false
0001723128
DEF 14A
0001723128
2023-01-01
2023-12-31
0001723128
amrx:ChiragPatelMember
2023-01-01
2023-12-31
0001723128
amrx:ChintuPatelMember
2023-01-01
2023-12-31
0001723128
amrx:ChiragPatelMember
2022-01-01
2022-12-31
0001723128
amrx:ChintuPatelMember
2022-01-01
2022-12-31
0001723128
2022-01-01
2022-12-31
0001723128
amrx:ChiragPatelMember
2021-01-01
2021-12-31
0001723128
amrx:ChintuPatelMember
2021-01-01
2021-12-31
0001723128
2021-01-01
2021-12-31
0001723128
amrx:ChiragPatelMember
2020-01-01
2020-12-31
0001723128
amrx:ChintuPatelMember
2020-01-01
2020-12-31
0001723128
2020-01-01
2020-12-31
0001723128
ecd:PeoMember
amrx:ChiragPatelMember
amrx:ExclusionOfStockAwardsMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChiragPatelMember
amrx:InclusionOfEquityValuesMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChintuPatelMember
amrx:ExclusionOfStockAwardsMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChintuPatelMember
amrx:InclusionOfEquityValuesMember
2023-01-01
2023-12-31
0001723128
ecd:NonPeoNeoMember
amrx:AverageExclusionOfStockAwardsAndOptionAwardsMember
2023-01-01
2023-12-31
0001723128
ecd:NonPeoNeoMember
amrx:AverageInclusionOfEquityValuesMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChiragPatelMember
amrx:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChiragPatelMember
amrx:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChiragPatelMember
amrx:ChangeInFairValueFromLastDayOfPriorYearToVestingDateOfUnvestedEquityAwardsThatVestedDuringYearMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChiragPatelMember
amrx:FairValueAtLastDayOfPriorYearOfEquityAwardsForfeitedDuringYearMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChintuPatelMember
amrx:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedOutstandingAndUnvestedAsOfLastDayOfYearMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChintuPatelMember
amrx:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChintuPatelMember
amrx:ChangeInFairValueFromLastDayOfPriorYearToVestingDateOfUnvestedEquityAwardsThatVestedDuringYearMember
2023-01-01
2023-12-31
0001723128
ecd:PeoMember
amrx:ChintuPatelMember
amrx:FairValueAtLastDayOfPriorYearOfEquityAwardsForfeitedDuringYearMember
2023-01-01
2023-12-31
0001723128
ecd:NonPeoNeoMember
amrx:AverageYearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedOutstandingAndUnvestedAsOfLastDayOfYearMember
2023-01-01
2023-12-31
0001723128
ecd:NonPeoNeoMember
amrx:AverageChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2023-01-01
2023-12-31
0001723128
ecd:NonPeoNeoMember
amrx:AverageChangeInFairValueFromLastDayOfPriorYearToVestingDateOfUnvestedEquityAwardsThatVestedDuringYearMember
2023-01-01
2023-12-31
0001723128
ecd:NonPeoNeoMember
amrx:AverageFairValueAtLastDayOfPriorYearOfEquityAwardsForfeitedDuringYearMember
2023-01-01
2023-12-31
0001723128
1
2023-01-01
2023-12-31
0001723128
2
2023-01-01
2023-12-31
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
SCHEDULE 14A
PROXY STATEMENT PURSUANT TO SECTION 14(a)
OF THE SECURITIES EXCHANGE ACT OF 1934
(Amendment No. )
 |
Filed by the Registrant |
 |
Filed by a Party other than the Registrant |
| Check the appropriate box: |
 |
Preliminary Proxy Statement |
 |
Confidential, for Use of the Commission Only (as permitted by Rule 14A-6(E)(2)) |
 |
Definitive Proxy Statement |
 |
Definitive Additional Materials |
 |
Soliciting Material under §240.14a-12 |

Amneal Pharmaceuticals, Inc.
(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement, if
other than the Registrant)
| Payment of Filing Fee (Check all boxes that apply): |
 |
No fee required. |
 |
Fee paid previously with preliminary materials. |
 |
Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11. |

Notice
of 2024
Annual Meeting of Stockholders
and Proxy Statement
You are cordially invited to attend the Amneal Pharmaceuticals,
Inc. 2024 Annual Meeting of Stockholders.
THURSDAY, MAY 2, 2024
9:00 a.m., Eastern Daylight Time
Virtual Meeting at www.virtualshareholdermeeting.com/AMRX2024
Items to be Voted On
1.
Elect as directors the 11 nominees named in the accompanying
proxy statement;
2.
Approve the compensation of our named executive officers
on an advisory basis;
3.
Ratify the appointment of Ernst & Young LLP as
our independent registered public accounting firm for fiscal year 2024; and
4.
Transact such other business as may properly come before
the annual meeting or any adjournment or postponement of the meeting.
Record Date
You are eligible to vote if you were a stockholder of record at the close of business on March 11,
2024. A list of stockholders of record will be made available to stockholders during the meeting at www.virtualshareholdermeeting.com/AMRX2024
when you enter your 16-Digit Control Number. |
|
Voting
Your vote is important, and you are invited to attend the annual meeting. Whether or not you expect
to attend the annual meeting, we encourage you to vote as soon as possible. To ensure your shares are voted, you may vote
your shares in advance of the meeting over the internet, by telephone or, if you requested to receive printed proxy materials,
by mailing a proxy or voting instruction card. Voting over the internet, by telephone or by mail will ensure your representation
at the annual meeting regardless of whether you attend the meeting.
Virtual Annual Meeting
We are once again hosting a virtual meeting this year. The live audio
webcast will be available at www.virtualshareholdermeeting.com/AMRX2024.
This proxy statement and the related materials are first being distributed or made available to stockholders
on or about March 22, 2024.
By Order of the Board of Directors,
Jason B. Daly
Senior Vice President, Chief Legal Officer & Corporate
Secretary
Bridgewater, New Jersey
March 22, 2024 |
| |
| Review
your proxy statement and vote in advance of the meeting in one of three ways: |
| |
|
|
 |
 |
 |
| INTERNET |
BY TELEPHONE |
BY MAIL |
| Visit the website
on your proxy card |
Call the telephone number
on your proxy card |
Sign, date
and return your proxy card in the enclosed envelope |
| |
|
|
Please
refer to the enclosed proxy materials or the information forwarded by your bank, broker
or other holder of record to see which voting methods are available to you.
Important Notice Regarding the Availability of Proxy Materials for the Annual
Meeting of Stockholders to be held on May 2, 2024:
The notice of annual meeting, this proxy statement and our annual report on Form
10-K for the fiscal year ended December 31, 2023 are available at www.proxyvote.com.
|
| |
To
Our Fellow
Stockholders, Stakeholders and Colleagues,
 |
|
 |
| |
|
|
Chintu Patel
Co-Founder, Co-Chief
Executive Officer,
and Director |
|
Chirag Patel
Co-Founder, Co-Chief
Executive Officer,
President and Director |
2023 was a robust year of growth, outstanding
execution, and further diversification of our business as we expanded in new high growth areas. We are a diversified and growing
pharmaceutical company across Retail, Injectables, Biosimilars, Specialty, Distribution and International. Our company’s
mission centers on improving access to affordable, high-quality, and innovative medicines across these areas. We are extremely
proud of our colleagues, and the passion they bring in delivering value as We make healthy possible.
2023 Highlights
Across Amneal, notable accomplishments in 2023
included:
| • |
Launched 39 new Generics products as the portfolio
continues to shift towards more complex, non-oral solid products; |
| • |
Successfully commercialized first three biosimilars,
ALYMSYS®, RELEUKO® and FYLNETRA® in first year post launch, and added two biosimilars
to the pipeline; |
| • |
Received approval for a number of high-value injectable
products as we further expansion of our injectables portfolio; |
| • |
Delivered continued growth in key Specialty products,
including RYTARY® and UNITHROID®, added ONGENTYS® to expand our portfolio, and
continued to advance our Specialty pipeline, including IPX203 for the treatment of Parkinson’s Disease; |
| • |
Drove continued, strong double-digit growth in our
durable AvKARE distribution business in the U.S.; and |
| • |
Expanded our international presence as we launched
three new business areas in India: Opthalmology, Diagnostics and Oncology, received approval for our first products in China
and finalized numerous global partnerships for distributing our medicines. |
These achievements contributed to strong 2023
performance and financial results, highlighted by revenue growth of 8% in 2023, and reflects durable growth as the top line has
grown consistently each year since 2019.
We believe the Company’s balance sheet
is strong as we successfully refinanced our debt and extended maturities to 2028. In addition, we reorganized our corporate structure,
which is expected to drive significant cash savings for the Company.
Making Healthy Possible and
Accessible Through Corporate Responsibility
At Amneal, we understand our important role in
the healthcare sector and society at large. Our mission statement “We make healthy possible” transcends our offering
of high-quality, accessible medicines to building healthy communities and a healthy planet. As a company, our deep commitment to
safeguarding the environment and serving humanity ensures we are building a resilient business well into the future.
We bring this vision to life in part through
our diversity and inclusion programming that nurtures the best and brightest in global talent. We are deepening our portfolio of
innovative products to help address unmet therapeutic needs. We are
directing our strong R&D capabilities to create more affordable
generics of complex products that help more patients access essential medicines. We routinely assess and optimize our manufacturing
operations to reduce our greenhouse gas emissions. We contribute to stronger health outcomes across our local communities in the
U.S., India and Ireland through aligned non-profit partnerships, corporate philanthropy efforts and employee volunteerism.
We invite you to read Amneal Pharmaceuticals’ “At a
Glance,” which follows this letter and describes what we do and how we improve access to medications for patients around
the world while creating long-term value for our stockholders.
We also encourage you to read the pages of this proxy statement to
inform your voting decisions. We ask for your voting support and welcome you to communicate with us via the various means described
in this proxy statement.
Sincerely,

Chintu Patel
Co-Founder, Co-CEO and Director
Chirag K. Patel
Co-Founder, Co-CEO, President and Director
March 22, 2024
AMNEAL PHARMACEUTICALS’
INC. AT-A-GLANCE
WHAT DO WE DO?
We founded Amneal Pharmaceuticals, Inc. (“Amneal”
or the “Company”) in 2002 to provide access to affordable medicines. Amneal is a global, vertically integrated, and
diversified pharmaceuticals company. We provide over 7,700 high-quality jobs and help consumers obtain access to the medicines
they need.
WHAT IS OUR STRATEGY?
We are focused on providing high-quality, affordable
medicines to patients and creating value for all stakeholders. Our strategy is sharply focused, yet we believe provides the appropriate
flexibility needed to navigate an unpredictable healthcare environment where cost pressures are acute. Key elements supporting
our value creation strategy include:
| • |
Operating large-scale, in-house best-in-class manufacturing facilities globally;
|
| • |
Creating a carefully funded research and development platform to feed a large
and diverse pipeline of over 160 pipeline programs to supplement our current portfolio of more than 270 marketed commercial
products; |
| • |
Executing on a strategy to steadily move up the value chain to more complex
and difficult-to-manufacture products that offset the steady erosion of our base Generics business and that have higher barriers
to entry and more defensible revenue streams, including: |
| |
• |
Our move into biosimilars and sterile injectables, which typically
have more durable revenue streams with institutional customers; |
| |
• |
Our participation in the federal healthcare sector through AvKARE,
which diversifies our channel mix; |
| |
• |
Our Specialty segment, which benefits from a truly differentiated
platform in neurology and endocrinology that helps us serve large populations with unmet needs and leverage our existing commercial
infrastructure; and |
| |
• |
Our accelerating entry into key international markets including Europe,
Asia-Pacific and other opportunistic emerging markets helping us strengthen the value of our high-value portfolio products
in new ways; |
| • |
Maintaining collaborative relationships with regulators and sustaining an excellent track record within the
industry, with no major observations at our sites to date; |
| • |
Steadily broadening our scientific and industry expertise through management of and partnership with adjacent
life-sciences areas; |
| • |
Making strategic acquisitions in adjacent life sciences areas to enable continual and rapid pivoting and learning;
and |
| • |
Responding to an evolving global regulatory environment that requires increased corporate disclosure of climate
and business risks by: |
| |
• |
Preparing the company for disclosing its global environmental footprint,
which has involved the introduction of a new carbon accounting software for tracking scope 1 and scope 2 greenhouse gas emissions
in all of our global manufacturing operations; |
| |
• |
Increasing governance around climate and business risk through oversight
of our Board of Directors and its committees, a cross-functional internal taskforce, and rigorous data management processes
and approvals; and |
| |
• |
Reporting on our corporate impact via our annual 2023 Environmental,
Social and Governance (“ESG”) report. The report was tracked against the United Nations Sustainable Development
Goals and the Sustainability Accounting Standards Board Biotechnology and Pharmaceuticals Standard. |
Further information about our Corporate Responsibility
programs are available at https://www.amneal.com/about/responsibility. Please note that this website and our ESG Reports are not
part of our public disclosures and are not part of our proxy solicitation materials.
WHAT DIFFERENTIATES AMNEAL
PHARMACEUTICALS?
We combine the best of public company accountability,
large scale vertical integration and a strong track record of agile execution with a long-term vision for the healthcare industry
and Amneal’s leadership role in it. We believe this is a combination that is particularly valuable in the global, affordable
medicines sector in these complex and challenging times.
Safe Harbor Statement
Certain statements contained herein, regarding
matters that are not historical facts, are forward-looking statements (as defined in the U.S. Private Securities Litigation Reform
Act of 1995). Such forward-looking statements include statements regarding management’s intentions, plans, beliefs, expectations
or forecasts for the future, including among other things: discussions of future operations; expected operating results and financial
performance; impact of planned acquisitions and dispositions; the Company’s strategy for growth; product development; regulatory
approvals; market position and expenditures, and other non-historical statements. Words such as “plans,” “expects,”
“will,” “anticipates,” “estimates” and similar words, or the negatives thereof, are intended
to identify estimates and forward-looking statements.
The reader is cautioned not to rely on these
forward-looking statements. These forward-looking statements are based on current expectations of future events, including with
respect to future market conditions, company performance and financial results, operational investments, business prospects, new
strategies and growth initiatives, the competitive environment, and other events. If the underlying assumptions prove inaccurate
or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections
of Amneal. Such risks and uncertainties include, but are not limited to: our ability to successfully develop, license, acquire
and commercialize new products on a timely basis; the competition we face in the pharmaceutical industry from brand and generic
drug product companies, and the impact of that competition on our ability to set prices; our ability to obtain exclusive marketing
rights for our products; our revenues are derived from the sales of a limited number of products, a substantial portion of which
are through a limited number of customers; the impact of a prolonged business interruption within our supply chain; the continuing
trend of consolidation of certain customer groups; our dependence on third-party suppliers and distributors for raw materials for
our products and certain finished goods; legal, regulatory and legislative efforts by our brand competitors to deter competition
from our generic alternatives; our dependence on information technology systems and infrastructure and the potential for cybersecurity
incidents; our ability to attract, hire and retain highly skilled personnel; risks related to federal regulation of arrangements
between manufacturers of branded and generic products; our reliance on certain licenses to proprietary technologies from time to
time; the significant amount of resources we expend on research and development; the risk of claims brought against us by third
parties; risks related to changes in the regulatory environment, including U.S. federal and state laws related to healthcare fraud
abuse and health information privacy and security and changes in such laws; changes to Food and Drug Administration product approval
requirements; the impact of healthcare reform and changes in coverage and reimbursement levels by governmental authorities and
other third-party payers; our dependence on third-party agreements for a portion of our product offerings; our substantial amount
of indebtedness and our ability to generate sufficient cash to service our indebtedness in the future, and the impact of interest
rate fluctuations on such indebtedness; our potential expansion into additional international markets subjecting us to increased
regulatory, economic, social and political uncertainties, including recent events affecting the financial services industry; our
ability to identify, make and integrate acquisitions or investments in complementary businesses and products on advantageous terms;
the impact of global economic, political or other catastrophic events; our obligations under a tax receivable agreement may be
significant; and the high concentration of ownership of our class A common stock and the fact that we are controlled by the Amneal
Group. The forward-looking statements contained herein are also subject generally to other risks and uncertainties that are described
from time to time in our filings with the Securities and Exchange Commission (the “SEC”), including under Item 1A,
“Risk Factors” in our most recent Annual Report on Form 10-K and in our subsequent reports on Forms 10-Q and 8-K. Investors
are cautioned not to place undue reliance on any such forward-looking statements, which speak only as of the date they are made,
and we undertake no obligation to revise or update such statements to reflect the occurrence of events or circumstances after the
date hereof.
Corporate Governance
Corporate Structure, Reorganization and Transfer
of Listing Exchange
The Company is a holding company, whose principal
assets are common units (“Amneal Common Units”) of Amneal Pharmaceuticals LLC (“Amneal LLC”). In 2018,
Amneal LLC completed the acquisition of Impax Laboratories, Inc. (“Impax”), a generic and specialty pharmaceutical
company (the “Combination”).
The group, together with their affiliates
and certain assignees, who owned Amneal LLC when it was a private company (the “Amneal Group”) held 50.1% of Amneal
Common Units and the Company held the remaining 49.9% as of December 31, 2022. On November 7, 2023, we implemented a plan pursuant
to which the Company and Amneal LLC reorganized and we simplified our corporate structure by eliminating our umbrella partnership-C-corporation
structure and converting to a more traditional structure whereby all stockholders hold their voting and economic interests directly
through the public company (the “Reorganization”). Effective with the Reorganization, the Company directly or indirectly
holds 100% of the Amneal Common Units and the Company remains Amneal LLC’s sole managing member, having the sole voting power
to make all of Amneal LLC’s business decisions and control its management. Shortly after we effected the Reorganization,
at market open on December 27, 2023, we transferred the listing of our Class A common stock to the Nasdaq Stock Market LLC (“Nasdaq”).
Stockholders Agreement
In connection with the Combination, we entered
into a stockholders agreement with the Amneal Group, which was amended and restated in August 2019 and again in November 2023 as
part of the Reorganization (the “Stockholders Agreement”), that sets forth, among other things, certain rights and
obligations of the Company and the Amneal Group with respect to the corporate governance of the Company.
Board Composition
The Stockholders Agreement provides that,
until the Amneal Group owns less than 10% of the outstanding shares of the Company’s common stock, the Board of Directors
of the Company (the “Board”) will consist of no more than 13 members.
As of the date hereof, the Board has fixed
its size at 11, composed as follows:
| • |
Amneal
Group Directors. Six directors, including
the Chairman of the Board, were designated by the Amneal Group. The directors designated by the Amneal Group, three of whom
qualify as and are considered independent under Nasdaq rules, are referred to herein as the “Amneal Group Directors.” |
| • |
Non-Amneal
Group Directors. Five directors were designated
as the Company Independent Directors (as defined in the Stockholders Agreement). The directors not designated by the Amneal
Group are referred to herein as the “Non-Amneal Group Directors.” |
Pursuant to, and effective as of, the amendment
to the Stockholders Agreement in August 2019, the Amneal Group representative who appoints the Amneal Group Directors will
not be (i) an employee of the Company or (ii) an individual controlled, directly or indirectly, by employees of the Company,
a Company subsidiary, or any person controlled by the Company. This change was made in connection with the appointment of
Chirag Patel and Chintu Patel as co-Chief Executive Officers in order to preserve the independence of those Amneal Group Directors
who are intended to be independent.
For so long as the Amneal Group continues
to beneficially own more than 50% of the outstanding shares of the Company, the Amneal Group Directors will have the right to designate
the Chairman or Co-Chairmen of the Board, and the Non-Amneal Group Directors will have the right to designate a lead Independent
Director of the Board (as applicable).
TPG Group Holdings has the right, subject
to certain ownership thresholds and other requirements, to designate a director for appointment to the Company’s Board (which
right TPG currently has not exercised) or to designate an observer to the Board.
There are currently two observers to the
Board, one of whom has been designated by the Board of Directors and one of whom has been designated by TPG Group Holdings.
For so long as the Amneal Group continues
to beneficially own more than 50% of the outstanding shares of the Company, the Amneal
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
10 |
| |
|
Group will have the right to designate for
nomination the lowest number of designees that constitute a majority of the total number of directors comprising the Board. The
Company will cause such nominee(s) to be included in any slate of nominees recommended by the Board to the stockholders of the
Company for election.
If the Amneal Group beneficially owns 50%
or less but more than 10% of the outstanding shares of the Company, the Amneal Group will have the right to designate a number
of directors proportionate to the beneficial ownership of outstanding shares of the Company held by the Amneal Group (rounded up
to the nearest whole number); provided, however, that such rounding shall not result in the Amneal Group having the right to designate
a majority of the total number of directors comprising the Board when the Amneal Group beneficially owns 50% or less of the outstanding
shares of the Company’s common stock.
With respect to the Amneal Group Directors, until the date on which the Amneal Group
ceases to beneficially own at least 10% of the outstanding shares of the Company (the “Trigger Date”), any vacancy
will be filled by the Board with a director designated by the Amneal Group, except when such vacancy is created when the number
of the Amneal Group Directors then serving on the Board is in excess of the number of Amneal Group designees the Amneal Group has
the right to designate under the Company’s Bylaws and the Stockholders Agreement.
With respect to the Non-Amneal Group Directors,
the Nominating and Corporate Governance Committee will recommend to the Company’s Board to fill any vacancy (other than the
CEO of the Company) with a person who satisfies all the qualifications of a Company Independent Director (as defined in the Stockholders
Agreement), subject to the prior written consent of the Conflicts Committee.
Board Committees
The Stockholders Agreement provides that
the Company’s Board shall have the following committees: (i) Audit Committee, (ii) Nominating and Corporate Governance Committee,
(iii) Compensation Committee, and (iv) Conflicts Committee. The formation of, composition of, and amendment to the charter of any
other committee requires the approval of 75% of the Board.
Until the Trigger Date, each committee of
the Company’s Board (other than the Conflicts Committee) will include at least one director designated by the Amneal Group,
subject to the applicable Nasdaq rules and requirements. If at any time, any committee (other than the Conflicts Committee) does
not have at least one such Amneal Group-designated director, the Amneal Group will be entitled to designate a director to have
observer rights with respect to such committee.
Amneal Group Agreement to Vote
Until the Trigger Date, the Amneal Group
must cause its shares to be present for quorum purposes at any stockholders meeting, vote in favor of all director nominees recommended
by the Company’s Board, and not vote in favor of the removal of any Non-Amneal Group Director, unless such removal is recommended
by the Nominating and Corporate Governance Committee.
Amneal Group Consent Rights
For so long as the Amneal Group beneficially
owns more than 25% of the outstanding shares of the Company, the Company will not take the following actions without obtaining
prior consent of the Amneal Group:
| • |
amend, modify, or repeal any provision of the Company’s Certificate of Incorporation or Bylaws in a manner that adversely impacts any Amneal Group member; |
| • |
effect any change in the authorized number of directors, except pursuant to the Stockholders Agreement; |
| • |
create or reclassify any new or existing class or series of capital stock to grant rights, preferences, or privileges with respect to voting, liquidation, redemption, conversion or dividends that are senior to or on parity with those of the shares held by the Amneal Group; or |
| • |
consummate any transaction as a result of which (a) more than 50% of the outstanding shares of the Company will be beneficially owned by any persons other than Amneal Group members and (b) any Amneal Group member receives an amount or form of consideration different from that which is granted to other holders of the Company’s shares. |
Bylaws Amendments
As previously disclosed and referenced above,
in light of the substantial investment of the Amneal Group prior to the Combination, the Company continues to have certain legacy
obligations and corporate governance features that were adopted in connection with the Combination. In particular, the Bylaws that
we adopted following the Combination contained a provision requiring the vote of not less than two-thirds of the voting power of
the issued and outstanding shares entitled to vote at a duly called and convened annual or special meeting of stockholders in order
to amend certain limited Bylaws provisions, including provisions related to stockholder meetings and proposals as well as to the
number, term and removal of and nominating
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
11 |
| |
|
process for directors. Given the consent
rights afforded to the Amneal Group described above pursuant to the Stockholders Agreement, coupled with the continued significant
ownership stake of the Company by the Amneal Group, as exemplified by the Company’s designation as a “controlled company”
as further discussed below, we expect that this provision will remain in place for the foreseeable future unless otherwise determined
by the Amneal Group.
Code of Business Conduct; Corporate Governance
Guidelines; Board Committee Charters
We are committed to conducting every aspect
of our business in an ethical, open and honest manner and in full compliance with the law, both in letter and in spirit. Our Code
of Business Conduct applies to all of our employees, officers and directors and lays out guidelines for our employees, officers
and directors to follow as they conduct business on behalf of our Company. We have also adopted Corporate Governance Guidelines,
which, together with our Certificate of Incorporation, Bylaws and Board committee charters, form the framework for the corporate
governance of the Company. In addition, the Stockholders Agreement between the Company and the Amneal Group and the limited liability
company agreement of Amneal LLC set forth a number of corporate governance requirements with respect to the Company.
The full text of the Code of Business Conduct
as well as our Corporate Governance Guidelines, Audit Committee Charter, Compensation Committee Charter, Nominating and Corporate
Governance Committee Charter and Conflicts Committee Charter are available at the investors section of our web site, http://investors.amneal.com.
We intend to satisfy our disclosure obligations, if any, with respect to any amendment to, or waiver from, a provision of the Code
of Business Conduct that applies to our directors or executive officers, including our principal executive officers, principal
financial officer or principal accounting officer, in the investors section of our web site. Stockholders may request free printed
copies of the Code of Business Conduct, Corporate Governance Guidelines and the Board committee charters by writing to: Amneal
Pharmaceuticals, Inc., Attention: Corporate Secretary, 400 Crossing Boulevard, Bridgewater, NJ 08807 or corporatesecretary@amneal.com.
Controlled Company Status
The Amneal Group holds a majority of the
voting power of our common stock. As a result, we are a “controlled company” as defined by Nasdaq rules and have the
option to elect to avail ourselves of exemptions from certain Nasdaq requirements. Despite our status as a controlled company,
however, we have chosen to govern ourselves without using these exemptions, which we believe sets the right tone with respect to
our approach to corporate governance. Generally speaking, under Nasdaq rules, a controlled company is exempt from the requirements
of (a) a majority of the board of directors consisting of independent directors; (b) independent director oversight of director
nominations; and (c) a compensation committee composed entirely of independent directors, with a written charter addressing specified
matters. Although we currently do not avail ourselves of any of these exemptions in the interest of corporate governance best practices,
if we were to take advantage of any of them, our stockholders would not have the same protections afforded to stockholders of companies
that are subject to all of the Nasdaq requirements. We believe that the Amneal Group’s control and its long-term stewardship
have provided a strategic advantage to our Company. For example, the alignment between our co-CEOs and the Amneal Group as our
largest stockholder provides management the flexibility to efficiently pivot as needed to address short-term matters while also
supporting transformational changes necessary for the Company’s long-term success. In addition, the Amneal Group’s
values have played an important role in our unique work culture that celebrates inclusion, diversity, and equity, which we believe
has helped us to attract and retain top talent.
Board Leadership Structure
Our Board of Directors is led by an independent
Chairman. The Chairman’s responsibilities include, but are not limited to, presiding over all meetings of the Board at which
members of management are not present, including any executive sessions of independent directors, reviewing Board meeting schedules
and agendas and acting as a liaison between the independent directors and the Co-Chief Executive Officers. Our Co-Chief Executive
Officers do not hold leadership positions on the Board, but our Corporate Governance Guidelines do not prohibit them from doing
so, as the Board retains the discretion to modify its leadership structure in the future as it deems appropriate. The Board has
determined that its leadership structure is in the best interests of the Company and our stockholders because it allows our Co-Chief
Executive Officers to focus on our day-to-day business and our Chairman to focus on managing Board operations and effectiveness
and other corporate governance matters, while providing independent Board leadership.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
12 |
| |
|
Meetings of the Board of Directors
In the fiscal year ended December 31, 2023
(“fiscal 2023”), the Board of Directors held five meetings. Each of the directors attended at least 75% of the aggregate
of all meetings held by the Board of Directors and each committee of the Board of Directors on which he or she served during fiscal
2023, in each case held during the period for which he or she was a director and committee member. Our independent directors meet
in executive session at least twice annually. The Chairman presides over executive sessions of the independent directors.
Communication with the Board of Directors;
Director Attendance at Annual Meetings
Stockholders, employees and all other interested
parties may communicate with a member or members or a committee of the Board of Directors by addressing their correspondence to
the Board member or members or committee c/o Corporate Secretary, Amneal Pharmaceuticals, Inc., 400 Crossing Boulevard, Bridgewater,
NJ 08807 or by email to corporatesecretary@amneal. com. Our corporate secretary will review the correspondence and will determine,
in his good faith judgment, which stockholder communications will be relayed to the Board of Directors, any committee or any director.
Our corporate secretary has the authority to discard or disregard any inappropriate communications or to take other appropriate
actions with respect to any such inappropriate communications. Subject to the foregoing, mail addressed to “Board of Directors”
or “non-management directors” will be forwarded to the Chairman.
Recognizing that director attendance at our
annual meetings can provide our stockholders with a valuable opportunity to communicate with Board members about issues affecting
our Company, we encourage our directors to attend each annual meeting of stockholders. The 2023 annual meeting was attended by
all eleven of the directors holding office at the time.
Director Independence
In making independence determinations, the
Board of Directors observes all criteria for independence established by the SEC, Nasdaq, other governing laws and regulations,
and the Stockholders Agreement, and considers all relevant facts and circumstances. In accordance with our Corporate Governance
Guidelines, to be considered independent:
| • |
the director must meet the bright-line independence tests under Nasdaq rules; and |
| • |
the Board must affirmatively determine that the director otherwise has no relationship that would interfere with the exercise of independent judgment in carrying out the responsibilties of a director. |
The Board of Directors, through its Conflicts
Committee, annually reviews all relevant business relationships any director may have with our Company. As a result of its annual
review, the Board has affirmatively determined that each of the following directors meets the independence tests under the Nasdaq
rules, none of them has a material relationship with the Company other than their service as directors and, as a result, such directors
are independent: Paul Meister, Deb Autor, J. Kevin Buchi, Jeff George, John Kiely, Ted Nark, Emily Peterson Alva and Shlomo Yanai.
Accordingly, even though we are a “controlled company” and therefore exempt from certain board independence requirements
under the Nasdaq rules, we nonetheless have a majority of independent directors on our Board, and each Board committee is comprised
entirely of independent directors.
In making the foregoing independence determination,
the Board of Directors considered the following relationship in light of the Company’s independence standards and determined
that it does not constitute a material relationship with the Company:
Emily Peterson Alva’s spouse was employed
by Avtar Investments, LLC (“Avtar”), a financial advisory firm that manages investments for Chirag Patel and Chintu
Patel, each a member of the Amneal Group and Co-CEOs of the Company. Ms. Alva’s spouse is no longer affiliated with Avtar.
In addition to the director independence
standards described above, the Stockholders Agreement imposes additional independence requirements that apply in certain circumstances.
The Stockholders Agreement defines a “Company Independent Director” as a director who:
| • |
meets the independence standards under the rules of Nasdaq; |
| • |
is a non-Amneal Group Director; |
| • |
is not a current or former member of the Board of Directors of any Amneal Group member or its affiliates or officer or employee of any Amneal Group member or its affiliates; |
| • |
does not have and has not had any other material relationship with the Company or its affiliates; and |
| • |
is designated as a “Company Independent Director.” |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
13 |
| |
|
Committees of the Board
of Directors
The Board of Directors has four standing
committees: an Audit Committee, a Compensation Committee, a Nominating and Corporate Governance Committee, and a Conflicts Committee.
The following table sets forth the current
members of each committee and the number of meetings held during fiscal 2023 for each of the Board’s committees, as well
as each director’s status as either independent or not independent and either an Amneal Group Director or Non-Amneal Group
Director.
| |
|
Independent |
|
Amneal
Group
Director |
|
Non-Amneal
Group
Director |
|
Audit
Committee |
|
Compensation
Committee |
|
Nominating
and Corporate
Governance
Committee |
|
Conflicts
Committee |
| Emily Peterson Alva |
|
 |
|
 |
|
|
|
 |
|
|
|
|
|
|
| Deb Autor |
|
 |
|
|
|
 |
|
 |
|
|
|
|
|
 |
| J. Kevin Buchi |
|
 |
|
|
|
 |
|
 |
|
|
|
 |
|
Chair |
| Jeff George |
|
 |
|
|
|
 |
|
 |
|
 |
|
|
|
 |
| John Kiely |
|
 |
|
|
|
 |
|
Chair |
|
|
|
 |
|
 |
| Paul Meister |
|
 |
|
 |
|
|
|
|
|
 |
|
Chair |
|
|
| Ted Nark |
|
 |
|
 |
|
|
|
|
|
Chair |
|
 |
|
|
| Chintu Patel |
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
| Chirag Patel |
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
| Gautam Patel |
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
| Shlomo Yanai |
|
 |
|
|
|
 |
|
|
|
 |
|
|
|
 |
| #
of Meetings in 2023 |
|
|
|
|
|
|
|
5 |
|
5 |
|
4 |
|
15 |
Audit Committee
The principal duties and responsibilities
of our Audit Committee are to assist the Board in its oversight of:
| • |
the quality and integrity of the Company’s financial statements; |
| • |
the Company’s compliance with legal and regulatory requirements; |
| • |
the implementation of the Company’s enterprise risk management program and policies with respect to risk assessment and risk management, including cyber risks and information security; |
| • |
the independent auditor’s qualifications, performance and independence; and |
| • |
the performance of the Company’s internal audit function. |
The Audit Committee has the power to investigate
any matter brought to its attention within the scope of its duties. It also has the authority to retain counsel and advisors to
fulfill its responsibilities and duties. Each director who serves on the Audit Committee is independent under the Nasdaq rules
and as that term is used in Section 10A(m)(3) of the Securities Exchange Act of 1934, as amended. The Board of Directors has determined
that each member of the Audit Committee meets the financial sophistication requirements of Nasdaq. Further, the Board of Directors
has determined that John Kiely qualifies as an Audit Committee financial expert as that term is defined by applicable SEC regulations
and has designated Mr. Kiely as the Audit Committee’s financial expert.
The Audit Committee operates under a written
charter adopted by the Board of Directors. A copy of the charter is available at the investor relations section of our website
at https://investors. amneal.com/corporate-governance/policies. The report of the Audit Committee begins on page 64 of this proxy
statement.
Compensation Committee
The principal duties and responsibilities
of the Compensation Committee are as follows:
| • |
review and oversee the Company’s
overall compensation philosophy and oversee the development and implementation of compensation programs aligned with the Company’s
business strategy; |
| • |
review and make recommendations
to the Board in overseeing the compensation of the Company’s directors and Co-Chief Executive Officers and review and
approve the compensation of the Company’s other executive officers; |
| • |
review and approve or make recommendations to the
Board regarding the Company’s annual cash incentive program and equity-based plan and arrangements; |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
14 |
| |
|
| • |
review and discuss with management
the Company’s “Compensation Discussion and Analysis” (“CD&A”) and recommend whether such
analysis should be included in the Proxy Statement filed with the SEC; |
| • |
produce an annual report on executive
compensation for inclusion in the Company’s Proxy Statement; |
| • |
review and discuss the results
of the stockholder advisory vote on “say-on-pay”, if any, and general market reactions to executive compensation
regard to the Company’s named executive officers; |
| • |
review and approve any stock
ownership guidelines for directors and executive officers of the Company and monitor complaiance therewith; |
| • |
review and approve any “clawback”
policy to be adopted by the Company to recoup compensation paid to employees, if and as the committee determines to be necessary
or appropriate, or as required by applicable law or Nasdaq requirements, and monitor compliance therewith; |
| • |
oversee the Company’s policies
and practices with respect to managing compensation-related risks; |
| • |
oversee and approve the management continuity process;
and otherwise comply with the committee’s responsibilities and duties as set forth in its charter. |
Each director who serves on the Compensation
Committee is independent under the Nasdaq rules and applicable SEC regulations with respect to Compensation Committees. In addition
to satisfying all other applicable independence requirements, all members of the Compensation Committee qualify as “non-employee
directors” for purposes of Rule 16b-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
The Compensation Committee operates under a written charter adopted by the Board of Directors, a copy of which is available at
the investor relations section of our website at https://investors.amneal.com/corporate-governance/ policies. The report of the
Compensation Committee is on page 40 of this proxy statement.
Pursuant to the Stockholders Agreement, the
Amneal Group has the right to nominate two of the four directors serving on the Compensation Committee for so long as the Amneal
Group beneficially owns more than 50% of the outstanding shares of the Company. The remaining directors are designated by a majority
of the Company Independent Directors of the Company’s Board.
Nominating and Corporate Governance Committee
The principal duties and responsibilities
of the Nominating and Corporate Governance Committee are as follows:
| • |
identify individuals qualified to become Board members consistent with the criteria approved by the Board; |
| • |
recommend to the Board director nominees for election by stockholders; |
| • |
take leadership role in overseeing management’s handling of ESG matters of importance to the Company; |
| • |
develop and recommend to the Board a set of Corporate Governance Guidelines; and |
| • |
oversee the evaluation of the Board and management. |
Each director who serves on the Nominating
and Corporate Governance Committee is independent under the Nasdaq rules. The Nominating and Corporate Governance Committee operates
under a written charter adopted by the Board of Directors, a copy of which is available at the investor relations section of our
website at https://investors.amneal.com/corporate-governance/policies.
Pursuant to the Stockholders Agreement, the
Amneal Group has the right to nominate two of the four directors serving on the Nominating and Corporate Governance Committee for
so long as the Amneal Group beneficially owns more than 50% of the outstanding shares of the Company. The remaining directors are
designated by a majority of the Company Independent Directors of the Company’s Board.
Conflicts Committee
The principal duties and responsibilities
of the Conflicts Committee are to provide leadership and guidance to the Board and the Company regarding transactions or situations
involving potential conflicts of interest between the Company and its related parties. The responsibilities of the Conflicts Committee
include approval of certain transfers of shares of the Company by an Amneal Group member to third parties, approval of qualifying
related party transactions, and approval of any material amendment to the Stockholders Agreement, as set forth in the Conflicts
Committee Charter.
The Conflicts Committee operates under a
written charter adopted by the Board of Directors, a copy of which is available at the investor relations section of our website
https://investors. amneal.com/corporate-governance/policies.
Pursuant to the Stockholders Agreement, until
the Trigger Date, the Board will have a Conflicts Committee comprised solely of Company Independent Directors. Any amendments to
the Conflicts Committee Charter will be approved by (i) 75% of the directors of the Company’s Board, (ii) a majority of the
Company Independent Directors, and (iii) a majority of the Conflicts Committee.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
15 |
| |
|
The Board’s Role
in Risk Oversight
Management is responsible for managing the
day-to-day risks our Company faces. Our Board is responsible for:
| • |
confirming that management has implemented an appropriate system to manage these risks, i.e., to identify, assess, mitigate, monitor and communicate about these risks; and |
| • |
providing effective risk oversight through the Board’s committee structure and oversight processes. |
Beyond these fundamental responsibilities
for risk oversight, our Board concentrates on the broader implications of our strategic plans and allows the committees to focus
on specific areas of risk. Our directors, through their risk oversight role, are responsible for confirming that the risk management
processes designed and implemented by the Company’s executive officers and other senior managers are consistent with the
Company’s corporate strategy and are functioning as intended.
The Board believes that full and open communication
between management and the Board of Directors is essential for effective risk management and oversight. In addition to making quarterly
presentations at our quarterly Board meetings, our executive officers are available to discuss any questions or concerns raised
by the Board relating to risk management and any other matters. In addition, management typically reports on cybersecurity matters
to our Audit Committee twice a year.
Audit Committee
In accordance with its charter, the Audit
Committee is required to, among other things, focus on the reasonableness of control processes for identifying and managing key
business, financial and regulatory reporting risks. The Audit Committee is also mandated by its charter to discuss with management
our Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures, including
our risk assessment and risk management policies. The Audit Committee monitors our Company’s credit risk, liquidity risk,
regulatory risk, operational risk and enterprise risk through regular reviews with management, external auditors and our Company’s
internal audit function. The Audit Committee reviews and discusses with management the implementation, execution and performance
of the Company’s enterprise risk management program and the strategies, processes and controls pertaining to the management
of the company’s information technology operations, including cyber risks and information security.
Compensation Committee
The Compensation Committee assists the Board
in fulfilling its oversight responsibilities with respect to the evaluation and management of risks arising from our compensation
policies and programs. The Compensation Committee has reviewed our incentive compensation programs, discussed the concept of risk
as it relates to our compensation program, considered various mitigating factors and reviewed these items with its independent
consultant, Meridian Compensation Partners, LLC (“Meridian”).
In addition, our Compensation Committee asked
Meridian to conduct an independent risk assessment of our executive compensation program. Based on these reviews and discussions,
the Compensation Committee does not believe our compensation program creates risks that are reasonably likely to have a material
adverse effect on our business.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee
assists the Board in fulfilling its oversight responsibilities with respect to the management of risks associated with corporate
governance, including Board structure, size, membership and succession planning for our directors, as well as ESG matters of importance
to the Company. The Committee also assists in carrying out the Company’s commitment to ESG principles and diverse representation
on its Board.
Conflicts Committee
The Conflicts Committee assists the Board
in fulfilling its oversight responsibilities with respect to the management of the risks associated with the conflicts of interest
that may arise from certain related party transactions.
Cybersecurity
While the Board is ultimately responsible
for risk oversight, committees of the Board assist in fulfilling the oversight responsibilities of the Board in certain areas of
risk. Our Audit Committee is responsible for overseeing risks from cybersecurity threats and receives cybersecurity updates from
Information Technology (“IT”) leadership at least twice a year. When meeting with the Audit Committee, the IT leadership
team highlights significant accomplishments and issues related to our IT infrastructure, including cybersecurity incidents, risks,
industry trends, notable incidents facing other companies, incident preparedness and other developments. The Audit Committee also
receives updates regarding progress on initiatives to further align with the five pillars of the NIST CSF. These briefings are
designed to provide visibility to the Audit Committee about the identification, assessment, and management of critical risks, audit
findings, and management’s risk mitigation strategies. Our global head of Cybersecurity, who reports to our CFO, participates
in H-ISAC (Health Information Sharing and Analysis Center), a global community and member forum for coordinating, collaborating
and sharing vital Physical and Cyber Threat Intelligence and best practices with each other. Our Board also receives regular quarterly
updates from our Vice President of Corporate Compliance who reports to our Chief Legal Officer.
We administer multiple cybersecurity related
training and awareness events annually. Our user awareness training program includes baseline cybersecurity training for all new
employees and an annual mandatory interactive cybersecurity training
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
16 |
| |
|
for all employees and contingent workers,
as well as multiple cybersecurity topic awareness broadcast emails and targeted training to specific user groups. We employ a third
party to conduct periodic penetration testing to scan different parts of the Company’s IT environment to identify potential
vulnerabilities. Critical or high vulnerabilities are prioritized for swift remediation. Additionally, we employ a third-party
service for continuous cyber risk monitoring and make continuous adjustments to system and network configurations to improve our
cyber risk rating score. For further information related to our cybersecurity risk management process and identified cybersecurity
risks, please refer to Part I, Item 1C of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
Director Nominations
The Nominating and Corporate Governance Committee
considers recommendations for directorships submitted by our stockholders on a substantially similar basis as it considers other
nominees. Stockholders who wish the Nominating and Corporate Governance Committee to consider their recommendations for nominees
for the position of director should submit their recommendations, in accordance with the procedures (including the information
requirements) set forth in our Bylaws, in writing to: Corporate Secretary, Amneal Pharmaceuticals, Inc., 400 Crossing Boulevard,
Bridgewater, NJ 08807. For the Nominating and Corporate Governance Committee to have adequate time to consider such candidate,
the stockholder’s notice should be received at the address above within the time period set forth in our Bylaws (see “Director
Nominations and Other Proposals to Be Presented at Our 2025 Annual Meeting” below).
In its assessment of each potential candidate,
the Nominating and Corporate Governance Committee reviews the nominee’s personal and professional integrity, ethics and values
and ability to make mature business decisions. The Nominating and Corporate Governance Committee may also consider the following
criteria, as well as any other factor the committee deems relevant:
| • |
the candidate’s experience in corporate management, such as serving as an officer of a publicly held company; |
| • |
the candidate’s experience as a board member of a publicly held company; |
| • |
the candidate’s professional and academic experience relevant to our industry; |
| • |
the strength of the candidate’s leadership skills; |
| • |
the candidate’s experience in finance and accounting and executive compensation practices; |
| • |
diversity of viewpoints, background, experience and other demographics; and |
| • |
whether the candidate has the time required for the preparation, participation and attendance at board and committee meetings. |
Nominees may also be recommended by directors,
members of management, or, in some cases, by a third-party firm. In identifying and considering candidates for nomination to the
Board, the Nominating and Corporate Governance Committee may consider, in addition to the requirements described above and set
out in its charter, quality of experience, our needs and the range of knowledge, experience and diversity represented on the Board.
Each director candidate is evaluated by the Nominating and Corporate Governance Committee based on the same criteria and in the
same manner, regardless of whether the candidate was recommended by a stockholder or by others.
The Board of Directors does not have a formal
policy on Board diversity as it relates to the selection of nominees for the Board. That said, the Board believes that diversity
and a variety of experiences and viewpoints should be represented on the Board. In selecting a director nominee, the Nominating
and Corporate Governance Committee focuses on skills, viewpoints, expertise or background that would complement the existing Board.
The Nominating and Corporate Governance Committee regularly assesses the composition of the Board and seeks to identify candidates
representing diverse experience at policy-making levels in business, management, marketing, finance, human resources, communications
and other areas that are relevant to our activities. In addition, one of the many factors that the Board and the Nominating and
Corporate Governance Committee carefully considers is the importance to the Company of ethnic and gender diversity. The Nominating
and Corporate Governance Committee assesses its effectiveness in this regard when evaluating the composition of the Board.
In the case of a recommendation submitted
by a stockholder, after full consideration, the stockholder proponent will be notified of the decision of the Nominating and Corporate
Governance Committee.
The Nominating and Corporate Governance Committee
conducts the appropriate and necessary inquiries with respect to the backgrounds and qualifications of all director nominees. Diversity
is essential to Amneal’s success. It starts at the top, with six out of ten of our executives identifying as diverse by race,
ethnicity, or gender, and permeates through the organization of Amneal employees. In 2023, women represented 19% of our global
workforce of Amneal employees. In the United States, they represented 41% of our workforce and held 32% of leadership roles at
the level of Director and above for Amneal employees. Approximately 74% of our U.S. workforce of Amneal employees identified as
diverse by race or ethnicity. The Nominating and Corporate Governance Committee also reviews the independence of each candidate
and other qualifications of all director candidates, as well as considers questions of possible conflicts of interest between director
nominees and our Company. After the Nominating and Corporate Governance Committee has completed its review of a nominee’s
qualifications and conducted the appropriate inquiries, the Nominating and Corporate Governance Committee makes a determination
whether to recommend the nominee for approval by the Board of Directors. If the Nominating and Corporate Governance Committee decides
to recommend the director nominee for nomination by the Board of Directors and such recommendation is accepted by the Board, the
form of our proxy solicitation will include the name of the director nominee.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
17 |
| |
|
Board and Organizational Diversity
The Company also places a high priority on
creating a Board that reflects expanded experiences and perspectives, including experiences and perspectives arising out of diversity
related to race, ethnicity, gender, sexual orientation and areas of expertise. Consistent with this philosophy, our Board reflects
a mix of ethnic, sexual orientation and gender diversity. As set forth in the matrix below, of our 11 Board members, ten have disclosed
their gender and demographic backgrounds, which consists of eight male and two female Board members, with three Board members who
identify as Asian, seven who identify as white and one who identifies as LGBTQ+.
| Board
Diversity Matrix (As of March 11, 2024) |
| Board
Size: |
|
|
|
|
|
|
|
|
| Total Number of Directors: |
|
|
|
|
|
11 |
|
|
| |
|
Female |
|
Male |
|
Non-Binary |
|
Did
not Disclose Gender |
| Gender: |
|
|
|
|
|
|
|
|
| Directors: |
|
2 |
|
8 |
|
|
|
1 |
| Number
of Directors who Identify in Any of the Categories Below: |
| Asian |
|
|
|
3 |
|
|
|
|
| White |
|
2 |
|
5 |
|
|
|
|
| LGBTQ+ |
|
|
|
|
|
1 |
|
|
Director Compensation
Each of our non-employee directors receives
an annual fee payable in cash. In addition, so that our non-employee directors have an ownership interest aligned with our stockholders,
each non-employee director also receives an annual grant of restricted stock units. Board members also receive an initial equity
grant when they join the Board. Members of our Board committees receive an additional annual fee for each committee on which they
serve. In addition to the annual grant of restricted stock units that all non-employee directors are eligible to receive, our Chairman
receives an additional annual award of restricted stock units with a grant date fair value of $100,000 in connection with his increased
duties. From time to time, the Compensation Committee reviews the compensation of our non-employee directors with our independent
compensation consultant and makes recommendations to the Board with respect to any changes; our non-employee director compensation
program was last reviewed in May 2023. In connection with this update, and as recommended by the independent compensation
consultant based on market practices, no changes were made to
the non-employee director compensation. No changes were made
to our non-employee director compensation structure during 2023
other than the use of a 12-month average stock price over 2022 in
setting the grant price for all equity awards to directors. This 2022
12-month average stock price was $3.27, as compared to an actual
date of grant closing price of $1.86. Consequently, grant date fair
values for awards to non-employee directors were only 57% of
targeted values. The Company expects to revert back to its historic
methodology of using the closing market price of our common stock
on the date of grant for setting the grant price with respect to future
equity grants to non-employee directors. This change in setting the
grant price was made in order to align with grant price methodology
used for awards made to NEOs and eligible employees.
Our director compensation program is summarized
in the table below.
| Annual
Cash Retainer |
|
$75,000 |
| Annual Equity Grant |
|
|
| Restricted stock units (target
value) |
|
$250,000/$350,000 for Chairman |
| Vesting Schedule |
|
1
year cliff vesting |
| Additional Cash Fees for
Committee Service |
|
|
| Audit Committee (Chair/Member) |
|
$25,000/$15,000 |
| Compensation Committee (Chair/Member) |
|
$20,000/$10,000 |
| Nominating and Corporate Governance
Committee (Chair/Member) |
|
$15,000/$7,500 |
| Conflicts Committee (Chair/Member) |
|
$25,000/$15,000 |
In addition to the annual retainers described
above, for each duly convened meeting of a committee that a member of such committee attends in excess of six duly convened meetings
per calendar year, such member shall be entitled to receive an additional Per Meeting Stipend equal to 1/6th of such
member’s annual cash stipend for service on that committee.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
18 |
| |
|
During fiscal 2023, our non-employee directors
received the following compensation:
| Name | |
Fees Earned or Paid in Cash | |
Stock Awards(10) | |
Total |
| Emily Peterson Alva(1) | |
$ | 75,000 | |
$ | 142,203 | |
$ | 217,203 |
| Deborah Autor(2) | |
$ | 124,833 | |
$ | 142,203 | |
$ | 267,036 |
| J. Kevin Buchi(3) | |
$ | 160,000 | |
$ | 142,203 | |
$ | 302,203 |
| Jeff George(4) | |
$ | 135,000 | |
$ | 142,203 | |
$ | 277,203 |
| John Kiely(5) | |
$ | 142,500 | |
$ | 142,203 | |
$ | 284,703 |
| Paul Meister(6) | |
$ | 100,000 | |
$ | 199,083 | |
$ | 299,083 |
| Ted Nark(7) | |
$ | 102,500 | |
$ | 142,203 | |
$ | 244,703 |
| Gautam Patel(8) | |
$ | 75,000 | |
$ | 142,203 | |
$ | 217,203 |
| Shlomo Yanai(9) | |
$ | 112,500 | |
$ | 142,203 | |
$ | 254,703 |
| (1) |
As of December 31, 2023, Ms. Alva had 76,453 RSUs outstanding
and 53,021 options outstanding. |
| (2) |
As of December 31, 2023, Ms. Autor had 76,453 RSUs outstanding. |
| (3) |
As of December 31, 2023, Mr. Buchi had 76,453 RSUs outstanding and 81,397
options outstanding. |
| (4) |
As of December 31, 2023, Mr. George had 76,453 RSUs outstanding and
28,506 options outstanding. |
| (5) |
As of December 31, 2023, Mr. Kiely had 76,453 RSUs outstanding and 28,506
options outstanding. |
| (6) |
As of December 31, 2023, Mr. Meister had 107,034 RSUs outstanding and
115,156 options outstanding. |
| (7) |
As of December 31, 2023, Mr. Nark had 76,453 RSUs outstanding and 53,021
options outstanding. |
| (8) |
As of December 31, 2023, Mr. Patel had 76,453 RSUs outstanding and 53,021
options outstanding. |
| (9) |
As of December 31, 2023, Mr. Yanai had 76,453 RSUs outstanding and 28,506
options outstanding. |
| (10) |
For equity grants made to our non-employee directors in 2023, the Company
utilized the 2022 12-month stock price average in setting the grant price. This 2022 12-month average stock price was $3.27,
as compared to an actual date of grant closing price of $1.86. Consequently, grant date fair values for awards to non-employee
directors were only 57% of targeted values. The Company expects to revert back to its historic methodology of using the closing
market price of our common stock on the date of grant for setting the grant price with respect to future equity grants to
non-employee directors. |
Employee directors do not receive any separate
compensation for their Board activities. Prior to their appointment in August 2019 as Co-Chief Executive Officers, Chintu Patel
and Chirag Patel received compensation for their service as non-employee directors in accordance with the program described above.
Following these appointments, they no longer receive compensation for service as directors. See “Compensation Discussion
and Analysis” beginning on page 31 for information about their compensation as Co-Chief Executive Officers.
Director Stock Ownership Guidelines
In order to further align the interests of
our non-employee directors with the interests of our stockholders, we require our non-employee directors to own our stock as set
forth below.
| Position |
Minimum
Ownership Guideline |
| Non-employee directors |
3x annual cash retainer |
We adopted our stock ownership
guidelines in May 2018, as amended in April 2022, and we expect our non-employee directors to be able to achieve the required
ownership thresholds by five years from the date of adoption of the guidelines. Newly elected directors will have five years
from the date of their appointment or election. For the purpose of determining stock ownership levels, we include shares
owned directly or by immediate family members residing in the same household (or through trusts for their benefit),
underlying restricted stock and restricted stock units (whether or not vested) but exclude shares underlying unexercised
option awards and unearned performance awards. All of our non-employee directors have achieved the minimum ownership
thresholds or are working towards compliance within five years from the date or their appointment or election.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
19 |
| |
|
Proposal 1 Election
of Directors
Introduction
Our Company’s Bylaws provide for the
annual election of directors. Upon the recommendation of our Nominating and Corporate Governance Committee, our Board has nominated
for election each of our current directors.
At the annual meeting, the 11 nominees for
director are to be elected to hold office until the next annual meeting of stockholders and until their successors have been elected
and qualified. Each of the nominees has consented to serve as a director if elected. If any of the nominees shall become unable
or unwilling to stand for election as a director (an event not now anticipated by the Board), proxies will be voted for such substitute
as designated by the Board, or, alternatively, the Board may reduce the number of directors on the Board, subject to the provisions
of the Stockholders Agreement.
Director Nominees
For each of the 11 director nominees standing
for election, the following sets forth certain biographical information, including a description of their business experience during
at least the past five years and the specific experience, qualifications, attributes or skills that qualify them to serve as directors
of the Company and/or members of the Board committees on which they serve. All eleven nominees were previously elected by stockholders
at the 2023 annual meeting.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
20 |
| |
|

