UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the Month of September 2023
001-36203
(Commission File Number)
CAN-FITE BIOPHARMA LTD.
(Exact name of Registrant as specified in its charter)
10 Bareket Street
Kiryat Matalon, P.O. Box 7537
Petach-Tikva 4951778, Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant
files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Can-Fite BioPharma Ltd. has
posted to its website an updated corporate presentation. A copy of the presentation is furnished with this Report of Foreign Private Issuer
on Form 6-K as Exhibit 99.1 and is incorporated herein by reference.
Exhibit Index
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| Date: September 7, 2023 |
By: |
/s/ Motti Farbstein |
| |
|
Motti Farbstein |
| |
|
Chief Executive Officer and Chief Financial Officer |
2
Exhibit 99.1

Small Molecules for Big Clinical Needs NYSE American:CANF | September 2023 Investor Presentation TM

Forward Looking Statement 2 This presentation contains forward - looking statements, about Can - Fite’s expectations, beliefs or intentions regarding, among other things, its product development efforts, business, financial condition, results of operations, strategies or prospects . All statements in this communication, other than those relating to historical facts, are “forward looking statements” . Forward - looking statements can be identified by the use of forward - looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters . Forward - looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made . Because forward - looking statements relate to matters that have not yet occurred, these statements are inherently subject to known and unknown risks, uncertainties and other factors that may cause Can - Fite’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements . Important factors that could cause actual results, performance or achievements to differ materially from those anticipated in these forward - looking statements include, among other things, our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all ; uncertainties of cash flows and inability to meet working capital needs ; the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts ; our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials ; our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals ; the clinical development, commercialization and market acceptance of our product candidates ; our ability to establish and maintain strategic partnerships and other corporate collaborations ; the implementation of our business model and strategic plans for our business and product candidates ; the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others ; competitive companies, technologies and our industry ; risks related to the COVID - 19 pandemic and the Russian invasion of Ukraine ; risks related to not satisfying the continued listing requirements of NYSE American ; and statements as to the impact of the political and security situation in Israel on our business . More information on these risks, uncertainties and other factors is included from time to time in the “Risk Factors” section of Can - Fite’s Annual Report on Form 20 - F filed with the SEC on March 30 , 2023 and other public reports filed with the SEC and in its periodic filings with the TASE . Existing and prospective investors are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof . Can - Fite undertakes no obligation to publicly update or review any forward - looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws .

Small Molecule Drugs for the Treatment of Oncological and Inflammatory Diseases Robust Clinical Proof of Concept & Excellent safety profile Successful Out - licensing Deals Company Overview 3 1 2 3 Financial Summary (Ticker: CANF) Listed on NYSE American and Tel - Aviv Stock Exchange ~4 M ADRs outstanding; ~1,225 M ordinary shares outstanding (1 ADR = 300 Ordinary Shares) Cash: ~$9.6 M as of June 30, 2023 4

