Filed
Pursuant to Rule 424(b)(5)
Registration
No. 333-269225
PROSPECTUS
SUPPLEMENT
(To
Prospectus dated January 13, 2020)

$5,000,000
Common
Stock
This
prospectus supplement relates to the issuance and sale of shares of our common stock, par value $0.001 per share, having an aggregate
offering price of up to $5.0 million, from time to time solely through Ladenburg Thalmann & Co. Inc., as exclusive sales agent (whom
we refer to herein as Ladenburg or the Sales Agent). Any sales consummated under this prospectus supplement will be made under an “at-the-market”
offering program under the terms of an At Market Issuance Sales Agreement between us and Ladenburg, dated February 24, 2023, or the Sales
Agreement, pursuant to which we may sell up to $5 million of our common stock. See “Plan of Distribution.”
Our
common stock is listed on the NYSE American under the symbol “OGEN.” The last reported sale price of our common stock on
the NYSE American on February 23, 2023 was $5.09 per share.
Sales
of our common stock, if any, under this prospectus supplement may be made in sales deemed to be “at the market offerings”
as defined in Rule 415 promulgated under the Securities Act of 1933, as amended, or the Securities Act. If authorized by us in writing,
Ladenburg may also sell shares of our common stock in negotiated transactions at market prices prevailing at the time of sale or at prices
related to such prevailing market prices. Ladenburg is not required to sell any specific number or dollar amount of securities, but will
act as a sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed
terms between Ladenburg and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
The
compensation to Ladenburg for sales of common stock sold pursuant to the sales agreement will be equal to 3.0% of the gross proceeds
of any shares of common stock sold under the sales agreement. In connection with the sale of the common stock on our behalf, Ladenburg
will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Ladenburg will be
deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification and contribution to Ladenburg with
respect to certain liabilities, including liabilities under the Securities Act or the Exchange Act of 1934, as amended, or the Exchange
Act.
As
of January 18, 2023, the aggregate market value of our outstanding common stock held by non-affiliates, or the public float, was approximately
$17,872,965, which was calculated based on 1,985,885 shares of our outstanding common stock held by non-affiliates and on a price of
$9.00 per share, the last reported sale price for our common stock on January 18, 2023. Pursuant to General Instruction I.B.6 of Form
S-3, in no event will we sell our securities in a public primary offering with a value exceeding one-third of our public float in any
12-month period unless our public float subsequently rises to $75.0 million or more.
Investing
in our securities involves a high degree of risk. Before buying any securities, you should carefully read the discussion of material
risks of investing in our common stock under the heading “Risk Factors” beginning on page S-7 of this prospectus supplement
and the documents incorporated by reference herein and the accompanying prospectus.
Neither
the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or determined if
this prospectus supplement or the prospectus to which it relates is truthful or complete. Any representation to the contrary is a criminal
offense.
Ladenburg
Thalmann
The
date of this prospectus supplement is February 24, 2023
TABLE
OF CONTENTS
Prospectus
Supplement
Prospectus
ABOUT
THIS PROSPECTUS SUPPLEMENT
This
prospectus supplement and the accompanying prospectus form part of a registration statement on Form S-3 that we filed with the Securities
and Exchange Commission, or the “SEC,” using a “shelf” registration process. This document contains two parts.
The first part consists of this prospectus supplement, which provides you with specific information about this offering. The second part,
the accompanying prospectus, provides more general information, some of which may not apply to this offering. Generally, when we refer
only to the “prospectus,” we are referring to both parts combined. This prospectus supplement, and the information incorporated
herein by reference, may add, update or change information in the accompanying prospectus. You should read the entire prospectus supplement
as well as the accompanying prospectus and the documents incorporated by reference herein that are described under the headings “Where
You Can Find More Information” and “Incorporation of Certain Documents by Reference.” If there is any inconsistency
between the information in this prospectus supplement and the accompanying prospectus, you should rely on the information in this prospectus
supplement.
This
prospectus supplement relates only to an offering of up to $5.0 million of shares of our common stock through Ladenburg. These sales,
if any, will be made pursuant to the terms of the sales agreement entered into between us and Ladenburg on February 24, 2023, a copy
of which is incorporated by reference into this prospectus supplement. The $5,000,000 of common stock that may be offered, issued and
sold under the prospectus is included in the $40,000,000 of securities that may be offered, issued and sold by us pursuant to our shelf
registration statement.
You
should rely only on the information contained in this prospectus supplement and the accompanying prospectus, including the information
incorporated by reference into this prospectus supplement and the accompanying prospectus, and any free writing prospectus that we have
authorized for use in connection with this offering. We have not authorized anyone to provide you with information that is different.
The
information contained in this prospectus supplement and the accompanying prospectus, including the information incorporated by reference
into this prospectus supplement and the accompanying prospectus, and any free writing prospectus that we have authorized for use in connection
with this offering is accurate only as of the respective dates thereof, regardless of the time of delivery of this prospectus supplement,
the accompanying prospectus or free writing prospectus, if any, or of any sale of our securities. It is important for you to read and
consider all information contained in this prospectus supplement and the accompanying prospectus, including the information incorporated
by reference into this prospectus supplement and the accompanying prospectus, and any free writing prospectus that we have authorized
for use in connection with this offering in making your investment decision. You should also read and consider the information in the
documents to which we have referred you in the sections entitled “Where You Can Find More Information” and “Incorporation
of Certain Documents by Reference” in this prospectus supplement.
We
are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The distribution
of this prospectus supplement and the accompanying prospectus and the offering of securities in certain jurisdictions may be restricted
by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying prospectus must
inform themselves about, and observe any restrictions relating to, the offering of our securities and the distribution of this prospectus
supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus do not
constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by
this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful for such person
to make such an offer or solicitation.
The
industry and market data and other statistical information contained in this prospectus supplement, the accompanying prospectus and the
documents we incorporate by reference are based on management’s estimates, independent publications, government publications, reports
by market research firms or other published independent sources, and, in each case, are believed by management to be reasonable estimates.
Although we believe these sources are reliable, we have not independently verified the information. None of the independent industry
publications used in this prospectus supplement, the accompanying prospectus or the documents we incorporate by reference were prepared
on our or our affiliates’ behalf and none of the sources cited by us consented to the inclusion of any data from its reports, nor
have we sought their consent.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document
that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases,
for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or
covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such
representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Any
portion of the $5.0 million included in this prospectus supplement that is not previously sold or included in an active placement notice
pursuant to the Sales Agreement is available for sale in other offerings pursuant to the base prospectus, and if no shares are sold under
the Sales Agreement, the full $5.0 million of securities may be sold in other offerings pursuant to the base prospectus and a corresponding
prospectus supplement, in accordance with securities laws.
References
to, “we,” “us,” “our company,” “Oragenics,” the “Company,” and similar terms
refer to Oragenics, Inc., a Florida corporation, unless the context otherwise requires.
CAUTIONARY
STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
Certain
statements in this prospectus supplement, the accompanying prospectus and documents incorporated by reference herein that look forward
in time or express management’s expectations or beliefs with respect to the occurrence of future events are forward-looking statements
as defined under within the meaning of Section 27A of the Securities Act of 1933, as amended, or Securities Act, and Section 21E of the
Securities Exchange Act of 1934, as amended, or Exchange Act, as amended, and are subject to the safe harbor created therein for forward-looking
statements. Such statements include, but are not limited to, (i) projections of revenue, earnings, capital structure and other financial
items, (ii) statements of our plans and objectives, (iii) statements of expected future economic performance, and (iv) assumptions underlying
statements regarding us or our business. Forward-looking statements can be identified by, among other things, the use of forward-looking
language, such as “believes,” “expects,” “estimates,” “may,” “will,” “should,”
“could,” “seeks,” “plans,” “intends,” “anticipates” or “scheduled to”
or the negatives of those terms, or other variations of those terms or comparable language, including, notably, language concerning the
“impact” or “limitations” relating to COVID-19, or by discussions of strategy or other intentions, particularly
as they relate to the development and funding of our new Terra CoV-2 vaccine product candidate.
We
caution investors that actual results or business conditions may differ materially from those projected or suggested in forward-looking
statements as a result of various factors including, but not limited to, the following risks and the other factors described in the Risk
Factors section of our annual report on Form 10-K, in our quarterly reports on Form 10-Q and in our Current Reports on Form 8-K incorporated
by reference. These factors include:
| |
● |
We
have incurred significant operating losses since our inception and cannot assure you that we will generate revenues or achieve profitability; |
| |
|
|
| |
● |
We
will need to raise additional capital to continue to implement our business strategy and we may not be able to do so; |
| |
|
|
| |
● |
Our
financial capacity and performance, including our ability to obtain funding, non-dilutive or otherwise, necessary to do the research,
development, manufacture and commercialization of any one or all of our product candidates; |
| |
|
|
| |
● |
The
timing, progress and results of clinical trials of our product candidates, including statements regarding the timing of initiation
and completion of pre-clinical studies or clinical trials or related preparatory work, the period during which the results of the
trials will become available and our research and development programs; |
| |
|
|
| |
● |
The
timing of any submission of filings for regulatory approval of our product candidates and our ability to obtain and maintain regulatory
approvals for our product candidates for any indication; |
| |
|
|
| |
● |
Our
expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates including as to administration,
distribution and storage; |
| |
|
|
| |
● |
Our
expectations regarding the size of the patient populations, market acceptance and opportunity for and clinical utility of our product
candidates, if approved for commercial use; |
| |
|
|
| |
● |
Our
manufacturing capabilities and strategy, including the scalability and commercial viability of our manufacturing methods and processes,
and those of our contractual partners; |
| |
|
|
| |
● |
Our
expectations regarding the scope of any approved indications for our product candidates; |
| |
|
|
| |
● |
Our
ability to successfully commercialize our product candidates; |
| |
|
|
| |
● |
The
potential benefits of, and our ability to maintain, our relationships and collaborations with the NIAID, the NIH, the NRC and other
potential collaboration or strategic relationships; |
| |
● |
Our
ability to use our lantibiotic platform to develop future product candidates; |
| |
|
|
| |
● |
Our
estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional
funding, including any application for future grants or funding; |
| |
|
|
| |
● |
Our
ability to identify, recruit and retain key personnel and consultants; |
| |
● |
Our
ability to obtain, retain, protect and enforce our intellectual property position for our product candidates, and the scope of such
protection; |
| |
|
|
| |
● |
Our
ability to advance the development of our new NT-CoV2-1 vaccine product candidate under the timelines and in accord with the milestones
projected; |
| |
|
|
| |
● |
Our
inability to achieve success in our identification of lantibiotic homologs or the manufacture and nonclinical testing of our lantibiotic
product candidates; |
| |
|
|
| |
● |
Our
need to comply with extensive and costly regulation by worldwide health authorities, who must approve our product candidates prior
to substantial research and development and could restrict or delay the future commercialization of certain of our product candidates; |
| |
|
|
| |
● |
Our
ability to successfully complete pre-clinical and clinical development of, and obtain regulatory approval of our product candidates
and commercialize any approved products on our expected timeframes or at all; |
| |
|
|
| |
● |
The
safety, efficacy and benefits of our product candidates; |
| |
|
|
| |
● |
The
content and timing of submissions to and decisions made by the FDA, other regulatory agencies and nongovernmental bodies and actors,
such as investigational review boards; |
| |
|
|
| |
● |
The
effects of government regulation and regulatory developments, and our ability and the ability of the third parties with whom we engage
to comply with applicable regulatory requirements; |
| |
|
|
| |
● |
The
capacities and performance of our suppliers and manufacturers and other third parties over whom we have limited control; |
| |
|
|
| |
● |
Our
ability to maintain our listing on the NYSE American and the effects of our contemplated 1 for 60 reverse stock split on our price
per share and the trading market of our common stock; |
| |
|
|
| |
● |
The
impact of the COVID-19 pandemic on our financial condition and business operations and our ability to continue research and development
for existing product candidates on previously-projected timelines or in accord with ordinary practices, as well as the broader governmental,
global health and macro- and microeconomic responses to and consequences of the pandemic; |
| |
|
|
| |
● |
We
may be adversely impacted by any significant broad-based financial crises and its impact on consumers, retailers and equity and debt
markets as well as our inability to obtain required additional funding to conduct our business; |
| |
|
|
| |
● |
As
a public company, we must implement additional and expensive finance and accounting systems, procedures and controls as we grow our
business and organization to satisfy reporting requirements, which add to our costs and require additional management time and resources; |
| |
|
|
| |
● |
Our
competitive position and the development of and projections relating to our competitors or our industry; and |
| |
|
|
| |
● |
The
impact of laws and regulations, including those that may not yet exist. |
We
cannot assure you that we have identified all the factors that create uncertainties. Moreover, new risks emerge from time to time and
it is not possible for our management to predict all risks, nor can we assess the impact of all risks on our business or the extent to
which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. Except
as required by law, we undertake no obligation to publicly release the result of any revision of these forward-looking statements to
reflect events or circumstances after the date of this prospectus or the respective dates of documents incorporated by reference herein
or therein that include forward-looking statements.
We
urge you to consider these factors before investing in our common stock. The forward-looking statements included in this prospectus supplement,
the accompanying prospectus and any other offering material, or in the documents incorporated by reference into this prospectus supplement,
the accompanying prospectus and any other offering material, are made only as of the date of the prospectus supplement, the accompanying
prospectus, any other offering material or the incorporated document. For more detail on these and other risks, please see “Risk
Factors” in this prospectus supplement, the accompanying prospectus, our Annual Report on Form 10-K for our fiscal year ended
December 31, 2021, filed with the SEC on March 24, 2022, our 10-K/A for our fiscal year ended December 31, 2021, filed with the SEC on
July 29, 2022, our Quarterly Report on Form 10-Q for our quarter ended March 31, 2022, filed with the SEC on May 13, 2022, our Quarterly
Report on Form 10-Q for our quarter ended June 30, 2022, filed with the SEC on August 9, 2022, our Quarterly Report on Form 10-Q for
our quarter ended September 30, 2022, filed with the SEC on November 14, 2020, our Current Reports on Form 8-K filed January 26, 2022,
February 28, 2022, March 10, 2022, April 6, 2022, April 19, 2022, May 17, 2022, June 23, 2022, July 8, 2022, August 3, 2022, August 24,
2022, September 30, 2022, October 3, 2022, November 11, 2022, December 16, 2022, December 19, 2022, December 20, 2022, December 22, 2022
, December 23, 2022, January 23, 2023 and February 2, 2023 and our other filings with the SEC.
This
prospectus supplement also contains estimates, projections and other information concerning our industry, the market and our business.
Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual
events or circumstances may differ materially from events and circumstances reflected in this information. We obtained the industry,
market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general
publications and research surveys and studies conducted by third parties.
PROSPECTUS
SUPPLEMENT SUMMARY
This
summary highlights selected information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying
prospectus. This summary may not contain all of the information that may be important to you. You should read this prospectus supplement,
the accompanying prospectus, the information incorporated by reference in each, and any related free writing prospectus before making
an investment decision. You should pay special attention to the “Risk Factors” section beginning on page S-7 of this prospectus
supplement and “Risk Factors” set forth in our most recent annual report on Form 10-K for the year ended December 31, 2021
our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2022, June 30, 2022, and September 30, 2022, respectively and in
the other documents which are incorporated by reference in this prospectus supplement and the accompanying prospectus in their entirety
to determine whether an investment in our common stock is appropriate for you.
Overview
We
are a biotechnology company dedicated to the research and development of potential therapies to fight infectious diseases including coronaviruses
and multidrug-resistant organisms. Our lead product (NT-CoV2-1) is an intranasal vaccine candidate to prevent coronavirus disease (“COVID-19”)
from the SARS-CoV-2 virus and variants thereof. The NT-CoV2-1 program leverages coronavirus spike protein research licensed from the
National Institutes of Health and the National Research Council of Canada with a focus on reducing viral transmission and offering a
more patient-friendly intranasal administration. Our proprietary lantibiotics program features a novel class of antibiotics against bacteria
our research has shown may be applicable to multiple antibiotic resistant organisms.
Our
SARS-CoV-2 Vaccine Product Candidate - NT-CoV2-1
Following
our May 2020 acquisition of one hundred percent (100%) of the total issued and outstanding common stock of Noachis Terra, Inc. (“Noachis
Terra”) we are focused on the development and commercialization of a vaccine product candidate to provide long-lasting immunity
from SARS-CoV-2, which causes COVID-19. Noachis Terra is a party to a worldwide, nonexclusive intellectual property and biological materials
license agreement with the National Institute of Allergy and Infectious Diseases (“NIAID”), an institute within the National
Institutes of Health (“NIH”), relating to certain research, patent applications and biological materials involving pre-fusion
stabilized coronavirus spike proteins and their use in the development and commercialization of a vaccine to provide specific, long lasting
immunity from SARS-CoV-2. Since the acquisition we have conducted testing in animal models, including SARS-CoV-2 challenge studies in
hamsters, using specific formulations for intramuscular administration (our Terra CoV-2 vaccine candidate) and intranasal administration
(our NT-CoV2-1 vaccine candidate), both based on the NIAID pre-fusion stabilized spike protein antigens. Following consideration of a
number of factors, including but not limited to the competitive landscape, we determined to bring the intranasal vaccine candidate NT-CoV2-1,
into further development due to the greater differentiation versus current COVID-19 vaccines and the potential benefits of intranasal
over intramuscular administration. We believe these benefits could include a higher reduction of transmission of SARS-CoV-2 and would
offer a needle-free delivery option. We therefore are currently focusing our development efforts on our more highly differentiated NT-CoV2-1
vaccine candidate.
