MedinCell’s Partner Teva Announces Successful Launch of UZEDY™
02 Août 2023 - 10:49PM
Business Wire
MedinCell (Paris:MEDCL):
Access here the complete press release
During today’s Q2 earnings call, Richard Francis, President and
CEO of Teva, and Eric Hughes, Executive Vice President, Global
R&D & Chief Medical Officer, commented the US launch of
UZEDY since May 2023 and the olanzapine Long-Acting Injectable
(LAI).
About UZEDY
Richard Francis said1:
“We are actually right on plan or slightly ahead of our market
access strategy targets and we are very happy with the launch.”
“The newest member of our innovative family is said risperidone,
our long-acting treatment for schizophrenia. Now, to remind
everybody, this is a $4 billion market and we've only just launched
UZEDY, but we're very pleased with the feedback we're getting from
healthcare professionals. And they're confirming that the product
profile that we have is unique and advantageous. Now we're seeing
this in the fact that our NBRX2 is 40%, so already we're getting
40% of the risperidone long-acting market. We're also seeing
hospitals look to use our free samples and free trial requests, and
we're having good discussions with our payers. So once again, I
think excitement around UZEDY early days, but initial feedback is
very positive.”
UZEDY, a long-acting injectable (LAI) risperidone for the
treatment of schizophrenia in adults, is the first FDA-approved
product based on MedinCell’s BEPO technology. MedinCell is eligible
for up to $105m commercial milestones and for royalties on net
sales.
About LAI olanzapine (mdc-TJK), initiated in January
2023
Eric Hughes said1:
“Our Olanzapine Phase 3 study is actually enrolling very
quickly.”
“Olanzapine as an oral agent account for 20% of the patients
being treated today, but only less than 1% of patients on the
long-acting form are being using that product. And that's primarily
because of the safety profile3.”
Richard Francis, said1:
“With olanzapine, I've already highlighted the fact that it's a
$4 billion market. But if we do manage to bring this to the market
with a favorable safety profile, I think we have a real opportunity
to have a significant product on our hands here.”
mdc-TJK is an investigational long-acting injectable olanzapine
also based on BEPO technology. If approved, it could be the first
olanzapine LAI with a favorable safety profile offering a valued
treatment option as a complement to UZEDY for severe schizophrenia
patients. MedinCell is eligible for $12m left out of $17m of
development milestones, for up to $105m commercial milestones and
for royalties on net sales.
Other programs
Teva and MedinCell also announce the initiation of preliminary
formulation activities for a new program in an undisclosed
indication, along with the decision to terminate the mdc-ANG
program at preclinical stage for strategic reasons.
1 Extracts from the Teva’s Q2 2023 earnings call, August 2,
2023. Full webcast, transcript and presentation are available on
ir.tevapharm.com 2 NBRx = new-to-brand prescriptions: new
prescriptions, as in the first time a patient is being prescribed a
particular drug 3 The only existing LAI of Olanzapine has a FDA
black box warning from for PDSS (Post injection Delirium/Sedation
Syndrome) that limits its use
View source
version on businesswire.com: https://www.businesswire.com/news/home/20230802242735/en/
MedinCell David Heuzé Head of Communications
david.heuze@medincell.com +33 (0)6 83 25 21 86
NewCap Louis-Victor Delouvrier/Alban Dufumier Investor
Relations medincell@newcap.eu +33 (0)1 44 71 94 94
NewCap Nicolas Merigeau Media Relations
medincell@newcap.eu +33 (0)1 44 71 94 94
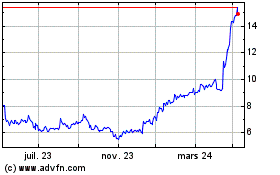
Medincell (EU:MEDCL)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
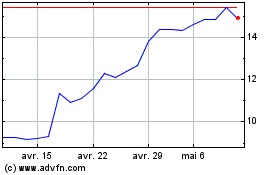
Medincell (EU:MEDCL)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024