0001609550False00016095502024-01-082024-01-08
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________
FORM 8-K
_________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 8, 2024
_________________________
INSPIRE MEDICAL SYSTEMS, INC.
(Exact name of registrant as specified in its charter)
_________________________ | | | | | | | | | | | | | | |
| Delaware | | 001-38468 | | 26-1377674 |
| (State or other jurisdiction of incorporation) | | (Commission File Number) | | (I.R.S. Employer Identification No.) |
5500 Wayzata Blvd., Suite 1600
Golden Valley, Minnesota 55416
(Address of principal executive offices) (Zip Code)
(844) 672-4357
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, $0.001 par value per share | | INSP | | New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition
period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02. Results of Operations and Financial Condition.
On January 8, 2024, Inspire Medical Systems, Inc. (the “Company”) issued a press release announcing certain preliminary and unaudited results for the quarter and full year ended December 31, 2023. The full text of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 7.01. Regulation FD Disclosure.
In January of 2024, the Company will be participating in various meetings with investors and analysts, and a copy of the Company’s presentation materials being used at these meetings is furnished as Exhibit 99.2 hereto and is incorporated herein by reference. These presentation materials are also available on the Investor Relations page of the Company’s website at https://investors.inspiresleep.com.
The information in each of Item 2.02 and Item 7.01 of this Current Report on Form 8-K and in the press release attached as Exhibit 99.1 and the presentation attached as Exhibit 99.2 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| | | | | | | | |
| Exhibit No. | | Description |
| | |
| 99.1 | | |
| | |
| 99.2 | | |
| | |
| 104 | | Cover Page Interactive Data File - the cover page XBRL tags are embedded within the Inline XBRL document. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| | INSPIRE MEDICAL SYSTEMS, INC. |
| | |
| Date: | January 8, 2024 | By: | /s/ Richard J. Buchholz |
| | | Richard J. Buchholz |
| | | Chief Financial Officer |
Exhibit 99.1
Inspire Medical Systems, Inc. Announces Preliminary Results
for the Fourth Quarter and Full Year 2023 and Provides Initial 2024 Guidance
MINNEAPOLIS, Minnesota - January 8, 2024 - Inspire Medical Systems, Inc. (NYSE: INSP) (Inspire), a medical technology company focused on the development and commercialization of innovative, minimally invasive solutions for patients with obstructive sleep apnea, today announced certain preliminary, unaudited results for the fourth quarter and full year ended December 31, 2023, and provided its initial full year 2024 guidance.
Preliminary, Unaudited Fourth Quarter and Full Year 2023 Revenue and Recent Business Highlights
•Revenue for the fourth quarter of 2023 is anticipated to be in the range of $192.3 million to $192.5 million, an approximately 40% increase over the same quarter of 2022
•Revenue for full year 2023 is anticipated to be in the range of $624.6 million to $624.8 million, an approximately 53% increase over full year 2022
•Activated 78 new centers in the U.S. in the fourth quarter of 2023, bringing the total to 1,180 U.S. medical centers implanting Inspire therapy
•Created 13 new sales territories in the U.S. in the fourth quarter of 2023, bringing the total to 287 U.S. sales territories
•Published 2023 Environmental, Social, and Governance (ESG) Report
Initial Full Year 2024 Revenue Guidance
•Revenue for full year 2024 is anticipated to be in the range of $775 million to $785 million, a 24% to 26% increase over full year 2023
“We are very pleased with our strong preliminary revenue performance in the fourth quarter as the team executed exceptionally well and finished the year with significant momentum,” said Tim Herbert, President and Chief Executive Officer of Inspire Medical Systems. “Throughout 2023, we demonstrated improved operating leverage as our sales growth outpaced operating expenses. As a result, we expect to announce a profitable fourth quarter when we report our full financial results, currently planned for February 6, 2024.”