On
July 26, 2021, we entered into a licensing agreement with the National Research Council (“NRC”) that enables us to pursue
the development of next-generation vaccines against the SARS-CoV-2 virus and its variants. The license was subsequently amended to: include
the Omicron variant, broaden the non-exclusive field of use to include all diseases caused by coronaviruses, and any genetic variants
thereof, to add a research protocol developed by the NRC, and to add reagents as part of the NRC Technology licensed by us. The NRC technologies,
in combination with the licensed technologies from the U.S. NIH used in our NT-CoV2-1 vaccine candidate, provide us with a platform that
can generate cell lines for high-yield production of spike protein antigens for existing and emerging variants of concern. This platform
should allow production of cell lines within six to eight weeks of spike gene sequence availability, compared with six to nine months
for traditional production of such cell lines. The NRC technologies, developed with support from the NRC’s Pandemic Response Challenge
Program, are expected to enable expedited evaluation of SARS-CoV-2 antigen candidates in pre-clinical and clinical studies.
Coronaviruses
are a family of viruses that can lead to upper-respiratory infections in humans. Recent clinical reports also suggest that the SARS-CoV-2
virus can affect other body-systems, including the nervous, cardiovascular, gastrointestinal and renal systems. Among the recent iterations
of coronaviruses to move from animal to human carriers is SARS-CoV-2, which, beginning in Wuhan, China, in late 2019, caused a global
pandemic due to its rapid spread and the relatively high mortality rate (as compared to the seasonal influenza). Pfizer/BioNTech received
FDA approval for their COVID-19 vaccines in August of 2021 and the Moderna vaccine in January 2022. The Janssen vaccine is currently
available in the United States under Emergency Use Authorizations (“EUA”) by the FDA. In July 2022, the FDA granted EUA for
the Novavax COVID-19 vaccine as well. Current vaccines have reduced the rates of hospitalization and death due to COVID-19 in vaccinated
individuals, but the transmission levels even in vaccinated individuals has allowed SARS-CoV-2 variants to continue to circulate. We
believe given the size of the worldwide spread of COVID-19 that even with additional vaccines available, there will be demand for the
highly differentiated NT-CoV2-1 vaccine, once development is successfully completed. We intend to combine the research, patent applications
and biological materials covered by our NIAID license and with our NRC license and our existing clinical research and manufacturing capabilities
to respond to this ongoing, global, public health issue. We believe our NT-CoV2-1 vaccine holds the possibility of playing an important
role in addressing this issue.
Coronaviruses,
such as SARS-CoV-2, possess signature protein spikes on their outer capsule. Our NIAID license covers patents and data on a vaccine candidate
that were created based on a stabilized pre-fusion spike trimeric protein. By stabilizing the spike protein in the pre-fusion state,
the number of immunogenic centers is increased thereby allowing for a greater likelihood of successful antibody binding, resulting in
an improved immunogenic response. Spike protein antigens stabilized in the pre-fusion state have been used successfully in the leading
COVID-19 vaccines from Pfizer/BioNTech and Moderna, which we believe reduces the risk of using the same approach in our NT-CoV2-1 vaccine
candidate. The genetic code, acquired from the NIH, for the stabilized pre-fusion spike protein was provided to Aragen Bioscience, Inc.
(“Aragen”) for the purpose of insertion of the spike protein gene sequence into a Chinese Hamster Ovary (“CHO”)
cell line. Aragen is a leading contract research organization focused on accelerating pre-clinical biologics product development, has
extensive experience building CHO cell lines for recombinant proteins, such as monoclonal antibodies. Aragen successfully inserted the
NIH pre-fusion spike protein gene sequence into a CHO cell line and Oragenics is currently producing Phase 1 clinical material based
upon this cell line.
We
entered into both a material transfer agreement and a non-exclusive research license agreement with Inspirevax for the use of intranasal
mucosal adjuvants in our NT-CoV2-1 vaccine candidates. Regarding the intranasal mucosal adjuvants of interest, BDX300 and BDX301 are
proteosome-based adjuvants comprised of proteins and lipopolysaccharides with improved attributes including enhanced immune response,
manufacturing efficiency and the benefits of intranasal vaccine administration. The non-exclusive license agreement allows for the collaboration
and research regarding the intranasal delivery of vaccine during clinical development with the opportunity to enter into a commercial
agreement upon regulatory approval of the intranasal vaccine. The NT-CoV2-1 vaccine containing Inspirevax’s intranasal mucosal
adjuvant BDX301 has been studied in pre-clinical animal studies, including hamster viral challenge studies and mouse immunogenicity studies.
A rabbit toxicology study has been initiated and is required for regulatory approval prior to the Phase 1 clinical study.
A
Non-Exclusive Research License Agreement with Inspirevax was executed in February 2022. This agreement granted the Company non-exclusive
rights to conduct non-clinical and clinical research and trials in relation to vaccines comprising the BDX300 or BDX301 adjuvants to
prevent or treat diseases caused by coronaviruses and genetic variants thereof.
We
began pre-clinical studies in June of 2021 through our collaboration and material transfer agreement with the NRC. We initiated an immunogenicity
study in mice to evaluate several adjuvant candidates. On August 30, 2021, we announced the successful completion of these mouse immunogenicity
studies that supported further development using either the intramuscular or intranasal routes of administration. A hamster challenge
study was initiated in September of 2021 to assess inhibition of viral replication using adjuvants specific for intramuscular and intranasal
administration. In December of 2021, we announced that both formulations generated robust immune responses and reduced the SARS-CoV-2
viral loads to undetectable levels in the nasal passages and lungs five days following a viral challenge. By contrast, hamsters in the
control groups that had received saline or adjuvants alone had no detectable immune response and substantial viral loads. The vaccines
delivered by intranasal and intramuscular routes generated immune responses as measured by multiple assays. On June 14, 2022, we announced
that the results of these studies were published in Nature Scientific Reports.
In
March of 2022, following a positive assessment of a rabbit-based pilot study, we initiated a Good Laboratory Practice toxicology study
to evaluate the safety profile and immunogenicity of NT-CoV2-1 in rabbits. This important preclinical study is designed to provide data
required to advance our intranasal vaccine candidate into human clinical studies. The study has concluded and we completed the full set
of toxicology data, which is needed to support the filing of an IND application for NT-CoV2-1. Based on the findings of the final toxicology
report, including a full histopathology evaluation, we were able to confirm a safety and immunogenicity profile that further support
our plan to submit regulatory filings required to progress to a Phase 1 clinical study.
While
we previously had a Type B Pre-IND Meeting with the FDA on our intramuscular vaccine product candidate, we again met with the FDA in
a Type B Pre-IND Meeting request to discuss our intranasal vaccine product candidate. As a result of this meeting, the FDA indicated
that the Company could file an IND application for NT-CoV2-1 following the availability of the final GLP toxicology report for inclusion
in the IND.
We
believe the benefits of our NT-CoV2-1 vaccine product candidate through its intranasal delivery mechanism to be:
| ● |
Targeted
Mucosal Immunity – Conventional injectable vaccines are poor inducers of mucosal immunity, whereas intranasal immunization
can induce strong mucosal immunity by enhancing the immune response at the entry sites of mucosal pathogens. When the SARS-CoV-2
virus enters the nasal cavity, the respiratory epithelial layer is the first barrier against viral infection. The intranasal route
of vaccination provides two additional layers of protection over intramuscular shots because (i) it produces immunoglobulin A and
resident memory B and T cells in the respiratory mucosa that are an effective barrier to infection at those sites, and (ii) cross-reactive
resident memory B and T cells can respond earlier than other immune cells should a viral variant start an infection. |
| |
|
| ● |
Needle-Free
Administration – As an obvious benefit, intranasal administration means needle-free delivery, resulting in meaningful differentiation
for children and needle-phobic populations, improved compliance and the potential for self-administration. |
| |
|
| ● |
Storage
& Transport – The currently available mRNA-based vaccines have been delivered globally via stringent storage and transport
requirements that strain distribution logistics under the best of circumstances. A key benefit of our NT-CoV2-1 vaccine candidate
is a significantly reduced handling burden, allowing transport at a more manageable refrigeration temperature (5°C) that improves
access globally including remote and under-vaccinated geographies. |
| |
|
| ● |
Durability
– Broad initial success with mRNA vaccines has significantly diminished COVID-19’s impact and death, but the trade-off
has been fleeting efficacy. By benefitting from the immunological properties of the hybrid NIH/NRC construct, NT-CoV2-1 is potentially
much more durable and long-lasting than currently available mRNA-based therapies. |
Through
assessment of a variety of factors including our pre-clinical testing to date, the expected benefits noted above, evolving variants and
available vaccines in use, we determined to focus our development efforts on the intranasal delivery of our vaccine product candidate,
NT-CoV2-1, which we believe is more highly differentiated than the currently available and late-stage COVID-19 vaccines. We are currently
evaluating formulation options and considering regulatory pathways to advance the program. In connection therewith, we are strategically
assessing multiple opportunities inclusive of further regulatory guidance and requirements and the potential implications thereof. As
a result, the Company now anticipates being in a position to file an IND application in the United States or Canada and to thereafter
commence a Phase 1 clinical study with NT-CoV2-1, the protocol for which is under development, during the second half of 2023.
We
expect to use our currently available cash resources to continue to advance the development of NT-CoV2-1 through IND-enabling studies
and commencement of a Phase 1 clinical trial with further clinical development being contingent upon the receipt of additional funding,
including non-dilutive government grant funding which we continue to pursue, or partnering or out-licensing opportunities.
Our
Antibiotic Product Candidate - Oragenics Derived Compound (ODC-x)
Members
of our scientific team discovered that a certain bacterial strain of Streptococcus mutans, produces Mutacin 1140 (MU1140), a molecule
belonging to the novel class of antibiotics known as lantibiotics. Lantibiotics, such as MU1140, are highly modified peptide antibiotics
made by a small group of Gram-positive bacterial species. Over 60 lantibiotics have been discovered, to date. We believe lantibiotics
are generally recognized by the scientific community to be potent antibiotic agents.
In
nonclinical testing, MU1140 has shown activity against all Gram-positive bacteria against which it has been tested, including those responsible
for a number of healthcare associated infections, or HAIs. A high percentage of hospital-acquired infections are caused by highly antibiotic-resistant
bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) or multidrug-resistant Gram-negative bacteria. We believe the need
for novel antibiotics is increasing as a result of the growing resistance of target pathogens to existing FDA approved antibiotics on
the market.
Lantibiotics
have been difficult to investigate for their clinical usefulness as therapeutic agents in the treatment of infectious diseases due to
a general inability to produce or synthesize sufficient quantities of pure amounts of these molecules. Traditional fermentation methods
can only produce minute amounts of the lantibiotic.
The
timing of the filing of an IND regarding any future lantibiotic candidate is subject to our having sufficient available human, material
and financing capital, which includes research subjects, both animal and human, given all of our anticipated needs and expected requirements
in connection with our ongoing research and development initiatives. We expect to continue to advance our lantibiotics program to an
IND filing based on the availability of both human and financial capital. Based upon the current funding we expect to continue to focus
on the identification of new potential product lantibiotic candidates, efficient and cost-effective improvements in the manufacturing
processes and pre-clinical studies required to support a first in human Phase 1 clinical study.
In
October 2021, we were awarded a small business innovation research grant in the amount of $250,000 (“Computer-aided Design for
Improved Lantibiotics”, R41GM136034) for the Company’s continued research and development of lantibiotics, including its
collaborative program with the Biomolecular Sciences Institute at Florida International University (FIU). The grant provides the Company
with funding to develop novel lantibiotics for the treatment of ESKAPE pathogens (defined as Enterococcus faecium, Staphylococcus
aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.).
Product
Candidates.
Through
our wholly-owned subsidiary, Noachis Terra, we began the research and development stage for our new Terra CoV-2 and NT-CoV2-1 vaccine
product candidates. We hold a nonexclusive, worldwide intellectual property license agreement for certain research, patent applications
and biological materials relating to the use of pre-fusion coronavirus spike proteins for the development and commercialization of a
vaccine against SARS-CoV-2. We also hold a non-exclusive license with the NRC that enables us to pursue the rapid development of next-generation
vaccines against the SARS-CoV-2 (the “NIH License”) virus and its variants (the “NRC License” and together with
the NIH License the “License Agreements”).
Additionally,
we are developing semi-synthetic lantibiotic analogs that may be effective against systemic Gram-positive multidrug infections, and analogs
that may be effective in treating Gram-negative infections. We seek to protect our product candidates through patents and patent applications
pursuant to the terms of our License Agreements.
| Product/Candidate |
|
Description |
|
Application |
|
Status |
| |
|
|
|
|
|
|
| NT-CoV2-1 |
|
Intranasal
vaccine candidate (recombinant protein + adjuvant) to provide long lasting immunity against SARS-CoV-2 |
|
Broad,
community-based vaccine immunity against SARS-CoV-2 |
|
Pre-clinical
|
| |
|
|
|
|
|
|
| Antibiotics |
|
Semi-synthetic
analogs of MU1140: Member of lantibiotic class of antibiotics |
|
Healthcare-associated
infections |
|
Pre-clinical |
Recent
Developments
On
January 20, 2023 we effected a 1 for 60 reverse stock split of our authorized, issued and outstanding shares of common stock. The par
value per common share remained unchanged. Except where the context otherwise requires, share numbers in this prospectus supplement reflect
the 1 for 60 reverse stock split of our common stock.
Our
Business Development Strategy
Success
in the biopharmaceutical and product development industry relies on the continuous development of novel product candidates. Most product
candidates do not make it past the clinical development stage, which forces companies to look externally for innovation. Accordingly,
we expect from time to time, to seek strategic opportunities through various forms of business development, which can include strategic
alliances, licensing deals, joint ventures, collaborations, equity-or debt-based investments, dispositions, mergers and acquisitions.
We view these business development activities as a necessary component of our strategies, and we seek to enhance shareholder value by
evaluating business development opportunities both within and complementary to our current business as well as opportunities that may
be new and separate from the development of our existing product candidates.
Corporate
and Other Information
We
were incorporated in November 1996 and commenced operations in 1999. We consummated our initial public offering in June 2003. Our executive
office is located at, 4902 Eisenhower Boulevard, Suite 125 Tampa, Florida, 33634 and our research facilities are located at 13700 Progress
Boulevard, Alachua, Florida 32615. Our telephone number is (813) 286-7900 and our website is http://www.oragenics.com. We make available
free of charge on our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments
to those reports as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange
Commission (the “SEC”). The reports are also available at www.sec.gov. We do not incorporate by reference into this prospectus
the information on, or accessible through, our website, and you should not consider it as part of this prospectus and it should not be
relied on in connection with this offering. We have included our website address as an inactive textual reference only.
THE
OFFERING
The
following summary contains basic information about our Common Stock and the offering and is not intended to be complete. It does not
contain all the information that may be important to you. For a more complete understanding of our Common Stock, you should read the
section of the accompanying prospectus entitled “Description of Capital Stock.”
| Common
stock offered by us: |
|
Shares
of our common stock having an aggregate offering price of up to $5.0 million. |
| |
|
|
| Manner
of Offering: |
|
“At
the market offering” that may be made from time to time through or to Ladenburg, as sales agent or principal. See “Plan
of Distribution” on page S-16 of this prospectus supplement |
| |
|
|
| Use
of proceeds: |
|
We
intend to use the net proceeds from this offering, if any, to continue funding, our pre-clinical development of our SARS-CoV-1 vaccine,
Terra CoV-1 and our lantibiotics program and for general corporate purposes, including research and development activities, capital
expenditures, and working capital. We reserve the right, at the sole discretion of our management, to reallocate the proceeds of
this offering in response to developments in our business and other factors. See “Use of Proceeds” on page S-13 of this
prospectus supplement. |
| |
|
|
Common
Stock to be outstanding immediately after this offering (1)
|
|
Up
to 2,912,758 shares of common stock, assuming sales of 888,099 shares of common stock in this offering at an assumed offering price
of $5.63 per share (the closing price on February 17, 2023). The actual number of shares sold will vary depending on the sales price
under this offering |
| |
|
|
| Risk
factors: |
|
Investing
in our securities involves a high degree of risk and purchasers of our securities may lose their entire investment. See “Risk
Factors” below and in our most recent Annual Report on Form 10-K, which are incorporated by reference and the other information
included elsewhere in this prospectus supplement and the accompanying prospectus for a discussion of factors you should carefully
consider before deciding to invest in our securities. |
| |
|
|
| Trading: |
|
Our
shares of Common Stock currently trade on NYSE American under the symbol “OGEN”. |
(1)
The number of shares of our common stock to be outstanding immediately after this offering is based on 2,204,657 shares of our common
stock outstanding as of September 30, 2022, on a pro forma basis and excludes as of that date:
| ● | 149,090
shares of our common stock issuable upon the exercise of outstanding options under our equity
incentive plans as of September 30, 2022 at a weighted average exercise price of $40.80 per
share; |
| ● | 299,870
shares of common stock reserved for issuance under outstanding warrants as of September 30,
2022 with a weighted average exercise price of $84.60 per share; |
| ● | 138,455
additional shares of common stock reserved for future issuance under our equity incentive
plans as of September 30, 2022; and |
| ● | 22,528
shares of common stock reserved for issuance under conversion of our outstanding shares of
preferred stock. |
Except
as otherwise indicated, all information in this prospectus supplement assumes no exercise of outstanding options or warrants to purchase
common stock since September 30, 2022.
RISK
FACTORS
Before
purchasing our common stock you should carefully consider the risk factors set forth below and under the heading “Risk Factors”
included in our most recent Annual Report on Form 10-K as revised or supplemented by our subsequent Quarterly Reports on Form 10-Q, each
of which are on file with the SEC and are incorporated herein by reference, as well as all other information contained in this prospectus
supplement and the accompanying prospectus and incorporated by reference and any free writing prospectus that we have authorized for
use in connection with this offering. The risks and uncertainties described below and in our most recent Annual Report on Form 10-K,
as revised or supplemented by our subsequent Quarterly Reports on Form 10-Q, are not the only risks and uncertainties we face. Additional
risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any
of the risks described below or in our most recent Annual Report on Form 10-K, as revised or supplemented by our subsequent Quarterly
Reports on Form 10-Q, actually occur, our business, financial condition and results of operations could suffer. As a result, the trading
price of our stock could decline, perhaps significantly, and you could lose all or part of your investment. The risks discussed below
and in most recent Annual Report on Form 10-K, as revised or supplemented by our subsequent Quarterly Reports on Form 10-Q, also include
forward-looking statements and our actual results may differ substantially from those discussed in these forward-looking statements.
