Alcon Reports Results for Dry-Eye-Treatment Trials
09 Janvier 2024 - 11:20PM
Dow Jones News
By Denny Jacob
Alcon reported topline results from two trials evaluating
AR-15512, a candidate treatment for dry-eye disease.
The eye-care company said the primary endpoint achieved
statistical significance in both trials. Additional data derived
from secondary endpoints demonstrated the rapid onset and sustained
tear production, said Alcon.
AR-15512 was well tolerated and no serious ocular adverse events
were reported.
Alcon expects to file a new drug application for AR-15512 with
the U.S. Food and Drug Administration in mid-2024.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
January 09, 2024 17:05 ET (22:05 GMT)
Copyright (c) 2024 Dow Jones & Company, Inc.
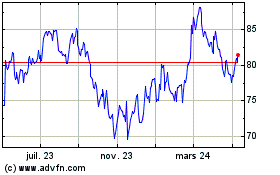
Alcon (NYSE:ALC)
Graphique Historique de l'Action
De Mar 2024 à Avr 2024
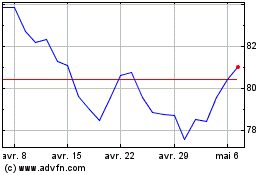
Alcon (NYSE:ALC)
Graphique Historique de l'Action
De Avr 2023 à Avr 2024