The preliminary, unaudited revenue results described in this press release are estimates only and are subject to revision until Inspire reports its full financial results for 2023 in its Annual Report on Form 10-K.
Inspire previously announced its participation in the 41st Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco, CA, including a formal company presentation at 7:30 a.m. P.T. on Monday, January 8, 2024.
About Inspire Medical Systems
Inspire is a medical technology company focused on the development and commercialization of innovative, minimally invasive solutions for patients with obstructive sleep apnea. Inspire’s proprietary Inspire therapy is the first and only FDA-approved neurostimulation technology that provides a safe and effective treatment for moderate to severe obstructive sleep apnea.
For additional information about Inspire, please visit www.inspiresleep.com.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward-looking statements, including, without limitation, statements regarding our preliminary, unaudited fourth quarter and full year results, and our expectations regarding full year 2024 financial outlook. In some cases, you can identify forward-looking statements by terms such as ‘‘may,’’ ‘‘will,’’ ‘‘should,’’ ‘‘expect,’’ ‘‘plan,’’ ‘‘anticipate,’’ ‘‘could,’’ “future,” “outlook,” “guidance,” ‘‘intend,’’ ‘‘target,’’ ‘‘project,’’ ‘‘contemplate,’’ ‘‘believe,’’ ‘‘estimate,’’ ‘‘predict,’’ ‘‘potential,’’ ‘‘continue,’’ or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words.
These forward-looking statements are based on management’s current expectations and involve known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, estimates regarding the annual total addressable market for our Inspire therapy in the U.S. and our market opportunity outside the U.S.; future results of operations, financial position, research and development costs, capital requirements and our needs for additional financing; commercial success and market acceptance of our Inspire therapy; the impact of the ongoing and global COVID-19 pandemic; general and international economic, political, and other risks, including currency and stock market fluctuations and the uncertain economic environment; our ability to achieve and maintain adequate levels of coverage or reimbursement for our Inspire system or any future products we may seek to commercialize; competitive companies and technologies in our industry; our ability to enhance our Inspire system, expand our indications and develop and commercialize additional products; our business model and strategic plans for our products, technologies and business, including our implementation thereof; our ability to accurately forecast customer demand for our Inspire system and manage our inventory; our dependence on third-party suppliers, contract manufacturers and shipping carriers; consolidation in the healthcare industry; our ability to expand, manage and maintain our direct sales and marketing organization, and to market and sell our Inspire system in markets outside of the U.S.; risks associated with international operations; our ability to manage our growth; our ability to increase the number of active medical centers implanting Inspire therapy; our ability to hire and retain our senior management and other highly qualified personnel; risk of product liability claims; risks related to information technology and cybersecurity; risk of damage to or interruptions at our facilities; our ability to commercialize or obtain regulatory approvals for our Inspire therapy and system, or the effect of delays in commercializing or obtaining regulatory approvals; FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry generally, including healthcare reform measures in the U.S. and international markets; and the timing or likelihood of regulatory filings and approvals. Other important factors that could cause actual results, performance or achievements to differ materially from those contemplated in this press release can be found under the captions “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations“ in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, as updated in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, and as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors page of our website at www.inspiresleep.com. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Thus, one should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Investor & Media Contact