See the section entitled “Forward-Looking Information.”
Risks
Related To Our Financial Condition and Need For Additional Capital
We
have incurred significant losses since our inception and expect to continue to experience losses for the foreseeable future.
We
have incurred significant net losses and negative cash flow in each year since our inception, including net losses of approximately and
$14.0 million and $13.4 million for the nine months ended September 30, 2022 and September 30, 2021, respectively, and approximately
$15.7 million and $26.4 million for the years ended December 31, 2021, and 2020, respectively. As of September 30, 2022, our accumulated
deficit was approximately $185.3 million. We have devoted a significant amount of our financial resources to research and development,
including our nonclinical development activities and clinical trials. We expect that the costs associated with our plans to begin preclinical
research, contract manufacturing and file an IND for our NT-CoV2-1 vaccine product candidate and the research and development of our
product candidates in the area of lantibiotics (“Lantibiotics Program”) will continue to increase the level of our overall
expenses significantly going forward. Additionally, our License Agreements also requires the payment of certain recurring and performance-based
royalties that may negatively impact our financial capabilities. As a result, we expect to continue to incur substantial net losses and
negative cash flow for the foreseeable future. These losses and negative cash flows have had, and will continue to have, an adverse effect
on our shareholders’ equity and working capital. Because of the numerous risks and uncertainties associated with product development
and commercialization, we are unable to accurately predict the timing or amount of substantial expenses or when, or if, we will be able
to generate the revenue necessary to achieve or maintain profitability.
We
will need to raise additional capital in the future to complete the development and commercialization of our product candidates and operate
our business.
Developing
and commercializing biopharmaceutical products, including conducting nonclinical studies and clinical trials and establishing
manufacturing capabilities, and the progress of our efforts to develop and commercialize our product candidates, is expensive, and
can cause us to use our limited, available capital resources faster than we currently anticipate. We anticipate that our cash
resources of approximately $11.4 million as of December 31, 2022, will be sufficient to fund our operations as presently structured
through the third quarter of 2023. We are currently evaluating cost-saving initiatives, including restructuring that could allow
further cash runway through 2023 to the extent such initiatives are undertaken. Our actual costs may ultimately vary from our
current expectations, which could materially impact our use of capital and our forecast of the period of time through which our
financial resources will be adequate to support our operations. Our current cash, cash equivalents and short-term investments are
not sufficient to fully implement our business strategy and sustain our operations. Accordingly, we will need to seek additional
sources of financing and such additional financing may not be available on favorable terms, if at all. Until we can generate a
sufficient amount of product revenue, if ever, we expect to finance future cash needs through public or private equity offerings,
debt financings or corporate or government collaboration and licensing arrangements. If we do not succeed in raising additional
funds on acceptable terms, we may be unable to complete existing nonclinical and planned clinical trials or obtain approval of our
product candidates from the FDA and other regulatory authorities. We expect capital outlays and operating expenditures to increase
over the next several years as we expand our infrastructure, and research and development activities. Specifically, we need to raise
additional capital to, among other things:
| |
● |
conduct
preclinical research for our NT-CoV-2-1 vaccine product candidate, file an IND with the FDA and, if approved, engage in Phase 1 clinical
trials; |
| |
|
|
| |
● |
engage
in GMP and non-GMP manufacturing for our product candidates at the preclinical research and clinical trial stages; |
| |
|
|
| |
● |
expand
our clinical laboratory operations and conduct further research and development on lantibiotics; |
| |
|
|
| |
● |
fund
our clinical validation study activities; |
| |
|
|
| |
● |
expand
our research and development activities; and |
| |
|
|
| |
● |
finance
our capital expenditures and general and administrative expenses. |
Our
present and future funding requirements will depend on many factors, including:
| |
● |
the
level of research and development investment budgeted to develop our current and future product candidates through each phase of
development; |
| |
|
|
| |
● |
the
timing, scope, progress, results and cost of research and development, testing, screening, manufacturing, preclinical and non-clinical
studies and clinical trials, including any impacts related to the COVID-19 pandemic; |
| |
|
|
| |
● |
costs
of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; |
| |
|
|
| |
● |
our
need or decision to acquire or license complementary technologies or acquire complementary businesses; |
| |
|
|
| |
● |
changes
in test development plans needed to address any difficulties in product candidate selection for commercialization; |
| |
|
|
| |
● |
competing
vaccine and technological and market developments; |
| |
|
|
| |
● |
our
interaction and relationship with the FDA, or other, regulatory agencies; and |
| |
|
|
| |
● |
changes
in regulatory policies or laws that affect our operations. |
Additional
capital may not be available on satisfactory terms, or at all. Furthermore, if we raise additional funds by issuing equity securities,
dilution to our existing stockholders could result. Any equity securities issued also may provide for rights, preferences or privileges
senior to those of holders of our common and preferred stock. If we raise additional funds by issuing debt securities, these debt securities
would have rights, preferences and privileges senior to those of holders of our common stock, and the terms of the debt securities issued
could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements,
we might be required to relinquish significant rights to our technologies or our products under development or grant licenses on terms
that are not favorable to us, which could lower the economic value of those programs to us. If adequate funds are not available, we may
have to scale back our operations or limit our research and development activities, which may cause us to grow at a slower pace, or not
at all, and our business could be adversely affected.
In
addition, we could be forced to discontinue product development and commercialization of one or more of our product candidates, curtail
or forego sales and marketing efforts, and/or forego licensing attractive business opportunities.
Risks
Relating to this Offering
The
market price of our common stock has been, and may continue to be volatile and fluctuate significantly, which could result in substantial
losses for investors.
The
trading price for our common stock has been, and we expect it to continue to be, volatile. The price at which our common stock trades
depends upon a number of factors, including our historical and anticipated operating results, our financial situation, announcements
by us or our competitors, our ability or inability to raise the additional capital we may need and the terms on which we raise it, and
general market and economic conditions. Some of these factors are beyond our control. Broad market fluctuations may lower the market
price of our common stock and affect the volume of trading in our stock, regardless of our financial condition, results of operations,
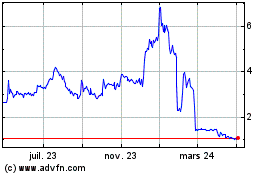
business or prospects. The closing price of our common stock as reported on the NYSE American had a high price of $33.60 and a low price
of $6.60 in the 52-week period ended December 31, 2022 and a high price of $9.00 and a low price of $5.09 from January 1, 2023 through
February 23, 2023. Among the factors that may cause the market price of our common stock to fluctuate are the risks described in this
“Risk Factors” section and other factors, including:
| |
● |
results
of preclinical and clinical studies of our product candidates or those of our competitors; |
| |
|
|
| |
● |
regulatory
or legal developments in the U.S. and other countries, especially changes in laws and regulations applicable to our product candidates; |
| |
|
|
| |
● |
actions
taken by regulatory agencies with respect to our product candidates, clinical studies, manufacturing process or sales and marketing
terms; |
| |
|
|
| |
● |
introductions
and announcements of new products by us or our competitors, and the timing of these introductions or announcements; |
| |
|
|
| |
● |
announcements
by us or our competitors of significant acquisitions or other strategic transactions or capital commitments; |
| |
|
|
| |
● |
fluctuations
in our quarterly operating results or the operating results of our competitors; |
| |
|
|
| |
● |
variance
in our financial performance from the expectations of investors; |
| |
|
|
| |
● |
changes
in the estimation of the future size and growth rate of our markets; |
| |
|
|
| |
● |
changes
in accounting principles or changes in interpretations of existing principles, which could affect our financial results; |
| |
|
|
| |
● |
failure
of our products to achieve or maintain market acceptance or commercial success; |
| |
|
|
| |
● |
conditions
and trends in the markets we serve; |
| |
|
|
| |
● |
changes
in general economic, industry and market conditions; |
| |
|
|
| |
● |
changes
in legislation or regulatory policies, practices or actions; |
| |
|
|
| |
● |
the
commencement or outcome of litigation involving our company, our general industry or both; |
| |
|
|
| |
● |
recruitment
or departure of key personnel; |
| |
|
|
| |
● |
changes
in our capital structure, such as future issuances of securities, redemption or conversion of preferred stock or the incurrence of
additional debt; |
| |
|
|
| |
● |
actual
or expected sales of our common stock by our stockholders; |
| |
|
|
| |
● |
acquisitions
and financings; and |
| |
|
|
| |
● |
the
trading volume of our common stock. |
In
addition, the stock markets, in general, NYSE American and the market for biotech companies in particular, may experience a loss of investor
confidence. Such loss of investor confidence may result in extreme price and volume fluctuations in our common stock that are unrelated
or disproportionate to the operating performance of our business, financial condition or results of operations. These broad market and
industry factors may materially harm the market price of our common stock and expose us to securities class action litigation. Such litigation,
even if unsuccessful, could be costly to defend and divert management’s attention and resources, which could further materially
harm our financial condition and results of operations.
You
may experience immediate and substantial dilution.
The
offering price per share in this offering may exceed the pro forma net tangible book value per share of our common stock outstanding
prior to this offering. Assuming that an aggregate of 888,099 shares of our common stock are sold during the term of the sales agreement
with Ladenburg at a price of $6.63 per share, one dollar above the last reported sale price of our common stock on the NYSE American
on February 17, 2023, for aggregate gross proceeds of approximately $5.0 million, after deducting commissions and estimated aggregate
offering expenses payable by us, you will experience immediate dilution of $0.26 per share, representing the difference between our pro
forma as adjusted net tangible book value per share as of September 30, 2022 after giving effect to this offering and the assumed offering
price. The exercise of outstanding stock options and warrants may result in further dilution of your investment. See the section entitled
“Dilution” below for a more detailed illustration of the dilution you may incur if you participate in this offering.
The
actual number of shares we will issue under the sales agreement with Ladenburg, at any one time or in total, is uncertain.
Subject
to certain limitations in the sales agreement with Ladenburg and compliance with applicable law, we have the discretion to deliver placement
notices to Ladenburg at any time throughout the term of the sales agreement. The number of shares that are sold by Ladenburg after delivering
a placement notice will fluctuate based on the market price of the common stock during the sales period and limits we set with Ladenburg
Our
management team may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield
a significant return.
Our
management will have broad discretion over the use of proceeds from this offering. We intend to use the net proceeds from this offering
to fund of our Terra CoV-1 research and clinical trials, and for working capital and general corporate purposes. Our management will
have considerable discretion in the application of the net proceeds, and you will not have the opportunity, as part of your investment
decision, to assess whether the proceeds are being used appropriately. The net proceeds may be used for corporate purposes that do not
increase our operating results or enhance the value of our common stock.
The
precise amount and timing of the application of these proceeds will depend upon a number of factors, such as the timing and progress
of our research and development efforts, our funding requirements and the availability and costs of other funds. As of the date of this
prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. Depending
on the outcome of our efforts and other unforeseen events, our plans and priorities may change and we may apply the net proceeds of this
offering in different manners than we currently anticipated.
The
failure by our management to apply these funds effectively could harm our business, financial condition and results of operations. Pending
their use, we may invest the net proceeds from this offering in short-term, interest-bearing instruments. These investments may not yield
a favorable return to our stockholders.
Future
sales of our common stock in the public market could cause our stock price to fall.
Sales
of a substantial number of shares of our common stock, or the perception by the market that those sales could occur, could cause the
market price of our common stock to decline or could make it more difficult for us to raise funds through the sale of equity in the future.
Future
issuances of common stock could further depress the market for our common stock. We expect to continue to incur drug development and
selling, general and administrative costs, and to satisfy our funding requirements, we will need to sell additional equity securities,
which may include sales of significant amounts of common stock to strategic investors, and which common stock may be subject to registration
rights and warrants with anti-dilutive protective provisions. The sale or the proposed sale of substantial amounts of our common stock
or other equity securities in the public markets or in private transactions may adversely affect the market price of our common stock
and our stock price may decline substantially. Our stockholders may experience substantial dilution and a reduction in the price that
they are able to obtain upon sale of their shares. Also, new equity securities issued may have greater rights, preferences or privileges
than our existing common stock. In addition, we have a significant number of shares of restricted stock, stock options and warrants outstanding.
To the extent that outstanding stock options or warrants have been or may be exercised or other shares issued, investors purchasing our
common stock in this offering may experience further dilution.
If
we make one or more significant acquisitions in which the consideration includes stock or other securities, our stockholders’ holdings
may be significantly diluted. In addition, stockholders’ holdings may also be diluted if we enter into arrangements with third
parties permitting us to issue shares of common stock in lieu of certain cash payments upon the achievement of milestones.
The
issuance of shares of our common stock under our 2021 Equity Incentive Plan is covered by Form S-8 registration statements we filed with
the Securities and Exchange Commission, or SEC, and upon exercise of the options, such shares may be resold into the market. We have
also issued shares of common stock and warrants in connection with previous private placements. Such shares are available for resale
as well as certain of the shares of common stock issuable upon exercise of the warrants. We have also issued shares of our common stock
in the private placement and financing transaction, which are deemed to be “restricted securities,” as that term is defined
in Rule 144 promulgated under the Securities Act of 1933, as amended, or Securities Act, and such shares may be resold pursuant to the
provisions of Rule 144. The resale of shares acquired from us in private transactions could cause our stock price to decline significantly.
In addition, the conversion of outstanding shares preferred stock into common stock and the subsequent sale of shares of common stock
could also cause our stock price to decline significantly.
In
addition, from time to time, certain of our shareholders may be eligible to sell all or some of their restricted shares of common stock
by means of ordinary brokerage transactions in the open market pursuant to Rule 144, subject to certain limitations. In general, pursuant
to Rule 144, after satisfying a six-month holding period: (i) affiliated shareholders, or shareholders whose shares are aggregated, may,
under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the
then-outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such
sale and (ii) non-affiliated shareholders may sell without such limitations, in each case provided we are current in our public reporting
obligations. Rule 144 also permits the sale of securities by non-affiliates that have satisfied a one-year holding period without any
limitation or restriction.
We
are unable to estimate the number of shares that may be sold because this will depend on the market price for our common stock, the personal
or business circumstances of sellers and other factors.
We
do not intend to pay cash dividends.
We
have not declared or paid any cash dividends on our common stock, and we do not anticipate declaring or paying cash dividends for the
foreseeable future. Any future determination as to the payment of cash dividends on our common stock will be at our Board of Directors’
discretion and will depend on our financial condition, operating results, capital requirements and other factors that our Board of Directors
considers to be relevant.
You
may experience future dilution as a result of future equity offerings.
To
raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable
for our common stock at prices that may not be the same as the prices per share in this offering. We may sell shares or other securities
in any other offering at a price per share that is less than the prices per share paid by investors in this offering, and investors purchasing
shares of our common stock or other securities in the future could have rights superior to existing stockholders. The price per share
at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions
may be higher or lower than the prices per share paid by investors in this offering.
We
cannot assure you that we will continue to be listed on the NYSE American.
Our
common stock commenced trading on the NYSE American (formerly the NYSE MKT) on April 10, 2013, and we are subject to certain NYSE American
continued listing requirements and standards. On December 19, 2022 we received notice of non compliance from the NYSE American due to
our share price being too low. We subsequently effected a 1 for 60 stock split on January 20, 2023 and received notice on February 1,
2023 from the NYSE American that we had regained compliance. We may also incur costs that we have not previously incurred for expenses
for compliance with the rules and requirements of the NYSE American. We cannot provide any assurance that we will be able to continue
to satisfy the requirements of the NYSE American’s continued listing standards. A delisting of our common stock from the NYSE American
could negatively affect the price and liquidity of our common stock and could impair our ability to raise capital in the future.
USE
OF PROCEEDS
We
intend to use the net proceeds from this offering to continue funding, our pre-clinical development of our SARS-CoV-2 vaccine, Terra
CoV-1 and our lantibiotics program and for general corporate purposes, including research and development activities, capital expenditures,
the redemption of all or a portion of our outstanding Series C Preferred Stock at its stated value and working capital.
The
precise amount and timing of the application of these proceeds will depend upon a number of factors, such as the timing and progress
of our research and development efforts, our funding requirements and the availability and costs of other funds. As of the date of this
prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. Accordingly,
our management will have broad discretion in the timing and application of these proceeds. Pending application of the net proceeds as
described above, we intend to temporarily invest the proceeds in short-term, interest-bearing instruments. Pending application of the
net proceeds for the purposes as described above, we expect to invest the net proceeds in short-term, interest-bearing securities, investment
grade securities, certificates of deposit or direct or guaranteed obligations of the U.S. government.
DIVIDEND
POLICY
To
date, we have neither declared nor paid any dividends on our common stock nor do we anticipate that such dividends will be paid in the
foreseeable future. Rather, we intend to retain any earnings to finance the growth and development of our business. Any payment of cash
dividends on our common stock in the future will be dependent, among other things, upon our earnings, financial condition, capital requirements
and other factors which the board of directors deems relevant. In addition, restrictive covenants contained in any financing agreements
entered into in the future may preclude us from paying any dividends.
DILUTION
If
you invest in our common stock in this offering, your ownership interest may be diluted to the extent of the difference between the price
per share of our common stock in this offering and the as adjusted net tangible book value per share of our common stock immediately
after this offering.
As
of September 30, 2022, our net tangible book value was $12.9 million, or $6.62 per share of our common stock, based upon 1,955,080 shares
of common stock outstanding as of that date. Our pro forma net tangible book value, as of September 30, 2022, was $13.0 million, or $6.43
per share of our common stock based upon 2,024,657 shares of common stock outstanding as of that date. Pro forma net tangible book value
gives effect to the issuance of shares under our prior ATM program after September 30, 2022 for net proceeds of $69,289 and 63,619 shares
issued without any proceeds due to rounding up of fractional shares as a result of our reverse stock split. Historical net tangible book
value per share is equal to our total tangible assets, less total liabilities, divided by the number of outstanding shares of our common
stock. Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers of shares
of common stock in this offering and the net tangible book value per share of common stock immediately after this offering.
