false
0001821825
0001821825
2025-02-13
2025-02-13
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date
of earliest event reported): February 13, 2025
Organon
& Co.
(Exact name of registrant
as specified in its charter)
| Delaware |
|
001-40235 |
|
46-4838035 |
| (State or other jurisdiction of |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
| incorporation) |
|
| |
|
| |
|
|
|
|
30
Hudson Street, Floor
33,
Jersey City,
NJ |
|
|
|
07302 |
(Address and principal executive
offices) |
|
|
|
(Zip Code) |
| Registrant’s telephone number, including area code: (551)
430-6900 |
Check the
appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any
of the following provisions:
| ¨ | Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of
each class |
|
Trading
Symbol(s) |
|
Name of
each exchange on which registered |
| Common
Stock, par value $0.01 per share |
|
OGN |
|
NYSE |
Indicate by check mark whether the registrant is
an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 2.02 | Results of Operations and Financial Condition. |
On February 13, 2025, Organon & Co.
(the “Company”) issued a press release (the “Earnings Release”) regarding its results for the quarter
and full year ended December 31, 2024. The Earnings Release is included as Exhibit 99.1 to this report.
The information contained in this Item 2.02, including
Exhibit 99.1 attached hereto, is considered to be “furnished” and shall not be deemed “filed” for purposes
of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to
liability under that Section. The information in this Current Report shall not be incorporated by reference into any filing or other document
pursuant to the Securities Act of 1933, as amended (the “Securities Act”) or the Exchange Act, except as shall be expressly
set forth by specific reference in such filing or document. The release contains forward-looking statements regarding the Company and
includes a cautionary statement identifying important factors that could cause actual results to differ materially from those anticipated.
| Item 7.01 | Regulation FD Disclosure. |
In connection with the conference call announced
in the Earnings Release, on February 13, 2025, the Company made available the Company Information Presentation relating to its financial
results for the quarter and full year ended December 31, 2024. The Company Information Presentation may be accessed within the investor
relations section of the Company’s website, https://www.organon.com. A copy of the Company Information Presentation is attached
hereto as Exhibit 99.2 and is incorporated herein by reference.
The information in this Item 7.01, including Exhibit 99.2
attached hereto, is considered to be “furnished” and shall not be deemed “filed” for purposes of Section 18
of the Exchange Act or otherwise subject to liability under that Section. The information in this Current Report shall not be incorporated
by reference into any filing or other document pursuant to the Securities Act or the Exchange Act, except as shall be expressly set forth
by specific reference in such filing or document. The Company Information Presentation contains forward-looking statements regarding the
Company and includes a cautionary statement identifying important factors that could cause actual results to differ materially from those
anticipated.
| Item 9.01 | Financial Statements and Exhibits. |
SIGNATURES
Pursuant to the requirements of
the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto
duly authorized.
| |
Organon & Co. |
| |
|
| |
By: |
/s/ Matthew Walsh |
| |
|
Name: |
Matthew Walsh |
| |
|
Title: |
Chief Financial Officer |
Dated: February 13, 2025
Exhibit 99.1

