As filed with the Securities and Exchange Commission on June 6, 2024
Registration Statement No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-3
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
AVALO THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| | | | | |
Delaware (State or other jurisdiction of incorporation or organization) | 45-0705648 (I.R.S. Employer Identification Number) |
540 Gaither Road, Suite 400
Rockville, Maryland 20850
Telephone: (410) 522-8707
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Christopher Sullivan
Chief Financial Officer
Avalo Therapeutics, Inc.
540 Gaither Road, Suite 400
Rockville, Maryland 20850
Telephone: (410) 522-8707
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Andrew J. Gibbons
Alexander M. Donaldson
Wyrick Robbins Yates & Ponton LLP
4101 Lake Boone Trail, Suite 300
Raleigh, North Carolina 27607
Telephone: (919) 781-4000
Fax: (919) 781-4865
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ¨
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ¨
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | |
Large accelerated filer ¨ | | Accelerated filer ¨ |
Non-accelerated filer þ | | Smaller reporting company þ |
| | Emerging growth company ¨ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. ¨
____________________________
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. The selling stockholders may not sell these securities or accept an offer to buy these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and the selling stockholders are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated June 6, 2024
PRELIMINARY PROSPECTUS
Up to 22,357,897 Shares of Common Stock Issuable Upon Conversion of Shares of Series C Non-Voting Convertible Preferred Stock Offered by Selling Stockholders
22,357.897 Shares of Series C Non-Voting Convertible Preferred Stock Offered by Selling Stockholders
Up to 11,967,526 Shares of Common Stock Issuable Upon the Exercise of Warrants Offered by Selling Stockholders
Warrants to Purchase up to 11,967,526 Shares of Common Stock (or 11,967.526 Shares of Series C Non-Voting Convertible Preferred Stock) Offered by Selling Stockholders
This prospectus relates to the sale or other disposition from time to time of (i) up to 22,357,897 shares of our common stock, $0.001 par value per share, issuable upon the conversion of shares of our Series C non-voting convertible preferred stock, (ii) 22,357.897 shares of our Series C non-voting convertible preferred stock, (iii) up to 11,967,526 shares of our common stock issuable upon the exercise of warrants,, and (iv) warrants to purchase up to 11,967,526 shares of our common stock (or 11,967.526 shares of Series C non-voting convertible preferred stock), all held by the selling stockholders named in this prospectus, including their transferees, pledgees, donees or successors. We are not selling any shares of common stock, shares of Series C non-voting convertible preferred stock or warrants to purchase common stock (or Series C non-voting convertible preferred stock) under this prospectus and will not receive any of the proceeds from the sale of such securities by the selling stockholders.
The shares of Series C non-voting convertible preferred stock and warrants were issued (i) to the former stockholders of AlmataBio, Inc. in connection with our acquisition of AlmataBio, Inc. on March 27, 2024, and (ii) to institutional investors in a related private placement of Series C non-voting convertible preferred stock and warrants that closed on March 28, 2024. Subject to receiving the requisite stockholder approval and certain beneficial ownership limitations, each share of Series C non-voting convertible preferred stock will automatically convert upon the requisite stockholder approval into shares of common stock in accordance with the terms of the Series C non-voting convertible preferred stock. See “Prospectus Summary – Recent Events” for more details.
The selling stockholders may sell or otherwise dispose of the securities covered by this prospectus in a number of different ways and at varying prices. While there is no established public trading market for our Series C non-voting convertible preferred stock, we believe the actual offering price in sales of our Series C non-voting convertible preferred stock by the selling stockholders will be derived from the prevailing market price of our common stock at the time of any such sale. We provide more information about how the selling stockholders may sell or otherwise dispose of their securities in the section entitled “Plan of Distribution” beginning on page 19. The selling stockholders will pay all brokerage fees and commissions and similar expenses. We will pay all expenses (except brokerage fees and commissions and similar expenses) relating to the registration of the securities with the Securities and Exchange Commission. No underwriter or other person has been engaged to facilitate the sale of the securities in this offering.
Our common stock is traded on The Nasdaq Capital Market under the symbol “AVTX.” On June 4, 2024, the last reported sale price of our common stock was $11.00 per share. We recommend that you obtain current market quotations for our common stock prior to making an investment decision. The Series C non-voting convertible preferred stock and the warrants are not listed on any exchange, and we do not intend to list the Series C non-voting convertible preferred stock or warrants on any exchange.
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 8 of this prospectus and in the documents incorporated by reference herein, to read about factors you should consider before investing in our securities.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
Prospectus dated , 2024
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
You should rely only on the information that we have provided or incorporated by reference in this prospectus and any prospectus supplement that we may authorize to be provided to you. We have not, and the selling stockholders have not, authorized anyone to provide you with different information. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus or any prospectus supplement that we may authorize to be provided to you. If anyone provides you with different or inconsistent information, you should not rely on it. You should assume that the information in this prospectus and any prospectus supplement or incorporated herein or therein is accurate only as of the date on the cover of such document, regardless of the time of delivery of this prospectus or any prospectus supplement or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates.
We urge you to carefully read this prospectus and any prospectus supplement, together with the information incorporated herein or therein by reference as described under the heading “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference.”
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed or are incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find Additional Information.”
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the information incorporated by reference in this prospectus include “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and Section 27A of the Securities Act of 1933, as amended, or the Securities Act. For these purposes, any statements contained or incorporated by reference herein regarding our strategy, future operations, financial position, product candidates, future revenues, projected costs, prospects, plans and objectives of management, other than statements of historical facts, are forward-looking statements. In some cases, you can identify forward-looking statements by the words “may,” “might,” “can,” “will,” “to be,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “likely,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future, although not all forward-looking statements contain these words. These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We cannot guarantee that we actually will achieve the plans, intentions or expectations expressed or implied in our forward-looking statements. There are a number of important factors that could cause actual results, levels of activity, performance or events to differ materially from those expressed or implied in the forward-looking statements we make.
Examples of risks and uncertainties that could cause actual results to differ materially from historical performance and any forward-looking statements include, but are not limited to, the risks described under the heading “Risk Factors” on page 8 of this prospectus, in our most recent Annual Report on Form 10-K, and subsequent reports filed with the SEC. Given these risks, uncertainties and other factors, many of which are beyond our control, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate, and you should not place undue reliance on these forward-looking statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all.
Any forward-looking statement speaks only as of the date on which it is made. Although we may elect to update forward-looking statements in the future, we specifically disclaim any obligation to do so, except as may be required by law, even if our estimates change, and readers should not rely on our forward-looking statements as representing our views as of any date subsequent to the date the statements were made.
PROSPECTUS SUMMARY
This summary highlights selected information from this prospectus and does not contain all of the information that you need to consider in making your investment decision. You should carefully read this entire prospectus and any prospectus supplement, including the risks of investing in our securities discussed under the heading “Risk Factors” contained in this prospectus and any prospectus supplement, and under similar headings in the other documents that are incorporated by reference into this prospectus or any prospectus supplement. You should also carefully read the information incorporated by reference into this prospectus, including our financial statements, and the exhibits to the registration statement of which this prospectus is a part. Unless the context indicates otherwise, references in this prospectus to “Avalo,” “Company,” “we,” “us” and “our” refer to Avalo Therapeutics, Inc. and its subsidiaries unless the context indicates otherwise.
Company Overview
Avalo Therapeutics, Inc. is a clinical stage biotechnology company focused on the treatment of immune dysregulation. Avalo’s lead asset is AVTX-009, an anti-IL-1β monoclonal antibody (“mAb”), targeting inflammatory diseases. Avalo’s pipeline also includes quisovalimab (anti-LIGHT mAb) and AVTX-008 (BTLA agonist fusion protein).
Recent Events
On March 27, 2024, the Company acquired AVTX-009, through a merger of AlmataBio Inc. (“AlmataBio”) with and into the Company’s wholly owned subsidiary (the “AlmataBio Transaction”). Avalo’s acquisition of AlmataBio was structured as a stock-for-stock transaction whereby all outstanding equity interests in AlmataBio were exchanged in a merger for a combination of Avalo common stock and shares of Avalo Series C non-voting convertible preferred stock, resulting in the issuance of 171,605 shares of Avalo common stock and 2,412 shares of Series C non-voting convertible preferred stock. The Company also made a cash payment of $7.5 million in April 2024 to the former AlmataBio stockholders, which was due upon the initial closing of the private placement investment (described below) on March 28, 2024. A portion of the consideration for the AlmataBio Transaction includes development milestones to the former AlmataBio stockholders, including $5.0 million due upon the first patient dosed in a Phase 2 trial in patients with hidradenitis suppurativa (“HS”) for AVTX-009 and $15.0 million due upon the first patient dosed in a Phase 3 trial for AVTX-009, both of which are payable in cash or common stock of Avalo (or a combination thereof) at the election of the former AlmataBio stockholders. In the absence of timely notice of such election, Avalo may elect to pay the second and third milestones in cash or common stock of Avalo or a combination thereof.
Additionally, on March 28, 2024, the Company closed a private placement investment for up to $185 million in gross proceeds, including an initial upfront gross investment of $115.6 million, whereby Avalo issued (i) 19,945.897 shares of Series C non-voting convertible preferred stock and (ii) warrants to purchase up to an aggregate of 11,967,526 shares of Avalo’s common stock (or up to an aggregate of 11,967.526 shares of Series C non-voting convertible preferred stock), at a per share exercise price of $5.796933 per share of common stock, for an aggregate exercise price of $69.4 million. Net proceeds were approximately $108.1 million after deducting transaction costs.
See the audited financial statements of AlmataBio for the period from April 28, 2023 (date of inception) to December 31, 2023, and the unaudited pro forma financial information of AlmataBio and Avalo for the year ended December 31, 2023, and for the three months ended March 31, 2024, contained in our Current Report on Form 8-K/A, as filed with SEC on June 3, 2024, which are incorporated in this prospectus by reference in their entirety.
The Series C non-voting convertible preferred stock is not convertible into shares of common stock unless and until the Company’s stockholders approve the issuance of the shares of common stock of the Company to be issued upon conversion of the shares of Series C non-voting convertible preferred stock and upon the exercise of the warrants (the “Required Stockholder Approval”). Pursuant to the merger agreement for the AlmataBio Transaction, the Company is obligated to file a proxy statement with the Securities and Exchange Commission (the “SEC”) as soon as practicable after March 27, 2024. Pursuant to the securities purchase agreement for the March 2024 private placement, the Company is obligated to file a proxy statement with SEC for a stockholder meeting to seek the Required Stockholder Approval not later than 75 days after March 28, 2024. If the Required Stockholder Approval is not obtained at that meeting, the Company must hold a stockholder meeting at least once every 90 days until the Required Stockholder Approval is obtained. To that end, the Company plans to file its preliminary proxy materials for the annual meeting prior to or on June 11, 2024 and plans to hold its stockholder meeting promptly thereafter.
Our Strategy
Our strategy for increasing stockholder value includes:
•Advancing our pipeline of compounds through development and to regulatory approval;
•Developing the go-to-market strategy to quickly and effectively market, launch, and distribute each of our compounds that receives regulatory approval;
•Opportunistically out-licensing rights to indications or geographies; and
•Acquiring or licensing rights to targeted, complementary differentiated preclinical and clinical stage compounds.
