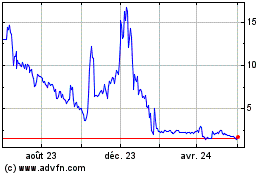
Processa Pharmaceuticals Provides Data Update Supporting a Potential Personalized Treatment Approach for Improved Cancer Care
17 Août 2023 - 2:05PM

Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) (“Processa” or the
“Company”), a clinical-stage pharmaceutical company focused on
developing the next generation of chemotherapeutic drugs to improve
the efficacy and safety for patients suffering from cancer,
provides an interim analysis from its ongoing Phase 1B trial of
Next Generation Capecitabine (NGC-Cap) in patients with
gastrointestinal cancer, which identifies a personalized treatment
approach that may yield improved safety and treatment efficacy.
NGC-Cap combines the administration of PCS6422, Processa’s
irreversible dihydropyrimidine dehydrogenase (DPD) enzyme
inhibitor, with low doses of the commonly used chemotherapy
capecitabine, which is metabolized to 5-fluorouracil (5-FU) in the
body. DPD promotes the further metabolism of 5-FU to
fluor-beta-alanine (FBAL), a metabolite that leads to dose-limiting
chemotherapy side effects.
Processa has found that regularly measuring the
concentrations of DPD, as expressed by the metabolite FBAL, may
provide a method to better understand how each patient responds to
different NGC-Cap dosage regimens. This insight potentially allows
physicians to develop and administer patient-specific treatment
protocols of NGC-Cap for each patient to inhibit the development of
side effects and promote broader drug efficacy, patient safety, and
tolerability.
Currently, capecitabine, among the most widely
used chemotherapy drugs, is dosed based on a standard dosage
regimen for all patients. Unfortunately, many patients cannot
tolerate that dose and must either have their dose reduced or have
their treatment interrupted. These modifications are often
associated with reduced efficacy in treating cancer.
Sian Bigora, Pharm.D., Processa's Chief
Development and Regulatory Officer, commented, “The presence of DPD
as expressed by the plasma concentration of FBAL provides us
important information that may confirm our hypothesis that we can
optimize our dosing of NGC-Cap to maintain efficacy and minimize
side effects by carefully tracking each individual patient
following an initial dosing of our novel chemotherapy. By following
the chemical concentration in the plasma in each patient, we may be
able to actually optimize therapy through individualization of each
patient’s dosing regimen. We find these results, albeit early
results, to be very encouraging.”
In the ongoing Phase 1B study, Processa followed
the activity of DPD following NGC-Cap dosing by measuring 5-FU and
FBAL in the plasma of patients. FBAL has no cancer-killing activity
but is known to cause dose-limiting side effects such as Hand-Foot
Syndrome. The data shows that DPD enzyme activity (as represented
by FBAL levels) was extremely low for approximately 24-48 hours in
patients who received PCS6422 plus capecitabine. The low level of
the DPD enzyme activity during the first 24-48 hours resulted in
capecitabine being metabolized mainly to its active cancer-killing
metabolites instead of FBAL. Over the next 3-6 days, the amount of
FBAL increased and the levels of 5-FU decreased as a result of the
increased DPD activity due to newly formed DPD enzyme (de novo
synthesis). This leads to the progressive reduction of the
cancer-killing metabolites and the anti-cancer activity of the
therapy.
David Young, Pharm. D., Ph.D., Processa’s
President of R&D, added, “As we continue to obtain data from
the Phase 1B trial, we are able to better understand the
relationship between the concentrations of FBAL and 5-FU in the
body relative to the time of administration and formation of de
novo DPD. This study has already demonstrated that the addition of
PCS6422 to low doses of capecitabine results in the formation of
more cancer-killing metabolites before the concentration of the
metabolites that only cause dose-limiting side effects
significantly increases. Part of our ongoing objectives for the
Phase 1B trial and our planned Phase 2 trial will be to determine
the optimal regimen of NGC-Cap to balance the safety and efficacy
profiles for each individual patient by monitoring DPD activity
through 5-FU and FBAL. We look forward to continuing to investigate
NGC-Cap and to providing updates as they develop.”