| Media Contacts: |
Karissa Peer |
Investor Contacts: |
Jennifer Halchak |
| |
(614) 314-8094 |
|
(201) 275-2711 |
| |
Kate Vossen |
|
Renee McKnight |
| |
(732) 675-8448 |
|
(551) 204-6129 |
Organon Reports Results for the Fourth Quarter
and Full Year Ended December 31, 2024
| · | Full year 2024 revenue of $6.4 billion, up 2% as-reported and 3% at constant currency |
| · | Full year 2024 diluted earnings per share of $3.33
and non-GAAP Adjusted diluted earnings per share of $4.11 |
| · | Full year 2024 Adjusted EBITDA of $1.96 billion inclusive
of $81 million of IPR&D, representing a 30.6% Adjusted EBITDA margin |
| · | Full year 2025 financial guidance ranges provided |
| · | Full year revenue range of $6.125 billion - $6.325 billion, inclusive of an approximate $200 million year-over-year negative impact
from foreign exchange |
| · | Adjusted EBITDA margin range of 31.0% - 32.0% |
Jersey City, N.J., February 13, 2025 – Organon (NYSE: OGN)
today announced its results for the fourth quarter and full year ended December 31, 2024.
“In 2024 we achieved our third year of constant currency revenue
growth and delivered Adjusted EBITDA margin expansion ex-IPR&D," said Kevin Ali, Organon's chief executive officer. "Our
2025 financial guidance reflects the potential for a fourth year of constant currency revenue growth despite the loss of exclusivity (LOE)
of our second largest product, Atozet, in certain markets. Further, we will continue to be extremely disciplined on operating costs to
support Adjusted EBITDA margins ex-IPR&D of 31.0% or better.”
Fourth Quarter 2024 Revenue
| in $ millions | |
Q4 2024 | | |
Q4 2023 | | |
VPY | | |
VPY ex-FX | |
| Women’s Health | |
$ | 466 | | |
$ | 465 | | |
| — | % | |
| — | % |
| Biosimilars | |
| 163 | | |
| 199 | | |
| (18 | )% | |
| (18 | )% |
| Established Brands | |
| 935 | | |
| 915 | | |
| 2 | % | |
| 2 | % |
| Other (1) | |
| 28 | | |
| 19 | | |
| 53 | % | |
| 52 | % |
| Revenue | |
$ | 1,592 | | |
$ | 1,598 | | |
| — | % | |
| — | % |
Totals may not foot due to rounding and percentages
are computed using unrounded amounts.
(1) Other includes manufacturing sales to third parties.
For the fourth quarter of 2024, total revenue
was $1.592 billion, flat on both an as-reported and ex-FX basis.
Women’s
Health revenue was flat on both an as-reported and ex-FX basis in the fourth quarter of 2024, compared with the fourth quarter of 2023.
Nexplanon® (etonogestrel implant) growth
of 12% ex-FX, offset a 37% ex-FX decline in
NuvaRing® (etonogestrel / ethinyl estradiol
vaginal ring) attributable to ongoing generic competition, as well as an 8% ex-FX decline
in the company's fertility portfolio. The fourth quarter decline in the fertility portfolio was primarily due to an unfavorable
year-over-year comparison to the fourth quarter of 2023 when the company benefited from a one-time buy-in associated with the exit of
a spin-related Interim Operating Model Agreement in the United States.
Biosimilars
revenue declined 18% on both an as-reported and ex-FX basis in the fourth quarter of 2024,
compared with the fourth quarter of 2023, primarily driven by the timing of tenders in Brazil
for Ontruzant® (trastuzumab-dttb) and Brenzys™ (etanercept) as
well as a 16% ex-FX decline in Renflexis® (infliximab-abda)
attributable to competitive pricing pressure in the U.S. Performance was partially offset by sales of Hadlima® (adalimumab-bwwd)
that have continued to ramp up since its July 2023 launch in the U.S.
Established
Brands revenue grew 2% both on an as-reported basis and ex-FX
in the fourth quarter of 2024, primarily related to the revenue contribution of Emgality®(1) (galcanezumab-gnlm)
and Vtama®(2) (tapinarof), which together more than offset the impact
of the loss of exclusivity (“LOE”) of Atozet™ (ezetimibe and atorvastatin) in key markets in Europe and
Japan.
(1) Emgality is a trademark registered in the United States
in the name of Eli Lilly and Company (used under license). Organon acquired certain European licensing and distribution rights to Emgality
and Rayvow from Eli Lilly beginning in early 2024.
(2) Vtama was acquired as part of Organon's acquisition
of Dermavant Sciences Inc., which closed on October 28, 2024.
Fourth Quarter 2024 Profitability
| in $ millions, except per share amounts | |
Q4 2024 | | |
Q4 2023 | | |
VPY | |
| Revenues | |
$ | 1,592 | | |
$ | 1,598 | | |
| — | % |
| Cost of sales | |
| 696 | | |
| 683 | | |
| 2 | % |
| Gross profit | |
| 896 | | |
| 915 | | |
| (2 | )% |
| Non-GAAP Adjusted gross profit (1) | |
| 965 | | |
| 964 | | |
| — | % |
| Net income | |
| 109 | | |
| 546 | | |
| (80 | )% |
| Non-GAAP Adjusted net income (1) | |
| 235 | | |
| 226 | | |
| 4 | % |
| Diluted Earnings per Share (EPS) | |
| 0.42 | | |
| 2.13 | | |
| (80 | )% |
| Non-GAAP Adjusted diluted EPS (1) | |
| 0.90 | | |
| 0.88 | | |
| 2 | % |
| Acquired IPR&D and milestones | |
| — | | |
| — | | |
| — | |
| Adjusted EBITDA (Non-GAAP) (1,2) | |
| 448 | | |
| 449 | | |
| — | % |
| | |
| | | |
| | | |
| | |
| | |
Q4 2024 | | |
Q4 2023 | | |
| | |
| Gross margin | |
| 56.3 | % | |
| 57.3 | % | |
| | |
| Non-GAAP Adjusted gross margin (1) | |
| 60.6 | % | |
| 60.3 | % | |
| | |
| Adjusted EBITDA margin (Non-GAAP) (1, 2) | |
| 28.1 | % | |
| 28.1 | % | |
| | |
| (1) | See Tables 4 and 5 for reconciliations of GAAP to non-GAAP financial measures. |
| (2) | There was no IPR&D or milestone expense impacting Adjusted EBITDA in the fourth quarter comparable periods. |
Gross margin was 56.3% as-reported and 60.6% on a non-GAAP adjusted
basis in the fourth quarter of 2024, compared with 57.3% as-reported and 60.3% on a non-GAAP adjusted basis in the fourth quarter of 2023.
Lower reported gross margin in the fourth quarter of 2024 was due to higher year- over-year amortization related to acquisitions completed
in 2024 and acquisition-related expense. The modest year-over-year improvement in non-GAAP Adjusted gross margin was primarily due to
favorable product mix partially offset by unfavorable price.
Net income for the fourth quarter of 2024 was $109 million, or $0.42
per diluted share, compared with $546 million, or $2.13 per diluted share, in the fourth quarter of 2023. In the fourth quarter of 2023,
a Swiss tax arrangement was terminated, resulting in a net benefit of $476 million to GAAP net income in that period, or a benefit of
$1.86 per share. For the fourth quarter of 2024, non-GAAP Adjusted net income was $235 million, or $0.90 per diluted share, compared with
$226 million, or $0.88 per diluted share, in 2023.
Non-GAAP Adjusted EBITDA margin was 28.1% in the fourth quarter of
2024 consistent with the fourth quarter of 2023 primarily due to flat year-over-year revenue, Adjusted gross profit and non-GAAP operating
expenses. There was no IPR&D or milestone expense impacting Adjusted EBITDA results in the fourth quarter comparable periods.
Full Year 2024 Revenue
| in $ millions | |
FY 2024 | | |
FY 2023 | | |
VPY | | |
VPY ex-FX | |
| Women’s Health | |
$ | 1,777 | | |
$ | 1,702 | | |
| 4 | % | |
| 5 | % |
| Biosimilars | |
| 662 | | |
| 593 | | |
| 12 | % | |
| 12 | % |
| Established Brands | |
| 3,849 | | |
| 3,847 | | |
| — | % | |
| 2 | % |
| Other (1) | |
| 115 | | |
| 121 | | |
| (6 | )% | |
| (6 | )% |
| Revenue | |
$ | 6,403 | | |
$ | 6,263 | | |
| 2 | % | |
| 3 | % |
(1)
Other includes manufacturing sales to third parties.
Full year 2024 revenue was $6.4 billion, an increase of 2% as-reported
and 3% ex-FX, compared with the full year 2023.
Women’s
Health revenue increased 4% as-reported and 5% ex-FX for full year 2024 compared with 2023. Nexplanon grew 17% ex-FX to record
revenue of $963 million. Jada® System grew 40% ex-FX to achieve $61 million
in revenue. Together these factors more than offset a 33% ex-FX decline in NuvaRing, which continues to be impacted by generic
competition as well as a 2% ex-FX decline in the company’s Fertility business.
Biosimilars
revenue increased 12% on both an as-reported and ex-FX basis for full year 2024, compared with the prior year, primarily driven
by growth in Hadlima, following its U.S. launch in July 2023. Renflexis
and Ontruzant declined 1% ex-FX and 9%
ex-FX, respectively, as both products are in the mature phase of their product life cycles and face significant competitive pricing pressure.
Revenue
for Established Brands was flat on an as-reported basis and increased 2% ex-FX for full year 2024. Contributions from Emgality
and Vtama, along with recovery in certain injectable steroid products following a market action in 2023 more than offset
the impact from the Atozet LOE in Europe and Japan and unfavorable pricing in Japan.
Full Year 2024 Profitability
| in $ millions, except per share amounts | |
2024 | | |
2023 | | |
VPY | |
| Revenues | |
$ | 6,403 | | |
$ | 6,263 | | |
| 2 | % |
| Cost of sales | |
| 2,688 | | |
| 2,515 | | |
| 7 | % |
| Gross profit | |
| 3,715 | | |
| 3,748 | | |
| (1 | )% |
| Non-GAAP Adjusted gross profit (1) | |
| 3,944 | | |
| 3,930 | | |
| — | % |
| Net income | |
| 864 | | |
| 1,023 | | |
| (16 | )% |
| Non-GAAP Adjusted net income (1) | |
| 1,065 | | |
| 1,061 | | |
| — | % |
| Diluted Earnings per Share (EPS) | |
| 3.33 | | |
| 3.99 | | |
| (17 | )% |
| Non-GAAP Adjusted diluted EPS (1) | |
| 4.11 | | |
| 4.14 | | |
| (1 | )% |
| Acquired in-process research & development (IPR&D) and milestones | |
| 81 | | |
| 8 | | |
| NM | |
| Adjusted EBITDA (1, 2) | |
| 1,958 | | |
| 1,944 | | |
| 1 | % |
| | |
| | | |
| | | |
| | |
| | |
2024 | | |
2023 | | |
| | |
| Gross margin | |
| 58.0 | % | |
| 59.8 | % | |
| | |
| Non-GAAP Adjusted gross margin (1) | |
| 61.6 | % | |
| 62.7 | % | |
| | |
| Adjusted EBITDA margin (1, 2) | |
| 30.6 | % | |
| 31.0 | % | |
| | |
| (1) | See Tables 4 and 5 for reconciliations of GAAP to non-GAAP financial measures. |
| (2) | Adjusted EBITDA and Adjusted EBITDA margin include $81 million in 2024 and $8 million in 2023 related to acquired IPR&D and milestones. |
Gross
margin was 58.0% as-reported and 61.6% on an adjusted basis for full year 2024, compared with 59.8% as-reported and 62.7% on an
adjusted basis for full year 2023. The year-over-year decline in reported gross margin was driven by higher year-over-year amortization
related to 2024 acquisitions as well as acquisition-related expense. The year-over-year decrease in Adjusted gross margin reflects unfavorable
price as well as higher inflation impacts to material and distribution costs.
Adjusted
EBITDA margin was 30.6% for full year 2024, compared with 31.0% for full year 2023. The year-over-year decrease was primarily a
result of higher IPR&D expense in full year 2024, followed by lower Adjusted gross margin. Non-GAAP operating expenses were contained
to 1% growth in the full year 2024, inclusive of $81 million of IPR&D and milestone expense in 2024, compared with $8 million in 2023.
Net
income for full year 2024 was $864 million, or $3.33 per diluted share, compared with $1,023 million,
or $3.99 per diluted share in 2023. Full year 2023 reported Net income benefited from the fourth quarter
termination of the aforementioned Swiss tax arrangement, which represented a benefit of $1.86 per share for the full year. Non-GAAP
Adjusted net income was $1,065 million for full year 2024, consistent with $1,061 million in
full year 2023.
Capital Allocation
Today, Organon’s Board of Directors declared a quarterly dividend
of $0.28 for each issued and outstanding share of the company's common stock. The dividend
is payable on March 13, 2025, to stockholders of record at the close of business on February 24, 2025.
As of December 31, 2024, cash and cash equivalents were $675 million,
and debt was $8.9 billion.
Full Year Guidance
Organon does not provide GAAP financial measures on a forward-looking
basis because the company cannot predict with reasonable certainty and without unreasonable effort, the ultimate outcome of legal proceedings,
unusual gains and losses, the occurrence of matters creating GAAP tax impacts, and acquisition-related expenses. These items are uncertain,
depend on various factors, and could be material to Organon’s results computed in accordance with GAAP.
Full year 2024 actual results and 2025 financial guidance are presented
below on a non-GAAP basis, except revenue.
| | |
Full Year 2024 Actuals | |
Full Year 2025 Guidance |
| Revenue | |
$6.403B | |
$6.125B-$6.325B |
| FX translation headwind | |
~$80M | |
~$200M |
| Adjusted gross margin | |
61.6% | |
60.0%-61.0% |
| SG&A | |
$1.57B/25% | |
Mid-20% range |
| R&D | |
$440M/6.9% | |
Upper single-digit |
| IPR&D* | |
$81M | |
- |
| Adjusted EBITDA margin (Non-GAAP) | |
30.6% | |
31.0%-32.0% |
| Interest | |
$520M | |
~$510M |
| Depreciation | |
$126M | |
~$135M |
| Effective non-GAAP tax rate | |
18.8% | |
22.5%-24.5% |
| Fully diluted weighted average shares outstanding | |
259M | |
~263M |
*The company does not provide guidance for forward-looking IPR&D
and milestone expense.
Webcast Information
Organon will host a conference call at 8:30 a.m. Eastern Time
today to discuss its fourth quarter and full year 2024 financial results. To listen to the event and view the presentation slides via
webcast, join from the Organon Investor Relations website at https://www.organon.com/investor-relations/events-and-presentations/.
A replay of the webcast will be available approximately two hours after the conclusion of the live event on the company’s website.
Institutional investors and analysts interested in participating in the call must register in advance by clicking on this link: https://registrations.events/direct/Q4I5851155
Following registration, participants will receive a confirmation email
containing details on how to join the conference call, including dial-in information and a unique passcode and registrant ID. Pre-registration
will allow participants to bypass an operator and be placed directly into the call.
About Organon
Organon is an independent global healthcare company with a primary
mission to help improve the health of women throughout their lives. Organon’s diverse portfolio offers over 70 medicines and
products in women’s health, biosimilars, and a large franchise of established medicines across a range of therapeutic areas. In
addition to Organon’s current products, the company invests in innovative solutions and research to drive future growth opportunities
in women’s health and biosimilars. In addition, Organon is pursuing opportunities to collaborate with biopharmaceutical partners
and innovators looking to commercialize their products by leveraging its scale and agile presence in fast growing international markets.
Organon has geographic scope with significant reach, world-class commercial
capabilities, and over 10,000 employees with headquarters located in Jersey City, New Jersey.
For more information, visit http://www.organon.com and
connect with us on LinkedIn, Instagram, X (formerly known as Twitter) and Facebook.
Cautionary Note Regarding Non-GAAP Financial Measures
This press release contains “non-GAAP financial measures,”
which are financial measures that either exclude or include amounts that are correspondingly not excluded or included in the most directly
comparable measures calculated and presented in accordance with U.S. generally accepted accounting principles (“GAAP”). Specifically,
the company makes use of the non-GAAP financial measures Adjusted EBITDA, Adjusted EBITDA margin, Adjusted gross margin, Adjusted gross
profit, Adjusted net income, and Adjusted diluted EPS, which are not recognized terms under GAAP and are presented only as a supplement
to the company’s GAAP financial statements. This press release also provides certain measures that exclude the impact of foreign
exchange. We calculate foreign exchange by converting our current-period local currency financial results using the prior period average
currency rates and comparing these adjusted amounts to our current-period results. The company believes that these non-GAAP financial
measures help to enhance an understanding of the company’s financial performance. However, the presentation of these measures has
limitations as an analytical tool and should not be considered in isolation, or as a substitute for the company’s results as reported
under GAAP. Because not all companies use identical calculations, the presentations of these non-GAAP measures may not be comparable to
other similarly titled measures of other companies. Please refer to Table 4 and Table 5 of this press release for additional information,
including relevant definitions and reconciliations of non-GAAP financial measures contained herein to the most directly comparable GAAP
measures.
In addition, the company’s full-year 2025 guidance measures (other
than revenue) are provided on a non-GAAP basis because the company is unable to reasonably predict certain items contained in the GAAP
measures. Such items include, but are not limited to, acquisition-related expenses, restructuring and related expenses, stock-based compensation,
the ultimate outcome of legal proceedings, unusual gains and losses, the occurrence of matters creating GAAP tax impacts and other items
not reflective of the company's ongoing operations.
The company’s management uses the non-GAAP financial measures
described above to evaluate the company’s performance and to guide operational and financial decision making. Further, the company’s
management believes that these non-GAAP financial measures, which exclude certain items, help to enhance its ability to meaningfully communicate
its underlying business performance, financial condition and results of operations.
Cautionary Note Regarding Forward-Looking Statements
Except for historical information, this press release includes “forward-looking
statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, including,
but not limited to, statements about management’s expectations about Organon’s future financial performance and prospects,
including expectations regarding clinical studies and regulatory approvals (including the timing and outcome thereof), full-year 2025
guidance estimates and predictions regarding other financial information and metrics, the expected impact of our ongoing restructuring
initiatives, expectations regarding our collaborations with third parties, and franchise and product performance and strategy expectations
for future periods. Forward-looking statements may be identified by words such as “prospects,” “opportunity,”
“objective,” “guidance,” potential,” “should,” “continue,” “will,” “continue,”
“pursuing,” “expects,” “intends,” “plans,” “believes,” “future,”
“estimates,” or words of similar meaning. These statements are based upon the current beliefs and expectations of the company’s
management and are subject to significant risks and uncertainties. If underlying assumptions prove inaccurate, or risks or uncertainties
materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include, but are not limited to, the uncertainty
of the clinical trial and regulatory approvals during the expected timeframe, if at all; an inability to adapt to the industry-wide trend
toward highly discounted channels; difficulties implementing or executing on Organon’s acquisition strategy, difficulties integrating
such acquisitions (including its recent acquisition of Dermavant Sciences Ltd.) or any other failure to recognize the benefits of such
acquisitions; changes in tax laws or other tax guidance which could adversely affect our cash tax liability, effective tax rates, and
results of operations and lead to greater audit scrutiny; expanded brand and class competition in the markets in which the company operates;
global tensions, which may result in disruptions in the broader global economic environment; governmental initiatives that adversely impact
