Novartis to Seek US Accelerated Approval of Atrasentan After Phase 3 Study Met Primary Goal
30 Octobre 2023 - 8:07AM
Dow Jones News
By Giulia Petroni
Novartis will submit an application next year for a possible
accelerated approval of atrasentan in the U.S. after its phase 3
study met the primary endpoint.
The Swiss pharmaceutical company said Monday that the study
showed a significant reduction in proteinuria, a condition causing
high levels of protein in urine, in patients with kidney disease
IgA nephropathy.
Novartis plans to review interim results with the U.S. Food and
Drug Administration for a potential regulatory submission for
accelerated approval. The study will continue, with final readout
expected in the first quarter of 2026, it said.
Write to Giulia Petroni at giulia.petroni@wsj.com
(END) Dow Jones Newswires
October 30, 2023 02:52 ET (06:52 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Novartis (NYSE:NVS)
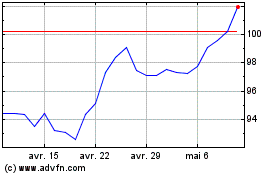
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
Novartis (NYSE:NVS)
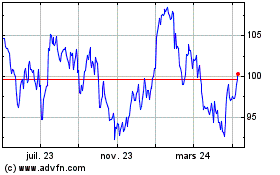
Graphique Historique de l'Action
De Mai 2023 à Mai 2024