Pipeline — Overview, Competition, and Intellectual Property
AVTX-009: Anti-IL-1β monoclonal antibody (“mAb”) targeting inflammatory diseases.
Overview: AVTX-009 is a humanized monoclonal antibody (IgG4) that binds to interleukin-1β (“IL-1β”) with high affinity and neutralizes its activity. IL-1β is a central driver in the inflammatory process. Overproduction or dysregulation of IL-1β is implicated in many autoimmune and inflammatory diseases. IL-1β is a major, validated target for therapeutic intervention. There is evidence that inhibition of IL-1β could be effective in HS and a variety of inflammatory diseases in dermatology, gastroenterology, and rheumatology.
Competition: As of the date of this prospectus, and to our knowledge, AVTX-009 is one of three anti-IL-1β antibodies in clinical development worldwide. Currently, worldwide there are two drugs approved for hidradenitis suppurativa (“HS”).
License: AVTX-009 is being developed through a world-wide exclusive license from Eli Lilly and Company (“Lilly”) (the “Lilly License Agreement”). Avalo obtained the rights to AVTX-009, including the world-wide exclusive license from Lilly, pursuant to its acquisition of AlmataBio in the first quarter of 2024. AlmataBio had previously purchased the rights, title and interest in the asset from Leap Therapeutics, Inc. (“Leap”) in 2023.
Avalo is required to pay up to $70 million based on the achievement of specified development and regulatory milestones. Upon commercialization, the Company is required to pay sales-based milestones aggregating up to $720 million. Additionally, Avalo is required to pay royalties during a country-by-country royalty term equal to a mid-single digit-to-low double digit of Avalo or its sublicensees’ annual net sales.
Pursuant to the AlmataBio Transaction, the Company made a cash payment of $7.5 million in April 2024 to the former AlmataBio stockholders which was due upon the initial closing of the private placement investment on March 28, 2024. Further, as noted above, a portion of the consideration for the AlmataBio Transaction includes development milestones to the former AlmataBio stockholders including $5.0 million due upon the first patient dosed in a Phase 2 trial in patients with hidradenitis suppurativa for AVTX-009 and $15.0 million due upon the first patient dosed in a Phase 3 trial for AVTX-009, both of which are payable in cash or stock of Avalo (or a combination thereof) at the election of the former AlmataBio stockholders. In the absence of timely notice of such election, Avalo may elect to pay the second and third milestones in cash or Common Stock of Avalo or a combination thereof.
Avalo is responsible for the development and commercialization of the AVTX-009 program.
Market, Data, and Patent Exclusivity: If we receive marketing approval, we expect to receive biologics data exclusivity in the United States, which would provide twelve years of data exclusivity in the United States from the date of FDA approval.
Quisovalimab (AVTX-002): Anti-LIGHT mAb targeting immune-inflammatory diseases.
Overview: Quisovalimab is a fully human mAb, directed against human LIGHT (Lymphotoxin-like, exhibits Inducible expression, and competes with HSV Glycoprotein D for Herpesvirus Entry Mediator (“HVEM”), a receptor expressed by T lymphocytes; also referred to as TNFSF14). There is increasing evidence that the dysregulation of the LIGHT-signaling network which includes LIGHT, its receptor HVEM and LTβR and the downstream checkpoint BTLA, is a disease-driving mechanism in autoimmune and inflammatory reactions in barrier organs. Therefore, Avalo believes reducing LIGHT levels can moderate immune dysregulation in many acute and chronic inflammatory disorders.
•Quisovalimab has shown a rapid and sustained reduction of LIGHT levels, as well as a favorable safety and tolerability profile, in all indications studied including COVID-19 acute respiratory distress syndrome (“ARDS”), Crohn’s Disease and non-eosinophilic asthma (“NEA”).
•Quisovalimab was statistically significant in reducing respiratory failure and mortality in patients hospitalized with COVID-19 ARDS. Quisovalimab also demonstrated positive trends in an open-label study of Crohn’s Disease.
•A post-hoc analysis of the Company’s Phase 2 randomized, double-blind placebo-controlled trial in patients with poorly controlled NEA showed a sub-population of NEA patients with baseline LIGHT levels of over 125 pg/mL, which represented over 50% of patients, and had an approximate 50% reduction in asthma-related events for patients treated with quisovalimab compared to placebo.
•Avalo is conducting a strategic review of the quisovalimab program.
Competition: As of the date of this prospectus, and to our knowledge, quisovalimab is the only anti-LIGHT mAb in clinical development in the United States.
License: On March 25, 2021, the Company entered into a license agreement with Kyowa Kirin Co., Ltd. (“KKC”) for exclusive worldwide rights to develop, manufacture and commercialize quisovalimab for all indications (the “KKC License Agreement”). The KKC License Agreement replaced the Amended and Restated Clinical Development and Option Agreement between the Company and KKC dated May 28, 2020.
Under the KKC License Agreement, the Company paid KKC an upfront license fee of $10 million. Avalo is also required to pay KKC up to an aggregate of $112.5 million based on the achievement of specified development and regulatory milestones. Upon commercialization, the Company is required to make milestone payments to KKC aggregating up to $75 million tied to the achievement of annual net sales targets.
Additionally, the Company is required to pay KKC royalties during a country-by-country royalty term equal to a mid-teen percentage of annual net sales. The Company is required to pay KKC a double-digit percentage (less than 30%) of the payments that the Company receives from sublicensing its rights under the KKC License Agreement, subject to certain exclusions. Avalo is responsible for the development and commercialization of quisovalimab in all indications worldwide (other than the option in the KKC License Agreement that, upon exercise by KKC, allows KKC to develop, manufacture and commercialize quisovalimab in Japan). In addition to the KKC License Agreement, Avalo is responsible for making additional royalty payments upon commercialization of up to an amount of less than 10% of net sales.
Market, Data, and Patent Exclusivity: If we receive marketing approval, we expect to receive biologics data exclusivity in the United States, which would provide twelve years of data exclusivity in the United States from the date of FDA approval. Additionally, patents exclusively licensed from KKC may provide exclusivity in the United States through 2028 absent any extension, and additional patent applications filed by us covering certain methods of using quisovalimab, if issued and properly maintained, should provide additional exclusivity in certain indications through 2043, absent any extension.
AVTX-008: Fully human B and T Lymphocyte Attenuator agonist fusion protein targeting immune dysregulation disorders.
Overview: AVTX-008 is a fully human B and T Lymphocyte Attenuator (“BTLA”) agonist fusion protein.
•AVTX-008 is uniquely positioned as a fusion protein with high-binding affinity and serum stability. AVTX-008 is differentiated by having specific binding to BTLA, with no binding to LIGHT or CD160.
•Avalo is conducting a strategic review of the AVTX-008 program.
Competition: As of the date of this prospectus, and to our knowledge, worldwide there are a total of five BTLA agonist antibodies in clinical development for the treatment of autoimmune diseases.
License: On June 21, 2021, the Company entered into an Exclusive Patent License Agreement with Sanford Burnham Prebys Medical Discovery Institute (the “Sanford Burnham Prebys License Agreement”) under which Avalo obtained an exclusive license to a portfolio of issued patents and patent applications covering AVTX-008.
Under the Sanford Burnham Prebys License Agreement, Avalo paid a mid-six digit upfront license fee and pays mid-five digit annual maintenance fees. Avalo is also required to pay Sanford Burnham Prebys up to approximately $24 million based on the achievement of specified development and regulatory milestones. Upon commercialization, the Company is required to pay Sanford Burnham Prebys sales-based milestones aggregating up to $50 million tied to the achievement of annual net sales targets. Additionally, the Company is required to pay Sanford Burnham Prebys royalties during a country-by-country royalty term equal to a low-to-mid single digit percentage of annual net sales. Avalo is also required to pay Sanford Burnham Prebys a tiered low-double digit percentage of payments that Avalo receives from sublicensing of its rights under the Sanford Burnham Prebys License Agreement, subject to certain exclusions. Avalo is responsible for the development and commercialization of the program.
Market, Data, and Patent Exclusivity: If we receive marketing approval, we expect to receive biologics data exclusivity in the United States, which would provide twelve years of data exclusivity in the United States from the date of FDA approval. Additionally, patents exclusively licensed from Sanford Burnham Prebys may provide exclusivity in the United States through 2036 absent any extension.
Corporate Information
We were incorporated in Delaware in 2011 and commenced operations in the second quarter of 2011. Our principal executive offices are located at 540 Gaither Road, Suite 400, Rockville, Maryland 20850 and our telephone number is (410) 522‑8707. Our website address is www.avalotx.com. The information contained on, or that can be accessed through, our website is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
“Avalo”, the Avalo logo and other trademarks or service marks of Avalo Therapeutics, Inc. appearing in this prospectus are the property of Avalo Therapeutics, Inc. Our company is a “smaller reporting company” as defined in Rule 12b-2 of the Exchange Act, and we have elected to take advantage of certain of the scaled disclosure available to smaller reporting companies under the Exchange Act.
THE OFFERING
Up to 22,357,897 Shares of Common Stock Issuable Upon Conversion of Shares of Series C Non-Voting Convertible Preferred Stock
22,357.897 Shares of Series C Non-Voting Convertible Preferred Stock
Up to 11,967,526 Shares of Common Stock Issuable Upon the Exercise of Warrants Offered by Selling Stockholders
Warrants to Purchase up to 11,967,526 Shares of Common Stock (or 11,967.526 Shares of Series C Non-Voting Convertible Preferred Stock)
This prospectus relates to the resale or other disposition from time to time of (i) up to 22,357,897 shares of our common stock issuable upon the conversion of shares of our Series C non-voting convertible preferred stock, (ii) 22,357.897 shares of our Series C non-voting convertible preferred stock, (iii) up to 11,967,526 shares of our common stock issuable upon the exercise of warrants, and (iv) warrants to purchase up to 11,967,526 shares of our common stock (or 11,967.526 shares of Series C non-voting convertible preferred stock), all held by the selling stockholders named in this prospectus, including their transferees, pledgees, donees or successors. All of the securities being offered were issued between March 27 and March 28, 2024, (i) to former stockholders of AlmataBio and (ii) to certain investors in our private placement, all of whom are identified as selling stockholders hereunder.
| | | | | | | | |
Common stock offered by the selling stockholders (1) | | 34,325,423 |
Common stock outstanding before the offering (2) | | 1,034,130 |
| Common stock to be outstanding after the offering | | 35,359,553 |
| Common stock Nasdaq Capital Market Symbol | | AVTX |
(1) Consists of shares of common stock issuable upon (i) the conversion of Series C non-voting convertible preferred stock and (ii) the exercise of warrants to purchase shares of our common stock held by the selling stockholders.
(2) Based on the number of shares outstanding as of May 15, 2024.
