false
0001727196
0001727196
2024-10-07
2024-10-07
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event Reported): October
7, 2024
Scholar Rock Holding Corporation
(Exact Name of Registrant as Specified in Charter)
| Delaware |
001-38501 |
82-3750435 |
(State or Other Jurisdiction of
Incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification
Number) |
| |
|
|
| 301 Binney Street, 3rd Floor, Cambridge, MA 02142 |
| (Address of Principal Executive Offices) (Zip Code) |
(857) 259-3860
(Registrant's telephone
number, including area code)
(Former name or
former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title
of each class |
Trading
Symbol(s) |
Name of each exchange
on which registered |
| Common Stock, par value $0.001 per share |
SRRK |
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as
defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17
CFR §240.12b-2). Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected
not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act. ¨
| Item 7.01. | Regulation
FD Disclosure. |
On October 7, 2024, Scholar Rock Holding
Corporation (the “Company”) issued a press release announcing positive topline data from its Phase 3 SAPPHIRE trial evaluating
apitegromab, an investigational fully human monoclonal antibody that inhibits myostatin, and plans to submit a U.S. Biologics License
Application and European Union marketing authorization application in the first quarter of 2025. A copy of the press release is attached
hereto as Exhibit 99.1.
The information in this Item 7.01 of Form 8-K,
including the accompanying Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of such section, nor shall such
information be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, regardless of the
general incorporation language of such filing, except as shall be expressly set forth by specific reference in such filing.
The Company has also made available a slide
presentation relating to topline data from its Phase 3 SAPPHIRE trial evaluating apitegromab for the potential treatment of spinal muscular
atrophy, a copy of which is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.2.
| Item 9.01. | Financial
Statements and Exhibits. |
(d) Exhibits
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
Scholar Rock Holding Corporation |
| |
|
|
| |
|
|
| Date: October 7, 2024 |
By: |
/s/ Junlin Ho |
| |
|
Junlin Ho |
| |
|
General Counsel and Corporate Secretary |
Exhibit 99.1
Scholar Rock Reports Apitegromab Meets Primary
Endpoint in Phase 3 SAPPHIRE Study in Patients with Spinal Muscular Atrophy (SMA)
| ● | Apitegromab met primary endpoint with statistically significant and clinically meaningful improvement in motor function as measured
by the gold standard Hammersmith Functional Motor Scale Expanded (HFMSE) for patients with SMA receiving apitegromab versus placebo (current
standard of care) at week 52 |
| ● | 30.4% of patients receiving apitegromab had >3 point improvement in HFMSE versus 12.5% of patients on placebo |
| ● | Patients receiving apitegromab demonstrated early motor function improvement compared to placebo from the first measured time point
at 8 weeks, benefit observed at 52 weeks as measured by HFMSE |
| ● | Patients receiving apitegromab experienced clinically meaningful benefit in motor function across all age groups (ages 2-21) |
| ● | Favorable safety profile in SAPPHIRE consistent with apitegromab’s long-term safety profile observed in the Phase 2 TOPAZ
trial with >48 months of treatment experience in SMA patients |
| ● | Scholar Rock plans to submit a U.S. Biologics License Application and European Union marketing authorisation application in Q1
2025 |
| ● | Scholar Rock to host Investor Call today at 8:00 AM ET |
CAMBRIDGE,
Mass.--(BUSINESS WIRE)--October 7, 2024--Scholar Rock (NASDAQ: SRRK), a late-stage biopharmaceutical company focused on advancing
innovative treatments for spinal muscular atrophy (SMA), cardiometabolic disorders, and other serious diseases where protein growth factors
play a fundamental role, today announced positive topline results from the Phase 3 SAPPHIRE clinical trial (NCT05156320)
evaluating the efficacy and safety of apitegromab, an investigational muscle-targeted therapy, in patients with SMA.
The study achieved its primary endpoint demonstrating a statistically
significant and clinically meaningful improvement for apitegromab versus placebo in motor function as measured by the gold standard HFMSE
in SMA patients on chronic dosing of standard of care therapies (either nusinersen or risdiplam). Based upon the similar pharmacological
profile of the 20 mg/kg and 10 mg/kg doses of apitegromab, the statistical analysis plan was prespecified to analyze both the combined
dose (10 mg/kg and 20 mg/kg) compared to placebo, and 20 mg/kg dose each compared to placebo. Statistical significance is achieved
per the prespecified statistical analysis plan (Hochberg multiplicity adjustment) where the p-value (≤0.025) is more rigorous
if only one prespecified analysis crosses the statistical significance boundary of ≤ 0.05.
| ● | In the main efficacy population (ages 2-12), the mean difference in change from baseline in HFMSE was
1.8 points (p=0.0192) for all patients receiving apitegromab 10 mg/kg and 20 mg/kg (n=106) compared to placebo (n=50). Patients receiving
20 mg/kg of apitegromab (n=53) showed a 1.4 point mean difference compared to placebo (p=0.1149). |
| | | |
| ● | The prespecified analysis of the 10 mg/kg dose showed that patients receiving 10 mg/kg of apitegromab
(n=53) showed an improvement of 2.2 points (nominal p=0.0121) compared to placebo. |
| ● | Based upon PK/PD data from the SAPPHIRE trial, similar levels of target engagement were observed for
the 10 mg/kg and 20 mg/kg dose groups. |
Motor function outcomes were meaningful and consistent across the main
efficacy population and in the ages 13-21 exploratory population, favored apitegromab (n=22) compared to placebo (n=10). Thirty
percent of patients receiving apitegromab had >3 point improvement in HFMSE versus 12.5% of patients on placebo. Patients receiving
apitegromab demonstrated early motor function improvement compared to placebo from the first measured time point at 8 weeks, benefit expanded
at 52 weeks as measured by HFMSE. Following trial completion, 98 percent of SAPPHIRE patients (185/188) enrolled in the ongoing ONYX open-label
expansion study.
“We are thrilled that apitegromab met the primary endpoint in
our Phase 3 SAPPHIRE clinical study. The results clearly demonstrate robust and clinically meaningful improvement in motor function in
patients with SMA,” said Jay Backstrom, M.D., MPH, President and Chief Executive Officer of Scholar Rock. “At Scholar
Rock, we are working with urgency to deliver the potentially transformative benefits of apitegromab to children and adults with SMA in
the US, Europe, and around the world.”
Treatment with apitegromab was well-tolerated across all age groups.
There were no clinically relevant differences in the adverse event profile by dose, 10 mg/kg versus 20 mg/kg. No new safety findings were
observed in the SAPPHIRE clinical trial; the profile was consistent with that observed in the Phase 2 TOPAZ clinical trial, including
an extension study which had over four years of treatment as of the cut-off date. Serious adverse events (SAEs) were consistent with the
underlying disease and current standard of care received by patients; no SAEs were assessed as related to apitegromab. There were no study
drug discontinuations due to adverse events.
“We are grateful to the families and investigators who participated
in our trials. The positive Phase 3 SAPPHIRE trial, along with over 4 years of TOPAZ clinical trial data, clearly demonstrate the potentially
transformative benefit of apitegromab to drive clinically meaningful improvements in motor function as measured by HFMSE in a broad SMA
population, where motor function would normally be expected to generally decline over time,” said Jing Marantz, M.D., Ph.D., Chief
Medical Officer at Scholar Rock. “We look forward to submitting our applications to the FDA and the EMA in Q1 2025.”
The U.S. Food and Drug Administration (FDA) has granted Fast Track,
Orphan Drug, and Rare Pediatric Disease designations, and the European Medicines Agency (EMA) has granted Priority Medicines (PRIME) and
Orphan Medicinal Product designations, to apitegromab for the treatment of SMA. The Company plans to submit a U.S. Biologics License Application
(BLA) and a European Union marketing authorisation application (MAA) in Q1 2025.
“It’s a great day for people living with SMA and their
families. These encouraging trial results mark a critical milestone for the SMA community,” said Kenneth Hobby, President of Cure
SMA. “Declining motor function and hopes for reversing losses associated with muscle weakness are significant unmet needs, impacting
activities of daily living, from breathing, eating, self-care, to working and social interactions. We need an approved therapy that can
support motor function and further improve daily activities for people with SMA.”
Analyses of the full Phase 3 SAPPHIRE data are ongoing, and Scholar
Rock plans to present detailed results at an upcoming medical conference in early 2025. Preliminary baseline characteristics from the
trial will be presented during a poster presentation at the upcoming 29th Annual Congress of the World Muscle Society on Friday,
October 11, 2024, being held in Prague, Czech Republic.
Conference Call Information
Scholar Rock
will hold an investor conference call today, October 7 at 8:00 am ET. To access the live conference call, participants may register
here. The live audio webcast of the call will be available under “Events and Presentations” in the Investor Relations section
of the Scholar Rock website at http://investors.scholarrock.com. To participate via telephone, please register here.
Upon registration, all telephone participants will receive a confirmation email detailing how to join the conference call, including
the dial-in number along with a unique passcode and registrant ID that can be used to access the call. An archived replay of the webcast
will be available on the Company’s website for approximately 90 days.
Presentation at Annual Congress of the World Muscle Society
Scholar Rock will present baseline characteristics from the SAPPHIRE
trial in a poster presentation at the 29th Annual Congress of the World Muscle Society. Details of the presentation are as
follows:
Title:
Apitegromab in Spinal Muscular Atrophy: baseline characteristics of participants enrolled in the phase 3 SAPPHIRE study
Presentation
type: Poster presentation
Presenter:
Thomas O. Crawford, M.D., Professor of Neurology and Pediatrics, Johns Hopkins University
Date and
time: Friday, October 11, 2024, 3:45 PM CET
Location:
Prague, Czech Republic
About Apitegromab
Apitegromab is an investigational fully human monoclonal antibody inhibiting
myostatin activation by selectively binding the pro- and latent forms of myostatin in the skeletal muscle. It is the first muscle-targeted
treatment candidate to demonstrate clinical proof-of-concept in spinal muscular atrophy (SMA). Myostatin, a member of the TGFβ superfamily
of growth factors, is expressed primarily by skeletal muscle cells, and the absence of its gene is associated with an increase in muscle
mass and strength in multiple animal species, including humans. Scholar Rock believes that its highly selective targeting of pro-
and latent forms of myostatin with apitegromab may lead to a clinically meaningful improvement in motor function in patients with SMA.
The U.S. Food and Drug Administration (FDA) has granted Fast Track, Orphan Drug and Rare Pediatric Disease designations, and
the European Medicines Agency (EMA) has granted Priority Medicines (PRIME) and Orphan Medicinal Product designations, to apitegromab
for the treatment of SMA. Apitegromab has not been approved for any use by the FDA or any other regulatory agency.
About SAPPHIRE
SAPPHIRE was a randomized, double-blind, placebo-controlled Phase 3
clinical trial that evaluated the safety and efficacy of apitegromab in nonambulatory patients with Types 2 and 3 SMA who are receiving
current standard of care (either nusinersen or risdiplam). SAPPHIRE enrolled 156 patients aged 2-12 years old in the main efficacy population.
These patients were randomized 1:1:1 to receive for 12 months either apitegromab 10 mg/kg, apitegromab 20 mg/kg, or placebo by intravenous
(IV) infusion every 4 weeks. An exploratory population that enrolled 32 patients aged 13-21 years old was also evaluated. These patients
were randomized 2:1 to receive either apitegromab 20 mg/kg or placebo.
About SMA
Spinal muscular atrophy (SMA) is a rare, genetic neuromuscular disease
that afflicts an estimated 30,000 to 35,000 people in the United States and Europe. The disease is characterized by the
loss of motor neurons, atrophy of the voluntary muscles of the limbs and trunk, and progressive muscle weakness. While there has been
progress in the development of therapeutics that address the loss of motor neurons, there continues to be a high unmet need for therapies
that directly address the progressive muscle weakness that leads to loss of motor function in SMA.
About Scholar Rock
Scholar Rock is a biopharmaceutical company that discovers, develops,
and delivers life-changing therapies for people with serious diseases that have high unmet need. As a global leader in the biology of
the transforming growth factor beta (TGFβ) superfamily of cell proteins and named for the visual resemblance of a scholar rock to
protein structures, the clinical-stage company is focused on advancing innovative treatments where protein growth factors are fundamental.
Over the past decade, Scholar Rock has created a pipeline with the potential to advance the standard of care for neuromuscular
disease, cardiometabolic disorders, cancer, and other conditions where growth factor-targeted drugs can play a transformational role.
This commitment
to unlocking fundamentally different therapeutic approaches is powered by broad application of a proprietary platform, which has developed
novel monoclonal antibodies to modulate protein growth factors with extraordinary selectivity. By harnessing cutting-edge science in
disease spaces that are historically under-addressed through traditional therapies, Scholar Rock works every day to create
new possibilities for patients. Learn more about our approach at ScholarRock.com and follow @ScholarRock and on LinkedIn.