| |
|
|
|
PAUL MEISTER
Age 71
Chairman
Paul Meister is a partner in Novalis LifeSciences,
a life science-focused venture firm, and is also co-founder and Chief Executive Officer of Liberty Lane Partners, LLC, a private
investment company with investment holdings in healthcare, technology and distribution-related industries. From 2014 to 2018, he
was President of MacAndrews & Forbes Incorporated, a private company that owns or controls a diverse set of businesses.
During 2018, he also served, on an interim basis, as Executive Vice Chairman of Revlon, Inc. a leading beauty products company.
He served as Chairman and Chief Executive Officer of inVentiv Health (now Syneos Health), a leading provider of commercial, consulting
and clinical research services to the pharmaceutical and biotech industries, from 2010 to 2014. He was Chairman of Thermo Fisher
Scientific, Inc., a scientific instruments equipment and supplies company, from November 2006 to April 2007. He was previously
an Executive Officer of Fisher Scientific International, Inc., a predecessor of Thermo Fisher Scientific from 1991 to 2006. Mr.
Meister has a bachelor’s degree from the University of Michigan and a master’s of business administration degree from
Northwestern University. Mr. Meister’s extensive public company experience, as both an executive and a board member, provides
the Board with significant expertise in management, strategy, finance and capital markets, operations and mergers and acquisitions.
Other Public Company Directorships include Aptiv PLC (since 2019); Oaktree Acquisition Corp. I (2019 – 2021), Oaktree Acquisition
Corp. II (2020 – 2022), Quanterix Corporation (since 2013), and Scientific Games Corporation (2012 – 2020).
Skills and Qualifications:
• CEO,
General Management, Commercial
• Corporate
Development, Business Development, M&A
• R&D,
Scientific
• Investment,
Venture Capital
• International
|
|
CHINTU PATEL
Age 52
Co-Chief
Executive Officer
Chintu Patel has served as Co-Chief Executive
Officer and director of the Company since August 2019. Mr. Patel served as a Co-Chairman of the Board from May 2018 to August 2019,
and previously served as Co-Chairman and Co-Chief Executive Officer of Amneal from 2002 until the Combination. With his brother,
Chirag Patel, Mr. Patel also co-founded and invested in several independent biopharmaceutical companies including Asana Biosciences,
Kashiv BioSciences and Prolong Pharmaceuticals, each of which specializes in innovative science and drug delivery technologies
and Mr. Patel serves on the board of each of these companies. Before founding Amneal, Mr. Patel was a pharmacist and senior-level
manager with Eckerd Pharmacy from 1994 to 2002, where he won numerous awards. Mr. Patel has been a featured speaker at the Hauppauge
Industrial Association in New York and serves on the boards of the Long Island Association and Long Island University, and is a
recipient of the 2011 Ernst & Young National Entrepreneur of the Year Life Sciences Award®. Mr. Patel and
his wife, Falguni Patel, run the Irada International Foundation, which focuses on health, education, and community outreach projects
in India and the United States. Mr. Patel holds a bachelor’s degree in Pharmacy from Rutgers College of Pharmacy and also
holds an honorary doctorate degree from Long Island University.
Skills and Qualifications:
• CEO,
General Management, Commercial
• Manufacturing
• R&D,
Scientific
• Regulatory
|
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
21 |
| |
|

| |
|
|
|
CHIRAG PATEL
Age 57
Co-Chief Executive Officer and President
|
|
EMILY PETERSON ALVA
Age 49
|
| |
|
|
|
Chirag Patel has served as Co-Chief Executive
Officer, President and director of the Company since August 2019. Mr. Patel served as a Co-Chairman of the Board from May 2018
to August 2019, and previously was Co-Founder of Amneal and served as Co-Chairman and Co-Chief Executive Officer of Amneal from
2005 to October 2017. With his brother, Chintu Patel, Mr. Patel has also invested in several independent biopharmaceutical
companies including Asana Biosciences, Kashiv Biosciences and Prolong Pharmaceuticals, each of which specializes in innovative
science and drug delivery technologies and Mr. Patel serves on the board of each of these companies. Earlier in his career, Mr.
Patel co-founded technology companies NextGen Technologies and Veriprise Wireless. Mr. Patel also serves as a Managing Trustee
for the Liberty Science Center of New Jersey, and a Trustee for the Foundation for Morristown Medical Center. He is also a recipient
of the 2011 Ernst & Young National Entrepreneur of the Year Life Sciences Award®. Mr. Patel supports various
philanthropic causes and, together with his wife, Priti Patel, established the Niswarth International Foundation in 2013. The Foundation
aims to bring fresh water, sanitation, nutrition and education to underprivileged children. Mr. Patel received his bachelor’s
degrees in Commerce from H.A. College of Commerce, India, and in Business Administration from New Jersey City University. He also
holds an honorary Doctorate of Humane Letters from New Jersey City University in recognition of his efforts to serve others.
Skills and Qualifications:
• CEO,
General Management, Commercial
• Corporate
Development, Business Development, M&A
• Investment,
Venture Capital
• International
• Regulatory
|
|
Emily Peterson Alva is an experienced corporate
board member and an accomplished finance and strategy executive. Ms. Alva has distinguished herself as a financial, strategic,
and business advisor to boards and leadership teams across industries. Over more than twenty five years, Ms. Alva has advised some
of the world’s largest public and private companies facing complex strategic decisions, and led the resulting wide range
of M&A transactions that followed. Ms. Alva served as an investment banker at Lazard, a global leader in financial advisory
and M&A, where she worked from 1997 to 2013, most recently as an M&A Partner. Today, Ms. Alva is a strategic advisor to
the CEO and the Board of Constellis, a defense contractor and provider of global security solutions, a role she has held since
2021. Ms. Alva is also currently on the corporate boards of Alkermes plc, a public pharmaceutical company, and Robotic Research,
LLC, a private autonomous vehicle technology company serving defense and commercial markets. Ms. Alva is also on the nonprofit
board of the Mission Society of New York City, a foundational charity in New York history, providing economic and educational
support to children and families over more than two centuries. Ms. Alva received a B.A. in Economics from Barnard College, Columbia
University.
Skills and Qualifications:
• M&A,
Complex Transactions and Corporate Development
• Capital
Structure, Corporate Finance and Capital Allocation
• Strategy
and Corporate Governance
|
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
22 |
| |
|

| |
|
|
|
DEB AUTOR
Age 57
|
|
J. KEVIN BUCHI
Age 68
|
| |
|
|
|
Deb Autor is CEO of Healthcare Innovation
Catalysts, a provider of regulatory affairs, clinical advisory, quality, compliance, federal partnership, and strategic advisory
services to global life sciences organizations; and Chair of the Board of the FDA Alumni Association. Ms. Autor has also served
as a Scientific Advisory Council Member of the Centre for Innovation in Regulatory Science and as a Board Member of the Antimicrobial
Resistance Industry Alliance, the United States Pharmacopoeia Quality Institute, and the Parenteral Drug Association.
From 2019-2021, Ms. Autor was Global Head
of Regulatory Excellence at AstraZeneca, where she led multiple regulatory functions for all AstraZeneca products globally. Before
joining AstraZeneca, Ms. Autor served at Mylan N.V. from 2013-2019, where she was Head of Strategic Global Quality and Regulatory
Policy and Head of Global Quality.
Ms. Autor was a senior leader at FDA from
2001-2013, most recently as Deputy Commissioner for Global Regulatory Operations and Policy where she oversaw all FDA inspections,
criminal investigations and international operations for human and veterinary drugs, biologics, medical devices, tobacco and food.
Before that, Ms. Autor served as Director of the Office of Compliance of the Center for Drug Evaluation and Research, leading enforcement
and policy making for compliance with all drug requirements, including drug approval; current good manufacturing practices (GMP);
human subject protection and bioresearch monitoring (GCP); import and export; and recalls. Before joining FDA, Ms. Autor was a
Trial Attorney in the Office of Consumer Litigation of the U.S. Department of Justice, where she litigated civil and criminal cases
on behalf of FDA.
Skills and Qualifications:
• General
Management
• Biosciences,
Pharmaceuticals
• Regulatory
• Compliance
• Legal
|
|
J. Kevin Buchi has served on our Board of
Directors since the Combination. He previously served as the CEO and a member of the board of directors of Biospecifics Technologies
Corp., a biopharmaceutical company from October 2019 until April 2020. He previously served as Impax’s Interim President
and Chief Executive Officer from December 2016 until March 2017, and as a member of the board of directors of Impax from 2016 until
the completion of the Combination. From August 2013 to December 2016, Mr. Buchi served as President and CEO and a member of
the board of directors of TetraLogic Pharmaceuticals Corporation, a biopharmaceutical company, whose assets were subsequently acquired
by Medivir AB in December 2016. Prior to TetraLogic Pharmaceuticals, Mr. Buchi served as Corporate Vice President, Global Branded
Products of Teva Pharmaceutical Industries Ltd., from October 2011 to May 2012. Prior to Teva, Mr. Buchi served as CEO of
Cephalon, Inc., which was subsequently acquired by Teva, from December 2010 to October 2011, and held various positions at Cephalon,
including Chief Operating Officer from January 2010 to December 2010 and Chief Financial Officer from 1996 to 2009. From September
2020 until March 2021, Mr. Buchi served as director and chairman of the Audit Committee of Ziopharm Oncology Inc. Since April 2013,
Mr. Buchi has served as a director and member of the remuneration and nominating committee, and audit committee of the board
of Benitec Biopharma Ltd., a biotechnology company headquartered in Australia. Since November 2021 he has served as a director
of Ampeo Pharmaceuticals. From August 2018 until December 2021, Mr. Buchi served as a director and chairman of the board of Dicerna
Pharmaceuticals. Mr. Buchi previously served as a director of Ziopharm Oncology. Mr. Buchi received his BA degree from Cornell
University and a Masters of Management degree from the J.L. Kellogg Graduate School of Management at Northwestern University. Mr. Buchi’s
extensive experience as a senior executive and board member in the pharmaceutical industry provides the Board with unique insights
into our business.
Skills and Qualifications:
• CEO,
General Management, Commercial
• Corporate
Development, Business Development, M&A
• Finance
and Accounting
• International
|
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
23 |
| |
|