4 Normal Cells • Global leader in discovering and developing drugs that target the A3 adenosine receptor (A3AR) Therapeutic Target • Demonstrated in >1600 patients across clinical trials Excellent Safety Profile • High efficacy and good safety ptofile with anti - inflammatory and anti - cancer effects shown in Phase 2 and Phase 3 studies Proven Therapeutic Effect • Small molecule, orally bioavailable drugs • Bind only to pathological cells, not normal cells Pipeline Drugs Unique Platform Technology Specific oral therapy aimed at diseased cells Pathological Cells A3 Adenosine Receptor (A3AR) NYSE: CANF A3 Adenosine Receptor Pathological Cell Normal Cell
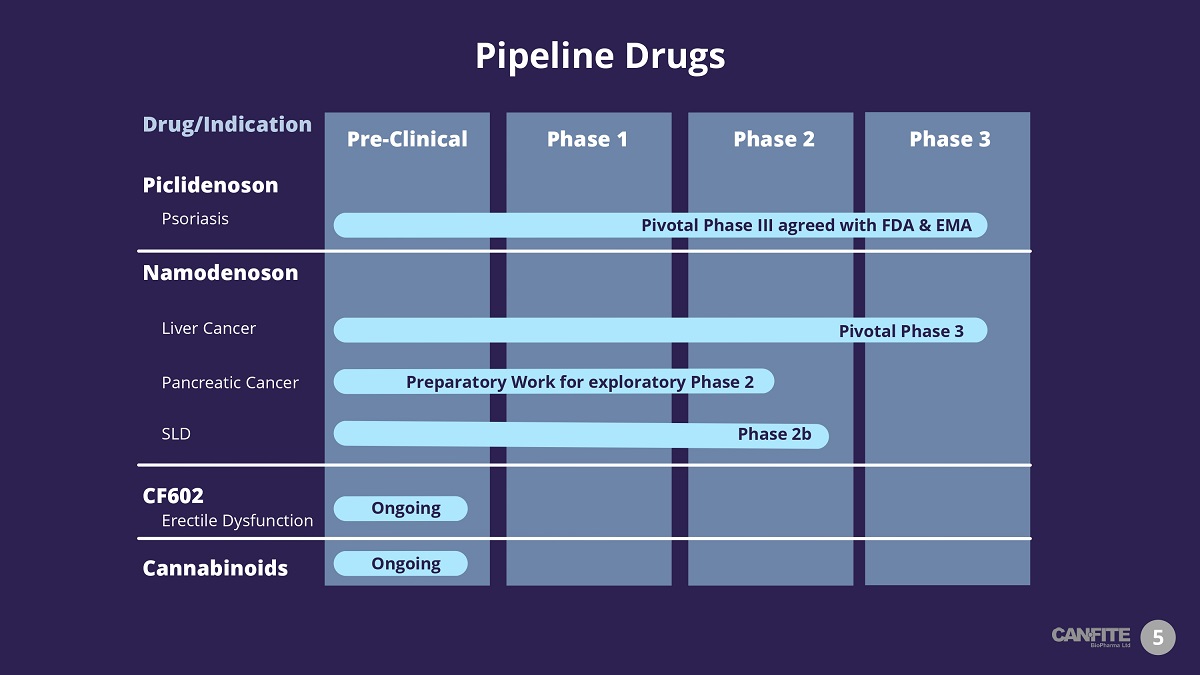
Pipeline Drugs 5 Piclidenoson Psoriasis Phase 1 Phase 3 Phase 2 Pre - Clinical Namodenoson Liver Cancer SLD Pivotal Phase III agreed with FDA & EMA Pivotal Phase 3 Phase 2b CF602 Erectile Dysfunction Cannabinoids Ongoing Drug/Indication Ongoing Pancreatic Cancer Preparatory Work for exploratory Phase 2

Corporate Partnerships: Current Out - Licensing Deals potential based on regulatory and sales milestones $20M received in upfront and milestone payments $130M *$8.5M was from a license with a Japanese company, SKK; the license was terminated due to SKK’s strategic change of focus to ind ications not related to autoimmune diseases. Psoriasis, Liver Cancer, SLD Liver Cancer, SLD Psoriasis Psoriasis Psoriasis, Liver Cancer, SLD Typical Deal Structure • Up - front money upon signing a distribution deal • Regulatory milestone payments • Royalties (double - digits) • Sales milestone payments 6 VETBIOLIX Psoriasis Piclidenoson - Pets’ Osteoarthritis Canada Spain, Switzerland, Austria South Korea South Korea China, Taiwan, Hong Kong, Macao Global Eastern Europe * Corporate Partnerships: Current Out - Licensing Deals * Estonia, Lithuania, Latvia, Bosnia, Bulgaria, Croatia, Czech Republic, Hungary, Moldavia, North Macedonia, Poland, Romania, S erb ia, Slovakia, Slovenia

Moderate to Severe Psoriasis Piclidenoson • Nucleoside derivative • Highly Selective A3AR Agonist • Molecular weight - 510.29 • Water insoluble • Half lifetime in blood – 8 - 9 hours Piclidenoson Drug Candidate Chemical Properties 7

Piclidenoson - Drug Profile • Overexpression of the A3AR target in Keratinocytes of psoriasis patients • Robust anti - inflammatory effect manifested by specific apoptosis of inflammatory cells • Piclidenoson inhibits IL - 17 & IL - 23 production in keratinocytes • Piclidenoson had significant anti - psoriatic effects and promising safety profile in a Phase 3 trial in patients with moderate - to - severe plaque psoriasis. Piclidenoson for the Treatment of Plaque Psoriasis IL - 23 & IL - 17 Rationale for Development 8

9 Phase III Study Endpoints - Achieved PASI 75 Significant Superiority of Piclidenoson 3 mg vs. Placebo Primary Endpoint Secondary Endpoint Subjects Achieving PGA2 for Piclidenoson vs Placebo I Study Design Excellent Safety Profile

10 An agreement for co - development has been signed with the Italian Theleton Fund Lowe Syndrome Rare Genetic Disease 10 • Lowe Syndrome - Known as oculo - cerebro - renal syndrome (OCRL), an X - linked genetic condition occurring in males • Piclidenoson - Correct this genetic syndrome in pre - clinical studies (3 rd party findings) • Prevalence - Approximately 1 in 500,000 • Rare genetic disease - Drug can be potentially registered based on its success in a limited number of patients

