--12-310001800315false0001800315srt:ProFormaMember2024-06-3000018003152024-01-012024-06-3000018003152024-10-072024-10-070001800315srt:ProFormaMember2024-01-012024-06-3000018003152023-01-012023-12-310001800315us-gaap:ScenarioAdjustmentMember2024-06-300001800315us-gaap:ScenarioAdjustmentMember2023-01-012023-12-310001800315us-gaap:ScenarioAdjustmentMember2024-01-012024-06-300001800315srt:ProFormaMember2023-01-012023-12-3100018003152024-06-30xbrli:purexbrli:sharesiso4217:USD
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 7, 2024
GALECTO, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
Delaware |
|
001-39655 |
|
37-1957007 |
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
75 State Street, Suite 100
Boston, MA 02109
(Address of principal executive offices, including zip code)
(+45) 70 70 52 10
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trade Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, $0.00001 par value per share |
|
GLTO |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01 Entry into a Material Definitive Agreement.
Asset Purchase Agreement
On October 7, 2024, Galecto, Inc. (the “Company”) and Bridge Medicines LLC, a Delaware limited liability company (“Bridge Medicines”), entered into an Asset Purchase Agreement (the “Purchase Agreement”) pursuant to which the Company acquired global rights to Bridge Medicines’ BRM-1420 program, a novel dual ENL-YEATS and FLT3 inhibitor for multiple genetic subsets of acute myeloid leukemia (AML), and assumed certain of Bridge Medicines’ liabilities associated with the acquired assets (the “Asset Purchase”). Pursuant to the Purchase Agreement, at the closing of the Asset Purchase (the “Closing”), as consideration to Bridge Medicines for the Asset Purchase, the Company (a) issued to Bridge Medicines (i) 62,594 shares (the “Common Stock Payment Shares”) of the Company’s common stock, par value $0.00001 per share (the “Common Stock”), and (ii) 160.562 shares (the “Preferred Stock Payment Shares” and together with the Common Stock Payment Shares and the Common Stock Payment Shares issuable upon conversion of the Preferred Stock Payment Shares, the “Payment Shares”) of the Company’s newly designated Series A non-voting convertible preferred stock, par value $0.00001 per share (the “Preferred Stock”) and (b) assumed specified liabilities. Closing of the Asset Purchase occurred on October 7, 2024.
The terms of the Preferred Stock are as set forth in the Certificate of Designation of Preferences, Rights and Limitations of Series A Non-Voting Convertible Preferred Stock, attached as Exhibit 3.1 to this Current Report on Form 8-K (the “Certificate of Designation”), filed with the Secretary of State of the State of Delaware on October 7, 2024. Each share of Preferred Stock is convertible into 1,000 shares of Common Stock at the election of the holder of such Preferred Stock, subject to, and contingent upon, the approval by the Company’s stockholders to approve, for purposes of the Nasdaq Stock Market Rules, the issuance of the Company’s Common Stock upon conversion of the Preferred Stock Payment Shares (the “Stockholder Approval”). Furthermore, on the third business day following the Company’s receipt of Stockholder Approval, each outstanding share of Preferred Stock shall, subject to certain beneficial ownership limitations, automatically convert into 1,000 shares of Common Stock upon the conversion terms set forth in the Certificate of Designation. Except as required by law, the Preferred Stock shall have no voting rights, provided that the Company shall not, without the affirmative vote or written consent of the holders of majority of then outstanding Preferred Stock, among other things, alter or change adversely the power, preferences or rights given to the Preferred Stock, amend the Certificate of Designation, issue additional shares of Preferred Stock, consummate certain transactions prior to Stockholder Approval, amend or terminate the Support Agreements (as defined below) or amend or fail to comply with certain provisions of the Purchase Agreement.
The Purchase Agreement also provides that until the twelve-month anniversary of the Closing, Bridge Medicines will hold and not sell any of the Payment Shares, subject to certain exceptions.
The Purchase Agreement contains customary representations, warranties, conditions and covenants.
Carl Goldfischer, Chairman of the board of directors (the “Board”) of the Company is also the Executive Chairman of Bridge Medicines.
Company Support Agreements
Concurrently with the execution of the Purchase Agreement, the executive officers, directors and certain equityholders of the Company, who collectively are the record or beneficial holders of 3.5% of the shares of Common Stock of the Company, entered into stockholder support agreements (the “Support Agreements”), providing among other things, that such officers, directors and stockholders will, among other things, vote in favor of the Buyer Stockholder Matters (as defined in the Purchase Agreement).
The Certificate of Designation, the Purchase Agreement and the form of Support Agreements have been included as exhibits hereto solely to provide investors with information regarding their terms. Neither is intended to be a source of financial, business or operational information about the Company or Bridge Medicines. The representations, warranties and covenants contained in the Purchase Agreement and Support Agreements were made only for the purposes of the Purchase Agreement and Support Agreements as of the dates specified therein and solely for the benefit of the parties thereto. In addition, the representations, warranties and covenants contained in the Purchase Agreement and Support Agreements may be subject to qualifications and limitations agreed upon by the parties in connection with negotiating the terms thereof, including Bridge Medicines’ representations, warranties and covenants in the Purchase Agreement being qualified by disclosure schedules made for the purpose of allocating contractual risk amongst the parties as opposed to establishing such matters as facts, and may further be subject to certain standards of materiality applicable to the parties that differ from those applicable to investors. As a result, investors should not rely on the representations, warranties and covenants included in the Purchase Agreement or the Support Agreements, or any descriptions thereof, as characterizations of the actual state of facts or condition of the Company or Bridge Medicines and each of their respective businesses. Moreover, information concerning the subject matter of the representations and warranties may change after the date of the Purchase Agreement and Support Agreements, which subsequent information may or may not be fully reflected in public disclosures.
The foregoing description of the terms of the Purchase Agreement, Certificate of Designations, and Support Agreements are not complete and are qualified in their entirety by reference to the Purchase Agreement and form of Support Agreement, copies of which are filed as Exhibit 2.1, Exhibit 3.1 and Exhibit 10.1 to this Current Report on Form 8-K and incorporated herein by reference.
Rockefeller University License Agreement
In accordance with the Purchase Agreement, the Company will acquire and assume Bridge Medicines’ rights in and obligations under a Bridge Medicines License Agreement, by and between Bridge Medicines and The Rockefeller University (“Rockefeller”), dated February 3, 2020 (the “License Agreement”).
Pursuant to the License Agreement, the Licensee (being Bridge Medicines before the assignment of its rights and interest in the License Agreement to the Company pursuant to the Purchase Agreement, and the Company after such assignment) has, subject to standard terms and conditions, an exclusive, worldwide, sublicensable, license to certain patent rights and a non-exclusive worldwide, sublicensable, license to certain know-how, materials, tools, techniques, or instruments related to Bridge Medicines’ ENL-YEATS program that are controlled by Rockefeller to use and commercially exploit products, processes, or services for the prevention, treatment, prognosis and/or diagnosis of conditions and diseases in humans (the “Licensed Products”). The License Agreement also contains a development plan which outlines a preclinical, clinical, and commercial strategy for the development of Licensed Products and governs the ownership and license of improvements to the licensed rights generated pursuant to any sponsored research agreements between the Licensee and Rockefeller. Rockefeller is a double-digit percentage holder of the Bridge Medicines equity rights associated with the Licensed Products.
Pursuant to the License Agreement, the Licensee shall pay Rockefeller, on a quarterly basis, royalties on net sales of Licensed Products by the Licensee, its affiliates, and its sublicensees, which are based on the amount of net sales of such Licensed Products during the calendar year in which the relevant quarterly period ends. Such royalties range from low single-digit percentages to mid-single-digit percentages and are subject to standard deductions and royalty anti-stacking provisions. The obligation to pay royalties shall expire on a Licensed Product-by-Licensed Product basis and country-by-country basis until the later of the expiration of licensed patents covering a Licensed Product in such country, the expiration of any market exclusivity period for such Licensed Product in such country, and 15 years from the first commercial sale of such Licensed Product in such country (the “Royalty Term”). In addition, the Licensee, in the event that a priority review voucher is issued by a governmental authority to the Licensee or its affiliates in connection with and in consideration of the development of a Licensed Product for the treatment of a specific type of cancer, and the Licensee sells such voucher to a third party, the Licensee shall pay Rockefeller a low double-digit percentage of the proceeds of such sale.
The License Agreement shall remain in effect on a country-by-country and Licensed Product-by-Licensed Product basis until the expiration of the Royalty Term for such Licensed Product in such country. Upon the expiration of the Royalty Term for a Licensed Product in a country, the Licensee shall be granted a completely paid-up, royalty-free license in respect of such Licensed Product in such country. The Licensee may terminate the License Agreement on 30 days’ notice to Rockefeller, and the License Agreement also contains other standard termination rights for material breach, bankruptcy, and patent challenge.
The foregoing description of the License Agreement does not purport to be complete and is subject to, and qualified in its entirety by reference to the full text of the License Agreement, a copy of which shall be filed with the Company’s annual report on Form 10-K for the period ending December 31, 2024.
Item 2.01 Completion of Acquisition or Disposition of Assets
See the description set forth under Item 1.01 about with respect to the Closing of the Asset Purchase, which is incorporated herein by reference.
The Company has determined that the Asset Purchase does not constitute the acquisition of a business as defined by Regulation S-X Rule 11-01(d). The unaudited pro forma condensed consolidated financial statements of the Company giving effect to the Asset Purchase are filed herewith as Exhibit 99.1.
Item 3.02 Unregistered Sales of Equity Securities
See the description set forth under Item 1.01 above with respect to the issuance of the Payment Shares, which is incorporated into this Item 3.02 by reference. At the Closing, the Payment Shares were issued to Bridge Medicines pursuant to the exemption from the registration requirements provided in Section 4(a)(2) of the Securities Act of 1933, as amended (the "Securities Act") for transactions by an issuer not involving any public offering. Accordingly, the Payment Shares were not registered under the Securities Act and may not be offered or sold in the United States except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act.
Item 5.02 Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
In connection with the Closing of the Asset Purchase, based on the recommendations of the Compensation Committee of the Board, the Board approved certain compensation-related matters effective as of the Closing of the Asset Purchase, for Hans Schambye, Ph.D., M.D., President and Chief Executive Officer of the Company, and Garrett Winslow, General Counsel of the Company, as described below.
The Board approved an increase in Mr. Winslow’s annual bonus target from 30% to 40%, applicable beginning with Mr. Winslow’s annual bonus with respect to 2024 performance. The Board also approved an acceleration of the retention bonus provided under Mr. Winslow’s Retention Bonus Agreement, dated October 19, 2023, based on his contributions to the Company during its strategic alternative process and the Asset Purchase, resulting in the payment of $115,500 to Mr. Winslow effective as of the Closing of the Asset Purchase.
In addition, while the Board determined that the performance criteria for payment of a bonus to Dr. Schambye identified in the Bonus Agreement, dated September 26, 2023, between Dr. Schambye and the Company (the “Bonus Agreement”) had not been achieved, the Board determined, based on changes in the Company’s strategic initiatives that occurred following the signing of the Bonus Agreement and Dr. Schambye’s contributions to the Company during its strategic alternative review process and the Asset Purchase, to pay Dr. Schambye a one-time bonus of 1,332,675 DKK effective as of the Closing of the Asset Purchase.
The Board also approved retention agreements for all employees that will be remaining with the Company following the Closing of the Asset Purchase, including Dr. Schambye and Mr. Winslow. Pursuant to the terms of Dr. Schambye’s and Mr. Winslow’s retention agreements, each executive shall be entitled to a cash bonus, separate from any annual bonus for 2024 or 2025, equal to 100% of his target bonus upon the earlier of (i) December 31, 2025, provided he remains employed by the Company through such date, (ii) a Sale Event (as defined in the Company’s Executive Separation Benefits Plan that is filed as Exhibit 10.4 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 (the “Separation Benefits Plan”)) provided he remains employed by the Company through such date, or (iii) his termination without Cause (as defined in the Separation Benefits Plan). Dr. Schambye’s target bonus is equal to 2,173,800 DKK and Mr. Winslow’s target bonus is equal to $160,160.
The foregoing summaries of the agreements for Dr. Schambye and Mr. Winslow do not purport to be complete, and are subject to, and qualified in their entirety by, the forms of such documents, copies of which shall be filed with the Company’s annual report on Form 10-K for the period ending December 31, 2024.
Item 5.03 Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year.
See the description set forth under Item 1.01 above with respect to the Certificate of Designation and the description of the Preferred Stock, which is incorporated into this Item 5.03 by reference.
Item 7.01 Regulation FD Disclosure.
On October 7, 2024, the Company issued a press release announcing the entry into the Purchase Agreement described by Item 1.01 above. A copy of the press release is attached as Exhibit 99.2 to this Current Report on Form 8-K.
Included as Exhibit 99.3 to this Current Report on Form 8-K is the Company’s corporate presentation, dated October 2024, which is incorporated herein by reference. The Company intends to utilize this presentation and its contents in various meetings with securities analysts, investors and others commencing on October 7, 2024.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.2 and 99.3, shall not be incorporated by reference into any filing of the Company, whether made before, on or after the date hereof, regardless of any general incorporation language in such filing, unless expressly incorporated by specific reference to such filing. The information contained in Item 7.01 of this Current Report on Form 8-K Report, including Exhibits 99.2 and 99.3, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section.
Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking statements” which include, but are not limited to, all statements that do not relate solely to historical or current facts, such as statements regarding the Company’s expectations, intentions or strategies regarding the future, or the effects of the Asset Purchase. In some cases, these statements include words like: “may,” “could,” “potential,” “will,” “plan,” “believe,” “goal,” “optimistic,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. The Company’s expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks, and changes in circumstances, including but not limited to risks and uncertainties related to: the contemplated benefits to the Company from the Asset Purchase; potential litigation relating to the transaction that could be instituted against the Company, Bridge Medicines or their respective directors; possible disruptions from the proposed transaction that could harm the Company’s and/or Bridge Medicines’ respective businesses; the Company’s ability to grow and successfully execute on its business plan, including the development and commercialization of its pipeline; changes in the applicable laws or regulations; the possibility that the Company may be adversely affected by other economic, business, and/or competitive factors; and other risks and uncertainties indicated from time to time described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 filed with Securities and Exchange Commission (“SEC”) on March 8, 2024 and in the Company’s other filings with the SEC. The Company cautions that the foregoing list of factors is not exclusive and not to place undue reliance upon any forward-looking statements which speak only as of the date made. Moreover, the Company operates in a very competitive and rapidly changing environment. New risks emerge from time to time. Except as required by law, the Company does not undertake any obligation to update publicly any forward-looking statements for any reason after the date of this Current Report on Form 8-K to conform these statements to actual results or to changes in their expectations.
Item 9.01 – Financial Statements and Exhibits.
(d) Exhibits
† Schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant undertakes to furnish supplemental copies of any of the omitted schedules upon request by the SEC.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Galecto, Inc. |
|
|
|
|
Date: October 7, 2024 |
|
|
|
By: |
|
/s/ Hans T. Schambye |
|
|
|
|
|
|
Hans T. Schambye, M.D., Ph.D. |
|
|
|
|
|
|
President and Chief Executive Officer |
ASSET PURCHASE AGREEMENT
between
Galecto, inc.
as Buyer,
and
bridge medicines llc
as Seller
dated as of
October 7, 2024
TABLE OF CONTENTS
Page
|
|
|
Article I DEFINITIONS |
2 |
1.1 |
Definitions |
2 |
1.2 |
Table of Definitions |
9 |
Article II PURCHASE AND SALE |
10 |
2.1 |
Purchase and Sale of Assets |
10 |
2.2 |
Excluded Assets |
11 |
2.3 |
Assumed Liabilities |
12 |
2.4 |
Excluded Liabilities |
12 |
2.5 |
Share Consideration |
12 |
2.6 |
Certificate of Designation; Directors. |
12 |
2.7 |
Purchased Asset Valuation |
13 |
2.8 |
Withholding Tax |
13 |
2.9 |
Consents, Authorizations, Waivers; Contract Assignments |
13 |
Article III CLOSING |
14 |
3.1 |
Closing |
14 |
3.2 |
Conditions Precedent to Obligations of Each Party; Closing Deliverables |
14 |
Article IV REPRESENTATIONS AND WARRANTIES OF SELLER |
16 |
4.1 |
Organization of Seller |
16 |
4.2 |
Capitalization; Subsidiaries |
16 |
4.3 |
Authority of Seller; Vote Required |
17 |
4.4 |
No Conflicts; Consents |
17 |
4.5 |
Financial Statements |
18 |
4.6 |
Undisclosed Liabilities |
18 |
4.7 |
Contracts |
18 |
4.8 |
Title to Purchased Assets |
20 |
4.9 |
Intellectual Property |
21 |
4.10 |
Data Privacy |
23 |
4.11 |
Legal Proceedings |
24 |
4.12 |
Compliance with Laws; Permits |
24 |
4.13 |
FDA and Health Care Regulatory Matters. |
24 |
4.14 |
Taxes |
26 |
|
|
|
4.15 |
Absence of Certain Changes or Events |
28 |
4.16 |
Accredited Investor; Investment Experience |
28 |
4.17 |
Supplier and Vendors |
29 |
4.18 |
Employment Matters; Benefit Plans |
29 |
4.19 |
Real Property |
29 |
4.20 |
Affiliate Interests and Transactions |
29 |
4.21 |
Insurance |
29 |
4.22 |
Brokers |
30 |
4.23 |
No Other Representations and Warranties |
30 |
Article V REPRESENTATIONS AND WARRANTIES OF BUYER |
30 |
5.1 |
Organization of Buyer |
30 |
5.2 |
Authority of Buyer |
31 |
5.3 |
No Conflicts; Consents |
31 |
5.4 |
Capitalization |
31 |
5.5 |
Brokers |
31 |
5.6 |
Legal Proceedings |
32 |
5.7 |
Securities Duly Issued |
32 |
5.8 |
Vote Required |
32 |
5.9 |
Buyer SEC Documents and Filings; Financial Statements |
32 |
5.10 |
Reporting Company |
33 |
5.11 |
Listing |
33 |
5.12 |
Solvency |
33 |
5.13 |
No Other Representations and Warranties |
33 |
Article VI COVENANTS |
33 |
6.1 |
Buyer Stockholders’ Meeting |
33 |
6.2 |
Proxy Statement |
35 |
6.3 |
Reservation of Buyer Common Stock; Issuance of Shares of Buyer Common Stock |
36 |
6.4 |
Lock-Up Period |
36 |
6.5 |
Nasdaq Listing Application |
36 |
6.6 |
Legends |
37 |
6.7 |
Private Placement |
37 |
6.8 |
Cooperation |
37 |
6.9 |
Confidentiality |
37 |
|
|
|
6.10 |
Public Announcements |
38 |
6.11 |
Insurance Proceeds |
38 |
6.12 |
Transfer Taxes |
38 |
6.13 |
Certain Tax Matters |
38 |
6.14 |
Further Assurances; Access to Records |
39 |
6.15 |
Release |
39 |
6.16 |
Confidentiality |
40 |
6.17 |
Service Providers |
40 |
6.18 |
Non-Competition |
40 |
Article VII MISCELLANEOUS |
40 |
7.1 |
Survival |
40 |
7.2 |
Expenses |
40 |
7.3 |
Notices |
41 |
7.4 |
Interpretation |
41 |
7.5 |
Headings |
42 |
7.6 |
Severability |
42 |
7.7 |
Entire Agreement |
42 |
7.8 |
Successors and Assigns |
42 |
7.9 |
No Third-Party Beneficiaries |
42 |
7.10 |
Amendment and Modification; Waiver |
43 |
7.11 |
Governing Law; Jurisdiction |
43 |
7.12 |
Specific Performance |
43 |
7.13 |
Waiver of Jury Trial |
43 |
7.14 |
Counterparts |
44 |
Schedule I – Knowledge parties
Appendix I - Officers, directors and stockholders of Buyer executing Buyer Stockholder Support Agreements
Exhibit A – Form of Certificate of Designation
Exhibit B – Form of Buyer Stockholder Support Agreement
ASSET PURCHASE AGREEMENT
This ASSET PURCHASE AGREEMENT (this “Agreement”), dated as of October 7, 2024, is made and entered into between Galecto, Inc., a Delaware corporation (“Buyer”), and Bridge Medicines LLC, a Delaware limited liability company (“Seller”, together with Buyer, the “Parties”, and each, a “Party”).
RECITALS
A. Seller wishes to sell to Buyer, and Buyer wishes to purchase from Seller, the Purchased Assets (as defined herein), and, in connection therewith, Buyer is willing to assume from Seller the Assumed Liabilities (as defined herein), all upon the terms and subject to the conditions set forth herein (such sale and purchase, together with all other transactions contemplated by this Agreement, the “Transaction”).
B. The board of directors of Buyer (the “Buyer Board”) has (i) approved and declared advisable this Agreement and the Transaction, including the issuance of shares of Buyer Common Stock (as defined herein) and shares of Buyer Convertible Preferred Stock (as defined herein) to Seller pursuant to the terms of this Agreement and (ii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Buyer vote to approve the Buyer Stockholder Matters (as defined herein) at the Buyer Stockholders’ Meeting (as defined herein) to be convened following the Closing.
C. The board of managers of Seller (the “Seller Board”) has (i) determined that the Transaction is fair to, advisable and in the best interests of Seller and the Seller Members (as defined herein) and (ii) approved and declared advisable this Agreement and the Transaction, including that Buyer acquire from Seller the Purchased Assets.
D. Concurrently with the execution and delivery of this Agreement, the members of Seller sufficient to adopt and approve this Agreement and sell the Purchased Assets as required under the laws of Delaware and the organizational documents of Seller are executing and delivering an action by written consent in form and substance reasonably acceptable to Buyer in order to obtain the Required Seller Member Vote (the “Seller Member Written Consent”).
E. Concurrently with the execution and delivery of this Agreement and as a condition and inducement to Seller’s willingness to enter into this Agreement, each of the officers, directors and stockholders set forth on Appendix I hereof (solely in their capacity as stockholders of Buyer) are executing support agreements in favor of Seller, each in substantially the form attached hereto as Exhibit B (each, a “Buyer Stockholder Support Agreement” and together, the “Buyer Stockholder Support Agreements”), pursuant to which such Persons have, subject to the terms and conditions set forth therein, agreed to vote all of their shares of capital stock of Buyer in favor of the Buyer Stockholder Matters.
AGREEMENT
NOW, THEREFORE, in consideration of the mutual covenants and agreements hereinafter set forth and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto agree as follows:
Article I
DEFINITIONS
1.1 Definitions. The following terms have the meanings specified or referred to in this Article I:
“Action” means any claim, action, cause of action, demand, lawsuit, arbitration, inquiry, audit, examination, notice of violation, proceeding, litigation, citation, summons, subpoena or investigation of any nature, civil, criminal, administrative, regulatory or otherwise, whether at law or in equity.
“Affiliate” of a Person means any other Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such Person. The term “control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by Contract or otherwise.
“Benefit Plan” means any retirement, pension, profit sharing, deferred compensation, equity bonus, savings, bonus, incentive, cafeteria, medical, dental, vision, hospitalization, life insurance, accidental death and dismemberment, medical expense reimbursement, dependent care assistance, tuition reimbursement, disability, sick pay, holiday, vacation, severance, change of control, equity purchase, equity option, restricted equity, phantom equity, equity appreciation rights, fringe benefit or other compensation or employee benefit plan, program, policy, practice, Contract or arrangement of any kind (including any “employee benefit plan,” as defined in Section 3(3) of ERISA, whether or not subject to ERISA) or any employment, consulting or personal services Contract, plan or arrangement, whether written or oral, qualified or nonqualified, funded or unfunded, or domestic or foreign, (a) sponsored, maintained, contributed to or required to be contributed to by Seller, any Affiliate of Seller or any ERISA Affiliate (or to which Seller, any Affiliate of Seller or any ERISA Affiliate) is a party and which covers or benefits any current or former officer, employee, director, consultant, independent contractor or any other service provider of or to Seller (or any spouse, dependent or beneficiary of any such individual); or (b) with respect to which Seller has any Liability (including any contingent Liability).
“Books and Records” means books, documents, records, files, agreements, manuals and other information in the possession or control of Seller, whether in hard copy, electronic or computer format, in each case, that are exclusively related to the Specified Program (whether or not required to be severed from any books, documents, records, files, agreements, manuals or other information not related to the Specified Program).
“Business Day” means any day except Saturday, Sunday or any other day on which commercial banks located in New York, New York are authorized or required by Law to be closed for business.
“Buyer Common Stock” means shares of common stock of Buyer, par value $0.00001 per share.
“Buyer Convertible Preferred Stock” means shares of Series A preferred stock of Buyer, par value $0.00001 per share, newly designated pursuant to the Certificate of Designation.
“Buyer SEC Documents” means all statements, reports, schedules, forms and other documents required to have been filed by Buyer with the SEC.
“Code” means the Internal Revenue Code of 1986, as amended.
“Contracts” means all legally binding contracts, purchase orders, leases, deeds, mortgages, licenses, instruments, notes, commitments, undertakings, indentures, joint ventures and all other agreements, commitments and legally binding arrangements.
“Current Assets” means those categories of current assets of Seller determined in accordance with GAAP, applied using the same accounting methods, practices, principles, policies and procedures, with consistent classifications, judgments and valuation and estimation methodologies that were used in the preparation of the balance sheet as of December 31, 2023 included in the Financial Statements.
“Current Liabilities” means those categories of current liabilities of Seller determined in accordance with GAAP, applied using the same accounting methods, practices, principles, policies and procedures, with consistent classifications, judgments and valuation and estimation methodologies that were used in the preparation of the balance sheet as of December 31, 2023 included in the Financial Statements.
“Encumbrance” means any charge, claim, community property interest, pledge, equitable interest, lien (statutory or other), option, security interest, mortgage, easement, encroachment, right of way, right of first refusal, or restriction of any kind, including any restriction on use, voting, transfer, receipt of income or exercise of any other attribute of ownership.
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended, and the regulations promulgated thereunder.
“ERISA Affiliate” means any Person that is (or, at any relevant time, was) treated, together with Seller, as a “single employer” within the meaning of Section 414 of the Code or Section 4001 of ERISA.
“Exchange Act” means the Security Exchange Act of 1934, as amended.
“Excluded Taxes” means: (i) all Taxes of Seller or any of their respective Affiliates, or for which Seller or its Affiliates is otherwise liable, for any taxable period, including all Taxes of any member of an affiliated, consolidated, combined or unitary group of which Seller (or any predecessor thereof) is or was a member on or prior to the Closing Date, including pursuant to Section 1.1502-6 of the Treasury Regulations or any analogous or similar state, local or non-U.S. Law or regulation; (ii) all Taxes relating to the Excluded Assets or Excluded Liabilities for any taxable period; (iii) all Taxes relating to the Purchased Assets or the Assumed Liabilities for any taxable period ending on or before the Closing Date and, with respect to any taxable period that includes (but does not end on) the Closing Date, the portion of such taxable period ending on and including the Closing Date; (iv) any Loss from the breach of any covenants and agreements of
Seller in respect of Taxes contained in Sections 6.12 and 6.13; (v) any Loss from the breach of representations and warranties concerning Seller contained in Section 4.14; (vi) any employment Taxes (including withholding Taxes) required with respect to any payments made under or contemplated by this Agreement with respect to Buyer’s purchase of the Purchased Assets pursuant to this Agreement; and (vii) Seller’s portion of any Transfer Taxes levied on the transfer of the Purchased Assets pursuant to Section 6.12.
“FDA” means the U.S. Food and Drug Administration or any successor agency or authority thereto.
“GAAP” means United States generally accepted accounting principles, as in effect from time to time.
“Governmental Authority” means any federal, state, local or foreign government or political subdivision thereof, or any agency or instrumentality of such government or political subdivision (including for the avoidance of doubt, any such government, subdivision, agency or instrumentality responsible for the administration or imposition of any Tax), or any quasi-governmental authority (to the extent that the rules, regulations or orders of such authority have the force of Law), or any arbitrator, court or tribunal of competent jurisdiction.
“Governmental Order” means any order, award, decision, injunction, judgment, ruling, decree, charge, writ, subpoena or verdict entered, issued, made or rendered by any Governmental Authority or arbitrator.
“Health Care Laws” means any applicable Laws relating to pharmaceutical products, good manufacturing practices (to the extent applicable), interactions with health care professionals, fraud and abuse matters, laboratory testing, genetic testing, genomic sequencing, biospecimen collection or testing, non-clinical testing, complaint handling, adverse event reporting, biohazards, and pharmacies. Health Care Laws includes, but are not limited to: (a) the Federal Food, Drug and Cosmetic Act of 1938, as amended (the “FDCA”); (b) the Public Health Service Act of 1944, as amended (the “PHSA”), and the regulations of the U.S. Food and Drug Administration (the “FDA”) promulgated thereunder, including 21 C.F.R. Parts 11, 50, 54, 56, 58, 312, and 812; (c) Medicare (Title XVIII of the Social Security Act) and Medicaid (Title XIX of the Social Security Act); (d) the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)); (e) the Stark Anti-Self-Referral Law (42 U.S.C. § 1395nn); (f) the Anti-Inducement Law (42 U.S.C. § 1320a-7a(a)(5)); (g) the civil False Claims Act (31 U.S.C. §§ 3729 et seq.); (h) the administrative False Claims Law (42 U.S.C. § 1320a-7b(a)); (i) the exclusion Laws (42 U.S.C. § 1320a-7); (j) any other applicable federal, state, local or non-U.S. laws, regulations and requirements having the force of law related to the design, development, testing, studying, manufacturing, processing, storing, importing or exporting, licensing, labeling or packaging of Seller’s products, or that is related to remuneration (including ownership) to or by physicians or other health care providers (including kickbacks) or the disclosure or reporting of the same, patient or program charges, record-keeping, claims processing, documentation requirements, medical necessity, referrals, the hiring of employees or acquisition of services or supplies from those who have been excluded from government health care programs, quality, safety, licensure, accreditation or any other material aspect of providing health care products or services; (k) HIPAA; and (l) all applicable Laws governing the licensure, accreditation, certification and operation of Seller’s business.
“HIPAA” means collectively: (a) the Health Insurance Portability and Accountability Act of 1996; (b) the Health Information Technology for Economic and Clinical Health Act (Title XIII of the American Recovery and Reinvestment Act of 2009); and (c) the Omnibus Rule effective March 26, 2013 (78 Fed. Reg. 5566), the implementing regulations at 45 CFR Parts 160 and 164, and related binding guidance from the United States Department of Health and Human Services, in each case, as the same may be amended, modified or supplemented from time to time.
“Indebtedness” means, without duplication, (i) indebtedness for borrowed money, (ii) indebtedness evidenced by any note, bond (other than any performance bond), debenture, mortgage or other debt instrument or debt security, (iii) payment obligations currently due and payable under any interest rate, currency or other hedging agreement, (iv) obligations under any performance bond or letter of credit (to the extent drawn), (v) any amounts for the deferred purchase price of goods and services (including, without limitation, trade payables and any earn out Liabilities associated with past acquisitions); (vi) all obligations of the type referred to in clauses (i) through (v) of other Persons for the payment of which Seller is responsible or liable, as obligor, guarantor, surety or otherwise, including any guarantee of such obligations; (vii) all obligations of the type referred to in clauses (i) through (vi) of other Persons secured by (or for which the holder of such obligations has an existing right, contingent or otherwise, to be secured by) any Encumbrance on any property or asset of Seller (whether or not such obligation is assumed by Seller); and (viii) all prepayment penalties, premiums or fees required to be paid in connection with the prepayment of any of the foregoing.
“Intellectual Property” means all intellectual property and industrial property rights and assets, and all rights, interests and protections that are associated with, similar to, or required for the exercise of, any of the foregoing, however arising, pursuant to the Laws of any jurisdiction throughout the world, whether registered or unregistered, including any and all: (a) trademarks, service marks, trade names, brand names, logos, trade dress, design rights and other similar designations of source, sponsorship, association or origin, together with the goodwill connected with the use of and symbolized by, and all registrations, applications and renewals for, any of the foregoing; (b) internet domain names, whether or not trademarks, registered in any top-level domain by any authorized private registrar or Governmental Authority, web addresses, web pages, websites and related content, accounts with Twitter, Facebook, Instagram and other social media platforms and the content found thereon and related thereto, and URLs; (c) works of authorship, expressions, designs and design registrations, whether or not copyrightable, including copyrights, author, performer, moral and neighboring rights, and all registrations, applications for registration and renewals of such copyrights; (d) inventions, discoveries, trade secrets, business and technical information and know-how, databases, data collections and other confidential and proprietary information and all rights therein; (e) patents (including all reissues, divisionals, provisionals, continuations and continuations-in-part, re-examinations, renewals, substitutions and extensions thereof), patent applications, and other patent rights and any other Governmental Authority-issued indicia of invention ownership (including inventor’s certificates, petty patents and patent utility models); (f) software and firmware, including data files, source code, object code, application programming interfaces, architecture, files, records, schematics, computerized databases and other related specifications and documentation; (g) royalties, fees, income, payments and other proceeds now or hereafter due or payable with respect to any and all of the foregoing; (h) rights of personality and publicity; and (i) all rights to any Actions of any nature available to or being pursued to the extent related to any of the foregoing, whether accruing before, on or after the
Closing Date, including all rights to and claims for damages, restitution and injunctive relief for infringement, dilution, misappropriation, violation, misuse, breach or default, with the right but no obligation to sue for such legal and equitable relief, and to collect, or otherwise recover, any such damages.
“Intellectual Property Assets” means all Intellectual Property that is owned by or licensed to or purported to be owned by or licensed to Seller that relate to, or are used or held for use in connection with the Specified Program.
“IRS” means the Internal Revenue Service of the United States.
“Know-How” means know-how, trade secrets and other confidential or proprietary information, including data, invention rights, materials, technical, pre-clinical and clinical data, results, instructions, dossiers, records, documents, applications, processes, methods, formulas, formulation information, packaging and chemical specifications, raw material specifications, chemical and finished goods analytical test methods, stability data, testing data and quality control data for biological, chemical, pharmacological, toxicological, physical, analytical, clinical and safety, in each case, that Seller has rights in, are owned by Seller, or in the possession of Seller of the date of the Agreement and as of the Closing that relate to, or are used, or held for use in connection with the Specified Program.
“Knowledge” means, (i) with respect to an individual, that such individual has actual knowledge of the relevant fact or such individual would reasonably be expected to have obtained knowledge of such fact in the ordinary course of the performance of such individual’s employment responsibilities, and (ii) with respect to Buyer or Seller, as applicable, any individual identified on Schedule I has actual knowledge of the relevant fact or such individual would reasonably be expected to have obtained knowledge of such fact in the ordinary course of the performance of such individual’s employment responsibilities.
“Law” means any statute, law, ordinance, regulation, rule, code, order, constitution, treaty, common law, judgment, decree, directive, other requirement or rule of law of any Governmental Authority.
“Legal Requirements” means any federal, state, foreign, local, municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Authority and any orders, writs, injunctions, awards, judgments and decrees.
“Liability(ies)” means any and all debts, liabilities, obligations, claims, Losses, costs, expenses or commitments of any kind or nature whatsoever, asserted or unasserted, known or unknown, absolute or contingent, accrued or unaccrued, matured or unmatured or otherwise.
“Licensed Registered Intellectual Property Assets” means all Registered Intellectual Property Assets that are licensed to Seller by a third party.
“Losses” means all losses, damages, Liabilities, deficiencies, Actions, judgments, costs or expenses of whatever kind, including reasonable attorneys’ fees and the cost of enforcing any right
to indemnification hereunder, but excluding punitive or exemplary damages (except to the extent the same are awarded to a third party).
“Nasdaq” means The Nasdaq Stock Market.
“Owned Registered Intellectual Property Assets” means all Registered Intellectual Property Assets that are owned, or purported to be owned by Seller.
“Operating Agreement” means that certain Amended and Restated Limited Liability Company Operation Agreement of Seller, dated October 31, 2016, by and among the Seller Members.
“Permits” means all permits, licenses, franchises, approvals, authorizations, registrations, certificates, variances and similar rights obtained, or required to be obtained, from Governmental Authorities.
“Person” means an individual, corporation, partnership, joint venture, limited liability company, Governmental Authority, unincorporated organization, trust, association or other entity.
“Personal Data” means, collectively, all data or information constituting the personal information relating to or identifying any natural person or household, including employees, customers, and other individuals, that has been collected or otherwise obtained by Seller. For purposes of this definition, an identifiable natural person is one who can be identified, directly or indirectly, in particular by reference to an identifier such as a name, an identification number, location data, an online identifier or to one or more factors specific to the physical, physiological, genetic, mental, economic, cultural or social identity of that natural person.
“Purchased Asset Valuation” means, the product obtained by multiplying (x) the arithmetic mean of the highest and lowest Nasdaq trading prices of the Buyer Common Stock on the Closing Date by (y) the number of shares of Buyer Common Stock underlying the Share Consideration on an as-converted basis.
“Registered Intellectual Property Assets” means all Intellectual Property Assets that are subject to any issuance, registration, application or other filing by, to or with any Governmental Authority or authorized private registrar in any jurisdiction, including registered trademarks, domain names and copyrights, issued and reissued patents and patent applications for any of the foregoing.
“Regulatory Authority” means, with respect to any country, federal, state, local, or other regulatory jurisdiction, the applicable Governmental Authority responsible for granting any registration, authorization or approval necessary to research, develop, test, manufacture, distribute, sell or market a pharmaceutical product in such country or regulatory jurisdiction, including, in the United States, the FDA.
“Regulatory Information and Documents” means (a) all Permits (including pricing and reimbursement approvals), and pending applications, agreements, for any thereof, and similar rights obtained from any Regulatory Authorities and/or Governmental Authorities, to take any action in connection with the Purchased Assets, including, without limitation, NDAs and INDs,
together with all supporting data, documents, submissions, correspondence, reports and clinical studies relating thereto (including, without limitation, documentation of pharmacovigilance, good clinical practice, good laboratory practice and good manufacturing practice); (b) all adverse event reports and other data, information and materials relating to adverse experiences with respect to the Purchased Assets; (c) all written notices, filings, communications or other correspondence between Seller, on the one hand, and any Governmental Authority and/or Regulatory Authorities, on the other hand, relating to the Purchased Assets, including any safety reports or updates, complaint files and product quality reviews, and clinical or pre-clinical data derived from clinical studies conducted or sponsored by Seller, which data relates to the Purchased Assets; and (d) all other information regarding development, testing, activities pertaining to each Purchased Assets and/or compliance with any law or regulation of any jurisdiction, including audit reports, corrective and preventive action documentation and reports, and relevant data and correspondence; in each case, as maintained by or otherwise that are owned by or in the possession of Seller as of the date of this Agreement and as of the Closing.
“Representative” means, with respect to any Person, any and all directors, officers, employees, consultants, financial advisors, counsel, accountants and other agents of such Person.
“Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002.
“SEC” means the United States Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended.
“Seller Disclosure Letter” means the disclosure letter delivered by Seller concurrently with the execution and delivery of this Agreement with respect to the Schedules and Exhibits provided in this Agreement.
“Seller Members” means all the members of Seller as of the Closing Date as provided in Schedule 4.2 of the Seller Disclosure Letter.
“Specified Program” means Seller’s ENL-YEATS program, as currently in effect or as proposed to be conducted, as described in the confidential slide deck presented by Seller to the Buyer Board on September 3, 2024.
“Tax Return” means any return, declaration, report, claim for refund, information return or statement or other document relating to Taxes filed or required to be filed with a Governmental Authority, including any schedule or attachment thereto, and including any amendment thereof.