our marketing activities, particularly in China; volatility in our stock price; political and social pressures, or regulatory developments,
that adversely impact demand for, availability of, or patient access to contraception or fertility products; recent United States Supreme
Court decisions and other developments impacting regulatory agencies and their rule making, including related financial market reactions,
tax planning and international trade practices; difficulties with performance of third parties we rely on for our business growth; the
failure of any supplier to provide substances, materials, or services as agreed; the increased cost of supply, manufacturing, packaging,
and operations; difficulties developing and sustaining relationships with commercial counterparties; competition from generic products
as our products lose patent protection; any failure by us to obtain an additional period of market exclusivity in the United States for
Nexplanon subsequent to the expiration of the rod patents in 2027; as well as the continued impact of our loss of data exclusivity
for Atozet; disruptions at the U.S. Food and Drug Administration, the U.S. Securities and Exchange Commission (the “SEC”)
and other U.S. and comparable foreign government agencies; pricing pressures globally, including rules and practices of managed care
groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and health care reform, pharmaceutical
reimbursement and pricing in general; an inability to fully execute on our product development and commercialization plans in the United
States, Europe, and elsewhere internationally; the failure by us or our third party collaborators and/or their suppliers to fulfill our
or their regulatory or quality obligations; the impact of higher selling and promotional costs; and the impact of cyberattacks or other
events that may affect Organon’s information technology systems or those of third parties.
The company undertakes no obligation to publicly update any forward-looking
statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially
from those described in the forward-looking statements can be found in the company’s filings with the SEC, including the company’s
most recent Annual Report on Form 10-K and subsequent SEC filings, available at the SEC’s Internet site (www.sec.gov).
TABLE 1
Organon & Co.
Condensed Consolidated Statement of Income
(Unaudited, $ in millions except shares in thousands and per share
amounts)
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Revenues | |
$ | 1,592 | | |
$ | 1,598 | | |
$ | 6,403 | | |
$ | 6,263 | |
| Cost of sales | |
| 696 | | |
| 683 | | |
| 2,688 | | |
| 2,515 | |
| Gross Profit | |
| 896 | | |
| 915 | | |
| 3,715 | | |
| 3,748 | |
| | |
| | | |
| | | |
| | | |
| | |
| Selling, general and administrative | |
| 470 | | |
| 469 | | |
| 1,760 | | |
| 1,893 | |
| Research and development | |
| 130 | | |
| 134 | | |
| 469 | | |
| 528 | |
| Acquired in-process research and development and milestones | |
| — | | |
| — | | |
| 81 | | |
| 8 | |
| Restructuring costs | |
| 8 | | |
| 58 | | |
| 31 | | |
| 62 | |
| Interest expense | |
| 132 | | |
| 129 | | |
| 520 | | |
| 527 | |
| Exchange losses | |
| 15 | | |
| 17 | | |
| 26 | | |
| 42 | |
| Other expense, net | |
| 12 | | |
| 4 | | |
| 21 | | |
| 15 | |
| Income before income taxes | |
| 129 | | |
| 104 | | |
| 807 | | |
| 673 | |
| Income tax expense (benefit) | |
| 20 | | |
| (442 | ) | |
| (57 | ) | |
| (350 | ) |
| Net income | |
$ | 109 | | |
$ | 546 | | |
$ | 864 | | |
$ | 1,023 | |
| | |
| | | |
| | | |
| | | |
| | |
| Earnings per share: | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | 0.42 | | |
$ | 2.14 | | |
$ | 3.36 | | |
$ | 4.01 | |
| Diluted | |
$ | 0.42 | | |
$ | 2.13 | | |
$ | 3.33 | | |
$ | 3.99 | |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding: | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 257,690 | | |
| 255,617 | | |
| 257,046 | | |
| 255,239 | |
| Diluted | |
| 259,878 | | |
| 256,590 | | |
| 259,152 | | |
| 256,270 | |
TABLE 2
Organon & Co.
Sales by top products
(Unaudited, $ in millions)
| | |
Three Months Ended December 31, | | |
Year Ended December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
U.S. | | |
Int’l | | |
Total | | |
U.S. | | |
Int’l | | |
Total | | |
U.S. | | |
Int’l | | |
Total | | |
U.S. | | |
Int’l | | |
Total | |
| Women’s Health | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Nexplanon/Implanon NXT | |
$ | 175 | | |
$ | 83 | | |
$ | 258 | | |
$ | 154 | | |
$ | 76 | | |
$ | 231 | | |
$ | 672 | | |
$ | 291 | | |
$ | 963 | | |
$ | 572 | | |
$ | 257 | | |
$ | 830 | |
| Follistim AQ | |
| 26 | | |
| 39 | | |
| 65 | | |
| 51 | | |
| 31 | | |
| 83 | | |
| 84 | | |
| 152 | | |
| 237 | | |
| 125 | | |
| 136 | | |
| 262 | |
| NuvaRing (1) | |
| 6 | | |
| 18 | | |
| 24 | | |
| 20 | | |
| 19 | | |
| 39 | | |
| 39 | | |
| 75 | | |
| 115 | | |
| 90 | | |
| 86 | | |
| 176 | |
| Ganirelix Acetate Injection | |
| 4 | | |
| 24 | | |
| 28 | | |
| 4 | | |
| 18 | | |
| 22 | | |
| 20 | | |
| 89 | | |
| 109 | | |
| 19 | | |
| 91 | | |
| 110 | |
| Marvelon/Mercilon | |
| — | | |
| 31 | | |
| 31 | | |
| — | | |
| 37 | | |
| 37 | | |
| — | | |
| 134 | | |
| 134 | | |
| — | | |
| 134 | | |
| 134 | |
| Jada | |
| 18 | | |
| — | | |
| 18 | | |
| 13 | | |
| — | | |
| 13 | | |
| 60 | | |
| 1 | | |
| 61 | | |
| 43 | | |
| — | | |
| 43 | |
| Other Women’s Health (1) (2) | |
| 15 | | |
| 27 | | |
| 42 | | |
| 16 | | |
| 26 | | |
| 40 | | |
| 56 | | |
| 104 | | |
| 158 | | |
| 48 | | |
| 101 | | |
| 147 | |
| Biosimilars | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Renflexis | |
| 52 | | |
| 13 | | |
| 65 | | |
| 63 | | |
| 14 | | |
| 77 | | |
| 219 | | |
| 55 | | |
| 274 | | |
| 234 | | |
| 43 | | |
| 278 | |
| Ontruzant | |
| 6 | | |
| 28 | | |
| 34 | | |
| 10 | | |
| 52 | | |
| 62 | | |
| 29 | | |
| 112 | | |
| 141 | | |
| 46 | | |
| 109 | | |
| 155 | |
| Brenzys | |
| — | | |
| 15 | | |
| 15 | | |
| — | | |
| 28 | | |
| 28 | | |
| — | | |
| 77 | | |
| 77 | | |
| — | | |
| 73 | | |
| 73 | |
| Aybintio | |
| — | | |
| 6 | | |
| 6 | | |
| — | | |
| 9 | | |
| 9 | | |
| — | | |
| 28 | | |
| 28 | | |
| — | | |
| 43 | | |
| 43 | |
| Hadlima | |
| 33 | | |
| 11 | | |
| 44 | | |
| 15 | | |
| 8 | | |
| 23 | | |
| 104 | | |
| 38 | | |
| 142 | | |
| 17 | | |
| 26 | | |
| 44 | |
| Established Brands | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cardiovascular | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Zetia (1) | |
| 2 | | |
| 75 | | |
| 77 | | |
| 3 | | |
| 65 | | |
| 67 | | |
| 7 | | |
| 310 | | |
| 317 | | |
| 8 | | |
| 314 | | |
| 322 | |
| Vytorin | |
| 2 | | |
| 24 | | |
| 26 | | |
| 1 | | |
| 28 | | |
| 29 | | |
| 6 | | |
| 102 | | |
| 108 | | |
| 6 | | |
| 124 | | |
| 129 | |
| Atozet | |
| — | | |
| 76 | | |
| 76 | | |
| — | | |
| 122 | | |
| 122 | | |
| — | | |
| 473 | | |
| 473 | | |
| — | | |
| 519 | | |
| 519 | |
| Rosuzet | |
| — | | |
| 13 | | |
| 13 | | |
| — | | |
| 18 | | |
| 18 | | |
| — | | |
| 49 | | |
| 49 | | |
| — | | |
| 70 | | |
| 70 | |
| Cozaar/Hyzaar | |
| 2 | | |
| 55 | | |
| 57 | | |
| 2 | | |
| 55 | | |
| 57 | | |
| 9 | | |
| 234 | | |
| 243 | | |
| 10 | | |
| 272 | | |
| 281 | |
| Other Cardiovascular (1) (2) | |
| — | | |
| 34 | | |
| 34 | | |
| — | | |
| 28 | | |
| 29 | | |
| 2 | | |
| 130 | | |
| 133 | | |
| 2 | | |
| 136 | | |
| 139 | |
| Respiratory | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Singulair | |
| 2 | | |
| 82 | | |
| 84 | | |
| 2 | | |
| 111 | | |
| 114 | | |
| 9 | | |
| 350 | | |
| 359 | | |
| 11 | | |
| 393 | | |
| 404 | |
| Nasonex (1) | |
| — | | |
| 76 | | |
| 76 | | |
| — | | |
| 69 | | |
| 69 | | |
| — | | |
| 276 | | |
| 276 | | |
| — | | |
| 266 | | |
| 266 | |
| Dulera | |
| 42 | | |
| 11 | | |
| 52 | | |
| 40 | | |
| 10 | | |
| 50 | | |
| 162 | | |
| 42 | | |
| 203 | | |
| 156 | | |
| 38 | | |
| 194 | |
| Clarinex | |
| — | | |
| 27 | | |
| 28 | | |
| 1 | | |
| 29 | | |
| 30 | | |
| 3 | | |
| 125 | | |
| 127 | | |
| 5 | | |
| 132 | | |
| 136 | |
| Other Respiratory (1) (2) | |
| 13 | | |
| 4 | | |
| 17 | | |
| 8 | | |
| 4 | | |
| 11 | | |
| 38 | | |
| 13 | | |
| 53 | | |
| 49 | | |
| 14 | | |
| 64 | |
| Non-Opioid Pain, Bone and Dermatology | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Arcoxia | |
| — | | |
| 58 | | |
| 58 | | |
| — | | |
| 51 | | |
| 51 | | |
| — | | |
| 270 | | |
| 270 | | |
| — | | |
| 257 | | |
| 257 | |
| Fosamax | |
| — | | |
| 38 | | |
| 38 | | |
| — | | |
| 35 | | |
| 36 | | |
| 3 | | |
| 147 | | |
| 151 | | |
| 3 | | |
| 156 | | |
| 159 | |
| Diprospan | |
| — | | |
| 36 | | |
| 36 | | |
| — | | |
| 33 | | |
| 33 | | |
| — | | |
| 139 | | |
| 139 | | |
| — | | |
| 91 | | |
| 91 | |
| Vtama | |
| 10 | | |
| 1 | | |
| 12 | | |
| — | | |
| — | | |
| — | | |
| 10 | | |
| 1 | | |
| 12 | | |
| — | | |
| — | | |
| — | |
| Other Non-Opioid Pain, Bone and Dermatology (2) | |
| 3 | | |
| 69 | | |
| 71 | | |
| 4 | | |
| 64 | | |
| 67 | | |
| 19 | | |
| 279 | | |
| 295 | | |
| 14 | | |
| 261 | | |
| 275 | |
| Other | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Emgality/Rayvow | |
| — | | |
| 38 | | |
| 38 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 107 | | |
| 107 | | |
| — | | |
| — | | |
| — | |
| Proscar | |
| — | | |
| 22 | | |
| 22 | | |
| — | | |
| 20 | | |
| 20 | | |
| 1 | | |
| 94 | | |
| 95 | | |
| 1 | | |
| 96 | | |
| 97 | |
| Propecia | |
| 1 | | |
| 31 | | |
| 32 | | |
| 2 | | |
| 31 | | |
| 33 | | |
| 6 | | |
| 105 | | |
| 111 | | |
| 7 | | |
| 118 | | |
| 125 | |
| Other (2) | |
| 3 | | |
| 83 | | |
| 87 | | |
| 1 | | |
| 78 | | |
| 79 | | |
| 14 | | |
| 314 | | |
| 328 | | |
| 13 | | |
| 308 | | |
| 319 | |
| Other (3) | |
| 1 | | |
| 28 | | |
| 28 | | |
| 1 | | |
| 18 | | |
| 19 | | |
| — | | |
| 115 | | |
| 115 | | |
| (1 | ) | |
| 121 | | |
| 121 | |
| Revenues | |
$ | 416 | | |
$ | 1,176 | | |
$ | 1,592 | | |
$ | 411 | | |
$ | 1,187 | | |
$ | 1,598 | | |
$ | 1,572 | | |
$ | 4,831 | | |
$ | 6,403 | | |
$ | 1,478 | | |
$ | 4,785 | | |
$ | 6,263 | |
Totals
may not foot due to rounding. Trademarks appearing above in italics are trademarks of, or are used under license by, the
Organon group of companies.
| (1) | Sales of the authorized generic versions of NuvaRing,
Zetia and Nasonex were previously included in other and have been reclassified to their respective brand name product. |
| (2) | Includes sales of products not listed separately. |
| (3) | Other includes manufacturing
sales to third parties. |
TABLE 3
Organon & Co.
Sales by geographic area
(Unaudited, $ in millions)
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Europe and Canada | |
$ | 420 | | |
$ | 414 | | |
$ | 1,763 | | |
$ | 1,673 | |
| United States | |
| 416 | | |
| 411 | | |
| 1,572 | | |
| 1,478 | |
| Asia Pacific and Japan | |
| 244 | | |
| 261 | | |
| 1,050 | | |
| 1,129 | |
| China | |
| 213 | | |
| 203 | | |
| 847 | | |
| 864 | |
| Latin America, Middle East, Russia, and Africa | |
| 266 | | |
| 279 | | |
| 1,034 | | |
| 965 | |
| Other (1) | |
| 33 | | |
| 30 | | |
| 137 | | |
| 154 | |
| Revenues | |
$ | 1,592 | | |
$ | 1,598 | | |
$ | 6,403 | | |
$ | 6,263 | |
(1) Other
includes manufacturing sales to third parties.
TABLE 4
Organon & Co.
Reconciliation of GAAP Reported to Non-GAAP Adjusted Metrics
(Unaudited, $ in millions)
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| GAAP Gross Profit | |
$ | 896 | | |
$ | 915 | | |
$ | 3,715 | | |
$ | 3,748 | |
| Adjusted for: | |
| | | |
| | | |
| | | |
| | |
| Spin-related costs (1) | |
| — | | |
| 17 | | |
| 6 | | |
| 47 | |
| Manufacturing network costs (2) | |
| 15 | | |
| — | | |
| 54 | | |
| — | |
| Stock-based compensation | |
| 4 | | |
| 4 | | |
| 17 | | |
| 17 | |
| Amortization | |
| 43 | | |
| 28 | | |
| 145 | | |
| 116 | |
| Acquisition-related costs (3) | |
| 7 | | |
| — | | |
| 7 | | |
| — | |
| Other | |
| — | | |
| — | | |
| — | | |
| 2 | |
| Adjusted Non-GAAP Gross Profit | |
$ | 965 | | |
$ | 964 | | |
$ | 3,944 | | |
$ | 3,930 | |
(1) Spin-related costs include costs from the separation of Merck & Co., Inc., Rahway, NJ, US. For additional details refer to Table 5.
(2) Manufacturing network related costs include costs from exiting manufacturing and supply agreements with Merck & Co., Inc., Rahway NJ, US. For additional details refer to Table 5.
(3) Acquisition-related costs relate to costs from the acquisition of Dermavant. For additional details refer to Table 5.
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| GAAP Gross Margin | |
| 56.3 | % | |
| 57.3 | % | |
| 58.0 | % | |
| 59.8 | % |
| Total impact of Non-GAAP adjustments | |
| 4.3 | % | |
| 3.0 | % | |
| 3.6 | % | |
| 2.9 | % |
| Adjusted Non-GAAP Gross Margin | |
| 60.6 | % | |
| 60.3 | % | |
| 61.6 | % | |
| 62.7 | % |
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| GAAP Selling, general and administrative expenses | |
$ | 470 | | |
$ | 469 | | |
$ | 1,760 | | |
$ | 1,893 | |
| Adjusted for: | |
| | | |
| | | |
| | | |
| | |
| Spin-related costs (1) | |
| (9 | ) | |
| (47 | ) | |
| (88 | ) | |
| (178 | ) |
| Stock-based compensation | |
| (17 | ) | |
| (18 | ) | |
| (70 | ) | |
| (68 | ) |
| Acquisition-related costs (2) | |
| (24 | ) | |
| — | | |
| (28 | ) | |
| — | |
| Other | |
| (3 | ) | |
| (3 | ) | |
| (3 | ) | |
| (91 | ) |
| Adjusted Non-GAAP Selling, general and administrative expenses | |
$ | 417 | | |
$ | 401 | | |
$ | 1,571 | | |
$ | 1,556 | |
(1) Spin-related costs include costs from the separation of Merck & Co., Inc., Rahway, NJ, US. For additional details refer to Table 5.
(2) Acquisition-related costs relate to costs from the acquisition of Dermavant. For additional details refer to Table 5.
TABLE 4
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
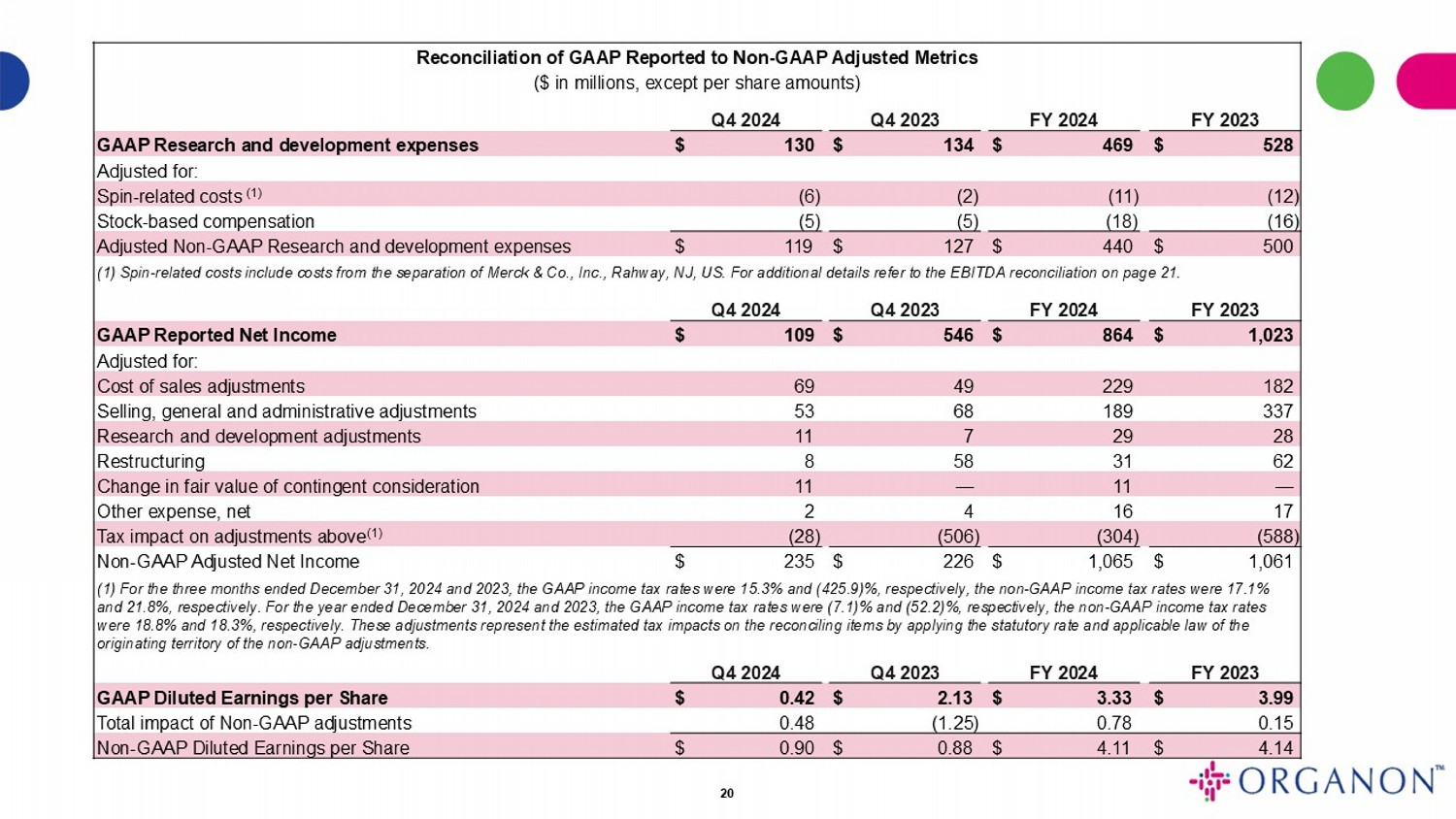
| GAAP Research and development expenses | |
$ | 130 | | |
$ | 134 | | |
$ | 469 | | |
$ | 528 | |
| Adjusted for: | |
| | | |
| | | |
| | | |
| | |
| Spin-related costs (1) | |
| (6 | ) | |
| (2 | ) | |
| (11 | ) | |
| (12 | ) |
| Stock-based compensation | |
| (5 | ) | |
| (5 | ) | |
| (18 | ) | |
| (16 | ) |
| Adjusted Non-GAAP Research and development expenses | |
$ | 119 | | |
$ | 127 | | |
$ | 440 | | |
$ | 500 | |
(1) Spin-related costs include costs from the separation of Merck & Co., Inc., Rahway, NJ, US. For additional details refer to Table 5.
TABLE 4
Organon & Co.
Reconciliation of GAAP Reported to Non-GAAP Adjusted Metrics (Continued)
(Unaudited, $ in millions except per share amounts)
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| GAAP Reported Net Income | |
$ | 109 | | |
$ | 546 | | |
$ | 864 | | |
$ | 1,023 | |
| Adjusted for: | |
| | | |
| | | |
| | | |
| | |
| Cost of sales adjustments | |
| 69 | | |
| 49 | | |
| 229 | | |
| 182 | |
| Selling, general and administrative adjustments | |
| 53 | | |
| 68 | | |
| 189 | | |
| 337 | |
| Research and development adjustments | |
| 11 | | |
| 7 | | |
| 29 | | |
| 28 | |
| Restructuring | |
| 8 | | |
| 58 | | |
| 31 | | |
| 62 | |
| Change in fair value of contingent consideration | |
| 11 | | |
| — | | |
| 11 | | |
| — | |
| Other expense, net | |
| 2 | | |
| 4 | | |
| 16 | | |
| 17 | |
| Tax impact on adjustments above(1) | |
| (28 | ) | |
| (506 | ) | |
| (304 | ) | |
| (588 | ) |
| Non-GAAP Adjusted Net Income | |
$ | 235 | | |
$ | 226 | | |
$ | 1,065 | | |
$ | 1,061 | |
(1) For the three months ended December 31, 2024 and 2023, the GAAP income tax rates were 15.3% and (425.9)%, respectively, and the non-GAAP income tax rates were 17.1% and 21.8%, respectively. For the year ended December 31, 2024 and 2023, the GAAP income tax rates were (7.1)% and (52.2)%, respectively, and the non-GAAP income tax rates were 18.8% and 18.3%, respectively. These adjustments represent the estimated tax impacts on the reconciling items by applying the statutory rate and applicable law of the originating territory of the non-GAAP adjustments.
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| GAAP Diluted Earnings per Share | |
$ | 0.42 | | |
$ | 2.13 | | |
$ | 3.33 | | |
$ | 3.99 | |
| Total impact of Non-GAAP adjustments | |
| 0.48 | | |
| (1.25 | ) | |
| 0.78 | | |
| 0.15 | |
| Non-GAAP Diluted Earnings per Share | |
$ | 0.90 | | |
$ | 0.88 | | |
$ | 4.11 | | |
$ | 4.14 | |
TABLE 5
Organon & Co.
Reconciliation of GAAP Net Income to Non-GAAP Adjusted EBITDA
(Unaudited, $ in millions)
| | |
Three Months Ended
December 31, | | |
Year Ended
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Net income | |
$ | 109 | | |
$ | 546 | | |
$ | 864 | | |
$ | 1,023 | |
| Depreciation (1) | |
| 33 | | |
| 30 | | |
| 126 | | |
| 118 | |
| Amortization | |
| 43 | | |
| 28 | | |
| 145 | | |
| 116 | |
| Interest expense | |
| 132 | | |
| 129 | | |
| 520 | | |
| 527 | |
| Income tax expense (benefit) | |
| 20 | | |
| (442 | ) | |
| (57 | ) | |
| (350 | ) |
| EBITDA (Non-GAAP) | |
$ | 337 | | |
$ | 291 | | |
$ | 1,598 | | |
$ | 1,434 | |
| Restructuring costs | |
| 8 | | |
| 58 | | |
| 31 | | |
| 62 | |
| Spin-related costs (2) | |
| 17 | | |
| 70 | | |
| 121 | | |
| 254 | |
| Manufacturing network related (3) | |
| 15 | | |
| — | | |
| 54 | | |
| — | |
| Acquisition-related costs (4) | |
| 31 | | |
| — | | |
| 35 | | |
| — | |
| Change in fair value of contingent consideration | |
| 11 | | |
| — | | |
| 11 | | |
| — | |
| Other costs (5) | |
| 3 | | |
| 3 | | |
| 3 | | |
| 93 | |
| Stock-based compensation | |
| 26 | | |
| 27 | | |
| 105 | | |
| 101 | |
| Adjusted EBITDA (Non-GAAP) | |
$ | 448 | | |
$ | 449 | | |
$ | 1,958 | | |
$ | 1,944 | |
| Adjusted EBITDA margin (Non-GAAP) | |
| 28.1 | % | |
| 28.1 | % | |
| 30.6 | % | |
| 31.0 | % |
(1) Excludes accelerated depreciation included in one-time costs.
(2) Spin-related costs reflect certain costs incurred in connection with activities taken to separate Organon from Merck & Co., Inc., Rahway, NJ, US. These costs include, but are not limited to, $6 million and $34 million for the three months ended December 31, 2024 and 2023, respectively, and $53 million and $134 million for the year ended December 31, 2024 and 2023, respectively, for information technology infrastructure, primarily related to the implementation of a stand-alone enterprise resource planning system and redundant software licensing costs, as well as $8 million for the three months ended December 31, 2023, and $20 million and $28 million for the year ended December 31, 2024 and 2023, respectively, associated with temporary transition service agreements with Merck & Co., Inc., Rahway, NJ, US.
(3) Manufacturing network related costs, including exiting of temporary manufacturing and supply agreements with Merck & Co., Inc., Rahway, NJ, US, reflect accelerated depreciation, exit premiums, technology transfer costs, stability and qualification batch costs, and third-party contractor costs.
(4) Acquisition-related costs for the three months ended December 31, 2024 reflect $8 million of transaction related costs, $10 million of Dermavant transaction bonuses and separation charges and $7 million of amortization pertaining to the fair value inventory purchase accounting adjustment. Acquisition related costs for the year ended December 31, 2024 reflect $12 million of transaction related costs, $10 million of Dermavant transaction bonuses and separation charges and $7 million of amortization pertaining to the fair value inventory purchase accounting adjustment.
(5) Other costs for the year ended December 31, 2023, include $80 million related to the Microspherix legal matter.
As the costs described in (1) through (5) above are directly related to the separation of Organon and acquisition related activities and therefore arise from a one-time event outside of the ordinary course of the company’s operations, the adjustment of these items provides meaningful, supplemental, information that the company believes will enhance an investor's understanding of the company's ongoing operating performance.
Exhibit 99.2