11 • FDA and EMA - agreed on the psoriasis registration Plan • Pivotal Phase III looking at Piclidenoson Vs. Placebo • Primary endpoint – PASI 75 & PGA 2 • Adolescents will be included Regulatory Status Psoriasis Pivotal Phase III 11 II

Oncology & SLD Namodenoson • MW: 544.73 g/mol • Water Insoluble • Half life: 12 hours • Nucleoside Derivative • Orally Bioavailable • High Stability in the Liver Namodenoson Drug Candidate Chemical Properties 12

Piclidenoson - Drug Profile • A3AR is over - expressed in human hepatocellular carcinoma (HCC) cells. • Namodenoson induces de - regulation of the Wnt and NF - kB signalling pathways resulting in apoptosis of HCC cells. • In Phase II study in patients with advanced HCC, Namodenoson was safe and well tolerated. Evidence of antitumor activity was observed. Rationale for Development Namodenoson for the Treatment of Advanced Liver Cancer 13

Piclidenoson - Phase II/III Study HCC Phase II Study – Recent Data • Patient was enrolled in Phase II liver cancer study • Continued treatment with Namodenoson for >6 years under Open Label Extension Program in Europe • Patient had Complete Response: Completely cleared all cancer lesions • Over the course of 5 years, clinical benefits included: • Disappearance of ascites • Return to normal liver function • Disappearance of peritoneal carcinomatosis Complete disappearance of tumor lesions Presented at the AASLD 2022 & ASCO - Breakthrough 2023 Meeting Complete Response in a Namodenoson Treated Patient 14

• FDA and EMA agreed on Pivotal Phase 3 study protocol • Interim analysis to be conducted by Independent Data Monitoring Committee (IDMC) after 50% of planned 450 patients are enrolled and treated • Namodenoson evaluated as a 2nd - or 3rd - line treatment for advanced liver cancer patients in whom other approved therapies have not been or are no longer effective • Primary endpoint - overall survival • Orphan Drug Status - granted by FDA and EMA • Fast Track Status - granted by FDA • Compassionate Use Program - currently treating liver cancer patients in Israel and Romania Orphan Drug Designation with FDA & EMA Fast Track Designation with FDA Enrollment – ongoing Advanced Liver Cancer Pivotal Phase 3 15

Piclidenoson - Drug Profile • Namodenoson induces 90% growth inhibition of pancreatic cancer cells • The molecular mechanism of action includes de - regulation of the Wnt and the Ras signaling pathways • In vivo studies showed robust inhibition of pancreatic tumor size Rationale for Development Namodenoson for the Treatment of Pancreatic Cancer 16

17 Pancreatic Cancer Second line therapy • Open label • Oral dose of Namodenoson: 25 mg twice daily • Primary End point: safety • Secondary Endpoints: objective response, progression - free survival, duration of response, disease control (defined as an objective response or stable disease), overall survival Exploratory Phase II Study

Piclidenoson - Drug Profile • Induction of anti - inflammatory effect manifested by reduction of NAFLD Activity Score (NAS) • Anti - fibrotic effect • Anti - steatotic effect: significant decrease in steatosis, ballooning and lobular inflammation • A decrease in ALT & AST and triglyceride levels • Liver protective effect: protects the liver against Ischemia/Reperfusion injury Rationale for Development Namodenoson for the Treatment of Steatotic (Fatty) Liver Disease (SLD; previously NASH) 18