On
a pro forma basis, after giving effect to our receipt of $4.7 million of estimated net proceeds (after deducting commissions and estimated
offering expenses payable by us) from our sale of 888,099 shares of common stock in this offering at an assumed offering price of $5.63
per share (the last reported sale price of our common stock on the NYSE American on February 17, 2023), our pro forma as adjusted net
tangible book value as of September 30, 2022 would have been $17.7 million, or $6.08 per share. This amount would represent an immediate
decrease in net tangible book value of $0.54 per share of our common stock to existing stockholders and an immediate increase in net
tangible book value of $0.45 per share of our common stock to new investors purchasing shares of common stock in this offering at the
assumed public offering price.
The
following table illustrates this hypothetical dilution on a per share basis:
| Public offering price per share | |
$ | 5.63 | |
| | |
| | |
| Historical net tangible book value per share as of September 30, 2022 | |
$ | 6.62 | |
| | |
| | |
| Pro forma decrease in net tangible book value per share as of September 30, 2022 | |
$ | (0.19 | ) |
| | |
| | |
| Pro forma net tangible book value per share as of September 30, 2022 | |
$ | 6.43 | |
| | |
| | |
| Decrease in net tangible book value per share attributable to new investors in this offering | |
$ | (0.54 | ) |
| | |
| | |
| Pro Forma as adjusted net tangible book value per share after giving effect to this offering | |
$ | 6.08 | |
| | |
| | |
| Increase in net tangible value per share to new investors participating in this offering | |
$ | 0.45 | |
The
information discussed above is illustrative only and will adjust based on the actual public offering price and other terms of this offering
determined at pricing and will also be affected by any securities sold by us, if any, pursuant the accompanying base prospectus. An increase
of $1.00 per share in the price at which the shares are sold from the assumed offering price of $5.63 per share shown in the table above,
assuming all of our common stock in the aggregate amount of 888,099 shares is sold at that price, would increase our pro forma as adjusted
net tangible book value per share after the offering to $6.37 per share and the dilution in net tangible book value per share to new
investors would be $0.26 per share, after deducting commissions and estimated aggregate offering expenses payable by us. A decrease
of $1.00 per share in the price at which the shares are sold from the assumed offering price of $5.63 per share shown in the table above,
assuming all of our common stock in the aggregate amount of 888,099 shares is sold at that price, would decrease our pro forma as adjusted
net tangible book value per share after the offering to $5.78 per share and the increase in net tangible book value per share to new
investors would be $1.15 per share, after deducting commissions and estimated aggregate offering expenses payable by us.
The
foregoing table assumes for illustrative purposes that an aggregate of 888,099 shares of our common stock are sold at a price of $5.63
per share, the last reported sale price of our common stock on the NYSE American on February 17, 2023, for aggregate gross proceeds of
$5.0 million. The shares sold in this offering, if any, will be sold from time to time at various prices. The foregoing table also excludes
the following as of that date:
| |
● |
149,090
shares of our common stock issuable upon the exercise of outstanding options under our equity incentive plans as of September 30,
2022 at a weighted average exercise price of $40.80 per share; |
| |
|
|
| |
● |
299,870
shares of common stock reserved for issuance under outstanding warrants as of September 30, 2022 with a weighted average exercise
price of $84.60 per share; and |
| |
|
|
| |
● |
138,455
additional shares of common stock reserved for future issuance under our equity incentive plans as of September 30, 2022; and |
| |
|
|
| |
● |
22,528
shares of common stock reserved for issuance under conversion of our outstanding shares of preferred stock as of September 30, 2022. |
To
the extent that any outstanding stock options or warrants are exercised, new stock options or warrants are issued, or we otherwise issue
additional shares of common stock in the future at a price less than the offering price, there will be further dilution to new investors.
In
addition, we may choose to raise additional capital due to market conditions or strategic considerations, even if we believe we have
sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity
or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
PLAN
OF DISTRIBUTION
Pursuant
to the Sales Agreement, entered into by and between the Company and Ladenburg Thalmann & Co. Inc., or Ladenburg, Ladenburg has agreed
to act as exclusive sales agent in connection with this offering of our common stock pursuant to this prospectus supplement and the accompanying
prospectus. Ladenburg is not purchasing or selling any of the shares of our common stock offered by this prospectus supplement, nor is
it required to arrange the purchase or sale of any specific number or dollar amount of shares of our common stock, but has agreed to
use their reasonable best efforts to arrange for the sale of all of the shares of our common stock offered hereby.
Upon
delivery of a placement notice and subject to the terms and conditions of the Sales Agreement, Ladenburg may sell shares of our common
stock by any method permitted by law deemed to be an “at-the-market” equity offering as defined in Rule 415 promulgated under
the Securities Act, including sales made directly on or through the NYSE American, the existing trading market for our common stock,
sales made to or through a market maker other than on an exchange or otherwise, in negotiated transactions at market prices prevailing
at the time of sale or at prices related to such prevailing market prices, and/or any other method permitted by law, including in privately
negotiated transactions.
We
will pay Ladenburg in cash, upon each sale of shares of our common stock pursuant to the Sales Agreement, a commission equal to 3.0%
of the gross sales price per share of common stock sold. Because there is no minimum offering amount required as a condition to this
offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. Ladenburg’s
initial legal fees paid by us for the offering shall be up to $75,000. In addition to such fees, at the end of each quarter in which
the offering is open we have agreed to pay Ladenburg’s legal counsel up to an additional $4,000. We estimate that the total expenses
for the offering, excluding compensation and reimbursements payable to the Sales Agent under the terms of the Sales Agreement, will be
approximately $75,000.
Settlement
for sales of shares of our common stock will occur on the second business day following the date on which any sales are made, or on some
other date that is agreed upon by us and Ladenburg in connection with a particular transaction, in return for payment of the net proceeds
to us. There is no arrangement for funds to be received in an escrow, trust or similar arrangement. Sales of shares of our common stock
as contemplated in this prospectus will be settled through the facilities of The Depository Trust Company or by such other means as we
and Ladenburg may agree upon.
We
have agreed to provide indemnification and contribution to Ladenburg and specified persons against certain civil liabilities, including
liabilities under the Securities Act, and the Exchange Act, and to contribute to payments that Ladenburg may be required to make in respect
of such liabilities.
Ladenburg
may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by them
and any profit realized on the resale of the shares sold by them while acting as principal might be deemed to be underwriting discounts
or commissions under the Securities Act. As an underwriter, Ladenburg would be required to comply with the requirements of the Securities
Act and the Exchange Act, including, without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation M under
the Exchange Act. These rules and regulations may limit the timing of purchases and sales of shares by the agent acting as principal.
Under these rules and regulations, Ladenburg:
| ● | may
not engage in any stabilization activity in connection with our securities; and |
| ● | may
not bid for or purchase any of our securities or attempt to induce any person to purchase
any of our securities, other than as permitted under the Exchange Act, until it has completed
its participation in the distribution. |
The
offering of our common stock pursuant to the Sales Agreement will terminate upon the earlier of (i) the sale of all shares of our common
stock subject to the Sales Agreement or (ii) termination of the Sales Agreement as permitted therein. Either we or the Sales Agent may
terminate the Sales Agreement at any time upon five (5) days’ prior notice.
Ladenburg
and its affiliates may in the future provide various investment banking, commercial banking and other financial services for us, for
which services they may in the future receive customary fees. To the extent required by Regulation M, Ladenburg will not engage in any
market making activities involving our shares of our common stock while the offering is ongoing under this prospectus.
This
prospectus supplement and the accompanying prospectus in electronic format may be made available on a website maintained by Ladenburg
and Ladenburg may distribute this prospectus supplement and the accompanying prospectus electronically.
The
foregoing does not purport to be a complete statement of the terms and conditions of the Sales Agreement. A copy of the Sales Agreement
is included as an exhibit to our Current Report on Form 8-K that will be filed with the SEC and incorporated by reference into the registration
statement of which this prospectus supplement and the accompanying base prospectus form a part.
LEGAL
MATTERS
The
validity of the issuance of the securities offered hereby will be passed upon for us by Shumaker, Loop & Kendrick, LLP. Certain legal
matters in connection with the offering will be passed upon for the sales agent Ellenoff Grossman
& Schole LLP.
EXPERTS
The
financial statements incorporated in this prospectus by reference from the Company’s Annual Report on Form 10-K for our fiscal
year ended December 31, 2021, filed with the SEC on March 24, 2022 have been audited by Mayer Hoffman McCann P.C., an independent registered
public accounting firm, as stated in their report which is incorporated herein by reference. Such financial statements have been so incorporated
in reliance upon the report of such firm given upon their authority as experts in accounting and auditing Mayer Hoffman McCann P.C. has
no interest in the shares being registered in this filing.
WHERE
YOU CAN FIND MORE INFORMATION
We
are a public company and file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange
Commission (“SEC”).You can request copies of these documents by writing to the SEC and paying a fee for the copying cost.
Our SEC filings are also available to the public at the SEC’s web site at http://www.sec.gov.
In
addition, we maintain a web site that contains information regarding our company, including copies of reports, proxy statements and other
information we file with the SEC. The address of our web site is www.oragenics.com. Except for the documents specifically incorporated
by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute
a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active
link to our website.
This
prospectus supplement and the accompanying prospectus are part of a registration statement on Form S-3 that we filed with the SEC registering
the securities that may be offered and sold hereunder. The registration statement, including exhibits thereto, contains additional relevant
information about us and these securities that, as permitted by the rules and regulations of the SEC, we have not included in this prospectus
supplement or the accompanying prospectus. A copy of the registration statement can be obtained at the address set forth above. You should
read the registration statement for further information about us and these securities.
INFORMATION
INCORPORATED BY REFERENCE
In
this document, we “incorporate by reference” certain information we file with the SEC, which means that we can disclose important
information to you by referring to that information. The information incorporated by reference is considered to be a part of this prospectus
supplement. Any statement contained in a document incorporated by reference herein shall be deemed to be modified or superseded for all
purposes to the extent that a statement contained in this prospectus supplement or in any other subsequently filed document that is also
incorporated or deemed to be incorporated by reference, modifies or supersedes such statement. Any statement so modified or superseded
shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement. We incorporate by reference
the documents listed below (other than, in each case, documents or information deemed to be furnished and not filed in accordance with
SEC rules):
| |
● |
Our
Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 24, 2022 and our Form 10-K/A for the
year ended December 31, 2021, filed with the SEC on July 29, 2022; |
| |
● |
Our
Quarterly Reports on Form 10-Q for the quarter ended March 31, 2022, filed with the SEC on May 13, 2022, for the quarter ended June 30, 2022 filed with the SEC on August 9, 2022 and for the quarter ended September 30, 2022 filed with the SEC on November 14, 2022;
|
| |
● |
Our
Definitive Proxy Statement on Schedule 14A, filed with the SEC on October 31, 2022; |
| |
● |
Our
Current Reports on Form 8-K, filed January 26, 2022, February 28, 2022, March
10, 2022, April
6, 2022, April
19, 2022, May
17, 2022, June
23, 2022, July
8, 2022, August
3, 2022, August
24, 2022, September
30, 2022, October
3, 2022, November 16,2022, December 16, 2022, December
19, 2022, December
20, 2022, December
22, 2022, December
23, 2022, January 23, 2023 and February 2, 2023; |
| |
● |
The
description of our common stock set forth in our registration statement on Form 8-A12B, filed April 8, 2013, including any amendments
or reports filed for purposes of updating such description. |
We
hereby undertake to provide without charge to each person, including any beneficial owner, to whom a copy of this prospectus supplement
is delivered, upon written or oral request of any such person, a copy of any and all of the information that has been or may be incorporated
by reference in this prospectus supplement, including any exhibits that are specifically incorporated by reference in such documents.
Requests for such copies should be directed as follows: Oragenics, Inc., 4902 Eisenhower Boulevard, Suite 125, Tampa, Florida 33634,
Attention: Investor Relations, Phone: (813) 276-7900
PROSPECTUS

$40,000,000
Common
Stock
Warrants
Units
From
time to time, we may offer, issue and sell up to $40,000,000 of any combination of the securities described in this prospectus. We may
also offer securities as may be issuable upon conversion, redemption, repurchase, exchange or exercise of any securities registered hereunder,
including any applicable antidilution provisions.
This
prospectus provides you with a general description of the securities we may offer. Each time we offer securities, we will provide the
specific terms of these offerings and securities in one or more supplements to this prospectus. We may also authorize one or more free
writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any related free writing
prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable
prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before buying any
of the securities being offered.
This
prospectus may not be used to consummate a sale of any securities unless accompanied by a prospectus supplement.
The
securities may be sold directly by us to investors, through agents designated from time to time or to or through underwriters or dealers,
on a continuous or delayed basis. For additional information on the methods of sale, you should refer to the section entitled “Plan
of Distribution” in this prospectus and in the applicable prospectus supplement. If any agents or underwriters are involved in
the sale of any securities with respect to which this prospectus is being delivered, the names of such agents or underwriters and any
applicable fees, commissions, discounts and over-allotment options will be set forth in a prospectus supplement. The price to the public
of such securities and the net proceeds that we expect to receive from such sale will also be set forth in a prospectus supplement.
Our
common stock is listed on the NYSE American under the symbol “OGEN.” The last reported sale price of our common stock on
January 12, 2023 was $7.63 per share. The applicable prospectus supplement will contain information, where applicable, as to any
other listing, if any, on the NYSE American or any securities market or other exchange of the securities covered by the applicable prospectus
supplement.
As
of January 12, 2023, the aggregate market value of our outstanding common stock held by non-affiliates, or the public float, was approximately
$14,662,868, which was calculated based on 1,921,739 shares of our outstanding common stock held by non-affiliates and
on a price of $7.63 per share, the last reported sale price for our common stock on January 12, 2023. Pursuant to General
Instruction I.B.6 of Form S-3, in no event will we sell our securities in a public primary offering with a value exceeding one-third
of our public float in any 12-month period unless our public float subsequently rises to $75.0 million or more.
Investing
in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the
heading “Risk Factors” beginning on page 8 of this prospectus, or contained in the applicable prospectus
supplement and any related free writing prospectus we have authorized for use in connection with a specific offering, and under
similar headings in the other documents that are incorporated by reference into this prospectus.
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED
IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The
date of this prospectus is January 25, 2023.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, utilizing
a “shelf” registration process. Under this shelf registration statement, we may, from time to time, sell any combination
of the securities referred to herein in one or more offerings for total gross proceeds of up to $40,000,000. This prospectus provides
you with a general description of the securities we may offer.
Until
such time, if ever, as we are eligible to use General Instruction I.B.1. of Form S-3, pursuant to General Instruction I.B.6. of Form
S-3, we are permitted to use the registration statement of which this prospectus forms a part to sell, via a primary offering, a maximum
amount of securities equal to one-third of the aggregate market value of our outstanding voting and non-voting common equity held by
non-affiliates of our company in any twelve month period.
Each
time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will contain more specific
information about the terms of the offered securities. We also may authorize one or more free writing prospectuses to be provided to
you that may contain material information relating to these offerings. This prospectus, together with applicable prospectus supplements
and any related free writing prospectuses, includes all material information relating to these offerings. We also may add, update or
change, in the prospectus supplement and in any related free writing prospectus that we may authorize to be provided to you, any of the
information contained in this prospectus or in the documents that we have incorporated by reference into this prospectus. We urge you
to read carefully this prospectus, any applicable prospectus supplement and any related free writing prospectus, together with the information
incorporated herein by reference as described under the section entitled “Where You Can Find Additional Information” and
“Incorporation of Certain Information by Reference” in this prospectus, before investing in any of the securities offered.
THIS
PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF SECURITIES UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
You
should rely only on the information that we have provided or incorporated by reference in this prospectus, any applicable prospectus
supplement and any related free writing prospectus that we may authorize to be provided to you. We have not authorized any other person
to provide you with different or additional information. No dealer, salesperson or other person is authorized to give any information
or to represent anything not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus
that we may authorize to be provided to you. You must not rely on any unauthorized information or representation. This prospectus, any
applicable supplement to this prospectus or any related free writing prospectus do not constitute an offer to sell or the solicitation
of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus, any applicable supplement
to this prospectus or any related free writing prospectus constitute an offer to sell or the solicitation of an offer to buy securities
in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
You
should not assume that the information appearing in this prospectus, any applicable prospectus supplement or any related free writing
prospectus is accurate on any date subsequent to the date on the front of the document and that any information we have incorporated
by reference is accurate as of the date of the document incorporated by reference, but not on any date subsequent to the date of the
document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any
related free writing prospectus or any sale of a security. Our business, financial condition, results of operations and prospectus may
have changed since those dates.
This
prospectus contains and incorporates by reference market data, industry statistics and other data that have been obtained from, or compiled
from, information made available by third parties. We have not independently verified their data. This prospectus and the information
incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks, service
marks and trade names included or incorporated by reference into this prospectus, any applicable prospectus supplement or any related
free writing prospectus are the property of their respective owners.
This
prospectus and the information incorporated herein by reference contains summaries of certain provisions contained in some of the documents
described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their
entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed, or will be incorporated
by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents
as described below under the section entitled “Where You Can Find Additional Information” and “Incorporation of Certain
Information by Reference.”
PROSPECTUS
SUMMARY
The
items in the following summary are described in more detail elsewhere in this prospectus and in the documents incorporated by reference
herein. This summary provides an overview of selected information and does not contain all the information you should consider before
investing in our common stock. Therefore, you should read the entire prospectus and any free writing prospectus that we have authorized
for use in connection with this offering carefully, including the “Risk Factors,” and information under similar headings
in the other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated
by reference into this prospectus, including our financial statements and related notes, and the exhibits to the registration statement
of which this prospectus is a part, before making any investment decision.
