KalVista Pharmaceuticals to Present at Upcoming Investor Conferences
12 Novembre 2024 - 12:30PM
Business Wire
KalVista Pharmaceuticals, Inc. (NASDAQ: KALV), today announced
that the Company will participate in a fireside chat at each of the
following investor conferences:
- Stifel 2024 Healthcare Conference on Tuesday, November 19,
2024, at 9:10 a.m. EST
- Jefferies London Healthcare Conference on Thursday, November
21, 2024, at 11:00 a.m. GMT
Each presentation will be live webcast on the Company’s website
at www.kalvista.com. After the presentations, an audio archive will
be available on KalVista’s website for 30 days.
About KalVista Pharmaceuticals, Inc.
KalVista Pharmaceuticals, Inc. is a global pharmaceutical
company whose mission is to develop and deliver life-changing oral
medicines for people affected by rare diseases with significant
unmet need. Sebetralstat, our novel, investigational candidate for
the oral, on-demand treatment of hereditary angioedema, is under
regulatory review by the U.S. FDA with a PDUFA goal date of June
17, 2025. In addition, we have completed marketing authorization
application (MAA) submissions for sebetralstat to the European
Medicines Agency as well as the United Kingdom, Switzerland,
Australia, and Singapore, and we anticipate filing a MAA in Japan
in late 2024.
For more information about KalVista, please visit
www.kalvista.com or follow on social media at
@KalVista and LinkedIn.
Forward-Looking Statements
This press release contains "forward-looking" statements within
the meaning of the safe harbor provisions of the U.S. Private
Securities Litigation Reform Act of 1995. Forward-looking
statements can be identified by words such as: "anticipate,"
"intend," "plan," "goal," "seek," "believe," "project," "estimate,"
"expect," "strategy," "future," "likely," "may," "should," "will"
and similar references to future periods. These statements are
subject to numerous risks and uncertainties that could cause actual
results to differ materially from what we expect. Examples of
forward-looking statements include, among others, timing or
outcomes of communications with the FDA, our expectations about
safety and efficacy of our product candidates and timing of
clinical trials and its results, our ability to commence clinical
studies or complete ongoing clinical studies, including our
KONFIDENT-S and KONFIDENT-KID trials, and to obtain regulatory
approvals for sebetralstat and other candidates in development, the
success of any efforts to commercialize sebetralstat, the ability
of sebetralstat and other candidates in development to treat HAE or
other diseases, and the future progress and potential success of
our oral Factor XIIa program. Further information on potential risk
factors that could affect our business and financial results are
detailed in our filings with the Securities and Exchange
Commission, including in our annual report on Form 10-K for the
year ended April 30, 2024, our quarterly reports on Form 10-Q, and
our other reports that we may make from time to time with the
Securities and Exchange Commission. We undertake no obligation to
publicly update any forward-looking statement, whether written or
oral, that may be made from time to time, whether as a result of
new information, future developments or otherwise.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241112749536/en/
Jenn Snyder Vice President, Corporate Affairs (617) 448-0281
jsnyder@kalvista.com Ryan Baker Head, Investor Relations (617)
771-5001 ryan.baker@kalvista.com
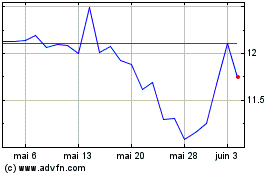
KalVista Pharmaceuticals (NASDAQ:KALV)
Graphique Historique de l'Action
De Nov 2024 à Déc 2024
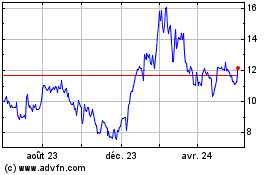
KalVista Pharmaceuticals (NASDAQ:KALV)
Graphique Historique de l'Action
De Déc 2023 à Déc 2024