- In the RESILIENT SMA study, taldefgrobep alpha showed
clinically meaningful improvements in motor function at all
timepoints on the Motor Function Measurement-32 scale (MFM-32), but
the treatment arm did not statistically separate on the primary
outcome at Week 48 compared to the placebo+standard of care (SOC)
group.
- Efficacy signals were observed in clinically relevant and
biomarker-defined subgroups including those related to age,
ambulatory status, background therapy, and baseline myostatin
level.
- Analyses of prespecified subgroups by race and ethnicity
demonstrated that the largest study population (87% Caucasian;
n=180) showed clinically meaningful improvements on the MFM-32 at
all timepoints, including Week 48, compared to the corresponding
placebo+SOC group (p < 0.05). Additional analyses of these
subjects (n=123) who had measurable baseline myostatin (the
pharmacological target of taldefgrobep) showed an improved efficacy
signal within this myostatin-positive population (p=0.02).
- Biohaven plans to engage the FDA regarding potential next steps
forward and will present the study data at an upcoming conference.
The optional long-term extension phase of the trial will remain
ongoing pending further data analysis as well as regulatory
discussions.
- Prespecified outcome measures in the overall study population
analyzing the change from baseline in body composition at Week 48
demonstrated a greater reduction in the percent change in total
body fat mass in the taldefgrobep arm compared to the placebo+SOC
arm (p=0.008) as measured by dual energy x-ray absorptiometry
(DXA). The taldefgrobep arm also showed numerically larger
increases in lean muscle mass and bone density compared to the
placebo+SOC arm.
- Given the overall strength and consistency of
the taldefgrobep-associated changes in body composition (i.e.,
fat mass, lean muscle mass, and bone density), Biohaven plans to
rapidly advance taldefgrobep into a placebo-controlled Phase 2
obesity study in 4Q2024 using a user-friendly, self-administered
autoinjector.
- Taldefgrobep demonstrated robust target engagement in the
RESILIENT study, reducing myostatin levels below detection in all
treated subjects over 48 weeks.
- Taldefgrobep was well-tolerated in the RESILIENT study with 97%
of subjects continuing into the optional long-term extension. There
were no taldefgrobep treatment-related serious adverse events
(SAEs).
NEW HAVEN,
Conn., Nov. 25, 2024 /PRNewswire/ -- Biohaven
Ltd. (NYSE: BHVN) (Biohaven), a global clinical-stage
biopharmaceutical company focused on the discovery, development and
commercialization of life-changing therapies to treat a broad range
of rare and common diseases, provided an update today regarding the
taldefgrobep alfa development programs in SMA and
obesity.
In the RESILIENT SMA study, taldefgrobep showed
clinically meaningful improvements in motor function at all
timepoints on the Motor Function Measurement-32 scale (MFM-32) but
the treatment arm did not statistically separate on the primary
outcome at 48 weeks compared to the placebo+standard of care (SOC)
group. Signals of efficacy were observed in clinically relevant and
biomarker-defined subgroups including those related to age,
ambulatory status, background therapy, and presence of myostatin at
baseline. Notably, there was an unexpectedly large subgroup (35%)
of subjects without measurable levels of myostatin at baseline and
imbalances in some genetic factors (SMN2 copy number, race) across
treatment arms.
Diverse populations are affected by SMA, but its
genetic epidemiology and carrier rates vary with race or ethnicity,
with more than a 2-fold higher prevalence in Caucasian compared to
non-Caucasian populations1. Analyses of prespecified
subgroups by race and ethnicity demonstrated that the largest study
population (87% Caucasian; n=180) showed a 2.2-point
change-from-baseline improvement with taldefgrobep treatment on the
MFM-32 at Week 48 compared to a 1.1-point change-from-baseline
improvement in the corresponding placebo+SOC group (p < 0.039).
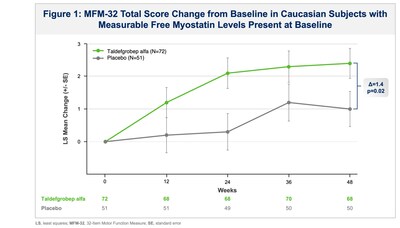
The improvement associated with taldefgrobep in this subgroup was
further increased to a 1.4-point placebo adjusted
change-from-baseline (p=0.02) when analyzed by subjects who had
measurable myostatin levels at baseline (see Figure 1). These
benefits on the MFM-32 continued to increase in the open-label
extension period based on preliminary data through Extension Week
24. Moreover, a responder analysis of this myostatin-positive
subgroup, with response defined as ≥ 3-point change from baseline
improvement on the MFM-32 at Week 48, showed that 50% of
taldefgrobep-treated subjects responded compared to 30% in the
placebo+SOC arm.
