As
filed with the Securities and Exchange Commission on May 8, 2024
Registration
Statement No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-3
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
PALISADE
BIO, INC.
(Exact
name of Registrant as Specified in its Charter)
| Delaware |
|
52-2007292 |
(State
or other jurisdiction of
incorporation
or organization) |
|
(I.R.S.
Employer
Identification
Number) |
7750
El Camino Real, Suite 2A
Carlsbad,
California 92009
(858)
704-4900
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Paracorp
Incorporated
2140
S Dupont highway
Camden,
DE 19934
(302)
697-4590
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Raul
Silvestre, Esq.
Dennis
Gluck, Esq.
2629
Townsgate Road #215
Westlake
Village, CA 91361
(818)
597-7552
If
the only securities being registered on this form are being offered pursuant to dividend or interest reinvestment plans, please check
the following box. ☐
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following
box. ☒
If
this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective
upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If
this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional
securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company,” and “emerging growth company” in Rule 12b–2 of the Exchange Act.
| Large
accelerated filer |
☐ |
Accelerated
filer |
☐ |
| |
|
|
|
| Non-accelerated
filer |
☒ |
Smaller
reporting company |
☒ |
| |
|
|
|
| |
|
Emerging
growth company |
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective
on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. The selling stockholders may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and
it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED MAY 8, 2024
PROSPECTUS

1,575,019
shares of Common Stock
This
prospectus covers the offer and resale by the selling stockholders identified in this prospectus of up to an aggregate of 1,575,019 shares
of our common stock and common stock underlying warrants issued pursuant to a private placement of our securities on May 6, 2024
(the “May 2024 Offering”), consisting of (i) 85,100 common shares (the “Shares”), (ii) 530,142 common shares
issuable upon the exercise of prefunded warrants with a perpetual term (the “Prefunded Warrants”), (iii) 922,863 common shares
issuable upon the exercise of common stock purchase warrants with a term of seven (7) years (the “Common Warrants”) and (iv)
36,914 common shares issuable upon the exercise of placement agent warrants with a term of five (5) years (the “Placement Agent
Warrants”). The Shares, Prefunded Warrants, and Common Warrants were issued in the May 2024 Offering pursuant to a securities purchase
agreement, dated May 1, 2024 (the “Purchase Agreement”), and the Placement Agent Warrants were issued as partial compensation
for placement agent fees. We are registering the Shares and common shares underlying the Prefunded Warrants, Common Warrants,
and Placement Agent Warrants (collectively “Warrant Shares”) issuable upon exercise of the Prefunded Warrants, Common Warrants,
and Placement Agent Warrants on behalf of the selling stockholders, to be offered and sold by each from time to time.
We
are not selling any shares of common stock under this prospectus and will not receive any proceeds from the sales by the selling stockholders
of such shares. We are paying the cost of registering the shares of common stock covered by this prospectus as well as various related
expenses. Each selling stockholder is responsible for all selling commissions, transfer taxes and other costs related to the offer and
sale of its shares.
Sales
of the shares by the selling stockholders may occur at fixed prices, at market prices prevailing at the time of sale, at prices related
to prevailing market prices, at negotiated prices and/or at varying prices determined at the time of sale. The selling stockholders may
sell shares directly or through underwriters, broker-dealers or agents, who may receive compensation in the form of discounts, concessions
or commissions from the selling stockholders, the purchasers of the common shares, or both. The selling stockholders may sell any, all
or none of the securities offered by this prospectus and we do not know when or in what amount the selling stockholders may sell their
shares of common stock hereunder following the effective date of the registration statement of which this prospectus forms a part. Additional
information about how the selling stockholders may sell or otherwise dispose of their shares of common stock is contained in the section
of this prospectus entitled “Plan of Distribution” on page 11.
Our
common stock is listed on The Nasdaq Capital Market under the symbol “PALI.” On May 7, 2024, the last reported sale price
of our common stock was $7.90 per share.
Investing
in our common stock involves a high degree of risk. Before making an investment decision, please read the information under “Risk
Factors” beginning on page 9 of this prospectus and under similar headings in any amendment or supplement to this prospectus
or in any filing with the Securities and Exchange Commission that is incorporated by reference herein.
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED
IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The
date of this prospectus is , 2024
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, using a
“shelf” registration process. Under this registration statement, the selling stockholders may sell from time to time in one
or more offerings the common stock described in this prospectus.
We
have not authorized anyone to provide you with information other than the information that we have provided or incorporated by reference
in this prospectus and your reliance on any unauthorized information or representation is at your own risk. This prospectus may be used
only in jurisdictions where offers and sales of these securities are permitted. You should assume that the information appearing in this
prospectus is accurate only as of the date of this prospectus and that any information we have incorporated by reference is accurate
only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus, or any sale of
our common stock. Our business, financial condition and results of operations may have changed since those dates.
Unless
otherwise stated, all references in this prospectus to “we,” “us,” “our,” “Palisade,”
the “Company” and similar designations refer to Palisade Bio, Inc. This prospectus contains references to trademarks belonging
to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other
visual displays, may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that
the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names.
We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement
or sponsorship of us by, any other companies.
A
prospectus supplement may add to, update or change the information contained in this prospectus. You should read both this prospectus
and any applicable prospectus supplement together with additional information described below under the heading “Where You Can
Find Additional Information.”
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and any applicable prospectus supplement or free writing prospectus, including the documents that we incorporate by reference
herein and therein, contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933,
as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These statements
relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other
factors which may cause our actual results, performance or achievements to be materially different from any future results, performances
or achievements expressed or implied by the forward-looking statements. Forward-looking statements may include, but are not limited to,
statements about:
| |
● |
estimates
about the size and growth potential of the markets for our product candidates, and our ability to serve those markets, including
any potential revenue; |
| |
|
|
| |
● |
future
regulatory, judicial, and legislative changes or developments in the United States (“U.S.”) and foreign countries and
the impact of these changes; |
| |
|
|
| |
● |
our
ability to successfully develop our technologies and proposed products; |
| |
|
|
| |
● |
our
ability to compete effectively in a competitive industry; |
| |
|
|
| |
● |
our
ability to identify and qualify manufacturers to provide active pharmaceutical ingredients (“API”) and manufacture drug
product; |
| |
|
|
| |
● |
our
ability to enter into commercial supply agreements; |
| |
|
|
| |
● |
the
success of competing technologies that are or may become available; |
| |
|
|
| |
● |
our
ability to attract and retain key scientific or management personnel; |
| |
|
|
| |
● |
the
accuracy of our estimates regarding expenses, capital requirements and needs for additional financing; |
| |
|
|
| |
● |
our
ability to obtain funding for our operations and the development of our product candidates; |
| |
|
|
| |
● |
our
ability to attract collaborators and partners; and |
| |
|
|
| |
● |
the
impact of pandemic, foreign or domestic conflicts, or other global disruptions on our business, our operations, and our supply. |
In
some cases, you can identify forward-looking statements by terms such as “may,” “will,” “intend,”
“should,” “could,” “would,” “expects,” “plans,” “anticipates,”
“believes,” “estimates,” “projects,” “predicts,” “potential” and similar
expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events
and are based on assumptions and are subject to risks and uncertainties. As such, our actual results may differ significantly from those
expressed in any forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking
statements.