Unless
otherwise mentioned or unless the context requires otherwise, all references in this prospectus to “Oragenics” the “Company,”
“we,” “our” and “us” or similar references mean Oragenics, Inc. When we refer to “you,”
we mean the holders of the applicable securities. We own various U.S. federal trademark applications and unregistered trademarks, including
our company name. All other trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely
for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but such references
should not be construed as any indication that their respective owners will not assert, to the fullest extent under applicable law, their
rights thereto.
Overview
We
are a development-stage company dedicated to fighting infectious diseases including coronaviruses and multidrug-resistant organisms.
Our lead product (NT-CoV2-1) is an intranasal vaccine candidate to prevent coronavirus disease (“COVID-19”) from the SARS-CoV-2
virus and variants thereof. The NT-CoV2-1 program leverages coronavirus spike protein research licensed from the National Institutes
of Health and the National Research Council of Canada with a focus on reducing viral transmission and offering a more patient-friendly
intranasal administration. Our lantibiotics program features a novel class of antibiotics against bacteria that have developed resistance
to commercial antibiotics.
Our
SARS-CoV-2 Vaccine Product Candidate - NT-CoV2-1
Following
our May 2020 acquisition of one hundred percent (100%) of the total issued and outstanding common stock of Noachis Terra, Inc. (“Noachis
Terra”) we are focused on the development and commercialization of a vaccine product candidate to provide long-lasting immunity
from SARS-CoV-2, which causes COVID-19. Noachis Terra is a party to a worldwide, nonexclusive intellectual property and biological materials
license agreement with the National Institute of Allergy and Infectious Diseases (“NIAID”), an institute within the National
Institutes of Health (“NIH”), relating to certain research, patent applications and biological materials involving pre-fusion
stabilized coronavirus spike proteins and their use in the development and commercialization of a vaccine to provide specific, long lasting
immunity from SARS-CoV-2. Since the acquisition we have conducted testing in animal models, including SARS-CoV-2 challenge studies in
hamsters, using specific formulations for intramuscular administration (our Terra CoV-2 vaccine candidate) and intranasal administration
(our NT-CoV2-1 vaccine candidate), both based on the NIAID pre-fusion stabilized spike protein antigens. Following consideration of a
number of factors, including but not limited to the competitive landscape, we determined to bring the intranasal vaccine candidate NT-CoV2-1,
into further development due to the greater differentiation versus current COVID-19 vaccines and the potential benefits of intranasal
over intramuscular administration. We believe these benefits could include a higher reduction of transmission of SARS-CoV-2 and would
offer a needle-free delivery option. We therefore are currently focusing our development efforts on our more highly differentiated NT-CoV2-1
vaccine candidate.
On
July 26, 2021, we entered into a licensing agreement with the National Research Council (“NRC”) that enables us to pursue
the development of next-generation vaccines against the SARS-CoV-2 virus and its variants. The license was subsequently amended to: include
the Omicron variant, broaden the non-exclusive field of use to include all diseases caused by coronaviruses, and any genetic variants
thereof, to add a research protocol developed by the NRC, and to add reagents as part of the NRC Technology licensed by us. The NRC technologies,
in combination with the licensed technologies from the U.S. NIH used in our NT-CoV2-1 vaccine candidate, provide us with a platform that
can generate cell lines for high-yield production of spike protein antigens for existing and emerging variants of concern. This platform
should allow production of cell lines within six to eight weeks of spike gene sequence availability, compared with six to nine months
for traditional production of such cell lines. The NRC technologies, developed with support from the NRC’s Pandemic Response Challenge
Program, are expected to enable expedited evaluation of SARS-CoV-2 antigen candidates in pre-clinical and clinical studies.
Coronaviruses
are a family of viruses that can lead to upper-respiratory infections in humans. Recent clinical reports also suggest that the SARS-CoV-2
virus can affect other body-systems, including the nervous, cardiovascular, gastrointestinal and renal systems. Among the recent iterations
of coronaviruses to move from animal to human carriers is SARS-CoV-2, which, beginning in Wuhan, China, in late 2019, caused a global
pandemic due to its rapid spread and the relatively high mortality rate (as compared to the seasonal influenza). Pfizer/BioNTech received
FDA approval for their COVID-19 vaccines in August of 2021 and the Moderna vaccine in January 2022. The Janssen vaccine is currently
available in the United States under Emergency Use Authorizations (“EUA”) by the FDA. In July 2022, the FDA granted EUA for
the Novavax COVID-19 vaccine as well. Current vaccines have reduced the rates of hospitalization and death due to COVID-19 in vaccinated
individuals, but the transmission levels even in vaccinated individuals has allowed SARS-CoV-2 variants to continue to circulate. We
believe given the size of the worldwide spread of COVID-19 that even with additional vaccines available, there will be demand for the
highly differentiated NT-CoV2-1 vaccine, once development is successfully completed. We intend to combine the research, patent applications
and biological materials covered by our NIAID license and with our NRC license and our existing clinical research and manufacturing capabilities
to respond to this ongoing, global, public health issue. We believe our NT-CoV2-1 vaccine holds the possibility of playing an important
role in addressing this issue.
Coronaviruses,
such as SARS-CoV-2, possess signature protein spikes on their outer capsule. Our NIAID license covers patents and data on a vaccine candidate
that were created based on a stabilized pre-fusion spike trimeric protein. By stabilizing the spike protein in the pre-fusion state,
the number of immunogenic centers is increased thereby allowing for a greater likelihood of successful antibody binding, resulting in
an improved immunogenic response. Spike protein antigens stabilized in the pre-fusion state have been used successfully in the leading
COVID-19 vaccines from Pfizer/BioNTech and Moderna, which we believe reduces the risk of using the same approach in our NT-CoV2-1 vaccine
candidate. The genetic code, acquired from the NIH, for the stabilized pre-fusion spike protein was provided to Aragen Bioscience, Inc.
(“Aragen”) for the purpose of insertion of the spike protein gene sequence into a Chinese Hamster Ovary (“CHO”)
cell line. Aragen is a leading contract research organization focused on accelerating pre-clinical biologics product development, has
extensive experience building CHO cell lines for recombinant proteins, such as monoclonal antibodies. Aragen successfully inserted the
NIH pre-fusion spike protein gene sequence into a CHO cell line and Oragenics is currently producing Phase 1 clinical material based
upon this cell line.
We
entered into both a material transfer agreement and a non-exclusive research license agreement with Inspirevax for the use of intranasal
mucosal adjuvants in our NT-CoV2-1 vaccine candidates. Regarding the intranasal mucosal adjuvants of interest, BDX300 and BDX301 are
proteosome-based adjuvants comprised of proteins and lipopolysaccharides with improved attributes including enhanced immune response,
manufacturing efficiency and the benefits of intranasal vaccine administration. The non-exclusive license agreement allows for the collaboration
and research regarding the intranasal delivery of vaccine during clinical development with the opportunity to enter into a commercial
agreement upon regulatory approval of the intranasal vaccine. The NT-CoV2-1 vaccine containing Inspirevax’s intranasal mucosal
adjuvant BDX301 has been studied in pre-clinical animal studies, including hamster viral challenge studies and mouse immunogenicity studies.
A rabbit toxicology study has been initiated and is required for regulatory approval prior to the Phase 1 clinical study.
A
Non-Exclusive Research License Agreement with Inspirevax was executed in February 2022. This agreement granted the Company non-exclusive
rights to conduct non-clinical and clinical research and trials in relation to vaccines comprising the BDX300 or BDX301 adjuvants to
prevent or treat diseases caused by coronaviruses and genetic variants thereof.
We
began pre-clinical studies in June of 2021 through our collaboration and material transfer agreement with the NRC. We initiated an immunogenicity
study in mice to evaluate several adjuvant candidates. On August 30, 2021, we announced the successful completion of these mouse immunogenicity
studies that supported further development using either the intramuscular or intranasal routes of administration. A hamster challenge
study was initiated in September of 2021 to assess inhibition of viral replication using adjuvants specific for intramuscular and intranasal
administration. In December of 2021, we announced that both formulations generated robust immune responses and reduced the SARS-CoV-2
viral loads to undetectable levels in the nasal passages and lungs five days following a viral challenge. By contrast, hamsters in the
control groups that had received saline or adjuvants alone had no detectable immune response and substantial viral loads. The vaccines
delivered by intranasal and intramuscular routes generated immune responses as measured by multiple assays. On June 14, 2022, we announced
that the results of these studies were published in Nature Scientific Reports.
In
March of 2022, following a positive assessment of a rabbit-based pilot study, we initiated a Good Laboratory Practice toxicology study
to evaluate the safety profile and immunogenicity of NT-CoV2-1 in rabbits. This important preclinical study is designed to provide data
required to advance our intranasal vaccine candidate into human clinical studies. The study has concluded and we completed the full set
of toxicology data, which is needed to support the filing of an IND application for NT-CoV2-1. Based on the findings of the final toxicology
report, including a full histopathology evaluation, we were able to confirm a safety and immunogenicity profile that further support
our plan to submit regulatory filings required to progress to a Phase 1 clinical study.
While
we previously had a Type B Pre-IND Meeting with the FDA on our intramuscular vaccine product candidate, we again met with the FDA in
a Type B Pre-IND Meeting request to discuss our intranasal vaccine product candidate. As a result of this meeting, the FDA indicated
that the Company could file an IND application for NT-CoV2-1 following the availability of the final GLP toxicology report for inclusion
in the IND.
We
believe the benefits of our NT-CoV2-1 vaccine product candidate through its intranasal delivery mechanism to be:
| ● | Targeted
Mucosal Immunity – Conventional injectable vaccines are poor inducers of mucosal
immunity, whereas intranasal immunization can induce strong mucosal immunity by enhancing
the immune response at the entry sites of mucosal pathogens. When the SARS-CoV-2 virus enters
the nasal cavity, the respiratory epithelial layer is the first barrier against viral infection.
The intranasal route of vaccination provides two additional layers of protection over intramuscular
shots because (i) it produces immunoglobulin A and resident memory B and T cells in the respiratory
mucosa that are an effective barrier to infection at those sites, and (ii) cross-reactive
resident memory B and T cells can respond earlier than other immune cells should a viral
variant start an infection. |
| ● | Needle-Free
Administration – As an obvious benefit, intranasal administration means needle-free
delivery, resulting in meaningful differentiation for children and needle-phobic populations,
improved compliance and the potential for self-administration. |
| ● | Storage
& Transport – The currently available mRNA-based vaccines have been delivered
globally via stringent storage and transport requirements that strain distribution logistics
under the best of circumstances. A key benefit of our NT-CoV2-1 vaccine candidate is a significantly
reduced handling burden, allowing transport at a more manageable refrigeration temperature
(5°C) that improves access globally including remote and under-vaccinated geographies. |
| ● | Durability
– Broad initial success with mRNA vaccines has significantly diminished COVID-19’s
impact and death, but the trade-off has been fleeting efficacy. By benefitting from the immunological
properties of the hybrid NIH/NRC construct, NT-CoV2-1 is potentially much more durable and
long-lasting than currently available mRNA-based therapies. |
Through
assessment of a variety of factors including our pre-clinical testing to date, the expected benefits noted above, evolving variants and
available vaccines in use, we determined to focus our development efforts on the intranasal delivery of our vaccine product candidate,
NT-CoV2-1, which we believe is more highly differentiated than the currently available and late-stage COVID-19 vaccines. We expect to
seek to file an IND application with the FDA and to thereafter commence a Phase 1 clinical study with NT-CoV2-1, the protocol for which
is currently under development.
We
expect to use our currently available cash resources to continue to advance the development of NT-CoV2-1 through IND-enabling studies
and commencement of a Phase 1 clinical trial with further clinical development being contingent upon the receipt of additional funding,
including non-dilutive government grant funding which we continue to pursue, or partnering or out-licensing opportunities.
Our
Antibiotic Product Candidate - Oragenics Derived Compound (ODC-x)
Members
of our scientific team discovered that a certain bacterial strain of Streptococcus mutans, produces Mutacin 1140 (MU1140), a molecule
belonging to the novel class of antibiotics known as lantibiotics. Lantibiotics, such as MU1140, are highly modified peptide antibiotics
made by a small group of Gram-positive bacterial species. Over 60 lantibiotics have been discovered, to date. We believe lantibiotics
are generally recognized by the scientific community to be potent antibiotic agents.
In
nonclinical testing, MU1140 has shown activity against all Gram-positive bacteria against which it has been tested, including those responsible
for a number of healthcare associated infections, or HAIs. A high percentage of hospital-acquired infections are caused by highly antibiotic-resistant
bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) or multidrug-resistant Gram-negative bacteria. We believe the need
for novel antibiotics is increasing as a result of the growing resistance of target pathogens to existing FDA approved antibiotics on
the market.
Lantibiotics
have been difficult to investigate for their clinical usefulness as therapeutic agents in the treatment of infectious diseases due to
a general inability to produce or synthesize sufficient quantities of pure amounts of these molecules. Traditional fermentation methods
can only produce minute amounts of the lantibiotic.
The
timing of the filing of an IND regarding any future lantibiotic candidate is subject to our having sufficient available human, material
and financing capital, which includes research subjects, both animal and human, given all of our anticipated needs and expected requirements
in connection with our ongoing research and development initiatives. We expect to continue to advance our lantibiotics program to an
IND filing based on the availability of both human and financial capital. Based upon the current funding we expect to continue to focus
on the identification of new potential product lantibiotic candidates, efficient and cost-effective improvements in the manufacturing
processes and pre-clinical studies required to support a first in human Phase 1 clinical study.
In
October 2021, we were awarded a small business innovation research grant in the amount of $250,000 (“Computer-aided Design for
Improved Lantibiotics”, R41GM136034) for the Company’s continued research and development of lantibiotics, including its
collaborative program with the Biomolecular Sciences Institute at Florida International University (FIU). The grant provides the Company
with funding to develop novel lantibiotics for the treatment of ESKAPE pathogens (defined as Enterococcus faecium, Staphylococcus
aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.).
Product
Candidates.
Through
our wholly-owned subsidiary, Noachis Terra, we began the research and development stage for our new Terra CoV-2 and NT-CoV2-1 vaccine
product candidates. We hold a nonexclusive, worldwide intellectual property license agreement for certain research, patent applications
and biological materials relating to the use of pre-fusion coronavirus spike proteins for the development and commercialization of a
vaccine against SARS-CoV-2. We also hold a non-exclusive license with the NRC that enables us to pursue the rapid development of next-generation
vaccines against the SARS-CoV-2 (the “NIH License”) virus and its variants (the “NRC License” and together with
the NIH License the “License Agreements”).
Additionally,
we are developing semi-synthetic lantibiotic analogs that may be effective against systemic Gram-positive multidrug infections, and analogs
that may be effective in treating Gram-negative infections. We seek to protect our product candidates through patents and patent applications
pursuant to the terms of our License Agreements.
| Product/Candidate |
|
Description |
|
Application |
|
Status |
| |
|
|
|
|
|
|
| NT-CoV2-1 |
|
Intranasal
vaccine candidate (recombinant protein + adjuvant) to provide long lasting immunity against SARS-CoV-2 |
|
Broad,
community-based vaccine immunity against SARS-CoV-2 |
|
Pre-clinical
|
| |
|
|
|
|
|
|
| Antibiotics |
|
Semi-synthetic
analogs of MU1140: Member of lantibiotic class of antibiotics |
|
Healthcare-associated
infections |
|
Pre-clinical |
Recent
Developments
On
December 22, 2022 our board of directors approved a 1 for 60 reverse stock split of our authorized, issued and outstanding of common
stock to be effective on January 20, 2023. The par value per common shares will remain unchanged. Except where the context otherwise
requires, share numbers in this prospectus reflect the 1 for 60 reverse stock split of our common stock.
Our
Business Development Strategy
Success
in the biopharmaceutical and product development industry relies on the continuous development of novel product candidates. Most product
candidates do not make it past the clinical development stage, which forces companies to look externally for innovation. Accordingly,
we expect from time to time, to seek strategic opportunities through various forms of business development, which can include strategic
alliances, licensing deals, joint ventures, collaborations, equity-or debt-based investments, dispositions, mergers and acquisitions.
We view these business development activities as a necessary component of our strategies, and we seek to enhance shareholder value by
evaluating business development opportunities both within and complementary to our current business as well as opportunities that may
be new and separate from the development of our existing product candidates.
Corporate
and Other Information
We
were incorporated in November 1996 and commenced operations in 1999. We consummated our initial public offering in June 2003. Our executive
office is located at, 4902 Eisenhower Boulevard, Suite 125 Tampa, Florida, 33634 and our research facilities are located at 13700 Progress
Boulevard, Alachua, Florida 32615. Our telephone number is (813) 286-7900 and our website is http://www.oragenics.com. We make available
free of charge on our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments
to those reports as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange
Commission (the “SEC”). The reports are also available at www.sec.gov. We do not incorporate by reference into this prospectus
the information on, or accessible through, our website, and you should not consider it as part of this prospectus and it should not be
relied on in connection with this offering. We have included our website address as an inactive textual reference only.
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company” as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934, as amended,
or the Exchange Act. We may remain a smaller reporting company until we have a non-affiliate public float in excess of $250 million and
annual revenues in excess of $100 million, or a non-affiliate public float in excess of $700 million, each as determined on an annual
basis. A smaller reporting company may take advantage of relief from some of the reporting requirements and other burdens that are otherwise
applicable generally to public companies. These provisions include:
| ● | being
permitted to provide only two years of audited financial statements, in addition to any required
unaudited interim financial statements, with correspondingly reduced “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| ● | not
being required to comply with the auditor attestation requirements in the assessment of our
internal control over financial reporting; and |
| ● | reduced
disclosure obligations regarding executive compensation in our periodic reports, proxy statements
and registration statements. |
Securities
We May Offer
We
may offer shares of our common stock, warrant shares of our common stock to purchase, either individually or in combination, and/or units
consisting of some or all of such securities for total gross proceeds of up to $40 million, from time to time under this prospectus,
together with the applicable prospectus supplement and any related free writing prospectus, at prices and on terms to be determined by
market conditions at the time of any offering. This prospectus provides you with a general description of the securities we may offer.