Availability of Other Information About Scholar Rock
Investors and others should note that we communicate with our investors
and the public using our company website www.scholarrock.com, including, but not limited to, company disclosures, investor presentations
and FAQs, Securities and Exchange Commission filings, press releases, public conference call transcripts and webcast transcripts, as well
as on Twitter and LinkedIn. The information that we post on our website or on Twitter or LinkedIn could be deemed to be material information.
As a result, we encourage investors, the media and others interested to review the information that we post there on a regular basis.
The contents of our website or social media shall not be deemed incorporated by reference in any filing under the Securities Act of 1933,
as amended.
Scholar Rock® is a registered trademark of Scholar Rock, Inc.
Forward-Looking Statements
This press release contains "forward-looking statements"
within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Scholar
Rock’s future expectations, plans and prospects, including without limitation, Scholar Rock’s expectations regarding its growth,
strategy, progress and timing of its clinical trials for apitegromab, and indication selection and development timing, including the timing
of any regulatory submissions, the therapeutic potential, clinical benefits and safety of any product candidates, expectations regarding
timing, success and data announcements of current ongoing preclinical and clinical trials, its cash runway, expectations regarding the
achievement of important milestones, the ability of any product candidate to perform in humans in a manner consistent with earlier nonclinical,
preclinical or clinical trial data, and the potential of its product candidates and proprietary platform. The use of words such as “may,”
“might,” “could,” “will,” “should,” “expect,” “plan,” “anticipate,”
“believe,” “estimate,” “project,” “intend,” “future,” “potential,”
or “continue,” and other similar expressions are intended to identify such forward-looking statements. All such forward-looking
statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that
could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These
risks and uncertainties include, without limitation, whether the results from the Phase 3 SAPPHIRE trial will be sufficient to support
regulatory approval, that the full results from the Phase 3 SAPPHIRE trial may differ from the topline data, that preclinical and clinical
data, including the results from the Phase 2 or Phase 3 clinical trial of apitegromab, are not predictive of, may be inconsistent with,
or more favorable than, data generated from future or ongoing clinical trials of the same product candidates; the data generated from
Scholar Rock’s nonclinical and preclinical studies and clinical trials; information provided or decisions made by regulatory authorities;
competition from third parties that are developing products for similar uses; Scholar Rock’s ability to obtain, maintain and protect
its intellectual property; Scholar Rock’s dependence on third parties for development and manufacture of product candidates including,
without limitation, to supply any clinical trials; and Scholar Rock’s ability to manage expenses and to obtain additional funding
when needed to support its business activities and establish and maintain strategic business alliances and new business initiatives, and
our ability to continue as a going concern; as well as those risks more fully discussed in the section entitled "Risk Factors"
in Scholar Rock’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, as well as discussions of potential
risks, uncertainties, and other important factors in Scholar Rock’s subsequent filings with the Securities and Exchange Commission.
Any forward-looking statements represent Scholar Rock’s views only as of today and should not be relied upon as representing its
views as of any subsequent date. All information in this press release is as of the date of the release, and Scholar Rock undertakes no