We
discuss many of these risks in greater detail under the heading “Risk Factors” in this prospectus, in the “Business”
and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections incorporated by
reference from our most recent Annual Report on Form 10-K filed on March 26, 2024 with the SEC, as well as any amendments thereto reflected
in subsequent filings with the SEC.
The
discussion of risks and uncertainties set forth in those filings is not necessarily a complete or exhaustive list of all risks facing
us at any particular point in time. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus
will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In
light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation
or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. Forward-looking
statements represent our estimates and assumptions only as of the date of the document containing the applicable statement. Unless required
by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or
developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied
in such forward-looking statements. You should read this prospectus, any applicable prospectus supplement, together with the documents
that we have filed with the SEC that are incorporated by reference and any free writing prospectus we have authorized for use in connection
with this offering, completely and with the understanding that our actual future results may be materially different from what we expect.
We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
PROSPECTUS
SUMMARY
This
summary highlights certain information about us, this offering and selected information contained elsewhere in or incorporated by reference
into this prospectus. This summary is not complete and does not contain all of the information that you should consider before making
an investment decision. For a more complete understanding of our company, you should read and consider carefully the more detailed information
included or incorporated by reference in this prospectus and any applicable prospectus supplement, including the factors described under
the heading “Risk Factors” beginning on page 9 of this prospectus, as well as the information incorporated by reference
from our most recent Annual Report on Form 10-K, before making an investment decision.
Company
Overview
We
are a pre-clinical stage biotechnology company focused on developing and advancing novel therapies for patients living with autoimmune,
inflammatory, and fibrotic diseases. Our lead product candidate, PALI-2108, is being developed as a therapeutic for patients living with
inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”) and Crohn’s disease (“CD”).
Strategy
Our
objective is to establish ourselves as a leader in the development of differentiated product candidates targeting the autoimmune, inflammatory,
and fibrotic disease markets, which we believe will address a large, well-established need among patients living with autoimmune and
inflammatory diseases.
We
believe the key elements of our strategy include:
| |
● |
advancing
our lead product candidate, PALI-2108 into human clinical trials; |
| |
● |
leveraging
our drug development platform infrastructure to identify product candidates that target autoimmune, inflammatory, and fibrotic diseases; |
| |
● |
pursuing
strategic partnerships to further expand our programs and maximize the worldwide potential of our product candidates and platform;
and |
| |
● |
pursuing
strategy of in-licensing/acquisition or out-licensing/sale of our product candidates. |
PALI-2108
PALI-2108,
our lead asset, is currently in the pre-clinical stage of development. We have commenced pivotal pre-clinical studies and anticipate
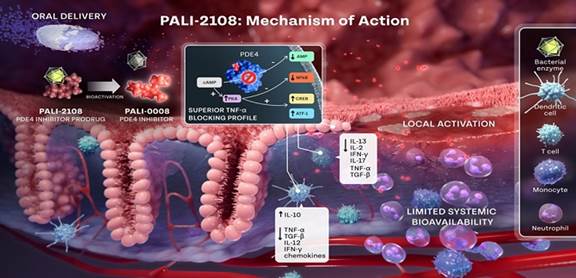
commencing Phase 1 clinical trials during the fourth quarter of 2024. PALI-2108 is a prodrug PDE4 inhibitor that operates through a sophisticated
mechanism within colon tissues, targeting the key enzyme phosphodiesterase-4 (PDE4). This enzyme is pivotal in cAMP hydrolysis, and by
inhibiting PDE4, intracellular cAMP levels are elevated. This elevation leads to the downregulation of inflammatory cytokines and a reduction
in the expression of cell adhesion molecules. By modulating these processes, PALI-2108 effectively prevents the local infiltration and
activation of inflammatory cells in the colon tissues, offering a targeted approach for UC treatment. With a galactose-derived sugar
moiety, PALI-2108 remains minimally absorbed until activated by the colonic bacterium enzyme β-glucuronidase. This prodrug exhibits
colon preference, as demonstrated in DSS-induced UC mouse models and oxazolone colitis-induced mice, showcasing its localized bioactivation
and colon-specific distribution.
Inflammatory
Bowel Disease
IBD
is a chronic condition characterized by inflammation within the gastrointestinal tract. It encompasses two main disorders: UC and CD.
UC primarily affects the colon and the rectum. Inflammation occurs in the innermost lining of the colon leading to ulcers. Symptoms include
bloody diarrhea, abdominal pain, bowel urgency, and frequent bowel movements. CD can affect any part of the gastrointestinal tract, from
the mouth to the anus. It is characterized by inflammation that extends through multiple layers of the bowel wall. Symptoms include abdominal
pain, diarrhea, weight loss, fatigue, and complications such as strictures or fistulas. Both conditions can significantly impact patients’
quality of life in terms of physical health, emotional well-being, and the unpredictability of symptom onset.
IBD
affects millions of individuals worldwide, with increasing prevalence and incidence in both developed and developing countries. In the
United States, it is estimated that approximately 2.4 million individuals currently have IBD, with approximately 70,000 patients newly
diagnosed every year. The prevalence of UC in the United States is approximately 900,000 individuals, and the prevalence of CD in the
United States is approximately 800,000 individuals.
Selected
Risks Affecting Our Business
Below
is a summary of the factors that make an investment in our securities speculative or risky. This summary does not address all the risks
that we face nor discusses such risks in detail. Additional discussion of the risks and uncertainties summarized in this risk factor
summary, and other risks and uncertainties that we face, are set forth below under the heading “Risk Factors” in the documents
that are incorporated by reference into this prospectus, including our Annual Report on Form 10-K filed with the SEC on March 26, 2024.
Risks
Related to Our Development, Commercialization and Regulatory Approval of Our Investigational Therapies
| ● | Our
business depends on the successful pre-clinical and clinical development, regulatory approval
and commercialization of our recently licensed therapeutic compound, including our lead asset
PALI-2108. |
| ● | There
are substantial risks inherent in drug development, and, as a result, we may not be able
to successfully develop PALI-2108. |
| ● | We
depend on our license agreement with Giiant Pharma, Inc. (“Giiant”) to permit
us to use patents and patent applications relating to PALI-2108. Termination of these rights
or the failure to comply with obligations under the license agreement could materially harm
our business and prevent us from developing or commercializing PALI-2108, our lead product
candidate. |
| ● | We
expect that our operations and development of PALI-2108 will require substantially more capital
than we currently have, and we cannot guarantee when or if we will be able to secure such
additional funding. |
| ● | There
can be no assurance that our product candidates will obtain regulatory approval. |
| ● | If
pre-clinical and clinical studies of PALI-2108 do not yield successful results, we may decide
to not continue the development of PALI-2108. |
| ● | Even
if our clinical studies are successful and achieve regulatory approval, the approved product
label may be more limited than we anticipate, which could limit the commercial prospects
of PALI-2108. |
| ● | We
may in the future conduct clinical trials for PALI-2108 outside the United States (“U.S.”),
and the U.S. Food and Drug Administration (“FDA”) and applicable foreign regulatory
authorities may not accept data from such trials. |
| ● | We
anticipate relying on third-party Contract Research Organizations (“CROs”) and
other third parties to conduct and oversee our pre-clinical studies and clinical trials.