Fourth Quarter and Full Year 2024 Earnings Organon

Disclaimer statement Cautionary Note Regarding Forward - Looking Statements Except for historical information, this presentation includes “forward - looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, including, but not limited to, statements about management’s expectations about Organon’s future financial performance and pr osp ects, including expectations regarding clinical studies and regulatory approvals (including the timing and outcome thereof), full - year 2025 guidance estimates and predictions regarding other financia l information and metrics, the expected impact of our ongoing restructuring initiatives, expectations regarding our collaborations with third parties, and franchise and product performance and strategy ex pectations for future periods. Forward - looking statements may be identified by words such as “prospects,” “opportunity,” “objective,” “guidance,” potential,” “should,” “continue,” “will,” “continue,” “pur sui ng,” “expects,” “intends,” “plans,” “believes,” “future,” “estimates,” or words of similar meaning. These statements are based upon the current beliefs and expectations of the company’s management and are subject to sig nificant risks and uncertainties. If underlying assumptions prove inaccurate, or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward - look ing statements. Risks and uncertainties include, but are not limited to, the uncertainty of the clinical trial and regulatory approvals durin g t he expected timeframe, if at all; an inability to adapt to the industry - wide trend toward highly discounted channels; difficulties implementing or executing on Organon’s acquisition strategy, difficulties integratin g s uch acquisitions (including its recent acquisition of Dermavant Sciences Ltd.) or any other failure to recognize the benefits of such acquisitions; changes in tax laws or other tax guidance which could adver sel y affect our cash tax liability, effective tax rates, and results of operations and lead to greater audit scrutiny; expanded brand and class competition in the markets in which the company operates; global ten sio ns, which may result in disruptions in the broader global economic environment; governmental initiatives that adversely impact our marketing activities, particularly in China; volatility in ou r s tock price; political and social pressures, or regulatory developments, that adversely impact demand for, availability of, or patient access to contraception or fertility products; recent United States Supreme Co urt decisions and other developments impacting regulatory agencies and their rule making, including related financial market reactions, tax planning and international trade practices; difficulties with perfo rma nce of third parties we rely on for our business growth; the failure of any supplier to provide substances, materials, or services as agreed; the increased cost of supply, manufacturing, packaging, and operations; di fficulties developing and sustaining relationships with commercial counterparties; competition from generic products as our products lose patent protection; any failure by us to obtain an addi tio nal period of market exclusivity in the United States for Nexplanon subsequent to the expiration of the rod patents in 2027; as well as the continued impact of our loss of data exclusivity for Atozet; disruptions at the U.S. Food and Drug Administration, the U.S. Securities and Exchange Commission (the “SEC”) and other U.S. and comparable foreign government agencies; pricing pressures globally, including rules an d practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and health care reform, pharmaceutical reimbursement and pricing in genera l; an inability to fully execute on our product development and commercialization plans in the United States, Europe, and elsewhere internationally; the failure by us or our third party col lab orators and/or their suppliers to fulfill our or their regulatory or quality obligations; the impact of higher selling and promotional costs; and the impact of cyberattacks or other events that may affect Organon’s inf ormation technology systems or those of third parties. The company undertakes no obligation to publicly update any forward - looking statement, whether as a result of new information, f uture events or otherwise. Additional factors that could cause results to differ materially from those described in the forward - looking statements can be found in the company’s filings with the SEC, inc luding the company’s most recent Annual Report on Form 10 - K and subsequent SEC filings, available at the SEC’s Internet site (www.sec.gov). 2