“Tax” or “Taxes” means, without limitation, any and all (a) U.S. federal, state, local, non-U.S. and other income, gross receipts, sales, use, production, ad valorem, transfer, documentary, franchise, registration, profits, license, lease, service, service use, withholding, payroll, employment, unemployment, estimated, excise, escheat or unclaimed property, severance, environmental, stamp, occupation, premium, property (real or personal), real property gains, windfall profits, customs, duties or other taxes, fees, assessments or charges of any kind whatsoever, together with any interest, additions or penalties with respect thereto and any interest in respect of such additions or penalties and (b) liability for any amounts of the type described in
clause (a) above payable by reason of Treasury Regulations Section 1.1502-6 (or similar provision of state, local or non-U.S. Law), transferee or successor liability, by Contract or otherwise.
“Transaction Documents” means this Agreement, the Bill of Sale, the Assignment and Assumption Agreement, and the other agreements, instruments and documents required to be delivered at or in connection with the Closing. For the avoidance of doubt, the term “Transaction Documents” does not include any consulting agreement or employment related materials executed by Matthew Kronmiller relating to a position as a member of Buyer's senior management team.
1.2 Table of Definitions. The following terms have the meanings set forth in the Sections referenced below:
|
|
Definition |
Location |
Agreement |
Preamble |
Annual Financial Statements |
4.5(a) |
Assigned Contracts |
2.1(d) |
Assignment and Assumption Agreement |
3.2(b)(ii) |
Assumed Liabilities |
2.3 |
Bill of Sale |
3.2(b)(i) |
Buyer |
Preamble |
Buyer Board |
Recitals |
Buyer Common Stock Payment Shares |
2.5 |
Buyer Disclosure Letter |
Article V |
Buyer Preferred Stock Payment Shares |
2.5 |
Buyer Releasees |
6.13 |
Buyer Stockholder Matters |
6.1(a)(ii) |
Buyer Stockholders’ Meeting |
6.1(a)(ii) |
Certificate of Designation |
2.5 |
Charter Amendment Proposal |
6.1(a)(ii) |
Closing |
3.1 |
Closing Date |
3.1 |
Confidential Information |
4.9(l) |
Confidentiality Agreement |
6.16 |
Excluded Assets |
2.2 |
Excluded Liabilities |
2.4 |
Financial Statements |
4.5(a) |
Fraud Policy |
4.13(e) |
IRB |
4.13(d) |
Key Suppliers and Vendors |
4.17 |
Last Balance Sheet |
4.5(a) |
Last Balance Sheet Date |
4.5(a) |
Last Interim Financial Statements |
4.5(a) |
|
|
Materials |
2.1(c) |
New Hire Documents |
6.15 |
Party |
Preamble |
Patent Assignment |
3.2(b)(v) |
Permitted Encumbrances |
4.8(a) |
Personal Data Obligations |
4.10(a) |
Preferred Stock Conversion Proposal |
2.5 |
Privacy Laws |
4.10(a) |
Process |
4.10(a) |
Processing |
4.10(a) |
Proxy Statement |
6.2(a) |
Purchased Assets |
2.1 |
Purchased Asset Valuation Calculation |
2.7 |
Purchased Asset Valuation Schedule |
2.7 |
Releasing Parties |
6.15 |
Required Buyer Stockholder Vote |
5.8 |
Required Seller Member Vote |
4.3(b) |
Seller |
Preamble |
Seller Board |
Recitals |
Seller Designee |
2.6(b) |
Seller IP Rights Agreements |
4.9(h) |
Seller Member Written Consent |
Recitals |
Seller Related Party |
4.20 |
Share Consideration |
2.5 |
Specified Excluded Liabilities |
2.4 |
Transaction |
Recitals |
Transfer Taxes |
6.10 |
Transferred Data |
2.1(b) |
USPTO |
4.9(g) |
Article II
PURCHASE AND SALE
2.1 Purchase and Sale of Assets. The term “Purchased Assets” means all of Seller’s right, title and interest in, to and under all of the assets, properties and rights of every kind and nature, whether real, personal or mixed, tangible or intangible, wherever located and whether now existing or hereafter acquired prior to the Closing Date, except for the Excluded Assets described in Section 2.2 below, that relate to, or are used or held for use in connection with, the Specified Program, including all of Seller’s right, title and interest in, to and under the following assets as of the Closing Date:
(a) all Intellectual Property Assets listed on Schedule 2.1(a) of the Seller Disclosure Letter;
(b) any data regarding the Intellectual Property Assets, including preclinical or clinical data regarding the Intellectual Property Assets, owned by Seller (the “Transferred Data”);
(c) all materials listed on Schedule 2.1(c) of the Seller Disclosure Letter (the “Materials”);
(d) the Contracts and rights and interests, therein, to which Seller is a party or by which Seller is bound (including any and all such Contracts relating to the Materials, Know‑How and/or the Regulatory Documents and Information) listed on Schedule 2.1(d) of the Seller Disclosure Letter (the “Assigned Contracts”);
(e) all Know-How owned by Seller;
(f) all Permits listed on Schedule 2.1(f) of the Seller Disclosure Letter, but only to the extent such Permits may be transferred under applicable Law;
(g) the Books and Records;
(h) all rights or causes of action against third parties arising out of occurrences to the extent relating to the Purchased Assets, including, all rights under express or implied warranties relating to the Purchased Assets and all rights, claims, credits, causes of action or rights of set-off against third parties relating to the Purchased Assets, including all rights to seek and obtain injunctive relief and to recover damages for past, present and future infringement relating to the Purchased Assets;
(i) all rights to any Actions of any nature available to or being pursued by Seller to the extent related to the Purchased Assets or the Assumed Liabilities (as defined herein), whether arising by way of counterclaim or otherwise;
(j) any and all Regulatory Information and Documents;
(k) all rights under any non-competition, non-solicitation, invention assignment, confidentiality or other restrictive covenant agreement with any Service Provider; and
(l) all goodwill associated with the Purchased Assets.
2.2 Excluded Assets. Seller is not selling, and Buyer is not purchasing, any of the following assets of Seller, all of which shall be retained by Seller and excluded from the Purchased Assets (collectively, the “Excluded Assets”):
(a) all rights which accrue or will accrue to Seller or any Seller Member under the Transaction Documents; and
(b) books, documents, records, files, agreements, manuals and other information in the possession or control of Seller, whether in hard copy, electronic or computer format, in each case, that are not related to the Specified Program in whole or in part;
(c) cash held in Seller's bank accounts as of the Closing,
(d) the assets, properties, contracts and rights specifically set forth on Schedule 2.2(d) of the Seller Disclosure Letter.
2.3 Assumed Liabilities. On the terms and subject to the conditions set forth in this Agreement, at the Closing, Buyer will assume the Liabilities of Seller specifically identified on Schedule 2.3 of the Seller Disclosure Letter (collectively, the “Assumed Liabilities”), and no other Liabilities.
2.4 Excluded Liabilities. Notwithstanding the provisions of Section 2.3 or any other provision of this Agreement, any schedule or exhibit hereto or any Transaction Document to the contrary, Buyer does not assume and shall not be responsible to pay, perform or discharge (and Seller shall retain, pay, perform or otherwise discharge without recourse to Buyer) any Liabilities of Seller or any of its Affiliates of any kind or nature whatsoever, including, without limitation, Excluded Taxes and those Liabilities set forth on Schedule 2.4 of the Seller Disclosure Letter (the “Specified Excluded Liabilities”) other than the Assumed Liabilities, including, for the avoidance of doubt, any Liabilities of Seller for any present or former employees, officers, directors, retirees, independent contractors or consultants of Seller, including, without limitation, any Liabilities associated with any claims for wages or other benefits, bonuses, accrued vacation, workers’ compensation, severance, retention, termination or other payments (collectively, the “Excluded Liabilities”).
2.5 Share Consideration. The aggregate share consideration (the “Share Consideration”) to be paid by Buyer for the Purchased Assets and Assumed Liabilities at the Closing shall be (a) 62,594 shares of Buyer Common Stock (“Buyer Common Stock Payment Shares”) and (b) 160.562 shares of Buyer Convertible Preferred Stock (“Buyer Preferred Stock Payment Shares”). Each Buyer Preferred Stock Payment Share shall be convertible into 1,000 shares of Buyer Common Stock, subject to and contingent upon the affirmative vote of a majority of the Buyer Common Stock votes properly cast for or against the proposal at a meeting of stockholders of Buyer to approve, for purposes of the Nasdaq Stock Market Rules, the issuance of shares of Buyer Common Stock to Seller upon conversion of any and all shares of Buyer Convertible Preferred Stock in accordance with the terms of the certificate of designation (the “Certificate of Designation”) in substantially the form attached hereto as Exhibit A (the “Preferred Stock Conversion Proposal”).
2.6 Certificate of Designation; Directors.
(a) On or prior to the Closing Date, Buyer will file the Certificate of Designation with the office of the Secretary of State of the State of Delaware.
(b) Within four (4) Business Days of the Closing Date, Buyer shall take all actions necessary to appoint the individual identified on Schedule 2.6(b) of the Seller Disclosure Letter (the “Seller Designee”) as a director on the Buyer Board.
(c) On or prior to the Closing Date, Buyer shall take all actions necessary such that Matthew Kronmiller is offered a position as a member of Buyer’s senior management team contingent upon and effective immediately following the Closing.
2.7 Purchased Asset Valuation. For all U.S. federal income tax purposes, the Parties shall treat Buyer’s acquisition of the Purchased Assets and Assumed Liabilities as a taxable sale of assets by Seller to Buyer in exchange for the Share Consideration (and the Assumed Liabilities and any other relevant items treated as consideration for applicable income Tax purposes) (as finally determined). The Share Consideration (and the Assumed Liabilities and any other relevant items treated as consideration for applicable income Tax purposes) shall be allocated among the Purchased Assets in accordance with the provisions of GAAP and Section 1060 of the Code and the Treasury Regulations promulgated thereunder. No later than the Closing Date, Seller will deliver to Buyer a schedule (the “Purchased Asset Valuation Schedule”) prepared in accordance with GAAP and applicable Treasury regulations, setting forth, in reasonable detail, Seller’s good faith, estimated calculations of the components of the Purchased Asset Valuation (the “Purchased Asset Valuation Calculation”) as of immediately prior to the Closing prepared and certified by Seller’s chief financial officer (or if there is no chief financial officer at such time, the principal financial and accounting officer for Seller). Buyer shall provide Seller, within twenty (20) days following Buyer’s receipt of the Purchased Asset Valuation Schedule, any comments on the Purchased Asset Valuation Schedule, and Seller shall incorporate any such reasonable comments in good faith. If Buyer does not provide Seller with any comments on the Purchased Asset Valuation Schedule within twenty (20) days following Buyer’s receipt of the Purchased Asset Valuation Schedule, then the Purchased Asset Valuation Schedule shall be deemed final. Seller shall make available to Buyer, as reasonably requested by Buyer, the work papers and back-up materials used or useful in preparing the Purchased Asset Valuation Schedule and, if reasonably requested by Buyer, Seller’s accountants and counsel at reasonable times and upon reasonable notice.
2.8 Withholding Tax. Buyer shall be entitled to deduct and withhold from any portion of any consideration payable or otherwise deliverable pursuant to this Agreement to any Person all Taxes that Buyer is required to deduct and withhold under any provision of Tax Law. All such withheld amounts shall be treated as delivered to Seller hereunder.
2.9 Consents, Authorizations, Waivers; Contract Assignments. Nothing in this Agreement or the other Transaction Documents shall be construed as an agreement to assign any Assigned Contract, Permit, right or other Purchased Asset that by its terms or pursuant to applicable Law is not capable of being sold, assigned, transferred, novated or delivered without the consent or waiver of a third party or Governmental Authority unless and until such consent or waiver shall be given in a form and substance reasonably acceptable to Buyer. For purposes of this Section 2.9, it shall be reasonable for Buyer not to accept the form and substance of any consent, waiver, authorization, novation or notice if it (a) changes or modifies, in any material respect, any Assigned Contract; (b) results in any material incremental cost to Buyer; or (c) makes any representations concerning, or attempts to impose any conditions on, Buyer (other than the obligation to pay or perform the obligations expressly set forth in such Assigned Contract to the extent such obligations constitute Assumed Liabilities hereunder). Seller and Buyer shall use commercially reasonable efforts (provided, however, that Seller shall not be required to pay any consideration therefor which is not required under a Contract or arrangement in place as of the
Closing) to obtain such consents, authorizations and waivers and to resolve the impediments to the sale, assignment, transfer, novation or delivery contemplated by this Agreement or the other Transaction Documents and to obtain any other consents, authorizations and waivers necessary to convey to Buyer all of the Purchased Assets. In the event any such consents, authorizations or waivers are not obtained prior to the Closing Date in a form and substance reasonably acceptable to Buyer, Seller and Buyer shall (unless requested otherwise by Buyer) continue to use commercially reasonable efforts to obtain the relevant consents, authorizations or waivers until such consents, authorizations or waivers are obtained. To the extent that any Purchased Asset, Assumed Liability, or Permit cannot be transferred to Buyer following the Closing pursuant to this Section 2.9, Buyer and Seller shall use commercially reasonable efforts to enter into such arrangements to provide to the parties the economic (taking into account all burdens and benefits, including Tax costs and benefits) and, to the extent permitted under applicable Law, operational equivalent of the transfer of such Purchased Asset, Assumed Liability, or Permit, including (a) enforcing, at Buyer’s request, any rights of Seller arising with respect thereto, including the right to terminate such Assigned Contract upon the request of Buyer and (b) permitting Buyer to enforce any rights arising with respect thereto. Seller will pay to Buyer, when received, all income, proceeds and other monies received by Seller from third parties to the extent related to Buyer’s intended rights under any Assigned Contract, as contemplated by this Agreement, including this Section 2.9. Once any such consent, waiver, authorization or novation is obtained or notice is properly made in form and substance reasonably acceptable to Buyer, Seller will assign such Assigned Contract to Buyer at no additional cost to Buyer. Any expenses incurred by Seller, and any reasonable expenses incurred by Buyer, in connection with the arrangements contemplated by this Section 2.9 will be borne by Seller. If following the Closing, Seller becomes aware of any Contract to which Seller is a party, which is not an Assigned Contract or otherwise disclosed on the Seller Disclosure Letter, Seller shall promptly notify Buyer in writing of the existence of such Contract. In such case, or if Buyer otherwise becomes aware of any such Contract, Buyer may, at its election, agree to assume such Contract, and Seller shall assign such Contract to Buyer at no additional cost to Buyer.
Article III
CLOSING
3.1 Closing. Subject to the terms and conditions of this Agreement, the consummation of the Transaction shall take place at a closing (the “Closing”) to be held via e-mail exchange of .pdf documents on the date of this Agreement (the “Closing Date”). The Closing shall be deemed effective as of 12:01 a.m. Eastern time on the Closing Date.
3.2 Conditions Precedent to Obligations of Each Party; Closing Deliverables.
(a) The obligations of each Party to enter into this Agreement and the Transaction Documents and consummate the Transaction at the Closing are subject to the satisfaction or, to the extent permitted by applicable Law, the written waiver by each of the Parties, at or prior to the Closing, of each of the following conditions:
(i) No temporary restraining order, preliminary or permanent injunction or other Governmental Order preventing the consummation of the Transaction shall have been issued by any court of competent jurisdiction or other
Governmental Authority of competent jurisdiction and remain in effect and there shall not be any Law which has the effect of making the consummation of the Transaction illegal; and
(ii) Buyer shall have filed with Nasdaq a Notification Form: Listing of Additional Shares for the listing of the Buyer Common Stock Payment Shares, Buyer Preferred Stock Payment Shares and the Buyer Common Stock to be issued upon conversion of the Buyer Preferred Stock Payment Shares, and Nasdaq shall not have notified Buyer that it has any objections to such notification that are continuing.
(b) At the Closing, Seller shall deliver to Buyer the following, each in form and substance reasonably satisfactory to Buyer:
(i) a bill of sale (the “Bill of Sale”) duly executed by Seller, transferring the Purchased Assets to Buyer;
(ii) an assignment and assumption agreement (the “Assignment and Assumption Agreement”) duly executed by Seller, effecting the assignment to and assumption by Buyer of the Assigned Contracts and the Assumed Liabilities;
(iii) written consents from each of the parties identified on Schedule 3.2(b)(iii) of the Seller Disclosure Letter in form and substance satisfactory to Buyer;
(iv) a patent assignment agreement (the “Patent Assignment”) duly executed by Seller;
(v) a properly completed IRS Form W-9 duly executed by Seller;
(vi) a certificate of an authorized officer of Seller, certifying that attached thereto are true and complete copies of Seller’s Certificate of Formation, Operating Agreement, a Certificate of Good Standing from the Secretary of State of the State of Delaware, the authorizing resolutions of Seller Board, and the Seller Member Written Consent;
(vii) the Purchased Asset Valuation Schedule; and
(c) At the Closing, Buyer shall deliver to Seller the following:
(i) the Assignment and Assumption Agreement duly executed by Buyer;
(ii) the Patent Assignment duly executed by Buyer;
(iii) a copy of the Certificate of Designation, certified by the Secretary of State of the State of Delaware;
(iv) evidence of the issuance of the Share Consideration to Seller; and
(v) a certificate of an authorized officer of Buyer, certifying that attached thereto are true and complete copies of the resolutions duly adopted by the Buyer Board which shall be in full force and effect as of the Closing authorizing the execution and delivery of the Transaction Documents and the performance of Buyer’s obligations thereunder.
Article IV
REPRESENTATIONS AND WARRANTIES OF SELLER
Seller hereby represents and warrants to Buyer, as of the Closing Date or, if a representation or warranty is made as of a specified date, as of such date, as follows, except as set forth on the Seller Disclosure Letter delivered separately and concurrently with the execution of this Agreement (it being understood and agreed that: (i) such exceptions shall be deemed to be part of the representations and warranties made hereunder, and (ii) each item in a particular section of the Seller Disclosure Letter applies to the corresponding section hereof and to any other section to the extent its relevance is reasonably apparent on the face of such item).
4.1 Organization of Seller. Seller is a limited liability company duly organized and validly existing under the Laws of Delaware and has full power and authority to own, operate or lease the properties and assets now owned, operated or leased by it and to carry on its business as currently conducted. Seller is duly qualified or licensed as a foreign entity to do business, and is in good standing (or the equivalent), in each jurisdiction where the ownership or operation of the Purchased Assets or the conduct of the business makes such qualification or licensing necessary, except for any such failures to be so qualified or licensed and in good standing that are not, individually or in the aggregate, material to Seller. Schedule 4.1 of the Seller Disclosure Letter lists each jurisdiction in which Seller is licensed or qualified to do business. Seller has not violated, and is not in violation of, any of the provisions of Seller’s governing documents.
4.2 Capitalization; Subsidiaries. Schedule 4.2 of the Seller Disclosure Letter lists all of the Seller Members and their outstanding percentage interests. The Seller Members are the record and beneficial owners of all of the equity interests of Seller. There are no equity interests, or rights, options or warrants to acquire equity interests, of Seller other than the equity interests set forth on Schedule 4.2 of the Seller Disclosure Letter. Except for Seller’s Certificate of Formation and the Operating Agreement of Seller, there are no voting trusts, voting agreements, proxies, equityholder agreements or other similar agreements applicable to Seller. Seller is not a party to, is not otherwise bound by, and has not granted, any equity appreciation rights, participations, phantom equity or similar rights. All the issued and outstanding equity interests of Seller are duly authorized, validly issued, and free of preemptive rights and there are no unsatisfied capital contributions with respect thereto. Seller does not currently have, nor has it ever had, any direct or indirect stock or other equity or ownership interest (whether controlling or not) in any corporation, association, partnership, joint venture or other entity.
4.3 Authority of Seller; Vote Required.
(a) Seller has all requisite power and authority to enter into this Agreement and the other Transaction Documents to which Seller is a party, to carry out its obligations hereunder and thereunder and to consummate the transactions contemplated hereby and thereby. The execution and delivery by Seller of this Agreement and any other Transaction Document to which Seller is a party, the performance by Seller of its obligations hereunder and thereunder and the consummation by Seller of the transactions contemplated hereby and thereby have been duly authorized by all requisite action on the part of Seller. Seller Board, by resolutions duly adopted (and not thereafter modified or rescinded) by unanimous written consent of the managers, including the managers not affiliated with Buyer, has (i) determined that the Transaction is fair to, advisable and in the best interests of Seller and its members and (ii) approved and declared advisable this Agreement and the Transaction, including that Buyer acquire from Seller the Purchased Assets. This Agreement and each other Transaction Document to which Seller is a party have been duly executed and delivered by Seller, and (assuming due authorization, execution and delivery by each other party hereto and thereto) this Agreement and such other Transaction Documents each constitutes a legal, valid and binding obligation of Seller enforceable against Seller in accordance with its terms, subject to the effect of any applicable bankruptcy, insolvency (including Laws relating to fraudulent transfers), reorganization, moratorium or similar Laws affecting creditors’ rights generally and subject to the effect of general principles of equity (regardless of whether considered in a proceeding at law or in equity).
(b) The affirmative vote of a majority of holders of the equity interests outstanding on the record date for the Seller Member Written Consent (the “Required Seller Member Vote”), is the only vote of the holders of any class or series of Seller equity interests necessary to adopt and approve this Agreement, the Transaction Documents and the Transaction. No interest in Seller is subject to any appraisal or dissenters rights in connection with the Transaction. Seller has obtained approval by written consent from Seller Members sufficient for the Required Seller Member Vote in lieu of a meeting pursuant to Section 18-302 of the DLLCA, for purposes of adopting and approving this Agreement, the Transaction Documents and the Transaction.
4.4 No Conflicts; Consents. Except as set forth on Schedule 4.4 of the Seller Disclosure Letter, the execution, delivery and performance by Seller of this Agreement and the other Transaction Documents to which they are a party, and the consummation of the transactions contemplated hereby and thereby, do not and will not: (a) result in a violation or breach of, or default under, any provision of the Articles of Organization of Seller, the Operating Agreement of Seller, or other organizational documents of Seller; (b) result in a violation or breach by Seller of any provision of any Law applicable to Seller or the Purchased Assets; (c) require any notice, authorization, approval, order, permit or consent of or with any Governmental Authority; (d) result in a material violation or breach of, or default under (with or without notice or lapse of time, or both), or give rise to a right of termination, cancellation or acceleration of any obligation or loss of any benefit under, or require the consent, notice or other action by any Person under, any Assigned Contract, (e) result in a violation or breach of, or default under (with or without notice or lapse of time, or both), or give rise to a right of termination, cancellation or acceleration of any obligation or loss of any benefit under, or require the consent, notice or other action by any Person under any Contract (other than the Assigned Contracts) to which Seller is a party, except where the violation, breach, conflict, default, acceleration or failure to give notice would not be material
to Seller; or (f) result in the creation or imposition of any Encumbrance, other than Permitted Encumbrances, on the Purchased Assets.
4.5 Financial Statements; Solvency.
(a) Schedule 4.5(a) of the Seller Disclosure Letter sets forth copies of (i) the internally prepared unaudited financial statements of Seller, consisting of the balance sheet of Seller as at December 31 in each of the years 2022 and 2023 and the related statements of operations and cash flows for the years then ended (the “Annual Financial Statements”), (ii) the internally prepared unaudited balance sheet of Seller as of June 30, 2024 and the related internally prepared unaudited statement of operations for the six (6) month period then ended (the “Last Interim Financial Statements” and, together with the Annual Financial Statements, collectively, the “Financial Statements”). The Financial Statements have been prepared in accordance with GAAP, consistent with past practice throughout the periods involved, subject to the absence of footnote disclosures and year-end adjustments for interim periods. The Financial Statements are based on the books and records of Seller and fairly present in all material respects the financial condition and the results of the operations of Seller for the periods indicated. The balance sheet of Seller as of June 30, 2024, is referred to herein as the “Last Balance Sheet” and the date thereof as the “Last Balance Sheet Date.”
(b) As of the Closing Date, upon the consummation of the Transaction, Seller’s Current Assets are equal to or greater than its Current Liabilities and Seller shall not be declared judicially insolvent.
4.6 Undisclosed Liabilities. Seller has no Liabilities with respect to Seller or the Purchased Assets required to be reflected on a balance sheet prepared in accordance with GAAP, except (a) those that are adequately reflected or reserved against in the Last Balance Sheet as of the Last Balance Sheet Date, (b) those that have been incurred in the ordinary course of business consistent with past practice since the Last Balance Sheet Date and that are not, individually or in the aggregate, material in amount, and (c) those set forth on Schedule 4.6 of the Seller Disclosure Letter.
4.7 Contracts.
(a) Schedule 4.7 of the Seller Disclosure Letter sets forth all Contracts to which Seller is a party or otherwise bound that are of the following nature and are directly related or relevant to the Purchased Assets, the Assumed Liabilities or the Specified Program (excluding, for clarity, Contracts that have expired or been terminated with no surviving provisions):
(i) any Contract for the purchase of services, equipment, materials, products, or supplies that relate to, or are used or held for use in connection with, the Specified Program;
(ii) any Contract relating to or evidencing Indebtedness;
(iii) any Contract with any Governmental Authority;
(iv) any Contract with any Affiliate of Seller;
(v) any Contract regarding any material indemnification provided by Seller;
(vi) any Contract with a noncompetition, nonsolicitation, “most-favored-nation” pricing or exclusivity agreement or other arrangement that would prevent, restrict or limit in any way Seller or, to the extent that such Contract is an Assigned Contract, Buyer, from carrying on its business in any manner or in any geographic location, including with respect to the Specified Program;
(vii) any employment, independent contractor or consulting Contract related to the Specified Program (excluding offer letters on Seller’s standard form made available to Buyer);
(viii) any Contract of more than $10,000 pursuant to which Seller is the lessee or lessor of, or holds, uses, or makes available for use to any Person, (x) any real property or (y) any tangible personal property;
(ix) any Contract of more than $10,000 for the sale or purchase (including any option to purchase or right of first refusal or right of first negotiation) of any real or tangible personal property that relates to, or is used or held for use in connection with, the Specified Program;
(x) any license agreement related to the Specified Program providing for the payment or receipt of royalties or other compensation by Seller, or the license of any Intellectual Property Assets;
(xi) any license, sublicense or other Contract related to the Specified Program to which Seller is a party and pursuant to which any Person is authorized to use any Intellectual Property Asset (other than (i) by employees and contractors for the sole purpose of fulfilling their job functions or providing services for Seller’s benefit, and (ii) any confidential information related to the Specified Program provided under confidentiality agreements);
(xii) any joint venture or partnership, merger, asset or equity purchase or divestiture Contract related to or involving the Purchased Assets, the Assumed Liabilities or the Specified Program;
(xiii) any confidentiality, secrecy, or non-disclosure agreement entered into outside the ordinary course of business;
(xiv) any Contract that results in any Person holding a power of attorney that relates to Seller, the Purchased Assets or the Assumed Liabilities;
(xv) any Contract granting any Person any Encumbrance on all or any part of the Purchased Assets, other than Permitted Encumbrances;
(xvi) any Contract with respect to the settlement of any litigation, proceeding or claim, to which Seller is a party;
(xvii) any Contract pursuant to which rights of any third party are triggered or become exercisable, or under which any other consequence, result or effect arises, in connection with or as a result of the execution of this Agreement or the consummation the Transaction, either alone or in combination with any other event; and
(xviii) any Contract, whether or not made in the ordinary course of business, that is material to the Purchased Assets, Assumed Liabilities or Specified Program and not previously disclosed pursuant to this Section 4.7(a).
(b) Seller is not in material breach of, or material default under, any Assigned Contract. To Seller’s Knowledge, each other Person that has or had any obligation or liability under any Assigned Contract is in full compliance with all applicable terms and requirements of such Assigned Contract. To Seller’s Knowledge, no event has occurred or circumstance exists that (with or without notice or lapse of time, but excluding the impact of the transactions contemplated by this Agreement to the extent set forth on Schedule 4.4 of the Seller Disclosure Letter) may contravene, conflict with, or result in a violation or breach of, or give Seller or any other Person, the right to declare a default or exercise any remedy under, or to accelerate the maturity or performance of, or to cancel, terminate, or modify, any Assigned Contract. Within the one (1)‑year period immediately preceding the Closing Date, Seller has not given to or received from any other Person any notice or other communication (whether oral or written) regarding any actual, alleged, possible, or potential violation or breach of, or default under, any Assigned Contract. Seller has made available to Buyer, true correct and complete copies of any contract set forth on Schedule 4.7 of the Seller Disclosure Letter, including any and all amendments thereto.
4.8 Title to Purchased Assets; Sufficiency of Assets.
(a) Seller has good and valid title to, or a valid leasehold interest in, all of the Purchased Assets (excluding the Intellectual Property Assets, title to which is addressed in Section 4.9) and is transferring to Buyer, pursuant to the Transaction, all of its right, title and interest in such Purchased Assets (including leasehold interests), free and clear of Encumbrances except for (i) Encumbrances for Taxes not yet due and payable and (ii) Encumbrances imposed under applicable Law (collectively, the “Permitted Encumbrances”).
(b) To Seller’s Knowledge, the Purchased Assets are sufficient for the continued exploitation of the Specified Program after the Closing and constitute all of the rights, property, and assets necessary to exploit, or related to, the Specified Program, in each case, in substantially the same manner as conducted by Seller immediately prior to the Closing. None of the Excluded Assets are material to the exploitation of, or related to, the Purchased Assets or Specified Program.
4.9 Intellectual Property.
(a) Schedule 4.9(a) of the Seller Disclosure Letter sets forth all Owned Registered Intellectual Property Assets. The entire right, title and interest in and to all Owned Registered Intellectual Property Assets is solely and exclusively owned by Seller and all are subsisting and recorded (or applied for) in the name of Seller, and to Seller’s Knowledge, valid
and enforceable (or in the case of applications applied for). To Seller’s Knowledge, all registration, maintenance and renewal fees currently due in connection with any Owned Registered Intellectual Property Assets have been paid and all documents, recordation and certificates in connection with such Owned Registered Intellectual Property Assets currently required to be filed have been filed with the relevant Governmental Authorities in the United States or foreign jurisdictions, as the case may be, for the purposes of prosecuting, maintaining and perfecting the registration of such Owned Registered Intellectual Property Assets and recording Seller’s ownership interests therein. To Seller’s Knowledge, there are no liens, encumbrances or security interests recorded at the USPTO or any other patent office against any of the Owned Registered Intellectual Property Assets for the benefit of a third party. Seller has delivered to Buyer’s counsel evidence of Seller’s ownership rights of registration of all Owned Registered Intellectual Property Assets.
(b) Schedule 4.9(b) of the Seller Disclosure Letter lists all Licensed Registered Intellectual Property Assets, such Schedule sets forth the contract under which such Licensed Registered Intellectual Property Asset is licensed to Seller, and all such Licensed Registered Intellectual Property Assets are duly and validly licensed pursuant to contracts disclosed on Schedule 4.7 of the Seller Disclosure Letter.
(c) Seller has not transferred ownership of, granted any exclusive license of or exclusive right to use, or granted any exclusive rights in or to joint ownership of, any Intellectual Property Assets to any other Person. To Seller’s Knowledge, no Person other than Seller possesses any material current or contingent rights to any Owned Registered Intellectual Property Assets. All Owned Registered Intellectual Property Assets are fully transferable, alienable or licensable by Seller without restriction and without payment of any kind to any Person (excluding payments required to record transfer with the United States Patent and Trademark Office).
(d) Except as set forth on Schedule 4.9(d) of the Seller Disclosure Letter, there are no Contracts to which Seller is a party with respect to (i) the in-license to Seller of any third party Intellectual Property, excluding commercially available off-the-shelf software, or (ii) the out-license by Seller of any Intellectual Property Asset to a third party.
(e) Seller has obtained from all individuals who participated in any material respect in the invention or authorship of, or who are under any obligation to assign any Intellectual Property Assets that Seller purports to own effective written assignments of all ownership rights of such individuals in such Intellectual Property Assets.
(f) The Intellectual Property Assets that Seller purports to own and the Intellectual Property Assets licensed that Seller purports to license from third parties constitute all Intellectual Property Assets used in or otherwise necessary for the operation of the Specified Program as of the date of the Agreement.
(g) All information to the Knowledge of Seller relating to the subject matter of the claims of the Owned Registered Intellectual Property Assets has been disclosed to the United States Patent and Trademark Office (“USPTO”) to the extent required by 37 C.F.R. § 1.56 or any applicable patent office in any other jurisdiction to the extent required by the applicable rules and regulations in such jurisdiction. All material information submitted to the USPTO and any applicable patent office in any other jurisdiction in connection with the Owned Registered
Intellectual Property Assets, and in connection with the prosecution thereof, was accurate in all material respects at the time it was submitted. Seller, with respect to any of the Registered Intellectual Property Assets, has not made any material misrepresentation or concealed any material information from the USPTO in violation of 37 C.F.R. Section § 1.56 or from any applicable patent office in any other jurisdiction in violation of the applicable rules and regulations in such jurisdiction. Each Registered Intellectual Property Asset is valid, subsisting, enforceable and in full force and effect; and has not been cancelled, expired, or abandoned.
(h) Seller is not in material breach of any Contract governing any Intellectual Property Assets included in the Assigned Contracts (the “Seller IP Rights Agreements”) and the consummation of the Transaction will not result in the modification, cancellation, termination, suspension of, or acceleration of any payments with respect to the Seller IP Rights Agreements in any material respect, or give any non-Seller party to any Seller IP Rights Agreement the right to do any of the foregoing.
(i) To Seller’s Knowledge, the conduct of Seller’s business related to the Specified Program, including the use and other exploitation of the Intellectual Property Assets, has not infringed, misappropriated, diluted or violated, and does not infringe, misappropriate, dilute or violate, any Intellectual Property rights of any Person. Seller has not received any written notice or claim asserting that any such infringement, misappropriation, dilution or violation has occurred, and no facts or circumstances exist that would provide a reasonable basis for any such claim. Seller has not received any offer for a license of Intellectual Property from any Person in connection with an allegation by such Person that Seller has infringed or misappropriated any of the Intellectual Property of such Person.
(j) There is no written claim, suit, action, or proceeding by any third party pending or, to Seller’s Knowledge, threatened against Seller contesting the validity, enforceability, or ownership of, or rights to use any Intellectual Property Asset. To Seller’s Knowledge, the conduct of Seller in relation to the Specified Program, including the use and other exploitation of the Intellectual Property Assets, does not constitute unfair competition or trade practices under the Laws of any jurisdiction. Seller has not received any written notice or claim asserting any such unfair competition or trade practices and no facts or circumstances exist that would provide a reasonable basis for any such claim.
(k) No funding, facilities or personnel of any Governmental Authority were used to develop, manufacture, formulate, or create, in whole or in part, any Owned Registered Intellectual Property Asset that would lead to such Governmental Authority claiming ownership or other interest in or option to such Intellectual Property Asset owned by Seller.
(l) Seller has taken commercially reasonable steps to protect and preserve the confidentiality of all confidential, proprietary or non-public information included in the Purchased Assets (“Confidential Information”). All use, disclosure or appropriation of Confidential Information owned by Seller by or to a third party has been pursuant to the terms of a written Contract between Seller and such third party that includes language protecting the confidentiality of such Confidential Information.
4.10 Data Privacy.
(a) Seller is and at all relevant times has been in compliance in all material respects with all Privacy Laws applicable to the conduct of Seller and Seller contractual obligations governing any operation or set of operations performed by or, to Seller’s Knowledge, on behalf of Seller on Personal Data or on sets of Personal Data, whether or not by automated means, such as collection, recording, organization, structuring, storage, adaptation or alteration, retrieval, consultation, use, disclosure by transmission, dissemination or otherwise making available, alignment or combination, restriction, erasure or destruction (collectively, “Processing”, including “Process” and related correlatives, as applicable). Seller’s Processing of such Personal Data is in compliance in all material respects with (i) applicable Privacy Laws and guidelines relating to the Processing of Personal Data to which Seller is bound; (ii) Seller’s privacy policy (and applicable terms of use) as published on its website (in a manner readily accessible to visitors and current or potential customers), (iii) any written privacy policies (or applicable terms of use), documents, or promises or representations presented by Seller in writing to employees, consumers or customers, or other persons or entities to whom such Personal Data relates and to which Seller is bound, and (iv) any written contractual obligations of Seller to its customers (actual or potential), employees, or other Persons or entities regarding the privacy or security of Personal Data (collectively, “Personal Data Obligations”). The execution or delivery of this Agreement or any Transaction Documents, or the performance of Seller’s obligations hereunder or thereunder or transfer of the Personal Data to Buyer will not materially violate any of Seller’s Personal Data Obligations. Seller has not received written notice of and Seller has no Knowledge of any claims or investigations related to its Processing of Personal Data or its Personal Data Obligations, nor, to Seller’s Knowledge, are there valid grounds for any such bona fide claims or investigations of such kind. “Privacy Laws” mean applicable Legal Requirements relating to privacy, security and/or collection, use or other Processing of the Transferred Data that constitutes Personal Data of any individual (including, as applicable, clinical trial participants).
(b) Seller has taken reasonable actions (including implementing reasonable technical, physical or administrative safeguards) designed to protect Personal Data in its possession or under its control against unauthorized use, processing, access, disclosure modification, loss or theft. Seller has contractually obligated all third parties processing Personal Data on its behalf to comply with applicable Privacy Laws and has taken commercially reasonable measures to ensure that such third parties comply with these obligations. There has been, to Seller’s Knowledge, no material unauthorized: (i) use, processing, access, modification, loss or theft of Personal Data or security breach relating to Personal Data in the custody of Seller; or (ii) unintended or improper disclosure of or access to any Personal Data in the custody of Seller. Seller has taken reasonable steps to detect during all relevant times any unauthorized use, processing, access, disclosure, modification, loss, or theft of Personal Data. The transactions contemplated hereby will not violate any Personal Data Obligations relating to the use, dissemination, or transfer or any Transferred Data that constitutes Seller Processed Personal Data.
4.11 Legal Proceedings.
(a) There are, and for the past three (3) years there have been, no Actions pending or, to Seller’s Knowledge, threatened against or by Seller or any of its managers or officers (in their capacities as such or relating to their service or relationship with Seller) (a) relating to or affecting the Purchased Assets or the Assumed Liabilities; or (b) that challenge or seek to prevent, enjoin or otherwise delay the Transaction.
(b) There are no outstanding orders or unsatisfied judgments, penalties or awards against, relating to or affecting the Purchased Assets or the Assumed Liabilities.
4.12 Compliance with Laws; Permits.
(a) Seller has complied at all times, and is now complying, in all material respects with all Laws applicable to the ownership and use of the Purchased Assets. Seller has not received any written notice of any violation of any such Law or Legal Requirement which cannot be remedied prior to the Closing.
(b) All material Permits required for the ownership and use of the Purchased Assets required by FDA or any other Governmental Authority have been obtained by Seller and are valid and in full force and effect. All fees and charges currently due with respect to such Permits have been paid in full. Schedule 4.12(b) of the Seller Disclosure Letter lists all current material Permits issued to Seller that are related to the ownership and use of the Purchased Assets. To Seller’s Knowledge, no event has occurred that, with or without notice or lapse of time or both, would reasonably be expected to result in the revocation, suspension, lapse or limitation of any Permit set forth in Schedule 4.12(b) of the Seller Disclosure Letter.
4.13 FDA and Health Care Regulatory Matters.
(a) Seller is in compliance, in all material respects, with all applicable statutes, Laws, rules, regulations, and guidance administered or issued by FDA or other Regulatory Authorities with jurisdiction over Seller’s activities and products with respect to the Specified Program, including, but not limited to the Federal Food, Drug, and Cosmetic Act, and all other statutes, laws, rules and regulations regarding the development, storage, handling, testing and clinical trials, manufacturing, packaging, labeling, marketing, distributing, advertising and promoting the products, recalls and product withdrawals, and complaint handling or adverse event reporting; in each case, to the extent applicable to Seller and the Specified Program. No recall actions have been commenced in the past or are currently pending or, to Seller’s Knowledge, threatened in writing with respect to the Purchased Assets.