We will describe in the applicable prospectus supplement relating to any securities the particular terms of the securities offered by
that prospectus supplement. If we indicate in the applicable prospectus supplement, the terms of the securities may differ from the terms
we have summarized below. We may also include in the prospectus supplement information about material United States federal income tax
considerations relating to the securities, and the securities exchange, if any, on which the securities will be listed.
We
may sell from time to time, in one or more offerings:
| |
● |
Common
stock; |
| |
● |
Warrants
to purchase shares of common stock; and |
| |
● |
Units
consisting of any combination of the securities listed above. |
In
this prospectus, we refer to the common stock, warrants and units collectively as “securities.” The total dollar amount of
all securities that we may sell pursuant to this prospectus will not exceed $40,000,000.
This
prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. You should carefully review the risks and uncertainties described under the heading
“Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar
headings in our Annual Report on Form 10-K for the year ended December 31, 2021, as updated or supplemented by any subsequently
filed periodic reports and other documents as filed with the SEC and incorporated by reference into this prospectus, before deciding
whether to purchase any of the securities being registered pursuant to the registration statement of which this prospectus is a part.
Each of the risk factors described in the documents referenced above could adversely affect our business, operating results and financial
condition, as well as adversely affect the value of an investment in our securities, and the occurrence of any of these risks might cause
you to lose all or part of your investment. Additional risks not presently known to us or that we currently believe are immaterial may
also significantly impair our business operations.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference herein contain forward-looking statements. These are based on our management’s
current beliefs, expectations and assumptions about future events, conditions and results and on information currently available to us.
Discussions containing these forward-looking statements may be found, among other places, in the sections entitled “Business,”
“Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
contained in the documents incorporated by reference herein.
Any
statements in this prospectus, or incorporated herein, about our expectations, beliefs, plans, objectives, assumptions or future events
or performance are not historical facts and are forward-looking statements. Within the meaning of Section 27A of the Securities Act and
Section 21E of the Exchange Act, these forward-looking statements include statements regarding:
| |
● |
We
have incurred significant operating losses since our inception and cannot assure you that we will generate revenues or achieve profitability; |
| |
|
|
| |
● |
We
will need to raise additional capital to fully implement our business strategy and we may not be able to do so; |
| |
|
|
| |
● |
Our
financial capacity and performance, including our ability to obtain funding, non-dilutive or otherwise, necessary to do the research,
development, manufacture and commercialization of any one or all of our product candidates; |
| |
|
|
| |
● |
The
timing, progress and results of clinical trials of our product candidates, including statements regarding the timing of initiation
and completion of pre-clinical studies or clinical trials or related preparatory work, the period during which the results of the
trials will become available and our research and development programs; |
| |
|
|
| |
● |
The
timing of any submission of filings for regulatory approval of our product candidates and our ability to obtain and maintain regulatory
approvals for our product candidates for any indication; |
| |
|
|
| |
● |
Our
expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates including as to administration,
distribution and storage; |
| |
|
|
| |
● |
Our
expectations regarding the size of the patient populations, market acceptance and opportunity for and clinical utility of our product
candidates, if approved for commercial use; |
| |
|
|
| |
● |
Our
manufacturing capabilities and strategy, including the scalability and commercial viability of our manufacturing methods and processes,
and those of our contractual partners; |
| |
|
|
| |
● |
Our
expectations regarding the scope of any approved indications for our product candidates; |
| |
|
|
| |
● |
Our
ability to successfully commercialize our product candidates; |
| |
|
|
| |
● |
The
potential benefits of, and our ability to maintain, our relationships and collaborations with the NIAID, the NIH, the NRC and other
potential collaboration or strategic relationships; |
| |
|
|
| |
● |
Our
ability to use our lantibiotic platform to develop future product candidates; |
| |
● |
Our
estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional
funding, including any application for future grants or funding; |
| |
|
|
| |
● |
Our
ability to identify, recruit and retain key personnel and consultants; |
| |
● |
Our
ability to obtain, retain, protect and enforce our intellectual property position for our product candidates, and the scope of such
protection; |
| |
|
|
| |
● |
Our
ability to advance the development of our new NT-CoV2-1 vaccine product candidate under the timelines and in accord with the milestones
projected; |
| |
|
|
| |
● |
Our
inability to achieve success in our identification of lantibiotic homologs or the manufacture and nonclinical testing of our lantibiotic
product candidates; |
| |
|
|
| |
● |
Our
need to comply with extensive and costly regulation by worldwide health authorities, who must approve our product candidates prior
to substantial research and development and could restrict or delay the future commercialization of certain of our product candidates; |
| |
|
|
| |
● |
Our
ability to successfully complete pre-clinical and clinical development of, and obtain regulatory approval of our product candidates
and commercialize any approved products on our expected timeframes or at all; |
| |
|
|
| |
● |
The
safety, efficacy and benefits of our product candidates; |
| |
|
|
| |
● |
The
content and timing of submissions to and decisions made by the FDA, other regulatory agencies and nongovernmental bodies and actors,
such as investigational review boards; |
| |
|
|
| |
● |
The
effects of government regulation and regulatory developments, and our ability and the ability of the third parties with whom we engage
to comply with applicable regulatory requirements; |
| |
|
|
| |
● |
The
capacities and performance of our suppliers and manufacturers and other third parties over whom we have limited control; |
| |
|
|
| |
● |
Our
ability to maintain our listing on the NYSE American and the effects of our contemplated 1 for 60 reverse stock split on
our price per share and the trading market of our common stock; |
| |
|
|
| |
● |
The
impact of the COVID-19 pandemic on our financial condition and business operations and our ability to continue research and development
for existing product candidates on previously-projected timelines or in accord with ordinary practices, as well as the broader governmental,
global health and macro- and microeconomic responses to and consequences of the pandemic; |
| |
|
|
| |
● |
We
may be adversely impacted by any significant broad-based financial crises and its impact on consumers, retailers and equity and debt
markets as well as our inability to obtain required additional funding to conduct our business; |
| |
|
|
| |
● |
As
a public company, we must implement additional and expensive finance and accounting systems, procedures and controls as we grow our
business and organization to satisfy reporting requirements, which add to our costs and require additional management time and resources; |
| |
|
|
| |
● |
Our
competitive position and the development of and projections relating to our competitors or our industry; and |
| |
|
|
| |
● |
The
impact of laws and regulations, including those that may not yet exist. |
In
some cases, you can identify forward-looking statements by the words “may,” “might,” “can,” “will,”
“to be,” “could,” “would,” “should,” “expect,” “intend,” “plan,”
“objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,”
“potential,” “likely,” “continue” and “ongoing,” or the negative of these terms, or other
comparable terminology intended to identify statements about the future, although not all forward-looking statements contain these words.
These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity,
performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.
You
should refer to the “Risk Factors” section contained in the applicable prospectus supplement and any related free writing
prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus, for a discussion
of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements.
Given these risks, uncertainties and other factors, many of which are beyond our control, we cannot assure you that the forward-looking
statements in this prospectus will prove to be accurate, and you should not place undue reliance on these forward-looking statements.
Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties
in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person
that we will achieve our objectives and plans in any specified time frame, or at all.
Except
as required by law, we assume no obligation to update these forward-looking statements publicly, or to revise any forward-looking statements
to reflect events or developments occurring after the date of this prospectus, even if new information becomes available in the future.
USE
OF PROCEEDS
We
will retain broad discretion over the use of the net proceeds from the sale of the securities offered hereby. Except as described in
any applicable prospectus supplement or in any free writing prospectuses that we may authorize to be provided to you in connection with
a specific offering, we currently intend to use the net proceeds from the sale of the securities offered hereby for working capital,
capital expenditures and general corporate purposes, which may include, without limitation, funding
research, clinical and process development and manufacturing of our product candidates. We may also use a portion of the net proceeds
to invest in, collaborate with, acquire, or in-licensing of products or product candidates, business or technologies that we believe
are complementary to our own, although we have no current plans, commitments or agreements with respect to any acquisitions as of the
date of this prospectus. We will set forth in the applicable prospectus supplement or free writing prospectus our intended use for the
net proceeds received from the sale of any securities sold pursuant to the prospectus supplement or free writing prospectus. Pending
these uses, we intend to invest the net proceeds in investment-grade, interest-bearing securities.
DIVIDEND
POLICY
We
have never paid cash dividends on our common stock. Moreover, we do not anticipate paying periodic cash dividends on our common stock
for the foreseeable future. We intend to use all available cash and liquid assets in the operation and growth of our business. Any future
determination about the payment of dividends will be made at the discretion of our board of directors and will depend upon our earnings,
if any, capital requirements, operating and financial conditions and on such other factors as our board of directors deems relevant.
DESCRIPTION
OF CAPITAL STOCK
The
following descriptions are summaries of the material terms that are included in our amended and restated articles of incorporation (as
amended) and our bylaws (as amended) as well as the specific agreements such descriptions relate to. This summary is qualified in its
entirety by the specific terms and provisions contained in our restated articles of incorporation, bylaws and the specific agreements
described herein, copies of which we have filed as exhibits to the registration statement of which this prospectus is a part, and by
the provisions of applicable law.
Overview
Authorized
Capital Stock
Our
authorized capital stock consists of 250,000,000 (4,166,666 following the effectiveness of our 1 for 60 reverse stock on January 20,
2023) shares of common stock, par value $0.001, and 50,000,000 shares of preferred stock, without par value.
Common
Stock
Voting
The
holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the shareholders.
Approval of an amendment of our articles of incorporation, a merger, a share exchange, a sale of all our property or dissolution must
be approved by a majority of all votes entitled to be cast. Such votes may be cast in person or by proxy as provided in Article I Section
8 of our bylaws. One third of our shares entitled to vote constitute a quorum for purposes of a meeting of our shareholders.
Dividends
Subject
to preferences that may be applicable to any outstanding preferred stock, the holders of our common stock are entitled to receive ratably
all dividends, if any, as may be declared from time to time by our Board of Directors out of the funds legally available.
In
the event of the liquidation, dissolution or winding up of the Company, the holders of our common stock are entitled to share ratably
in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding.
The common stock has no preemptive or conversion rights. There are no redemption or sinking fund provisions applicable to the common
stock. All outstanding shares of common stock are fully paid and non-assessable.
Rights
upon Liquidation
Upon
our liquidation, dissolution or winding-up, after payment in full of our liabilities and the amounts required to be paid to holders of
any outstanding shares of preferred stock, if any, all holders of our common stock, along with the holders of our Series A Convertible
Preferred Stock and Series B Convertible Preferred Stock on an “as if” converted basis, will be entitled to receive a pro
rata distribution of all of our assets and funds legally available for distribution.
Redemption
and Pre-Emptive Rights
No
shares of our common stock are subject to redemption or have preemptive rights to purchase additional shares of our common stock or any
of our other securities.
Fully
Paid and Nonassessable
All
of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Preferred
Stock
Our
Board of Directors has the authority, without action by our shareholders, to designate and issue up to 50,000,000 shares of preferred
stock in one or more series or classes and to designate the rights, preferences and privileges of each series or class, which may be
greater than the rights of our common stock. These rights, preferences and privileges could include dividend rights, conversion rights,
voting rights, redemption rights, liquidation preferences, the number of shares constituting any class or series and the designation
of the class or series. Terms selected by our Board of Directors in the future could decrease the amount of earnings and assets available
for distribution to holders of shares of common stock or adversely affect the rights and powers, including voting rights, of the holders
of shares of common stock without any further vote or action by the stockholders. As a result, the rights of holders of our common stock
will be subject to, and may be adversely affected by, the rights of the holders of the Series A Convertible Preferred Stock, and Series
B Convertible Preferred Stock or any other preferred stock that may be issued by us in the future, which could have the effect of decreasing
the market price of our common stock.
Series
A Convertible Preferred Stock
On
May 10, 2017 and on July 25, 2017, we issued an aggregate of 12,000,000 shares of convertible preferred stock, designated as the Series
A Convertible Preferred Stock pursuant to the certificate of designation and rights filed by us with the Secretary of State of the State
of Florida, with an aggregate original purchase price and initial liquidation preference of $3.0 million. Each share of Series A Convertible
Preferred Stock was issued for an amount equal to $0.25 per share, which we refer to as the original purchase price. On March 9, 2018
and August 26, 2022, certain holders of Series A Convertible Preferred Stock elected to convert to common stock and, as a result of such
conversions, 5,417,000 shares of Series A Preferred remain outstanding.
The
following description is a summary of the material provisions of the Series A Convertible Preferred Stock and the certificate of designation
and rights and does not purport to be complete. This summary is subject to and is qualified by reference to all the provisions of the
Series A Convertible Preferred Stock and certificate of designation and rights of Series A Convertible Preferred Stock, including the
definitions of certain terms used in the certificate of designation and rights. We urge you to read this document because it, and not
this description, defines the rights of a holder of the Series A Convertible Preferred Stock. A copy of the form of certificate of designation
and rights that we filed with the Secretary of State of the State of Florida effective May 10, 2017 as amended and restated effective
November 8, 2017 has been incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part.
No
Mandatory Redemption Date or Sinking Fund
The
shares of Series A Convertible Preferred Stock do not have a mandatory redemption date and are not subject to any sinking fund. The shares
of Series A Convertible Preferred Stock will remain outstanding indefinitely unless we elect to redeem them under the circumstances described
below in “Redemption” or we otherwise repurchase them or they are converted into shares of our common stock as described
below under “Conversion Rights.”
Dividends
The
shares of Series A Convertible Preferred Stock are entitled to participate in all dividends declared and paid on shares of company common
stock on an “as if” converted basis.
Liquidation
Preference
Upon
any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary that is not a Fundamental Transaction (as
defined in the certificate of designation), the holders of Series A Convertible Preferred Stock shall be entitled to receive out of the
assets, the greater of (i) the product of the number of shares of Series A Preferred Stock then held by such holder, multiplied by the
original issue price; and (ii) the amount that would be payable to such holder in the liquidation in respect of Common Stock issuable
upon conversion of such shares of Series A Preferred Stock if all outstanding shares of Series A Preferred Stock were converted into
Common Stock immediately prior to the Liquidation.
Ranking
The
Series A Convertible Preferred Stock ranks (i) on par with the Common Stock and Series B Convertible Preferred Stock and junior to Series
C Non-Convertible Preferred Stock as to dividend rights and (ii) on par with Series B Convertible Preferred Stock, junior to Series C
Non-Convertible Preferred Stock and senior to Common Stock as to rights upon liquidation, dissolution or winding up of the Company, whether
voluntarily or involuntarily.
See
“Voting Rights—Matters Requiring Approval of Holders of Series A Convertible Preferred Stock” for a description of
the types of issuances of equity securities and other securities of our company requiring approval of holders of a majority of shares
of Series A Convertible Preferred Stock then outstanding, voting together as a class.
Redemption
To
the extent we have funds legally available therefor, at any time after the fifth anniversary of the original issue date of the Series
A Convertible Preferred Stock, we have the right to redeem all or any portion of the outstanding shares of Series A Convertible Preferred
Stock at the original issue price of $0.25 by providing at least seventy five (75) days written notice of such redemption to all holders
of the then outstanding shares of Series A Convertible Preferred Stock.
Conversion
Rights
The
holders of shares of Series A Convertible Preferred Stock will, at any time, be entitled to convert some or all of their Series A Convertible
Preferred Stock into the number of shares of our common stock obtained by dividing the original purchase price of the shares to be converted
by the aggregate Series A conversion price (which originally equaled the original purchase price, but is subject to adjustment), which
amount we refer to as the conversion price.
The
conversion price will be adjustable upon the occurrence of certain events and transactions to prevent dilution as described under “Adjustments
to Conversion Price to Prevent Dilution.” Any shares of our common stock issued upon conversion of the shares of Series A Convertible
Preferred Stock shall be validly issued, fully paid and non-assessable. The Company shall in lieu of fractional shares rounded up to
the next whole share. The initial conversion price was $0.25 but was adjusted to $2.50 as a result of the Company’s reverse split
of 1 for 10 on January 19, 2018 and will be subject to further adjustment following the Company’s contemplated 1 for 60 reverse
stock split expected to be effective on January 20, 2023.
Adjustments
to Conversion Price to Prevent Dilution
The
Series A Convertible Preferred Stock is subject to provisions that protect the holders against dilution by adjustment of the conversion
price and/or number of shares of common stock issuable upon conversion in certain events such as a subdivision, combination or reclassification
of our outstanding common stock.
Voting
Rights—Matters Requiring Approval of Holders of Series A Convertible Preferred Stock
Except
as otherwise required by law, the Series A Convertible Preferred Stock shall have no voting rights. However, as long as any shares of
Series A Convertible Preferred Stock are outstanding, we shall not, without the affirmative vote of the holders of a majority of the
then outstanding shares of the Series A Convertible Preferred Stock, (a) alter or change adversely the powers, preferences or rights
given to the Series A Convertible Preferred Stock or alter or amend the certificate of designation, (b) amend its articles of incorporation
or other charter documents in any manner that adversely affects any rights of the holders of Series A Convertible Preferred Stock, (c)
increase the number of authorized shares of Series A Convertible Preferred Stock, or (d) enter into any agreement with respect to any
of the foregoing.
Registration
Rights
The
holders of the Series A Convertible Preferred Stock were granted certain demand registration rights and piggyback registration rights
with respect to the shares of our Common Stock issuable upon conversion of the Series A Preferred Stock and exercise of their associated
warrants, subject to customary cutbacks, blackout periods and other exceptions.