Disclaimer statement, cont. Cautionary Note Regarding Non - GAAP Financial Measures This presentation contains “non - GAAP financial measures,” which are financial measures that either exclude or include amounts t hat are correspondingly not excluded or included in the most directly comparable measures calculated and presented in accordance with U.S. generally accepted accounting principles (“GAAP”). Speci fic ally, the company makes use of the non - GAAP financial measures Adjusted EBITDA, Adjusted EBITDA margin, Adjusted gross margin, Adjusted gross profit, Adjusted net income, and Adjusted dilu ted EPS, which are not recognized terms under GAAP and are presented only as a supplement to the company’s GAAP financial statements. This presentation also provides certain measures t hat exclude the impact of foreign exchange. We calculate foreign exchange by converting our current - period local currency financial results using the prior period average currency rates and com paring these adjusted amounts to our current - period results. The company believes that these non - GAAP financial measures help to enhance an understanding of the company’s financial performance. However, the presentation of these measures has limitations as an analytical tool and should not be considered in isolation, or as a substitute for the company’s results as reported under GAA P. Because not all companies use identical calculations, the presentations of these non - GAAP measures may not be comparable to other similarly titled measures of other companies. Please refer to pages 19 - 21 of this presentation for additional information, including relevant definitions and reconciliations of non - GAAP financial measures contained herein to the most directly comparable GAAP measures. In addition, the company’s full - year 2025 guidance measures (other than revenue) are provided on a non - GAAP basis because the co mpany is unable to reasonably predict certain items contained in the GAAP measures. Such items include, but are not limited to, acquisition - related expenses, restructuring and related expenses, stock - based compensation, the ultimate outcome of legal proceedings, unusual gains and losses, the occurrence of matters creating GAAP tax impacts and other items not reflective of the company's on going operations. The company’s management uses the non - GAAP financial measures described above to evaluate the company’s performance and to guide operational and financial decision making. Further, the company’s management believes that these non - GAAP financial measures, which exclude certain items, help to enhance its ability t o meaningfully communicate its underlying business performance, financial condition and results of operations. 3 See Slides 19 - 21 of this presentation for a reconciliation of non - GAAP measures.

Full year 2024 highlights 4 • Revenue of $6.4 billion, up 3% ex - FX • All three franchises grew at constant currency • Diluted EPS of $3.33; Adj. Diluted EPS of $4.11 • Adj. EBITDA of $1.96 billion, inclusive of $81 million of IPR&D and milestones, representing 30.6% Adjusted EBITDA margin See Slides 19 - 21 of this presentation for a reconciliation of non - GAAP measures.

Women’s Health Women’s Health Ex - FX VPY Act VPY FY 2023 FY 2024 Ex - FX VPY Act VPY Q4 - 23 Q4 - 24 Revenues $ mil 17% 16% 830 963 12% 12% 231 258 Nexplanon ® (contraception) (33)% (35)% 176 115 (37)% (38)% 39 24 NuvaRing ® (contraception) 2% — % 134 134 (16)% (16)% 37 31 Marvelon / Mercilon (contraception) (9)% (10)% 262 237 (21)% (21)% 83 65 Follistim AQ ® (fertility) 1% (1)% 110 109 26% 27% 22 28 Ganirelix Acetate Injection (fertility) 40% 40% 43 61 39% 39% 13 18 Jada ® (device) 10% 9% 147 158 2% — % 40 42 Other Women's Health products 5% 4% 1,702 1,777 — % — % 465 466 Total Women's Health • Franchise growth of 5% • Record year in Nexplanon, on track to eclipse $1 billion of revenue in 2025 Totals may not foot due to rounding . Trademarks appearing above in italics are trademarks of, or are used under license by, the Organon group of companies . 5

Biosimilars Biosimilars Ex - FX VPY Act VPY FY 2023 FY 2024 Ex - FX VPY Act VPY Q4 - 23 Q4 - 24 Revenues $ mil (1)% (1)% 278 274 (16)% (16)% 77 65 Renflexis ® (9)% (9)% 155 141 (45)% (46)% 62 34 Ontruzant ® 6% 6% 73 77 (47)% (47)% 28 15 Brenzys (35)% (34)% 43 28 (33)% (32)% 9 6 Aybintio NM NM 44 142 NM NM 23 44 Hadlima ® 12% 12% 593 662 (18)% (18)% 199 163 Biosimilars 6 • Third consecutive year of double - digit revenue growth • Hadlima achieves over $100 million of sales in U.S. • Renflexis and Ontruzant at mature phase of lifecycle Totals may not foot due to rounding . Trademarks appearing above in italics are trademarks of, or are used under license by, the Organon group of companies .
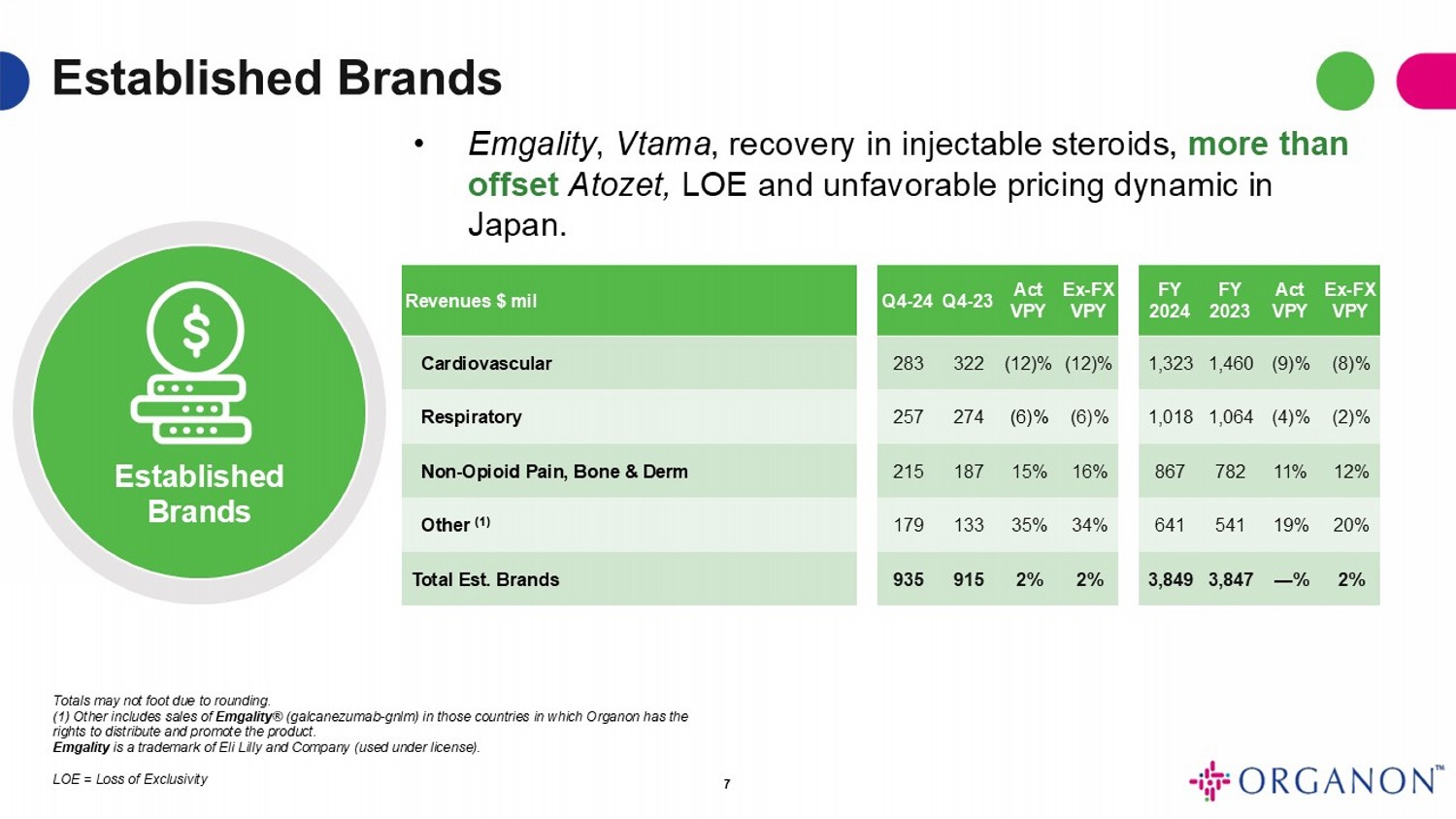
Established Brands Established Brands Ex - FX VPY Act VPY FY 2023 FY 2024 Ex - FX VPY Act VPY Q4 - 23 Q4 - 24 Revenues $ mil (8)% (9)% 1,460 1,323 (12)% (12)% 322 283 Cardiovascular (2)% (4)% 1,064 1,018 (6)% (6)% 274 257 Respiratory 12% 11% 782 867 16% 15% 187 215 Non - Opioid Pain, Bone & Derm 20% 19% 541 641 34% 35% 133 179 Other (1) 2% — % 3,847 3,849 2% 2% 915 935 Total Est. Brands 7 • Emgality , Vtama , recovery in injectable steroids, more than offset Atozet, LOE and unfavorable pricing dynamic in Japan. Totals may not foot due to rounding . (1) Other includes sales of Emgality ® (galcanezumab - gnlm) in those countries in which Organon has the rights to distribute and promote the product. Emgality is a trademark of Eli Lilly and Company (used under license) . LOE = Loss of Exclusivity
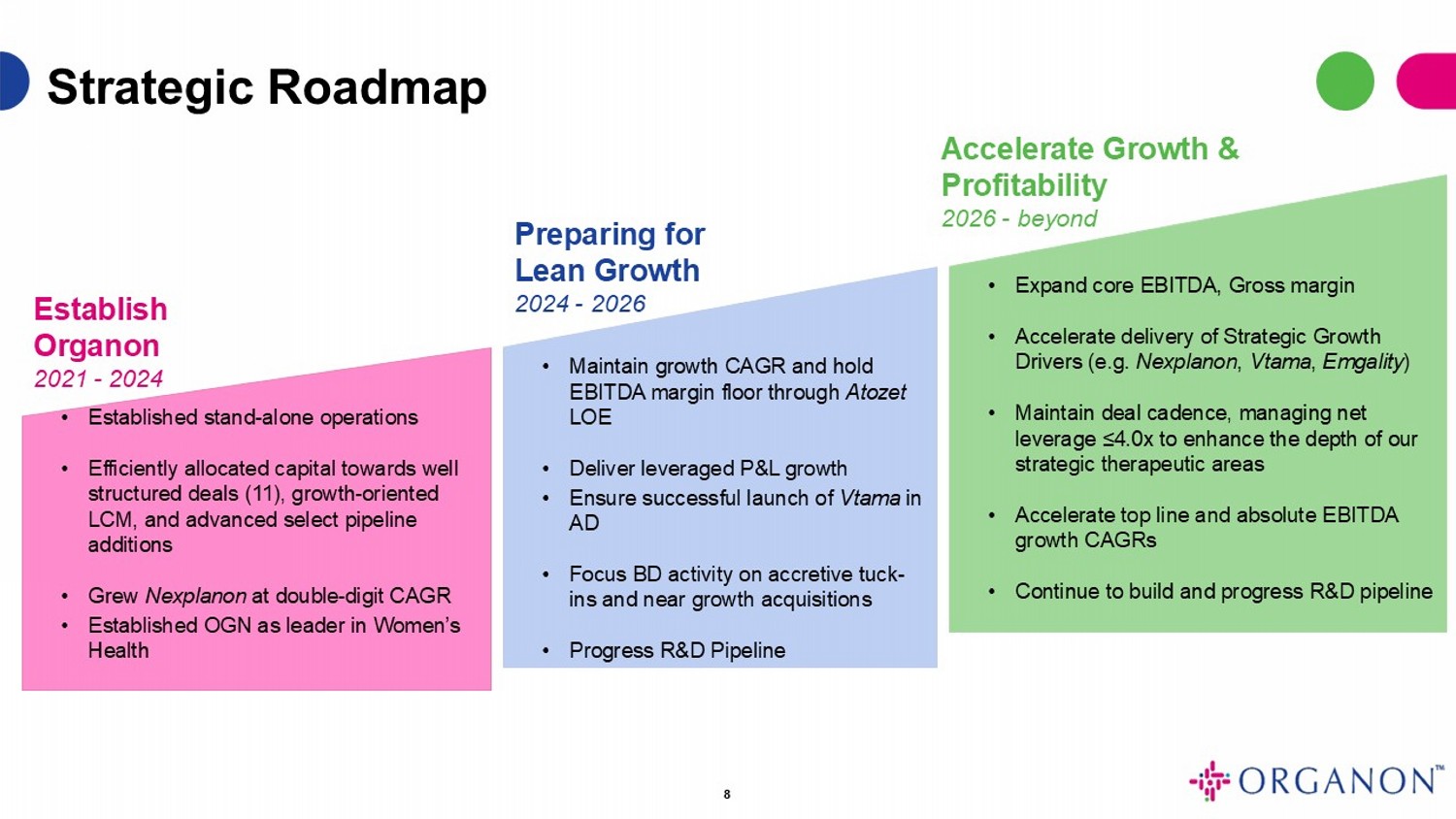
8 Strategic Roadmap • Maintain growth CAGR and hold EBITDA margin floor through Atozet LOE • Deliver leveraged P&L growth • Ensure successful launch of Vtama in AD • Focus BD activity on accretive tuck - ins and near growth acquisitions • Progress R&D Pipeline • Established stand - alone operations • Efficiently allocated capital towards well structured deals (11), growth - oriented LCM, and advanced select pipeline additions • Grew Nexplanon at double - digit CAGR • Established OGN as leader in Women’s Health • Expand core EBITDA, Gross margin • Accelerate delivery of Strategic Growth Drivers (e.g. Nexplanon , Vtama , Emgality ) • Maintain deal cadence, managing net leverage ≤4.0x to enhance the depth of our strategic therapeutic areas • Accelerate top line and absolute EBITDA growth CAGRs • Continue to build and progress R&D pipeline Establish Organon 2021 - 2024 Preparing for Lean Growth 2024 - 2026 Accelerate Growth & Profitability 2026 - beyond

