Annual Information Update
10 Novembre 2005 - 8:01AM
UK Regulatory
RNS Number:9131T
Provalis PLC
10 November 2005
10 November 2005
Provalis plc
Annual Information Update 2005
Provalis plc ("the Company") is pleased to provide its' annual information
update, in accordance with the requirements of the Prospectus Rule 5.2. This
update refers to information that has been published or made available by the
Company to the public over the twelve months ending today. To avoid an
unnecessarily lengthy document, information is referred to in this update rather
than included in full.
The information referred to in this update was up to date at the time the
information was published but some information may now be out-of-date.
(1) Announcements made via RNS, a Regulatory Information Service
All of the documents listed below were published via RNS, a Regulatory
Information Service on or around the dates indicated.
Date of publication Brief description of announcement
19/10/2005 Preliminary Results
13/09/2005 Notice of Preliminary Results
10/08/2005 Board Change
03/08/2005 NGSP Certification for in2it(TM)A1C
26/07/2005 Update on progress of in2it(TM)A1C
10/06/2005 Appointment of director
10/06/2005 Trading Update
01/04/2005 Retirement of director
02/03/2005 Director Shareholding
01/03/2005 Interim Results
25/02/2005 Intention to terminate US Listing
18/02/2005 Board Changes
25/01/2005 Notice of Interim Results
07/01/2005 Half Year Trading Update
13/12/2004 Vaccine Option Agreement
03/12/2004 First Commercial Shipment of in2it(TM)A1C
22/11/2004 Final Payment for Diclomax
Copies of the above announcements can be downloaded from the Company's website
(www.provalis.com) or obtained from the Company Secretary at the Company's
Registered Office, Newtech Square, Deeside Industrial Park, Deeside, Flintshire,
CH5 2NT.
(2) Documents filed at Companies House
All of the documents listed below were filed with Companies House in Cardiff on
or around the dates indicated.
Date of filing Brief description of document
13/09/05 Form 403A Declaration of satisfaction in full of guarantee and
debenture in favour of Barclays Bank plc
06/09/05 Form 288b relating to the resignation of Dr Philip Gould as a
director
01/07/05 Form 288a relating to the appointment of Peter Woodford as a
director
04/04/05 Form 288b relating to the resignation of Dr David Bloxham as a
director
23/03/05 Form 363s Annual Return
22/02/05 Form 288a relating to appointment of Dr Alan Aikman as a
director
9/11/04 Annual Report and Accounts 2004
Copies of documents filed at Companies House can be obtained from Companies
House or from the Company Secretary at the Company's Registered Office, Newtech
Square, Deeside Industrial Park, Deeside, Flintshire, CH5 2NT.
Contacts:
Provalis plc 01244 833 402
Lee Greenbury, Company Secretary
College Hill 0207 457 2020
Adrian Dufield/ Corinna Dorward
"Safe Harbor" Statement under the US Private Securities Litigation Reform Act of
1995: Statements in this announcement that relate to future plans, expectations,
events, performances and the like are forward-looking statements as defined in
the US Private Securities Litigation Reform Act of 1995. Actual results of
events could differ materially from those described in the forward-looking
statements due to a variety of factors. Such factors include, among others: the
viability of the Group's products, which are at various stages of development;
the generation of sufficient operating cash flow by the Group's pharmaceutical
and medical diagnostic businesses to finance the ongoing development of these
businesses as well as the Group's research and development activities; the
success of the Group's research and development strategy and activities;
uncertainties related to future clinical trial results and the associated
regulatory process; the execution and success of collaborative agreements with
third parties; availability and level of reimbursement for the Group's products
from government health administration authorities or other third-party payors;
the rate of net cash utilisation within the Group and, hence, the Group's
possible need for additional capital in the short, medium and/or long term; the
Group's intellectual property position and the success of patent applications
for its products and technologies; the Group's dependence on key personnel;
general business and economic conditions; the impact of future laws, regulations
and policies; stock market trends in the Group's sector; and other factors
beyond the Group's control that may cause the Group's available capital
resources to be used more quickly than expected. These and other factors that
could affect the Company's future results are more fully described in its
filings with the US Securities and Exchange Commission, in particular the latest
20-F filing, copies of which are available from the Company Secretary at the
Company's registered address.
This information is provided by RNS
The company news service from the London Stock Exchange
END
AIUDGMGMNLGGKZM
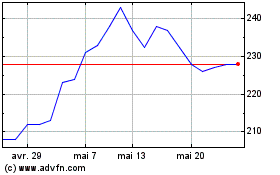
Globaldata (LSE:DATA)
Graphique Historique de l'Action
De Juin 2024 à Juil 2024
Globaldata (LSE:DATA)
Graphique Historique de l'Action
De Juil 2023 à Juil 2024