Product Launch
23 Novembre 2000 - 8:30AM
UK Regulatory
RNS Number:5656U
Provalis PLC
23 November 2000
Provalis' GlycosalTM Product to be used by US Government
Health Agency in long term landmark diabetes care trial
Provalis Diagnostics and its US distributor Bio-Rad are pleased to announce
that the GlycosalTM point-of-care test will be used in one of the most
extensive, long term, diabetes care trials ever to be carried out. GlycosalTM
is a Point-of-Care diagnostic product for measuring the long term level of
glucose (glycated haemoglobin or HbA1c) in the blood of diabetics.
This landmark clinical trial studying the treatment of diabetes has been named
the 'Action to Control Cardiovascular Risk in Diabetes' (ACCORD) trial. The
National Heart, Lung and Blood Institute (a division of the US National
Institute of Health) is sponsoring the ACCORD trial. It will last for 8 years
and involve up to 10,000 patients, and will involve GlycosalTM being used to
test each patient monthly.
The overall goal of the ACCORD trial is to address the challenge of reducing
the rate of cardiovascular disease in patients with Type 2 diabetes. Glycaemic
control, as measured by blood glucose and HbA1c levels, is one of the three
cardiovascular disease risk factors that will be assessed during the trial.
HbA1c testing will be done at approximately 60 clinical sites in the United
States and Canada.
John Curtis, Managing Director of Provalis Diagnostics said "We are delighted
to be part of such an important and prestigious trial. The decision by the US
government to use GlycosalTM, so soon after regulatory approval (510k) of it
was granted, represents a confirmation of confidence in the quality of our
product and the ability of Provalis to supply their extensive needs over the
next eight years.
Phil Gould, CEO of Provalis added, "The awarding of this contract shows great
confidence in our product and will generate significant additional secure
business for the product over an extended period. This contract supports the
continued move of our Medical Diagnostic division towards profitability, and
justifies the extensive R&D activity and resource used in the development of
the product ".
Commenting on the award of this US government contract John Hertia, New
Business Development Manager of Bio-Rad Laboratories Inc., said "We are
pleased to be part of the ACCORD trial. Starting from the land mark Diabetes
Control and Complications (DCCT) trial, Bio-Rad has always been actively
involved in diabetes clinical research, providing research teams with
high-performance systems for HbA1c measurement. Provalis' GlycosalTM product,
in the Bio-Rad format Micromat II, was selected over other systems for its
technical performance and ease of use. We look forward to working with the NIH
and Provalis on supplying the product for this important trial".
Provalis' Internet Website ; http://www.provalis.com
"Safe Harbor" Statement under the US Private Securities Litigation Reform Act
of 1995: Some or all of the statements in this document that relate to future
plans, expectations, events, performances and the like are forward looking
statements as defined in the US Private Securities Litigation Reform Act of
1995. Actual results of events could differ materially from those described
in the forward looking statements due to a variety of factors, including those
set forth in Provalis plc's filings with the US Securities and Exchange
Commission.
National Heart, Lung and Blood Institute (NHLBI) Statement on private sector
participation in a clinical trial: Neither the conduct of the trial nor the
results should be represented as a NHLBI endorsement of the drug or product
under study.
For further information:-
Dr Phil Gould, Provalis plc, Tel: 01244 833463
Mr John Curtis, Provalis Diagnostics Limited, Tel: 01244 833527
Lisa Baderoon, Buchanan Communications, Tel: 020 7466 5000
Notes to Editors
Provalis plc (LSE.PRO and NASDAQ.PVLS) is an integrated healthcare business
with three separate divisions focused on the supply and sale of prescription
medicines, the development, manufacture and sale of medical diagnostics
worldwide, and the development of new therapeutic products, such as vaccines
to combat infectious diseases.
The three divisions are:-
Healthcare - This division sells and markets branded, third party,
prescription medicines in the UK to GPs and hospitals through its own
regionally managed sales force. This division sells products in the areas of
gastroenterology, osteoporosis, migraine and dermatology.
Medical Diagnostics - This division develops and sells medical diagnostic
products to world markets through distributors. The division has an
established business in diagnostic products for infectious diseases and has
recently launched the innovative products GlycosalTM and OsteosalTM in the
areas of diabetes and osteoporosis respectively.
Therapeutic Research & Development - This division develops a range of vaccine
candidates for infectious diseases through a network of research
collaborators.
Diabetes
Diabetes is a chronic disease that has no cure and affects 5 to10 % of the
population. The disease is on the increase and is reaching epidemic
proportions in the Western World. A recent study in the United States showed a
massive 33 % increase in the incidence of the disease over an eight-year
period from 1990 to 1998. This trend is set to continue with an ageing and
increasingly overweight population. Diabetes leads to many serious
complications, which include blindness, amputations and kidney disease.
However, one of the most serious complications is an increase in the incidence
of coronary heart disease (heart attacks) and stroke.
Accord Trial
The ACCORD trial aims to tightly control the blood glucose (sugar), blood
pressure and cholesterol levels of diabetics, within normal levels. It is
expected that this will markedly decrease the incidence of heart disease and
lead to an improved quality of life for those suffering from the disease.
The trial will monitor 10,000 patients over an 8 year period at approximately
60 centres across the United States and Canada. The GlycosalTM test will be
used to monitor the glycated haemoglobin (HbA1c) level of up to10,000 patients
on a monthly basis, as part of their clinical examination. It is intended that
progress papers will be published by the National Heart Lung and Blood
Institute throughout the period of the trial.
The ACCORD trial contract has been placed with Bio-Rad, one of the two US
distributors of GlycosalTM, which is sold by Bio-Rad in the US under the
Micromat II brand name. Bio-Rad are the world's leading suppliers of HbA1c
tests with worldwide sales in excess of 30,000,000 tests in 1999.
The current $800m HbA1c market is set to increase dramatically over the next
few years as the number of Diabetics increase. The ACCORD trial is a landmark
event in the care and treatment of people with Diabetes and follows on from
the Diabetes Control and Complications Trial (DCCT) and the UK Prospective
Diabetes Study (UKPDS), which are already having a major impact on the health
and well-being of diabetics. These trials have demonstrated that frequent
HbA1c testing offers a significant reduction in the long term costs of
treating diabetes which currently accounts for 10% of all Western Healthcare
costs."
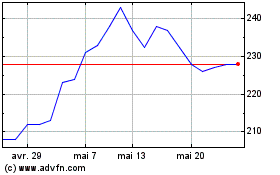
Globaldata (LSE:DATA)
Graphique Historique de l'Action
De Juin 2024 à Juil 2024
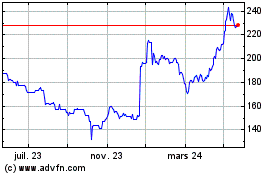
Globaldata (LSE:DATA)
Graphique Historique de l'Action
De Juil 2023 à Juil 2024