If these third parties do not meet our requirements or otherwise conduct the studies or trials
as required, we may not be able to satisfy our contractual obligations or obtain regulatory
approval for, or commercialize, our product candidates. |
| ● | We
have entered into a collaborative research agreement with Giiant related to pre-clinical
development, which will require the efforts of Giiant and its personnel, which are out of
our control. |
Risks
Related to Our Business
| ● | We
have a limited operating history and have never generated any revenue from product sales. |
| ● | Our
business model assumes revenue from, among other activities, marketing or out-licensing the
products we develop. PALI-2108 is in the early stages of development and because we have
a short development history with PALI-2108, there is a limited amount of information about
us upon which you can evaluate our business and prospects. |
| ● | Our
common stock could be delisted from the Nasdaq Stock Market if we are unable to maintain
compliance with the Nasdaq Stock Market’s continued listing standards. |
| ● | We
have received a notification from the Nasdaq Stock Market that our audit committee does not
have three (3) independent members as a result of recent director resignations. If we fail
to timely appoint an independent director that meets the Nasdaq Stock Market Requirements
for audit committees, Nasdaq could delist our common stock. |
| ● | Our
success depends on the attracting and retaining of senior management and scientists with
relevant expertise. |
| ● | We
may choose to discontinue developing or commercializing any of our product candidates, or
may choose to not commercialize product candidates in approved indications, at any time during
development or after approval, which could adversely affect us and our operations. |
| ● | Our
inability to successfully in-license, acquire, develop and market additional product candidates
or approved products could impair our ability to grow our business. |
Risks
Related to Our Dependence on Third Parties
| ● | We
expect to rely on collaborations with third parties for the successful development and commercialization
of our product candidates. |
| ● | We
anticipate relying completely on third-party contractors to supply, manufacture and distribute
clinical drug supplies for our product candidates. |
Risks
Related to Our Financial Operations
| ● | We
have expressed substantial doubt about our ability to continue as a going concern. |
| ● | We
have a history of net operating losses, and we expect to continue to incur net operating
losses and may never achieve profitability. |
| ● | Failure
to remediate a material weakness in internal controls over financial reporting could result
in material misstatements in our consolidated financial statements. |
Risks
Related to Our Intellectual Property
| ● | We
may not be able to obtain, maintain or enforce global patent rights or other intellectual
property rights that cover our product candidates and technologies that are of sufficient
breadth to prevent third parties from competing against us. |
| ● | If
we fail to comply with our obligations under our intellectual property license agreements,
we could lose license rights that are important to our business. |
Other
Risks Related to Our Securities
| ● | We
will need to raise additional capital in the future to fund our operations, which may not
be available to us on favorable terms or at all. |
| ● | Our
common stock price may be highly volatile. |
| ● | If
we fail to maintain proper and effective internal controls, our ability to produce accurate
financial statements on a timely basis could be impaired. |
| ● | Our
Board of Directors has broad discretion to issue additional securities, which might dilute
the net tangible book value per share of our common stock for existing stockholders. |
Corporate
Information
The
registrant was originally incorporated in 2001 in the State of Delaware under the name Neuralstem, Inc. In October of 2019, Neuralstem,
Inc. changed its name to Seneca Biopharma, Inc. In April of 2021, we effected a merger, whereby Leading BioSciences, Inc. (our operations
prior to the completion of a merger with Seneca) became a wholly owned subsidiary of Seneca. In April of 2021, we changed our name from
Seneca Biopharma, Inc. to Palisade Bio, Inc. Our principal executive offices are located at 7750 El Camino Real, Suite 2A, Carlsbad,
California 92009, our telephone number is (858) 704-4900 and our website address is www.palisadebio.com.
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage
of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We
will remain a smaller reporting company until the last day of any fiscal year for so long as either (1) the market value of our shares
of common stock held by non-affiliates does not equal or exceed $250 million as of the prior June 30th, or (2) our annual
revenues did not equal or exceed $100 million during such completed fiscal year and the market value of our shares of common stock held
by non-affiliates did not equal or exceed $700.0 million as of the prior June 30th. To the extent we take advantage of any
reduced disclosure obligations, it may make comparison of our financial statements with other public companies difficult or impossible.
Reverse
Stock Split
On
April 5, 2024, we effected a reverse stock split of our outstanding common stock (“Reverse Split”). As a result of the Reverse
Split, each of the Company’s shareholders received one (1) new share of common stock for every fifteen (15) shares such shareholder
held immediately prior to the effective time of the Reverse Split. The Reverse Split affected all the Company’s issued and outstanding
shares of common stock equally. The Reverse Split also affected the Company’s outstanding stock options, warrants and other exercisable
or convertible securities and resulted in the shares underlying such instruments being reduced and the exercise price or conversion price
being increased proportionately. No fractional shares were issued because of the Reverse Split. Any fractional shares that would have
otherwise resulted from the Reverse Split were paid in cash, at an amount equal to the resulting fractional interest in one (1) share
of the common stock to which the shareholder would otherwise be entitled, multiplied by the closing trading price of the Common Stock
on April 5, 2024. All common stock shares, common stock per share data and shares of common stock underlying convertible preferred stock,
stock options and common stock warrants included in the financial statements, including the exercise price or conversion price of such
equity instruments, as applicable, were retrospectively adjusted to reflect the effect of the Reverse Split.
May
2024 Private Placement
Securities
Purchase Agreement
On
May 1, 2024, we entered into a securities purchase agreement (the “Securities Purchase Agreement”) with an institutional
investor, pursuant to which the Company agreed to sell and issue, in a private placement (the “May 2024 Offering”): (i) 85,100
shares of common stock (the “Shares”) and (ii) 530,142 prefunded warrants with an exercise price of $0.0001 per share, with
a perpetual term (the “Prefunded Warrants”), at a purchase price per Share or Prefunded Warrant of $6.5015 (less the applicable
exercise price of $0.0001 for each Prefunded Warrant purchased). We additionally issued the investor, 922,863 common stock warrants with
an exercise price of $6.314 per share and a term of seven (7) years (the “Common Warrants”). All of the Prefunded Warrants
and Common Warrants issued in the May 2024 Offering are immediately exercisable from their date of issuance.