| |
|
|
|
JEFF GEORGE
Age 50
Jeff George has served on our Board of Directors
since December 2019. Mr. George has over 20 years of global healthcare and corporate leadership experience. He is currently
the Managing Partner of Maytal Capital, a healthcare-focused private equity investment and advisory firm he founded in 2017, and
an Operating Partner at Revival Healthcare Capital, a medical device-focused private equity firm. Between 2008 and 2016, he served
on the Executive Committee of Novartis Group AG, one of the largest global pharmaceutical companies, first as Division Head and
CEO of Sandoz, Novartis’ $10 billion generic pharmaceuticals and biosimilars subsidiary, and then as Division Head and CEO
of Alcon, Novartis’ then $10 billion branded eye care subsidiary. In both roles, he was responsible for leading over 25,000
associates globally across more than 160 countries. Mr. George previously headed Emerging Markets for the Middle East, Africa,
Southeast Asia and CIS at Novartis Pharmaceuticals and served as Vice President and Head of Western and Eastern Europe for Novartis
Vaccines. Prior to this, he held leadership roles at Gap Inc. and McKinsey & Co. Mr. George serves on the boards
of 908 Devices, a pioneer in life science diagnostics, Dorian Therapeutics, a cell therapy biotech spun out of Stanford University,
and MAPS PBC, late -stage CNS-focused private biopharma company where he serves as Chairman. He also serves on several non-profit
boards including Education Opens Doors, where he is Chairman, the North Texas Food Bank, and YPO of Dallas. He previously served
on the board of directors of AdvaMed, the medical device industry association, and Roam Analytics, an AI-driven healthcare software
firm, and Wishbone Medical, a leader in pediatric orthopedic medical devices. He holds an M.B.A. from Harvard Business School,
an M.A. from Johns Hopkins University’s School of Advanced International Studies (SAIS), and a B.A. degree from Carleton
College.
Skills and Qualifications:
• CEO,
General Management, Commercial
• Corporate
Development, Business Development, M&A
• Investment,
Private Equity
• International
|
|
JOHN KIELY
Age 65
John Kiely has served on our Board of Directors
since December 2019. Mr. Kiely has more than 35 years of financial leadership and advisory experience serving public companies,
including multinational corporations. From July 1991 until July 2019, when he retired, he served as a Senior Assurance Partner
at Pricewaterhouse Coopers, a multinational professional services firm, where he focused on the pharmaceutical, manufacturing,
chemical, medical device and private equity sectors. He held various roles of increasing responsibility at Pricewaterhouse Coopers,
including as Private Equity Assurance Leader, U.S. Pharmaceutical Leader and Global Pharmaceutical Assurance Leader. Mr. Kiely
is currently and has been since July 2019 on the board of directors of Zovio, Inc., an education technology services company.
In addition, Mr. Kiely has served on the board of directors of Covis Pharmaceutical, Inc. since April 2020. Mr. Kiely holds a B.S.
degree from Saint Francis University.
Skills and Qualifications:
• General
Management, Commercial
• Finance
and Accounting
• M&A
• International
|
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
24 |
| |
|

| |
|
|
|
TED NARK
Age 65
Ted Nark has served on our Board of Directors
since the Combination. Mr. Nark serves as Chairman of West Edge Partners, a lower middle market private equity firm based out of
Los Angeles. Mr. Nark has served as a Managing Director of KRG Capital Partners, a Denver-based $2 billion private equity fund,
since 2007. In that role, he has led the identification, negotiation and due diligence of new acquisitions and has worked with
portfolio companies and maintained relationships with limited partners. While at KRG, Mr. Nark has led the acquisition and successful
monetization of companies, including Convergint Technologies, Diversified Food Services and Petrochoice. From 2006 to 2007, Mr.
Nark was Partner at Leonard Green & Partners and, from 2002 to 2006, served as CEO and Chairman of the Board of White
Cap Construction Supply, a Leonard Green-owned distributor of construction hardware, tools and materials to professional contractors
in the United States. Previously, Mr. Nark served as CEO of Corporate Express Australia and Group President at Corporate Express,
Inc. Mr. Nark is currently a board member of several private companies such as Convergint Technologies, Resource Label Group,
and Coastal Farm and Ranch Supply. Mr. Nark has previously served on the boards of Corporate Express Australia, Fort Dearborn,
White Cap Construction Supply, FTD, Leslies Pools, Gaiam, Real Goods Solar, Western Windows, SavAtree, Trafficware, the Maroon
Group, and Claim Jumper. Mr. Nark received a B.S. degree from Washington State University. Mr. Nark’s strong background in
finance and corporate development combined with his service in executive leadership roles within complex corporate organizations
contribute strategic and management insight to our Board.
Skills and Qualifications:
• CEO,
General Management, Commercial
• Corporate
Development, Business Development, M&A
• Investment,
Venture Capital
• International
|
|
GAUTAM PATEL
Age 51
Gautam Patel has served on our Board of Directors
since the Combination. Mr. Patel has been a Managing Director of Tarsadia Investments, a private investment firm based in Newport
Beach, California, since 2012. In that role, he has led a team of investment professionals to identify, evaluate and execute principal
control over equity investments across sectors including life sciences, financial services and technology. Prior to joining Tarsadia,
Mr. Patel served as a Managing Director at Lazard from 2008 to 2012, where he led financial and strategic advisory efforts in sectors
including transportation and logistics, private equity, and healthcare. Prior to that, Mr. Patel served in a variety of advisory
roles at Lazard from 1999 to 2008, including multiple restructuring, bankruptcy and corporate reorganization assignments in 2001
and 2008. From 1994 to 1997, Mr. Patel was an Analyst at Donaldson, Lufkin & Jenrette, where he worked on M&A as well
as high-yield and equity financings. Mr. Patel is currently a Board Member of Spectrum Brands and several private companies
such as Kashiv Biosciences, Asana Biosciences and Prolong Pharmaceuticals. Mr. Patel also serves on the board of Casita Maria Center
for Arts & Education, a New York based non-profit organization that aims to empower children through arts-based education.
Mr. Patel received a Bachelor of Arts degree from Claremont McKenna College, a Bachelor of Science degree from Harvey Mudd College,
an MSc from the London School of Economics and an M.B.A. from the University of Chicago. Mr. Patel brings an extensive knowledge
of the Company’s business and operations combined with deep experience in finance, corporate development and healthcare investing
to the Board.
Skills and Qualifications:
• Corporate
Development, Business Development, M&A
• Finance
and Accounting
• Investment,
Venture Capital
|
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
25 |
| |
|

| |
|
|
|
SHLOMO YANAI
Age 71
Shlomo Yanai has served on our Board of Directors
since December 2019. Mr. Yanai has more than 17 years of corporate leadership experience, primarily in the pharmaceutical
industry. Mr. Yanai served as President and Chief Executive Officer at Teva Pharmaceutical Industries Ltd. from 2007 to 2012. Prior
to that, Mr. Yanai was the CEO of Adama Industries from 2002 to 2006. Mr. Yanai currently serves as the Chairman of the Board
of Lumenis Ltd., a Board member at Philip Morris, and a senior advisor to Moelis & Company. Mr. Yanai previously served
as Vice Chairman of the Rothschild Caesarea Foundation, Chairman of the Board of Cambrex Corporation and director of Protalix Biotherapeutics,
PDL BioPharma Inc., Perrigo Company, Sagent Pharmaceuticals, Elisra, Bank Leumi Lelsreal, and I.T.L. Optronics Ltd. and a Board
member of W. R. Grace from 2018-2021. Mr. Yanai also held various leadership positions in the Israel Defense Forces. Mr. Yanai
is a graduate of Harvard Business School’s AMP program. He holds a Master of Public Administration degree from George Washington
University and a B.A. degree from Tel Aviv University and is also a graduate of the U.S. National Defense University – War
College.
Skills and Qualifications:
• CEO,
General Management, Commercial
• Investment,
Venture Capital
|
|
|
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
26 |
| |
|
Required Vote
Our Bylaws provide for a majority vote standard
in uncontested elections of directors. Therefore, to be elected at our 2024 annual meeting, which is an uncontested election, each
nominee for director must receive the affirmative vote of the majority of the votes cast with respect to such nominee by the holders
of the shares of common stock voting in person, by remote communication or by proxy at the annual meeting. A majority of the votes
cast means that the number of votes cast “for” a nominee must exceed the number of votes cast “against”
that nominee.
Recommendation of the Board of Directors
 |
THE BOARD OF DIRECTORS RECOMMENDS THAT THE STOCKHOLDERS VOTE “FOR”
EACH OF THE BOARD OF DIRECTORS’ NOMINEES SET FORTH IN PROPOSAL NO. 1. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
27 |
| |
|
Our Management
Executive Officers and Directors
Our executive officers and directors, their
positions and their ages as of March 11, 2024, are as set forth in the table below. Each of our directors holds office until the
next annual meeting of our stockholders or until his or her successor has been elected and qualified. Our executive officers serve
at the discretion of the Board of Directors.
| Name |
|
Age |
|
Position |
| Chintu Patel |
|
52 |
|
Co-Chief Executive Officer, Director |
| Chirag Patel |
|
57 |
|
Co-Chief Executive Officer and President, Director |
| Andrew Boyer |
|
58 |
|
Executive Vice President, Chief Commercial Officer-Generics |
| Anastasios Konidaris |
|
57 |
|
Executive Vice President, Chief Financial Officer |
| Jason B. Daly |
|
50 |
|
Senior Vice President, Chief Legal Officer and Corporate Secretary |
| Nikita Shah |
|
46 |
|
Executive Vice President, Chief Human Resources Officer |
| Paul Meister |
|
71 |
|
Chairman of the Board of Directors |
| Emily Peterson Alva |
|
49 |
|
Director |
| Deb Autor |
|
57 |
|
Director |
| J. Kevin Buchi |
|
68 |
|
Director |
| Jeff George |
|
50 |
|
Director |
| John Kiely |
|
65 |
|
Director |
| Ted Nark |
|
65 |
|
Director |
| Gautam Patel |
|
51 |
|
Director |
| Shlomo Yanai |
|
71 |
|
Director |
For a description of the business experience
of the above individuals who are director nominees standing for election, see “Proposal No. 1-Election of Directors.”
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
28 |
| |
|

|
ANDREW BOYER
Executive Vice President, Chief
Commercial Officer-Generics
|
|
ANASTASIOS KONIDARIS
Executive Vice President, Chief
Financial Officer
|
| |
|
|
| Andrew Boyer has served as our Executive Vice President, Chief Commercial Officer – Generics since August 2020. Prior to that, he served as our Senior Vice President, Commercial Operations since the Combination, and from February 2018, to the completion of the Combination, as Executive Vice President, Commercial Operations of Amneal. Prior to joining Amneal, Mr. Boyer served as President & CEO of North America Generics, Teva Pharmaceutical Industries Ltd., a multinational pharmaceuticals company, from August 2016 to February 2018. Before that, Mr. Boyer was Senior Vice President of Sales and Marketing for the U.S. Generics Division at Allergan, a global pharmaceutical company, from September 2006 to August 2016. Mr. Boyer joined Allergan (then known as Watson Pharmaceuticals) in 1998 as Associate Director of Marketing in Generics. Before joining Allergan, Mr. Boyer served as National Accounts Manager for Lederle/American Cyanamid as well as Marketing Manager for Barr Laboratories. Mr. Boyer received his degree in Business Administration and Management from State University of New York at Albany. |
|
Anastasios Konidaris has served as our Executive Vice President and Chief Financial Officer since August 2020. Prior to that, he was Senior Vice President and Chief Financial Officer since March, 2020. Mr. Konidaris previously served as Executive Vice President and Chief Financial Officer of Alcresta Pharmaceuticals, a specialty pharmaceutical company, since March 2016. Prior to joining Alcresta, Mr. Konidaris served as Senior Vice President and Chief Financial Officer of Ikaria, a biotherapeutics company, from October 2011 to May 2015. From 2007 to May 2011, Mr. Konidaris served as Senior Vice President and Chief Financial Officer at Dun & Bradstreet Corporation, a leading commercial information services company, where he also served as Principal Accounting Officer and led the Global Finance Operations from 2005 to 2007. Earlier in his career, Mr. Konidaris held senior financial and operational positions of increasing responsibility at Schering-Plough, Pharmacia, Rhone-Poulenc Rorer, Novartis, and Bristol-Myers Squibb. Since 2015, Mr. Konidaris has served as a director and chairman of the audit committee of Zep, Inc. He previously served as chairman of Kadmon Holdings, director of Pernix Therapeutics and Delcath Systems. Mr. Konidaris received an MBA from Drexel University and a B.S. from Gwynedd Mercy College. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
29 |
| |
|