Use of Proceeds
The (i) up to 22,357,897 shares of our common stock issuable upon the conversion of shares of our Series C non-voting convertible preferred stock, (ii) 22,357.897 shares of our Series C non-voting convertible preferred stock, (iii) up to 11,967,526 shares of our common stock issuable upon the exercise of warrants, and (iv) warrants to purchase up to 11,967,526 shares of our common stock (or 11,967.526 shares of Series C non-voting convertible preferred stock)that are being offered for resale by the selling stockholders will be sold for the accounts of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of such securities offered for resale hereby will go to the selling stockholders and we will not receive any proceeds from the resale of those securities by the selling stockholders.
We may receive up to a total of $69,374,946 in gross proceeds if all of the warrants to purchase up to 11,967,526 shares of our common stock are exercised for cash for shares of common stock. Of the gross proceeds we may receive, we will owe a commission fee of 2.5% of such proceeds to our placement agent. However, as we are unable to predict the timing or amount of potential exercises of the warrants, we have not allocated any proceeds of such exercises to any particular purpose. Accordingly, all such proceeds are allocated to working capital.
We will incur all costs associated with this registration statement and prospectus.
Dividend Policy
We have never paid dividends on our capital stock and do not anticipate paying any dividends for the foreseeable future.
Risk Factors
Investing in our securities involves a high degree of risk. Please read the information contained under the heading “Risk Factors” on page 8 of this prospectus and in any report incorporated by reference herein.
RISK FACTORS
Investing in our securities involves a high degree of risk. Before purchasing any securities, you should consider carefully the risks and uncertainties described in the section entitled “Risk Factors” contained in our Annual Report on Form 10-K for the year ended December 31, 2023, as filed with SEC on March 29, 2024, which are incorporated in this prospectus by reference in their entirety, as well as in subsequently filed SEC reports and any prospectus supplement. These risks and uncertainties are not the only risks and uncertainties we face. Additional risks and uncertainties not currently known to us, or that we currently view as immaterial, may also impair our business. If any of the risks or uncertainties described in our SEC filings or any additional risks and uncertainties actually occur, our business, financial condition, results of operations and cash flow could be materially and adversely affected. In that case, the trading price of our common stock and the value of our Series C non-voting convertible preferred stock and the warrants to purchase shares of our common stock could decline and you might lose all or part of your investment. Please also refer to the section entitled “Special Note Regarding Forward-Looking Statements.”
Risks Related to this Offering
Pursuant to the terms of the securities purchase agreement for the March 2024 private placement, we are required to recommend that our stockholders approve the conversion of all outstanding shares of our Series C non-voting convertible preferred stock into shares of our common stock and the ability to issue shares of common stock upon the exercise of the warrants. We cannot guarantee that our stockholders will approve this matter, and if they fail to do so, it might have an adverse impact on our operations and the value and liquidity of your investment might be impaired.
Under the terms of the securities purchase agreement for the March 2024 private placement, we agreed to use our best efforts to obtain the Required Stockholder Approval to allow (i) the conversion of all outstanding shares of Series C non-voting convertible preferred stock issued in the AlmataBio Transaction and the March 2024 private placement and (ii) the ability of all warrants issued in the March 2024 private placement to be exercised for shares of common stock, as required by the Nasdaq listing rules, at a meeting of our stockholders and, if such approval is not obtained at that meeting, to seek to obtain such approval at a stockholders meeting to be held at least every 90 days thereafter until such approval is obtained. If the Required Stockholder Approval is not obtained at a meeting, the need to hold one or more additional meetings would be time consuming and costly. In addition, the failure to receive the Required Stockholder Approval could impair the public perception of our Company and our securities and could have an adverse impact on the liquidity and value of the Series C non-voting convertible preferred stock and the warrants, as well as on the value of our common stock.
We do not intend to apply for any listing of the Series C non-voting convertible preferred stock or the warrants on any exchange or nationally recognized trading system, and we do not expect a robust trading market to develop for the Series C non-voting convertible preferred stock or the warrants.
We do not intend to apply for any listing of the Series C non-voting convertible preferred stock or the warrants on the Nasdaq Capital Market or any other securities exchange or nationally recognized trading system, and we do not expect a robust trading market to develop for the Series C non-voting convertible preferred stock or the warrants. Without an active market, the liquidity of the Series C non-voting convertible preferred stock and the warrants will be limited. Further, the existence of the Series C non-voting convertible preferred stock and the warrants may act to reduce both the trading volume and the trading price of our common stock.
The Series C non-voting convertible preferred stock and the warrants are not convertible or exercisable for shares of our common stock without the approval of our stockholders and therefore may not have any value.
The Series C non-voting convertible preferred stock is convertible into shares of our common stock and the warrants are exercisable for shares of our common stock only upon the approval of the Stock Issuance Proposal. If we do not obtain approval of the Stock Issuance Proposal, then the Series C Preferred Stock will remain outstanding in accordance with its terms, the Warrants will remain outstanding and exercisable for shares of Series C Preferred Stock, and the Milestone payments would only be payable in cash. If the Series C Preferred Stock cannot convert to shares of Common Stock, there may be less reason for the holders of the Warrants to exercise them as to our knowledge there currently is no market for the Series C Preferred Stock nor do we expect a robust market to develop. This would reduce the ability of the Company to benefit from the receipt of cash proceeds from the exercise of the Warrants.
The warrants are speculative in nature and may not have any value.
The warrants will expire in accordance with their terms (see “Description of Securities to be Registered – Warrants – March 2024 Warrants”), and during that time the holders of the warrants may exercise their right to acquire our common stock and
pay an exercise price of $5.796933 per share. There can be no assurance that the market price of our common stock will exceed the exercise price of the warrants, and consequently, whether it will be profitable for holders of the warrants to exercise the warrants.
Except as provided in the terms of the Series C non-voting convertible preferred stock and the warrants, holders of Series C non-voting convertible preferred stock and warrants purchased in this offering will have no rights as stockholders of common stock until such holders convert their shares of Series C non-voting convertible preferred stock or exercise their warrants and acquire our common stock.
Except as provided therein, the Series C non-voting convertible preferred stock and the warrants offered in this offering do not confer voting rights, but rather represent (i) the right to acquire shares of our common stock at a conversion ratio or exercise price following the Required Stockholder Approval and (ii) the right to receive dividends equal to and in the same form, and in the same manner, based on the then-current conversion ratio as dividends actually paid on shares of Common Stock. Only upon any conversion of the Series C non-voting convertible preferred stock or any exercise of the warrants would the holders thereof be entitled to exercise the voting rights of a holder of common stock and then only as to matters for which the record date occurs after the conversion or exercise date.
A substantial number of shares of our common stock may be sold in this offering, which could cause the price of our common stock to decline.
In this offering, the 34,325,423 shares of Common Stock issuable upon conversion of the Series C Preferred Stock and upon the exercise of the Warrants will represent approximately 97% of the shares of Common Stock outstanding on May 15, 2024 on an as converted-basis. The sale of a substantial number of shares of our securities in the public market, or the perception that such sales may occur, could adversely affect the price of our common stock on the Nasdaq Capital Market. We cannot predict the effect, if any, that market sales of those shares of common stock or the availability of those shares of common stock for sale will have on the market price of our common stock.
In addition, in the future, we may also issue shares of our common stock in connection with investments or acquisitions. The amount of shares of our common stock issued in connection with an investment or acquisition could substantially increase our shares of common stock outstanding, which could adversely affect the price of our common stock on the Nasdaq Capital Market.
The price of our common stock could be subject to rapid and substantial volatility. Such volatility, including any stock run-ups, may be unrelated to our actual or expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our common stock. Volatility in our common stock price may subject us to securities litigation.
The market for our common stock may have, when compared to seasoned issuers, significant price volatility and we expect that the price of our shares of common stock may continue to be more volatile than that of a seasoned issuer for the indefinite future. As a relatively small-capitalization company with a relatively small public float, we may experience greater share price volatility, extreme price run-ups, lower trading volume, and less liquidity than large-capitalization companies. In particular, our common stock may be subject to rapid and substantial price volatility, low volumes of trades, and large spreads in bid and ask prices. Such volatility, including any stock run-ups, may be unrelated to our actual or expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our common stock.
In addition, if the trading volumes of our common stock are low, persons buying or selling in relatively small quantities may easily influence the price of our common stock. This low volume of trades could also cause the price of our common stock to fluctuate greatly, with large percentage changes in price occurring in any trading day session. Holders of our common stock may also not be able to readily liquidate their investment or may be forced to sell at depressed prices due to low volume trading. Broad market fluctuations and general economic and political conditions may also adversely affect the market price of our common stock. As a result of this volatility, investors may experience losses on their investment in our common stock. A decline in the market price of our common stock also could adversely affect our ability to issue additional common stock or other securities and our ability to obtain additional financing in the future.
To the extent that a secondary market for the Series C non-voting convertible preferred stock or the warrants develops, we believe that the market price of the Series C non-voting convertible preferred stock and the warrants would be significantly affected by the market price of our common stock. No assurance can be given that an active market in our Series C non-voting convertible preferred stock or the warrants will develop or be sustained. If an active market does not develop, holders of our Series C non-voting convertible preferred stock or the warrants may be unable to readily sell the securities they hold or may not be able to sell their securities at all.
In addition, in the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities to the Company and could divert our management’s attention and resources.
Because we do not intend to declare cash dividends on our shares of common stock in the foreseeable future, stockholders must rely on appreciation of the value of our common stock for any return on their investment.
We have never declared or paid cash dividends on our common stock. The continued operation and expansion of our business will require substantial funding. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. Accordingly, we do not anticipate that we will pay any cash dividends on shares of our common stock for the foreseeable future. Consequently, stockholders must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any gains on their investment. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend upon results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant. Further, the expected lack of a liquid market for the Series C non-voting convertible preferred stock and the warrants might impair their value and their ability to appreciate in value.
If we are not able to comply with the applicable continued listing requirements or standards of The Nasdaq Stock Market, Nasdaq could delist our common stock.
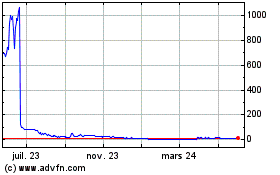
Our common stock is currently listed on The Nasdaq Stock Market. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, a minimum closing bid price of $1.00 per share, and certain corporate governance requirements. There can be no assurances that we will be able to comply with the applicable listing standards. For example, on August 8, 2023, Nasdaq notified us that we failed the $1.00 minimum bid price requirement and the $35 million minimum Market Value of Listed Securities (“MVLS”) requirement. The Company affected a 1-for-240 reverse stock split on December 28, 2023, which has allowed its common stock to trade above $1.00 since December 29, 2023. On January 30, 2024, the Company received written notification from Nasdaq confirming that the Company had regained compliance with the Bid Price Rule. Nasdaq also notified the Company that it is subject to a mandatory panel monitor for a period of one year from January 30, 2024. If, within the one-year monitoring period, Nasdaq finds the Company again out of compliance with the Bid Price Rule, then notwithstanding Nasdaq Rule 5810(c)(2), the Company will not be permitted to provide Nasdaq with a plan of compliance with respect to that deficiency and Nasdaq will not be permitted to grant additional time for the Company to regain compliance with respect to that deficiency, nor will the Company be afforded an applicable cure or compliance period pursuant to Nasdaq Rule 5810(c)(3). Instead, Nasdaq will issue a Delist Determination Letter and the Company will have an opportunity to request a new hearing with the initial Nasdaq panel assigned to the Company for its recent noncompliance or newly convened hearings panel if the initial panel is unavailable. The Company will have the opportunity to respond to the hearings panel as provided by Nasdaq Rule 5815(d)(4)(C). If the Company fails to satisfy the Nasdaq panel, its securities would be delisted from Nasdaq. There can be no assurance that we will continue to maintain such requirement or remain in compliance with any other Nasdaq listing requirements.
