Boston Scientific's Watchman Flx Pro Device Gets FDA Approval
06 Septembre 2023 - 10:47PM
Dow Jones News
By Sabela Ojea
Boston Scientific said the U.S. Food and Drug Administration has
granted approval to its Watchman Flx Pro Left Atrial Appendage
Closure device for use in nonvalvular atrial fibrillation
patients.
The biotechnology engineering company said the device is
designed for patients that are eligible for anticoagulation
therapy.
Its technology is indicated to reduce stroke risk in patients
with nonvalvular atrial fibrillation who need an alternative to
oral anticoagulation therapy, the company added.
Write to Sabela Ojea at sabela.ojea@wsj.com; @sabelaojeaguix
(END) Dow Jones Newswires
September 06, 2023 16:32 ET (20:32 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
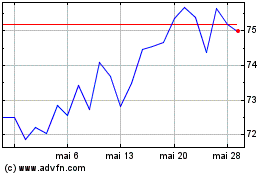
Boston Scientific (NYSE:BSX)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
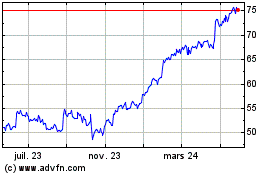
Boston Scientific (NYSE:BSX)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024