
If the arbitrators selected by the parties are unable or fail to agree upon the third arbitrator, the third arbitrator shall be selected by the ICC. Either party may apply to the arbitrator seeking injunctive relief until the arbitration award is
rendered or the controversy is otherwise resolved. Either party also may, without waiving any remedy under this undertaking, seek from any court having jurisdiction any interim or provisional relief that is necessary to protect the rights or
property of that party, pending the arbitration panel’s determination of the merits of the controversy. The arbitration shall be conducted in English in London, United Kingdom, and judgment on the award rendered by the arbitrators may be
entered in any court having jurisdiction thereof. In the event of a conflict between this paragraph and the ICC Rules, this paragraph shall govern.
We
agree and give our consent that a copy of this undertaking may be provided to the Board of Directors of the Target and that this undertaking may be referred to and the main terms and conditions hereof may be disclosed in any public announcement that
the Bidder or the Target may make in connection with the Tender Offer or in the offer document related thereto.
This undertaking is given on the
understanding that nothing in this undertaking shall cause us to be deemed to be acting in concert with the Bidder or any other holder of Shares, ADSs, Warrants or Equity Instruments of the Company.
|
| Yours faithfully, |
|
| [INSERT NAME OF HOLDER OF EQUITY INSTRUMENTS] |
|
| By:
|
| Name: |
|
|
|
| Number of Shares (not including Shares represented by ADSs or Warrants): |
|
|
|
|
|
| Number of ADSs: |
|
|
|
|
|
| Number of Warrants: |
|
|
|
|
|
| Number of 2011 Option Rights: |
|
|
|
|
|
| Number of 2014 Option Rights: |
|
|
|
|
|
| Number of 2016 Option Rights |
|
|
|
|
|
| Number of Swiss Option Rights: |
|
|
|
|
|
| Number of 2011 Share Rights: |
|
|
|
|
|
| Number of 2014 Share Rights: |
|
|
|
|
|
|
| Acknowledged and accepted. |
|
| Acorda Therapeutics, Inc. |
|
| By:
|
| Name: |
Exhibit (d)(3)
EXECUTION VERSION
Confidentiality Agreement
dated 30 November 2015
Between
ACORDA THERAPEUTICS INC.
And
BIOTIE THERAPIES CORP.
This confidentiality agreement (the “Agreement”) is entered into on 30 November 2015
by and between:
| (1) |
Acorda Therapeutics Inc., with its registered address at 420 Saw Mill River Road, Ardsley, New York 10502, United States (the “Offeror”); and |
| (2) |
Biotie Therapies Corp., with its registered address at Joukahaisenkatu 6, 20520 Turku, Finland (the “Company”); the Company and the Offeror also referred to individually as a Party and jointly as
the Parties. |
BACKGROUND
| (A) |
The Offeror has expressed an interest in exploring the possibility of acquiring 100% of the equity securities in the Company pursuant to a negotiated transaction (the “Offer”). |
| (B) |
In connection with potential discussions relating to the Offer, the Offeror may have received and may receive certain Confidential Information (as defined below). |
| (C) |
This Agreement sets out the terms and conditions on which the Company will provide any Confidential Information and on which the Offeror, in consideration of the Company agreeing to disclose any Confidential
Information, may use such Confidential Information, and similarly, the terms and conditions on which the Parties may disclose any Transaction Information. |
| (D) |
Both Parties are companies with their shares listed on a regulated market and acknowledge that the fact that discussions between the Offeror and the Company may take place, are taking place and/or have taken place may
qualify as insider information/material non-public information pursuant to applicable securities laws. |
NOW,
THEREFORE, the Parties agree as follows:
| 1 |
|
CONFIDENTIAL INFORMATION; REPRESENTATIVES |
| 1.1 |
For the purposes of this Agreement, “Confidential Information” shall mean any and all market, financial, commercial, technical and other information, whether written or oral and regardless of the manner
of disclosure and format: |
| |
(a) |
which is confidential and furnished or to be furnished by the Company or its Representatives (as defined below) to the Offeror or its Representatives (as defined below) before or after the date hereof, relating to the
Company, any of its affiliates or any of the businesses, properties, assets, operations, products, product candidates, services, liabilities, financial or other condition, employees, prospects and/or the results of operations of the Company or its
affiliates, and including, but not limited to, data, formulas, know-how, calculations, compilations, programs, drawings, products, devices, proven technology, equipment configurations, technical studies, research and development efforts, trial
results, and knowledge relating thereto, any information on costs or expenses, price lists and price breakdowns as well as client lists; and |
| |
(b) |
including all analyses, studies, presentations, information and other documents or media to the extent that they contain or otherwise reflect any information referred to in item (a) above. |
Information about the fact that discussions between the Offeror and the Company may take place, are taking
place and/or have taken place and about the contents and existence of this Agreement shall constitute “Transaction Information”.
