Teva Gets FDA Approval for Movement Disorders Treatment
18 Février 2023 - 12:03AM
Dow Jones News
By Sabela Ojea
Teva Pharmaceutical Industries Ltd. has received U.S. Food and
Drug Administration approval for its Austedo Xr medication, which
treats a range of uncontrollable muscle movements associated with
Huntington's Disease.
The generic-drug maker said the FDA has approved Austedo Xr's
extended-release tablets, a new once-daily formula for adults
diagnosed with tardive dyskinesia and chorea.
Huntington's Disease is a rare and inherited progressive
neurological disorder that causes nerve cells in parts of the brain
to gradually break down and die.
Shares inched up 0.5% to $10.11 in after-hours trading.
Write to Sabela Ojea at sabela.ojea@wsj.com; @sabelaojeaguix
(END) Dow Jones Newswires
February 17, 2023 17:48 ET (22:48 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
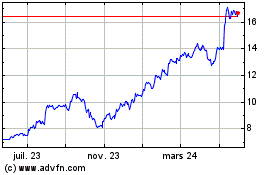
Teva Pharmaceutical Indu... (NYSE:TEVA)
Graphique Historique de l'Action
De Juin 2024 à Juil 2024
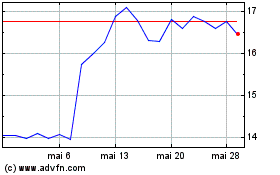
Teva Pharmaceutical Indu... (NYSE:TEVA)
Graphique Historique de l'Action
De Juil 2023 à Juil 2024