Registration
Rights Agreement
In
connection with the May 2024 Offering we also entered into a registration rights agreement (“Registration Rights Agreement”)
with the Purchaser, whereby the Company agreed to file a registration statement on Form S-3 within 10 days of the date of the closing
of the May 2024 Offering, which shall provide for the resale by holder of the Shares and Warrants Shares and to have such registration
statement declared effective within 60 days of the date of the Registration Rights Agreement, and to use best efforts to keep such registration
statement effective at all times until such date that the Shares and Warrant Shares either (i) have been sold, or (ii) may be sold without
volume or manner-of-sale restrictions pursuant to Rule 144 and without the requirement for the Company to be in compliance with the current
public information requirement under Rule 144. The Registration Rights Agreement also provides for the payment of liquidated damages
in the event the Registration Statement is not filed on time, is not declared effective within 60 days and for certain other occurrences.
Placement
Agent Agreement
Pursuant
to a placement agency agreement dated May 1, 2024, we engaged Ladenburg Thalmann & Co. Inc. (the “Placement Agent”),
to act as our exclusive placement agent in connection with the May 2024 Offering. As partial consideration for its services, we issued
the Placement Agent common stock purchase warrants to purchase an aggregate of 36,914 shares of our Common Stock (the “Placement
Agent Warrants”). The Placement Agent Warrants have an exercise price of $10.727 per share and a term of five (5) years. The Placement
Agent Warrants are immediately exercisable.
The
Offering
The
registration statement of which this prospectus is a part relates to the resale of the (i) Shares sold to the selling stockholder in
the May 2024 Offering, (ii) shares of common stock that may be issued to the selling stockholder in connection with the exercise of the
Prefunded Warrants and Common Warrants issued in May 2024 Offering, and (iii) shares of common stock that may be issued in connection
with the exercise of the Placement Agent Warrants issued to the Placement Agent in the May 2024 Offering.
| Common
stock offered by the selling stockholders: |
|
1,575,019
shares |
| |
|
|
| Terms
of the offering: |
|
The
selling stockholders will determine when and how they will sell the common stock offered in this prospectus, as described in the
section of this prospectus entitled “Plan of Distribution.” |
| |
|
|
| Use
of proceeds: |
|
We
will not receive any proceeds from the sale of shares of our common stock by the selling stockholders. |
| |
|
|
| Risk
factors: |
|
See
“Risk Factors” beginning on page 9, for a discussion of factors you should carefully consider before deciding to invest
in our common stock. |
| |
|
|
| Nasdaq
Capital Market symbol: |
|
PALI |
The
selling stockholders named in this prospectus may offer and sell up to 1,575,019 shares of our common stock.
The
selling stockholders are prohibited, subject to certain exceptions, from exercising the Prefunded Warrants, Common Warrants, and Placement
Agent Warrants, as applicable, to the extent that immediately prior to or after giving effect to such exercise, the selling stockholder,
together with its affiliates and other attribution parties, would own more than 4.99% (or 9.99% with respect to certain selling stockholders
and / or certain warrants) of the total number of shares of the Company’s common stock then issued and outstanding, which percentage
may be changed at a selling stockholder’s election to a lower percentage at any time or to a higher percentage not to exceed 9.99%
upon 61 days’ notice to the Company.
Our
common stock is currently listed on The Nasdaq Capital Market under the symbol “PALI.”
Shares
of common stock that may be offered under this prospectus will be fully paid and non-assessable. We will not receive any proceeds from
the sales by the selling stockholders of any of the common stock covered by this prospectus. Throughout this prospectus, when we refer
to the shares of our common stock being registered on behalf of the selling stockholders for offer and resale, we are referring to the
Shares sold in the May 2024 Offering and shares of common stock issued to the selling stockholders in connection with the exercise of
the Prefunded Warrants, Common Warrants, and Placement Agent Warrants, respectively. When we refer to the selling stockholders in this
prospectus, we are referring to the selling stockholders identified in this prospectus and, as applicable, their permitted transferees
or other successors-in-interest that may be identified in a supplement to this prospectus or, if required, a post-effective amendment
to the registration statement of which this prospectus is a part.
RISK
FACTORS
Investing
in our common stock involves a high degree of risk. Before making an investment decision, you should carefully consider the risks described
below and described in the sections entitled “Risk Factors” in our most recent Annual Report on Form 10-K, as filed with
the SEC on March 26, 2024, which is incorporated herein by reference in its entirety, as well as any amendment or updates to our risk
factors reflected in subsequent filings with the SEC, including any applicable prospectus supplement. Our business, financial condition,
results of operations or prospects could be materially adversely affected by any of these risks. The trading price of our securities
could decline due to any of these risks, and you may lose all or part of your investment. This prospectus and the documents incorporated
herein by reference also contain forward-looking statements that involve risks and uncertainties. Our actual results could differ materially
from those anticipated in these forward-looking statements as a result of certain factors, including the risks mentioned elsewhere in
this prospectus. For more information, see the section of this prospectus entitled “Where You Can Find Additional Information.”
Please also read carefully the section of this prospectus entitled “Special Note Regarding Forward-Looking Statements.”
USE
OF PROCEEDS
We
will not receive any of the proceeds from the sale or other disposition of shares of our common stock held by the selling stockholders
pursuant to this prospectus. We will bear the out-of-pocket costs, expenses and fees incurred in connection with the registration of
shares of our common stock to be sold by the selling stockholders, including registration, listing and qualifications fees, printers
and accounting fees, and fees and disbursements of counsel, or collectively, the Registration Expenses. Other than Registration Expenses,
the selling stockholders will bear underwriting discounts, commissions, placement agent fees or other similar expenses payable with respect
to sales of their shares.
SELLING
STOCKHOLDERS
We
are registering the resale of 1,575,019 shares of common stock consisting of (i) 85,100 Shares, (ii) 530,142 common shares issuable upon
the exercise of Prefunded Warrants, (iii) 922,863 common shares issuable upon the exercise of Common Warrants and (iv) 36,914 common
shares issuable upon the exercise of Placement Agent Warrants held by the selling stockholders identified below to permit such selling
stockholders, or their permitted transferees or other successors-in-interest that may be identified in a supplement to this prospectus
or, if required, a post-effective amendment to the registration statement of which this prospectus is a part, to resell or otherwise
dispose of these shares in the manner described under the section of this prospectus entitled “Plan of Distribution” (as
may be supplemented and amended).
The
selling stockholders may sell some, all or none of their shares. We do not know how long the selling stockholders will hold the shares
before selling them, and we currently have no agreements, arrangements or understandings with any of the selling stockholders regarding
the sale or other disposition of any of the shares. The shares covered hereby may be offered from time to time by the selling stockholders.
As a result, we cannot estimate the number of shares of common stock the selling stockholders will beneficially own after their sales
under this prospectus. In addition, the selling stockholders may have sold, transferred or otherwise disposed of all or a portion of
its shares of common stock since the date on which it provided information for this table.