duty to update this information unless required by law.
Scholar Rock:
Investors
Rushmie Nofsinger
Scholar Rock
rnofsinger@scholarrock.com
ir@scholarrock.com
857-259-5573
Media
Molly MacLeod
Scholar Rock
mmacleod@scholarrock.com
media@scholarrock.com
802-579-5995
Exhibit 99.2

© 2024 Scholar Rock, Inc. All rights reserved. Positive Topline Results from Pivotal Phase 3 SAPPHIRE Trial of Apitegromab in Spinal Muscular Atrophy (SMA) October 7, 2024 1

© 2024 Scholar Rock, Inc. All rights reserved. Agenda 2 Jay Backstrom, M.D., MPH, President & Chief Executive Officer Introduction Jing Marantz , M.D. Ph.D., Chief Medical Officer SAPPHIRE Results Jay Backstrom, M.D., MPH, President & Chief Executive Officer Concluding Remarks Q&A Session

© 2024 Scholar Rock, Inc. All rights reserved. Forward - Looking Statements Various statements in this presentation concerning the future expectations, plans and prospects of Scholar Rock Holding Corporation and Scholar Rock, Inc . (collectively, “Scholar Rock”), including, without limitation, Scholar Rock’s expectations regarding its growth, strategy, progress and timing of its clinical trials for apitegromab, and indication selection and development timing, including the timing of any regulatory submissions or commercial launch, the therapeutic potential, clinical benefits and safety of any product candidates, expectations regarding timing, success and data announcements of current ongoing preclinical and clinical trials, its cash runway, expectations regarding the achievement of important milestones, the ability of any product candidate to perform in humans in a manner consistent with earlier nonclinical, preclinical or clinical trial data, and the potential of its product candidates and proprietary platform . The use of words such as “may,” “might,” “could,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify such forward - looking statements . All such forward - looking statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward - looking statements . These risks and uncertainties include, without limitation, whether the results from the Phase 3 SAPPHIRE trial will be sufficient to support regulatory approval, that the full results from the Phase 3 SAPPHIRE trial may differ from the topline data, that preclinical and clinical data, including the results from the Phase 2 or Phase 3 clinical trial of apitegromab, are not predictive of, may be inconsistent with, or more favorable than, data generated from future or ongoing clinical trials of the same product candidates ; the data generated from Scholar Rock’s nonclinical and preclinical studies and clinical trials ; information provided or decisions made by regulatory authorities ; competition from third parties that are developing products for similar uses ; Scholar Rock’s ability to obtain, maintain and protect its intellectual property ; Scholar Rock’s dependence on third parties for development and manufacture of product candidates including, without limitation, to supply any clinical trials ; and Scholar Rock’s ability to manage expenses and to obtain additional funding when needed to support its business activities and establish and maintain strategic business alliances and new business initiatives, and our ability to continue as a going concern ; as well as those risks more fully discussed in the section entitled "Risk Factors" in Scholar Rock’s Quarterly Report on Form 10 - Q for the quarter ended June 30 , 2024 , as well as discussions of potential risks, uncertainties, and other important factors in Scholar Rock’s subsequent filings with the Securities and Exchange Commission . Any forward - looking statements represent Scholar Rock’s views only as of today and should not be relied upon as representing its views as of any subsequent date . All information in this presentation is as of the date of the presentation, and Scholar Rock undertakes no duty to update this information unless required by law . This presentation discusses product candidates that are under clinical study, and which have not yet been approved for