(b) At all times during the last two (2) years, Seller has not received any written notice, warning, administrative proceeding order, complaint, or other written communication of any actual or threatened enforcement Action, adverse inspectional finding, or investigation by any Regulatory Authority or other Governmental Authority with respect to the Purchased Assets that Seller has violated any applicable Legal Requirements, including any FDA Form 483, warning letter or untitled letter, in each case, that have not been complied with or closed to the satisfaction of the relevant Regulatory Authority or other Governmental Authority. To the Knowledge of Seller, neither the FDA nor any other Governmental Authority is considering such action nor do circumstances exist that would reasonably be expected to lead to any such action. Seller is not a party to, and Seller does not have any ongoing reporting obligations pursuant to, any corporate integrity agreements, deferred prosecution agreements, monitoring agreements, consent decrees, settlement orders, plans of correction or similar agreements with or imposed by any Governmental Authority.
(c) Neither Seller, nor any of its employees, officers or directors or, to the Knowledge of Seller, its third-party vendors and contractors has been, is, or is in anticipation of being (based on a conviction by the courts or a finding of fault by a regulatory authority): (a) debarred pursuant to the Generic Drug Enforcement Act of 1992 (21 U.S.C. § 335a); (b) disqualified from participating in clinical trials pursuant to 21 C.F.R. §312.70; (c) disqualified as a testing facility under 21 C.F.R. Part 58, Subpart K, or otherwise restricted from the conduct of research; (d) excluded, debarred or suspended from or otherwise ineligible to participate in a “Federal Health Care Program” as defined in 42 U.S.C. 1320a-7b, or any other governmental payment, procurement or non-procurement program; or (e) included on the HHS/OIG List of Excluded Individuals/Entities, the General Services Administration’s List of Parties Excluded from Federal Programs, or the FDA Debarment List.
(d) All clinical, pre-clinical, and other studies and tests sponsored or conducted by or on behalf of Seller and directly related to the Purchased Assets have been and, if still pending, are being conducted in compliance in all material respects with all applicable Laws and Legal Requirements relating to the Specified Program, including, the Federal Food, Drug, and Cosmetic Act and 21 C.F.R. Parts 50, 54, 56, 58, and 312. No clinical or preclinical trial, study, or test conducted by or on behalf of Seller and directly related to the Purchased Assets has been terminated, suspended, or materially modified for safety or non-compliance reasons by FDA, an Institutional Review Board (“IRB”), or comparable authority. No investigational new drug (IND) application or New Drug Application (NDA) filed by or on behalf of Seller with the FDA or other Regulatory Authority and directly related to the Purchased Assets has been terminated or suspended by the FDA or any other Regulatory Authority, and neither the FDA nor any applicable Regulatory Authority has commenced, or, to the Knowledge of Seller, threatened in writing to initiate, any Action to place a clinical hold order on, or otherwise terminate, delay or suspend, any ongoing clinical investigation directly related to the Purchased Assets or the Specified Program and being conducted by or on behalf of Seller. Except as set forth on Schedule 4.13(d) of the Seller Disclosure Letter, to the Knowledge of Seller, there are not any studies, tests, development or trials the results of which reasonably call into question the results of the studies, tests, development and trials conducted by or on behalf of Seller and directly related to the Purchased Assets in any material respect.
(e) All reports, documents, forms, claims, applications, records submissions, supplements, amendments, and notices required to be filed with, maintained for or furnished to the FDA or any other Governmental Authority by Seller and directly related to the Purchased Assets, have been so filed, maintained or furnished by Seller. Neither Seller nor any of its officers, employees, or to Seller’s Knowledge, any of its contractors or agents has made any materially false statements on, or material omissions from, any notifications, applications, approvals, reports and other submissions to the FDA or any similar Governmental Authority directly pertaining to the Purchased Assets. Seller has not committed any act, made any statement or failed to make any statement, in each case in respect of Seller that violates the “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy, as stated at 56 Fed. Reg. 46191 (September 10, 1991) (“Fraud Policy”). To its Knowledge, Seller is not the subject of any pending or threatened investigation by the FDA pursuant to its Fraud Policy. The descriptions of, protocols for, and data and other results of, the studies, tests, development and trials conducted by or on behalf of Seller, and records related to the manufacturing, testing, packaging, labeling, marketing, storage, and
distribution of Purchased Assets that have been furnished or made available to Buyer are accurate and complete in all material respects.
(f) At all times during the last two (2) years, Seller has operated in material compliance with all Health Care Laws applicable to the Specified Program, and has timely filed all material reports, applications, statements, documents, registrations, filings, corrections, updates, amendments, supplements, and submissions directly related to the Purchased Assets required to be filed by them under applicable Health Care Laws. Each such filing was true and correct in all material respects as of the date of submission, or was corrected in or supplemented by a subsequent filing, and any material and legally necessary or required updates, changes, corrections, amendments, supplements, or modifications to such filings have been submitted to the applicable governmental authorities.
4.14 Taxes.
(a) All income and other material Tax Returns required to be filed by Seller have been timely filed. Such Tax Returns are true, complete and correct in all material respects. All Taxes due and owing by Seller (whether or not shown on any Tax Return) have been timely paid.
(b) Seller has withheld and paid each Tax required to have been withheld and paid in connection with amounts paid or owing to any employee, independent contractor, creditor, customer, equityholder or other party, and complied with all information reporting and backup withholding provisions of applicable Law.
(c) Seller has collected all sales, use, value added, goods and services, and similar Taxes required to be collected and timely remitted all such Taxes collected to the appropriate Governmental Authority in accordance with applicable Law.
(d) No extensions or waivers of statutes of limitations have been given or requested with respect to any Taxes of Seller.
(e) All deficiencies asserted, or assessments made, against Seller as a result of any examinations by any Governmental Authority have been fully paid.
(f) There is no audit or pending or threatened audit of, or Tax controversy associated with, any Tax Return of Seller currently being conducted by a Governmental Authority.
(g) Seller is not a party to any Action by any Governmental Authority. There are no pending or threatened Actions by any Governmental Authority. No Governmental Authority in a jurisdiction where Seller does not file Tax Returns or pay Taxes has made any claim that Seller is or may be subject to Tax or required to file Tax Returns in that jurisdiction.
(h) There are no Encumbrances for Taxes upon any of the Purchased Assets nor is any Governmental Authority in the process of imposing any Encumbrances for Taxes on any of the Purchased Assets (other than Permitted Encumbrances).
(i) Seller is not and has never been, a member of any “affiliated group” of corporations within the meaning of Section 1504 of the Code (or any similar affiliated, combined, consolidated, or unitary group or arrangement for group relief for state, local, or foreign income Tax purposes). Seller has no Liability for Taxes of any other Person (i) under Treasury Regulation Section 1.1502-6 or any comparable provision of other Tax Law or (ii) arising under Contract, by operation of Law, by reason of being a successor or transferee, or otherwise. None of the Assumed Liabilities is a Contract regarding the sharing or allocation of either Liability for Taxes or payment of Taxes.
(j) Seller, from the date of its formation through the Closing Date, has been properly classified as either a partnership or disregarded entity for U.S. federal and applicable state and local income Tax purposes.
(k) Seller is not a “foreign person” as that term is used in Treasury Regulations Section 1.1445-2.
(l) None of the Assumed Liabilities is an obligation to (i) make or provide any payment or benefit that, either alone or together with any other payments or benefits, constitutes or could constitute a “parachute payment” within the meaning of Section 280G(b)(2) of the Code, or (ii) pay or reimburse any person for any Taxes imposed under Section 4999 of the Code as a result of the consummation of the transactions contemplated in this Agreement, either alone or in connection with any other event. None of the Assumed Liabilities is a “nonqualified deferred compensation plan” within the meaning of Section 409A(d)(1) of the Code.
(m) None of the assets held by Seller constitute a “United States real property interest” within the meaning of Section 897(c) of the Code.
(n) No power of attorney with respect to any Tax matter is currently in force with respect to the Purchased Assets.
(o) Seller is not a party to or bound by any Tax sharing, Tax indemnity or Tax allocation contract or other similar arrangement with any Person.
(p) Seller is not and has never been a party to any “reportable transaction,” as defined in Treasury Regulation Section 1.6011-4(b).
4.15 Absence of Certain Changes or Events. Since the Last Balance Sheet Date, Seller has conducted its business in all material respects in the ordinary course of business consistent with past practice.
4.16 Accredited Investor; Investment Experience; Restricted Securities.
(a) Seller is an “accredited investor” and represents that each Seller Member is an “accredited investor” as such term is defined in Section 501(a) of Regulation D promulgated under the Securities Act and acknowledges that it is informed as to the risks of the transactions contemplated hereby and of ownership of the shares of Buyer Common Stock and Buyer Convertible Preferred Stock. Seller is acquiring the shares of Buyer Common Stock and Buyer Convertible Preferred Stock for its own account with the present intention of holding such
securities for investment purposes and not with a view to, or for sale in connection with, any distribution of cash securities in violation of any federal or state securities Laws until the expiration of the Lock-Up Period (as defined herein). Seller has not been formed for the specific purpose of acquiring the Share Consideration. Seller is not acquiring the Share Consideration as a result of any advertisement, article, notice or other communication regarding the Share Consideration published in any newspaper, magazine or similar media or broadcast over television or radio or presented at any seminar or any other general advertisement. Seller, either alone or together with its representatives, has such knowledge, sophistication and experience in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the Stock Consideration, and has so evaluated the merits and risks of such investment. Seller, and each Seller Member, is able to bear the economic risk of an investment in the Share Consideration and, at the present time, is able to afford a complete loss of such investment.
(b) Seller, and each Seller Member, understands that the Share Consideration has not been, and will not be, registered under the Securities Act, by reason of a specific exemption from the registration provisions of the Securities Act which depends upon, among other things, the bona fide nature of the investment intent and the accuracy of Seller’s representations as expressed herein. Seller understands that the Buyer Common Stock Payment Shares and Buyer Preferred Stock Payment Shares comprising the Share Consideration are “restricted securities” under applicable U.S. federal and state securities laws and that, pursuant to these laws, Seller must hold the Share Consideration indefinitely unless they are registered with the SEC and qualified by state authorities, or an exemption from such registration and qualification requirements is available and the book-entry position representing the Share Consideration delivered at the Closing shall contain a legend or restrictive notation to such effect. Seller acknowledges that Buyer has no obligation to register or qualify the Share Consideration for resale and that Buyer does not intend to do so. Seller further acknowledges that if an exemption from registration or qualification is available, it may be conditioned on various requirements including, but not limited to, the time and manner of sale, the holding period for the Share Consideration, and on requirements relating to Buyer which are outside of Seller’s control, and which Buyer is under no obligation and may not be able to satisfy. Seller has been advised to consult legal counsel prior to making any offer, resale, pledge or transfer of any of the Share Consideration.
4.17 Supplier and Vendors. Schedule 4.17 of the Seller Disclosure Letter sets forth the names of the five (5) largest suppliers, licensors and vendors (“Key Suppliers and Vendors”) of Seller from which Seller purchased or licensed products or services relating to the Purchased Assets during the twelve (12) months ending on the last full calendar month preceding the date hereof. Seller has not received any notice that there has been any material adverse change in the price or terms of such products or services provided by any Key Supplier and Vendor, or that any such Key Supplier and Vendor intends to materially change the price or terms in a manner adverse to Seller.
4.18 Employment Matters; Benefit Plans. Seller has not, and no ERISA Affiliate of Seller has, maintained or made any contribution to (or had any obligation to make any contribution to) any defined benefit pension plan or multiemployer pension plan that is subject to Title IV of ERISA at any time. Seller has satisfied all obligations applicable to Seller or any of its ERISA Affiliates under section 4980B of the Code, Part 6 of Subtitle B of Title I of ERISA and each other Applicable Law relating to continuation of health or other coverage (or similar requirement) to
any employee or former employee of Seller (or any dependent or former dependent of such an employee or former employee) with respect to any qualifying event that has occurred. No Benefit Plan contains any provision or requirement that will cause Buyers or any Affiliate of Buyers to have any Liability with respect thereto (including any successor liability). Seller has properly fulfilled all obligations in all material respects with respect to each Benefit Plan under all Laws, including the Code, ERISA, the Affordable Care Act and all other Laws relating to benefit plans.
4.19 Real Property. Seller does not and has never owned or leased, any real property material to the Purchased Assets.
4.20 Affiliate Interests and Transactions. Except as set forth on Schedule 4.20 of the Seller Disclosure Letter, no director, manager, employee, consultant or Affiliate of Seller or any family member of Seller (including any Seller Members) (a “Seller Related Party”) has any direct or indirect right, title or interest in any properties or assets of any kind or character (whether real, personal or mixed, tangible or intangible, contingent or otherwise) related to, used or held for use in connection with the business of Seller or the Specified Program other than the Excluded Assets. No Seller Related Party conducts any part of the business of Seller for or on behalf of Seller or otherwise. No Seller Related Party of Seller is a party to, or has any direct or indirect rights in, to or under, any Assigned Contract or is otherwise indebted to Seller. No Seller Related Party owns or holds, directly or indirectly, any interest in (excepting holdings solely for passive investment purposes of securities of publicly held and traded entities constituting less than one percent of the equity of any such entity), or is a shareholder, officer, director, manager, employee or consultant of any Person that is a competitor, lessor, lessee, licensor, licensee, customer, supplier or distributor of Seller or conducts a business similar to Seller.
4.21 Insurance. Schedule 4.21 of the Seller Disclosure Letter sets forth a true and complete list of all casualty, directors’ and officers’ liability, general liability, product liability, workers’ compensation and all other types of insurance policies maintained with respect to the Purchased Assets. All such policies are in full force and effect in all material respects. There are no pending claims under such policies which are reasonably likely to exhaust the applicable limits of liability. Seller has not (a) received any written notice regarding any cancellation or invalidation of any insurance policy, refusal of any coverage or rejection of any claim under any insurance policy or material adjustment in the premiums payable with respect to any insurance policy, or (b) any written self-insurance or co-insurance plan.
4.22 Brokers. No broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission or other similar charges in connection with the Transaction based upon arrangements made by or on behalf of Seller. Buyer and its Affiliates will not incur any liability, either directly or indirectly, for any such brokerage, finder’s fees, agents’ commission or other similar charges as a result of this Agreement, any other Transaction Documents, the Transaction or such other transactions contemplated by any Transaction Documents, or for any act or omission of Seller, any of its Affiliates or any of their respective directors, officers, employees, stockholders or agents.
4.23 No Other Representations and Warranties. Seller acknowledges and agrees that none of Buyer or any of its Representatives has made or makes any other express or implied representation or warranty, whether written or oral, on behalf of Buyer, including any
representation or warranty as to the accuracy or completeness of any information regarding Buyer furnished or made available to Seller and its Representatives, other than the representations and warranties expressly set forth in Article V, and Seller has not relied on any representation, warranty or other statement relating to Buyer.
Article V
REPRESENTATIONS AND WARRANTIES OF BUYER
Except (i) as set forth in the written disclosure schedule delivered by Buyer to Seller (the “Buyer Disclosure Letter”) or (ii) as disclosed in the Buyer SEC Documents filed with the SEC since January 1, 2023 and prior to the date hereof and publicly available on the SEC’s Electronic Data Gathering Analysis and Retrieval system (but (A) without giving effect to any amendment thereof filed with, or furnished to the SEC on or after the date hereof and (B) excluding any disclosures contained under the heading “Risk Factors” and any disclosure of risks included in any “forward-looking statements” disclaimer or in any other section to the extent they are forward-looking statements or cautionary, predictive or forward-looking in nature), it being understood that any matter disclosed in the Buyer SEC Documents shall be deemed to be disclosed in a section of the Buyer Disclosure Letter only to the extent that is readily apparent from a reading of such Buyer SEC Documents that is applicable to such section or subsection of the Buyer Disclosure Letter, Buyer hereby represents and warrants to Seller, as of the Closing Date or, if a representation or warranty is made as of a specified date, as of such date, as follows:
5.1 Organization of Buyer. Each of Buyer and its subsidiaries is a corporation or limited liability company duly incorporated or formed, validly existing and in good standing under the Laws of the jurisdiction of its incorporation or organization and has all necessary corporate power and authority: (i) to conduct its business in the manner in which its business is currently being conducted, (ii) to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used and (iii) to perform its obligations under all Contracts by which it is bound. All of Buyer’s subsidiaries are wholly owned by Buyer.
5.2 Authority of Buyer. Buyer has full power and authority to enter into this Agreement and the other Transaction Documents to which Buyer is a party, to carry out its obligations hereunder and thereunder and to consummate the transactions contemplated hereby and thereby. The execution and delivery by Buyer of this Agreement and any other Transaction Documents to which Buyer is a party, the performance by Buyer of its obligations hereunder and thereunder and the consummation by Buyer of the transactions contemplated hereby and thereby have been duly authorized by all requisite action on the part of Buyer. The Buyer Board, by resolutions duly adopted (and not thereafter modified or rescinded) by unanimous vote of the members not affiliated with Seller, has (i) approved and declared advisable this Agreement and the Transaction, including the issuance of shares of Buyer Common Stock and shares of Buyer Convertible Preferred Stock to Seller pursuant to the terms of this Agreement and (ii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Buyer vote to approve the Buyer Stockholder Matters at the Buyer Stockholders’ Meeting to be convened following the Closing. This Agreement and each other Transaction Document has been duly executed and delivered by Buyer, and (assuming due authorization, execution and delivery by each other party hereto) this Agreement constitutes a legal, valid and binding obligation of Buyer enforceable against Buyer in accordance with its terms, subject to the
effect of any applicable bankruptcy, insolvency (including Laws relating to fraudulent transfers), reorganization, moratorium or similar Laws affecting creditors’ rights generally and subject to the effect of general principles of equity (regardless of whether considered in a proceeding at law or in equity).
5.3 No Conflicts; Consents. The execution, delivery and performance by Buyer of this Agreement and the other Transaction Documents to which it is a party, and the consummation of the transactions contemplated hereby and thereby, do not and will not: (a) conflict with or result in a violation or breach of, or default under, any provision of the certificate of incorporation or bylaws of Buyer; or (b) conflict with or result in a violation or breach of any provision of any Law or Governmental Order applicable to Buyer. No consent, approval, Permit, Governmental Order, declaration or filing with, or notice to, any Governmental Authority is required by or with respect to Buyer in connection with the execution and delivery of this Agreement and the other Transaction Documents and the consummation of the transactions contemplated hereby and thereby, except for any consents, approvals, Permits, Governmental Orders, declarations, and notices which have not been obtained and which, in the aggregate, would not impede the consummation of the transactions contemplated hereby and the performance by Buyer of its obligations hereunder.
5.4 Capitalization.
(a) The authorized capital stock of Buyer, as of October 3, 2024, consists of (i) 300,000,000 shares of Buyer Common Stock, 1,254,393 of which are issued and outstanding, and (ii) 10,000,000 authorized shares of Buyer Preferred Stock, none of which are issued and outstanding.
5.5 Brokers. Except for Leerink Partners LLC, no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the transactions contemplated by this Agreement or any other Transaction Document based upon arrangements made by or on behalf of Buyer.
5.6 Legal Proceedings.
(a) There are, and for the past five (5) years there have been, no Actions pending or, to Buyer’s Knowledge, threatened against or by Buyer or any of its directors or officers (in their capacities as such or relating to their service or relationship with Buyer) (a) relating to or affecting the business of Buyer; or (b) that challenge or seek to prevent, enjoin or otherwise delay the Transaction.
(b) There are no outstanding orders or unsatisfied judgments, penalties or awards against, relating to or affecting the business of Buyer.
5.7 Securities Duly Issued. All Buyer Common Stock and Buyer Preferred Stock issuable to Seller under this Agreement to be issued hereunder upon issuance in accordance with the terms of this Agreement and the Certificate of Designation will be validly issued, fully paid and non-assessable, and free and clear of all Encumbrances and preemptive rights, except for (i) restrictions on transfer arising under applicable securities Laws and (ii) the applicable terms and conditions of the organizational documents of Buyer.
5.8 Vote Required. The approval of holders of Buyer Common Stock is not required in order to approve this Agreement or, except with respect to the Buyer Stockholder Matters, the transactions contemplated hereby. The affirmative vote of a majority of the votes properly cast for or against the Preferred Stock Conversion Proposal at the Buyer Stockholders’ Meeting by the holders of Buyer Common Stock (other than the Buyer Common Stock Payment Shares to be issued at Closing pursuant to this Agreement) is the only vote of the holders of any class or series of Buyer’s capital stock necessary to approve the Preferred Stock Conversion Proposal (the “Required Buyer Stockholder Vote”).
5.9 Buyer SEC Documents and Filings; Financial Statements.
(a) All Buyer SEC Documents since January 1, 2021 have been so filed on a timely basis. A true and complete copy of each Buyer SEC Document is available on the web site maintained by the SEC at http://www.sec.gov, other than portions in respect of which confidential treatment was not objected to by the SEC. As of their respective filing dates (or, if amended or superseded by a filing prior to the date of this Agreement, then on the date of such later filing): (i) each of the Buyer SEC Documents has complied in all material respects with the requirements of the Securities Act, the Exchange Act and the Sarbanes-Oxley Act, as the case may be, and the rules and regulations of the SEC promulgated thereunder applicable to such Buyer SEC Documents and (ii) none of the Buyer SEC Documents contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading, except to the extent corrected (A) in the case of the Buyer SEC Documents filed on or prior to the date of this Agreement that were amended or superseded on or prior to the date of this Agreement, by the filing of the applicable amending or superseding Buyer SEC Document, and (B) in the case of the Buyer SEC Documents filed after the date of this Agreement that are amended or superseded prior to the Closing, by the filing of the applicable amending or superseding Buyer SEC Document.
(b) The financial statements of Buyer included or incorporated by reference in the Buyer SEC Documents (including the notes thereto) complied as to form in all material respects with the published rules and regulations of the SEC with respect thereto, were prepared in accordance with GAAP consistently applied throughout the periods presented (except as may be indicated in the notes thereto, except in the case of pro forma statements, or, in the case of unaudited financial statements, except as permitted under Form 10-Q under the Exchange Act) and fairly presented in all material respects the consolidated financial position of Buyer and its consolidated subsidiaries as of the respective dates thereof and the consolidated results of Buyer’s operations and cash flows for the periods indicated (subject to, in the case of unaudited statements, normal and recurring year-end audit adjustments).
5.10 Reporting Company. Buyer is a publicly-held company subject to reporting obligations pursuant to Section 13 of the Exchange Act, and Buyer Common Stock is registered pursuant to Section 12(b) of the Exchange Act.
5.11 Listing. (i) Buyer Common Stock is listed on Nasdaq, (ii) Buyer has not received any oral or written notice that Buyer Common Stock is ineligible or will become ineligible for listing on Nasdaq nor that Buyer Common Stock does not meet all requirements for the
continuation of such listing and (iii) Buyer satisfies all the requirements for the continued listing of Buyer Common Stock on Nasdaq. Buyer is in material compliance with all applicable Nasdaq listing and corporate governance rules.
5.12 Solvency. Immediately after giving effect to the Transaction, Buyer shall (a) be able to pay its debts as they become due and shall own property having a fair saleable value greater than the amounts required to pay its debts (including a reasonable estimate of the amount of all contingent liabilities) as they become due and (b) have adequate capital to carry on its businesses. No transfer of property is being made and no obligation is being incurred in connection with the Transaction with the intent to hinder, delay or defraud either present or future creditors of Buyer or its Affiliates.
5.13 No Other Representations and Warranties. Buyer acknowledges and agrees that none of Seller or any of its Representatives has made or makes any other express or implied representation or warranty, whether written or oral, on behalf of Seller, including any representation or warranty as to the accuracy or completeness of any information regarding Seller furnished or made available to Buyer and its Representatives, other than the representations and warranties expressly set forth in Article IV, and Buyer has not relied on any representation, warranty or other statement relating to Seller.
Article VI
COVENANTS
6.1 Buyer Stockholders’ Meeting.
(a) As promptly as practicable following the execution of this Agreement, Buyer shall take all action necessary under applicable Law to call, give notice of and hold a meeting of the holders of Buyer Common Stock for the purpose of seeking:
(i) approval of the Preferred Stock Conversion Proposal; and
(ii) if deemed necessary or appropriate by the Buyer Board or as otherwise required by applicable Law or Contract, approval of an amendment to Buyer’s certificate of incorporation to authorize sufficient Buyer Common Stock in Buyer’s certificate of incorporation for the conversion of the Buyer Convertible Preferred Stock issued pursuant to this Agreement (the “Charter Amendment Proposal”) (the matters contemplated by the clauses 6.2(a)(i)–(ii) and such other proposals that Buyer and Seller may mutually agree upon, including without limitation for purposes of approval under Nasdaq Rule 5635(a)(2), are referred to as the “Buyer Stockholder Matters,” and such meeting, the “Buyer Stockholders’ Meeting”).
(b) Buyer agrees to use reasonable best efforts (i) to call and hold the Buyer Stockholders’ Meeting as soon as practicable after the date hereof, and (ii) to solicit and obtain the Required Buyer Stockholder Vote, including without limitation, (A) engaging a proxy solicitation firm and information agent, and (B) actively attempting to contact and obtain votes from the Buyer's stockholders. If the approval of the Buyer Stockholder Matters is not obtained at the Buyer Stockholders’ Meeting or if on a date preceding the Buyer Stockholders’ Meeting, Buyer
reasonably believes that (x) it will not receive proxies sufficient to obtain the Required Buyer Stockholder Vote, whether or not quorum would be present or (y) it will not have sufficient shares of Buyer Common Stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the Buyer Stockholders’ Meeting, then, in each case, Buyer will use its reasonable best efforts to adjourn the Buyer Stockholders’ Meeting one or more times to a date or dates no more than 30 days after the scheduled date for such meeting, and to obtain such approvals at such time. If the Buyer Stockholders’ Meeting is not so adjourned, and/or if the approval of the Buyer Stockholder Matters is not then obtained, Buyer will use its reasonable best efforts to obtain such approvals as soon as practicable thereafter (including engaging a third party proxy solicitor), and in any event to obtain such approvals at the next occurring annual meeting of the stockholders of Buyer or, if such annual meeting is not scheduled to be held within six months after the Buyer Stockholders’ Meeting, a special meeting of the stockholders of Buyer to be held within six months after the Buyer Stockholders’ Meeting. Buyer will hold an annual meeting or special meeting of its stockholders, at which a vote of the stockholders of Buyer to approve the Buyer Stockholder Matters will be solicited and taken, at least once every six months until Buyer obtains approval of the Buyer Stockholder Matters.
(c) Buyer agrees that (i) the Buyer Board shall recommend that the holders of Buyer Common Stock vote to approve the Buyer Stockholder Matters and shall use its reasonable best efforts to solicit and obtain such approval within the time frames set forth in Section 6.1(b), and (ii) the Proxy Statement (and the accompanying letter to stockholders, if any) shall include a statement to the effect that the Buyer Board recommends that the Buyer’s stockholders vote to approve the Buyer Stockholder Matters.
(d) Seller and Buyer acknowledge that, under the Nasdaq Stock Market Rules, the Buyer Common Stock Payment Shares and the Buyer Preferred Stock Payment Shares will not be entitled to vote on the Buyer Stockholder Matters. Accordingly, until the earlier of (x) such time as the Required Buyer Stockholder Vote is obtained or (y) the twelve month anniversary of the Closing Date: (i) Seller shall not vote the Buyer Common Stock Payment Shares on any proposal at any Buyer Stockholders’ Meeting seeking approval from the stockholders of the Buyer Stockholder Matters, including the Preferred Stock Conversion Proposal; (ii) Seller understands and agrees that Buyer shall be entitled to enforce the voting restriction in clause (i) hereof at any such Buyer Stockholders’ Meeting; (iii) the Buyer Common Stock Payment Shares shall remain in book-entry or certificated form, and book-entry statements or stock certificates evidencing the Buyer Common Stock Payment Shares shall bear a restrictive legend referencing this Section 6.1(d), which legend shall be removed promptly following such time as the Required Buyer Stockholder Vote is obtained; (iv) in addition to any other requirements under this Agreement, as a condition of transfer of the Buyer Common Stock Payment Shares or any interest therein, any transferee shall agree in writing to be bound by the terms of this Section 6.1(d); and (v) such transferee understands and confirms that the Company shall be relying on the foregoing covenants in entering into this Agreement and issuing the Buyer Common Stock Payment Shares.
6.2 Proxy Statement.
(a) As promptly as practicable after the Closing Date, Buyer shall prepare and file with the SEC a proxy statement in preliminary form relating to the Buyer Stockholders’ Meeting to be held in connection with the Buyer Stockholder Matters (together with any
amendments thereof or supplements thereto, the “Proxy Statement”). Buyer shall use its commercially reasonable efforts to (i) cause the Proxy Statement to comply with applicable rules and regulations promulgated by the SEC and (ii) respond promptly to any comments or requests of the SEC or its staff related to the Proxy Statement. Buyer shall not file the Proxy Statement, or any amendment or supplement thereto, or respond to SEC comments or requests, without providing Seller a reasonable opportunity to review and comment thereon (which comments shall be reasonably considered in good faith by Buyer).
(b) Buyer covenants and agrees that the Proxy Statement (and the letters to stockholders, notice of meeting and form of proxy included therewith) will (i) comply as to form in all material respects with the requirements of applicable U.S. federal securities Laws and the DGCL, and (ii) will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading.
(c) Buyer shall use commercially reasonable efforts to cause the Proxy Statement to be mailed to Buyer’s stockholders as promptly as practicable after the Proxy Statement has been filed with the SEC and either (i) the SEC has indicated that it does not intend to review the Proxy Statement or that its review of the Proxy Statement has been completed or (ii) at least ten (10) days shall have passed since the Proxy Statement was filed with the SEC without receiving any correspondence from the SEC commenting upon, or indicating that it intends to review, the Proxy Statement, all in compliance with applicable U.S. federal securities laws and the DGCL. If either Party (A) becomes aware of any event or information that, pursuant to the Securities Act or the Exchange Act, should be disclosed in an amendment or supplement to the Proxy Statement, (B) receives notice of any SEC request for an amendment or supplement to the Proxy Statement or for additional information related thereto, or (C) receives SEC comments on the Proxy Statement, as the case may be, then such Party, as the case may be, shall promptly inform the other Parties thereof and shall cooperate and consult with such other Parties in Buyer filing such amendment or supplement with the SEC and, if appropriate, in mailing such amendment or supplement to the Buyer stockholders. Seller will cooperate with Buyer as reasonably requested by Buyer with respect to the Proxy Statement and use commercially reasonable efforts to promptly furnish to Buyer all information concerning Seller and its Seller Members that may be required or reasonably requested in connection with any action contemplated by this Section 6.2.
6.3 Reservation of Buyer Common Stock; Issuance of Shares of Buyer Common Stock. For as long as any Buyer Preferred Stock Payment Shares remain outstanding, Buyer shall at all times reserve and keep available, free from preemptive rights, out of its authorized but unissued Buyer Common Stock or shares of Buyer Common Stock held in treasury by Buyer, for the purpose of effecting the conversion of the Buyer Preferred Stock Payment Shares, the full number of shares of Buyer Common Stock then issuable upon the conversion of all Buyer Preferred Stock Payment Shares then outstanding. All shares of Buyer Common Stock delivered upon conversion of the Buyer Preferred Stock Payment Shares shall be newly issued shares or shares held in treasury by Buyer, shall have been duly authorized and validly issued and shall be fully paid and nonassessable, and shall be free from preemptive rights and free of any Encumbrance.
6.4 Lock-Up Period. Without the prior written consent of Buyer, Seller shall not, and shall not cause any direct or indirect Affiliate to, during the period commencing on the Closing
Date and ending on the twelve (12) month anniversary thereof (the “Lock-up Period”), (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any of the Buyer Common Stock Payment Shares, the Buyer Preferred Stock Payment Shares and the Buyer Common Stock to be issued upon the conversion of Buyer Preferred Stock Payment Shares and (2) enter into any hedging, swap or other agreement or transaction that transfers, in whole or in part, any of the economic consequences of ownership of the Buyer Common Stock Payment Shares, the Buyer Preferred Stock Payment Shares and the Buyer Common Stock to be issued upon the conversion of Buyer Preferred Stock Payment Shares, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of the Buyer Common Stock Payment Shares, the Buyer Preferred Stock Payment Shares and the Buyer Common Stock to be issued upon the conversion of Buyer Preferred Stock Payment Shares, in cash or otherwise. Notwithstanding the foregoing, Seller may transfer or otherwise dispose of the Buyer Common Stock Payment Shares, the Buyer Preferred Stock Payment Shares and the Buyer Common Stock to be issued upon the conversion of Buyer Preferred Stock Payment Shares in a transaction exempt from registration under the Securities Act without Buyer’s written consent to holders of Seller’s promissory notes (each a “Seller Noteholder”), provided that such Seller Noteholder agrees to sign and deliver a lock-up agreement reflecting the conditions and terms of this Section 6.4 for the balance of the Lock-up Period, and provided further that Buyer may require Seller to provide to Buyer and Buyer’s transfer agent a legal opinion required by Buyer’s transfer agent with respect to such transfer being exempt from registration under the Securities Act.
6.5 Nasdaq Listing Application. Buyer shall use its reasonable best efforts to (a) maintain its existing listing on Nasdaq; and (b) prepare and submit to Nasdaq a notification form for the listing of the Buyer Common Stock Payment Shares and the Buyer Common Stock to be issued upon conversion of the Buyer Preferred Stock Payment Shares to be issued in connection with the consummation of the Transaction, to cause such shares to be approved for listing (subject to official notice of issuance). The Parties will use reasonable best efforts to coordinate with respect to compliance with Nasdaq rules and regulations. Each Party will promptly inform the other Party of all verbal or written communications between Nasdaq and such Party or its representatives.
6.6 Legends. Buyer shall be entitled to place appropriate legends, including the legend noted in Section 6.1(d), on the book entries and/or certificates evidencing any shares of Buyer Common Stock or shares of Buyer Convertible Preferred Stock to be received in the Transaction by equity holders of Seller who may be considered “affiliates” of Buyer for purposes of Rule 144 under the Securities Act reflecting the restrictions set forth in Rule 144 and to issue appropriate stop transfer instructions to the transfer agent for the Buyer Common Stock and the Buyer Convertible Preferred Stock.
6.7 Private Placement. Each of Seller and Buyer shall take all reasonably necessary action on its part such that the issuance of Buyer Common Stock Payment Shares and Buyer Preferred Stock Payment Shares pursuant to this Agreement constitutes a transaction exempt from registration under the Securities Act in compliance with Rule 506 of Regulation D promulgated thereunder. Each certificate representing Buyer Common Stock Payment Shares and the Buyer Preferred Stock Payment Shares comprising Share Consideration shall, until such time that such shares are not so restricted under the Securities Act, bear a legend identical or similar in effect to
the following legend (together with any other legend or legends required by applicable state securities applicable Law or otherwise, if any):
“THE SHARES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933 (THE ‘ACT’) AND MAY NOT BE OFFERED, SOLD OR OTHERWISE TRANSFERRED, ASSIGNED, PLEDGED OR HYPOTHECATED UNLESS REGISTERED UNDER THE ACT OR UNLESS AN EXEMPTION FROM THE REGISTRATION REQUIREMENTS OF THE ACT IS AVAILABLE.”
6.8 Cooperation. Each Party shall use reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary, proper or advisable under applicable Law, to consummate and make effective the transactions contemplated under this Agreement as promptly as practicable. Each Party shall cooperate reasonably with the other Party and shall provide the other Party with such assistance as may be reasonably requested for the purpose of facilitating the performance by each Party of its respective obligations under this Agreement and to enable the combined entity to continue to meet its obligations following the Closing.
6.9 Confidentiality. Except with Buyer’s prior written consent or as reasonably required in furtherance of an Seller Member’s duties as a director, officer or employee of Buyer, for a period of four (4) years commencing on the Closing Date, Seller shall, and shall cause its Affiliates to, hold in confidence any and all confidential and proprietary information, whether written or oral, concerning the Purchased Assets, this Agreement, the other Transaction Documents, or the transactions consummated pursuant hereto and thereto, except to the extent that Seller or such Affiliate can show that such information, at any time during such four (4) year period (a) is generally available to and known by the public through no fault of Seller or any Affiliate or Representative of Seller; (b) is required or compelled to be disclosed by Law (including applicable securities Law, or any judicial, administrative or regulatory process (including by interrogatories, subpoena, civil investigative demand, formal or informal investigative demand or similar process)); or (c) is lawfully acquired by Seller or an Affiliate or Representative of Seller after the Closing from sources that are not restricted from disclosing such information by a legal, contractual or fiduciary obligation. If, during the four (4) year period commencing on the Closing Date, Seller or any Affiliate or Representative of Seller is compelled to disclose any information by judicial or administrative process or by other requirements of Law, Seller shall, or shall cause its Affiliate or Representative to, promptly notify Buyer in writing and shall disclose only that portion of such information that Seller or its Affiliate or Representative is advised by its counsel in writing is legally required to be disclosed, provided that Seller shall, and shall cause its Affiliates and Representatives to, at Buyer’s expense, use commercially reasonable efforts to obtain an appropriate protective order or other reasonable assurance that confidential treatment will be accorded such information.
6.10 Public Announcements. Unless otherwise required by applicable Law (based upon the reasonable advice of counsel), neither Seller, Buyer nor their respective Affiliates and Representatives shall make any public announcements in respect of this Agreement or the transactions contemplated hereby or otherwise communicate with any news media without the prior written consent of the other party (which consent shall not be unreasonably withheld or
delayed) except as reasonably required in furtherance of the duties of a director, officer or employee of Buyer.
6.11 Insurance Proceeds. If Seller or any of its Affiliates receives or collects any insurance proceeds with respect to the Purchased Assets or the Assumed Liabilities, Seller shall, or shall cause such Affiliate to, remit such proceeds to Buyer within five (5) Business Days after its receipt thereof. In addition, if requested by Buyer, Seller shall, if its insurance policies remain in effect, cooperate with Buyer to make and pursue recovery of any claim under an “occurrence” based insurance policy of Seller that relates to the Purchased Assets or the Assumed Liabilities and is based upon an event, state of facts or condition that first existed or occurred prior to the Closing Date; provided that nothing in this Section 6.9 shall require Seller to maintain its insurance policies for any duration after the Closing.
6.12 Transfer Taxes. Fifty percent (50%) of all transfer, documentary, recording, sales, use, stamp, registration, value added or other similar Taxes, charges or fees (including any penalties and interest) incurred in connection with this Agreement and the other Transaction Documents (“Transfer Taxes”) shall be borne and paid by Seller and Buyer, respectively. The party responsible for preparing any Tax Returns or other documents under applicable Law with respect to such Transfer Taxes shall, at its own expense and in the manner prescribed by applicable Law, timely file any Tax Return or other document with respect to such Transfer Taxes or fees (and the other party shall cooperate with respect thereto as may be reasonably necessary), and the parties agree to cooperate to minimize or eliminate any such Transfer Taxes to the extent permitted by applicable Law.
6.13 Certain Tax Matters.
(a) At or after the Closing, all ad valorem, property or other Taxes imposed on a periodic basis pertaining to the Purchased Assets shall be prorated on the basis of the number of days of the relevant Tax year or period which have elapsed through the Closing Date, determined without reference to any change of ownership occasioned by the consummation of the transactions contemplated by this Agreement. Seller shall be responsible for that portion of such amounts relating to the period on or prior to the Closing Date and Buyer shall be responsible for that portion of such amounts relating to the period after the Closing Date. Within one hundred twenty (120) days after the Closing, Seller and Buyer shall each present a statement to the other setting forth the amount of reimbursement to which each is entitled under this Section 6.11, together with such supporting evidence as is reasonably necessary to calculate such amount to be reimbursed. Such amount shall be paid by the Party owing it to the other Party within ten (10) Business Days after delivery of such statement.