| 1.2 |
For the purposes of this Agreement, “Representative” shall mean a Party’s directors, officers, employees, professional advisors and providers of financing relating to the Offer (which in the case
of the Offeror, shall mean any financing provider listed in Annex A and any future additions to that Annex with the consent of the Company (not to be unreasonably withheld or delayed)), as well as a Party’s subsidiaries and affiliated companies
and their respective directors, officers and employees. |
| 2 |
NON-DISCLOSURE AND USE OF CONFIDENTIAL INFORMATION |
| |
(a) |
treat, and cause and ensure that all of its Representatives to whom Confidential Information and Transaction Information is disclosed treat, any and all Confidential Information and Transaction Information as secret and
strictly confidential and otherwise in accordance with the provisions of this Agreement, and consequently not disclose any Confidential Information or Transaction Information to any third party, provided, however, that the Offeror may disclose
Confidential Information and Transaction Information to its Representatives to the extent such disclosure is necessary for the Offeror to be able to evaluate and negotiate the contemplated Offer (it being understood that the number of persons
receiving Confidential Information and Transaction Information shall be kept to a minimum); |
| |
(b) |
not make use of, and cause and ensure that any of its Representatives to whom Confidential Information is disclosed will not make use of, any part of the Confidential Information for any other purpose than the
contemplated Offer; |
| |
(c) |
instruct each of its Representatives to whom Confidential Information and Transaction Information is disclosed in accordance with Section 2.1(a) of the confidential nature of the Confidential Information and
Transaction Information and the restrictions set forth in this Agreement prior to any such disclosure; and |
| |
(d) |
be responsible for any disclosure or use of the Confidential Information or Transaction Information by the Offeror or its Representatives that does not comply with the terms of this Agreement. |
| 2.2 |
The obligations of the Offeror and its Representatives under Section 2.1 will not restrict the use or disclosure of Confidential Information by the Offeror or its Representatives to the extent that the Offeror
demonstrates that: |
| |
(a) |
such Confidential Information was already lawfully and other than by reason of any breach of this Agreement in the possession of the Offeror (as evidenced by written or electronic records of the Offeror) at the time of
its disclosure to the Offeror or any of its Representatives by the Company or any of its Representatives and was not, to the knowledge of the Offeror or its Representatives, obtained through a source subject to any other confidentiality undertaking
to the Company; |
| |
(b) |
such Confidential Information is or becomes generally publicly available other than as a result of its unauthorised disclosure by the Offeror or its Representatives; |
| |
(c) |
such Confidential Information becomes available to it or its Representatives lawfully and independently from a source that was not, to the knowledge of the Offeror or its Representatives, subject to any other
confidentiality undertaking to the Company; or |
| |
(d) |
such information is independently developed by employees of the Offeror. |
| |
It is expressly acknowledged and agreed to by the Offeror that the fact that discussions between the Offeror and the Company may take place, are taking place and/or have taken place is not information that falls within
this Section 2.2. |
| |
(a) |
treat, and cause and ensure that all of its Representatives to whom such information is disclosed treat, the Transaction Information as secret and strictly confidential and otherwise in accordance with the provisions of
this Agreement, and consequently not disclose any Transaction Information to any third party, provided, however, that the Company may disclose such information to its Representatives to the extent such disclosure is necessary for the Company to be
able to evaluate and negotiate the contemplated Offer (it being understood that the number of persons receiving such information shall be kept to a minimum); |
| |
(b) |
instruct each of its Representatives to whom Transaction Information is disclosed in accordance with Section 2.3(a) of the confidential nature of the Transaction Information, and the restrictions set forth in this
Agreement prior to any such disclosure; |
| |
(c) |
be responsible for any disclosure of the Transaction Information by the Company or its Representatives that does not comply with the terms of this Agreement; and |
| |
(d) |
comply, with respect to such Transaction Information, with the same confidentiality measures as set out for the Offeror in Sections 3 and 12 hereof. |
| 2.4 |
This Agreement shall not prohibit the Offeror from disclosing Confidential Information or either Party from disclosing the Transaction Information that is required to be disclosed by mandatory provisions of law or