Beneficial
ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to our common stock.
Generally, a person “beneficially owns” shares of our common stock if the person has or shares with others the right to vote
those shares or to dispose of them, or if the person has the right to acquire voting or disposition rights within 60 days.
Under
the terms of the Prefunded Warrants, Common Warrants, and Placement Agent Warrants, respectively, the selling stockholders may not exercise
such warrants or any other warrants to the extent such exercise would cause such selling stockholder, together with its affiliates, to
beneficially own a number of shares of common stock which would exceed 4.99% (or 9.99% with respect to the elections of certain selling
stockholders / warrants held) of our then outstanding common stock following such exercise, excluding for purposes of such determination
common stock issuable upon exercise of the warrants and such other warrants, which have not been exercised. The number of shares in the
second and fourth columns and the percentage in the fourth column reflect this limitation.
The
information in the table below and the footnotes thereto regarding shares of common stock to be beneficially owned after the offering
assumes the exercise of the Prefunded Warrants, Common Warrants, and Placement Agent Warrants, as applicable by the selling stockholders
and sale of all shares being offered by the selling stockholders under this prospectus. The percentage of shares owned after the offering
is based on 937,562 shares of common stock outstanding as of May 7, 2024.
| | |
Common Shares
Owned Before Sale (1) | | |
| | |
Common Shares
Owned After Sale (2) | |
| | |
Held
Outright | | |
Convertible
Securities | | |
Amount | | |
% of
class | | |
Shares being
registered | | |
Amount | | |
% of
Class | |
| Armistice Capital, LLC. (3) | |
| 85,100 | | |
| 9,384 | | |
| 94,484 | | |
| 9.99 | % | |
| 1,538,105 | | |
| 117,354 | | |
| 4.68 | % |
| Ladenburg Thalmann & Co. Inc. (4) | |
| - | | |
| 47,609 | | |
| 47,609 | | |
| 4.83 | % | |
| 14,766 | | |
| 32,843 | | |
| 3.33 | % |
| Nicholas Stergis (5) | |
| - | | |
| 30,361 | | |
| 30,361 | | |
| 3.14 | % | |
| 22,148 | | |
| 8,213 | | |
| * | |
| TOTALS | |
| 85,100 | | |
| 87,364 | | |
| 172,454 | | |
| 16.85 | % | |
| 1,575,019 | | |
| 158,410 | | |
| 6.98 | % |
*
Less than 1%.
(1)
Pursuant to Rules 13d-3 and 13d-5 of the Exchange Act, beneficial ownership includes any common shares (“Common Shares”)
as to which a shareholder has sole or shared voting power or investment power, and also any Common Shares which the shareholder has the
right to acquire within 60 days, including upon exercise of Common Share purchase options or warrants. There were 937,562 Common
Shares outstanding as of May 7, 2024. All shares referenced below are Common Shares.
(2)
Assumes the exercise of the Prefunded Warrants, Common Warrants, and Placement Agent Warrants, each to the extent applicable, and sale
of all shares available for sale under this prospectus and no further acquisitions of shares by the selling stockholder.
(3)
The shares being registered include (i) 85,100 Common Shares issued in the May 2024 Offering, (ii) 530,142 Common Shares underlying Prefunded
Warrants issued in the May 2024 Offering, and (iii) 922,863 Common Shares underlying Common Warrants issued in the May 2024 Offering.
The Prefunded Warrants are subject to a 9.99% maximum beneficial ownership limitation and the Common Warrants are subject to a 4.99%
maximum beneficial ownership limitation (subject to increase to 9.99% on 61 days notice). The total shares owned before the sale excludes
(i) 520,758 Common Shares underlying Prefunded Warrants that would be in excess of the 9.99% beneficial ownership limitation, (ii) 922,863
Common Warrants that would be in excess of the 4.99% beneficial ownership limitation and (iii) 117,354 Common Shares underlying previously
owned warrants from other offerings of the Company’s securities. The securities are directly held by Armistice Capital Master Fund
Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be beneficially owned by: (i) Armistice
Capital, LLC (“Armistice Capital”), as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member
of Armistice Capital. The Prefunded Warrants are subject to a beneficial ownership limitation of 9.99% and the Common Warrants are subject
to a beneficial ownership limitation of 4.99%, which such limitation restricts the Selling Stockholder from exercising that portion of
the respective warrants that would result in the Selling Stockholder and its affiliates owning, after exercise, a number of shares of
common stock in excess of the beneficial ownership limitation. The address of Armistice Capital Master Fund Ltd. is c/o Armistice Capital,
LLC, 510 Madison Avenue, 7th Floor, New York, NY 10022.
(4)
The shares being registered include 14,766 Common Shares underlying Placement Agent Warrants issued to the selling stockholder in the
May 2024 Offering. The Placement Agent Warrants are subject to a 4.99% maximum beneficial ownership limitation (subject to increase to
9.99% on 61 days notice). Ladenburg is a registered broker-dealer that received its Placement Agent Warrants pursuant to investment banking
services in the May 2024 Offering. Peter Blum, CEO of Ladenburg, has voting and dispositive control with respect to the securities being
offered.
(5)
The shares being registered include 22,148 Common Shares underlying Placement Agent Warrants issued to the selling stockholder in the
May 2024 Offering. The Placement Agent Warrants are subject to a 4.99% maximum beneficial ownership limitation (subject to increase to
9.99% on 61 days notice). The selling stockholder is an affiliate of Ladenburg Thalmann & Co. Inc., a registered broker-dealer that
received its Placement Agent Warrants pursuant to investment banking services in the May 2024 Offering. Nicholas Stergis has voting and
dispositive control with respect to the securities being offered.
Relationship
with Selling Stockholders
As
discussed in greater detail above under the section of this prospectus entitled “Prospectus Summary” we entered into agreements
with the selling stockholders pursuant to which they acquired the Shares, Prefunded Warrants, Common Warrants, and Placement Agent Warrants,
as applicable, and agreed, pursuant to a registration rights agreement to file a registration statement to enable the resale of the shares
of common stock and shares of common stock issuable upon the exercise of the respective warrants.
Except
for Ladenburg Thalman & Co. Inc. who served as our placement agent with respect to the May 2024 Offering, as well as placement agent
or underwriter in a number of our prior offerings, none of the selling stockholders nor any persons having control over such selling
stockholders have held any position or office with us or our affiliates within the last three years nor has had a material relationship
with us or any of our predecessors or affiliates within the past three years, other than as a result of the ownership of our shares or
other securities.
PLAN
OF DISTRIBUTION
We
are registering the Shares or shares of common stock issued and issuable upon exercise of the Prefunded Warrants, Common Warrants, and
Placement Agent Warrants, to permit the resale of these shares of common stock by the holders from time to time after the date of this
prospectus. We will not receive any of the proceeds from the sale by the selling stockholders of the shares of common stock. We will
bear all fees and expenses incident to our obligation to register the shares of common stock.
