false 0001576263 0001576263 2023-08-07 2023-08-07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 7, 2023
MIRATI THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-35921 |
|
46-2693615 |
| (State of incorporation) |
|
(Commission File No.) |
|
(IRS Employer Identification No.) |
3545 Cray Court, San Diego, California 92121
(Address of principal executive offices and zip code)
Registrant’s telephone number, including area code: (858) 332-3410
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock |
|
MRTX |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
On August 8, 2023, Mirati Therapeutics, Inc. (the “Company”) issued a press release announcing its financial results for the three and six months ended June 30, 2023. A copy of this press release is attached hereto as Exhibit 99.1.
The information in this Item 2.02 and the exhibit hereto are being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section, nor shall they be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such a filing.
| Item 5.02 |
Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. |
Mr. Meek Separation
On August 7, 2023 (the “Meek Separation Date”), David Meek resigned as a member of the Company’s board of directors and as the Company’s Chief Executive Officer and principal executive officer. Mr. Meek’s resignation was not the result of a disagreement with the Company on any matter relating to the Company’s operations, policies or practices.
The Company and Mr. Meek entered into a separation agreement, pursuant to which Mr. Meek will receive the severance compensation provided for under Section 2.1 of the Company’s Executive Severance Plan (the “Severance Plan”) in exchange for Mr. Meek providing the Company with a standard release.
In addition to the severance compensation provided under Section 2.1 of the Severance Plan, if the Company publicly announces the execution of a definitive agreement to consummate a Change in Control (as defined in the Severance Plan) within three months following the termination of the Consulting Period (as defined below) and consummates the Change in Control within 12 months of the date of such public announcement, then in lieu of any additional compensation otherwise provided for in Section 2.2 of the Severance Plan, and provided that Mr. Meek signs and returns to the Company an additional release, the Company will pay Mr. Meek a cash lump sum payment in an amount equal to the sum of (i) the product of the number of restricted stock units Mr. Meek forfeited as of the Meek Separation Date and the per share merger consideration as defined within the definitive Change in Control agreement plus (ii) the in-the-money value of all outstanding options as of the Meek Separation Date.
Additionally, the Company and Mr. Meek will enter into a consulting relationship through October 15, 2023, unless earlier terminated (such period, the “Consulting Period”), whereby Mr. Meek will provide strategic advice and counseling as requested by the Company’s Board. As the sole consideration for Mr. Meek’s consulting services, the Company will extend the period during which Mr. Meek may exercise his outstanding stock options to the date that is 90 days following the termination of the Consulting Period. Mr. Meek will not continue to vest as to any of his outstanding equity awards as of the Meek Separation Date as a result of the consulting relationship.
Appointment of Dr. Baum as Interim Chief Executive Officer
The Company’s board of directors has, effective as of the Meek Separation Date, appointed Charles M. Baum, M.D., Ph.D., in addition to his other titles, as Interim Chief Executive Officer of the Company and as the Company’s principal executive officer.
Dr. Baum, age 64, has served as our President, Founder, and Head of Research and Development since September 2021 and previously served as our Chief Executive Officer from November 2012 to September 2021. Dr. Baum also has served as a member of our Board of Directors since November 2012. Dr. Baum received his M.D. and Ph.D. (Immunology) degrees from Washington University School of Medicine in St. Louis, Missouri and completed his post-doctoral training at Stanford University.
In connection with Dr. Baum’s appointment, the Company has agreed, effective as of the Meek Separation Date, to (i) increase Dr. Baum’s annual base salary to $750,000 through the later of December 31, 2023 or the date his successor is duly appointed by the Company’s board of directors, (ii) increase Dr. Baum’s target bonus percentage to 65% of his annual base salary (prorated for his service as Interim Chief Executive Officer from the Meek Separation Date through the later of December 31, 2023 or the date his successor is duly appointed by the Company’s board of directors), and (iii) grant Dr. Baum a restricted stock unit award with a $2,000,000 target value, with the number of shares to be calculated based on the Company’s closing price on August 9, 2023 (the “RSU Grant”). The RSU Grant will vest in full on August 9, 2024, subject to Dr. Baum’s continuous service through each such date.
There are no family relationships between Dr. Baum and any of the Company’s current or former directors or executive officers. Dr. Baum is not a party to any transaction that would require disclosure under Item 404(a) of Regulation S-K promulgated under the Securities Act.
Executive Retention Awards
The compensation committee of the Company’s board of directors granted restricted stock units (the “Retention Grants”) with a $825,000 target value, with the number of shares to be calculated based on the Company’s closing price on August 9, 2023, to the following executive officers: James Christensen, Ph.D., the Company’s Chief Scientific Officer, Ben Hickey, the Company’s Chief Commercial Officer, John Moriarty, the Company’s Chief Legal Officer and Corporate Secretary, Alan Sandler, M.D., the Company’s Chief Medical Officer, and Laurie Stelzer, the Company’s Chief Financial Officer. The Retention Grants vest in full on August 9, 2024, subject to such recipient’s continuous service through the vesting date.
Reduction in Board Size
In connection with Mr. Meek’s resignation from the Company’s board of directors, the Company’s board of directors reduced the size of the board to nine directors.
| Item 7.01 |
Regulation FD Disclosure. |
On August 8, 2023, the Company issued a press release announcing Mr. Meek’s resignation and the appointment of Dr. Baum. A copy of this press release is attached hereto as Exhibit 99.2.
The information in this Item 7.01, including the press release attached as Exhibit 99.2, shall not be deemed “filed” for the purposes of or otherwise subject to the liabilities under Section 18 of the Exchange Act. Unless expressly incorporated into a filing of the Company under the Securities Act or the Exchange Act, the information contained in this Item 7.01, including the press release attached as Exhibit 99.2, shall not be incorporated by reference into any Company filing, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
On August 8, 2023, the Company released a corporate presentation, which provides updated interim data from KRYSTAL-7 and initial data on MRTX1719. The revised corporate presentation was made available on the Company’s website. A copy of the corporate presentation is attached as Exhibit 99.3 and is incorporated into this Current Report on Form 8-K by reference.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: August 8, 2023 |
|
MIRATI THERAPEUTICS, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ John B. Moriarty, Jr. |
|
|
|
|
|
|
John B. Moriarty, Jr. |
|
|
|
|
|
|
Chief Legal Officer & Corporate Secretary |
Exhibit 99.1

Mirati Therapeutics Reports Second Quarter 2023 Financial Results and Recent Corporate Updates
Shares plans to initiate Phase 3 combination study evaluating adagrasib with pembrolizumab in first line
non-small cell lung cancer in patients with TPS ≥ 50%
Announces compelling initial clinical data for MRTX1719 in MTAP-deleted cancers demonstrating favorable safety profile and early signs of
strong clinical activity
David Meek to depart Company, Charles M. Baum, M.D., Ph.D., President and Founder, to assume role of
interim CEO during search for permanent CEO
Company will announce financial results for the second quarter 2023 along with
recent corporate updates during a conference call at 5:30 p.m. ET / 2:30 p.m. PT
SAN DIEGO – August 8, 2023 – Mirati
Therapeutics, Inc.® (NASDAQ: MRTX), a commercial stage biotechnology company, today announced financial results for the second
quarter 2023 along with recent pipeline and corporate updates.
“We are pleased to share the significant progress made during the second quarter of
2023, highlighted by an update to the clinical data and articulation of our path to develop KRAZATI® in front line non-small cell lung cancer (NSCLC). Coupled with strong second quarter KRAZATI sales performance, these data reinforce our belief that KRAZATI is the
best-in-class KRASG12C inhibitor with significant potential to positively impact the lives of patients living with
cancer,” said Charles Baum, M.D, Ph.D., interim CEO, president and founder, Mirati Therapeutics, Inc. “Further, we advanced our robust pipeline of targeted oncology programs, including MRTX1719, our potentially first-in-class MTA cooperative PRMT5 inhibitor, where we demonstrated a favorable safety profile, proof of mechanism and compelling early clinical activity in patients with
MTAP-deleted cancers. Looking ahead, we are confident in the potential of our broad pipeline of innovative, potentially best-in-class programs including MRTX1133 and our
next generation of KRAS inhibitors, as well as MRTX1719 and MRTX0902, our SOS1 inhibitor. We continue to demonstrate our proven expertise in the discovery and development of transformational treatments to people living with cancer.”
Pipeline Updates
Adagrasib (Potent and selective KRASG12C inhibitor)
| |
• |
|
In August, the Company shared updated clinical data in first-line NSCLC for the combination of adagrasib
with pembrolizumab. The Company announced plans to begin enrolling patients with TPS ≥ 50% in a Phase 3 clinical study by year-end 2023. |
| |
• |
|
In August, the Company shared that KRYSTAL 17, a Phase 2 clinical study evaluating adagrasib in
combination with chemo-immunotherapy for patients with TPS < 50% has been initiated. The Company intends to provide an update on this study and outline potential registrational development plans in 2024. |
| |
• |
|
In July, the company announced the European Medicine Agency’s (EMA) Committee for Medicinal Products for
Human Use (CHMP) issued a negative opinion on the Conditional Marketing Authorisation Application (MAA) for KRAZATI® (adagrasib) for the treatment of patients with KRASG12C -mutated advanced NSCLC. The evaluation of the full MAA is not impacted and will be considered by the CHMP following the data from KRYSTAL-12, a Phase 3
clinical study of adagrasib versus docetaxel in second line NSCLC patients. The Company has requested a formal reexamination of the Conditional MAA. (View Release) |
| |
• |
|
In June, the Journal of Clinical Oncology published clinical results from the
KRYSTAL-1 study of adagrasib, a potent and selective KRASG12C inhibitor, demonstrating durable intracranial activity in patients living
with KRASG12C-mutated NSCLC with untreated central nervous system metastases. (View Release) |
| |
• |
|
In June, the Company presented updated clinical data for adagrasib as a targeted treatment for KRASG12C-mutated advanced pancreatic ductal adenocarcinoma (PDAC), biliary tract cancer and other solid tumors at the American Society of Clinical Oncology (ASCO) Annual Meeting. Based on these results,
the Company plans to discuss a tumor agnostic Accelerated Approval approach with FDA by year-end 2023. (View Release) |
| |
• |
|
The Company is on track to complete a supplemental New Drug Application (sNDA) for third-line and beyond
colorectal cancer in patients with a KRASG12C mutation by year-end 2023. |
| |
• |
|
The Company continues to enroll in KRYSTAL-10, a Phase 3 registrational
clinical study in second-line colorectal cancer patients, evaluating the combination of adagrasib plus cetuximab versus chemotherapy. The Company expects to complete enrollment by year-end 2023 and
plans to share top line results in 2024. |
| |
• |
|
The Company continues to enroll in KRYSTAL-12, a Phase 3 clinical study
of adagrasib versus docetaxel in second line NSCLC patients. The Company plans to share data from this study in 2024. |
MRTX1719 (MTA cooperative PRMT5 inhibitor)
| |
• |
|
In August, the Company presented initial clinical data from the Phase 1 dose escalation clinical study evaluating
MRTX1719, an MTA cooperative PRMT5 inhibitor, in patients with solid tumors harboring MTAP-gene deletions, demonstrating a favorable safety profile and early signs of clinical activity. A manuscript characterizing the Company’s preclinical
and early clinical experience with 1719 including case studies from the Phase 1 study was accepted by Cancer Discovery and will publish online on August 8, 2023. The Company expects to initiate a Phase 2 study in the first half of 2024.
|
MRTX1133 (Potent and selective KRASG12D inhibitor)
| |
• |
|
The Company continues to enroll patients in the Phase 1/2 clinical study with plans to share initial clinical
data in the first half of 2024. |
MRTX0902 (Potent SOS1 inhibitor)
| |
• |
|
In July, the Company initiated a cohort within the Phase 1/2 trial evaluating the combination of MRTX0902 plus
adagrasib with plans to share initial clinical data in 2024. |
Sitravatinib (Potent TAM receptor inhibitor)
| |
• |
|
In May, the Company announced that the Phase 3 SAPPHIRE study evaluating sitravatinib plus nivolumab
(OPDIVO®)1 in second or third line non-squamous NSCLC study did not meet its primary endpoint of
overall survival at the final analysis. The Company plans to disclose study data at an upcoming medical meeting. (View Release) |
Recent Corporate Updates
| |
• |
|
In August, the Company announced that David Meek departed as CEO. Charles Baum, M.D., Ph.D., will assume the role
of interim CEO while the Company searches for a permanent CEO. |
| |
• |
|
In June, the Company announced Carol Gallagher, Pharm.D. was appointed to the Company Board of Directors as an
independent director. (View Release) |
Second Quarter Financial Results
| |
• |
|
Cash, cash equivalents and short-term investments of approximately $779.4 million as of June 30, 2023.