|
JASON B. DALY
Senior Vice President, Chief Legal
Officer
and Corporate Secretary
|
|
NIKITA SHAH
Executive Vice President, Chief
Human Resources Officer
|
| |
|
|
| Jason Daly is our Senior Vice President, Chief Legal Officer and Corporate Secretary, a position he has held since January 2022. Mr. Daly is responsible for leading Amneal’s global legal and corporate compliance strategies with a focus on delivering value as Amneal drives into more complex commercial categories. Mr. Daly brings substantial experience leading the legal and commercial strategies for global pharmaceutical & medical device companies, including Teva Pharmaceuticals, Inc., where he most recently served as Senior Vice President, U.S. Market Access and previously as Vice President & Chief of Staff, North American Commercial as well as General Counsel – U.S. Generics & North American Commercial. In his seven-year tenure with Teva, Mr. Daly was responsible for driving the company’s multi-billion-dollar U.S. managed-care customer accounts, including go-to-market tactics for biosimilars and expanding formulary access for key branded products. He also served as a key executive, overseeing a team of commercial attorneys responsible for legal strategies related to patent settlements and authorized generics, pricing policies, REMS programs, the commercialization of the company’s brand and generic medicines, and government affairs. Prior to Teva, Mr. Daly served nearly a decade in various legal and commercial leadership roles with Straumann Group. Mr. Daly previously worked for law firms HinckleyAllen LLP and WilmerHale LLP (f/k/a Hale & Dorr LLP) and was a law clerk to Judge Mary Lisi in the United States District Court in Providence, Rhode Island. Mr. Daly holds academic degrees from the University of Pennsylvania Law School as well as the University of Rhode Island. He holds professional certificates from The Wharton School of Business, The Kellogg School of Management and the Boston University School of Management. He is also a member of the Massachusetts and Rhode Island Bar Associations. |
|
Nikita Shah has served as our Executive Vice President, Chief Human Resources Officer since August 2020. Ms. Shah is responsible for overseeing Amneal’s Human Resources, Internal Communications and ESG (Environmental, Social and Governance) functions, and partners with the Co-CEOs to lead the creation and execution of the Company’s long-term Corporate Strategy. Prior to this position, Ms. Shah served as the Company’s Senior Vice President, Chief Human Resources Officer from May 2018 to July 2020 and as Senior Vice President, Human Resources and Corporate Affairs from January 2014 to May 2018. Prior to joining Amneal, Ms. Shah led the internal audit and human resources functions for Warner Chilcott, a global specialty pharmaceutical company. She also supported corporate Mergers & Acquisitions, process improvements and systems efficiencies across the organization. Prior to Warner Chilcott, Ms. Shah held roles of increasing responsibilities at AT&T and Deloitte. Ms. Shah received her Master’s degrees in Accounting and Auditing from Gujarat University, India. She is a certified public accountant. |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
30 |
| |
|
Executive Compensation
Compensation Discussion and Analysis
The following Compensation Discussion
and Analysis contains statements regarding historical and future Company performance targets or goals. We have disclosed these
targets or goals in the limited context of our compensation programs and they should not be understood to be statements of management’s
expectations or estimates of results. We specifically caution investors not to apply these statements to other contexts.
Executive Summary
In the paragraphs that follow, we provide
an overview and analysis of our compensation programs and policies, the material compensation decisions we have made under those
programs and policies, and the material factors that we considered in making those decisions. Following this section, you will
find a series of tables containing specific information about the compensation earned or paid in fiscal 2023 to the following executive
officers:
2023 NAMED EXECUTIVE OFFICERS
| Name |
Position |
| Chirag Patel |
Co-Chief Executive Officer and President |
| Chintu Patel |
Co-Chief Executive Officer |
| Anastasios Konidaris |
Executive Vice President and Chief Financial Officer |
| Andrew Boyer |
Executive Vice President, Chief Commercial Officer - Generics |
| Nikita Shah |
Executive Vice President, Chief Human Resources Officer |
| Jason Daly |
Senior Vice President, Chief Legal Officer and Corporate Secretary |
Throughout this proxy statement we refer
to these individuals as our “named executive officers” or “NEOs.” The discussion below is intended to help
you understand the detailed information provided in those tables and put that information into context within our overall compensation
program.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
31 |
| |
|
The primary objective of our executive compensation program is
to provide compensation designed to:
| • |
attract, motivate and retain executive officers of outstanding ability and potential; |
| • |
reinforce the execution of our business strategy and the achievement of our business objectives; and |
| • |
align the interests of our executive officers with the interests of our stockholders, with the ultimate objective of increasing stockholder value. |
The Compensation Committee aims to provide
incentives for performance in a given year and over a sustained period by paying reasonable and competitive compensation, and by
basing a significant portion of our total compensation package upon achieving that performance (i.e., “pay for performance”).
We also aim for simplicity in our compensation
program so that it is easy for our employees and our stockholders to understand the various components of our compensation program
and the incentives designed to drive Company performance. The three key components of our executive compensation program are base
salary, annual cash performance-based incentive and equity-based long-term incentive awards.
We believe that the compensation program
has been instrumental in helping the Company achieve financial and strategic goals, as evidenced by the following:
2023 Financial Performance and Operational
Execution
We are a diversified and growing pharmaceutical company across
each of our three business segments, Generic (which includes Retail, Injectables and Biosimilars), Specialty and AvKARE both in
the U.S. and Internationally. Our Company’s mission centers on improving access to affordable, high-quality, and innovative
medicines across these areas. 2023 was a robust year of growth, outstanding execution, and further diversification of our business
as we expanded into new high growth areas. During 2023, we:
| • |
Launched 39 new Generics products as the portfolio continues to shift towards more complex, non-oral solid products; |
| • |
Successfully commercialized our first three biosimilars, ALYMSYS®, RELEUKO® and FYLNETRA®, in their first year post launch, and added two biosimilars to the pipeline; |
| • |
Received approval for a number of high-value injectable products as we further expand our injectables portfolio; |
| • |
Delivered continued growth in key Specialty products, including RYTARY® and UNITHROID®, added ONGENTYS® to expand our portfolio, and continued to advance our Specialty pipeline, including IPX203 for the treatment of Parkinson’s Disease; |
| • |
Drove continued, strong double-digit growth in our durable AvKARE distribution business in the U.S.; and |
| • |
Expanded our international presence as
we launched three new business areas in India: Opthalmology, Diagnostics and Oncology, received approval for our first products
in China and finalized numerous global partnerships for distributing our medicines |
These achievements contributed to strong 2023 performance, highlighted by revenue growth of 8.0% and Adjusted EBITDA growth of 8.6%, in both cases as compared to 2022, and reflects durable growth as both top and bottom-line metrics have grown consistently each year since 2019.
The Company’s balance sheet is strong
as we successfully refinanced our debt and extended maturities to 2028. In addition, during 2023, we reorganized our corporate
structure which is expected to drive significant cash savings for the Company. Furthermore, we finished 2023 with net leverage
of 4.8x, which is down from 7.4x in 2019. We look to achieve our target of below 4.0x net leverage in 2025.
| | |
Full-Year 2023
Results* | | |
Full-Year 2022
Results* | |
| Net Revenue | |
$ | 2,394 | | |
$ | 2,212 | |
| Net Loss | |
$ | (84 | ) | |
$ | (130 | ) |
| Diluted EPS | |
$ | (0.48 | ) | |
$ | (0.86 | ) |
| Adjusted Net Income | |
$ | 198 | | |
$ | 208 | |
| Adjusted EBITDA | |
$ | 558 | | |
$ | 514 | |
| Adjusted Diluted EPS | |
$ | 0.64 | | |
$ | 0.68 | |
| * |
In millions, except per share data |
Adjusted net income, adjusted EBITDA, adjusted
diluted EPS and net leverage are not financial measures in conformity with U.S. generally accepted accounting principles (“GAAP”).
Please see “Appendix A – Non-GAAP Measures” for more information, including reconciliations to the most directly
comparable GAAP measures along with an explanation for why we use these measures and how they are useful to investors.
Amneal delivered positive TSR for the year
on a percentage basis, exceeding the Russell 2000 Index, Nasdaq Composite Total Return Index and Dow Jones U.S. Select Pharmaceuticals
Index during 2023. Set forth below is a line graph comparing the change in the cumulative total shareholder return on our Class
A common stock with the cumulative total returns of the Nasdaq Composite Total Return Index, the Russell 2000 Index and the Dow
Jones U.S. Select Pharmaceuticals Index for the period from December 31, 2018 to December 31, 2023, assuming the investment
of $100 on December 31, 2018, and the reinvestment of dividends.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
32 |
| |
|
Comparison of Cumulative Five Year Total Return
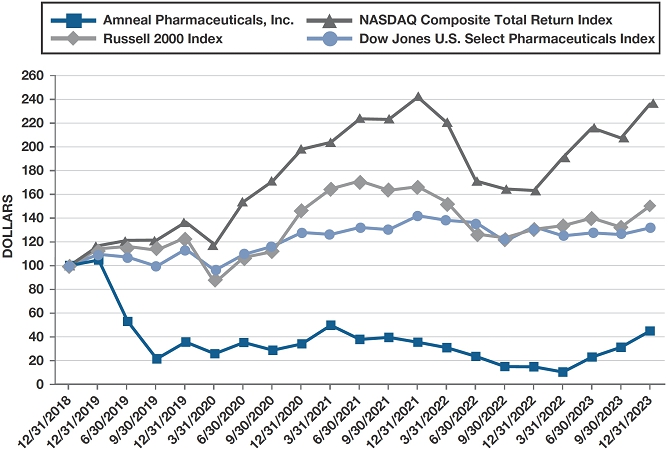
Role of the Compensation Committee
The Compensation Committee of our Board of Directors
is responsible for setting and administering the policies that govern salary, annual and long-term incentive programs and other compensation
and benefits for our executive officers. The Compensation Committee annually evaluates the performance of, and evaluates the compensation
of, our Co-Chief Executive Officers based upon a combination of the achievement of corporate goals and individual performance. As part
of its performance review process, the Compensation Committee solicits the input of the full Board of Directors and makes recommendations
to the full Board, which makes compensation decisions with respect to our Co-Chief Executive Officers on the basis of those recommendations.
The Compensation Committee oversees various executive and employee compensation plans and programs, and it has responsibility for continually
monitoring these plans and programs to confirm that they adhere to our compensation philosophy and objectives. Our Compensation Committee
determines the appropriate compensation levels of executives, evaluates officer and director compensation plans, policies and programs,
and reviews benefit plans for officers. Our Compensation Committee believes that the total compensation paid to our named executive officers
should be reasonable and competitive, and that a significant portion of the total compensation should be tied to our Company’s annual
and long-term performance.
The Compensation Committee has the authority to
engage the services of outside advisers, experts and others to assist the Compensation Committee, and believes that it is important to
do so from time to time. See “Peer Group Surveys and the Role of Our Compensation Consultant” below.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
33 |
| |
|
Role of Our Co-Chief Executive Officers in Compensation
Decisions
Regarding most compensation matters, including executive
compensation and our annual and long-term incentive plans, Chirag Patel, our Co-Chief Executive Officer and President, and Chintu Patel,
our Co-Chief Executive Officer, evaluate the performance of our other executive officers and make recommendations to the Compensation
Committee on the basis of these reviews, including with respect to salary adjustments and incentive plan award amounts for the other executive
officers. The Compensation Committee, however, does not delegate any of its functions to others in setting compensation for our named
executive officers and exercises its judgment to make final compensation determinations.
Neither of the Co-Chief Executive Officers participates
in the decision making regarding his own compensation. Our Compensation Committee reports the compensation decisions it has made with
respect to our executive officers other than our Co-Chief Executive Officers to the Board of Directors.
Peer Group Surveys and the Role of Our Compensation
Consultant
The Compensation Committee engages an independent
compensation consultant who acts as a strategic advisor to the Compensation Committee, providing objective analysis and expertise on matters
related to executive compensation programs and governance. Since 2022, the Compensation Committee has engaged Meridian as the committee’s
independent compensation consultant.
Each year the Compensation Committee reviews and
evaluates the independence of its compensation consultant and considers several factors in determining their independence. For 2023, the
Compensation Committee evaluated the following factors in determining Meridian’s independence:
| • |
Meridian’s services are limited to their scope of work as the Compensation Committee’s independent
compensation consultant. |
| • |
The fees paid to Meridian represented less than 1% of Meridian’s total annual revenue. |
| • |
Meridian maintains policies and procedures which are designed to prevent conflicts of interest including a Code of Business Conduct
and Ethics Policy as well as an Insider Trading and Stock Ownership Policy. |
| • |
There were no business or personal relationships between Meridian’s individual compensation advisor with any Compensation
Committee member nor any executive officer of the Company. |
Further, in assessing Meridian’s independence
from management in providing executive compensation services to the Compensation Committee, the Compensation Committee considered that
Meridian is only engaged by, takes direction from, and reports to, the Compensation Committee and only the Compensation Committee has
the right to terminate or replace Meridian as its compensation consultant. For 2023, the Compensation Committee evaluated whether any
work provided by Meridian raised any conflict of interest and, based on the foregoing, determined that it did not.
Peer Group Companies and Survey Data
Our Compensation Committee finds comparative data
from our peer group to be helpful in setting and adjusting executive compensation, but it does not target our programs or any particular
element of compensation to be at or within a particular percentile or range compared to our peers. Our Compensation Committee uses the
peer group data along with other relevant compensation survey sources to ensure that our executive compensation program and its constituent
elements are and remain competitive in relation to our peers, and applies judgment and discretion in establishing targeted compensation
levels taking into account not only competitive market data but also the experience of the executive, scope of responsibility, critical
skill sets and expertise.
The Compensation Committee reviews the composition
of our peer group annually to ensure that the companies constituting the peer group continue to provide meaningful and relevant compensation
comparisons, including representation from appropriate industries, financial metric comparisons (such as revenue, EBITDA, market capitalization
and financial growth), number of employees and location. With respect to the composition of the peer group used to evaluate 2023 executive
compensation, the Compensation Committee also sought representation from companies with generic, specialty and biosimilar products as
our own mix of products across these categories continues to evolve. In September of 2022, in consideration of these factors and the recommendation
of its independent compensation consultant, the Compensation Committee removed OPKO Health and added ANI Pharmaceuticals, Inc., Coherus
BioSciences, Inc., Eagle Pharmaceuticals, Inc., Mallinckrodt plc, Neurocrine Biosciences, Inc., Organon & Co, Pacira BioSciences,
Inc., Teva Pharmaceutical Industries Limited and Viatris Inc. to the 2023 compensation peer group.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
34 |
| |
|
Our 2023 peer group consisted of the following publicly
traded companies:
| • Alkermes plc |
|
• Mallinckrodt plc* |
| • Amphastar Pharmaceuticals, Inc. |
|
• Neurocrine Biosciences, Inc.* |
| • ANI Pharmaceuticals, Inc.* |
|
• Organon & Co.* |
| • Coherus BioSciences, Inc.* |
|
• Pacira BioSciences, Inc.* |
| • Eagle Pharmaceuticals, Inc.* |
|
• Perrigo Company plc |
| • Emergent BioSolutions Inc. |
|
• Prestige Consumer Healthcare Inc. |
| • Endo International plc |
|
• Supernus Pharmaceuticals, Inc. |
| • Horizon Therapeutics Public Limited Company |
|
• Teva Pharmaceutical Industries Limited* |
| • Jazz Pharmaceuticals plc |
|
• United Therapeutics Corporation |
| • Lannett
Company, Inc. |
|
• Viatris
Inc.* |
| * |
New addition in September 2022. |
At the time of the Compensation Committee’s
September 2022 peer group review, Amneal approximated the 65th, 25th and 40th percentiles for revenue,
market capitalization and enterprise value, respectively, relative to the 2023 compensation peer group.
Components of Executive Compensation
Consistent with its pay for performance philosophy,
the Compensation Committee believes that it is important to place a greater percentage of executives’ and senior managers’
compensation at risk than that of non-executives and non-senior managers. This is done by tying executives’ and senior managers’
compensation directly to the performance of the Company. Accordingly, as set forth in the charts below, a significant portion of executive
compensation consists of annual and long-term incentives linked to the Company’s financial performance and/or the performance of
the Company’s stock.
Base Salaries
The base salary component of our executive compensation
program is fixed cash compensation that is based on role, scope of responsibility, experience, previous performance, and competitive pay
practices. On an annual basis, the Compensation Committee reviews peer group as well as other compensation survey data as an independent
measure to confirm that base salary levels remain reasonable and competitive.
The base salaries of our named executive officers
as of December 31, 2022 and December 31, 2023, respectively, are reflected in the accompanying table. After two years of no change
in the base salaries of the named executive officers, and taking into account factors, including market data regarding base salary changes,
the Compensation Committee approved increases to the base salary of each of our named executive officers excluding our Co-CEOs and Mr.
Boyer.
| Name | |
2022
Base Salary | | |
2023
Base Salary | | |
%
Increase | | |
Reason for Change |
| Chirag Patel | |
$ | 750,000 | | |
$ | 750,000 | | |
| — | | |
|
| Chintu Patel | |
$ | 750,000 | | |
$ | 750,000 | | |
| — | | |
|
| Anastasios Konidaris | |
$ | 550,000 | | |
$ | 566,500 | | |
| 3.0 | % | |
Merit |
| Andrew Boyer | |
$ | 600,000 | | |
$ | 600,000 | | |
| — | | |
|
| Nikita Shah | |
$ | 485,000 | | |
$ | 499,550 | | |
| 3.0 | % | |
Merit |
| Jason Daly | |
$ | 435,000 | | |
$ | 478,500 | | |
| 10.0 | % | |
Merit, Market Adjustment |
Annual and Long-Term Incentive Awards
In order to align the interests of our stockholders
with our compensation plans, we tie significant portions of our named executive officers’ compensation to our annual and long-term
financial, operating and stock price performance through annual cash and long-term equity incentives. The Compensation Committee’s
philosophy is that named executive officers should expect the level of their compensation to vary with performance, with compensation
increasing when performance exceeds our internal targets and budgets and compensation decreasing when performance falls below these expectations.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
35 |
| |
|
Annual Performance-Based Cash Incentive Compensation
The Compensation Committee believes that, to reward
performance and overall Company success, a portion of an executive officer’s annual cash compensation should be tied to the achievement
of the Company’s goals and that individual’s performance goals. The annual incentive program is designed as a pay-for-performance
plan and includes components for both Company and individual performance.
Company performance is based on achievement of adjusted
EBITDA with minimum, target and maximum milestones from which a Company performance multiplier is derived. The minimum performance threshold
is 85% of target and the maximum performance level is 125% of target; the associated company performance multiplier ranges from 25% to
150%.
Individual performance is also a component of the
annual incentive program measured based on achievement of personal/team performance goals and assessment against the Company’s core
values. The individual performance multiplier can range from 0-150%. This annual cash incentive payment is calculated as set forth below.
Annual Incentive
Target Amount |
x |
Company Performance
Multiplier |
x |
Individual Performance
Multiplier |
= |
Incentive
Payment |
| Targets vary based on level and are expressed as a percentage of base salary |
|
Full year 2023 Company performance is measured based on achievement against adjusted EBITDA goal |
|
Individual performance multiplier can range from 0%-150% |
|
|
The Compensation Committee historically has chosen
adjusted EBITDA as the target performance objective for the payment of awards under our annual cash incentive plan. Adjusted EBITDA is
not a term defined under U.S. GAAP. We define adjusted EBITDA as net income before net interest expense, income taxes, and depreciation
and amortization (“EBITDA”), as adjusted for certain other items described in our SEC filings, including stock-based compensation
expense, acquisition, site closure and idle facility expenses, restructuring and other charges, net charges related to legal matters,
asset impairment charges, foreign exchange losses or gains, change in fair value of contingent consideration, and insurance recoveries
for property losses and associated expenses. Please see “Appendix A – Non-GAAP Measures” for more information, including
reconciliations to the most directly comparable GAAP measure.
The Compensation Committee believes that adjusted
EBITDA growth most closely reflects our operating performance and is a key metric for driving the long-term strategic direction that the
Board of Directors has set for our Company. Further, adjusted EBITDA growth is highly correlated to or reflective of our Company’s
financial and operational improvements, ability to generate cash flow from operations, growth and long-term return to stockholders. We
believe that adjusted EBITDA is helpful in assessing the overall performance of our business and is helpful in highlighting trends in
our overall business because the extraordinary or one-time items excluded in calculating adjusted EBITDA have little or no bearing on
our day-to-day operating performance. We also believe that adjusted EBITDA is an important non-GAAP valuation tool that potential investors
use to measure our profitability against other companies in our industry.
Our adjusted EBITDA target for a given year is determined
by the Compensation Committee based upon recommendations from and discussions with management, a review of current economic conditions
and recent acquisition activity and aligns with our external targets. Factors used by the Compensation Committee in setting adjusted EBITDA
targets include, among others, the following:
| • |
reasonable growth expectations taking into account a variety of circumstances faced by our Company; |
| • |
market conditions, including the related impact on cost and our ability to offset any cost increases with pricing increases or
other cost savings measures; and |
| • |
prior fiscal year adjusted EBITDA. |
After the Compensation Committee reviews the final
full year financial results of our Company, the Compensation Committee approves annual cash incentive payouts for the prior year. Annual
cash incentive awards are generally paid in March.
No annual cash incentive payments are made unless
the threshold adjusted EBITDA target has been achieved. Adjusted EBITDA targets under the annual cash incentive plan may be reset periodically
within a fiscal year by the Compensation Committee to take into account acquisitions, divestitures and other unplanned events. Subject
to the provisions of an applicable employment agreement, executives generally must be employed at the time of payout to receive an annual
cash incentive award. The Compensation Committee may, however, at its discretion, prorate awards in the event of certain circumstances
such as the executive’s promotion, demotion, death or retirement.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
36 |
| |
|
The Company performance component of the annual
award each executive is eligible to receive is based upon a percentage of the executive’s annualized base salary, with such percentage
varying depending upon the level of adjusted EBITDA as compared to threshold, target and maximum adjusted EBITDA performance objectives
as set forth in the table below.
| |
|
Company
Performance Component of Annual
Bonus Award as a Percentage of Base Salary |
| Name |
|
Threshold |
|
Target |
|
Maximum |
| Chirag Patel |
|
25% |
|
100% |
|
150% |
| Chintu Patel |
|
25% |
|
100% |
|
150% |
| Anastasios Konidaris |
|
13.75% |
|
55% |
|
82.5% |
| Andrew Boyer |
|
20% |
|
80% |
|
120% |
| Nikita Shah |
|
13.75% |
|
55% |
|
82.5% |
| Jason Daly |
|
12.5% |
|
50% |
|
75% |
The fiscal 2023 combined adjusted EBITDA threshold,
target and maximum performance objectives were $437.75 million, $515 million and $643.75 million, respectively. Our Company’s
fiscal 2023 reported adjusted EBITDA was $558 million; furthermore, for purposes of determining performance under the 2023 annual cash
incentive plan, the Compensation Committee reviewed and approved, as permitted under the plan, an additional $12 million adjustment to
the reported 2023 adjusted EBITDA measure for certain regulatory developments deemed to be outside of management’s control. This
resulted in achievement of 110.7% of target and a 121.4% Company performance multiplier.
The 2023 annual performance-based cash incentive
program also provides for an individual performance multiplier, which ranges from 0% to 150% based on an individual’s performance
during the year. In assessing the performance of our NEOs, other than our co-CEOs, the Compensation Committee, with the input of our co-CEOs,
considered each officer’s performance against the goals of their respective functions, as well as the executive’s contribution
to achievement of our corporate goals. The individual multipliers ranged from 100% to 120%.
| Name |
|
2023 Base
Salary |
|
Annual
Incentive
Target % |
|
Annual
Incentive
Target $ |
|
X |
|
Company
Performance
Multiplier % |
|
X |
|
Individual
Performance
Multiplier % |
|
= |
|
Final AIP
Payout
$ |
|
AIP Payout %
of Target |
| Chirag K. Patel |
|
$ |
750,000 |
|
100% |
|
$ |
750,000 |
|
|
|
121.4% |
|
|
|
100% |
|
|
|
$ |
910,500 |
|
121.4% |
| Chintu Patel |
|
$ |
750,000 |
|
100% |
|
$ |
750,000 |
|
|
|
121.4% |
|
|
|
100% |
|
|
|
$ |
910,500 |
|
121.4% |
| Anastasios Konidaris |
|
$ |
566,500 |
|
55% |
|
$ |
311,575 |
|
|
|
121.4% |
|
|
|
120% |
|
|
|
$ |
453,902 |
|
145.7% |
| Andrew Boyer |
|
$ |
600,000 |
|
80% |
|
$ |
480,000 |
|
|
|
121.4% |
|
|
|
100% |
|
|
|
$ |
582,720 |
|
121.4% |
| Nikita Shah |
|
$ |
499,550 |
|
55% |
|
$ |
274,753 |
|
|
|
121.4% |
|
|
|
100% |
|
|
|
$ |
333,550 |
|
121.4% |
| Jason Daly |
|
$ |
478,500 |
|
50% |
|
$ |
239,250 |
|
|
|
121.4% |
|
|
|
100% |
|
|
|
$ |
290,450 |
|
121.4% |
In determining the individual performance modifier
for the named executive officers other than Mr. Konidaris, the Compensation Committee considered that, for 2023, each of the executives
met the goals of their respective functions and contributed to the achievement of the Company’s corporate goals. In approving the
120% individual performance modifier for Mr. Konidaris, the Compensation Committee took into consideration, among other things, (1) that
Amneal exceeded all of its budgeted financial metrics for 2023, (2) the successful refinancing of a $2.35 billion term loan, the
effect of which is to extend the maturity date of an equivalent principal amount of term loans under Amneal’s existing term loan
credit agreement to May 4, 2028, and (3) the work performed in support of the Company’s closing of the Reorganization.
Long-Term Incentive Compensation
Our long-term incentive compensation program is
designed to promote a balanced focus on driving performance, retaining talent and aligning the interests of our executives with those
of our other stockholders. The Amneal Pharmaceuticals, Inc. Amended and Restated 2018 Incentive Award Plan (the “2018 Incentive
Award Plan”) authorizes the grant of stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards,
other stock or cash-based awards and dividend equivalent awards to employees, non-employee directors and consultants.
Our long-term incentive compensation program for
2023 for the named executive officers was comprised of two components: performance-based restricted stock units (“PSUs”) and
restricted stock units (“RSUs”).
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
37 |
| |
|
| • |
Performance-based restricted stock units.
We grant PSUs, which vest upon the attainment of certain performance metrics, in order to further incentivize our executive
officers to deliver superior long-term results. |
| • |
Restricted stock units. We grant restricted stock
unit awards as a retention tool as they provide the opportunity to receive stock only if the recipient is still employed by us on
the date the restrictions lapse. |
In 2023, the total value of each named executive
officer’s equity grants was divided between restricted stock units and PSUs, other than the Co-Chief Executive Officers who received
all of their long-term incentive compensation in the form of PSUs. The mix of different types of awards is generally intended to combine
the retention and downside risk benefits inherent in restricted stock units with the incentive and stockholder value creation benefits
inherent in performance-based restricted stock units.
In addition, in order to preserve share capacity
under the 2018 Incentive Award Plan, for our 2023 long-term incentive program, the Company utilized the 2022 12-month stock price average
in setting the grant price for all equity awards. This 2022 12-month average stock price was $3.27, as compared to an actual date of grant
closing price of $1.92. Consequently, grant date fair values for awards under our 2023 long-term incentive plan were only 59% of targeted
values. The Company expects to revert back to its historic methodology of using the closing market price of our common stock on the date
of grant for setting the grant price with respect to awards under the 2024 long-term incentive plan.
Considerations Regarding the Co-Chief Executive Officers’
2023 Long-Term Incentive Compensation Awards
The Compensation Committee forms its recommendation
regarding the long-term incentive compensation for the Co-Chief Executive Officers following an in-depth assessment of numerous factors
and developments and in consideration of its “pay for performance” philosophy. In making its determinations, the Compensation
Committee has historically approached the Co-Chief Executive structure by evaluating the total compensation of the top two executives
at peer companies.
With respect to the long-term incentive awards made
to the Co-Chief Executive Officers in 2023, the Compensation Committee initially targeted a total long-term incentive compensation package
for each of the Co-Chief Executive Officers of $2.4 million solely in the form of PSUs, representing a below-market value lower than the
25th percentile of the Company’s peer group companies. Given the economic headwinds impacting the Company’s share
price development, for fiscal year 2023, the Co-Chief Executive Officers proposed, and the Compensation Committee agreed, that the Co-Chief
Executive Officers would be awarded half of the targeted long-term incentive award, or $1.2 million, an amount that resulted in total
annual compensation significantly below the Company’s peer group median. This proactive proposal to reduce the value of the Co-Chief
Executive Officers’ long-term incentive award for 2023 was further affected by the Company’s decision to use the 12-month
average stock price for FY 2022 ($3.27) in setting the grant price for 2023 long-term incentive awards. This award pricing methodology,
coupled with the use of a Monte Carlo valuation, further reduced the fair market value of the Co-Chief Executive Officers’ long-term
incentive awards to approximately $664,000 each, as reflected in the Summary Compensation Table.
The following table sets forth the total grant value
and components mix determined for issuance to our named executive officers in 2023:
| Name | |
Value of 2023
Awards(1) | | |
% PSUs | |
% RSUs |
| Chirag Patel | |
$ | 1,200,000 | | |
100% | |
|
| Chintu Patel | |
$ | 1,200,000 | | |
100% | |
|
| Anastasios Konidaris | |
$ | 1,500,000 | | |
50% | |
50% |
| Andrew Boyer | |
$ | 1,300,000 | | |
50% | |
50% |
| Nikita Shah | |
$ | 1,200,000 | | |
50% | |
50% |
| Jason Daly | |
$ | 1,000,000 | | |
50% | |
50% |
| (1) |
Values in the table represent the determined grant value. Actual awards were based on this value using
the 2022 twelve-month average closing stock price, which was $3.27. |
Restricted Stock Unit Awards
In 2023, annual restricted stock unit grants were
made in March for all of the named executive officers, other than our Co-Chief Executive Officers, who received no restricted stock unit
grants. All of the restricted stock unit awards vest in four equal installments on the first, second, third and fourth anniversary of
the grant date subject to cancellation or acceleration as provided in the individual restricted stock unit award agreements. The number
of restricted stock unit awards granted to each named executive officer in 2023 was based upon the 2022 twelve-month average closing stock
price. For additional details on this award, see “Executive Compensation—Management Employment & Separation Agreements”
below.
Performance-Based Restricted Stock Unit Awards
In 2023, our named executive officers were granted
PSU awards in March. These awards will be earned at a rate of 0% and 200% of the target award amounts based on the Company’s achievement
of a stock price growth target relative to the 30-day average closing stock price preceding the grant date, which was $2.34, during the
performance period from March 1, 2023 until February 28, 2026. A payout of 75% of the target award is earned at the achievement of
150% of the stock price growth target based on the trailing average closing stock price of our common stock over the 60 calendar days
preceding February 28, 2026, and a payout of 200% of the target award if the average closing stock price is 300% or greater of the stock
price growth target. Any earned awards vest in full at the end of such performance period, subject to cancellation or acceleration as
provided in the individual performance-based restricted stock unit award agreements. The number of PSUs granted to each named executive
officer is based upon the 2022 twelve-month average closing stock price.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
38 |
| |
|
Performance-Based Restricted Stock Unit Award Determinations
for 2023
In 2021, Messrs. Chirag Patel, Chintu Patel, Boyer
and Konidaris and Ms. Shah were granted Performance-Based Restricted Stock that would vest based on achievement of an absolute stock price
target. Actual performance during the three-year performance period of March 1, 2021 through February 28, 2024 was below the absolute
stock price threshold of $8.00, resulting in no payout. As a result, the underlying awards were cancelled.
Other Compensation and Benefits
Benefits offered to our named executive officers
serve a different purpose than do the other elements of total compensation. In general, they are provided for the convenience of the Company
or are designed to provide a safety net of protection against the financial catastrophes that can result from illness, disability or death.
Most benefits offered to our named executive officers are generally the same as those offered to the general employee population.
Under the Company’s 401(k) plan, the Company
makes a 100% matching contribution with respect to each participant’s elective contributions, up to 5% percent of such participant’s
compensation (provided that for fiscal 2022, matching contributions were based only on the first $305,000 of such participant’s
compensation in accordance with Internal Revenue Code limitations). Matching contributions generally become fully vested after three years
of employment with the Company.
Executive Severance and Change in Control Severance Benefits
For a discussion of executive severance and change
in control severance benefits, our rationale for offering those benefits and the triggers for payments, see “Management Employment &
Separation Agreements—Severance Upon a Change in Control” below.
Consideration of 2023 Say-on-Pay Vote
At the 2023 Annual Meeting of Stockholders, our
“say-on-pay” advisory vote received 98.4% support. The Compensation Committee regarded this vote, as well as feedback from
our engagement with stockholders, as demonstrating strong support for our executive compensation program and did not make any significant
changes to our compensation programs as a result of this vote.
Accounting and Tax Considerations
Financial reporting and income tax consequences
to our Company of individual compensation elements are important considerations for our Compensation Committee when it is analyzing the
overall level of compensation and the mix of compensation. Overall, the Compensation Committee seeks to balance its objective of maintaining
a fair, reasonable and competitive compensation package for our named executive officers with enabling the deductibility of compensation.
Executive Compensation Clawback Policy
Effective October 2, 2023, the Compensation Committee
adopted our Clawback Policy to comply with applicable listing standards, which implements the final rule promulgated by the SEC for recovery
of erroneously awarded compensation. The Clawback Policy requires recovery of any excess incentive-based compensation from any current
or former executive officers subject to Section 16 of the Exchange Act (“Section 16”) following a restatement of financial
information that affects a financial measure used to determine such incentive-based compensation.
The excess amount is any such amount by which the
affected incentive-based compensation exceeds the compensation that would have been received by the executive officer, without regard
to any taxes paid, had the applicable financial measure not been subject to error. Under the Clawback Policy, our Compensation Committee
determines the method of recovery, and recovery may be deemed impractical only in limited circumstances enumerated in the Nasdaq listing
standards.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
39 |
| |
|
Stock Ownership Guidelines for Executive Officers
In order to further align the interests of our management with the interests
of our stockholders, we require our executive officers to own shares of our stock as set forth below.
| Position |
Minimum Ownership
Guideline |
| Co-Chief Executive Officers |
6x base salary |
| Other Executive Officers |
2x base salary |
We adopted our stock ownership guidelines in May
2018, and we expect our executive officers to be able to achieve the required ownership thresholds by five years from the date of adoption
of the guidelines. Newly appointed officers will have five years from the date they became subject to the stock ownership guidelines to
comply with them. For the purpose of determining stock ownership levels, we include shares owned directly or by immediate family members
residing in the same household (or through trusts for their benefit) and underlying restricted stock and restricted stock units (whether
or not vested), but exclude shares underlying unexercised stock option awards and unearned performance awards. All of our named executive
officers are in compliance with these requirements.
See “Corporate Governance—Director Compensation—Director
Stock Ownership Guidelines” above for a description of stock ownership guidelines we have adopted for our non-employee directors.
Anti-Hedging Policy
To prevent speculation or hedging, our insider trading
policy prohibits our named executive officers (and our directors and all other employees) from engaging in short sales of our Company’s
stock. Company policy also prohibits our directors, executive officers and certain other employees from purchasing or selling any financial
instrument that is designed to hedge or offset any decrease in the market value of our Company’s stock, including prepaid variable
forward contracts, equity swaps, collars and other derivative securities that are directly linked to our Company’s stock. All other
employees are discouraged, but not expressly prohibited, from entering into hedging transactions related to Company stock. In addition,
pursuant to our Corporate Governance Guidelines, directors and executive officers who are not Amneal Group Members (as defined in the
Stockholders Agreement) are prohibited from pledging Company stock as collateral for a loan.
Report of the Compensation Committee
The Compensation Committee of the Board of Directors
has reviewed and discussed the foregoing Compensation Discussion and Analysis with management. Based on this review and discussion, the
committee recommended to our Board of Directors that the Compensation Discussion and Analysis be included in this proxy statement and
incorporated by reference into the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023. This report
is provided by the following directors, who comprise the committee.
Compensation Committee:
Ted Nark (Chair)
Jeff George
Paul Meister
Shlomo Yanai
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
40 |
| |
|
Summary Compensation Table
The following table shows the compensation of our
named executive officers for the periods presented. The sum and/or computation of individual numerical amounts disclosed in the following
table and related footnotes may not equal the total due to rounding.
Name and
Principal Position | |
Year | |
Salary
($) | |
Bonus
($) | |
Stock
Awards(1)
($) | |
Option
Awards
($) | |
Non-Equity
Incentive Plan
Compensation
($) | |
All Other
Compensation(2)
($) | |
Total
($) |
| Chirag Patel | |
2023 | |
750,000 | |
— | |
664,219 | |
— | |
910,500 | |
45,666 | |
2,370,386 |
| Co-Chief Executive Officer and President | |
2022 | |
750,000 | |
— | |
3,605,796 | |
— | |
652,500 | |
39,814 | |
5,048,110 |
| |
2021 | |
724,038 | |
— | |
3,266,406 | |
— | |
757,500 | |
37,172 | |
4,785,116 |
| Chintu Patel | |
2023 | |
750,000 | |
— | |
664,219 | |
— | |
910,500 | |
42,833 | |
2,367,553 |
| Co-Chief Executive Officer | |
2022 | |
750,000 | |
— | |
3,605,796 | |
— | |
652,500 | |
54,436 | |
5,062,732 |
| | |
2021 | |
724,038 | |
— | |
3,266,406 | |
— | |
757,500 | |
50,881 | |
4,798,825 |
| Anastasios Konidaris | |
2023 | |
563,327 | |
— | |
855,505 | |
— | |
453,902 | |
18,531 | |
1,891,266 |
| EVP & Chief Financial Officer | |
2022 | |
550,000 | |
— | |
1,876,807 | |
— | |
289,493 | |
16,886 | |
2,733,186 |
| | |
2021 | |
550,000 | |
— | |
1,770,757 | |
— | |
326,912 | |
16,327 | |
2,663,996 |
| Andrew Boyer | |
2023 | |
600,000 | |
— | |
741,438 | |
— | |
582,720 | |
18,531 | |
1,942,689 |
| EVP, Chief Commercial Officer - Generics | |
2022 | |
600,000 | |
— | |
1,626,572 | |
— | |
438,480 | |
16,886 | |
2,681,937 |
| |
2021 | |
600,000 | |
— | |
1,534,657 | |
— | |
484,800 | |
16,406 | |
2,635,863 |
| Nikita Shah | |
2023 | |
496,752 | |
— | |
684,403 | |
— | |
333,550 | |
18,528 | |
1,533,232 |
| EVP, Chief Human Resources Officer | |
2022 | |
485,000 | |
— | |
1,501,454 | |
— | |
255,519 | |
16,886 | |
2,258,859 |
| | |
2021 | |
483,789 | |
— | |
1,416,601 | |
— | |
307,136 | |
16,169 | |
2,223,694 |
| Jason Daly | |
2023 | |
470,135 | |
— | |
570,336 | |
— | |
290,450 | |
18,476 | |
1,349,396 |
| Senior Vice President, Chief Legal Officer & Secretary | |
2022 | |
401,538 | |
175,000 | |
938,409 | |
— | |
189,225 | |
16,765 | |
1,720,937 |
| (1) |
These amounts reflect the aggregate grant date fair value of each
restricted stock unit award and PSU award granted during the fiscal year, computed in accordance with FASB ASC Topic 718. The valuation
assumptions used in determining such amounts are described in Note 23 to the financial statements included in our Annual Report on
Form 10-K for the year ended December 31, 2023. For the PSU awards granted in 2023, the value of the awards as of the grant date,
assuming that the highest level of performance achievement would be achieved (which is 200% of target), for Mr. Chirag Patel, Mr.
Chintu Patel, Mr. Konidaris, Mr. Boyer, Ms. Shah and Mr. Daly would be $1,328,439, $1,328,439, $830,276, $719,573, $664,219 and $553,516,
respectively. |
| (2) |
The amounts shown in this column for 2023 consist of the following components: |
| |
Name | |
Company
401(k)
Match
($) | |
Life and
Disability
Insurance
Premiums Paid
by Company
($) | |
Cost for
Personal Use
of Driver and
Company Car(1)
($) | |
Total
($) |
| |
Chirag Patel | |
16,500 | |
2,031 | |
27,135 | |
45,666 |
| |
Chintu Patel | |
16,500 | |
2,031 | |
24,302 | |
42,833 |
| |
Anastasios Konidaris | |
16,500 | |
2,031 | |
— | |
18,531 |
| |
Andrew Boyer | |
16,500 | |
2,031 | |
— | |
18,531 |
| |
Nikita Shah | |
16,500 | |
2,028 | |
— | |
18,528 |
| |
Jason Daly | |
16,500 | |
1,976 | |
— | |
18,476 |
| |
(1) |
We own a car and employ a driver for the exclusive use of each of Mr. Chirag Patel and Mr. Chintu
