Gilead, Galapagos Say Arthritis Drug Gets Recommendation to Start Marketing in Europe
24 Juillet 2020 - 3:14PM
Dow Jones News
By Dave Sebastian
Gilead Sciences Inc. and Galapagos NV said their arthritis drug
got a scientific recommendation from the European Medicines Agency
to grant marketing authorization in Europe.
The recommendation comes after phase 2 and 3 studies for the
drug, Jyseleca, met primary endpoints, the companies said
Friday.
The European Commission will now review the recommendation, with
an expected decision in the third quarter, the companies said. If
authorized, the company can market the drug in the European Union's
27 countries.
The companies said the once-daily filgotinib had a consistent
clinical safety profile when applied as monotherapy or in
combination with methotrexate during the trials.
Write to Dave Sebastian at dave.sebastian@wsj.com
(END) Dow Jones Newswires
July 24, 2020 08:59 ET (12:59 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
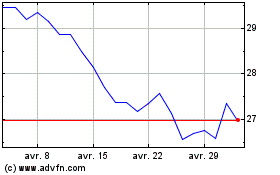
Galapagos (EU:GLPG)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
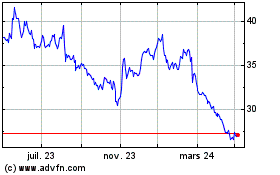
Galapagos (EU:GLPG)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024