Further, on May 20, 2024, we received a written notice from Nasdaq indicating that the Company no longer complies with the requirement under Nasdaq Listing Rule 5550(b)(1) to maintain a minimum of $2,500,000 in stockholders equity for continued listing on the Nasdaq Capital Market (the “Stockholders’ Equity Requirement) because the Company reported stockholders’ equity of negative $112.6 million in its Form 10-Q for the period ended March 31, 2024, and, as of the date of the written notice, the Company did not meet the alternatives of market value of listed securities or net income from continuing operations (together with the Stockholders’ Equity Requirement, the “Listing Rule”). In accordance with the Nasdaq Listing Rules, the Company has 45 calendar days, until July 5, 2024, to submit a plan to regain compliance, which the Company plans to timely submit for consideration by the Nasdaq Listing Qualification staff (“Staff”). If the plan is accepted, the Staff may grant the Company an extension period of up to 180 calendar days from the date of the notice to evidence compliance. Although this notice from Nasdaq has no immediate effect on the listing of the Company’s common stock, if the Staff does not accept the Company’s plan or if the Company is unable to regain compliance within any extension period granted by the Staff, the Staff would be required to issue a delisting determination.
There can be no assurance that Nasdaq will accept the Company’s plan to regain compliance with the Listing Rule or, if accepted, that the Company will evidence compliance with the Listing Rule during any extension period that Nasdaq may grant.
In the event that our common stock is delisted from The Nasdaq Stock Market and is not eligible for quotation or listing on another market or exchange, trading of our common stock could be conducted only in the over‑the‑counter market or on an
electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on an exchange.
A delisting would also likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we may take actions to restore our compliance with The Nasdaq Stock Market’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below The Nasdaq Stock Market minimum bid price requirement or prevent non‑compliance with The Nasdaq Stock Market’s listing requirements.
USE OF PROCEEDS
The (i) up to 22,357,897 shares of our common stock issuable upon the conversion of shares of our Series C non-voting convertible preferred stock, (ii) 22,357.897 shares of our Series C non-voting convertible preferred stock, (iii) up to 11,967,526 shares of our common stock issuable upon the exercise of warrants, and (iv) warrants to purchase up to 11,967,526 shares of our common stock (or 11,967.526 shares of Series C non-voting convertible preferred stock) are being offered for resale by the selling stockholders and will be sold for the accounts of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of such securities offered for resale hereby will go to the selling stockholders and we will not receive any proceeds from the resale of those securities by the selling stockholders.
We may receive up to a total of $69,374,946 in gross proceeds if all of the warrants to purchase up to 11,967,526 shares of our common stock are exercised for cash for shares of common stock. Of the gross proceeds we may receive, we will owe a commission fee of 2.5% of such proceeds to our placement agent. However, as we are unable to predict the timing or amount of potential exercises of the warrants, we have not allocated any proceeds of such exercises to any particular purpose. Accordingly, all such proceeds are allocated to working capital.
We will incur all costs associated with this registration statement and prospectus.
SELLING STOCKHOLDERS
The following table sets forth certain information regarding the selling stockholders and the shares of common stock beneficially owned by them, which information is available to us as of May 15, 2024. The selling stockholders may offer the shares of common stock, the shares of Series C non-voting convertible preferred stock and the warrants under this prospectus from time to time and may elect to sell under this prospectus some, all or none of the shares and warrants offered for resale by this prospectus. However, for the purposes of the table below, we have assumed that, after completion of the offering, none of the securities covered by this prospectus will be held by the selling stockholders. In addition, a selling stockholder may have sold, transferred or otherwise disposed of all or a portion of that holder’s shares of common stock, Series C non-voting convertible preferred stock or warrants since the date on which the selling stockholder provided information for this table. We have not made independent inquiries about such transfers or dispositions. See the section entitled “Plan of Distribution” beginning on page 19.
Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act. The percentage of shares beneficially owned prior to the offering is based on 1,034,130 shares of our common stock outstanding as of May 15, 2024.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Warrants | | Shares of Series C Non-Voting Convertible Preferred Stock | | Shares of Common Stock |
| Name | | Warrants with the following number of underlying shares beneficially owned prior to offering | Warrants with the following number of underlying shares registered for sale hereby | Warrants with the following number of underlying shares owned after this offering | | Number of shares of preferred stock beneficially owned prior to offering | Maximum number of shares of preferred stock registered for sale hereby | Number of shares of preferred stock owned after this offering | Percentage of preferred stock beneficially owned after offering(1) | | Number of shares of common stock beneficially owned prior to offering | Maximum Number of shares of common stock registered for sale hereby | Number of shares of common stock beneficially owned after offering | Percentage of common stock beneficially owned after offering(1) |
Justin DiMartino (2) | | — | — | — | | 292 | 292 | — | * | | 312,779 | 292,000 | 20,779 | * |
Patrick J. Crutcher (2) | | — | — | — | | 513 | 513 | — | * | | 549,467 | 513,000 | 36,467 | * |
Mellisa Huhn (2) | | — | — | — | | 9 | 9 | — | * | | 9,641 | 9,000 | 641 | * |
Naveen Daryani (2) | | — | — | — | | 7 | 7 | — | * | | 7,504 | 7,000 | 504 | * |
Navneet Kumar (2) | | — | — | — | | 8 | 8 | — | * | | 8,605 | 8,000 | 605 | * |
Tatyana Touzova (2) | | — | — | — | | 101 | 101 | — | * | | 108,213 | 101,000 | 7,213 | * |
Emily Nixon(2) | | — | — | — | | 5 | 5 | — | * | | 5,403 | 5,000 | 403 | * |
Emerald Bioventures, LLC (2) | | — | — | — | | 821 | 821 | — | * | | 879,345 | 821,000 | 58,345 | * |
Boothbay Absolute Return Strategies, LP (3) | | 138,797 | 138,797 | — | | 313.330 | 313.330 | — | * | | 459,430 | 452,127 | 7,303 | * |
Boothbay Diversified Alpha Master Fund, LP (4) | | 67,613 | 67,613 | — | | 155.689 | 155.689 | — | * | | 227,085 | 223,302 | 3,783 | * |
Ikarian Healthcare Master Fund, LP (5) | | 569,861 | 569,861 | — | | 1,480.770 | 1,480.770 | — | * | | 2,094,274 | 2,050,631 | 43,643 | * |
Commodore Capital Master LP (6) | | 2,264,128 | 2,264,128 | — | | 3,773.547 | 3,773.547 | — | * | | 6,077,675 | 6,037,675 | 40,000 | * |
TCG Crossover Fund II, L.P. (7) | | 2,264,128 | 2,264,128 | — | | 3,773.547 | 3,773.547 | — | * | | 6,037,675 | 6,037,675 | — | * |
Biotechnology Value Fund, L.P. (8) | | 1,027,637 | 1,027,637 | — | | 1,712.730 | 1,712.730 | — | * | | 2,740,367 | 2,740,367 | — | * |
Biotechnology Value Fund II, L.P. (9) | | 809,027 | 809,027 | — | | 1,348.379 | 1,348.379 | — | * | | 2,157,406 | 2,157,406 | — | * |
Biotechnology Value Trading Fund OS LP (10) | | 75,842 | 75,842 | — | | 126.405 | 126.405 | — | * | | 202,247 | 202,247 | — | * |
MSI BVF SPV, LLC (11) | | 28,173 | 28,173 | — | | 46.956 | 46.956 | — | * | | 75,129 | 75,129 | — | * |
OrbiMed Private Investments IX, LP (12) | | 1,035,030 | 1,035,030 | — | | 1,725.050 | 1,725.050 | — | * | | 2,760,080 | 2,760,080 | — | * |
OrbiMed Genesis Master Fund, L.P. (13) | | 517,515 | 517,515 | — | | 862.525 | 862.525 | — | * | | 1,380,040 | 1,380,040 | — | * |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
RA Capital Healthcare Fund, L.P. (14) | | 1,293,787 | 1,293,787 | — | | 2,156.313 | 2,156.313 | — | * | | 3,450,100 | 3,450,100 | — | * |
Deep Track Biotechnology Master Fund, Ltd. (15) | | 1,164,408 | 1,164,408 | — | | 1,940.682 | 1,940.682 | — | * | | 3,105,090 | 3,105,090 | — | * |
Petrichor Opportunities Fund I LP (16) | | 224,827 | 224,827 | — | | 374.714 | 374.714 | — | * | | 599,541 | 599,541 | — | * |
Petrichor Opportunities Fund I Intermediate LP (17) | | 98,618 | 98,618 | — | | 164.365 | 164.365 | — | * | | 262,983 | 262,983 | — | * |
Logos Opportunities Fund IV LP (18) | | 323,446 | 323,446 | — | | 539.079 | 539.079 | — | * | | 862,525 | 862,525 | — | * |
Dellora Investments Master Fund LP (19) | | 64,689 | 64,689 | — | | 107.816 | 107.816 | — | * | | 172,505 | 172,505 | — | * |
| | | | | | | | | | | | | | | | | | | | |
| * | | Represents beneficial ownership of less than one percent of the outstanding shares of our common stock. |
| | | | | | |
| (1) | | | The Series C non-voting convertible preferred stock and the warrants held by the certain of the selling stockholder are subject to a beneficial ownership limitation of 4.99% or 9.99% as may be designated in the selling stockholder’s related footnote, which does not permit that portion of the Series C non-voting convertible preferred stock or the warrants that would result in the selling stockholder and its affiliates owning, after conversion or exercise, a number of shares of common stock in excess of the beneficial ownership limitation. The amount of shares beneficially owned by the selling stockholder before the offering does not give effect to the beneficial ownership limitation. |
| | | | | | |
| (2) | | | Consists of shares of (i) shares of common stock issued pursuant to the Almata Merger and (ii) common stock issuable upon the conversion of Series C non-voting convertible preferred stock issued to the selling stockholder on March 27, 2024, which the holder cannot dispose of until September 27, 2024. |
| | | | | | |
| (3) | | | Consists of (i) 82 shares of Series C non-voting convertible preferred stock, which are convertible into 82,000 shares of common stock, issued to the selling stockholder on March 27, 2024, which the holder cannot dispose of until September 27, 2024, (ii) 231.330 shares of Series C non-voting convertible preferred stock, which are convertible into 231,330 shares of common stock, and warrants to purchase 138,797 shares of common stock purchased by the selling stockholder on March 28, 2024, (iii) 5,859 shares of common stock issued pursuant to the Almata Merger, and (iv) 1,445 shares of common stock purchased on the open market. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Boothbay Diversified Alpha Master Fund, LP, and Ikarian Healthcare Master Fund, LP, which are related entities. |
| | | | | | |
| (4) | | | Consists of (i) 43 shares of Series C non-voting convertible preferred stock, which are convertible into 43,000 shares of common stock, issued to the selling stockholder on March 27, 2024, which the holder cannot dispose of until September 27, 2024, (ii) 112.689 shares of Series C non-voting convertible preferred stock, which are convertible into 112,689 shares of common stock, and warrants to purchase 67,613 shares of common stock purchased by the selling stockholder on March 28, 2024, (iii) 3,079 shares of common stock issued pursuant to the Almata Merger, and (iv) 704 shares of common stock purchased on the open market. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Boothbay Absolute Return Strategies, LP and Ikarian Healthcare Master Fund, LP , which are related entities. |
| | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| (5) | | | Consists of (i) 531 shares of Series C non-voting convertible preferred stock, which are convertible into 531,000 shares of common stock, issued to the selling stockholder on March 27, 2024, which the holder cannot dispose of until September 27, 2024, (ii) 949.770 shares of Series C non-voting convertible preferred stock, which are convertible into 949,770 shares of common stock, and warrants to purchase 569,861 shares of common stock purchased by the selling stockholder on March 28, 2024, (iii) 37,710 shares of common stock issued pursuant to the Almata Merger, and (iv) 5,933 shares of common stock purchased on the open market. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Boothbay Diversified Alpha Master Fund, LP, and Boothbay Absolute Return Strategies, LP, which are related entities. |
| | | | | | |
| (6) | | | Consists of 3,773.547 shares of Series C non-voting convertible preferred stock, which are convertible into 3,773,547 shares of common stock, warrants to purchase 2,264,128 shares of common stock purchased by the selling stockholder on March 28, 2024, and 40,000 shares of common stock purchased on the open market. Subject to a beneficial ownership limitation of 4.99%. |
| | | | | | |
| (7) | | | Consists of 3,773.547 shares of Series C non-voting convertible preferred stock, which are convertible into 3,773,547 shares of common stock, and warrants to purchase 2,264,128 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 4.99%. |
| | | | | | |
| (8) | | | Consists of 1,712.730 shares of Series C non-voting convertible preferred stock, which are convertible into 1,712,730 shares of common stock, and warrants to purchase 1,027,637 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Biotechnology Value Fund II, L.P., Biotechnology Value Trading Fund OS LP, and MSI BVF SPV, LLC, which are related entities. |
| | | | | | |
| (9) | | | Consists of 1,348.379 shares of Series C non-voting convertible preferred stock, which are convertible into 1,348,379 shares of common stock, and warrants to purchase 809,027 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Biotechnology Value Fund, L.P., Biotechnology Value Trading Fund OS LP, and MSI BVF SPV, LLC, which are related entities. |
| | | | | | |
| (10) | | | Consists of 126.405 shares of Series C non-voting convertible preferred stock, which are convertible into 126,405 shares of common stock, and warrants to purchase 75,842 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Biotechnology Value Fund, L.P., Biotechnology Value Trading Fund II, L.P., and MSI BVF SPV, LLC, which are related entities. |
| | | | | | |
| (11) | | | Consists of 46.956 shares of Series C non-voting convertible preferred stock, which are convertible into 46,956 shares of common stock, and warrants to purchase 28,173 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Biotechnology Value Fund, L.P., Biotechnology Value Fund II, L.P., and Biotechnology Value Trading Fund OS LP, which are related entities. |
| | | | | | |
| (12) | | | Consists of 1,725.050 shares of Series C non-voting convertible preferred stock, which are convertible into 1,725,050 shares of common stock, and warrants to purchase 1,035,030 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with OrbiMed Genesis Master Fund, L.P., which is a related entity. OrbiMed Capital GP IX LLC (“OrbiMed GP”) is the general partner of OrbiMed Private Investments IX, LP (“OPI IX”). OrbiMed Advisors LLC (“OrbiMed Advisors”) is the managing member of OrbiMed GP. By virtue of such relationships, OrbiMed GP and OrbiMed Advisors may be deemed to have voting and investment power with respect to the securities held by OPI IX and as a result, may be deemed to have beneficial ownership over such securities. OrbiMed Advisors exercises voting and investment power through a management committee comprised of Carl L. Gordon, Sven H. Borho, and W. Carter Neild, each of whom disclaims beneficial ownership over the shares held by OPI IX. |
| | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| (13) | | | Consists of 862.525 shares of Series C non-voting convertible preferred stock, which are convertible into 862,525 shares of common stock, and warrants to purchase 517,515 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with OrbiMed Private Investments IX, LP, which is a related entity. OrbiMed Genesis GP LLC (“Genesis GP”) is the general partner of OrbiMed Genesis Master Fund, L.P. (“Genesis Master Fund”). OrbiMed Advisors is the managing member of Genesis GP. By virtue of such relationships, Genesis GP and OrbiMed Advisors may be deemed to have voting and investment power with respect to the securities held by Genesis Master Fund and as a result, may be deemed to have beneficial ownership over such securities. OrbiMed Advisors exercises voting and investment power through a management committee comprised of Carl L. Gordon, Sven H. Borho, and W. Carter Neild, each of whom disclaims beneficial ownership over the shares held by Genesis Master Fund. |
| | | | | | |
| (14) | | | Consists of 2,156.313 shares of Series C non-voting convertible preferred stock, which are convertible into 2,156,313 shares of common stock, and warrants to purchase 1,293,787 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99%. RA Capital Management, L.P. is the investment manager for RA Capital Healthcare Fund, L.P. The general partner of RA Capital Management, L.P. is RA Capital Management GP, LLC, of which Peter Kolchinsky and Rajeev Shah are the managing members. Each of Dr. Kolchinsky and Mr. Shah may be deemed to have voting or investment control over the shares. Dr. Kolchinsky and Mr. Shah disclaim beneficial ownership of such shares, except to the extent of any pecuniary interest therein. The principal business address of the selling stockholder is c/o RA Capital Management, L.P., 200 Berkeley Street, 18th Floor, Boston, MA 02116. |
| | | | | | |
| (15) | | | Consists of 1,940.682 shares of Series C non-voting convertible preferred stock, which are convertible into 1,940,682 shares of common stock, and warrants to purchase 1,164,408 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99%. |
| | | | | | |
| (16) | | | Consists of 374.714 shares of Series C non-voting convertible preferred stock, which are convertible into 374,714 shares of common stock, and warrants to purchase 224,827 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Petrichor Opportunities Fund I Intermediate LP, which is a related entity. |
| | | | | | |
| (17) | | | Consists of 164.365 shares of Series C non-voting convertible preferred stock, which are convertible into 164,365 shares of common stock, and warrants to purchase 98,618 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99% on an aggregated basis with Petrichor Opportunities Fund I LP, which is a related entity. |
| | | | | | |
| (18) | | | Consists of 539.079 shares of Series C non-voting convertible preferred stock, which are convertible into 539,079 shares of common stock, and warrants to purchase 323,446 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99%. Logos Opportunities IV GP LLC (“GP IV”) is the general partner of Logos Opportunities Fund IV LP and may be deemed to have beneficial ownership of these shares. Arsani William and Graham Walmsley are the members of GP IV. Mr. William and Mr. Walmsley each disclaim beneficial ownership of these shares, except to the extent of each’s pecuniary interest therein. |
| | | | | | |
| (19) | | | Consists of 107.816 shares of Series C non-voting convertible preferred stock, which are convertible into 107,816 shares of common stock, and warrants to purchase 64,689 shares of common stock purchased by the selling stockholder on March 28, 2024. Subject to a beneficial ownership limitation of 9.99%. |
Information about any other selling stockholders will be included in prospectus supplements or post-effective amendments, if required. Information about the selling stockholders may change from time to time. Any changed information with respect to which we are given notice will be included in a prospectus supplement.
Registration Rights Agreement
In connection with the securities purchase agreement for the March 2024 private placement, on March 28, 2024, the Company entered into a registration rights agreement with the March 2024 investors (the “Registration Rights Agreement”). Pursuant to the Registration Rights Agreement, the Company has agreed to file a registration statement registering for resale (i) the shares of common stock underlying the Series C non-voting convertible preferred stock issued to the former AlmataBio stockholders in the AlmataBio Transaction and the investors in the March 2024 private placement, (ii) the shares of common stock underlying the warrants issued in the March 2024 private placement, (iii) the shares of Series C non-voting convertible preferred stock issued to the former AlmataBio stockholders in the AlmataBio Transaction and the investors in the March 2024 private placement, and (iv) the warrants issued to the investors in the March 2024 private placement (collectively, the “Registrable Securities”). The Company agreed to file such registration statement within 75 days of March 28, 2024, and have such registration statement declared effective with 135 days of March 28, 2024. The Company also agreed to use its best efforts to keep the registration statement effective under the Securities Act until all Registrable Securities have been publicly sold by the holders thereof. The registration statement of which this prospectus is a part is being filed in order to satisfy our obligations under the Registration Rights Agreement.
We have also agreed, among other things, to indemnify the selling stockholders, and each of their respective officers, directors, agents, partners, members, managers, stockholders, affiliates, investment advisers and employee and any person who controls a selling stockholder and the officers, directors, partners, managers, stockholders, agents, investment advisers and employees of each such controlling person from certain liabilities and pay all fees and expenses (including any reasonable legal fees) incident to our obligations under the Registration Rights Agreement.
PLAN OF DISTRIBUTION
We are registering the shares of Series C non-voting convertible preferred stock, warrants, and shares of common stock of Avalo Therapeutics, Inc., par value of $0.001 per share, or the Common Stock, which we refer to herein as “Shares,” issued to the selling stockholders or issuable upon conversion of our Series C non-voting convertible preferred stock and exercise of the warrants to permit the sale, transfer or other disposition of the Shares, shares of Series C non-voting convertible preferred stock and warrants by the selling stockholders or their donees, pledgees, distributees, transferees or other successors-in-interest from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale by the selling stockholders of the Shares. We will, however, to the extent the warrants are exercised for cash, receive proceeds from such exercises; to the extent we receive such proceeds, they are expected to be used for general corporate and working capital purposes. We will, or will procure to, bear all fees and expenses incident to our obligation to register the Shares.