9 Strategic Pillars for 2025 Demonstrate resilience in base business Portfolio managed entrepreneurially since spin, driving cash flow for re - investment and growth Enhance value capture from efficiency Consistent capital allocation Deliver promise of growth products and pipeline Offset incremental Dermavant expenses, managing to best op - ex efficiency since spin - off Continue capital allocation priorities servicing capex and dividend first Nexplanon blockbuster status, >$1B global sales ~$300M from new business development including $150M in Vtama

Women's Health Established Brands Biosimilars Key milestones in our R&D pipeline Clinical Regulatory Clinical development ongoing 1 Ph2b topline results OG - 6219 OG - 8276A (Mercilon Japan) Anticipated Ph3 study completion Atopic Dermatitis U.S. approval (1) Organon has option to acquire “ Claria ”, not currently owned by Organon. (2) Organon has exclusive commercialization rights in China for Bao Pharma’s investigational asset, SJ02, in fertility. (3) Prolia and Xgeva are trademarks registered in the U.S. in the name of Amgen Inc.; Perjeta is a trademark registered in the U.S. in the name of Genentech, Inc. SJ02 HA reviews (ongoing) (bPerjeta) 3 HLX - 11 (bProlia/bXgeva) 3 HLX - 14 Anticipated U.S. approval 5 year - indication Anticipated China approval 2 4Q24 1H25 2H25 Anticipated approval timelines subject to final health authority review/approva l 10

+2% reported +3% at constant currency $ mil 11 Continued strength in volume growth (1) LOE = Loss of Exclusivity (2) VBP = Volume Based Procurement (3) Other includes manufacturing sales to third parties. (3) (1) (2) ~ ~ ~ ~ ~ ~ ~(130) bps headwind 415
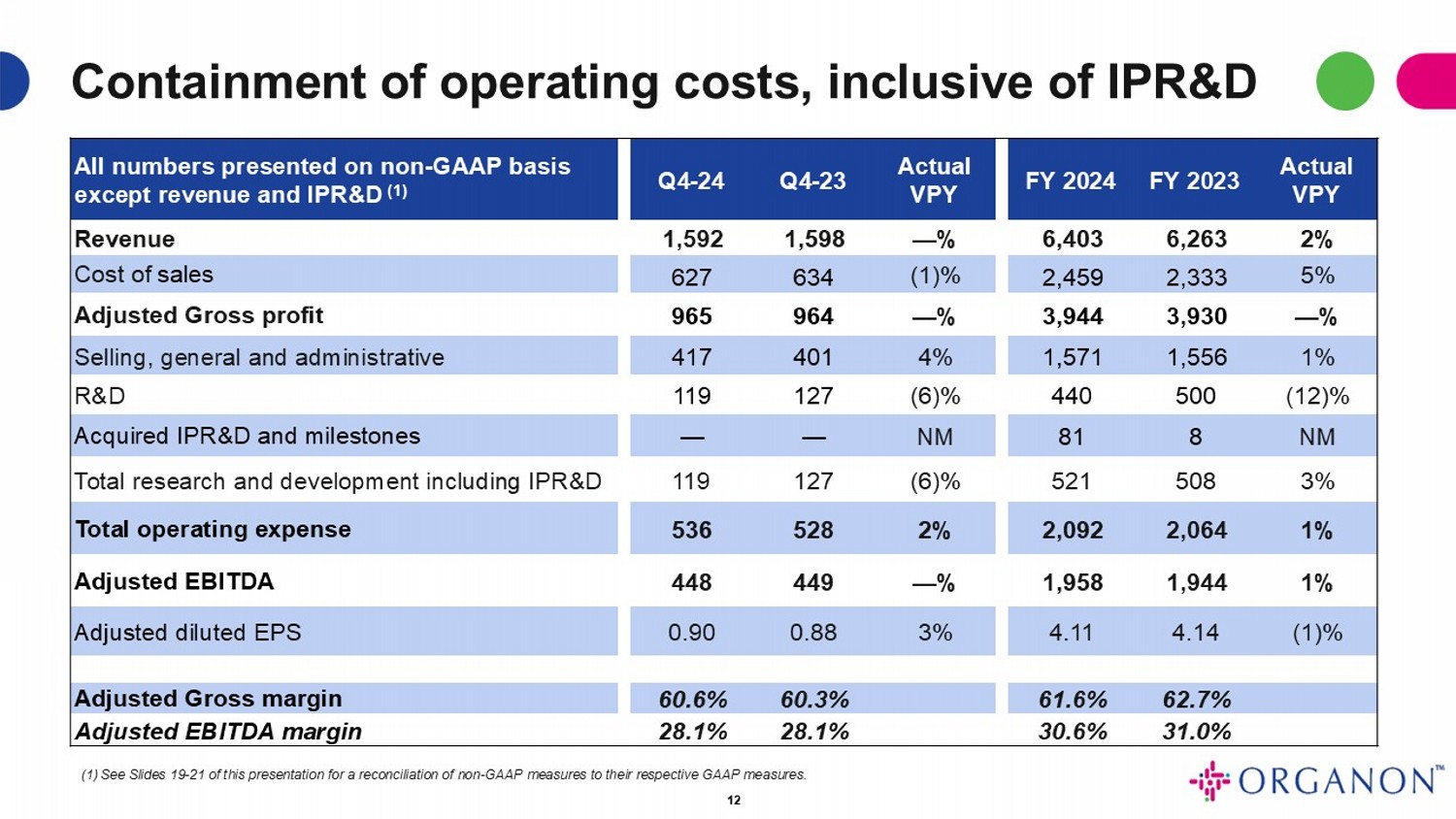
Containment of operating costs, inclusive of IPR&D Actual VPY FY 2023 FY 2024 Actual VPY Q4 - 23 Q4 - 24 All numbers presented on non - GAAP basis except revenue and IPR&D (1) 2% 6,263 6,403 — % 1,598 1,592 Revenue 5% 2,333 2,459 (1)% 634 627 Cost of sales — % 3,930 3,944 — % 964 965 Adjusted Gross profit 1% 1,556 1,571 4% 401 417 Selling, general and administrative (12)% 500 440 (6)% 127 119 R&D NM 8 81 NM — — Acquired IPR&D and milestones 3% 508 521 (6)% 127 119 Total research and development including IPR&D 1% 2,064 2,092 2% 528 536 Total operating expense 1% 1,944 1,958 — % 449 448 Adjusted EBITDA (1)% 4.14 4.11 3% 0.88 0.90 Adjusted diluted EPS 62.7% 61.6% 60.3% 60.6% Adjusted Gross margin 31.0% 30.6% 28.1% 28.1% Adjusted EBITDA margin (1) See Slides 19 - 21 of this presentation for a reconciliation of non - GAAP measures to their respective GAAP measures. 12

13 Full year 2023 Full year 2024 (USD millions) $1,944 $1,958 Adjusted EBITDA (496) (486) Less: Net cash interest expense (135) (293) Less: Cash taxes (225) (89) Less: Change in net working capital (148) (123) Less: CapEx $940 $967 Free Cash Flow Before One - Time Costs (344) (160) Less: One - time spin - related costs (35) (190) Less: Other one - time costs (1) $561 $617 Free Cash Flow (2) Delivered on ~$1B of free cash flow before one - time costs for full year 2024 (1) For 2024, includes cash payments associated with restructuring initiatives ($87M), planned exits from supply agreements with Merck & Co., Inc., Rahway, NJ. ($60M), one - time acquisition costs ($18M) and the second payment on the Microspherix settlement ($25M). The $35M in 2023 was the first payment on Microspherix. (2) Free cash flow represents net cash flows provided by operating activities plus capital expenditures and the effect of exchange rate changes on cash and cash equivalents. • Cash taxes normalized compared with unusually low 2023 • Active net working capital management • Significant decline in spin related one - time costs • Increase in other one - time costs due to OpEx restructuring and MSA exits

14 Net leverage ratio ~4.2x at December 31, 2024 Dec 2024 Dec 2023 Dec 2022 Dec 2021 $ mil $675 $693 $706 $737 Cash and cash equivalents 8,889 8,760 8,913 9,134 Gross Debt (1) 8,214 8,067 8,207 8,397 Net Debt (1) * The definition of net debt is in the company's credit agreement and excludes unamortized fees; but includes capitalized lea se obligations. Additionally, the LTM EBITDA calculation excludes acquired IPR&D and milestone expense. (1) Debt figures are net of discounts and unamortized fees of $124 million, $105 million, $84 million, and $97 million as of Dec ember 31, 2021, December 31, 2022, December 31, 2023 and December 31, 2024, respectively.