Non-Caucasian subjects (n=26) had a higher than
expected placebo response and did not separate from placebo on the
MFM-32 at Week 48 (p=0.24). Significant enrichment of known, common
genetic polymorphisms in non-Caucasian subjects, considered likely
to confer myostatin-inhibitory independence for myostatin
therapies, are probable in explaining this ethnic separation of
responsiveness to therapy. This provides a potentially facile
biomarker for identifying SMA patients likely to benefit from
taldefgrobep and other myostatin therapies. Further analysis is
ongoing to better understand the likely potential impact of these
readily monitorable biomarker and genetic factors, including
myostatin allele frequencies, in both the potential lower overall
response in treated subjects and the overall higher response
observed in placebo subjects.
In the overall study population, and relevant to
activity of taldefgrobep on muscle and fat, prespecified outcome
measures analyzing the change from baseline in body composition at
Week 48 demonstrated a greater reduction in the percent change in
total body fat mass in the taldefgrobep arm compared to the
placebo+SOC arm (p=0.008) as measured by dual energy x-ray
absorptiometry (DXA). The taldefgrobep arm also showed
numerically larger increases in lean muscle mass and bone density
compared to the placebo+SOC arm. Importantly, variables such
as race or baseline myostatin levels did not alter the impact of
taldefgrobep on the favorable effects on fat mass over the course
of the study. These results suggest that the body composition
changes observed in subjects treated with taldefgrobep, including
lower fat mass, are not solely mediated by taldefgrobep's effects
on myostatin but also through its effects on other targets such as
activin A. The favorable effects observed on overall body
composition (fat and lean mass) is consistent with nonclinical data
showing that taldefgrobep has direct effects on adipose tissue
mediated by activin receptor signaling through multiple ligands,
including activin A, myostatin and others. Notably, taldefgrobep is
the first and only myostatin blocking agent that has shown the
ability to favorably change fat mass in people with SMA. This
finding has important implications for the potential of
taldefgrobep as a treatment for obesity in a broad population.
Based upon data from this study demonstrating
target engagement (i.e., myostatin reduction) and
taldefgrobep-associated changes in body composition (i.e., fat
mass, lean muscle mass and bone density), Biohaven intends to
accelerate taldefgrobep clinical plans in 4Q24, advancing a
user-friendly, self-administered autoinjector in adults living
with overweight and obesity, medical conditions defined by excess,
abnormal body fat.
Biomarker analyses demonstrated that taldefgrobep
achieved robust target engagement, reducing myostatin levels in all
treated subjects below the level of detection at all time points
measured through Week 48. Post hoc efficacy analyses in subjects
with measurable baseline myostatin (the pharmacological target of
taldefgrobep) showed an improved efficacy signal in this
myostatin-positive population.
Cliff Bechtold,
Taldefgrobep Development Lead and President of Biohaven Ireland,
commented, "SMA is a devastating rare disease and although we are
disappointed that taldefgrobep did not achieve a statistically
significant difference in the broad study population on the MFM-32,
we are encouraged that a majority subgroup did show a treatment
benefit compared to the placebo arm. The observed treatment effect
on motor function, which had a similar magnitude on the MFM-32
after 1 year of treatment as approved therapies (i.e., risdiplam),
along with the strong biomarker evidence of target engagement,
suggests that taldefgrobep may play a potentially beneficial role
in a majority subgroup population of SMA patients. Additionally,
taldefgrobep demonstrated an important beneficial effect on body
composition which supports our plans to accelerate development in
broader populations with obesity."
Taldefgrobep was well-tolerated in the RESILIENT
trial with 97% of subjects continuing into the optional long-term
extension, which will remain open and ongoing pending discussion
with FDA. There were no taldefgrobep treatment-related SAEs.
Evaluation of additional RESILIENT clinical and
biomarker data is ongoing, and Biohaven plans to engage with FDA
regarding these emerging data to discuss a path forward. Full
topline data will be presented at an upcoming scientific
meeting.
