Smith+Nephew to highlight breakthrough Sports Medicine technologies for joint repair at AAOS 2025
10 Mars 2025 - 4:00PM
UK Regulatory
Smith+Nephew to highlight breakthrough Sports Medicine technologies
for joint repair at AAOS 2025
Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology
company, today announces it will showcase the latest advancements
in Sports Medicine for joint repair at the American Academy of
Orthopaedic Surgeons Annual Meeting in San Diego this week. Some of
the highlighted technologies will include:
Spatial Surgery
Smith+Nephew continues to pioneer in Sports Medicine and is
excited to introduce a new category called Spatial Surgery - a
revolutionary new frontier in arthroscopic surgical innovation.
This 510(k)-pending technology called the TESSA◊ Spatial
Surgery System (Tracking Enabled Spatial Surgery Assistant) plans
to combine personalized operative planning with a real-time,
tracking enabled device using advanced imaging and augmented
reality guidance to assist a surgeon in decision making. Learn more
at www.spatialsurgery.com
CARTIHEAL◊ AGILI-C◊ Cartilage Repair
Implant
Smith+Nephew’s CARTIHEAL AGILI-C Cartilage Repair Implant is an
FDA approved device that was previously granted breakthrough
designation and is now evolving the cartilage repair landscape. The
unique properties of the implant enable physicians to surgically
treat patients that previously had no access to cartilage repair
procedures.1-3 In a large randomized controlled trial,
when compared to the surgical standard of care,* the
CARTIHEAL AGILI-C implant demonstrated:
- Proven clinical superiority: Patients treated with the
CARTIHEAL AGILI-C Implant reported significantly better knee
function, pain relief, and mobility improvements over a 4-year
period.1,4 **
- Significantly lower risk of TKA or osteotomy: Patients’
risk of additional knee reconstruction/realignment surgery at 4
years.4
- Different patient profiles – same great results: The
scaffold treats a broad group of patients across age, lesion size,
and presence of osteoarthritis while delivering clinically
meaningful results.1,4, **
The use of the CARTIHEAL AGILI-C Implant in the presence of
osteoarthritis will be featured during OrthoDome at AAOS on
Wednesday, March 12, from 11:16-11:31 am PST. You can learn
more by visiting the CARTIHEAL AGILI-C webpage here.
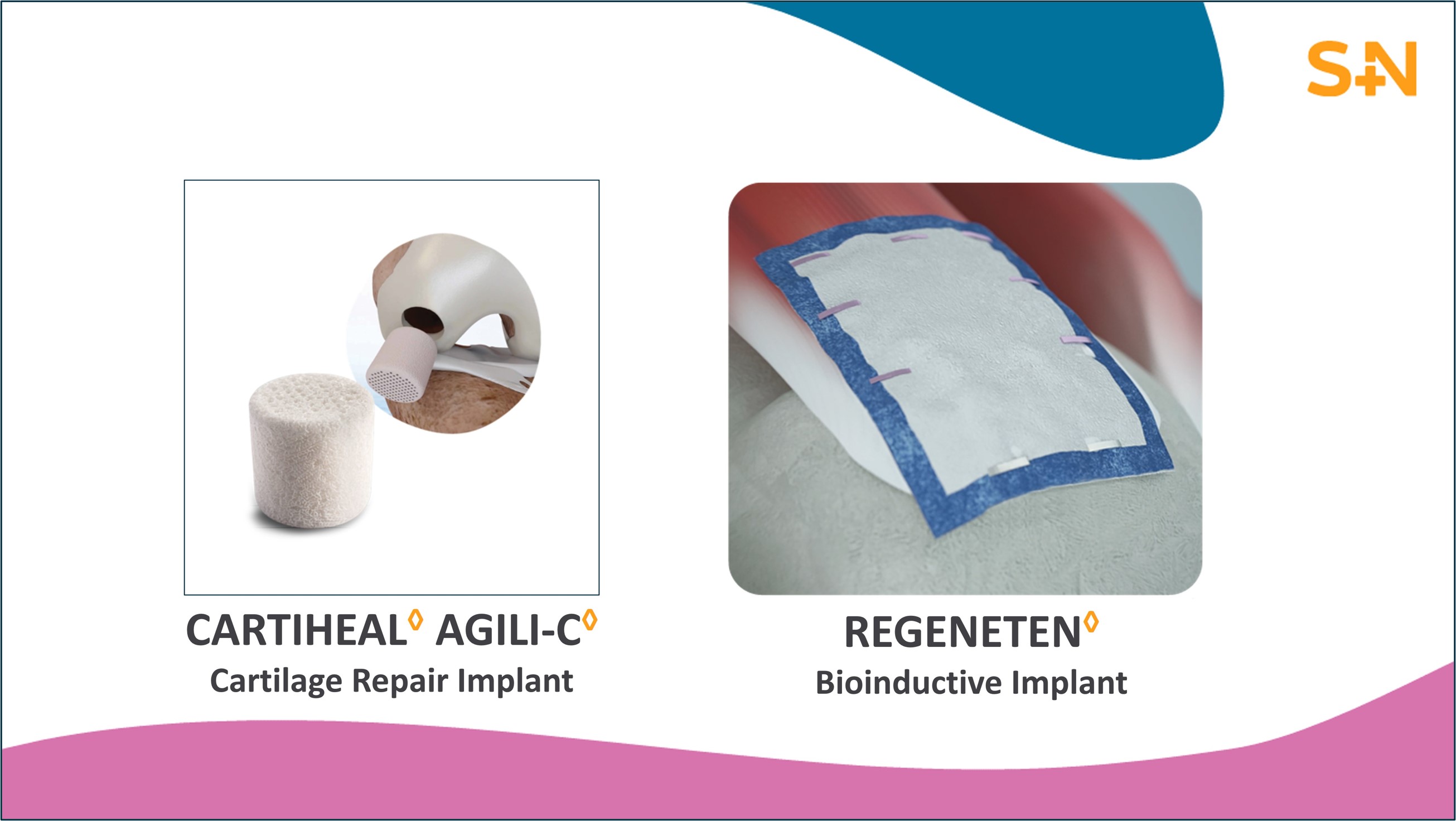
REGENETEN◊ Bioinductive Implant
The REGENETEN Bioinductive Implant is a scaffold made from
highly purified type I collagen fibers and has changed the way