Each
selling stockholder (each hereafter, the “Selling Stockholder”) and any of their pledgees, assignees and successors-in-interest
may, from time to time, sell any or all of their securities covered hereby on the Nasdaq Capital Market or any other stock exchange,
market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices.
A Selling Stockholder may use any one or more of the following methods when selling securities:
| ● | ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block
trades in which the broker-dealer will attempt to sell the securities as agent but may position
and resell a portion of the block as principal to facilitate the transaction; |
| ● | purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | an
exchange distribution in accordance with the rules of the applicable exchange; |
| ● | privately
negotiated transactions; |
| ● | settlement
of short sales; |
| ● | in
transactions through broker-dealers that agree with the Selling Stockholders to sell a specified
number of such securities at a stipulated price per security; |
| ● | through
the writing or settlement of options or other hedging transactions, whether through an options
exchange or otherwise; |
| ● | a
combination of any such methods of sale; or |
| ● | any
other method permitted pursuant to applicable law. |
The
Selling Stockholder may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933,
as amended (the “Securities Act”), if available, rather than under this prospectus.
Broker-dealers
engaged by the Selling Stockholder may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions
or discounts from the Selling Stockholder (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser)
in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in
excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or
markdown in compliance with FINRA Rule 2121.
In
connection with the sale of the securities or interests therein, the Selling Stockholder may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they
assume. The Selling Stockholder may also sell securities short and deliver these securities to close out their short positions, or loan
or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholder may also enter into option
or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the
delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The
Selling Stockholder and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. The Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
The
Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company
has agreed to indemnify the Selling Stockholder against certain losses, claims, damages and liabilities, including liabilities under
the Securities Act.
We
agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders
without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for
the Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar
effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule
of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable
state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is
complied with.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the Selling Stockholder will be subject to applicable provisions of the Exchange
Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common
stock by the Selling Stockholder or any other person. We will make copies of this prospectus available to the Selling Stockholder and
have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
EXPERTS
The
consolidated financial statements of Palisade Bio, Inc. as of December 31, 2023 and 2022 and for the years then ended, incorporated by
reference in this Prospectus, have been audited by Baker Tilly US, LLP, an independent registered public accounting firm, as stated in
their report, which is incorporated herein by reference. Such consolidated financial statements have been so incorporated by reference
in reliance upon the report of such firm given their authority as experts in accounting and auditing. The report on the consolidated
financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
LEGAL
MATTERS
Certain
legal matters, including the validity of the shares of common stock offered pursuant to this registration statement, will be passed upon
for us by Silvestre Law Group, P.C., Westlake Village, California.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus is part of the registration statement on Form S-3 we filed with the SEC under the Securities Act and does not contain all
the information set forth or incorporated by reference in the registration statement. Whenever a reference is made in this prospectus
to any of our contracts, agreements or other documents, the reference may not be complete and you should refer to the exhibits that are
a part of the registration statement or the exhibits to the reports or other documents incorporated by reference into this prospectus
for a copy of such contract, agreement or other document. Because we are subject to the information and reporting requirements of the
Exchange Act, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are
available to the public over the Internet at the SEC’s website at www.sec.gov. Our Annual Report on Form 10-K, Quarterly Reports
on Form 10-Q and Current Reports on Form 8-K, including any amendments to those reports, and other information that we file with or furnish
to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act can also be accessed free of charge on the “Investor” section
of our website. These filings will be available as soon as reasonably practicable after we electronically file such material with, or
furnish it to, the SEC. Our website address is www. palisadebio.com. Information contained on or accessible through our website is not
a part of this prospectus and is not incorporated by reference herein, and the inclusion of our website address in this prospectus is
an inactive textual reference only.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information
to you by referring to those documents. The information incorporated by reference is an important part of this prospectus, and information
that we file later with the SEC will automatically update and supersede this information.
We
incorporate by reference the following documents we filed with the SEC pursuant to Section 13 of the Exchange Act and any future filings
we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act after the date of this prospectus until the termination
of the offering of the shares covered by this prospectus (other than information furnished under Item 2.02 or Item 7.01 of Form 8-K):
| |
● |
our
Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 26, 2024, including the Part III information
contained therein; |
| |
|
|
| |
● |
our
Current Reports on Form 8-K filed on January 29, 2024, February 1, 2024, February 13, 2024, March 6, 2024, March 27, 2024, April 5, 2024, April 10, 2024, May 3, 2024; and |
| |
|
|
| |
● |
the
description of our common stock which is registered under Section 12 of the Exchange Act, in our registration statement on Form 8-A
filed with the SEC on July 1, 2015, including any amendments or reports filed for the purpose of updating such description, including
Exhibit 4.2 to our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 17, 2022. |
You
may access our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, Proxy Statement, and amendments,
if any, to those documents filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at the
SEC’s website or our website as soon as reasonably practicable after such material is electronically filed with, or furnished to,
the SEC. The reference to our website does not constitute incorporation by reference of the information contained in our website. We
do not consider information contained on, or that can be accessed through, our website to be part of this prospectus or the related registration
statement.
We
will provide to each person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request,
a copy of any or all of the information that is incorporated by reference into this prospectus but not delivered with the prospectus,
including exhibits which are specifically incorporated by reference into such documents. You should direct any requests for documents
to Palisade Bio, Inc. at 7750 El Camino Real, Suite 2A, Carlsbad, California 92009 Attn: Secretary, or by telephone (858) 704-4900.
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
14. Other Expenses of Issuance and Distribution.
The
following is a statement of the estimated expenses to be incurred by us in connection with the registration of the securities under this
registration statement, all of which will be borne by us.
| Securities and Exchange Commission Registration Fee | |
$ | 1,681 | |
| Legal Fees and Expenses | |
$ | 50,000 | |
| Accountants’ Fees and Expenses | |
$ | 15,000 | |
| Miscellaneous | |
$ | 10,000 | |
| | |
| | |
| Total | |
$ | 76,681 | |
Item
15. Indemnification of Directors and Officers.
Our
amended and restated certificate of incorporation contains provisions that eliminate, to the maximum extent permitted by the General
Corporation Law of the State of Delaware, the personal liability of directors and executive officers for monetary damages for breach
of their fiduciary duties as a director or officer. Our amended and restated certificate of incorporation and bylaws provide that we
shall indemnify our directors and executive officers and may indemnify our employees and other agents to the fullest extent permitted
by the General Corporation Law of the State of Delaware.
Sections
145 and 102(b)(7) of the General Corporation Law of the State of Delaware provide that a corporation may indemnify any person made a
party to an action by reason of the fact that he or she was a director, executive officer, employee or agent of the corporation or is
or was serving at the request of the corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid
in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a
manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal
action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or
in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to
be liable to the corporation.