(b) Buyer and Seller shall cooperate, as and to the extent reasonably requested by either Party, in connection with the filing of any Tax Returns and any Action with respect to Taxes relating to the Purchased Assets, in each case with respect to any taxable period ending on or before the Closing Date and, with respect to any taxable period that includes (but does not end on) the Closing Date, the portion of such taxable period ending on and including the Closing Date. Such cooperation shall include the retention and (upon a Party’s reasonable request) the provision of records and information which are reasonably relevant to any such Tax Return or Action, making employees available on a mutually convenient basis to provide additional information and
explanation of any material provided hereunder, and timely notification of receipt of any notice of an Action or notice of deficiency relating to any Tax or Tax Return with respect to which the non-recipient Party may have liability hereunder.
6.14 Further Assurances; Access to Records. Each of the parties hereto shall, and shall cause their respective Affiliates to, execute and deliver such additional documents, instruments, conveyances and assurances and take such further actions as may be reasonably required to carry out the provisions of, and give effect to the transactions contemplated by, this Agreement and the other Transaction Documents, including as may be necessary or appropriate to assure fully to Buyer all of the properties, rights, titles, interests, estates, remedies, powers and privileges intended to be conveyed to Buyer under this Agreement and the other Transaction Documents and to assure fully to Seller the assumption of the liabilities and obligations intended to be assumed by Buyer pursuant to this Agreement and the other Transaction Documents. Without limiting the foregoing, Seller agrees to provide to Buyer and its Representatives with, and will cooperate with Buyer as reasonably requested to obtain, access to all documents, books, records (including Tax records), agreements, workpapers and financial data that relate to the Purchased Assets or the Assumed Liabilities for reasonable business purposes, including as reasonably requested in connection with the preparation of financial statements, securities or debt offerings of Buyer or its Affiliates, the conduct of any field exam by a lender or other third party, preparation of Tax Returns, or any Action; provided, however, that such access may be restricted to the extent required by applicable Law or disclosure of any such information would result in the loss or waiver of the attorney-client privilege. In connection with any securities or debt offerings of Buyer or its Affiliates, Seller shall, if requested by Buyer and at Buyer’s expense, request that its pre-Closing accountants deliver customary comfort letters with respect to historical business financial information and consent to, and request that Seller’s pre-Closing accountants deliver consents to, the inclusion of historical business financial information and accountant reports in any offering or debt document or registration statement.
6.15 Release. Seller, on behalf of itself and its respective heirs, legal representatives, successors, and assigns (collectively, the “Releasing Parties”), hereby, irrevocably and unconditionally, fully and forever acquits, releases, covenants not to sue, and discharges and agrees to hold harmless Buyer and its Affiliates, and its and their managers, directors, officers, members, shareholders, partners, employees, agents, legal representatives, predecessors, successors, and assigns (collectively, the “Buyer Releasees”), from any and all actions, claims, charges, demands, damages, Losses, obligations, liabilities, costs, expenses (including reasonable attorneys’ fees and court costs), causes of action, debts, contracts, torts, covenants, fiduciary duties, responsibilities, suits and judgments, at law or in equity, of every nature and kind that Seller, or any of the other Releasing Parties have, may have had, or may have in the future against the Buyer Releasees, whether known or unknown, except obligations arising pursuant to this Agreement and any other Transaction Documents. The release set forth in this Section 6.13 will be binding upon Seller, and its respective heirs, legal representatives, successors, and assigns and will inure to the benefit of the Buyer Releasees and their respective successors and assigns.
6.16 Confidentiality. The parties hereto acknowledge that Buyer and Seller have previously executed that certain Reciprocal Confidential Disclosure Agreement, dated November 1, 2023 (as amended and/or restated from time to time, the “Confidentiality Agreement”) which shall continue in full force and effect in accordance with its terms.
6.17 Service Providers. If Buyer, in its sole discretion, offers any service relationship to any Service Provider, Seller shall use reasonable best efforts to cause such Service Provider(s) to execute any Service Provider Agreement required by Buyer in its discretion (the “New Hire Documents”). Any such Service Provider’s service relationship with Buyer shall be contingent on such Service Provider’s execution and return, within the time period required by Buyer but in no event later than the Closing Date, of the applicable New Hire Documents. Seller (and not Buyer) shall be solely and fully liable for any Liability of any kind related to any Service Provider’s service relationship with Seller for any period of time up to and including the Closing Date. Such Liability expressly includes any vacation, paid time off, severance, salary, wages, bonuses, equity compensation, employment Law Liability and Liability for any other compensation or benefits.
6.18 Non-Competition. Neither Seller nor any of its Affiliates shall (alone or with others, including by supporting, promoting or encouraging) research, develop, manufacture or commercialize the Specified Program. The terms of this Section 6.18 will be binding as to Seller and its Affiliates until the third (3rd) anniversary of the Closing Date. Notwithstanding anything to the contrary herein, the holding as passive investment of up to two percent (2%) of the share capital and/or voting power (determined as if all options, warrants and other convertible securities held by Seller and its Affiliates were exercised, exchanged or converted) of any enterprise shall not be deemed to be a contravention of this Section 6.18. For the avoidance of doubt, none of the Seller Members shall be considered an Affiliate of Seller for the purposes of this Section 6.18.
Article VII
MISCELLANEOUS
7.1 Survival. The representations and warranties of Seller and Buyer contained in this Agreement or any certificate or instrument delivered pursuant to this Agreement shall terminate at the Closing, and only the covenants that by their terms survive the Closing and this Section 7.1 shall survive the Closing.
7.2 Expenses. All costs and expenses, including, without limitation, fees and disbursements of counsel, financial advisors and accountants, incurred in connection with this Agreement and the transactions contemplated hereby shall be paid by the Party incurring such costs and expenses, except that substantially concurrently with the Closing, Buyer shall pay an aggregate of $330,000 in the amounts and to the persons and entities set forth on Section 7.2 of the Seller Disclosure Letter.
7.3 Notices. All notices, requests, consents, claims, demands, waivers and other communications hereunder shall be in writing and shall be deemed to have been given (a) when delivered by hand (with written confirmation of receipt); (b) when received by the addressee if sent by a nationally recognized overnight courier (receipt requested); (c) on the date sent by e-mail of a PDF document (with confirmation of transmission) if sent during normal business hours of the recipient, and on the next Business Day if sent after normal business hours of the recipient or (d) on the third (3rd) day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective parties at the following addresses (or at such other address for a party as shall be specified in a notice given in accordance with this Section 7.3):
|
|
If to Seller: |
Bridge Medicines LLC 420 East 70th Street, Suite 510 New York, NY 10021 with a copy (which shall not constitute notice) to: Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP One Marina Park Drive, Suite 900 Boston, MA 02210 |
If to Buyer: |
Galecto, Inc. 75 State Street, Suite 100
Boston, MA 02109 with a copy (which shall not constitute notice) to: Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. 919 Third Avenue, New York, NY 10022 |
7.4 Interpretation. For purposes of this Agreement, (i) the words “include,” “includes” and “including” shall be deemed to be followed by the words “without limitation”; (ii) the word “or” is not exclusive; and (iii) the words “herein,” “hereof,” “hereby,” “hereto” and “hereunder” refer to this Agreement as a whole. Unless the context otherwise requires, references herein: (a) to Exhibits and Schedules mean the Exhibits and Schedules contained in the Seller Disclosure Letter and Buyer Disclosure Letter attached to this Agreement; (b) to an agreement, instrument or other document means such agreement, instrument or other document as amended, supplemented and modified from time to time to the extent permitted by the provisions thereof and (c) to a statute means such statute as amended from time to time and includes any successor legislation thereto and any regulations promulgated thereunder. This Agreement shall be construed without regard to any presumption or rule requiring construction or interpretation against the party drafting an instrument or causing any instrument to be drafted. The schedules, the Seller Disclosure Letter and the Buyer Disclosure Letter referred to herein shall be construed with, and as an integral part of, this Agreement to the same extent as if they were set forth verbatim herein.
7.5 Headings. The headings in this Agreement are for reference only and shall not affect the interpretation of this Agreement.
7.6 Severability. If any term or provision of this Agreement is invalid, illegal or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other term or provision of this Agreement or invalidate or render unenforceable such term or provision in any other jurisdiction. Upon such determination that any term or other provision is invalid, illegal or unenforceable, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the transactions contemplated hereby be consummated as originally contemplated to the greatest extent possible.
7.7 Entire Agreement. This Agreement (including the Seller Disclosure Letter and the Buyer Disclosure Letter) and the other Transaction Documents constitute the sole and entire agreement of the parties to this Agreement with respect to the subject matter contained herein and therein, and supersede all prior and contemporaneous understandings and agreements, both written and oral, with respect to such subject matter. In the event of any inconsistency between the statements in the body of this Agreement and those in the other Transaction Documents, the Seller Disclosure Letter and the Buyer Disclosure Letter (other than an exception expressly set forth as such in the Seller Disclosure Letter or the Buyer Disclosure Letter), the statements in the body of this Agreement will control.
7.8 Successors and Assigns. This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties and their respective successors and permitted assigns; provided, however, that neither this Agreement nor any of a Party’s rights or obligations hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s prior written consent shall be void and of no effect.
7.9 No Third-Party Beneficiaries. This Agreement is for the sole benefit of the parties hereto and their respective successors and permitted assigns and nothing herein, express or implied, is intended to or shall confer upon any other Person or entity any legal or equitable right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
7.10 Amendment and Modification; Waiver. This Agreement may only be amended, modified or supplemented by an agreement in writing signed by each party hereto. No waiver by any party of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any right, remedy, power or privilege arising from this Agreement shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
7.11 Governing Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflict of laws. In any action or proceeding between any of the Parties arising out of or relating to this Agreement or any of the Transaction, each of the Parties:
(a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the United States District Court for the District of Delaware or, to the extent that neither of the foregoing courts has jurisdiction, the Superior Court of the State of Delaware; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of this Section 7.11; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any Party; (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given in accordance with Section 7.3 of this Agreement; and (f) irrevocably and unconditionally waives the right to trial by jury.
7.12 Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a Party will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise of any other remedy. The Parties agree that irreparable damage for which monetary damages, even if available, would not be an adequate remedy, would occur in the event that any Party does not perform the provisions of this Agreement (including failing to take such actions as are required of it hereunder to consummate this Agreement) in accordance with its specified terms or otherwise breaches such provisions. Accordingly, the Parties acknowledge and agree that the Parties shall be entitled to an injunction, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof, in addition to any other remedy to which they are entitled at law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance or other equitable relief on the basis that any other Party has an adequate remedy at law or that any award of specific performance is not an appropriate remedy for any reason at law or in equity. Any Party seeking an injunction or injunctions to prevent breaches of this Agreement shall not be required to provide any bond or other security in connection with any such order or injunction.
7.13 Waiver of Jury Trial. EACH OF THE PARTIES HEREBY IRREVOCABLY WAIVES ALL RIGHT TO A TRIAL BY JURY IN ANY ACTION, PROCEEDING OR COUNTERCLAIM (INCLUDING ANY ACTION OR LIABILITY INVOLVING ANY OF THE FINANCING SOURCES) ARISING OUT OF OR RELATING TO THIS AGREEMENT, THE OTHER TRANSACTION DOCUMENTS, OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY.
7.14 Counterparts. This Agreement may be executed in any number of counterparts, each of which when so executed and delivered shall be deemed to be an original and all of which taken together constitute one and the same instrument. This Agreement may be (i) transmitted for reproduction and execution by any means now known or hereafter devised, including facsimile or electronic file transmission, and (ii) converted from its original software program to another and/or printed on different paper formats or in different fonts, any or all of which may result in variations to the pagination and appearance of the counterpart versions of this Agreement. The execution and delivery of counterparts of this Agreement, by facsimile, by electronic file transmission or by original manual signature, regardless of the means or any variation in pagination or appearance, shall be binding upon the parties. Any party delivering an executed counterpart of this Agreement
by facsimile or by electronic file transmission shall also deliver a manually executed counterpart of this Agreement to each other party, but failure to do so shall not affect the validity, enforceability or binding effect of this Agreement.
[SIGNATURE PAGES FOLLOW]
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be executed as of the date first written above.
GALECTO, INC.
By: /s/Hans T. Schambye
Name: Hans T. Schambye
Title: Chief Executive Officer
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be executed as of the date first written above.
BRIDGE MEDICINES LLC
By: /s/ Matt Kronmiller
Name: Matt Kronmiller
Title: Chief Executive Officer
GALECTO, INC.
CERTIFICATE OF DESIGNATION OF PREFERENCES,
RIGHTS AND LIMITATIONS
OF
SERIES A NON-VOTING CONVERTIBLE PREFERRED STOCK
Pursuant to Section 151 of the
General Corporation Law of the State of Delaware
THE UNDERSIGNED DOES HEREBY CERTIFY, on behalf of Galecto, Inc., a Delaware corporation (the “Corporation”), that the following resolution was duly adopted by the Board of Directors of the Corporation (the “Board of Directors”), in accordance with the provisions of Section 151 of the General Corporation Law of the State of Delaware (the “DGCL”), at a meeting duly called and held on October 2, 2024, which resolution provides for the creation of a series of the Corporation’s Preferred Stock, par value $0.00001 per share, which is designated as “Series A Non-Voting Convertible Preferred Stock”, with the preferences, rights and limitations set forth therein relating to dividends, conversion, redemption, dissolution and distribution of assets of the Corporation.
WHEREAS: the Amended and Restated Certificate of Incorporation of the Corporation (as amended from time to time, the “Certificate of Incorporation”), provides for a class of its authorized stock known as Preferred Stock, consisting of 10,000,000 shares, $0.00001 par value per share (the “Preferred Stock”), issuable from time to time in one or more series.
RESOLVED: that, pursuant to authority conferred upon the Board of Directors by the Certificate of Incorporation, (i) a series of Preferred Stock of the Corporation be, and hereby is authorized by the Board of Directors, (ii) the Board of Directors hereby authorizes the issuance of 200 shares of “Series A Non-Voting Convertible Preferred Stock” pursuant to the terms of the Asset Purchase Agreement, dated as of the date hereof, by and between the Corporation and Bridge Medicines LLC, a Delaware limited liability company (the “Asset Purchase Agreement”), and (iii) the Board of Directors hereby fixes the designations, powers, preferences and relative, participating, optional or other special rights, and the qualifications, limitations or restrictions thereof, of such shares of Preferred Stock, in addition to any provisions set forth in the Certificate of Incorporation that are applicable to the Preferred Stock of all classes and series, as follows:
TERMS OF SERIES A NON-VOTING CONVERTIBLE PREFERRED STOCK
1. Definitions. For the purposes hereof, the following terms shall have the following meanings:
“Business Day” means any day except any Saturday, any Sunday, any day which is a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Buy-In” shall have the meaning set forth in Section 6.5.4.
“Closing Sale Price” means, for any security as of any date, the last closing trade price for such security immediately prior to 4:00 p.m., New York City time, on the principal Trading Market where such security is listed or traded, as reported by Bloomberg, L.P. (or an equivalent, reliable reporting service), or if the foregoing do not apply, the last trade price of such security in the over-the-counter market on the electronic bulletin board for such security as reported by Bloomberg, L.P., or, if no last trade price is reported for such security by Bloomberg, L.P., the average of the bid prices of any market makers for such security as reported on the OTC Pink Market by OTC Markets Group, Inc.
“Commission” means the United States Securities and Exchange Commission.
“Common Stock” means the Corporation’s common stock, par value $0.00001 per share, and stock of any other class of securities into which such securities may hereafter be reclassified or changed.
“Conversion Shares” means, collectively, the shares of Common Stock issuable upon conversion of the shares of Series A Non-Voting Preferred Stock in accordance with the terms hereof.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Holder” means a holder of shares of Series A Non-Voting Preferred Stock.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Standard Settlement Period” means the standard settlement period, expressed in a number of Trading Days, for the Corporation’s Trading Market or quotation system with respect to the Common Stock that is in effect on the date of delivery of an applicable Notice of Conversion.
“Trading Day” means a day on which the principal Trading Market is open for business.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, or the New York Stock Exchange (or any successors to any of the foregoing).
2. Designation, Amount and Par Value. The series of Preferred Stock shall be designated as the Corporation’s Series A Non-Voting Convertible Preferred Stock (the “Series A Non-Voting Preferred Stock”) and the number of shares so designated shall be 200. Each share of Series A Non-Voting Preferred Stock shall have a par value of $0.00001 per share.
3. Dividends. Holders shall be entitled to receive, and the Corporation shall pay, dividends on shares of the Series A Non-Voting Preferred Stock (on an as-if-converted-to-Common-Stock basis, without regard to the Beneficial Ownership Limitation (as defined below)) equal to and in the same form, and in the same manner, as dividends (other than dividends on shares of the Common Stock payable in the form of Common Stock) actually paid on shares of the Common Stock when, as and if such dividends (other than dividends payable in the form of Common Stock) are paid on shares of the Common Stock. Other than as set forth in the previous sentence, no other dividends shall be paid on shares of Series A Non-Voting Preferred Stock, and the Corporation shall pay no dividends (other than dividends payable in the form of Common Stock) on shares of the Common Stock unless it simultaneously complies with the previous sentence.
4. Voting Rights.
4.1 Except as otherwise provided herein or as otherwise required by the DGCL, the Series A Non-Voting Preferred Stock shall have no voting rights. However, as long as any shares of Series A Non-Voting Preferred Stock are outstanding, the Corporation shall not, without the affirmative vote or written waiver of the holders of a majority of the then outstanding shares of the Series A Non-Voting Preferred Stock: (i) alter or change adversely the powers, preferences or rights given to the Series A Non-Voting Preferred Stock or alter or amend this Certificate of Designation of Preferences, Rights and Limitations of Series A Non-Voting Convertible Preferred Stock (the “Certificate of Designation”), amend or repeal any provision of, or add any provision to, the Certificate of Incorporation or Amended and Restated Bylaws of the Corporation, or file any articles of amendment, certificate of designations, preferences, limitations and relative rights of any series of Preferred Stock, in each case if such action would adversely alter or change the preferences, rights, privileges or powers of, or restrictions provided for the benefit of the Series A Non-Voting Preferred Stock, regardless of whether any of the foregoing actions shall be by means of amendment to the Certificate of Incorporation or by merger, consolidation, domestication, transfer, continuance, recapitalization, reclassification, waiver, statutory conversion or otherwise, (ii) issue further shares of Series A Non-Voting Preferred Stock or increase or decrease (other than by conversion) the number of authorized shares of Series A Non-Voting Preferred Stock, (iii) prior to the Stockholder Approval (as defined below), consummate either: (A)
any Fundamental Transaction (as defined below) or (B) any merger or consolidation of the Corporation with or into another entity or any stock sale to, or other business combination in which the stockholders of the Corporation immediately before such transaction do not hold at least a majority of the voting power of the capital stock of the Corporation or such other entity immediately after such transaction, (iv) amend, in any manner that would be reasonably likely to prevent, impede or materially delay the Stockholder Approval or the Automatic Conversion (as defined below), or terminate, any of those certain Support Agreements entered into by the Corporation, Bridge Medicines LLC and certain holders of shares of Common Stock, or agree to any transfer, sale or disposition of such shares subject to the Support Agreements (except for such transfers, sales or dispositions with respect to which the approval of the Corporation is not required pursuant to the applicable Support Agreement), (v) amend, or fail to comply with, in each case in any manner that would be reasonably likely to prevent, impede or materially delay the Stockholder Approval, Sections 6.1 or 6.2 of the Asset Purchase Agreement, or (vi) enter into any agreement with respect to any of the foregoing that does not explicitly require the approval contemplated herein to consummate such transaction. Holders of shares of Common Stock acquired upon the conversion of shares of Series A Non-Voting Preferred Stock shall be entitled to the same voting rights as each other holder of Common Stock; except that such holders may not vote such shares upon the proposal for the Stockholder Approval in accordance with Rule 5635 of the listing rules of The Nasdaq Stock Market LLC.
4.2 Any vote required or permitted under Section 4.1 may be taken at a meeting of the Holders or through the execution of an action by written consent in lieu of such meeting or other written waiver by such stockholders, provided that the consent or waiver is executed by Holders representing a majority of the outstanding shares of Series A Non-Voting Preferred Stock, unless a higher percentage is required by the DGCL, in which case the written consent of the Holders of not less than such higher percentage shall be required.
5. Rank; Liquidation.
5.1 The Series A Non-Voting Preferred Stock shall rank on parity with the Common Stock as to distributions of assets upon liquidation, dissolution or winding up of the Corporation, whether voluntarily or involuntarily.
5.2 Upon any liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary (a “Liquidation”), each Holder shall be entitled to receive out of the assets, whether capital or surplus, of the Corporation the same amount that a holder of Common Stock would receive if the Series A Non-Voting Preferred Stock were fully converted (disregarding for such purpose any Beneficial Ownership Limitations and assuming receipt of Stockholder Approval) to Common Stock which amounts shall be paid pari passu with all holders of Common Stock, plus an additional amount equal to any dividends declared on but unpaid to such shares. If, upon any such Liquidation, the assets of the Corporation shall be insufficient to pay the Holders of shares of the Series A Non-Voting Preferred Stock the amount required under the preceding sentence, then all remaining assets of the Corporation shall be distributed ratably to the Holders and the holders of Common Stock in accordance with the respective amounts that would be payable on all such securities if all amounts payable thereon were paid in full. For the avoidance of any doubt, a Fundamental Transaction shall not be deemed a Liquidation unless the Corporation expressly declares that such Fundamental Transaction shall be treated as if it were a Liquidation.
6. Conversion.
6.1 Automatic Conversion on Stockholder Approval. Effective as of 5:00 p.m. Eastern time on the third Business Day after the date that the Corporation’s stockholders approve the conversion of the Series A Non-Voting Preferred Stock into shares of Common Stock in accordance with the listing rules of the Nasdaq Stock Market, as set forth in Section 6.1 of the Asset Purchase Agreement (the “Stockholder Approval”), each share of Series A Non-Voting Preferred Stock then outstanding shall automatically convert into a number of shares of Common Stock equal to the Conversion Ratio (as defined below), subject to the Beneficial Ownership Limitation (the “Automatic Conversion”). The Corporation shall inform each Holder of the occurrence of the Stockholder Approval within one Business Day of such Stockholder Approval, it being understood that the filing of a Current Report on Form 8-K reporting the Stockholder Approval shall be deemed sufficient to inform the Holders. In determining the application of the Beneficial Ownership Limitations solely with respect to the Automatic Conversion, the Corporation shall calculate beneficial ownership for each Holder assuming beneficial ownership by such Holder of: (x) the number of shares of Common Stock issuable to such Holder in such Automatic Conversion, plus (y) any additional shares of
Common Stock for which a Holder has provided the Corporation with prior written notice of beneficial ownership no less than five days prior to the date of Stockholder Approval (a “Beneficial Ownership Statement”) and assuming the conversion of all shares of Series A Non-Voting Preferred Stock held by all other Holders less the aggregate number of shares of Series A Non-Voting Preferred Stock held by all other Holders that will not convert into shares of Common Stock on account of the application of any Beneficial Ownership Limitations applicable to any such other Holders. If a Holder fails to provide the Corporation with a Beneficial Ownership Statement at least five days prior to the date of Stockholder Approval, then the Corporation shall presume the Holder’s beneficial ownership of Common Stock (excluding the Conversion Shares) to be zero or such other number of shares of Common Stock as the Corporation shall have reason to believe are beneficially owned by such Holder. The shares of Series A Non-Voting Preferred Stock that are converted in the Automatic Conversion are referred to as the “Converted Stock”. For the avoidance of doubt, any shares of Series A Non-Voting Preferred Stock that are not automatically converted pursuant to the Automatic Conversion as a result of a Beneficial Ownership Limitation shall remain outstanding until such shares of Series A Non-Voting Preferred Stock are converted pursuant to Section 6.2. The Conversion Shares shall be issued as follows:
6.1.1 Converted Stock that is registered in book entry form shall be automatically cancelled upon the Automatic Conversion and converted into the corresponding Conversion Shares, which shares shall be issued in book entry form and without any action on the part of the Holders and shall be delivered to the Holders within one (1) Business Day of the effectiveness of the Automatic Conversion.
6.1.2 Converted Stock that is issued in certificated form shall be deemed converted into the corresponding Conversion Shares on the date of Automatic Conversion and the Holder’s rights as a holder of such shares of Converted Stock shall cease and terminate on such date, excepting only the right to receive the Conversion Shares upon the Holder tendering to the Corporation (or its designated agent) the stock certificate(s) (duly endorsed) representing such certificated Converted Stock.
6.1.3 Notwithstanding the cancellation of the Converted Stock upon the Automatic Conversion, Holders of Converted Stock shall continue to have any remedies provided herein or otherwise available at law or in equity to such Holder because of a failure by the Corporation to comply with the terms of this Certificate of Designation. In all cases, the Holder shall retain all of its rights and remedies for the Corporation’s failure to convert the Converted Stock.
6.2 Conversion at Option of Holder. Subject to Section 6.1, Section 6.4 and Section 6.5.3, each share of Series A Non-Voting Preferred Stock then outstanding that is not otherwise converted into Common Stock upon the Automatic Conversion as a result of the applicability of a Beneficial Ownership Limitation shall be convertible, at any time and from time to time following 5:00 p.m. Eastern time on the third Business Day after the date that the Stockholder Approval is obtained by the Corporation, at the option of the Holder thereof, into a number of shares of Common Stock equal to the Conversion Ratio, subject to the Beneficial Ownership Limitation (each, an “Optional Conversion”). Holders shall effect conversions by providing the Corporation with the form of conversion notice attached hereto as Annex A (a “Notice of Conversion”), duly completed and executed. Provided the Corporation’s transfer agent is participating in the Depository Trust Company (“DTC”) Fast Automated Securities Transfer program, following the expiration of the lock-up period as set forth in Section 6.4 of the Asset Purchase Agreement (the “Lock-up Period”), the Notice of Optional Conversion may specify, at the Holder’s election, whether the applicable Conversion Shares shall be credited to the account of the Holder’s prime broker with DTC through its Deposit Withdrawal Agent Commission system (a “DWAC Delivery”). The date on which an Optional Conversion shall be deemed effective (the “Conversion Date”) shall be the Trading Day that the Notice of Conversion, completed and executed, is sent via email to, and received during regular business hours by, the Corporation; provided, that the original certificate(s) (if any) representing such shares of Series A Non-Voting Preferred Stock being converted, duly endorsed, and the accompanying Notice of Conversion, are received by the Corporation within two (2) Trading Days thereafter. In all other cases, the Conversion Date shall be defined as the Trading Day on which the original certificate(s) (if any) representing such shares of Series A Non-Voting Preferred Stock being converted, duly endorsed, and the accompanying Notice of Conversion, are received by the Corporation. The calculations set forth in the Notice of Conversion shall control in the absence of manifest or mathematical error.
6.3 Conversion Ratio. The “Conversion Ratio” for each share of Series A Non-Voting Preferred Stock shall be 1,000 shares of Common Stock issuable upon the conversion (the “Conversion”) of each share of Series A Non-Voting Preferred Stock (corresponding to a ratio of 1,000:1), subject to adjustment as provided herein.
6.4 Beneficial Ownership Limitation. Notwithstanding anything herein to the contrary, the Corporation shall not effect any conversion of any share of Series A Non-Voting Preferred Stock, including pursuant to Section 6.1, and a Holder shall not have the right to convert any portion of the Series A Non-Voting Preferred Stock pursuant to Section 6.2, to the extent that, after giving effect to such attempted conversion set forth on an applicable Notice of Conversion with respect to the Series A Non-Voting Preferred Stock, such Holder (or any of such Holder’s Affiliates or any other Person who would be a beneficial owner of Common Stock beneficially owned by the Holder for purposes of Section 13(d) or Section 16 of the Exchange Act and the applicable rules and regulations of the Commission, including any “group” of which the Holder is a member (the foregoing, “Attribution Parties”)) would beneficially own a number of shares of Common Stock in excess of the Beneficial Ownership Limitation. For purposes of the foregoing sentence, the aggregate number of shares of Common Stock beneficially owned by such Holder and its Attribution Parties shall include the number of shares of Common Stock issuable upon conversion of the Series A Non-Voting Preferred Stock subject to the Notice of Conversion or the Automatic Conversion, as applicable, with respect to which such determination is being made, but shall exclude the number of shares of Common Stock which are issuable upon (A) conversion of the remaining, unconverted Series A Non-Voting Preferred Stock beneficially owned by such Holder or any of its Attribution Parties, and (B) exercise or conversion of the unexercised or unconverted portion of any other securities of the Corporation (including any warrants) beneficially owned by such Holder or any of its Attribution Parties that are subject to and would exceed a limitation on conversion or exercise similar to the limitation contained herein. Except as set forth in the preceding sentence, for purposes of this Section 6.4, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the applicable rules and regulations of the Commission, and the terms “beneficial ownership” and “beneficially own” have the meanings ascribed to such terms therein. In addition, for purposes hereof, “group” has the meaning set forth in Section 13(d) of the Exchange Act and the applicable rules and regulations of the Commission. For purposes of this Section 6.4, in determining the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as stated in the most recent of the following: (A) the Corporation’s most recent periodic or annual filing with the Commission, as the case may be, (B) a more recent public announcement by the Corporation that is filed with the Commission, or (C) a more recent notice by the Corporation or the Corporation’s transfer agent to the Holder setting forth the number of shares of Common Stock then outstanding. Upon the written request of a Holder (which may be by email), the Corporation shall, within two (2) Trading Days thereof, confirm in writing to such Holder (which may be via email) the number of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to any actual conversion or exercise of securities of the Corporation, including shares of Series A Non-Voting Preferred Stock, by such Holder or its Attribution Parties since the date as of which such number of outstanding shares of Common Stock was last publicly reported or confirmed to the Holder. The “Beneficial Ownership Limitation” shall initially be set at 4.9% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock pursuant to such Notice of Conversion or Automatic Conversion (as applicable) to the extent permitted pursuant to this Section 6.4. The Corporation shall be entitled to rely on representations made to it by the Holder in any Notice of Conversion regarding its Beneficial Ownership Limitation. Notwithstanding the foregoing, by written notice to the Corporation (which may be via email), (i) the Holder may reset the Beneficial Ownership Limitation percentage to a higher percentage, not to exceed 19.9%, which increase will not be effective until the sixty-first (61st) day after such written notice is delivered to the Corporation, and (ii) the Holder may reset the Beneficial Ownership Limitation percentage to a lower percentage effective immediately after the delivery of such notice to the Corporation, provided that such decrease shall not become effective until the later of (x) 5:00 p.m. Eastern time on the third Business Day after the date of the Stockholder Approval and (y) if Stockholder Approval is not obtained within six months after the initial issuance of the Series A Non-Voting Preferred Stock, the date that is three Business Days after the date that is six months after the initial issuance of the Series A Non-Voting Preferred Stock. Upon such a change by a Holder of the Beneficial Ownership Limitation, not to exceed 19.9%, the Beneficial Ownership Limitation may not be further amended by such Holder without first providing the minimum notice required by this Section 6.4. Notwithstanding the foregoing, at any time following notice of a Fundamental Transaction, the Holder may waive and/or change the Beneficial Ownership Limitation effective immediately upon written notice to the Corporation and may reinstitute a Beneficial Ownership Limitation at any time thereafter effective immediately upon written notice to the Corporation. The provisions of this Section 6.4 shall be construed, corrected and implemented in a manner so as to effectuate the intended Beneficial Ownership Limitation herein contained and the shares of Common
Stock underlying the Series A Non-Voting Preferred Stock in excess of the Beneficial Ownership Limitation shall not be deemed to be beneficially owned by the Holder for any purpose including for purposes of Section 13(d) or Rule 16a-1(a)(1) of the Exchange Act.
6.5 Mechanics of Conversion.
6.5.1 Delivery of Certificate or Electronic Issuance. Upon Conversion not later than the lesser of two (2) Trading Days and the number of Trading Days comprising the Standard Settlement Period after, in the case of the Automatic Conversion, the Stockholder Approval, or in the case of an Optional Conversion, the applicable Conversion Date, or if the Holder requests the issuance of physical certificate(s), not later than the lesser of two (2) Trading Days and the number of Trading Days comprising the Standard Settlement Period after receipt by the Corporation of the original certificate(s) representing such shares of Series A Non-Voting Preferred Stock being converted, duly endorsed, and the accompanying Notice of Conversion (the “Share Delivery Date”), the Corporation shall either (a) deliver, or cause to be delivered, to the converting Holder a physical certificate or certificates representing the number of Conversion Shares being acquired upon the conversion of shares of Series A Non-Voting Preferred Stock, or (b) following the expiration of the Lock-up Period in the case of a DWAC Delivery (if so request by the Holder), electronically transfer such Conversion Shares by crediting the account of the Holder’s prime broker with DTC through its DWAC system. If in the case of any Notice of Conversion such certificate or certificates for the Conversion Shares are not delivered to or as directed by or, following the expiration of the Lock-up Period, in the case of a DWAC Delivery, such shares are not electronically delivered to or as directed by, the applicable Holder by the Share Delivery Date, the applicable Holder shall be entitled to elect to rescind such Notice of Conversion by written notice to the Corporation at any time on or before its receipt of such certificate or certificates for Conversion Shares or electronic receipt of such shares, as applicable, in which event the Corporation shall promptly return to such Holder any original Series A Non-Voting Preferred Stock certificate delivered to the Corporation and such Holder shall promptly return to the Corporation any Common Stock certificates or, following the expiration of the Lock-up Period, otherwise direct the return of any shares of Common Stock delivered to the Holder through the DWAC system, representing the shares of Series A Non-Voting Preferred Stock unsuccessfully tendered for conversion to the Corporation.
6.5.2 Obligation Absolute. Subject to Section 6.4 and subject to Holder’s right to rescind a Notice of Conversion pursuant to Section 6.5.1, the Corporation’s obligation to issue and deliver the Conversion Shares upon conversion of Series A Non-Voting Preferred Stock in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by a Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any Person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by such Holder or any other Person of any obligation to the Corporation or any violation or alleged violation of law by such Holder or any other Person, and irrespective of any other circumstance which might otherwise limit such obligation of the Corporation to such Holder in connection with the issuance of such Conversion Shares. Subject to Section 6.4 and subject to Holder’s right to rescind a Notice of Conversion pursuant to Section 6.5.1, in the event a Holder shall elect to convert any or all of its Series A Non-Voting Preferred Stock, the Corporation may not refuse conversion based on any claim that such Holder or anyone associated or affiliated with such Holder has been engaged in any violation of law, agreement or for any other reason, unless an injunction from a court, on notice to Holder, restraining and/or enjoining conversion of all or part of the Series A Non-Voting Preferred Stock of such Holder shall have been sought and obtained by the Corporation, and the Corporation posts a surety bond for the benefit of such Holder in the amount of 150% of the value of the Conversion Shares into which would be converted the Series A Non-Voting Preferred Stock which is subject to such injunction, which bond shall remain in effect until the completion of arbitration/litigation of the underlying dispute and the proceeds of which shall be payable to such Holder to the extent it obtains judgment. In the absence of such injunction, the Corporation shall, subject to Section 6.4 and subject to Holder’s right to rescind a Notice of Conversion pursuant to Section 6.5.1, issue Conversion Shares upon a properly noticed conversion.
6.5.3 Cash Settlement. If, at any time after the earlier of Stockholder Approval or six months after the initial issuance of the Series A Non-Voting Preferred Stock, the Corporation fails to deliver to a Holder such certificate or certificates, pursuant to Section 6.5.1 on or prior to the third (3rd) Trading Day after the Share Delivery Date applicable to such conversion other than a failure caused by (i) incorrect or incomplete information provided by Holder to the Corporation or (ii) the application of the Beneficial Ownership Limitation after
Stockholder Approval (but, prior to the Stockholder Approval, disregarding for such purpose any Beneficial Ownership Limitation), then, unless the Holder has rescinded the applicable Notice of Conversion pursuant to Section 6.5.1, the Corporation shall, at the request of the Holder, pay an amount equal to the Fair Value (as defined below) of such undelivered shares, with such payment to be made within three Business Days from the date of request by the Holder, whereupon the Corporation’s obligations to deliver such shares underlying the Notice of Conversion shall be extinguished upon payment in full of the Fair Value of such undelivered shares. For purposes of this Section 6.5.3, the “Fair Value” of shares shall be fixed with reference to the last reported Closing Sale Price on the principal Trading Market on which the Common Stock is listed as of the Trading Day immediately prior to the date on which the Notice of Conversion is delivered to the Corporation. For the avoidance of doubt, the cash settlement provisions set forth in this Section 6.5.3 shall be available irrespective of the reason for the Corporation’s failure to timely deliver Conversion Shares (other than a failure caused by (i) incorrect or incomplete information provided by Holder to the Corporation or (ii) the application of the Beneficial Ownership Limitation after Stockholder Approval (but, prior to the Stockholder Approval, disregarding for such purpose any Beneficial Ownership Limitation)), including due to limitations set forth in Section 6.5.6, the lack of obtaining Stockholder Approval, or due to applicable Trading Market rules.
6.5.4 Buy-In on Failure to Timely Deliver Certificates. If the Corporation fails to deliver to a Holder the applicable certificate or certificates or, following the expiration of the Lock-up Period, to effect a DWAC Delivery, as applicable, by the Share Delivery Date pursuant to Section 6.5.1 (other than due to a failure caused by materially incorrect or incomplete information provided by Holder to the Corporation or the application of the Beneficial Ownership Limitation), and if after such Share Delivery Date such Holder is required by its brokerage firm to purchase (in an open market transaction or otherwise), or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by such Holder of the Conversion Shares which such Holder was entitled to receive upon the conversion relating to such Share Delivery Date (a “Buy-In”), then the Corporation shall (A) pay in cash to such Holder (in addition to any other remedies available to or elected by such Holder) the amount by which (x) such Holder’s total purchase price (including any brokerage commissions) for the shares of Common Stock so purchased exceeds (y) the product of (1) the aggregate number of shares of Common Stock that such Holder was entitled to receive from the conversion at issue multiplied by (2) the actual sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of such Holder, either reissue (if surrendered) the shares of Series A Non-Voting Preferred Stock equal to the number of shares of Series A Non-Voting Preferred Stock submitted for conversion or deliver to such Holder the number of shares of Common Stock that would have been issued if the Corporation had timely complied with its delivery requirements under Section 6.5.1. For example, if a Holder purchases shares of Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted conversion of shares of Series A Non-Voting Preferred Stock with respect to which the actual sale price (including any brokerage commissions) giving rise to such purchase obligation was a total of $10,000 under clause (A) of the immediately preceding sentence, the Corporation shall be required to pay such Holder $1,000. The Holder shall provide the Corporation written notice, within three (3) Trading Days after the occurrence of a Buy-In, indicating the amounts payable to such Holder in respect of such Buy-In together with applicable confirmations and other evidence reasonably requested by the Corporation. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Corporation’s failure to timely deliver certificates representing shares of Common Stock upon conversion of the shares of Series A Non-Voting Preferred Stock as required pursuant to the terms hereof or the cash settlement remedy set forth in Section 6.5.3; provided, however, that the Holder shall not be entitled to both (i) require the reissuance of the shares of Series A Non-Voting Preferred Stock submitted for conversion for which such conversion was not timely honored and (ii) receive the number of shares of Common Stock that would have been issued if the Corporation had timely complied with its delivery requirements under Section 6.5.1.
