MedinCell: Publication of the Activity Report and Financial Information for the First Half-year (April - Sept. 2019)
03 Décembre 2019 - 5:54PM
Business Wire
MedinCell (Paris:MEDCL):
Progression of the portfolio with FDA IND clearance to initiate
clinical activities for mdc-TJK and mdc-ANG entering
preclinical
Launch of Animal Health activities
Revenue increase and strong cash position in line with
expectations
Post-closing events
- Up to 19 M$ grant from the Bill & Melinda Gates Foundation
for mdc-WWM
- Antipsychotic programs: confirmation of clinical activities
start for the second long-acting injectable antipsychotic mdc-TJK
and interim analysis for mdc-IRM Phase 3 in the second half of
2020
The information contained in this press release will be
commented by the MedinCell management team during an online
conference on December 3, 2019. The comments and answers to
participants' questions will be available on
invest.medincell.com
Press release related to half-year events is available on
MedinCell’s website: https://invest.medincell.com/#news
View source
version on businesswire.com: https://www.businesswire.com/news/home/20191203005830/en/
MedinCell David Heuzé Communication leader
david.heuze@medincell.com +33 (0)6 83 25 21 86
NewCap Louis-Victor Delouvrier / Alexia Faure Investor
relations medincell@newcap.eu +33 (0)1 44 71 94 94
NewCap Nicolas Merigeau Media relations
medincell@newcap.eu +33 (0)1 44 71 94 94
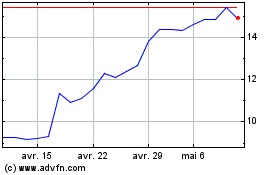
Medincell (EU:MEDCL)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
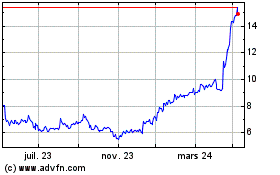
Medincell (EU:MEDCL)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024