19 Phase IIa Study Successfully Concluded: • Reduced liver fat content (LFC) • Anti - Inflammatory effect • Dose selection for Phase 2b determined • Decrease in body weight • Excellent safety profile Designed to Address Severe Unmet Need; No FDA - Approved Treatment Currently Enrolling Patients for a Phase IIb Study Phase IIb Study • Multicenter, randomized, double - blind, placebo - controlled study in 140 subjects with biopsy - confirmed SLD • Subjects are randomly assigned in a 2:1 ratio to oral doses of Namodenoson 25 mg every 12 hours or a matching placebo for 36 weeks • Regular evaluation for safety and efficacy biomarkers baseline measurements at weeks 6, 12, 24, and 36 • Primary efficacy endpoint will be determined by liver biopsy at week 36 SLD
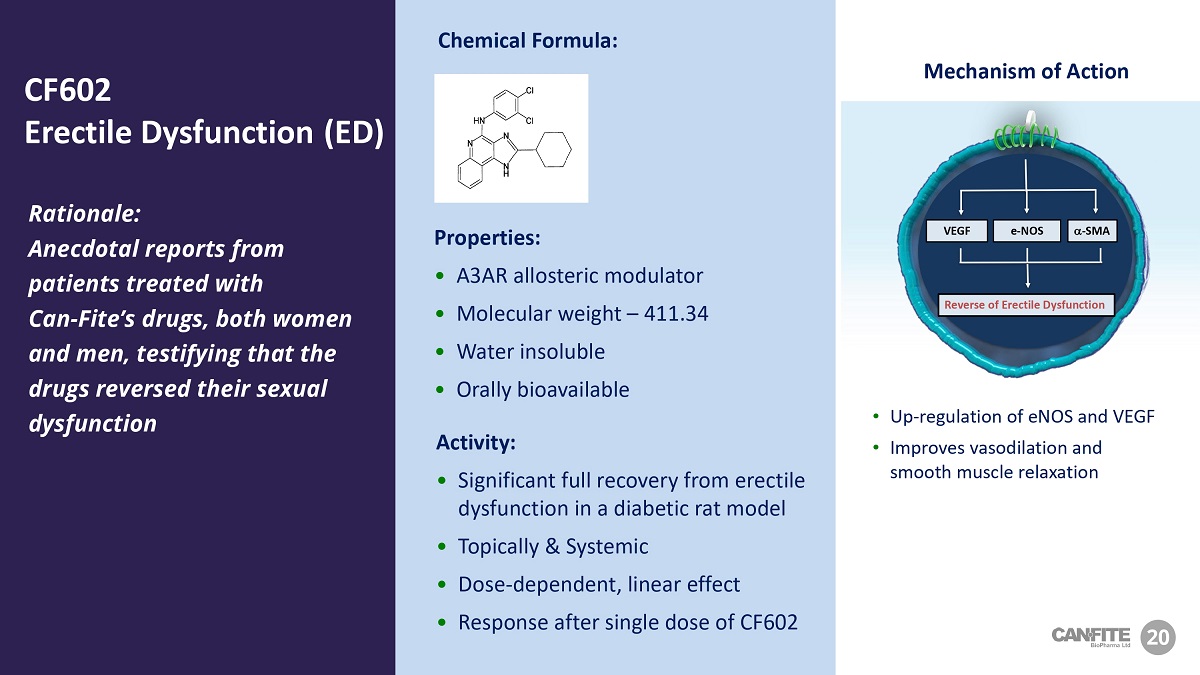
VEGF a - SMA e - NOS Reverse of Erectile Dysfunction Mechanism of Action • Up - regulation of eNOS and VEGF • Improves vasodilation and smooth muscle relaxation Rationale: Anecdotal reports from patients treated with Can - Fite’s drugs, both women and men, testifying that the drugs reversed their sexual dysfunction Properties: • A3AR allosteric modulator • Molecular weight – 411.34 • Water insoluble • Orally bioavailable Activity: • Significant full recovery from erectile dysfunction in a diabetic rat model • Topically & Systemic • Dose - dependent, linear effect • Response after single dose of CF602 20 CF 602 Erectile Dysfunction (ED) Chemical Formula:

Rationale: Cannabinoids are known to bind to A3 adenosine receptor (A3AR) and mediate clinical effects 21 Cannabinoids Can - Fite filed a patent protecting the discovery of cannabinoid - based treatment of diseases where A3AR is overexpressed including liver cancer, other cancers, autoimmune inflammatory and metabolic diseases Intellectual Property Based on this assay, Can - Fite has demonstrated how CBD - rich T3/C15 binds to A3AR to inhibit the growth of hepatocellular carcinoma and liver stellate via de - regulation of the Wnt /β - catenin pathway Pre - clinical Data in Liver Cancer and Fibrosis Can - Fite developed a biological assay that identifies clinically active cannabinoids based on the binding to A3AR Assay to Identify Clinically Active Cannabinoids A3AR CBD

Monetizing advanced portfolio through c orporate partnerships – Piclidenoson and Namodenoson have been out - licensed in select territories with ~$20 million received to date and potentially up an additional $130 million plus royalties Oral drugs with proven safety and efficacy in Phase 2 & 3 – Piclidenoson and Namodenoson are Phase 3 assets in psoriasis and liver cancer; Namodenoson showed strong efficacy in a Phase 2 SLD study and is headed into an exploratory Phase 2a study in pancreatic cancer Novel therapeutic approach – Unique technology for the treatment of cancer, liver and inflammatory diseases; addressing multi - billion dollar markets Intellectual property portfolio – Consists of 15 patent families issued and pending to protect the different indications Financially well positioned – To conduct all clinical development programs and G&A for > 1 year 22 Closing Highlights
Can Fite BioPharma (AMEX:CANF)
Graphique Historique de l'Action
De Oct 2024 à Nov 2024

Can Fite BioPharma (AMEX:CANF)
Graphique Historique de l'Action
De Nov 2023 à Nov 2024
