Form 8-K - Current report
13 Janvier 2025 - 1:00PM
Edgar (US Regulatory)
false
0001850119
0001850119
2025-01-13
2025-01-13
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
January 13, 2025
Century Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware |
|
001-40498 |
|
84-2040295 |
(State or other jurisdiction of
incorporation or organization) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification No.) |
|
25
North 38th Street, 11th Floor
Philadelphia, Pennsylvania |
|
19104 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: (267) 817-5790
Not Applicable
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2.
below):
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
|
Trading Symbol |
|
Name
of Exchange on Which Registered |
| Common Stock, par value $0.0001 per share |
|
IPSC |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange
Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act.
| Item
2.02 |
Results of Operations and Financial Condition |
On January 13, 2025, Century Therapeutics,
Inc. (the “Company”) announced that, as of December 31, 2024, the Company had approximately $220 million of cash, cash equivalents
and investments. This unaudited, preliminary amount has been prepared by and is the responsibility of management. This amount is based
upon information available to management as of the date of this Current Report on Form 8-K and subject to completion of financial closing
procedures that could result in changes to the amount. Furthermore, this amount does not present all information necessary for an understanding
of the Company’s financial condition as of December 31, 2024. The Company’s independent registered public accounting firm,
Ernst & Young LLP, has not audited, reviewed, compiled or performed any procedures with respect to this preliminary financial data
and, accordingly, Ernst & Young LLP does not express an opinion or any other form of assurance with respect thereto. The Company’s
actual results for the year ended December 31, 2024 will be included in the Company’s Annual Report on Form 10-K for the year ended
December 31, 2024 and may differ materially from the above estimate.
The information furnished pursuant to this Item 2.02 is intended to
be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended
(the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference
in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly
set forth by specific reference in such filing.
| Item 7.01 |
Regulation FD Disclosure |
On January 13, 2025, the Company updated information
reflected in a slide presentation, which is attached hereto as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein
by reference. Representatives of the Company will use the updated presentation in various meetings with investors from time to time.
The information contained in this Item 7.01 (including Exhibit 99.1)
is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject
to the liabilities of that section and shall not be deemed incorporated by reference in any filing under the Securities Act or
the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01 |
Financial Statements and Exhibits |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
CENTURY THERAPEUTICS, INC. |
| |
|
|
| |
By: |
/s/ Brent Pfeiffenberger, Pharm.D. |
| |
Name: |
Brent Pfeiffenberger, Pharm.D. |
| |
Title: |
President and Chief Executive Officer |
Date: January 13, 2025
Exhibit 99.1
| January 2025
Corporate Overview |

| 2
Forward-looking statements
This presentation contains forward-looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995. All
statements contained in this presentation, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements
regarding our clinical development plans and timelines and the initial safety and efficacy profiles of CNTY-101 are forward-looking statements. These statements involve known and
unknown risks, uncertainties and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance or
achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “should,”
“expect,” “plan,” “aim,” “seek,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “forecast,” “potential” or “continue” or the negative of these
terms or other similar expressions. The forward-looking statements in this presentation are only predictions. We have based these forward-looking statements largely on our current