Series
B Convertible Preferred Stock
On
November 8, 2017, we issued 6,600,000 shares of convertible preferred stock, designated as the Series B Convertible Preferred Stock pursuant
to the certificate of designation and rights filed by us with the Secretary of State of the State of Florida, with an aggregate original
purchase price and initial liquidation preference of $3.3 million. Each share of Series B Convertible Preferred Stock was issued for
an amount equal to $0.50 per share, which we refer to as the original purchase price. On August 26, 2022 a certain holder of Series B
Convertible Preferred Stock elected to convert to common stock and, as a result of such conversion, 4,050,000 shares of Series B Convertible
Preferred Stock remain outstanding.
The
following description is a summary of the material provisions of the Series B Convertible Preferred Stock and the certificate of designation
and rights and does not purport to be complete. This summary is subject to and is qualified by reference to all the provisions of the
Series B Convertible Preferred Stock and certificate of designation and rights of Series B Convertible Preferred Stock, including the
definitions of certain terms used in the certificate of designation and rights. We urge you to read this document because it, and not
this description, defines the rights of a holder of the Series B Convertible Preferred Stock. A copy of the form of certificate of designation
and rights that we filed with the Secretary of State of the State of Florida effective November 8, 2017 has been incorporated by reference
as an exhibit to the registration statement of which this prospectus forms a part.
No
Mandatory Redemption Date or Sinking Fund
The
shares of Series B Convertible Preferred Stock do not have a mandatory redemption date and are not subject to any sinking fund. The shares
of Series B Convertible Preferred Stock will remain outstanding indefinitely unless we elect to redeem them under the circumstances described
below in “Redemption” or we otherwise repurchase them or they are converted into shares of our common stock as described
below under “Conversion Rights.”
Dividends
The
shares of Series B Convertible Preferred Stock are entitled to participate in all dividends declared and paid on shares of company common
stock on an “as if” converted basis.
Liquidation
Preference
Upon
any liquidation, dissolution or winding-up of the Company (any such event, a “Liquidation”), whether voluntary or involuntary,
each holder of shares of Series B Convertible Preferred Stock shall be entitled to receive, after payment to the Series C Non-Convertible
Preferred Stock as provided in the Certificate of Designation of Series C Non-Convertible Preferred Stock, but on par with Series A Convertible
Preferred Stock and in preference to the holders of Common Stock, an amount of cash equal to the greater of (i) the product of the number
of shares of Series B Convertible Preferred Stock then held by such holder, multiplied by the original issue price; and (ii) the amount
that would be payable to such holder in the Liquidation in respect of Common Stock issuable upon conversion of such shares of Series
B Convertible Preferred Stock if all outstanding shares of Series B Convertible Preferred Stock were converted into Common Stock immediately
prior to the Liquidation (disregarding for this purpose any and all limitations of any kind on such conversion).
Ranking
The
Series B Convertible Preferred Stock ranks (i) on par with the Common Stock and Series A Convertible Preferred Stock and junior to Series
C Non-Convertible Preferred Stock as to dividend rights and (ii) junior to Series C Non-Convertible Preferred Stock, on par with Series
A Convertible Preferred Stock and senior to the Common Stock as to distributions of assets upon liquidation, dissolution or winding up
of the Corporation, whether voluntarily or involuntarily.
See
“Voting Rights—Matters Requiring Approval of Holders of Series B Convertible Preferred Stock” for a description of
the types of issuances of equity securities and other securities of our company requiring approval of holders of a majority of shares
of Series B Convertible Preferred Stock then outstanding, voting together as a class.
Redemption
To
the extent we have funds legally available therefor, at any time after the fifth anniversary of the original issue date of the Series
B Convertible Preferred Stock, we have the right to redeem all or any portion of the outstanding shares of Series B Convertible Preferred
Stock at the original issue price of $0.50 by providing at least seventy five (75) days written notice of such redemption to all holders
of the then outstanding shares of Series B Convertible Preferred Stock.
Conversion
Rights
The
holders of shares of Series B Convertible Preferred Stock will, at any time, be entitled to convert some or all of their Series B Convertible
Preferred Stock into the number of shares of our common stock obtained by dividing the original purchase price of the shares to be converted
by the aggregate Series B conversion price (which originally equaled the original purchase price, but is subject to adjustment), which
amount we refer to as the conversion price and then multiplying such product by two (2).
The
conversion price will be adjustable upon the occurrence of certain events and transactions to prevent dilution as described under “Adjustments
to Conversion Price to Prevent Dilution.” Any shares of our common stock issued upon conversion of the shares of Series B Convertible
Preferred Stock shall be validly issued, fully paid and non-assessable. The Company shall either pay cash in lieu of fractional shares
or round up to the next whole share. The initial conversion price was $0.50 but was adjusted to $5.00 as a result of the Company’s
reverse split of 1 for 10 on January 19, 2018 and will be subject to further adjustment following the Company’s contemplated 1
for 60 reverse stock split expected to be effective on January 20, 2023.
Adjustments
to Conversion Price to Prevent Dilution
The
Series B Convertible Preferred Stock is subject to provisions that protect the holders against dilution by adjustment of the conversion
price and/or number of shares of common stock issuable upon conversion in certain events such as a subdivision, combination or reclassification
of our outstanding common stock.
Voting
Rights—Matters Requiring Approval of Holders of Series B Convertible Preferred Stock
Except
as otherwise required by law, the Series B Convertible Preferred Stock shall have no voting rights. However, as long as any shares of
Series B Convertible Preferred Stock are outstanding, we shall not, without the affirmative vote of the holders of a majority of the
then outstanding shares of the Series B Convertible Preferred Stock, (a) amend, alter, repeal, restate or supplement (in each case, whether
by reclassification, merger, consolidation, reorganization or otherwise) the certificate of designation in any manner that would adversely
affect the holders of the Series B Convertible Preferred Stock, (b) authorize or agree to authorize any increase in the number of shares
of Series B Convertible Preferred Stock or issue any additional shares of Series B Convertible Preferred Stock, (c) amend, alter or repeal
any provision of the Certificate of Incorporation or Bylaws of the Company which would adversely affect any right, preference, privilege
or voting power of the Series B Convertible Preferred Stock or the holders thereof or (d) agree to take any of the foregoing actions.
Registration
Rights
The
holders of the Series B Convertible Preferred Stock were granted certain demand registration rights and piggyback registration rights
with respect to the shares of our Common Stock issuable upon conversion of the Series B Preferred Stock and exercise of their associated
warrants, subject to customary cutbacks, blackout periods and other exceptions.
Series
C Non-Voting, Non-Convertible Preferred Stock
On
November 8, 2017, we issued to a single older 100 shares of non-convertible preferred stock, designated as the Series C Non-Voting, Non-Convertible
Preferred Stock pursuant to the certificate of designation and rights filed by us with the Secretary of State of the State of Florida,
with a stated value and liquidation preference equal to $33,847.9874 per share, which we refer to as the Stated Value. The shares of
Series C Non-Voting, Non-Convertible Preferred Stock were entitled to payment-in-kind (“PIK”) dividends thereon at the annual
rate of twelve percent (12%) (the “Initial Rate”) of its Stated Value, payable by issuing additional shares of Series C Non-Voting,
Non-Convertible Preferred Stock within thirty days after the end of each calendar year, pro-rata for partial years. During the three
months ended March 31, 2021, the Company provided a notice of redemption, to the holder of the Company’s Series C Preferred Stock
to redeem all outstanding Series C Preferred Stock (which included the dividend of 26.697 shares paid on January 28, 2021 and any accrued
dividends due through the redemption date of March 13, 2021). The Series C Preferred Stock redemption amount of approximately $5.6 million
was paid on March 15, 2021 and all outstanding shares of Series C Preferred Stock were cancelled.
Series
D Preferred Stock-Converted to Common Stock
On
July 13, 2018, our board of directors designated 9,364,000 shares of our preferred stock as Series D Convertible Preferred Stock (“Series
D Preferred Stock”), which were subsequently issued on July 17, 2018, none of which are currently issued and outstanding. The preferences
and rights of the Series D Preferred Stock was set forth in a Certificate of Designation (the “Series D Certificate of Designation”).
Pursuant to a transfer agency agreement between us and Continental Stock Transfer & Trust Company, as transfer agent, the Series
D Preferred Stock was issued in book-entry form and represented only by one or more global certificates deposited with The Depository
Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC. Prior to the
end of 2018, all of 9,364,000 shares of Series D Preferred Stock had converted to common stock and as such, the Company no longer has
any Series D Preferred Stock outstanding.
Registration
Rights
Series
A Preferred Stock Private Placement. Pursuant to the May 10, 2017 Registration Rights Agreement, we granted certain demand registration
rights and piggyback registration rights with respect to the shares of our Common Stock issuable upon conversion of the Series A Preferred
Stock and the exercise of the common stock warrants that were issued commensurate with the issuance of the Series A Preferred Stock.
Series
B Preferred Stock Private Placement. Pursuant to the November 8, 2017 Amended and Restated Registration Right Agreement, we granted
certain demand registration rights and piggyback registration rights with respect to the shares of our Common Stock issuable upon conversion
of the Series B Preferred Stock and the exercise of the common stock warrants that were issued commensurate with the issuance of the
Series B Preferred Stock.. The Amended and Restated Registration Rights Agreement amended the previous registration rights agreement
entered into in connection with our Series A Preferred Stock Financing in May 2017.
The
following descriptions are summaries of the material terms that are included in our amended and restated articles of incorporation (as
amended) and our bylaws (as amended) as well as the specific agreements such descriptions relate to. This summary is qualified in its
entirety by the specific terms and provisions contained in our restated articles of incorporation, bylaws and the specific agreements
described herein, copies of which we have filed as exhibits to the registration statement of which this prospectus is a part, and by
the provisions of applicable law.
Certain
Anti-Takeover Provisions
Florida
Law
We
are not subject to the statutory anti-takeover provisions under Florida law because in our articles of incorporation we have specifically
elected to opt out of both the “control-share acquisitions” (F.S. 607.0902) and the “affiliated transactions”
(F.S. 607.0901) statutes. Since these anti-takeover statutes do not apply to a corporation that has specifically elected to opt out of
such provisions, we would not be able to invoke the protection of such statutes in the event of a hostile takeover attempt.
Articles
of Incorporation and Bylaw Provisions
Our
articles of incorporation and bylaws contain provisions that could have an anti-takeover effect. These provisions include
| |
● |
authorization
of the issuance of “blank check” preferred stock that could be issued by our Board of Directors without shareholder approval
and that may be substantially dilutive or contain preferences or rights objectionable to an acquiror; |
| |
● |
the
ability of the Board of Directors to amend the bylaws without shareholder approval; |
| |
● |
vacancies
on our board may only be filled by the remaining Directors and not our shareholders; and |
| |
● |
requirements
that only our Board, our President or holders of more than 10% of our shares can call a special meeting of shareholders. |
These
provisions in our articles of incorporation and bylaws could delay or discourage transactions involving an actual or potential change
in control of us, including transactions in which shareholders might otherwise receive a premium for their shares over their current
prices. Such provisions could also limit the ability of shareholders to approve transactions that shareholders may deem to be in their
best interests and could adversely affect the price of our common stock.
Listing
of Common Stock
Our
common stock is currently listed on the NYSE American under the trading symbol “OGEN.”
Transfer
Agent and Registrar
The
transfer agent and registrar of our common stock is Continental Stock Transfer & Trust Company, 1 State Street 30th Floor, New York,
New York 10004, telephone: (212) 509-4000.
DESCRIPTION
OF WARRANTS
The
following description, together with the additional information that we include in any applicable prospectus supplement and in any related
free writing prospectus that we may authorize to be distributed to you, summarizes the material terms and provisions of the warrants
that we may offer under this prospectus, which may be issued in one or more series. Warrants may be offered independently or in combination
with other securities offered by any prospectus supplement. While the terms we have summarized below will apply generally to any warrants
that we may offer under this prospectus, we will describe the particular terms of any series of warrants in more detail in the applicable
prospectus supplement. The following description of warrants will apply to the warrants offered by this prospectus unless we provide
otherwise in the applicable prospectus supplement. The applicable prospectus supplement for a particular series of warrants may specify
different or additional terms.
Any
warrants issued under this prospectus may be evidenced by warrant certificates. Warrants also may be issued under an applicable warrant
agreement that we enter into with a warrant agent. We will indicate the name and address of the warrant agent, if applicable, in the
prospectus supplement relating to the particular series of warrants being offered.
We
will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports
that we file with the SEC, the form of warrant and/or the warrant agreement and warrant certificate, as applicable, that contain the
terms of the particular series of warrants we are offering, and any supplemental agreements, before the issuance of such warrants. The
following description summarizes the material terms and provisions of the warrants and is subject to, and qualified in its entirety by
reference to, all the provisions of the form of warrant and/or the warrant agreement and warrant certificate, as applicable, and any
supplemental agreements applicable to a particular series of warrants that we may offer under this prospectus. We urge you to read the
applicable prospectus supplement related to the particular series of warrants that we may offer under this prospectus, as well as any
related free writing prospectuses, and the complete form of warrant and/or the warrant agreement and warrant certificate, as applicable,
and any supplemental agreements, that contain the terms of the warrants.
General
We
will describe in the applicable prospectus supplement the terms of the series of warrants being offered, including:
| |
● |
the
title of such securities; |
| |
● |
the
offering price and aggregate number of warrants offered; |
| |
● |
the
currency or currencies for which the warrants may be purchased; |
| |
● |
if
applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with
each such security or each principal amount of such security; |
| |
● |
if
applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| |
● |
if
applicable, the minimum or maximum amount of such warrants which may be exercise at any one time; |
| |
● |
in
the case of warrants to purchase common stock, the number of shares of common stock, purchasable upon the exercise of one warrant
and the price at which, and the currency in which, these shares may be purchased upon such exercise; |
| |
● |
the
effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants; |
| |
● |
the
dates on which the right to exercise the warrants shall commence or expire; |
| |
● |
the
terms of any rights to redeem or call the warrants; |
| |
● |
the
terms of any rights to force the exercise of the warrants; |
| |
● |
any
provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| |
● |
the
dates on which the right to exercise the warrants will commence and expire; |
| |
● |
the
manner in which the warrant agreements and warrants may be modified; |
| |
● |
a
discussion of any material or special U.S. federal income tax considerations of holding or exercising the warrants; |
| |
● |
the
antidilution provisions of the warrant, if any; |
| |
● |
the
terms of the securities issuable upon exercise of the warrants; and |
| |
● |
any
other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before
exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise,
including: in the case of warrants to purchase common stock, the right to receive dividends, if any, or, payments upon our liquidation,
dissolution or winding up or to exercise voting rights, if any.
Exercise
of Warrants
Each
warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price
that we describe in the applicable prospectus supplement. The warrants may be exercised as set forth in the prospectus supplement relating
to the warrants offered. Unless we otherwise specify in the applicable prospectus supplement, warrants may be exercised at any time up
to the close of business on the expiration date set forth in the prospectus supplement relating to the warrants offered thereby. After
the close of business on the expiration date, unexercised warrants will become void.
Unless
we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants by delivering the warrant
certificate representing the warrants to be exercised together with specified information, and paying the required amount to the warrant
agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the
warrant certificate and in the applicable prospectus supplement the information that the holder of the warrant will be required to deliver
to the warrant agent in connection with the exercise of the warrant.
Upon
receipt of payment and the warrant or warrant certificate, as applicable, properly completed and duly executed at the corporate trust
office of the warrant agent, if any, or any other office, including ours, indicated in the prospectus supplement, we will, as soon as
practicable, issue and deliver the securities purchasable upon such exercise. If less than all of the warrants (or the warrants represented
by such warrant certificate) are exercised, a new warrant or a new warrant certificate, as applicable, will be issued for the remaining
warrants.
Governing
Law
Unless
we otherwise specify in the applicable prospectus supplement, the warrants and any warrant agreements will be governed by and construed
in accordance with the laws of the State of New York.
Enforceability
of Rights by Holders of Warrants
Each
warrant agent, if any, will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship
of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of
warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or
warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder
of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action
its right to exercise, and receive the securities purchasable upon exercise of, its warrants.
DESCRIPTION
OF UNITS
Units
We
may issue units consisting of any combination of our common stock and warrants. We will issue each unit so that the holder of the unit
is also the holder of each security included in the unit. As a result, the holder of a unit will have the rights and obligations of a
holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit
may not be held or transferred separately, at any time or at any time before a specified date.
The
summary below and that contained in any prospectus supplement is qualified in its entirety by reference to all of the provisions of the
unit agreement and/or unit certificate, and depositary arrangements, if applicable. We urge you to read the applicable prospectus supplements
and any related free writing prospectuses related to the units that we may offer under this prospectus, as well as the complete unit
agreement and/or unit certificate, and depositary arrangements, as applicable, that contain the terms of the units.
We
will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports
that we file with the SEC, the form of unit agreement and/or unit certificate, and depositary arrangements, as applicable, that contain
the terms of the particular series of units we are offering, and any supplemental agreements, before the issuance of such units.
The
applicable prospectus supplement, information incorporated by reference or free writing prospectus may describe:
| |
● |
the
designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those
securities may be held or transferred separately; |
| |
● |
any
provisions for the issuance, payment, settlement, transfer, or exchange of the units or of the securities composing the units; |
| |
● |
whether
the units will be issued in fully registered or global form; and |
| |
● |
any
other terms of the units. |
The
applicable provisions described in this section, as well as those described under “Common Stock” and “Warrants”
above, will apply to each unit and to each security included in each unit, respectively
LEGAL
OWNERSHIP OF SECURITIES
We
can issue securities in registered form or in the form of one or more global securities. We describe global securities in greater detail
below. We refer to those persons who have securities registered in their own names on the books that we or any applicable trustee, depositary
or warrant agent maintain for this purpose as the “holders” of those securities. These persons are the legal holders of the
securities. We refer to those persons who, indirectly through others, own beneficial interests in securities that are not registered
in their own names, as “indirect holders” of those securities. As we discuss below, indirect holders are not legal holders,
and investors in securities issued in book-entry form or in street name will be indirect holders.