6.5.5 Reservation of Shares Issuable Upon Conversion. The Corporation covenants that at all times it will reserve and keep available out of its authorized and unissued shares of Common Stock for the sole purpose of issuance upon conversion of the Series A Non-Voting Preferred Stock, free from preemptive rights or any other actual contingent purchase rights of Persons other than the Holders of the Series A Non-Voting Preferred Stock, not less than such aggregate number of shares of the Common Stock as shall be issuable (taking into account the adjustments of Section 7) upon the conversion of all outstanding shares of Series A Non-Voting Preferred Stock. The Corporation covenants that all shares of Common Stock that shall be so issuable shall, upon issue, be duly authorized, validly issued, fully paid and non-assessable.
6.5.6 Fractional Shares. No fractional shares of Common Stock shall be issued upon conversion of the Series A Non-Voting Preferred Stock and no certificates or scrip for any such fractional shares shall be issued. Any fractional shares of Common Stock that a Holder of Series A Non-Voting Preferred Stock would otherwise be entitled to receive shall be aggregated with all fractional shares of Common Stock issuable to such Holder and any remaining fractional shares shall be rounded down to the nearest whole share. In lieu of any fractional shares to which a Holder would otherwise be entitled, the Corporation shall pay cash equal to such fraction multiplied by the closing price of the Common Stock on the Trading Market on the Trading Day immediately preceding the applicable Conversion Date. Whether or not fractional shares would be issuable upon such conversion shall be determined on the basis of the total number of shares of Series A Non-Voting Preferred Stock the Holder is at the time converting into Common Stock and the aggregate number of shares of Common Stock issuable upon such conversion.
6.5.7 Transfer Taxes. The issuance of certificates for shares of the Common Stock upon conversion of the Series A Non-Voting Preferred Stock shall be made without charge to any Holder for any documentary stamp or similar taxes that may be payable in respect of the issue or delivery of such certificates, provided that the Corporation shall not be required to pay any tax that may be payable in respect of any transfer involved in the issuance and delivery of any such certificate upon conversion in a name other than that of the registered Holder(s) of such shares of Series A Non-Voting Preferred Stock and the Corporation shall not be required to issue or deliver such certificates unless or until the Person or Persons requesting the issuance thereof shall have paid to the Corporation the amount of such tax or shall have established to the satisfaction of the Corporation that such tax has been paid.
6.6 Status as Stockholder. Upon the date of conversion, (i) the shares of Series A Non-Voting Preferred Stock being converted shall be deemed converted into shares of Common Stock and (ii) the Holder’s rights as a holder of such converted shares of Series A Non-Voting Preferred Stock shall cease and terminate, excepting only the right to receive certificates for such shares of Common Stock and to any remedies provided herein or otherwise available at law or in equity to such Holder because of a failure by the Corporation to comply with the terms of this Certificate of Designation. In all cases, the Holder shall retain all of its rights and remedies for the Corporation’s failure to convert Series A Non-Voting Preferred Stock. In no event shall the Series A Non-Voting Preferred Stock convert into shares of Common Stock prior to the Stockholder Approval.
7. Certain Adjustments.
7.1 Stock Dividends and Stock Splits. If the Corporation, at any time while this Series A Non-Voting Preferred Stock is outstanding: (A) pays a stock dividend or otherwise makes a distribution or distributions payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Corporation upon conversion of this Series A Non-Voting Preferred Stock) with respect to the then outstanding shares of Common Stock; (B) subdivides outstanding shares of Common Stock into a larger number of shares; or (C) combines (including by way of a reverse stock split) outstanding shares of Common Stock into a smaller number of shares, then the Conversion Ratio shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding any treasury shares of the Corporation) outstanding immediately after such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately before such event (excluding any treasury shares of the Corporation). Any adjustment made pursuant to this Section 7.1 shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision or combination.
7.2 Fundamental Transaction. If, at any time while this Series A Non-Voting Preferred Stock is outstanding, (A) the Corporation effects any merger or consolidation of the Corporation with or into another Person or any stock sale to, or other business combination (including, without limitation, a reorganization, recapitalization, spin-off, share exchange or scheme of arrangement) with or into another Person (other than such a transaction in which the Corporation is the surviving or continuing entity and its Common Stock is not exchanged for or converted into other securities, cash or property), (B) the Corporation effects any sale, lease, transfer or exclusive license of all or substantially all of its assets in one transaction or a series of related transactions, (C) any tender offer or exchange offer (whether by the Corporation or another Person) is completed pursuant to which more than 50% of the Common Stock not held by the Corporation or such Person is exchanged for or converted into other securities, cash or property, or (D) the Corporation effects any reclassification of the Common Stock or any compulsory share exchange pursuant (other than as a result of a dividend, subdivision or combination covered by Section 7.1) to which the Common Stock
is effectively converted into or exchanged for other securities, cash or property (in any such case, a “Fundamental Transaction”), then, upon any subsequent conversion of this Series A Non-Voting Preferred Stock, the Holders shall have the right to receive, in lieu of the right to receive Conversion Shares, for each Conversion Share that would have been issuable upon such conversion (without regard to any Beneficial Ownership Limitations and assuming receipt of Stockholder Approval) immediately prior to the occurrence of such Fundamental Transaction, the same kind and amount of securities, cash or property as it would have been entitled to receive upon the occurrence of such Fundamental Transaction if it had converted the Series A Non-Voting Preferred Stock to Conversion Shares immediately prior to such Fundamental Transaction (without regard to any Beneficial Ownership Limitations and assuming receipt of Stockholder Approval) (the “Alternate Consideration”). For purposes of any such subsequent conversion, the determination of the Conversion Ratio shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Corporation shall adjust the Conversion Ratio in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holders shall be given the same choice as to the Alternate Consideration it receives upon any conversion of this Series A Non-Voting Preferred Stock following such Fundamental Transaction. To the extent necessary to effectuate the foregoing provisions, any successor to the Corporation or surviving entity in such Fundamental Transaction shall file a new certificate of designations with the same terms and conditions and issue to the Holders new preferred stock consistent with the foregoing provisions and evidencing the Holders’ right to convert such preferred stock into Alternate Consideration. The terms of any agreement to which the Corporation is a party and pursuant to which a Fundamental Transaction is effected shall include terms requiring any such successor or surviving entity to comply with the provisions of this Section 7.2 and ensuring that this Series A Non-Voting Preferred Stock (or any such replacement security) will be similarly adjusted upon any subsequent transaction analogous to a Fundamental Transaction. The Corporation shall cause to be delivered to each Holder, at its last address as it shall appear upon the stock books of the Corporation, written notice of any Fundamental Transaction at least 20 calendar days prior to the date on which such Fundamental Transaction is expected to become effective or close.
7.3 Calculations. All calculations under this Section 7 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 7, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding any treasury shares of the Corporation) issued and outstanding.
8. Redemption. The shares of Series A Non-Voting Preferred Stock shall not be redeemable; provided, however, that the foregoing shall not limit the ability of the Corporation to purchase or otherwise deal in such shares to the extent otherwise permitted hereby and by law, nor shall the foregoing limit the Holder’s rights under Section 6.5.3.
9. Transfer. A Holder may transfer any shares of Series A Non-Voting Preferred Stock together with the accompanying rights set forth herein, held by such Holder without the consent of the Corporation; provided that such transfer is in compliance with applicable securities laws. The Corporation shall in good faith (i) do and perform, or cause to be done and performed, all such further acts and things, and (ii) execute and deliver all such other agreements, certificates, instruments and documents, in each case, as any holder of Series A Non-Voting Preferred Stock may reasonably request in order to carry out the intent and accomplish the purposes of this Section 9. The transferee of any shares of Series A Non-Voting Preferred Stock shall be subject to the Beneficial Ownership Limitation applicable to the transferor as of the time of such transfer.
10. Series A Non-Voting Preferred Stock Register. The Corporation shall maintain at its principal executive offices (or such other office or agency of the Corporation as it may designate by notice to the Holders in accordance with Section 11), a register for the Series A Non-Voting Preferred Stock, in which the Corporation shall record (i) the name, address, and electronic mail address of each holder in whose name the shares of Series A Non-Voting Preferred Stock have been issued and (ii) the name, address, and electronic mail address of each transferee of any shares of Series A Non-Voting Preferred Stock. The Corporation may deem and treat the registered Holder of shares of Series A Non-Voting Preferred Stock as the absolute owner thereof for the purpose of any conversion thereof and for all other purposes. The Corporation shall keep the register open and available at all times during business hours for inspection by any holder of Series A Non-Voting Preferred Stock or his, her or its legal representatives.
11. Notices. Any notice required or permitted by the provisions of this Certificate of Designation to be given to a Holder of shares of Series A Non-Voting Preferred Stock shall be mailed, postage prepaid, to the post office address last shown on the records of the Corporation, or given by electronic communication in compliance with the provisions of the DGCL, and shall be deemed sent upon such mailing or electronic transmission.
12. Book-Entry; Certificates. The Series A Non-Voting Preferred Stock will be issued in book-entry form; provided that, if a Holder requests that such Holder’s shares of Series A Non-Voting Preferred Stock be issued in certificated form, the Corporation will instead issue a stock certificate to such Holder representing such Holder’s shares of Series A Non-Voting Preferred Stock. To the extent that any shares of Series A Non-Voting Preferred Stock are issued in book-entry form, references herein to “certificates” shall instead refer to the book-entry notation relating to such shares.
13. Lost or Mutilated Series A Non-Voting Preferred Stock Certificate. If a Holder’s Series A Non-Voting Preferred Stock certificate shall be mutilated, lost, stolen or destroyed, the Corporation shall execute and deliver, in exchange and substitution for and upon cancellation of a mutilated certificate, or in lieu of or in substitution for a lost, stolen or destroyed certificate, a new certificate for the shares of Series A Non-Voting Preferred Stock so mutilated, lost, stolen or destroyed, but only upon receipt of evidence of such loss, theft or destruction of such certificate, and of the ownership hereof reasonably satisfactory to the Corporation.
14. Waiver. Any waiver by the Corporation or a Holder of a breach of any provision of this Certificate of Designation shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other provision of this Certificate of Designation or a waiver by any other Holders. The failure of the Corporation or a Holder to insist upon strict adherence to any term of this Certificate of Designation on one or more occasions shall not be considered a waiver or deprive that party (or any other Holder) of the right thereafter to insist upon strict adherence to that term or any other term of this Certificate of Designation. Any waiver by the Corporation or a Holder must be in writing. Notwithstanding any provision in this Certificate of Designation to the contrary, any provision contained herein and any right of the Holders of Series A Non-Voting Preferred Stock granted hereunder may be waived as to all shares of Series A Non-Voting Preferred Stock (and the Holders thereof) upon the written consent of the Holders of not less than a majority of the shares of Series A Non-Voting Preferred Stock then outstanding, provided, however, that the Beneficial Ownership Limitation applicable to a Holder, and any provisions contained herein that are related to such Beneficial Ownership Limitation, cannot be modified, waived or terminated without the consent of such Holder, provided further, that any proposed waiver that would, by its terms, have a disproportionate and materially adverse effect on any Holder shall require the consent of such Holder(s).
15. Severability. Whenever possible, each provision hereof shall be interpreted in a manner as to be effective and valid under applicable law, but if any provision hereof is held to be prohibited by or invalid under applicable law, then such provision shall be ineffective only to the extent of such prohibition or invalidity, without invalidating or otherwise adversely affecting the remaining provisions hereof.
16. Status of Converted Series A Non-Voting Preferred Stock. If any shares of Series A Non-Voting Preferred Stock shall be converted, redeemed, repurchased or otherwise acquired by the Corporation, such shares shall, to the fullest extent permitted by applicable law, be retired and cancelled upon such acquisition, and shall not be reissued as a share of Series A Non-Voting Preferred Stock. Any share of Series A Non-Voting Preferred Stock so acquired shall, upon its retirement and cancellation, and upon the taking of any action required by applicable law, resume the status of authorized but unissued shares of preferred stock and shall no longer be designated as Series A Non-Voting Preferred Stock.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, Galecto, Inc. has caused this Certificate of Designation of Preferences, Rights and Limitations of Series A Non-Voting Convertible Preferred Stock to be duly executed by its President and Chief Executive Officer on October 7, 2024.
|
|
|
|
|
|
GALECTO, INC. |
|
|
By: |
|
/s/ Hans T. Schambye |
|
|
|
|
|
|
Name: |
|
Hans T. Schambye, M.D., Ph.D. |
Title: |
|
President and Chief Executive Officer |
[Signature Page to Certificate of Designations]
ANNEX A
NOTICE OF CONVERSION
(TO BE EXECUTED BY THE REGISTERED HOLDER IN ORDER TO CONVERT SHARES OF SERIES A NON-VOTING CONVERTIBLE PREFERRED STOCK)
The undersigned Holder hereby irrevocably elects to convert the number of shares of Series A Non-Voting Preferred Stock indicated below, represented in book-entry form, into shares of common stock, par value $0.00001 per share (the “Common Stock”), of Galecto, Inc., a Delaware corporation (the “Corporation”), as of the date written below. If securities are to be issued in the name of a Person other than the undersigned, the undersigned will pay all transfer taxes payable with respect thereto. Capitalized terms utilized but not defined herein shall have the meaning ascribed to such terms in that certain Certificate of Designation of Preferences, Rights and Limitations of Series A Non-Voting Convertible Preferred Stock (the “Certificate of Designation”) filed by the Corporation with the Secretary of State of the State of Delaware on October 7, 2024.
As of the date hereof, the number of shares of Common Stock beneficially owned by the undersigned Holder (together with such Holder’s Attribution Parties), including the number of shares of Common Stock issuable upon conversion of the Series A Non-Voting Preferred Stock subject to this Notice of Conversion, but excluding the number of shares of Common Stock which are issuable upon (A) conversion of the remaining, unconverted Series A Non-Voting Preferred Stock beneficially owned by such Holder or any of its Attribution Parties, and (B) exercise or conversion of the unexercised or unconverted portion of any other securities of the Corporation (including any warrants) beneficially owned by such Holder or any of its Attribution Parties that are subject to a limitation on conversion or exercise similar to the limitation contained in Section 6.4 of the Certificate of Designation, is _____%. For purposes hereof, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the applicable regulations of the Commission. In addition, for purposes hereof, “group” has the meaning set forth in Section 13(d) of the Exchange Act and the applicable regulations of the Commission.
CONVERSION CALCULATIONS:
|
|
|
|
|
|
Date to Effect Conversion: |
|
|
|
|
Number of shares of Series A Non-Voting Preferred Stock owned prior to Conversion: |
|
|
|
|
Number of shares of Series A Non-Voting Preferred Stock to be Converted: |
|
|
|
|
Number of shares of Common Stock to be Issued: |
|
|
|
|
Address for delivery of physical certificates: |
|
|
|
|
|
|
By:__________________________________ |
GALECTO, INC.
SUPPORT AGREEMENT
THIS SUPPORT AGREEMENT (this “Agreement”), dated as of October 7, 2024 is made by and among Galecto, Inc., a Delaware corporation (“Buyer”), Bridge Medicines LLC, a Delaware limited liability company (the “Seller”), and the undersigned holder (“Stockholder”) of shares of capital stock (the “Shares”) of the Buyer.
WHEREAS, concurrently with the entry into this Agreement, Buyer and Seller are entering into an Asset Purchase Agreement, dated of even date herewith (the “Asset Purchase Agreement”), providing for, among other things, the Buyer to purchase from Seller the Purchased Assets (as defined in the Asset Purchase Agreement) in consideration of the issuance of shares of Buyer Common Stock (as defined in the Asset Purchase Agreement) and shares of Buyer Convertible Preferred Stock (as defined in the Asset Purchase Agreement) to Seller pursuant to the terms of the Asset Purchase Agreement;
WHEREAS, as of the date hereof, Stockholder beneficially owns and has sole or shared voting power with respect to the number of Shares, holds options to purchase Shares (“Buyer Options”) and/or holds restricted stock units to acquire Shares (“Buyer Restricted Stock Units”), and such other rights to acquire Shares, as the case may be, in each case in the number of Shares indicated opposite Stockholder’s name on Schedule 1 attached hereto;
WHEREAS, as an inducement and a condition to the willingness of Buyer and Seller to enter into the Asset Purchase Agreement, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder has agreed to enter into and perform this Agreement; and
WHEREAS, all capitalized terms used in this Agreement without definition herein shall have the meanings ascribed to them in the Asset Purchase Agreement.
NOW, THEREFORE, in consideration of, and a condition to, Buyer’s and Seller’s entering into the Asset Purchase Agreement, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder, Buyer and Seller agree as follows:
1. Agreement to Vote Shares. Stockholder agrees that, prior to the Expiration Date (as defined in Section 2 below), at any meeting of the stockholders of Buyer or any adjournment or postponement thereof, with respect to the Buyer Stockholder Matters, Stockholder shall, or shall cause the holder of record on any applicable record date to:
(a) appear at such meeting (in person or by proxy) or otherwise cause the Shares and New Shares (as defined in Section 3 below) to be counted as present thereat for purposes of calculating a quorum;
(b) from and after the date hereof until the Expiration Date, vote (or cause to be voted) all of the Shares and any New Shares that Stockholder shall be entitled to so vote (the “Covered Shares”): (i) in favor of (A) the Buyer Stockholder Matters, and (B) against any proposals, or any
agreement, transaction or other matter that is intended to, or would reasonably be expected to impede, interfere with, delay, postpone or materially and adversely affect the approval of the Buyer Stockholder Matters; and (ii) to approve any proposal to adjourn or postpone the applicable meeting to a later date, if there are not sufficient votes for the approval of the Buyer Stockholder Matters on the date on which such meeting is held. Stockholder shall not take or commit or agree to take any action inconsistent with the foregoing.
2. Expiration Date. As used in this Agreement, the term “Expiration Date” shall mean the earlier of (a) the Stockholder Approval (as defined in the Certificate of Designations), (b) the mutual written agreement of the parties to terminate this Agreement, or (c) the first anniversary of the Closing Date.
3. Additional Acquisitions. Stockholder agrees that any shares of capital stock or other equity voting securities of Buyer that Stockholder acquires or with respect to which Stockholder otherwise acquires sole or shared voting power (including any proxy) after the execution of this Agreement and prior to the Expiration Date, whether by the exercise of any Buyer Options, settlement of Buyer Restricted Stock Units or otherwise, including, without limitation, by gift, succession, in the event of a stock split or as a dividend or distribution of any Shares (“New Shares”), shall be subject to the terms and conditions of this Agreement to the same extent as if they constituted the Shares.
4. Agreement to Retain Shares. From and after the date hereof until the Expiration Date, Stockholder shall not, directly or indirectly (a) sell, assign (directly or indirectly), transfer, tender, pledge, exchange, gift, grant, or placement in trust or otherwise dispose of (including, without limitation, by the creation of any Liens (as defined in Section 5(c) below)), or offer to do any of the foregoing (each, a “Transfer”) any right, title, or interest (including any right or power to vote to which the holder thereof may be entitled, whether such right or power is granted by proxy or otherwise) to any Covered Shares, (b) deposit any Covered Shares into a voting trust or enter into a voting agreement or similar arrangement with respect to such Covered Shares or grant any proxy or power of attorney with respect thereto inconsistent with Stockholder’s obligations under Section 1 of this Agreement, (c) enter into any Contract, option, commitment or other arrangement or understanding with respect to the direct or indirect Transfer any right, title, or interest (including any right or power to vote to which the holder thereof may be entitled, whether such right or power is granted by proxy or otherwise) to any Covered Shares, or (d) take any action that would reasonably be expected to make any representation or warranty of Stockholder contained herein untrue or incorrect or have the effect of restricting the Stockholder’s legal power, authority and right to vote all of the Covered Shares or would otherwise prevent or disable Stockholder from performing any of Stockholder’s obligations under this Agreement. Any action taken in violation of the foregoing sentence shall be null and void ab initio. Notwithstanding the foregoing, Stockholder may make (1) Transfers by will or by operation of Law or other Transfers for estate-planning purposes, (2) with respect to Stockholder’s Buyer Options (and any Shares underlying such Buyer Options) which expire on or prior to the Expiration Date, Transfers of Shares to Buyer (or effecting a “net exercise” of a Buyer Option) as payment for the (i) exercise price of Stockholder’s Buyer Options and (ii) taxes applicable to the exercise of Stockholder’s Buyer Options, (3) with respect to Stockholder’s Buyer Restricted Stock Units, (i) transfers for the net settlement of Stockholder’s Buyer Restricted Stock Units settled in Shares (to pay tax withholding obligations) or (ii) transfers for receipt upon settlement of Stockholder’s Buyer Restricted Stock
Units, and the sale of a sufficient number of such Shares acquired upon settlement of such securities as would generate sales proceeds sufficient to pay the aggregate taxes payable by Stockholder as a result of such settlement, (4) if Stockholder is an entity, partnership or limited liability company, a Transfer to one or more equityholders, partners or members of Stockholder or to an affiliated person, corporation, trust or other entity controlling or under common control with Stockholder, or if Stockholder is a trust, a transfer to a beneficiary, provided that in each such case the applicable transferee has signed a support agreement in substantially the form hereof, (5) make Transfers that occur by operation of law pursuant to a qualified domestic relations order or in connection with a divorce settlement, and (6) Transfers as Seller may otherwise agree in writing in its sole discretion. If any voluntary or involuntary Transfer of any Shares covered hereby shall occur (including a Transfer permitted by Section 4(1) through Section 4(5), sale by a Stockholder’s trustee in bankruptcy, or a sale to a purchaser at any creditor’s or court sale), the transferee (which term, as used herein, shall include any and all transferees and subsequent transferees of the initial transferee) shall take and hold such Shares subject to all of the restrictions, liabilities and rights under this Agreement, which shall continue in full force and effect, and as a condition of receipt if such Transfer or sale, the transferee shall sign a written acknowledgement of such applicability or a joinder hereto.
5. Representations and Warranties of Stockholder. Stockholder hereby represents and warrants to Buyer and Seller as follows:
(a) If Stockholder is an entity: (i) Stockholder is duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is incorporated, organized or constituted, (ii) Stockholder has all necessary power and authority to execute and deliver this Agreement, to perform Stockholder’s obligations hereunder and to consummate the transactions contemplated hereby, and (iii) the execution and delivery of this Agreement, performance of Stockholder’s obligations hereunder and the consummation of the transactions contemplated hereby by Stockholder have been duly authorized by all necessary action on the part of Stockholder and no other proceedings on the part of Stockholder are necessary to authorize this Agreement, or to consummate the transactions contemplated hereby. If Stockholder is an individual, Stockholder has the legal capacity to execute and deliver this Agreement, to perform Stockholder’s obligations hereunder and to consummate the transactions contemplated hereby;
(b) this Agreement has been duly executed and delivered by or on behalf of Stockholder and, assuming this Agreement constitutes a valid and binding agreement of Buyer and Seller, constitutes a valid and binding agreement with respect to Stockholder, enforceable against Stockholder in accordance with its terms, except as enforcement may be limited by general principles of equity whether applied in a court of Law or a court of equity and by bankruptcy, insolvency and similar Laws affecting creditors’ rights and remedies generally;
(c) Stockholder beneficially owns the number of Shares and other rights with respect to Shares indicated opposite Stockholder’s name on Schedule 1, free and clear of any liens, claims, charges or other encumbrances or restrictions of any kind whatsoever (“Liens”), and has sole or shared, and otherwise unrestricted, voting power with respect to such Shares and none of the Shares is subject to any voting trust or other agreement, arrangement or restriction with respect to the voting of the Shares, except as contemplated by this Agreement;
(d) the execution and delivery of this Agreement by Stockholder does not, and the performance by Stockholder of his, her or its obligations hereunder and the compliance by Stockholder with any provisions hereof will not, violate or conflict with, result in a material breach of or constitute a default (or an event that with notice or lapse of time or both would become a material default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of any Liens on any Shares pursuant to, any agreement, instrument, note, bond, mortgage, Contract, lease, license, permit or other obligation or any order, arbitration award, judgment or decree to which Stockholder is a party or by which Stockholder is bound, or any Law, statute, rule or regulation to which Stockholder is subject or, in the event that Stockholder is a corporation, partnership, trust or other entity, any bylaw or other organizational document of Stockholder; except for any of the foregoing as would not reasonably be expected to prevent or delay the performance by Stockholder of his, her or its obligations under this Agreement in any material respect;
(e) the execution and delivery of this Agreement by Stockholder does not, and the performance of this Agreement by Stockholder does not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority or regulatory authority by Stockholder except for applicable requirements, if any, of the Exchange Act, and except where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not prevent or delay the performance by Stockholder of his, her or its obligations under this Agreement in any material respect;
(f) no investment banker, broker, finder or other intermediary is entitled to a fee or commission from Buyer or Seller in respect of this Agreement based upon any Contract made by or on behalf of Stockholder; and
(g) as of the date of this Agreement, there is no Action pending or, to the knowledge of Stockholder, threatened against Stockholder that would reasonably be expected to prevent or delay the performance by Stockholder of his, her or its obligations under this Agreement in any material respect.
6. Irrevocable Proxy. By execution of this Agreement, Stockholder does hereby appoint the Buyer and any of its designees with full power of substitution and resubstitution, as Stockholder’s true and lawful attorney-in-fact and irrevocable proxy, to the fullest extent of Stockholder’s rights with respect to the Covered Shares, to vote or cause to be voted, if Stockholder fails to vote his, her or its Covered Shares, solely with respect to the matters and in the manner set forth in Section 1 hereof. Stockholder intends this proxy to be irrevocable and coupled with an interest hereunder until the Expiration Date, hereby revokes (or agrees to cause to be revoked) any proxy previously granted by Stockholder with respect to the Covered Shares and represents that none of such previously-granted proxies are irrevocable. The Stockholder hereby affirms that the proxy set forth in this Section 6 is given in connection with, and granted in consideration of, and as an inducement to the Buyer and Seller to enter into the Asset Purchase Agreement and that such proxy is given to secure the obligations of the Stockholders under Section 1. The irrevocable proxy and power of attorney granted herein shall survive the death or incapacity of Stockholder and the obligations of Stockholder shall be binding on Stockholder’s heirs, personal representatives, successors, transferees and assigns. Stockholder hereby agrees not to grant any subsequent powers of attorney or proxies with respect to any Shares inconsistent with its obligations under Section 1
of this Agreement until after the Expiration Date. Notwithstanding anything contained herein to the contrary, this irrevocable proxy and power of attorney shall automatically terminate upon the Expiration Date.
7. [Reserved]
8. No Legal Actions. Stockholder will not in its capacity as a stockholder of Buyer bring, commence, institute, maintain, prosecute or voluntarily aid any Action which (i) challenges the validity or seeks to enjoin the operation of any provision of this Agreement or (ii) alleges that the execution and delivery of this Agreement by Stockholder, either alone or together with the other voting agreements and proxies to be delivered in connection with the execution of the Asset Purchase Agreement constitutes a breach of any fiduciary duty of the Buyer Board or any member thereof.
9. Other Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a party will be deemed cumulative with, and not exclusive of, any other remedy conferred hereby, or by Law or equity upon such party, and the exercise by a party of any one remedy will not preclude the exercise of any other remedy. The parties hereto agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that the parties shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof without the need of posting bond in any court of the United States or any state having jurisdiction, this being in addition to any other remedy to which they are entitled at Law or in equity.
10. Directors and Officers. This Agreement shall apply to Stockholder solely in Stockholder’s capacity as a stockholder of Buyer and/or holder of Buyer Options and/or Buyer Restricted Stock Units and not in Stockholder’s capacity as a director, officer or employee of Buyer or any of its Subsidiaries or in Stockholder’s capacity as a trustee or fiduciary of any employee benefit plan or trust. Notwithstanding any provision of this Agreement to the contrary, nothing in this Agreement shall (or require Stockholder to attempt to) limit or restrict a director and/or officer of Buyer in the exercise of his or her fiduciary duties consistent with the terms of the Asset Purchase Agreement as a director and/or officer of Buyer or in his or her capacity as a trustee or fiduciary of any employee benefit plan or trust or prevent or be construed to create any obligation on the part of any director and/or officer of Buyer or any trustee or fiduciary of any employee benefit plan or trust from taking any action in his or her capacity as such director, officer, trustee and/or fiduciary.
11. No Ownership Interest. Nothing contained in this Agreement shall be deemed to vest in Buyer or Seller any direct or indirect ownership or incidence of ownership of or with respect to any Covered Shares. All rights, ownership and economic benefits of and relating to the Covered Shares shall remain vested in and belong to Stockholder.
12. Termination. This Agreement shall terminate and shall have no further force or effect as of the Expiration Date. Notwithstanding the foregoing, upon termination or expiration of this Agreement, no party shall have any further obligations or liabilities under this Agreement; provided, however, nothing set forth in this Section 12 or elsewhere in this Agreement shall relieve
any party from liability for any fraud or for any willful and material breach of this Agreement prior to termination hereof.
13. Further Assurances. Stockholder shall, from time to time, execute and deliver, or cause to be executed and delivered, such additional or further consents, documents and other instruments as Buyer or Seller may reasonably request for the purpose of effectively obtaining approval of the Buyer Stockholder Matters.
14. Disclosure. Stockholder hereby agrees that Buyer and Seller may publish and disclose in the Proxy Statement and any related documents filed with such regulatory authority and as otherwise required by Law, Stockholder’s identity and ownership of the Covered Shares and the nature of Stockholder’s commitments, arrangements and understandings under this Agreement and may further file this Agreement as an exhibit to the Proxy Statement or in any other filing made by Buyer or Seller as required by Law or the terms of the Asset Purchase Agreement, including with the SEC or other regulatory authority, relating to the transactions contemplated by the Asset Purchase Agreement, all subject to prior review and a reasonable opportunity to comment by Stockholder’s counsel. Prior to the Closing, Stockholder shall not, and shall use its reasonable best efforts to cause its representatives not to, directly or indirectly, make any press release, public announcement or other public communication about the transactions contemplated by the Asset Purchase Agreement without the prior written consent of Buyer and Seller, provided that the foregoing shall not limit or affect any actions taken by Stockholder (or any affiliated officer or director of Stockholder) that would be permitted to be taken by Stockholder, Buyer or Seller pursuant to the Asset Purchase Agreement; provided, further, that the foregoing shall not affect any actions of Stockholder the prohibition of which would be prohibited under applicable Law and shall not prohibit Stockholder or its affiliates from making any publicly-available filings required by applicable law, regulation or legal process.
15. Notice. All notices and other communications hereunder shall be in writing and shall be deemed given if delivered personally or sent by overnight courier (providing proof of delivery), or by electronic transmission (upon confirmation of receipt of transmission) to Seller or Buyer, as the case may be, in accordance with Section 7.3 of the Asset Purchase Agreement and to Stockholder at his, her or its address or email address (upon confirmation of receipt of transmission) set forth on Schedule 1 attached hereto (or at such other address for a party as shall be specified by like notice).
16. Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or provision of this Agreement is invalid or unenforceable, the parties hereto agree that the court making such determination shall have the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent
possible, the economic, business and other purposes of such invalid or unenforceable term or provision.
17. Assignability. This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the parties hereto and their respective successors and assigns; provided, however, that neither this Agreement nor any of a party’s rights or obligations hereunder may be assigned or delegated by such party without the prior written consent of the other parties hereto, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by such party without the other party’s prior written consent shall be void and of no effect. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person (other than the parties hereto) any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
18. No Waivers. No waivers of any breach of this Agreement extended by Buyer or Seller to Stockholder shall be construed as a waiver of any rights or remedies of Buyer or Seller, as applicable, with respect to any other stockholder of Buyer who has executed an agreement substantially in the form of this Agreement or with respect to any subsequent breach of Stockholder or any other stockholder of Buyer. No waiver of any provisions hereof by any party shall be deemed a waiver of any other provisions hereof by any such party, nor shall any such waiver be deemed a continuing waiver of any provision hereof by such party.
19. Applicable Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the state of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of Laws. In any Action between any of the parties arising out of or relating to this Agreement, each of the parties: (i) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the state of Delaware or to the extent such court does not have subject matter jurisdiction, the Superior Court of the State of Delaware or the United States District Court for the District of Delaware, (ii) agrees that all claims in respect of such Action shall be heard and determined exclusively in accordance with clause (i) of this Section 19, (iii) waives any objection to laying venue in any such Action in such courts, (iv) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any party, and (v) agrees that service of process upon such party in any such Action shall be effective if notice is given in accordance with Section 15 of this Agreement.
20. Waiver of Jury Trial. The parties hereto hereby waive any right to trial by jury with respect to any Action related to or arising out of this Agreement, any document executed in connection herewith and the matters contemplated hereby and thereby.
21. No Agreement Until Executed. Irrespective of negotiations among the parties or the exchanging of drafts of this Agreement, this Agreement shall not constitute or be deemed to evidence a Contract, agreement, arrangement or understanding between the parties hereto unless and until (a) the Buyer Board has approved, for purposes of any applicable anti-takeover Laws and regulations and any applicable provision of the certificate of incorporation of Buyer, this Agreement, the Asset Purchase Agreement and the transactions contemplated in the Asset Purchase Agreement, (b) the Asset Purchase Agreement is executed by all parties thereto, and (c) this Agreement is executed by all parties hereto.
22. Entire Agreement; Counterparts; Exchanges by Electronic Transmission. This Agreement and the other agreements referred to in this Agreement constitute the entire agreement and supersede all prior agreements and understandings, both written and oral, among or between any of the parties with respect to the subject matter hereof and thereof. This Agreement may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Agreement (in counterparts or otherwise) by all parties by electronic transmission via “.pdf” shall be sufficient to bind the parties to the terms and conditions of this Agreement.
23. Amendment. This Agreement may not be amended, supplemented or modified, and no provisions hereof may be modified or waived, except by an instrument in writing signed on behalf of each party hereto; provided, however, that the rights or obligations of any Stockholder may be waived, amended or otherwise modified in a writing signed by Buyer, Seller and Stockholder.
24. Fees and Expenses. Except as otherwise specifically provided herein, the Asset Purchase Agreement or any other agreement contemplated by the Asset Purchase Agreement to which a party hereto is a party, each party hereto shall bear its own expenses in connection with this Agreement and the transactions contemplated hereby.
25. Voluntary Execution of Agreement. This Agreement is executed voluntarily and without any duress or undue influence on the part or behalf of the parties. Each of the parties hereby acknowledges, represents and warrants that (i) it has read and fully understood this Agreement and the implications and consequences thereof; (ii) it has been represented in the preparation, negotiation, and execution of this Agreement by legal counsel of its own choice, or it has made a voluntary and informed decision to decline to seek such counsel; and (iii) it is fully aware of the legal and binding effect of this Agreement.
26. Definition of Asset Purchase Agreement. For purposes of this Agreement, the term “Asset Purchase Agreement” may include such agreement as amended or modified as long as such amendments or modifications (a) do not change the form of consideration payable under the Asset Purchase Agreement, in a manner materially adverse to Stockholder or (b) have been agreed to in writing by Stockholder.
27. Construction.
(a) For purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include masculine and feminine genders.
(b) The parties hereto agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting party shall not be applied in the construction or interpretation of this Agreement.
(c) As used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
(d) Except as otherwise indicated, all references in this Agreement to “Sections,” and “Schedules” are intended to refer to Sections of this Agreement and Schedules to this Agreement, respectively.
(e) The underlined headings contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement and shall not be referred to in connection with the construction or interpretation of this Agreement.
[Remainder of Page has Intentionally Been Left Blank]
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EXECUTED as of the date first above written. |
|
|
|
|
|
|
[STOCKHOLDER] |
|
|
|
|
|
|
Signature: |
|
Signature Page to Buyer Support Agreement
EXECUTED as of the date first above written.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GALECTO, INC. |
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
|
|
|
|
|
|
|
BRIDGE MEDICINES LLC |
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
|
|
|
Signature Page to Buyer Support Agreement
SCHEDULE 1
|
|
|
|
Name, Address and Email Address of Stockholder |
Shares of Buyer Common Stock |
Buyer Options |
Buyer Restricted Stock Units |
|
|
|
|
0.001
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
On October 7, 2024, the Company completed the Closing of the Asset Purchase as further described under Item 1.01 of this Current Report on Form 8-K.
The following unaudited pro forma condensed consolidated financial information is based upon the historical financial statements of the Company, adjusted to reflect the Closing of the Asset Purchase. The following unaudited pro forma condensed consolidated financial information of the Company should be read in conjunction with the related notes herein and with the historical consolidated financial statements of the Company and the related notes thereto included in previous filings with the U.S. Securities and Exchange Commission ("SEC").
To provide a better understanding of the impact of the Asset Purchase, the following unaudited pro forma condensed consolidated financial information is presented to reflect how the Asset Purchase might have affected the historical financial statements had the transactions been consummated at an earlier date. The unaudited pro forma condensed consolidated statements of operations and comprehensive loss that follow are presented as if the Closing of the Asset Purchase had occurred on January 1, 2023, the beginning of the earliest period presented. The unaudited pro forma condensed consolidated balance sheet as of June 30, 2024 is presented as if the Closing of the Asset Purchase had occurred on that date.
On August 29, 2024, the Company executed a reverse stock split of the Company’s common stock at a ratio of 1-for-25 (the “Reverse Stock Split”). The historical financial information included in the pro forma information has been adjusted to reflect the impact of the Reverse Stock Split.
The unaudited pro forma condensed consolidated financial information presented in accordance with Article 11 of the SEC Regulation S-X (“Article 11 of Regulation S-X”), are for informational purposes only and do not purport to show the results that would have occurred had such transaction been completed as of the date and for the periods presented or which may occur in the future. The unaudited pro forma condensed consolidated financial information constitutes forward-looking information and is subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated. Accordingly, such information should not be relied upon as an indicator of future performance, financial condition or liquidity. For example, the financial information does not reflect any potential ongoing earnings or costs associated with the Asset Purchase, nor the conversion of the Preferred Stock into shares of Common Stock which is subject to, and contingent upon, Stockholder Approval.
GALECTO, INC.
Pro Forma Condensed Consolidated Balance Sheet
As of June 30, 2024
(in thousands, except share and per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Galecto, Inc. |
|
|
Asset
Acquisition
Adjustments |
|
|
|
Pro Forma |
|
Assets |
|
|
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
22,862 |
|
|
$ |
(2,680 |
) |
(a) |
|
$ |
20,182 |
|
Marketable securities |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
Prepaid expenses and other current assets |
|
|
2,306 |
|
|
|
— |
|
|
|
|
2,306 |
|
Total current assets |
|
|
25,168 |
|
|
|
(2,680 |
) |
|
|
|
22,488 |
|
Operating lease right-of-use asset |
|
|
76 |
|
|
|
— |
|
|
|
|
76 |
|
Equipment, net |
|
|
67 |
|
|
|
— |
|
|
|
|
67 |
|
Other assets, non-current |
|
|
1,986 |
|
|
|
— |
|
|
|
|
1,986 |
|
Total assets |
|
$ |
27,297 |
|
|
$ |
(2,680 |
) |
|
|
$ |
24,617 |
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
724 |
|
|
$ |
— |
|
|
|
$ |
724 |
|
Accrued expenses and other current liabilities |
|
|
2,985 |
|
|
|
— |
|
|
|
|
2,985 |
|
Total current liabilities |
|
|
3,709 |
|
|
|
— |
|
|
|
|
3,709 |
|
Operating lease liabilities, non-current |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
Total liabilities |
|
|
3,709 |
|
|
|
— |
|
|
|
|
3,709 |
|
Mezzanine equity |
|
|
|
|
|
|
|
|
|
|
Preferred stock, par value of $0.00001 per share; 10,000,000
shares authorized at June 30, 2024; no shares issued or
outstanding as of June 30, 2024, actual; 161 shares issued
and outstanding as of June 30, 2024, pro forma |
|
|
— |
|
|
|
1,876 |
|
(b) |
|
|
1,876 |
|
Stockholders’ equity |
|
|
|
|
|
|
|
|
|
|
Common stock, par value of $0.00001 per share; 300,000,000
shares authorized at June 30, 2024; 1,084,841 issued and
outstanding at June 30, 2024; actual, 1,147,435 issued and
outstanding at June 30, 2024, pro forma |
|
|
— |
|
|
|
— |
|
(c) |
|
|
— |
|
Additional paid-in capital |
|
|
290,291 |
|
|
|
732 |
|
(c) |
|
|
291,023 |
|
Accumulated deficit |
|
|
(266,900 |
) |
|
|
(5,288 |
) |
(d) |
|
|
(272,188 |
) |
Accumulated other comprehensive gain |
|
|
197 |
|
|
|
— |
|
|
|
|
197 |
|
Total stockholders’ equity |
|
|
23,588 |
|
|
|
(2,680 |
) |
|
|
|
20,908 |
|
Total liabilities and stockholders' equity |
|
$ |
27,297 |
|
|
$ |
(2,680 |
) |
|
|
$ |
24,617 |
|
See accompanying notes to the unaudited pro forma condensed consolidated financial statements.