We
have purchased and intend to maintain insurance on behalf of any person who is or was a director or officer of our company against any
loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
We
have entered, and intend to continue to enter, into separate indemnification agreements with our directors and executive officers to
provide these directors and executive officers additional contractual assurances regarding the scope of the indemnification set forth
in the registrant’s amended and restated certificate of incorporation and amended and restated bylaws and to provide additional
procedural protections. At present, there is no pending litigation or proceeding involving a director or executive officer of the Company
regarding which indemnification is sought. The indemnification provisions in our amended and restated certificate of incorporation, amended
and restated bylaws and the indemnification agreements entered into or to be entered into between us and each of our directors and executive
officers may be sufficiently broad to permit indemnification of our directors and executive officers for liabilities arising under the
Securities Act. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and
controlling persons of ours pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities
and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
We carry liability insurance for our directors and officers.
Item
16. Exhibit Index.
| Exhibit
No. |
|
Description |
| |
|
|
| 4.1 |
|
Form of Prefunded Common Stock Warrant issued in May 2024 Private Placement (Incorporated by reference to Exhibit 4.01 to the Registrant’s Current Report on Form 8-K filed with the SEC on May 3, 2024). |
| |
|
|
| 4.2 |
|
Form of Common Stock Warrant issued in May 2024 Private Placement (Incorporated by reference to Exhibit 4.02 to the Registrant’s Current Report on Form 8-K filed with the SEC on May 3, 2024). |
| |
|
|
| 4.3 |
|
Form of Placement Agent Warrant issued in May 2024 Private Placement (Incorporated by reference to Exhibit 4.03 to the Registrant’s Current Report on Form 8-K filed with the SEC on May 3, 2024). |
| |
|
|
| 5.1* |
|
Opinion of Silvestre Law Group, P.C. |
| |
|
|
| 10.1+ |
|
Form of Securities Purchase Agreement entered into pursuant to the May 2024 Private Placement (Incorporated by reference to Exhibit 10.01 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 3, 2024). |
| |
|
|
| 10.2+ |
|
Form of Registration Right Agreement entered into Pursuant to the May 2024 Private Placement (Incorporated by reference to Exhibit 10.02 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 3, 2024). |
| |
|
|
| 10.3+ |
|
Form of Placement Agency Agreement entered into Pursuant to the May 2024 Private Placement (Incorporated by reference to Exhibit 10.03 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 3, 2024. |
| |
|
|
| 23.1* |
|
Consent of Baker Tilly US, LLP. |
| |
|
|
| 23.2* |
|
Consent of Silvestre Law Group, P.C. (included in legal opinion filed as Exhibit 5.1). |
| |
|
|
| 24.1* |
|
Power of Attorney (included on signature page). |
| |
|
|
| 107* |
|
Filing Fee Table |
| |
*
Filed herewith |
| |
+
Schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. Palisade undertakes to furnish supplemental
copies of any of the omitted schedules upon request by the U.S. Securities and Exchange Commission. |
Item
17. Undertakings.
| (3) |
The
undersigned registrant hereby undertakes: (1) To file, during any period in which offers or sales are being made, a post-effective
amendment to this registration statement: |
| |
(i) |
to
include any prospectus required by Section 10(a)(3) of the Securities Act; |
| |
|
|
| |
(ii) |
to
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end
of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission
(the “SEC”) pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20%
change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective
registration statement; |
| |
|
|
| |
(iii) |
to
include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement; |
provided,
however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the registration statement is on Form
S-3 and the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with
or furnished to the SEC by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 (the “Exchange
Act”) that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant
to Rule 424(b) that is part of the registration statement.
| |
(2) |
That,
for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a
new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be
deemed to be the initial bona fide offering thereof. |
| |
|
|
| |
(3) |
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. |
| |
(4) |
That,
for the purpose of determining liability under the Securities Act to any purchaser: |
| |
(i) |
each
prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the
date the filed prospectus was deemed part of and included in the registration statement; and |
| |
(ii) |
each
prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule
430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required
by Section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier
of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in
the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at
that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities
in the registration statement to which the prospectus relates, and the offering of such securities at that time shall be deemed to
be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior
to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part
of the registration statement or made in any such document immediately prior to such effective date. |
| |
(b) |
The
undersigned registrant undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s
annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit
plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement
shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof. |
| |
(c) |
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons
of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the
SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event
that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid
by a director, officer, or controlling person of the registrant in the successful defense of any action, suit, or proceeding) is
asserted by such director, officer, or controlling person of the registrant in connection with the securities being registered, the
registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities
Act and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant
to the requirements of the Securities Act, the registrant certifies that it has reasonable grounds to believe that it meets all of the
requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto
duly authorized, in the city of Carlsbad, State of California, on May 8, 2024.
| |
PALISADE
BIO, INC. |
| |
|
|
| Date:
May 8, 2024 |
By: |
/s/
J.D. Finley |
| |
|
J.D.
Finley |
| |
|
Chief
Executive Officer, |
| |
|
Chief
Financial Officer and Director |
POWER
OF ATTORNEY
Each
person whose signature appears below constitutes and appoints J.D. Finley as his or her true and lawful attorneys-in-fact and agent,
each acting alone, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any
and all capacities, to sign any or all amendments (including post-effective amendments) to this registration statement on Form S-3, and
to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission,
granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and
necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby
ratifying and confirming all that said attorneys-in-fact and agent, or their substitute or substitutes, may lawfully do or cause to be
done by virtue hereof.
Pursuant
to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and
on the dates indicated.
| Name |
|
Title |
|
Date |
| |
|
|
|
|
| |
|
Chief
Executive Officer, |
|
|
| /s/
J.D. Finley |
|
Chief
Financial Officer and Director |
|
May
8, 2024 |
| J.D.
Finley |
|
(Principal
Executive and Financial Officer) |
|
|
| |
|
|
|
|
| |
|
|
|
|
| /s/
Donald A. Williams |
|
Chairman
of the Board of Directors |
|
May
8, 2024 |
| Donald
A. Williams |
|
|
|
|
| |
|
|
|
|
| /s/
Binxian Wei |
|
Director |
|
May
8, 2024 |
| Binxian
Wei |
|
|
|
|
Exhibit
5.1
SILVESTRE
LAW GROUP, P.C.
2629
Townsgate Road, Suite 215
Westlake
Village, CA 91361
(818)
597-7552
Fax
(805) 553-9783
May
8, 2024
Palisade
Bio, Inc.
7750
El Camino Real, Suite 2A
Carlsbad,
CA 92009
Ladies
and Gentlemen:
We
have acted as counsel to Palisade Bio, Inc., a Delaware corporation (the “Company”), in connection with the
Registration Statement on Form S-3 (the “Registration Statement”) filed by the Company under the Securities
Act of 1933, as amended, including a related prospectus (the “Prospectus”), covering the registration for resale
of 1,575,019 shares of the Company’s common stock, par value $0.01 per share (the “Common Stock”), consisting
of (i) 85,100 shares of outstanding Common Stock (“Shares”), (ii) 530,142 shares of Common Stock issuable upon the
exercise of outstanding prefunded warrants (“Prefunded Warrants”), (iii) 922,863 shares of Common Stock issuable upon
the exercise of outstanding common stock warrants (“Common Warrants”), and (iv) 36,914 shares of Common Stock issuable
upon exercise of outstanding placement agent warrants (“Placement Agent Warrants”) held by the selling stockholders
identified in the Prospectus (collectively, such shares of Common Stock underlying Prefunded Warrants, Common Warrants, and Placement
Agent Warrants, the “Warrant Shares”).