Ezgi Yagci
Vice President, Investor Relations
ezgiyagci@inspiresleep.com
617-549-2443

1 Inspire Medical Systems, Inc. JP Morgan Healthcare Conference January 8, 2024 NYSE: INSP

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward- looking statements. In some cases, you can identify forward-looking statements by terms such as ‘‘may,’’ ‘‘will,’’ ‘‘should,’’ ‘‘expect,’’ ‘‘plan,’’ ‘‘anticipate,’’ ‘‘could,’’ “future,” “outlook,” ‘‘intend,’’ ‘‘target,’’ ‘‘project,’’ ‘‘contemplate,’’ ‘‘believe,’’ ‘‘estimate,’’ ‘‘predict,’’ ‘‘potential,’’ ‘‘continue,’’ or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. The forward-looking statements in this presentation relate to, among other things, statements regarding the impact of the COVID-19 pandemic on our business operations, financial results and financial condition, investments in our business, our growth strategies, our expectation that a substantial portion of postponed Inspire therapy procedures will be rescheduled, our expectations regarding the final reimbursement levels for Inspire therapy procedures, the activity of our commercial team once circumstances allow, full year 2023 financial and operational outlook, and positive insurance coverage of Inspire therapy and improvements in market access, clinical data growth, product development, indication expansion, market development, and prior authorization approvals. These forward-looking statements are based on management’s current expectations and involve known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, estimates regarding the annual total addressable market for our Inspire therapy in the U.S. and our market opportunity outside the U.S.; future results of operations, financial position, research and development costs, capital requirements and our needs for additional financing; commercial success and market acceptance of our Inspire therapy; the impact of the ongoing and global COVID-19 pandemic; general and international economic, political, and other risks, including currency and stock market fluctuations and the uncertain economic environment; our ability to achieve and maintain adequate levels of coverage or reimbursement for our Inspire system or any future products we may seek to commercialize; competitive companies and technologies in our industry; our ability to enhance our Inspire system, expand our indications and develop and commercialize additional products; our business model and strategic plans for our products, technologies and business, including our implementation thereof; our ability to accurately forecast customer demand for our Inspire system and manage our inventory; our dependence on third-party suppliers, contract manufacturers and shipping carriers; consolidation in the healthcare industry; our ability to expand, manage and maintain our direct sales and marketing organization, and to market and sell our Inspire system in markets outside of the U.S.; risks associated with international operations; our ability to manage our growth; our ability to increase the number of active medical centers implanting Inspire therapy; our ability to hire and retain our senior management and other highly qualified personnel; risk of product liability claims; risks related to information technology and cybersecurity; risk of damage to or interruptions at our facilities; our ability to commercialize or obtain regulatory approvals for our Inspire therapy and system, or the effect of delays in commercializing or obtaining regulatory approvals; FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry generally, including healthcare reform measures in the U.S. and international markets; the timing or likelihood of regulatory filings and approvals; risks related to our debt and capital structure; our ability to establish and maintain intellectual property protection for our Inspire therapy and system or avoid claims of infringement; tax risks; risks that we may be deemed an investment company under the Investment Company Act of 1940; regulatory risks; the volatility of the trading price of our common stock; and our expectations about market trends. Other important factors that could cause actual results, performance or achievements to differ materially from those contemplated in this presentation can be found under the captions “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2022, as updated in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, and as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors page of our website at www.inspiresleep.com. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Thus, one should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. We do not intend our use or display of other parties' trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties. 2

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL JP Morgan Conference Agenda • Inspire 2023 Year-in-Review • Preliminary, unaudited FY23 Revenue and Initial FY24 Revenue Guidance • Product Pipeline • Education and Awareness • Mechanism of Action and GLP-1s • Reimbursement • Our Growth Strategy 3

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire 2023 Year in Review *video 1
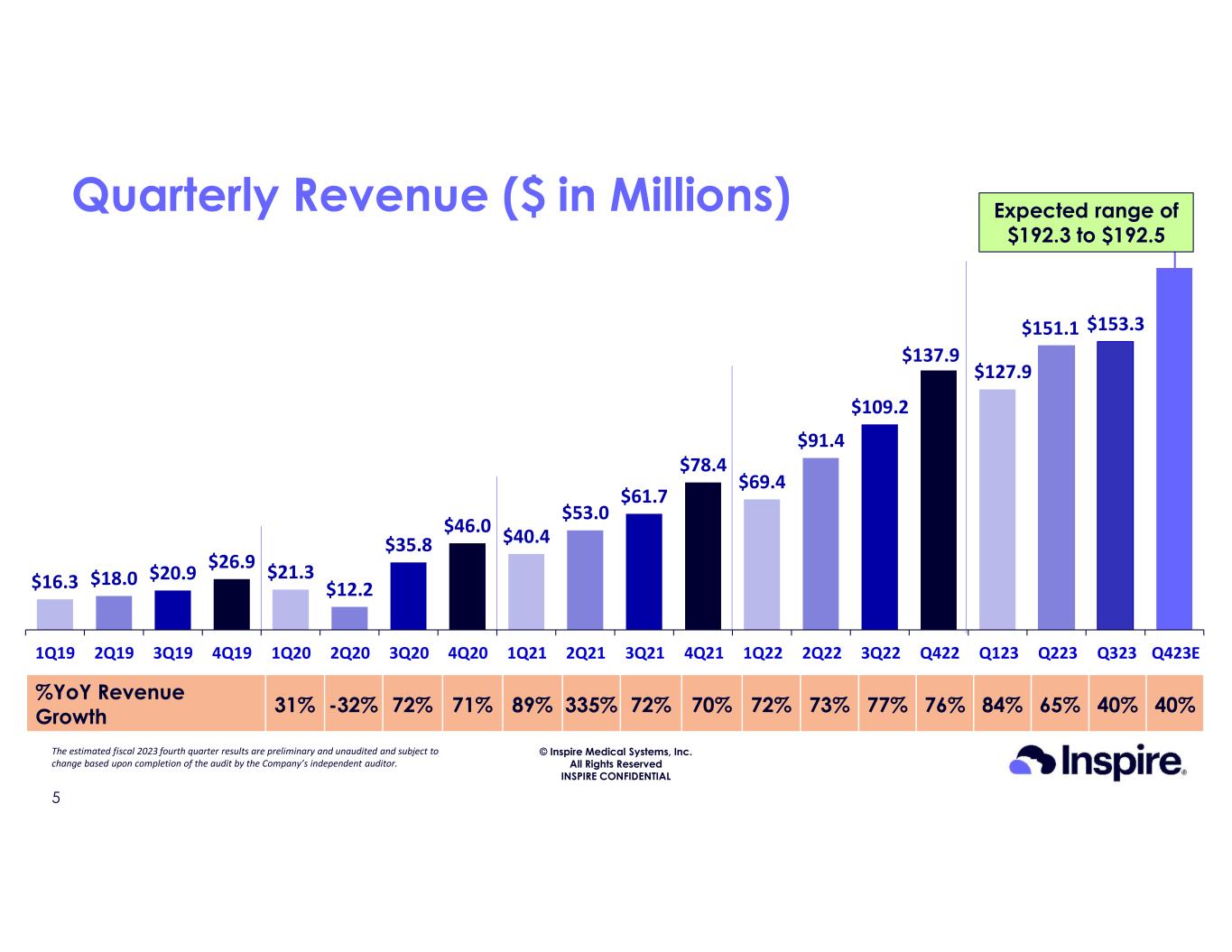
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL $16.3 $18.0 $20.9 $26.9 $21.3 $12.2 $35.8 $46.0 $40.4 $53.0 $61.7 $78.4 $69.4 $91.4 $109.2 $127.9 $151.1 $153.3 1Q19 2Q19 3Q19 4Q19 1Q20 2Q20 3Q20 4Q20 1Q21 2Q21 3Q21 4Q21 1Q22 2Q22 3Q22 Q422 Q123 Q223 Q323 Q423E Quarterly Revenue ($ in Millions) 40%40%65%84%76%77%73%72%70%72%335%89%71%72%-32%31% %YoY Revenue Growth $137.9 5 $192.4E The estimated fiscal 2023 fourth quarter results are preliminary and unaudited and subject to change based upon completion of the audit by the Company’s independent auditor. Expected range of $192.3 to $192.5
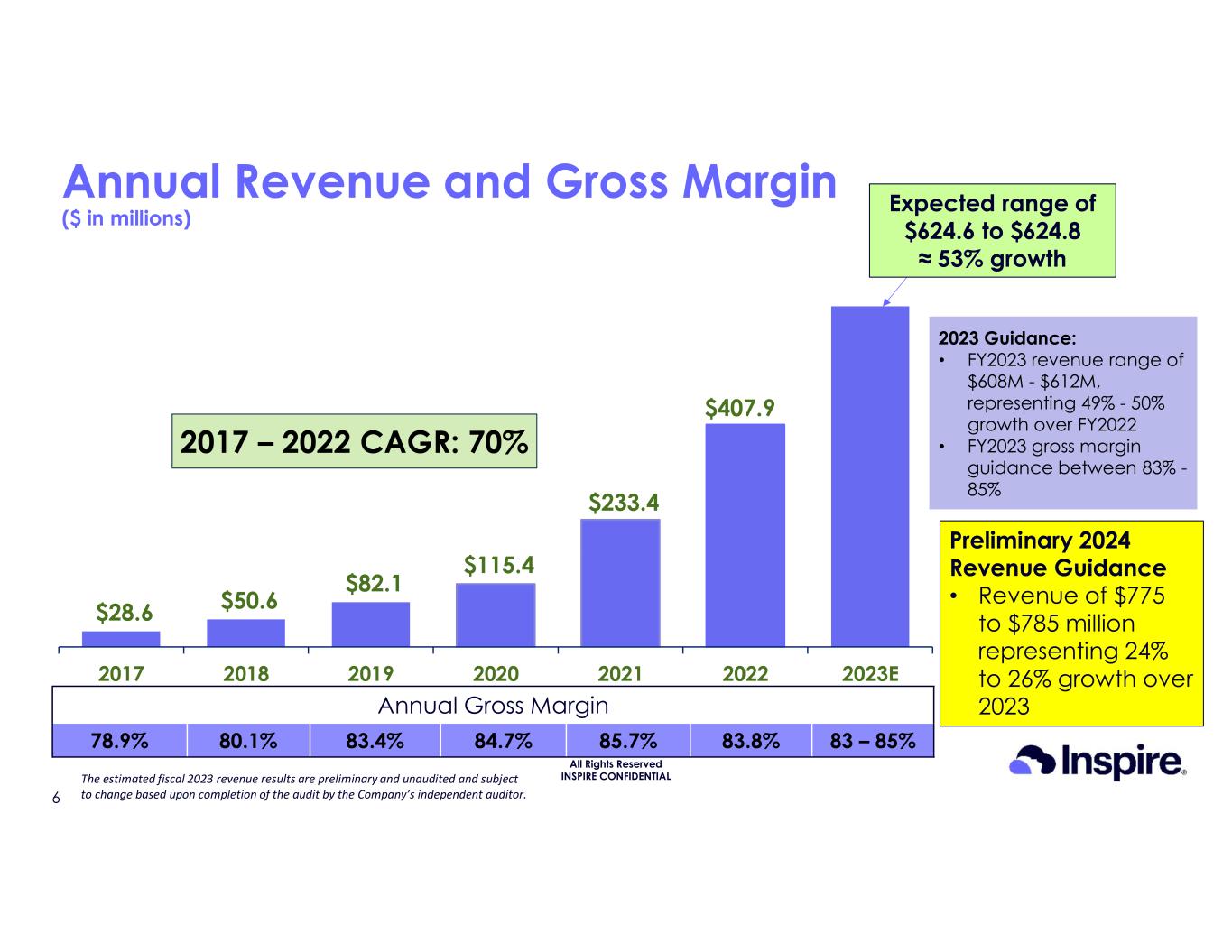
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL $28.6 $50.6 $82.1 $115.4 $233.4 $624.7 2017 2018 2019 2020 2021 2022 2023E 2017 – 2022 CAGR: 70% Annual Revenue and Gross Margin ($ in millions) Annual Gross Margin 83 – 85%83.8%85.7%84.7%83.4%80.1%78.9% 2023 Guidance: • FY2023 revenue range of $608M - $612M, representing 49% - 50% growth over FY2022 • FY2023 gross margin guidance between 83% - 85% $407.9 6 Preliminary 2024 Revenue Guidance • Revenue of $775 to $785 million representing 24% to 26% growth over 2023 The estimated fiscal 2023 revenue results are preliminary and unaudited and subject to change based upon completion of the audit by the Company’s independent auditor. Expected range of $624.6 to $624.8 ≈ 53% growth
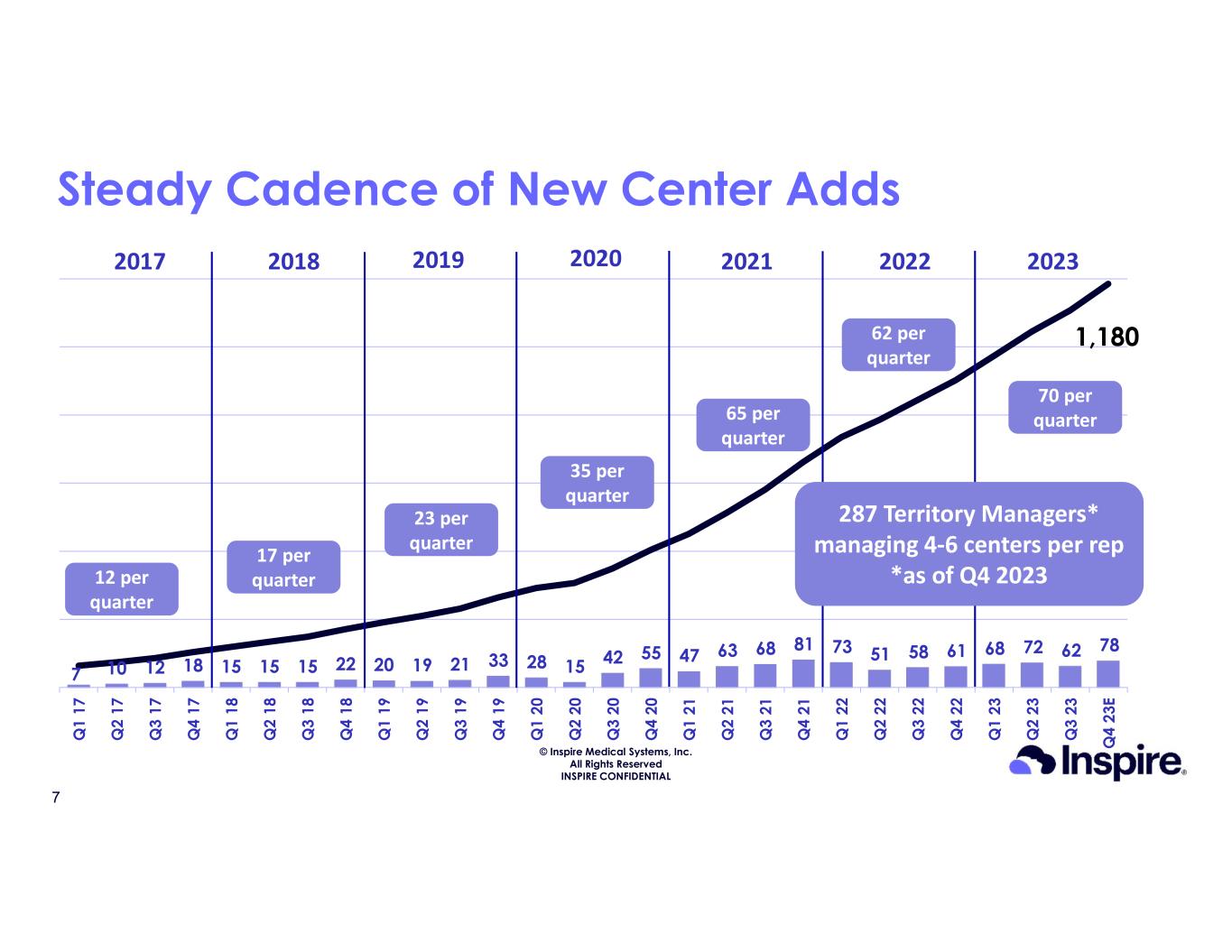
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL 7 10 12 18 15 15 15 22 20 19 21 33 28 15 42 55 47 63 68 81 73 51 58 61 68 72 62 78 Q 1 17 Q 2 17 Q 3 17 Q 4 17 Q 1 18 Q 2 18 Q 3 18 Q 4 18 Q 1 19 Q 2 19 Q 3 19 Q 4 19 Q 1 20 Q 2 20 Q 3 20 Q 4 20 Q 1 21 Q 2 21 Q 3 21 Q 4 21 Q 1 22 Q 2 22 Q 3 22 Q 4 22 Q 1 23 Q 2 23 Q 3 23 Q 4 23 E 1,180 Steady Cadence of New Center Adds 2017 2018 2019 2020 12 per quarter 17 per quarter 23 per quarter 35 per quarter 2021 65 per quarter 2022 287 Territory Managers* managing 4-6 centers per rep *as of Q4 2023 62 per quarter 2023 70 per quarter 7

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire Therapy is a Proven Solution for Patients with OSA Inspire System 1 2 3 Three implanted devices and patient remote: Pressure sensing lead: detects when the patient is attempting to breathe Neurostimulator: houses the electronics and battery power for the device Stimulation lead: delivers electrical stimulation to the hypoglossal nerve Inspire Procedure Typically, a 60-90 min outpatient procedure Requires two small incisions Patients typically recover quickly and resume normal activities in just a few days Patient turns on the device each night with the remote control before going to sleep 2 1 Hypoglossal Nerve Neurostimulator Stimulation Lead Pressure Sensing Lead 3 Inspire Algorithm Active while patients sleep Closed-loop system which senses breathing patterns Inspire system turned on Airflow Breathing Oxygen Saturation No OSA eventsOSA events Provides stimulation during inspiratory phase of respiration maintaining open airway Over 60,000 Inspire procedures completed to date 8 Inspire V Neurostimulator in final qual and follow-up response to FDA early in 2024 • Incorporates sensing function • Provides software-based platform for future feature additions