Net reduction in cash, cash equivalents and short-term investments for the second quarter of 2023 was $122.9 million. The Company expects 2023 net cash burn to annualize within a range of $560 million to $580 million.
|
| |
• |
|
Net KRAZATI® product revenue for the three and six
months ended June 30, 2023 was $13.4 million and $19.7 million, respectively. Net product revenue during the three months ended June 30, 2023 was comprised of $11.7 million of commercial sales and $1.7 million of sales
to a third-party commercial customer for its clinical trials. There was no product revenue for the same periods in 2022. |
| |
• |
|
License and collaboration revenue for the three and six months ended June 30, 2023 was $0.3 million and
$1.2 million, respectively, related to clinical supply revenue earned under the agreement with Zai Lab. License and collaboration revenue for the same periods in 2022 was $5.4 million and $6.1 million, respectively, related to a
$5 million milestone payment from Zai Lab for the initiation of the first pivotal clinical trial of adagrasib for the first indication in China, and clinical supply revenue earned under the agreement with Zai Lab. |
| |
• |
|
Cost of product revenue for the three and six months ended June 30, 2023 was $1.3 million and
$2.1 million, respectively, of which $1.0 million and $1.6 million, respectively, related to product manufacturing and distribution costs, and royalties incurred on net sales of
KRAZATI®, and the remainder represented non-cash amortization expense for our intangible asset. There was no cost of product revenue for the same
periods in 2022. |
| |
• |
|
Research and development expenses for three and six months ended June 30, 2023 were $124.2 million and
$250.9 million, respectively, compared to $128.3 million and $259.3 million for the same periods in 2022, respectively. The decrease was primarily driven by a reduction in clinical development costs for sitravatinib as enrollment was
completed in the SAPPHIRE Phase 3 clinical study in the second quarter of 2022, and a decrease in share-based compensation due to a decrease in the fair value of equity awards granted during the period, partially offset by increases in costs for
earlier stage clinical development programs such as MRTX1133, and an increase in salaries and other employee related expense to support portfolio advancement. |
| |
• |
|
Selling, general and administrative expenses for the three and six months ended June 30, 2023 were
$75.5 million and $149.0 million, respectively, compared to $54.2 million and $108.2 million, respectively for the same periods in 2022. The increases were primarily due to an increase in headcount-related costs, including
share-based compensation and salaries, and commercial-related costs to support the marketing and sales of KRAZATI®. |
| |
• |
|
Net loss for the three months ended June 30, 2023 was $176.9 million, or $3.04 per share basic and
diluted, compared to a net loss of $176.4 million, or $3.18 per share basic and diluted for the same period in 2022. Net loss for the six months ended June 30, 2023 was $361.5 million, or $6.22 per share basic and diluted, compared to
a net loss of $364.8 million, or $6.57 per share basic and diluted for the same period in 2022. |
Conference Call Information
There will be a conference call on August 8, 2023 at 5:30 p.m. ET / 2:30 p.m. PT during which company executives will review financial
information for the second quarter and provide corporate updates.
Investors and the general public are invited to listen to a live webcast of the call at
the “Investors and Media” section on Mirati.com or by dialing the U.S. toll free +1 773-305-6853 or international +1 888-394-8218, confirmation code: 6674271.
A replay of the call will be available approximately 2 hours after the
event has ended at the same website.
About Mirati Therapeutics, Inc.®
Mirati Therapeutics, Inc. is a commercial stage biotechnology company whose mission is to discover, design and deliver breakthrough therapies to transform the
lives of patients with cancer and their loved ones. The company is relentlessly focused on bringing forward therapies that address areas of high unmet need, including lung cancer, and advancing a pipeline of novel therapeutics targeting the genetic
and immunological drivers of cancer. Unified for patients, Mirati’s vision is to unlock the science behind the promise of a life beyond cancer.
For more information about Mirati, visit us at Mirati.com or follow us on Twitter,
LinkedIn, and Facebook.
Forward Looking Statements
This press release includes forward-looking statements regarding Mirati’s business, financial guidance and the therapeutic and commercial potential of
KRAZATI® (adagrasib), sitravatinib (TAM receptor inhibitor), MRTX1719 (MTA-cooperative PRMT5 inhibitor), MRTX0902 (SOS1 inhibitor), and
MRTX1133 (selective KRASG12D inhibitor), Mirati’s technologies and Mirati’ other products in development. Any statement describing Mirati’s goals, expectations, intentions or
beliefs, financial or other projections, is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, including those inherent
in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines.
Mirati’s forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ
materially from those expressed or implied by such forward-looking statements. Although Mirati’s forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known
by Mirati. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Mirati’s programs are described in additional detail in Mirati’ annual report on Form 10-K, and most recent Form 10-Q, which are on file with the Securities and Exchange Commission and available at the SEC’s Internet site (www.sec.gov). These
forward-looking statements are made as of the date of this press release, and Mirati assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking
statements, except as required by law.
Mirati Contacts
Investor Relations: ir@mirati.com
Media Relations:
media@mirati.com
+++
1OPDIVO® (nivolumab) and the related logo are registered trademarks of Bristol-Myers Squibb Company
Mirati Therapeutics, Inc.
Consolidated Balance Sheets
(unaudited)
(in
thousands)
|
|
|
|
|
|
|
|
|
| |
|
June 30,
2023 |
|
|
December 31,
2022 |
|
| Assets |
|
|
|
|
|
|
|
|
| Current assets |
|
|
|
|
|
|
|
|
| Cash, cash equivalents and short-term investments |
|
$ |
779,434 |
|
|
$ |
1,083,837 |
|
| Accounts receivable, net |
|
|
10,523 |
|
|
|
865 |
|
| Inventory |
|
|
8,644 |
|
|
|
3,020 |
|
| Other current assets |
|
|
20,316 |
|
|
|
21,239 |
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
818,917 |
|
|
|
1,108,961 |
|
| Property and equipment, net |
|
|
16,867 |
|
|
|
17,540 |
|
| Intangible asset, net |
|
|
14,397 |
|
|
|
14,914 |
|
| Long-term investment |
|
|
4,565 |
|
|
|
3,465 |
|
| Right-of-use
asset |
|
|
35,431 |
|
|
|
36,122 |
|
| Other long-term assets |
|
|
24,866 |
|
|
|
21,645 |
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
915,043 |
|
|
$ |
1,202,647 |
|
|
|
|
|
|
|
|
|
|
| Liabilities and Shareholders’ Equity |
|
|
|
|
|
|
|
|
| Current liabilities |
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
32,507 |
|
|
$ |
38,861 |
|
| Accrued liabilities |
|
|
107,483 |
|
|
|
120,587 |
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
139,990 |
|
|
|
159,448 |
|
| Lease liability |
|
|
42,722 |
|
|
|
43,661 |
|
| Other liabilities |
|
|
3,380 |
|
|
|
3,022 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
186,092 |
|
|
|
206,131 |
|
|
|
|
|
|
|
|
|
|
| Shareholders’ equity |
|
|
728,951 |
|
|
|
996,516 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities and shareholders’ equity |
|
$ |
915,043 |
|
|
$ |
1,202,647 |
|
|
|
|
|
|
|
|
|
|
Mirati Therapeutics, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(unaudited)
(in
thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
| |
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
| |
|
(unaudited) |
|
|
(unaudited) |
|
| Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Product revenue, net |
|
$ |
13,365 |
|
|
$ |
— |
|
|
$ |
19,656 |
|
|
$ |
— |
|
| License and collaboration revenues |
|
|
325 |
|
|
|
5,362 |
|
|
|
1,201 |
|
|
|
6,071 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total revenue |
|
|
13,690 |
|
|
|
5,362 |
|
|
|
20,857 |
|
|
|
6,071 |
|
| Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cost of product revenue |
|
|
1,025 |
|
|
|
— |
|
|
|
1,583 |
|
|
|
— |
|
| Cost of product revenue—intangible asset amortization |
|
|
258 |
|
|
|
— |
|
|
|
517 |
|
|
|
— |
|
| Research and development |
|
|
124,187 |
|
|
|
128,339 |
|
|
|
250,870 |
|
|
|
259,315 |
|
| Selling, general and administrative |
|
|
75,490 |
|
|
|
54,228 |
|
|
|
148,980 |
|
|
|
108,179 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
200,960 |
|
|
|
182,567 |
|
|
|
401,950 |
|
|
|
367,494 |
|
| Loss from operations |
|
|
(187,270 |
) |
|
|
(177,205 |
) |
|
|
(381,093 |
) |
|
|
(361,423 |
) |
| Other income (expense), net |
|
|
10,357 |
|
|
|
760 |
|
|
|
19,594 |
|
|
|
(3,408 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(176,913 |
) |
|
$ |
(176,445 |
) |
|
$ |
(361,499 |
) |
|
$ |
(364,831 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Unrealized gain (loss) on
available-for-sale investments |
|
|
26 |
|
|
|
(909 |
) |
|
|
2,069 |
|
|
|
(5,711 |
) |
| Foreign currency translation adjustment |
|
|
(34 |
) |
|
|
— |
|
|
|
(65 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Comprehensive loss |
|
$ |
(176,921 |
) |
|
$ |
(177,354 |
) |
|
$ |
(359,495 |
) |
|
$ |
(370,542 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share, basic and diluted |
|
$ |
(3.04 |
) |
|
$ |
(3.18 |
) |
|
$ |
(6.22 |
) |
|
$ |
(6.57 |
) |
| Weighted average common shares outstanding, basic and diluted |
|
|
58,232 |
|
|
|
55,552 |
|
|
|
58,132 |
|
|
|
55,511 |
|
Exhibit 99.2

Mirati Therapeutics Announces Departure of Chief Executive Officer David Meek
Charles M. Baum, M.D., Ph.D. assumes role of interim CEO
SAN DIEGO, Calif., August 8, 2023 /PRNewswire/ — Mirati Therapeutics, Inc.® (NASDAQ:
MRTX), a commercial stage biotechnology company today announced that David Meek and the Company mutually agreed for Mr. Meek to step down from his role as CEO and as a member of the Board of Directors. His responsibilities have been assumed by
former CEO, president, and founder Charles M. Baum, M.D., Ph.D., acting as interim CEO, while the company conducts a search for a permanent CEO. Mr. Meek will remain as a consultant to the Company through October 15, 2023.
“David has made a significant impact during his tenure at Mirati, most notably guiding the company through the successful launch of KRAZATI®, the rapid advancement of our robust and innovative pipeline and the expansion of the Mirati team,” said Faheem Hasnain, chairman of the board of directors, Mirati Therapeutics, Inc.
“We are grateful for his contributions to the company.”
“I am proud of the accomplishments Mirati has achieved with the successful launch
of KRAZATI and advancing an innovative pipeline,” said David Meek. “I had the pleasure of working with an accomplished team, and together we have helped to improve the lives of many people which is and will continue to be a purpose and
passion going forward.”
“We extend our sincere gratitude to David for helping to advance Mirati as a leading biotech, delivering targeted
therapies to people living with cancer,” said Charles M. Baum, M.D., Ph.D., interim CEO, founder, and president, Mirati Therapeutics, Inc. “We look optimistically to the future and the ability of our robust pipeline of potentially best-in-class or first-in-class targeted oncology programs to offer the promise of a life
beyond cancer for patients and their loved ones.”
About Mirati Therapeutics, Inc.