The selling stockholders may sell all or a portion of the Shares beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the Shares are sold through underwriters or broker-dealers, the selling stockholders will be responsible for underwriting discounts (it being understood that the selling stockholders shall not be deemed to be underwriters solely as a result of their participation in this offering) or commissions or agent’s commissions. The Shares may be sold on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, in the over-the-counter market or in transactions otherwise than on these exchanges or systems or in the over-the-counter market and in one or more transactions at fixed prices, at prices related to prevailing market prices, or at negotiated prices, or, in the case of sales of our common stock, at market prices prevailing at the time of sale. While there is no established public trading market for our Series C non-voting convertible preferred stock, we believe the actual offering price in sales of our Series C non-voting convertible preferred stock by the selling stockholders will be derived from the prevailing market price of our common stock at the time of any such sale. These prices, as well as the timing, manner and size of each sale, will be determined by the selling stockholders or by agreement between such holders and underwriters or dealers who may receive fees or commissions in connection with such sale. These sales may be effected in transactions, which may involve crosses or block transactions. The selling stockholders may use any one or more of the following methods when selling Shares:
•ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
•block trades in which the broker-dealer will attempt to sell the Shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction;
•to or through underwriters or purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
•an exchange distribution in accordance with the rules of the applicable exchange;
•through the distribution of such securities by any selling stockholder to its equity holders;
•privately negotiated transactions;
•settlement of short sales entered into after the effective date the registration statement of which this prospectus is a part;
•broker-dealers may agree with the selling stockholders to sell a specified number of such securities at a stipulated price per Share;
•through the writing or settlement of options or other hedging transactions, whether such options are listed on an options exchange or otherwise;
•a combination of any such methods of sale; and
•any other method permitted pursuant to applicable law.
The selling stockholders also may resell all or a portion of the Shares in open market transactions in reliance upon Rule 144 under the Securities Act, as amended, or the Securities Act, as permitted by that rule, or Section 4(a)(1) under the Securities Act, if available, rather than under this prospectus, provided that they meet the criteria and conform to the requirements of those provisions.
Broker-dealers engaged by the selling stockholders may arrange for other broker-dealers to participate in sales. If the selling stockholders effect such transactions by selling Shares to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the Shares for whom they may act as agent or to whom they may sell as principal. Such commissions will be in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction will not be in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2121.01.
In connection with sales of the Shares or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the Shares in the course of hedging in positions they assume. The selling stockholders may also sell Shares short and if such short sale takes place after the date that the registration statement of which this prospectus is a part is declared effective by the SEC, the selling stockholders may deliver Shares covered by this prospectus to close out short positions and to return borrowed Shares in connection with such short sales. The selling stockholders may also loan or pledge Shares to broker-dealers that in turn may sell such Shares, to the extent permitted by applicable law. The selling stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction). Notwithstanding the foregoing, the selling stockholders have been advised that they may not use Shares the resale of which has been registered on this registration statement to cover short sales of our Common Stock made prior to the date the registration statement, of which this prospectus forms a part, has been declared effective by the SEC.
The selling stockholders may, from time to time, pledge or grant a security interest in some or all of the Shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the Shares from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act of 1933, amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the Shares in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling stockholders and any broker-dealer or agents participating in the distribution of the Shares may be deemed to be “underwriters” within the meaning of Section 2(11) of the Securities Act in connection with such sales. In such event, any commissions paid, or any discounts or concessions allowed to, any such broker-dealer or agent and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Selling Stockholders who are “underwriters” within the meaning of Section 2(11) of the Securities Act will be subject to the applicable prospectus delivery requirements of the Securities Act including Rule 172 thereunder and may be subject to certain statutory liabilities of, including but not limited to, Sections 11, 12 and 17 of the Securities Act and Rule 10b-5 under the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Each selling stockholder has informed the Company that it is not a registered broker-dealer and does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the Shares. Upon the Company being notified in writing by a selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of Common Stock through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to Rule 424(b) under the Securities Act, disclosing (i) the name of each such selling stockholder and of the participating broker-dealer(s), (ii) the number of Shares involved, (iii) the price at which such the Shares were sold, (iv) the commissions paid or discounts or concessions allowed to such broker-dealer(s), where applicable, (v) that such broker-dealer(s) did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus, and (vi) other facts material to the transaction. In no event shall any broker-dealer receive fees, commissions and markups, which, in the aggregate, would exceed eight percent (8.0%).
Under the securities laws of some U.S. states, the Shares may be sold in such states only through registered or licensed brokers or dealers. In addition, in some U.S. states the Shares may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling stockholder will sell any or all of the Shares registered pursuant to the shelf registration statement, of which this prospectus forms a part.
Each selling stockholder and any other person participating in such distribution will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including, without limitation, to the extent applicable, Regulation M
of the Exchange Act, which may limit the timing of purchases and sales of any of the Shares by the selling stockholder and any other participating person. To the extent applicable, Regulation M may also restrict the ability of any person engaged in the distribution of the Shares to engage in market-making activities with respect to the Shares. All of the foregoing may affect the marketability of the Shares and the ability of any person or entity to engage in market-making activities with respect to the Shares.
We will pay all expenses of the registration of the Shares pursuant to the registration rights agreement, including, without limitation, SEC filing fees and expenses of compliance with state securities or “blue sky” laws; provided, however, that each selling stockholder will pay all underwriting discounts and selling commissions, and any related legal or other advisory fees and expenses (in excess of the up to $50,000, per registration in reasonable fees and expenses of legal counsel to the selling stockholders which we agreed to reimburse) incurred by it, if any. We will indemnify the selling stockholders against certain liabilities, including some liabilities under the Securities Act, in accordance with the Registration Rights Agreement, or the selling stockholders will be entitled to contribution. We may be indemnified by the selling stockholders against certain civil liabilities set forth in the Registration Rights Agreement, including liabilities under the Securities Act, which may arise from any written information furnished to us by the selling stockholders specifically for use in this prospectus, in accordance with the Registration Rights Agreement, or we may be entitled to contribution.
DESCRIPTION OF SECURITIES TO BE REGISTERED
The following description of our securities to be offered for resale hereby and provisions of our amended and restated certificate of incorporation, as amended (the “A&R Certificate of Incorporation”) and our fifth amended and restated bylaws (the “A&R Bylaws”) are summaries with respect to the securities offered hereby. This description also summarizes relevant provisions of the General Corporation Law of the State of Delaware, which we refer to as the DGCL. The terms of our A&R Certificate of Incorporation and A&R Bylaws and the terms of the DGCL are more detailed than the general information provided below. Therefore, please carefully consider the actual provisions of the A&R Certificate of Incorporation and the A&R Bylaws, which have been filed with the SEC as exhibits to the registration statement of which this prospectus forms a part, as well as the DGCL.
General
Under our A&R Certificate of Incorporation, we are authorized to issue up to 200,000,000 shares of common stock, $0.001 par value per share, and 5,000,000 shares of preferred stock, $0.001 par value per share. Of the preferred shares, we have designated 34,326 shares as Series C non-voting convertible preferred stock, one share as Series D non-voting convertible preferred stock, and one share as Series E non-voting convertible preferred stock. Our board of directors may establish the rights and preferences of additional series of the preferred stock from time to time. As of May 15, 2024, we had 1,034,130 shares of common stock outstanding, 22,357.897 shares of Series C non-voting convertible preferred stock outstanding, one share of Series D non-voting non-convertible preferred stock outstanding, and one share of Series E non-voting non-convertible preferred stock outstanding.
Common Stock
Voting
Each holder of common stock is entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors, and does not have cumulative voting rights. Accordingly, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election.
Dividends
Subject to preferences that may be applicable to any then-outstanding preferred stock, the holders of common stock are entitled to receive dividends, if any, as may be declared from time to time by the board of directors out of legally available funds.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities, and the satisfaction of any liquidation preference granted to the holders of any then-outstanding shares of preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any then-outstanding shares of preferred stock.
Fully Paid and Nonassessable
All of our outstanding shares of common stock are fully paid and nonassessable.
Warrants
As of May 15, 2024, we had outstanding warrants to purchase an aggregate of 11,969,063 shares of our common stock at a weighted average exercise price of $10.07 per share, subject to adjustment upon occurrence of certain conditions. The warrants offered hereby and their terms are described below. There is no established public trading market for the warrants and we do not intend to list the warrants on any national securities exchange or nationally recognized trading system.
March 2024 Warrants
On March 28, 2024, in connection with the AlmataBio Transaction and related private placement financing, we issued warrants to purchase up to an aggregate of 11,967,526 shares of our common stock or shares of Series C non-voting convertible preferred stock, at the holders’ option, for an exercise price equal to $5.796933 per share of common stock. The warrants became exercisable on March 28, 2024, if exercised for shares of Series C non-voting convertible preferred stock, and will become exercisable for shares of common stock upon the date that the Required Stockholder Approval is received. The warrants will expire on the earlier of (i) March 28, 2029 or (ii) the thirty-first day following the public announcement of the first patient being dosed in a Phase 2 trial for the indication of HS (the “Dosing Date”), provided that if the Required Stockholder Approval has not been received by the Dosing Date, then the warrants will expire on the earlier of the (A) the fifth anniversary of the date of issuance or (B) thirty-first day following receipt of the Required Stockholder Approval.
Preferred Stock
General
Pursuant to our A&R Certificate of Incorporation, our board of directors has the authority, without further action by the stockholders (unless such stockholder action is required by applicable law or stock exchange listing rules), to designate and issue up to 5,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the designations, powers, preferences, privileges and relative participating, optional or special rights and the qualifications, limitations or restrictions thereof, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights of the common stock, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors, without stockholder approval, can issue preferred stock with voting, conversion or other rights that could adversely affect the voting power and other rights of the holders of common stock. Preferred stock could be issued quickly with terms designed to delay or prevent a change in control of our company or make removal of management more difficult. Additionally, the issuance of preferred stock may have the effect of decreasing the market price of the common stock and may adversely affect the voting power of holders of common stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation.
Our board of directors will fix the designations, voting powers, preferences and rights of each series, as well as the qualifications, limitations or restrictions thereof, of any series of preferred stock that we offer in the certificate of designation relating to that series. We will file as an exhibit to a report that we file with the SEC the form of any certificate of designation that describes the terms of the series of preferred stock. This description will include:
•the title and stated value;
•the number of shares authorized;
•the liquidation preference per share;
•the purchase price per share;
•the dividend rate per share, if any, dividend period and payment dates and method of calculation for dividends;
•whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
•our right, if any, to defer payment of dividends and the maximum length of any such deferral period;
•the procedures for any auction and remarketing, if any;
•the provisions for a sinking fund, if any;
•the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights;
•any listing of the preferred stock on any securities exchange or market;
•whether the preferred stock will be convertible into our common stock or other securities of ours, including warrants, and, if applicable, the conversion period, the conversion price, or how it will be calculated, and under what circumstances it may be adjusted;
•whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange period, the exchange price, or how it will be calculated, and under what circumstances it may be adjusted;
•voting rights, if any, of the preferred stock;
•preemption rights, if any;
•restrictions on transfer, sale or other assignment, if any;
•a discussion of any material or special U.S. federal income tax considerations applicable to the preferred stock;
•the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs;
•any limitations on issuances of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock being issued as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and
•any other specific terms, rights, preferences, privileges, qualifications or restrictions of the preferred stock.
The DGCL, the corporate law of our state of our incorporation, which is Delaware, provides that the holders of preferred stock will have the right to vote separately as a class (or, in some cases, as a series) on an amendment to our A&R Certificate of Incorporation if the amendment would change the par value or, unless the A&R Certificate of Incorporation provides otherwise, the number of authorized shares of the class or change the powers, preferences or special rights of the class or series so as to adversely affect the class or series, as the case may be. This right is in addition to any voting rights that may be provided for in the applicable certificate of designation.