rules, by the order of any court or other competent tribunal or regulatory authority of competent jurisdiction or by the rules and regulations of any stock exchange on which its shares or other securities are listed, provided that the disclosing
Party shall to the extent legally permissible provide the other Party with prompt written notification prior to any disclosure of the circumstances of the required disclosure and of the information that the other Party (or its Representative, as
applicable) proposes to be disclosed, in order to enable the other Party to evaluate and contest such disclosure or seek a protective order or other appropriate remedy in that connection, and if so requested, assist the other Party in seeking such
order or remedy, and to the extent that no such protective order or remedy is obtained, disclose only that portion of Confidential Information or Transaction Information and only to the authority and in the manner that is required under law or the
relevant order of the court or other competent tribunal as set out above, and use its reasonable best efforts to ensure continued confidential treatment for the Confidential Information or Transaction Information so disclosed. Notwithstanding any
disclosure pursuant to this Section 2.4, the disclosing Party and its Representatives will continue to be bound by this Agreement with respect to the information disclosed pursuant to this Section 2.4 For the avoidance of doubt, the costs
and expenses associated with requesting a protective order or other appropriate remedy in accordance with this Section 2.4 shall be borne by the Party requesting such protective order or remedy. |
Before making any public announcement, press release or other
public disclosure relating to the Offer, this Agreement or the fact that discussions between the Offeror and the Company may take place, are taking place and/or have taken place, the Party making such public disclosure, in the case of both
(i) and (ii) to the extent legally permissible and practically possible shall: (i) consult with the other Party on the content and timing of any such public disclosure, and (ii) no such public disclosure shall be made without the
prior written consent of the other Party. Without consulting and obtaining the prior written consent the other Party, a Party may only make a public announcement, press release or other public disclosure that is required by the mandatory laws or
regulations or the rules and regulations of a stock exchange on which its shares or other securities are listed, and only provided that it is not practically possible to consult and obtain the consent of the other Party prior to such public
disclosure, and that in such disclosure, the identity of the other Party shall not be disclosed unless required by mandatory law or regulation or the rules and regulations of a stock exchange on which the shares or other securities of the disclosing
Party are listed. The circumstances, timing, content and manner of any such public announcement, press release or other public disclosure made by a Party without consulting or obtaining the prior written consent of the other Party, shall be
disclosed to the other Party immediately following such public announcement, press release or other public disclosure, to the extent legally permissible and practically possible. Specifically and notwithstanding anything else in this Agreement, the
Offeror shall not take a decision to make the Offer which would require that an announcement of the launch of an Offer is made without the prior written consent of the Company, except that, for the avoidance of doubt, the aforementioned and this
Section shall not apply to potential announcements by the Offeror should the standstill obligations set forth in Section 11 of this Agreement be terminated pursuant to Section 11.3 below.
| 4 |
CONFIDENTIALITY MEASURES |
To secure the confidentiality of the Confidential Information
and Transaction Information, the Offeror shall, and shall use commercially reasonable efforts to ensure that its Representatives shall, without limitation, (i) store all documents or materials containing Confidential Information or Transaction
Information safely and to protect them against theft and any unauthorised access; (ii) only make copies of the Confidential Information or Transaction Information to the extent required for allowing the Offeror and its Representatives to
evaluate the Offer; (iii) not use, reproduce, transform or store any of the Confidential Information or Transaction Information in a computer or electronic information retrieval system that is known to be accessible by third parties;
(iv) inform the Company immediately upon becoming aware of any actual or imminent unauthorised use or disclosure of Confidential Information or Transaction Information, and take all reasonable steps to assist the Company in preventing or
stopping such unauthorised use or disclosure; and (v) respond forthwith to any reasonable query by the Company regarding the use, storage or alleged disclosure of the Confidential Information or Transaction Information received by it or any of
its Representatives.