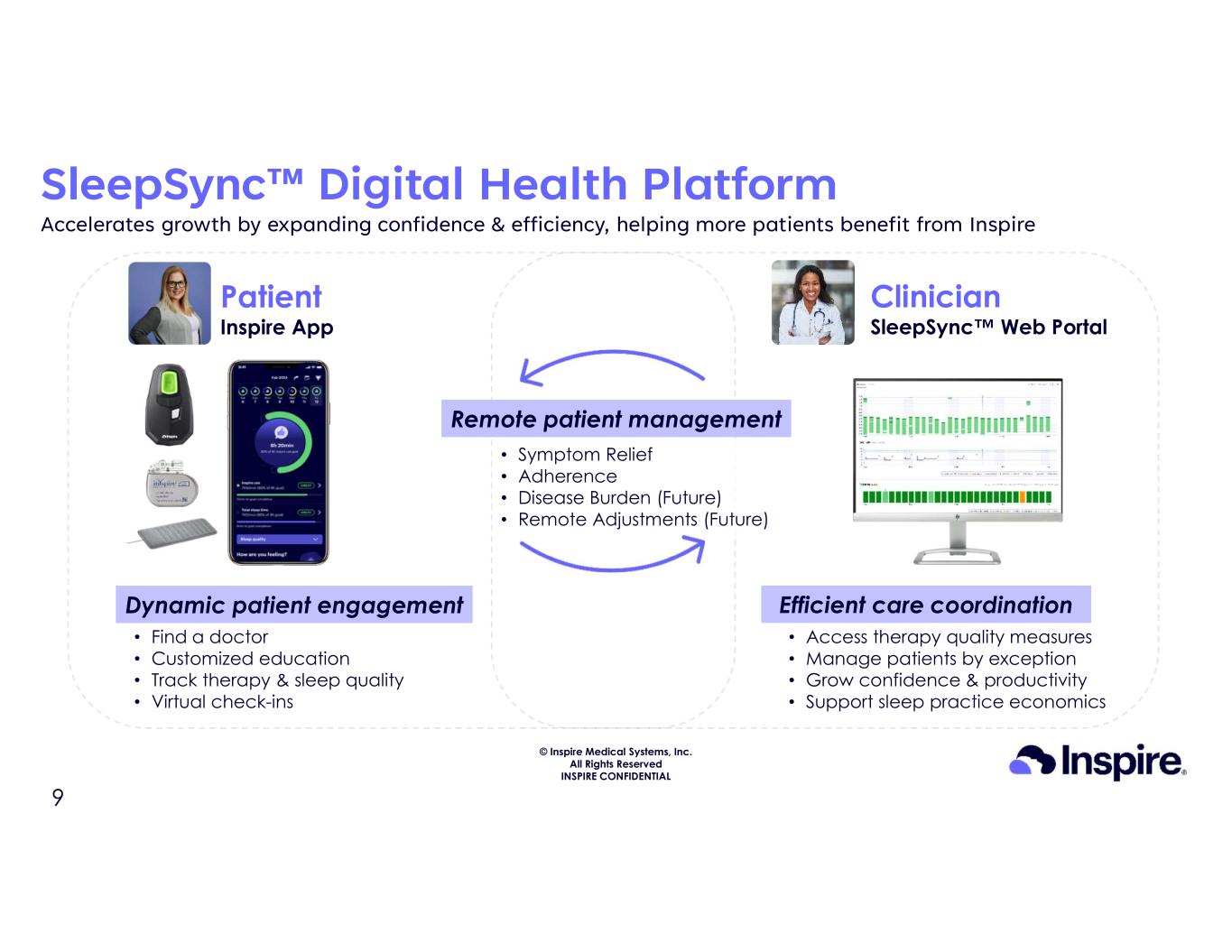
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL SleepSync™ Digital Health Platform Accelerates growth by expanding confidence & efficiency, helping more patients benefit from Inspire Patient Inspire App Clinician SleepSync™ Web Portal Remote patient management Dynamic patient engagement Efficient care coordination • Find a doctor • Customized education • Track therapy & sleep quality • Virtual check-ins • Access therapy quality measures • Manage patients by exception • Grow confidence & productivity • Support sleep practice economics • Symptom Relief • Adherence • Disease Burden (Future) • Remote Adjustments (Future) 9
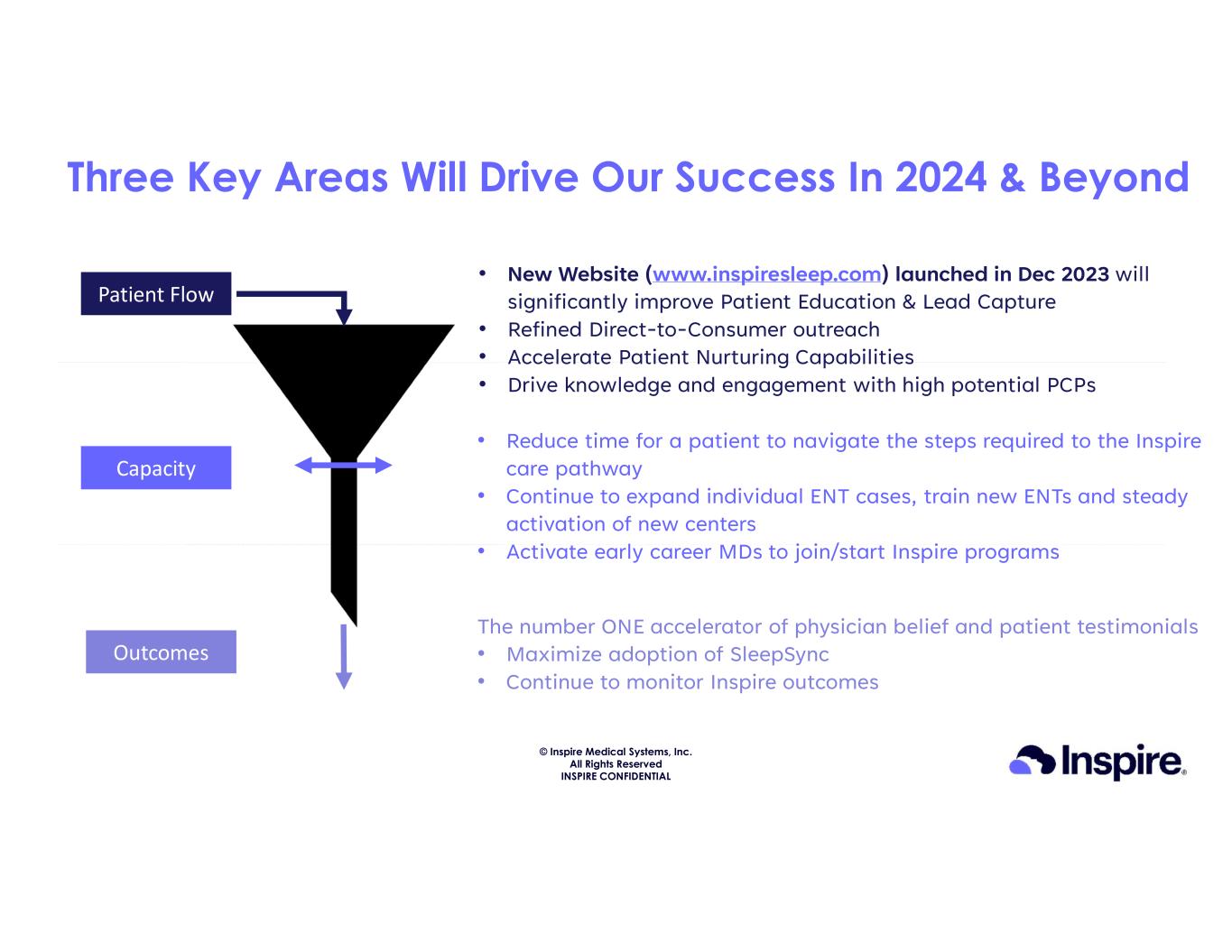
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Three Key Areas Will Drive Our Success In 2024 & Beyond 10 Patient Flow Capacity Outcomes • New Website (www.inspiresleep.com) launched in Dec 2023 will significantly improve Patient Education & Lead Capture • Refined Direct-to-Consumer outreach • Accelerate Patient Nurturing Capabilities • Drive knowledge and engagement with high potential PCPs • Reduce time for a patient to navigate the steps required to the Inspire care pathway • Continue to expand individual ENT cases, train new ENTs and steady activation of new centers • Activate early career MDs to join/start Inspire programs The number ONE accelerator of physician belief and patient testimonials • Maximize adoption of SleepSync • Continue to monitor Inspire outcomes

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL *video 2 Patient Outreach Program
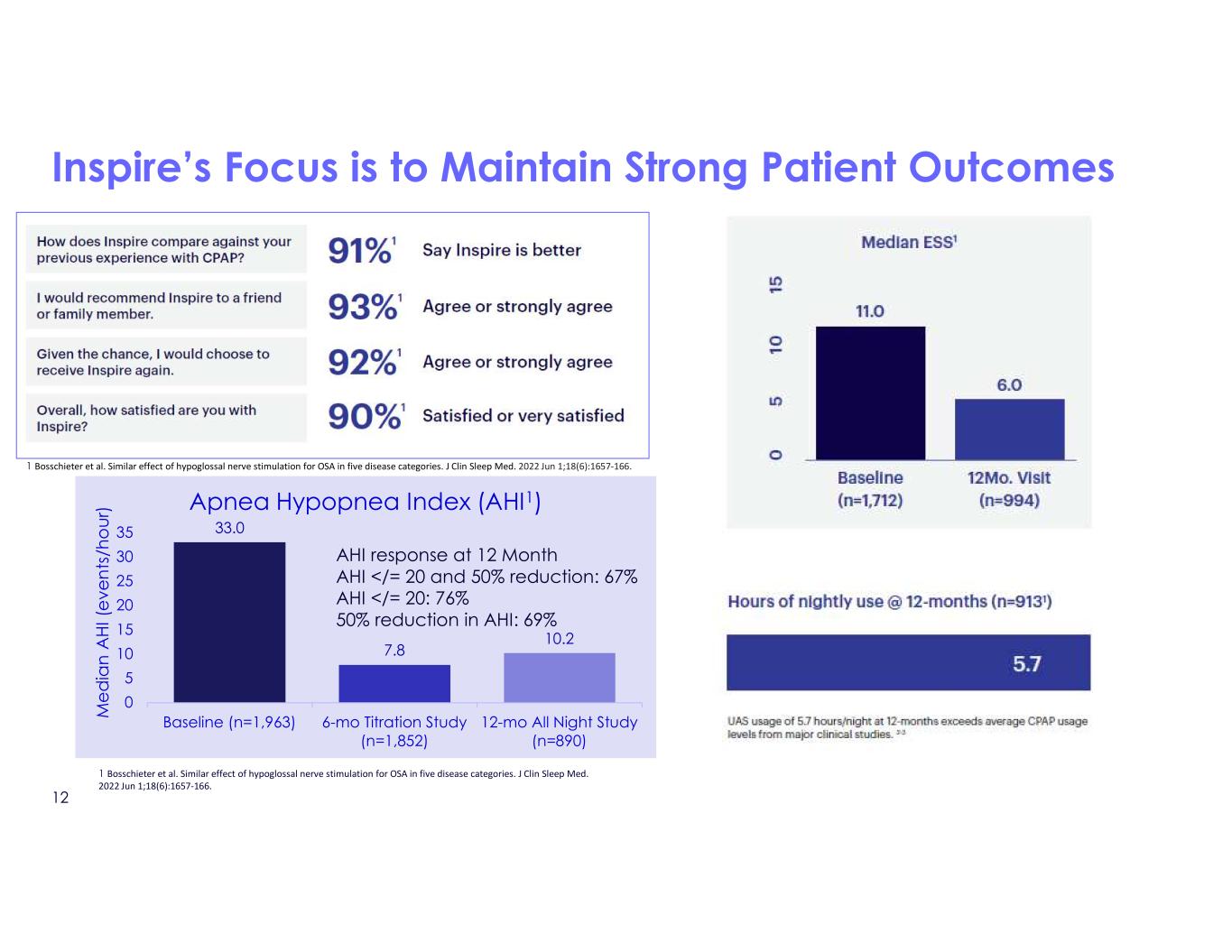
Inspire’s Focus is to Maintain Strong Patient Outcomes 33.0 7.8 10.2 0 5 10 15 20 25 30 35 Baseline (n=1,963) 6-mo Titration Study (n=1,852) 12-mo All Night Study (n=890) M e d ia n A H I ( e ve n ts /h o u r) Apnea Hypopnea Index (AHI1) AHI response at 12 Month AHI </= 20 and 50% reduction: 67% AHI </= 20: 76% 50% reduction in AHI: 69% 1 Bosschieter et al. Similar effect of hypoglossal nerve stimulation for OSA in five disease categories. J Clin Sleep Med. 2022 Jun 1;18(6):1657-166. 1 Bosschieter et al. Similar effect of hypoglossal nerve stimulation for OSA in five disease categories. J Clin Sleep Med. 2022 Jun 1;18(6):1657-166. 12
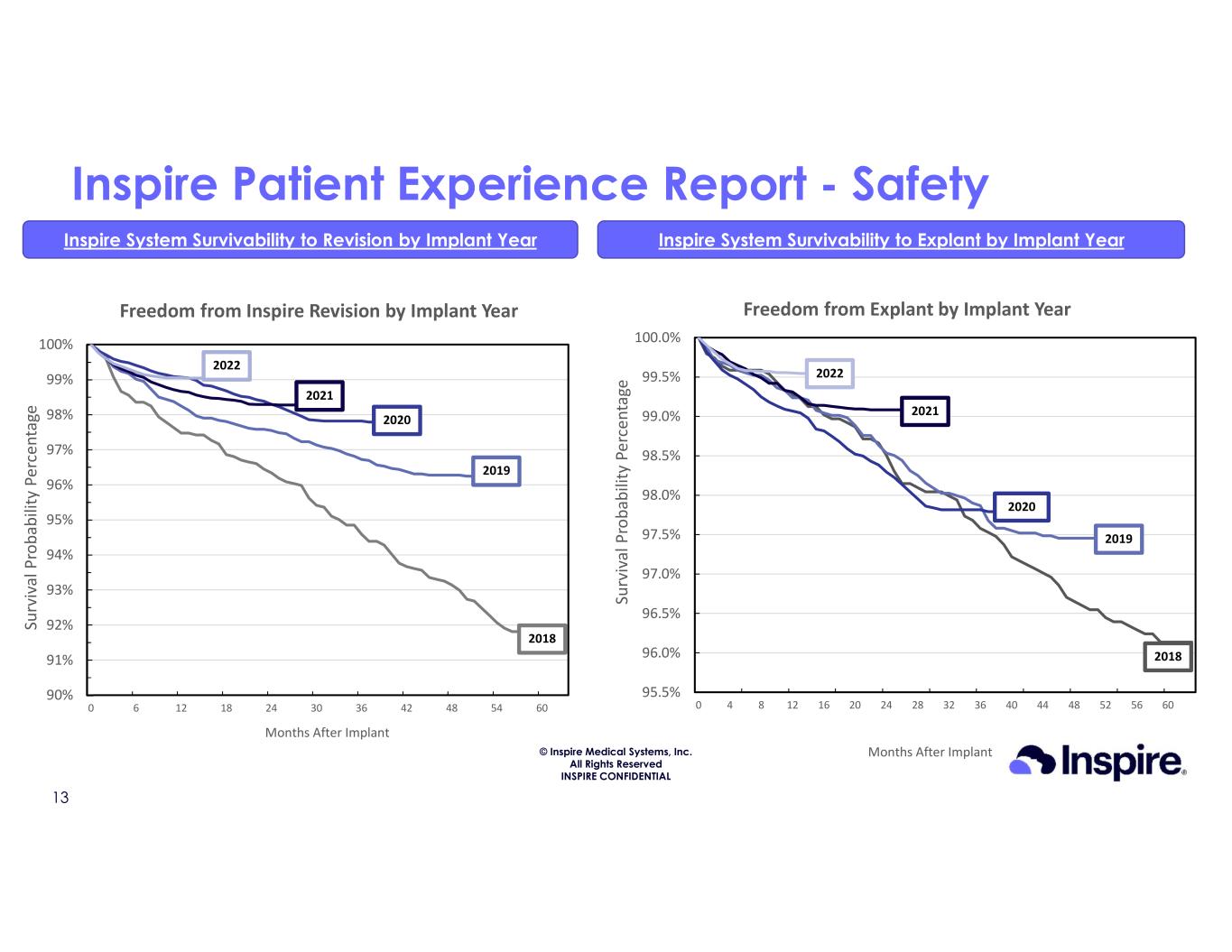
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire Patient Experience Report - Safety Inspire System Survivability to Revision by Implant Year Inspire System Survivability to Explant by Implant Year 13 90% 91% 92% 93% 94% 95% 96% 97% 98% 99% 100% 0 6 12 18 24 30 36 42 48 54 60 Su rv iv al P ro ba bi lit y Pe rc en ta ge Months After Implant Freedom from Inspire Revision by Implant Year 2018 2020 2019 2021 2022 95.5% 96.0% 96.5% 97.0% 97.5% 98.0% 98.5% 99.0% 99.5% 100.0% 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 Su rv iv al P ro ba bi lit y Pe rc en ta ge Months After Implant Freedom from Explant by Implant Year 2018 2020 2019 2021 2022
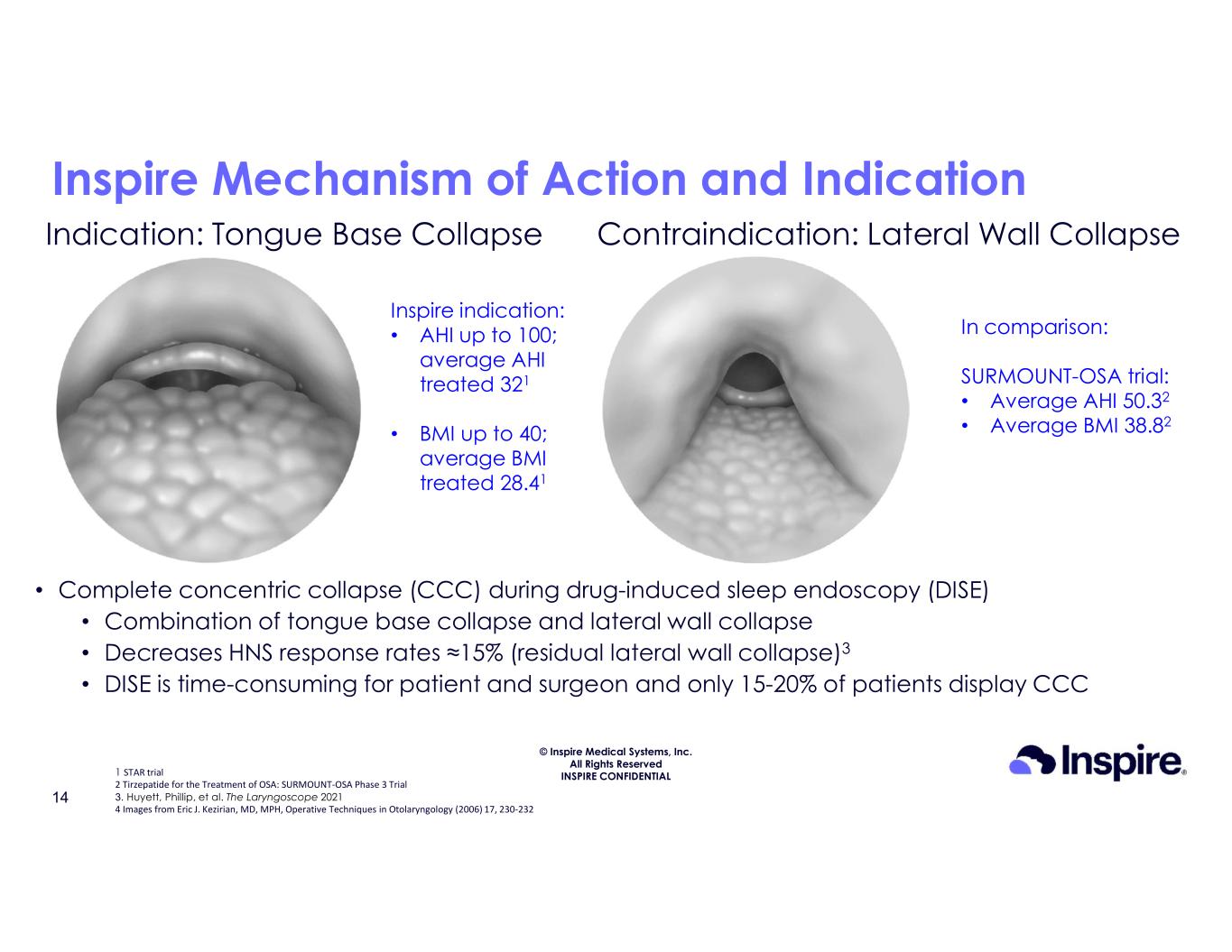
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Inspire Mechanism of Action and Indication Indication: Tongue Base Collapse Contraindication: Lateral Wall Collapse Inspire indication: • AHI up to 100; average AHI treated 321 • BMI up to 40; average BMI treated 28.41 In comparison: SURMOUNT-OSA trial: • Average AHI 50.32 • Average BMI 38.82 14 1 STAR trial 2 Tirzepatide for the Treatment of OSA: SURMOUNT-OSA Phase 3 Trial 3. Huyett, Phillip, et al. The Laryngoscope 2021 4 Images from Eric J. Kezirian, MD, MPH, Operative Techniques in Otolaryngology (2006) 17, 230-232 • Complete concentric collapse (CCC) during drug-induced sleep endoscopy (DISE) • Combination of tongue base collapse and lateral wall collapse • Decreases HNS response rates ≈15% (residual lateral wall collapse)3 • DISE is time-consuming for patient and surgeon and only 15-20% of patients display CCC
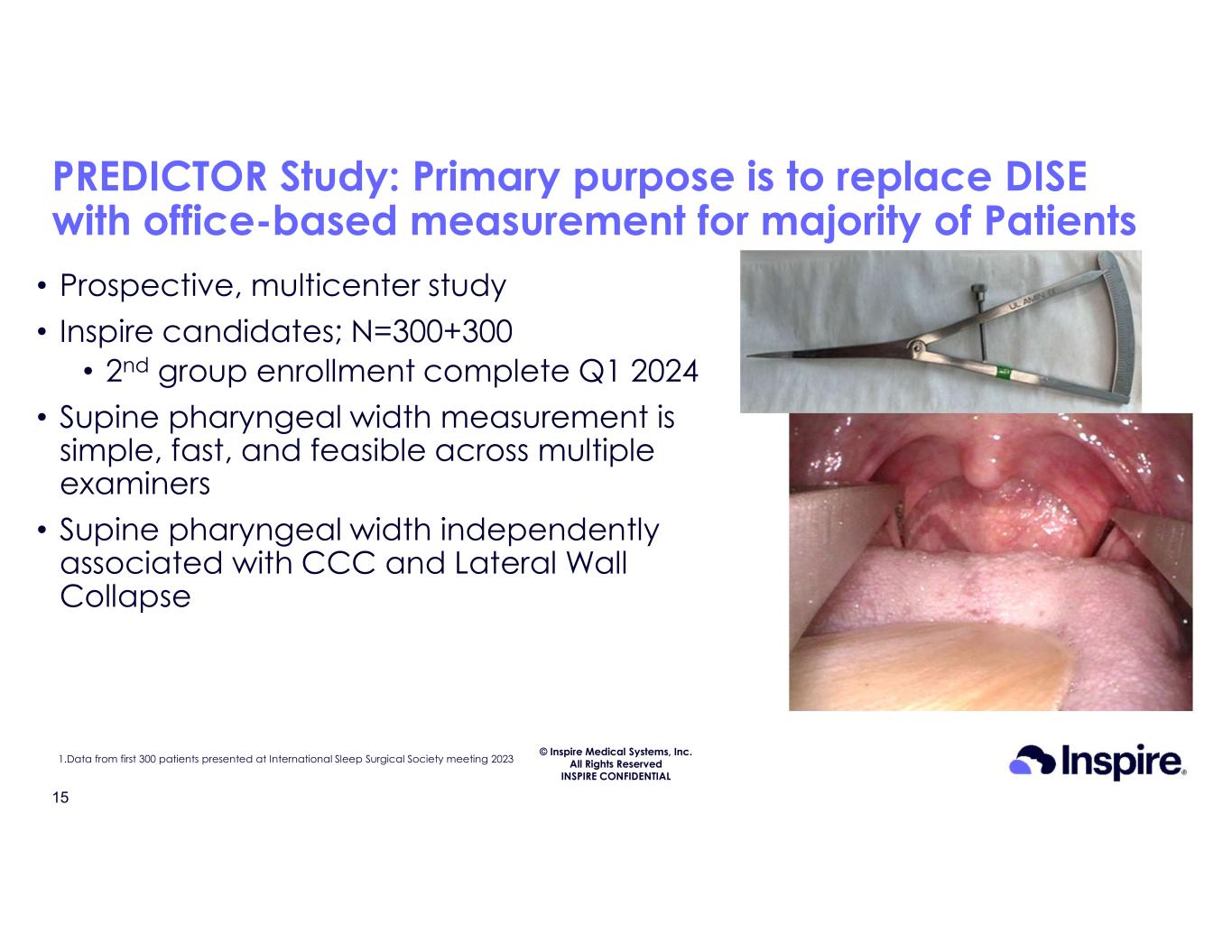