expectations and projections about future events and financial trends that we believe may affect our business, financial condition, and results of operations. These forward-looking
statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some
of which are beyond our control, including, among others: our ability to successfully advance our current and future product candidates through development activities, preclinical studies,
and clinical trials; our dependence on the success of our lead product candidate, CNTY-101; our ability to progress CNTY-101 through our CALiPSO and ELiPSE Phase 1 clinical trials; our
ability to meet development milestones on anticipated timelines; uncertainties inherent in the results of preliminary data, pre-clinical studies and earlier-stage clinical trials, which may not
be predictive of final results or the results of later-stage clinical trials; our ability to obtain FDA clearance of our future IND submissions and commence and complete clinical trials on
expected timelines, or at all; our reliance on the maintenance of certain key collaborative relationships for the manufacturing and development of our product candidates; the timing, scope
and likelihood of regulatory filings and approvals, including final regulatory approval of our product candidates; the impact of geopolitical issues, banking instability and inflation on our
business and operations, supply chain and labor force; the performance of third parties in connection with the development of our product candidates, including third parties conducting
our clinical trials as well as third-party suppliers and manufacturers; our ability to successfully commercialize our product candidates and develop sales and marketing capabilities, if our
product candidates are approved; our ability to recruit and maintain key members of management and our ability to maintain and successfully enforce adequate intellectual property
protection. These and other risks and uncertainties are described more fully in the “Risk Factors” section of our most recent filings with the Securities and Exchange Commission and
available at www.sec.gov. You should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking
statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in a dynamic industry
and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face.
Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future
events, changed circumstances or otherwise. |
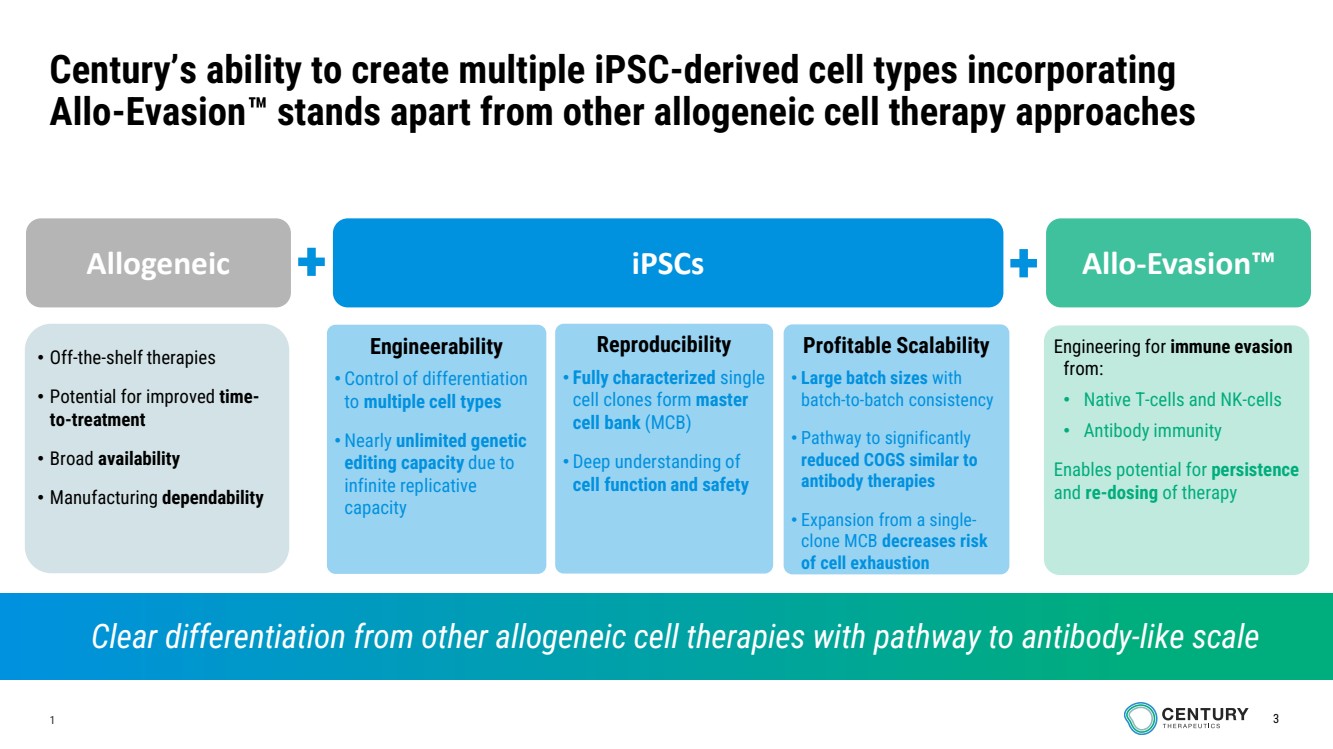
| 1 3
Century’s ability to create multiple iPSC-derived cell types incorporating
Allo-Evasion stands apart from other allogeneic cell therapy approaches
• Off-the-shelf therapies
• Potential for improved time-to-treatment
• Broad availability
• Manufacturing dependability
Engineerability
• Control of differentiation
to multiple cell types
• Nearly unlimited genetic
editing capacity due to
infinite replicative
capacity
Engineering for immune evasion
from:
• Native T-cells and NK-cells
• Antibody immunity
Enables potential for persistence
and re-dosing of therapy
Reproducibility
• Fully characterized single
cell clones form master
cell bank (MCB)
• Deep understanding of
cell function and safety
Profitable Scalability
• Large batch sizes with
batch-to-batch consistency
• Pathway to significantly
reduced COGS similar to
antibody therapies
• Expansion from a single-clone MCB decreases risk
of cell exhaustion
Allogeneic iPSCs Allo-Evasion
Clear differentiation from other allogeneic cell therapies with pathway to antibody-like scale |
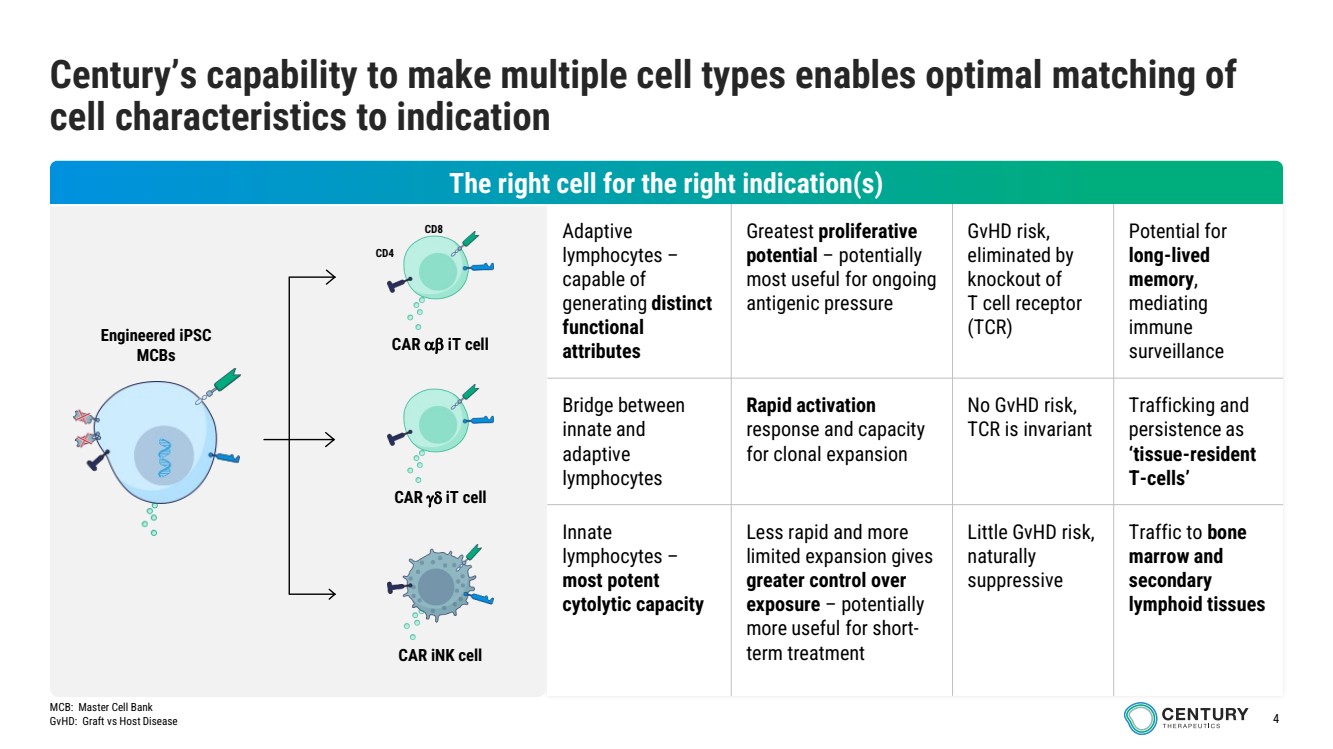
| 4
Adaptive
lymphocytes –
capable of
generating distinct
functional
attributes
Greatest proliferative
potential – potentially
most useful for ongoing
antigenic pressure
GvHD risk,
eliminated by
knockout of
T cell receptor
(TCR)
Potential for
long-lived
memory,
mediating
immune
surveillance
Bridge between
innate and
adaptive
lymphocytes
Rapid activation
response and capacity
for clonal expansion
No GvHD risk,
TCR is invariant
Trafficking and
persistence as
‘tissue-resident
T-cells’
Innate
lymphocytes –
most potent
cytolytic capacity
Less rapid and more
limited expansion gives
greater control over
exposure – potentially
more useful for short-term treatment
Little GvHD risk,
naturally
suppressive
Traffic to bone
