Altimmune Gets FDA Fast-Track Designation for Pemvidutide in NASH
26 Octobre 2023 - 2:15PM
Dow Jones News
By Colin Kellaher
Altimmune has won U.S. Food and Drug Administration fast-track
designation for its lead product candidate pemvidutide in the
treatment of nonalcoholic steatohepatitis, the chronic liver
condition commonly known as NASH.
The Gaithersburg, Md., clinical-stage biopharmaceutical company
on Thursday said it is studying the efficacy and safety of
pemvidutide in NASH in a Phase 2b placebo-controlled biopsy-driven
trial.
The FDA's fast-track program is designed to facilitate the
development and expedite the review of treatments for serious or
potentially life-threatening illnesses with high unmet medical
needs.
There are currently no FDA-approved drugs to treat NASH, which
is caused by a buildup of fat in the liver and is estimated to
affect 17 million Americans.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
October 26, 2023 08:00 ET (12:00 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
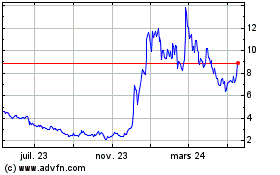
Altimmune (NASDAQ:ALT)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
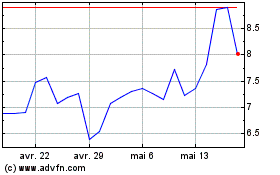
Altimmune (NASDAQ:ALT)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024