| 5 |
CONTACTS WITH THE COMPANY |
The Offeror shall ensure that any contacts by the Offeror
and its Representatives with the Company relating or referring to the Offer, this Agreement or the actions referred to herein shall be directed only to the Company’s President and CEO, Timo Veromaa, the Chairman of the Board of Directors of the
Company, William M. Burns and the Chief Financial Officer of the Company, David Cook as well as the Company’s advisors Davis Polk & Wardwell LLP, Guggenheim Securities, LLC and Hannes Snellman Attorneys, Ltd. For the avoidance of
doubt,
no contact shall be made with any other Representatives or shareholders of the Company without
the prior written consent of the Company. For the sake of clarity, the above provisions of this Section 5 do not apply to the extent that a contact is made in the ordinary course of business and not in any way relating or referring to the
Offer, this Agreement or the actions referred to herein.
| 6 |
NON-SOLICITATION OF EMPLOYEES |
The Offeror agrees that for a period of twelve
(12) months after the date hereof, it shall not, and shall use its reasonable best efforts to ensure that its Representatives shall not, without the prior written consent of the Company, directly or indirectly employ, engage or seek to employ
or engage, through solicitation, recruitment or otherwise, any director, officer or employee of the Company or its controlled affiliates, with the exception of (and only to the extent) hiring (i) a person who is responding to a general
advertisement, provided that the advertising is not specifically directed to persons referred to herein, (ii) a person who contacts the Offeror on her or his own initiative without any direct or indirect solicitation and (iii) a person has
not been employed by the Company or its controlled affiliates during the six (6) months prior to such employment, engagement or seeking to employ or engage. For the purposes of this Section 6, the term Representatives shall not include the
Offeror’s professional advisors and providers of financing.
All Confidential Information shall remain the sole property of the
Company, and nothing in this Agreement shall be considered, by implication or otherwise, as a grant of any right or licence by the Company to the Offeror or any of its Representatives to any of the Confidential Information or to any intellectual
property embodied in such Confidential Information.
The Offeror acknowledges and agrees that (i) no express or implied
representation or warranty is being made hereunder by the Company or any other party as to the correctness, completeness or accuracy of the Confidential Information or other information provided; and (ii) the Company and its Representatives are
under no obligation to update the Confidential Information provided to the Offeror or its Representatives or to notify the Offeror or its Representative of or to correct any inaccuracies in the Confidential Information (even if such inaccuracies are
discovered subsequent to the provision of the Confidential Information). The Company or its Representatives shall have no liability to the Offeror or any other person resulting from the use of such Confidential Information or other information by
the Offeror, its Representatives or any other parties, whether permitted under this Agreement or not, or with respect to their reliance upon the completeness and accuracy of any such information.
The Offeror agrees that it will not place any reliance on any statement,
representation, warranty or undertaking (written or oral or in any other form) made by the Company or its Representatives in connection with the Confidential Information or the Offer, and will be responsible for making its own decisions in relation
to the Confidential Information and the Offer.