Growth in Nexplanon , Emgality and Vtama expected to offset LOE, pricing $ mil 15 ( 1) LOE = Loss of Exclusivity (2) VBP = Value Based Procurement (3) Other includes manufacturing sales to third parties. Emgality is a trademark of Eli Lilly and Company (used under license) . (1) (2) (3) (4.3%) to (1.2%) reported (1.2%) to +1.9% constant currency ~(300) bps headwind to growth in 2025 ~ ~ ~ ~ ~ ~ $6,125 - $6,325
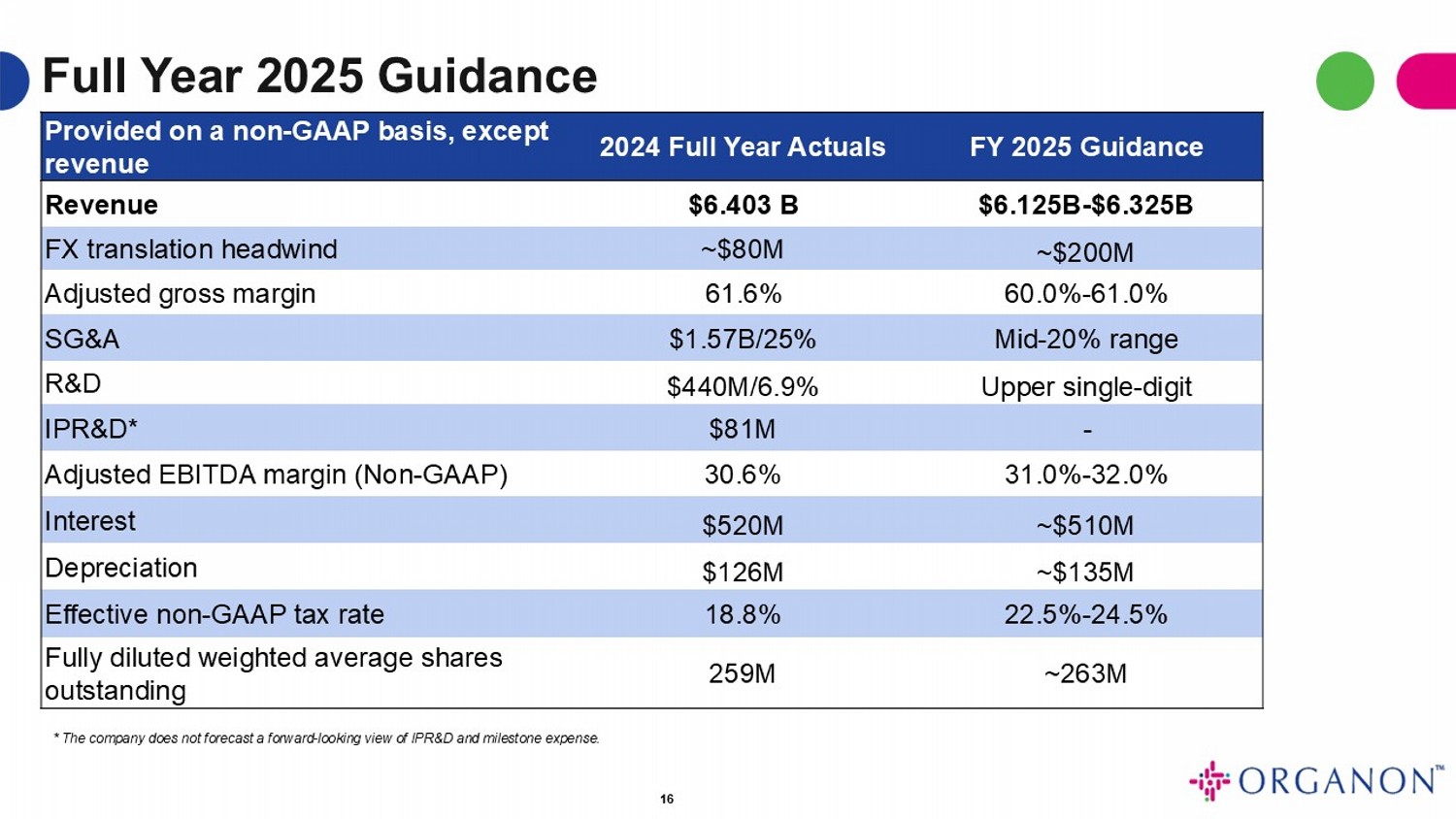
Full Year 2025 Guidance 16 FY 2025 Guidance 2024 Full Year Actuals Provided on a non - GAAP basis, except revenue $6.125B - $6.325B $6.403 B Revenue ~$200M ~$80M FX translation headwind 60.0% - 61.0% 61.6% Adjusted gross margin Mid - 20% range $1.57B/25% SG&A Upper single - digit $440M/6.9% R&D - $81M IPR&D* 31.0% - 32.0% 30.6% Adjusted EBITDA margin (Non - GAAP) ~$510M $520M Interest ~$135M $126M Depreciation 22.5% - 24.5% 18.8% Effective non - GAAP tax rate ~263M 259M Fully diluted weighted average shares outstanding * The company does not forecast a forward - looking view of IPR&D and milestone expense.
Q&A
Appendix
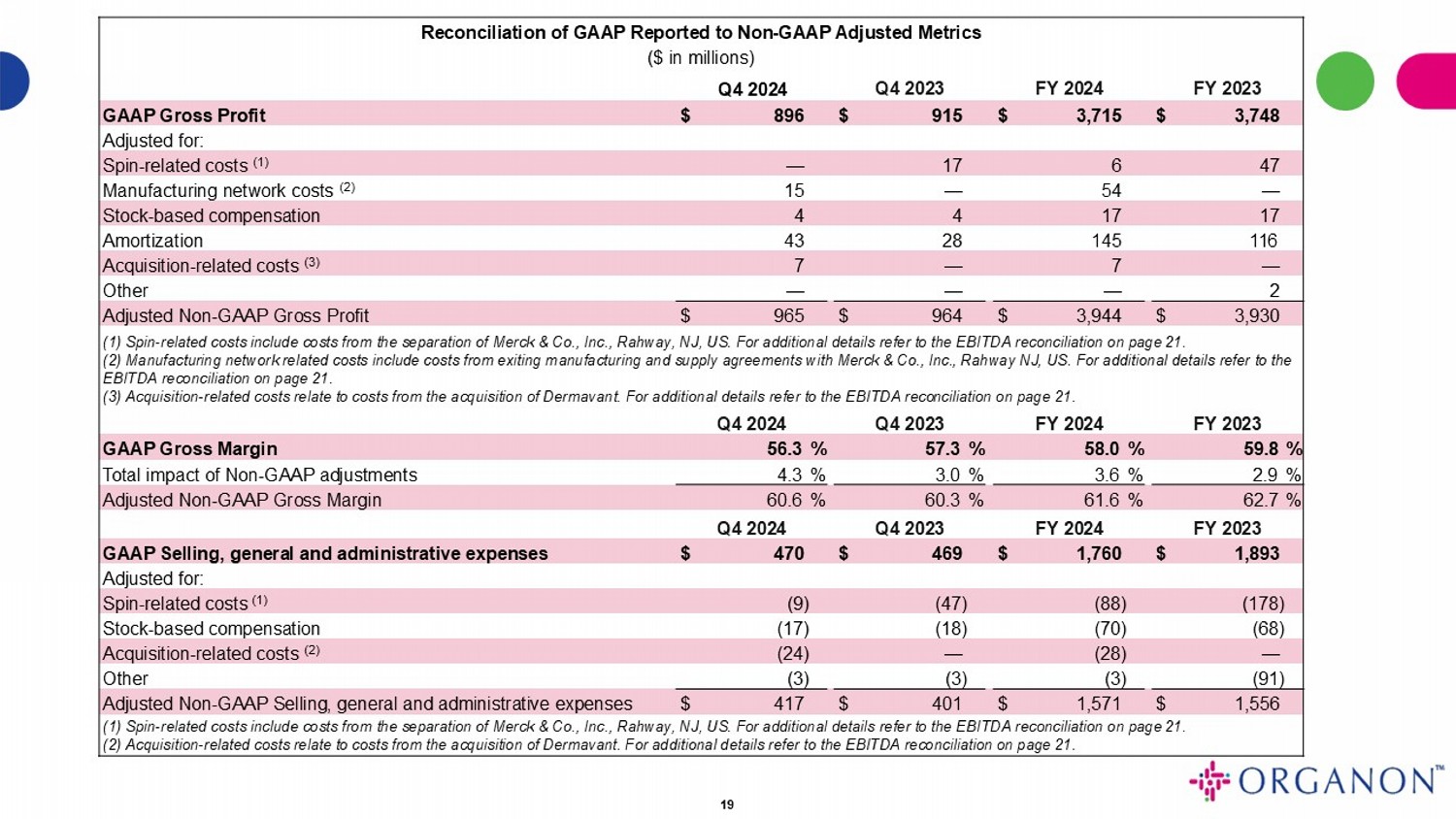
Reconciliation of GAAP Reported to Non - GAAP Adjusted Metrics ($ in millions) FY 2023 FY 2024 Q4 2023 Q4 2024 $ 3,748 $ 3,715 $ 915 $ 896 GAAP Gross Profit Adjusted for: 47 6 17 — Spin - related costs (1) — 54 — 15 Manufacturing network costs (2) 17 17 4 4 Stock - based compensation 116 145 28 43 Amortization — 7 — 7 Acquisition - related costs (3) 2 — — — Other $ 3,930 $ 3,944 $ 964 $ 965 Adjusted Non - GAAP Gross Profit (1) Spin - related costs include costs from the separation of Merck & Co., Inc., Rahway, NJ, US. For additional details refer to t he EBITDA reconciliation on page 21 . (2) Manufacturing network related costs include costs from exiting manufacturing and supply agreements with Merck & Co., Inc. , R ahway NJ, US. For additional details refer to the EBITDA reconciliation on page 21 . (3) Acquisition - related costs relate to costs from the acquisition of Dermavant. For additional details refer to the EBITDA reco nciliation on page 21 . FY 2023 FY 2024 Q4 2023 Q4 2024 59.8 % 58.0 % 57.3 % 56.3 % GAAP Gross Margin 2.9 % 3.6 % 3.0 % 4.3 % Total impact of Non - GAAP adjustments 62.7 % 61.6 % 60.3 % 60.6 % Adjusted Non - GAAP Gross Margin FY 2023 FY 2024 Q4 2023 Q4 2024 $ 1,893 $ 1,760 $ 469 $ 470 GAAP Selling, general and administrative expenses Adjusted for: (178) (88) (47) (9) Spin - related costs (1) (68) (70) (18) (17) Stock - based compensation — (28) — (24) Acquisition - related costs (2) (91) (3) (3) (3) Other $ 1,556 $ 1,571 $ 401 $ 417 Adjusted Non - GAAP Selling, general and administrative expenses (1) Spin - related costs include costs from the separation of Merck & Co., Inc., Rahway, NJ, US. For additional details refer to t he EBITDA reconciliation on page 21 . (2) Acquisition - related costs relate to costs from the acquisition of Dermavant . For additional details refer to the EBITDA reconciliation on page 21 . 19
Reconciliation of GAAP Reported to Non - GAAP Adjusted Metrics ($ in millions, except per share amounts) FY 2023 FY 2024 Q4 2023 Q4 2024 $ 528 $ 469 $ 134 $ 130 GAAP Research and development expenses Adjusted for: (12) (11) (2) (6) Spin - related costs (1) (16) (18) (5) (5) Stock - based compensation $ 500 $ 440 $ 127 $ 119 Adjusted Non - GAAP Research and development expenses (1) Spin - related costs include costs from the separation of Merck & Co., Inc., Rahway, NJ, US. For additional details refer to t he EBITDA reconciliation on page 21 . FY 2023 FY 2024 Q4 2023 Q4 2024 $ 1,023 $ 864 $ 546 $ 109 GAAP Reported Net Income Adjusted for: 182 229 49 69 Cost of sales adjustments 337 189 68 53 Selling, general and administrative adjustments 28 29 7 11 Research and development adjustments 62 31 58 8 Restructuring — 11 — 11 Change in fair value of contingent consideration 17 16 4 2 Other expense, net (588) (304) (506) (28) Tax impact on adjustments above (1) $ 1,061 $ 1,065 $ 226 $ 235 Non - GAAP Adjusted Net Income (1) For the three months ended December 31, 2024 and 2023, the GAAP income tax rates were 15.3% and (425.9)%, respectively, the non - GAAP income tax rates were 17.1% and 21.8%, respectively. For the year ended December 31, 2024 and 2023, the GAAP income tax rates were (7.1)% and (52.2)%, respe ctively, the non - GAAP income tax rates were 18.8% and 18.3%, respectively. These adjustments represent the estimated tax impacts on the reconciling items by applyin g t he statutory rate and applicable law of the originating territory of the non - GAAP adjustments. FY 2023 FY 2024 Q4 2023 Q4 2024 $ 3.99 $ 3.33 $ 2.13 $ 0.42 GAAP Diluted Earnings per Share 0.15 0.78 (1.25) 0.48 Total impact of Non - GAAP adjustments $ 4.14 $ 4.11 $ 0.88 $ 0.90 Non - GAAP Diluted Earnings per Share 20

GAAP Net Income to Adjusted EBITDA FY 2023 FY 2024 Q4 2023 Q4 2024 Unaudited, $ in millions $ 1,023 $ 864 $ 546 $ 109 Net income 118 126 30 33 Depreciation (1) 116 145 28 43 Amortization 527 520 129 132 Interest expense (350) (57) (442) 20 Income tax expense (benefit) $ 1,434 $ 1,598 $ 291 $ 337 EBITDA (Non - GAAP) 62 31 58 8 Restructuring costs 254 121 70 17 Spin - related costs (2) — 54 — 15 Manufacturing network related (3) — 35 — 31 Acquisition - related costs (4) — 11 — 11 Change in fair value of contingent consideration 93 3 3 3 Other costs (5) 101 105 27 26 Stock - based compensation $ 1,944 $ 1,958 $ 449 $ 448 Adjusted EBITDA (Non - GAAP) 31.0 % 30.6 % 28.1 % 28.1 % Adjusted EBITDA margin (Non - GAAP) 21 (1) Excludes accelerated depreciation included in one - time costs. (2) Spin - related costs reflect certain costs incurred in connection with activities taken to separate Organon from Merck & Co., Inc., Rahway, NJ, US. These costs include, but are not limited to, $6 million and $34 million for the three months ended December 31, 2024 and 2023, respectively, and $53 million and $134 million for the year ended December 31, 2024 and 2023, respectively, for information technology infrastructure, primarily related to the implementation of a stand - alone enterprise resource planning system and redundant softw are licensing costs, as well as $8 million for the three months ended December 31, 2023 and $20 million and $28 million for the year ended December 31, 2024 and 2023, respectively, associated with temporary tran sition service agreements with Merck & Co., Inc., Rahway, NJ, US. (3) Manufacturing network related costs, including exiting of temporary manufacturing and supply agreements with Merck & Co., In c., Rahway, NJ, US, reflect accelerated depreciation, exit premiums, technology transfer costs, stability and qualification batch costs, and third - party contractor costs. (4) Acquisition - related costs for the three months ended December 31, 2024 reflect $8 million of transaction related costs, $10 million of Dermavant transaction bonuses and separation charges and $7 million of amortization pertaining to the fair value inventory purchase accounting adjustment. Acquisition related costs for the year en ded December 31, 2024 reflect $12 million of transaction related costs, $10 million of Dermavant transaction bonuses and separation charges and $7 million of amortization pertaining to the fair value inventory pu rch ase accounting adjustment. (5) Other costs for the year ended December 31, 2023, include $80 million related to the Microspherix legal matter. As the costs described in (1) through (5) above are directly related to the separation of Organon and acquisition related act ivi ties and therefore arise from a one - time event outside of the ordinary course of the company’s operations, the adjustment of these items provides meaningful, supplemental, information that the company believes wil l enhance an investor's understanding of the company's ongoing operating performance.

