FDA Recalls Several Medicines That Contain Valsartan
14 Juillet 2018 - 2:05AM
Dow Jones News
By Maria Armental
Several medicines used to treat high-blood pressure and heart
failure are being recalled in the U.S. over concerns they may be
contaminated by a carcinogen.
The recall in the U.S. affects valsartan and
valsartan/hydrochlorothiazide products from Teva Pharmaceutical
Industries Ltd. (TEVA) and privately held Major Pharmaceuticals and
Solco Healthcare, the Food and Drug Administration said Friday.
The FDA said it was looking into the recalled products' levels
of N-nitrosodimethylamine, classified as a substance that could
cause cancer, and assessing the possible effect on patients who had
taking them.
The ingredient valsartan was supplied by a third party, the FDA
said without identifying the supplier. Similar recalls last week,
including in Europe and Canada, identified the supplier as China's
Zhejiang Huahai Pharmaceutical Co. (600521.SH).
Solco on Friday said it would recall affected lots of the
medications listed. Teva Pharmaceutical, Major Pharmaceuticals and
Zhejiang Huahai couldn't be immediately reached for comment
Friday.
Write to Maria Armental at maria.armental@wsj.com
(END) Dow Jones Newswires
July 13, 2018 19:50 ET (23:50 GMT)
Copyright (c) 2018 Dow Jones & Company, Inc.
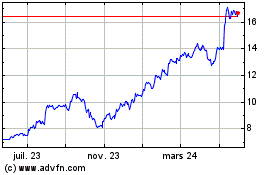
Teva Pharmaceutical Indu... (NYSE:TEVA)
Graphique Historique de l'Action
De Juin 2024 à Juil 2024
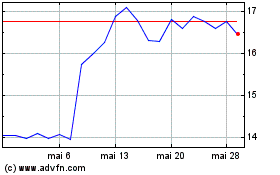
Teva Pharmaceutical Indu... (NYSE:TEVA)
Graphique Historique de l'Action
De Juil 2023 à Juil 2024