Patel. Although the majority of the driver’s services (and, therefore, the costs associated with the car) are for business
purposes, we allow for use of the car and driver for personal purposes - generally for daily commute - as we believe this accommodation
enables increased productivity during this time. We calculated the incremental cost to us for the personal use of the Company car
and driver based on the costs associated with the car, its operation, and a portion of the driver’s compensation equivalent
in proportion to the time the car is used for personal purposes. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
41 |
| |
|
Grants of Plan Based Awards in 2023
The following tables set forth information about non-equity and equity
awards granted to the named executive officers in fiscal 2023.
| | |
| |
Estimated Future Payouts
Under Non-Equity Incentive
Plan Awards(1) | |
Estimated Future Payouts Under
Equity Incentive Plan Awards(2) | |
All Other
Stock
Awards:
Number of
Shares of | |
Grant
Date Fair
Value of
Stock and |
| Name | |
Grant
Date | |
Threshold
($) | |
Target
($) | |
Maximum
($) | |
Threshold
(#) | |
Target
(#) | |
Maximum
(#) | |
Stock or
Units(3)
(#) | |
Option
Awards(4)
($) |
| Chirag Patel | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| 2023 Annual Cash Incentive | |
| |
187,500 | |
750,000 | |
1,125,000 | |
| |
| |
| |
| |
|
| PSU Grant | |
3/3/23 | |
| |
| |
| |
183,486 | |
366,972 | |
733,944 | |
| |
664,219 |
| Chintu Patel | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| 2023 Annual Cash Incentive | |
| |
187,500 | |
750,000 | |
1,125,000 | |
| |
| |
| |
| |
|
| PSU Grant | |
3/3/23 | |
| |
| |
| |
183,486 | |
366,972 | |
733,944 | |
| |
664,219 |
| Anastasios Konidaris | |
| |
| |
| |
| |
| |
| |
| |
|
| 2022 Annual Cash Incentive | |
| |
77,894 | |
311,575 | |
467,363 | |
| |
| |
| |
| |
|
| PSU Grant | |
3/3/23 | |
| |
| |
| |
114,679 | |
229,358 | |
458,716 | |
| |
415,138 |
| RSU Grant | |
3/3/23 | |
| |
| |
| |
| |
| |
| |
229,358 | |
440,367 |
| Andrew Boyer | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| 2023 Annual Cash Incentive | |
| |
120,000 | |
480,000 | |
720,000 | |
| |
| |
| |
| |
|
| PSU Grant | |
3/3/23 | |
| |
| |
| |
99,389 | |
198,777 | |
397,554 | |
| |
359,786 |
| RSU Grant | |
3/3/23 | |
| |
| |
| |
| |
| |
| |
198,777 | |
381,652 |
| Nikita Shah | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| 2023 Annual Cash Incentive | |
| |
68,688 | |
274,753 | |
412,129 | |
| |
| |
| |
| |
|
| PSU Grant | |
3/3/23 | |
| |
| |
| |
91,743 | |
183,486 | |
366,972 | |
| |
332,110 |
| RSU Grant | |
3/3/23 | |
| |
| |
| |
| |
| |
| |
183,486 | |
352,293 |
| Jason Daly | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| 2023 Annual Cash Incentive | |
| |
59,813 | |
239,250 | |
358,875 | |
| |
| |
| |
| |
|
| PSU Grant | |
3/3/23 | |
| |
| |
| |
76,453 | |
152,905 | |
305,810 | |
| |
276,758 |
| RSU Grant | |
3/3/23 | |
| |
| |
| |
| |
| |
| |
152,905 | |
293,578 |
| (1) |
The amounts shown in these columns reflect
the corporate performance targets under our annual performance-based cash incentive plan. “Threshold” equals 25% of Target,
“Target” equals 100% and “Maximum” equals 150% of Target. |
| (2) |
The number of shares shown reflects the “Threshold,” “Target”
and “Maximum” payout levels under the 2023 performance-based restricted stock unit awards granted under the 2018 Incentive
Award Plan. “Threshold” equals 50% of Target, “Target” equals 100% of Target and “Maximum” equals
200% of Target. |
| (3) |
The number of shares shown reflects the 2023 restricted stock unit awards under
the 2018 Incentive Award Plan. The restricted stock unit awards made in 2023 vest in four equal installments on the first, second,
third and fourth anniversary of the date of grant, assuming continued employment. |
| (4) |
These amounts reflect the aggregate grant date fair value of each stock award
granted during the fiscal year, computed in accordance with FASB ASC Topic 718. The valuation assumptions used in determining such
amounts are described in Note 23 to the financial statements included in our Annual Report on Form 10-K for the year ended December
31, 2023. As the PSUs were subject to market-based vesting conditions, the grant date fair value was determined using a Monte-Carlo
simulation model, which is a probabilistic approach for estimating the grant date fair value of the PSUs for purposes of accounting
under FASB ASC Topic 718. |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
42 |
| |
|
Outstanding Equity Awards at December 31, 2023
| | |
Option Awards | |
Stock Awards |
| Name | |
Number of
Securities
Underlying
Options
that are
Exercisable
(#) | |
Number of
Securities
Underlying
Options
that are
Unexercisable
(#) | |
Option
Exercise
Price
($) | |
Option
Expiration
Date | |
Number
of Shares
or Units of
Stock that
Have Not
Vested
(#) | |
Market Value
of
Shares or
Units of Stock
that Have Not
Vested(1)
($) | |
Equity
Incentive
Plan Awards:
Number of
Unearned
Shares, Units,
or Other Rights
that Have Not
Vested
(#) | |
Equity Incentive
Plan Awards:
Market or
Payout Value
of Unearned
Shares, Units,
or Other Rights
that Have Not
Vested
($) |
| Chirag Patel | |
24,977 | |
— | |
15.01 | |
5/7/28 | |
| |
| |
| |
|
| | |
28,044 | |
— | |
14.05 | |
5/6/29 | |
| |
| |
| |
|
| | |
| |
| |
| |
| |
| |
| |
0(7) | |
— |
| | |
| |
| |
| |
| |
| |
| |
289,855(8) | |
1,759,420 |
| | |
| |
| |
| |
| |
| |
| |
733,944(9) | |
4,455,040 |
| Chintu Patel | |
24,977 | |
— | |
15.01 | |
5/7/28 | |
| |
| |
| |
|
| | |
28,044 | |
— | |
14.05 | |
5/6/29 | |
| |
| |
| |
|
| | |
| |
| |
| |
| |
| |
| |
0(7) | |
— |
| | |
| |
| |
| |
| |
| |
| |
289,855(8) | |
1,759,420 |
| | |
| |
| |
| |
| |
| |
| |
733,944(9) | |
4,455,040 |
| Anastasios | |
| |
| |
| |
| |
87,720(2) | |
532,460 | |
| |
|
| Konidaris | |
| |
| |
| |
| |
72,394(3) | |
439,432 | |
0(7) | |
— |
| | |
| |
| |
| |
| |
135,870(4) | |
824,731 | |
90,580(8) | |
549,821 |
| | |
| |
| |
| |
| |
229,358(5) | |
1,392,203 | |
458,716(9) | |
2,784,406 |
| Andrew Boyer | |
272,480 | |
— | |
2.75 | |
5/7/28 | |
| |
| |
| |
|
| | |
100,553 | |
— | |
2.75 | |
3/1/29 | |
| |
| |
| |
|
| | |
| |
| |
| |
| |
31,663(6) | |
192,194 | |
| |
|
| | |
| |
| |
| |
| |
62,742(3) | |
380,844 | |
0(7) | |
— |
| | |
| |
| |
| |
| |
117,754(4) | |
714,767 | |
78,503(8) | |
476,513 |
| | |
| |
| |
| |
| |
198,777(5) | |
1,206,576 | |
397,554(9) | |
2,413,153 |
| Nikita Shah | |
36,331 | |
— | |
2.75 | |
5/7/28 | |
| |
| |
| |
|
| | |
95,525 | |
23,882 | (2) |
2.75 | |
3/1/29 | |
| |
| |
| |
|
| | |
| |
| |
| |
| |
35,620(6) | |
216,213 | |
| |
|
| | |
| |
| |
| |
| |
57,915(3) | |
351,544 | |
0(7) | |
— |
| | |
| |
| |
| |
| |
108,696(4) | |
659,785 | |
72,464(8) | |
439,856 |
| | |
| |
| |
| |
| |
183,486(5) | |
1,113,760 | |
366,972(9) | |
2,227,520 |
| Jason Daly | |
| |
| |
| |
| |
67,935(4) | |
412,365 | |
45,290(8) | |
274,910 |
| | |
| |
| |
| |
| |
152,905(5) | |
928,133 | |
305,810(9) | |
1,856,267 |
| (1) |
Based on the closing price of our Class A common stock of $6.07 on December 29, 2023, the last trading
day of the year. |
| (2) |
Restricted stock units vest in one installment on March 12, 2024. |
| (3) |
Restricted stock units vest in two equal installments on March 1, 2024 and March 1, 2025. |
| (4) |
Restricted stock units vest in three equal installments on March 3, 2024, March, 3, 2025, and March 3, 2026. |
| (5) |
Restricted stock units vest in four equal installments on March 3, 2024, March 3, 2025, March, 3, 2026, and March 3, 2027. |
| (6) |
Restricted stock units vest in one installment on February 27, 2024. |
| (7) |
The performance-based restricted stock units vest following the conclusion of the March 1, 2021 through February 29, 2024
performance period based upon the level of attainment of absolute stock price (based on the closing stock price of the Company’s
stock on NASDAQ). Payouts for this performance period are estimated at 0% of the target level. |
| (8) |
The performance-based restricted stock units vest following the conclusion of the March 1, 2022 through February 28, 2025
performance period based upon the level of attainment of stock price growth (based on the closing stock price of the Company’s
stock on NASDAQ). The amounts listed represent the threshold number of units under each award. Total shares earned under the performance-based
restricted stock units will range from 0% to 200% of the target number of units based on actual performance. |
| (9) |
The performance-based restricted stock units vest following the conclusion of the March 1, 2023 through February 28, 2026
performance period based upon the level of attainment of stock price growth (based on the closing stock price of the Company’s
stock on NASDAQ). The amounts listed represent the maximum number of units under each award as the estimated payout as of the end
of 2023 is above target level performance. Total shares earned under the performance-based restricted stock units will range from
0% to 200% of the target number of units based on actual performance. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
43 |
| |
|
2023 Option Exercises and Stock Vested
| | |
Option Awards | |
Stock Awards |
| Name | |
Number of
Shares Acquired
on Exercise
(#) | |
Value Realized
on Exercise
($) | |
Number of
Shares Acquired
on Vesting
(#) | |
Value Realized
on Vesting(1)
($) |
| Chirag Patel | |
— | |
— | |
— | |
— |
| Chintu Patel | |
— | |
— | |
— | |
— |
| Anastasios Konidaris | |
— | |
— | |
169,205 | |
296,817 |
| Andrew Boyer | |
— | |
— | |
137,725 | |
306,715 |
| Nikita Shah | |
— | |
— | |
112,539 | |
231,540 |
| Jason Daly | |
— | |
— | |
22,645 | |
43,478 |
| (1) |
Amounts reported are based on the closing price of our Class A common stock on the New York Stock Exchange
on the vesting date. |
Management Employment & Separation Agreements
Among our named executive officers, we have or had employment agreements
with Andrew Boyer, Anastasios Konidaris and Nikita Shah.
Andrew Boyer
Andrew Boyer is party to an Employment Agreement,
effective as of February 5, 2018, with Amneal (the “Boyer Employment Agreement”).
The initial term of the Boyer Employment Agreement
began on February 5, 2018 and was scheduled to expire on June 30, 2022, unless further extended or earlier terminated as provided in the
Boyer Employment Agreement.
On July 31, 2020, the Company entered into Modification
No. 1 to the Boyer Employment Agreement, effective as of August 1, 2020 (the “Effective Date”). Pursuant to the Modification,
the term of Mr. Boyer’s Employment Agreement was extended until June 30, 2023 (the “Term”) and will automatically be
renewed thereafter for single one-year periods unless written notice of non-renewal is provided by any party at least 90 days prior to
the end of the Term or the agreement is earlier terminated in accordance with its terms. Under the Modification, as of the Effective Date,
Mr. Boyer began serving as the Executive Vice President, Chief Commercial Officer – Generics and receiving an annual base salary
of $600,000 (reduced from $661,917), which amount is subject to increase by the Company’s Board of Directors. Further, in connection
with the Modification, Mr. Boyer received an award of restricted stock units having a grant date fair value equal to $300,000 with a third
of such units vesting on each of June 30, 2021, June 30, 2022 and June 30, 2023, subject to Mr. Boyer’s continued employment through
the applicable vesting date.
On February 21, 2023, the Company entered into
Modification No. 2 to the Boyer Employment Agreement, effective as of March 1, 2023 (the “Effective Date”). Pursuant to the
Modification, the term of Mr. Boyer’s Employment Agreement was extended until March 31, 2025 (the “Term”) and will automatically
be renewed thereafter for single one-year periods unless written notice of non-renewal is provided by any party at least 90 days prior
to the end of the Term or the agreement is earlier terminated in accordance with its terms. As described in further detail under “Certain
Related Parties and Related Party Transactions – The Reorganization,” we entered into an administrative amendment to the Boyer
Employment Agreement in light of the Reorganization, namely to clarify that (i) Mr. Boyer will remain directly employed by Amneal LLC,
and (ii) all references in such employment agreement to Old Amneal (as defined herein) shall be deemed to instead refer to New Amneal.
Mr. Boyer is also eligible to receive an annual
bonus targeted at 80% of his base salary under the annual bonus program, and a personal performance multiplier based on his performance
and as determined by the Board in its discretion of between zero and 150% of this amount.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
44 |
| |
|
Severance
The Boyer Employment Agreement provides for severance
payments and benefits if (i) Mr. Boyer resigns for “good reason” (as defined in the Boyer Employment Agreement) or (ii) the
Board of Directors terminates Mr. Boyer’s employment without “cause” (as defined in the Boyer Employment Agreement),
in each case other than during the period that is within three months preceding or 24 months following a “change in control”
(as defined in the Boyer Employment Agreement). In addition to payment of earned and vested payments and benefits, these severance payments
and benefits include: (A) two times his base salary as then in effect; (B) a pro rata portion of his annual bonus for the fiscal year
in which the termination occurs, based on actual performance for such fiscal year, and the prior year’s bonus to the extent not
then already paid (based on the higher of target or actual performance of the relevant goals); (C) continuation of healthcare benefits
until the second anniversary of his termination date; (D) the vesting and, if applicable, exercisability of each outstanding equity award
granted to Mr. Boyer will be accelerated to the extent such equity award would have vested had Mr. Boyer’s employment continued
until the first anniversary of his termination date (and, to the extent applicable, each outstanding equity award granted to Mr. Boyer
will remain exercisable until the first anniversary of his termination date); and (E) outplacement services by a reputable national outplacement
service for up to two years following his termination date.
Severance Upon a Change in Control
The Boyer Employment Agreement also provides for
severance payments and benefits if (i) Mr. Boyer resigns for good reason, (ii) the Board of Directors terminates Mr. Boyer’s employment
without cause or (iii) Mr. Boyer’s employment terminates by reason of death or disability (as defined in the Boyer Employment Agreement),
in each case within three months preceding or 24 months following a change in control. In addition to payment of earned and vested payments
and benefits, these severance payments and benefits include: (A) the sum of (x) two times his base salary as then in effect plus (y) two
times his target annual bonus as then in effect; (B) a pro rata portion of his annual bonus for the fiscal year in which the termination
occurs, based on actual performance for such fiscal year, and the prior year’s bonus to the extent not then already paid (based
on the higher of target or actual performance of the relevant goals); (C) continuation of healthcare benefits until the second anniversary
of his termination date; (D) the vesting and, if applicable, exercisability of each equity award granted to Mr. Boyer will be fully accelerated
(and, to the extent applicable, each outstanding equity award granted to Mr. Boyer will remain exercisable until the first anniversary
of his termination date); and (E) outplacement services by a reputable national outplacement service for up to two years following his
termination date.
The Boyer Employment Agreement requires Mr. Boyer
to maintain the confidentiality of information relating to the Company during and after the term of such agreement and also contains non-competition,
non-solicitation and non-disparagement covenants as well as other provisions customary for this type of employment agreement.
Anastasios Konidaris
Anastasios Konidaris is party to an Employment
Agreement, dated March 11, 2020, by and among Amneal, the Company and Mr. Konidaris (the “Konidaris Employment Agreement”).
The Konidaris Employment Agreement provides that
Mr. Konidaris will be employed as the Company’s Executive Vice President and Chief Financial Officer at an annual base salary of
$550,000. Further, Mr. Konidaris is eligible to earn annual incentive compensation under the Company’s annual bonus plan, with the
target amount of his annual bonus being equal to 55% of his base salary and a personal performance multiplier based on his performance
and as determined by the Board in its discretion of between zero and 150% of this amount. Pursuant to the Konidaris Employment Agreement,
not later than 30 days following the effective date of the agreement, the Company granted to Mr. Konidaris an award of restricted stock
units having a value equal to $1,000,000 and an award of PSUs having a value equal to $1,000,000. Subject to Mr. Konidaris’s continuous
services to the Company through each vesting date, the restricted stock units will vest in four equal installments beginning on the first
anniversary of the effective date of the Konidaris Employment Agreement, and the PSUs will be earned and will vest based on the same vesting
and performance conditions as the performance-based restricted stock units awarded to the Company’s other named executive officers
in 2020.
On February 21, 2023, the Company entered into
Modification No. 2 to the Konidaris Employment Agreement, effective as of March 1, 2023 (the “Effective Date”). Pursuant to
the Modification, the term of Mr. Konidaris’ Employment Agreement was extended until March 31, 2025 (the “Term”) and
will automatically be renewed thereafter for single one-year periods unless written notice of non-renewal is provided by any party at
least 90 days prior to the end of the Term or the agreement is earlier terminated in accordance with its terms. As described in further
detail under “Certain Related Parties and Related Party Transactions—The Reorganization,” we entered into an administrative
amendment to the Konidaris Employment Agreement in light of the Reorganization, namely to clarify that (i) Mr. Konidaris will be directly
employed by Amneal LLC rather than Old Amneal, and (ii) all references in such employment agreement to Old Amneal shall be deemed to instead
refer to New Amneal (other than references relating to Old Amneal as employer which shall be deemed to instead refer to Amneal LLC as
employer as of the effective time of the Reorganization).
Severance
In the case of termination by the Company without
cause or a termination by Mr. Konidaris for good reason (each as defined in the Konidaris Employment Agreement), Mr. Konidaris will be
entitled to receive the following severance benefits: (1) an amount equal to 150% of his then-current annual base salary; (2) a prorated
portion of the annual bonus award for the year during which the termination occurs, based on actual performance for such fiscal year;
(3) benefits continuation for a period of 18 months following the date of termination; and (4) outplacement assistance for a period of
12 months following the date of termination.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
45 |
| |
|
Severance Upon a Change in Control
In the case of a termination by the Company without
cause or a termination by Mr. Konidaris for good reason within three months prior to or 12 months following a change in control (as defined
in the Konidaris Employment Agreement), Mr. Konidaris will be entitled to receive the severance benefits described above. In addition,
the vesting and exercisability of each equity award granted to Mr. Konidaris will accelerate effective as of the date of termination,
with any performance conditions determined based on actual achievement as of the date of termination, and, if applicable, will remain
exercisable for a period of not less than 12 months following the termination.
Nikita Shah
Nikita Shah is party to an Employment Agreement,
dated July 29, 2020, by and among Amneal, the Company and Ms. Shah (the “Shah Employment Agreement”).
The Shah Employment Agreement provides that Ms.
Shah will be employed as the Company’s Executive Vice President, Chief Human Resources Officer at an annual base salary of (i) $450,000
beginning on August 1, 2020; and (ii) $485,000 beginning on January 1, 2021. Further, Ms. Shah is eligible to earn annual incentive compensation
under the Company’s annual bonus plan with the target amount of her annual bonus being equal to 55% of her base salary and a personal
performance multiplier based on her performance and as determined by the Board in its discretion of between zero and 150% of this amount.
Ms. Shah will be eligible to participate in the Company’s long term incentive plan.
On February 21, 2023, the Company entered into
Modification No. 1 to the Shah Employment Agreement, effective as of March 1, 2023 (the “Effective Date”). Pursuant to the
Modification, the term of Ms. Shah’s Employment Agreement was extended until March 31, 2025 (the “Term”) and will automatically
be renewed thereafter for single one-year periods unless written notice of non-renewal is provided by any party at least 90 days prior
to the end of the Term or the agreement is earlier terminated in accordance with its terms. As described in further detail under “Certain
Related Parties and Related Party Transactions—The Reorganization,” we entered into an administrative amendment to the Shah
Employment Agreement in light of the Reorganization, namely to clarify that (i) Ms. Shah will be directly employed by Amneal LLC rather
than Old Amneal, and (ii) all references in such employment agreement to Old Amneal shall be deemed to instead refer to New Amneal (other
than references relating to Old Amneal as employer which shall be deemed to instead refer to Amneal LLC as employer as of the effective
time of the Reorganization.
Severance
In the case of termination by the Company without
cause or a termination by Ms. Shah for good reason (each as defined in the Shah Employment Agreement), Ms. Shah will be entitled to receive
the following severance benefits: (1) an amount equal to 150% of her then-current annual base salary; (2) a pro-rated portion of the annual
bonus award for the year during which the termination occurs, based on actual performance for such fiscal year; (3) benefits continuation
for a period of 18 months following the date of termination; (4) the vesting and, if applicable, exercisability of each outstanding equity
award granted to Ms. Shah will be accelerated to the extent such equity award would have vested had Ms. Shah’s employment continued
until the first anniversary of her termination date (and, to the extent applicable, each outstanding equity award granted to Ms. Shah
will remain exercisable until the first anniversary of her termination date); and (5) outplacement assistance for a period of 12 months
following the date of termination.
Severance Upon a Change in Control
In June 2020, the Compensation Committee approved
severance benefits for Ms. Shah in the case of a termination by the Company without cause or a termination by Ms. Shah for good reason
within three months prior to or 12 months following a change in control (as defined in the Shah Employment Agreement); in such case, Ms.
Shah will be entitled to receive the severance benefits described above. In addition, the vesting and exercisability of each outstanding
equity award granted to Ms. Shah will accelerate effective as of the date of termination, with any performance conditions determined based
on actual achievement as of the date of termination, and, if applicable, will remain exercisable for a period of not less than 12 months
following the termination.
Severance Plan
On June 17, 2020, the Compensation Committee adopted
the Amneal Pharmaceuticals LLC Severance Plan (the “Severance Plan”). The Severance Plan is intended to constitute an “employee
welfare benefit plan” under Section 3(1) of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”).
Under the Severance Plan, in the event of (a) a participant’s involuntary termination of employment without Cause (as defined below)
due to (i) a reduction-in-force; (ii) a layoff; (iii) the elimination of a participant’s role; (iv) the reorganization of the Company,
or a business unit, division, or department of the Company; (v) a change in business plan or structure that results in the participant’s
separation from employment; or (vi) any other reason as determined by the Company in its sole discretion or (b) a mandatory relocation
of a participant’s primary workplace to a location that is more than fifty (50) miles from a participant’s prior primary workplace,
on an individualized basis the participant will be eligible to receive up to a maximum of (depending on his or her position) (A) a lump
sum payment of 52 weeks of his or her base
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
46 |
| |
|
pay, (B) a prorated portion of his or her incentive
award, (C) fully subsidized COBRA premiums for 52 weeks, and (D) outplacement services for 52 weeks. Under the Severance Plan, in the
event of a participant’s termination in connection with a change of control, the participant will be eligible to receive up to a
maximum of (depending on his or her position) (A) a lump sum payment of 64 weeks of his or her base pay, (B) prorated target bonus, (C)
fully subsidized COBRA premiums up to a maximum of 64 weeks, and (D) outplacement services up to a maximum for 64 weeks.
Executive officers who are subject to an individual
employment agreement or contract with the Company or any member of the Company Group are excluded from participation in Severance Plan.
Mr. Daly is not subject to an individual employment agreement or contract with the Company, and as such, Mr. Daly participates in the
Severance Plan.
As used in the Severance Plan, “Cause”
means (i) any failure or neglect by the participant to perform his or her duties or responsibilities to the Company or any of its subsidiaries
(the “Company Group”), (ii) any act of fraud, embezzlement, theft, misappropriation, or material dishonesty by the participant
relating to the Company Group or its business or assets, (iii) the participant’s commission of a felony or other crime involving
moral turpitude, (iv) any gross negligence or intentional misconduct on the part of the participant in the conduct of his or her duties
and responsibilities or services, as applicable, with the Company Group or its affiliates or that adversely affects the image, reputation
or business of the Company Group or its affiliates, or (v) any material breach by the participant of any written agreement between the
Company Group and the participant or any written policy applicable generally to employees of the Company Group.
Potential Payments Upon Termination or Change in Control
To enable us to offer a competitive executive compensation
program, we believe it is important to provide reasonable severance benefits to our executive officers.
In addition to the employment agreements between
the Company and certain of our named executive officers and the Severance Plan, discussed above, from time to time, we may explore potential
transactions that could result in a change in control of our Company. We believe that when a transaction is perceived as imminent, or
is taking place, we should be able to receive and rely on the disinterested service of our executive officers, without them being distracted
or concerned by the personal uncertainties and risks associated with such a situation. We further believe that our stockholders are best
served if their interests are aligned with the interests of our executives, and providing change in control benefits should eliminate,
or at least reduce, the reluctance of senior management to pursue potential transactions that may enhance the value of our stockholders’
investments. Consistent with this, we provide change-in-control benefits to our named executive officers only if the officer’s employment
terminates in connection with the change in control (often referred to as “double-trigger” change-in-control benefits).
The estimated severance and other benefits for
each named executive officer, either pursuant to the Severance Plan or an applicable employment agreement, in the event of a termination
of employment are set forth below. The amounts assume that the termination was effective as of December 29, 2023 (the last business day
of fiscal 2023) and thus are based upon amounts earned through such date and are only estimates of the amounts that would actually be
paid to such named executive officers upon their termination. Since many factors (e.g., the time of year when the event occurs, the Company’s
stock price) could affect the nature and amount of benefits a named executive officer could potentially receive, any amounts paid or distributed
upon a future termination may be different from those shown in the table below. The amounts shown are in addition to benefits generally
available to salaried employees. Mr. Chirag Patel and Mr. Chintu Patel do not participate in the Severance Plan nor do they have an applicable
employment agreement with severance terms in the event of a termination of employment.
Under our performance-based restricted stock unit
agreements, upon an NEO’s “Qualifying Termination” prior to the end of the applicable performance period, the number
of performance-based restricted stock units earned by the employee would be determined as of the employee’s termination date based
on actual performance through such date. For purposes of this paragraph, a “Qualifying Termination” shall mean such employee’s
termination of service prior to the end of the applicable performance period due to death, permanent disability or involuntary termination
by the Company without “cause.” In the event of a Change in Control prior to the end of the three-year performance period,
the number of performance-based restricted stock units earned will be determined as of the date of the Change in Control based on actual
performance through such date. Such performance-based restricted stock units that are deemed earned pursuant to the preceding sentence
will vest on the date of the Change in Control, subject to the employee’s continued employment or service to the Company or its
subsidiaries through the date of the Change in Control.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
47 |
| |
|
| | |
| Without Cause or for Good
Reason Termination | |
| | |
| |
| Name | |
Benefit | |
Without Change
in Control | | |
With Change in
Control | | |
Termination for
Death | | |
Termination for
Disability | |
| Chirag Patel | |
Cash | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated options | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated RSUs(1) | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
PSUs(2) | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | |
| | |
Health Care & Outplacement | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
TOTAL | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | |
| Chintu Patel | |
Cash | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated options | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated RSUs(1) | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
PSUs(2) | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | |
| | |
Health Care & Outplacement | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
TOTAL | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | | |
$ | 2,784,400 | |
| Anastasios Konidaris | |
Cash | |
$ | 1,303,652 | | |
$ | 1,303,652 | | |
$ | — | | |
$ | — | |
| | |
Accelerated options | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated RSUs(1) | |
$ | — | | |
$ | 3,188,826 | | |
$ | — | | |
$ | — | |
| | |
PSUs(2) | |
$ | 1,740,254 | | |
$ | 1,740,254 | | |
$ | 1,740,254 | | |
$ | 1,740,254 | |
| | |
Health Care & Outplacement | |
$ | 58,413 | | |
$ | 58,413 | | |
$ | — | | |
$ | — | |
| | |
TOTAL | |
$ | 3,102,319 | | |
$ | 6,291,145 | | |
$ | 1,740,254 | | |
$ | 1,740,254 | |
| Andrew Boyer | |
Cash | |
$ | 1,782,720 | | |
$ | 2,742,720 | | |
$ | — | | |
$ | — | |
| | |
Accelerated options | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated RSUs(1) | |
$ | 922,516 | | |
$ | 2,494,382 | | |
$ | — | | |
$ | — | |
| | |
PSUs(2) | |
$ | 1,508,220 | | |
$ | 1,508,220 | | |
$ | 1,508,220 | | |
$ | 1,508,220 | |
| | |
Health Care & Outplacement | |
$ | 122,203 | | |
$ | 122,203 | | |
$ | — | | |
$ | — | |
| | |
TOTAL | |
$ | 4,335,660 | | |
$ | 6,857,525 | | |
$ | 1,508,220 | | |
$ | 1,508,220 | |
| Nikita Shah | |
Cash | |
$ | 1,082,875 | | |
$ | 1,082,875 | | |
$ | — | | |
$ | — | |
| | |
Accelerated options | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated RSUs(1) | |
$ | 657,785 | | |
$ | 2,341,302 | | |
$ | — | | |
$ | — | |
| | |
PSUs(2) | |
$ | 1,392,200 | | |
$ | 1,392,200 | | |
$ | 1,392,200 | | |
$ | 1,392,200 | |
| | |
Health Care & Outplacement | |
$ | 58,413 | | |
$ | 58,413 | | |
$ | — | | |
$ | — | |
| | |
TOTAL | |
$ | 3,191,273 | | |
$ | 4,874,790 | | |
$ | 1,392,200 | | |
$ | 1,392,200 | |
| Jason Daly | |
Cash | |
$ | 768,950 | | |
$ | 879,373 | | |
$ | — | | |
$ | — | |
| | |
Accelerated options | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Accelerated RSUs(1) | |
$ | — | | |
$ | 1,340,499 | | |
$ | — | | |
$ | — | |
| | |
PSUs(2) | |
$ | 1,160,167 | | |
$ | 1,160,167 | | |
$ | 1,160,167 | | |
$ | 1,160,167 | |
| | |
Health Care & Outplacement | |
$ | 61,105 | | |
$ | 72,206 | | |
$ | — | | |
$ | — | |
| | |
TOTAL | |
$ | 1,990,221 | | |
$ | 3,452,244 | | |
$ | 1,160,167 | | |
$ | 1,160,167 | |
| (1) |
Represents unvested restricted stock units that would accelerate
in connection with the applicable termination event valued based on the closing price of our Class A common stock on December
29, 2023, which was $6.07. |
| (2) |
Represents unvested performance-based restricted stock units that would
be earned in connection with the applicable termination event valued based on the closing price of our Class A common stock
on December 29, 2023, which was $6.07. These amounts reflect probable achievement as of December 29, 2023. |
Release
The obligation of the Company to provide the salary continuation and
other severance benefits described above is contingent upon and subject to the execution and delivery by the executive officer
of a general release of claims against the Company.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
48 |
| |
|
Pay Ratio Disclosure
The 2023 annual total compensation of the median
compensated of all our employees who were employed as of December 1, 2023, other than our Co-CEOs, Mr. Chintu Patel and Mr.
Chirag Patel, was $11,024. The median employee is located in India. Since the Company operated with Co-CEOs for 2023, the annual
total compensation of $2,370,386, for Mr. Chirag Patel, which was moderately higher than Mr. Chintu Patel, was used. Had we used
Mr. Chintu Patel, our pay ratio would not have changed significantly. The ratio of the compensation of the median employee to Mr.
Chirag Patel’s compensation was 1 to 215.
The SEC’s rules for identifying the median
compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to
adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their
employee populations and compensation practices. As a result, the pay ratio reported by other companies may not be comparable to
the pay ratio reported above, as other companies have different employee populations and compensation practices and may utilize
different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
The pay ratio reported above is a reasonable
estimate calculated in a manner consistent with SEC rules based on our payroll and employment records and the methodology described
below.
As of December 1, 2023, we employed approximately
2,420 U.S.-based employees and 5,282 non-U.S. employees. For purposes of calculating our median employee compensation, in addition
to excluding our Co-Chief Executive Officers, we excluded all 78 employees from Ireland, which accounted for 1% of our total employee
population, using the de minimis exception. For these purposes, we identified the median compensated employee among an employee
population of approximately 2,418 U.S. employees (which excludes our Co-CEOs) and 5,204 Indian employees. We identified the median
compensated employee using target total cash compensation (comprised of base salary equivalent and target bonus), which we annualized
for any employee, other than temporary or seasonal employees, who did not work for the entire year. The average annual exchange
rate as of December 1, 2023 was used to convert amounts paid in foreign currencies to U.S. Dollars in order to identify the median
employee, while the average annual exchange rate as of December 31, 2023 was used to calculate the 2023 annual total compensation
of the median employee.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
49 |
| |
|
Pay Versus Performance Disclosure
In accordance with rules adopted by the
Securities and Exchange Commission pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, we are
providing the following disclosure regarding executive compensation for our principal executive officer (“PEO”)
and Non-PEO NEOs and Company performance for the fiscal years listed below. The Compensation Committee did not consider the
pay versus performance disclosure below in making its pay decisions for any of the years shown. The sum and/or computation of
individual numerical amounts disclosed in the following tables and related footnotes may not equal the total due to
rounding.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Value of Initial
Fixed $100
Investment
based on(4) |
|
|
|
| Year |
|
Summary
Compensation
Table Total for
Chirag Patel(1)
($) |
|
Summary
Compensation
Table Total for
Chintu Patel(1)
($) |
|
Compensation
Actually Paid
to Chirag
Patel(1)(2)(3)
($) |
|
Compensation
Actually Paid
to Chintu
Patel(1)(2)(3)
($) |
|
Average
Summary
Compensation
Table Total
for Non-PEO
NEOs(1)
($) |
|
Average
Compensation
Actually Paid
to Non-PEO
NEOs(1)(2)(3)
($) |
|
TSR
($) |
|
Peer
Group
TSR
($) |
|
Net
Income
(Loss)
($ Millions) |
|
Adjusted
EBITDA
($ Millions)(5) |
| (a) |
|
(b) |
|
(b) |
|
(c) |
|
(c) |
|
(d) |
|
(e) |
|
(f) |
|
(g) |
|
(h) |
|
(i) |
| 2023 |
|
2,370,386 |
|
2,367,553 |
|
9,458,710 |
|
9,455,877 |
|
1,679,146 |
|
5,801,457 |
|
125.93 |
|
124.97 |
|
(89) |
|
558 |
| 2022 |
|
5,048,110 |
|
5,062,732 |
|
(1,835,706) |
|
(1,821,083) |
|
2,348,670 |
|
(121,995) |
|
41.29 |
|
123.43 |
|
(255) |
|
514 |
| 2021 |
|
4,785,116 |
|
4,798,825 |
|
1,383,918 |
|
1,397,627 |
|
2,420,041 |
|
1,421,310 |
|
99.38 |
|
129.31 |
|
20 |
|
538 |
| 2020 |
|
2,320,716 |
|
2,328,528 |
|
3,422,575 |
|
3,430,387 |
|
1,922,858 |
|
1,985,608 |
|
94.81 |
|
114.32 |
|
69 |
|
433 |
| 2020 |
2021 |
2022 |
2023 |
| Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
| Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
| Nikita Shah |
Nikita Shah |
Nikita Shah |
Nikita Shah |
| Joseph Todisco |
Joseph Todisco |
Jason Daly |
Jason Daly |
| Todd Branning |
|
|
|
| (1) |
Chirag Patel and Chintu Patel were our Co-PEOs for each year presented.
The individuals comprising the Non-PEO NEOs for each year presented are listed below. |
| 2020 |
2021 |
2022 |
2023 |
| Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
| Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
| Nikita Shah |
Nikita Shah |
Nikita Shah |
Nikita Shah |
| Joseph Todisco |
Joseph Todisco |
Jason Daly |
Jason Daly |
| Todd Branning |
|
|
|
| (2) |
The amounts shown for Compensation Actually Paid have been
calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually earned, realized, or
received by the Company’s NEOs. These amounts reflect the Summary Compensation Table Total with certain adjustments
as described in footnote 3 below. |
| (3) |
Compensation Actually Paid reflects the exclusions and inclusions of certain
amounts for the PEOs and the Non-PEO NEOs as set forth below. Equity values are calculated in accordance with FASB ASC Topic
718. Amounts in the Exclusion of Stock Awards column are the amounts from the Stock Awards column set forth in the Summary
Compensation Table. |
| Year |
|
Summary Compensation
Table Total for Chirag
Patel
($) |
|
Exclusion of Stock
Awards for Chirag Patel
($) |
|
Inclusion of Equity
Values for Chirag Patel
($) |
|
Compensation Actually
Paid to Chirag Patel
($) |
| 2023 |
|
2,370,386 |
|
(664,219) |
|
7,752,544 |
|
9,458,710 |
| |
|
|
|
|
|
|
|
|
| Year |
|
Summary Compensation
Table Total for Chintu
Patel
($) |
|
Exclusion of Stock
Awards for Chintu Patel
($) |
|
Inclusion of Equity
Values for Chintu Patel
($) |
|
Compensation Actually
Paid to Chintu Patel
($) |
| 2023 |
|
2,367,553 |
|
(664,219) |
|
7,752,544 |
|
9,455,877 |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
50 |
| |
|
| Year |
|
Average Summary
Compensation Table
Total for Non-PEO NEOs
($) |
|
Average Exclusion
of Stock Awards and
Option Awards for Non-
PEO NEOs
($) |
|
Average Inclusion of
Equity Values for Non-
PEO NEOs
($) |
|
Average Compensation
Actually Paid to Non-
PEO NEOs
($) |
| 2023 |
|
1,679,146 |
|
(712,920) |
|
4,835,231 |
|
5,801,457 |
The amounts in the Inclusion of Equity Values in the tables above
are derived from the amounts set forth in the following tables:
| Year |
|
Year-End
Fair Value
of Equity Awards
Granted During
Year That Remained
Unvested as of
Last Day of Year for
Chirag Patel
($) |
|
Change in
Fair
Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Chirag
Patel
($) |
|
Change in
Fair Value
from Last Day of
Prior Year to Vesting
Date of Unvested
Equity Awards that
Vested During Year
for Chirag Patel
($) |
|
Fair Value
at Last
Day of Prior Year
of Equity Awards
Forfeited During
Year for Chirag Patel
($) |
|
Total -
Inclusion of
Equity Values for
Chirag Patel
($) |
| 2023 |
|
3,526,601 |
|
4,225,943 |
|
— |
|
— |
|
7,752,544 |
| |
|
|
|
|
|
|
|
|
|
|
| Year |
|
Year-End Fair Value
of Equity Awards
Granted During
Year That Remained
Outstanding and
Unvested as of
Last Day of Year for
Chintu Patel
($) |
|
Change in Fair
Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Chintu
Patel
($) |
|
Change in Fair Value
from Last Day of
Prior Year to Vesting
Date of Unvested
Equity Awards that
Vested During Year
for Chintu Patel
($) |
|
Fair Value at Last
Day of Prior Year
of Equity Awards
Forfeited During
Year for Chintu Patel
($) |
|
Total - Inclusion of
Equity Values for
Chintu Patel
($) |
| 2023 |
|
3,526,601 |
|
4,225,943 |
|
— |
|
— |
|
7,752,544 |
| |
|
|
|
|
|
|
|
|
|
|
| Year |
|
Average Year-
End Fair Value of
Equity Awards
Granted During
Year That Remained
Outstanding and
Unvested as of Last
Day of Year for Non-
PEO NEOs
($) |
|
Average Change in
Fair Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Non-PEO
NEOs
($) |
|
Average Change in
Fair Value from Last
Day of Prior Year
to Vesting Date of
Unvested Equity
Awards that Vested
During Year for Non-
PEO NEOs
($) |
|
Average Fair Value
at Last Day of
Prior Year of Equity
Awards Forfeited
During Year for Non-
PEO NEOs
($) |
|
Total - Average
Inclusion of
Equity Values for
Non-PEO NEOs
($) |
| 2023 |
|
2,996,942 |
|
1,838,427 |
|
(137) |
|
— |
|
4,835,231 |
| (4) |
The Peer Group TSR set forth in this table utilizes the Dow
Jones U.S. Select Pharmaceuticals Index, which we also utilize in the stock performance graph required by Item 201(e) of Regulation
S-K, included in our Annual Report for the year ended December 31, 2023. The comparison assumes $100 was invested for the
period starting December 31, 2019 (the last trading day of fiscal 2019), through the end of the listed year in the Company
and in the Dow Jones U.S. Select Pharmaceuticals Index, respectively. Historical stock performance is not necessarily indicative
of future stock performance. |
| (5) |
We determined adjusted EBITDA to be the most important financial performance
measure used to link Company performance to Compensation Actually Paid to our PEOs and Non-PEO NEOs in 2023. We may determine
a different financial performance measure to be the most important financial performance measure in future years. Adjusted
EBITDA is not a term defined under U.S. GAAP. We define adjusted EBITDA as net income before net interest expense, income
taxes, and depreciation and amortization (“EBITDA”), as adjusted for certain other items described in our SEC
filings, including stock-based compensation expense, acquisition, site closure and idle facility expenses, restructuring and
other charges, net charges related to legal matters, asset impairment charges, foreign exchange losses or gains, change in
fair value of contingent consideration, and insurance recoveries for property losses and associated expenses. Prior to January
1, 2022, research and development milestone expenses related to license and collaboration agreements were excluded from our
calculation of adjusted EBITDA. The amounts shown in this column for the fiscal years ended December 31, 2021 and December
31, 2020 reflect this historical approach. Effective January 1, 2022, we no longer exclude research and development milestone
expenses related to license and collaboration agreements from adjusted EBITDA. The amounts shown in this column for the fiscal
year ended December 31, 2023 and December 31, 2022 reflect this modified approach; for prior periods, refer to our Form
8-K filed with the Securities and Exchange Commission on May 4, 2022 for a full reconciliation of previously reported non-GAAP
results, including adjusted EBITDA, to revised non-GAAP results. As a result of this modified approach, our reported adjusted
EBITDA for the fiscal years ended December 31, 2021 and December 31, 2020 was revised to $512 million and $433 million, respectively. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
51 |
| |
|
Description of Relationship Between PEO and Non-PEO
NEO Compensation Actually Paid and Company Total Shareholder Return (“TSR”)
The following chart sets forth the relationship
between Compensation Actually Paid to our PEOs, the average of Compensation Actually Paid to our Non-PEO NEOs, and the Company’s
cumulative TSR over the four most recently completed fiscal years.
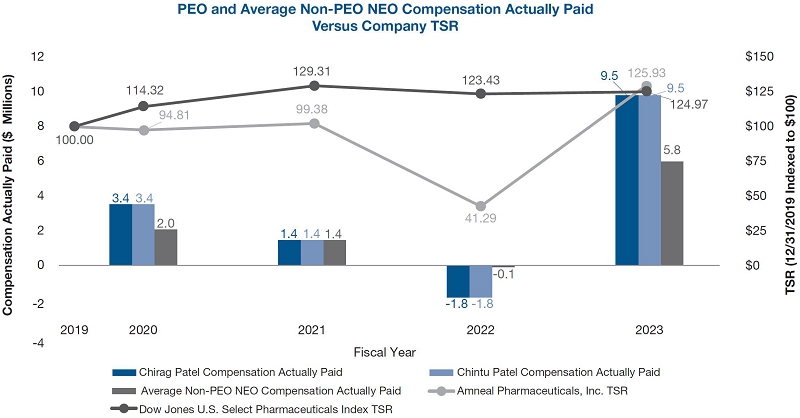
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
52 |
| |
|
Description of Relationship Between PEO and Non-PEO
NEO Compensation Actually Paid and Net Income
The following chart sets forth the relationship
between Compensation Actually Paid to our PEOs, the average of Compensation Actually Paid to our Non-PEO NEOs, and our net income
during the four most recently completed fiscal years.

| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
53 |
| |
|
Description of Relationship Between PEO and Non-PEO
NEO Compensation Actually Paid and Adjusted EBITDA
The following chart sets forth the relationship
between Compensation Actually Paid to our PEO, the average of Compensation Actually Paid to our Non-PEO NEOs, and our Adjusted
EBITDA during the four most recently completed fiscal years.
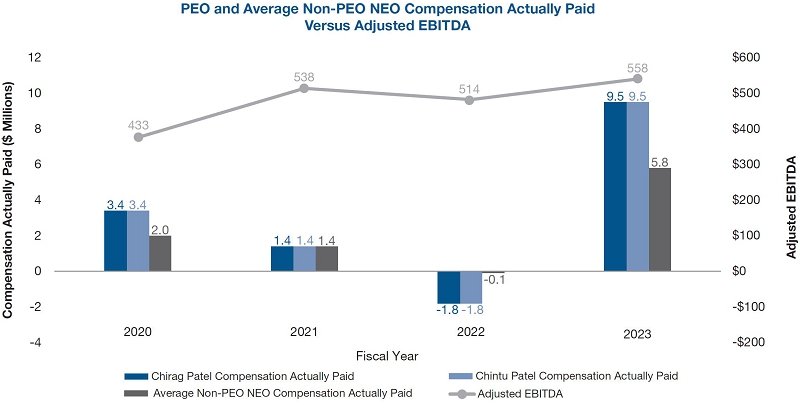
Tabular List of Most Important Financial Performance
Measures
The following table presents the financial performance
measures that the Company considers to have been the most important in linking Compensation Actually Paid to our PEOs and other
NEOs for 2023 to Company performance. The measures in this table are not ranked.
Adjusted EBITDA
Stock Price |
| |
| # # # |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
54 |
| |
|
Proposal 2 Advisory
Vote on Executive Compensation
Introduction
The Dodd-Frank Wall Street Reform and Consumer
Protection Act of 2010 enables our stockholders to vote to approve, on an advisory (non-binding) basis, the compensation of our
named executive officers as disclosed in this proxy statement in accordance with the SEC’s rules.
As described in detail under the heading “Compensation
Discussion and Analysis,” our executive compensation programs, which are guided by the principle of “pay for performance,”
are designed to attract, motivate, and retain our named executive officers, reinforce the execution of our business strategy and
the achievement of our business objectives, and align the interests of our executive officers with the interests of our stockholders,
with the ultimate objective of improving stockholder value. Under these programs, our named executive officers are rewarded for
the achievement of annual and long-term goals and the realization of increased stockholder value. Please read the “Compensation
Discussion and Analysis” beginning on page 31 for additional details about our executive compensation programs, including
information about the fiscal 2023 compensation of our named executive officers.
We believe that our compensation program has
been instrumental in helping the Company achieve strong financial performance.
Therefore, we are asking our stockholders to
indicate their support for our named executive officer compensation as described in this proxy statement. This proposal, commonly
known as a “say on pay” proposal, gives our stockholders the opportunity to express their views on our named executive
officers’ compensation. This vote is not intended to address any specific item of compensation, but rather the overall compensation
of our named executive officers and the philosophy, policies and practices described in this proxy statement.
The say on pay vote is advisory, and therefore
not binding on our Company, the Compensation Committee or our Board of Directors. However, our Board of Directors and our Compensation
Committee value the opinions of our stockholders and will consider the outcome of the vote and the concerns of our stockholders
when making future decisions on the compensation of our named executive officers and our Company’s compensation principles,
policies and procedures.
In accordance with the Board’s policy of
holding annual say on pay votes, we expect that the next advisory vote to approve executive compensation will occur at our 2025
Annual Meeting.
Required Vote
Approval of this proposal requires the affirmative
vote of a majority in voting power of the shares of common stock present in person, by remote communication or by proxy at the
annual meeting and entitled to vote on the proposal.
Recommendation of the Board
of Directors
 |
THE BOARD OF DIRECTORS RECOMMENDS THAT THE STOCKHOLDERS VOTE “FOR”
THE PROPOSAL TO APPROVE, IN AN ADVISORY MANNER, THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS, AS DISCLOSED IN THIS PROXY
STATEMENT PURSUANT TO THE COMPENSATION DISCLOSURE RULES OF THE SEC. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
55 |
| |
|
Security Ownership
of Certain Beneficial Owners and Management
Beneficial Ownership
The following table sets forth information as
of March 11, 2024, with respect to the beneficial ownership of our Class A common stock and shows the number of and percentage
owned by:
| • |
each person or entity our Company believes to be the beneficial owner of more
than five percent of any class of our common stock based solely on management’s review of SEC filings; |
| • |
each named executive officer listed in the summary compensation table; |
| • |
each director; and |
| • |
all of our current directors and executive officers as a group. |
Beneficial ownership of shares is determined
under the rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment
power. Except as indicated by footnote, and subject to applicable community property laws, each person identified in the table
possesses sole voting and investment power with respect to all shares of stock held by such person. As of March 11, 2024, 308,554,228
shares of Class A common stock were outstanding.
| Name(1) | |
Shares of
Common Stock | |
Options(2) | |
RSUs(2) | |
Total | |
% of
Class | |
| Emily Peterson Alva | |
174,318 | |
53,021 | |
76,453 | |
303,792 | |
* | |
| Deborah M Autor | |
54,949 | |
— | |
76,453 | |
131,402 | |
* | |
| Andrew Boyer | |
319,381 | |
373,033 | |
— | |
692,414 | |
* | |
| J. Kevin Buchi | |
185,619 | |
81,397 | |
76,453 | |
343,469 | |
* | |
| Jason B. Daly | |
57,322 | |
— | |
— | |
57,322 | |
* | |
| Jeff George | |
193,084 | |
28,506 | |
76,453 | |
298,043 | |
* | |
| John Kiely | |
179,274 | |
28,506 | |
76,453 | |
284,233 | |
* | |
| Anastasios Konidaris | |
470,386 | |
— | |
87,719 | |
558,105 | |
* | |
| Paul Meister | |
620,658 | |
115,156 | |
107,034 | |
842,848 | |
* | |
| Ted Nark | |
224,318 | |
53,021 | |
76,453 | |
353,792 | |
* | |
| Chintu Patel | |
25,265,818 | |
53,021 | |
— | |
25,318,839 | |
8.21 | |
| Chirag Patel | |
21,781,986 | |
53,021 | |
— | |
21,835,007 | |
7.08 | |
| Gautam Patel | |
1,972,433 | |
53,021 | |
76,453 | |
2,060,860 | |
* | |
| Nikita Shah | |
391,666 | |
131,856 | |
— | |
523,522 | |
* | |
| Shlomo Yanai | |
169,274 | |
28,506 | |
76,453 | |
274,233 | |
* | |
| Total Current Directors and Executive Officers as
a Group (15 Persons) | |
52,060,486 | |
1,052,065 | |
765,330 | |
53,877,881 | |
17.46 | |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
56 |
| |
|
Set forth below is a table demonstrating the
ownership of the Amneal Group(8) as of March 11, 2024:
| Name(1) | |
Shares of Class A Common Stock(2) | |
% of Class | |
| Akram Mahesh(3) | |
30,384,769 | |
9.85 | % |
| Tushar Patel(4) | |
53,578,209 | |
17.36 | % |
| Dipan Patel(5) | |
26,905,073 | |
8.72 | % |
| Chintu Patel(6) | |
25,318,839 | |
8.21 | % |
| Chirag Patel(7) | |
21,835,007 | |
7.08 | % |
| Other Members of the Amneal Group | |
8,113,600 | |
2.63 | % |
| TOTAL
AMNEAL GROUP(8) | |
166,135,497 | |
53.84 | % |
| Set forth below is a table demonstrating the ownership of certain other beneficial owners as of March 11, 2024: |
|
| |
|
|
|
|
|
| Name | |
Shares
of Class A Common Stock | |
%
of Class | |
| Funds affiliated with Fosun International Limited(9) | |
18,407,656 | |
5.97 | % |
| Funds affiliated with TPG Inc.(10) | |
12,328,767 | |
4.00 | % |
| * |
Less than 1% |
| (1) |
Unless otherwise noted, the address for each beneficial owner listed on the table is Amneal Pharmaceuticals, Inc.,
400 Crossing Boulevard, Bridgewater, NJ 08807. |
| (2) |
Column includes shares underlying exercisable stock options and stock options and restricted stock unit awards that
will vest within 60 days of March 11, 2024. |
| (3) |
Akram Mahesh, c/o Tattva Fiduciary Company, 100 West Liberty Street, 10th Floor, Reno, NV 89501. As reported
in the Schedule 13G filed by Akram Mahesh on January 4, 2024, Akram Mahesh has the sole power to (a) vote or direct the vote
of and (b) dispose or direct the disposition of 30,384,769 shares. |
| (4) |
c/o Tarsadia Investments, LLC, 520 Newport Center Drive, Twenty-First Floor, Newport Beach, CA 92660. Tushar Patel
may be deemed to beneficially own 53,578,209 shares of Class A common stock held of record by Tushar Patel Family Trust. |
| (5) |
c/o Buckhead America Hospitality, 2855 Springhill Parkway, Smyrna, GA 30080. Dipan Patel may be deemed to beneficially
own 26,905,073 shares of Class A common stock held of record by Dipan Patel Living Trust, AP-1 Trust, AP-2 Trust, AP-3 Trust,
AP-5 Trust, AP-7 Trust, and AP-9 Trust. |
| (6) |
Chintu Patel may be deemed to beneficially own 24,753,252 shares of Class A common stock held of record by The Chintu
Patel Revocable Trust and The Falguni Patel Revocable Trust. |
| (7) |
Chirag Patel may be deemed to beneficially own 21,269,420 shares of Class A common stock held of record by The Chirag
Patel Revocable Trust and The Priti Patel Revocable Trust. Mr. Patel has pledged to Credit Suisse AG 21,269,420 shares of
Class A common stock to secure the obligations of those certain borrowers to the Promissory Note and Collateral Agreement
dated December 10, 2021 (as modified from time to time). |
| (8) |
See “Corporate Governance – Corporate Structure” above for a discussion of the relationship between
the Amneal Group and the Company. Messrs. Chintu Patel, Chirag Patel and Gautam Patel are members of the Amneal Group
and also members of our Board, and their shares are reported in the “Executive Officers and Directors” table above.
Shares of Class A common stock held by members of the Amneal Group other than Messrs. Akram Mahesh, Chintu Patel, Chirag Patel,
Dipan Patel and Tushar Patel are not reported in this table. |
| (9) |
Fosun International Limited, Room 808, ICBC Tower, 3 Garden Road, Central, Hong Kong. As reported in the Form 13G/A
filed on December 12, 2023 with respect to its holdings as of December 12, 2023, Fosun has the shared power to (a) vote or
direct the vote of and (b) dispose or direct the disposition of 18,407,656 shares. |
| (10) |
TPG Inc., 301 Commerce Street, Suite 3300, Fort Worth, TX 76102. As reported in the Form 13D/A filed by TPG GP A, LLC
on December 14, 2022 with respect to its holdings as of December 12, 2022, the reporting persons have the shared power to
vote or direct the vote and the shared power to dispose or direct the disposition of 12,328,767 shares. |
To our knowledge, except as noted above, no person
or entity is the beneficial owner of more than five percent of the voting power of the Company’s stock.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
57 |
| |
|
Certain Related Parties
and Related Party Transactions
Review and Approval of Related
Party Transactions
Our Board of Directors recognizes that transactions
involving our Company and related parties present heightened risk of potential or actual conflicts of interest that may interfere—or
even appear to interfere—with the interests of our Company. Therefore, it is the policy of our Company (as set forth in our
written Related Person Transaction Policy and Procedures and Conflicts Committee Charter) that the Conflicts Committee of the Board
of Directors shall review, approve or ratify any transaction with related parties required to be reported by our Company under
the applicable rules and regulations governing related party transactions promulgated by the SEC. As discussed more fully in our
Related Person Transaction Policy, in determining whether to approve a transaction, the Conflicts Committee considers all relevant
facts and circumstances, including, among other factors, the material terms of the transaction, the approximate dollar value of
the amount involved in the transaction, the nature of the related person’s interest in the transaction, the approximate dollar
value of the amount of the related person’s direct or indirect interest in the transaction, whether the transaction is on
terms comparable to those that could be obtained in arm’s length dealings with an unrelated third party, the importance of
the transaction both to the Company and to the Related Person and whether the transaction would likely impair the judgment of a
director or executive officer to act in the best interest of the Company. Copies of our Related Person Transaction Policy and Procedures
and Conflicts Committee Charter are available on our website.
Related
Party Transactions
See the footnotes to the tables in the section
entitled “Security Ownership of Certain Beneficial Owners and Management” for a discussion of related party transactions
between the Company and trusts controlled by Chirag Patel relating to pledging arrangements.
Fosun International Limited
Fosun is a Chinese international conglomerate
and investment company that is a stockholder of the Company. On June 6, 2019, the Company entered into a license and supply agreement
with a subsidiary of Fosun, which is a Chinese pharmaceutical company. Under the terms of the agreement, the Company will hold
the import drug license required for pharmaceutical products manufactured outside of China and will supply Fosun with finished,
packaged products for Fosun to then sell in the China market. Fosun will be responsible for obtaining regulatory approval in China
and for shipping the product from Amneal’s facility to Fosun’s customers in China. In consideration for access to the
Company’s U.S. regulatory filings to support its China regulatory filings and for the supply of product, Fosun paid the Company
a $1 million non-refundable fee, net of tax, in July 2019 and will be required to pay the Company $0.3 million for each of eight
products upon the first commercial sale of each in China in addition to a supply price and a profit share. On August 11, 2023,
the Company and Fosun amended the license and supply agreement to, among other things, (i) increase the products subject to the
agreement from eight to ten, (ii) eliminate the first commercial sales milestone of $0.3 million for each product and (iii) decrease
the profit share percentage applicable to all products. For the year ended December 31, 2023, the Company recognized $0.1 million
in revenue from this agreement.
On August 12, 2021, the Company entered into
an active pharmaceutical ingredient (“API”) co-development agreement with a subsidiary of Fosun. Under the terms of
the agreement, the Company granted Fosun an exclusive license to manufacture and sell two pharmaceutical products outside of the
U.S. Fosun will be responsible for obtaining regulatory approvals outside of the U.S. Fosun paid the Company a $0.2 million non-refundable
fee, which was recognized in 2021 as revenue and will be required to pay the Company $0.1 million for each of the two products
upon the first commercial sale of each in China in addition to a profit share. For the year ended December 31, 2023, the Company
did not earn any revenue from this agreement.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
58 |
| |
|
TPG Operations LLC and TPG Capital BD, LLC
TPG Operations LLC (“TPG”) is a private
investment firm that provides financial services and is a significant stockholder of the Company. Jeff Schilling, an observer of
our Board, is a managing director of TPG. TPG offers capital and strategic support for companies with substantial growth potential
primarily in the healthcare, financial services, real estate, and clean technology sectors.
TPG Capital BD, LLC (“TPG Capital”),
a subsidiary of TPG, provided the Company with advice and assistance with respect to the refinancing of the Company’s Term
Loan. Upon closing of the refinancing in November 2023, the Company paid a success fee of $3.0 million to TPG Capital based on
customary market terms.
Transactions Involving Our Co-CEOs (Mr. Chirag
Patel and Mr. Chintu Patel)
Kanan, LLC
Kanan, LLC (“Kanan”) is an independent
real estate company and the landlord of Amneal’s leased manufacturing facilities located at 65 Readington Road, Branchburg,
New Jersey, 131 Chambers Brook Road, Branchburg, New Jersey and 1 New England Avenue, Piscataway, New Jersey, pursuant
to certain lease agreements entered into by Amneal and Kanan (the “New Jersey Lease Agreements”). Pursuant to
the New Jersey Lease Agreements, rent expense paid to Kanan for the year ended December 31, 2023 was approximately $2.5 million.
Chirag Patel and Chintu Patel beneficially own,
through certain revocable trusts, 28.0% in the aggregate of the equity securities of Kanan. In addition, each of Chintu Patel and
Chirag Patel serves on the Board of Managers of Kanan.
Kashiv Biosciences, LLC
Kashiv Biosciences, LLC (“Kashiv”)
is an independent contract development organization that has historically been focused primarily on the development of 505(b)(2)
NDA products utilizing its own proprietary technology platforms, particularly in the areas of abuse deterrence and bioavailability
enhancement. Kashiv possesses deep knowledge and patented novel technologies for developing complex generic and specialty products,
and Amneal has collaborated with Kashiv to develop a number of those products.
In connection with the Acquisition, the Company
and Kashiv entered into a Storage Agreement pursuant to which the Company agreed to provide storage and inventory transfer services
at the KSP-acquired facility for a period of one year post-Acquisition, which has been extended for an additional one year period.
This Storage Agreement expired during 2023 and was not extended.
The Company and Kashiv are party to a master
services agreement, dated February 20, 2020, covering certain services that Kashiv provides the Company for commercial product
support, with such services charged at an hourly rate of $100 per hour for actual time spent plus third party costs.
In April 2022, Amneal and Kashiv entered into
a consulting agreement whereby Kashiv will provide advice relating to its Specialty programs, with such services to be charged
at an hourly rate of $200 per hour for clinical consulting and $240 per hour for non-clinical consulting.
In late 2018, Adello Biologics, LLC (“Adello”)
contributed substantially all of its assets to Kashiv as of January 1, 2019, and transferred all agreements to Kashiv, including
agreements between Amneal and Adello. The following agreements are now between Kashiv and Amneal.
Amneal and Kashiv are party to a license and
commercialization agreement (the “Kashiv License Agreement”) pursuant to which Kashiv and Amneal have agreed to cooperate
with respect to certain development activities in connection with two biologic pharmaceutical products (the “Kashiv Products”).
In addition, under the Kashiv License Agreement, Kashiv has appointed Amneal as its exclusive marketing partner for the Kashiv
Products in the United States. In connection with the Kashiv License Agreement, Kashiv received an upfront payment of $1.5 million
from Amneal in October 2017 and is entitled to share in Amneal’s net profits on the products if and when commercialized.
The Kashiv License Agreement provided for potential future milestone payments to Kashiv of up to $183.0 million, as follows: (i)
up to $22.5 million relating to regulatory approval and execution, (ii) up to $43.0 million for successful delivery of commercial
launch inventory, (iii) up to $50.0 million depending on the number of competitors at launch for one product, and (iv) between
$15.0 million and $67.5 million for the achievement of cumulative net sales for both products.
In July 2022, the Company and Kashiv amended
the Kashiv License Agreement to, among other things, (i) eliminate milestones related to the manufacturing and delivery of the
Kashiv products, (ii) revise the net sales milestones to provide for future milestone payments by the Company to Kashiv of up to
$37.5 million for the achievement of cumulative combined net sales goals for both products, and (iii) adjust the supply price of
product that Kashiv manufacturers and supplies to the Company, which will lower the cost per unit of both products. The remaining
milestones are subject to reaching certain commercial sales volume objectives. In addition, the agreement provides for Amneal to
pay a profit share equal to 50% of net profits, after considering manufacturing and marketing costs.
On May 27, 2022, the FDA approved the biologic
license application, associated with the amended Kashiv License Agreement, for Pegfilgrastim-pbbk. In connection with this regulatory
approval and associated activity, the Company incurred a milestone of $15.0 million during the third quarter of 2022, which was
paid to Kashiv at that time. The milestone was capitalized as an intangible asset and will be amortized to cost of sales over an
estimated useful life of 8.3 years.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
59 |
| |
|
On May 12, 2023, the Company and Kashiv amended
the Kashiv License Agreement to provide for, among other things, (i) Kashiv to have the right to develop either or both an auto-injector
delivery system or an on-body injector for the biosimilar PEG-filgrastim, and (ii) the Company to have a right of first negotiation
to launch and commercialize either or both of such products.
In August 2020, Amneal and Kashiv entered into
a product development agreement for the development and commercialization of two generic peptide products, Ganirelix Acetate and
Cetrorelix Acetate. Under the agreement, the intellectual property and ANDA for these products are owned by Amneal, and Kashiv
is to receive a profit share for all sales of the products made by Amneal.
Pursuant to the agreements described above, total
payments for milestones, services and reimbursement of expenses paid to Kashiv for the year ended December 31, 2023 was $5.1 million.
As of December 31, 2023, the Company has $1.0 million of inventory on hand associated with the amended Kashiv License Agreement.
In addition, as of December 31, 2023, $3.2 million was outstanding and payable to Kashiv.
In December 2022, Amneal and Kashiv entered into
a development supply agreement specific to four generic product candidates. Amneal will be responsible for manufacturing batch
products, as well as to perform certain developmental activities on behalf of Kashiv. Kashiv, as owner of the IP, will be responsible
for regulatory filings, obtaining FDA approval, marketing, selling, and pricing activities. Kashiv is also responsible to reimburse
Amneal for sourcing developmental materials at cost. Pursuant to the terms of the development supply agreement, Amneal is eligible
to earn up to $2.4 million related to the aforementioned services. For the year ended December 31, 2023, the Company recognized
$2.8 million related to reimbursable costs associated with developmental work for this agreement, as a reduction to research and
development expenses.
The Company and Kashiv are also party to an arrangement
whereby the Company leases parking spaces from Kashiv, pursuant to which the Company pays to Kashiv approximately $99,000 per year.
Chirag Patel and Chintu Patel beneficially own,
directly and through certain revocable or irrevocable trusts for the benefit of their immediate families, 50% in the aggregate
of the outstanding equity securities of Kashiv. In addition, each of Chintu Patel and Chirag Patel serve on the Board of Managers
of Kashiv.
Nava Pharma, LLC and PharmaSophia,
LLC
PharmaSophia, LLC (“PharmaSophia”)
is a joint venture formed by Nava Pharma, LLC (“Nava”) and Oakwood Laboratories, LLC for the purpose of developing
certain products. PharmaSophia is currently actively developing one injectable product. PharmaSophia and Nava are parties to a
research and development agreement pursuant to which Nava provides research and development services to PharmaSophia. Nava subcontracted
this obligation to Amneal. Amneal, PharmaSophia and Oakwood also entered into a manufacturing agreement whereby Amneal agreed to
manufacture exhibit and commercial batches of products under development.
In October 2022, PharmaSophia and Amneal entered
into an exclusive license and commercialization agreement (the “PharmaSophia Agreement”) to develop, manufacture, and
sell one injectable product. Under the terms of the agreement, Amneal committed to spend up to $6.0 million to further develop
the product, including all related expenses up to submission of the ANDA, which will be owned by Amneal. In addition,
under the terms of the PharmaSophia Agreement, PharmaSophia settled a liability of $1.1 million payable to Amneal under the terms
of the Nava Agreement by reducing the amount of Amneal’s committed spending under the terms of the PharmaSophia Agreement
to $4.9 million. PharmaSophia will receive a 50% profit share for all sales of product made by Amneal under the PharmaSophia Agreement.
Chirag Patel and Chintu Patel beneficially own,
directly and through certain revocable trusts, equity securities in Nava. Nava beneficially owns 50% of the outstanding equity
securities of PharmaSophia. In addition, each of Chintu Patel and Chirag Patel serve on Nava and PharmaSophia’s Board of
Managers.
Avtar Investments, LLC and
Avtar Enterprise, LLC
Avtar Investments, LLC is a private investment
firm and Avtar Enterprise, LLC is a private portfolio company (collectively, “Avtar”). Chirag Patel and Chintu Patel
beneficially own, directly and through certain revocable trusts, outstanding equity securities of Avtar. In July 2020, the Company
entered into an agreement with Avtar Enterprise under which Avtar agreed to provide consulting services relating to certain clinical
studies and regulatory advice for generic pharmaceutical products in development by the Company. Avtar and Amneal amended the agreement
in 2022 to include several inhalation products and specialty programs. For the year ended December 31, 2023, the Company recorded
$0.3 million in expenses related to the consulting agreement. As of December 31, 2023, $0.1 million was due to Avtar.
Employment and Stockholder
Arrangements with Immediate Family Members
Kanubhai Patel, the father of Chirag and Chintu
Patel, is employed by us and serves as an operational and strategic advisor to the Company and its Indian subsidiaries, including
but not limited to Amneal Pharmaceuticals India Private Limited. During the fiscal year ended December 31, 2023, Mr. Kanubhai Patel
had total cash compensation of approximately $458,428. In addition, he received a long-term incentive award in 2024 with a determined
value of $195,000.
Bindu Patel, a sister of Chirag and Chintu Patel,
is employed by us as a manager in the information technology department. During the fiscal year ended December 31, 2023, Ms. Bindu
Patel had total compensation of approximately $159,494.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
60 |
| |
|
The Reorganization
As disclosed above under “Corporate Governance—Corporate
Structure, Reorganization and Transfer of Listing Exchange,” on November 7, 2023, we implemented a plan pursuant to which
the Company and Amneal LLC reorganized and we simplified our corporate structure by eliminating our umbrella partnership-C-corporation
structure and converting to a more traditional structure whereby all stockholders hold their voting and economic interests directly
through the public company, which we refer to as the Reorganization. Effective with the Reorganization, the Company holds directly
or indirectly 100% of the Amneal Common Units and the Company remains Amneal LLC’s sole managing member, having the sole
voting power to make all of Amneal LLC’s business decisions and control its management. Following the implementation of the
Reorganization, Amneal Intermediate, Inc., a Delaware corporation (formerly known as Amneal Pharmaceuticals, Inc.) (for purposes
of the Reorganization, “Old Amneal”), became a wholly-owned subsidiary of a new holding company, Amneal Pharmaceuticals,
Inc., a Delaware corporation (formerly known as Amneal NewCo Inc.) (for purposes of the Reorganization, “New Amneal”).
Organizational Agreements and Documents
To effectuate the Reorganization, we entered
into and/or amended various organizational agreements and documents, including agreements and plans of merger, certificates of
incorporation and bylaws.
Assumption of Old Amneal Equity Plan and
Agreements
In connection with the Reorganization, on November
7, 2023, New Amneal assumed the 2018 Incentive Award Plan and all outstanding stock options and equity awards granted thereunder
as well as the indemnification agreements between Old Amneal and the directors and executive officers of Old Amneal, in each case
to make administrative amendments related to the Reorganization.
Letter Agreement Amendments to Executive
Employment Agreements
In connection with the Reorganization, on November
7, 2023, each of (i) the Boyer Employment Agreement, (ii) the Konidaris Employment Agreement, and (iii) the Shah Employment Agreement
was amended. For further details, please refer to “Executive Compensation—Management Employment & Separation
Agreements” herein.
Stock Surrender Agreement
In connection with the Reorganization, the Company
and each member of the Amneal Group entered into a Stock Surrender Agreement, dated as of November 7, 2023 (“Stock Surrender
Agreement”) which had the effect of each member of the Amneal Group agreeing to irrevocably surrender to the Company each
share of Old Amneal Class B Common Stock held by such Amneal Group member immediately prior to the Reorganization.
Other Agreements
In connection with the Reorganization, the Company
entered into amendments to other agreements that are described more fully in this section.
Expenses
We incurred approximately $5.9 million in expenses
related to the Reorganization during the year ended December 31, 2023, comprised of professional fees.
Agreements Entered into in Connection with
the Combination and the PIPE Investment
In 2018, Amneal entered into an important strategic
transaction that we refer to as the Combination. The Combination was a transformative event that established Amneal as an industry
leader with high-value generic product pipelines and a growing specialty business. The following summarizes the agreements we entered
in connection with the Combination.
Stockholders Agreement
See the “Corporate Governance” section
of this proxy statement for a summary of the material terms of the Stockholders Agreement. In connection with the Reorganization,
on November 7, 2023, Old Amneal amended and restated the Stockholders Agreement in order to facilitate the Reorganization.
PIPE Investment
In connection with the Combination, on May 4,
2018, members of the Amneal Group entered into definitive purchase agreements (the “PIPE Purchase Agreement”) that
provided for a private placement of certain shares of Class A common stock and Class B-1 common stock (the “PIPE Investment”)
with select institutional investors (the “PIPE Investors”). Pursuant to the PIPE Purchase Agreement, upon the closing
of the Combination (the “Closing”), members of the Amneal Group exercised their right to cause Amneal LLC to redeem
units held by such members pursuant to the Third Amended and Restated LLC Agreement (dated as of May 4, 2018 and defined herein).
In connection with such redemption, such members of the Amneal Group received shares of Class A common stock or shares of Class
B-1 common stock
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
61 |
| |
|
in exchange for such redeemed units, in each
case pursuant to the LLC Agreement (such redemption and issuance of Class A common stock and Class B-1 common stock to the members
of the Amneal Group, the “Redemption”). Following the Redemption, the members of the Amneal Group sold such shares
of Class A common stock and Class B-1 common stock to the PIPE Investors at a per share purchase price of $18.25 for gross proceeds
of approximately $855,000,000. Following the PIPE Investment, the PIPE Investors owned collectively approximately 15% of the Company’s
shares on a fully diluted and as converted basis, with TPG owning all outstanding shares of Class B-1 common stock. As a result
of the Conversion in 2019, there are no longer any shares of Class B-1 common stock outstanding.
In connection with the Combination and in furtherance
of the PIPE Investment, TPG, the Amneal Group and the Company entered into the PIPE Side Letter providing for certain rights and
obligations of each in connection with the PIPE Investment. Pursuant to the PIPE Side Letter, TPG has customary registration rights
with respect to the Company’s shares owned by TPG. The PIPE Side Letter also provides TPG the right to designate a Board
observer with respect to the Company’s Board of Directors, as well as the right, subject to certain ownership thresholds
discussed herein, to designate a director for appointment to the Company’s Board of Directors.
Registration Rights Agreement
We entered into a Registration Rights Agreement
with the PIPE Investors in connection with the Closing. The Registration Rights Agreement provides the PIPE Investors certain registration
rights whereby the Company and Impax were required to jointly prepare and file with the SEC a shelf registration statement on Form
S-1 with respect to resales of all shares of Class A common stock beneficially owned by the Amneal Group. We agreed to use our
reasonable best efforts to become eligible to use Form S-3 and, upon becoming eligible, we agreed to promptly file a shelf registration
statement on Form S-3.
Tax Receivable Agreement
Pursuant to the LLC Agreement, in effect at the
Combination, the Amneal Group and its permitted transferees had the right to redeem all or a portion of their Amneal Common Units
(defined herein) for Class A common stock. In connection with such redemption, Old Amneal received a “step-up” in its
share of the tax basis in the Amneal LLC assets, and Old Amneal agreed to pay the Members (as defined below) for the value of such
benefits.
In connection with the Reorganization, on November
7, 2023, Old Amneal amended that certain Tax Receivable Agreement, dated as of May 4, 2018 (as amended, the “Tax Receivable
Agreement”), among Old Amneal and the Amneal Group (such amendment, the “TRA Amendment”), in order to (i) provide
that the percentage of the applicable tax savings the Amneal Group will be entitled to thereunder is decreased from 85% to 75%
and (ii) facilitate the Reorganization.
The following summary of the terms of the Tax
Receivable Agreement is not a complete description thereof and is qualified in its entirety by the full text thereof.
The Tax Receivable Agreement continues to govern
the administration and allocation between the parties of tax liabilities and benefits arising prior to, as a result of, and subsequent
to the Combination, and the respective rights, responsibilities and obligations of the Members and the Company with respect to
various other tax matters. The term “Members” includes the then existing members of Amneal LLC at Closing (other than
Old Amneal) and any persons who have executed and delivered a joinder in accordance with the Tax Receivable Agreement. Chirag Patel,
Chintu Patel and Gautam Patel are Members.
Determination of Realized Tax Benefit
Under the Tax Receivable Agreement, the Company
and Old Amneal will ensure that Amneal LLC and its subsidiaries that are treated as a partnership for U.S. federal income tax purposes
will have in effect an election under Section 754 of the Code.
Basis Schedules
Within 90 days after the filing of the U.S. federal
income tax return of Old Amneal for each relevant taxable year, Old Amneal will at its own expense deliver to the Members a schedule
that shows (a) the basis adjustments with respect to the reference assets as a result of the relevant exchanges through the Reorganization,
calculated (i) in the aggregate and (ii) solely with respect to exchanges by the applicable Member; (b) the period (or periods)
over which the reference assets are amortizable and/or depreciable; and (c) the period (or periods) over which each basis adjustment
is amortizable and/or depreciable.
Tax Benefit Schedules
Within 90 days after the filing of the U.S. federal
income tax return of Old Amneal for any taxable year in which there is a realized tax benefit or realized tax detriment, Old Amneal
shall, at its own expense, deliver to the Members a schedule showing the calculation of the realized tax benefit or realized tax
detriment for such taxable year.
Tax Benefit Payments
Each Member is entitled to receive an amount
equal to the sum of (1) 75% of the cumulative net realized tax benefit attributable to such Member as of the end of such taxable
year over the aggregate amount of all tax benefit payments previously made to such Member, and (2) the interest calculated at the
agreed rate from the due date for filing the U.S. federal income tax return of Old Amneal for such taxable year until the date
on which Old Amneal makes a timely tax benefit payment to the Member.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
62 |
| |
|
Termination
Old Amneal may terminate the Tax Receivable Agreement
with the written approval of a majority of the independent directors of the Company’s Board of Directors by making a payment
to the Members, equal to the present value of the tax benefit payments to be paid to each such Member, discounted at the lesser
of ICE LIBOR plus 100 basis points or 6.50% per annum, compounded annually (an “Early Termination Payment”). The Tax
Receivable Agreement will also be deemed to be terminated by Old Amneal and an Early Termination Payment by Old Amneal will be
required in the event of either (a) a Change of Control (as defined below) or (b) a material breach by Old Amneal of any of its
material obligations under the Tax Receivable Agreement.
A “Change of Control” includes (a)
any person other than the Amneal Group and their permitted transferees beneficially owning more than 50% of the combined voting
power of the Company; (b) the liquidation or dissolution of the Company, or the sale of all or substantially all of the assets
of the Company, unless the sale is to an entity of which at least 50% of the combined voting power is owned by the Company’s
Stockholders who owned the Company immediately prior to such sale in substantially the same proportions; (c) a business combination
of the Company or any of its subsidiaries with any other entity, after which the Company’s Board of Directors immediately
prior to such combination does not constitute at least a majority of the Board of Directors of the surviving company or its parent,
or all of the beneficial owners of the voting securities of the Company prior to such combination do not beneficially own more
than 50% of the combined voting power of the surviving entity; and (d) the following individuals ceasing to constitute a majority
of the Company’s Board of Directors: (i) the directors of the Company as of November 7, 2023 (“Initial Directors”)
and (ii) any new director whose appointment or nomination was approved by at least two-thirds of the directors who were (x) Initial
Directors or (y) whose appointment or nomination was approved by at least two-thirds of the Initial Directors.
LLC Agreement
In connection with the Combination, Amneal LLC,
the Company and the Existing Amneal Members (and the Amneal Group following the assignment and transfer by the Existing Amneal
Members (as defined in the LLC Agreement) of Amneal Common Units to the Amneal Group) entered into and are governed by the LLC
Agreement, which sets forth, among other things, certain transfer restrictions on Amneal Common Units, and rights to redeem Amneal
Common Units in certain circumstances. The following summary of the terms of the LLC Agreement is not a complete description thereof
and is qualified in its entirety by the full text thereof. Undefined capitalized terms in this section have the meaning ascribed
to them in the LLC Agreement.
In connection with the Reorganization, on November
7, 2023, Amneal LLC entered into the Fourth A&R LLC Agreement, in order to (i) remove the requirement for Amneal LLC to make
tax distributions, (ii) reflect the addition of New Amneal as a member and the removal as members of the former holders of Old
Amneal Class B Common Stock after conversion of the Common Units paired with Old Amneal Class B Common Stock to New Amneal Class
A common Stock and (iii) to facilitate the Reorganization.
Appointment of the Company as Manager
Under the LLC Agreement, the Company is admitted
as the sole managing member of Amneal LLC. As the managing member, the Company will conduct, direct and exercise full control over
all activities of Amneal LLC, including day-to-day business affairs and decision-making of Amneal LLC, without the approval of
any other member. As such, the Company, through Amneal’s LLC officers, will be responsible for all operational and administrative
decisions of Amneal LLC and the day-to-day management of Amneal’s LLC business.
Pursuant to the terms of the LLC Agreement, the
Company will not be permitted, under any circumstances, to be removed as managing member by the members of Amneal LLC. The Company
will not resign or cease to be the managing member unless proper provision is made for the obligations of the Company to remain
in full force and effect.
The managing member may cause Amneal LLC to contract
with the managing member or any affiliate of the managing member as long as the contracts are on terms comparable to those available
to others dealing at arm’s length or are approved by the members (other than the managing member and its controlled affiliates)
holding a majority of the Amneal Common Units.
Officers
The managing member will appoint the officers
of Amneal LLC to implement the day-to-day business and operations of Amneal LLC. In the event of a vacancy, the managing member
has the right to appoint a new officer to fill the vacancy.
Compensation
The Company will not be entitled to compensation
for its services as managing member. It will be entitled to reimbursement by Amneal LLC for reasonable fees and expenses incurred
on behalf of Amneal LLC, except for payment obligations of the Company under the Tax Receivable Agreement.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
63 |
| |
|
Report of
the Audit Committee
Under the guidance of a written charter adopted
by our Board of Directors, the Audit Committee oversees our management’s conduct of the financial reporting process on behalf
of the Board of Directors. A copy of the charter is available at the investor relations section of our company’s website,
http://investors.amneal.com. The Audit Committee also appoints the independent registered public accounting firm to be retained
to audit our company’s consolidated financial statements and internal control over financial reporting, and once retained,
the independent registered public accounting firm reports directly to the Audit Committee. The Audit Committee is responsible for
pre-approving both audit and non-audit services to be provided by the independent registered public accounting firm. The Audit
Committee’s charter reflects the above-mentioned responsibilities, and the Audit Committee and the Board of Directors periodically
review and revise the charter.
Management is responsible for our company’s
financial reporting process, including the system of internal controls, and for the preparation of consolidated financial statements
in accordance with accounting principles generally accepted in the United States of America. Our company’s independent registered
public accounting firm is responsible for auditing those consolidated financial statements and expressing an opinion on the conformity
of the consolidated financial statements with accounting principles generally accepted in the United States of America. In addition,
our company’s independent registered public accounting firm will express its own opinion on the effectiveness of the company’s
internal control over financial reporting. The Audit Committee’s responsibility is to monitor and review these processes.
It is not the Audit Committee’s duty or responsibility to conduct auditing or accounting reviews.
The Audit Committee meets at least four times
annually, or more frequently as circumstances dictate. During fiscal 2023, the Audit Committee met five times. The Audit Committee
also met with management periodically to consider the adequacy of our company’s internal controls, and discussed these matters
and the overall scope and plans for the audit of our company with our independent registered public accounting firm, Ernst &
Young LLP. The Audit Committee met with the independent registered public accounting firm, with and without management present,
to discuss the results of its examination, its evaluation of the effectiveness of our internal control over financial reporting,
and the overall quality of our financial reporting. The Audit Committee also discussed with senior management our company’s
disclosure controls and procedures and the certifications by our co-chief executive officers and chief financial officer, which
are required by the SEC under the Sarbanes-Oxley Act of 2002 for certain of our company’s filings with the SEC. The Audit
Committee also met separately from time to time with our chief financial officer and, at least quarterly, the Audit Committee met
in executive session.
In fulfilling its oversight responsibilities,
the Audit Committee reviewed and discussed with management and the independent registered public accounting firm the audited consolidated
financial statements in the Annual Report on Form 10-K for the fiscal year ended December 31, 2023, management’s assessment
of the effectiveness of our company’s internal control over financial reporting and the independent registered public accounting
firm’s evaluation of the effectiveness of our company’s internal control over financial reporting as of December 31,
2023. The Audit Committee reviewed with the independent registered public accounting firm, who is responsible for expressing an
opinion on the conformity of the consolidated financial statements with accounting principles generally accepted in the United
States of America, its judgments as to the quality, not just the acceptability, of our company’s accounting principles, the
reasonableness of significant judgments and the clarity of disclosures in the financial statements and such other matters as are
required to be discussed with the Audit Committee under auditing standards of the Public Company Accounting Oversight Board (PCAOB).
In addition, the Audit Committee has discussed with the independent registered public accounting firm its independence from our
company and our management, including the matters in the written disclosures and letter which were received by the Audit Committee
from the independent registered public accounting firm as required by the applicable requirements of the PCAOB, and considered
the compatibility of non-audit services with Ernst & Young LLP’s independence. In reliance on the reviews and discussions
referred to above, the Audit Committee recommended to the Board of Directors (and the board approved) that the audited consolidated
financial statements be included in the Annual Report on Form 10-K for the fiscal year ended December 31, 2023 for filing
with the SEC.
Audit Committee:
John Kiely (Chair)
Deb Autor
J. Kevin Buchi
Jeff George
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
64 |
| |
|
Proposal 3 Appointment
of Independent Registered Public Accounting Firm
Introduction
The Audit Committee has appointed Ernst &
Young LLP as the independent registered public accounting firm to audit our consolidated financial statements and the effectiveness
of our internal control over financial reporting for the fiscal year ending December 31, 2024.
We are asking our stockholders to ratify the
selection of Ernst & Young as our independent registered public accounting firm. Although ratification is not required
by our Bylaws or otherwise, our Board of Directors is submitting the selection of Ernst & Young to our stockholders for
ratification as a matter of good corporate practice. If the selection is not ratified, the Audit Committee will consider whether
it is appropriate to select another registered public accounting firm. Even if the selection is ratified, the Audit Committee in
its discretion may select a different registered public accounting firm at any time during the year if it determines that such
a change would be in the best interests of the Company and our stockholders.
One or more representatives of Ernst &
Young are expected to be present at the annual meeting. They will have an opportunity to make a statement if they desire to do
so and are expected to be available to respond to appropriate stockholder questions.
Independent Registered Public
Accounting Firm Fees
In addition to performing the audit of our consolidated
financial statements, Ernst & Young has provided various other services during fiscal 2023 and 2022. The aggregate fees
billed or expected to be billed for fiscal 2023 and 2022 for each of the following categories of services are as follows:
| Type of Fees | |
Fiscal 2023 | |
Fiscal 2022 |
| Audit Fees | |
$ | 5,748,100 | |
$ | 4,553,300 |
| Audit-Related Fees | |
| 100,000 | |
| 200,000 |
| Tax Fees | |
| 352,700 | |
| 423,300 |
| All Other Fees | |
| 7,200 | |
| 3,000 |
| TOTAL | |
$ | 6,208,000 | |
$ | 5,179,600 |
In accordance with the SEC’s definitions
and rules, the terms in the above table have the following meanings:
“Audit Fees” are the aggregate fees billed or
expected to be billed for each of fiscal 2023 and 2022 for professional services rendered by Ernst & Young for the audit
of our consolidated financial statements included in our annual reports on Form 10-K and review of the unaudited consolidated financial
statements included in our quarterly reports on Form 10-Q; SEC registration statements, including consents and review of documents
filed with the SEC, consultation on accounting standards or transactions, and for services in connection with statutory or regulatory
filings or engagements; and for services that are normally provided by Ernst & Young in connection with statutory and
regulatory filings or engagements for fiscal 2023 and 2022.
“Audit-Related Fees” are the aggregate
fees billed in fiscal 2023 and 2022 for assurance and related services by Ernst & Young that are reasonably related to
the performance of the audit or review of our consolidated financial statements.
“Tax Fees” are the aggregate fees
billed in each of fiscal 2023 and 2022 for professional services rendered by Ernst & Young for tax compliance, tax advice
and tax planning.
“All Other Fees” are the aggregate
fees billed in each of fiscal 2023 and 2022 for products and services provided by Ernst & Young not included in the first
three categories.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
65 |
| |
|
The Audit Committee has reviewed summaries of
the services provided by Ernst & Young and the related fees, and the Audit Committee has determined that the provision
of the non-audit services described above is compatible in maintaining the independence of Ernst & Young.
All of the services described above that required
pre-approval were pre-approved by the Audit Committee in accordance with its pre-approval policy. The Audit Committee pre-approval
policy provides that all auditing services and all non-audit services to be provided by Ernst & Young be pre-approved
by the Audit Committee, provided that the Audit Committee shall not approve any non-audit services prohibited by Section 10A(g)
of the Exchange Act.
Required Vote
Ratification of the appointment of our independent
registered public accounting firm requires the affirmative vote of a majority in voting power of the shares of common stock present
in person, by remote communication or by proxy at the annual meeting and entitled to vote on the proposal.
Recommendation of the Board
of Directors
 |
THE BOARD OF DIRECTORS RECOMMENDS THAT THE STOCKHOLDERS VOTE “FOR”
THE RATIFICATION OF THE APPOINTMENT OF ERNST & YOUNG LLP AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FOR
THE FISCAL YEAR ENDING DECEMBER 31, 2024. |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
66 |
| |
|
Other Matters
Our management is not aware of any other
matters to be presented for action at the annual meeting; however, if any such matters are properly presented for action, it is
the intention of the proxy appointees to vote in accordance with their best judgment on such matters.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
67 |
| |
|
About the Meeting
Why am I receiving these materials?
This proxy statement is provided to
the stockholders of the Company as of the close of business on March 11, 2024 (the “Record Date”) in connection
with the solicitation of proxies by our Board of Directors to be voted at our annual meeting of stockholders to be held
virtually at www.virtualshareholdermeeting.com/AMRX2024 at 9:00 a.m., Eastern Daylight Time, on Thursday, May 2, 2024, and at
any adjournment or postponement of the meeting. This proxy statement provides important information that you should consider
in deciding how to vote on the matters to be voted on at the annual meeting.
Why is the annual meeting being webcast online?
This year, the annual meeting will be a virtual
meeting of stockholders held via a live audio webcast. The format of the virtual meeting has been designed to ensure that our stockholders
who attend our annual meeting will be afforded the same rights and opportunities to participate as they would at an in-person meeting
and to enhance stockholder access, participation and communication through online tools. Stockholders will be able to present questions
online during the meeting through www.virtualshareholdermeeting.com/AMRX2024, providing our stockholders with the opportunity for
meaningful engagement with the Company. In addition, stockholders will be permitted to submit a question in advance of the meeting
at www.proxyvote.com after logging in with your 16-digit Control Number.
How do I participate in the virtual meeting?
Our annual meeting will be a completely virtual
meeting of stockholders, which will be conducted exclusively by live audio webcast. No physical in-person meeting will be held.
The online meeting will begin promptly at
9:00 a.m. EDT. We encourage you to access the meeting prior to the start time leaving ample time for the check in. To participate
in the meeting, you must have your 16-digit Control Number that is shown on your Notice or, if you received a printed copy of the
proxy materials, on your proxy card or the instructions that accompanied your proxy materials. You may access the annual meeting
online, vote and submit your questions during the meeting by visiting www.virtualshareholdermeeting.com/AMRX2024. Stockholders
will be able to submit questions during the meeting by typing in your question into the “ask a question” box on the
meeting page. If you lose your 16-digit Control Number, you may join the annual meeting as a “guest” but you will not
be able to vote, ask questions or access the list of stockholders as of the close of business on the Record Date.
Will I be able to participate in the virtual meeting on the
same basis as I would be able to participate in a live meeting?
The virtual meeting format for the annual
meeting will enable full and equal participation by all of our stockholders from any place in the world at little to no cost.
The format of the virtual meeting has been
designed to ensure that our stockholders who attend our annual meeting will be afforded the same rights and opportunities to participate
as they would at an in-person meeting and to enhance stockholder access, participation and communication through online tools.
We will take the following steps to ensure such an experience:
| • |
providing stockholders with the ability to submit appropriate questions in advance of the
meeting to ensure thoughtful responses; |
| • |
providing stockholders with the ability to submit appropriate questions real-time via the
meeting website, limiting questions to one per stockholder unless time otherwise permits; and |
| • |
answering as many questions submitted in accordance with the meeting rules of conduct as possible
in the time allotted for the meeting without discrimination. |
Questions pertinent to meeting matters will
be answered during the meeting, subject to time constraints. Questions regarding personal matters, including those related to employment
issues, are not pertinent to meeting matters and therefore will not be answered.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
68 |
| |
|
What if during the check-in time or during the meeting I have
technical difficulties or trouble accessing the virtual meeting website?
Should you require technical assistance,
support will be available by dialing 1-844-986-0822 (U.S.) or 1-303-562-9302 (International) during the meeting; these telephone
numbers will also be displayed on the meeting webpage. If there are any technical issues in convening or hosting the meeting, we
will promptly post information to our website, including information on when the meeting will be reconvened.
What items will be voted on at the annual
meeting?
At the annual meeting, the stockholders will consider and vote
upon:
| • |
the election of 11 directors named in this proxy statement to hold office until
the next annual meeting of stockholders (Proposal No. 1); |
| • |
an advisory vote to approve executive compensation, commonly referred to as a “say on
pay” proposal (Proposal No. 2); and |
| • |
the ratification of the appointment of Ernst & Young LLP as our independent registered
public accounting firm for fiscal 2024 (Proposal No. 3). |
If you received a paper copy of these materials
by mail, the proxy materials also include a proxy card or a voting instruction card for the annual meeting.
What is a proxy statement? What information is contained in
this proxy statement?
It is a document that SEC regulations require
us to give you when we ask you to designate proxies to vote on your behalf. The information in this proxy statement relates to
the proposals to be voted on at the annual meeting, the voting process, the Company’s Board of Directors and Board committees,
the compensation of our directors and executive officers for fiscal 2023 and other required information.
Why did I receive a notice in the mail regarding the Internet
availability of the proxy materials instead of a paper copy of the proxy materials?
We are pleased to be using the SEC rule that
allows companies to furnish their proxy materials to stockholders over the Internet. As a result, we are mailing to most of our
stockholders a Notice of Internet Availability of the proxy materials instead of a paper copy of the proxy materials. We believe
that this process allows us to provide our stockholders with the information they need in a timelier manner, while reducing the
environmental impact and lowering the costs of printing and distributing our proxy materials. All stockholders receiving the notice
will have the ability to access the proxy materials over the Internet and request to receive a paper copy of the proxy materials
by mail.
How can I access the proxy materials over the Internet?
The notice of annual meeting, proxy statement
and annual report are available at www.proxyvote.com. Instead of receiving future copies of the proxy materials by mail, most beneficial
owners can elect to receive an email that will provide electronic links to these documents. Opting to receive your proxy materials
online will save us the cost of producing and mailing documents to your home or business and will also give you an electronic link
to the proxy voting site. If you received a Notice of Internet Availability, that notice will contain additional instructions on
how to view our proxy materials on the Internet.
How may I obtain a paper copy of the proxy materials?
Stockholders receiving a Notice of Internet
Availability will find instructions about how to obtain a paper copy of the proxy materials on that notice. All stockholders who
do not receive a notice will receive a copy of the proxy materials by mail or email.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
69 |
| |
|
What is the difference between holding shares as a stockholder
of record and as a beneficial owner?
If your shares are registered directly in
your name with the Company’s registrar and transfer agent, Computershare, you are considered a stockholder of record with
respect to those shares.
If your shares are held in a brokerage account
or with a bank or other nominee, you are considered the “beneficial owner” of those shares.
Who is entitled to vote at the annual meeting?
Each holder of record of our Class A
common stock at the close of business on the Record Date is entitled to vote at the annual meeting (Class A common stock is
referred to herein as the “voting common stock”). As of the Record Date, a total of 308,554,228 shares of Class A
common stock were outstanding and are eligible to vote at the annual meeting. Each share of our Class A common stock is
entitled to one vote per share on all matters with respect to which holders are entitled to vote.
How do I vote during the meeting?
We will be hosting the annual meeting live
online. You can participate in the annual meeting live online at www.virtualshareholdermeeting. com/AMRX2024. The webcast will
start at 9:00 a.m. EDT. Stockholders may vote and submit questions while attending the meeting online. You will need the 16-digit
Control Number included on your Notice or, if you received a printed copy of the proxy materials, on your proxy card or the instructions
that accompanied your proxy materials in order to be able to vote and submit questions during the meeting.
Your shares may only be voted at the annual
meeting if you are present by remote communication or are represented by proxy. Whether or not you plan to attend the annual meeting,
we encourage you to vote by proxy in advance of the annual meeting to assure that your shares will be represented. Voting by proxy
will in no way limit your right to vote at the annual meeting if you later decide to participate in the online meeting. Only your
latest executed vote will count.
How do I vote my shares in advance without attending the Annual
Meeting?
If you are a stockholder of record, you may
vote by granting a proxy. Specifically, you may vote:
| • |
By Internet: If you have Internet access, you may submit your proxy by
going to www.proxyvote.com and by following the instructions on how to complete an electronic proxy card. You will need the
16-digit number included on your Notice of Internet Availability or your proxy card in order to vote by Internet. |
| • |
By Telephone: You may submit your proxy by dialing 1-800-690-6903 and by following
the recorded instructions. You will need the 16-digit number included on your Notice of Internet Availability or your proxy
card in order to vote by telephone. |
| • |
By Mail: You may vote by mail by requesting a proxy card from us, indicating your vote
by completing, signing and dating the card where indicated and by mailing or otherwise returning the card in the envelope
that will be provided to you. You should sign your name exactly as it appears on the proxy card. If you are signing in a representative
capacity (for example, as guardian, executor, trustee, custodian, attorney or officer of a corporation), indicate your name
and title or capacity. |
If you hold your shares in street name, you
may submit voting instructions to your broker, bank or other nominee. In most instances, you will be able to do this over the Internet,
by telephone or by mail. Please refer to information from your bank, broker or other nominee on how to submit voting instructions.
What can I do if I change my mind after I vote my shares?
Stockholders of Record
If you are a stockholder of record, you may
revoke your proxy at any time before it is exercised by timely submission of a written revocation to our corporate secretary at
our principal executive offices located at 400 Crossing Boulevard, Bridgewater, New Jersey 08807, submission of a properly executed
later-dated proxy, or by voting online at the annual meeting. Attendance at the annual meeting will not by itself constitute a
revocation of a proxy.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
70 |
| |
|
Beneficial Owners
If your shares are held in the name of a
broker, bank or other holder of record, that institution will instruct you as to how your vote may be changed and the deadline
for doing so.
If I am a stockholder of record, how will my shares be voted
if I sign, date and return my proxy card? What if I do not specify a choice for a matter when returning my signed proxy card?
All shares entitled to vote that are represented
by properly completed proxy cards received prior to the annual meeting and not revoked will be voted at the meeting in accordance
with your instructions. If you sign and return a proxy card but do not indicate how your shares should be voted, the shares represented
by your proxy card will be voted in accordance with the Board of Directors’ recommendations on Proposals 1-3 and in the discretion
of the persons designated as proxies as to any other matter that may properly come before the annual meeting.
What if I am a beneficial owner and do not give voting instructions
to my broker?
As a beneficial owner, to make sure your
shares are voted in the way you would like, you must provide voting instructions to your bank, broker or other nominee by the deadline
provided in the materials you receive from your bank, broker or other nominee.
If you do not provide voting instructions
to your bank, broker or other nominee, your shares cannot be voted on any “non-routine” matters, which is commonly
referred to as a “broker non-vote.”
Under current interpretations that govern
broker non-votes, Proposal Nos. 1 and 2 are considered non-routine matters, and a broker will lack the authority to vote uninstructed
shares at their discretion on such proposals. Proposal No. 3 is considered a routine matter, and a broker will be permitted to
exercise its discretion to vote uninstructed shares on the proposal.
What constitutes a quorum?
The presence by remote communication or represented
by proxy of the holders of a majority of the issued and outstanding shares of stock entitled to vote at the meeting shall constitute
a quorum for the transaction of business. Abstentions and broker non-votes will be counted as present and entitled to vote for
purposes of determining a quorum. A broker “non-vote” occurs when a nominee, such as a bank or broker, holding shares
for a beneficial owner, does not vote on a particular proposal because the nominee does not have discretionary voting power with
respect to that item and has not received instructions from the beneficial owner (see “What if I am a beneficial owner and
do not give voting instructions to my broker?” above).
How will votes be counted?
With respect to Proposal No. 1, to be elected,
a nominee for director must receive the affirmative vote of a majority of the votes cast with respect to such nominee by the holders
of the shares of common stock voting in person, by remote communication or by proxy at the annual meeting. Approval of each of
Proposal Nos. 2 and 3 requires the affirmative vote of a majority in voting power of the shares of common stock present in person,
by remote communication or by proxy at the annual meeting and entitled to vote on the subject matter. Abstentions will not affect
the outcome of the vote for Proposal No. 1 but will have the same effect as a vote “AGAINST” for Proposal Nos. 2 and
3. Broker non-votes will not affect the outcome of the vote for Proposal Nos. 1 through 3.
Who will count the votes?
A representative of American Election Services
LLC will tally the vote and serve as inspector of the annual meeting.
How are proxies being solicited and who will pay for the solicitation
of proxies?
We will bear the expense of the solicitation
of proxies. In addition to the solicitation of proxies by mail, solicitation may be made by our directors, officers and employees
by other means, including telephone, over the Internet or in person. No special compensation will be paid to our directors, officers
or employees for the solicitation of proxies. To solicit proxies, we will also request the assistance of brokerage houses, banks
and other custodians, nominees or fiduciaries, and, upon request, will reimburse such organizations or individuals for their reasonable
expenses in forwarding soliciting materials to beneficial owners and in obtaining authorization for the execution of proxies.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
71 |
| |
|
Additional Information
Stockholder Proposals for Inclusion in Our
2025 Annual Meeting Proxy Statement and Proxy Card
Under Exchange Act Rule 14a-8
(“Rule 14a-8”), any stockholder proposal to be considered by us for inclusion in our 2025 proxy statement and
form of proxy card for next year’s annual meeting of stockholders, expected to be held in May 2025, must be received by
our corporate secretary at our principal executive offices located at 400 Crossing Boulevard, Bridgewater, New Jersey 08807,
not later than November 22, 2024 and must otherwise comply with Rule 14a-8. While the Board will consider stockholder
proposals that we receive, we reserve the right to omit from our proxy statement stockholder proposals that do not satisfy
applicable SEC rules.
Director Nominations and Other Proposals to
Be Presented at Our 2025 Annual Meeting
In addition, our Bylaws establish advance
notice and other procedures with regard to stockholder nominations for director or any other stockholder proposals to be brought
before an annual meeting of stockholders that will not be included in our proxy statement. In general, notice must be received
by our corporate secretary not less than 90 days nor more than 120 days prior to the first anniversary of the preceding year’s
annual meeting of stockholders and must contain specified information concerning the matters to be brought before the meeting and
concerning the stockholder making the proposal. If no annual meeting was held in the previous year or if the annual meeting is
called for a date that is more than 30 calendar days earlier or more than 60 calendar days later than such anniversary date, notice
must be received not later than close of business on the 90th day prior, nor earlier than close of business on the 120th
day prior, to the date of such annual meeting or, if the first public disclosure of the date of such annual meeting is less
than 100 calendar days prior to the date of such annual meeting, the 10th calendar day following the day on which public
disclosure of the date of such annual meeting is first made by the Company. Therefore, to be presented at next year’s annual
meeting, director nominations and stockholder proposals that will not be included in our proxy statement must be received by our
corporate secretary at the address above on or after close of business on January 2, 2025 but not later than close of business
on February 1, 2025 and must contain the information specified in our Bylaws. A nomination or proposal also must comply with the
additional procedures set forth in our Bylaws.
In addition to satisfying the foregoing requirements
under our Bylaws, to comply with the universal proxy rules, stockholders who intend to solicit proxies in support of director nominees
other than the Company’s nominees must provide notice that sets forth the information required by Rule 14a-19 under the Exchange
Act no later than March 3, 2025.
Householding
Some brokers, banks and other nominee record
holders may be participating in the practice of “householding” proxy statements and annual reports or notices of Internet
availability of proxy materials, as applicable. This means that only one copy of such items may have been sent to multiple stockholders
in your household. We will promptly deliver, without charge, a separate copy of these documents to you if you so request by
writing or calling as follows: Amneal Pharmaceuticals, Inc., Attention: Corporate Secretary, 400 Crossing Boulevard, Bridgewater,
NJ 08807; telephone, (908) 947-3120. If you want to receive separate copies of the annual report and proxy statement or notice
of Internet availability of proxy materials, as applicable, in the future, or if you are receiving multiple copies and would like
to receive only one copy for your household, you should contact your broker, bank or other nominee record holder, or you may contact
us at the above address and phone number.
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
72 |
| |
|
Appendix A Non-GAAP
Financial Measures
Non-GAAP Financial Measures
This Proxy Statement includes certain non-GAAP
financial measures, including EBITDA, adjusted EBITDA, adjusted net income, adjusted diluted earnings per share and net leverage,
which are intended as supplemental measures of the Company’s performance that are not required by or presented in accordance
with GAAP. Management uses these non-GAAP historical measures internally to evaluate and manage the Company’s operations
and to better understand its business because they facilitate a comparative assessment of the Company’s operating performance
relative to its performance based on results calculated under GAAP. These non-GAAP measures also isolate the effects of some items
that vary from period to period without any correlation to core operating performance and eliminate certain charges that management
believes do not reflect the Company’s operations and underlying operational performance. The Compensation Committee of the
Company’s Board of Directors also uses certain of these measures to evaluate management’s performance and set its compensation.
The Company believes that these non-GAAP measures also provide useful information to investors regarding certain financial and
business trends relating to the Company’s financial condition and operating results. Providing this information therefore
allows investors to make independent assessments of the Company’s financial performance, results of operations and trends
while viewing the information through the eyes of management.
The calculation of non-GAAP adjusted diluted
earnings per share assumes the conversion of all outstanding shares of Class B common stock to shares of Class A common stock.
These non-GAAP measures are subject to limitations.
The non-GAAP measures presented herein may not be comparable to similarly titled measures used by other companies because other
companies may not calculate one or more in the same manner.
Additionally, the non-GAAP performance measures
exclude significant expenses and income that are required by GAAP to be recorded in the Company’s financial statements; do
not reflect changes in, or cash requirements for, working capital needs; and do not reflect interest expense, or the requirements
necessary to service interest or principal payments on debt. To compensate for these limitations, management presents and considers
these non-GAAP measures in conjunction with the Company’s GAAP results; no non-GAAP measure should be considered in isolation
from or as alternatives to net income, diluted earnings per share or any other measure determined in accordance with GAAP. Readers
should review the reconciliations included below, and should not rely on any single financial measure to evaluate the Company’s
business.
This Proxy Statement also includes certain
non-GAAP forward-looking information, such as net leverage. The Company cannot, however, provide a reconciliation between non-GAAP
targets and the most directly comparable GAAP measures without unreasonable efforts because it is unable to predict with reasonable
certainty the ultimate outcome of certain significant items required for the reconciliation. The items include, but are not limited
to, pandemic-related expenses, gains or losses related to changes in our tax receivable agreement liability, acquisition and site
closure expenses, restructuring and other charges, inventory-related charges, charges related to legal matters, gains and losses
on the sale of assets, impairment charges, and foreign exchange gains or losses. These items are uncertain, depend on various factors,
and could have a material impact on U.S. GAAP reported results.
A reconciliation of each non-GAAP measure
to the most directly comparable GAAP measure is set forth below.
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
73 |
| |
|
Reconciliation of Net Loss to Adjusted Net
Income and Calculation of Adjusted Diluted EPS
| (unaudited, in thousands, except EPS) | |
Year Ended
December 31, 2023 | | |
Year Ended
December 31, 2022 | |
| Net loss | |
$ | (48,722 | ) | |
$ | (254,789 | ) |
| Adjusted to add (deduct): | |
| | | |
| | |
| Non-cash interest | |
| 7,017 | | |
| 7,715 | |
| GAAP provision for income taxes | |
| 8,452 | | |
| 6,662 | |
| Amortization | |
| 157,219 | | |
| 164,997 | |
| Stock-based compensation expense | |
| 26,822 | | |
| 31,847 | |
| Acquisition, site closure expenses, and idle facility expenses(1) | |
| 7,017 | | |
| 15,682 | |
| Restructuring and other charges | |
| 1,650 | | |
| 1,378 | |
| Loss on refinancing | |
| 40,805 | | |
| 291 | |
| Charges related to legal matters, including interest, net(2) | |
| 14,784 | | |
| 273,226 | |
| Asset impairment charges(3) | |
| 70,015 | | |
| 26,843 | |
| Regulatory approval milestone | |
| - | | |
| 5,000 | |
| Change in fair value of contingent consideration | |
| (14,497 | ) | |
| 731 | |
| Insurance recoveries for property losses and associated expenses | |
| - | | |
| (1,911 | ) |
| Increase in tax receivable agreement liability | |
| 3,124 | | |
| 631 | |
| System implementation expense(4) | |
| 5,363 | | |
| 2,818 | |
| Reorganization expenses(5) | |
| 5,927 | | |
| 393 | |
| Other | |
| 2,466 | | |
| (2,235 | ) |
| Provision for income taxes(6) | |
| (60,014 | ) | |
| (56,450 | ) |
| Net income attributable to non-controlling interests not associated with our class B common stock | |
| (29,873 | ) | |
| (15,121 | ) |
| ADJUSTED NET INCOME (NON-GAAP) | |
$ | 197,555 | | |
$ | 207,708 | |
| Weighted average diluted shares outstanding (Non-GAAP)(7) | |
| 310,234 | | |
| 304,598 | |
| ADJUSTED DILUTED EARNINGS PER SHARE (NON-GAAP) | |
$ | 0.64 | | |
$ | 0.68 | |
| (1) |
Acquisition, site closure, and idle facility expenses for the year ended December
31, 2023 primarily included site closure costs associated with the planned cessation of manufacturing at our Hauppauge, NY
facility. Acquisition, site closure, and idle facility expenses for the year ended December 31, 2022 primarily included (i)
transaction and integration costs associated with the acquisition of the baclofen franchise from certain entities affiliated
with Saol International Limited, which closed on February 9, 2022; (ii) integration costs associated with the acquisition
of Puniska Healthcare Pvt. Ltd., which closed on November 2, 2021; and (iii) site closure costs associated with the planned
cessation of manufacturing at our Hauppauge, NY facility. |
| (2) |
For the year ended December 31, 2023, charges related to legal matters, net were primarily
comprised of (i) charges associated with civil prescription opioid litigation, (ii) a settlement of a customer claim, (iii)
a settlement of commercial antitrust litigation, and (iv) a settlement of a stockholder derivative lawsuit. For the year ended
December 31, 2022, charges related to legal matters, net, primarily included charges for (i) the settlements of the Opana
ER® antitrust litigation and (ii) prescription opioid litigation, offset in part by insurance recoveries associated
with class action shareholder lawsuits. |
| (3) |
Asset impairment charges for the year ended December 31, 2023 were primarily associated
with the write-offs of intangible assets. Asset impairment charges for the year ended December 31, 2022 were associated with
the write-offs of intangible assets and equipment. |
| (4) |
System implementation expense for the years ended December 31, 2023 and 2022 was primarily
for the implementation of indirect procurement software, sales deduction software, and financial statement consolidation software.
System implementation expenses were associated with the further integration of our acquired businesses. |
| (5) |
On November 7, 2023, the Company implemented a plan to reorganize and simplify its corporate
structure by eliminating its umbrella partnership-C-corporation structure and converting to a more traditional C-corporation
structure, whereby all stockholders hold their voting and economic interests directly through the public company (“Reorganization”).
For the years ended December 31, 2023 and 2022, Reorganization expenses were comprised of professional fees. |
| (6) |
The non-GAAP effective tax rates for the years ended December 31, 2023 and 2022 were 23.3%
and 21.4%, respectively. |
| (7) |
Weighted average diluted shares outstanding consisted of class A common stock and class
B common stock, as if all shares of class B common stock were converted to class A common stock as of January 1, 2022. |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
74 |
| |
|
Reconciliation of Net Loss to EBITDA and Adjusted
EBITDA
| (unaudited, in thousands) | |
Year Ended
December 31, 2023 | | |
Year Ended
December 31, 2022 | | |
Year Ended
December 31, 2019(1) | |
| Net loss | |
$ | (48,722 | ) | |
$ | (254,789 | ) | |
$ | (603,573 | ) |
| Adjusted to add: | |
| | | |
| | | |
| | |
| Interest expense, net | |
| 210,629 | | |
| 158,377 | | |
| 168,205 | |
| Provision for income taxes | |
| 8,452 | | |
| 6,662 | | |
| 383,331 | |
| Depreciation and amortization | |
| 229,400 | | |
| 240,175 | | |
| 207,235 | |
| EBITDA (Non-GAAP) | |
$ | 399,759 | | |
$ | 150,425 | | |
$ | 155,198 | |
| Adjusted to add (deduct): | |
| | | |
| | | |
| | |
| Stock-based compensation expense | |
| 26,822 | | |
| 31,847 | | |
| 21,679 | |
| Acquisition, site closure, and idle facility expenses(2) | |
| 7,017 | | |
| 15,682 | | |
| 73,471 | |
| Restructuring and other charges(3) | |
| 1,650 | | |
| 1,378 | | |
| 34,345 | |
| Loss on refinancing | |
| 40,805 | | |
| 291 | | |
| - | |
| Inventory related charges (4) | |
| - | | |
| - | | |
| 25,677 | |
| Charges related to legal matters, net(5) | |
| 11,824 | | |
| 269,930 | | |
| 12,591 | |
| Asset impairment charges(6) | |
| 70,107 | | |
| 26,909 | | |
| 175,210 | |
| Foreign exchange (gain) loss | |
| (1,671 | ) | |
| 12,364 | | |
| 4,962 | |
| Change in fair value of contingent consideration | |
| (14,497 | ) | |
| 731 | | |
| - | |
| Amortization of upfront payment(7) | |
| - | | |
| - | | |
| 36,393 | |
| Gain on sale of international businesses, net(8) | |
| - | | |
| - | | |
| (7,258 | ) |
| Insurance recoveries for property losses and associated expenses | |
| - | | |
| (1,911 | ) | |
| - | |
| Regulatory approval milestone | |
| - | | |
| 5,000 | | |
| - | |
| Increase (decrease) in tax receivable agreement liability(9) | |
| 3,124 | | |
| 631 | | |
| (192,884 | ) |
| System implementation expense(10) | |
| 5,363 | | |
| 2,818 | | |
| - | |
| Reorganization expenses(11) | |
| 5,927 | | |
| 393 | | |
| - | |
| Other | |
| 1,984 | | |
| (2,378 | ) | |
| (446 | ) |
| ADJUSTED EBITDA (NON-GAAP) | |
$ | 558,214 | | |
$ | 514,110 | | |
$ | 338,938 | |
| (1) |
Beginning in the first quarter of 2022, we no longer excluded research and
development milestone expenses related to license and collaboration agreements from our non-GAAP financial measures and our
line item components, including adjusted EBITDA. Adjusted results for the year ended December 31, 2019 have been revised to
reflect this change. |
| (2) |
Acquisition, site closure, and idle facility expenses for the year ended December 31, 2023
primarily included site closure costs associated with the planned cessation of manufacturing at our Hauppauge, NY facility.
Acquisition, site closure, and idle facility expenses for the year ended December 31, 2022 primarily included (i) transaction
and integration costs associated with the acquisition of the baclofen franchise from certain entities affiliated with Saol
International Limited, which closed on February 9, 2022; (ii) integration costs associated with the acquisition of Puniska
Healthcare Pvt. Ltd., which closed on November 2, 2021; and (iii) site closure costs associated with the planned cessation
of manufacturing at our Hauppauge, NY facility. Acquisition, site closure, and idle facility expenses for the year ended December
31, 2019 primarily included costs related to (i) plant closure and redundant employee costs and (ii) third party costs associated
with the combination with Impax Laboratories, Inc. ("Impax") and related integration including legal, investment
banking, accounting and information technology. |
| (3) |
For the year ended December 31, 2019, restructuring and other charges were primarily associated
with cash severance provided pursuant to our severance programs for employees at our Hauppauge, NY, Hayward, CA and other
facilities as well as asset-related charges associated with the impairment of property, plant and equipment and the right
of use asset associated with our Hauppauge, NY facility. |
| (4) |
For the year ended December 31, 2019, inventory related charges primarily represented inventory
obsolescence resulting from new initiatives and policies adopted with our restructuring efforts. |
| (5) |
For the year ended December 31, 2023, charges related to legal matters, net were primarily
comprised of (i) charges associated with civil prescription opioid litigation, (ii) a settlement of a customer claim, (iii)
a settlement of commercial antitrust litigation, and (iv) a settlement of a stockholder derivative lawsuit. For the year ended
December 31, 2022, charges related to legal matters, net, primarily included charges for (i) the settlements of the Opana
ER® antitrust litigation and (ii) prescription opioid litigation, offset in part by insurance recoveries associated with
class action shareholder lawsuits. For the year ended December 31, 2019, charges related to legal matters, net were primarily
associated with a settlement agreement with Teva Pharmaceuticals, Inc. regarding a matter associated with Impax prior to the
business combination. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
75 |
| |
|
| (6) |
Asset impairment charges for the year ended December 31, 2023 were primarily
associated with the write-offs of intangible assets. Asset impairment charges for the year ended December 31, 2022 were associated
with the write-offs of intangible assets and equipment. Asset impairment charges for the year ended December 31, 2019 were
primarily associated with the write-off of in process research and development and intangible asset impairment charges primarily
related to products acquired in the Impax business combination. |
| (7) |
Amortization of upfront payment for the year ended December 31, 2019 represented the amortization
of the upfront payment made to Lannett Company in connection with our transition agreement for Levothyroxine. |
| (8) |
For the year ended December 31, 2019 gain on sale of international business, net represented
the gain from the sale of our Creo Pharma Holding Limited subsidiary, which comprised substantially all of the Company's operations
in the United Kingdom, partially offset by the loss from the sale of our Amneal Deutschland GmbH subsidiary, which comprised
substantially all of the Company's operations in Germany. |
| (9) |
During the year ended December 31, 2019, we recorded a valuation allowance to reduce our
deferred tax assets (“DTAs”) to zero. In conjunction with the valuation allowance on our DTAs, we reversed the
accrued TRA liability, which resulted in a $193 million gain to our statement of operations. |
| (10) |
System implementation expense for the years ended December 31, 2023 and 2022 was primarily
for the implementation of indirect procurement software, sales deduction software, and financial statement consolidation software.
System implementation expenses were associated with the further integration of our acquired businesses. |
| (11) |
On November 7, 2023, the Company implemented a plan to reorganize and simplify its corporate
structure by eliminating its umbrella partnership-C-corporation structure and converting to a more traditional C-corporation
structure, whereby all stockholders hold their voting and economic interests directly through the public company (“Reorganization”).
For the years ended December 31, 2023 and 2022, Reorganization expenses were comprised of professional fees. |
| www.amneal.com |
|
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
76 |
| |
|
Calculation of Gross Leverage and Net Leverage
| | |
Year Ended December 31, |
| (unaudited, in thousands) | |
2023 | | |
2019 | |
| EBITDA | |
$ | 399,759 | | |
$ | 155,198 | |
| Adjusted EBITDA | |
$ | 558,214 | | |
$ | 338,938 | |
| (unaudited, in thousands) | |
As of
December 31, 2023 | | |
As of
December 31, 2019 | |
| Term Loan Due 2025(1) | |
$ | 191,979 | | |
$ | 2,658,876 | |
| Term Loan Due 2028(1) | |
| 2,351,647 | | |
| — | |
| Amended New Revolving Credit Facility(1) | |
| 179,000 | | |
| — | |
| Sellers Notes(1) | |
| 44,200 | | |
| — | |
| Other | |
| — | | |
| 624 | |
| Gross debt | |
$ | 2,766,826 | | |
$ | 2,659,500 | |
| Less: Cash and cash equivalents | |
| (91,542 | ) | |
| (151,197 | ) |
| NET DEBT | |
$ | 2,675,284 | | |
$ | 2,508,303 | |
| | |
Year Ended December 31, |
| (unaudited) | |
2023 | |
2019 |
| Gross leverage (Gross debt divided by Adjusted EBITDA) | |
5.0x | |
7.8x |
| Net leverage (Net debt divided
by Adjusted EBITDA) | |
4.8x | |
7.4x |
| (1) |
As defined in Note 16 in our 2023 Annual Report on Form 10-K. |
| |
AMNEAL
PHARMACEUTICALS, INC. | 2024 Proxy Statement |
|
77 |
| |
|