marketing by the FDA or any other regulatory agency . No representation or warranty, express or implied, is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied . This presentation may also contain estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry . This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we compete are necessarily subject to a high degree of uncertainty and risk . Apitegromab is an investigational drug candidate under evaluation . Apitegromab has not been approved for any use by the FDA or any other regulatory agency and the safety and efficacy of apitegromab has not been established .

© 2024 Scholar Rock, Inc. All rights reserved. Introduction Jay Backstrom, M.D., MPH President & Chief Executive Officer 4

© 2024 Scholar Rock, Inc. All rights reserved. Our Purpose: Create Possibilities for Those Living with Spinal Muscular Atrophy (SMA) 5 Muscle is everything. I want to live knowing that I have the strength to take care of myself if left alone. - Lyza
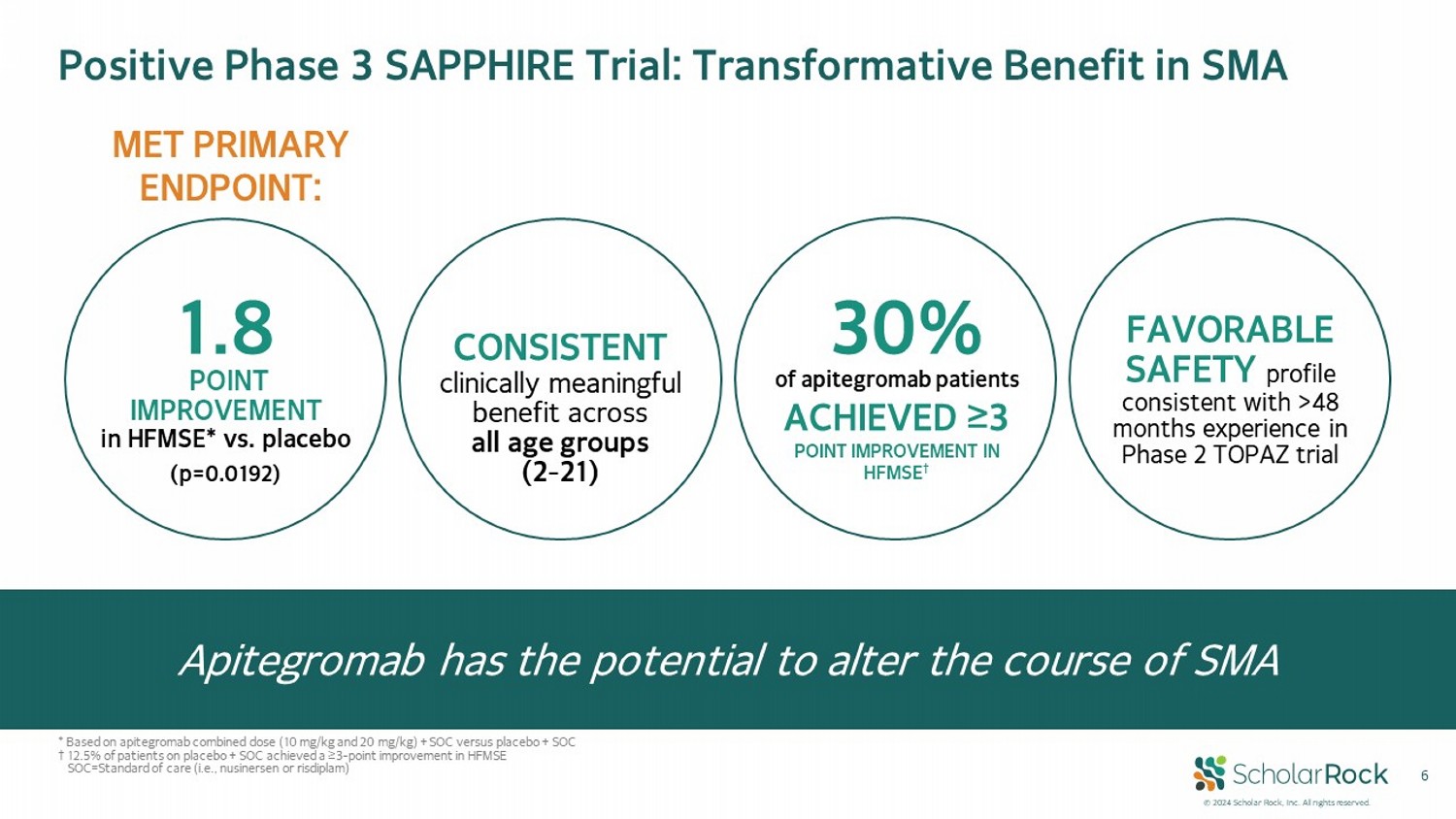
© 2024 Scholar Rock, Inc. All rights reserved. 6 CONSISTENT clinically meaningful benefit across all age groups (2 - 21) FAVORABLE SAFETY profile consistent with >48 months experience in Phase 2 TOPAZ trial 30 % of apitegromab patients ACHIEVED ≥3 POINT IMPROVEMENT IN HFMSE † 1.8 (p=0.0192) POINT IMPROVEMENT in HFMSE* vs. placebo MET PRIMARY ENDPOINT: Positive Phase 3 SAPPHIRE Trial: Transformative Benefit in SMA Apitegromab has the potential to alter the course of SMA * Based on apitegromab combined dose (10 mg/kg and 20 mg/kg) + SOC versus placebo + SOC † 12.5% of patients on placebo + SOC achieved a ≥3 - point improvement in HFMSE SOC = Standard of care (i.e., nusinersen or risdiplam)

© 2024 Scholar Rock, Inc. All rights reserved. Jing Marantz, M.D., Ph.D. Chief Medical Officer Phase 3 SAPPHIRE Topline Results
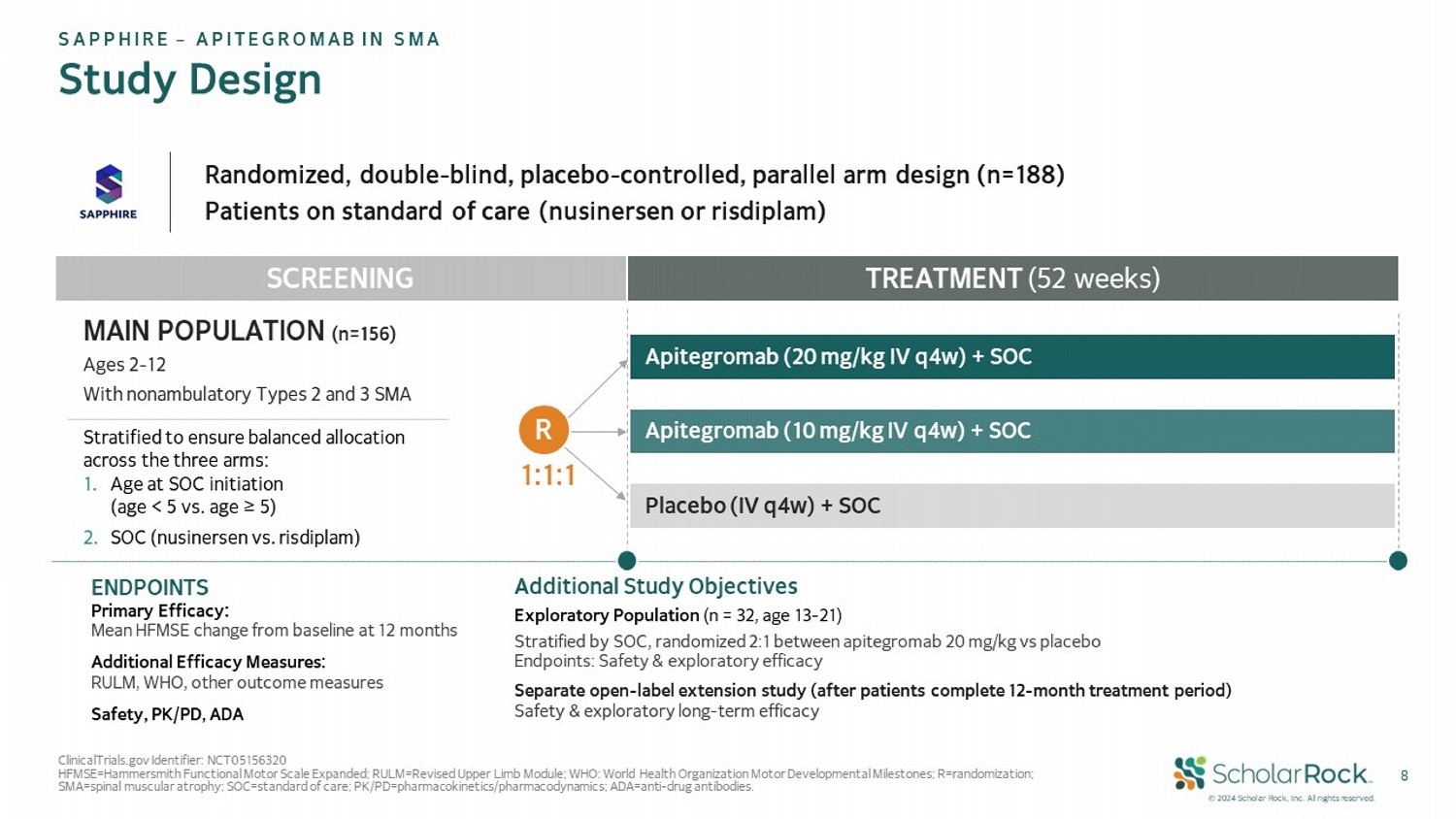
© 2024 Scholar Rock, Inc. All rights reserved. 8 Randomized, double - blind, placebo - controlled, parallel arm design (n=188) Patients on standard of care (nusinersen or risdiplam) TREATMENT (52 weeks) Apitegromab (20 mg/kg IV q4w) + SOC Apitegromab (10 mg/kg IV q4w) + SOC Placebo (IV q4w) + SOC SCREENING R ENDPOINTS Primary Efficacy: Mean HFMSE change from baseline at 12 months Additional Efficacy Measures: RULM, WHO, other outcome measures Safety, PK/PD, ADA Additional Study Objectives Exploratory Population (n = 32, age 13 - 21) S tratified by SOC, randomized 2:1 between apitegromab 20 mg/kg vs placebo Endpoints: Safety & exploratory efficacy Separate open - label extension study (after patients complete 12 - month treatment period) Safety & exploratory long - term efficacy ClinicalTrials.gov Identifier: NCT05156320 HFMSE=Hammersmith Functional Motor Scale Expanded; RULM=Revised Upper Limb Module; WHO: World Health Organization Motor Devel opm ental Milestones; R=randomization; SMA=spinal muscular atrophy; SOC=standard of care; PK/PD=pharmacokinetics/pharmacodynamics; ADA=anti - drug antibodies. SAPPHIRE – APITEGROMAB IN SMA Study Design MAIN POPULATION (n=156) Ages 2 - 12 With nonambulatory Types 2 and 3 SMA Stratified to ensure balanced allocation across the three arms: 1. Age at SOC initiation (age < 5 vs . age ≥ 5) 2. SOC (nusinersen vs. risdiplam) 1:1:1
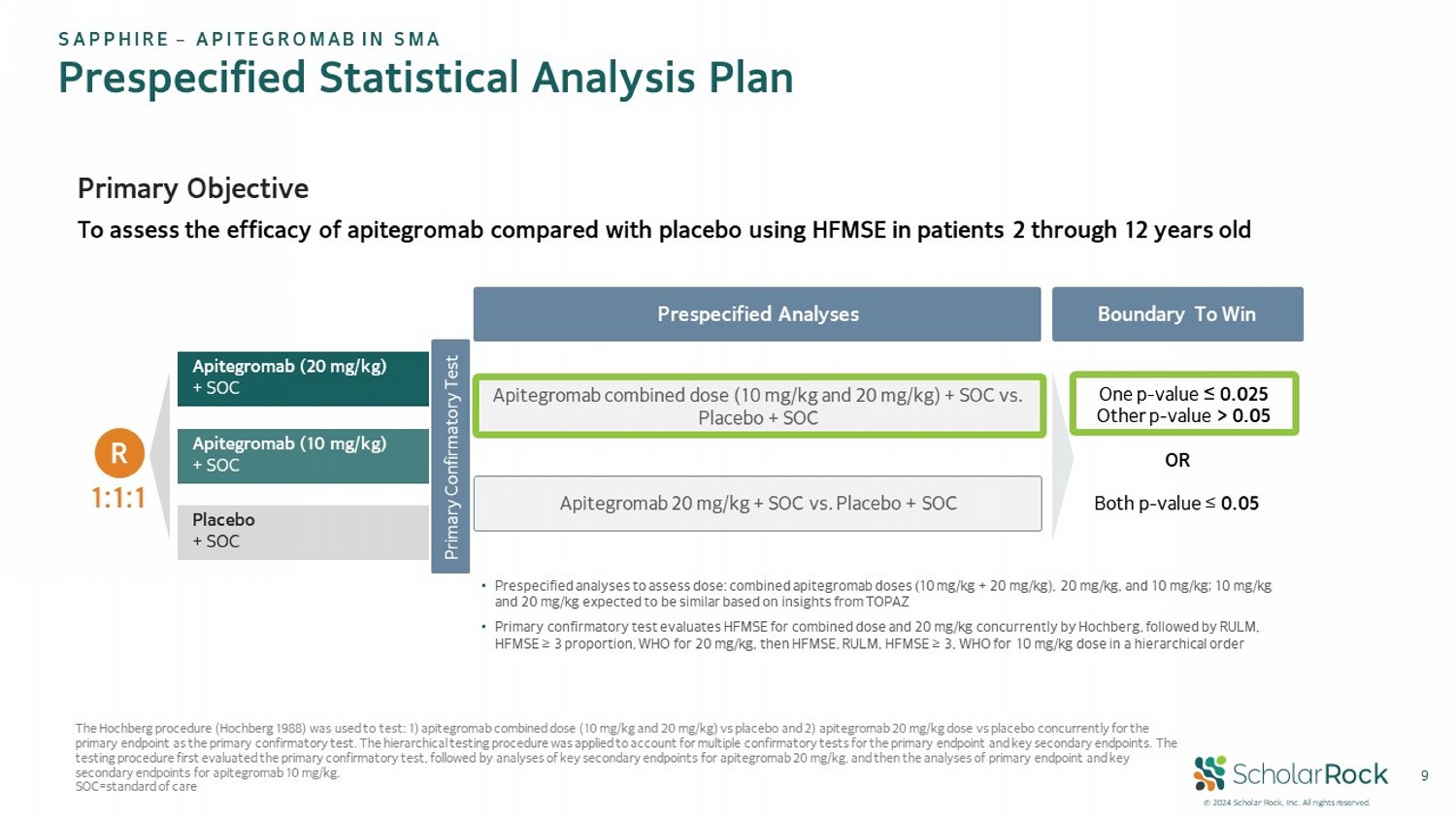
© 2024 Scholar Rock, Inc. All rights reserved. 9 Prespecified Statistical Analysis Plan SAPPHIRE – APITEGROMAB IN SMA Apitegromab (20 mg/kg) + SOC Apitegromab (10 mg/kg) + SOC Placebo + SOC R 1:1:1 • Prespecified analyses to assess dose: combined apitegromab doses (10 mg/kg + 20 mg/kg), 20 mg/kg, and 10 mg/kg; 10 mg/kg and 20 mg/kg expected to be similar based on insights from TOPAZ • Primary confirmatory test evaluates HFMSE for combined dose and 20 mg/kg concurrently by Hochberg, followed by RULM, HFMSE ≥ 3 proportion, WHO for 20 mg/kg, then HFMSE, RULM, HFMSE ≥ 3, WHO for 10 mg/kg dose in a hierarchical order Primary Objective To assess the efficacy of apitegromab compared with placebo using HFMSE in patients 2 through 12 years old Prespecified Analyses