marrow and
secondary
lymphoid tissues
Century’s capability to make multiple cell types enables optimal matching of
cell characteristics to indication
Engineered iPSC
MCBs
CAR iNK cell
CAR iT cell
CAR iT cell
CD4
CD8
The right cell for the right indication(s)
MCB: Master Cell Bank
GvHD: Graft vs Host Disease |
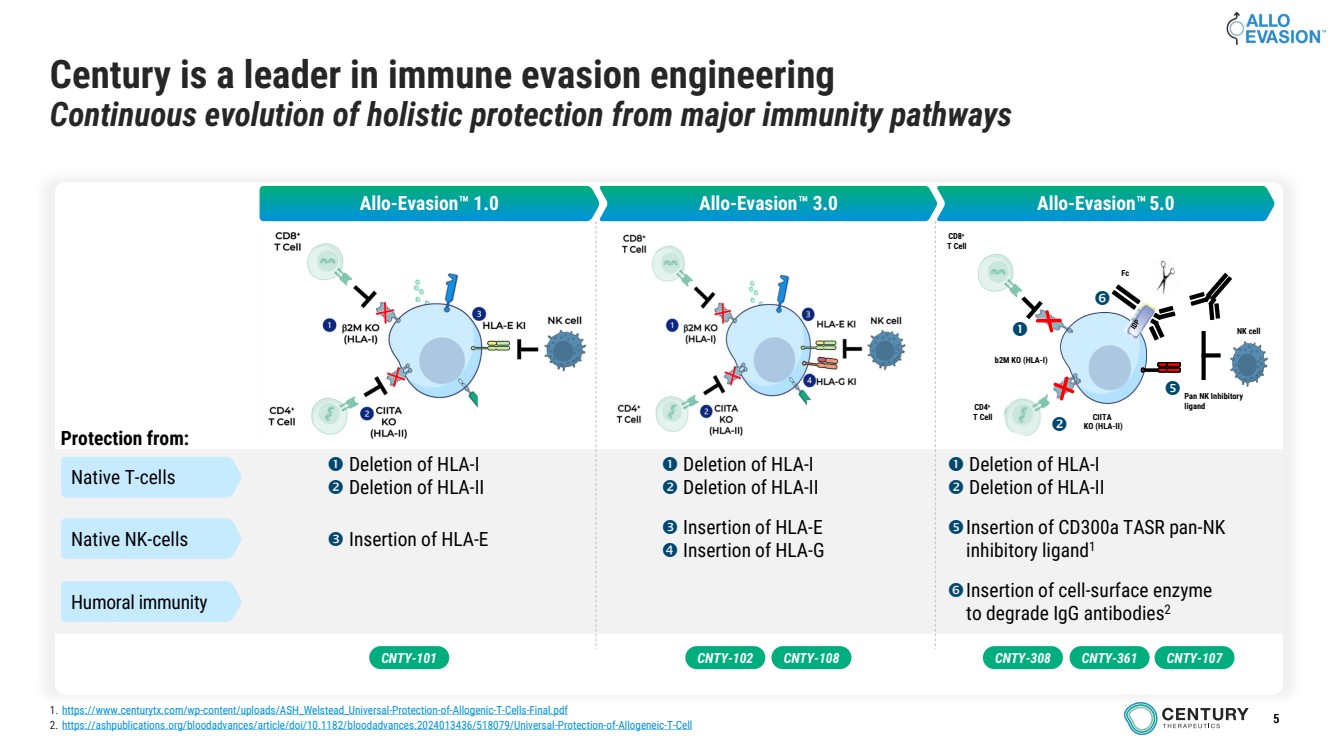
| 5
Century is a leader in immune evasion engineering
Continuous evolution of holistic protection from major immunity pathways
Allo-Evasion 1.0 Allo-Evasion 3.0 Allo-Evasion 5.0
Protection from:
Native T-cells Deletion of HLA-I
Deletion of HLA-II
Deletion of HLA-I
Deletion of HLA-II
Deletion of HLA-I
Deletion of HLA-II
Native NK-cells Insertion of HLA-E
Insertion of HLA-E
Insertion of HLA-G
Insertion of CD300a TASR pan-NK
inhibitory ligand1
Humoral immunity Insertion of cell-surface enzyme
to degrade IgG antibodies2
CNTY-101
1. https://www.centurytx.com/wp-content/uploads/ASH_Welstead_Universal-Protection-of-Allogenic-T-Cells-Final.pdf
2. https://ashpublications.org/bloodadvances/article/doi/10.1182/bloodadvances.2024013436/518079/Universal-Protection-of-Allogeneic-T-Cell
b2M KO (HLA-I)
CIITA
KO (HLA-II)
CD8+
T Cell
CD4+
T Cell
Pan NK Inhibitory
ligand
Fc
NK cell
CNTY-102 CNTY-108 CNTY-308 CNTY-361 CNTY-107
|
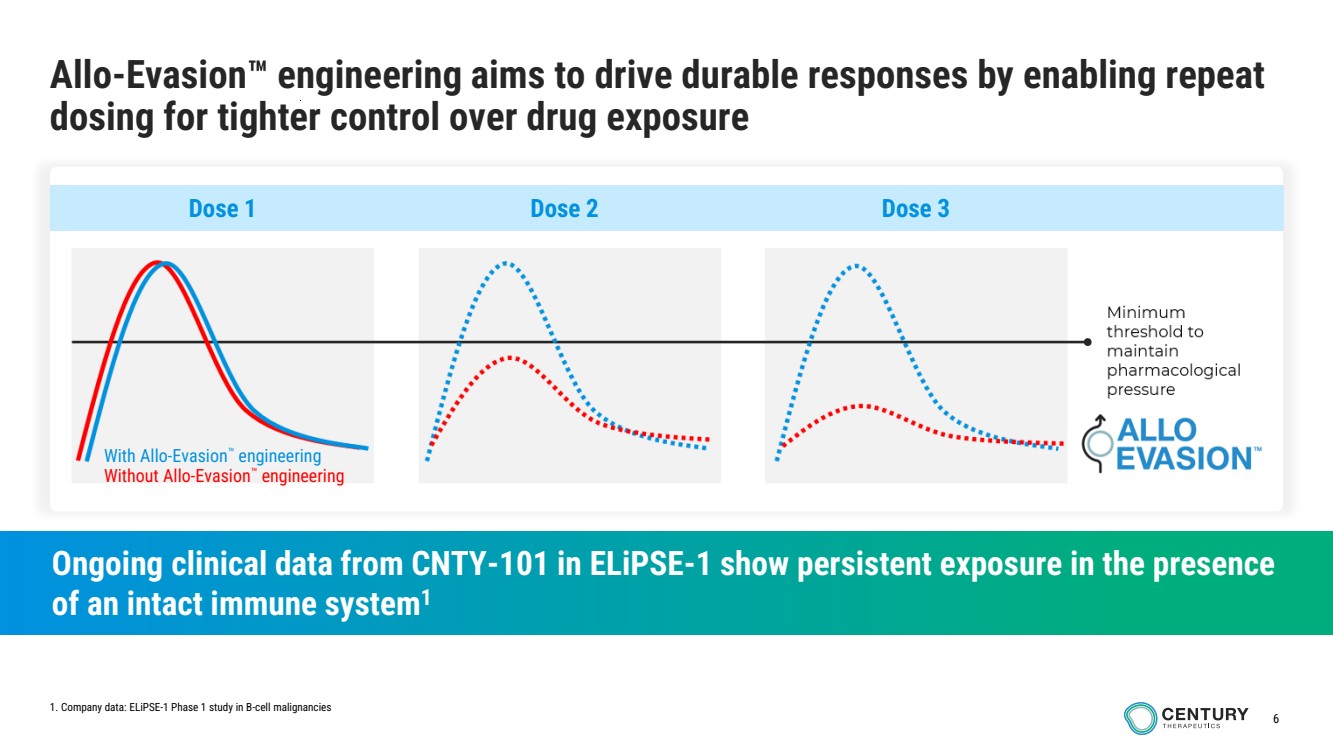
| 6
Allo-Evasion engineering aims to drive durable responses by enabling repeat
dosing for tighter control over drug exposure
Ongoing clinical data from CNTY-101 in ELiPSE-1 show persistent exposure in the presence
of an intact immune system1
With Allo-Evasion engineering
Without Allo-Evasion engineering
Dose 1 Dose 2 Dose 3
1. Company data: ELiPSE-1 Phase 1 study in B-cell malignancies |
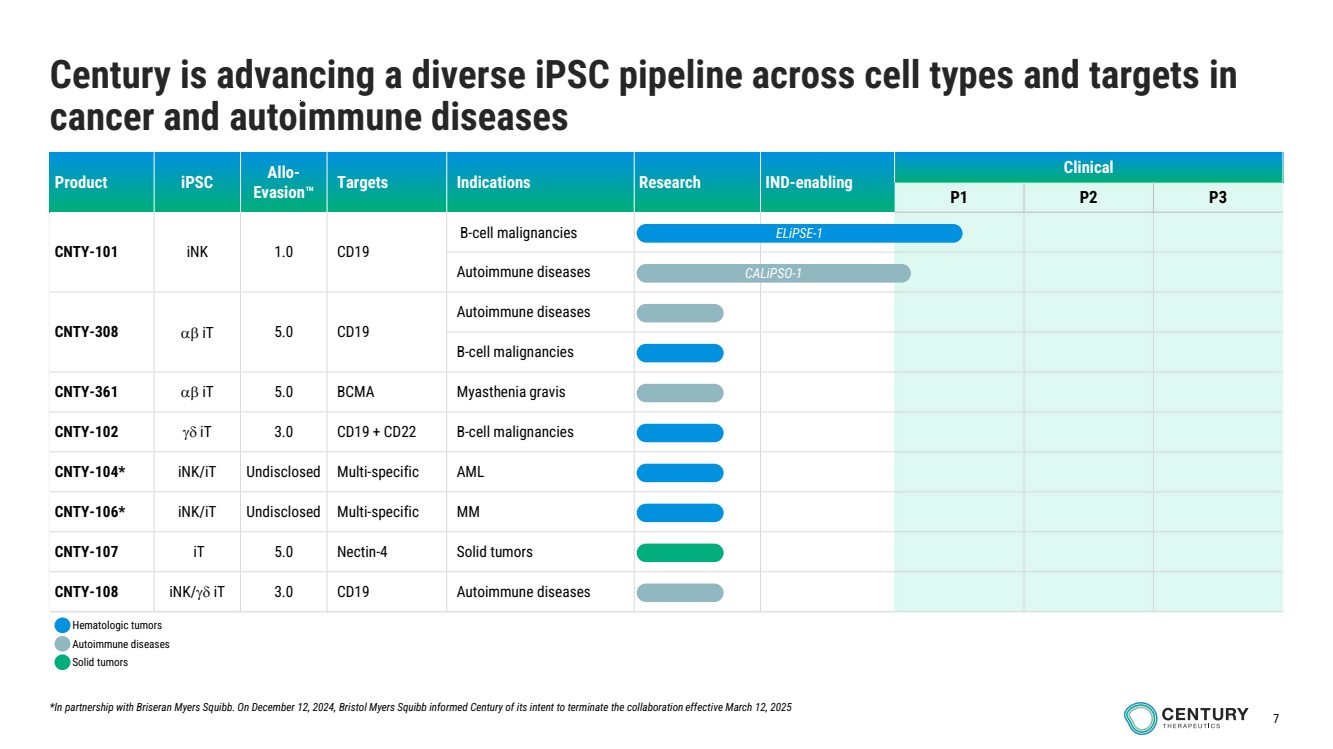
| 7
Product iPSC Allo-Evasion Targets Indications Research IND-enabling
Clinical
P1 P2 P3
CNTY-101 iNK 1.0 CD19
B-cell malignancies
Autoimmune diseases
CNTY-308 iT 5.0 CD19
Autoimmune diseases
B-cell malignancies
CNTY-361 iT 5.0 BCMA Myasthenia gravis
CNTY-102 iT 3.0 CD19 + CD22 B-cell malignancies
CNTY-104* iNK/iT Undisclosed Multi-specific AML
CNTY-106* iNK/iT Undisclosed Multi-specific MM
CNTY-107 iT 5.0 Nectin-4 Solid tumors
CNTY-108 iNK/ iT 3.0 CD19 Autoimmune diseases
Century is advancing a diverse iPSC pipeline across cell types and targets in
cancer and autoimmune diseases
CALiPSO-1
ELiPSE-1
*In partnership with Briseran Myers Squibb. On December 12, 2024, Bristol Myers Squibb informed Century of its intent to terminate the collaboration effective March 12, 2025
Autoimmune diseases
Hematologic tumors
Solid tumors |

| CNTY-101
CAR-iNK cell therapy with Allo-Evasion 1.0 |
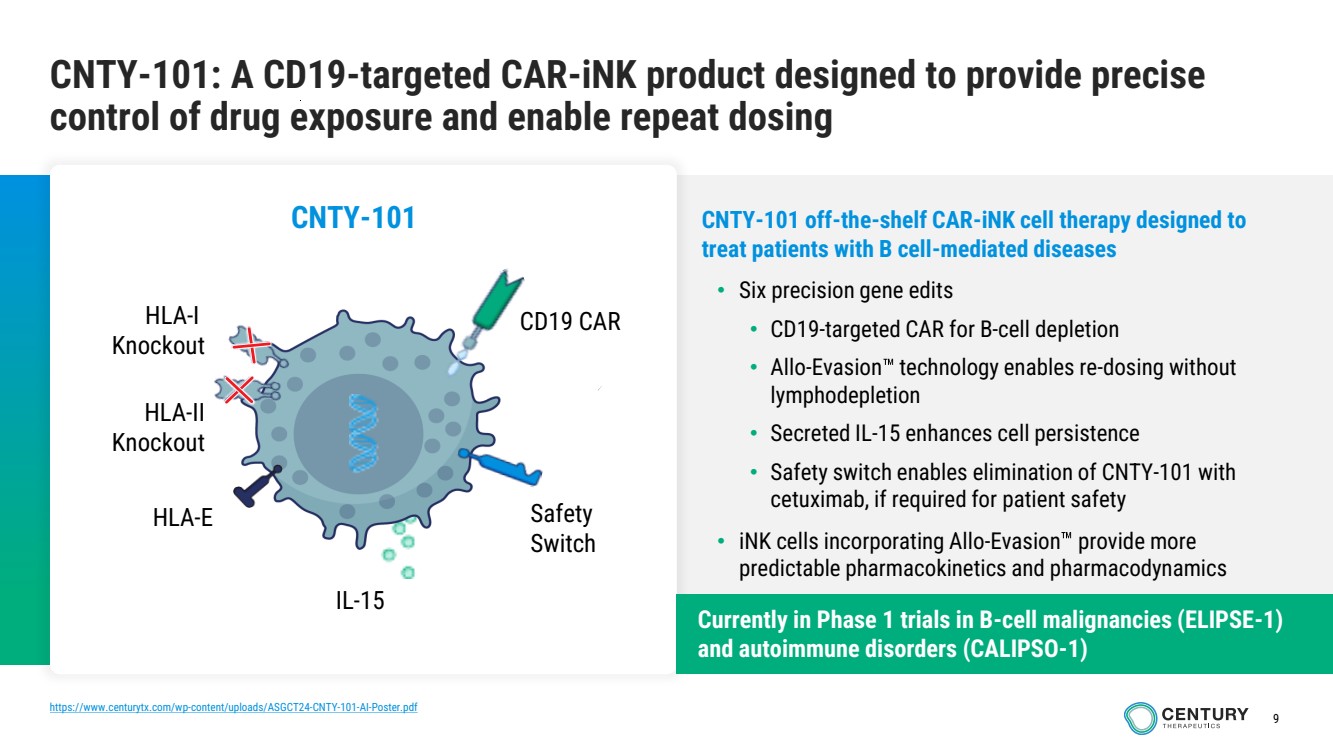
| 9
CNTY-101: A CD19-targeted CAR-iNK product designed to provide precise
control of drug exposure and enable repeat dosing
CNTY-101 off-the-shelf CAR-iNK cell therapy designed to
treat patients with B cell-mediated diseases
• Six precision gene edits
• CD19-targeted CAR for B-cell depletion
• Allo-Evasion technology enables re-dosing without
lymphodepletion
• Secreted IL-15 enhances cell persistence
• Safety switch enables elimination of CNTY-101 with
cetuximab, if required for patient safety
• iNK cells incorporating Allo-Evasion provide more
predictable pharmacokinetics and pharmacodynamics
CNTY-101
HLA-I
Knockout
IL-15
HLA-II
Knockout
CD19 CAR
HLA-E Safety
Switch
Currently in Phase 1 trials in B-cell malignancies (ELIPSE-1)
and autoimmune disorders (CALIPSO-1)
https://www.centurytx.com/wp-content/uploads/ASGCT24-CNTY-101-AI-Poster.pdf |
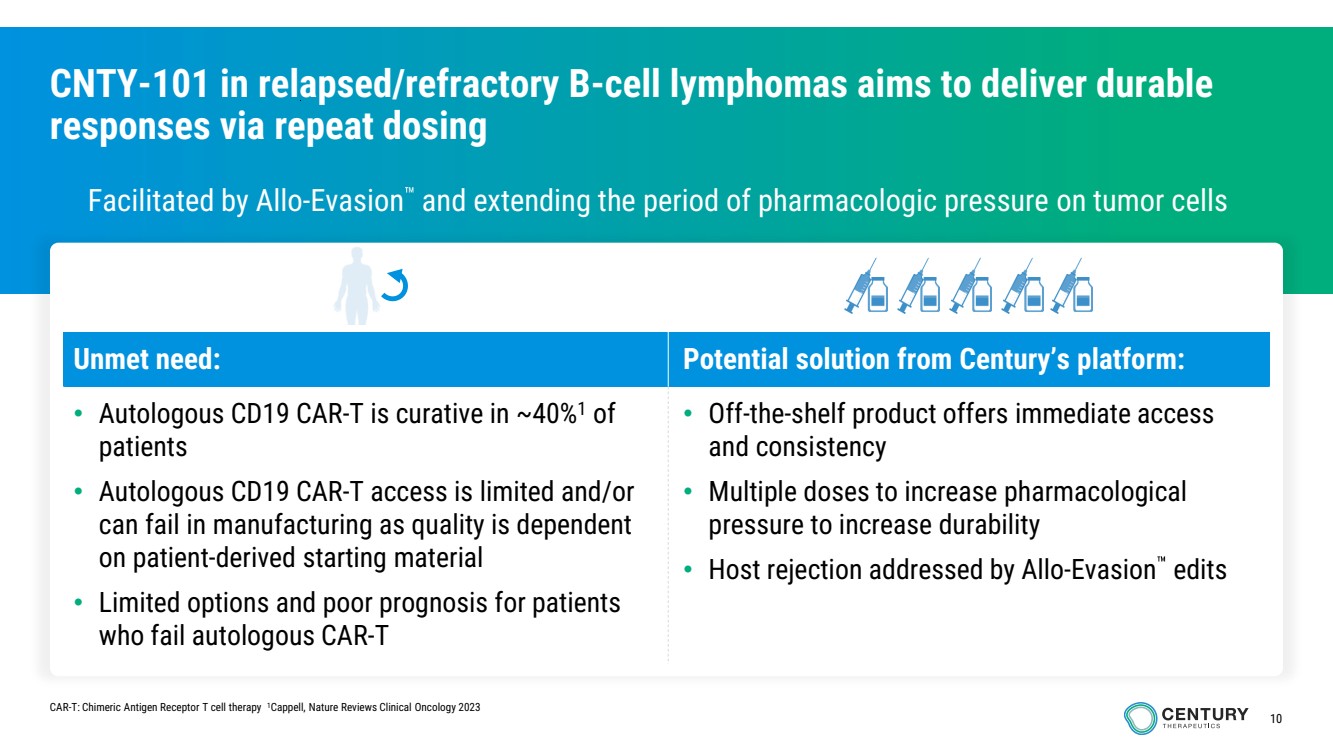
| 10
CNTY-101 in relapsed/refractory B-cell lymphomas aims to deliver durable
responses via repeat dosing
Facilitated by Allo-Evasion and extending the period of pharmacologic pressure on tumor cells
Unmet need: Potential solution from Century’s platform:
• Autologous CD19 CAR-T is curative in ~40%1 of
patients
• Autologous CD19 CAR-T access is limited and/or
can fail in manufacturing as quality is dependent
on patient-derived starting material
• Limited options and poor prognosis for patients
who fail autologous CAR-T
• Off-the-shelf product offers immediate access
and consistency
• Multiple doses to increase pharmacological
pressure to increase durability
• Host rejection addressed by Allo-Evasion edits
CAR-T: Chimeric Antigen Receptor T cell therapy 1Cappell, Nature Reviews Clinical Oncology 2023 |
