Philogen Announces Clinical Trial Collaboration with MSD
01 Juin 2023 - 8:30AM

Philogen Announces Clinical Trial Collaboration with MSD
The Phase II clinical trial investigates
Philogen's immunocytokines (i) L19IL2 (ii) L19TNF, and (iii)
Nidlegy™ in combination with MSD’s anti-PD-1 therapy, KEYTRUDA®
(pembrolizumab) in stage III and IV unresectable melanoma patients
who previously failed treatment with checkpoint inhibitors
Siena, Italy, June 1st, 2023 -
Philogen S.p.A., a clinical-stage biotechnology company focused on
the development of innovative medicines based on tumor-targeting
antibodies and small molecule ligands, announces that it has
entered into a Clinical Trial Collaboration and Supply Agreement
with MSD (Merck & Co., Inc., Rahway, NJ, USA). Under the terms
of the supply agreement, MSD provides their anti-PD-1 therapy,
KEYTRUDA® (pembrolizumab), to be evaluated in combination with
Philogen's immunocytokines L19IL2, L19TNF, and Nidlegy™ in a
randomized Phase II clinical trial. The study provides an
opportunity to explore the combination of immunocytokines and PD-1
blockade in stage III and IV unresectable melanoma patients who
failed prior checkpoint inhibitor therapies.
Nidlegy™ is also investigated in two Phase III
randomized clinical trials for the treatment of stage III B/C
melanoma in Europe and in the United States, as well as in two
Phase II clinical trials in High-Risk Basal Cell Carcinoma and
other non-melanoma skin cancers.
Dario Neri, CEO and CSO of Philogen,
commented: "KEYTRUDA® has shown impressive response rates
and long-term benefits for a substantial number of patients with
advanced melanoma. However, only a minor proportion of patients who
fail checkpoint inhibitors typically benefit from a subsequent
re-challenge with single-agent PD-1 blockade. Clinical experience
with intralesional recombinant IL2 has shown encouraging response
rates when combined with systemic anti-PD-1 antibodies in advanced
melanoma patients who progressed on or are resistant to checkpoint
inhibitors. This provides a strong rationale to combine Nidlegy™
(L19IL2 + L19TNF) with KEYTRUDA® in this setting, and we are
pleased to partner with MSD, a global leader in immuno-oncology, to
explore this opportunity."
KEYTRUDA® is a registered trademark of Merck
Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc.,
Rahway, NJ, USA.Nidlegy™ is a registered trademark of Philogen
S.p.A. of La Lizza 7, 53100 Siena, ITALY.
* * *
Philogen Group Description
Philogen is an Italian-Swiss company active in
the biotechnology sector, specialized in the research and
development of pharmaceutical products for the treatment of highly
lethal diseases. The Group mainly discovers and develops targeted
anticancer drugs, exploiting high-affinity ligands for tumor
markers (also called tumor antigens). These ligands - human
monoclonal antibodies or small organic molecules - are identified
using Antibody Phage Display Libraries and DNA-Encoded Chemical
Library technologies.
The Group's main therapeutic strategy for the
treatment of these diseases is represented by the so-called tumor
targeting. This approach is based on the use of ligands capable of
selectively delivering very potent therapeutic active ingredients
(such as pro-inflammatory cytokines) to the tumor mass, sparing
healthy tissues. Over the years, Philogen has mainly developed
monoclonal antibody-based ligands that are specific for antigens
expressed in tumor-associated blood vessels, but not expressed in
blood vessels associated with healthy tissues. These antigens are
usually more abundant and more stable than those expressed directly
on the surface of tumor cells. This approach, so called vascular
targeting, is used for most of the projects pursued by the
Group.
The Group's objective is to generate, develop
and market innovative products for the treatment of diseases for
which medical science has not yet identified satisfactory
therapies. This is achieved by exploiting (i) proprietary
technologies for the isolation of ligands that react with antigens
present in certain diseases, (ii) experience in the development of
products targeted at the tissues affected by the disease, (iii)
experience in drug manufacturing and development, and (iv) an
extensive portfolio of patents and intellectual property
rights.
Although the Group's drugs are primarily
oncology applications, the targeting approach is also potentially
applicable to other diseases, such as certain chronic inflammatory
diseases.
* * *
FOR MORE INFORMATION:
Philogen - Investor
Relations
IR@philogen.com - Emanuele Puca | Investor
Relations
Consilium Strategic Communications contacts
Mary-Jane Elliott, Davide Salvi
Philogen@consilium-comms.com
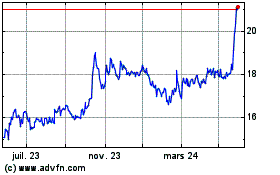
Philogen (BIT:PHIL)
Graphique Historique de l'Action
De Jan 2025 à Fév 2025
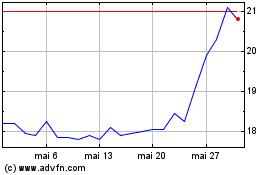
Philogen (BIT:PHIL)
Graphique Historique de l'Action
De Fév 2024 à Fév 2025