| 10.1 |
The Company is a company listed on the Nasdaq OMX Helsinki Oy stock exchange and the NASDAQ Global Select Market and, as such, any and all inside information concerning the Company is subject to the provisions and
restrictions of the Finnish Securities Markets Act and the U.S. securities laws. The Offeror acknowledges the possibility of having become or becoming an insider under the Finnish Securities Markets Act or other applicable laws and regulations and
undertakes to: |
| |
(a) |
advise itself and, before any disclosure of Confidential Information to such persons, its Representatives of the applicable securities laws and regulations and the disclosure and trading restrictions imposed thereunder;
|
| |
(b) |
refrain from, and to use commercially reasonable efforts to ensure that its Representatives to whom Confidential Information is disclosed shall refrain from, acting in any manner that could breach such laws or
regulations; |
| |
(c) |
limit access to Confidential Information in accordance with this Agreement; |
| |
(d) |
keep a list identifying all persons, and procure that each of its professional advisers shall keep a list identifying all persons at their respective organisations, to whom Confidential Information is disclosed or who
are aware of the preparations relating to the Offer (such lists also to include information on when each of the relevant persons has obtained information for the first time); and |
| |
(e) |
disclose the lists referred to above in sub-section (d) to the Company or the Finnish Financial Supervisory Authority or other competent authorities when and if so requested by the Company due to applicable
regulatory requirements or such authority (with the understanding that the Company may be obliged to further disclose the list to the relevant authorities). |
| 10.2 |
The Offeror acknowledges and agrees that it and any of its Representatives is or may be entered as an insider in the relevant insider register(s) of the Company, as applicable, and will comply with the statutory
requirements relating to such entry. |
| 10.3 |
The Company acknowledges the possibility of having become or becoming an insider under applicable laws and regulations and undertakes to refrain from, and to use commercially reasonable efforts to ensure that its
Representatives shall refrain from, acting in any manner that could breach such laws or regulations. |
| 10.4 |
For the avoidance of doubt and unless otherwise agreed between the Parties, the Company will use its reasonable efforts to ensure that Confidential
Information (other than information relating to the contemplated Offer) to be provided by the Company or its Representatives to the Offeror or its Representatives in connection with the discussions relating to the Offer will not include inside
information as defined in the Finnish Securities Markets Act and in the U.S. securities laws. Before disclosing any inside information (or information that may, in the Company’s reasonable opinion, constitute inside information) to the Offeror
or its Representatives, the Company shall ask whether or not the Offeror wishes to receive such information. Should the Offeror agree to receive such information and such information is disclosed to the Offeror or its Representatives, the Parties
shall use their reasonable efforts to negotiate in good faith measures to enable the |
Offeror to announce, launch and complete the Offer without breaching any applicable insider laws
or regulations.
| 11.1 |
Until the earliest of (i) the receipt by the Offeror of the prior written consent of the Company or (ii) the expiration of a period of twelve (12) months from the date of this Agreement, the Offeror shall
not (whether alone or in concert with others, or in one or a series of transactions), and shall cause its affiliates and associates (as such terms are defined in Rule 12b-2 of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange
Act”)) not to: |
| |
(a) |
purchase or effect any transaction in the securities or assets of the Company or any warranty, option or other right to acquire such securities or assets or entice any person to do so; |
| |
(b) |
do or omit to do any act as a result of which the Offeror (whether alone or in concert with others) purchases any interests in the securities of the Company; |
| |
(c) |
make, or procure or induce any other person to make, any offer for all or any of the securities of the Company or do or omit to do any act as a result of which the Offeror (whether alone or in concert with others) may
become obliged to make an offer for all or any of the securities in the Company; |
| |
(d) |
enter, agree to enter, propose, seek or offer to enter into or facilitate, advise or assist any person in examining or effecting, any merger, business combination, recapitalization, restructuring or other extraordinary
transaction involving the Company or any subsidiary of the Company; |
| |
(e) |
obtain any direct or indirect interest in such securities; |
| |
(f) |
make, or in any way participate in, any solicitation of voting rights attaching to the securities of the Company, or vote, or seek to advise or to influence any person with respect to the voting of, the securities of
the Company; |
| |
(g) |
form, join or in any way participate in a “group” (within the meaning of Section 13(d)(3) of the Exchange Act) with respect to any voting securities of the Company; |
| |
(h) |
call, request the calling of, or otherwise seek or assist in the calling of a special meeting of the shareholders of the Company; |
| |
(i) |
otherwise act to seek control over or to influence the Board of Directors or the policies of the Company; disclose publicly any intention, plan or arrangement to undertake any of the foregoing, or |
| |
(j) |
advise, assist or knowingly encourage any other person in connection with, or enter into an agreement to undertake, any of the foregoing. |
For the avoidance of doubt, the Offeror may, however, always make a tender offer for all the securities of the Company if such tender offer is
supported by the Board of Directors of the Company.