Mirati Therapeutics, Inc. is a commercial stage biotechnology company whose mission is to discover, design and deliver breakthrough therapies to transform the
lives of patients with cancer and their loved ones. The company is relentlessly focused on bringing forward therapies that address areas of high unmet need, including lung cancer, and advancing a pipeline of novel therapeutics targeting the genetic
and immunological drivers of cancer. Unified for patients, Mirati’s vision is to unlock the science behind the promise of a life beyond cancer.
For
more information about Mirati, visit us at Mirati.com or follow us on Twitter, LinkedIn and Facebook.
Forward Looking Statements
This press release includes forward-looking statements regarding Mirati’s business, financial guidance and the therapeutic and commercial potential of
KRAZATI® (adagrasib), MRTX1719 (MTA-cooperative PRMT5 inhibitor), MRTX0902 (SOS1 inhibitor), and MRTX1133 (selective KRASG12D inhibitor),
Mirati’s technologies and Mirati’s other products in development. Any statement describing Mirati’s goals, expectations, intentions or beliefs, financial or other projections, is a forward-looking statement and should be considered an
at-risk statement. Such statements are subject to certain risks and uncertainties, including those related to the impact COVID-19 could have on our business, and
including those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines.
Mirati’s forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ
materially from those expressed or implied by such forward-looking statements. Although Mirati’s forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known
by Mirati. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Mirati’s programs are described in additional detail in Mirati’s annual report on Form 10-K, and most recent Form 10-Q, which are on file with the Securities and Exchange Commission and available at the SEC’s Internet site (https://www.sec.gov/).
These forward-looking statements are made as of the date of this press release, and Mirati assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the
forward-looking statements, except as required by law.
Mirati Contacts
Investor Relations: ir@mirati.com
Media Relations:
media@mirati.com

Exhibit 99.3 Mirati Presentation August 2023 1

Forward Looking Statements and Disclaimers This presentation includes
forward-looking statements regarding Mirati's business, financial guidance and the therapeutic and commercial ® potential of KRAZATI (adagrasib), MRTX1719 (MTA cooperative PRMT5 inhibitor), MRTX0902 (SOS1 inhibitor), and MRTX1133 (selective
G12D KRAS inhibitor), Mirati’s technologies and Mirati’s other products in development. Any statement describing Mirati’s goals, expectations, intentions or beliefs, financial or other projections, including those related to cash
burn, is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, including those related to the impact COVID- 19 could have on our business, the inherent risks in the
process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines, and that interim results of a clinical trial are not
necessarily indicative of final results and one or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data and as more patient data becomes available, including the risk
that unconfirmed responses may not ultimately result in confirmed responses to treatment after follow-up evaluations. Mirati's forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its
results to differ materially from those expressed or implied by such forward-looking statements. Although Mirati's forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors
currently known by Mirati. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Mirati's programs are described in additional detail in Mirati’s annual report on Form 10-K, and most
recent Form 10-Q, which are on file with the Securities and Exchange Commission and available at the SEC's Internet site (www.sec.gov). These forward-looking statements are made as of the date of this presentation, and Mirati assumes no obligation
to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward- looking statements, except as required by law. This presentation shall not constitute an offer to sell or the
solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities
laws of any such state or jurisdiction Confidential 2

Corporate Overview 3