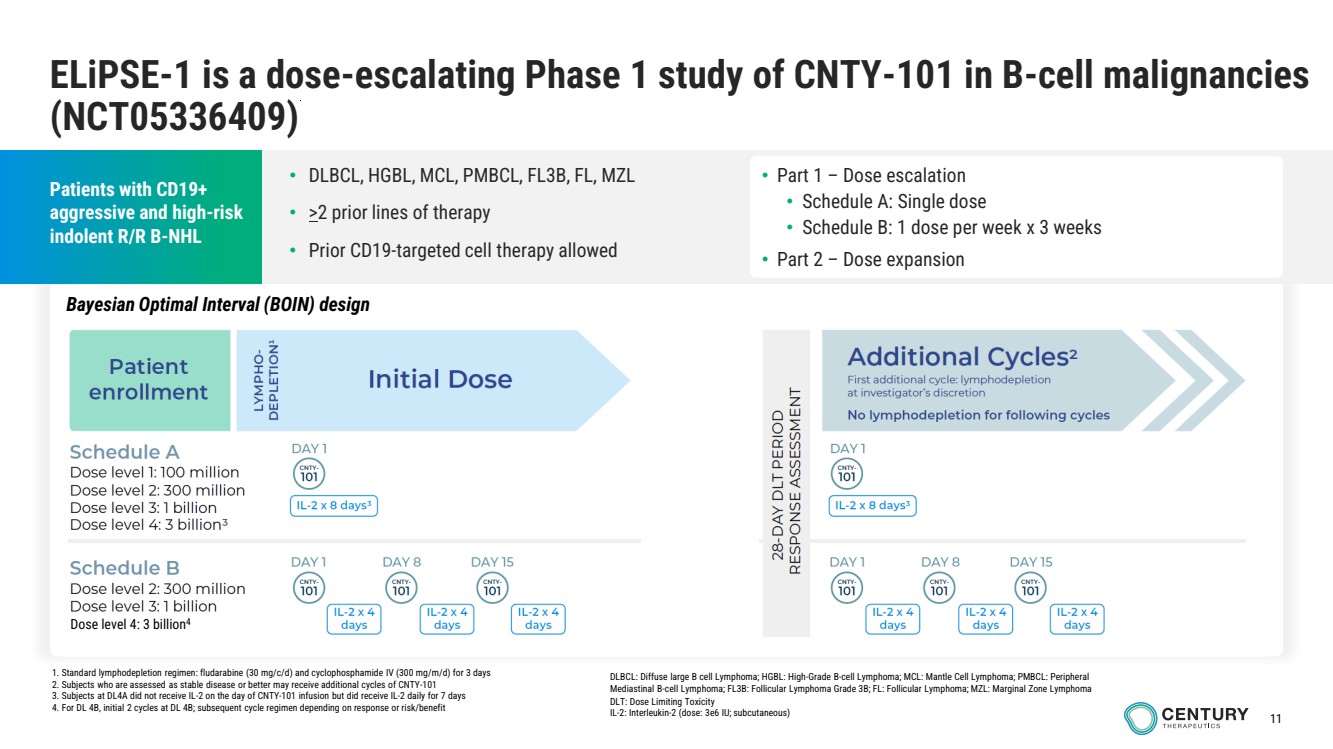
| 11
ELiPSE-1 is a dose-escalating Phase 1 study of CNTY-101 in B-cell malignancies
(NCT05336409)
Patients with CD19+
aggressive and high-risk
indolent R/R B-NHL
• Part 1 – Dose escalation
• Schedule A: Single dose
• Schedule B: 1 dose per week x 3 weeks
• Part 2 – Dose expansion
DLBCL: Diffuse large B cell Lymphoma; HGBL: High-Grade B-cell Lymphoma; MCL: Mantle Cell Lymphoma; PMBCL: Peripheral
Mediastinal B-cell Lymphoma; FL3B: Follicular Lymphoma Grade 3B; FL: Follicular Lymphoma; MZL: Marginal Zone Lymphoma
DLT: Dose Limiting Toxicity
IL-2: Interleukin-2 (dose: 3e6 IU; subcutaneous)
• DLBCL, HGBL, MCL, PMBCL, FL3B, FL, MZL
• >2 prior lines of therapy
• Prior CD19-targeted cell therapy allowed
Dose level 4: 3 billion4
Bayesian Optimal Interval (BOIN) design
1. Standard lymphodepletion regimen: fludarabine (30 mg/c/d) and cyclophosphamide IV (300 mg/m/d) for 3 days
2. Subjects who are assessed as stable disease or better may receive additional cycles of CNTY-101
3. Subjects at DL4A did not receive IL-2 on the day of CNTY-101 infusion but did receive IL-2 daily for 7 days
4. For DL 4B, initial 2 cycles at DL 4B; subsequent cycle regimen depending on response or risk/benefit |
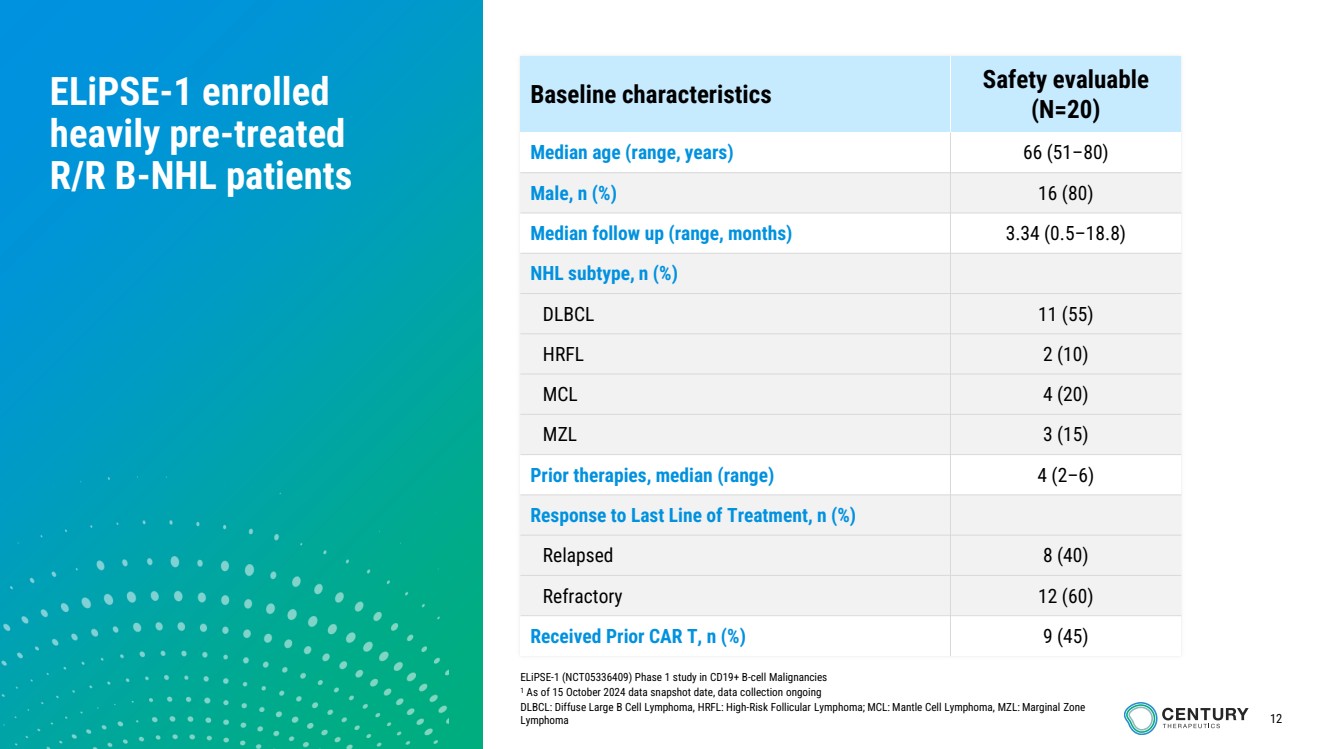
| 12
ELiPSE-1 enrolled
heavily pre-treated
R/R B-NHL patients
ELiPSE-1 (NCT05336409) Phase 1 study in CD19+ B-cell Malignancies
1 As of 15 October 2024 data snapshot date, data collection ongoing
DLBCL: Diffuse Large B Cell Lymphoma, HRFL: High-Risk Follicular Lymphoma; MCL: Mantle Cell Lymphoma, MZL: Marginal Zone
Lymphoma
Baseline characteristics Safety evaluable
(N=20)
Median age (range, years) 66 (51–80)
Male, n (%) 16 (80)
Median follow up (range, months) 3.34 (0.5–18.8)
NHL subtype, n (%)
DLBCL 11 (55)
HRFL 2 (10)
MCL 4 (20)
MZL 3 (15)
Prior therapies, median (range) 4 (2–6)
Response to Last Line of Treatment, n (%)
Relapsed 8 (40)
Refractory 12 (60)
Received Prior CAR T, n (%) 9 (45) |
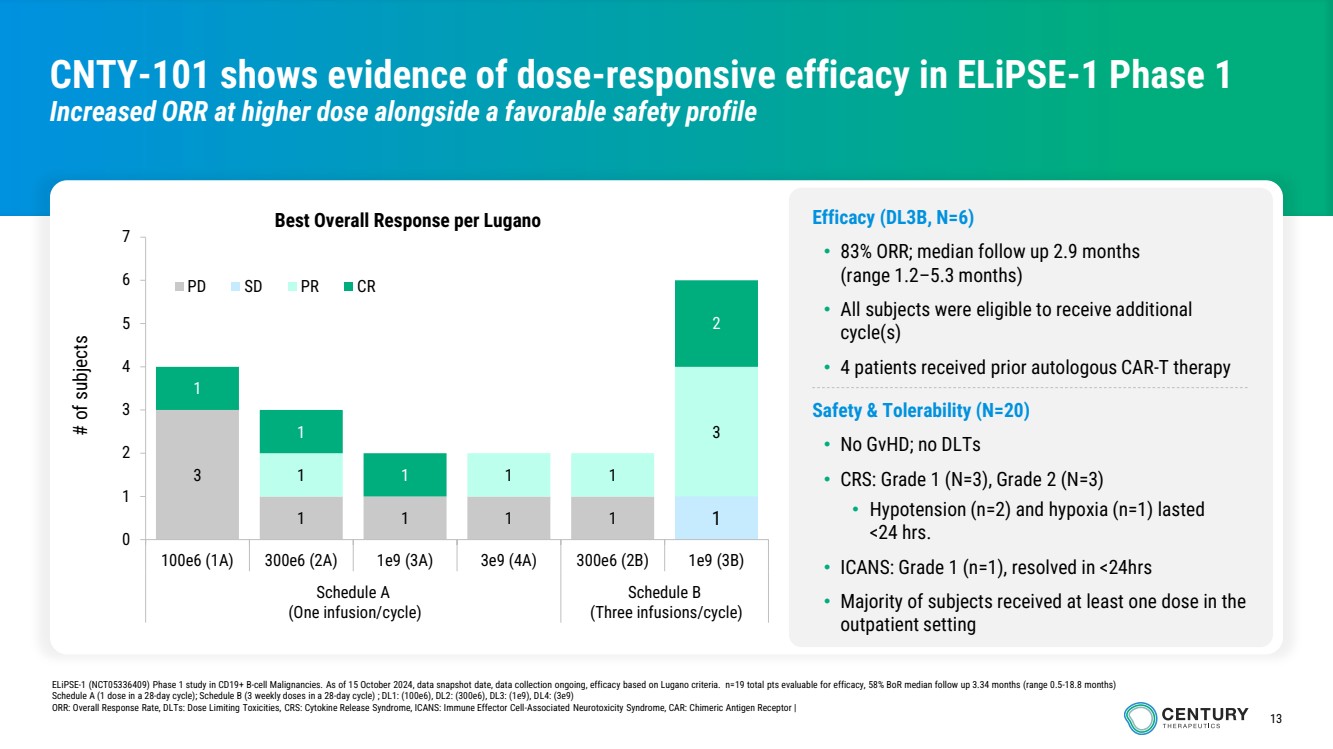
| 13
CNTY-101 shows evidence of dose-responsive efficacy in ELiPSE-1 Phase 1
Increased ORR at higher dose alongside a favorable safety profile
ELiPSE-1 (NCT05336409) Phase 1 study in CD19+ B-cell Malignancies. As of 15 October 2024, data snapshot date, data collection ongoing, efficacy based on Lugano criteria. n=19 total pts evaluable for efficacy, 58% BoR median follow up 3.34 months (range 0.5-18.8 months)
Schedule A (1 dose in a 28-day cycle); Schedule B (3 weekly doses in a 28-day cycle) ; DL1: (100e6), DL2: (300e6), DL3: (1e9), DL4: (3e9)
ORR: Overall Response Rate, DLTs: Dose Limiting Toxicities, CRS: Cytokine Release Syndrome, ICANS: Immune Effector Cell-Associated Neurotoxicity Syndrome, CAR: Chimeric Antigen Receptor |
Efficacy (DL3B, N=6)
• 83% ORR; median follow up 2.9 months
(range 1.2–5.3 months)
• All subjects were eligible to receive additional
cycle(s)
• 4 patients received prior autologous CAR-T therapy
Safety & Tolerability (N=20)
• No GvHD; no DLTs
• CRS: Grade 1 (N=3), Grade 2 (N=3)
• Hypotension (n=2) and hypoxia (n=1) lasted
<24 hrs.
• ICANS: Grade 1 (n=1), resolved in <24hrs
• Majority of subjects received at least one dose in the
outpatient setting
3
1 1 1 1 1
1 1 1
3
1
1
1
2
0
1
2
3
4
5
6
7
100e6 (1A) 300e6 (2A) 1e9 (3A) 3e9 (4A) 300e6 (2B) 1e9 (3B)
Schedule A
(One infusion/cycle)
Schedule B
(Three infusions/cycle)
# of subjects
Best Overall Response per Lugano
PD SD PR CR |
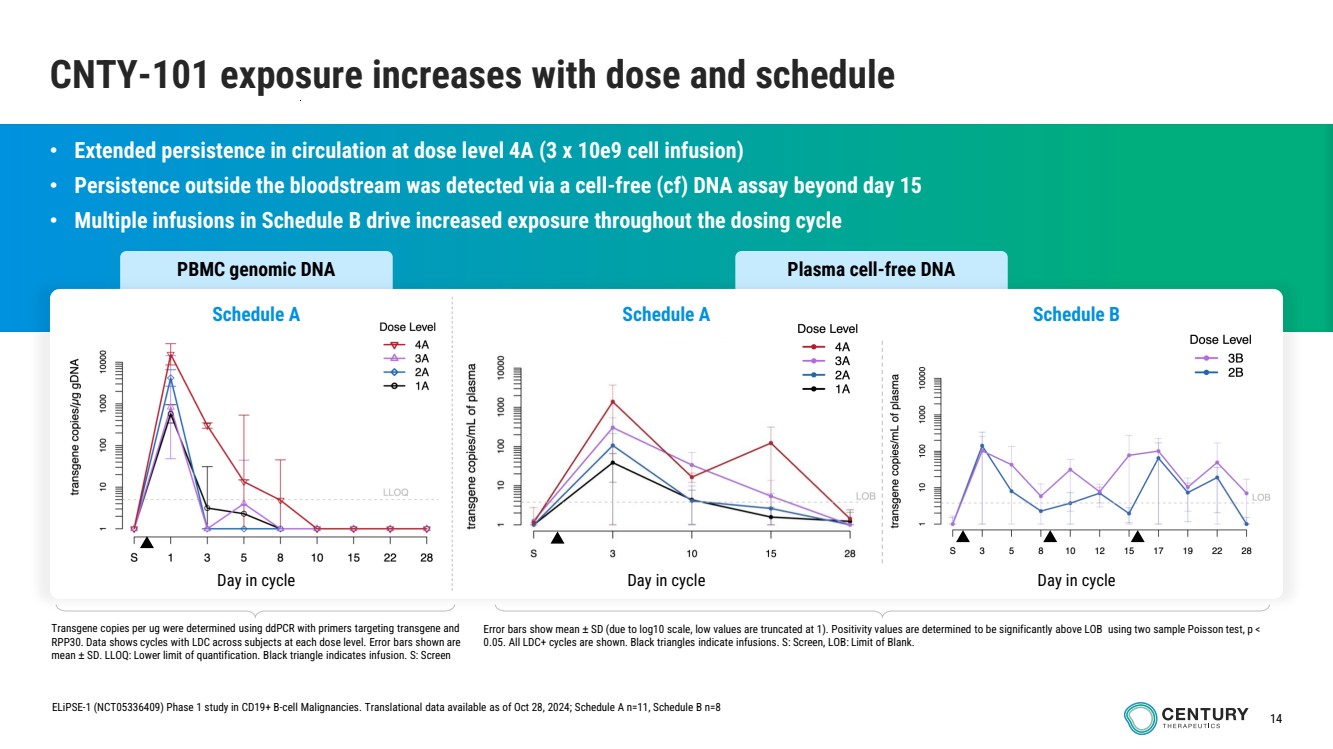
| 14
PBMC genomic DNA Plasma cell-free DNA
CNTY-101 exposure increases with dose and schedule
• Extended persistence in circulation at dose level 4A (3 x 10e9 cell infusion)
• Persistence outside the bloodstream was detected via a cell-free (cf) DNA assay beyond day 15
• Multiple infusions in Schedule B drive increased exposure throughout the dosing cycle
Transgene copies per ug were determined using ddPCR with primers targeting transgene and
RPP30. Data shows cycles with LDC across subjects at each dose level. Error bars shown are
mean ± SD. LLOQ: Lower limit of quantification. Black triangle indicates infusion. S: Screen
Error bars show mean ± SD (due to log10 scale, low values are truncated at 1). Positivity values are determined to be significantly above LOB using two sample Poisson test, p <
