Eon Labs Receives Final Approval for Bupropion HCl, ER 200mg Tablets and Will Begin Shipping Immediately
23 Juin 2005 - 11:13PM
Business Wire
Eon Labs, Inc. (Nasdaq: ELAB) announced today that it received
final approval for Bupropion HCl, ER 200mg tablets, the generic
equivalent of Wellbutrin SR(R)* 200mg tablets. The Company will
begin shipping immediately. Eon Labs is a generic pharmaceutical
company specializing in developing, licensing, manufacturing,
selling and distributing a broad range of prescription
pharmaceutical products. For press releases and other company
information, visit the Eon Labs, Inc. website at www.eonlabs.com.
Safe Harbor Statement under the U.S. Private Securities Litigation
Reform Act of 1995: This release contains statements that are
forward-looking in nature which express the beliefs and
expectations of management. Such statements are based on current
plans, estimates and expectations and involve a number of known and
unknown risks, uncertainties and other factors that could cause the
Company's future results, performance or achievements to differ
significantly from the results, performance or achievements
expressed or implied by such forward-looking statements. These
factors and additional information are discussed in the Company's
filings with the Securities and Exchange Commission and statements
in this release should be evaluated in light of these important
factors. Although we believe that these statements are based upon
reasonable assumptions, we cannot guarantee future results.
Forward-looking statements speak only as of the date on which they
are made, and the Company undertakes no obligation to update
publicly or revise any forward-looking statement, whether as a
result of new information, future developments or otherwise. *
Wellbutrin SR(R) is a registered trademark of GlaxoSmithKline and
is not affiliated with Eon Labs, Inc.
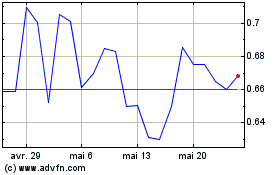
Elevai Labs (NASDAQ:ELAB)
Graphique Historique de l'Action
De Mai 2024 à Juin 2024
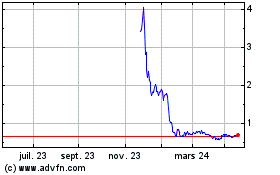
Elevai Labs (NASDAQ:ELAB)
Graphique Historique de l'Action
De Juin 2023 à Juin 2024