Mirati Well Positioned to Deliver Best-in-Class / First-in-Class
Treatments and Drive Growth A commercial-stage targeted oncology company with a demonstrated ability to deliver innovative, potentially best-in-class treatments to patients with a high unmet need. Proven Leader in Scalable and Synergistic Innovative
Portfolio KRAS Discovery & Commercial Organization, Targeting Areas of Development Particularly in Lung Cancer Unmet Need Backed by cash runway into 2025, our people, pipeline and selective partnering enable a data-driven approach to pipeline
advancement, investment in high value opportunities and commercial success to drive sustainable growth. 4
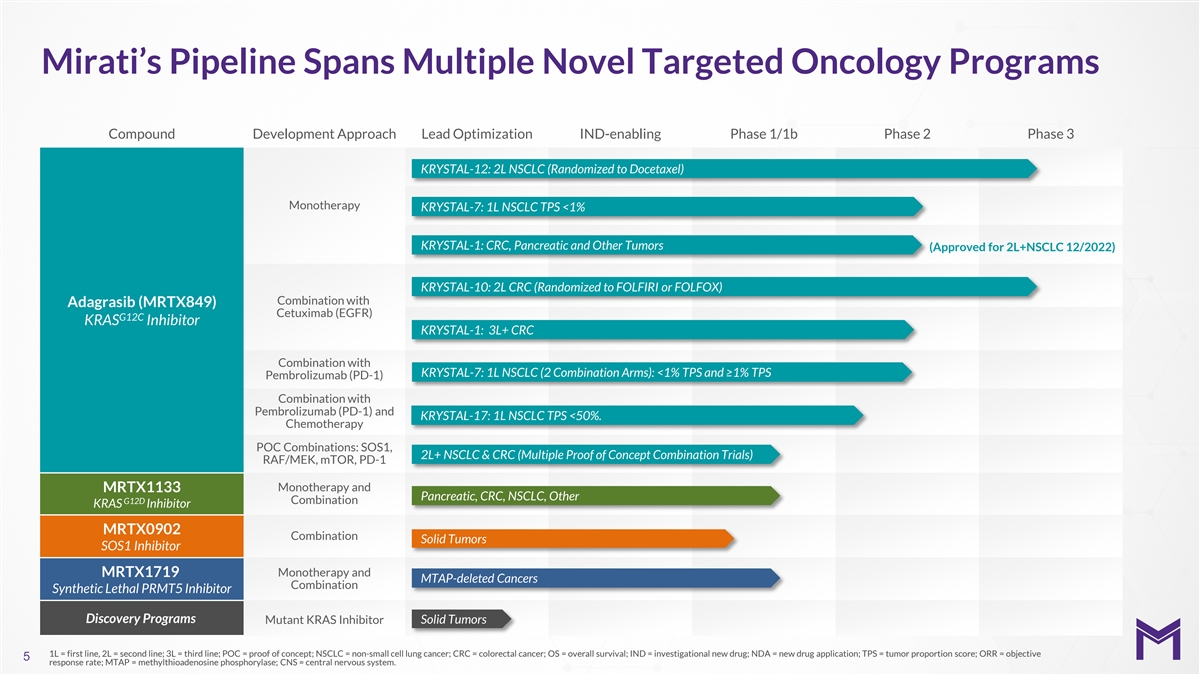
Mirati’s Pipeline Spans Multiple Novel Targeted Oncology Programs
Compound Development Approach Lead Optimization IND-enabling Phase 1/1b Phase 2 Phase 3 KRYSTAL-12: 2L NSCLC (Randomized to Docetaxel) Monotherapy KRYSTAL-7: 1L NSCLC TPS <1% KRYSTAL-1: CRC, Pancreatic and Other Tumors (Approved for 2L+NSCLC
12/2022) KRYSTAL-10: 2L CRC (Randomized to FOLFIRI or FOLFOX) Combination with Adagrasib (MRTX849) Cetuximab (EGFR) G12C KRAS Inhibitor KRYSTAL-1: 3L+ CRC Combination with KRYSTAL-7: 1L NSCLC (2 Combination Arms): <1% TPS and ≥1% TPS
Pembrolizumab (PD-1) Combination with Pembrolizumab (PD-1) and KRYSTAL-17: 1L NSCLC TPS <50%. Chemotherapy POC Combinations: SOS1, 2L+ NSCLC & CRC (Multiple Proof of Concept Combination Trials) RAF/MEK, mTOR, PD-1 Monotherapy and MRTX1133
Pancreatic, CRC, NSCLC, Other G12D Combination KRAS Inhibitor MRTX0902 Combination Solid Tumors SOS1 Inhibitor Monotherapy and MRTX1719 MTAP-deleted Cancers Combination Synthetic Lethal PRMT5 Inhibitor Discovery Programs Mutant KRAS Inhibitor Solid
Tumors 1L = first line, 2L = second line; 3L = third line; POC = proof of concept; NSCLC = non-small cell lung cancer; CRC = colorectal cancer; OS = overall survival; IND = investigational new drug; NDA = new drug application; TPS = tumor proportion
score; ORR = objective 5 response rate; MTAP = methylthioadenosine phosphorylase; CNS = central nervous system.
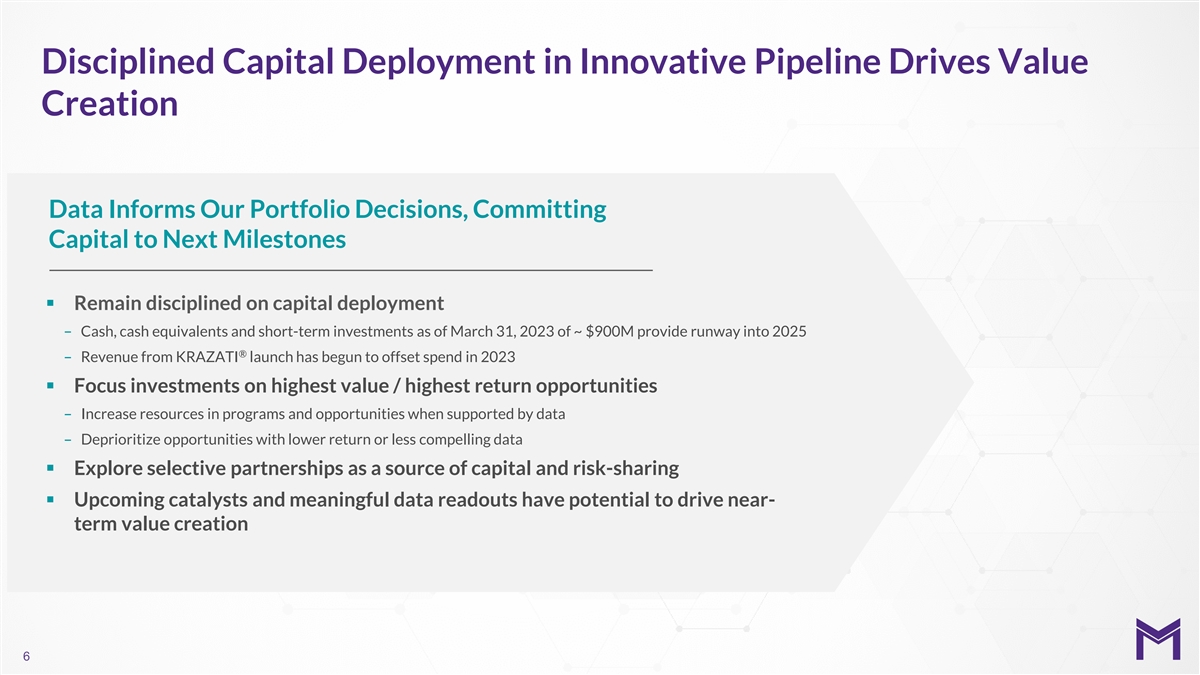
Disciplined Capital Deployment in Innovative Pipeline Drives Value
Creation Data Informs Our Portfolio Decisions, Committing Capital to Next Milestones § Remain disciplined on capital deployment – Cash, cash equivalents and short-term investments as of March 31, 2023 of ~ $900M provide runway into 2025
® – Revenue from KRAZATI launch has begun to offset spend in 2023 § Focus investments on highest value / highest return opportunities – Increase resources in programs and opportunities when supported by data –
Deprioritize opportunities with lower return or less compelling data § Explore selective partnerships as a source of capital and risk-sharing § Upcoming catalysts and meaningful data readouts have potential to drive near- term value
creation 6

Adagrasib: 1L NSCLC Updated Interim Data from KRYSTAL-7 and Path Forward
7
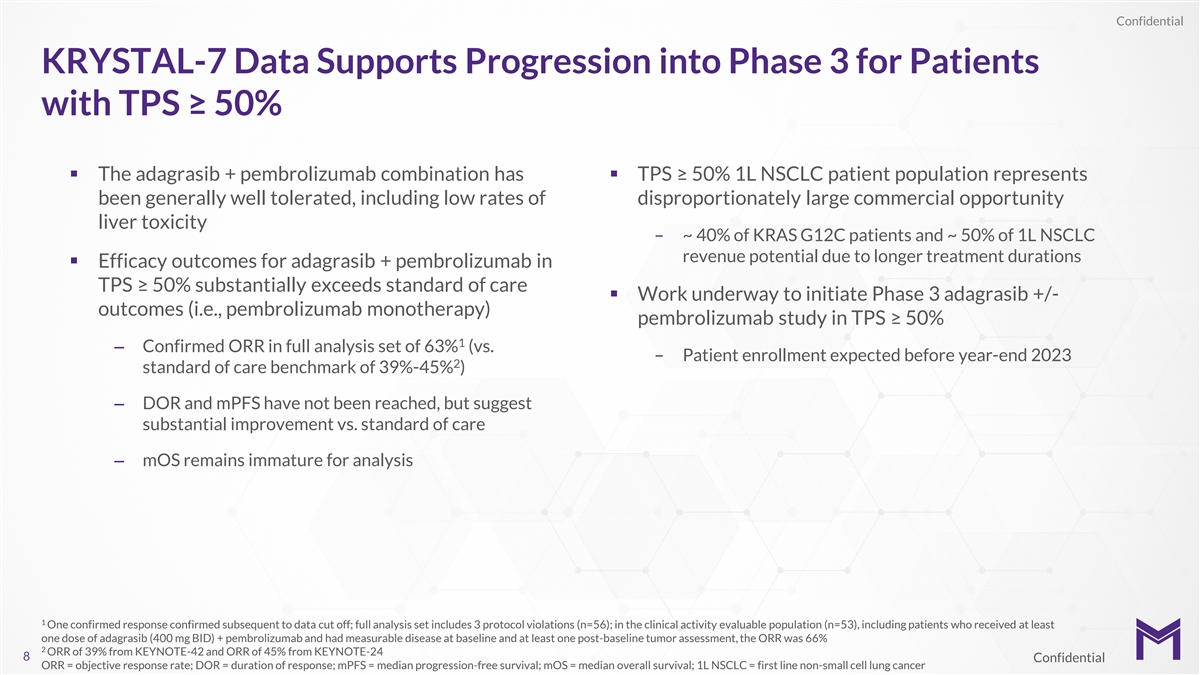
Confidential KRYSTAL-7 Data Supports Progression into Phase 3 for
Patients with TPS ≥ 50% § The adagrasib + pembrolizumab combination has § TPS ≥ 50% 1L NSCLC patient population represents been generally well tolerated, including low rates of disproportionately large commercial opportunity
liver toxicity – ~ 40% of KRAS G12C patients and ~ 50% of 1L NSCLC revenue potential due to longer treatment durations § Efficacy outcomes for adagrasib + pembrolizumab in TPS ≥ 50% substantially exceeds standard of care § Work
underway to initiate Phase 3 adagrasib +/- outcomes (i.e., pembrolizumab monotherapy) pembrolizumab study in TPS ≥ 50% 1 – Confirmed ORR in full analysis set of 63% (vs. – Patient enrollment expected before year-end 2023 2 standard
of care benchmark of 39%-45% ) – DOR and mPFS have not been reached, but suggest substantial improvement vs. standard of care – mOS remains immature for analysis 1 One confirmed response confirmed subsequent to data cut off; full
analysis set includes 3 protocol violations (n=56); in the clinical activity evaluable population (n=53), including patients who received at least one dose of adagrasib (400 mg BID) + pembrolizumab and had measurable disease at baseline and at least
one post-baseline tumor assessment, the ORR was 66% 2 ORR of 39% from KEYNOTE-42 and ORR of 45% from KEYNOTE-24 8 Confidential ORR = objective response rate; DOR = duration of response; mPFS = median progression-free survival; mOS = median overall