0.05. All LDC+ cycles are shown. Black triangles indicate infusions. S: Screen, LOB: Limit of Blank.
Schedule A Schedule B
Day in cycle Day in cycle
ELiPSE-1 (NCT05336409) Phase 1 study in CD19+ B-cell Malignancies. Translational data available as of Oct 28, 2024; Schedule A n=11, Schedule B n=8
Schedule A
Day in cycle |
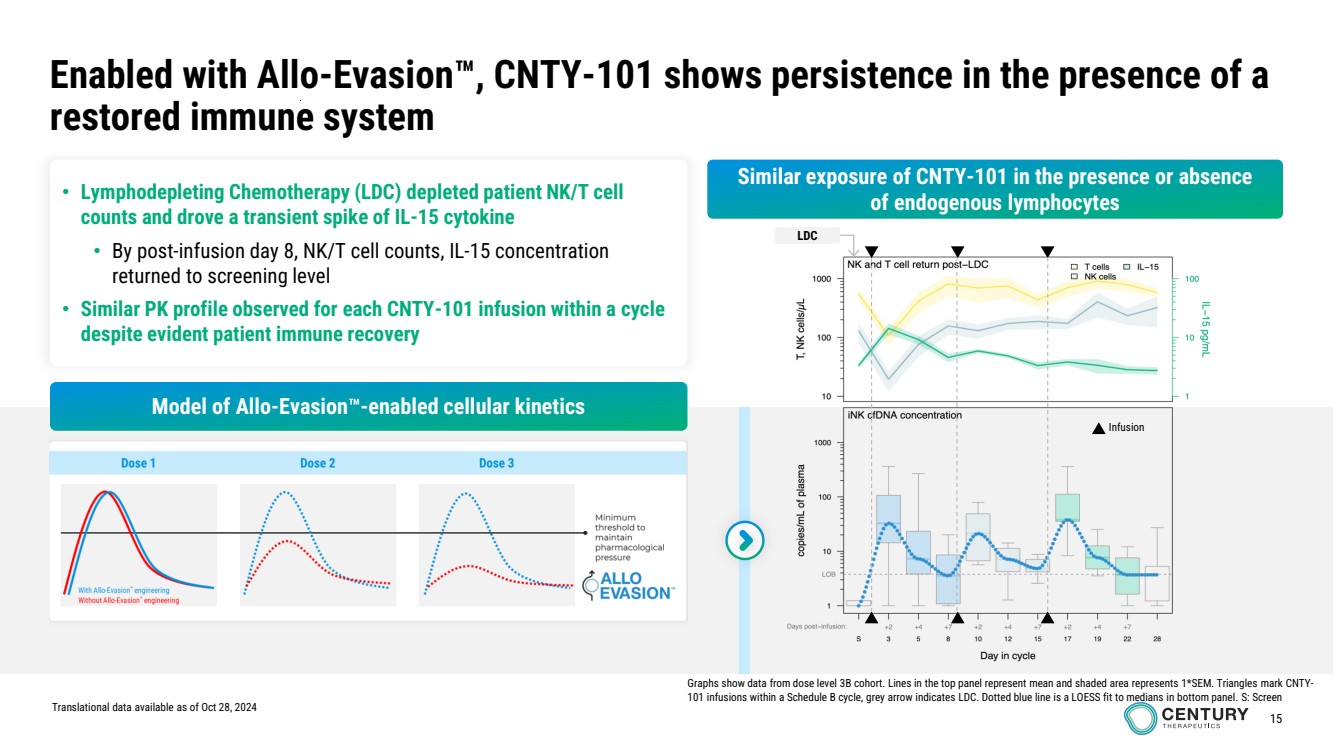
| 15
• Lymphodepleting Chemotherapy (LDC) depleted patient NK/T cell
counts and drove a transient spike of IL-15 cytokine
• By post-infusion day 8, NK/T cell counts, IL-15 concentration
returned to screening level
• Similar PK profile observed for each CNTY-101 infusion within a cycle
despite evident patient immune recovery
Enabled with Allo-Evasion , CNTY-101 shows persistence in the presence of a
restored immune system
Translational data available as of Oct 28, 2024
Graphs show data from dose level 3B cohort. Lines in the top panel represent mean and shaded area represents 1*SEM. Triangles mark CNTY-101 infusions within a Schedule B cycle, grey arrow indicates LDC. Dotted blue line is a LOESS fit to medians in bottom panel. S: Screen
Similar exposure of CNTY-101 in the presence or absence
of endogenous lymphocytes
Infusion
LDC
Model of Allo-Evasion -enabled cellular kinetics |
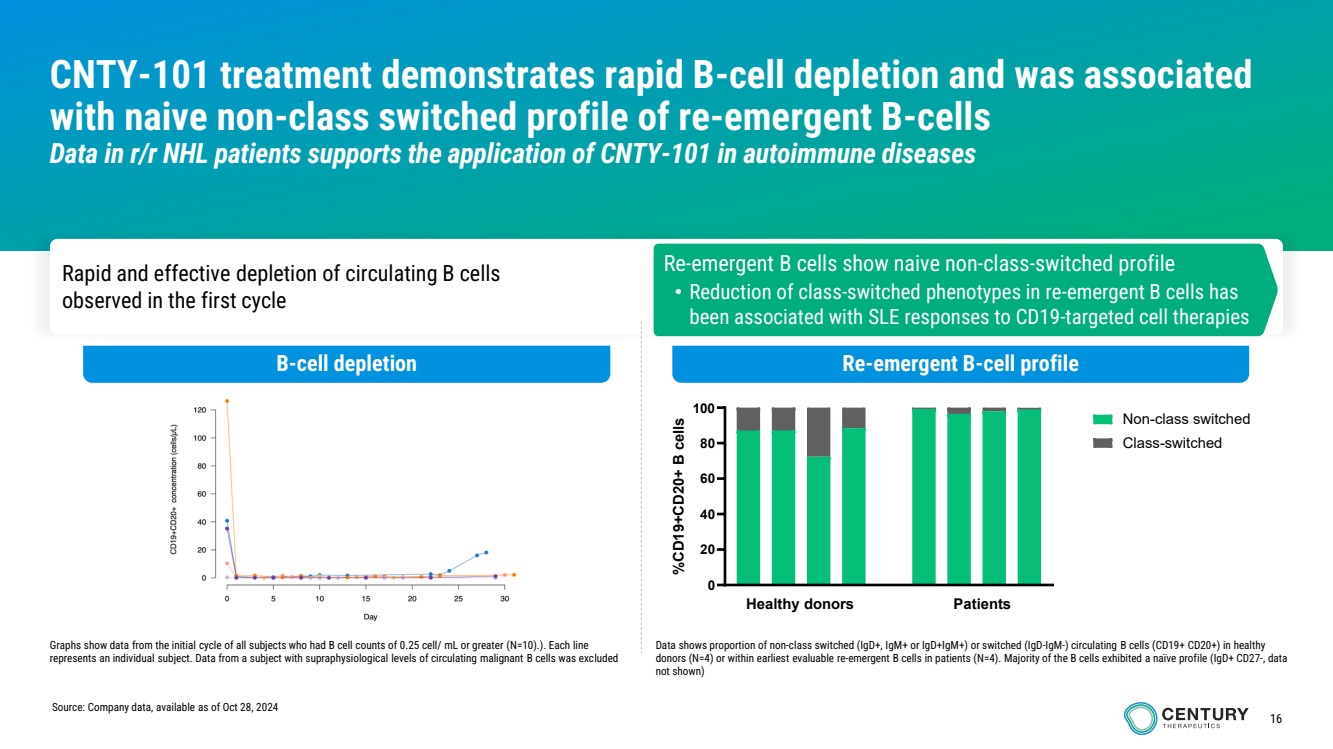
| 16
CNTY-101 treatment demonstrates rapid B-cell depletion and was associated
with naive non-class switched profile of re-emergent B-cells
Data in r/r NHL patients supports the application of CNTY-101 in autoimmune diseases
B-cell depletion Re-emergent B-cell profile
Rapid and effective depletion of circulating B cells
observed in the first cycle
0
20
40
60
80
100
%CD19+CD20+ B cells
Non-class switched Class-switched
Healthy donors Patients
Re-emergent B cells show naive non-class-switched profile
• Reduction of class-switched phenotypes in re-emergent B cells has
been associated with SLE responses to CD19-targeted cell therapies
Graphs show data from the initial cycle of all subjects who had B cell counts of 0.25 cell/ mL or greater (N=10).). Each line
represents an individual subject. Data from a subject with supraphysiological levels of circulating malignant B cells was excluded
Data shows proportion of non-class switched (IgD+, IgM+ or IgD+IgM+) or switched (IgD-IgM-) circulating B cells (CD19+ CD20+) in healthy
donors (N=4) or within earliest evaluable re-emergent B cells in patients (N=4). Majority of the B cells exhibited a naïve profile (IgD+ CD27-, data
not shown)
Source: Company data, available as of Oct 28, 2024 |
| 17
ELiPSE-1 initial data validates Century’s iPSC platform
Heavily pretreated and refractory patient population treated in first-in-human dose escalation
trial, including ~50% patients who had received prior CAR T treatments
Favorable initial safety profile; can be delivered in an outpatient setting
Increased response rates at higher doses and observations of deepening responses with additional
cycles.
83% ORR at Dose Level 3B
Dose-dependent increase in CNTY-101 exposure observed
Data for CNTY-101 continues to support the potential for Allo-Evasion to enable a multi-dosing regimen
in the presence of a restored endogenous immune system
ORR: Overall Response Rate |
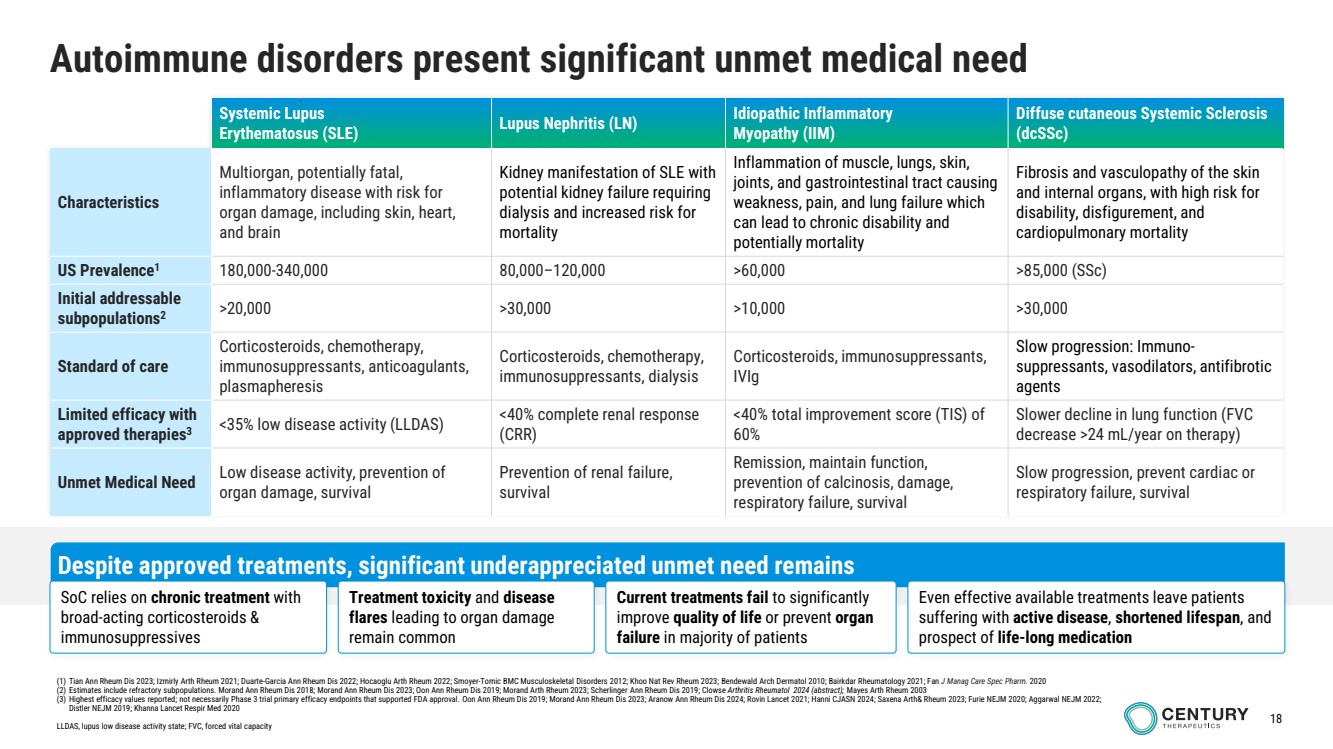
| 18
Autoimmune disorders present significant unmet medical need
Systemic Lupus
Erythematosus (SLE) Lupus Nephritis (LN) Idiopathic Inflammatory
Myopathy (IIM)
Diffuse cutaneous Systemic Sclerosis
(dcSSc)
Characteristics
Multiorgan, potentially fatal,
inflammatory disease with risk for
organ damage, including skin, heart,
and brain
Kidney manifestation of SLE with
potential kidney failure requiring
dialysis and increased risk for
mortality
Inflammation of muscle, lungs, skin,
joints, and gastrointestinal tract causing
weakness, pain, and lung failure which
can lead to chronic disability and
potentially mortality
Fibrosis and vasculopathy of the skin
and internal organs, with high risk for
disability, disfigurement, and
cardiopulmonary mortality
US Prevalence1 180,000-340,000 80,000–120,000 >60,000 >85,000 (SSc)
Initial addressable
subpopulations2
>20,000 >30,000 >10,000 >30,000
Standard of care
Corticosteroids, chemotherapy,
immunosuppressants, anticoagulants,
plasmapheresis
Corticosteroids, chemotherapy,
immunosuppressants, dialysis
Corticosteroids, immunosuppressants,
IVIg
Slow progression: Immuno-suppressants, vasodilators, antifibrotic
agents
Limited efficacy with
approved therapies3
<35% low disease activity (LLDAS) <40% complete renal response
(CRR)
<40% total improvement score (TIS) of
60%
Slower decline in lung function (FVC
decrease >24 mL/year on therapy)
Unmet Medical Need Low disease activity, prevention of
organ damage, survival
Prevention of renal failure,
survival