GALECTO, INC.
Pro Forma Condensed Consolidated Statement of Operations and Comprehensive Loss
Year Ended December 31, 2023
(in thousands, except share and per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Galecto, Inc. |
|
|
Asset
Acquisition
Adjustments |
|
|
|
Pro Forma |
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
23,770 |
|
|
$ |
5,288 |
|
(e) |
|
$ |
29,058 |
|
General and administrative |
|
|
12,687 |
|
|
|
— |
|
|
|
|
12,687 |
|
Restructuring costs |
|
|
3,448 |
|
|
|
— |
|
|
|
|
3,448 |
|
Total operating expenses |
|
|
39,905 |
|
|
|
5,288 |
|
|
|
|
45,193 |
|
Loss from operations |
|
|
(39,905 |
) |
|
|
(5,288 |
) |
|
|
|
(45,193 |
) |
Other income, net |
|
|
|
|
|
|
|
|
|
|
Interest income, net |
|
|
1,689 |
|
|
|
— |
|
|
|
|
1,689 |
|
Gain on sale of equipment |
|
|
64 |
|
|
|
— |
|
|
|
|
64 |
|
Foreign exchange transaction loss, net |
|
|
(197 |
) |
|
|
— |
|
|
|
|
(197 |
) |
Total other income, net |
|
|
1,556 |
|
|
|
— |
|
|
|
|
1,556 |
|
Net loss |
|
$ |
(38,349 |
) |
|
$ |
(5,288 |
) |
|
|
$ |
(43,637 |
) |
Net loss attributable to common shares |
|
$ |
(38,349 |
) |
|
$ |
160 |
|
(f) |
|
$ |
(38,189 |
) |
Net loss per common share, basic and diluted |
|
$ |
(36.08 |
) |
|
$ |
— |
|
|
|
$ |
(33.93 |
) |
Weighted-average number of shares used in computing net loss per common share, basic and diluted |
|
|
1,062,872 |
|
|
|
62,594 |
|
(g) |
|
|
1,125,466 |
|
Net loss attributable to preferred shares |
|
$ |
— |
|
|
$ |
(5,448 |
) |
(f) |
|
$ |
(5,448 |
) |
Net loss per preferred share, basic and diluted |
|
$ |
— |
|
|
$ |
— |
|
|
|
$ |
(33,932 |
) |
Weighted-average number of shares used in computing net loss per preferred share, basic and diluted |
|
|
— |
|
|
|
161 |
|
(g) |
|
|
161 |
|
Other comprehensive loss, net of tax |
|
|
|
|
|
|
|
|
|
|
Currency translation gain |
|
|
393 |
|
|
|
— |
|
|
|
|
393 |
|
Unrealized gain on marketable securities |
|
|
231 |
|
|
|
— |
|
|
|
|
231 |
|
Other comprehensive gain (loss), net of tax |
|
|
624 |
|
|
|
— |
|
|
|
|
624 |
|
Total comprehensive loss |
|
$ |
(37,725 |
) |
|
$ |
(5,288 |
) |
|
|
$ |
(43,013 |
) |
See accompanying notes to the unaudited pro forma condensed consolidated financial statements.
GALECTO, INC.
Pro Forma Condensed Consolidated Statement of Operations and Comprehensive Loss
Six Months Ended June 30, 2024
(in thousands, except share and per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Galecto, Inc. |
|
|
Asset
Acquisition
Adjustments |
|
|
|
Pro Forma |
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
4,297 |
|
|
$ |
— |
|
|
|
$ |
4,297 |
|
General and administrative |
|
|
6,053 |
|
|
|
— |
|
|
|
|
6,053 |
|
Restructuring costs |
|
|
968 |
|
|
|
— |
|
|
|
|
968 |
|
Total operating expenses |
|
|
11,318 |
|
|
|
— |
|
|
|
|
11,318 |
|
Loss from operations |
|
|
(11,318 |
) |
|
|
— |
|
|
|
|
(11,318 |
) |
Other income, net |
|
|
|
|
|
|
|
|
|
|
Interest income, net |
|
|
470 |
|
|
|
— |
|
|
|
|
470 |
|
Foreign exchange transaction gain, net |
|
|
75 |
|
|
|
— |
|
|
|
|
75 |
|
Total other income, net |
|
|
545 |
|
|
|
— |
|
|
|
|
545 |
|
Loss before income tax expense |
|
|
(10,773 |
) |
|
|
— |
|
|
|
|
(10,773 |
) |
Income tax expense |
|
|
42 |
|
|
|
— |
|
|
|
|
42 |
|
Net loss |
|
$ |
(10,815 |
) |
|
$ |
— |
|
|
|
$ |
(10,815 |
) |
Net loss attributable to common shares |
|
$ |
(10,815 |
) |
|
$ |
1,328 |
|
(f) |
|
$ |
(9,487 |
) |
Net loss per common share, basic and diluted |
|
$ |
(9.97 |
) |
|
$ |
— |
|
|
|
$ |
(8.27 |
) |
Weighted-average number of shares used in computing net loss per common share, basic and diluted |
|
|
1,084,565 |
|
|
|
62,594 |
|
(g) |
|
|
1,147,159 |
|
Net loss attributable to preferred shares |
|
$ |
— |
|
|
$ |
(1,328 |
) |
(f) |
|
$ |
(1,328 |
) |
Net loss per preferred share, basic and diluted |
|
$ |
— |
|
|
$ |
— |
|
|
|
$ |
(8,270 |
) |
Weighted-average number of shares used in computing net loss per preferred share, basic and diluted |
|
|
— |
|
|
|
161 |
|
(g) |
|
|
161 |
|
Other comprehensive loss, net of tax |
|
|
|
|
|
|
|
|
|
|
Currency translation loss |
|
|
(217 |
) |
|
|
— |
|
|
|
|
(217 |
) |
Unrealized gain on marketable securities |
|
|
34 |
|
|
|
— |
|
|
|
|
34 |
|
Other comprehensive gain (loss), net of tax |
|
|
(183 |
) |
|
|
— |
|
|
|
|
(183 |
) |
Total comprehensive loss |
|
$ |
(10,998 |
) |
|
$ |
— |
|
|
|
$ |
(10,998 |
) |
See accompanying notes to the unaudited pro forma condensed consolidated financial statements.
GALECTO, INC.
Notes to the Pro Forma Condensed Consolidated Financial Statements
(Unaudited)
1. Basis of Presentation
The unaudited pro forma condensed consolidated financial information was prepared with the Asset Purchase being accounted for as an asset acquisition by the Company under Accounting Standards Codification (“ASC”) Topic 805-50. The Company further concluded that the set of assets acquired in the transaction does not represent a business under Securities and Exchange Commission Regulation S-X Rule 11-01.
Upon completion of the Asset Purchase, the Company obtained control of assets consisting primarily of in-process research and development (IPR&D) related to Bridge Medicine’s BRM-1420 program. In accordance with U.S. GAAP, the Company must first assess whether an integrated set of assets and activities should be accounted for as an acquisition of a business or an asset acquisition. An initial screen test is completed to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single asset or group of similar assets. If that screen is met, the set is not considered a business and is accounted for as an asset acquisition. The Company will account for the Asset Purchase as an asset acquisition as substantially all of the fair value of the gross assets being acquired is concentrated within Bridge Medicine’s programs and development candidates which are considered a group of similar assets.
2. Transaction Adjustments
The unaudited pro forma condensed consolidated financial statements reflect the following notes and adjustments:
|
(a)Represents the cash paid to Bridge Medicines to reimburse transaction costs as well as the estimated transaction costs associated with the Asset Purchase incurred by the Company. (b)Represents the fair value of the Preferred Stock Payment Shares issued to Bridge Medicines in consideration of the assets acquired in the Asset Purchase. (c)Represents the fair value of the Common Stock Payment Shares issued to Bridge Medicines in consideration of the assets acquired in the Asset Purchase. (d)Represents the earnings adjustments associated with pro forma adjustment (e). (e)Represents the expense to be recorded associated with the Asset Purchase. The assets acquired do not have an alternative future use to the Company as defined in Accounting Standards Codification (“ASC”) 730, Research and development. Accordingly, the Company recorded the fair value of the consideration transferred for the assets acquired, which is comprised of the cash paid for transaction costs under pro forma adjustment (a), and the fair value of the Payment Shares under pro forma adjustments (b) and (c), as Research and development expense as opposed to capitalizing the costs as an asset. (f)Represents the allocation of pro forma net loss between the pro forma common shares and pro forma preferred shares of the Company. The preferred shares participate in earnings and losses of the Company with the Common Stock and are therefore treated as a separate class of common stock for purposes of calculating net loss per share. Net loss per share is provided separately for each class of stock. Subject to receipt of Stockholder Approval, the preferred stock is convertible into common stock at a conversion ratio of 1-to-1,000 and participate in losses on an as-converted basis. Accordingly, the net loss per preferred share is 1,000 times the net loss per common share. (g)Represents the issuance of shares of Common Stock Payment Shares and Preferred Stock Payment Shares to Bridge Medicines in consideration of the assets acquired in the Asset Purchase. |
Galecto Completes Strategic Review to Focus on Oncology and Liver Disease and Acquires Acute Myeloid Leukemia Preclinical Asset from Bridge Medicines
-Galecto will focus on cancer and liver disease, leveraging existing clinical stage asset GB1211
- Bolsters pipeline by obtaining global rights to BRM-1420, a novel dual ENL-YEATS and FLT3 inhibitor for multiple genetic subsets of acute myeloid leukemia (AML)
-BRM-1420 has the potential for enhanced clinical effectiveness compared to FLT3 inhibitors alone and has shown synergistic effects with SOC in preclinical models
BOSTON, October 7, 2024 (GLOBE NEWSWIRE) -- Galecto, Inc. (NASDAQ: GLTO), a clinical-stage biotechnology company focused on the development of novel treatments for cancer and fibrosis, today announced that, following an intensive strategic review process, Galecto has determined to focus on cancer and liver disease, leveraging its existing clinical stage asset GB1211, which has shown positive results in non-small cell lung cancer (NSCLC) and decompensated cirrhosis clinical studies. Galecto further announced that it has bolstered its pipeline with the acquisition of the global rights to BRM-1420, a novel, first-in-class asset developed by Bridge Medicines, a company co-founded by Takeda.
“Our strategic review process concluded that our best opportunity for building value and changing the lives for patients with severe diseases was to focus on our existing clinical stage compound GB1211 and increase our chance for success by acquiring complementary assets. The addition of BRM-1420 represents a significant advancement in our mission to develop and deliver breakthrough treatments for oncology and liver conditions,” said Dr. Hans Schambye, CEO of Galecto. “We are particularly optimistic about BRM-1420's potential to address challenging genetic subsets of AML and its observed synergistic effects with standard-of-care therapies and menin inhibitors.”
“AML is the most common acute leukemia in adults, yet despite available treatments, patient prognosis remains poor with significant unmet needs,” said Miles Gerson, Head of Takeda Ventures and Takeda’s Representative to Bridge Medicines. “Bridge Medicines has made considerable progress in recent years developing this new class of drugs and Galecto’s team is well positioned to continue advancing BRM-1420.”
As consideration for the acquisition of the global rights of BRM-1420, Galecto issued 62,594 shares of common stock to Bridge Medicines, representing 4.99% of the outstanding shares of Galecto’s common stock as of the date of the asset purchase, and 160.562 shares of a newly-issued Series A preferred stock convertible into 160,562 shares of common stock, or approximately 12.8% of Galecto’s common stock, upon receipt of stockholder approval.
Matthew Kronmiller, Bridge Medicine’s Chief Executive Officer, will be joining Galecto’s management team as the Executive Vice President of Strategy and Chief Business Officer. The transaction was approved by the Boards of Directors of both companies.
Leerink Partners served as the exclusive financial advisor to Galecto and Lazard served as exclusive financial advisor to Bridge Medicines. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. is serving as legal counsel to Galecto. Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP is serving as legal counsel to Bridge Medicines.
About BRM-1420
BRM-1420 is a potent and selective ENL-YEATS and FLT3 inhibitor of multiple genetic subsets of AML. It disrupts key oncogenic pathways by inhibiting these domains, showing potent activity in MLLr and NPM1c cell lines. Promising preclinical and in vivo results highlight its efficacy in inhibiting leukemia cell growth and
extending survival in AML models. In animal models, BRM-1420 exhibited superior efficacy to both FLT3 and menin inhibitors and was shown to inhibit cell proliferation in primary AML patient samples across multiple genotypes, including MLL-r, NPM1m, cKIT+, FLT3+, TET2+, and TP53+. These mutations are often seen in AML and, in total, could account for greater than 30% of the AML patient population. Many of these mutations have proven difficult to treat with currently available regimens and therefore represent a significant unmet medical need. The Company believes, based on preclinical data, that BRM-1420 could be additive or synergistic when used in combination with the current standard of care (azacitidine, venetoclax, cytarabine, gilteritinib), as well as current therapies under development, such as menin inhibitors.
Galecto plans to file an IND for BRM-1420 in the US in late 2025 or early 2026 and initiate clinical studies in patients with AML thereafter. Exclusive global rights to the program were assigned by Bridge Medicines to Galecto through a license with The Rockefeller University. The pioneering discoveries were a result of collaboration between the Rockefeller University and the Tri-Institutional Therapeutics Discovery Institute (Tri-I TDI), followed by licensing by Bridge Medicines.
About Galecto’s Pipeline
Galecto continues to develop GB1211, its first-in-class, oral small molecule, galectin-3 inhibitor for the treatment of oncology and severe liver cirrhosis. GB1211 is currently being studied in an investigator-initiated Phase 2 trial at Providence Portland Medical Center’s Earle A. Chiles Research Institute (EACRI). GB1211 is being administered in combination with the standard therapeutic dose of pembrolizumab (Keytruda®) in patients with unresectable or metastatic melanoma or recurrent or metastatic HNSCC progressing during or after platinum-containing chemotherapy. This trial is designed to evaluate the safety and efficacy of GB1211 in combination with pembrolizumab and determine whether the addition of GB1211 increases the response rate of pembrolizumab in metastatic melanoma and HNSCC patients. This trial was initiated and enrolled its first patient in the second quarter of 2024.
In addition, during the second half of 2023, Galecto concluded its Phase 1b/2a trial examining GB1211 in combination with atezolizumab, a PD-L1 checkpoint inhibitor, for the treatment of first-line non-small cell lung cancer (NSCLC). Four patients in this trial showed a partial response according to RECIST criteria (version 1.1), and two of these four patients continue to receive treatment in the extension phase of the trial.
As part of the strategic alternative review process, Galecto has determined not to further advance GB2064, its LOXL-2 inhibitor candidate, at this time.
About Galecto
Galecto is a clinical-stage biopharmaceutical company committed to realizing the promise of novel treatments for cancer and liver diseases. The Company’s pipeline consists of first-in-class small molecule drug candidates that target cancer and fibrosis signaling pathways, including (i) an orally active galectin-3 inhibitor (GB1211) for the treatment of liver cirrhosis; (ii) an orally active galectin-3 inhibitor (GB1211) in combination with a checkpoint inhibitor for various oncology indications; and (iii) a preclinical dual inhibitor of ENL-YEATS and FLT3 (BRM-1420) for multiple genetic subsets of AML. Galecto intends to use its website as a means of disclosing material non-public information. For regular updates about Galecto, visit www.galecto.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include statements about Galecto’s preclinical and clinical development plans for BRM-1420; BRM-1420's potential to address challenging genetic subsets of AML and its observed synergistic effects with standard-of-care therapies and menin inhibitors; that BRM-1420 has the potential to transform the treatment of AML; that the MLL-r, NPM1m, cKIT+, FLT3+, TET2+, and TP53+ mutations could account for greater than 30% of the AML patient population; and that BRM-1420 could be additive or synergistic when used in combination with current standard of care as well as current therapies under development. Such forward-looking statements include statements about Galecto’s focus and plans for preclinical and clinical development of its product candidates and pipeline. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. For such statements, Galecto claims the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from Galecto's expectations. Factors that could cause actual results to differ materially from the forward-looking statements include risks and uncertainties related to the development of Galecto’s product candidates and their therapeutic potential, having adequate funds and their use, and those disclosed in Galecto’s filings with the Securities and Exchange Commission (SEC), including, but not limited to, Galecto’s Annual Report on Form 10-K, as filed with the SEC on March 8, 2024. These forward-looking statements represent Galecto's judgment as of the time of this release. Galecto disclaims any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
For more information, contact:
|
|
Investors/US |
Media/EU |
Ashley R. Robinson
arr@lifesciadvisors.com |
Sandya von der Weid
svonderweid@lifesciadvisors.com |
+1 617 430 7577 |
+41 78 680 0538 |

TRANSFORMING �TREATMENT �of cancer and liver disease with �first-in-class small molecule agents Exhibit 99.3

Forward-looking statements This presentation contains forward-looking statements about Galecto, Inc.’s (“Galecto” or the “Company”) strategy, future plans, operations and prospects, including, but not limited to, statements regarding the development of Galecto’s compounds and potential opportunities, including BRM-1420; the expected timing and reporting of results of Galecto’s clinical trials; and Galecto’s expected cash runway. These statements involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation are forward-looking statements. The words ‘‘anticipate,’’ ‘‘believe,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘target,’’ ‘‘should,’’ ‘‘would,’’ and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. For such statements, Galecto claims the protection of the Private Securities Litigation Reform Act of 1995. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that could cause actual results to differ materially from such statements include, without limitation: that drug development is expensive, time consuming, uncertain and susceptible to change, interruption, delay or termination; enrolling patients in clinical trials is competitive and challenging and the expected timing of Galecto’s planned readouts for its ongoing clinical trials may be delayed as a result; that the timing and outcome of research, development and regulatory review and feedback is uncertain; Galecto’s need to raise additional capital to advance all of its programs; the amount of Galecto’s future losses is uncertain and could cause our stock price to fluctuate or decline; top-line data may not accurately reflect the complete results of a particular study or trial; results of clinical trials and other studies are subject to different interpretation and may not be predictive of future results; Galecto’s clinical trials may fail to demonstrate adequately the safety and efficacy of any of its drug candidates; Galecto’s drug candidates may not advance in development or be approved for marketing; clinical trial and other studies may not proceed at the time or in the manner expected or at all; clinical and nonclinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Galecto or others, request additional information, have additional recommendations or change their guidance or requirements; data and information related to the Company’s programs may not meet regulatory requirements or otherwise be sufficient for further development at all or on the Company’s projected timeline; and other risks related to developing, seeking regulatory approval of and commercializing drugs, including regulatory, manufacturing, supply and marketing issues and drug availability. Additional factors that could cause results to differ materially from those stated or implied by Galecto’s forward-looking statements are disclosed in its Securities and Exchange Commission (SEC) filings, including its most recent Annual Report on Form 10-K, filed with the SEC on March 8, 2024, under the headings “Risk Factors.” In addition, the forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so.
is working to create a world �where patients suffering �from cancer and severe liver diseases have effective �treatment solutions

Galecto enhances pipeline following strategic review Cash balance of ~$22.9M as of June 30, 2024 funds ongoing extension trials and acquired program with runway into 2026 Completed Strategic Review Identifies Core Assets and Direction Following an intensive strategic review process, Galecto has determined to focus on cancer and liver disease existing clinical stage asset GB1211 Pipeline is bolstered by acquisition of BRM-1420 for AML, which brings novelty and breakthrough potential Superior activity in preclinical AML models Identified after review of multiple assets, assisted by Leerink Enhanced Pipeline Focused On Oncology and Liver Disease Innovative platforms targeting core disease processes Pioneers in ENL-YEATS and galectin-3 �based pharmacology First-in-class highly specific, oral small-molecule inhibitors All programs address: Diseases characterized by clear unmet medical need Multi-billion-dollar market opportunities Early Data �Supportive of Drug Activity ENL-YEATS inhibitor: MoA estimated to cover 30%+ of AML population Rapid and durable anti-tumor activity in AML models Galectin-3 Inhibitors: Target engagement shown Positive biomarker data Evidence of tumor microenvironment transformation Significant clinical data in cirrhosis
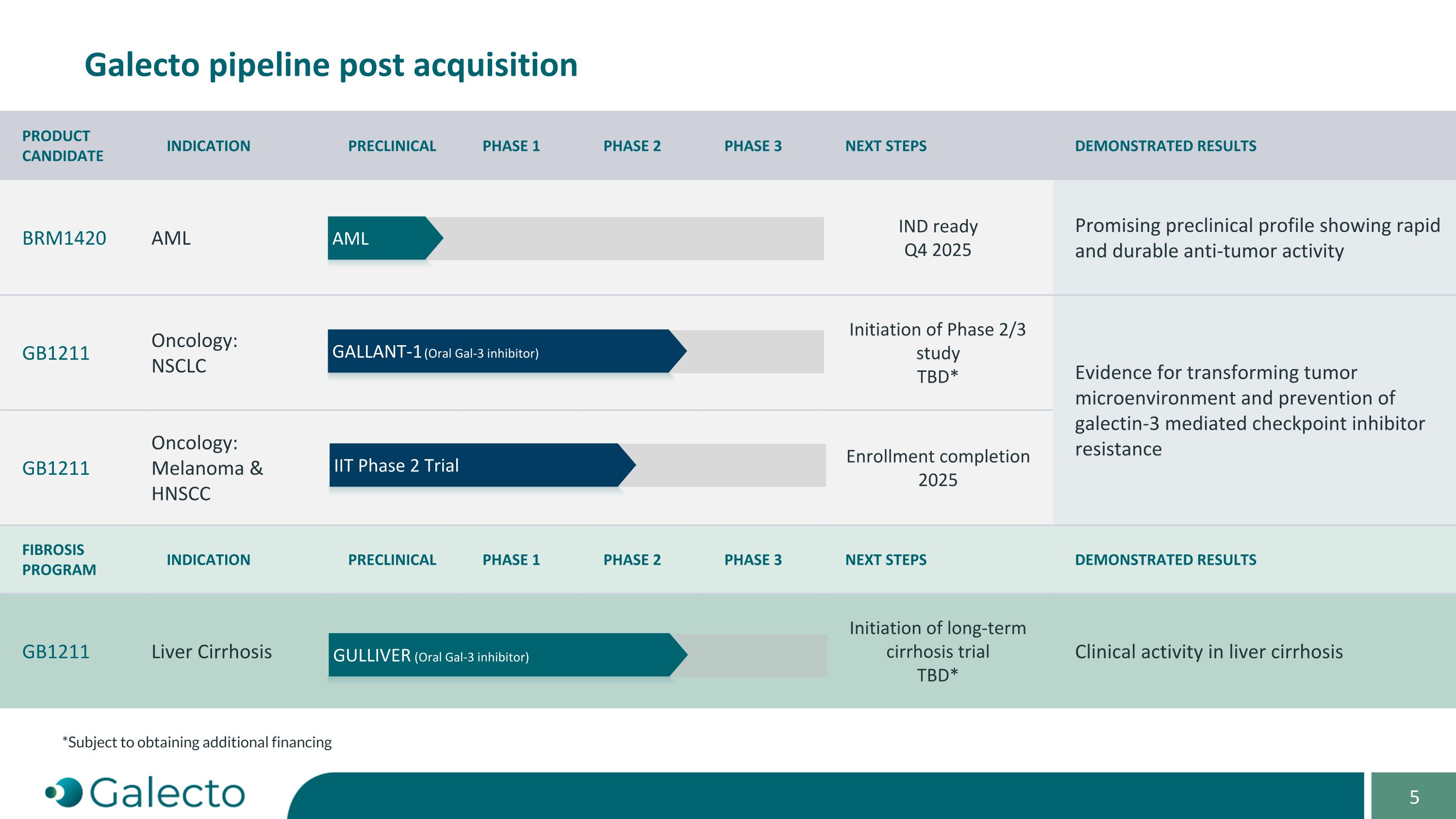
PRODUCT CANDIDATE INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NEXT STEPS DEMONSTRATED RESULTS BRM1420 AML IND ready�Q4 2025 Promising preclinical profile showing rapid and durable anti-tumor activity GB1211 Oncology: �NSCLC Initiation of Phase 2/3 study TBD* Evidence for transforming tumor microenvironment and prevention of galectin-3 mediated checkpoint inhibitor resistance GB1211 Oncology: Melanoma & HNSCC Enrollment completion 2025 FIBROSIS PROGRAM INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NEXT STEPS DEMONSTRATED RESULTS GB1211 Liver Cirrhosis Initiation of long-term cirrhosis trial TBD* Clinical activity in liver cirrhosis Galecto pipeline post acquisition GALLANT-1 (Oral Gal-3 inhibitor) AML IIT Phase 2 Trial *Subject to obtaining additional financing GULLIVER (Oral Gal-3 inhibitor)

Experienced management team & board of directors CEO Garrett Winslow Hans �Schambye Matt Kronmiller Lori�Firmani EVP, Strategy & CBO CFO Carl Goldfischer, M.D. Chairman Bay City Capital Partner Former CFO of ImClone1 Jayson Dallas, M.D. Former CEO Aimmune2 Amit Munshi, MBA CEO Orna Therapeutics Former CEO Arena3 Anne Prener, M.D., Ph.D. Former CEO Imbria Venture Partner SV Health Investors David Shapiro, M.D., FRCP, FFPM Retired CMO Intercept�Former CMO Idun4 Hans Schambye, M.D., Ph.D. CEO Galecto BOARD OF DIRECTORS 1Acquired by Eli Lilly 2Acquired by Nestlé 3Acquired by Pfizer GC 2

BRM-1420 �ENL-YEATS / FLT3 inhibitor for AML
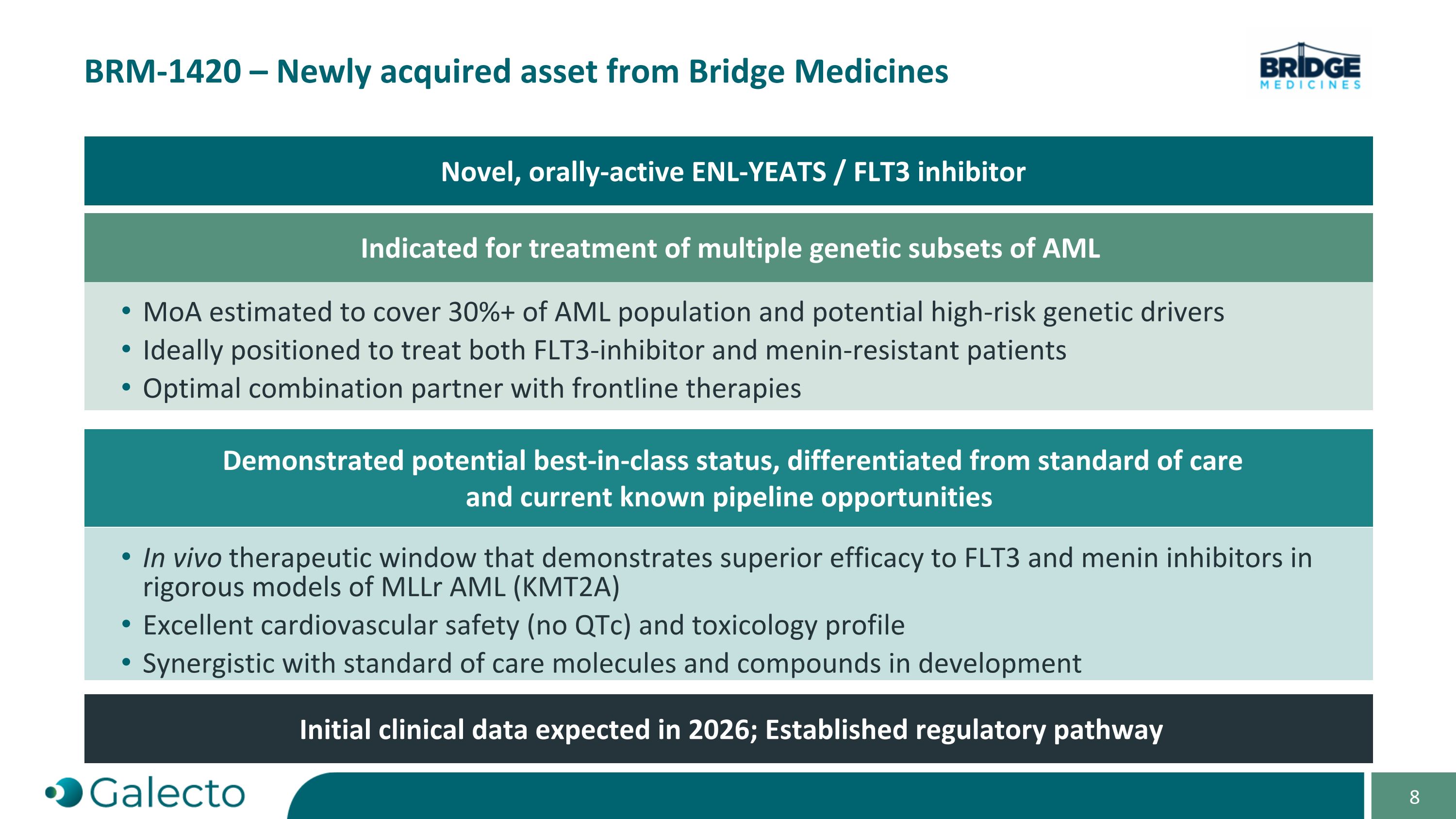
BRM-1420 – Newly acquired asset from Bridge Medicines MoA estimated to cover 30%+ of AML population and potential high-risk genetic drivers Ideally positioned to treat both FLT3-inhibitor and menin-resistant patients Optimal combination partner with frontline therapies In vivo therapeutic window that demonstrates superior efficacy to FLT3 and menin inhibitors in rigorous models of MLLr AML (KMT2A) Excellent cardiovascular safety (no QTc) and toxicology profile Synergistic with standard of care molecules and compounds in development Novel, orally-active ENL-YEATS / FLT3 inhibitor Indicated for treatment of multiple genetic subsets of AML Demonstrated potential best-in-class status, differentiated from standard of care and current known pipeline opportunities Initial clinical data expected in 2026; Established regulatory pathway
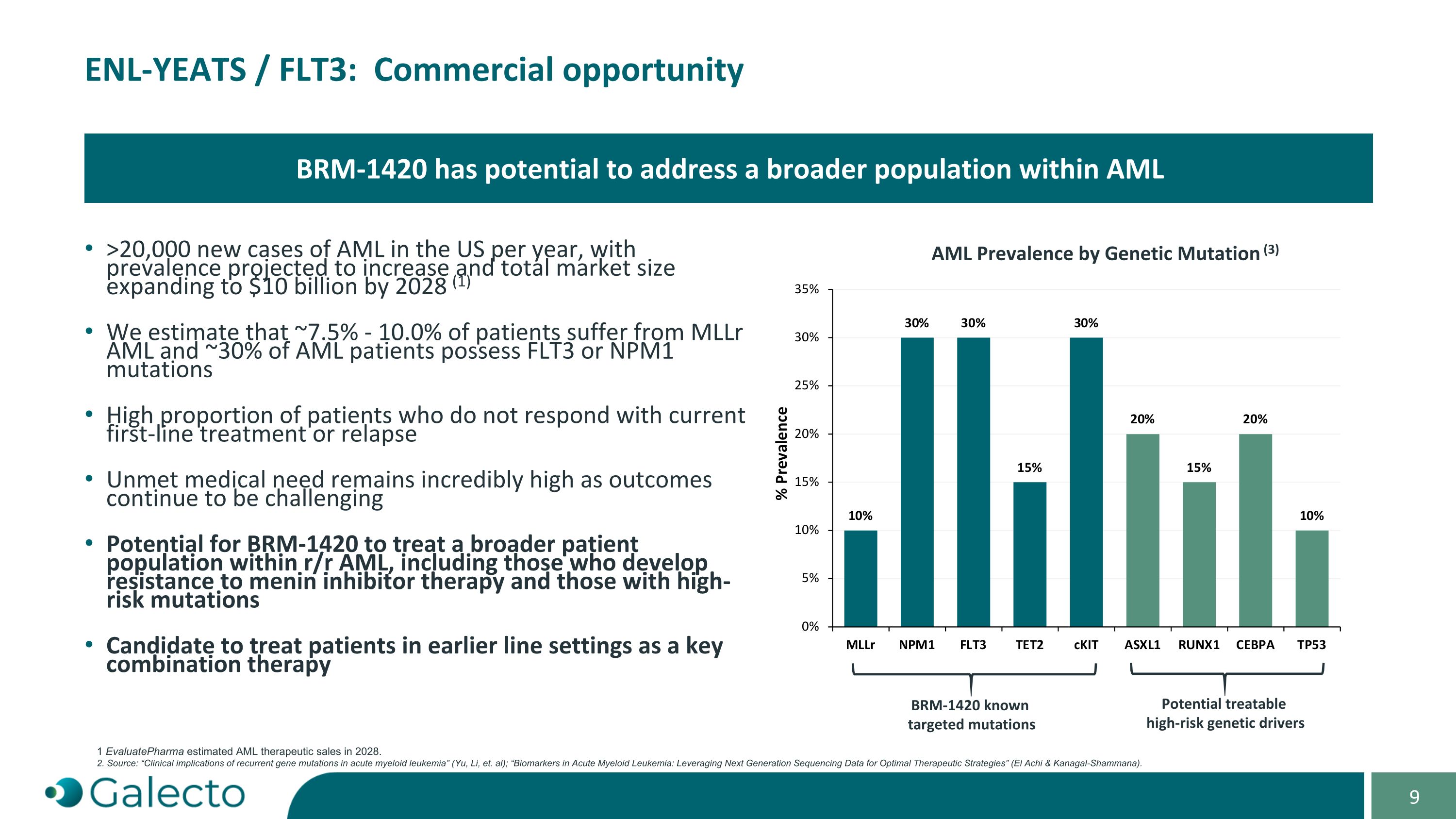
ENL-YEATS / FLT3: Commercial opportunity >20,000 new cases of AML in the US per year, with prevalence projected to increase and total market size expanding to $10 billion by 2028 (1) We estimate that ~7.5% - 10.0% of patients suffer from MLLr AML and ~30% of AML patients possess FLT3 or NPM1 mutations High proportion of patients who do not respond with current first-line treatment or relapse Unmet medical need remains incredibly high as outcomes continue to be challenging Potential for BRM-1420 to treat a broader patient population within r/r AML, including those who develop resistance to menin inhibitor therapy and those with high-risk mutations Candidate to treat patients in earlier line settings as a key combination therapy BRM-1420 has potential to address a broader population within AML AML Prevalence by Genetic Mutation (3) BRM-1420 known targeted mutations Potential treatable high-risk genetic drivers 1 EvaluatePharma estimated AML therapeutic sales in 2028. 2. Source: “Clinical implications of recurrent gene mutations in acute myeloid leukemia” (Yu, Li, et. al); “Biomarkers in Acute Myeloid Leukemia: Leveraging Next Generation Sequencing Data for Optimal Therapeutic Strategies” (El Achi & Kanagal-Shammana).