survival; 1L NSCLC = first line non-small cell lung cancer
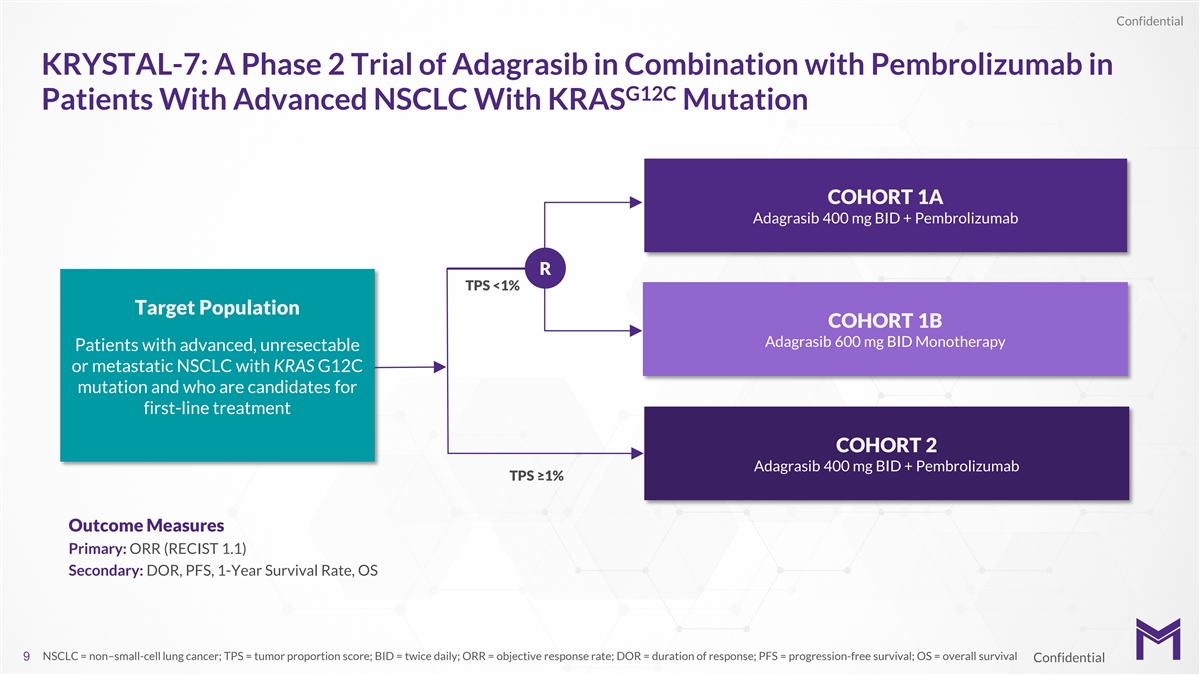
Confidential KRYSTAL-7: A Phase 2 Trial of Adagrasib in Combination with
Pembrolizumab in G12C Patients With Advanced NSCLC With KRAS Mutation COHORT 1A Adagrasib 400 mg BID + Pembrolizumab R TPS <1% Target Population COHORT 1B Adagrasib 600 mg BID Monotherapy Patients with advanced, unresectable or metastatic NSCLC
with KRAS G12C mutation and who are candidates for first-line treatment COHORT 2 Adagrasib 400 mg BID + Pembrolizumab TPS ≥1% Outcome Measures Primary: ORR (RECIST 1.1) Secondary: DOR, PFS, 1-Year Survival Rate, OS NSCLC = non–small-cell
lung cancer; TPS = tumor proportion score; BID = twice daily; ORR = objective response rate; DOR = duration of response; PFS = progression-free survival; OS = overall survival 9 Confidential
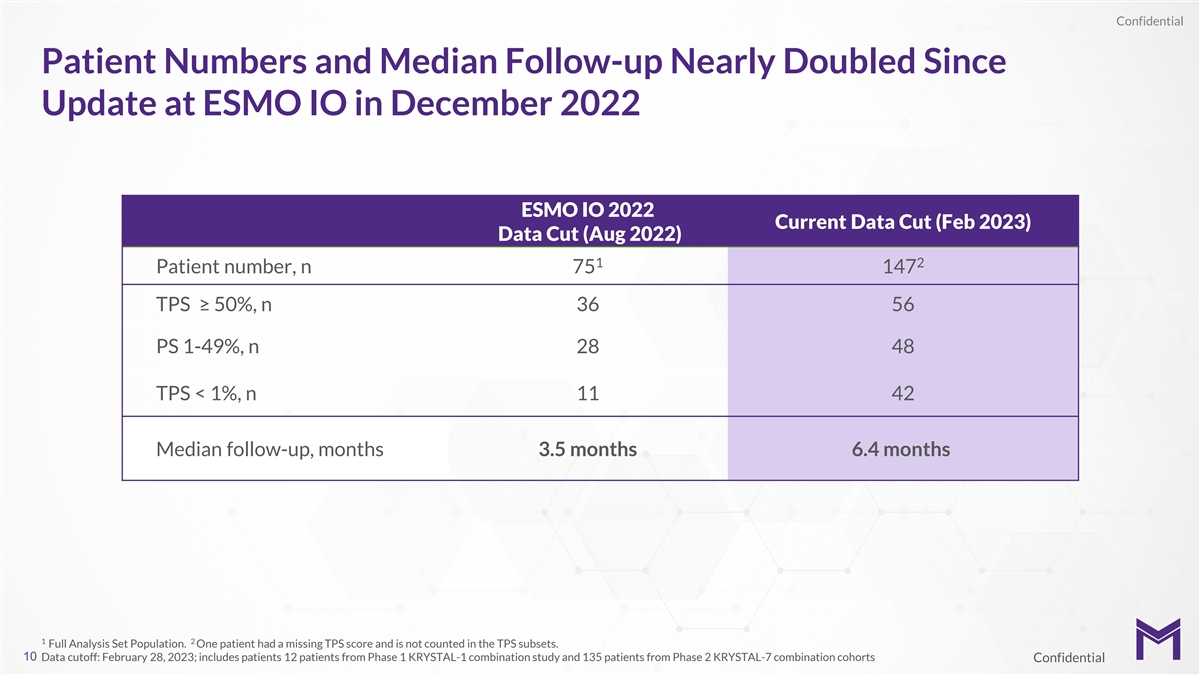
Confidential Patient Numbers and Median Follow-up Nearly Doubled Since
Update at ESMO IO in December 2022 ESMO IO 2022 Current Data Cut (Feb 2023) Data Cut (Aug 2022) 1 2 Patient number, n 75 147 TPS ≥ 50%, n 36 56 PS 1-49%, n 28 48 TPS < 1%, n 11 42 Median follow-up, months 3.5 months 6.4 months 1 2 Full
Analysis Set Population. One patient had a missing TPS score and is not counted in the TPS subsets. 10 Data cutoff: February 28, 2023; includes patients 12 patients from Phase 1 KRYSTAL-1 combination study and 135 patients from Phase 2 KRYSTAL-7
combination cohorts Confidential
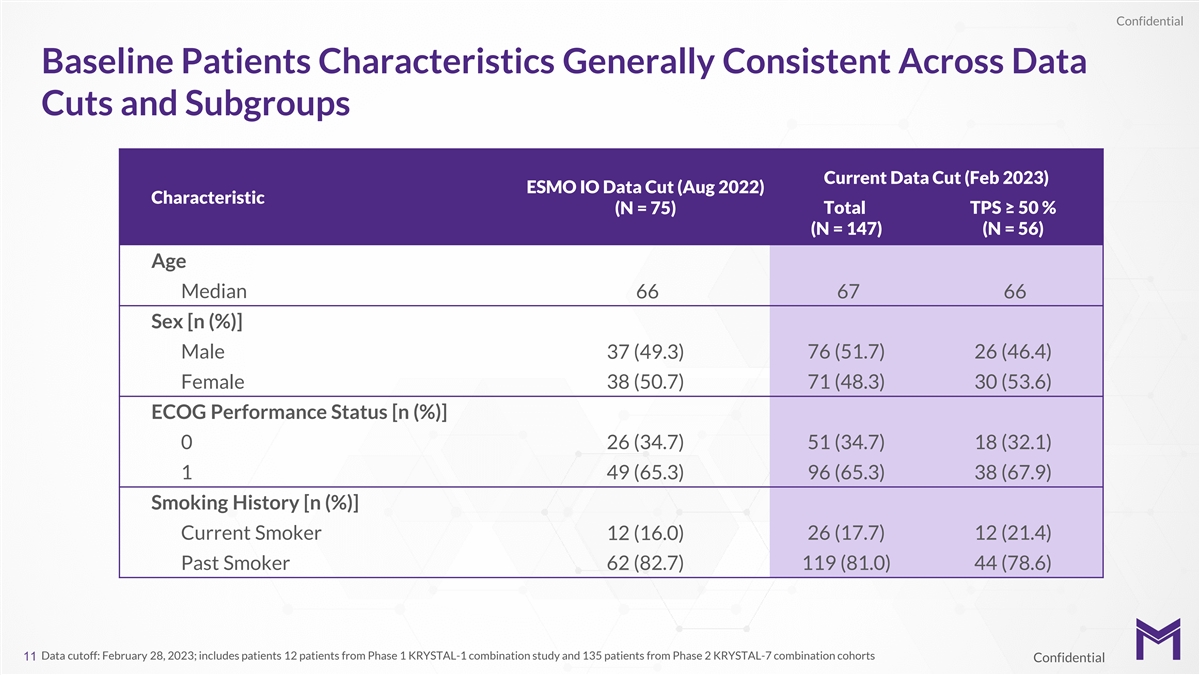
Confidential Baseline Patients Characteristics Generally Consistent
Across Data Cuts and Subgroups Current Data Cut (Feb 2023) ESMO IO Data Cut (Aug 2022) Characteristic Total TPS ≥ 50 % (N = 75) (N = 147) (N = 56) Age Median 66 67 66 Sex [n (%)] Male 37 (49.3) 76 (51.7) 26 (46.4) Female 38 (50.7) 71 (48.3) 30
(53.6) ECOG Performance Status [n (%)] 0 26 (34.7) 51 (34.7) 18 (32.1) 1 49 (65.3) 96 (65.3) 38 (67.9) Smoking History [n (%)] Current Smoker 12 (16.0) 26 (17.7) 12 (21.4) Past Smoker 62 (82.7) 119 (81.0) 44 (78.6) Data cutoff: February 28, 2023;
includes patients 12 patients from Phase 1 KRYSTAL-1 combination study and 135 patients from Phase 2 KRYSTAL-7 combination cohorts 11 Confidential
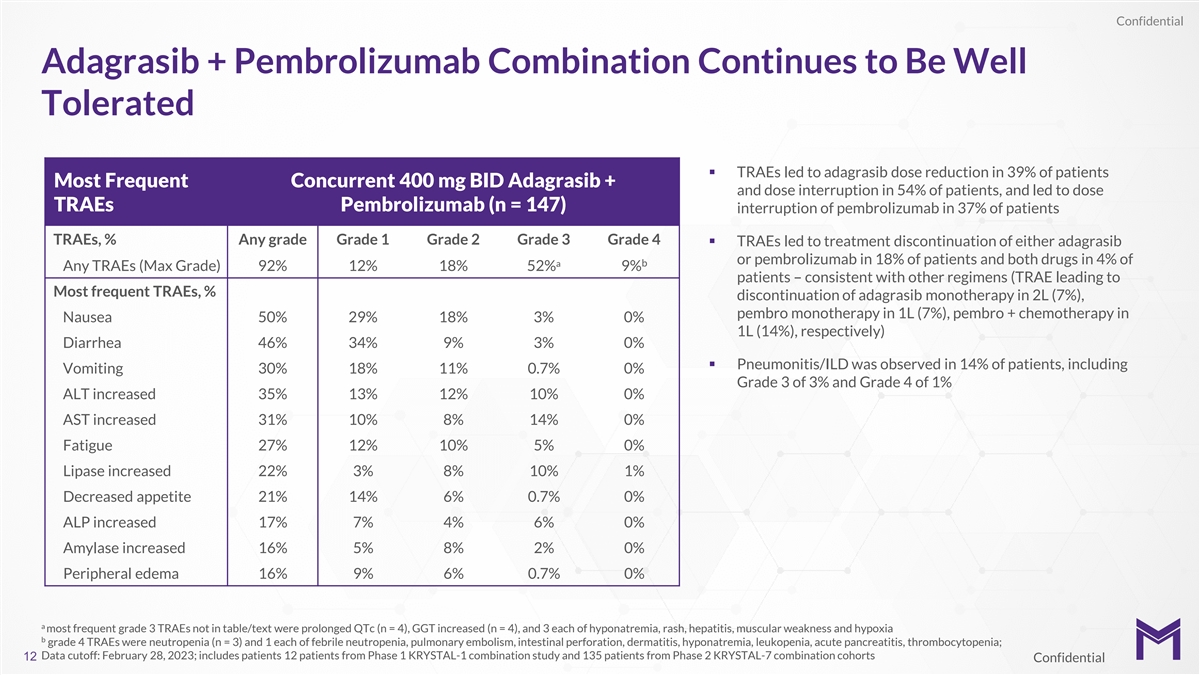
Confidential Adagrasib + Pembrolizumab Combination Continues to Be Well
Tolerated § TRAEs led to adagrasib dose reduction in 39% of patients Most Frequent Concurrent 400 mg BID Adagrasib + and dose interruption in 54% of patients, and led to dose TRAEs Pembrolizumab (n = 147) interruption of pembrolizumab in 37% of
patients TRAEs, % Any grade Grade 1 Grade 2 Grade 3 Grade 4 § TRAEs led to treatment discontinuation of either adagrasib or pembrolizumab in 18% of patients and both drugs in 4% of b a Any TRAEs (Max Grade) 92% 12% 18% 52% 9% patients –
consistent with other regimens (TRAE leading to Most frequent TRAEs, % discontinuation of adagrasib monotherapy in 2L (7%), pembro monotherapy in 1L (7%), pembro + chemotherapy in Nausea 50% 29% 18% 3% 0% 1L (14%), respectively) Diarrhea 46% 34% 9%
3% 0% § Pneumonitis/ILD was observed in 14% of patients, including Vomiting 30% 18% 11% 0.7% 0% Grade 3 of 3% and Grade 4 of 1% ALT increased 35% 13% 12% 10% 0% AST increased 31% 10% 8% 14% 0% Fatigue 27% 12% 10% 5% 0% Lipase increased 22% 3%
8% 10% 1% Decreased appetite 21% 14% 6% 0.7% 0% ALP increased 17% 7% 4% 6% 0% Amylase increased 16% 5% 8% 2% 0% Peripheral edema 16% 9% 6% 0.7% 0% a most frequent grade 3 TRAEs not in table/text were prolonged QTc (n = 4), GGT increased (n = 4), and
3 each of hyponatremia, rash, hepatitis, muscular weakness and hypoxia b grade 4 TRAEs were neutropenia (n = 3) and 1 each of febrile neutropenia, pulmonary embolism, intestinal perforation, dermatitis, hyponatremia, leukopenia, acute pancreatitis,
thrombocytopenia; Data cutoff: February 28, 2023; includes patients 12 patients from Phase 1 KRYSTAL-1 combination study and 135 patients from Phase 2 KRYSTAL-7 combination cohorts 12 Confidential
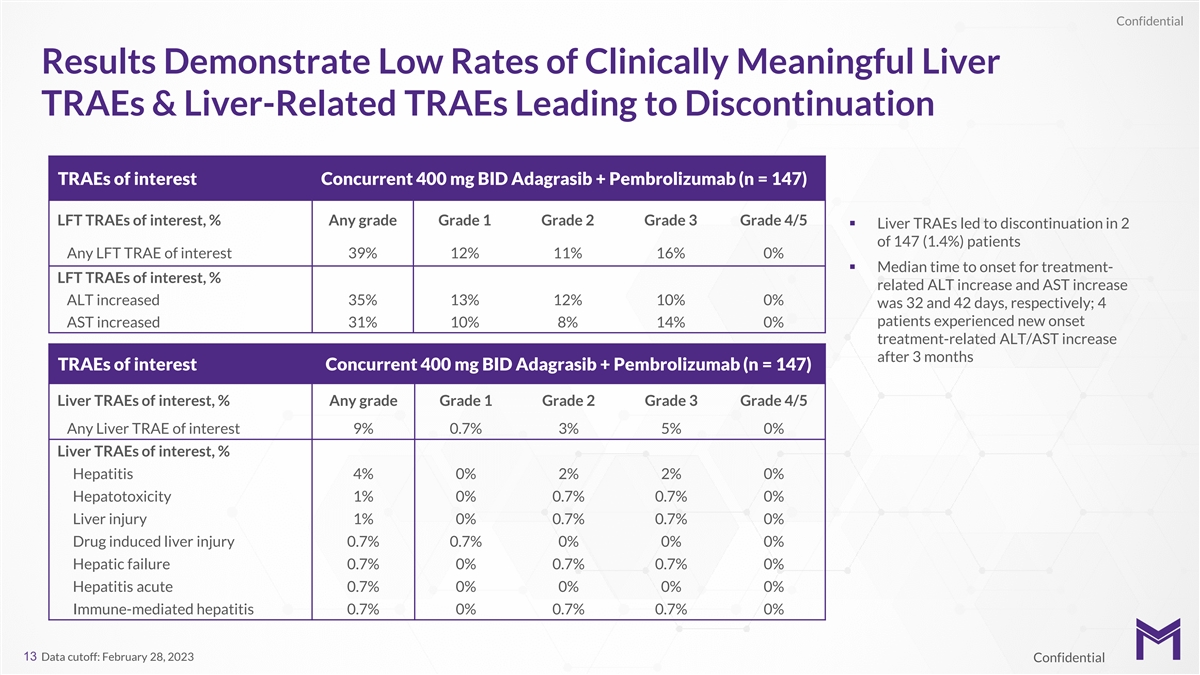
Confidential Results Demonstrate Low Rates of Clinically Meaningful
Liver TRAEs & Liver-Related TRAEs Leading to Discontinuation TRAEs of interest Concurrent 400 mg BID Adagrasib + Pembrolizumab (n = 147) LFT TRAEs of interest, % Any grade Grade 1 Grade 2 Grade 3 Grade 4/5 § Liver TRAEs led to