Series C Non-Voting Convertible Preferred Stock
We have authorized the issuance of 34,326 shares of Series C non-voting convertible preferred stock, each with a par value of $0.001 per share. There is no established public trading market for the Series C non-voting convertible preferred stock and we do not intend to list the Series C non-voting convertible preferred stock on any national securities exchange or nationally recognized trading system.
Each share of Series C non-voting convertible preferred stock is initially convertible into 1,000 shares of common stock, subject to adjustment as described below. The Series C non-voting convertible preferred stock will convert automatically on the second trading day after the receipt of the Required Stockholder Approval in accordance with Nasdaq rules, subject to Beneficial Ownership Limitation described below. No fractional shares will be issued upon conversion; rather any fractional share will be rounded up to the next whole share.
In all cases, conversion of the Series C non-voting convertible preferred stock will be subject to the Beneficial Ownership Limitation. The “Beneficial Ownership Limitation” prevents the conversion of any portion of a holder’s Series C non-voting convertible preferred stock if such conversion would cause the holder, together with its affiliates, to beneficially own more than 9.99% (or 4.99% in the case of certain holders) of the outstanding shares of common stock after giving effect to the conversion.
Except as required by the DGCL and the Series C Non-Voting Convertible Preferred Stock Certificate of Designation, the Series C non-voting convertible preferred stock has no voting rights. The Series C non-voting convertible preferred stock is entitled to receive dividends (on an as-if-converted-to-common-stock basis) equal to and in the same form as dividends (other than dividends in the form of common stock) actually paid on shares of the common stock when, as and if declared by the board (other than dividends in the form of common stock).
The Series C non-voting convertible preferred stock ranks in parity with the common stock as to dividends, distributions of assets upon liquidation, dissolution or winding up of the Company, whether voluntarily or involuntarily.
The Series C non-voting convertible preferred stock is subject to broad-based weighted average anti-dilution protection for certain issuances of common stock and securities convertible into common stock.
Registration Rights
Holders of our Series C non-voting convertible preferred stock and warrants offered hereby are entitled to certain rights with respect to the registration of such securities as further provided under the heading “Selling Stockholders – Registration Rights Agreements.”
Anti-Takeover Effects of Delaware Law and Our A&R Certificate of Incorporation and A&R Bylaws
Provisions of DGCL, our A&R Certificate of Incorporation, and our A&R Bylaws could make it more difficult to acquire us by means of a tender offer, a proxy contest, open market purchases, removal of incumbent directors and otherwise. It is possible that these provisions also could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests. These provisions, summarized below, are expected to discourage types of coercive takeover practices and inadequate takeover bids and to encourage persons to acquire control of us to first negotiate with us. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging takeover or acquisition proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Law
We are subject to section 203 of the DGCL, or Section 203. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
•prior to such time the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
•upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
•at or subsequent to such time the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder.
Section 203 defines a business combination to include:
•any merger or consolidation involving the corporation and the interested stockholder;
•any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation;
•subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder;
•subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or
•the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation.
Subject to certain exceptions, Section 203 defines an “interested stockholder” as an entity or person (other than the corporation or any direct or indirect majority-owned subsidiary of the corporation) who, together with the entity or person’s affiliates and associates, beneficially owns, or is an affiliate or associate of the corporation and within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
The existence of this provision generally will have an anti-takeover effect for transactions not approved in advance by our board of directors, including discouraging attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
A&R Certificate of Incorporation and A&R Bylaws
Provisions of our A&R Certificate of Incorporation and A&R Bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock and other securities. Among other things, our A&R Certificate of Incorporation and A&R Bylaws:
•permit our board of directors to issue up to 5,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other change in our control);
•provide that the authorized number of directors may be changed only be resolution of our board of directors;
•provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
•require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent;
•provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of such a stockholder’s notice;
•do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); and
•provide that special meetings of our stockholders may be called only by the chairman of the board, our chief executive officers or by our board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors.
The amendment of any of these provisions, with the exception of the ability of our board of directors to issue shares of preferred stock and designate any rights, preferences and privileges thereto, would require approval by the holders of at least 66 2/3% of our then outstanding common stock.
Choice of Forum
Our A&R Certificate of Incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware is the exclusive forum for:
•any derivative action or proceeding brought on our behalf;
•any action asserting a claim of breach of a fiduciary duty;
•any action asserting a claim against us arising pursuant to any provision of the DGCL, our A&R Certificate of Incorporation or our A&R Bylaws; or
•any action asserting a claim against us that is governed by the internal affairs doctrine.
The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any action, a court could find the choice of forum provisions contained in our A&R Certificate of Incorporation to be inapplicable or unenforceable in such action. These provisions would not apply to suits brought to enforce a duty or liability created by the Exchange Act, Securities Act or any other claim for which the federal courts have exclusive or concurrent jurisdiction. Any person or entity purchasing or otherwise acquiring any interest in our securities shall be deemed to have notice of and consented to these provisions. Our exclusive forum provision will not relieve us of our duties to comply with the federal securities laws and the rules and regulations thereunder, and our stockholders will not be deemed to have waived our compliance with these laws, rules and regulations. The choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with the Company or our directors and officers, which may discourage such lawsuits against the Company and our directors and officers or could result in increased costs for our stockholders to bring a claim in the chosen forum.
Nasdaq Capital Market Listing of Common Stock
Our common stock is listed on The Nasdaq Capital Market under the symbol “AVTX.”
Neither the Series C non-voting convertible preferred stock nor the March 2024 warrants are listed on The Nasdaq Capital Market or any other securities exchange or nationally recognized trading system.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Equiniti Trust Company, LLC. The transfer agent and registrar’s address is 6201 15th Avenue, Brooklyn, NY 11219. We serve as the transfer agent and registrar for the Series C non-voting convertible preferred stock.
LEGAL MATTERS
The validity of the securities being offered hereby will be passed upon for us by Wyrick Robbins Yates & Ponton LLP, Raleigh, North Carolina.
EXPERTS
Ernst & Young LLP, independent registered public accounting firm, has audited (1) our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023, and (2) the financial statements of AlmataBio, Inc. included in our Form 8-K/A dated June 3, 2024 for the period from April 28, 2023 (date of inception) to December 31, 2023, as set forth in their reports, which are incorporated by reference in this prospectus and elsewhere in the registration statement. Our financial statements and the financial statements of AlmataBio, Inc. are incorporated by reference in reliance on Ernst & Young LLP’s reports, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding registrants. Our SEC filings, including our registration statement of which this prospectus is a part and the exhibits and schedules thereto, are available on the SEC website at www.sec.gov.
We also maintain a website at www.avalotx.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained in or accessible through our website does not constitute a part of this prospectus. You may also request a copy of these filings, at no cost, by writing or telephoning us at: 540 Gaither Road, Suite 400, Rockville, Maryland 20850, (410) 522-8707.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The SEC file number for the documents incorporated by reference in this prospectus is 001-37590. The documents incorporated by reference into this prospectus contain important information that you should read about us.
The following documents are incorporated by reference into this document:
•our Current Reports on Form 8-K filed with the SEC on January 31, 2024, March 28, 2024, (as amended by that Current Report on Form 8-K/A filed on June 3, 2024), and May 23, 2024, in each case only to the extent the information in such report is filed and not furnished; and
We also incorporate by reference into this prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of the offering. These documents include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy statements.
Any statement in a document incorporated by reference or deemed to be incorporated by reference in this prospectus shall be deemed to be modified or superseded for the purposes of this prospectus to the extent that a statement contained herein or therein or in any other subsequently filed document which also is incorporated or deemed to be incorporated by reference herein or therein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Avalo Therapeutics, Inc., 540 Gaither Road, Suite 400 Rockville, Maryland 20850; telephone: (410) 522-8707.
Up to 22,357,897 Shares of Common Stock Issuable Upon Conversion of Shares of Series C Non-Voting Convertible Preferred Stock Offered by Selling Stockholders
22,357.897 Shares of Series C Non-Voting Convertible Preferred Stock Offered by Selling Stockholders
Up to 11,967,526 Shares of Common Stock Issuable Upon the Exercise of Warrants Offered by Selling Stockholders
Warrants to Purchase up to 11,967,526 Shares of Common Stock (or 11,967.526 Shares of Series C Non-Voting Convertible Preferred Stock) Offered by Selling Stockholders
, 2024
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution.
The following table sets forth an itemization of the various expenses, all of which we will pay, in connection with the issuance and distribution of the securities being registered. The selling stockholder will not be responsible for any of the expenses of this offering. All of the amounts shown are estimated except the SEC registration fee.
| | | | | | | | | | | |
| SEC registration fee | | $ | 65,976 | |
| Legal fees and expenses | | $ | 100,000 | |
| Accounting fees and expenses | | $ | 20,000 | |
| Printing expenses | | $ | — | |
| Total | | $ | 185,976 | |
Item 15. Indemnification of Directors and Officers.
We are incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law (“DGCL”) provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, parties to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer, director, employee, or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee, or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit, or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any person who is, or is threatened to be made, a party to any threatened, pending, or completed action or suit by or in the right of the corporation by reason of the fact that such person was a director, officer, employee, or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee, or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests, except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses which such officer or director has actually and reasonably incurred. Our amended and restated certificate of incorporation, as amended (the “A&R Certificate of Incorporation”) and our fifth amended and restated bylaws (the “A&R Bylaws”) provide for the indemnification of our directors and officers to the fullest extent permitted under the DGCL.
Section 102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
•breach of a director’s duty of loyalty to the corporation or its stockholders;
•act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
•unlawful payment of dividends, stock purchase or redemption of shares; or
•transaction from which the director derives an improper personal benefit.
Our A&R Certificate of Incorporation includes a provision providing for the limitation of liability to the maximum extent permitted under the DGCL. Expenses incurred by any officer or director in defending any proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking by or on behalf of such director or officer, to repay all amounts advanced if it should ultimately be determined that such director or officer is not entitled to be indemnified by us.
Section 174 of the DGCL provides, among other things, that a director, who willfully or negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption, may be held liable for such actions. A director who was either absent when the unlawful actions were approved, or dissented at the time, may avoid liability by causing his or her
dissent to such actions to be entered on the books containing minutes of the meetings of the board of directors at the time such action occurred or immediately after such absent director receives notice of the unlawful acts.
We maintain a directors’ and officers’ liability insurance policy. The policy insures directors and officers against unindemnified losses arising from certain wrongful acts in their capacities as directors and officers and reimburses us for those losses for which we have lawfully indemnified the directors and officers. The policy contains various exclusions.
We have entered into indemnification agreements with each of our directors and certain of our executive officers. These agreements require us to indemnify these individuals and, in certain cases, affiliates of such individuals, to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to the Company, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified.
Item 16. Exhibits and Financial Statement Schedules.
