SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
PURSUANT
TO RULE 13a-16 OR 15d-163
UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For
the month of November 2024
Alterity
Therapeutics Limited
(Name
of Registrant)
Level 14, 350 Collins Street,
Melbourne, Victoria 3000 Australia
(Address
of Principal Executive Office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
This
Form 6-K is being incorporated by reference into our Registration Statement on Form S-8 (Files No. 333-251073, 333-248980
and 333-228671) and our
Registration Statements on Form F-3 (Files No. 333-274816, 333-251647, 333-231417
and 333-250076)
ALTERITY
THERAPEUTICS LIMITED
(a
development stage enterprise)
The
following exhibits are submitted:
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
Alterity Therapeutics Limited |
| |
|
|
| |
By: |
/s/ Geoffrey P. Kempler |
| |
|
Geoffrey P. Kempler |
| |
|
Chairman |
Date:
November 22, 2024
2
Exhibit 99.1

Results of 2024 Annual General
Meeting
MELBOURNE,
AUSTRALIA AND SAN FRANCISCO, USA – 22 November 2024: In accordance with ASX Listing Rule 3.13.2 and Section 251AA of the Corporations
Act 2001 (Cth), the attached information is provided in relation to the resolutions passed by the shareholders of Alterity Therapeutics
(ASX: ATH, NASDAQ: ATHE) (“Alterity” or “the Company”) at its Annual General Meeting held on 22 November 2024.
About Alterity Therapeutics Limited
Alterity Therapeutics is a clinical
stage biotechnology company dedicated to creating an alternate future for people living with neurodegenerative diseases. The Company’s
lead asset, ATH434, has the potential to treat various Parkinsonian disorders and is currently being evaluated in two Phase 2 clinical
trials in Multiple System Atrophy. Alterity also has a broad drug discovery platform generating patentable chemical compounds to treat
the underlying pathology of neurological diseases. The Company is based in Melbourne, Australia, and San Francisco, California, USA. For
further information please visit the Company’s web site at www.alteritytherapeutics.com.
Authorisation & Additional information
This announcement was authorised by David
Stamler, CEO of Alterity Therapeutics Limited.
Investor and Media Contacts:
Australia
Ana Luiza Harrop
we-aualteritytherapeutics@we-worldwide.com
+61 452 510 255
U.S.
Remy Bernarda remy.bernarda@iradvisory.com
+1 (415) 203-6386
Forward Looking Statements
This press release contains
“forward-looking statements” within the meaning of section 27A of the identify such forward-looking statements by use of such
words as “expects,” “intends,” “hopes,” “anticipates,” “believes,” “could,”
“may,” “evidences” and “estimates,” and other similar expressions, but these words are not the exclusive
means of identifying such statements.
Important factors that could
cause actual results to differ materially from those indicated by such forward- looking statements are described in the sections titled
“Risk Factors” in the Company’s filings with the SEC, including its most recent Annual Report on Form 20-F as well as
reports on Form 6-K, including, but not limited to the following: statements relating to the Company’s drug development program, including,
but not limited to the initiation, progress and outcomes of clinical trials of the Company’s drug development program, including, but
not limited to, ATH434, and any other statements that are not historical facts. Such statements involve risks and uncertainties, including,
but not limited to, those risks and uncertainties relating to the difficulties or delays in financing, development, testing, regulatory
approval, production and marketing of the Company’s drug components, including, but not limited to, ATH434, the ability of the Company
to procure additional future sources of financing, unexpected adverse side effects or inadequate therapeutic efficacy of the Company’s
drug compounds, including, but not limited to, ATH434, that could slow or prevent products coming to market, the uncertainty of obtaining
patent protection for the Company’s intellectual property or trade secrets, the uncertainty of successfully enforcing the Company’s
patent rights and the uncertainty of the Company freedom to operate.
Any forward-looking statement
made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is
made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time
to time, whether as a result of new information, future developments or otherwise.