| 11.2 |
The Offeror shall not (and will ensure that its affiliates and associates and any person acting on behalf of or in concert with the Offeror or any of its affiliates or associates will not) directly or indirectly without
the prior written consent of the Company (x) make any request, directly or indirectly, to amend or waive any provision of this Section 11 or (y) take any action that would reasonably be expected to require the Company to make a public
announcement regarding the possibility of a business combination, merger or other type of transaction described in this Section 11 with the Offeror or any of its affiliates or associates; provided that the foregoing shall not prevent the
Offeror from submitting a private, confidential proposal to the Company’s Board of Directors. To the extent that the Offeror (whether alone or in concert with others) purchases an interest in securities of the Company in breach of this
Agreement, the Offeror must immediately dispose or procure the disposal of such interest (including holdings of any parties acting in concert with the Offeror to the extent within the control of the Offeror) to independent third parties. Pending
such disposal, the Offeror shall not, and shall procure that any other party acting in concert with it (to the extent within the control of the Offeror) shall not, exercise any rights attached to any such interest in securities. The aforementioned
shall not affect or limit any other remedies available for such breach. |
| 11.3 |
Notwithstanding the above, the standstill restrictions contained in this Section 11 shall terminate immediately in the event that: (i) any third party unaffiliated with the Offeror announces a tender offer or
exchange offer for the securities of the Company and, within the time periods required under applicable law, the Company’s Board of Directors has not publicly recommended that the shareholders of the Company reject such offer, (ii) the
Company enters into and announces an agreement with respect to a tender offer or exchange offer for the securities of the Company with a third party offeror; or (iii) the Company enters into and announces an agreement to combine or merge with,
or sell or dispose of 50% or more of its assets or equity securities (however structured), to any party not affiliated with the Offeror or any of its affiliates. |
| 11.4 |
For the sake of clarity, the expiry of the obligations of the Offeror or any of its Representatives under this Section 11 shall in no manner affect the obligations of the Offeror or any of its Representatives set
out in Section 10 above or any other obligation or requirement following from laws or regulations relating to the possession of inside information or otherwise. |
| 12 |
DESTRUCTION OF CONFIDENTIAL INFORMATION |
| 12.1 |
Offeror agrees to inform the Company promptly of any determination by it not to proceed with its consideration of the Offer. Following a request by the Company, the Offeror shall promptly, and in any case within ten
(10) days, destroy or return to the Company (as instructed by the Company) all written Confidential Information and any other media or written material containing or reflecting any of the Confidential Information in the possession of the
Offeror or its Representatives, together with any reproductions and copies, and to confirm any such destruction or return to the Company in writing. |
| 12.2 |
As the only exception to Section 12.1, (i) the Offeror and its Representatives shall not be required to expunge Confidential Information
from automatic computer back-up archive systems maintained as part of established written or electronic record retention policies (provided that the foregoing shall not be deemed to permit the accessing, retrieval or use thereof) and (ii) the
Offeror and its Representatives shall be entitled to retain such part of the Confidential Information which is required to be retained by them in order to comply with applicable mandatory laws, regulations and professional standards imposed by
relevant governmental, supervisory or regulatory bodies or with internal procedures for legal, compliance and regulatory purposes, provided in each case that the obligations of the Offeror and its Representatives
|
regarding confidentiality and use of such Confidential Information shall remain in force under
the terms of this Agreement until such Confidential Information has been destroyed.
The Parties recognise and acknowledge the confidential nature of the
Confidential Information and the Transaction Information, and that strict compliance with this Agreement is of utmost importance for the Parties, and that any unauthorised disclosure of Confidential Information or Transaction Information may cause
considerable and irreparable damage to the Company or the Offeror, as the case may be. If a Party or any of its Representatives breaches this Agreement, such Party shall be liable for any loss incurred by, or damage caused to the other Party or to
its directors, officers or employees due to such breach. Each Party furthermore acknowledges that monetary damages may not be a sufficient remedy for a breach of this Agreement and that the Parties shall be entitled, without prejudice to any other
rights or remedies available to it under applicable law or otherwise, to injunctive (in Finnish: turvaamistoimenpide) or equitable relief for any threatened or actual breach of this Agreement as may be deemed appropriate by a court of
competent jurisdiction.