Book-Entry
Holders
We
may issue securities in book-entry form only, as we will specify in the applicable prospectus supplement. This means securities may be
represented by one or more global securities registered in the name of a financial institution that holds them as depositary on behalf
of other financial institutions that participate in the depositary’s book-entry system. These participating institutions, which
are referred to as participants, in turn, hold beneficial interests in the securities on behalf of themselves or their customers.
Only
the person in whose name a security is registered is recognized as the holder of that security. Global securities will be registered
in the name of the depositary or its participants. Consequently, for global securities, we will recognize only the depositary as the
holder of the securities, and we will make all payments on the securities to the depositary. The depositary passes along the payments
it receives to its participants, which in turn pass the payments along to their customers who are the beneficial owners. The depositary
and its participants do so under agreements they have made with one another or with their customers; they are not obligated to do so
under the terms of the securities.
As
a result, investors in a global security will not own securities directly. Instead, they will own beneficial interests in a global security,
through a bank, broker or other financial institution that participates in the depositary’s book-entry system or holds an interest
through a participant. As long as the securities are issued in global form, investors will be indirect holders, and not legal holders,
of the securities.
Street
Name Holders
We
may terminate a global security or issue securities that are not issued in global form. In these cases, investors may choose to hold
their securities in their own names or in “street name.” Securities held by an investor in street name would be registered
in the name of a bank, broker or other financial institution that the investor chooses, and the investor would hold only a beneficial
interest in those securities through an account he or she maintains at that institution.
For
securities held in street name, we or any applicable trustee or depositary will recognize only the intermediary banks, brokers and other
financial institutions in whose names the securities are registered as the holders of those securities, and we or any such trustee or
depositary will make all payments on those securities to them. These institutions pass along the payments they receive to their customers
who are the beneficial owners, but only because they agree to do so in their customer agreements or because they are legally required
to do so. Investors who hold securities in street name will be indirect holders, not legal holders, of those securities.
Legal
Holders
Our
obligations, as well as the obligations of any applicable trustee or third party employed by us or a trustee, run only to the legal holders
of the securities. We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any
other indirect means. This will be the case whether an investor chooses to be an indirect holder of a security or has no choice because
we are issuing the securities only in global form.
For
example, once we make a payment or give a notice to the holder, we have no further responsibility for the payment or notice even if that
holder is required, under agreements with its participants or customers or by law, to pass it along to the indirect holders but does
not do so. Similarly, we may want to obtain the approval of the holders to amend an indenture, to relieve us of the consequences of a
default or of our obligation to comply with a particular provision of an indenture, or for other purposes. In such an event, we would
seek approval only from the holders, and not the indirect holders, of the securities. Whether and how the legal holders contact the indirect
holders is up to the legal holders.
Special
Considerations for Indirect Holders
If
you hold securities through a bank, broker or other financial institution, either in book-entry form because the securities are represented
by one or more global securities or in street name, you should check with your own institution to find out:
| |
● |
how
it handles securities payments and notices; |
| |
● |
whether
it imposes fees or charges; |
| |
● |
how
it would handle a request for the holders’ consent, if ever required; |
| |
● |
whether
and how you can instruct it to send you securities registered in your own name so you can be a holder, if that is permitted in the
future; |
| |
● |
how
it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to protect
their interests; and |
| |
● |
if
the securities are in book-entry form, how the depositary’s rules and procedures will affect these matters. |
Global
Securities
A
global security is a security that represents one or any other number of individual securities held by a depositary. Generally, all securities
represented by the same global securities will have the same terms.
Each
security issued in book-entry form will be represented by a global security that we issue to, deposit with and register in the name of
a financial institution or its nominee that we select. The financial institution that we select for this purpose is called the depositary.
Unless we specify otherwise in the applicable prospectus supplement, The Depository Trust Company, New York, New York, known as DTC,
will be the depositary for all securities issued in book-entry form.
A
global security may not be transferred to or registered in the name of anyone other than the depositary, its nominee or a successor depositary,
unless special termination situations arise. We describe those situations below under “—Special Situations When a Global
Security Will Be Terminated.” As a result of these arrangements, the depositary, or its nominee, will be the sole registered owner
and legal holder of all securities represented by a global security, and investors will be permitted to own only beneficial interests
in a global security. Beneficial interests must be held by means of an account with a broker, bank or other financial institution that
in turn has an account with the depositary or with another institution that does. Thus, an investor whose security is represented by
a global security will not be a legal holder of the security, but only an indirect holder of a beneficial interest in the global security.
If
the prospectus supplement for a particular security indicates that the security will be issued as a global security, then the security
will be represented by a global security at all times unless and until the global security is terminated. If termination occurs, we may
issue the securities through another book-entry clearing system or decide that the securities may no longer be held through any book-entry
clearing system.
Special
Considerations for Global Securities
As
an indirect holder, an investor’s rights relating to a global security will be governed by the account rules of the investor’s
financial institution and of the depositary, as well as general laws relating to securities transfers. We do not recognize an indirect
holder as a holder of securities and instead deal only with the depositary that holds the global security.
If
securities are issued only as global securities, an investor should be aware of the following:
| |
● |
an
investor cannot cause the securities to be registered in his or her name, and cannot obtain non-global certificates for his or her
interest in the securities, except in the special situations we describe below; |
| |
● |
an
investor will be an indirect holder and must look to his or her own bank or broker for payments on the securities and protection
of his or her legal rights relating to the securities, as we describe above; |
| |
● |
an
investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are required
by law to own their securities in non-book-entry form; |
| |
● |
an
investor may not be able to pledge his or her interest in the global security in circumstances where certificates representing the
securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective; |
| |
● |
the
depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating
to an investor’s interest in the global security; |
| |
● |
we
and any applicable trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership
interests in the global security, nor will we or any applicable trustee supervise the depositary in any way; |
| |
● |
the
depositary may, and we understand that DTC will, require that those who purchase and sell interests in the global security within
its book-entry system use immediately available funds, and your broker or bank may require you to do so as well; and |
| |
● |
financial
institutions that participate in the depositary’s book-entry system, and through which an investor holds its interest in the
global security, may also have their own policies affecting payments, notices and other matters relating to the securities. |
There
may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for
the actions of any of those intermediaries.
Special
Situations When a Global Security Will Be Terminated
In
a few special situations described below, a global security will terminate and interests in it will be exchanged for physical certificates
representing those interests. After that exchange, the choice of whether to hold securities directly or in street name will be up to
the investor. Investors must consult their own banks or brokers to find out how to have their interests in securities transferred to
their own names, so that they will be direct holders. We have described the rights of holders and street name investors above.
Unless
we provide otherwise in the applicable prospectus supplement, a global security will terminate when the following special situations
occur:
| |
● |
if
the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security
and we do not appoint another institution to act as depositary within 90 days; |
| |
● |
if
we notify any applicable trustee that we wish to terminate that global security; or |
| |
● |
if
an event of default has occurred with regard to securities represented by that global security and has not been cured or waived. |
The
applicable prospectus supplement may also list additional situations for terminating a global security that would apply only to the particular
series of securities covered by the prospectus supplement. When a global security terminates, the depositary, and neither we nor any
applicable trustee, is responsible for deciding the names of the institutions that will be the initial direct holders.
PLAN
OF DISTRIBUTION
We
may sell the securities from time to time pursuant to underwritten public offerings, direct sales to the public, direct sales to the
public, negotiated transactions, block trades or a combination of these methods. We may sell the securities to or through underwriters
or dealers, through one or more agents, or directly to one or more purchasers. We may distribute securities from time to time in one
or more transactions:
| |
● |
at
a fixed price or prices, which may be changed; |
| |
● |
at
market prices prevailing at the time of sale; |
| |
● |
at
prices related to such prevailing market prices; |
| |
● |
at
varying prices determined at the time of sale; or |
We
may also sell equity securities covered by this registration statement in an “at the market” offering as defined in Rule
415(a)(4) under the Securities Act. Such offering may be made into an existing trading market for such securities in transactions at
other than a fixed price on or through the facilities of NYSE American or any other securities exchange or quotation or trading service
on which such securities may be listed, quoted or traded at the time of sale. Such at the market offerings, if any, may be conducted
by underwriters acting as principal or agent.
A
prospectus supplement or (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms
of the offering of the securities, including, to the extent applicable:
| |
● |
the
name or names of any underwriters, dealers or agents, if any; |
| |
● |
the
purchase price of the securities and the proceeds we will receive from the sale; |
| |
● |
any
over-allotment options under which underwriters may purchase additional securities from us; |
| |
● |
any
agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation; |
| |
● |
any
public offering price; |
| |
● |
any
discounts or concessions allowed or reallowed or paid to dealers; and |
| |
● |
any
securities exchange or market on which the securities may be listed. |
Only
the agents or underwriters named in each prospectus supplement will be agents or underwriters in connection with the securities offered
by a prospectus supplement.
Offers
to purchase the securities being offered by this prospectus may be solicited directly. Agents may also be designated to solicit offers
to purchase the securities from time to time. Any agent involved in the offer or sale of our securities will be identified in a prospectus
supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
If
a dealer is utilized in the sale of the securities being offered by this prospectus, the securities will be sold to the dealer, as principal.
The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale.
If
an underwriter is utilized in the sale of the securities being offered by this prospectus, an underwriting agreement will be executed
with the underwriter at the time of sale and the name of any underwriter will be provided in the prospectus supplement that the underwriter
will use to make resales of the securities to the public. In connection with the sale of the securities, we, or the purchasers of securities
for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts or commissions. The underwriter
may sell the securities to or through dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions
from the underwriters and/or commissions from the purchasers for which they may act as agent. Unless otherwise indicated in a prospectus
supplement, an agent will be acting on a best efforts basis and a dealer will purchase securities as a principal, and may then resell
the securities at varying prices to be determined by the dealer.
Any
compensation paid to underwriters, dealers or agents in connection with the offering of the securities, and any discounts, concessions
or commissions allowed by underwriters to participating dealers will be provided in the applicable prospectus supplement. Underwriters,
dealers and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities
Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to
be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil liabilities,
including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect thereof and to reimburse
those persons for certain expenses.
Any
common stock will be listed on the NYSE American, but any other securities may or may not be listed on a national securities exchange.
To facilitate the offering of securities, certain persons participating in the offering may engage in transactions that stabilize, maintain
or otherwise affect the price of the securities. This may include over-allotments or short sales of the securities, which involve the
sale by persons participating in the offering of more securities than were sold to them. In these circumstances, these persons would
cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment option, if
any. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the
open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed
if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to
stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. These
transactions may be discontinued at any time.
We
may authorize underwriters, dealers or other persons acting as our agents to solicit offers by certain institutions or other suitable
purchasers to purchase securities from us at the public offering price set forth in the prospectus supplement, pursuant to delayed delivery
contracts providing for payment and delivery on the date stated in each applicable prospectus supplement. Each contract will be for an
amount not less than, and the aggregate amount of securities sold pursuant to such contracts shall not be less nor more than, the respective
amounts stated in each applicable prospectus supplement. Institutions with whom the contracts, when authorized, may be made include commercial
and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and other institutions,
but shall in all cases be subject to our approval. Delayed delivery contracts will be subject only to those conditions set forth in each
applicable prospectus supplement and include the condition that the purchase of the securities covered by the delayed delivery contracts
will not at the time of delivery be prohibited under the laws of any jurisdiction in the United States to which the purchaser is subject.
Each prospectus supplement will set forth any commissions we pay for solicitation of these contracts. The underwriters and agents will
not have any responsibility with respect to the validity or performance of these contracts.
All
securities we may offer, other than common stock, will be new issues of securities with no established trading market. Any agents or
underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time
without notice. We cannot guarantee the liquidity of the trading markets for any securities. There is currently no market for any of
the offered securities, other than our common stock which is listed on the on the NYSE American. Any common stock will be listed on the
NYSE American but any other securities may or may not be listed on a national securities exchange. We have no current plans for listing
of the, warrants on any securities exchange or quotation system; any such listing with respect to any particular warrants will be described
in the applicable prospectus supplement or other offering materials, as the case may be.
Any
agents and underwriters who are qualified market makers on the NYSE American may engage in passive market making transactions in the
securities on the NYSE American in accordance with Regulation M, during the business day prior to the pricing of the offering, before
the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations
and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of
the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however,
the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize
the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued
at any time.
In
addition, we may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties
in privately negotiated transactions. If the applicable prospectus supplement so indicates, in connection with those derivatives, the
third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions.
If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related
open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings
of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be named in
the applicable prospectus supplement (or a post-effective amendment). In addition, we may otherwise loan or pledge securities to a financial
institution or other third party that in turn may sell the securities short using this prospectus and an applicable prospectus supplement.
Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection
with a concurrent offering of other securities.
The
specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
The
underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for
which they receive compensation.
In
compliance with guidelines of the Financial Industry Regulatory Authority, Inc., or FINRA, the maximum compensation to be received by
any FINRA member or independent broker dealer may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus
and any applicable prospectus supplement.
LEGAL
MATTERS
Unless
otherwise indicated in the applicable prospectus supplement, certain legal matters in connection with the offering and the validity of
the securities offered by this prospectus, and any supplement thereto, will be passed upon for us by Shumaker, Loop & Kendrick, LLP.
Additional legal matters may be passed upon for us or any underwriters, dealers or agents, by counsel that we will name in the applicable
prospectus supplement.
EXPERTS
The
audited financial statements of Oragenics, Inc. as of December 31, 2021 and 2020, and for the years ended December 31, 2021 and
2020, as set forth in its report included in our Annual Report on Form 10-K for the year ended December 31, 2021, incorporated by reference
in this prospectus have been audited by Mayer Hoffman McCann P.C., an independent registered public accounting firm, as stated in their
report dated March 24, 2022, which is incorporated by reference herein, and has been so incorporated in reliance upon the report of such
firm given upon their authority as experts in accounting and auditing.
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
This
prospectus is part of a registration statement we filed with the SEC. This prospectus does not contain all of the information set forth
in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities
we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the
registration statement. You should rely only on the information contained in this prospectus or incorporated by reference in this prospectus.
We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any state
where the offer is not permitted. You should not assume that the information in this prospectus is accurate as of any date other than
the date on the front page of this prospectus, regardless of the time of delivery of this prospectus or any sale of the securities offered
by this prospectus.
We
file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the
public at the SEC’s website at http://www.sec.gov.
Copies
of certain information filed by us with the SEC are also available on our website at www.Oragenics.com Information contained in
or accessible through our website does not constitute a part of this prospectus and is not incorporated by reference in this prospectus.
We have included our website address as an inactive textual reference only.
INCORPORATION
OF CERTAIN DOCUMENTS BY REFERENCE
The
SEC allows us to incorporate by reference the information we file with it, which means that we can disclose important information to
you by referring you to another document that we have filed separately with the SEC. You should read the information incorporated by
reference because it is an important part of this prospectus. We incorporate by reference the following information or documents that
we have filed with the SEC, excluding any portions of any Current Report on Form 8-K that are not deemed “filed” pursuant
to the General Instructions of Form 8-K:
| |
● |
Our
Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 24, 2022 and our Form 10-K/A for the
year ended December 31, 2021, filed with the SEC on July 29, 2022; |
| |
● |
Our
Quarterly Reports on Form 10-Q for the quarter ended March 31, 2022, filed with the SEC on May 13, 2022, for the quarter ended June 30, 2022 filed with the SEC on August 9, 2022 and for the quarter ended September 30, 2022 filed with the SEC on November 14, 2022;
|
| |
● |
Our
Definitive Proxy Statement on Schedule 14A, filed with the SEC on October 31, 2022; |
| |
● |
Our
Current Reports on Form 8-K, filed January 26, 2022, February 28, 2022, March 10, 2022, April 6, 2022, April 19, 2022, May 17, 2022,
June 23, 2022, July 8, 2022, August 3, 2022, August 24, 2022, September 30, 2022, October 3, 2022, November 16, 2022, December 15, 2022, December 19, 2022, December 20, 2022, December 22, 2022 and December 23, 2022; |
| |
● |
The
description of our common stock set forth in our registration statement on Form 8-A12B, filed April 8, 2013, including any amendments
or reports filed for purposes of updating such description. |
Any
information in any of the foregoing documents will automatically be deemed to be modified or superseded to the extent that information
in this prospectus or in a later filed document that is incorporated or deemed to be incorporated herein by reference modifies or replaces
such information.
We
also incorporate by reference into this prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of
Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a),
13(c), 14 or 15(d) of the Exchange Act (i) after the date of the initial filing of the registration statement of which this prospectus
forms a part and prior to effectiveness of the registration statement, or (ii) after the date of this prospectus but prior to the termination
of the offering. These documents include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current
Reports on Form 8-K, as well as proxy statements.
We
will provide to each person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request,
a copy of any or all of the documents that are incorporated by reference into this prospectus but not delivered with the prospectus,
including exhibits which are specifically incorporated by reference into such documents. You may request a copy of these filings at no
cost, by writing to or telephoning us at the following address: Oragenics, Inc., 4902 Eisenhower Boulevard, Suite 125, Tampa, Florida
33634, Attention: Corporate Secretary.
Any
statement contained in this prospectus or contained in a document incorporated or deemed to be incorporated by reference into this prospectus
will be deemed to be modified or superseded to the extent that a statement contained in this prospectus or any subsequently filed supplement
to this prospectus, or document deemed to be incorporated by reference into this prospectus, modifies or supersedes such statement.

Up
to $5,000,000 Shares of Common Stock
PROSPECTUS
SUPPLEMENT
February
24, 2023
Ladenburg
Thalmann
Oragenics (AMEX:OGEN)
Graphique Historique de l'Action
De Avr 2024 à Mai 2024
Oragenics (AMEX:OGEN)
Graphique Historique de l'Action
De Mai 2023 à Mai 2024