surgeons treat tendon injuries for more than ten
years.5-8 Clinical studies in rotator cuff repair have
demonstrated results that surpass the current standard of care:
- 3x reduction in the risk of re-tear in a randomized
controlled trial versus standard repair alone augmenting repair of
medium-to-large full-thickness tears (at
1-year).9***
- Accelerated recovery and return to activity when used as
an isolated treatment (compared with takedown and suture anchor
repair),10,11 while leading to consistent tendon healing
for partial-thickness tears.9,12
- Well established technique used in >150,000 patients, with a
proprietary delivery and fixation system resulting in a
15-minute procedure.9
In addition to continued value in rotator cuff repair, usage of
the REGENETEN Implant continues to increase in tendons around the
body, including those in the hip, knee, and foot & ankle, where
surgeons recognize its potential to improve tendon healing. You can
learn more by visiting the REGENETEN webpage here.
To learn more about Smith+Nephew’s Sports Medicine joint repair
solutions and enabling technologies, please visit our booth (#3729)
at the American Academy of Orthopaedic Surgeons Annual Meeting in
San Diego March 11-13, 2025, or
visit www.smith-nephew.com.
- ends –
Media Enquiries
Dave Snyder +1
(978) 749-1440
Smith+Nephew
david.snyder@smith-nephew.com
* Debridement or microfracture
** Over a 2- and 4-year follow-up
*** Re-tear: 8.3% vs 25.8%; Relative risk=0.32 [95%
Confidence Interval 0.13-
0.83]; p=0.0106.
References
- Altschuler N, Zaslav KR, Di Matteo B, et al. Aragonite-Based
Scaffold Versus Microfracture and Debridement for the Treatment of
Knee Chondral and Osteochondral Lesions: Results of a Multicenter
Randomized Controlled Trial. Am J Sports Med.
2023;51(4):957-967
- Kon E, Di Matteo B, Verdonk P, et al. Aragonite-Based Scaffold
for the Treatment of Joint Surface Lesions in Mild to Moderate
Osteoarthritic Knees: Results of a 2-Year Multicenter Prospective
Study. Am J Sports Med. 2021;49(3):588-598
- Kon E, Filardo G, Shani J, et al. Osteochondral regeneration
with a novel aragonite-hyaluronate biphasic scaffold: up to
12-month follow-up study in a goat model. J Orthop Surg Res.
2015;10:81
- Conte P, Anzillotti G, Crawford DC, et al. Differential
analysis of the impact of lesions' location on clinical and
radiological outcomes after the implantation of a novel
aragonite-based scaffold to treat knee cartilage defects. Int
Orthop. 2024;48(12):3117-3126
- Bokor DJ, et al. Muscles Ligaments Tendons J.