The Hochberg procedure (Hochberg 1988) was used to test: 1) apitegromab combined dose (10 mg/kg and 20 mg/kg) vs placebo and 2) apitegromab 20 mg/kg dose vs placebo concurrently for the primary endpoint as the primary confirmatory test. The hierarchical testing procedure was applied to account for multiple con fir matory tests for the primary endpoint and key secondary endpoints. The testing procedure first evaluated the primary confirmatory test, followed by analyses of key secondary endpoints for apitegro mab 20 mg/kg, and then the analyses of primary endpoint and key secondary endpoints for apitegromab 10 mg/kg. SOC=standard of care Boundary To Win Primary Confirmatory Test Apitegromab combined dose (10 mg/kg and 20 mg/kg) + SOC vs. Placebo + SOC Apitegromab 20 mg/kg + SOC vs. Placebo + SOC One p - value ≤ 0.025 Other p - value > 0.05 OR Both p - value ≤ 0.05
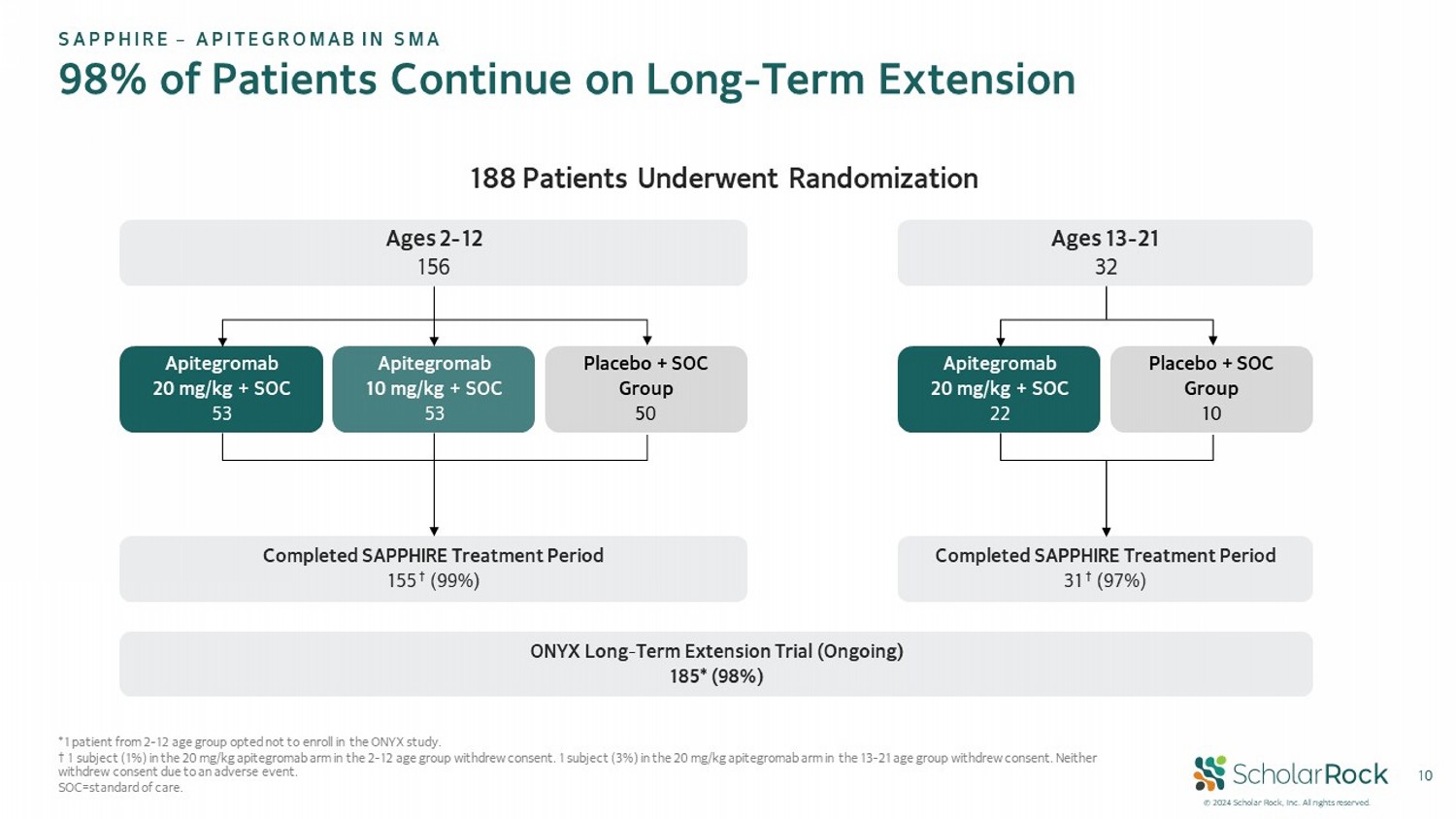
© 2024 Scholar Rock, Inc. All rights reserved. 10 *1 patient from 2 - 12 age group opted not to enroll in the ONYX study. † 1 subject (1%) in the 20 mg/kg apitegromab arm in the 2 - 12 age group withdrew consent. 1 subject (3%) in the 20 mg/kg apitegro mab arm in the 13 - 21 age group withdrew consent. Neither withdrew consent due to an adverse event. SOC=standard of care. 188 Patients Underwent Randomization Ages 2 - 12 156 Completed SAPPHIRE Treatment Period 155 † (99%) Apitegromab 20 mg/kg + SOC 53 Apitegromab 10 mg/kg + SOC 53 Placebo + SOC Group 50 Ages 13 - 21 32 Completed SAPPHIRE Treatment Period 31 † (97%) Apitegromab 20 mg/kg + SOC 22 Placebo + SOC Group 10 SAPPHIRE – APITEGROMAB IN SMA 98% of Patients Continue on Long - Term Extension ONYX Long - Term Extension Trial (Ongoing) 185* (98%)
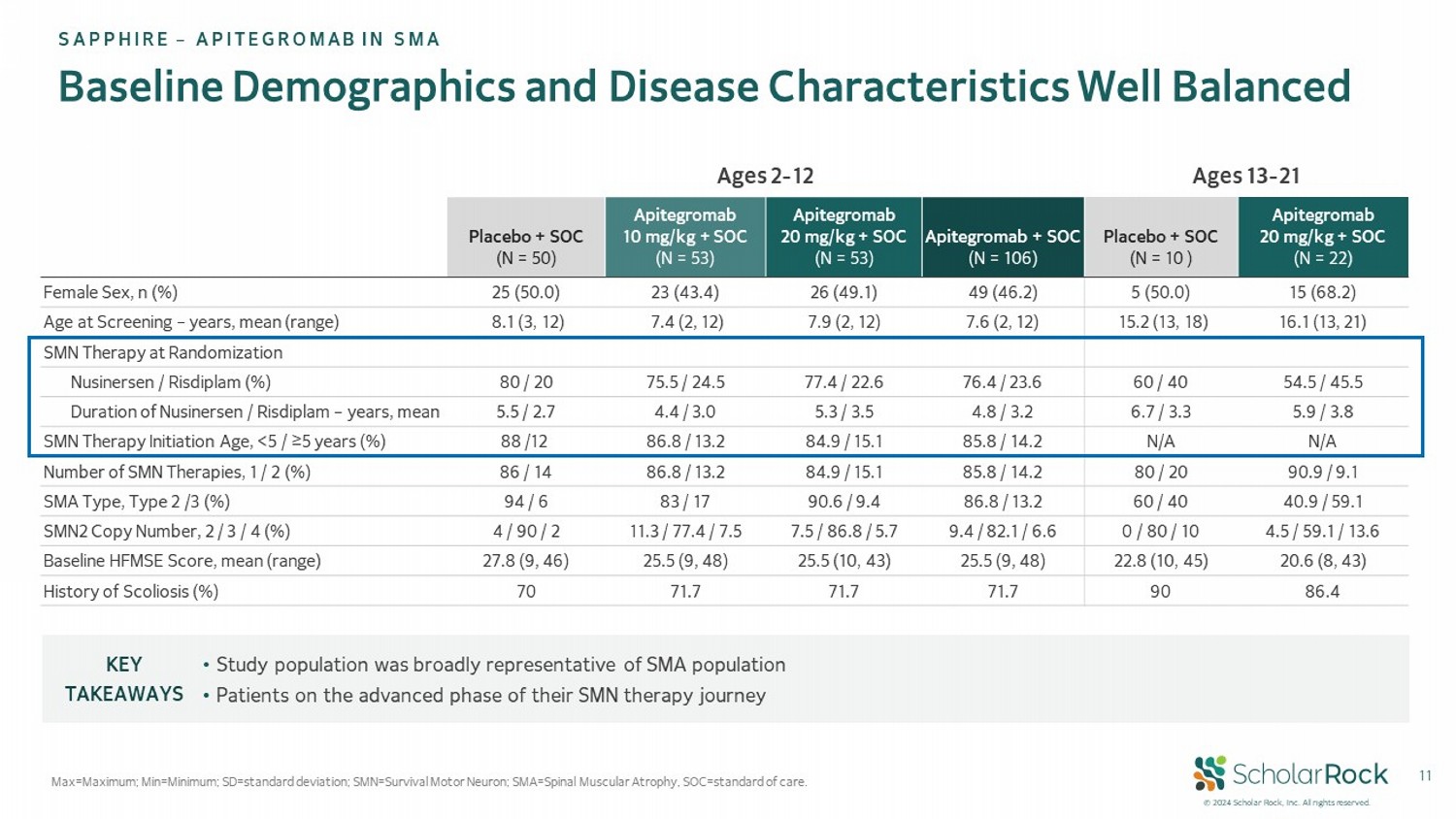
© 2024 Scholar Rock, Inc. All rights reserved. 11 Baseline Demographics and Disease Characteristics Well Balanced Max=Maximum; Min = Minimum; SD = standard deviation; SMN = Survival Motor Neuron; SMA = Spinal Muscular Atrophy , SOC=standard of care. SAPPHIRE – APITEGROMAB IN SMA Ages 13 - 21 Ages 2 - 12 Apitegromab 20 mg/kg + SOC (N = 22) Placebo + SOC (N = 10 ) Apitegromab + SOC (N = 106) Apitegromab 20 mg/kg + SOC (N = 53) Apitegromab 10 mg/kg + SOC (N = 53) Placebo + SOC (N = 50) 15 (68.2) 5 (50.0) 49 (46.2) 26 (49.1) 23 (43.4) 25 (50.0) Female Sex, n (%) 16.1 (13, 21) 15.2 (13, 18) 7.6 (2, 12) 7.9 (2, 12) 7.4 (2, 12) 8.1 (3, 12) Age at Screening – years, mean (range) SMN Therapy at Randomization 54.5 / 45.5 60 / 40 76.4 / 23.6 77.4 / 22.6 75.5 / 24.5 80 / 20 Nusinersen / Risdiplam (%) 5.9 / 3.8 6.7 / 3.3 4.8 / 3.2 5.3 / 3.5 4.4 / 3.0 5.5 / 2.7 Duration of Nusinersen / Risdiplam – years, mean N/A N/A 85.8 / 14.2 84.9 / 15.1 86.8 / 13.2 88 /12 SMN Therapy Initiation Age, <5 / ≥5 years (%) 90.9 / 9.1 80 / 20 85.8 / 14.2 84.9 / 15.1 86.8 / 13.2 86 / 14 Number of SMN Therapies, 1 / 2 (%) 40.9 / 59.1 60 / 40 86.8 / 13.2 90.6 / 9.4 83 / 17 94 / 6 SMA Type, Type 2 /3 (%) 4.5 / 59.1 / 13.6 0 / 80 / 10 9.4 / 82.1 / 6.6 7.5 / 86.8 / 5.7 11.3 / 77.4 / 7.5 4 / 90 / 2 SMN2 Copy Number, 2 / 3 / 4 (%) 20.6 (8, 43) 22.8 (10, 45) 25.5 (9, 48) 25.5 (10, 43) 25.5 (9, 48) 27.8 (9, 46) Baseline HFMSE Score, mean (range) 86.4 90 71.7 71.7 71.7 70 History of Scoliosis (%) • Study population was broadly representative of SMA population • Patients on the advanced phase of their SMN therapy journey KEY TAKEAWAYS
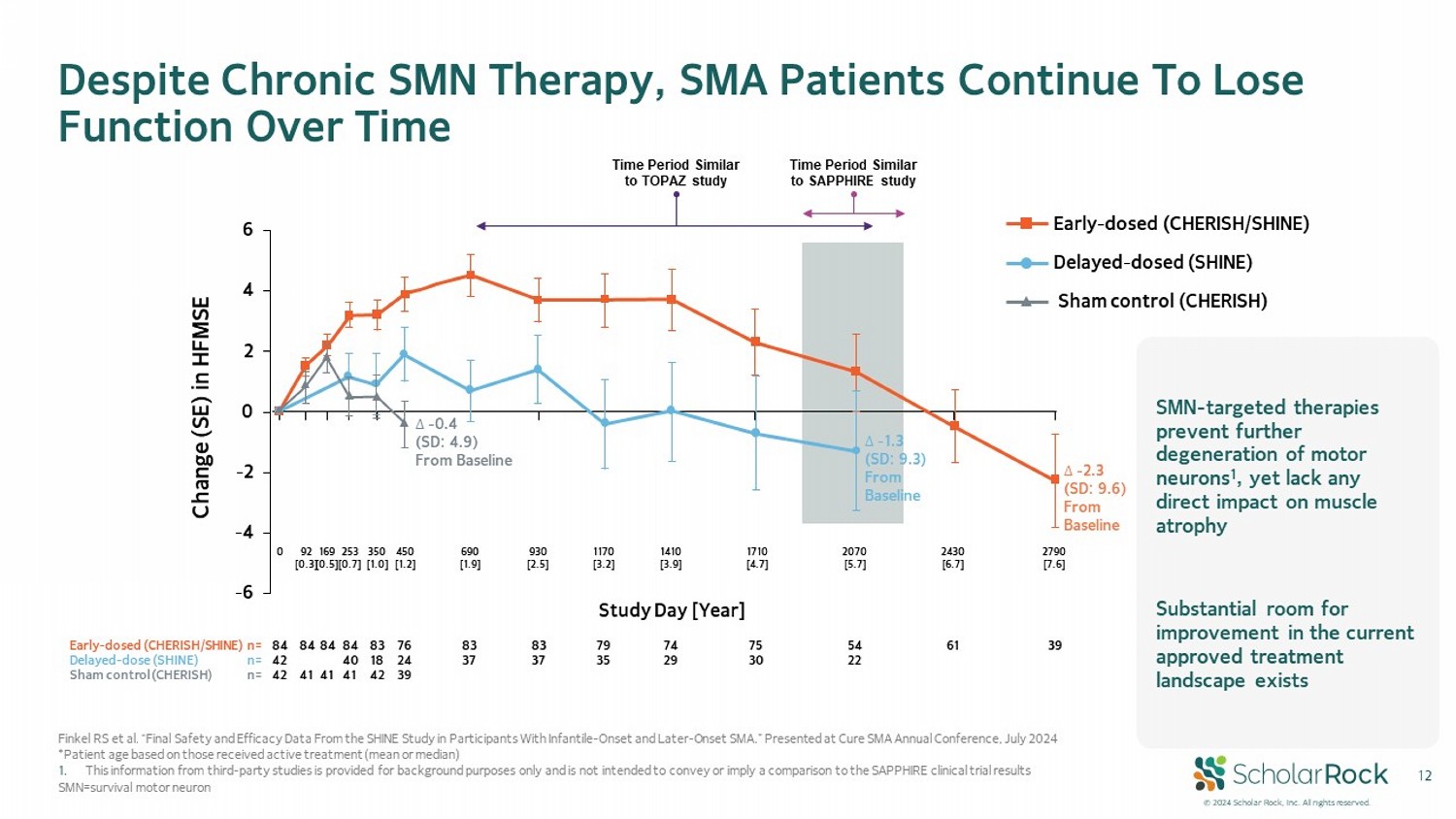
© 2024 Scholar Rock, Inc. All rights reserved. 12 Finkel RS et al. “Final Safety and Efficacy Data From the SHINE Study in Participants With Infantile - Onset and Later - Onset SMA.” Presented at Cure SMA Annual Conference, July 2024 *Patient age based on those received active treatment (mean or median) 1. This information from third - party studies is provided for background purposes only and is not intended to convey or imply a comp arison to the SAPPHIRE clinical trial results SMN=survival motor neuron Despite Chronic SMN Therapy, SMA Patients Continue To Lose Function Over Time Early - dosed (CHERISH/SHINE) Delayed - dosed (SHINE) Sham control (CHERISH) -6 -4 -2 0 2 4 6 ο - 1.3 (SD: 9.3) From Baseline ο - 2.3 (SD: 9.6) From Baseline ο - 0.4 (SD: 4.9) From Baseline Study Day [Year] Change (SE) in HFMSE Early - dosed (CHERISH/SHINE) n= Delayed - dose (SHINE) n= Sham control (CHERISH) n= 84 42 42 84 41 84 41 84 40 41 83 18 42 76 24 39 83 37 83 37 79 35 74 29 75 30 54 22 61 39 0 92 [0.3] 169 [0.5] 253 [0.7] 350 [1.0] 450 [1.2] 690 [1.9] 930 [2.5] 1170 [3.2] 1410 [3.9] 1710 [4.7] 2070 [5.7] 2430 [6.7] 2790 [7.6] Time Period Similar to TOPAZ study Time Period Similar to SAPPHIRE study SMN - targeted therapies prevent further degeneration of motor neurons 1 , yet lack any direct impact on muscle atrophy Substantial room for improvement in the current approved treatment landscape exists
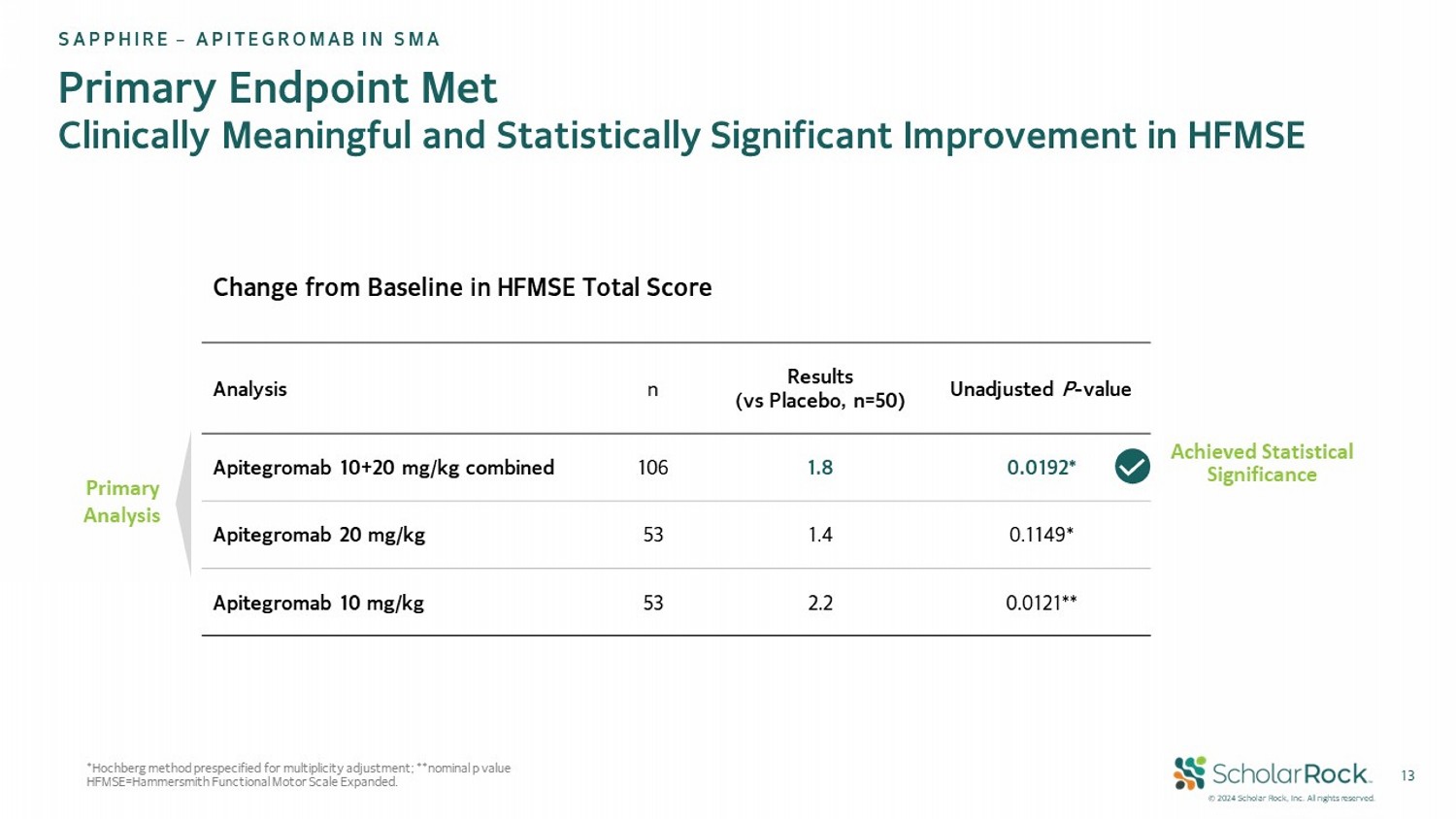
© 2024 Scholar Rock, Inc. All rights reserved. 13 *Hochberg method prespecified for multiplicity adjustment; **nominal p value HFMSE=Hammersmith Functional Motor Scale Expanded. Unadjusted P - value Results (vs Placebo, n=50) n Analysis 0.0192* 1.8 106 Apitegromab 10+20 mg/kg combined 0.1149* 1.4 53 Apitegromab 20 mg/kg 0.0121** 2.2 53 Apitegromab 10 mg/kg Change from Baseline in HFMSE Total Score Primary Endpoint Met Clinically Meaningful and Statistically Significant Improvement in HFMSE SAPPHIRE – APITEGROMAB IN SMA Achieved Statistical Significance Primary Analysis
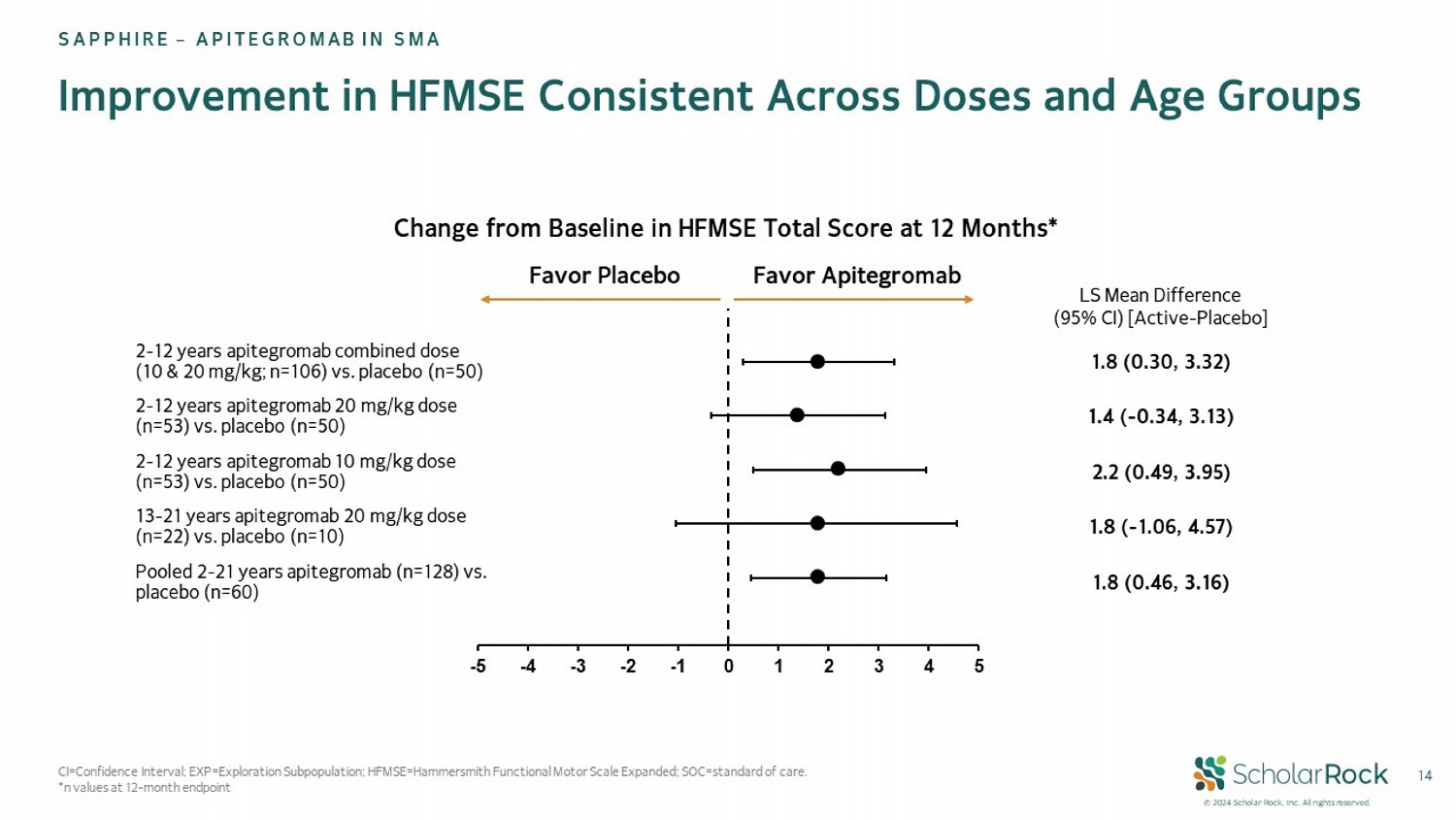
© 2024 Scholar Rock, Inc. All rights reserved. -5 -4 -3 -2 -1 0 1 2 3 4 5 14 Improvement in HFMSE Consistent Across Doses and Age Groups SAPPHIRE – APITEGROMAB IN SMA LS Mean Difference (95% CI) [Active - Placebo] 1.8 (0.30, 3.32) 2 - 12 years apitegromab combined dose (10 & 20 mg/kg; n=106) vs. placebo (n=50) 1.4 ( - 0.34, 3.13) 2 - 12 years apitegromab 20 mg/kg dose (n=53) vs. placebo (n=50) 2.2 (0.49, 3.95) 2 - 12 years apitegromab 10 mg/kg dose (n=53) vs. placebo (n=50) 1.8 ( - 1.06, 4.57) 13 - 21 years apitegromab 20 mg/kg dose (n=22) vs. placebo (n=10) 1.8 (0.46, 3.16) Pooled 2 - 21 years apitegromab (n=128) vs. placebo (n=60) Favor Apitegromab Favor Placebo Change from Baseline in HFMSE Total Score at 12 Months* CI=Confidence Interval; EXP=Exploration Subpopulation; HFMSE=Hammersmith Functional Motor Scale Expanded; SOC=standard of car e. *n values at 12 - month endpoint
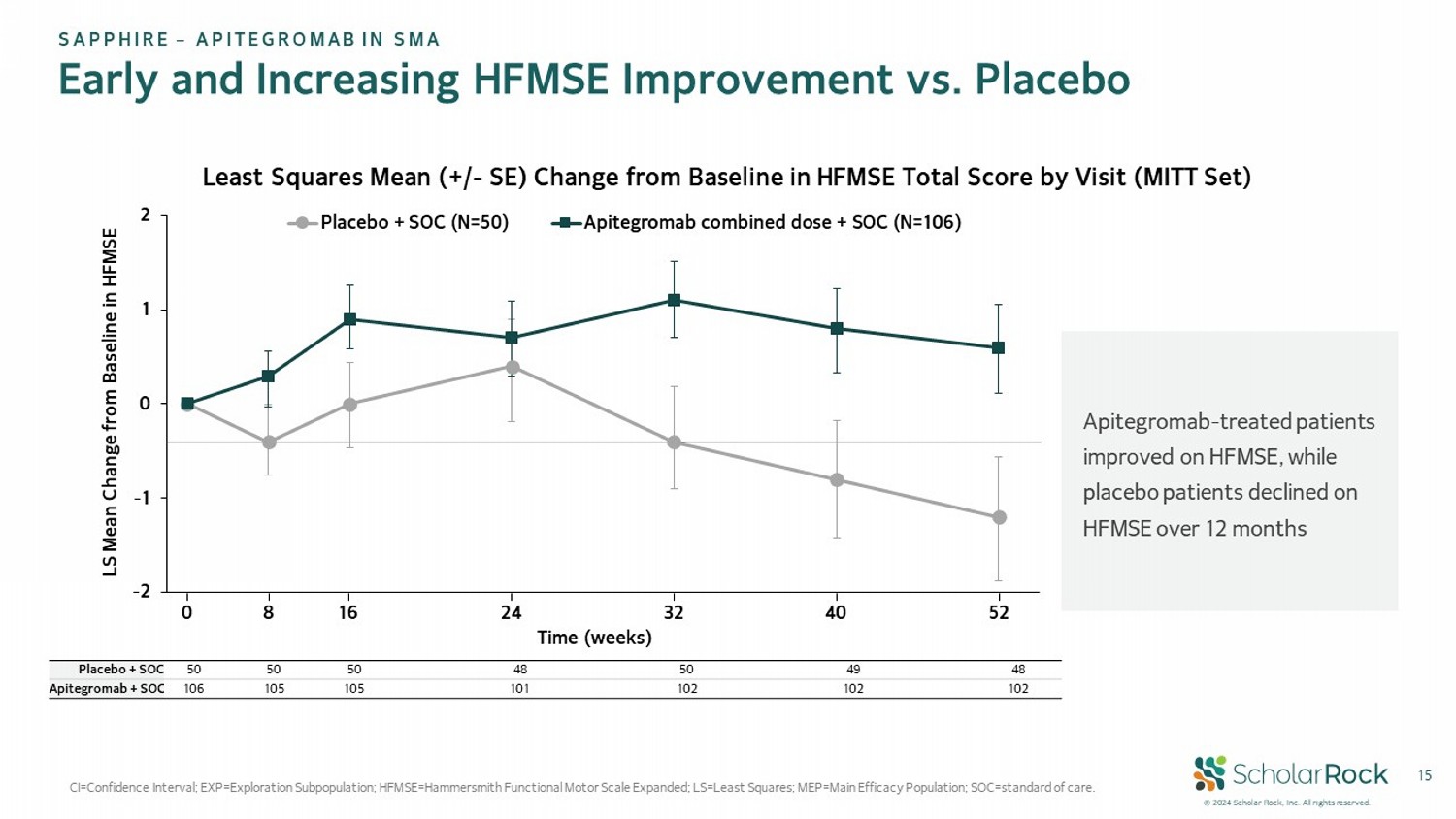
© 2024 Scholar Rock, Inc. All rights reserved. 15 CI=Confidence Interval; EXP=Exploration Subpopulation; HFMSE=Hammersmith Functional Motor Scale Expanded; LS=Least Squares; M EP= Main Efficacy Population; SOC=standard of care. SAPPHIRE – APITEGROMAB IN SMA Early and Increasing HFMSE Improvement vs. Placebo Least Squares Mean (+/ - SE) Change from Baseline in HFMSE Total Score by Visit (MITT Set) Apitegromab - treated patients improved on HFMSE, while placebo patients declined on HFMSE over 12 months -2 -1 0 1 2 Placebo + SOC (N=50) Apitegromab combined dose + SOC (N=106) 0 8 16 24 32 40 Time (weeks) 52 LS Mean Change from Baseline in HFMSE 48 49 50 48 50 50 50 Placebo + SOC 102 102 102 101 105 105 106 Apitegromab + SOC
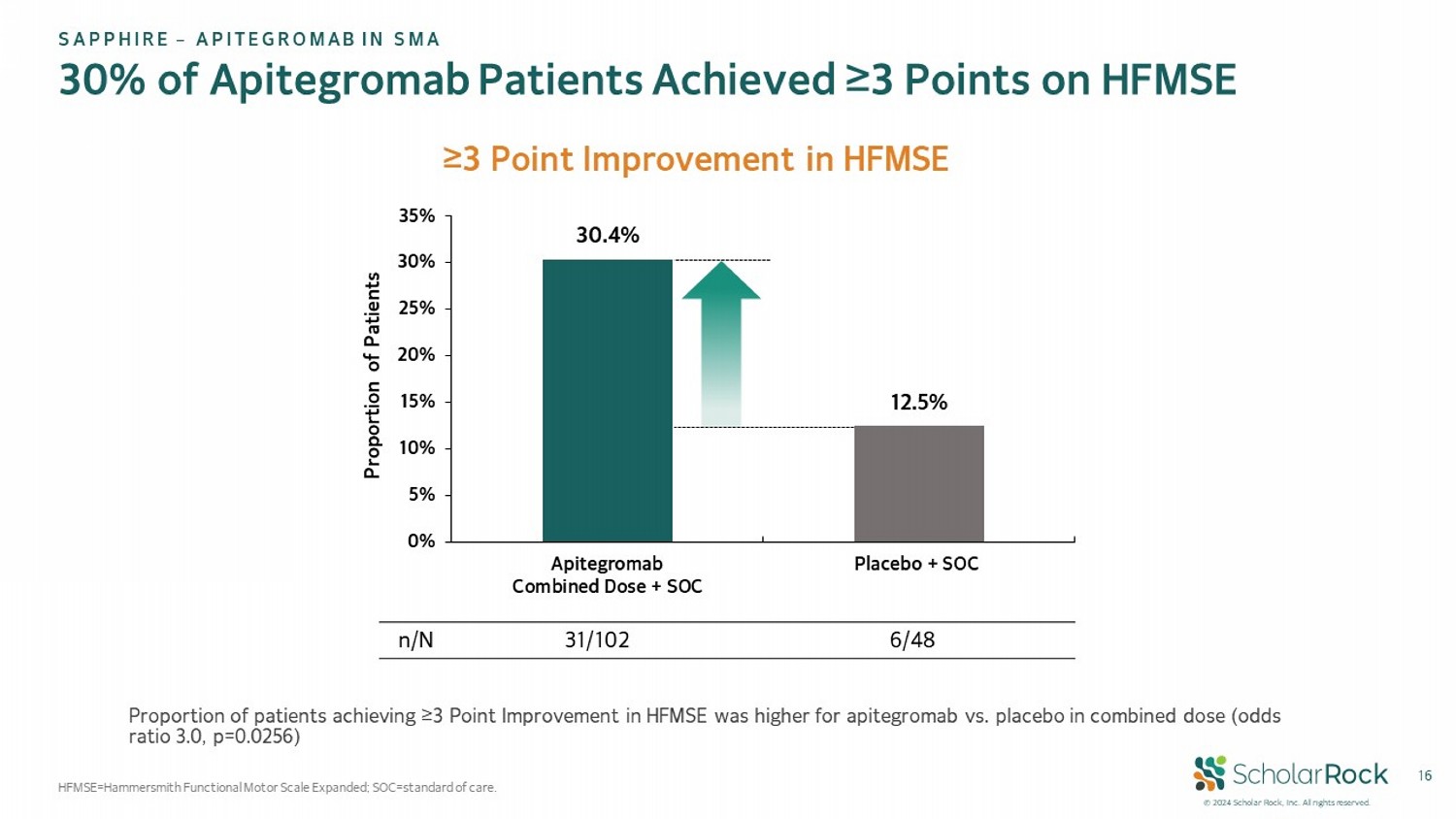
© 2024 Scholar Rock, Inc. All rights reserved. 16 30% of Apitegromab Patients Achieved ≥3 Points on HFMSE HFMSE=Hammersmith Functional Motor Scale Expanded; SOC = standard of care. SAPPHIRE – APITEGROMAB IN SMA ≥3 Point Improvement in HFMSE 30.4% 12.5% 0% 5% 10% 15% 20% 25% 30% 35% Apitegromab Combined Dose + SOC Placebo + SOC Proportion of Patients 6/48 31/102 n/N Proportion of patients achieving ≥3 Point Improvement in HFMSE was higher for apitegromab vs. placebo in combined dose (odds ratio 3.0, p=0.0256)
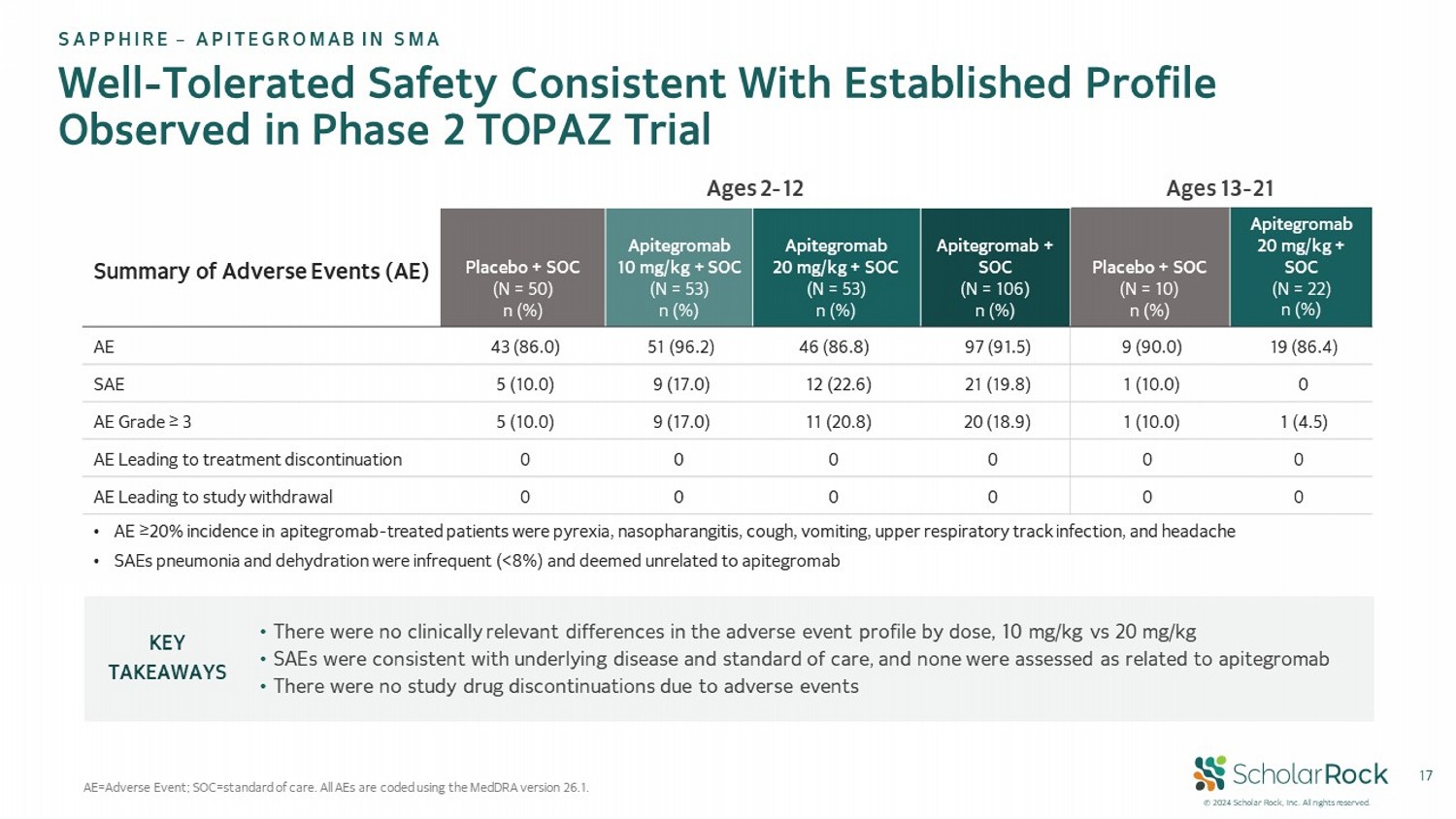