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL PREDICTOR Study: Primary purpose is to replace DISE with office-based measurement for majority of Patients 15 • Prospective, multicenter study • Inspire candidates; N=300+300 • 2nd group enrollment complete Q1 2024 • Supine pharyngeal width measurement is simple, fast, and feasible across multiple examiners • Supine pharyngeal width independently associated with CCC and Lateral Wall Collapse 1.Data from first 300 patients presented at International Sleep Surgical Society meeting 2023
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL High BMI and PREDICTOR Study: Lateral Wall Collapse (LWC) and Complete Concentric Collapse (CCC) • Independent variables significant in univariate analysis in first 300 patients showed: • Each unit increase in BMI 14% decrease in the odds of absence of LWC1 • Each unit increase in BMI 13% decrease in the odds of absence of CCC1 • Higher BMI associated with increased Lateral Wall Collapse and therefore additional Complete Concentric Collapse • PREDICTOR data being provided to insurance companies for incorporation into policies • GLP-1s – Reduction in BMI expected to be associated with lower LWC, reducing CCC and enabling patients to qualify for Inspire • Limited data show weight loss in lower BMI patients will resolve OSA • Recent small GLP-1 study showed strong weight loss but minimal reduction in AHI compared with arm using CPAP which demonstrated statistically significant reduction in AHI2 1.Data from first 300 patients presented at International Sleep Surgical Society meeting 2023 2.O’Donnell et al, CPAP but Not GLP1-mediated Weight Loss Improves Early Cardiovascular Disease in Obstructive Sleep Apnea: A Randomized Proof-of- Concept StudyAnn Am Thorac Soc. 2023 Dec 14. doi: 10.1513/AnnalsATS.202309-821OC. Online ahead of print

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Policy Reviews with Inspire Indication Expansions • Several payers have already reviewed and updated policies for: • AHI increase to 100 from 65; BMI warning to 40 from 32; & Pediatrics with Down syndrome • Alternative evaluations instead of DISE, such as supine pharyngeal width measurement • United Healthcare • Incorporated all revised elements including high AHI, high BMI and pediatric Down syndrome • New policy does require additional documentation for Oral Appliance therapy • Documentation for Oral Appliance therapy has been an element in prior authorizations to United Healthcare for some time and is not necessarily a new requirement • New policy describes documentation as: • Treatments tried, failed, or contraindicated; include the dates, duration of treatment, and reason for discontinuation, including: • Failed trial, intolerance, or refusal of positive airway pressure (PAP) treatments • Failed trial, intolerance, or inappropriate patient anatomy for Oral Appliance therapy • Each payer has specific documentation requirements, and the Inspire Prior Authorization team closely tracks updates and ensures conformity to policy

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Our Growth Strategy Through planned and controlled market expansion and robust physician training Ensure Strong Clinical Outcomes 1 By enhancing interconnectivity, simplifying the care pathway, and closely tracking outcomes Improve the Customer Experience 2 Amongst patients, ENT/Sleep physicians, and general practitioners Promote Widespread Consumer Awareness 3 Commensurate with new center additions and leveraging consumer outreach programs Drive Continued Commercial Scale 4 Driving breakthrough technology innovation and expanded indications Invest in Research & Development 5 Further penetrating existing markets and entering into new geographical locations Facilitate International Market Expansion 6 18

™

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Appendix 20