Remission, maintain function,
prevention of calcinosis, damage,
respiratory failure, survival
Slow progression, prevent cardiac or
respiratory failure, survival
Despite approved treatments, significant underappreciated unmet need remains
Even effective available treatments leave patients
suffering with active disease, shortened lifespan, and
prospect of life-long medication
SoC relies on chronic treatment with
broad-acting corticosteroids &
immunosuppressives
Treatment toxicity and disease
flares leading to organ damage
remain common
Current treatments fail to significantly
improve quality of life or prevent organ
failure in majority of patients
(1) Tian Ann Rheum Dis 2023; Izmirly Arth Rheum 2021; Duarte-Garcia Ann Rheum Dis 2022; Hocaoglu Arth Rheum 2022; Smoyer-Tomic BMC Musculoskeletal Disorders 2012; Khoo Nat Rev Rheum 2023; Bendewald Arch Dermatol 2010; Bairkdar Rheumatology 2021; Fan J Manag Care Spec Pharm. 2020
(2) Estimates include refractory subpopulations. Morand Ann Rheum Dis 2018; Morand Ann Rheum Dis 2023; Oon Ann Rheum Dis 2019; Morand Arth Rheum 2023; Scherlinger Ann Rheum Dis 2019; Clowse Arthritis Rheumatol 2024 (abstract); Mayes Arth Rheum 2003
(3) Highest efficacy values reported; not necessarily Phase 3 trial primary efficacy endpoints that supported FDA approval. Oon Ann Rheum Dis 2019; Morand Ann Rheum Dis 2023; Aranow Ann Rheum Dis 2024; Rovin Lancet 2021; Hanni CJASN 2024; Saxena Arth& Rheum 2023; Furie NEJM 2020; Aggarwal NEJM 2022;
Distler NEJM 2019; Khanna Lancet Respir Med 2020
LLDAS, lupus low disease activity state; FVC, forced vital capacity |
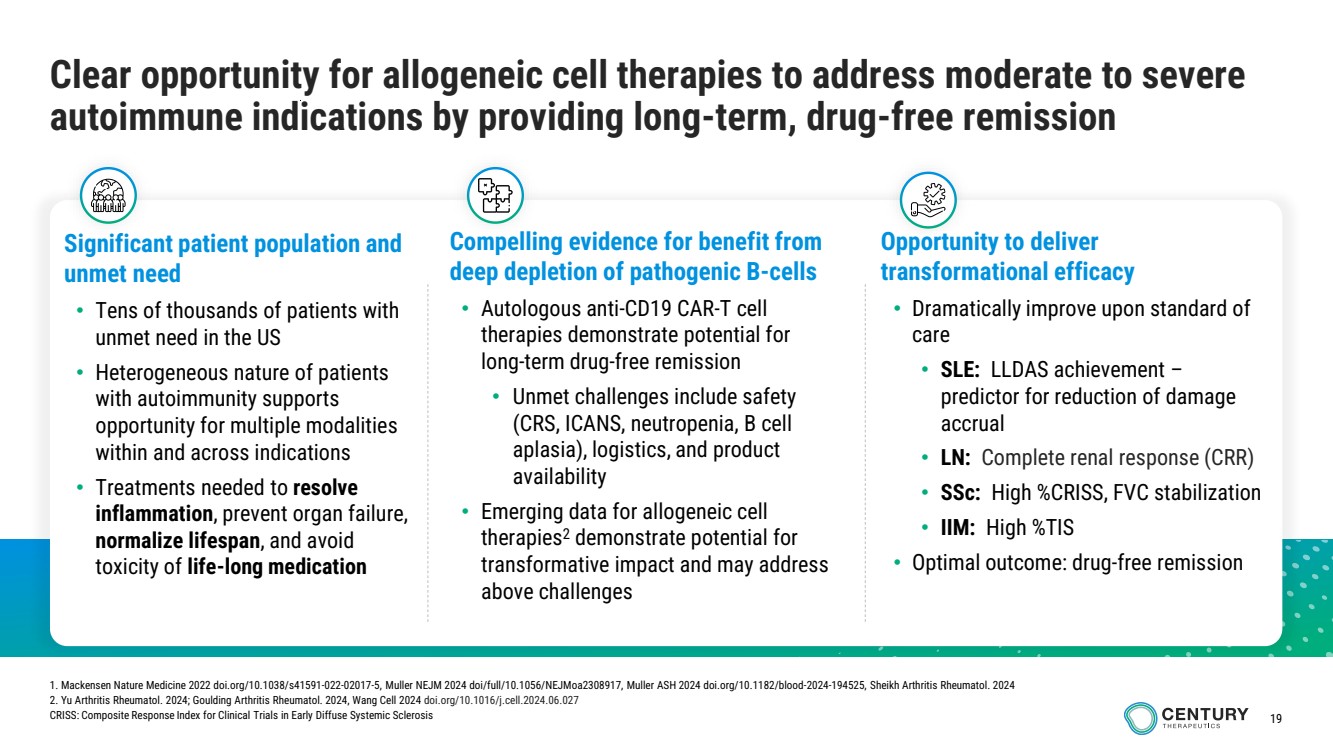
| 19
Clear opportunity for allogeneic cell therapies to address moderate to severe
autoimmune indications by providing long-term, drug-free remission
Significant patient population and
unmet need
• Tens of thousands of patients with
unmet need in the US
• Heterogeneous nature of patients
with autoimmunity supports
opportunity for multiple modalities
within and across indications
• Treatments needed to resolve
inflammation, prevent organ failure,
normalize lifespan, and avoid
toxicity of life-long medication
Opportunity to deliver
transformational efficacy
• Dramatically improve upon standard of
care
• SLE: LLDAS achievement –
predictor for reduction of damage
accrual
• LN: Complete renal response (CRR)
• SSc: High %CRISS, FVC stabilization
• IIM: High %TIS
• Optimal outcome: drug-free remission
Compelling evidence for benefit from
deep depletion of pathogenic B-cells
• Autologous anti-CD19 CAR-T cell
therapies demonstrate potential for
long-term drug-free remission
• Unmet challenges include safety
(CRS, ICANS, neutropenia, B cell
aplasia), logistics, and product
availability
• Emerging data for allogeneic cell
therapies2 demonstrate potential for
transformative impact and may address
above challenges
1. Mackensen Nature Medicine 2022 doi.org/10.1038/s41591-022-02017-5, Muller NEJM 2024 doi/full/10.1056/NEJMoa2308917, Muller ASH 2024 doi.org/10.1182/blood-2024-194525, Sheikh Arthritis Rheumatol. 2024
2. Yu Arthritis Rheumatol. 2024; Goulding Arthritis Rheumatol. 2024, Wang Cell 2024 doi.org/10.1016/j.cell.2024.06.027
CRISS: Composite Response Index for Clinical Trials in Early Diffuse Systemic Sclerosis |
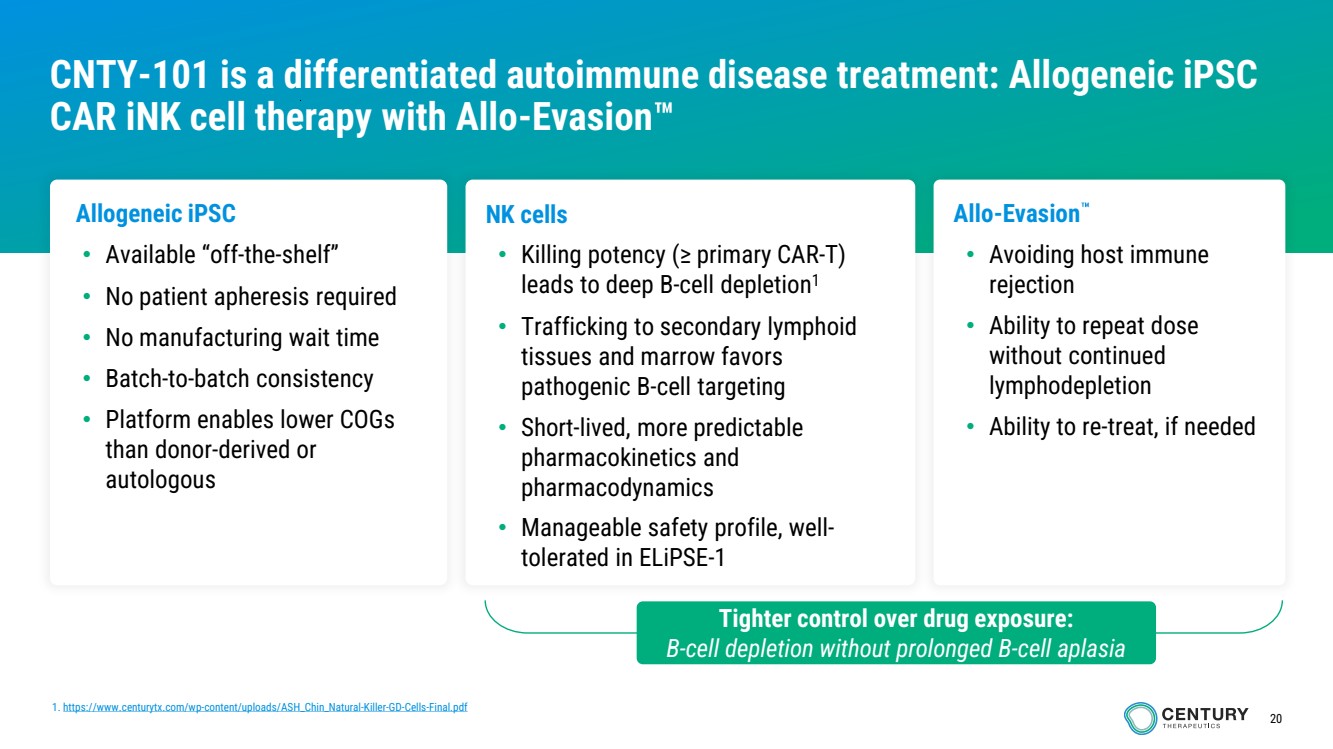
| 20
Allogeneic iPSC
• Available “off-the-shelf”
• No patient apheresis required
• No manufacturing wait time
• Batch-to-batch consistency
• Platform enables lower COGs
than donor-derived or
autologous
NK cells
• Killing potency (≥ primary CAR-T)
leads to deep B-cell depletion1
• Trafficking to secondary lymphoid
tissues and marrow favors
pathogenic B-cell targeting
• Short-lived, more predictable
pharmacokinetics and
pharmacodynamics
• Manageable safety profile, well-tolerated in ELiPSE-1
Allo-Evasion
• Avoiding host immune
rejection
• Ability to repeat dose
without continued
lymphodepletion
• Ability to re-treat, if needed
CNTY-101 is a differentiated autoimmune disease treatment: Allogeneic iPSC
CAR iNK cell therapy with Allo-Evasion
1. https://www.centurytx.com/wp-content/uploads/ASH_Chin_Natural-Killer-GD-Cells-Final.pdf
Tighter control over drug exposure:
B-cell depletion without prolonged B-cell aplasia |
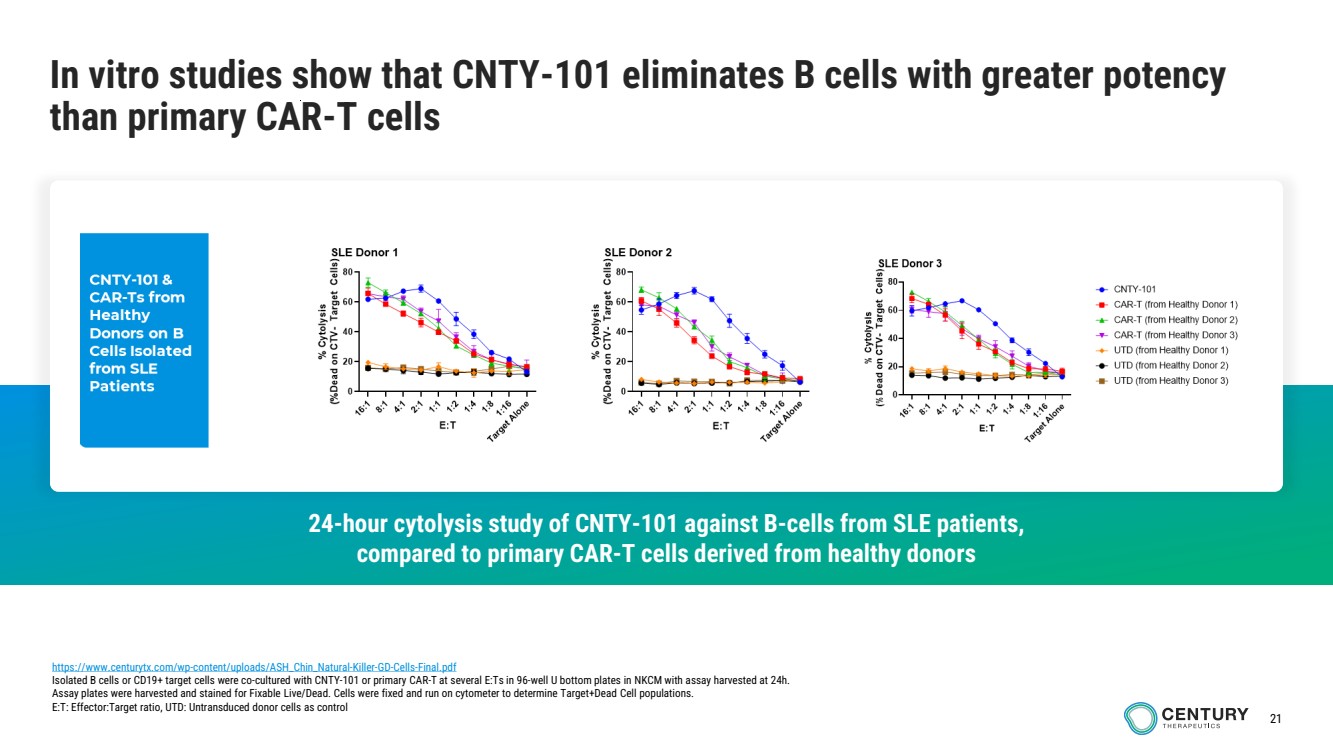
| 21
In vitro studies show that CNTY-101 eliminates B cells with greater potency
than primary CAR-T cells
https://www.centurytx.com/wp-content/uploads/ASH_Chin_Natural-Killer-GD-Cells-Final.pdf
Isolated B cells or CD19+ target cells were co-cultured with CNTY-101 or primary CAR-T at several E:Ts in 96-well U bottom plates in NKCM with assay harvested at 24h.
Assay plates were harvested and stained for Fixable Live/Dead. Cells were fixed and run on cytometer to determine Target+Dead Cell populations.