discontinuation in 2 of 147 (1.4%) patients Any LFT TRAE of interest 39% 12% 11% 16% 0% § Median time to onset for treatment- LFT TRAEs of interest, % related ALT increase and AST increase ALT increased 35% 13% 12% 10% 0% was 32 and 42 days,
respectively; 4 patients experienced new onset AST increased 31% 10% 8% 14% 0% treatment-related ALT/AST increase after 3 months TRAEs of interest Concurrent 400 mg BID Adagrasib + Pembrolizumab (n = 147) Liver TRAEs of interest, % Any grade Grade 1
Grade 2 Grade 3 Grade 4/5 Any Liver TRAE of interest 9% 0.7% 3% 5% 0% Liver TRAEs of interest, % Hepatitis 4% 0% 2% 2% 0% Hepatotoxicity 1% 0% 0.7% 0.7% 0% Liver injury 1% 0% 0.7% 0.7% 0% Drug induced liver injury 0.7% 0.7% 0% 0% 0% Hepatic failure
0.7% 0% 0.7% 0.7% 0% Hepatitis acute 0.7% 0% 0% 0% 0% Immune-mediated hepatitis 0.7% 0% 0.7% 0.7% 0% 13 Data cutoff: February 28, 2023 Confidential

Interim Efficacy Results in TPS ≥ 50% 14
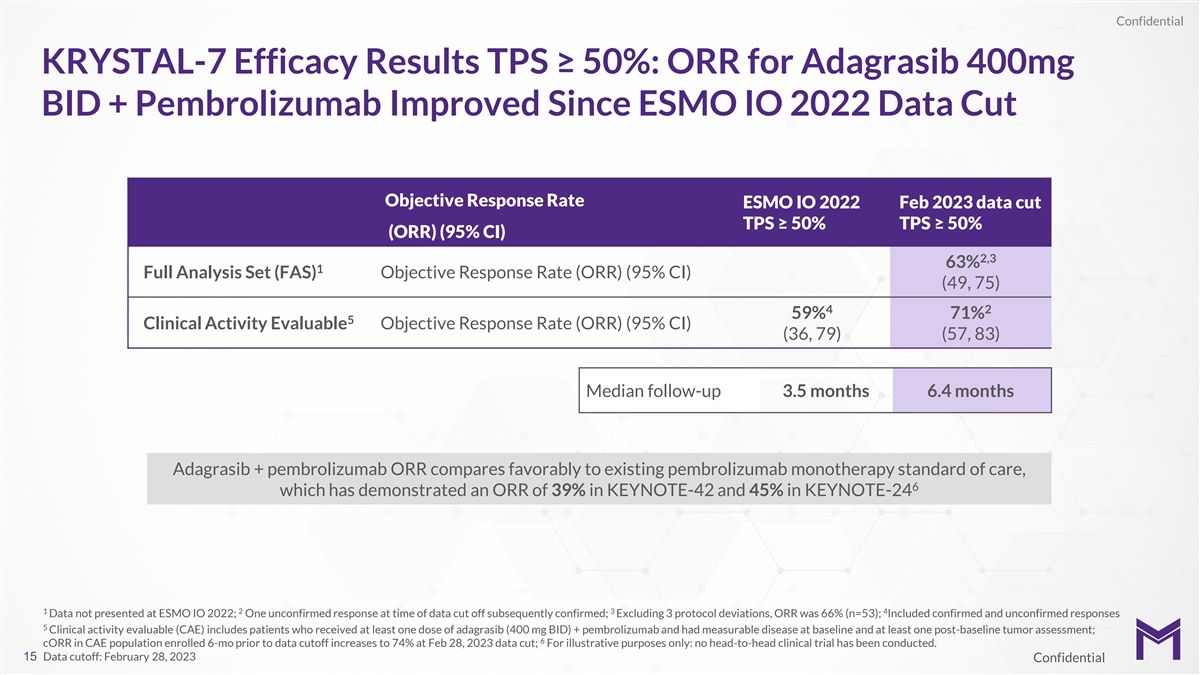
Confidential KRYSTAL-7 Efficacy Results TPS ≥ 50%: ORR for
Adagrasib 400mg BID + Pembrolizumab Improved Since ESMO IO 2022 Data Cut Objective Response Rate ESMO IO 2022 Feb 2023 data cut TPS ≥ 50% TPS ≥ 50% (ORR) (95% CI) 2,3 63% 1 Full Analysis Set (FAS) Objective Response Rate (ORR) (95% CI)
(49, 75) 4 2 59% 71% 5 Clinical Activity Evaluable Objective Response Rate (ORR) (95% CI) (36, 79) (57, 83) Median follow-up 3.5 months 6.4 months Adagrasib + pembrolizumab ORR compares favorably to existing pembrolizumab monotherapy standard of
care, 6 which has demonstrated an ORR of 39% in KEYNOTE-42 and 45% in KEYNOTE-24 1 2 3 4 Data not presented at ESMO IO 2022; One unconfirmed response at time of data cut off subsequently confirmed; Excluding 3 protocol deviations, ORR was 66%
(n=53); Included confirmed and unconfirmed responses 5 Clinical activity evaluable (CAE) includes patients who received at least one dose of adagrasib (400 mg BID) + pembrolizumab and had measurable disease at baseline and at least one post-baseline
tumor assessment; 6 cORR in CAE population enrolled 6-mo prior to data cutoff increases to 74% at Feb 28, 2023 data cut; For illustrative purposes only: no head-to-head clinical trial has been conducted. 15 Data cutoff: February 28, 2023
Confidential
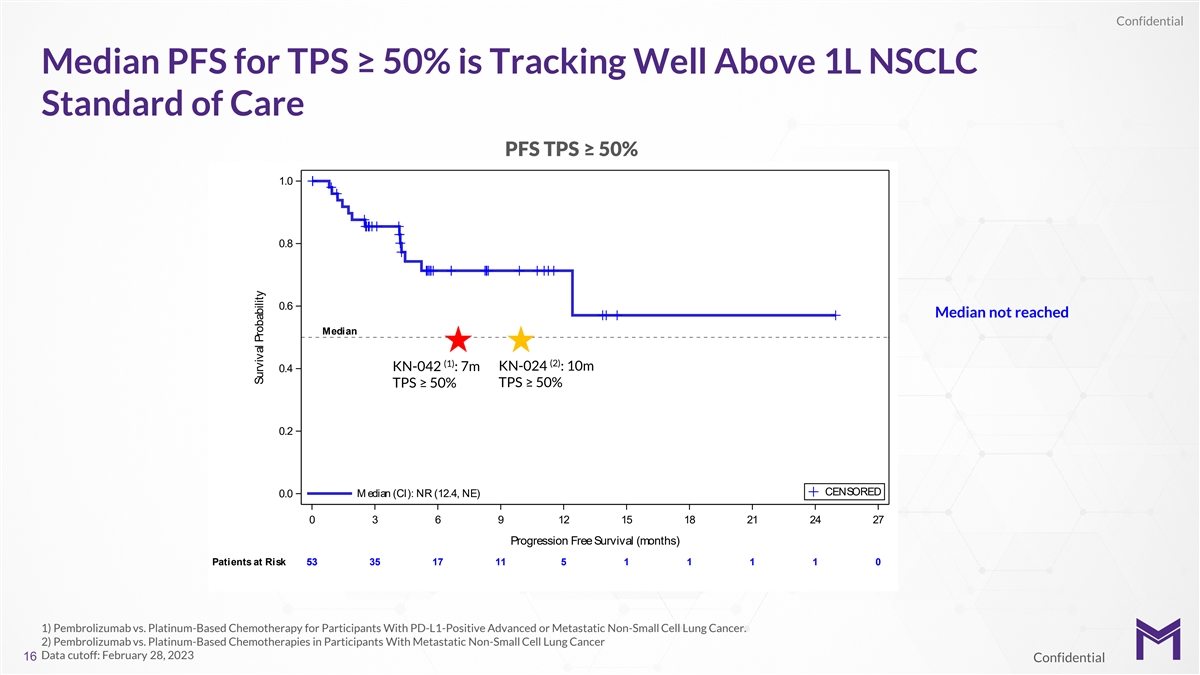
Confidential Median PFS for TPS ≥ 50% is Tracking Well Above 1L
NSCLC Standard of Care PFS TPS ≥ 50% 1.0 0.8 0.6 Median not reached Median (2) (1) KN-042 : 7m KN-024 : 10m 0.4 TPS ≥ 50% TPS ≥ 50% 0.2 CENSORED 0.0 M edian (CI): NR (12.4, NE) 0 3 6 9 12 15 18 21 24 27 Progression Free Survival
(months) Patients at Risk 53 53 35 17 11 5 1 1 1 1 0 1) Pembrolizumab vs. Platinum-Based Chemotherapy for Participants With PD-L1-Positive Advanced or Metastatic Non-Small Cell Lung Cancer. 2) Pembrolizumab vs. Platinum-Based Chemotherapies in
Participants With Metastatic Non-Small Cell Lung Cancer Data cutoff: February 28, 2023 16 Confidential Survival Probability
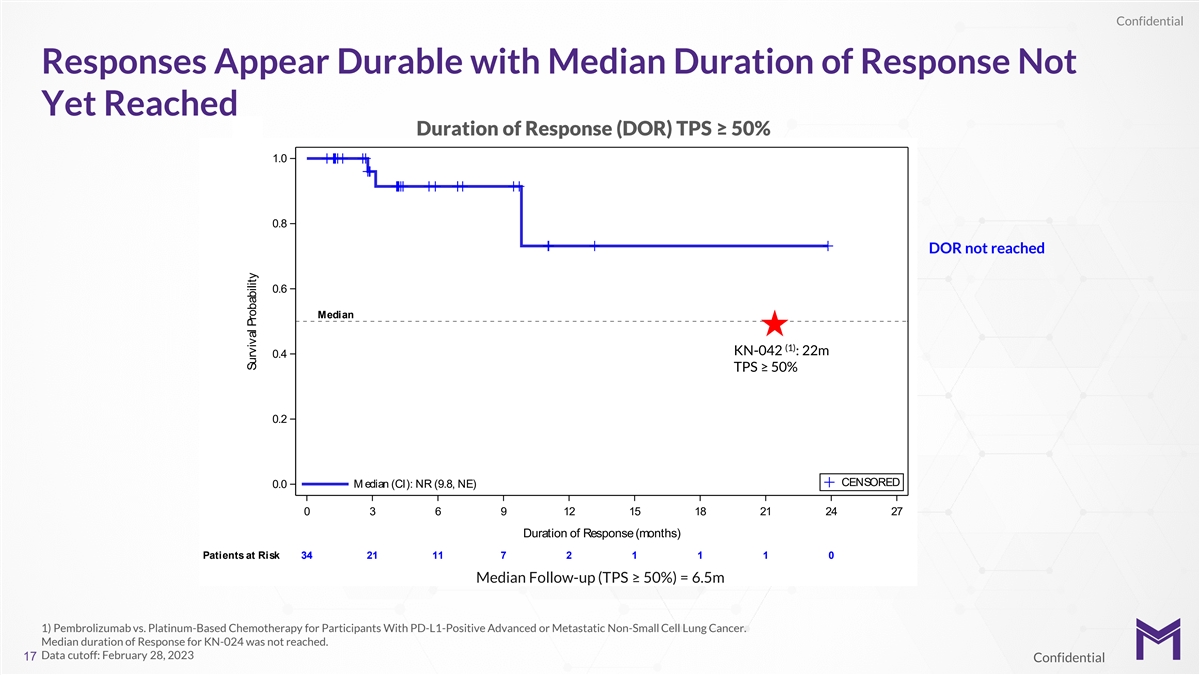
Confidential Responses Appear Durable with Median Duration of Response
Not Yet Reached Duration of Response (DOR) TPS ≥ 50% 1.0 0.8 DOR not reached 0.6 Median (1) KN-042 : 22m 0.4 TPS ≥ 50% 0.2 CENSORED M edian (CI): NR (9.8, NE) 0.0 0 3 6 9 12 15 18 21 24 27 Duration of Response (months) Patients at Risk
34 34 21 11 7 2 1 1 1 0 Median Follow-up (TPS ≥ 50%) = 6.5m 1) Pembrolizumab vs. Platinum-Based Chemotherapy for Participants With PD-L1-Positive Advanced or Metastatic Non-Small Cell Lung Cancer. Median duration of Response for KN-024 was not
reached. Data cutoff: February 28, 2023 17 Confidential Probability Response Maintained Survival Probability
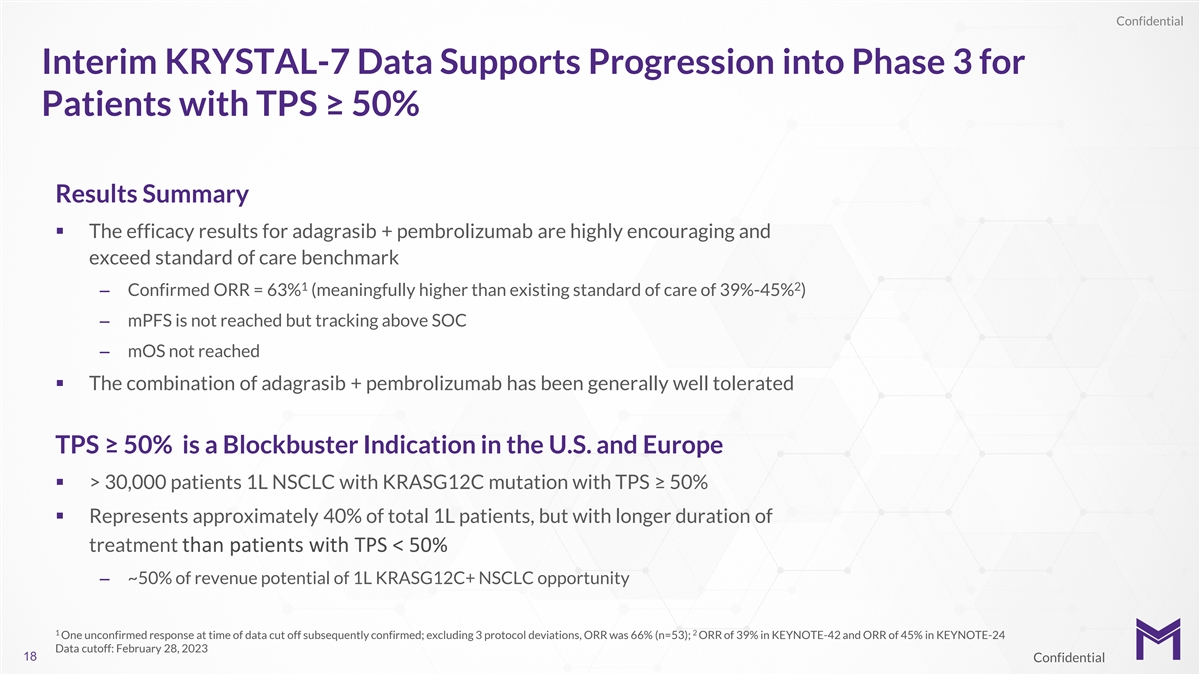
Confidential Interim KRYSTAL-7 Data Supports Progression into Phase 3
for Patients with TPS ≥ 50% Results Summary § The efficacy results for adagrasib + pembrolizumab are highly encouraging and exceed standard of care benchmark 1 2 – Confirmed ORR = 63% (meaningfully higher than existing standard of
care of 39%-45% ) – mPFS is not reached but tracking above SOC – mOS not reached § The combination of adagrasib + pembrolizumab has been generally well tolerated TPS ≥ 50% is a Blockbuster Indication in the U.S. and Europe
§ > 30,000 patients 1L NSCLC with KRASG12C mutation with TPS ≥ 50% § Represents approximately 40% of total 1L patients, but with longer duration of treatment than patients with TPS < 50% – ~50% of revenue potential of 1L