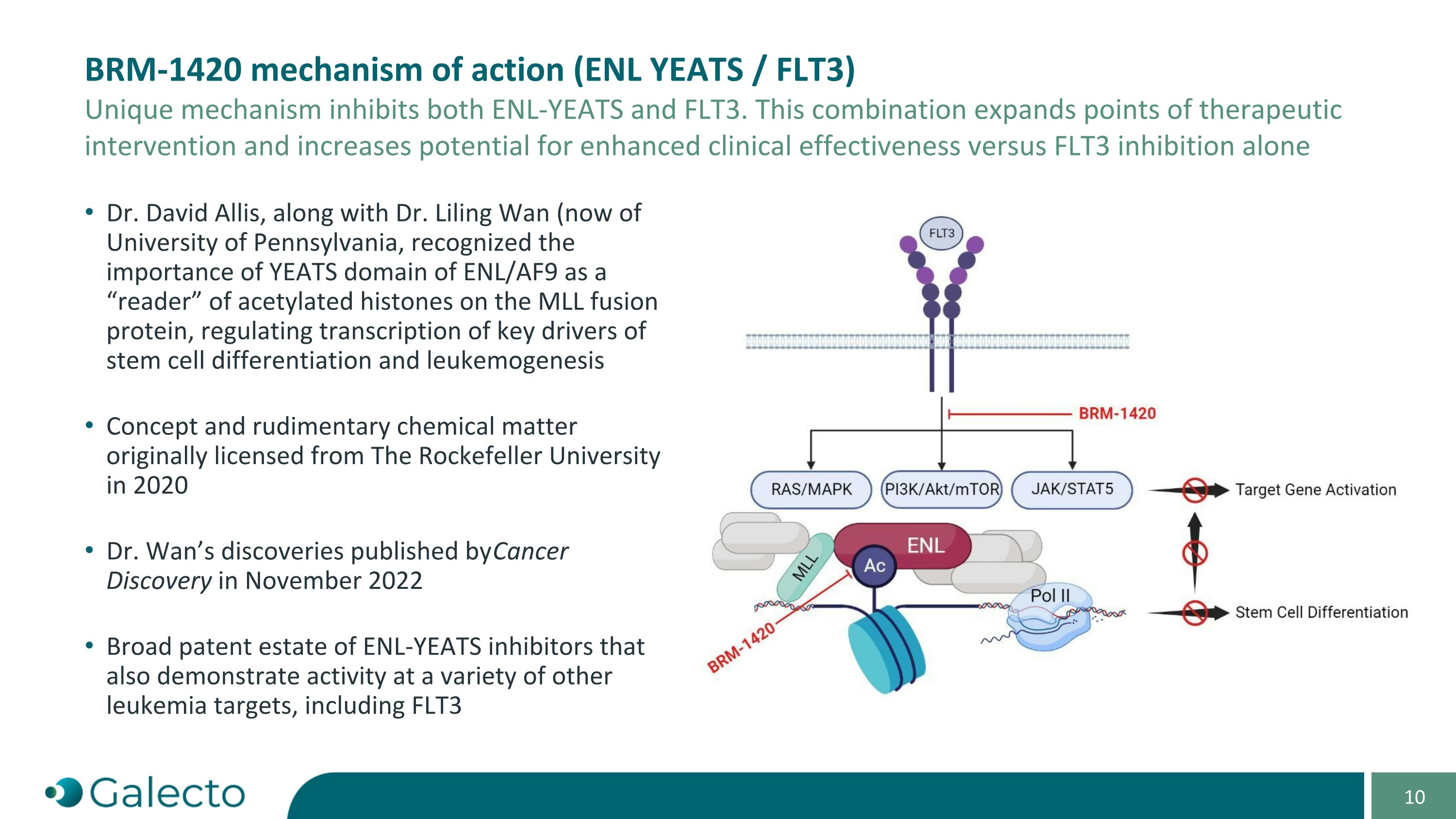
BRM-1420 mechanism of action (ENL YEATS / FLT3)�Unique mechanism inhibits both ENL-YEATS and FLT3. This combination expands points of therapeutic intervention and increases potential for enhanced clinical effectiveness versus FLT3 inhibition alone Dr. David Allis, along with Dr. Liling Wan (now of University of Pennsylvania, recognized the importance of YEATS domain of ENL/AF9 as a “reader” of acetylated histones on the MLL fusion protein, regulating transcription of key drivers of stem cell differentiation and leukemogenesis Concept and rudimentary chemical matter originally licensed from The Rockefeller University in 2020 Dr. Wan’s discoveries published by Cancer Discovery in November 2022 Broad patent estate of ENL-YEATS inhibitors that also demonstrate activity at a variety of other leukemia targets, including FLT3
Preclinical data and potential clinical implications Rapid and durable anti-tumor activity with survival > comparators Tolerability > comparators Pronounced effects on key genetic drivers of leukemogenesis and maintenance (HOXA9, MEIS1, MYC) Substantial reduction/elimination of blast cells in peripheral blood, bone marrow and spleen Immediate cell cycle arrest, differentiation of blasts and apoptosis Preclinical data shows potential for rapid onset of action, complete and durable responses, efficacy potentially superior to competitors, and a better safety/tolerability profile
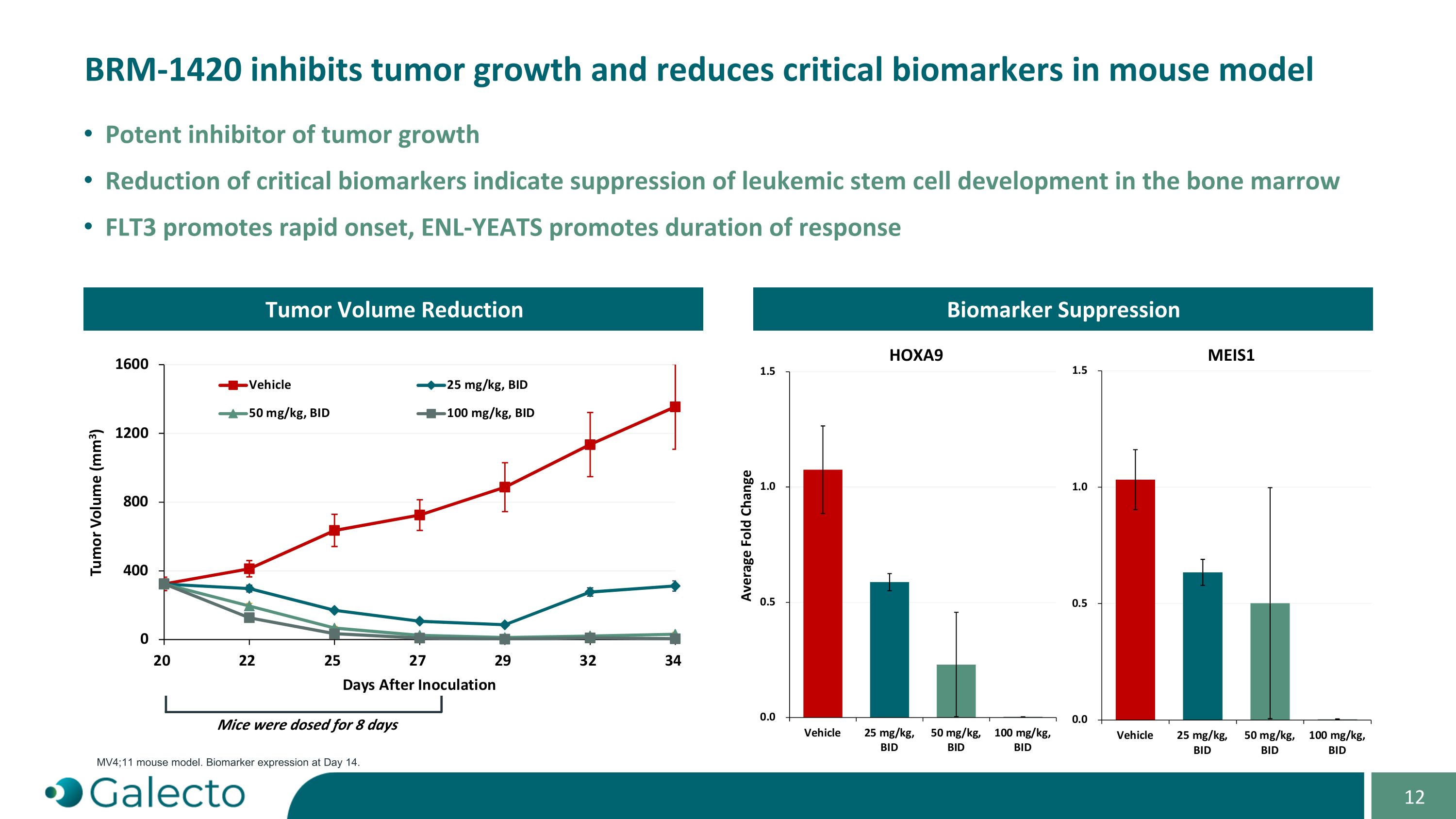
BRM-1420 inhibits tumor growth and reduces critical biomarkers in mouse model Potent inhibitor of tumor growth Reduction of critical biomarkers indicate suppression of leukemic stem cell development in the bone marrow FLT3 promotes rapid onset, ENL-YEATS promotes duration of response Tumor Volume Reduction Mice were dosed for 8 days MV4;11 mouse model. Biomarker expression at Day 14. Biomarker Suppression HOXA9 MEIS1 Average Fold Change
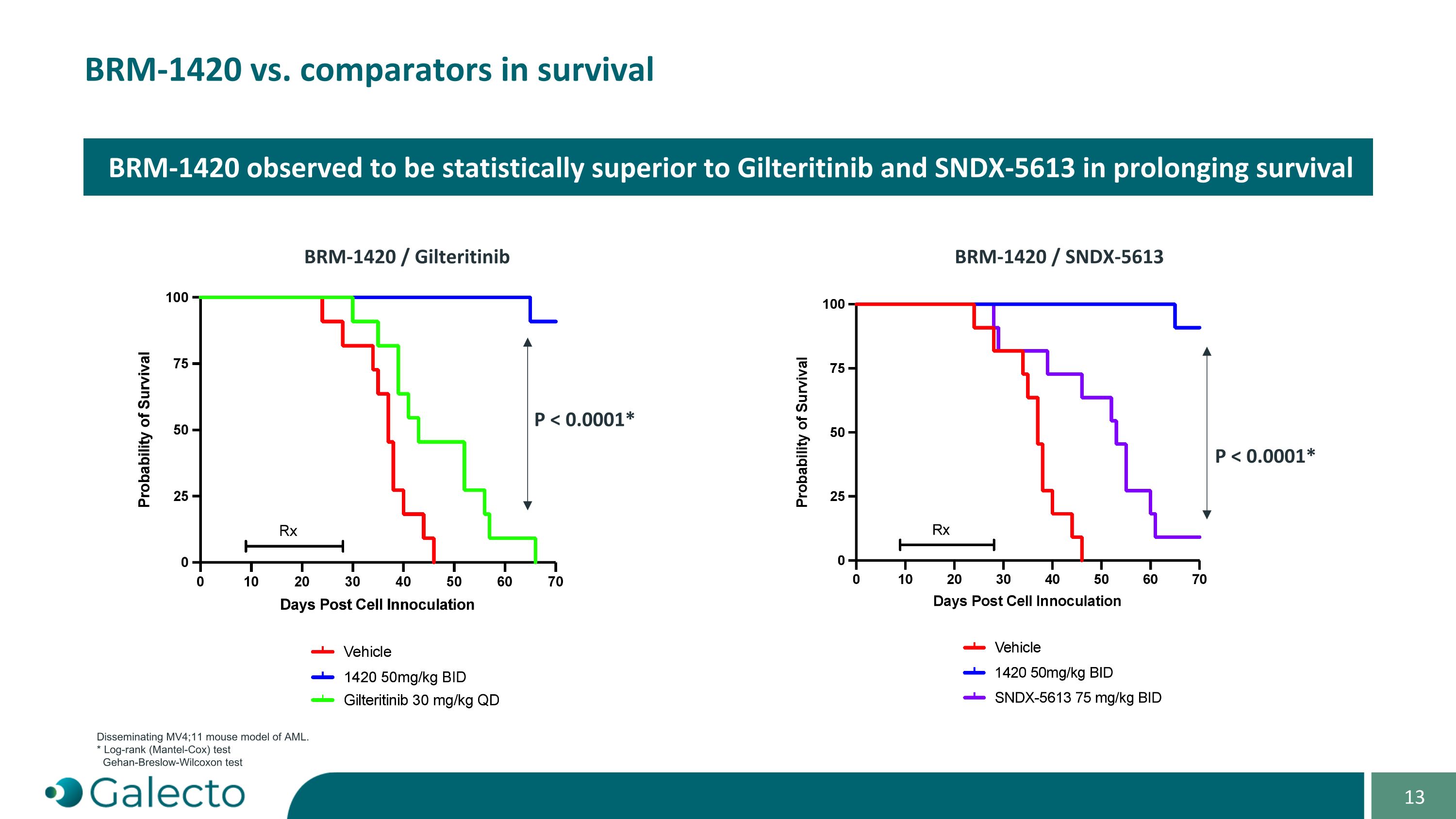
BRM-1420 vs. comparators in survival Disseminating MV4;11 mouse model of AML. * Log-rank (Mantel-Cox) test Gehan-Breslow-Wilcoxon test BRM-1420 observed to be statistically superior to Gilteritinib and SNDX-5613 in prolonging survival BRM-1420 / Gilteritinib BRM-1420 / SNDX-5613 P < 0.0001* P < 0.0001*
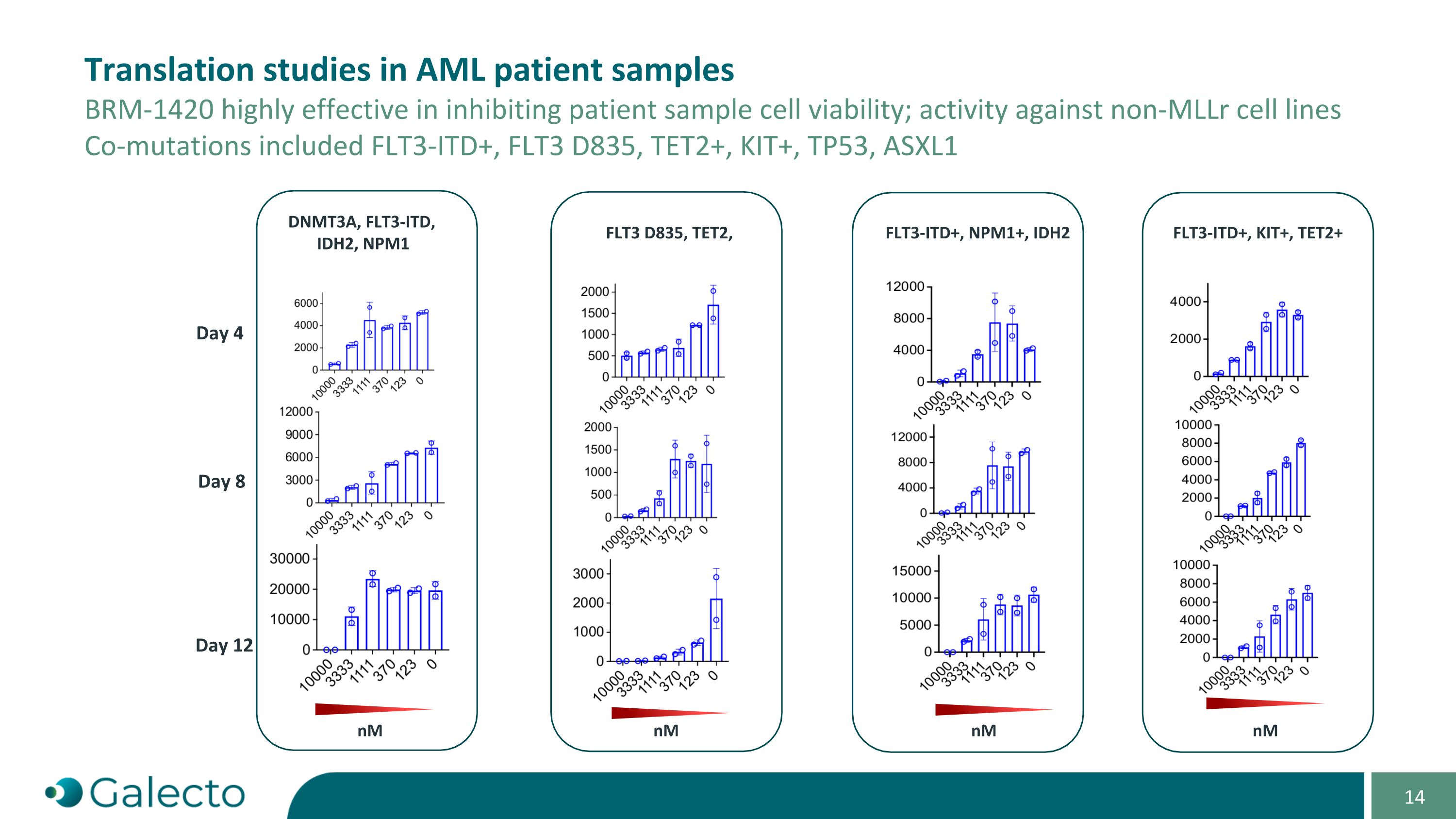
Translation studies in AML patient samples�BRM-1420 highly effective in inhibiting patient sample cell viability; activity against non-MLLr cell lines�Co-mutations included FLT3-ITD+, FLT3 D835, TET2+, KIT+, TP53, ASXL1 DNMT3A, FLT3-ITD, IDH2, NPM1 Day 4 Day 8 Day 12 FLT3 D835, TET2, FLT3-ITD+, NPM1+, IDH2 FLT3-ITD+, KIT+, TET2+ nM nM nM nM
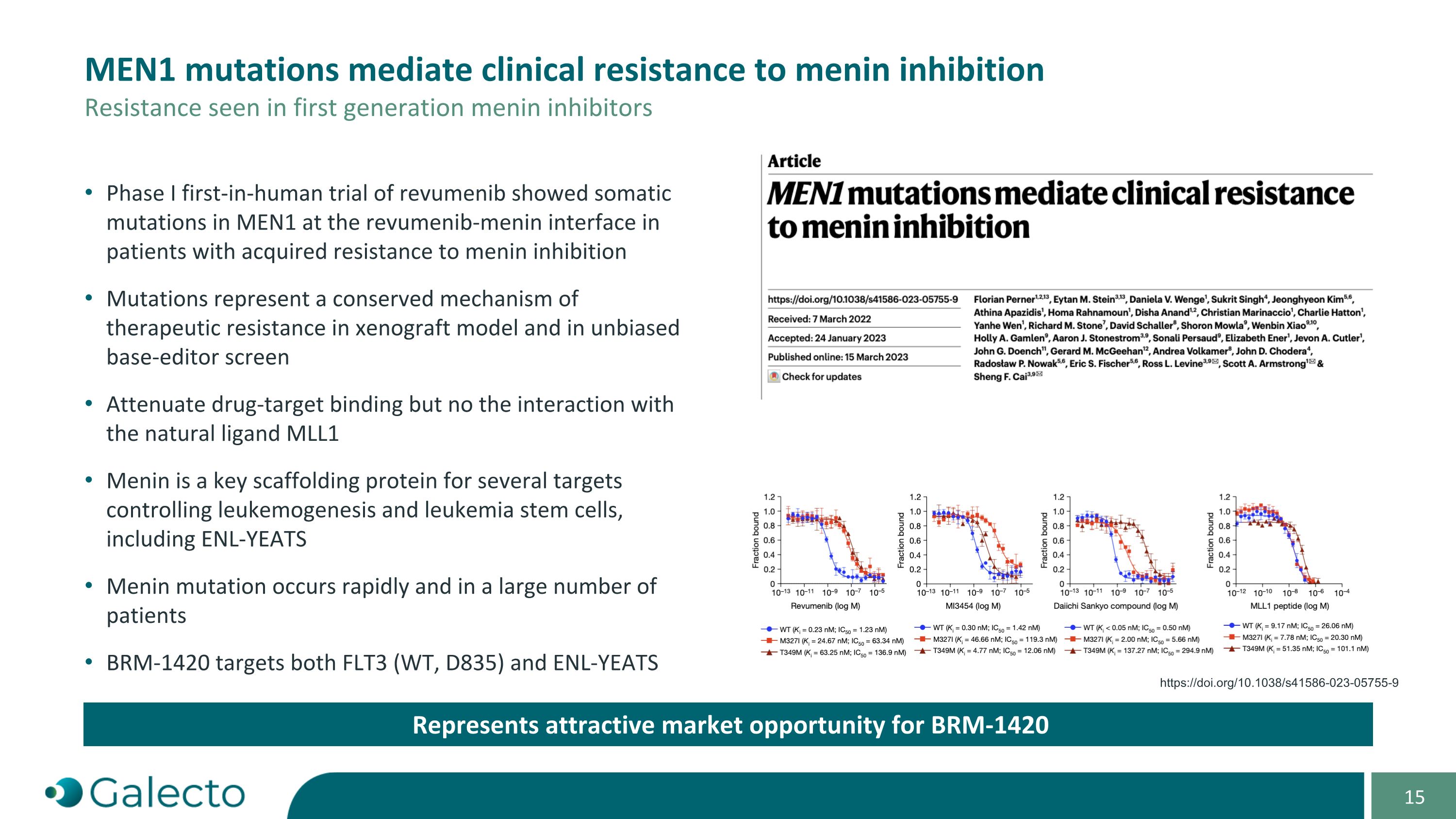
MEN1 mutations mediate clinical resistance to menin inhibition�Resistance seen in first generation menin inhibitors Represents attractive market opportunity for BRM-1420 Phase I first-in-human trial of revumenib showed somatic mutations in MEN1 at the revumenib-menin interface in patients with acquired resistance to menin inhibition Mutations represent a conserved mechanism of therapeutic resistance in xenograft model and in unbiased base-editor screen Attenuate drug-target binding but no the interaction with the natural ligand MLL1 Menin is a key scaffolding protein for several targets controlling leukemogenesis and leukemia stem cells, including ENL-YEATS Menin mutation occurs rapidly and in a large number of patients BRM-1420 targets both FLT3 (WT, D835) and ENL-YEATS https://doi.org/10.1038/s41586-023-05755-9
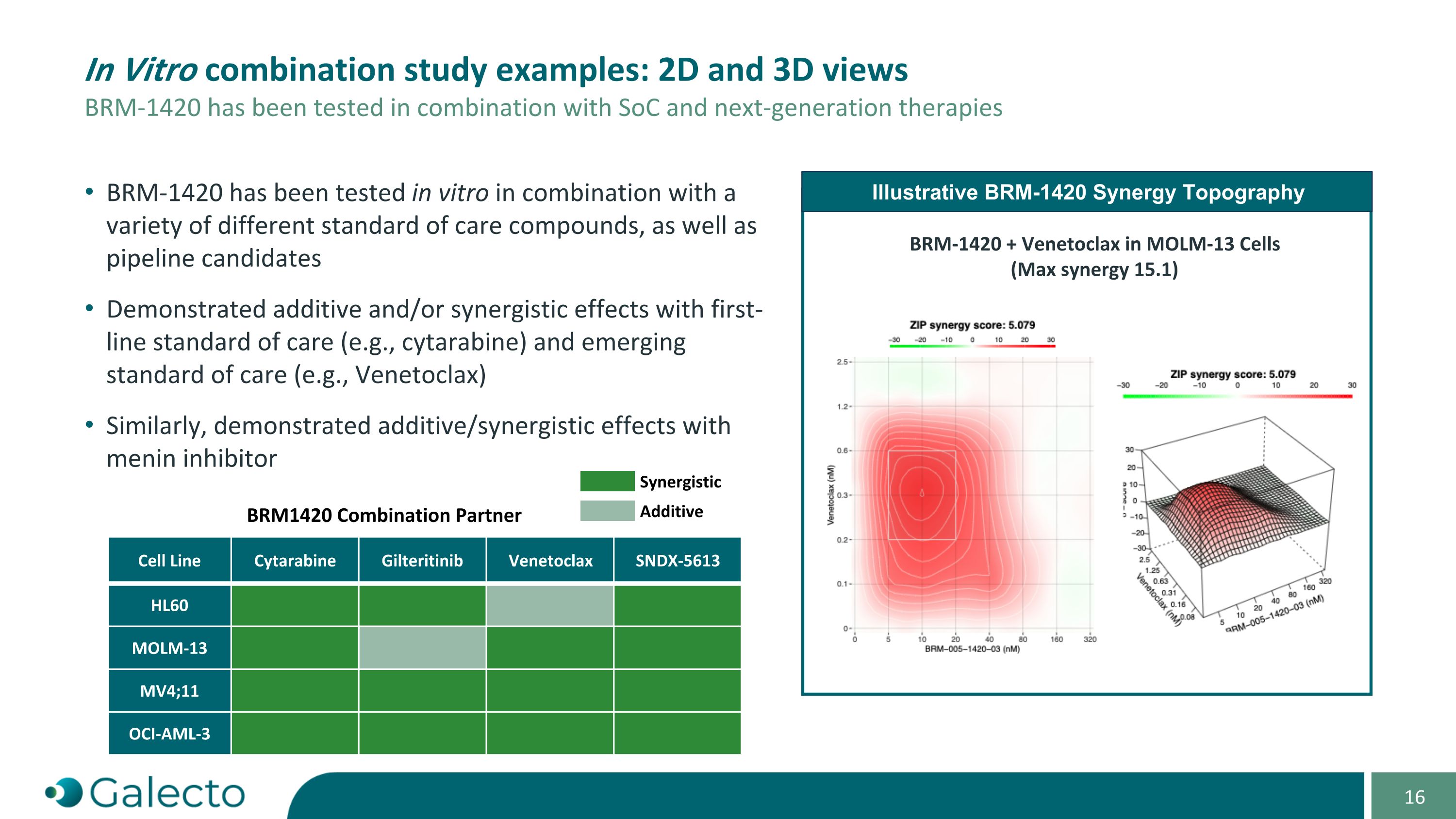
In Vitro combination study examples: 2D and 3D views�BRM-1420 has been tested in combination with SoC and next-generation therapies Illustrative BRM-1420 Synergy Topography BRM-1420 + Venetoclax in MOLM-13 Cells (Max synergy 15.1) BRM-1420 has been tested in vitro in combination with a variety of different standard of care compounds, as well as pipeline candidates Demonstrated additive and/or synergistic effects with first-line standard of care (e.g., cytarabine) and emerging standard of care (e.g., Venetoclax) Similarly, demonstrated additive/synergistic effects with menin inhibitor Cell Line Cytarabine Gilteritinib Venetoclax SNDX-5613 HL60 MOLM-13 MV4;11 OCI-AML-3 BRM1420 Combination Partner Synergistic Additive

BRM-1420 toxicology summary�Well-tolerated in both rats and dogs with no QTc liability Current standard of care and compounds in development have encountered safety / PK liabilities Common side effects of FLT3-inhibitor gilteritinib include diarrhea, anemia, fatigue and elevated LFT; warning included about QT prolongation Syndax’s menin-inhibitor has issues with tolerability (QT prolongation) and drug-drug interactions (CYP3A4 inhibitors) No statistically significant QTc prolongation seen with BRM-1420 up to 100 mg/kg in dog cardiovascular study No deaths, target organ toxicity, or changes in clinical chemistry seen in 14-day pilot studies in rats and dogs Some effects on WBC counts, hematocrit and hemoglobin that were mild and manageable in a clinical setting Effects on bone marrow / blood cell counts typically more severe with SoC in AML Reduction in reticulocytes observed but is easily monitorable in a clinical setting and reversable Effect on reticulocytes may be attributable to inhibition of AF9 and is an indirect surrogate for target engagement In dog study, study operator considered 200 mg/kg to be an MTD based on recurrent vomits, presence of soft to liquid feces and body weight loss

GB1211 �Oral Galectin-3 Inhibitor �for Cancer
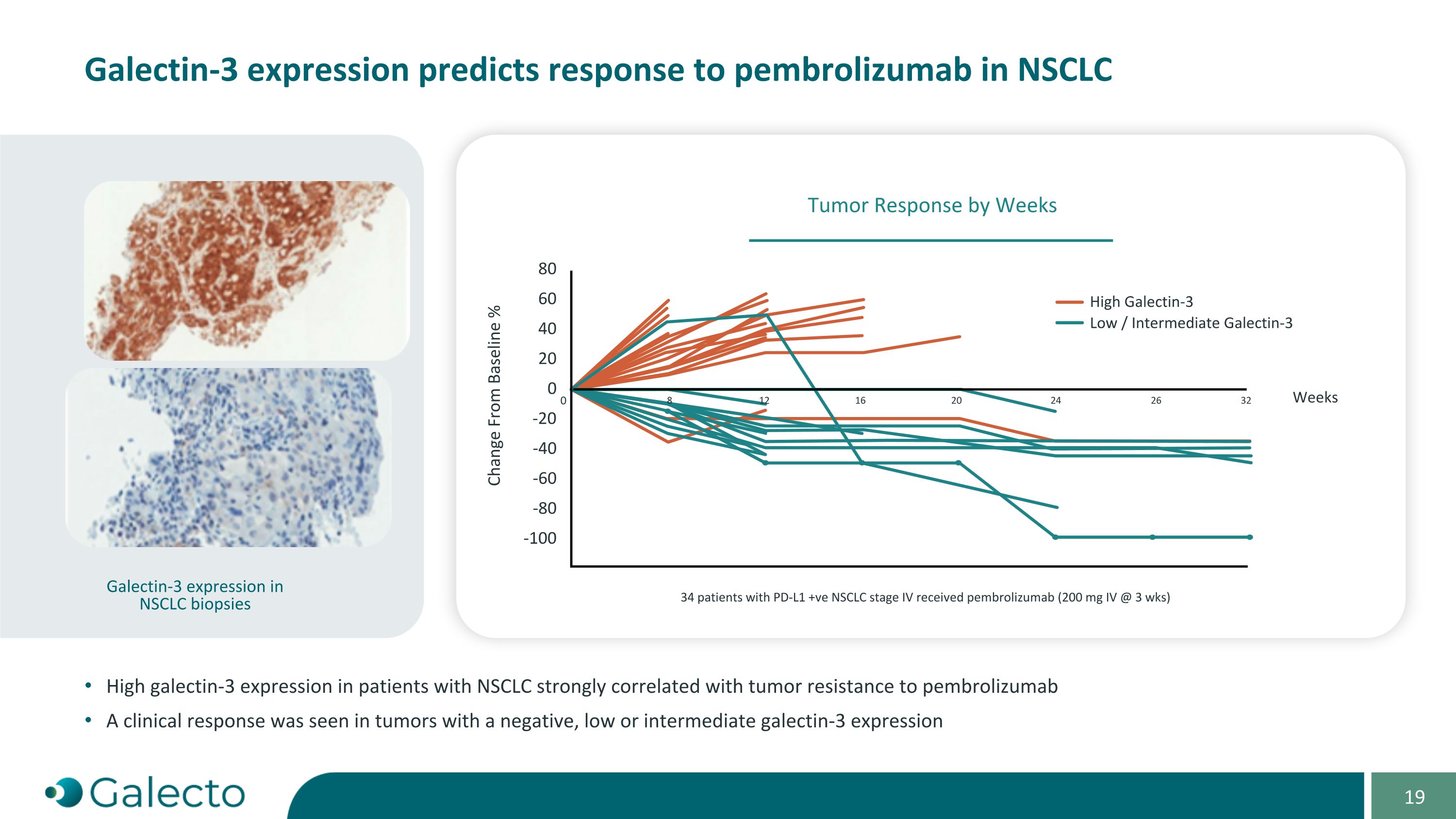
Galectin-3 expression predicts response to pembrolizumab in NSCLC High galectin-3 expression in patients with NSCLC strongly correlated with tumor resistance to pembrolizumab A clinical response was seen in tumors with a negative, low or intermediate galectin-3 expression Galectin-3 expression in NSCLC biopsies 34 patients with PD-L1 +ve NSCLC stage IV received pembrolizumab (200 mg IV @ 3 wks) -20 80 60 40 20 Change From Baseline % 0 0 8 12 16 20 24 26 32 High Galectin-3 Low / Intermediate Galectin-3 Weeks -40 -60 -80 -100 Tumor Response by Weeks
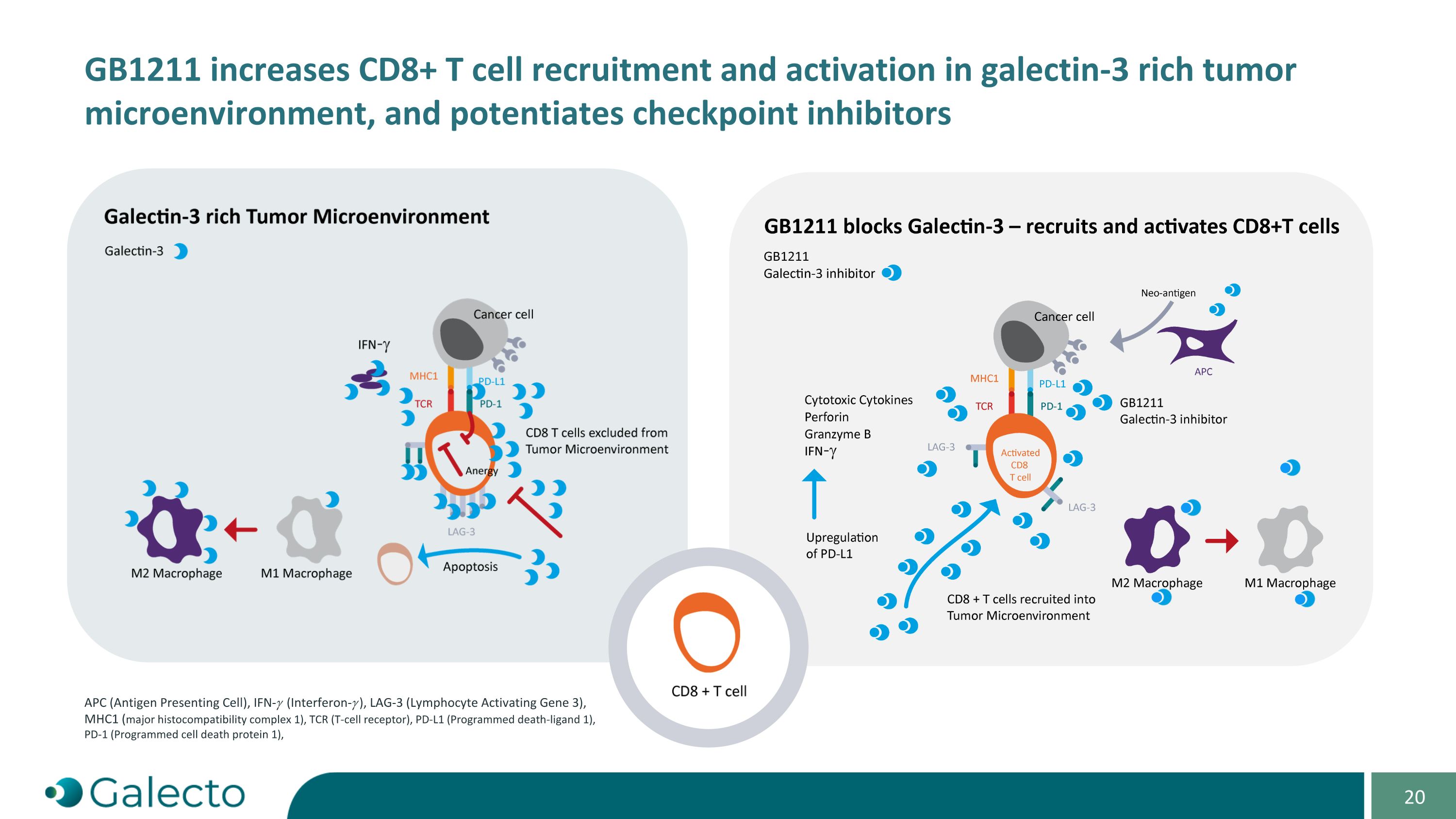
GB1211 increases CD8+ T cell recruitment and activation in galectin-3 rich tumor microenvironment, and potentiates checkpoint inhibitors APC (Antigen Presenting Cell), IFN-𝛾 (Interferon-𝛾), LAG-3 (Lymphocyte Activating Gene 3), MHC1 (major histocompatibility complex 1), TCR (T-cell receptor), PD-L1 (Programmed death-ligand 1), PD-1 (Programmed cell death protein 1),
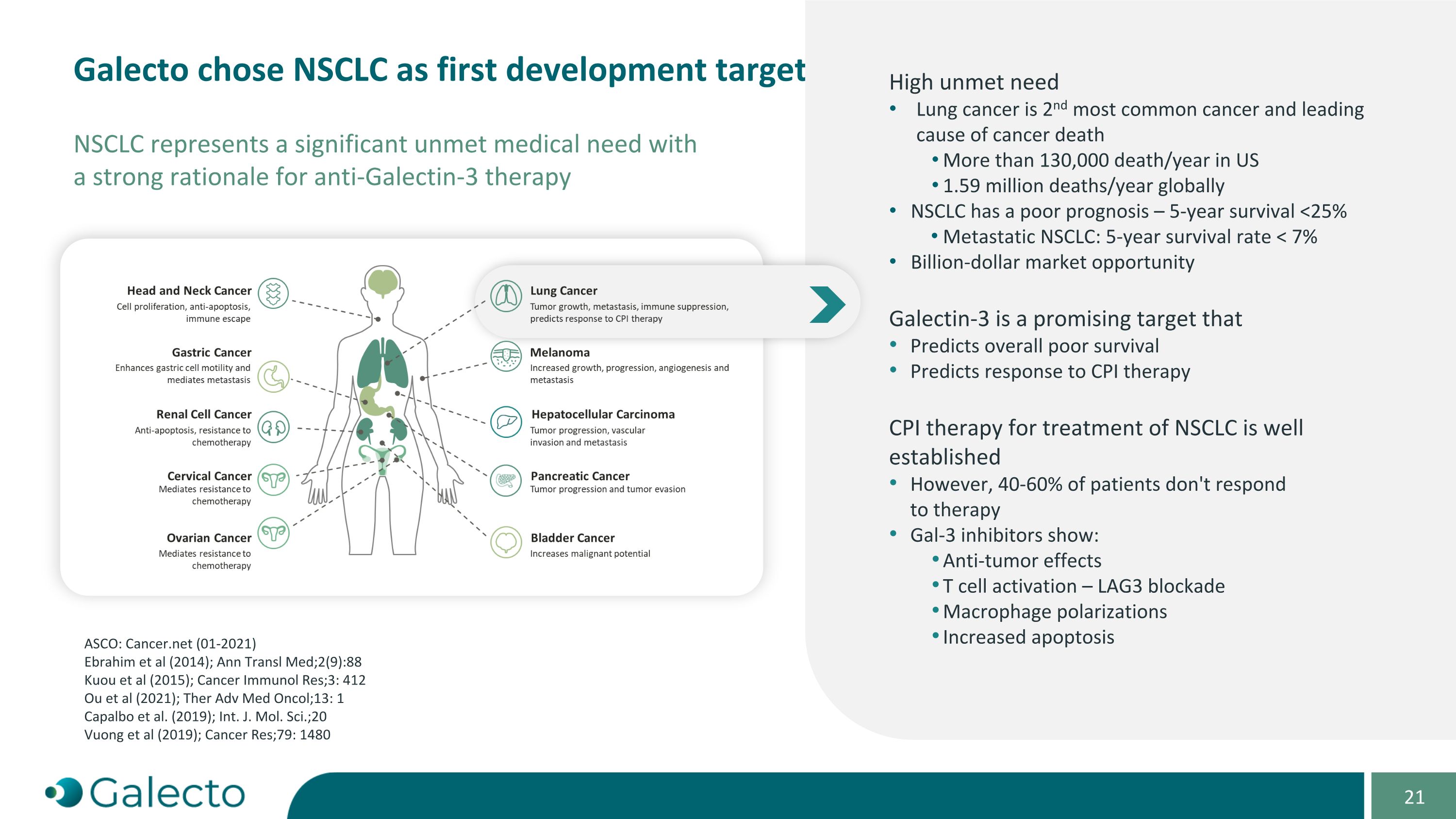
Galecto chose NSCLC as first development target��NSCLC represents a significant unmet medical need with �a strong rationale for anti-Galectin-3 therapy ASCO: Cancer.net (01-2021) Ebrahim et al (2014); Ann Transl Med;2(9):88 Kuou et al (2015); Cancer Immunol Res;3: 412�Ou et al (2021); Ther Adv Med Oncol;13: 1 Capalbo et al. (2019); Int. J. Mol. Sci.;20�Vuong et al (2019); Cancer Res;79: 1480 High unmet need Lung cancer is 2nd most common cancer and leading cause of cancer death More than 130,000 death/year in US 1.59 million deaths/year globally NSCLC has a poor prognosis – 5-year survival <25% Metastatic NSCLC: 5-year survival rate < 7% Billion-dollar market opportunity Galectin-3 is a promising target that Predicts overall poor survival Predicts response to CPI therapy CPI therapy for treatment of NSCLC is well established However, 40-60% of patients don't respond �to therapy Gal-3 inhibitors show: Anti-tumor effects T cell activation – LAG3 blockade Macrophage polarizations Increased apoptosis
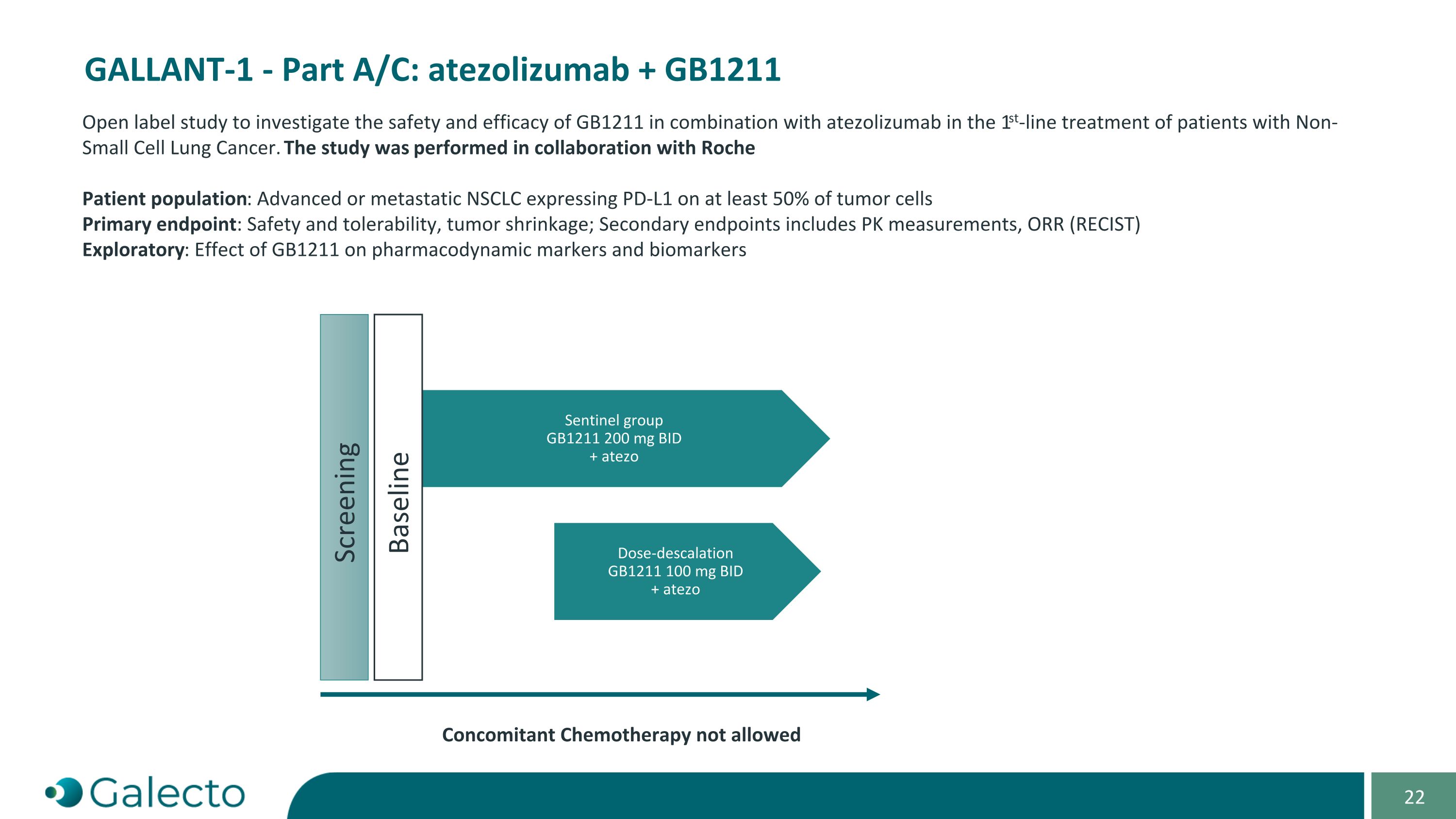
GALLANT-1 - Part A/C: atezolizumab + GB1211 Open label study to investigate the safety and efficacy of GB1211 in combination with atezolizumab in the 1st-line treatment of patients with Non-Small Cell Lung Cancer. The study was performed in collaboration with Roche Patient population: Advanced or metastatic NSCLC expressing PD-L1 on at least 50% of tumor cells Primary endpoint: Safety and tolerability, tumor shrinkage; Secondary endpoints includes PK measurements, ORR (RECIST) Exploratory: Effect of GB1211 on pharmacodynamic markers and biomarkers Dose-descalation GB1211 100 mg BID + atezo Concomitant Chemotherapy not allowed Sentinel group GB1211 200 mg BID + atezo Screening Baseline
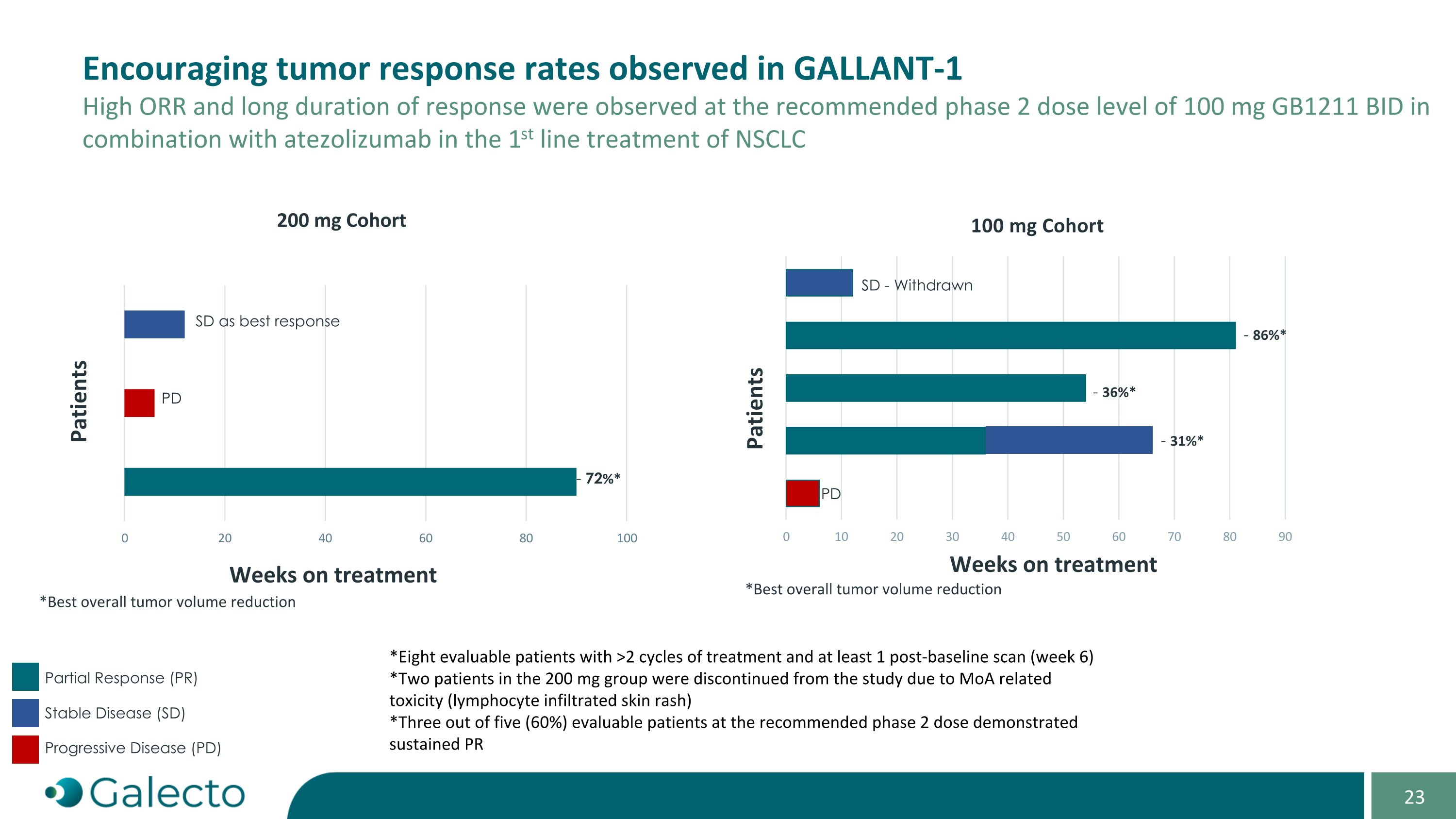
Encouraging tumor response rates observed in GALLANT-1�High ORR and long duration of response were observed at the recommended phase 2 dose level of 100 mg GB1211 BID in combination with atezolizumab in the 1st line treatment of NSCLC DP *Eight evaluable patients with >2 cycles of treatment and at least 1 post-baseline scan (week 6) *Two patients in the 200 mg group were discontinued from the study due to MoA related toxicity (lymphocyte infiltrated skin rash) *Three out of five (60%) evaluable patients at the recommended phase 2 dose demonstrated sustained PR SD as best response PD SD - Withdrawn - 36%* - 31%* - 86%* PD - 72%* Weeks on treatment Patients Patients *Best overall tumor volume reduction 200 mg Cohort Weeks on treatment *Best overall tumor volume reduction Partial Response (PR) Stable Disease (SD) Progressive Disease (PD)
Dose-limiting skin reactions observed at 200 mg dose indicate lymphocyte activation and hence support MoA for GB1211 in combination with atezolizumab Patients included in GALLANT-1 are not suited for chemotherapy-CPI combination and despite ECOG 0-1 performance status, the study population may represent a segment of hard-to-treat patients The 100 mg GB1211 BID dose on top of atezolizumab appears safe and well-tolerated No safety concerns in patients on long-term treatment 100 mg dose seems adequate based on dose-scaling modeling Responses seem to exceed the expected efficacy of atezolizumab monotherapy ORR at R2PD 60% vs. anticipated 30% The duration of response and PFS already now appears longer than anticipated, with all responding patients receiving treatment for more than 1Y As of September 30, 2024, two patients are being treated with GB1211+atezo in the extension phase of the trial Confidential Conclusions - GALLANT-1�GB1211 appears to be well-tolerated with encouraging efficacy in combination with atezolizumab monotherapy

GB1211 �Oral Galectin-3 Inhibitor �for Liver Cirrhosis
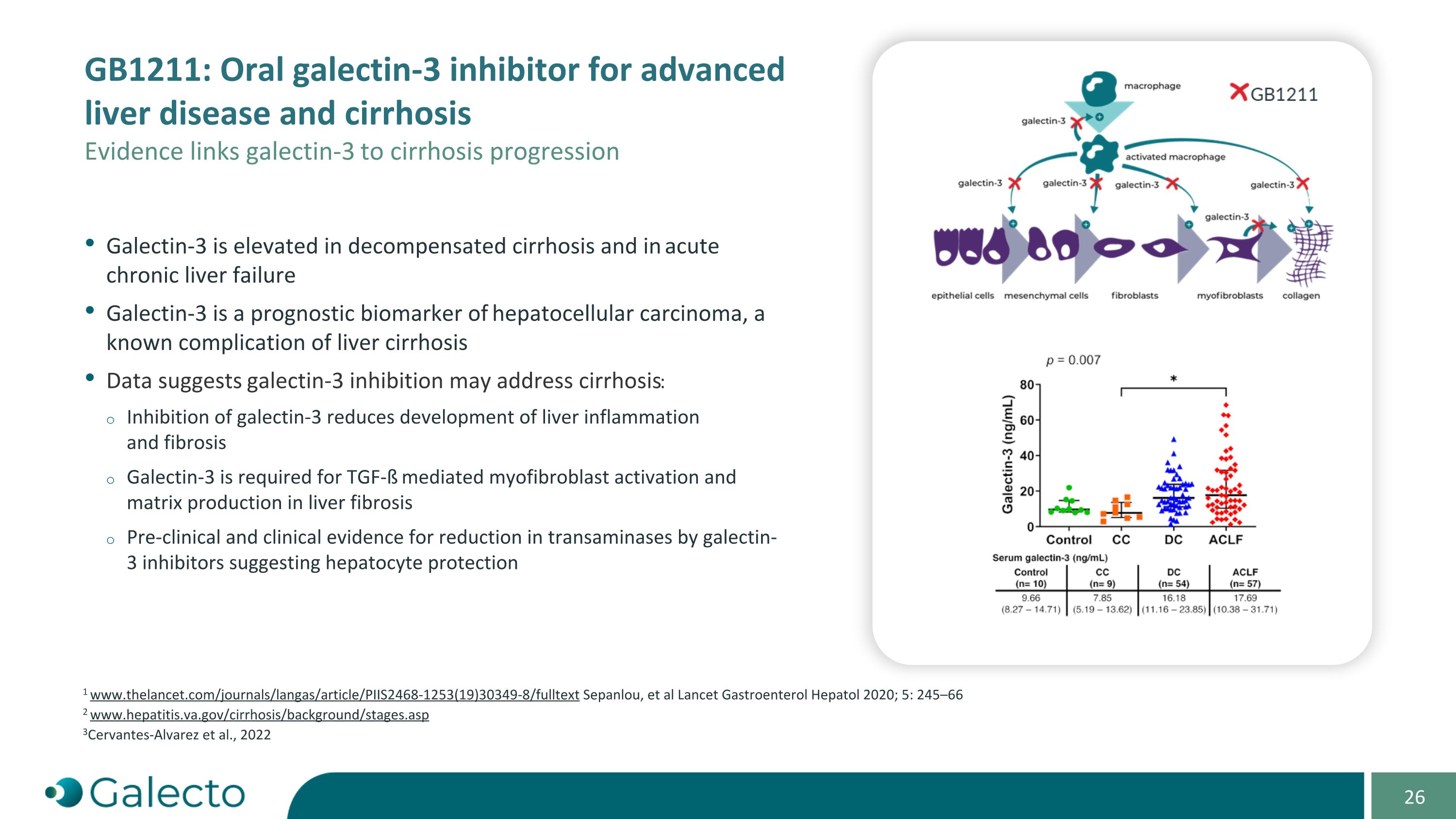
GB1211: Oral galectin-3 inhibitor for advanced�liver disease and cirrhosis�Evidence links galectin-3 to cirrhosis progression Galectin-3 is elevated in decompensated cirrhosis and in acute chronic liver failure Galectin-3 is a prognostic biomarker of hepatocellular carcinoma, a known complication of liver cirrhosis Data suggests galectin-3 inhibition may address cirrhosis: Inhibition of galectin-3 reduces development of liver inflammation �and fibrosis Galectin-3 is required for TGF-ß mediated myofibroblast activation and matrix production in liver fibrosis Pre-clinical and clinical evidence for reduction in transaminases by galectin-3 inhibitors suggesting hepatocyte protection 1 www.thelancet.com/journals/langas/article/PIIS2468-1253(19)30349-8/fulltext Sepanlou, et al Lancet Gastroenterol Hepatol 2020; 5: 245–66 2 www.hepatitis.va.gov/cirrhosis/background/stages.asp 3Cervantes-Alvarez et al., 2022
GULLIVER-2 – Part 2�A randomized, double blind, placebo-controlled 12-week study in Child-Pugh B patients BID, twice a day; CAP, controlled attenuation parameter; PK, pharmacokinetics; VCTE, vibration controlled transient elastography Part 1: Single dose hepatic �impairment study (Child-Pugh B) Part 3: Single dose hepatic �impairment study (Child-Pugh C) GB1211 100mg BID (n=15) Placebo BID (n=15) PK samples on Day 1, 7, 21, 42, 63, 84 Biochemistry on Day 1, 7, 42, and follow up Day 96 Part 2: Repeat dose hepatic impairment study (Child-Pugh B) Primary endpoints: Safety and tolerability PK Exploratory endpoints Biochemistry Liver fibrosis (VCTE) Steatosis (CAP) Exploratory biomarkers
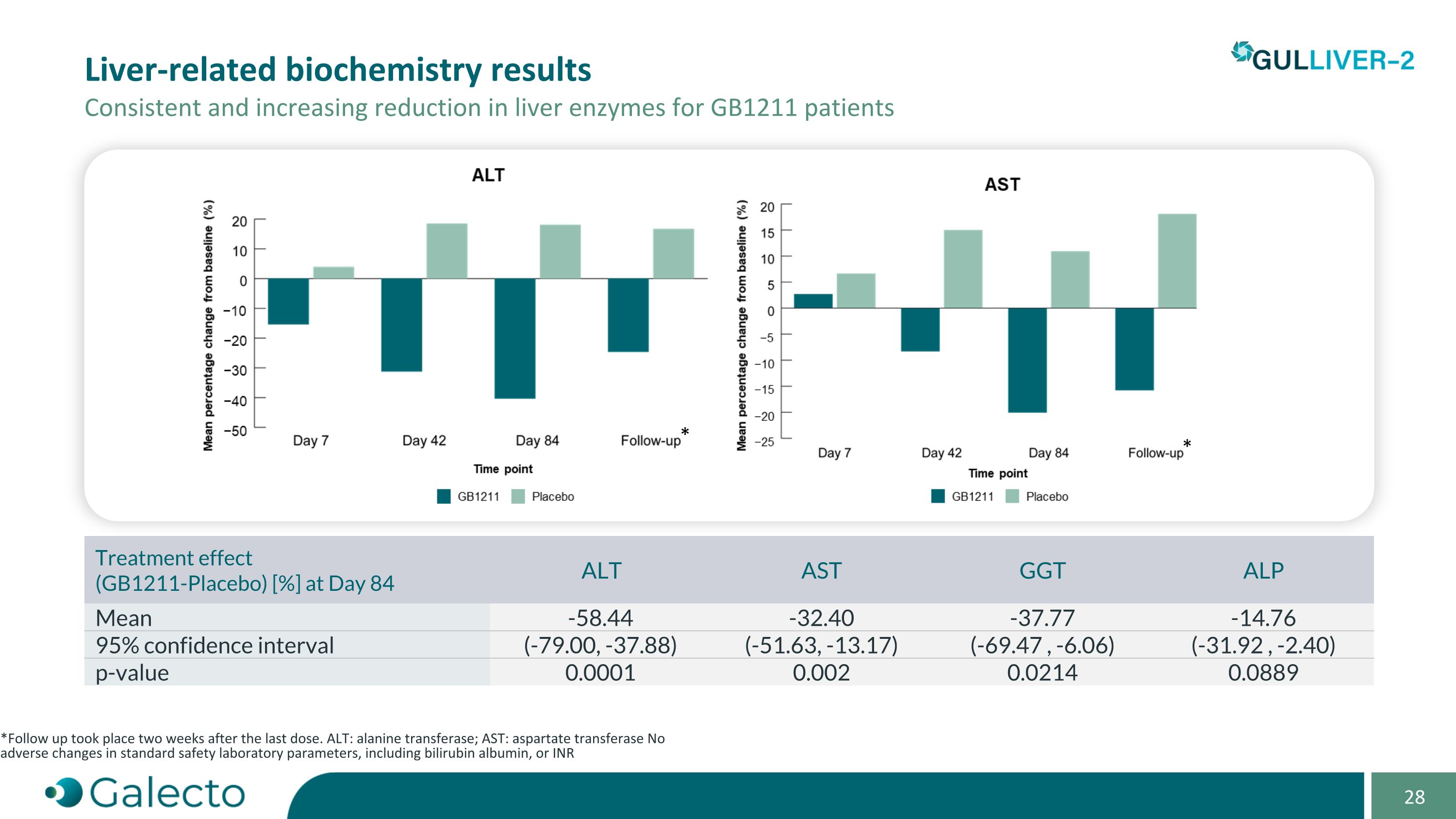
Liver-related biochemistry results�Consistent and increasing reduction in liver enzymes for GB1211 patients *Follow up took place two weeks after the last dose. ALT: alanine transferase; AST: aspartate transferase No adverse changes in standard safety laboratory parameters, including bilirubin albumin, or INR * * Treatment effect (GB1211-Placebo) [%] at Day 84 ALT AST GGT ALP Mean -58.44 -32.40 -37.77 -14.76 95% confidence interval (-79.00, -37.88) (-51.63, -13.17) (-69.47 , -6.06) (-31.92 , -2.40) p-value 0.0001 0.002 0.0214 0.0889
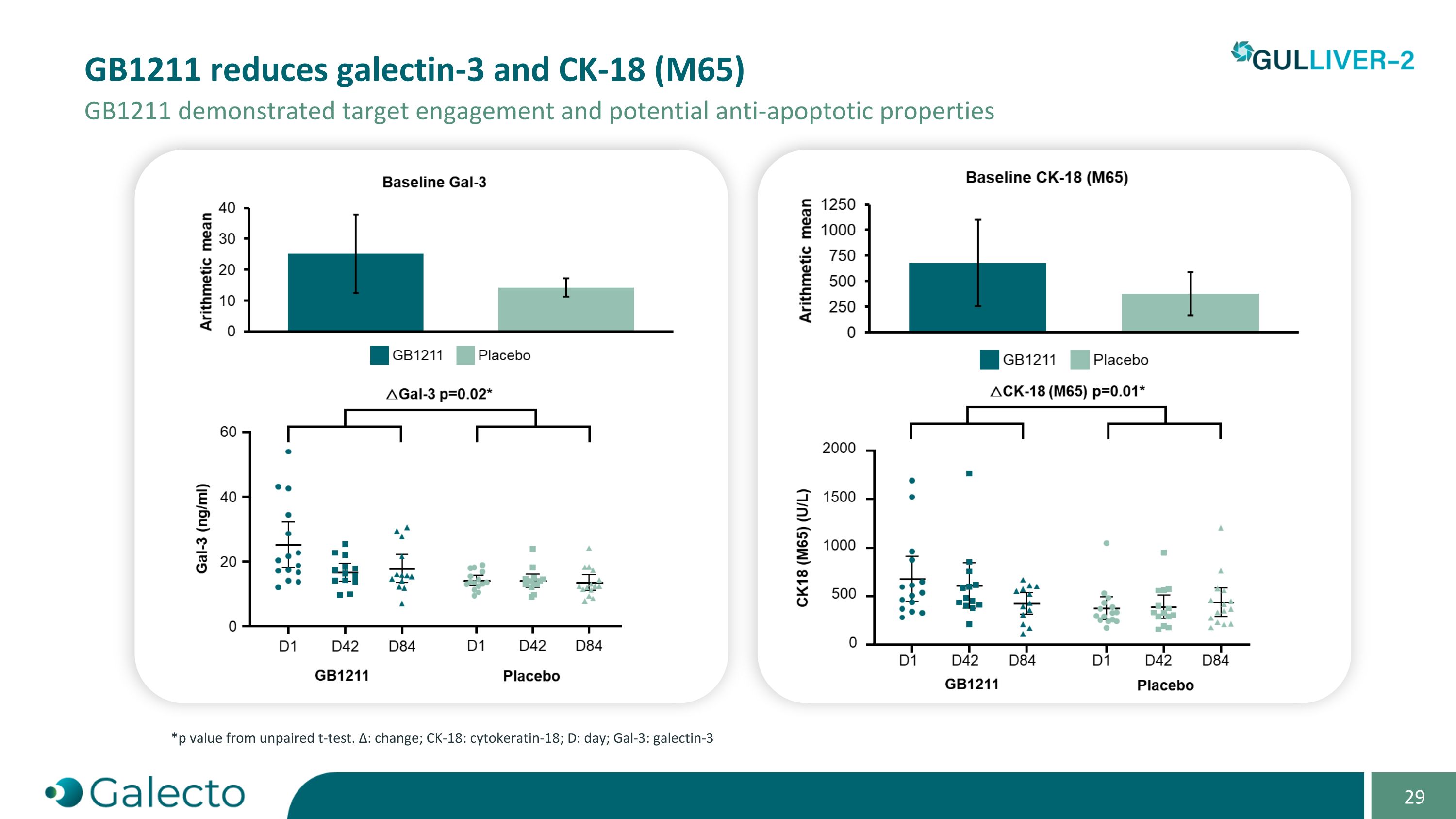
GB1211 reduces galectin-3 and CK-18 (M65) GB1211 demonstrated target engagement and potential anti-apoptotic properties *p value from unpaired t-test. Δ: change; CK-18: cytokeratin-18; D: day; Gal-3: galectin-3
GULLIVER-2 - topline results�Promising study results in a decompensated cirrhosis patient population Results strongly support progressing to phase 2/3 studies in severe liver diseases Galectin-3 in liver disease Carbohydrate binding protein shown to drive fibrosis via TGF-ß receptor Elevated in decompensated cirrhosis, alcoholic hepatitis and ACLF GB1211 is a potent, selective, oral inhibitor of Galectin-3 Well-tolerated GB1211 was well-tolerated with no drug-related adverse events identified Predictable PK profile consistent with the option of repeated dosing in patients with hepatic impairment Clinical Galectin-3 reduction demonstrates target engagement Consistent and statistically significant reductions in ALT, AST and GGT Concordant changes in liver biochemistry, liver stiffness & steatosis observed Data suggests that GB1211 improves liver inflammation and reduces liver injury

Summary
Galecto enhances pipeline following strategic review Cash balance of ~$22.9M as of June 30, 2024 funds ongoing trials and acquired program with runway into 2026 Completed Strategic Review Identifies Core Assets and Direction Following an intensive strategic review process, Galecto has determined to focus on cancer and liver disease leveraging its existing clinical stage asset GB1211 Pipeline is bolstered by acquisition of BRM-1420 for AML, which brings novelty and breakthrough potential Superior activity in preclinical AML models Identified after review of multiple assets, assisted by Leerink Enhanced Pipeline Focused On Oncology and Liver Disease Innovative platforms targeting core disease processes Pioneers in ENL-YEATS and galectin-3 �based pharmacology First-in-class highly specific, oral small-molecule inhibitors All programs address: Diseases characterized by clear unmet medical need Multi-billion-dollar market opportunities Early Data �Supportive of Drug Activity ENL-YEATS inhibitor: MoA estimated to cover 30%+ of AML population Rapid and durable anti-tumor activity in AML models Galectin-3 Inhibitors: Target engagement shown Positive biomarker data Evidence of tumor microenvironment transformation Significant clinical data in cirrhosis
v3.24.3
Document and Entity Information
|
Oct. 07, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 07, 2024
|
| Entity Registrant Name |
GALECTO, INC.
|
| Entity Central Index Key |
0001800315
|
| Entity Emerging Growth Company |
true
|
| Current Fiscal Year End Date |
--12-31
|
| Entity Ex Transition Period |
false
|
| Entity File Number |
001-39655
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
37-1957007
|
| Entity Address, Address Line One |
75 State Street
|
| Entity Address, Address Line Two |
Suite 100
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02109
|
| City Area Code |
+45
|
| Local Phone Number |
70 70 52 10
|
| Title of 12(b) Security |
Common Stock, $0.00001 par value per share
|
| Trading Symbol |
GLTO
|
| Security Exchange Name |
NASDAQ
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Pro Forma Condensed Consolidated Balance Sheet
$ in Thousands |
Jun. 30, 2024
USD ($)
|
| Current assets |
|
| Cash and cash equivalents |
$ 22,862
|
| Prepaid expenses and other current assets |
2,306
|
| Total current assets |
25,168
|
| Operating lease right-of-use asset |
76
|
| Equipment, net |
67
|
| Other assets, non-current |
1,986
|
| Total assets |
27,297
|
| Current liabilities |
|
| Accounts payable |
724
|
| Accrued expenses and other current liabilities |
2,985
|
| Total current liabilities |
3,709
|
| Total liabilities |
3,709
|
| Mezzanine equity |
|
| Preferred stock, par value of $0.00001 per share; 10,000,000 shares authorized at June 30, 2024; no shares issued or outstanding as of June 30, 2024, actual; 161 shares issued and outstanding as of June 30, 2024, pro forma |
|
| Stockholders’ equity |