Lindsey Lair, MD,
MBA, Vice President of Neurology and Clinical Lead for SMA at
Biohaven added, "Biohaven remains committed to fighting rare
diseases and will engage SMA experts and regulatory authorities
regarding the full dataset from the RESILIENT study. We are
extremely grateful to the international SMA community - especially
the participants and their families, investigators and their teams,
and patient advocacy groups who made the trial possible."
About Taldefgrobep alfa
Taldefgrobep
alfa is a fully human recombinant protein specifically designed to
inhibit both myostatin and activin receptor signaling. By blocking
the formation of the myostatin-activin receptor complex,
taldefgrobep prevents downstream activity that leads to muscle
atrophy and accumulation of fat mass. More information about
taldefgrobep alfa can be found at the Biohaven website.
About SMA
SMA is a rare genetic
neurodegenerative disorder characterized by the loss of motor
neurons, atrophy of the voluntary muscles of the limbs and trunk
and progressive muscle weakness that is often fatal and typically
diagnosed in young children. The underlying pathology of SMA is
caused by insufficient production of the SMN (survival of motor
neuron) protein, essential for the survival of motor neurons, and
is encoded by two genes, SMN1 and SMN2. Globally, SMA affects
approximately 1 in 10,000 births, and about 1 in every 50
individuals is a genetic carrier.
About Biohaven
Biohaven is a
biopharmaceutical company focused on the discovery, development,
and commercialization of life-changing treatments in key
therapeutic areas, including immunology, neuroscience, and
oncology. The company is advancing its innovative portfolio of
therapeutics, leveraging its proven drug development experience and
multiple proprietary drug development platforms.
Biohaven's extensive clinical and preclinical programs include
Kv7 ion channel modulation for epilepsy and mood disorders;
extracellular protein degradation for immunological diseases; TRPM3
antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition
for neuroinflammatory disorders; glutamate modulation for OCD and
SCA (spinocerebellar ataxia); myostatin inhibition for
neuromuscular and metabolic diseases, including SMA and obesity;
antibody recruiting bispecific molecules and antibody drug
conjugates for cancer. For more information, visit
www.biohaven.com.
Forward-looking Statements
This news
release includes forward-looking statements within the meaning of
the Private Securities Litigation Reform Act of 1995, including
statements about Biohaven Ltd. and our planned and ongoing clinical
trials, the timing of and the availability of data from those
trials (including full Phase 3 RESILIENT data for taldefgrobep
alfa), the timing and our decisions with our planned regulatory
filings, the timing of and our ability to obtain regulatory
approvals for our product candidates the clinical potential utility
of our product candidates, alone and as compared to other existing
potential treatment options, and the potential advancement of our
early phase programs. The use of certain words, including
"continue", "plan", "will", "believe", "may", "expect",
"anticipate" and similar expressions, is intended to identify
forward-looking statements. Investors are cautioned that any
forward-looking statements, including statements regarding the
future development, timing and potential marketing approval and
commercialization of our development candidates, are not guarantees
of future performance or results and involve substantial risks and
uncertainties. Actual results, developments and events may differ
materially from those in the forward-looking statements as a result
of various factors including: the expected timing, commencement and
outcomes of Biohaven's planned and ongoing clinical trials
(including further clinical trials for taldefgrobep alfa); the
timing of planned interactions and filings with the FDA; complying
with applicable U.S. regulatory requirements; the potential
commercialization of Biohaven's product candidates, and the
effectiveness and safety of Biohaven's product candidates.
Additional important factors to be considered in connection with
forward-looking statements are described in the Biohaven's filings
with the Securities and Exchange Commission, including within the
sections titled "Risk Factors" and "Management's Discussion and
Analysis of Financial Condition and Results of Operations". The
forward-looking statements are made as of the date of this news
release, and Biohaven does not undertake any obligation to update
any forward-looking statements, whether as a result of new
information, future events or otherwise, except as required by law.
This news release may also contain references to published
independent sources which are provided in good faith.
(1:
https://www.ncbi.nlm.nih.gov/books/NBK1352/)
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/biohaven-provides-update-on-taldefgrobep-alfa-development-program-for-spinal-muscular-atrophy-and-obesity-302314979.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/biohaven-provides-update-on-taldefgrobep-alfa-development-program-for-spinal-muscular-atrophy-and-obesity-302314979.html
SOURCE Biohaven Ltd.