© 2024 Scholar Rock, Inc. All rights reserved. 17 Well - Tolerated Safety Consistent With Established Profile Observed in Phase 2 TOPAZ Trial AE=Adverse Event; SOC = standard of care. All AEs are coded using the MedDRA version 26.1. Ages 13 - 21 Ages 2 - 12 Summary of Adverse Events (AE) Apitegromab 20 mg/kg + SOC (N = 22) n (%) Placebo + SOC (N = 10) n (%) Apitegromab + SOC (N = 106) n (%) Apitegromab 20 mg/kg + SOC (N = 53) n (%) Apitegromab 10 mg/kg + SOC (N = 53) n (%) Placebo + SOC (N = 50) n (%) 19 (86.4) 9 (90.0) 97 (91.5) 46 (86.8) 51 (96.2) 43 (86.0) AE 0 1 (10.0) 21 (19.8) 12 (22.6) 9 (17.0) 5 (10.0) SAE 1 (4.5) 1 (10.0) 20 (18.9) 11 (20.8) 9 (17.0) 5 (10.0) AE Grade ≥ 3 0 0 0 0 0 0 AE Leading to treatment discontinuation 0 0 0 0 0 0 AE Leading to study withdrawal • There were no clinically relevant differences in the adverse event profile by dose, 10 mg/kg vs 20 mg/kg • SAEs were consistent with underlying disease and standard of care, and none were assessed as related to apitegromab • There were no study drug discontinuations due to adverse events KEY TAKEAWAYS SAPPHIRE – APITEGROMAB IN SMA • AE ≥20% incidence in apitegromab - treated patients were pyrexia, nasopharangitis , cough, vomiting, upper respiratory track infection, and headache • SAEs pneumonia and dehydration were infrequent (<8%) and deemed unrelated to apitegromab
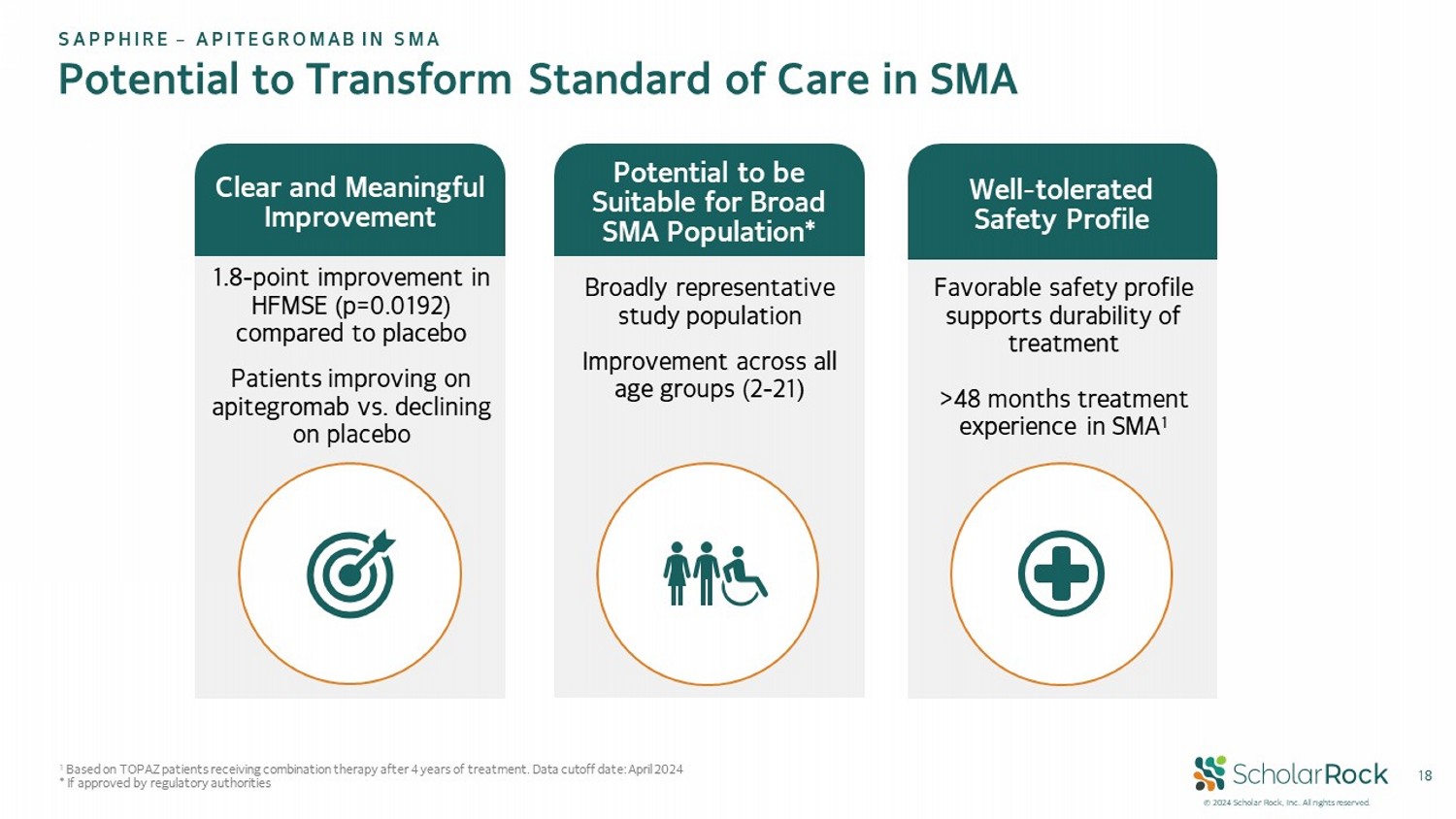
© 2024 Scholar Rock, Inc. All rights reserved. 18 Potential to Transform Standard of Care in SMA 1.8 - point improvement in HFMSE (p=0.0192) compared to placebo Patients improving on apitegromab vs. declining on placebo Favorable safety profile supports durability of treatment >48 months treatment experience in SMA 1 Clear and Meaningful Improvement Well - tolerated Safety Profile Broadly representative study population Improvement across all age groups (2 - 21) Potential to be Suitable for Broad SMA Population* 1 Based on TOPAZ patients receiving combination therapy after 4 years of treatment. Data cutoff date: April 2024 * If approved by regulatory authorities SAPPHIRE – APITEGROMAB IN SMA

© 2024 Scholar Rock, Inc. All rights reserved. Conclusion Jay Backstrom, M.D., MPH President & Chief Executive Officer

© 2024 Scholar Rock, Inc. All rights reserved. TGFβ=Transforming growth factor - beta. O UR MISSI ON To discover, develop, and deliver life - changing therapies by harnessing cutting - edge science to create new possibilities for people living with serious diseases We are a global leader in harnessing the life - changing potential of the TGFβ superfamily

© 2024 Scholar Rock, Inc. All rights reserved. Scholar Rock has an industry - leading, highly selective antibody engineering platform that has succeeded where others have failed. Apitegromab is the first and only muscle targeted therapy to show clinically meaningful and statistically significant functional improvement in SMA. Apitegromab is also the first and only anti - myostatin therapy to demonstrate a functional improvement in a pivotal Phase 3 study. 21 Innovating a New Era in the Treatment of Spinal Muscular Atrophy
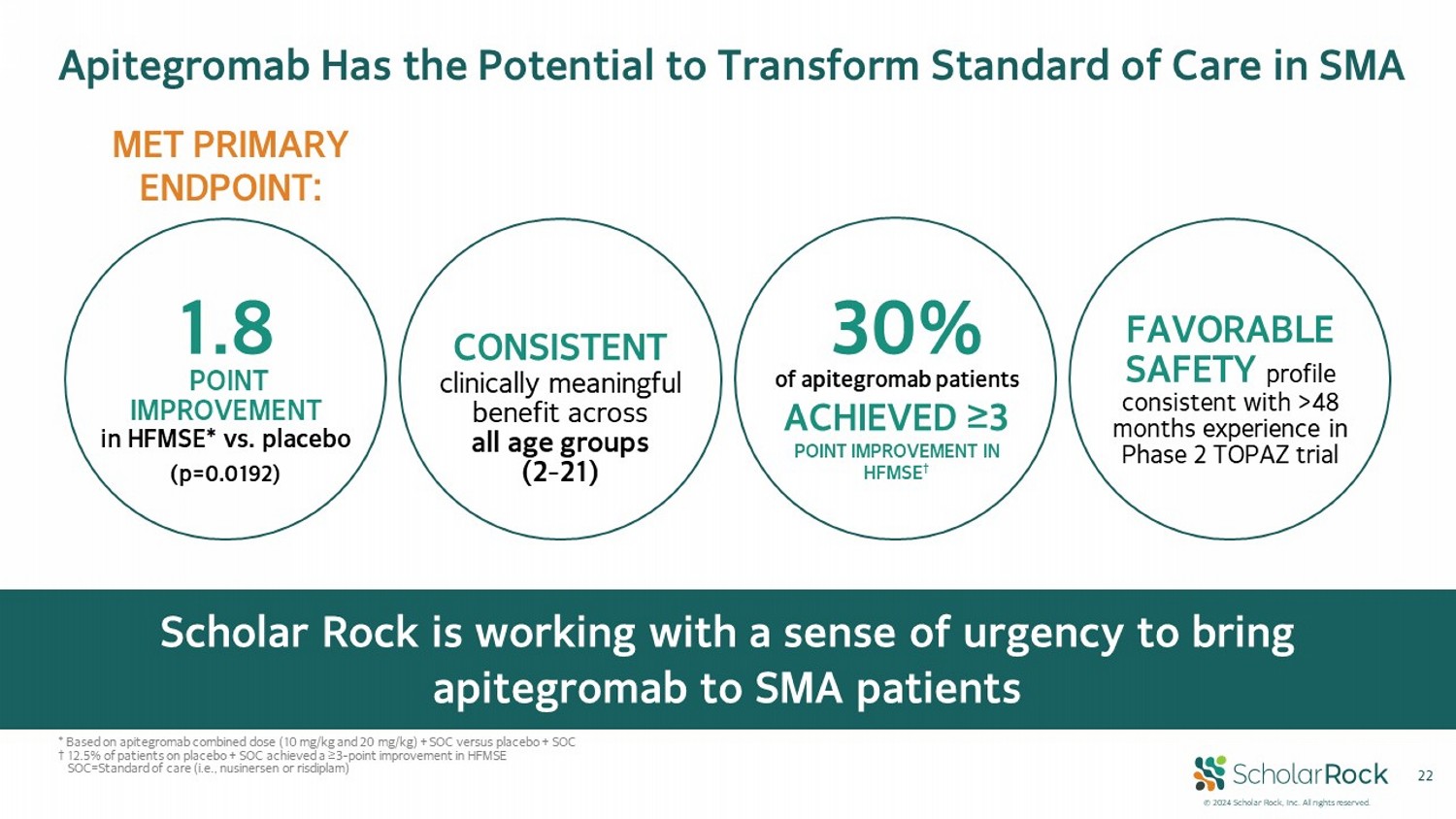
© 2024 Scholar Rock, Inc. All rights reserved. 22 CONSISTENT clinically meaningful benefit across all age groups (2 - 21) FAVORABLE SAFETY profile consistent with >48 months experience in Phase 2 TOPAZ trial 30% of apitegromab patients ACHIEVED ≥3 POINT IMPROVEMENT IN HFMSE † 1.8 (p=0.0192) POINT IMPROVEMENT in HFMSE* vs. placebo MET PRIMARY ENDPOINT: Apitegromab Has the Potential to Transform Standard of Care in SMA Scholar Rock is working with a sense of urgency to bring apitegromab to SMA patients * Based on apitegromab combined dose (10 mg/kg and 20 mg/kg) + SOC versus placebo + SOC † 12.5% of patients on placebo + SOC achieved a ≥3 - point improvement in HFMSE SOC = Standard of care (i.e., nusinersen or risdiplam)
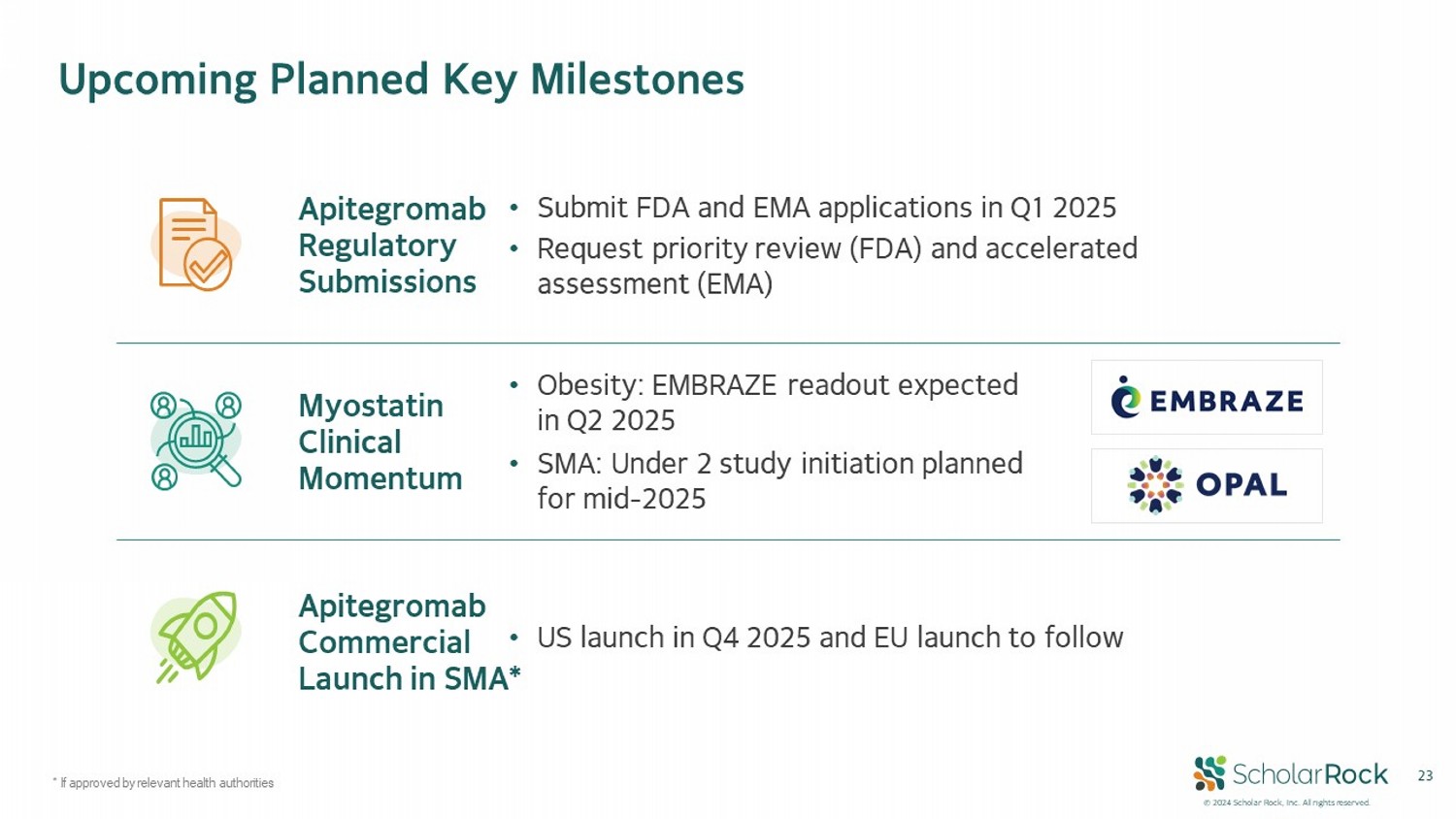
© 2024 Scholar Rock, Inc. All rights reserved. 23 Upcoming Planned Key Milestones * If approved by relevant health authorities • Submit FDA and EMA applications in Q1 2025 • Request priority review (FDA) and accelerated assessment (EMA) Apitegromab Regulatory Submissions • US launch in Q4 2025 and EU launch to follow Apitegromab Commercial Launch in SMA* • Obesity: EMBRAZE readout expected in Q2 2025 • SMA: Under 2 study initiation planned for mid - 2025 Myostatin Clinical Momentum

© 2024 Scholar Rock, Inc. All rights reserved. Q&A Session 24

© 2024 Scholar Rock, Inc. All rights reserved. 25 Thank you!