KRASG12C+ NSCLC opportunity 1 2 One unconfirmed response at time of data cut off subsequently confirmed; excluding 3 protocol deviations, ORR was 66% (n=53); ORR of 39% in KEYNOTE-42 and ORR of 45% in KEYNOTE-24 Data cutoff: February 28, 2023 18
Confidential
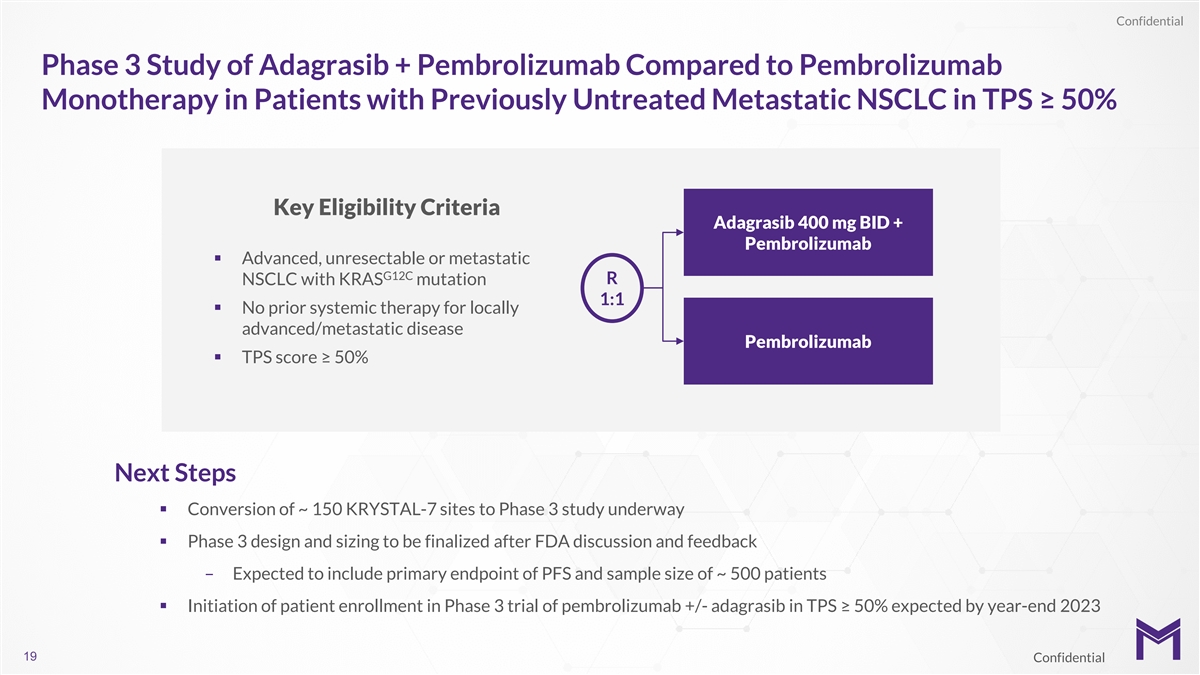
Confidential Phase 3 Study of Adagrasib + Pembrolizumab Compared to
Pembrolizumab Monotherapy in Patients with Previously Untreated Metastatic NSCLC in TPS ≥ 50% Key Eligibility Criteria Adagrasib 400 mg BID + Pembrolizumab § Advanced, unresectable or metastatic G12C NSCLC with KRAS mutation R 1:1 §
No prior systemic therapy for locally advanced/metastatic disease Pembrolizumab § TPS score ≥ 50% Next Steps § Conversion of ~ 150 KRYSTAL-7 sites to Phase 3 study underway § Phase 3 design and sizing to be finalized after FDA
discussion and feedback – Expected to include primary endpoint of PFS and sample size of ~ 500 patients § Initiation of patient enrollment in Phase 3 trial of pembrolizumab +/- adagrasib in TPS ≥ 50% expected by year-end 2023 19
Confidential

TPS < 50% 20

Confidential TPS < 50%: Approach to be Confirmed Following Initial
Data from Adagrasib Add-On to Standard of Care Chemo-Immunotherapy § Clear signals of clinical activity with adagrasib + pembrolizumab in full TPS < 50% population, but data suggest doublet approach is not sufficient to displace existing
standard of care chemo-immunotherapy – Safety/tolerability profile of adagrasib + pembrolizumab continues to be favorable, potentially enabling add-on to standard of care chemo-immunotherapy approach – Correlative analysis of
co-mutations and TPS ongoing which could be informative for phase 3 design § Phase 2 adagrasib + chemo-immunotherapy combination study (KRYSTAL-17) underway to confirm optimal Phase 3 approach for patients with TPS < 50% – Given the
non-overlapping safety profile with chemotherapy we anticipate the combination to be well tolerated § Decision about Phase 3 registrational plans in TPS < 50% patient population expected in 1H 2024 pending interpretation of KRYSTAL-17 data
§ TPS < 50% 1L NSCLC patient population represents ~ 60% of patients 21 Confidential

MRTX1719: MTA Cooperative PRMT5 22

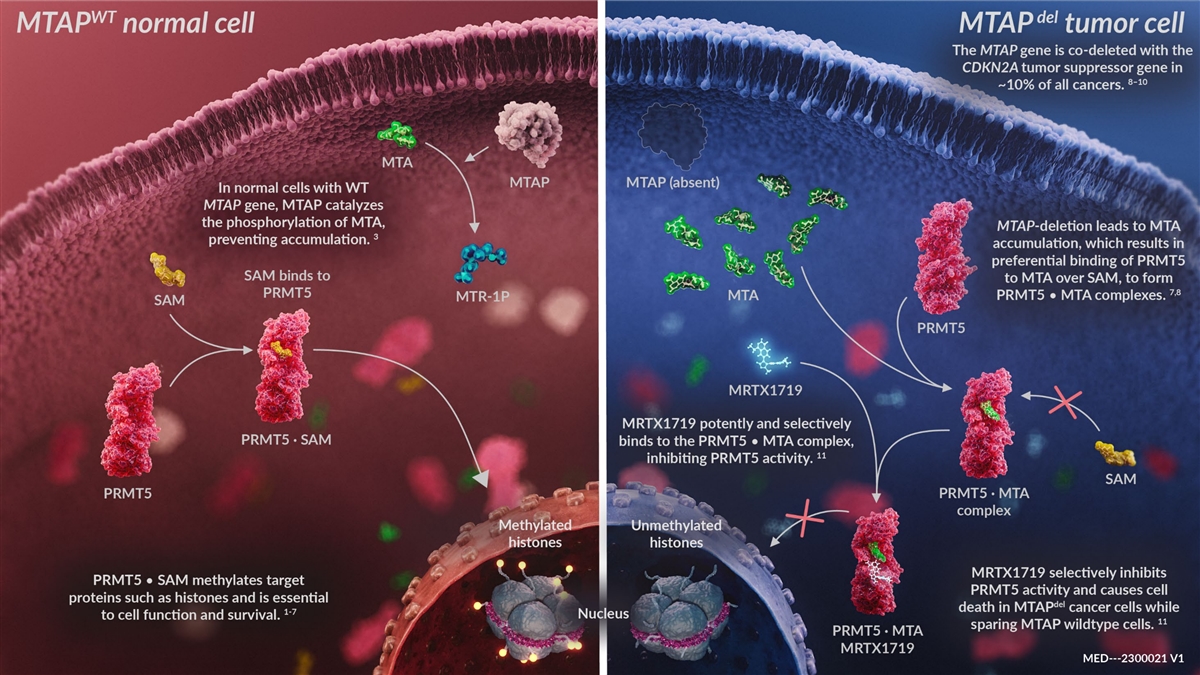
Confidential A Differentiated Opportunity to Selectively Target PRMT5
in MTAP Deleted Cancers Results in Deeper Target Inhibition & Tumor Response § PRMT5 is essential for cell growth and survival in a number of epithelial and bone marrow cell types st § Non-selective 1 generation PRMT5 inhibitors
indiscriminately inhibit PRMT5 in all cell types and target both healthy and tumor cells and were discontinued due to dose-limiting hematologic toxicities – GSK, JNJ, PFE, PRLD demonstrated single digit ORR with >50% Gr3+ toxicities §
MRTX1719 is a differentiated MTA-cooperative PRMT5 inhibitor that demonstrates 70-fold+ selectivity for MTAP deleted tumor cells while sparing bone marrow toxicity § MRTX1719 demonstrates regression of tumors harboring a MTAP gene deletion with
maximal antitumor activity requiring>95-98% inhibition of SDMA target biomarker at doses that spare normal cells and bone marrow – SDMA can be measured as a PD biomarker in clinical settings § The MTAP gene is deleted in ~10% of all
cancers, including NSCLC, PDAC, Bladder, Head & Neck, with an estimated annual incidence in the U.S. + Europe of >250,000 patients across lines of therapy – MTAP deleted cancers represent a particularly significant unmet medical need
with poor prognoses 23 Confidential
24
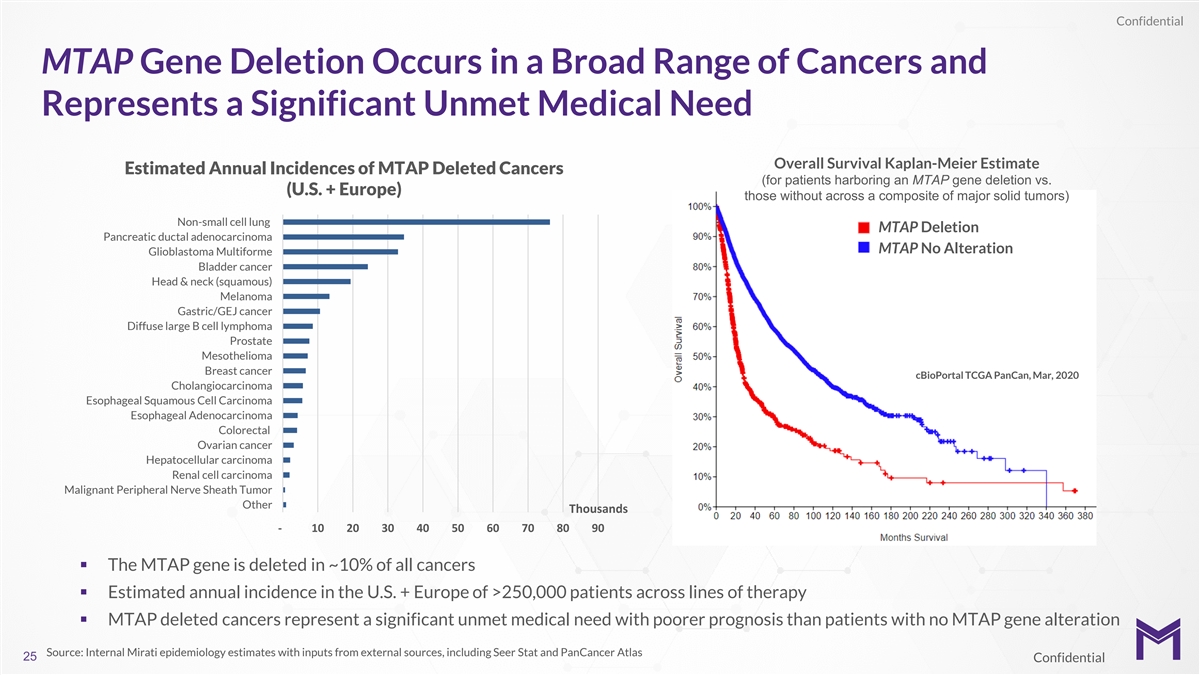
Confidential MTAP Gene Deletion Occurs in a Broad Range of Cancers and
Represents a Significant Unmet Medical Need Overall Survival Kaplan-Meier Estimate Estimated Annual Incidences of MTAP Deleted Cancers (for patients harboring an MTAP gene deletion vs. (U.S. + Europe) those without across a composite of major solid
tumors) Non-small cell lung MTAP Deletion Pancreatic ductal adenocarcinoma MTAP No Alteration Glioblastoma Multiforme Bladder cancer Head & neck (squamous) Melanoma Gastric/GEJ cancer Diffuse large B cell lymphoma Prostate Mesothelioma Breast
cancer cBioPortal TCGA PanCan, Mar, 2020 Cholangiocarcinoma Esophageal Squamous Cell Carcinoma Esophageal Adenocarcinoma Colorectal Ovarian cancer Hepatocellular carcinoma Renal cell carcinoma Malignant Peripheral Nerve Sheath Tumor Other Thousands