(a)Exhibits.
| | | | | |
Exhibit
Number | Description of Document |
| 2.1# | Agreement and Plan of Merger and Reorganization, dated March 27, 2024, by and among Avalo Therapeutics, Inc., Project Athens Merger Sub, Inc., Second Project Athens Merger Sub, LLC and AlmataBio, Inc. (incorporated by reference to Exhibit 2.1 to the Current Report on Form 8-K filed on March 28, 2024). |
| 3.1 | |
| 3.1.1 | |
| 3.1.2 | |
| 3.1.3 | |
| 3.1.4 | |
| 3.1.5 | |
| 3.1.6 | |
| 3.1.7 | |
| 3.1.8 | |
| 3.2 | |
| 4.1 | |
| 4.2 | |
| 5.1 | |
| 10.1# | |
| 10.2 | |
| 23.1 | |
| 23.2 | |
| 24.1 | |
| 107 | |
| |
| * | Portions of this exhibit have been redacted in compliance with Regulation S-K Item 601(b)(2). The Company will furnish copies of the unredacted exhibit to the SEC upon request. |
| # | Certain exhibits and schedules to this exhibit have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company hereby undertakes to furnish supplemental copies of any of the omitted exhibits or schedules upon request by the U.S. Securities and Exchange Commission. |
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(h) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit
to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Rockville, State of Maryland, on the 6th day of June, 2024.
| | | | | | | | |
|
| | AVALO THERAPEUTICS, INC. |
| | | |
| | By: | /s/ Garry Neil, M.D. |
| | Name: | Garry Neil, M.D. |
| | Title: | Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Garry Neil, M.D. and Christopher Sullivan, and each or any one of them, as his or her true and lawful agent, proxy and attorney-in-fact, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments (including post-effective amendments) to this registration statement together with all schedules and exhibits thereto and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, together with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, (iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and (iv) take any and all actions, which may be necessary or appropriate to be done, as fully for all intents and purposes as he or she might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or any of his or her substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| | | | | |
| /s/ Garry Neil, M.D. | | President and Chief Executive Officer, Chairman of the Board of Directors and Director | | June 6, 2024 |
| Garry Neil, M.D. | | (Principal Executive Officer) | | |
| | | | |
| /s/ Christopher Sullivan | | Chief Financial Officer | | June 6, 2024 |
| Christopher Sullivan | | (Principal Financial and Accounting Officer) | | |
| | | | |
| /s/ June Almenoff, M.D., Ph.D. | | Director | | June 6, 2024 |
| June Almenoff, M.D., Ph.D. | | | | |
| | | | |
| /s/ Mitchell Chan | | Director | | June 6, 2024 |
| Mitchell Chan | | | | |
| | | | | |
| /s/ Jonathan Goldman, M.D. | | Director | | June 6, 2024 |
| Jonathan Goldman, M.D. | | | | |
| | | | |
| /s/ Aaron Kantoff | | Director | | June 6, 2024 |
| Aaron Kantoff | | | | |
| | | | |
| /s/ Gilla Kaplan, Ph.D. | | Director | | June 6, 2024 |
| Gilla Kaplan, Ph.D. | | | | |
| | | | |
| /s/ Magnus Persson, M.D., Ph.D | | Director | | June 6, 2024 |
| Magnus Persson, M.D., Ph.D | | | | |
| | | | |
| /s/ Samantha Truex | | Director | | June 6, 2024 |
| Samantha Truex | | | | |
| | | | |
Calculation of Filing Fee Tables
Form S-3
(Form Type)
Avalo Therapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward Securities
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Security Type | Security Class Title | Fee Calculation or Carry Forward Rule | Amount Registered (1) | Proposed Maximum Offering Price Per Unit | Maximum Aggregate Offering Price | Fee Rate | Amount of Registration Fee |
Newly Registered Securities |
Fees to Be Paid | Equity | Series C Non-Voting Convertible Preferred Stock, par value $0.001 per share | 457(i) | 22,357.897 | (2) | $ | — | | (3) | $ | — | | $ | — | | $ | — | |
| Equity | Common Stock underlying the Series C Non-Voting Convertible Preferred Stock | 457(c) | 22,357,897 | | $ | 11.00 | | (4) | $ | 245,936,867 | | $ | 0.00014760 | | $ | 36,300 | |
| Equity | Warrants to purchase Common Stock | 457(i) | 11,967,526 | (5) | $ | — | | (3) | $ | — | | $ | — | | $ | — | |
| Equity | Common Stock underlying the Warrants | 457(c) | 11,967,526 | | $ | 16.80 | | (6) | $ | 201,054,437 | | $ | 0.00014760 | | $ | 29,676 | |
Fees Previously Paid | | | | | | | | |
Carry Forward Securities |
Carry Forward Securities | — | — | — | — | — | — | — | — |
| Total Offering Amounts | | $ | 446,991,304 | | | $ | 65,976 | |
| Total Fees Previously Paid | | — | | $ | — | |
| Total Fee Offsets | | — | | $ | — | |
| Net Fee Due | | | | $ | 65,976 | |
(1) Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), this registration statement also registers an indeterminate number of shares of common stock, $0.001 par value per share (the “Common Stock”), of Avalo Therapeutics, Inc. (the “Registrant”) which may become issuable by reason of any stock dividend, stock split or other similar transaction effected without the receipt of consideration that results in an increase in the number of the outstanding shares of the Registrant’s Common Stock.
(2) Represents the maximum number of shares of Series C non-voting convertible preferred stock, par value $0.001 per share, each convertible for 1,000 shares of common stock being registered for resale.
(3) No additional consideration will be received upon conversion of the Series C non-voting convertible preferred stock and therefore, no registration fee is required pursuant to Rule 457(i) under the Securities Act. Consistent with the response to Question 240.06 of the Securities Act Rules Compliance and Disclosure Interpretations, the registration fee with respect to the Series C non-voting convertible preferred stock and the warrants, including the exercise price of the warrants, has been allocated to the underlying common stock included in the registration fee.
(4) Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(c) under the Securities Act, based on the average of the high and low prices of the common stock as reported on Nasdaq Capital Market on June 4, 2024 of $11.00 per share.
(5) Represents the maximum number of warrants being registered for resale, each with an exercise price of $5.796933 per share of common stock.
(6) Pursuant to Rules 457(c) and 457(i) promulgated under the Securities Act and solely for the purpose of calculating the registration fee, the proposed maximum offering price per unit is the sum of the average of the high and low prices of the Common Stock as reported on Nasdaq Capital Market on June 4, 2024 ($11.00) and the warrant exercise price per share of common stock ($5.796933).
Wyrick Robbins Yates & Ponton LLP
4101 Lake Boone Trail, Suite 300
Raleigh, North Carolina 27607
June 6, 2024
Board of Directors
Avalo Therapeutics, Inc.
540 Gaither Road, Suite 400
Rockville, Maryland 20850
Re: Shelf Registration Statement on Form S-3
Ladies and Gentlemen:
We have acted as counsel to Avalo Therapeutics, Inc., a Delaware corporation (the “Company”), in connection with its registration statement on Form S-3 (the “Registration Statement”) filed on even date herewith with the Securities and Exchange Commission (the “Commission”) under the Securities Act of 1933, as amended (the “Act”). The Registration Statement relates to the resale from time to time by the selling stockholders listed in the Registration Statement under the heading “Selling Stockholders” of (i) 22,357,897 shares (the “Common Shares”) of our common stock, $0.001 par value per share (the “Common Stock”), issuable upon the conversion of issued and outstanding shares of our Series C non-voting convertible preferred stock (the “Preferred Stock”), (ii) 22,357.897 shares of our Series C non-voting convertible preferred stock (the “Preferred Shares ”), (iii) warrants to purchase up to 11,967,526 shares of our Common Stock (or 11,967.526 shares of Series C non-voting convertible preferred stock) (the “Warrants”), and (iv) 11,967,526 shares of Common Stock issuable upon the exercise of the Warrants (the “Warrant Shares”).
This opinion is being furnished in accordance with the requirements of Item 16 of Form S-3 and Item 601(b)(5)(i) of Regulation S-K.
In connection with the foregoing, we have relied upon, among other things, our examination of such documents, records of the Company and certificates of its officers and public officials as we deemed necessary for purposes of the opinions expressed below. In our examination of documents for purposes of this opinion, we have assumed, and express no opinion as to, the authenticity and completeness of all documents submitted to us as originals, the conformity to originals and completeness of all documents submitted to us as copies.
Based upon and subject to the foregoing and the additional limitations, qualifications, exceptions and assumptions set forth below, it is our opinion that:
1.The Common Shares, when issued and paid for in accordance with the provisions of the Preferred Stock, will be validly issued, fully paid and non-assessable.
| | | | | | | | |
Avalo Therapeutics, Inc. June 6, 2024 Page 2 | | |
2.The Preferred Shares have been validly issued, fully paid, and are nonassessable.
3.The Warrants constitute valid and binding obligations of the Company, enforceable against the Company in accordance with their terms, except as enforcement thereof may be limited by applicable bankruptcy, insolvency, reorganization, arrangement, moratorium or other similar laws affecting creditors’ rights, and subject to general equity principles and to limitations on availability of equitable relief, including specific performance.
4.The Warrant Shares, when issued and paid for in accordance with the provisions of the Warrants, will be validly issued, fully paid and non-assessable.
This opinion is limited to the Delaware General Corporation Law, including the statutory provisions of the Delaware General Corporate Law and all applicable provisions of the Delaware Constitution and reported judicial decisions interpreting these laws.
We hereby consent to the filing of this opinion with the Commission as Exhibit 5.1 to the Registration Statement and reference to our firm under the heading “Legal Matters” in the prospectus included therein. In giving this consent, we do not admit that we are within the category of persons whose consent is required by Section 7 of the Act or the rules and regulations promulgated thereunder by the Commission.
This opinion is intended solely for use in connection with the sale of the Securities subject to the Registration Statement and is not to be relied upon for any other purpose. This opinion is rendered as of the date first written above and based solely on our understanding of facts in existence as of such date after the aforementioned examination.
We assume no obligation to advise you of any fact, circumstance, event or change in the law or the facts that may hereafter be brought to our attention whether or not such occurrence would affect or modify any of the opinions expressed herein.
| | | | | |
| Sincerely, | |
|
|
| /s/ Wyrick Robbins Yates & Ponton LLP |
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the reference to our firm under the caption "Experts" in the Registration Statement (Form S-3) and related Prospectus of Avalo Therapeutics, Inc. for the registration of 34,325,423 shares of its common stock, 22,357.897 shares preferred stock, and 11,967,526 warrants, and to the incorporation by reference therein of our report dated March 29, 2024, with respect to the consolidated financial statements of Avalo Therapeutics, Inc. included in its Annual Report (Form 10-K) for the year ended December 31, 2023 and of our report dated June 3, 2024, with respect to the financial statements of AlmataBio, Inc., for the period from April 28, 2023 (date of inception) to December 31, 2023, included in Avalo Therapeutics Inc’s Form 8-K/A dated June 3, 2024, filed with the Securities and Exchange Commission.
/s/ Ernst & Young LLP
Tysons, Virginia
June 6, 2024
Avalo Therapeutics (NASDAQ:AVTX)
Graphique Historique de l'Action
De Mai 2024 à Juin 2024
Avalo Therapeutics (NASDAQ:AVTX)
Graphique Historique de l'Action
De Juin 2023 à Juin 2024