Teva Pharmaceutical Industries Shares Retreat After FDA Rejection Letter
20 Avril 2022 - 12:10AM
Dow Jones News
By Denny Jacob
Teva Pharmaceutical Industries Ltd. shares fell 0.7% to $10.11
after hours Tuesday after the Food and Drug Administration sent a
rejection letter to its U.S. affiliate and MedinCell on the New
Drug Application for TV-46000/mdc-IRM (risperidone extended-release
injectable suspension) in the treatment of schizophrenia.
Teva said it is reviewing next steps based on the Complete
Response Letter and will work closely with the FDA to address their
recommendations. Its application included Phase 3 data from two
TV-46000 studies that evaluated its efficacy and long-term safety
as a treatment for patients with schizophrenia.
Upon receiving a Complete Response Letter, the company has the
option to refile its application if it meets the criteria set out
in the letter.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
April 19, 2022 17:55 ET (21:55 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Teva Pharmaceutical Indu... (NYSE:TEVA)
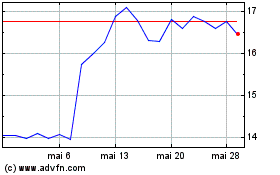
Graphique Historique de l'Action
De Juin 2024 à Juil 2024
Teva Pharmaceutical Indu... (NYSE:TEVA)
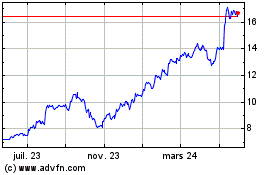
Graphique Historique de l'Action
De Juil 2023 à Juil 2024