© 2024 Scholar Rock, Inc. All rights reserved. 26 Appendix
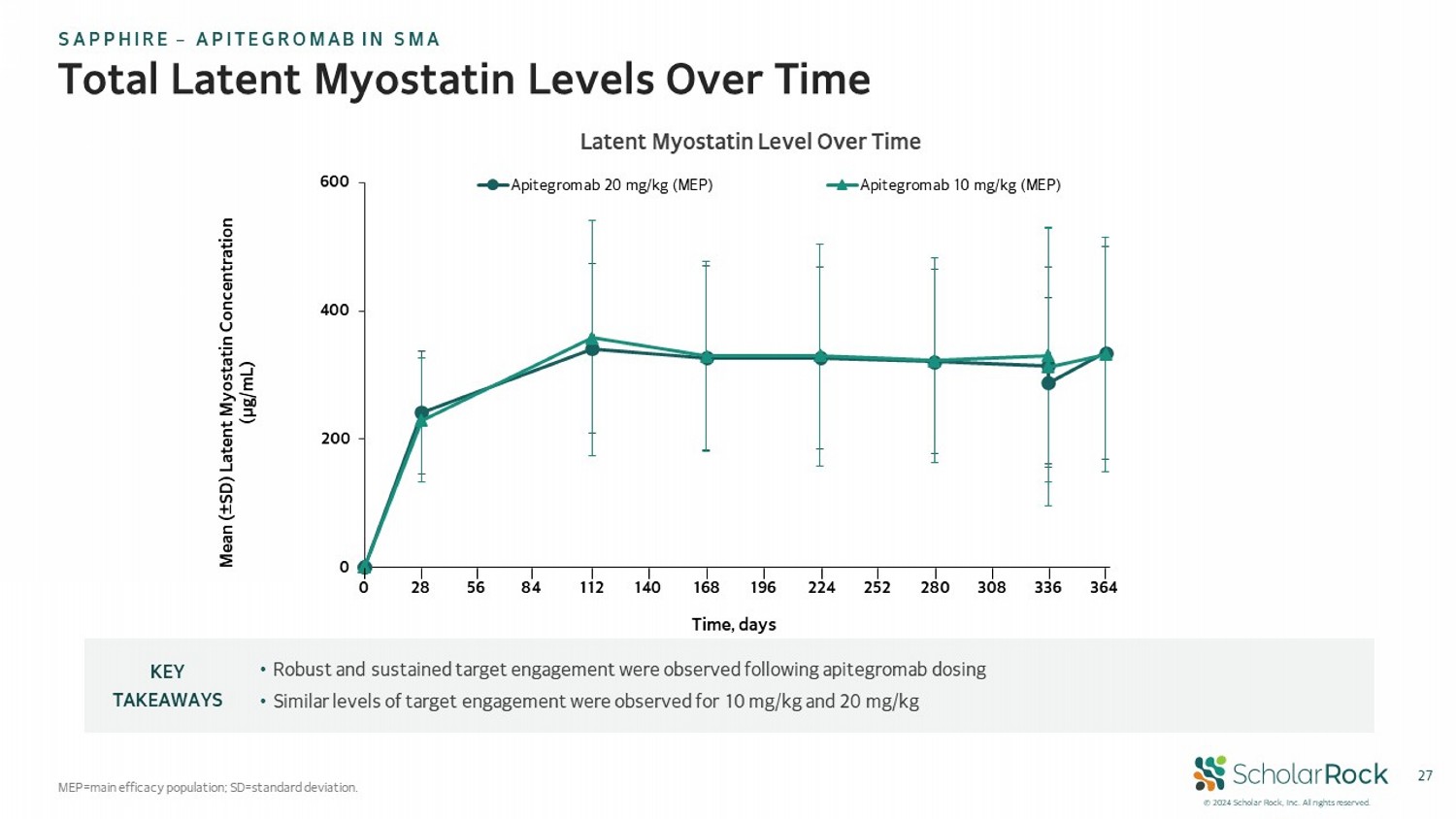
© 2024 Scholar Rock, Inc. All rights reserved. 27 Total Latent Myostatin Levels Over Time MEP=main efficacy population; SD=standard deviation. • Robust and sustained target engagement were observed following apitegromab dosing • Similar levels of target engagement were observed for 10 mg/kg and 20 mg/kg KEY TAKEAWAYS Latent Myostatin Level Over Time 0 200 400 600 Apitegromab 20 mg/kg (MEP) Apitegromab 10 mg/kg (MEP) Mean ( ± SD) Latent Myostatin Concentration (µg/mL) Time, days 0 28 168 308 336 364 56 84 112 140 196 224 252 280 SAPPHIRE – APITEGROMAB IN SMA
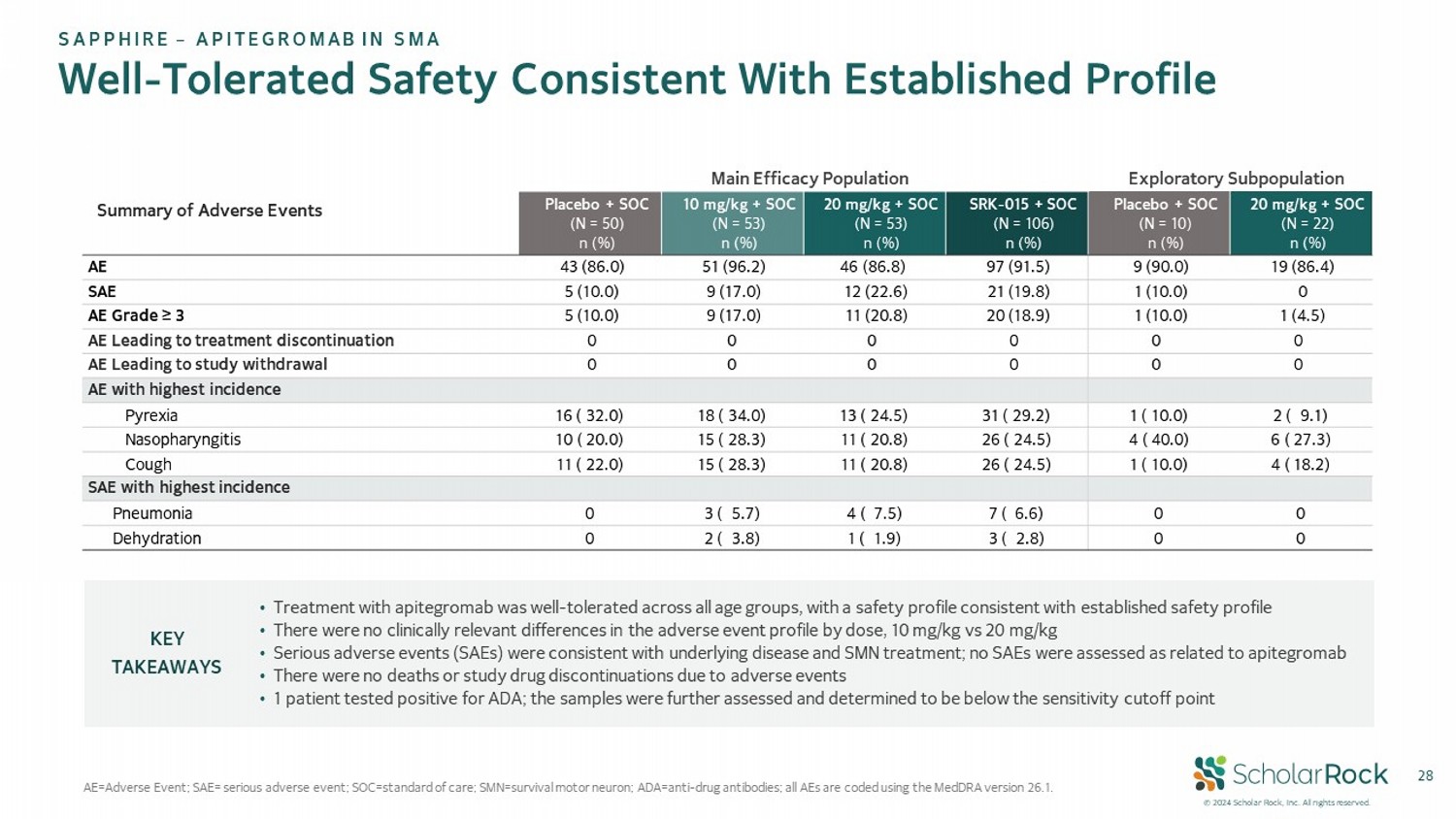
© 2024 Scholar Rock, Inc. All rights reserved. 28 Well - Tolerated Safety Consistent With Established Profile AE = Adverse Event; SAE= serious adverse event; SOC=standard of care; SMN=survival motor neuron; ADA=anti - drug antibodies; all AEs are coded using the MedDRA version 26.1. Exploratory Subpopulation Main Efficacy Population Summary of Adverse Events 20 mg/kg + SOC (N = 22) n (%) Placebo + SOC (N = 10) n (%) SRK - 015 + SOC (N = 106) n (%) 20 mg/kg + SOC (N = 53) n (%) 10 mg/kg + SOC (N = 53) n (%) Placebo + SOC (N = 50) n (%) 19 (86.4) 9 (90.0) 97 (91.5) 46 (86.8) 51 (96.2) 43 (86.0) AE 0 1 (10.0) 21 (19.8) 12 (22.6) 9 (17.0) 5 (10.0) SAE 1 (4.5) 1 (10.0) 20 (18.9) 11 (20.8) 9 (17.0) 5 (10.0) AE Grade ≥ 3 0 0 0 0 0 0 AE Leading to treatment discontinuation 0 0 0 0 0 0 AE Leading to study withdrawal AE with highest incidence 2 ( 9.1) 1 ( 10.0) 31 ( 29.2) 13 ( 24.5) 18 ( 34.0) 16 ( 32.0) Pyrexia 6 ( 27.3) 4 ( 40.0) 26 ( 24.5) 11 ( 20.8) 15 ( 28.3) 10 ( 20.0) Nasopharyngitis 4 ( 18.2) 1 ( 10.0) 26 ( 24.5) 11 ( 20.8) 15 ( 28.3) 11 ( 22.0) Cough SAE with highest incidence 0 0 7 ( 6.6) 4 ( 7.5) 3 ( 5.7) 0 Pneumonia 0 0 3 ( 2.8) 1 ( 1.9) 2 ( 3.8) 0 Dehydration • Treatment with apitegromab was well - tolerated across all age groups, with a safety profile consistent with established safety pr ofile • There were no clinically relevant differences in the adverse event profile by dose, 10 mg/kg vs 20 mg/kg • Serious adverse events (SAEs) were consistent with underlying disease and SMN treatment; no SAEs were assessed as related to api tegromab • There were no deaths or study drug discontinuations due to adverse events • 1 patient tested positive for ADA; the samples were further assessed and determined to be below the sensitivity cutoff point KEY TAKEAWAYS SAPPHIRE – APITEGROMAB IN SMA
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Scholar Rock (NASDAQ:SRRK)
Graphique Historique de l'Action
De Oct 2024 à Nov 2024

Scholar Rock (NASDAQ:SRRK)
Graphique Historique de l'Action
De Nov 2023 à Nov 2024