E:T: Effector:Target ratio, UTD: Untransduced donor cells as control
24-hour cytolysis study of CNTY-101 against B-cells from SLE patients,
compared to primary CAR-T cells derived from healthy donors |
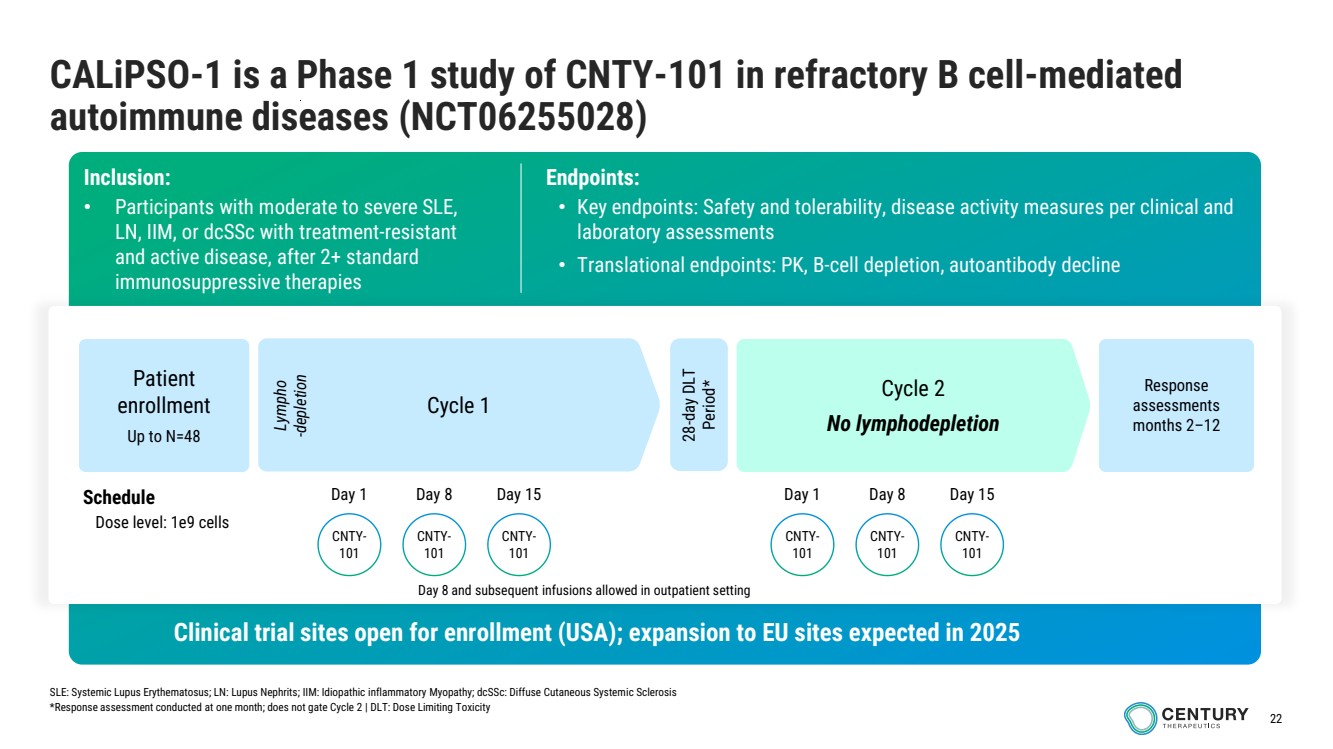
| 22
CALiPSO-1 is a Phase 1 study of CNTY-101 in refractory B cell-mediated
autoimmune diseases (NCT06255028)
infusions in outpatient setting
Inclusion:
• Participants with moderate to severe SLE,
LN, IIM, or dcSSc with treatment-resistant
and active disease, after 2+ standard
immunosuppressive therapies
Endpoints:
• Key endpoints: Safety and tolerability, disease activity measures per clinical and
laboratory assessments
• Translational endpoints: PK, B-cell depletion, autoantibody decline
Cycle 1
Cycle 2
No lymphodepletion
Schedule
Dose level: 1e9 cells
Lympho
-depletion
28-day DLT
Period*
Patient
enrollment
Up to N=48
Response
assessments
months 2–12
Clinical trial sites open for enrollment (USA); expansion to EU sites expected in 2025
SLE: Systemic Lupus Erythematosus; LN: Lupus Nephrits; IIM: Idiopathic inflammatory Myopathy; dcSSc: Diffuse Cutaneous Systemic Sclerosis
*Response assessment conducted at one month; does not gate Cycle 2 | DLT: Dose Limiting Toxicity
CNTY-101
CNTY-101
CNTY-101
Day 1 Day 8 Day 15
CNTY-101
CNTY-101
CNTY-101
Day 1 Day 8 Day 15
Day 8 and subsequent infusions allowed in outpatient setting |

| CNTY-308
CAR αβ-iT cell with Allo-Evasion 5.0 |
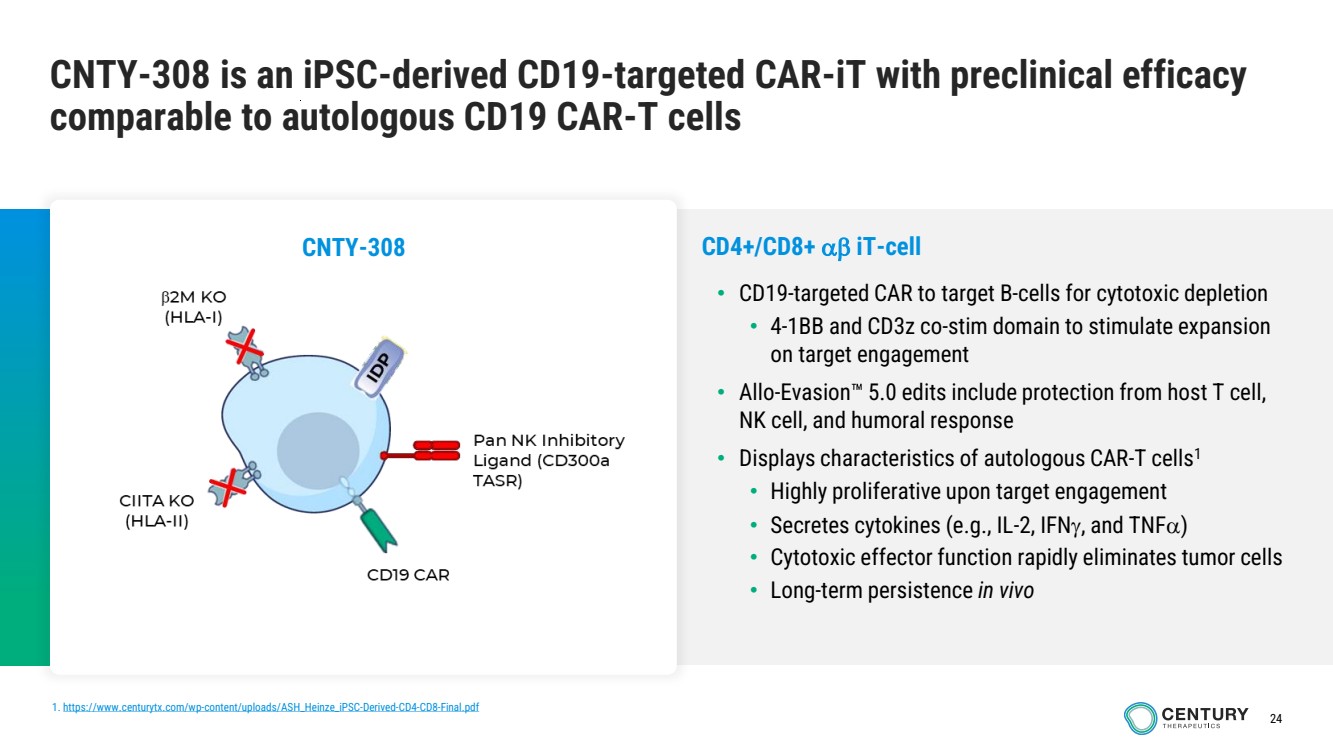
| 24
CNTY-308 is an iPSC-derived CD19-targeted CAR-iT with preclinical efficacy
comparable to autologous CD19 CAR-T cells
1. https://www.centurytx.com/wp-content/uploads/ASH_Heinze_iPSC-Derived-CD4-CD8-Final.pdf
CD4+/CD8+ iT-cell
• CD19-targeted CAR to target B-cells for cytotoxic depletion
• 4-1BB and CD3z co-stim domain to stimulate expansion
on target engagement
• Allo-Evasion 5.0 edits include protection from host T cell,
NK cell, and humoral response
• Displays characteristics of autologous CAR-T cells1
• Highly proliferative upon target engagement
• Secretes cytokines (e.g., IL-2, IFN, and TNF)
• Cytotoxic effector function rapidly eliminates tumor cells
• Long-term persistence in vivo
CNTY-308 |
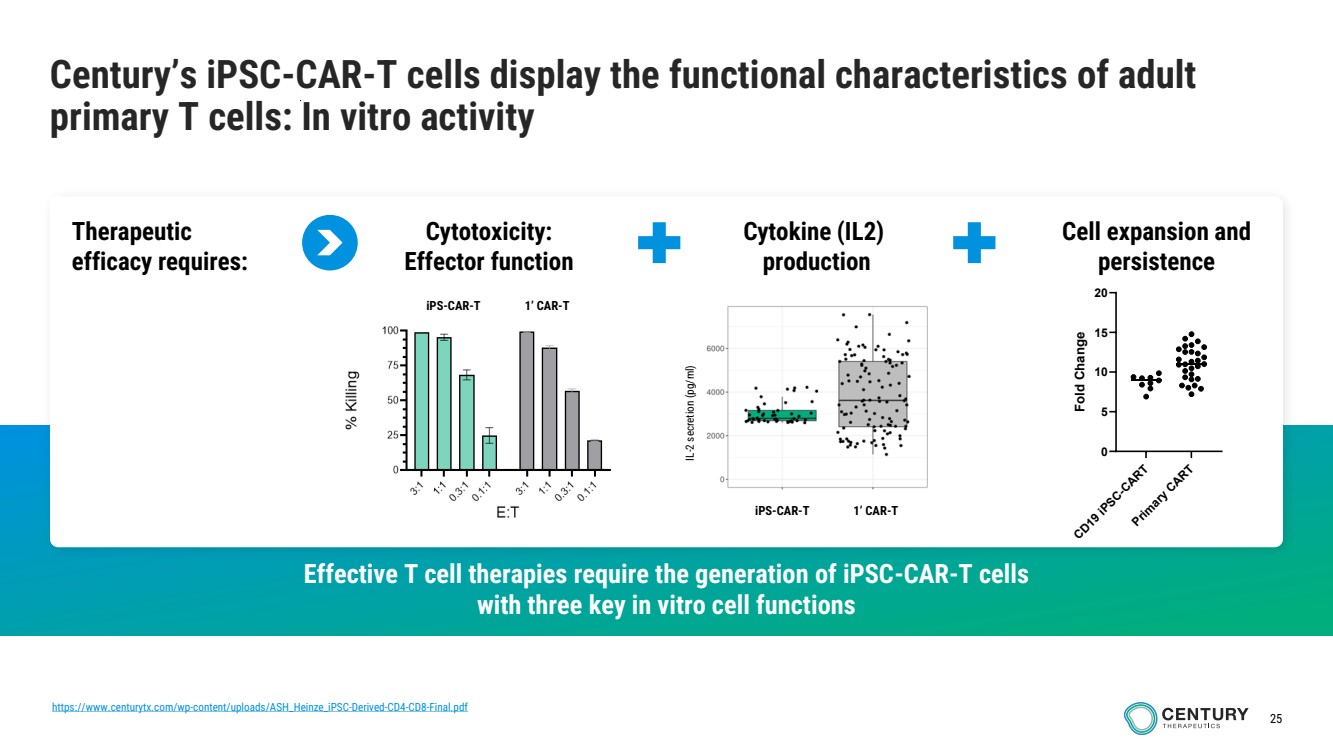
| 25
Century’s iPSC-CAR-T cells display the functional characteristics of adult
primary T cells: In vitro activity
Effective T cell therapies require the generation of iPSC-CAR-T cells
with three key in vitro cell functions
Cytotoxicity:
Effector function
Cell expansion and
persistence
Therapeutic
efficacy requires:
Cytokine (IL2)
production
iPS-CAR-T 1’ CAR-T
IL-2 secretion (pg/ml)
iPS-CAR-T 1’ CAR-T CD19 iPSC-CART Primary CART
0
5
10
15
20
Fold Change
https://www.centurytx.com/wp-content/uploads/ASH_Heinze_iPSC-Derived-CD4-CD8-Final.pdf |
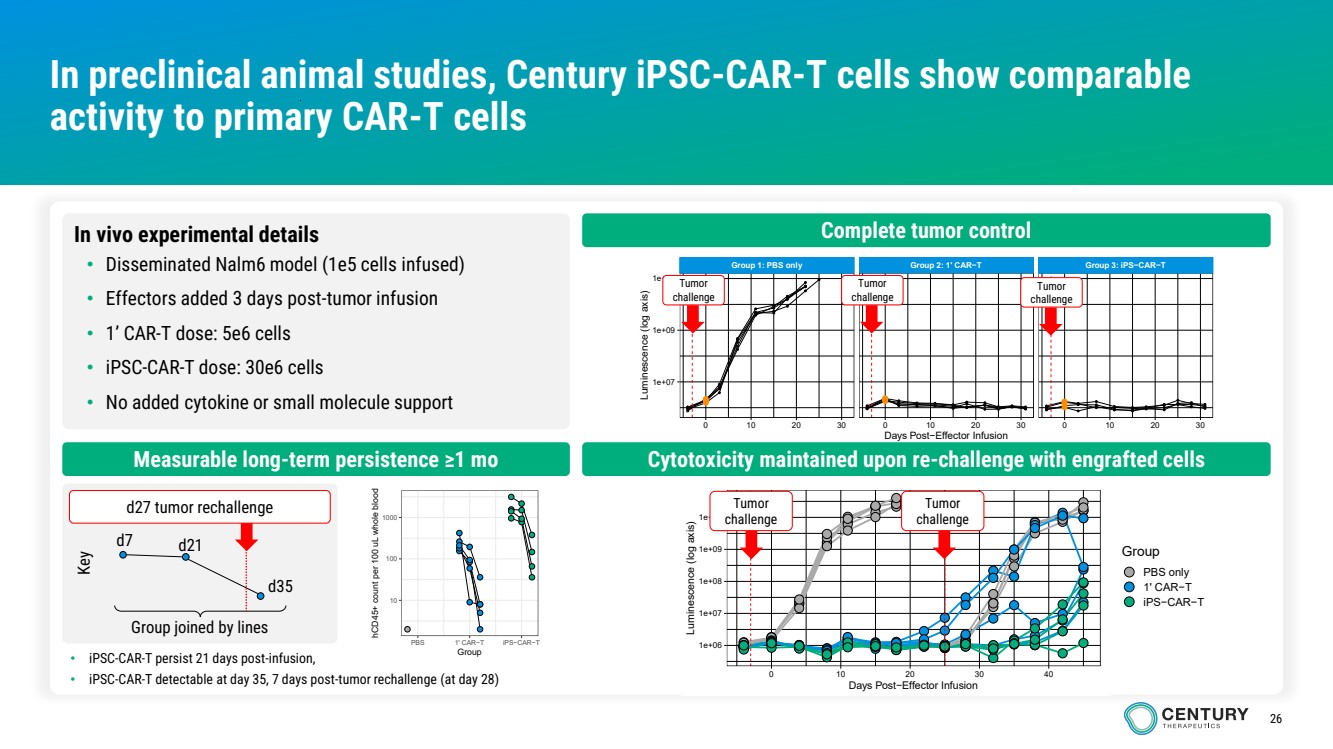
| 26
1e+06
1e+07
1e+08
1e+09
1e+10
0 10 20 30 40 Days Post−Effector Infusion
Luminescence (log axis)
Group
PBS only
1' CAR−T
iPS−CAR−T
Group 1: PBS only Group 2: 1' CAR−T Group 3: iPS−CAR−T
0 10 20 30 0 10 20 30 0 10 20 30
1e+07
1e+09
1e+11
Days Post−Effector Infusion
Luminescence (log axis)
In preclinical animal studies, Century iPSC-CAR-T cells show comparable
activity to primary CAR-T cells
Complete tumor control
Measurable long-term persistence ≥1 mo Cytotoxicity maintained upon re-challenge with engrafted cells
10
100
1000
PBS 1' CAR−T iPS−CAR−T Group
hCD45+ count per 100 uL whole blood
In vivo experimental details
• Disseminated Nalm6 model (1e5 cells infused)
• Effectors added 3 days post-tumor infusion
• 1’ CAR-T dose: 5e6 cells
• iPSC-CAR-T dose: 30e6 cells
• No added cytokine or small molecule support
Group joined by lines
d7 d21
d35
d27 tumor rechallenge
Key
Tumor
challenge
• iPSC-CAR-T persist 21 days post-infusion,
• iPSC-CAR-T detectable at day 35, 7 days post-tumor rechallenge (at day 28)
Tumor
challenge
Tumor
challenge
Tumor
challenge
Tumor
challenge
1e+06
1e+07
1e+08
1e+09
1e+10
0 10 20 30 40
Days Post−Effector Infusion
L
u
min
e
s
c
e
n
c
e (lo
g
a
xis)
Class
PBS only
1' CAR−T
iPS−CAR−T
Group
PBS only
1' CAR−T
iPS−CAR−T |

| Platform: iPSC cell foundry |
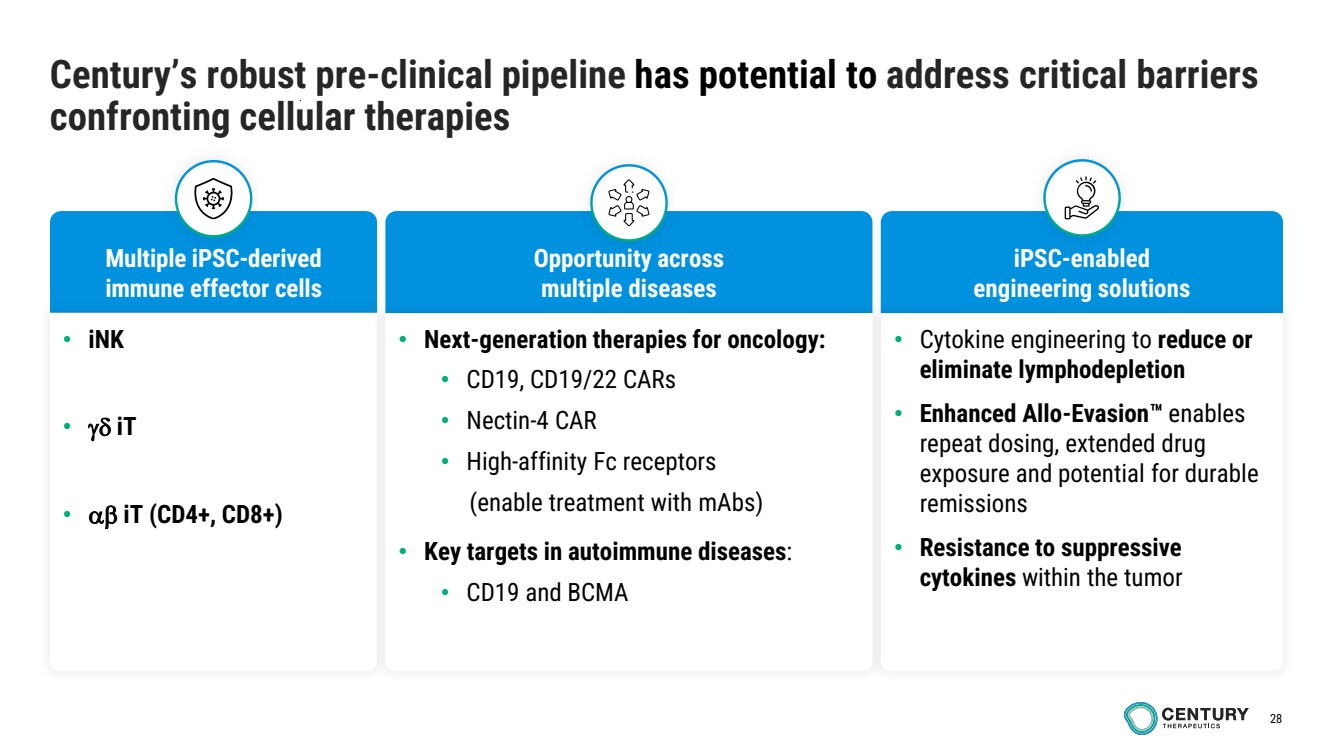
| 28
Century’s robust pre-clinical pipeline has potential to address critical barriers
confronting cellular therapies
Multiple iPSC-derived
immune effector cells
iPSC-enabled
engineering solutions
Opportunity across
multiple diseases
• Cytokine engineering to reduce or
eliminate lymphodepletion
• Enhanced Allo-Evasion enables
repeat dosing, extended drug
exposure and potential for durable
remissions
• Resistance to suppressive
cytokines within the tumor
• iNK
• iT
• iT (CD4+, CD8+)
• Next-generation therapies for oncology:
• CD19, CD19/22 CARs
• Nectin-4 CAR