The first three of five cohorts in the Phase 1B
trial have completed enrollment and the initial safety evaluation.
The fourth cohort has completed enrollment, and the safety
evaluation is ongoing. Enrollment in the last cohort is expected to
be completed in 4Q23, leading to an assessment of the safety
profile and potentially preliminary efficacy of NGC-Capecitabine in
totality across all cohorts by year-end. This cumulative view of
the safety profile will allow Processa to determine the appropriate
dose regimens to be used in its planned Phase 2 study in patients
with colorectal cancer to determine the Optimal Dose Regimen, as
mandated by the FDA’s new Project Optimus Initiative.
About Next Generation
Capecitabine (NGC-Cap)
NGC-Cap combines the administration of PCS6422,
the Company’s irreversible dihydropyrimidine dehydrogenase (DPD)
enzyme inhibitor, with the administration of low doses of the
commonly used chemotherapy Capecitabine.
PCS6422 is an uracil analog that irreversibly
inhibits dihydropyrimidine dehydrogenase (DPD). PCS6422 is neither
toxic nor active as a single agent in animals at comparable dose
levels. However, when administered in combination with Capecitabine
or 5-FU, PCS6422 decreases the metabolism of 5-FU to the
catabolites that only cause side effects.
About Processa Pharmaceuticals,
Inc.
Processa is a clinical stage pharmaceutical
company focused on developing the Next Generation Chemotherapy
drugs to improve the safety and efficacy of cancer treatment. By
combining Processa’s novel oncology pipeline with proven
cancer-killing active molecules and the Processa Regulatory Science
Approach as well as experience in defining Optimal Dosage Regimens
for FDA approvals, Processa not only will be providing better
therapy options to cancer patients but also increase the
probability of FDA approval for its Next Generation Chemotherapy
drugs. Processa’s NGC drugs are modifications of existing
FDA-approved oncology drugs resulting in an alteration of the
metabolism and/or distribution of drugs while maintaining the
existing mechanisms of killing the cancer cells. Our approach to
drug development is based on more than 30 years of drug development
expertise to efficiently design and conduct clinical trials that
demonstrate a positive benefit/risk relationship. Using its proven
Regulatory Science Approach, we have experience defining the
Optimal Dosage Regimen using the principles of the FDA’s Project
Optimus Oncology initiative. The advantages of Processa’s Next
Generation Chemotherapy drugs are expected to include fewer
patients experiencing side effects that lead to dose
discontinuation; more significant cancer response; and a greater
number of patients who will benefit from each Next Generation
Chemotherapy drug. Currently in our pipeline are three Next
Generation Chemotherapy drugs: Next Generation Capecitabine
(PCS6422 and capecitabine to treat metastatic colorectal,
gastrointestinal, breast, pancreatic, and other cancers), Next
Generation Gemcitabine (PCS3117 to treat pancreatic, lung, ovarian,
breast, and other cancers), and Next Generation Irinotecan (PCS11T
to treat lung, colorectal, gastrointestinal, pancreatic, and other
cancers).
For more information, visit our website
at www.processapharma.com.
Forward-Looking Statements
This release contains forward-looking
statements. The statements in this press release that are not
purely historical are forward-looking statements which involve
risks and uncertainties. Actual future performance outcomes and
results may differ materially from those expressed in
forward-looking statements. Please refer to the documents filed by
Processa Pharmaceuticals with the SEC, specifically the most recent
reports on Forms 10-K and 10-Q, which identify important risk
factors which could cause actual results to differ from those
contained in the forward-looking statements.
For More
Information:Investors:Bret ShapiroCORE
IRir@processapharma.com
Company Contact:Patrick
Lin(925) 683-3218plin@processapharma.com
Processa Pharmaceuticals (NASDAQ:PCSA)
Graphique Historique de l'Action
De Déc 2024 à Jan 2025
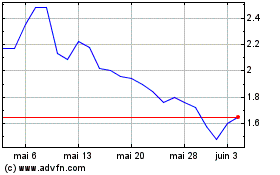
Processa Pharmaceuticals (NASDAQ:PCSA)
Graphique Historique de l'Action
De Jan 2024 à Jan 2025