| 14.1 |
All notices and other communication arising out of or relating to this Agreement shall be in writing in the English language and shall be sent by courier, first class mail, fax or e-mail to the relevant Party at the
following address or fax number or at such other address or fax number which has been provided in accordance with this Section 14.1: |
|
|
|
| If to the Company: |
|
|
| Biotie Therapies Corp. address: |
|
Joukahaisenkatu 6, 20520 Turku, Finland |
| fax: |
|
+358 2 274 8910 |
| e-mail: |
|
timo.veromaa@biotie.com |
| attention: |
|
Timo Veromaa |
|
|
| If to the Offeror: |
|
|
| Acorda Therapeutics Inc. address: |
|
420 Saw Mill River Road, Ardsley, New York 10502, United States |
| fax: |
|
914-606-9737 (U.S.) |
| e-mail: |
|
jwasman@acorda.com |
| attention: |
|
Jane Wasman, President, International and General Counsel |
| 14.2 |
The obligations under this Agreement, except as provided in Section 5 (Non-Solicitation of Employees), Section 11 (Standstill), Section 13 (Remedies), and Section 15 (Governing Law and Arbitration),
shall terminate upon the date occurring three (3) years from the date hereof regardless of any termination of this Agreement. |
| 14.3 |
Nothing herein shall be deemed to (i) prevent the Company or the Offeror from discontinuing or terminating any discussions relating to the Offer
at any time; (ii) prevent the Company from withholding any Confidential Information for whatever reason at whatever time; (iii) create any obligation upon the Offeror to make any Offer; or (iv) create any right of exclusivity for the
Offeror for conducting negotiations with the Company in relation to the possible acquisition of the Company. Each Party further agrees that notwithstanding anything in this Agreement to the contrary, neither Party nor its Representatives will be
under any legal obligation of any kind with |
respect to the Offer by virtue of this Agreement except for the matters specifically agreed to
herein nor shall the Company have any obligation to provide any Confidential Information to the Offeror, and the Company may terminate the Offeror’s and its Representatives’ access to Confidential Information at any time.
| 14.4 |
Each Party shall bear all its own costs and expenses, including but not limited to the costs of its advisors, in connection with any access to information and the negotiations for and the preparation of possible
agreements relating to the Offer. |
| 14.5 |
This Agreement constitutes the entire agreement between the Parties relating to the subject matter hereof and may not be amended except in writing and duly executed by both Parties. |
| 14.6 |
If any provision of this Agreement is declared to be invalid or unenforceable, the remaining provisions of this Agreement shall not be affected thereby but shall remain in full force and effect and be binding upon the
Parties. Without prejudice to the aforesaid, the Parties shall attempt through negotiations in good faith to replace the invalid or unenforceable provision with a provision closest to the mutually intended meaning of such provision and the spirit of
this Agreement. |
| 15 |
GOVERNING LAW AND ARBITRATION |
| 15.1 |
This Agreement shall be governed by and construed in accordance with the laws of Finland, without giving effect to its conflict of laws principles. |
| 15.2 |
Any dispute, controversy or claim arising out of or relating to this Agreement, or the breach, termination or validity thereof, shall be finally settled by arbitration in accordance with the Arbitration Rules of the
Finland Chamber of Commerce (it being understood and agreed that any injunctive or equitable relief may also be sought in the relevant district court with appropriate jurisdiction). The seat of arbitration shall be Helsinki, Finland, and the
language of the arbitration shall be English. |
IN WITNESS WHEREOF, the Parties have duly executed this Agreement in two (2) identical counterparts
on the day and year first above written.
|
|
|
| ACORDA THERAPEUTICS INC. |
|
|
|
|
/s/ Andrew Hindman |
| By: |
|
Andrew Hindman |
| Title: |
|
Chief Business Development Officer |
|
| BIOTIE THERAPIES CORP. |
|
|
|
|
/s/ Timo Veromaa |
| By: |
|
Timo Veromaa |
| Title: |
|
Chief Executive Officer |
BIOTIE THERAPIES CORP. (NASDAQ:BITI)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024

BIOTIE THERAPIES CORP. (NASDAQ:BITI)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024