- 10 20 30 40 50 60 70 80 90 § The MTAP gene is deleted in ~10% of all cancers § Estimated annual incidence in the U.S. + Europe of >250,000 patients across lines of therapy § MTAP deleted cancers represent a significant unmet
medical need with poorer prognosis than patients with no MTAP gene alteration Source: Internal Mirati epidemiology estimates with inputs from external sources, including Seer Stat and PanCancer Atlas 25 Confidential
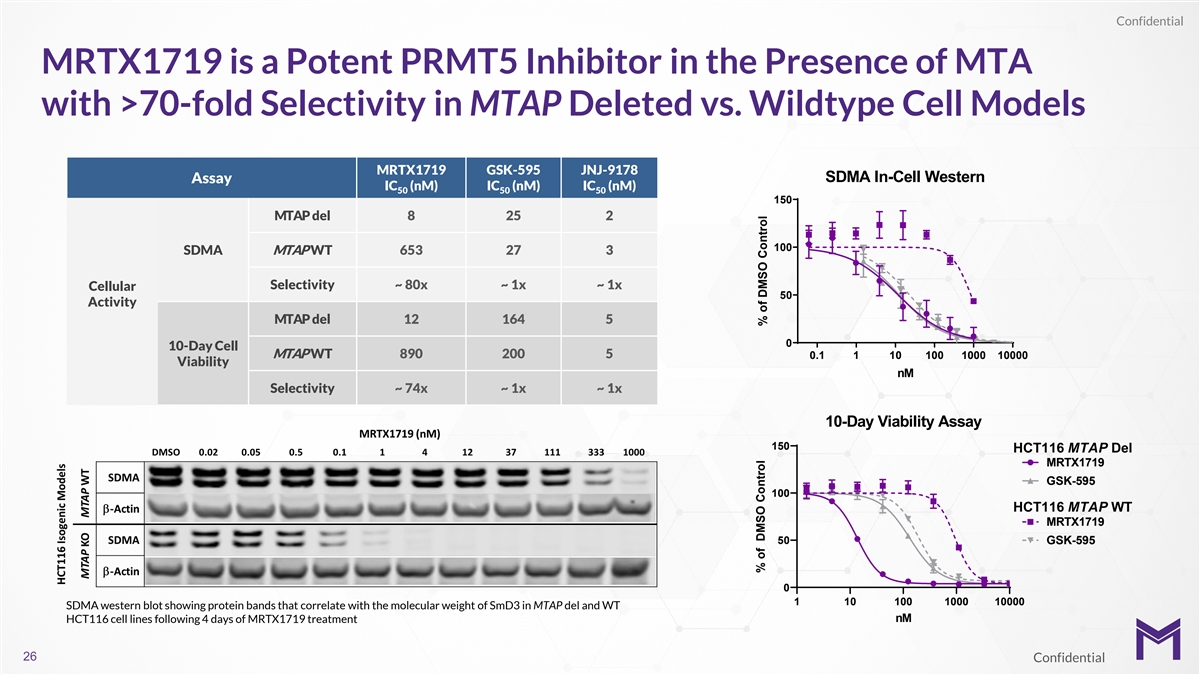
Confidential MRTX1719 is a Potent PRMT5 Inhibitor in the Presence of
MTA with >70-fold Selectivity in MTAP Deleted vs. Wildtype Cell Models MRTX1719 GSK-595 JNJ-9178 SDMA In-Cell Western Assay IC (nM) IC (nM) IC (nM) 50 50 50 150 MTAP del 8 25 2 100 SDMA MTAP WT 653 27 3 Selectivity ~ 80x ~ 1x ~ 1x Cellular 50
Activity MTAP del 12 164 5 0 10-Day Cell MTAP WT 890 200 5 0.1 1 10 100 1000 10000 Viability nM Selectivity ~ 74x ~ 1x ~ 1x 10-Day Viability Assay 150 HCT116MTAP Del MRTX1719 GSK-595 100 HCT116MTAP WT MRTX1719 50 GSK-595 0 1 10 100 1000 10000 SDMA
western blot showing protein bands that correlate with the molecular weight of SmD3 in MTAP del and WT HCT116 cell lines following 4 days of MRTX1719 treatment nM 26 Confidential % of DMSO Control % of DMSO Control
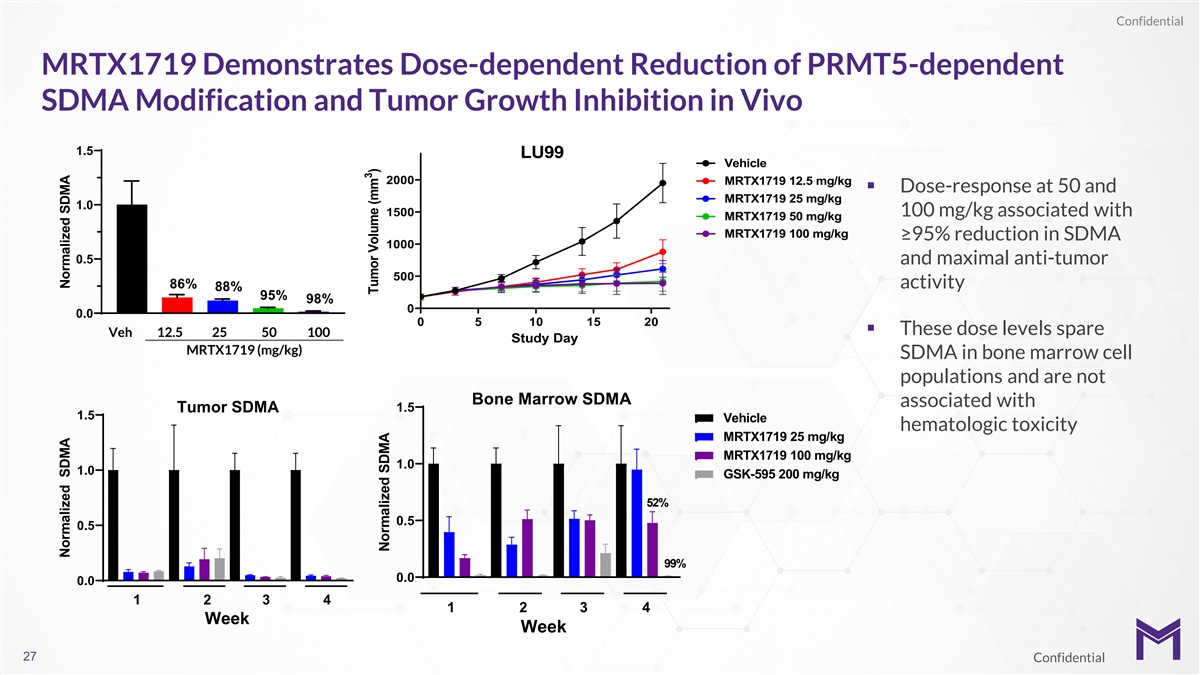
Confidential MRTX1719 Demonstrates Dose-dependent Reduction of
PRMT5-dependent SDMA Modification and Tumor Growth Inhibition in Vivo 1.5 LU99 Vehicle 2000 MRTX1719 12.5 mg/kg § Dose-response at 50 and MRTX1719 25 mg/kg 1.0 1500 100 mg/kg associated with MRTX1719 50 mg/kg MRTX1719 100 mg/kg ≥95%
reduction in SDMA 1000 0.5 and maximal anti-tumor 500 86% activity 88% 95% 98% 0 0.0 0 5 10 15 20 § These dose levels spare Veh 12.5 25 50 100 Study Day MRTX1719 (mg/kg) SDMA in bone marrow cell populations and are not Bone Marrow SDMA
associated with Tumor SDMA 1.5 1.5 Vehicle hematologic toxicity MRTX1719 25 mg/kg MRTX1719 100 mg/kg 1.0 1.0 GSK-595 200 mg/kg 52% 0.5 0.5 99% 0.0 0.0 1 2 3 4 1 2 3 4 Week Week 27 Confidential Normalized SDMA Normalized SDMA 3 Tumor Volume (mm )
Normalized SDMA

Confidential Encouraging Early Clinical Experience in Heavily
Pretreated Patients in Phase 1 Dose Escalation Study ▪ Dose escalation in 100% increments from 50 mg → 800 mg once-daily (QD) – 33 patients evaluable for safety across 6 dose levels, having received at least one dose of MRTX1719 1
– Of those 33 patients, 21 were evaluable for clinical response, having had at least one post-baseline tumor assessment (6 weeks +/- 10 days) 2 – 18 of 21 patients evaluable for clinical response had been treated at dose levels in the
therapeutic range of ≥100 mg QD – 800 mg defined as MAD (maximum administered dose) for QD schedule – 600 mg QD is under evaluation ▪ Early proof of concept achieved with 6 PRs (5 confirmed PR, 1 unconfirmed PR) per RECIST
v1.1 out of 18 patients evaluable for response 2 at therapeutic dose levels ≥100 mg QD across multiple dose levels and tumor types, including NSCLC, mesothelioma, biliary tract tumors, melanoma. ─ Note: the unconfirmed response (mPNST)
was observed shortly after data cutoff date ─ Observed deepening of, or sustained tumor regression in, responding patients over time ▪ Safety results remain encouraging at dose levels up to 800 mg QD – No dose limiting heme-related
toxicities that were observed with non-selective PRMT5 inhibitors – Most frequently reported TRAEs were nausea, vomiting, and fatigue with all TRAEs, except one Grade 3 event of fatigue, reported as Grade 1 or Grade 2 ▪ Emerging
preliminary PK results remain favorable – Effective half-life supports QD dosing – Average exposure at 200 mg QD comparable to the maximal pre-clinical target efficacious exposure ▪ SDMA (PD biomarker) completely extinguished in
patient tumor biopsies following MRTX1719 treatment at 200 mg QD Data cutoff: June 13, 2023 1 Note: 12 patients defined as not evaluable for clinical response include 7 patients on study < 6 weeks, 1 patient with scan data not evaluable, 2
patients MTAP WT, 2 patients off study due to AE prior to 1st scan 28 Confidential 2 Therapeutic dose level defined at ≥ 100 mg QD, as low dose nonlinear plasma exposure at 50 mg QD was markedly below therapeutic threshold.
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Mirati Therapeutics (NASDAQ:MRTX)
Graphique Historique de l'Action
De Déc 2024 à Jan 2025

Mirati Therapeutics (NASDAQ:MRTX)
Graphique Historique de l'Action
De Jan 2024 à Jan 2025