Flat as reported and at constant currency $ mil 22 Volume growth offsets price erosion and LOE impacts (1) LOE = Loss of Exclusivity (2) VBP = Volume Based Procurement (3) Other includes manufacturing sales to third parties. (3) (1) (2) ~ ~ ~ ~ ~ ~
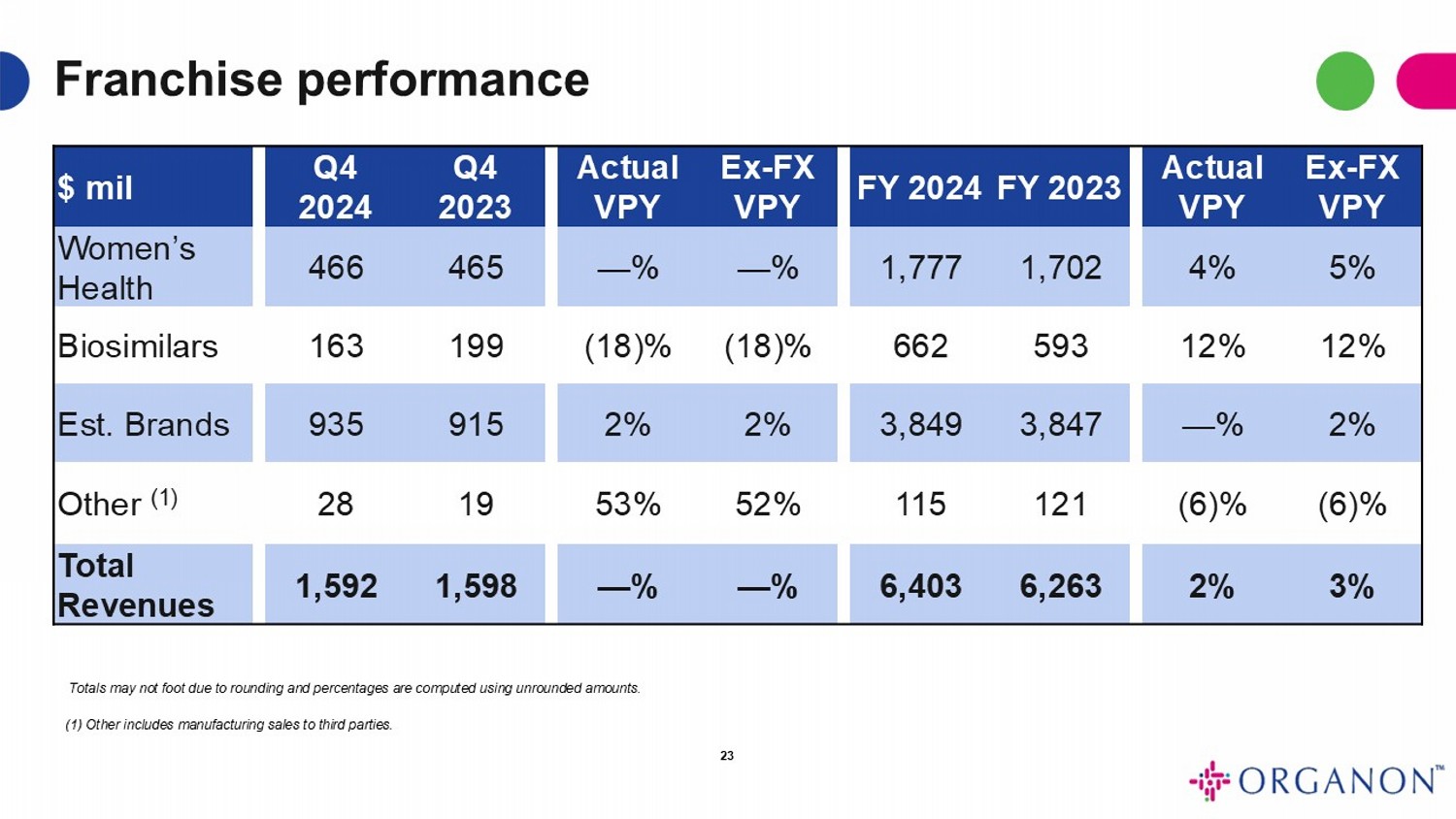
Franchise performance Ex - FX VPY Actual VPY FY 2023 FY 2024 Ex - FX VPY Actual VPY Q4 2023 Q4 2024 $ mil 5% 4% 1,702 1,777 — % — % 465 466 Women’s Health 12% 12% 593 662 (18)% (18)% 199 163 Biosimilars 2% — % 3,847 3,849 2% 2% 915 935 Est. Brands (6)% (6)% 121 115 52% 53% 19 28 Other (1) 3% 2% 6,263 6,403 — % — % 1,598 1,592 Total Revenues Totals may not foot due to rounding and percentages are computed using unrounded amounts. (1) Other includes manufacturing sales to third parties. 23
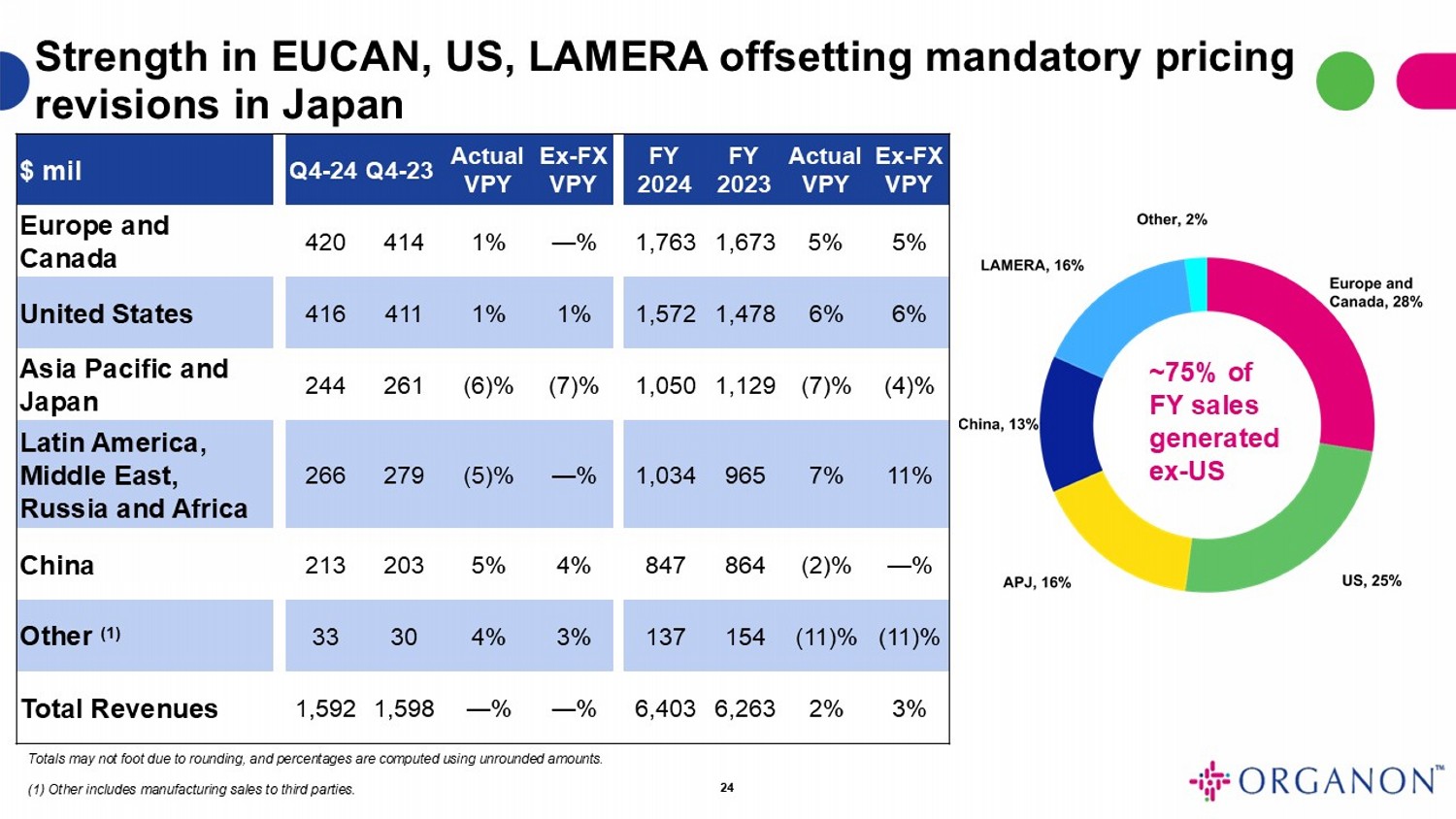
Ex - FX VPY Actual VPY FY 2023 FY 2024 Ex - FX VPY Actual VPY Q4 - 23 Q4 - 24 $ mil 5% 5% 1,673 1,763 — % 1% 414 420 Europe and Canada 6% 6% 1,478 1,572 1% 1% 411 416 United States (4)% (7)% 1,129 1,050 (7)% (6)% 261 244 Asia Pacific and Japan 11% 7% 965 1,034 — % (5)% 279 266 Latin America, Middle East, Russia and Africa — % (2)% 864 847 4% 5% 203 213 China (11)% (11)% 154 137 3% 4% 30 33 Other (1) 3% 2% 6,263 6,403 — % — % 1,598 1,592 Total Revenues Totals may not foot due to rounding, and percentages are computed using unrounded amounts. (1) Other includes manufacturing sales to third parties. ~75% of FY sales generated ex - US Strength in EUCAN, US, LAMERA offsetting mandatory pricing revisions in Japan 24
Women's Health 25 AhR = aryl - hydrocarbon receptor agonist, PDE - 4 = phosphodiesterase 4 inhibitor, JAK = janus kinase inhibitor, IL - 4 = interleukin 4 receptor alpha antagonist Biosimilars Women's Health Approved Vtama label vs non - steroidal treatments IL - 4 JAK PDE 4 AhR Dupixent * 16 Weeks Opzelura * 8 Weeks Zoryve * 4 Weeks Eucrisa * 4 Weeks Vtama ® 8 Weeks Once Every 2 Weeks - Once Every 4 Weeks Subcutaneous injection Twice Daily Topical cream Short term, ≤20% Body Surface Area Once Daily Topical Cream Twice Daily Topical Ointment Once Daily Topical Cream Dosing regimen Second line Second line First line First line First line Indication >6 months >12 years >6 years >3 months >2 years Population Mod. - Severe Mild - Moderate Mild - Moderate Mild - Moderate Mild, Moderate, Severe Disease severity No Yes Yes No No Limitations No Yes No No No Boxed Warning No Yes No Yes No Warnings & Precautions 36% / 38% 10% / 9% 54% / 51% 15% / 8% 32% / 29% 15% / 12% 33% / 32% 25% / 18% 45% / 46% 14% / 18% Investigator Global Assessment Score placebo 41% / 36% 12% / 10% 52% / 51% 15% / 16% 34% / 30% 21% / 12% N/A 56 % / 53% 34% / 24% Peak Pruritus placebo *Eucrisa is a trademark registered in the United States in the name of Anacor Pharmaceuticals, LLC. * Zoryve is a trademark registered in the United States in the name of Arcutis Biotherapeutics, Inc. *Opzelura is a trademark registered in the United States in the name of Incyte Holdings Corporation. *Dupixent is a trademark registered in the United States in the name of Sanofi Biotechnology. *Information based on product labeling as of January 28, 2025, and does not reflect results from a head - to - head study. Differenc es exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studie s.

Number of products 14 5 56 Women’s Health Biosimilars Established Brands Broad and diverse portfolio TM 26 Emgality is a trademark of Eli Lilly and Company (used under license) .
v3.25.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Organon (NYSE:OGN)
Graphique Historique de l'Action
De Jan 2025 à Fév 2025
Organon (NYSE:OGN)
Graphique Historique de l'Action
De Fév 2024 à Fév 2025