In
connection with this opinion, we have examined and relied upon (i) the Registration Statement and the Prospectus, (ii) the Company’s
Amended and Restated Certificate of Incorporation, as amended, and Amended and Restated Bylaws, each as currently in effect, (iii) the
Prefunded Warrants, Common Warrants, and Placement Agent Warrants, and (iv) such other documents, records, certificates, memoranda and
other instruments as in our judgment are necessary or appropriate to enable us to render the opinion expressed below. We have assumed
the genuineness of all documents submitted to us as originals, the conformity to originals of all documents submitted to us as copies,
the accuracy, completeness and authenticity of certificates of public officials and the due execution and delivery of documents by all
persons other than the Company where execution and delivery are prerequisites to the effectiveness thereof. As to certain factual matters,
we have relied upon a certificate of an officer of the Company and have not independently verified such matters.
Our
opinion is expressed only with respect to the General Corporation Law of the State of Delaware. We express no opinion as to whether any
particular laws other than those identified above are applicable to the subject matter hereof. We are not rendering any opinion as to
compliance with any federal or state antifraud law, rule or regulation relating to securities, or to the sale or issuance thereof.
On
the basis of the foregoing, and in reliance thereon, we are of the opinion that the Shares have been validly issued, fully paid, and
non-assessable. Additionally, we are of the opinion that the Warrant Shares underlying the Prefunded Warrants, Common Warrants, and Placement
Agent Warrants, as applicable, when issued and sold against payment therefor in accordance with the terms thereof, will be validly issued,
fully paid and non-assessable.
We
hereby consent to the reference to our firm under the caption “Legal Matters” in the prospectus included in the Registration
Statement and to the filing of this opinion as an exhibit to the Registration Statement.
Sincerely,
Silvestre
Law Group, P.C.
| By: |
/s/
Raul Silvestre |
|
| |
Raul
Silvestre |
|
Exhibit
23.1
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
hereby consent to the incorporation by reference in the Registration Statement on Form S-3 of Palisade Bio, Inc. of our report dated
March 26, 2024, relating to the consolidated financial statements, which includes an explanatory paragraph relating to the Company’s
ability to continue as a going concern and appears on page 66 of this annual report on Form 10-K for the year ended December 31, 2023.
We
also consent to the reference to us under the heading “Experts” in such Registration Statement.
/s/
BAKER TILLY US, LLP
New
York, New York
May
8, 2024
Exhibit
107
Calculation
of Filing Fee Tables
Form
S-3
(Form
Type)
Palisade
Bio, Inc.
(Exact
Name of Registrant as Specified in its Charter)
Table
1: Newly Registered and Carry Forward Securities
| | |
Security
Type | |
Security Class
Title | |
Fee
Calculation
or Carry
Forward
Rule | |
Amount
Registered
(1) | |
Proposed
Maximum
Offering
Price Per
Unit | |
Maximum
Aggregate
Offering
Price (1) | |
Fee Rate | |
Amount of
Registration
Fee |
| Fees to be Paid | |
Equity | |
Common Stock, $0.01 par value per share (2) | |
| 457 | (c) | |
| 85,100 | | |
$ | 7.14 | | |
$ | 607,954 | | |
| 0.0001476 | | |
| 90.00 | |
| Fees to be Paid | |
Equity | |
Common Stock, $0.01 par value per share, issuable upon exercise of Prefunded Warrants ($0.0001 exercise price) (3)(6) | |
| 457 | (g) | |
| 530,142 | | |
$ | 7.14 | | |
$ | 3,787,334 | | |
| 0.0001476 | | |
| 560.00 | |
| Fees to be Paid | |
Equity | |
Common Stock, $0.01 par value per share, issuable upon exercise of Warrants ($6.314 exercise price) (4)(6) | |
| 457 | (g) | |
| 922,863 | | |
$ | 7.14 | | |
$ | 6,592,933 | | |
| 0.0001476 | | |
| 974.00 | |
| Fees to be Paid | |
Equity | |
Common Stock, $0.01 par value per share, issuable upon exercise of Placement Agent Warrants ($10.73 exercise price) (5)(6) | |
| 457 | (g) | |
| 36,914 | | |
$ | 10.73 | | |
$ | 396,087 | | |
| 0.0001476 | | |
| 59.00 | |
| | |
| |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| |
TOTALS | |
| | | |
| 1,575,019 | | |
| | | |
$ | 11,384,309 | | |
| 0.0001476 | | |
| 1,681.00 | |
(1)
Represents the shares of Common Stock, $0.01 par value per share (the “Common Stock”) of Palisade Bio, Inc. (“Registrant”)
that will be offered for resale by the selling stockholders pursuant to the prospectus to which this exhibit is attached. Pursuant to
Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the shares of Common Stock being registered
hereunder include such indeterminate number of shares of Common Stock as may be issuable with respect to the shares of Common Stock being
registered hereunder as a result of stock splits, stock dividends or similar transactions.
(2)
Represents shares of common stock sold at a price per share of $6.5015, issued by the Registrant in a private placement on May 6, 2024.
Calculated based on the average of the high and low prices per share of the Registrant’s Common Stock reported on the Nasdaq Capital
Market on May 2, 2024, a date within five business days prior to the initial filing of the registration statement to which this exhibit
is attached.
(3)
Represents shares of common stock issuable upon the exercise of Prefunded Warrants to purchase common stock at an exercise price of $0.0001
per share issued by the Registrant in a private placement on May 6, 2024.
(4)
Represents shares of common stock issuable upon the exercise of Warrants to purchase common stock at an exercise price of $6.314 per
share issued by the Registrant in a private placement on May 6, 2024.
(5)
Represents shares of common stock issuable upon the exercise of Placement Agent Warrants to purchase common stock at an exercise price
of $10.73 per share issued by the Registrant in a private placement on May 6, 2024.
(6)
Pursuant to Rule 457(g) under the Securities Act, calculated based on the greater of (i) on the basis of the average of the high and
low prices per share of the Registrant’s Common Stock reported on the Nasdaq Capital Market on May 2, 2024, a date within five
business days prior to the initial filing of the registration statement to which this exhibit is attached or (ii) the exercise price
of the applicable warrant.
Palisade Bio (NASDAQ:PALI)
Graphique Historique de l'Action
De Fév 2025 à Mar 2025

Palisade Bio (NASDAQ:PALI)
Graphique Historique de l'Action
De Mar 2024 à Mar 2025