Disclosure of Proxy Votes
Alterity Therapeutics Limited
Annual General Meeting
Friday, 22 November 2024 |
 |
|
GPO Box 5193, Sydney, NSW 2001 |
|
P 1300 288 664 (aus) or +61 (0)2 9698 5414 (world) |
|
F +61 (0)2 8583 3040 E hello@automic.com.au |
| |
ABN 27 152 260 814 |
In accordance with section 251AA of the Corporations Act 2001, the
following information is provided in relation to resolutions put to members at the meeting.
| |
|
|
Proxy
Votes |
Poll
Results
(if applicable) |
Results |
| Resolution |
Decided
by
Show of
Hands (S) or
Poll (P) |
Total
Number of
Proxy Votes
exercisable by
proxies validly
appointed |
FOR |
AGAINST |
ABSTAIN |
PROXY’S
DISCRETION |
FOR |
AGAINST |
ABSTAIN |
OUTCOME |
| 1
Non-binding resolution to adopt Remuneration Report |
P |
360,278,902 |
247,282,902
68.64% |
87,766,213
24.36% |
27,512,053 |
25,229,787
7.00% |
272,623,588
75.65% |
87,766,213
24.35% |
27,512,053 |
- |
| 2
Re-Election of Director – Mr Geoffrey Kempler |
P |
393,912,798 |
326,330,105
82.84% |
42,974,739
10.91% |
2,862,414 |
24,607,954
6.25% |
376,435,438
89.75% |
42,974,739
10.25% |
2,862,414 |
Carried |
| 3
Ratification of prior issue of shares (Placement July 2024) |
P |
292,690,098 |
184,971,185
63.20% |
82,614,126
28.23% |
1,286,814 |
25,104,787
8.58% |
253,554,351
75.42% |
82,614,126
24.58% |
1,286,814 |
Carried |
| 4
Approval for issue of options – Mr Geoffrey Kempler |
P |
336,743,973 |
251,964,801
74.82% |
63,859,918
18.96% |
57,757,839 |
20,919,254
6.21% |
298,381,434
82.37% |
63,859,918
17.63% |
57,757,839 |
Carried |
| 5
Approval for issue of options – Mr Lawrence Gozlan |
P |
337,583,973 |
251,873,001
74.61% |
64,288,385
19.04% |
57,194,439 |
21,422,587
6.35% |
316,773,967
83.13% |
64,288,385
16.87% |
57,194,439 |
Carried |
| 6
Approval for issue of options – Mr Peter Marks |
P |
337,356,373 |
251,667,001
74.60% |
64,266,785
19.05% |
57,953,639 |
21,422,587
6.35% |
309,381,999
82.80% |
64,266,785
17.20% |
57,953,639 |
Carried |
| 7
Approval for issue of options – Mr Brian Meltzer |
P |
330,247,116 |
244,620,144
74.07% |
64,204,385
19.44% |
57,941,639 |
21,422,587
6.49% |
309,521,110
82.82% |
64,204,385
17.18% |
57,941,639 |
Carried |
| 8
Approval of 10% Placement Issue |
P |
391,033,484 |
266,912,465
68.26% |
99,500,946
25.45% |
5,255,128 |
24,620,073
6.30% |
335,010,917
77.10% |
99,500,946
22.90% |
5,255,128 |
Carried |
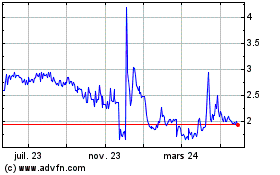
Alterity Therapeutics (NASDAQ:ATHE)
Graphique Historique de l'Action
De Oct 2024 à Nov 2024
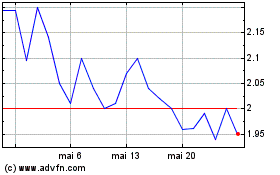
Alterity Therapeutics (NASDAQ:ATHE)
Graphique Historique de l'Action
De Nov 2023 à Nov 2024