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Obstructive Sleep Apnea is a Serious and Chronic Disease OSA is Caused by a Blocked or Partially Blocked Airway • Blockage prevents airflow to the lungs • Results in repeated arousals and oxygen de-saturations • Severity of sleep apnea is measured by frequency of apnea or hypopnea events per hour, which is referred to as the Apnea-Hypopnea Index (AHI) Airway obstruction during breathing Normal range: AHI < 5 events per hour Mild sleep apnea: 5 ≤ AHI < 15 events per hour Moderate sleep apnea: 15 ≤ AHI < 30 events per hour Severe sleep apnea: AHI ≥ 30 events per hour Most Patients Are Unaware of Their Condition… …and Untreated OSA Multiplies Serious Health Risks • High risk patients: obese, male or of advanced age • Common first indicator: heavy snoring • Other indicators: • Lack of energy • Headaches • Depression • Nighttime gasping • Dry mouth • Memory or concentration problems • Excessive daytime sleepiness Source: Company Website 1. Redline et al, The Sleep Heart Health Study. Am J Res and Crit Care Med 2010. 2. Gami et al, J Am Coll Cardiol 2013. 3. Young et al, J Sleep 2008. 2x The risk for stroke1 2x The risk for sudden cardiac death2 57% Increased risk for recurrence of Atrial Fibrillation after ablation4 Years of Follow-up % S ur vi vi ng Increased Risk of Mortality 5 4. Li et al, Europace 2014. 5. Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure from SHHS and Wisconsin Sleep Cohort Study. 5x The risk for cardiovascular mortality3 21
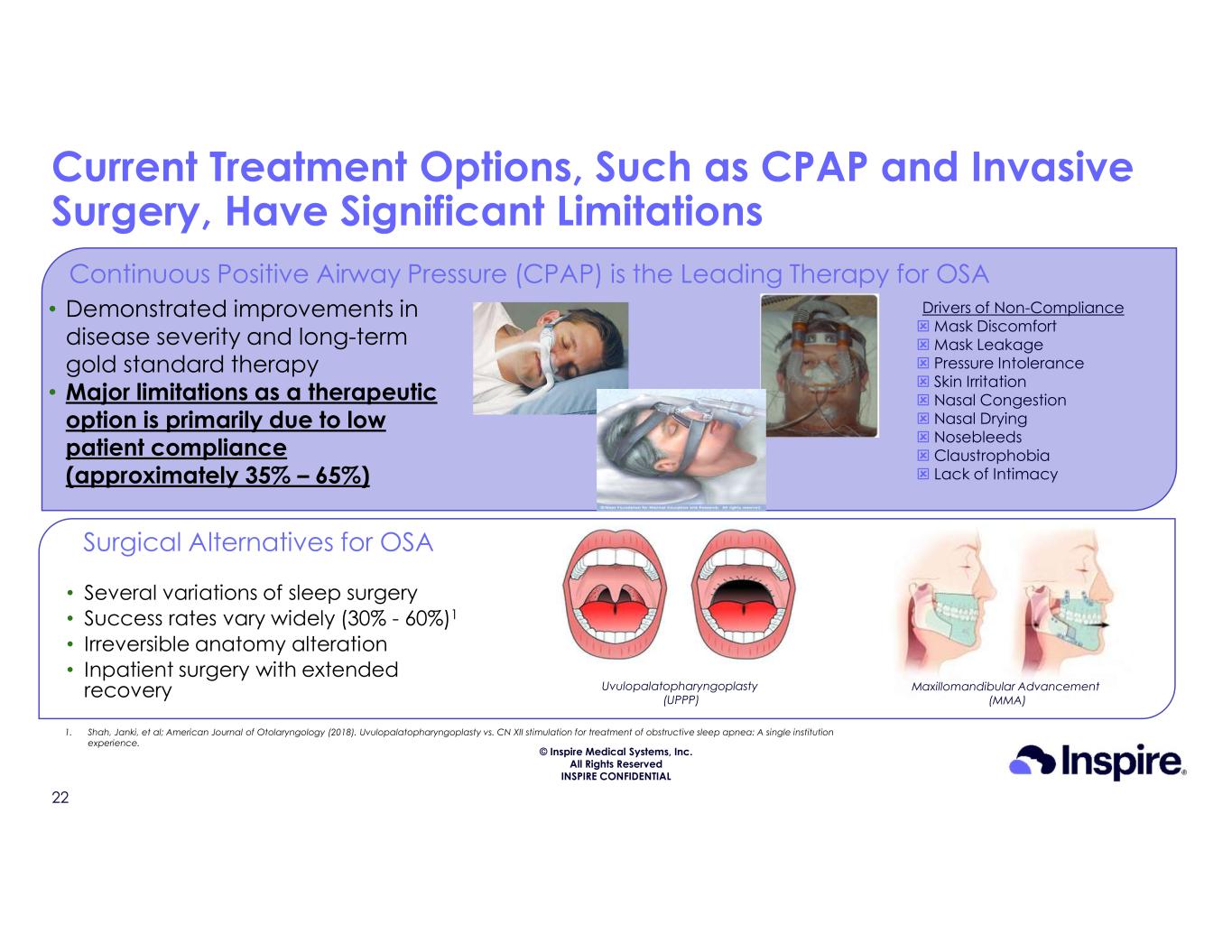
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Current Treatment Options, Such as CPAP and Invasive Surgery, Have Significant Limitations Ina Uvulopalatopharyngoplasty (UPPP) Maxillomandibular Advancement (MMA) • Several variations of sleep surgery • Success rates vary widely (30% - 60%)1 • Irreversible anatomy alteration • Inpatient surgery with extended recovery Surgical Alternatives for OSA Ina Continuous Positive Airway Pressure (CPAP) is the Leading Therapy for OSA 1. Shah, Janki, et al; American Journal of Otolaryngology (2018). Uvulopalatopharyngoplasty vs. CN XII stimulation for treatment of obstructive sleep apnea: A single institution experience. Drivers of Non-Compliance Mask Discomfort Mask Leakage Pressure Intolerance Skin Irritation Nasal Congestion Nasal Drying Nosebleeds Claustrophobia Lack of Intimacy • Demonstrated improvements in disease severity and long-term gold standard therapy • Major limitations as a therapeutic option is primarily due to low patient compliance (approximately 35% – 65%) 22

Company Overview • Inspire is the first and only FDA-approved neurostimulation technology for Obstructive Sleep Apnea (OSA) with significant first-mover advantage • Innovative, closed-loop, primary cell solution with ~11-year battery life • ~90-minute, safe, convenient, minimally invasive outpatient procedure • Full-body MRI compatibility • Over 60,000 patients treated with Inspire therapy • ~$10 billion annual U.S. market opportunity • Significant body of clinical evidence involving thousands of patients across more than 90 studies • Established reimbursement with 260 million U.S. covered lives including commercial payers, Medicare coverage in all 50 states, and Veterans Affairs • Proven management team leading 1,000+ employees • Strong financial profile with 83-85% gross margin • Well-capitalized balance sheet with $467 million in net cash (as of September 30, 2023) Our History & Key Milestones 1990s: Medtronic (MDT) begins early work on the development of Inspire 2001: Initial clinical results published by MDT 2007: Inspire is founded after being spun-out of MDT 2011: Initiated Phase III pivotal STAR trial; CE mark received in Europe 2014: STAR results published in the New England Journal of Medicine; received PMA approval from the FDA 2015: 18-month STAR data published; revenues of $8.0M 2016: 1,000th implant milestone; revenues of $16.4M 2017: Launched Inspire IV neurostimulator in U.S.; announced 5-year STAR results; 2,000th implant; revenues of $28.6M 2018: Inspire IV CE mark; 5-year STAR results publication; IPO on NYSE; Aetna begins covering the Inspire therapy; revenues of $50.6M 2019: 7,500th patient receives Inspire therapy; many BCBS plans and other large insurers write positive coverage; revenues of $82.1M 2020: Medicare coverage in all 50 states; FDA approved age range expansion of 18 to 21; Inspire Sleep app released; 10,000th implant; revenues of $115.4M 2021: Anthem policy issued; FDA approved 2-incision approach and Bluetooth® remote; 20,000th implant; revenues of $230.4M 2022: First implants in Japan, Singapore, and the U.K.; strategic investments in digital health platform capabilities; FDA approved full-body MRI compatibility; 36,000th implant; revenues of $407.9M 2023E: Expanded AHI, BMI and pediatric Down syndrome indications; first implants in Hong Kong; reimbursement in Belgium; 60,000th implant; expected revenues of $624.6M to $624.8M 2024E: SleepSync™ physician programmer expected to launch in the U.S.; expected FDA approval of Inspire V; expected reimbursement in France 23