v3.24.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1
Pay vs Performance Disclosure - USD ($)
|
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
Dec. 31, 2020 |
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Pay vs Performance [Table Text Block] |
|
Pay Versus Performance Disclosure
In accordance with rules adopted by the
Securities and Exchange Commission pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, we are
providing the following disclosure regarding executive compensation for our principal executive officer (“PEO”)
and Non-PEO NEOs and Company performance for the fiscal years listed below. The Compensation Committee did not consider the
pay versus performance disclosure below in making its pay decisions for any of the years shown. The sum and/or computation of
individual numerical amounts disclosed in the following tables and related footnotes may not equal the total due to
rounding.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Value of Initial
Fixed $100
Investment
based on(4) |
|
|
|
| Year |
|
Summary
Compensation
Table Total for
Chirag Patel(1)
($) |
|
Summary
Compensation
Table Total for
Chintu Patel(1)
($) |
|
Compensation
Actually Paid
to Chirag
Patel(1)(2)(3)
($) |
|
Compensation
Actually Paid
to Chintu
Patel(1)(2)(3)
($) |
|
Average
Summary
Compensation
Table Total
for Non-PEO
NEOs(1)
($) |
|
Average
Compensation
Actually Paid
to Non-PEO
NEOs(1)(2)(3)
($) |
|
TSR
($) |
|
Peer
Group
TSR
($) |
|
Net
Income
(Loss)
($ Millions) |
|
Adjusted
EBITDA
($ Millions)(5) |
| (a) |
|
(b) |
|
(b) |
|
(c) |
|
(c) |
|
(d) |
|
(e) |
|
(f) |
|
(g) |
|
(h) |
|
(i) |
| 2023 |
|
2,370,386 |
|
2,367,553 |
|
9,458,710 |
|
9,455,877 |
|
1,679,146 |
|
5,801,457 |
|
125.93 |
|
124.97 |
|
(89) |
|
558 |
| 2022 |
|
5,048,110 |
|
5,062,732 |
|
(1,835,706) |
|
(1,821,083) |
|
2,348,670 |
|
(121,995) |
|
41.29 |
|
123.43 |
|
(255) |
|
514 |
| 2021 |
|
4,785,116 |
|
4,798,825 |
|
1,383,918 |
|
1,397,627 |
|
2,420,041 |
|
1,421,310 |
|
99.38 |
|
129.31 |
|
20 |
|
538 |
| 2020 |
|
2,320,716 |
|
2,328,528 |
|
3,422,575 |
|
3,430,387 |
|
1,922,858 |
|
1,985,608 |
|
94.81 |
|
114.32 |
|
69 |
|
433 |
| (1) |
Chirag Patel and Chintu Patel were our Co-PEOs for each year presented.
The individuals comprising the Non-PEO NEOs for each year presented are listed below. |
| 2020 |
2021 |
2022 |
2023 |
| Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
| Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
| Nikita Shah |
Nikita Shah |
Nikita Shah |
Nikita Shah |
| Joseph Todisco |
Joseph Todisco |
Jason Daly |
Jason Daly |
| Todd Branning |
|
|
|
| (2) |
The amounts shown for Compensation Actually Paid have been
calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually earned, realized, or
received by the Company’s NEOs. These amounts reflect the Summary Compensation Table Total with certain adjustments
as described in footnote 3 below. |
| (3) |
Compensation Actually Paid reflects the exclusions and inclusions of certain
amounts for the PEOs and the Non-PEO NEOs as set forth below. Equity values are calculated in accordance with FASB ASC Topic
718. Amounts in the Exclusion of Stock Awards column are the amounts from the Stock Awards column set forth in the Summary
Compensation Table. |
| (4) |
The Peer Group TSR set forth in this table utilizes the Dow
Jones U.S. Select Pharmaceuticals Index, which we also utilize in the stock performance graph required by Item 201(e) of Regulation
S-K, included in our Annual Report for the year ended December 31, 2023. The comparison assumes $100 was invested for the
period starting December 31, 2019 (the last trading day of fiscal 2019), through the end of the listed year in the Company
and in the Dow Jones U.S. Select Pharmaceuticals Index, respectively. Historical stock performance is not necessarily indicative
of future stock performance. |
| (5) |
We determined adjusted EBITDA to be the most important financial performance
measure used to link Company performance to Compensation Actually Paid to our PEOs and Non-PEO NEOs in 2023. We may determine
a different financial performance measure to be the most important financial performance measure in future years. Adjusted
EBITDA is not a term defined under U.S. GAAP. We define adjusted EBITDA as net income before net interest expense, income
taxes, and depreciation and amortization (“EBITDA”), as adjusted for certain other items described in our SEC
filings, including stock-based compensation expense, acquisition, site closure and idle facility expenses, restructuring and
other charges, net charges related to legal matters, asset impairment charges, foreign exchange losses or gains, change in
fair value of contingent consideration, and insurance recoveries for property losses and associated expenses. Prior to January
1, 2022, research and development milestone expenses related to license and collaboration agreements were excluded from our
calculation of adjusted EBITDA. The amounts shown in this column for the fiscal years ended December 31, 2021 and December
31, 2020 reflect this historical approach. Effective January 1, 2022, we no longer exclude research and development milestone
expenses related to license and collaboration agreements from adjusted EBITDA. The amounts shown in this column for the fiscal
year ended December 31, 2023 and December 31, 2022 reflect this modified approach; for prior periods, refer to our Form
8-K filed with the Securities and Exchange Commission on May 4, 2022 for a full reconciliation of previously reported non-GAAP
results, including adjusted EBITDA, to revised non-GAAP results. As a result of this modified approach, our reported adjusted
EBITDA for the fiscal years ended December 31, 2021 and December 31, 2020 was revised to $512 million and $433 million, respectively. |
| (1) |
Chirag Patel and Chintu Patel were our Co-PEOs for each year presented.
The individuals comprising the Non-PEO NEOs for each year presented are listed below. |
| 2020 |
2021 |
2022 |
2023 |
| Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
| Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
| Nikita Shah |
Nikita Shah |
Nikita Shah |
Nikita Shah |
| Joseph Todisco |
Joseph Todisco |
Jason Daly |
Jason Daly |
| Todd Branning |
|
|
|
| (2) |
The amounts shown for Compensation Actually Paid have been
calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually earned, realized, or
received by the Company’s NEOs. These amounts reflect the Summary Compensation Table Total with certain adjustments
as described in footnote 3 below. |
| (3) |
Compensation Actually Paid reflects the exclusions and inclusions of certain
amounts for the PEOs and the Non-PEO NEOs as set forth below. Equity values are calculated in accordance with FASB ASC Topic
718. Amounts in the Exclusion of Stock Awards column are the amounts from the Stock Awards column set forth in the Summary
Compensation Table. |
| Year |
|
Summary Compensation
Table Total for Chirag
Patel
($) |
|
Exclusion of Stock
Awards for Chirag Patel
($) |
|
Inclusion of Equity
Values for Chirag Patel
($) |
|
Compensation Actually
Paid to Chirag Patel
($) |
| 2023 |
|
2,370,386 |
|
(664,219) |
|
7,752,544 |
|
9,458,710 |
| |
|
|
|
|
|
|
|
|
| Year |
|
Summary Compensation
Table Total for Chintu
Patel
($) |
|
Exclusion of Stock
Awards for Chintu Patel
($) |
|
Inclusion of Equity
Values for Chintu Patel
($) |
|
Compensation Actually
Paid to Chintu Patel
($) |
| 2023 |
|
2,367,553 |
|
(664,219) |
|
7,752,544 |
|
9,455,877 |
| Year |
|
Average Summary
Compensation Table
Total for Non-PEO NEOs
($) |
|
Average Exclusion
of Stock Awards and
Option Awards for Non-
PEO NEOs
($) |
|
Average Inclusion of
Equity Values for Non-
PEO NEOs
($) |
|
Average Compensation
Actually Paid to Non-
PEO NEOs
($) |
| 2023 |
|
1,679,146 |
|
(712,920) |
|
4,835,231 |
|
5,801,457 |
The amounts in the Inclusion of Equity Values in the tables above
are derived from the amounts set forth in the following tables:
| Year |
|
Year-End
Fair Value
of Equity Awards
Granted During
Year That Remained
Unvested as of
Last Day of Year for
Chirag Patel
($) |
|
Change in
Fair
Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Chirag
Patel
($) |
|
Change in
Fair Value
from Last Day of
Prior Year to Vesting
Date of Unvested
Equity Awards that
Vested During Year
for Chirag Patel
($) |
|
Fair Value
at Last
Day of Prior Year
of Equity Awards
Forfeited During
Year for Chirag Patel
($) |
|
Total -
Inclusion of
Equity Values for
Chirag Patel
($) |
| 2023 |
|
3,526,601 |
|
4,225,943 |
|
— |
|
— |
|
7,752,544 |
| |
|
|
|
|
|
|
|
|
|
|
| Year |
|
Year-End Fair Value
of Equity Awards
Granted During
Year That Remained
Outstanding and
Unvested as of
Last Day of Year for
Chintu Patel
($) |
|
Change in Fair
Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Chintu
Patel
($) |
|
Change in Fair Value
from Last Day of
Prior Year to Vesting
Date of Unvested
Equity Awards that
Vested During Year
for Chintu Patel
($) |
|
Fair Value at Last
Day of Prior Year
of Equity Awards
Forfeited During
Year for Chintu Patel
($) |
|
Total - Inclusion of
Equity Values for
Chintu Patel
($) |
| 2023 |
|
3,526,601 |
|
4,225,943 |
|
— |
|
— |
|
7,752,544 |
| |
|
|
|
|
|
|
|
|
|
|
| Year |
|
Average Year-
End Fair Value of
Equity Awards
Granted During
Year That Remained
Outstanding and
Unvested as of Last
Day of Year for Non-
PEO NEOs
($) |
|
Average Change in
Fair Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Non-PEO
NEOs
($) |
|
Average Change in
Fair Value from Last
Day of Prior Year
to Vesting Date of
Unvested Equity
Awards that Vested
During Year for Non-
PEO NEOs
($) |
|
Average Fair Value
at Last Day of
Prior Year of Equity
Awards Forfeited
During Year for Non-
PEO NEOs
($) |
|
Total - Average
Inclusion of
Equity Values for
Non-PEO NEOs
($) |
| 2023 |
|
2,996,942 |
|
1,838,427 |
|
(137) |
|
— |
|
4,835,231 |
| (4) |
The Peer Group TSR set forth in this table utilizes the Dow
Jones U.S. Select Pharmaceuticals Index, which we also utilize in the stock performance graph required by Item 201(e) of Regulation
S-K, included in our Annual Report for the year ended December 31, 2023. The comparison assumes $100 was invested for the
period starting December 31, 2019 (the last trading day of fiscal 2019), through the end of the listed year in the Company
and in the Dow Jones U.S. Select Pharmaceuticals Index, respectively. Historical stock performance is not necessarily indicative
of future stock performance. |
| (5) |
We determined adjusted EBITDA to be the most important financial performance
measure used to link Company performance to Compensation Actually Paid to our PEOs and Non-PEO NEOs in 2023. We may determine
a different financial performance measure to be the most important financial performance measure in future years. Adjusted
EBITDA is not a term defined under U.S. GAAP. We define adjusted EBITDA as net income before net interest expense, income
taxes, and depreciation and amortization (“EBITDA”), as adjusted for certain other items described in our SEC
filings, including stock-based compensation expense, acquisition, site closure and idle facility expenses, restructuring and
other charges, net charges related to legal matters, asset impairment charges, foreign exchange losses or gains, change in
fair value of contingent consideration, and insurance recoveries for property losses and associated expenses. Prior to January
1, 2022, research and development milestone expenses related to license and collaboration agreements were excluded from our
calculation of adjusted EBITDA. The amounts shown in this column for the fiscal years ended December 31, 2021 and December
31, 2020 reflect this historical approach. Effective January 1, 2022, we no longer exclude research and development milestone
expenses related to license and collaboration agreements from adjusted EBITDA. The amounts shown in this column for the fiscal
year ended December 31, 2023 and December 31, 2022 reflect this modified approach; for prior periods, refer to our Form
8-K filed with the Securities and Exchange Commission on May 4, 2022 for a full reconciliation of previously reported non-GAAP
results, including adjusted EBITDA, to revised non-GAAP results. As a result of this modified approach, our reported adjusted
EBITDA for the fiscal years ended December 31, 2021 and December 31, 2020 was revised to $512 million and $433 million, respectively. |
|
|
|
|
| Company Selected Measure Name |
|
adjusted EBITDA
|
|
|
|
| Named Executive Officers, Footnote [Text Block] |
|
| (1) |
Chirag Patel and Chintu Patel were our Co-PEOs for each year presented.
The individuals comprising the Non-PEO NEOs for each year presented are listed below. |
| 2020 |
2021 |
2022 |
2023 |
| Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
Andrew Boyer |
| Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
Anastasios Konidaris |
| Nikita Shah |
Nikita Shah |
Nikita Shah |
Nikita Shah |
| Joseph Todisco |
Joseph Todisco |
Jason Daly |
Jason Daly |
| Todd Branning |
|
|
|
|
|
|
|
| Peer Group Issuers, Footnote [Text Block] |
|
The Peer Group TSR set forth in this table utilizes the Dow
Jones U.S. Select Pharmaceuticals Index, which we also utilize in the stock performance graph required by Item 201(e) of Regulation
S-K, included in our Annual Report for the year ended December 31, 2023. The comparison assumes $100 was invested for the
period starting December 31, 2019 (the last trading day of fiscal 2019), through the end of the listed year in the Company
and in the Dow Jones U.S. Select Pharmaceuticals Index, respectively. Historical stock performance is not necessarily indicative
of future stock performance.
|
|
|
|
| Adjustment To PEO Compensation, Footnote [Text Block] |
|
| Year |
|
Summary Compensation
Table Total for Chirag
Patel
($) |
|
Exclusion of Stock
Awards for Chirag Patel
($) |
|
Inclusion of Equity
Values for Chirag Patel
($) |
|
Compensation Actually
Paid to Chirag Patel
($) |
| 2023 |
|
2,370,386 |
|
(664,219) |
|
7,752,544 |
|
9,458,710 |
| |
|
|
|
|
|
|
|
|
| Year |
|
Summary Compensation
Table Total for Chintu
Patel
($) |
|
Exclusion of Stock
Awards for Chintu Patel
($) |
|
Inclusion of Equity
Values for Chintu Patel
($) |
|
Compensation Actually
Paid to Chintu Patel
($) |
| 2023 |
|
2,367,553 |
|
(664,219) |
|
7,752,544 |
|
9,455,877 |
| Year |
|
Year-End
Fair Value
of Equity Awards
Granted During
Year That Remained
Unvested as of
Last Day of Year for
Chirag Patel
($) |
|
Change in
Fair
Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Chirag
Patel
($) |
|
Change in
Fair Value
from Last Day of
Prior Year to Vesting
Date of Unvested
Equity Awards that
Vested During Year
for Chirag Patel
($) |
|
Fair Value
at Last
Day of Prior Year
of Equity Awards
Forfeited During
Year for Chirag Patel
($) |
|
Total -
Inclusion of
Equity Values for
Chirag Patel
($) |
| 2023 |
|
3,526,601 |
|
4,225,943 |
|
— |
|
— |
|
7,752,544 |
| |
|
|
|
|
|
|
|
|
|
|
| Year |
|
Year-End Fair Value
of Equity Awards
Granted During
Year That Remained
Outstanding and
Unvested as of
Last Day of Year for
Chintu Patel
($) |
|
Change in Fair
Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Chintu
Patel
($) |
|
Change in Fair Value
from Last Day of
Prior Year to Vesting
Date of Unvested
Equity Awards that
Vested During Year
for Chintu Patel
($) |
|
Fair Value at Last
Day of Prior Year
of Equity Awards
Forfeited During
Year for Chintu Patel
($) |
|
Total - Inclusion of
Equity Values for
Chintu Patel
($) |
| 2023 |
|
3,526,601 |
|
4,225,943 |
|
— |
|
— |
|
7,752,544 |
|
|
|
|
| Non-PEO NEO Average Total Compensation Amount |
[1] |
$ 1,679,146
|
$ 2,348,670
|
$ 2,420,041
|
$ 1,922,858
|
| Non-PEO NEO Average Compensation Actually Paid Amount |
[1],[2],[3] |
$ 5,801,457
|
(121,995)
|
1,421,310
|
1,985,608
|
| Adjustment to Non-PEO NEO Compensation Footnote [Text Block] |
|
| Year |
|
Average Summary
Compensation Table
Total for Non-PEO NEOs
($) |
|
Average Exclusion
of Stock Awards and
Option Awards for Non-
PEO NEOs
($) |
|
Average Inclusion of
Equity Values for Non-
PEO NEOs
($) |
|
Average Compensation
Actually Paid to Non-
PEO NEOs
($) |
| 2023 |
|
1,679,146 |
|
(712,920) |
|
4,835,231 |
|
5,801,457 |
| |
|
|
|
|
|
|
|
|
|
|
| Year |
|
Average Year-
End Fair Value of
Equity Awards
Granted During
Year That Remained
Outstanding and
Unvested as of Last
Day of Year for Non-
PEO NEOs
($) |
|
Average Change in
Fair Value from Last
Day of Prior Year
to Last Day of Year
of Unvested Equity
Awards for Non-PEO
NEOs
($) |
|
Average Change in
Fair Value from Last
Day of Prior Year
to Vesting Date of
Unvested Equity
Awards that Vested
During Year for Non-
PEO NEOs
($) |
|
Average Fair Value
at Last Day of
Prior Year of Equity
Awards Forfeited
During Year for Non-
PEO NEOs
($) |
|
Total - Average
Inclusion of
Equity Values for
Non-PEO NEOs
($) |
| 2023 |
|
2,996,942 |
|
1,838,427 |
|
(137) |
|
— |
|
4,835,231 |
|
|
|
|
| Compensation Actually Paid vs. Total Shareholder Return [Text Block] |
|
The following chart sets forth the relationship
between Compensation Actually Paid to our PEOs, the average of Compensation Actually Paid to our Non-PEO NEOs, and the Company’s
cumulative TSR over the four most recently completed fiscal years.

