Nuvation Bio Gets New Drug Application Clearance From FDA for Solid Tumor Treatment
08 Janvier 2024 - 10:58PM
Dow Jones News
By Dean Seal
Nuvation Bio said federal regulators have cleared an
investigational new drug application for its treatment of advanced
solid tumors, NUV-1511.
The biopharmaceutical company said Monday that the Food and Drug
Administration's clearance makes NUV-1511 the first clinical
candidate from the company's novel drug-drug conjugate
platform.
"This IND clearance expands our clinical pipeline and validates
the approach of our proprietary DDC platform to design potent
oncology-focused chimeric small molecules which combine
tumor-targeting specificity with the anti-cancer activity of known
oncology agents," Chief Executive David Hung said.
The company said it expects to launch a Phase 1/2 study of
NUV-1511 in the first half of this year.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
January 08, 2024 16:43 ET (21:43 GMT)
Copyright (c) 2024 Dow Jones & Company, Inc.
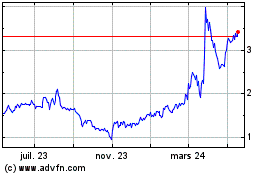
Panacea Acquisition (NYSE:NUVB)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
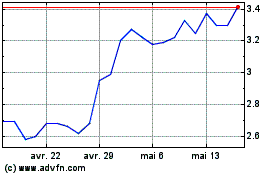
Panacea Acquisition (NYSE:NUVB)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024