• High-affinity Fc receptors
(enable treatment with mAbs)
• Key targets in autoimmune diseases:
• CD19 and BCMA |
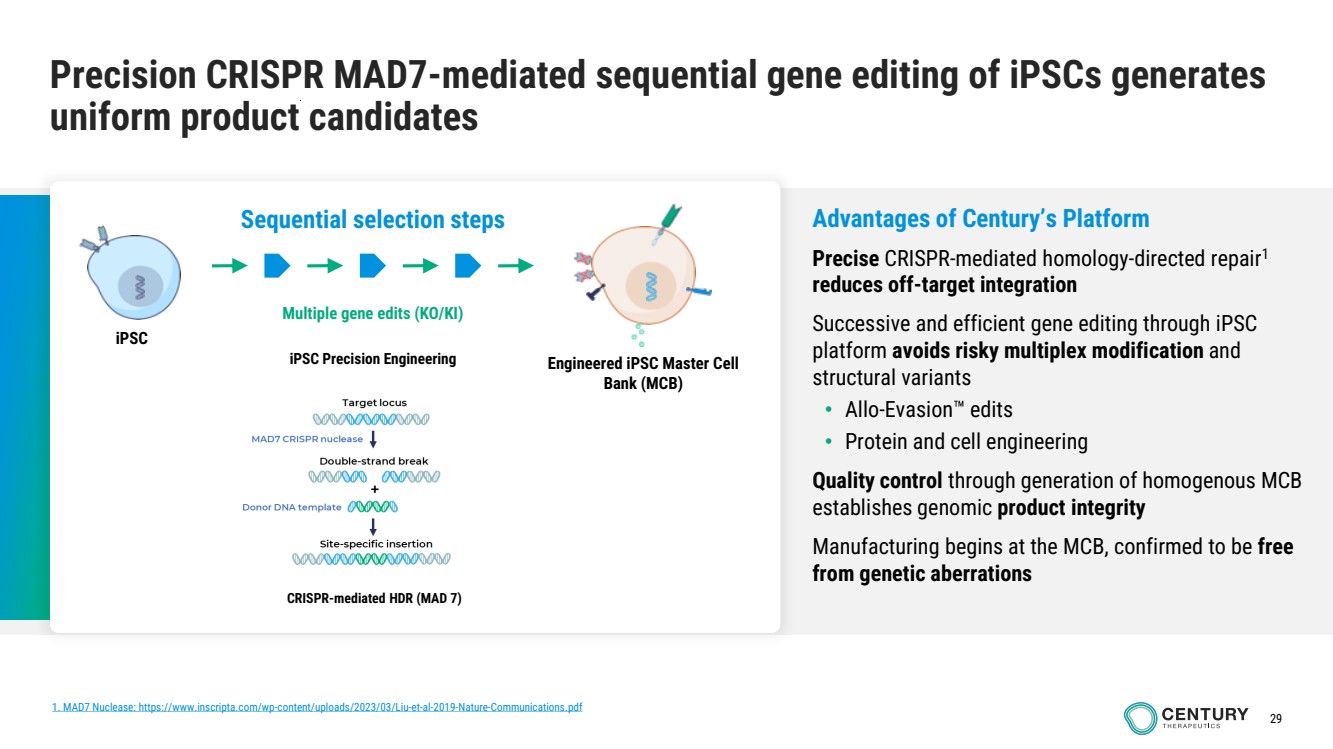
| 29
Precision CRISPR MAD7-mediated sequential gene editing of iPSCs generates
uniform product candidates
Multiple gene edits (KO/KI)
iPSC
Engineered iPSC Master Cell
Bank (MCB)
Sequential selection steps
iPSC Precision Engineering
CRISPR-mediated HDR (MAD 7)
Advantages of Century’s Platform
Precise CRISPR-mediated homology-directed repair1
reduces off-target integration
Successive and efficient gene editing through iPSC
platform avoids risky multiplex modification and
structural variants
• Allo-Evasion edits
• Protein and cell engineering
Quality control through generation of homogenous MCB
establishes genomic product integrity
Manufacturing begins at the MCB, confirmed to be free
from genetic aberrations
1. MAD7 Nuclease: https://www.inscripta.com/wp-content/uploads/2023/03/Liu-et-al-2019-Nature-Communications.pdf |

| 30
Century platform and in-house manufacturing: Pathway to scalable, profitable
cell therapy
Established in-house manufacturing from development to launch Quality product at disruptive scale and cost of goods
• Built-for-purpose 53,000 ft2 cGMP facility
• Key leaders each with 1–2 decades of cell therapy manufacturing
expertise, from leading commercial cell therapies
• In-house team facilitates aligned priorities, learnings, faster
product iteration for efficiency, speed, and product quality
• Builds and protects proprietary know-how
• Optionality with redundant sites (in-house, active CDMO)
• Consistency: Control of manufacturing and single-donor master-cell-bank over product lifetime for batch-to-batch reproducibility
• Increased cell fitness: Differentiated immune cells do not undergo
excessive expansion cycles which often result in cell exhaustion
• Product homogeneity: Clonal origin enables a well-characterized
product
• Potential to manufacture at antibody-like scale: Scalable platforms and
optimized processes to maximize yield, reduce COGs, and meet demand |
| 31
Century Therapeutics is advancing next-generation iPSC-derived allogeneic
NK and T cell therapy candidates for the treatment of cancer and autoimmunity
Ended FY24 with cash, cash equivalents,
and investments of ~$220M (unaudited*)
Cash runway into 2H26
Differentiated pipeline based on iPSC and Allo-Evasion technology
✓ Potential to overcome limitations of conventional allogeneic cell therapy
✓ Preclinical demonstration of CD4+/CD8+ iT cells with characteristics of primary T cells
Encouraging preliminary clinical data from Phase 1 trial of CNTY-101 in
R/R B-cell lymphomas
✓ 83% ORR at dose level 3B, with favorable safety profile
✓ Data supports the ability to re-dose in the presence of a restored endogenous immune system
✓ Study continuing with escalation to dose level 4B
Expansion into additional autoimmune indications
✓ CALiPSO-1 trial initiated in SLE, LN, IIM, and dcSSc participants
✓ CNTY-101 has differentiated profile in AID (allogeneic, iNK with Allo-Evasion )
✓ Multiple pipeline opportunities in AID
In-house manufacturing capabilities
✓ Efficient, scalable manufacturing
Phase 1 ELiPSE-1 trial of CNTY-101 in B-cell
malignancies
• Updated clinical data expected by mid-2025
Phase 1 CALiPSO-1 trial of CNTY-101 in B-cell mediated
autoimmune diseases
• Enrollment of patients across indications
Pre-clinical pipeline prioritization
• Prioritized pipeline to be announced in 1Q25
Multiple near-term milestones
*This estimate is unaudited and preliminary and actual results may differ due to the completion of our fiscal 2024 closing procedures. As such, this estimate should not be viewed as a substitute
for our full audited financial statements prepared in accordance with U.S. generally accepted accounting principles. |

| www.centurytx.com |
Cover
|
Jan. 13, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 13, 2025
|
| Entity File Number |
001-40498
|
| Entity Registrant Name |
Century Therapeutics, Inc.
|
| Entity Central Index Key |
0001850119
|
| Entity Tax Identification Number |
84-2040295
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
25
North 38th Street
|
| Entity Address, Address Line Two |
11th Floor
|
| Entity Address, City or Town |
Philadelphia
|
| Entity Address, State or Province |
PA
|
| Entity Address, Postal Zip Code |
19104
|
| City Area Code |
267
|
| Local Phone Number |
817-5790
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
IPSC
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Century Therapeutics (NASDAQ:IPSC)
Graphique Historique de l'Action
De Déc 2024 à Jan 2025

Century Therapeutics (NASDAQ:IPSC)
Graphique Historique de l'Action
De Jan 2024 à Jan 2025