2016;6(1):16-25.
- Warren JR, et al. J Shoulder Elbow Surg.
2024;33(11):2515-2529
- Bokor DJ, et al. Muscles Ligaments Tendons J.
2015;5(3):144-150.
- Smith+Nephew 2020 REGENETEN Collagen Implant Physical
Characteristics. Internal Report Internal Report. 15009769
- Ruiz Ibán MÁ, et al. Arthroscopy. 2024;40(6):1760-1773.
- Camacho Chacón JA, et al. J Shoulder Elbow Surg.
2024;33(9):1894-1904.
- Bushnell BD, et al. Orthop J Sports Med.
2021;9(8):23259671211027850.
- Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP.
Radiologic and clinical evaluation of a bioabsorbable collagen
implant to treat partial-thickness tears: a prospective multicenter
study. J Shoulder Elbow Surg. 2018 27(2):242-251
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused
on the repair, regeneration and replacement of soft and hard
tissue. We exist to restore people’s bodies and their self-belief
by using technology to take the limits off living. We call this
purpose ‘Life Unlimited’. Our 17,000 employees deliver this mission
every day, making a difference to patients’ lives through the
excellence of our product portfolio, and the invention and
application of new technologies across our three global business
units of Orthopaedics, Sports Medicine & ENT and Advanced Wound
Management.
Founded in Hull, UK, in 1856, we now operate in around 100
countries, and generated annual sales of $5.8 billion in 2024.
Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN).
The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith
& Nephew plc and its consolidated subsidiaries, unless the
context requires otherwise.
For more information about Smith+Nephew, please visit
www.smith-nephew.com and follow us on X, LinkedIn, Instagram or
Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may
or may not prove accurate. For example, statements regarding
expected revenue growth and trading profit margins, market trends
and our product pipeline are forward-looking statements. Phrases
such as "aim", "plan", "intend", "anticipate", "well-placed",
"believe", "estimate", "expect", "target", "consider" and similar
expressions are generally intended to identify forward-looking
statements. Forward-looking statements involve known and unknown
risks, uncertainties and other important factors that could cause
actual results to differ materially from what is expressed or
implied by the statements. For Smith+Nephew, these factors include:
conflicts in Europe and the Middle East, economic and financial
conditions in the markets we serve, especially those affecting
healthcare providers, payers and customers; price levels for
established and innovative medical devices; developments in medical
technology; regulatory approvals, reimbursement decisions or other
government actions; product defects or recalls or other problems
with quality management systems or failure to comply with related
regulations; litigation relating to patent or other claims; legal
and financial compliance risks and related investigative, remedial
or enforcement actions; disruption to our supply chain or
operations or those of our suppliers; competition for qualified
personnel; strategic actions, including acquisitions and disposals,
our success in performing due diligence, valuing and integrating
acquired businesses; disruption that may result from transactions
or other changes we make in our business plans or organisation to
adapt to market developments; relationships with healthcare
professionals; reliance on information technology and
cybersecurity; disruptions due to natural disasters, weather and
climate change related events; changes in customer and other
stakeholder sustainability expectations; changes in taxation
regulations; effects of foreign exchange volatility; and numerous
other matters that affect us or our markets, including those of a
political, economic, business, competitive or reputational nature.
Please refer to the documents that Smith+Nephew has filed with the
U.S. Securities and Exchange Commission under the U.S. Securities
Exchange Act of 1934, as amended, including Smith+Nephew's most
recent annual report on Form 20-F, which is available on the SEC’s
website at www. sec.gov, for a discussion of certain of these
factors. Any forward-looking statement is based on information
available to Smith+Nephew as of the date of the statement. All
written or oral forward-looking statements attributable to
Smith+Nephew are qualified by this caution. Smith+Nephew does not
undertake any obligation to update or revise any forward-looking
statement to reflect any change in circumstances or in
Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks
registered in US Patent and Trademark Office.


Smith & Nephew (LSE:SN.)
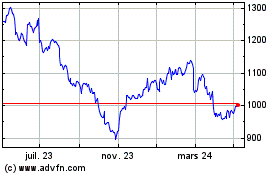
Graphique Historique de l'Action
De Fév 2025 à Mar 2025
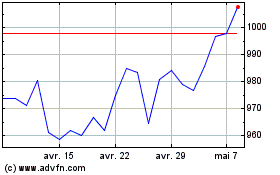
Smith & Nephew (LSE:SN.)
Graphique Historique de l'Action
De Mar 2024 à Mar 2025