|
| Common stock, par value of $0.00001 per share; 300,000,000 shares authorized at June 30, 2024; 1,084,841 issued and outstanding at June 30, 2024; actual, 1,147,435 issued and outstanding at June 30, 2024, pro forma |
|
| Additional paid-in capital |
290,291
|
| Accumulated deficit |
(266,900)
|
| Accumulated other comprehensive gain |
197
|
| Total stockholders’ equity |
23,588
|
| Total liabilities and stockholders' equity |
27,297
|
| Pro Forma |
|
| Current assets |
|
| Cash and cash equivalents |
20,182
|
| Prepaid expenses and other current assets |
2,306
|
| Total current assets |
22,488
|
| Operating lease right-of-use asset |
76
|
| Equipment, net |
67
|
| Other assets, non-current |
1,986
|
| Total assets |
24,617
|
| Current liabilities |
|
| Accounts payable |
724
|
| Accrued expenses and other current liabilities |
2,985
|
| Total current liabilities |
3,709
|
| Total liabilities |
3,709
|
| Mezzanine equity |
|
| Preferred stock, par value of $0.00001 per share; 10,000,000 shares authorized at June 30, 2024; no shares issued or outstanding as of June 30, 2024, actual; 161 shares issued and outstanding as of June 30, 2024, pro forma |
1,876
|
| Stockholders’ equity |
|
| Common stock, par value of $0.00001 per share; 300,000,000 shares authorized at June 30, 2024; 1,084,841 issued and outstanding at June 30, 2024; actual, 1,147,435 issued and outstanding at June 30, 2024, pro forma |
|
| Additional paid-in capital |
291,023
|
| Accumulated deficit |
(272,188)
|
| Accumulated other comprehensive gain |
197
|
| Total stockholders’ equity |
20,908
|
| Total liabilities and stockholders' equity |
24,617
|
| Asset Acquisition Adjustments |
|
| Current assets |
|
| Cash and cash equivalents |
(2,680)
|
| Total current assets |
(2,680)
|
| Total assets |
(2,680)
|
| Mezzanine equity |
|
| Preferred stock, par value of $0.00001 per share; 10,000,000 shares authorized at June 30, 2024; no shares issued or outstanding as of June 30, 2024, actual; 161 shares issued and outstanding as of June 30, 2024, pro forma |
1,876
|
| Stockholders’ equity |
|
| Common stock, par value of $0.00001 per share; 300,000,000 shares authorized at June 30, 2024; 1,084,841 issued and outstanding at June 30, 2024; actual, 1,147,435 issued and outstanding at June 30, 2024, pro forma |
|
| Additional paid-in capital |
732
|
| Accumulated deficit |
(5,288)
|
| Total stockholders’ equity |
(2,680)
|
| Total liabilities and stockholders' equity |
$ (2,680)
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid nor invoiced, and liabilities classified as other.
| Name: |
us-gaap_AccruedLiabilitiesAndOtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TemporaryEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount, attributable to parent, of an entity's issued and outstanding stock which is not included within permanent equity. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. Includes stock with a put option held by an ESOP and stock redeemable by a holder only in the event of a change in control of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.E.Q2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
| Name: |
us-gaap_TemporaryEquityCarryingAmountAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_StatementScenarioAxis=us-gaap_ScenarioAdjustmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Pro Forma Condensed Consolidated Balance Sheet (Parenthetical)
|
Jun. 30, 2024
$ / shares
shares
|
| Preferred stock, par value | $ / shares |
$ 0.00001
|
| Preferred stock, authorized |
10,000,000
|
| Preferred stock, issued |
0
|
| Preferred stock, outstanding |
0
|
| Common stock, par value | $ / shares |
$ 0.00001
|
| Common stock, authorized |
300,000,000
|
| Common stock, issued |
1,084,841
|
| Common stock, outstanding |
1,084,841
|
| Pro Forma |
|
| Preferred stock, issued |
161
|
| Preferred stock, outstanding |
161
|
| Common stock, issued |
1,147,435
|
| Common stock, outstanding |
1,147,435
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share amount of par value or stated value of stock classified as temporary equity. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 480
-SubTopic 10
-Section S99
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480244/480-10-S99-1
| Name: |
us-gaap_TemporaryEquityParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of securities classified as temporary equity that are permitted to be issued by an entity's charter and bylaws. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquitySharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of securities classified as temporary equity that have been sold (or granted) to the entity's shareholders. Securities issued include securities outstanding and securities held in treasury. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquitySharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of securities classified as temporary equity that have been issued and are held by the entity's shareholders. Securities outstanding equals securities issued minus securities held in treasury. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquitySharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
Pro Forma Condensed Consolidated Statement of Operations and Comprehensive Loss - USD ($)
$ in Thousands |
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Operating expenses |
|
|
| Research and development |
$ 4,297
|
$ 23,770
|
| General and administrative |
6,053
|
12,687
|
| Restructuring costs |
968
|
3,448
|
| Total operating expenses |
11,318
|
39,905
|
| Loss from operations |
(11,318)
|
(39,905)
|
| Other income, net |
|
|
| Interest income, net |
470
|
1,689
|
| Gain on sale of equipment |
|
64
|
| Foreign exchange transaction gain (loss), net |
75
|
(197)
|
| Total other income, net |
545
|
1,556
|
| Loss before income tax expense |
(10,773)
|
|
| Income tax expense |
42
|
|
| Net loss |
(10,815)
|
(38,349)
|
| Net loss attributable to common shares |
$ (10,815)
|
$ (38,349)
|
| Net loss per common share, basic |
$ (9.97)
|
$ (36.08)
|
| Net loss per common share, diluted |
$ (9.97)
|
$ (36.08)
|
| Weighted-average number of shares used in computing net loss per common share, basic |
1,084,565
|
1,062,872
|
| Weighted-average number of shares used in computing net loss per common share, diluted |
1,084,565
|
1,062,872
|
| Other comprehensive loss, net of tax |
|
|
| Currency translation gain (loss) |
$ (217)
|
$ 393
|
| Unrealized gain on marketable securities |
34
|
231
|
| Other comprehensive gain (loss), net of tax |
(183)
|
624
|
| Total comprehensive loss |
(10,998)
|
(37,725)
|
| Pro Forma |
|
|
| Operating expenses |
|
|
| Research and development |
4,297
|
29,058
|
| General and administrative |
6,053
|
12,687
|
| Restructuring costs |
968
|
3,448
|
| Total operating expenses |
11,318
|
45,193
|
| Loss from operations |
(11,318)
|
(45,193)
|
| Other income, net |
|
|
| Interest income, net |
470
|
1,689
|
| Gain on sale of equipment |
|
64
|
| Foreign exchange transaction gain (loss), net |
75
|
(197)
|
| Total other income, net |
545
|
1,556
|
| Loss before income tax expense |
(10,773)
|
|
| Income tax expense |
42
|
|
| Net loss |
(10,815)
|
(43,637)
|
| Net loss attributable to common shares |
$ (9,487)
|
$ (38,189)
|
| Net loss per common share, basic |
$ (8.27)
|
$ (33.93)
|
| Net loss per common share, diluted |
$ (8.27)
|
$ (33.93)
|
| Weighted-average number of shares used in computing net loss per common share, basic |
1,147,159
|
1,125,466
|
| Weighted-average number of shares used in computing net loss per common share, diluted |
1,147,159
|
1,125,466
|
| Net loss attributable to preferred shares |
$ (1,328)
|
$ (5,448)
|
| Net income (loss) per preferred share, basic |
$ (8,270)
|
$ (33,932)
|
| Net income (loss) per preferred share, diluted |
$ (8,270)
|
$ (33,932)
|
| Weighted-average number of shares used in computing net loss per preferred share, basic |
161
|
161
|
| Weighted-average number of shares used in computing net loss per preferred share, diluted |
161
|
161
|
| Other comprehensive loss, net of tax |
|
|
| Currency translation gain (loss) |
$ (217)
|
$ 393
|
| Unrealized gain on marketable securities |
34
|
231
|
| Other comprehensive gain (loss), net of tax |
(183)
|
624
|
| Total comprehensive loss |
(10,998)
|
(43,013)
|
| Asset Acquisition Adjustments |
|
|
| Operating expenses |
|
|
| Research and development |
|
5,288
|
| Total operating expenses |
|
5,288
|
| Loss from operations |
|
(5,288)
|
| Other income, net |
|
|
| Net loss |
|
(5,288)
|
| Net loss attributable to common shares |
$ 1,328
|
$ 160
|
| Weighted-average number of shares used in computing net loss per common share, basic |
62,594
|
62,594
|
| Weighted-average number of shares used in computing net loss per common share, diluted |
62,594
|
62,594
|
| Net loss attributable to preferred shares |
$ (1,328)
|
$ (5,448)
|
| Weighted-average number of shares used in computing net loss per preferred share, basic |
161
|
161
|
| Weighted-average number of shares used in computing net loss per preferred share, diluted |
161
|
161
|
| Other comprehensive loss, net of tax |
|
|
| Total comprehensive loss |
|
$ (5,288)
|
| X |
- DefinitionNet income (loss) attributable to common shares.
| Name: |
glto_NetIncomeLossAttributableToCommonShares |
| Namespace Prefix: |
glto_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNet income (loss) attributable to preferred shares.
| Name: |
glto_NetIncomeLossAttributableToPreferredShares |
| Namespace Prefix: |
glto_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNet income (loss) per preferred share, basic.
| Name: |
glto_NetIncomeLossPerPreferredShareBasic |
| Namespace Prefix: |
glto_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNet income (loss) per preferred share, diluted.
| Name: |
glto_NetIncomeLossPerPreferredShareDiluted |
| Namespace Prefix: |
glto_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted-average number of shares used in computing net loss per preferred share, basic.
| Name: |
glto_WeightedAverageNumberOfSharesUsedInComputingNetLossPerPreferredShareBasic |
| Namespace Prefix: |
glto_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted-average number of shares used in computing net loss per preferred share, diluted.
| Name: |
glto_WeightedAverageNumberOfSharesUsedInComputingNetLossPerPreferredShareDiluted |
| Namespace Prefix: |
glto_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized and unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482014/830-20-35-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481956/830-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481926/830-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481839/830-10-45-17
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of property, plant and equipment assets, including oil and gas property and timber property. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_GainLossOnSaleOfPropertyPlantEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income (expense) classified as operating. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(10))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_InterestIncomeExpenseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment in marketable security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_MarketableSecuritiesUnrealizedGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPeriodIncreaseDecreaseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of other comprehensive income (loss) attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after cash payment, of expenses associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses related to a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_RestructuringCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_StatementScenarioAxis=us-gaap_ScenarioAdjustmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Basis of Presentation
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Basis of Presentation |
1. Basis of Presentation The unaudited pro forma condensed consolidated financial information was prepared with the Asset Purchase being accounted for as an asset acquisition by the Company under Accounting Standards Codification (“ASC”) Topic 805-50. The Company further concluded that the set of assets acquired in the transaction does not represent a business under Securities and Exchange Commission Regulation S-X Rule 11-01. Upon completion of the Asset Purchase, the Company obtained control of assets consisting primarily of in-process research and development (IPR&D) related to Bridge Medicine’s BRM-1420 program. In accordance with U.S. GAAP, the Company must first assess whether an integrated set of assets and activities should be accounted for as an acquisition of a business or an asset acquisition. An initial screen test is completed to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single asset or group of similar assets. If that screen is met, the set is not considered a business and is accounted for as an asset acquisition. The Company will account for the Asset Purchase as an asset acquisition as substantially all of the fair value of the gross assets being acquired is concentrated within Bridge Medicine’s programs and development candidates which are considered a group of similar assets.
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Transaction Adjustments
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Transaction Adjustments [Abstract] |
|
| Transaction Adjustments |
2. Transaction Adjustments The unaudited pro forma condensed consolidated financial statements reflect the following notes and adjustments:
|
(a)Represents the cash paid to Bridge Medicines to reimburse transaction costs as well as the estimated transaction costs associated with the Asset Purchase incurred by the Company. (b)Represents the fair value of the Preferred Stock Payment Shares issued to Bridge Medicines in consideration of the assets acquired in the Asset Purchase. (c)Represents the fair value of the Common Stock Payment Shares issued to Bridge Medicines in consideration of the assets acquired in the Asset Purchase. (d)Represents the earnings adjustments associated with pro forma adjustment (e). (e)Represents the expense to be recorded associated with the Asset Purchase. The assets acquired do not have an alternative future use to the Company as defined in Accounting Standards Codification (“ASC”) 730, Research and development. Accordingly, the Company recorded the fair value of the consideration transferred for the assets acquired, which is comprised of the cash paid for transaction costs under pro forma adjustment (a), and the fair value of the Payment Shares under pro forma adjustments (b) and (c), as Research and development expense as opposed to capitalizing the costs as an asset. (f)Represents the allocation of pro forma net loss between the pro forma common shares and pro forma preferred shares of the Company. The preferred shares participate in earnings and losses of the Company with the Common Stock and are therefore treated as a separate class of common stock for purposes of calculating net loss per share. Net loss per share is provided separately for each class of stock. Subject to receipt of Stockholder Approval, the preferred stock is convertible into common stock at a conversion ratio of 1-to-1,000 and participate in losses on an as-converted basis. Accordingly, the net loss per preferred share is 1,000 times the net loss per common share. (g)Represents the issuance of shares of Common Stock Payment Shares and Preferred Stock Payment Shares to Bridge Medicines in consideration of the assets acquired in the Asset Purchase. |
|
| X |
- Definition
+ References
+ Details
| Name: |
glto_TransactionAdjustmentsAbstract |
| Namespace Prefix: |
glto_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
glto_TransactionAdjustmentsTextBlock |
| Namespace Prefix: |
glto_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
| X |
- Definition
+ References
+ Details
| Name: |
glto_TransactionAdjustmentsAbstract |
| Namespace Prefix: |
glto_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of common shares issuable upon conversion for each share of preferred stock to be converted. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockConvertibleConversionRatio |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:pureItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
Galecto (NASDAQ:GLTO)
Graphique Historique de l'Action
De Mar 2025 à Avr 2025

Galecto (NASDAQ:GLTO)
Graphique Historique de l'Action
De Avr 2024 à Avr 2025


