ABIONYX Announces Positive Clinical Results From CER-001 in the LCAT Deficiency Disease Published in the Annals of Internal M...
02 Mars 2021 - 2:00PM
Business Wire
- Demonstrated efficacy in renal and
ophthalmic indication
- Confirmation of the breakthrough therapy
status of CER-001 in kidney and ophthalmic diseases
- Systemic mechanism of action of
CER-001
ABIONYX Pharma (FR0012616852 - ABNX - PEA PME
eligible), a new generation biotech company dedicated to
innovative therapies for patients, today announces positive
clinical results from CER-001 in the LCAT (lecithin-cholesterol
acyltransferase) deficiency disease published in the Annals of
Internal Medicine.
The patient with inherited mutations in the lecithin-cholesterol
acyltransferase (LCAT) gene developed glomerulopathy and corneal
lipid deposits and displayed very low circulating levels of
high-density lipoprotein (HDL) and apoA-I. The patient was treated
with CER-001, an apoA-I-containing HDL mimetic, to help in
preventing rapidly progressive kidney failure.
The publication states that CER-001 prevented further kidney
function decline. The patient who was about to be dialysed due to
rapidly declining kidney function, was able to avoid the need for
dialysis during their treatment with CER-001.
No other treatment was introduced strongly suggesting that
stabilization of the renal function relied on the administration of
CER-001.
The publication mentions positive extra-renal clinical results
and the disappearance of visual blurring secondary to corneal
deposits. This clear improvement in visual function is still
observed after 1 year of follow-up.
Prof. Stanislas Faguer, nephrologist at Department of Nephrology
and Organ Transplantation Reference Center for Rare Kidney
Diseases, Hospital Rangueil, CHU Toulouse, states: “These positive
clinical data demonstrate that CER-001 prevented significantly
kidney function decline. ApoA-I containing HDL mimetic CER-001 is
the first treatment that has proven its ability to slow the
progression of renal failure and reduce visual discomfort secondary
to corneal lipid deposits in a patient with FLD. We look forward to
conducting new clinical studies of CER-001 to assess its ability to
improve the prognosis of other orphan or common kidney diseases for
which no effective treatment is currently available.”
Cyrille Tupin, Managing Director of ABIONYX Pharma concludes:
“This represents an important step forward in the clinical
development of CER-001, an HDL mimetic. We are committed to
advancing the clinical development of the mimetic HDL as quickly
and safely as possible while investigating new indications in
ophtalmology so that we can help address new rare or orphan
diseases with no existing treatment.”
View source
version on businesswire.com: https://www.businesswire.com/news/home/20210302005302/en/
NewCap Investor relations Louis-Victor Delouvrier
abionyx@newcap.eu +33 (0)1 44 71 98 53
NewCap Media relations Nicolas Merigeau abionyx@newcap.eu
+33 (0)1 44 71 94 98
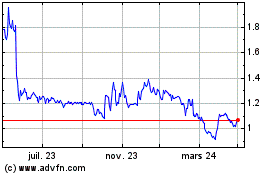
Abionyx Pharma (EU:ABNX)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
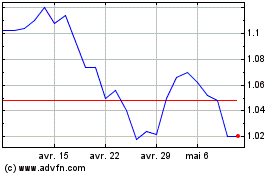
Abionyx Pharma (EU:ABNX)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024