|
|
|
|
| Compensation Actually Paid vs. Net Income [Text Block] |
|
The following chart sets forth the relationship
between Compensation Actually Paid to our PEOs, the average of Compensation Actually Paid to our Non-PEO NEOs, and our net income
during the four most recently completed fiscal years.

|
|
|
|
| Compensation Actually Paid vs. Company Selected Measure [Text Block] |
|
The following chart sets forth the relationship
between Compensation Actually Paid to our PEO, the average of Compensation Actually Paid to our Non-PEO NEOs, and our Adjusted
EBITDA during the four most recently completed fiscal years.

|
|
|
|
| Total Shareholder Return Vs Peer Group [Text Block] |
|
The following chart sets forth the relationship
between Compensation Actually Paid to our PEOs, the average of Compensation Actually Paid to our Non-PEO NEOs, and the Company’s
cumulative TSR over the four most recently completed fiscal years.

|
|
|
|
| Tabular List [Table Text Block] |
|
Tabular List of Most Important Financial Performance
Measures
The following table presents the financial performance
measures that the Company considers to have been the most important in linking Compensation Actually Paid to our PEOs and other
NEOs for 2023 to Company performance. The measures in this table are not ranked.
Adjusted EBITDA
Stock Price |
| |
| # # # |
|
|
|
|
| Total Shareholder Return Amount |
[4] |
$ 125.93
|
41.29
|
99.38
|
94.81
|
| Peer Group Total Shareholder Return Amount |
[4] |
124.97
|
123.43
|
129.31
|
114.32
|
| Net Income (Loss) Attributable to Parent |
|
$ (89,000,000)
|
$ (255,000,000)
|
$ 20,000,000
|
$ 69,000,000
|
| Company Selected Measure Amount |
[5] |
558,000,000
|
514,000,000
|
538,000,000
|
433,000,000
|
| Measure [Axis]: 1 |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Measure Name |
|
Adjusted EBITDA
|
|
|
|
| Measure [Axis]: 2 |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Measure Name |
|
Stock Price
|
|
|
|
| Non-PEO NEO [Member] | Average Exclusion of Stock Awards and Option Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
$ (712,920)
|
|
|
|
| Non-PEO NEO [Member] | Average Inclusion of Equity Values |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
4,835,231
|
|
|
|
| Non-PEO NEO [Member] | Average Year- End Fair Value of Equity Awards Granted During Year That Remained Outstanding and Unvested as of Last Day of Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
2,996,942
|
|
|
|
| Non-PEO NEO [Member] | Average Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
1,838,427
|
|
|
|
| Non-PEO NEO [Member] | Average Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
(137)
|
|
|
|
| Non-PEO NEO [Member] | Average Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
|
|
|
|
| Chirag Patel |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| PEO Total Compensation Amount |
[1] |
2,370,386
|
$ 5,048,110
|
$ 4,785,116
|
$ 2,320,716
|
| PEO Actually Paid Compensation Amount |
[1],[2],[3] |
$ 9,458,710
|
$ (1,835,706)
|
$ 1,383,918
|
$ 3,422,575
|
| PEO Name |
|
Chirag Patel
|
Chirag Patel
|
Chirag Patel
|
Chirag Patel
|
| Chirag Patel | PEO [Member] | Exclusion of Stock Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
$ (664,219)
|
|
|
|
| Chirag Patel | PEO [Member] | Inclusion of Equity Values |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
7,752,544
|
|
|
|
| Chirag Patel | PEO [Member] | Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
3,526,601
|
|
|
|
| Chirag Patel | PEO [Member] | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
4,225,943
|
|
|
|
| Chirag Patel | PEO [Member] | Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
|
|
|
|
| Chirag Patel | PEO [Member] | Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
|
|
|
|
| Chintu Patel |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| PEO Total Compensation Amount |
[1] |
2,367,553
|
$ 5,062,732
|
$ 4,798,825
|
$ 2,328,528
|
| PEO Actually Paid Compensation Amount |
[1],[2],[3] |
$ 9,455,877
|
$ (1,821,083)
|
$ 1,397,627
|
$ 3,430,387
|
| PEO Name |
|
Chintu Patel
|
Chintu Patel
|
Chintu Patel
|
Chintu Patel
|
| Chintu Patel | PEO [Member] | Exclusion of Stock Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
$ (664,219)
|
|
|
|
| Chintu Patel | PEO [Member] | Inclusion of Equity Values |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
7,752,544
|
|
|
|
| Chintu Patel | PEO [Member] | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
4,225,943
|
|
|
|
| Chintu Patel | PEO [Member] | Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
|
|
|
|
| Chintu Patel | PEO [Member] | Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
|
|
|
|
| Chintu Patel | PEO [Member] | Year-End Fair Value of Equity Awards Granted During Year That Remained Outstanding and Unvested as of Last Day of Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
$ 3,526,601
|
|
|
|
|
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_AdjToCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_AdjToNonPeoNeoCompFnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_AdjToPeoCompFnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph vi
| Name: |
ecd_CoSelectedMeasureAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:decimalItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph vi
| Name: |
ecd_CoSelectedMeasureName |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph iii
| Name: |
ecd_CompActuallyPaidVsCoSelectedMeasureTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph ii
| Name: |
ecd_CompActuallyPaidVsNetIncomeTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph i
| Name: |
ecd_CompActuallyPaidVsTotalShareholderRtnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph vi
| Name: |
ecd_MeasureName |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_NamedExecutiveOfficersFnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iii
| Name: |
ecd_NonPeoNeoAvgCompActuallyPaidAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph ii
| Name: |
ecd_NonPeoNeoAvgTotalCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iv
| Name: |
ecd_PeerGroupIssuersFnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iv
| Name: |
ecd_PeerGroupTotalShareholderRtnAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iii
| Name: |
ecd_PeoActuallyPaidCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_PeoName |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph ii
| Name: |
ecd_PeoTotalCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTableTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 6
| Name: |
ecd_TabularListTableTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iv
| Name: |
ecd_TotalShareholderRtnAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph iv
| Name: |
ecd_TotalShareholderRtnVsPeerGroupTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ecd_MeasureAxis=1 |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_MeasureAxis=2 |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_ExecutiveCategoryAxis=ecd_NonPeoNeoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_AverageExclusionOfStockAwardsAndOptionAwardsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_AverageInclusionOfEquityValuesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_AverageYearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedOutstandingAndUnvestedAsOfLastDayOfYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_AverageChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_AverageChangeInFairValueFromLastDayOfPriorYearToVestingDateOfUnvestedEquityAwardsThatVestedDuringYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_AverageFairValueAtLastDayOfPriorYearOfEquityAwardsForfeitedDuringYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_IndividualAxis=amrx_ChiragPatelMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_ExecutiveCategoryAxis=ecd_PeoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_ExclusionOfStockAwardsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_InclusionOfEquityValuesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_ChangeInFairValueFromLastDayOfPriorYearToVestingDateOfUnvestedEquityAwardsThatVestedDuringYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_FairValueAtLastDayOfPriorYearOfEquityAwardsForfeitedDuringYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_IndividualAxis=amrx_ChintuPatelMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=amrx_YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedOutstandingAndUnvestedAsOfLastDayOfYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Amneal Pharmaceuticals (NYSE:AMRX)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
Amneal Pharmaceuticals (NYSE:AMRX)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024