NGSP Certification
03 Août 2005 - 9:00AM
UK Regulatory
RNS Number:6515P
Provalis PLC
03 August 2005
Provalis plc
in2it(TM)A1c achieves NGSP Certification and Brand Creation Award
Provalis plc (LSE: PRO; NASDAQ: PVLS), the international medical diagnostics and
pharmaceuticals group, is pleased to announce that in2it(TM)A1c, its flagship
diabetes diagnostic product, has been certified by the National Glycohaemoglobin
Standardisation Program (NGSP).
NGSP is an independent quality assessment run by the University of Missouri. The
importance of the certification is such that the American Diabetes Association
only recommends, and many health care providers in the USA will only use, tests
that are NGSP certified.
During the last few months Provalis has begun to build brand awareness of in2it
(TM)A1c in the USA and is delighted to announce that its campaign in 2005 has
won first place in the recent Bio-medical Marketing Association Awards in the
category of Product Brand Creation.
Phil Gould, Chief Executive Officer of Provalis, said
"NGSP certification shows that in2it(TM) has achieved the gold standard in
independent technical assessments. This certification, coupled with increasing
product awareness, will accelerate sales of in2it(TM) in the USA".
3 August 2005
For further information:-
Provalis plc
Dr Phil Gould, Chief Executive Officer 01244 833463
Mr Peter Bream, Finance Director 01244 833552
Mr Lee Greenbury, Company Secretary 01244 833402
College Hill
Adrian Duffield 020 7457 2815
Corinna Dorward 020 7457 2803
Notes to Editors
Provalis plc (LSE: PRO; NASDAQ: PVLS) is an international healthcare group with
two operating businesses:-
* Medical Diagnostics - The business' principal products are in2it (TM)A1c and
Glycosal(R), both diabetes diagnostic tests. In2it(TM)A1c is a fully
automated, point of care, diabetes diagnostic, testing platform which has
marketing clearance from the FDA in the US for use in both physicians'
offices and at home on prescription.
* Pharmaceuticals - sells and markets its own, and third party, branded,
prescription medicines in the UK and Ireland to GPs and hospitals through
its regionally managed sales force. The business' principal product is
Diclomax(R), a medicine for use in the treatment of musculo-skeletal
disorders, and it also sells products in the areas of osteoporosis,
migraine and dermatology.
Visit Provalis' Revised Website at http://www.provalis.com
"Safe Harbor" Statement under the US Private Securities Litigation Reform Act of
1995: Statements in this announcement that relate to future plans, expectations,
events, performances and the like are forward-looking statements as defined in
the US Private Securities Litigation Reform Act of 1995. Actual results of
events could differ materially from those described in the forward-looking
statements due to a variety of factors. Such factors include, among others: the
viability of the Group's products, which are at various stages of development;
the generation of sufficient operating cash flow by the Group's pharmaceutical
and medical diagnostic businesses to finance the ongoing development of these
businesses as well as the Group's research and development activities; the
success of the Group's research and development strategy and activities;
uncertainties related to future clinical trial results and the associated
regulatory process; the execution and success of collaborative agreements with
third parties; availability and level of reimbursement for the Group's products
from government health administration authorities or other third-party payors;
the rate of net cash utilisation within the Group and, hence, the Group's
possible need for additional capital in the short, medium and/or long term; the
Group's intellectual property position and the success of patent applications
for its products and technologies; the Group's dependence on key personnel;
general business and economic conditions; the impact of future laws, regulations
and policies; stock market trends in the Group's sector; and other factors
beyond the Group's control that may cause the Group's available capital
resources to be used more quickly than expected. These and other factors that
could affect the Company's future results are more fully described in its
filings with the US Securities and Exchange Commission, in particular the latest
20-F filing, copies of which are available from the Company Secretary at the
Company's registered address.
This information is provided by RNS
The company news service from the London Stock Exchange
END
MSCPTMRTMMBMBTA
Globaldata (LSE:DATA)
Graphique Historique de l'Action
De Juin 2024 à Juil 2024
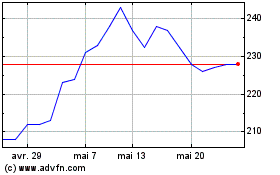
Globaldata (LSE:DATA)
Graphique Historique de l'Action
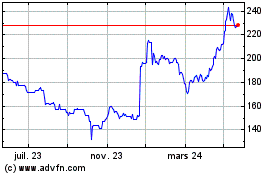
De Juil 2023 à Juil 2024