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Randy Ban Chief Commercial Officer Bryan Phillips SVP, General Counsel & Chief Compliance Officer Tim Herbert President, CEO & Founder Strong Management Team Ezgi Yagci Vice President, Investor Relations Rick Buchholz Chief Financial Officer John Rondoni Chief Technology Officer Kathy Sherwood Sr. Vice President, Market Access Phil Ebeling Chief Operating Officer Steve Jandrich Vice President, Human Resources Carlton Weatherby Chief Strategy Officer Dr. Charisse Sparks Chief Medical Officer 24
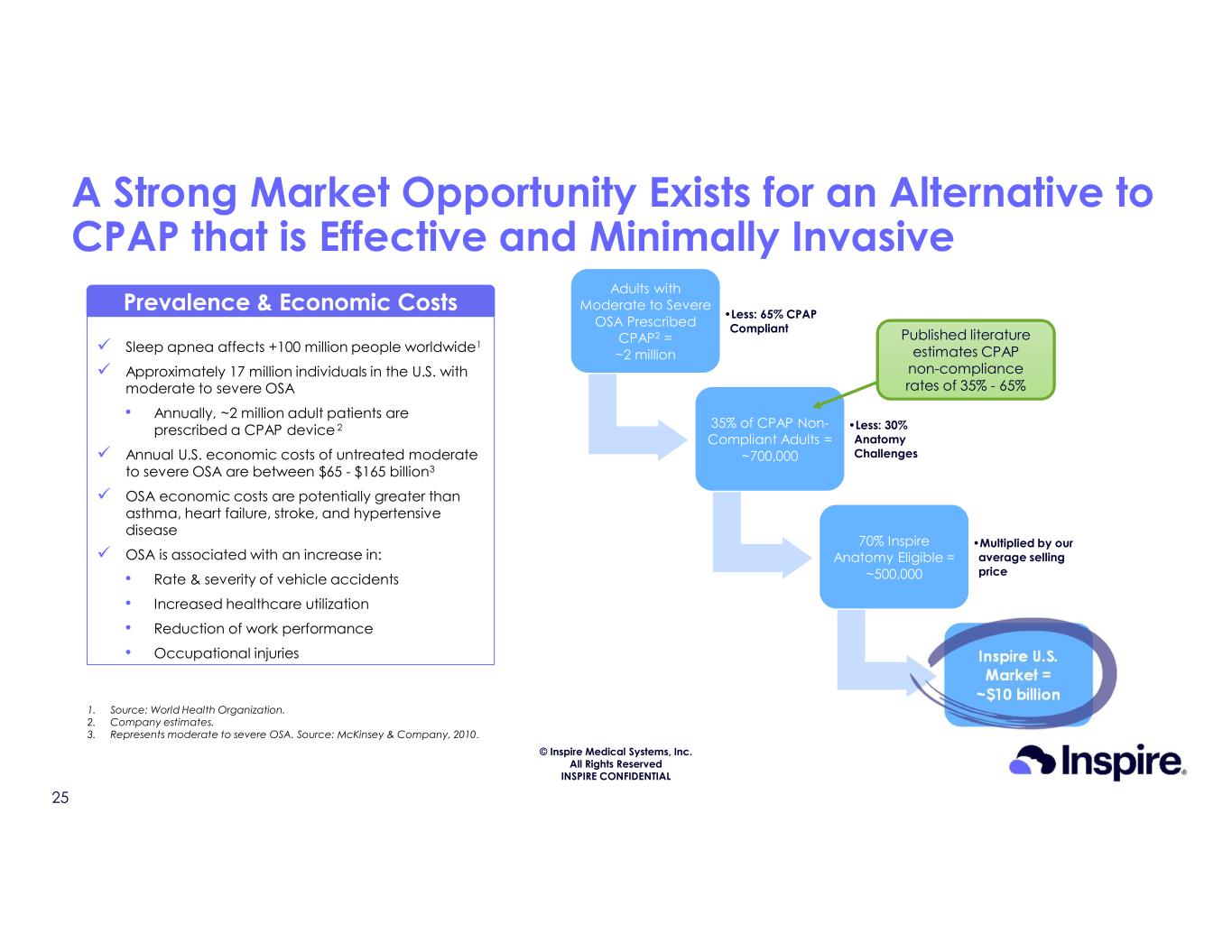
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL A Strong Market Opportunity Exists for an Alternative to CPAP that is Effective and Minimally Invasive Sleep apnea affects +100 million people worldwide1 Approximately 17 million individuals in the U.S. with moderate to severe OSA • Annually, ~2 million adult patients are prescribed a CPAP device 2 Annual U.S. economic costs of untreated moderate to severe OSA are between $65 - $165 billion3 OSA economic costs are potentially greater than asthma, heart failure, stroke, and hypertensive disease OSA is associated with an increase in: • Rate & severity of vehicle accidents • Increased healthcare utilization • Reduction of work performance • Occupational injuries Prevalence & Economic Costs 1. Source: World Health Organization. 2. Company estimates. 3. Represents moderate to severe OSA. Source: McKinsey & Company, 2010. Adults with Moderate to Severe OSA Prescribed CPAP2 = ~2 million •Less: 65% CPAP Compliant 35% of CPAP Non- Compliant Adults = ~700,000 •Less: 30% Anatomy Challenges 70% Inspire Anatomy Eligible = ~500,000 •Multiplied by our average selling price Inspire U.S. Market = ~$10 billion Published literature estimates CPAP non-compliance rates of 35% - 65% 25

© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL Continuous Data Collection and Outcomes Monitoring • Collect real-world, international outcomes data • Eligibility – ALL patients receiving Inspire therapy • ADHERE Registry - 5,000 enrollments at 62 medical centers (enrollment complete) • Transition to ADHERE 2.0 as part of Inspire SleepSync™ in the U.S. AHI = Apnea Hypopnea Index ESS = Epworth Sleepiness Scale Post Market Surveillance Data Real World Data Proactive Data Reactive Data Data Analysis for Signals 26
© Inspire Medical Systems, Inc. All Rights Reserved INSPIRE CONFIDENTIAL KEEPING CONTROL WHILE GROWING FAST Focus on the rapid scaling of commercialization (implanting centers and sales reps) while ensuring proper training to maintain control of high-quality therapy outcomes The Great Balancing Act Patient Outcomes Revenue Growth 27
v3.23.4
Document and Entity Information
|
Jan. 08, 2024 |
| Cover [Abstract] |
|
| Entity Registrant Name |
INSPIRE MEDICAL SYSTEMS, INC.
|
| Entity Central Index Key |
0001609550
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 08, 2024
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-38468
|
| Entity Tax Identification Number |
26-1377674
|
| Entity Address, Address Line One |
5500 Wayzata Blvd.
|
| Entity Address, Address Line Two |
Suite 1600
|
| Entity Address, City or Town |
Golden Valley
|
| Entity Address, State or Province |
MN
|
| Entity Address, Postal Zip Code |
55416
|
| City Area Code |
844
|
| Local Phone Number |
672-4357
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 par value per share
|
| Trading Symbol |
INSP
|
| Security Exchange Name |
NYSE
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Inspire Medical Systems (NYSE:INSP)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
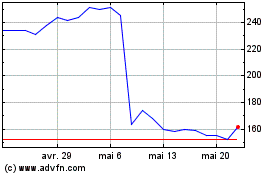
Inspire Medical Systems (NYSE:INSP)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024